
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Immunol., 05 March 2021
Sec. Cancer Immunity and Immunotherapy
Volume 12 - 2021 | https://doi.org/10.3389/fimmu.2021.624293
This article is part of the Research TopicThe Aryl Hydrocarbon Receptor as a Therapeutic Target in Cancer and AutoimmunityView all 5 articles
The aryl hydrocarbon receptor (AHR) is a ligand-activated transcription factor, which interacts with a wide range of organic molecules of endogenous and exogenous origin, including environmental pollutants, tryptophan metabolites, and microbial metabolites. The activation of AHR by these agonists drives its translocation into the nucleus where it controls the expression of a large number of target genes that include the AHR repressor (AHRR), detoxifying monooxygenases (CYP1A1 and CYP1B1), and cytokines. Recent advances reveal that AHR signaling modulates aspects of the intrinsic, innate and adaptive immune response to diverse microorganisms. This review will focus on the increasing evidence supporting a role for AHR as a modulator of the host response to viral infection.
Viral infectious diseases are a major cause of death and disability for millions of people throughout the world. Many factors, from host gender, age, genetics, up to nutritional status play a role in determining the susceptibility to and pathophysiological consequences of infection. In the end, the clinical outcome is variable and greatly dependent on the net result of the damage caused both by the pathogen as well as by the immune response of the host in response to the pathogen. Importantly, environmental factors, such as chemical exposures, also contribute to differential clinical outcomes of infections at the individual and populations level.
The aryl hydrocarbon receptor (AHR) is a ligand-activated transcription factor that interacts with a diverse array of anthropogenic and natural agonists (1–5). Because of the ubiquitous distribution of AHR agonists, we are constantly exposed to a diverse spectrum of AHR ligands; AHR signaling participates in our adaptation to changing environments as defined by alterations in the diet, the microbiome and metabolism.
Recent reports have shown a role for AHR as a modulator of the intrinsic, innate and adaptive immune response to viral infections (6, 7), with both positive and negative effects on host resistance and survival based on the experimental system used. In this review, we will evaluate the role of AHR on the host response to viral infection and its potential as a candidate target for therapeutic intervention.
AHR is a member of the basic helix–loop–helix (bHLH)/PER-ARNT-SIM (PAS) superfamily of transcription factors (8). AHR is a well-conserved protein, with an ubiquitous presence in mammalian tissues, and variable expression levels among tissues and throughout life (9–11) AHR controls a broad range of biological processes in response to environmental and metabolic cues (9–11). When inactive, AHR is part of a stable cytoplasmic multiprotein complex composed by the chaperone heat-shock protein 90 (HSP-90), the co-chaperone p23 (p23) and the hepatitis B virus X-associated protein (XAP2) (12–21). This complex has been proposed to stabilize the conformation of AHR, protect it from proteolitic degradation and contribute to its subcellular localization. AHR canonical signaling pathway is triggered by the binding of an agonist (A), which triggers a conformational change that exposes AHR nuclear localization signal (NLS), resulting in the nuclear translocation of the A-AHR-HSP90-p23 complex via β-importins. There is still some discrepancy over the role of XAP2 on nuclear translocation. Some studies suggest that XAP2 is involved in the cytoplasmic anchorage of the AHR complex. However, other studies suggest that XAP2 interferes with the interaction of the NLS with β-importins; this last interpretation would require the XAP2 to be released from the AHR complex before the nucleocytoplasmic shuttling occurs (12–16, 21–28). Inside the nucleus, the chaperones disassemble from the complex, the AHR-A structure heterodimerizes with the AHR nuclear translocator (ARNT) and interacts with specific sequences in DNA (xenobiotics response element, XRE) to control the expression of target genes (12, 13, 19, 21, 29, 30). Among these target genes are those encoding the Cytochrome P450 enzymes, specifically, members of the families 1 and 2: CYP1A1, CYP1B1, and CYP2A1 (21, 30) (Figure 1A).
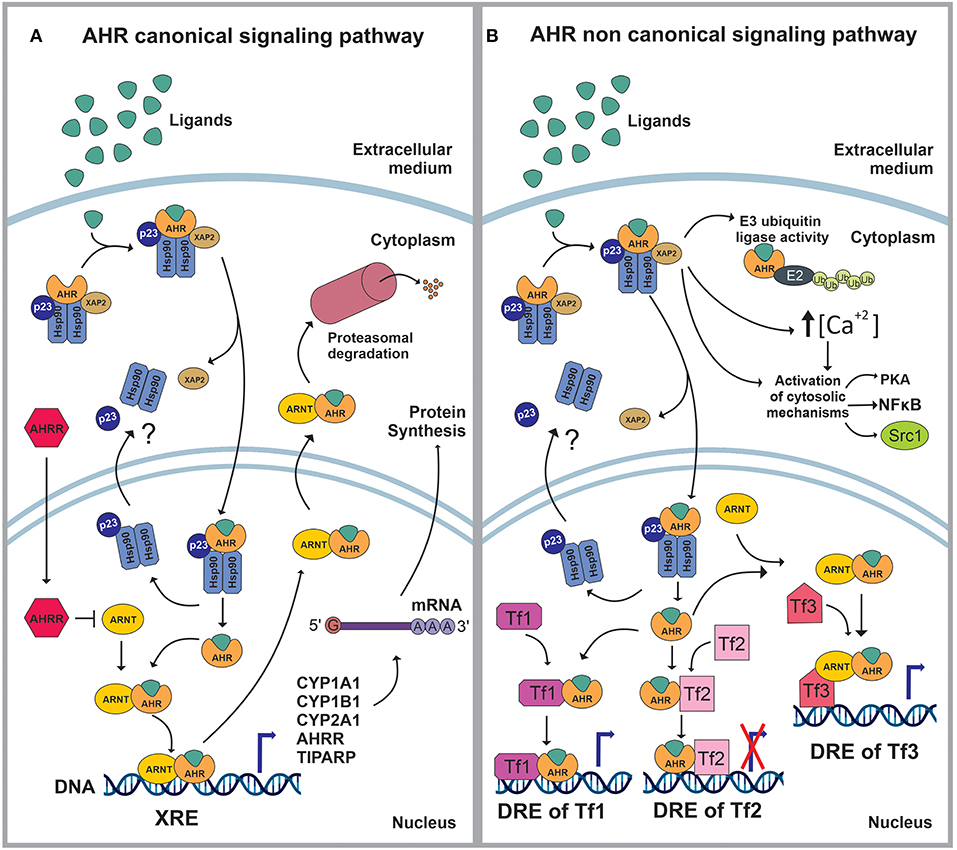
Figure 1. (A) In stationary state, the aryl hydrocarbon receptor (AHR) is part of a complex composed by the 90kDa heat shock protein (HSP90), the co-chaperone p23 (p23) and the hepatitis B virus X-associated protein (XAP2/AIP/ARA9). The complex stabilizes AHR conformation, protects AHR from proteolitic degradation and contributes to its subcellular localization. The ligand (L) binding triggers a conformational change in AHR exposing a nuclear localization signal (NLS). Then, XAP2 is released from the complex and the L-AHR-HSP90-p23 structure translocates into the nucleus via β-importins. Inside the nucleus, the chaperones are released from the complex and return to the cytoplasm whilst the AHR-L structure heterodimerizes with the aryl hydrocarbon receptor nuclear translocator (ARNT), interacts with its DNA- response-elements -xenobiotics response element (XRE)- and regulates the expression of different genes. The AHR canonical signaling pathway is characterized by the expression of CYP1A1, CYP1B1, CYP2A1, TIPARP and AHRR. Following the modulation of its target genes, the ARNT-AHR-L complex exits the nucleus and is targeted for proteasomal degradation. (B) The AHR non-canonical signaling pathway involves the regulation of cytoplasmic proteins as well as the control of gene expression. Within the cytoplasm, the AHR-L complex can function as an E3 ubiquitin ligase, promoting the proteasomal degradation of target proteins. It also increases the intracellular Ca2+ levels and interacts with different proteins such as PKA, NFκB and Src1. Once in the nucleus, AHR-L is capable of interacting and controlling the activity of other transcription factors (Tf) through transactivation/transrepression or it can exert a Co-activator/Co-Inhibitor role when it is associated with ARNT.
AHR also controls biological processes through a non-canonical signaling pathway, which involves multiple molecular mechanisms. For instance, AHR activation can result in the increase of the intracellular concentrations of Ca+2, or the activation of Src tyrosine kinase and focal adhesion kinase. Regarding the non-canonical genomic regulation, it has been reported that AHR can heterodimerize with other nuclear proteins, including transcription factors, to modulate their activity by transactivation/transrepression or protein-protein interactions. In this context, AHR has been shown to cross-talk with the nuclear factor kappa light chain enhancer of activated B cells (NFκB), activator protein-1 (AP-1), estrogen receptor (ER) and glucocorticoid receptor (GR), Krüppel-like Factor 4 and 6 (KLF4, KLF6), signal transducers and activators of transcription (STAT) proteins and members of the CCAAT-enhancer-binding proteins (C/EBP) family (2, 20, 21, 30–34) (Figure 1B).
The first AHR agonists identified were the non-halogenated polycyclic aromatic hydrocarbons (PAH) and halogenated aromatic hydrocarbons (HAH), which are major anthropogenic pollutants. These compounds are quite abundant and persistent in the environment due to their long half-life and bioaccumulation in the trophic chain. Their toxicity has been largely documented in humans as well as in other species with estimates indicating that more than 90% of the human exposures occur via contaminated food (21, 35–41). However, over the last decades, a wide variety of agonists from multiple sources such as the environment, the microbiome, the diet and metabolism have been shown to activate AHR. Currently they are classified into natural or synthetic, endogenous or exogenous agonists depending on their nature and sources (Figures 2, 3) (2, 21, 42, 43).
Multiple AHR modulators are used to study the function of this pathway in the control of immunity and other biological processes. The most utilized AHR agonists include the 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), an industrial by-product that persists as contaminant in the environment and is well-known for its toxicity in humans and other species; the 6-formylindolo(3,2b)carbazole (FICZ), an endogenous tryptophan (Trp) photoproduct; the endogenous indole derivative indoxyl 3-sulfate (I3S) and kynurenine (Kyn), a metabolite from the Trp catabolic pathway product from the enzymatic activity of the tryptophan 2,3-dioxygenase-2 (TDO2) and indoleamine 2,3-dioxygenase−1/2 (IDO-1/IDO-2) (Figures 2, 3). IDO-1 is expressed in response to IFN-γ stimulation and is thought to contribute to IFN-γ antiviral activity by the depletion of L-Trp during Kyn generation (44–47). Kyn produced by IDO-1 can then activate AHR, which can further boost IDO-1 expression, establishing an IDO1-AHR-IDO1 positive feedback loop to prolong AHR activation (21, 48).
Conversely 2-methyl-2H-pyrazole-3-carboxylic acid (2-methyl-4-o-tolylazo-phenyl)-amide (CH223191) is a synthetic potent and specific AHR competitive antagonist that preferentially inhibits the response to TCDD and related HAHs (Figure 3).
AHR agonists are incorporated from the environment via the oral route (e.g., dietary agonists), and also via their inhalation and absorption across mucosal barriers (1, 5, 49, 50). The environmental levels of AHR-activating pollutants (i.e., dioxins, PAH and HAH) are being reduced in the most developed countries, but their levels are increasing in the developing world (51–54). Therefore, these AHR agonists remain a continued threat to public health. Moreover, epidemiologic and animal-model studies suggest that environmental chemicals influence host responses to infectious diseases (55), and strong associations have been described between dioxin and HAH levels and viral respiratory tract infections, wheezing, and poor vaccine responses in infants and children (56–62). Hence, based on its multiple effects on the immune response, the modulation of AHR signaling by environmental chemicals is likely to have important effects on the host response to viral infection.
AHR regulates numerous genes associated with cellular homeostasis, proliferation, immune maturation and function, hematopoietic stem cell expansion, glucose tolerance, and the development of many human diseases. Indeed, accumulating evidence supporting a role for AHR signaling in various diseases has encouraged the investigation of the potential impact of AHR genetic variants in the susceptibility and development of human disorders. For instance, a splicing variant of AHR has been associated with retinitis pigmentosa (63). In addition, single-nucleotide polymorphisms (SNPs) in AHR were identified as risk factors for the development of lung cancer in a population of Chinese cigarette smokers (64); other AHR SNPs were recently associated with higher risk of Crohn's disease (65, 66). In addition, AHR genetic variants have been linked to endocrine disorders such as cyclical Cushing's disease (67) and to acromegaly or somatotropinoma (68, 69). Finally, AHR variants have been identified as risk factors for the development of coronary arterial (70) and heart disease (71). Taken together, these studies highlight the potential contributions of AHR polymorphisms to human disease, and call for future studies focused on the investigation of the role of these polymorphisms in viral infections.
Evidence showing that AHR activation has a strong influence on host resistance to viral infection was first reported over 40 years ago (72, 73). Those studies concluded that even very low doses of the AHR agonist TCDD enhance morbidity and mortality in rats and mice infected with lethal strains of influenza A virus (IAV) (74, 75). Influenza viruses are negative-sense single- stranded RNA viruses belonging to the Orthomyxoviridae family. IAVs cause acute respiratory infections in humans and are a great burden to public health and the global economy. As a result of the 2009 H1N1 pandemic, more than 120,000 people died worldwide, the majority of which were in the young age range (<65 years old) (reported by CDC). Avian influenza strains such as the H5N1 and H7N9 have also raised concern for future pandemics due to their capacity to cross the species barrier and cause lethal infections in humans. Although vaccination against seasonal influenza is an essential part of the public health strategy, its efficacy is variable, and there are only few therapeutic options for people who become infected.
Host defense during a primary IAV infection is mainly promoted by virus-specific CD8+ cytotoxic T lymphocytes (CTL), which kill infected cells in the lung (76). At this stage, B cells do not play a major role as they do not produce virus-specific antibodies. However, class-switched B cells, antibody-secreting plasma cells, and memory B cells participate in the generation of antibodies that protect against repeated infection with homotypic virus strains (77). It has been reported that AHR is highly induced upon B cell activation and has a critical role in regulating activation-induced cell fate outcomes. Of note, AHR suppresses antibody class switching in vivo after IAV infection and immunization with model antigens (78).
In addition, IAV-specific CD4+ T cells are key for the generation of virus-specific antibodies by B cells and the establishment of immunologic memory; additional functions are played by Foxp3+CD25+ regulatory CD4+ T cells (Tregs), Th17, and T follicular helper (TFH) cells during virus infection (79–82). Importantly, early life exposure to chemical AHR ligands alters the CD4+ T cell responses to IAV infection in adulthood (83). Finally, upon successful viral clearance, a pool of memory lymphocytes remains, which secures a rapid response if the same or similar influenza virus strains are later encountered. The proper regulation of these cell populations is required to generate an immune response which can successfully resolve the viral infection while averting an excessive inflammatory response with its associated immunopathology.
Treatment with a single oral dose of TCDD has been shown to increase morbidity and mortality in mice infected with a sublethal inoculum of IAV. The adaptive immune response is impaired upon AHR activation, with a marked reduction in dendritic cell (DC) function, the expansion and differentiation of CD4+ and CD8+ T cells, and virus-specific IgG titers (84). Recent genome wide transcriptional analyses of DCs isolated from lungs of IAV-infected mice treated with TCDD detected a strong down-regulation of CD209a and CCL17 expression. Indeed, Ingenuity Pathway Analysis (IPA) revealed that the most altered signaling pathways in these DCs are related to immune cell trafficking, cellular movement and hematological system development and function (85). Interestingly, although AHR activation increases morbidity and mortality caused by IAV infection, the kinetics of viral growth and the efficacy of the viral clearance, are not significantly different between control and TCDD-treated mice in primary infections nor even during homotypic reinfections (86–89). These findings suggest that the dysregulated expansion of the virus itself is not to blame for the poorer outcomes of TCDD-treated mice. Indeed, AHR activation increases the recruitment of neutrophils to the infected lung, and elevates the expression of IFNγ and the inducible nitric oxide synthase (iNOS) in the lungs during IAV infection (90, 91). These molecules could participate in antiviral mechanisms, but their dysregulated activity promote immunopathology which results in poorer survival. Indeed, neutrophil depletion markedly improves survival and abrogates the enhancement of broncho-pulmonary inflammation triggered by AHR activation with TCDD during acute IAV infection (92).
Studies using AHR mutant mice suggest that changes in the host response to IAV are mainly driven by direct interactions of AHR with XREs (85, 91). However, the specific AHR gene targets involved in the altered host responses to IAV induced by TCDD remain to be determined. Recent reports based on adoptive transfer, bone marrow transplantation, and conditional gene ablation have shown that AHR modulates both the response of hematopoietic cells, endothelial cells, and lung epithelial cells to IAV (93), suggesting that CD8+ T cell responses to IAV are suppressed by AHR signaling via indirect mechanisms (94). In addition, the increase in neutrophils and iNOS and IFNγ expression in the lungs of TCDD-treated mice is independent of AHR expression in hematopoietic cells (93). Instead, arh knockout mice revealed that the increased pulmonary neutrophilia induced by TCDD requires AHR activation in the respiratory epithelium, while the increase in iNOS expression is dependent on AHR activation in endothelial cells (91). Hence, multiple cell types participate in the AHR-mediated alterations of the host response to IAV.
Further insights were provided by the comparison of the alterations induced in the immune response to IAV by four representative AHR agonists: (1) TCDD, (2) 3,3′,4,4′,5-pentachlorobiphenyl (PCB126), a pollutant with documented human exposure, (3) 2-(1′H-indole-3′-carbonyl)-thiazole-4-carboxilic acid methyl ester (ITE), an AHR agonist isolated from mucosal tissues, and (4) FICZ, a degradation product of Trp. All these AHR ligands diminished virus-specific IgM levels and increased the proportion of regulatory T cells (7). TCDD, PCB126 and ITE, but not FICZ, reduced virus-specific IgG levels and CD8+ T cell responses. Similarly, ITE, PCB126, and TCDD reduced Th1 and T follicular helper cells, whereas FICZ increased their frequency. In Cyp1a1-deficient mice, all compounds reduced the response to IAV. Ahr knockout mice denoted that these compounds require AHR within hematopoietic cells to exert their effects (7). These findings suggest that the differential effects of specific AHR agonists on the immune response to IAV reflect differences in the half-life of the agonists, and potentially the induction of different AHR conformations. A deeper understanding of the mechanisms behind these ligand-specific effects will pave the way for the design of AHR-targeted therapeutics.
Coronaviruses (CoVs) are a family of positive-sense single-stranded RNA viruses with public health and agricultural importance. They mostly cause enteric or respiratory disease, which can be severe and life threatening. Human CoVs first come under the spotlight when outbreaks of the severe acute respiratory syndrome (SARS-CoV-1) and the Middle East respiratory disease (MERS-CoV) were reported in 2002-03 and 2012, respectively. At the end of 2019, a new human CoV (SARS-CoV-2) was identified in Wuhan, China, and associated with a severe respiratory infection, known today as CoVs disease 2019 (COVID-19). Compared to other positive-sense RNA viruses, CoVs have an exceptionally large genome (30 kb) and employ a complex genome expression strategy (95); our knowledge of host factors involved in CoVs replication is still extremely limited.
It was recently reported that AHR is activated in cells infected with a prototypic CoV, mouse hepatitis virus (MHV), resulting in the expression of several effector genes (96). Indeed, AHR was shown to be important for modulation of the host immune response to MHV, playing a role in the expression of the downstream effector TCDD-inducible poly(ADP-ribose) polymerase (TiPARP), which is required for maximal viral replication. In accordance with this, knockdown of TiPARP reduced viral replication and increased IFN expression, suggesting that TiPARP is a proviral factor for MHV infection. Moreover, MHV replication induced the expression of other AHR-driven genes in macrophages and DCs of infected mice. The pharmacologic modulation of AHR activity regulated the expression levels of cytokines induced by infection, specifically, interleukin 1β (IL-1β), IL-10, and TNF-α, supporting a role for AHR activation in the host response to MHV infection. Of note, while IDO-1 drives AHR activation in the context of other infections, MHV induced a similar expression level of downstream genes in wild-type and IDO-1-/- macrophages, suggesting that additional pathways besides IDO-1 are involved in AHR activation.
In the context of the explosive amount of research performed on SARS-CoV-2, a model was proposed where SARS-CoV-2 would activate AHR by an IDO1-independent mechanism, initially bypassing the IDO1-Kyn-AHR pathway, and then AHR would enhance its own activity through an IDO1-AHR-IDO1 positive feedback loop prolonging activation induced by this novel pathogen (97). In this sense, researchers discussed the possibility of a direct activation of AHRs by CoVs inducing immediate and simultaneous up-regulation of diverse AHR-dependent downstream effectors, which in turn, would result in AHR-related syndromes, consisting of inflammation, thromboembolism, and fibrosis, finally culminating in multiple organ injuries, and death (98).
The activation of AHRs by CoVs may lead to a diverse set of phenotypic disease scenarios depending on the time after infection, overall state of health, hormonal balance, age, gender, co-morbidities, diet and environmental factors modulating AHR signaling. In addition, infection by SARS-CoV-2 or the non-related respiratory syncytial virus (RSV), results in increased AHR and IDO-1 lung expression, concomitant with increased pro-inflammatory gene expression, activation of the Tissue Factor/Plasminogen Activator Inhibitor-1 (TF/PAI-1) signaling pathway, and up-regulation of CYP1A1 (98). Finally, bioinformatic screens of novel approaches for the therapeutic modulation of AHR signaling established that dexamethasone may down-regulate both AHR and IDO-1 expression, while calcitriol/vitamin D3 may down-regulate AHR, and tocopherol/vitamin E may down-regulate IDO-1 (98).
Flaviviruses comprise a group of positive-sense single-stranded RNA viruses of ~9–13 kb that cause severe endemic infection and epidemics on a global scale. Representative members of this group include dengue, West Nile, and Zika viruses. Flaviviruses constitute a significant health issue worldwide and many members of this family have shown potential to emerge and cause outbreaks in non-endemic geographical regions. Additionally, reemergence in areas where circulation was previously thought to be contained has been observed, such as the case of the 2018 outbreak of yellow fever virus in Brazil. Other medically-important flaviviruses such as Japanese encephalitis virus, which circulates mainly in Southern and Southeastern Asia, or tick borne encephalitis virus, which is endemic in parts of Eurasia, have not yet expanded globally. However, because their vectors are widely distributed, they do have potential for spreading. Of note, other worldwide human important diseases associated with this family include hepatitis C virus (99).
Zika virus (ZIKV) is a mosquito-vectored flavivirus isolated in 1947 in Uganda. Its circulation has been reported in humans from West Africa and Asia since the mid-1950s (100). ZIKV infection used to be a neglected disease for most of its history due to the mildness of its symptoms and the fact that it was geographically restricted. However, in 2015 Brazil registered an unprecedented epidemic (101) characterized by a high incidence of microcephaly cases, formally accepted to be linked to ZIKV in April 2016. The mechanism by which ZIKV crosses the placenta is still unclear, but its neurotropism and ability to destroy neural cells have been well-established (102). Neural progenitor cells (NPCs) are the primary target of the ZIKV, and this may partly explain the high number of abnormalities detected in neuroimaging examinations (103). It was reported that environmental factors strongly correlate with nutrition and socioeconomic position affecting the immune status and response to ZIKV infections (85). When analyzing this relationship for Recife (Pernambuco, Brazil), a city that was severely hit by ZIKV, it was found that cases of reported microcephaly in 2015 and 2016 were largely concentrated in areas with more impoverished living conditions (104). Noticeably, AHR is highly expressed in the human placenta and its expression is upregulated in placentas of women suffering unexplained miscarriages (105, 106). Hence, it is conceivable that sustained AHR activation in women exposed to pollutants and living in impoverished conditions with degraded housing and malnutrition (107, 108) might increase their susceptibility to ZIKV infection and ZIKV congenital syndrome.
Consistent with these observations, we recently showed that ZIKV infection up-regulates IDO-1 and AHR expression in first trimester trophoblast cells (109) and NPCs (106). Indeed, AHR was identified as a key proviral factor for ZIKV infection. In addition, it was found that ZIKV infection triggers AHR activation, limiting the production of IFN-I, involved in antiviral immunity and favoring viral replication in vitro. Importantly, the relevance of these findings was further evaluated using an in vivo murine model, in which AHR pharmacologic inhibition blocked ZIKV replication and ameliorated newborn microcephaly. These results suggest that AHR is a candidate target for host-directed therapies for flavivirus infection.
Dengue virus (DENV) is a flavivirus endemic in many tropical and sub-tropical countries where the transmission vectors Aedes spp. mosquitoes are present. There are four serotypes of DENV (DENV1-DENV4). Each serotype is antigenically different, meaning they elicit heterologous antibody responses. Infection with one serotype elicits neutralizing antibodies to that serotype. Cross-protection from infection with other serotypes is short lived; instead heterotypic infection can cause severe disease. After DENV infection, activation of innate immune pathways occurs, including IFN-I, complement, apoptosis, and autophagy, which the virus can evade or exploit to exacerbate disease.
With regards to the impact of AHR modulation on DENV infection, only in vitro data is available, suggesting that the pharmacological inhibition of AHR suppresses DENV replication (106). AHR activation in A549 cells with I3S increased DENV2 yield, as determined by standard plaque assay. Conversely, AHR inhibition with the antagonist CH223191 reduced viral RNA level, viral protein expression and viral titer in culture supernatants. Moreover, the knockdown of AHR diminished the production of DENV2 infectious viral particles. Of note, the treatment with the AHR antagonist CH223191 prior the infection with DENV1, DENV3, and DENV4, showed a comparable antiviral effect to the one already described for the best characterized serotype, DENV2 (106). The four dengue serotypes usually co-exist in the same geographical regions, which also leads to increased disease severity mediated by antibody-dependent enhancement (110). Also, different flaviviruses (e.g., ZIKV, yellow fever) may co-exist in the same regions as well. Therefore, the development of a single drug effective against many flaviviruses represents an example of a “one-drug, multiple bugs” approach (111) which may translate into major benefits, including: (i) the ability to treat future yet-unknown flavivirus outbreaks, (ii) the potential administration of a drug even before a differential diagnosis between flaviviruses can be made, and (iii) much lower research and development costs.
Hepatitis C virus (HCV) is a flavivirus belonging to the Hepacivirus genus. HCV infection can result in a persistent disease (112), remaining asymptomatic for years before the development of severe liver pathology including cirrhosis and hepatocellular carcinoma. HCV regulates critical signaling pathways in hepatocytes, and actively evades the antiviral immune response. In particular, HCV modulates cell metabolism and remodels specialized membrane structures and organelles such as double-membrane vesicles and lipid droplets, favoring virus replication and virion assembly. However, the molecular bases of these host-virus interactions are still unclear. Recently it was demonstrated that the benzamide derivative flutamide, which has been shown to act as an AHR antagonist (113), inhibits the cellular capacity to produce infectious HCV particles (113). Flutamide blocks the biogenesis of lipid structures in HCV-infected cells, disrupting virion assembly, indicating that AHR plays a key role in modulating LD storage and HCV infection. This novel role of AHR in lipid biogenesis was also confirmed in non-infected Huh-7 cells and primary human hepatocytes, suggesting that AHR regulates store lipid reserves independently of viral infection. Indeed, the product of the prototypic AHR target gene CYP1A1 was identified as the main regulator of AHR-mediated lipid biogenesis (113). Indeed, the inhibition of AHR-induced CYP1A1 up-regulation diminished the lipid droplet enlargement. Conversely, the enhanced expression of CYP1A1 restored lipid reserves in AHR-inhibited cells. Altogether, these data identify a role for AHR in the control of lipid biogenesis, a hallmark of HCV infection that boost the production of viral particles, identifying AHR as a candidate therapeutic target for HCV infection.
Human immunodeficiency viruses (HIV) belong to Retroviridae family, Orthoretroviridae subfamily in Lentivirus genus. They harbor spherical particles surrounded by an envelope whose outer part contains glycoproteins. The genome consists of two positive-sense single-stranded RNA copies and can be transcribed into double-stranded DNA by a reverse transcriptase, an enzyme contained in the viral particle. This provirus can integrate into the host cell chromosome until the initiation of a replication cycle (114).
The acquired immunodeficiency syndrome (AIDS) caused by HIV-1 is a progressive condition in which virus-induced immune dysfunction results in the development of serious opportunistic infections and cancers. Several metabolic pathways are altered by HIV-1 infection, with an impact on immune activation, inflammation, and acquisition of non-AIDS co-morbid diseases. Unusual high levels of Kyn have been detected in association with accelerated HIV-1 pathogenesis, but the molecular mechanism behind this observation remains unclear. However, it was recently reported that AHR is activated by Trp metabolites to favor HIV-1 infection and reactivation (115), suggesting a novel role for AHR in AIDS. AHR directly binds to the HIV-1 5′ long terminal repeat (5′-LTR) to activate viral transcription. Moreover, the binding of AHR with Tat viral protein facilitates the recruitment of positive transcription factors to viral promoters. These findings elucidate a previously unappreciated mechanism through which cellular Trp metabolites affect HIV pathogenesis, and also suggest that AHR signaling may be targeted to modulate HIV-1 infection (115).
In another set of studies, in vitro treatment with TCDD, benzo[a]pyrene, and 3-methycholanthrene increased HIV gene expression and the level of secreted p24 viral protein in several different cell lines; the use of mutant and dominant negative AHR constructs suggest that these effects on HIV-1 are AHR-dependent. Interestingly, the simian retrovirus SIV Vpx protein provides complete and partial resistance to the antiviral effects of AHR (116). Interactions between AHR and NF-κB have been implicated in some, but not all, of these studies (117–120), leaving the molecular mechanisms by which AHR impacts viral latency and viral replication, as well as their in vivo relevance, uncertain.
The large family of DNA genome Herpesviridae causes infections and diseases in multiple animal species, including humans. In particular, five herpesviruses are extremely widespread among humans: HSV-1 and HSV-2 (causing orolabial and genital herpes), varicella zoster virus or HHV-3 (causing chickenpox and shingles), Epstein–Barr virus or HHV-4 (responsible for several diseases, including mononucleosis and some cancers), and cytomegalovirus or HHV-5. More than 90% of adults have been infected with at least one of these pathogens (121–126). In addition, Kaposi's sarcoma-associated herpesvirus (KSHV), also known as HHV-8 is an important human health problem among immunocompromised people.
Ocular HSV-1 infection can result in chronic cornea inflammation driven by conventional CD4+ T cells and neutrophils, which ultimately leads to blindness. A recent study showed that TCDD reduces effector Th1 and Th17 cells, neutrophilic inflammation, and increases Foxp3+ Tregs in a mouse model of ocular HSV-1 infection (127). In this HSV-1 model, TCDD-treated mice harbored higher virus titers, and many succumbed to herpes encephalitis if AHR was activated before to infection. However, when AHR activation was triggered post HSV-1 infection, herpes encephalitis was reduced and there was improved pathology in the eye tissue. Hence, the timing of AHR activation seems to control the balance between limiting immunopathology and eliminating anti-virus protective immunity.
Human cytomegalovirus (HCMV), a beta-herpesvirus, causes severe birth defects in newborn infants and serious disease in immunocompromised patients (128). To replicate, HCMV must interfere with cellular DNA replication and block the cellular innate immune response, specifically IFN production and the expression of interferon-stimulated genes (ISGs). IDO-1 activation is believed to restrain HCMV multiplication by depriving the infected cells of Trp, while it can also limit virus-induced immunopathology via the production of Kyn. Indeed, elevated Kyn levels are detected during HCMV replication, high plasma Kyn levels have been correlated with HCMV reactivation in renal transplant recipients, and AHR activation has been shown to promote viral replication (129). Moreover, recent reports have described the interplay between AHR signaling and HCMV replication: (i) HCMV infection of primary human fibroblasts triggers the persistent induction of AHR transcriptional activity; (ii) Sustained AHR activity is associated with tightly balanced IDO-1 activity; (iii) AHR signaling is required for the efficient replication of virus; (iv) HCMV induced G1/S cell cycle arrest depends on AHR activity; and (v) HCMV exploits AHR signaling to counteract the innate antiviral immune response, in a negative feedback loop containing IDO1-Kyn-AHR (129). Follow up studies are needed to establish the molecular mechanisms by which endogenous AHR signaling modulates HCMV infection.
A relationship between Epstein Barr virus (EBV) and exposure to environmental AHR agonists has been proposed as a risk factor for non-Hodgkin lymphoma and other diseases (130, 131). Independent studies also detected a link between AHR and EBV genes and/or proteins. For example, the nuclear protein EBNA-3, which contributes to the transformation of EBV-infected B cells (132) interacts with AHR and the AHR chaperone protein XAP-2 (133, 134). However, the mechanistic roles of these associations in diseases such as non-Hodgkin lymphoma remains to be revealed. Surprisingly, it has been reported that EBNA-3 interacts with AHR in a ligand-independent manner. However, EBNA-3 boosts the TCDD-induced transcription of an AHR-driven reporter gene, suggesting that AHR activation synergizes with the effects of EBNA-3 during the control of AHR-target genes. The molecular mechanisms underlying the functional interactions between EBNA-3 and AHR might involve XAP2, which retains AHR in the cytoplasm in the absence of an exogenous ligand. Additional mechanisms might be involved, because XAP2 translocates to the nucleus in the presence of EBNA-3, which suggests that EBNA-3 might stabilize transcriptionally active AHR in the nucleus. Of note, these reports are the first to describe the physical interaction of AHR with a viral protein, identifying new roles and molecular mechanisms for AHR in virus-host interactions.
As discussed in the previous sections, the available data support a role for AHR in the regulation of the host immune response against many viruses. Because AHR activity can be regulated by small molecules, AHR is an attractive target for therapeutic immunomodulation in the context of virus infection. Indeed, in pre-clinical models, AHR antagonists were shown to ameliorate ZIKV congenital syndrome in mice (106), increased survival in IAV infected mice (135) and reduce lung pathology induced by SARS-CoV-2 in hACE2-transgenic mice (136). However, clinical studies evaluating the safety and efficacy of targeting AHR for the treatment of viral infections are lacking. Conversely, many studies have evaluated the AHR pathway as a candidate target for the treatment of other diseases such as cancer (137). Epacadostat (Incyte Corp), the first IDO1-selective inhibitor to be tested on large phase 3 trials, failed to demonstrate anticancer efficacy, but a second generation of IDO inhibitors are currently being investigated. Promising pre-clinical results have been obtained for many AHR antagonists, including HP163 (Hercules Pharmaceuticals, also successfully tested against ZIKV in vivo) and PX-A24590 (Phenex Pharmaceuticals). Moreover, two additional AHR antagonists are currently under clinical trials: BAY2416964 (Bayer) and IK-175 (Ikena Oncology). All the lessons being learnt from the development of drugs targeting AHR in the context of other diseases will likely impact the development of molecules targeting AHR to treat viral infections.
Antiviral drugs can be classified in two major categories: direct-acting antiviral agents (DAAs) or host-targeted antivirals (HTAs). While DAAs target virus components, HTAs target host molecules that impact viral replication. HTAs offer two major advantages over DAAs: i) They can target a broad range of viruses that require a specific host factor, and ii) They minimize the selection of drug-resistant virus strains. However, the major caveat of HTAs is the greater risk of cellular toxicity. In this context, the targeting of AHR is no different. In order to address the potential issue of cellular toxicity, the use of nanoparticles as a vehicle to deliver AHR modulators to specific cell types has been proposed (138, 139). This approach has been shown to minimize toxicity and maximize the therapeutic effect in the target cell types.
In summary, the AHR pathway has stepped into the spotlight for the treatment of several non-viral diseases. The growing amount of evidence on the role of AHR in virus-host interactions supports the development of AHR antagonists as a new family of HTAs.
Human migration, urbanization, people agglomeration, environmental factors and reassortment across species are considered strong driving forces for viral re-emergence (140). One of the more astonishing aspects of the recent SARS-CoV-2 pandemic is the high level of variability among patients in terms of disease severity: while some patients remain asymptomatic, others require intensive care. Environmental factors interact with genetic factors to control the response to multiple challenges including viral infections. The collective of all exposures throughout an individual's lifetime could represent a possible underestimated factor which may contribute to the variations in disease severity. This “exposome” includes environmental toxins, pharmacological treatments, lifestyle choices, diet, etc. Technological advances and interdisciplinary experimental systems combined with rational bioinformatic approaches (141) have allowed us to initiate a comprehensive assessment of the exposome and the pathways involved in sensing it, but our knowledge in this area is still scattered.
The well-defined AHR pathway provides an excellent model to investigate the effects of the exposome on multiple aspects of physiology such as the response to viral infections. In addition, a deep understanding of the complex interactions between the viral pathogen and the immunological host response is required in order to develop antiviral treatments.
In this context, multiple studies indicate a role for the AHR environmental sensing molecule in the control of the adaptive, innate and intrinsic response to multiple common human viruses, including both RNA and DNA viruses (Figure 4). These findings may shed new light on the effects of the exposome on viral infections, while identifying novel approaches for therapeutic intervention.
MT, FG, CG, and FQ contributed conception, design, and wrote sections of the manuscript. All authors contributed to manuscript revision, read and approved the submitted version.
This work was supported by Universidad de Buenos Aires (UBA) (20020160100091BA) and Consejo Nacional de Investigaciones Científicas y Tecnológicas (CONICET) (PIP11220170100171CO). CG is member of the Research Career CONICET. MT was supported by a doctoral fellow of Agencia Nacional para la Promoción Científica y Tecnológica (ANPCyT).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank all members of the laboratories involved for helpful advice and discussions.
1. Nguyen LP, Bradfield CA. The search for endogenous activators of the aryl hydrocarbon receptor. Chem Res Toxicol. (2008) 21:102–16. doi: 10.1021/tx7001965
2. Rothhammer V, Quintana FJ. The aryl hydrocarbon receptor: an environmental sensor integrating immune responses in health and disease. Nat Rev Immunol. (2019) 19:184–97. doi: 10.1038/s41577-019-0125-8
3. Gutiérrez-Vázquez C, Quintana FJ. Regulation of the immune response by the aryl hydrocarbon receptor. Immunity. (2018) 48:19–33. doi: 10.1016/j.immuni.2017.12.012
4. McIntosh BE, Hogenesch JB, Bradfield CA. Mammalian Per-Arnt-Sim proteins in environmental adaptation. Annu Rev Physiol. (2009) 72:625–45. doi: 10.1146/annurev-physiol-021909-135922
5. Denison MS, Nagy SR. Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annu Rev Pharmacol Toxicol. (2003) 43:309–34. doi: 10.1146/annurev.pharmtox.43.100901.135828
6. Head JL, Lawrence BP. The aryl hydrocarbon receptor is a modulator of anti-viral immunity. Biochem Pharmacol. (2009) 77:642–53. doi: 10.1016/j.bcp.2008.10.031
7. Boule LA, Burke CG, Jin GB, Lawrence BP. Aryl hydrocarbon receptor signaling modulates antiviral immune responses: ligand metabolism rather than chemical source is the stronger predictor of outcome. Sci Rep. (2018) 8:1–15. doi: 10.1038/s41598-018-20197-4
8. Kewley RJ, Whitelaw ML, Chapman-Smith A. The mammalian basic helix-loop-helix/PAS family of transcriptional regulators. Int J Biochem Cell Biol. (2004) 36:189–204. doi: 10.1016/S1357-2725(03)00211-5
9. Yi T, Wang J, Zhu K, Tang Y, Huang S, Shui X, et al. Aryl hydrocarbon receptor: a new player of pathogenesis and therapy in cardiovascular diseases. Biomed Res Int. (2018). doi: 10.1155/2018/6058784
10. Harper PA, Riddick DS, Okey AB. Regulating the regulator: Factors that control levels and activity of the aryl hydrocarbon receptor. Biochem Pharmacol. (2006) 72:267–79. doi: 10.1016/j.bcp.2006.01.007
11. Yamamoto J, Ihara K, Nakayama H, Hikino S, Satoh K, Kubo N, et al. Characteristic expression of aryl hydrocarbon receptor repressor gene in human tissues: organ-specific distribution and variable induction patterns in mononuclear cells. Life Sci. (2004) 74:1039–49. doi: 10.1016/j.lfs.2003.07.022
12. Kudo I, Hosaka M, Haga A, Tsuji N, Nagata Y, Okada H, et al. The regulation mechanisms of AhR by molecular chaperone complex. J Biochem. (2018) 163:223–32. doi: 10.1093/jb/mvx074
13. Seok SH, Lee W, Jiang L, Molugu K, Zheng A, Li Y, et al. Structural hierarchy controlling dimerization and target DNA recognition in the AHR transcriptional complex. Proc Natl Acad Sci USA. (2017) 114:5431–6. doi: 10.1073/pnas.1617035114
14. Petrulis JR, Perdew GH. The role of chaperone proteins in the aryl hydrocarbon receptor core complex. Chem Biol Interact. (2002) 141:25–40. doi: 10.1016/S0009-2797(02)00064-9
15. Cox MB, Miller CA. The p23 co-chaperone facilitates dioxin receptor signaling in a yeast model system. Toxicol Lett. (2002) 129:13–21. doi: 10.1016/S0378-4274(01)00465-9
16. Meyer BK, Perdew GH. Characterization of the AhR-hsp90-XAP2 core complex and the role of the immunophilin-related protein XAP2 in AhR stabilization. Biochemistry. (1999) 38:8907–17. doi: 10.1021/bi982223w
17. Meyer BK, Pray-Grant MG, Vanden Heuvel JP, Perdew GH. Hepatitis B Virus X-associated protein 2 is a subunit of the unliganded aryl hydrocarbon receptor core complex and exhibits transcriptional enhancer activity. Mol Cell Biol. (1998) 18:978–88. doi: 10.1128/MCB.18.2.978
18. Carver LA, Lapres JJ, Jain S, Dunham EE, Bradfield CA. Characterization of the Ah receptor-associated protein, ARA9. J Biol Chem. (1998) 273:33580–7. doi: 10.1074/jbc.273.50.33580
19. Uemura S, Nakajima Y, Yoshida Y, Furuya M, Matsutani S, Kawate S, et al. Biochemical properties of human full-length aryl hydrocarbon receptor (AhR). J Biochem. (2020) 168:285–94. doi: 10.1093/jb/mvaa047
20. Beischlag TV, Morales JL, Hollingshead BD, Perdew GH. The aryl hydrocarbon receptor complex and the control of gene expression. Crit Rev Eukaryot Gene Expr. (2008) 18:207–50. doi: 10.1615/CritRevEukarGeneExpr.v18.i3.20
21. Larigot L, Juricek L, Dairou J, Coumoul X. AhR signaling pathways and regulatory functions. Biochim Open. (2018) 7:1–9. doi: 10.1016/j.biopen.2018.05.001
22. Berg P, Pongratz I. Two parallel pathways mediate cytoplasmic localization of the dioxin (aryl hydrocarbon) receptor. J Biol Chem. (2002) 277:32310–9. doi: 10.1074/jbc.M203351200
23. Kazlauskas A, Sundström S, Poellinger L, Pongratz I. The hsp90 chaperone complex regulates intracellular localization of the dioxin receptor. Mol Cell Biol. (2001) 21:2594–607. doi: 10.1128/MCB.21.7.2594-2607.2001
24. Ikuta T, Eguchi H, Tachibana T, Yoneda Y, Kawajiri K. Nuclear localization and export signals of the human aryl hydrocarbon receptor. J Biol Chem. (1998) 273:2895–904. doi: 10.1074/jbc.273.5.2895
25. Pappas B, Yang Y, Wang Y, Kim K, Chung HJ, Cheung M, et al. P23 protects the human aryl hydrocarbon receptor from degradation via a heat shock protein 90-independent mechanism. Biochem Pharmacol. (2018) 152:34–44. doi: 10.1016/j.bcp.2018.03.015
26. Tsuji N, Fukuda K, Nagata Y, Okada H, Haga A, Hatakeyama S, et al. The activation mechanism of the aryl hydrocarbon receptor (AhR) by molecular chaperone HSP90. FEBS Open Bio. (2014) 4:796–803. doi: 10.1016/j.fob.2014.09.003
27. Nguyen PM, Wang D, Wang Y, Li Y, Uchizono JA, Chan WK. P23 co-chaperone protects the aryl hydrocarbon receptor from degradation in mouse and human cell lines. Biochem Pharmacol. (2012) 84:838–50. doi: 10.1016/j.bcp.2012.06.018
28. Petrulis JR, Kusnadi A, Ramadoss P, Hollingshead B, Perdew GH. The hsp90 co-chaperone XAP2 alters importin β recognition of the bipartite nuclear localization signal of the Ah receptor and represses transcriptional activity. J Biol Chem. (2003) 278:2677–85. doi: 10.1074/jbc.M209331200
29. Henry EC, Gasiewicz TA. Transformation of the aryl hydrocarbon receptor to a DNA-binding form is accompanied by release of the 90 kDa heat-shock protein and increased affinity for 2,3,7,8-tetrachlorodibenzo-p-dioxin. Biochem J. (1993) 294:95–101. doi: 10.1042/bj2940095
30. Wright EJ, Pereira De Castro K, Joshi AD, Elferink CJ. Canonical and non-canonical aryl hydrocarbon receptor signaling pathways. Curr Opin Toxicol. (2017) 2:87–92. doi: 10.1016/j.cotox.2017.01.001
31. Jin HL, Choi Y, Jeong KW. Crosstalk between aryl hydrocarbon receptor and glucocorticoid receptor in human retinal pigment epithelial cells. Int J Endocrinol. (2017) 2017:5679517. doi: 10.1155/2017/5679517
32. Wilson SR, Joshi AD, Elferink CJ. The tumor suppressor kruppel-like factor 6 is a novel aryl hydrocarbon receptor DNA binding partner. J Pharmacol Exp Ther. (2013) 345:419–29. doi: 10.1124/jpet.113.203786
33. Takenaka MC, Gabriely G, Rothhammer V, Mascanfroni ID, Wheeler MA, Chao CC, et al. Control of tumor-associated macrophages and T cells in glioblastoma via AHR and CD39. Nat Neurosci. (2019) 22:729–40. doi: 10.1038/s41593-019-0370-y
34. Quintana FJ, Murugaiyan G, Farez MF, Mitsdoerffer M, Tukpah AM, Burns EJ, et al. An endogenous aryl hydrocarbon receptor ligand acts on dendritic cells and T cells to suppress experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA. (2010) 107:20768–73. doi: 10.1073/pnas.1009201107
35. Sorg O. AhR signalling and dioxin toxicity. Toxicol Lett. (2014) 230:225–33. doi: 10.1016/j.toxlet.2013.10.039
36. Baccarelli A, Pesatori AC, Masten SA, Patterson DG, Needham LL, Mocarelli P, et al. Aryl-hydrocarbon receptor-dependent pathway and toxic effects of TCDD in humans: a population-based study in Seveso, Italy. Toxicol Lett. (2004) 149:287–93. doi: 10.1016/j.toxlet.2003.12.062
37. Billiard SM, Hahn ME, Franks DG, Peterson RE, Bols NC, Hodson PV. Binding of polycyclic aromatic hydrocarbons (PAHs) to teleost aryl hydrocarbon receptors (AHRs). Comp Biochem Physiol B Biochem Mol Biol. (2002) 133:55–68. doi: 10.1016/S1096-4959(02)00105-7
38. Mimura J, Fujii-Kuriyama Y. Functional role of AhR in the expression of toxic effects by TCDD. Biochim Biophys Acta Gen Subj. (2003) 1619:263–8. doi: 10.1016/S0304-4165(02)00485-3
39. Vanden Heuvel JP, Lucier G. Environmental toxicology of polychlorinated dibenzo-p-dioxins and polychlorinated dibenzofurans. Environ Health Perspect. (1993) 100:189–200. doi: 10.1289/ehp.93100189
40. Dolciami D, Ballarotto M, Gargaro M, López-Cara LC, Fallarino F, Macchiarulo A, et al. Targeting Aryl hydrocarbon receptor for next-generation immunotherapies: selective modulators (SAhRMs) versus rapidly metabolized ligands (RMAhRLs). Euro J Med Chem. (2020) 185:111842. doi: 10.1016/j.ejmech.2019.111842
41. Zack JA, Suskind RR. The mortality experience of workers exposed to tetrachlorodibenzodioxin in a trichlorophenol process accident. J Occup Med Off Publ Ind Med Assoc. (1980) 22:11–4. doi: 10.1097/00043764-198001000-00005
42. Murray IA, Patterson AD, Perdew GH. Aryl hydrocarbon receptor ligands in cancer: Friend and foe. Nat Rev Cancer. (2014) 14:801–14. doi: 10.1038/nrc3846
43. Denison MS, Soshilov AA, He G, Degroot DE, Zhao B. Exactly the same but different: Promiscuity and diversity in the molecular mechanisms of action of the aryl hydrocarbon (dioxin) receptor. Toxicol Sci. (2011) 124:1–22. doi: 10.1093/toxsci/kfr218
44. Favre D, Mold J, Hunt PW, Kanwar B, Loke P, Seu L, et al. Tryptophan catabolism by indoleamine 2,3-dioxygenase 1 alters the balance of TH17 to regulatory T cells in HIV disease. Sci Transl Med. (2010) 2:32ra36. doi: 10.1126/scitranslmed.3000632
45. Kane M, Zang TM, Rihn SJ, Zhang F, Kueck T, Alim M, et al. Identification of interferon-stimulated genes with antiretroviral activity. Cell Host Microbe. (2016) 20:392–405. doi: 10.1016/j.chom.2016.08.005
46. Mao R, Zhang J, Jiang D, Cai D, Levy JM, Cuconati A, et al. Indoleamine 2,3-dioxygenase mediates the antiviral effect of gamma interferon against hepatitis b virus in human hepatocyte-derived cells. J Virol. (2011) 85:1048–57. doi: 10.1128/JVI.01998-10
47. Obojes K, Andres O, Kim KS, Däubener W, Schneider-Schaulies J. Indoleamine 2,3-dioxygenase mediates cell type-specific anti-measles virus activity of gamma interferon. J Virol. (2005) 79:7768–76. doi: 10.1128/JVI.79.12.7768-7776.2005
48. Neavin DR, Liu D, Ray B, Weinshilboum RM. The role of the Aryl Hydrocarbon Receptor (AHR) in immune and inflammatory diseases. Int J Mol Sci. (2018) 19:3851. doi: 10.3390/ijms19123851
49. Behnisch PA, Hosoe K, Sakai SI. Combinatorial bio/chemical analysis of dioxin and dioxin-like compounds in waste recycling, feed/food, humans/wildlife and the environment. Environ Int. (2001) 27:495–519. doi: 10.1016/S0160-4120(01)00029-0
50. Schecter A, Cramer P, Boggess K, Stanley J, Päpke O, Olson J, et al. Intake of dioxins and related compounds from food in the U.S. population. J Toxicol Environ Heal - Part A. (2001) 63:1–18. doi: 10.1080/152873901750128326
51. Chan JKY, Guan HX, Xu Y, Liang Y, Ling XC, Sheng CW, et al. Body loadings and health risk assessment of polychlorinated dibenzo-p-dioxins and dibenzofurans at an intensive electronic waste recycling site in China. Environ Sci Technol. (2007) 41:7668–74. doi: 10.1021/es071492j
52. Li H, Yu L, Sheng G, Fu J, Peng P. Severe PCDD/F and PBDD/F pollution in air around an electronic waste dismantling area in China. Environ Sci Technol. (2007) 41:5641–6. doi: 10.1021/es0702925
53. Shen C, Huang S, Wang Z, Qiao M, Tang X, Yu C, et al. Identification of Ah receptor agonists in soil of E-waste recycling sites from Taizhou area in China. Environ Sci Technol. (2008) 42:49–55. doi: 10.1021/es071162z
54. Loutfy N, Fuerhacker M, Tundo P, Raccanelli S, El Dien AG, Ahmed MT. Dietary intake of dioxins and dioxin-like PCBs, due to the consumption of dairy products, fish/seafood and meat from Ismailia city, Egypt. Sci Total Environ. (2006) 370:1–8. doi: 10.1016/j.scitotenv.2006.05.012
55. Feingold BJ, Vegosen L, Davis M, Leibler J, Peterson A, Silbergeld EK. A niche for infectious disease in environmental health: rethinking the toxicological paradigm. Environ Health Perspect. (2010) 118:1165–72. doi: 10.1289/ehp.0901866
56. Heilmann C, Grandjean P, Weihe P, Nielsen F, Budtz-Jørgensen E. Reduced antibody responses to vaccinations in children exposed to polychlorinated biphenyls. PLoS Med. (2006) 3:1352–9. doi: 10.1371/journal.pmed.0030311
57. Stølevik SB, Nygaard UC, Namork E, Haugen M, Kvalem HE, Meltzer HM, et al. Prenatal exposure to polychlorinated biphenyls and dioxins is associated with increased risk of wheeze and infections in infants. Food Chem Toxicol. (2011) 49:1843–8. doi: 10.1016/j.fct.2011.05.002
58. Hochstenbach K, Van leeuwen DM, Gmuender H, Gottschalk RW, Stølevik SB, Nygaard UC, et al. Toxicogenomic profiles in relation to maternal immunotoxic exposure and immune functionality in newborns. Toxicol Sci. (2012) 129:315–24. doi: 10.1093/toxsci/kfs214
59. Stølevik SB, Nygaard UC, Namork E, Haugen M, Meltzer HM, Alexander J, et al. Prenatal exposure to polychlorinated biphenyls and dioxins from the maternal diet may be associated with immunosuppressive effects that persist into early childhood. Food Chem Toxicol. (2013) 51:165–72. doi: 10.1016/j.fct.2012.09.027
60. Dallaire F, Dewailly É, Muckle G, Vézina C, Jacobson SW, Jacobson JL, et al. Acute infections and environmental exposure to organochlorines in Inuit infants from Nunavik. Environ Health Perspect. (2004) 112:1359–64. doi: 10.1289/ehp.7255
61. Miyashita C, Sasaki S, Saijo Y, Washino N, Okada E, Kobayashi S, et al. Effects of prenatal exposure to dioxin-like compounds on allergies and infections during infancy. Environ Res. (2011) 111:551–8. doi: 10.1016/j.envres.2011.01.021
62. Jusko TA, De Roos AJ, Lee SY, Thevenet-Morrison K, Schwartz SM, Verner MA, et al. A birth cohort study of maternal and infant serum PCB-153 and DDE concentrations and responses to infant tuberculosis vaccination. Environ Health Perspect. (2016) 124:813–21. doi: 10.1289/ehp.1510101
63. Zhou Y, Li S, Huang L, Yang Y, Zhang L, Yang M, et al. A splicing mutation in aryl hydrocarbon receptor associated with retinitis pigmentosa. Hum Mol Genet. (2018) 27:2563–72. doi: 10.1093/hmg/ddy165
64. Chen D, Tian T, Wang H, Liu H, Hu Z, Wang Y, et al. Association of human aryl hydrocarbon receptor gene polymorphisms with risk of lung cancer among cigarette smokers in a Chinese population. Pharmacogenet Genomics. (2009) 19:25–34. doi: 10.1097/FPC.0b013e328316d8d8
65. Wu CQ, Cao SG, Xia XP, Xu CL, Xia SL, Lin XQ, et al. Association of Crohn's disease with aryl hydrocarbon receptor gene polymorphisms and haplotypes. Zhonghua Nei Ke Za Zhi. (2018) 57:37–43. doi: 10.3760/cma.j.issn.0578-1426.2018.01.007
66. Wu C qun, Lin Q ru, Ying S jie, Luo J kai, Hong W jun, Lin Z jian, et al. Association of crohn's disease with aryl hydrocarbon receptor gene polymorphisms in Patients from Southeast China. Immunol Invest. (2019) 48:809–21. doi: 10.1080/08820139.2019.1569677
67. De Sousa SMC, Manavis J, Feng J, Wang P, Schreiber AW, Scott HS, et al. A putative role for the aryl hydrocarbon receptor (AHR) gene in a patient with cyclical Cushing's disease. BMC Endocr Disord. (2020) 20:1–6. doi: 10.1186/s12902-020-0495-8
68. Cannavo S, Ragonese M, Puglisi S, Romeo PD, Torre ML, Alibrandi A, et al. Acromegaly is more severe in patients with AHR or AIP gene variants living in highly polluted areas. J Clin Endocrinol Metab. (2016) 101:1872–9. doi: 10.1210/jc.2015-4191
69. Re A, Ferraù F, Cafiero C, Spagnolo F, Barresi V, Romeo DP, et al. Somatic deletion in exon 10 of aryl hydrocarbon receptor gene in human GH-secreting pituitary tumors. Front Endocrinol (Lausanne). (2020) 11:1–8. doi: 10.3389/fendo.2020.591039
70. Huang S, Shui X, He Y, Xue Y, Li J, Li G, et al. AhR expression and polymorphisms are associated with risk of coronary arterial disease in Chinese population. Sci Rep. (2015) 5:8022. doi: 10.1038/srep08022
71. Pulignani S, Borghini A, Vecoli C, Foffa I, Ait-Ali L, Andreassi MG. A functional aryl hydrocarbon receptor genetic variant, alone and in combination with parental exposure, is a risk factor for congenital heart disease. Cardiovasc Toxicol. (2018) 18:261–7. doi: 10.1007/s12012-017-9436-9
72. Vos JG, Kreeftenberg JG, Engel HW, Minderhoud A, Van Noorle Jansen LM. Studies on 2,3,7,8-tetrachlorodibenzo-p-dioxin induced immune suppression and decreased resistance to infection: endotoxin hypersensitivity, serum zinc concentrations and effect of thymosin treatment. Toxicology. (1978) 9:75–86. doi: 10.1016/0300-483X(78)90033-1
73. Hinsdill RD, Couch DL, Speirs RS. Immunosuppression in mice induced by dioxin (TCDD) in feed. J Environ Pathol Toxicol. (1980) 4:401–25.
74. House R V, Lauer LD, Murray MJ, Thomas PT, Ehrlich JP, Burleson GR, et al. Examination of immune parameters and host resistance mechanisms in b6c3f1 mice following adult exposure to 2, 3, 7, 8 tetrachlorodibenzo-p-dioxin. J Toxicol Environ Health. (1990) 31:203–15. doi: 10.1080/15287399009531449
75. Burleson GR, Lebrec H, Yang YG, Ibanes JD, Pennington KN, Birnbaum LS. Effect of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) on influenza virus host resistance in mice. Fundam Appl Toxicol. (1996) 29:40–7. doi: 10.1093/toxsci/29.1.40
76. Kohlmeier JE, Woodland DL. Immunity to respiratory viruses. Annu Rev Immunol. (2009) 27:61–82. doi: 10.1146/annurev.immunol.021908.132625
77. Hikono H, Kohlmeier JE, Ely KH, Scott I, Roberts AD, Blackman MA, et al. T-cell memory and recall responses to respiratory virus infections. Immunol Rev. (2006) 211:119–32. doi: 10.1111/j.0105-2896.2006.00385.x
78. Vaidyanathan B, Chaudhry A, Yewdell WT, Angeletti D, Yen WF, Wheatley AK, et al. The aryl hydrocarbon receptor controls cell-fate decisions in B cells. J Exp Med. (2017) 214:197–208. doi: 10.1084/jem.20160789
79. Boyden AW, Legge KL, Waldschmidt TJ. Pulmonary infection with influenza A virus induces site-specific germinal center and T follicular helper cell responses. PLoS ONE. (2012) 7:e40733. doi: 10.1371/journal.pone.0040733
80. Betts RJ, Prabhu N, Ho AWS, Lew FC, Hutchinson PE, Rotzschke O, et al. Influenza a virus infection results in a robust, antigen-responsive, and widely disseminated foxp3+ regulatory t cell response. J Virol. (2012) 86:2817–25. doi: 10.1128/JVI.05685-11
81. Antunes I, Kassiotis G. Suppression of innate immune pathology by regulatory T cells during influenza a virus infection of immunodeficient mice. J Virol. (2010) 84:12564–75. doi: 10.1128/JVI.01559-10
82. Almansa R, Sanchez-Garcia M, Herrero A, Calzada S, Roig V, Barbado J, et al. Host response cytokine signatures in viral and nonviral acute exacerbations of chronic obstructive pulmonary disease. J Interf Cytokine Res. (2011) 31:409–13. doi: 10.1089/jir.2010.0131
83. Burke CG, Myers JR, Boule LA, Post CM, Brookes PS, Lawrence BP. Early life exposures shape the CD4+ T cell transcriptome, influencing proliferation, differentiation, and mitochondrial dynamics later in life. Sci Rep. (2019) 9:1–15. doi: 10.1038/s41598-019-47866-2
84. Jin GB, Moore AJ, Head JL, Neumiller JJ, Lawrence BP. Aryl hydrocarbon receptor activation reduces dendritic cell function during influenza virus infection. Toxicol Sci. (2010) 116:514–22. doi: 10.1093/toxsci/kfq153
85. Franchini AM, Lawrence BP. Environmental exposures are hidden modifiers of anti-viral immunity. Curr Opin Toxicol. (2018) 10:54–9. doi: 10.1016/j.cotox.2018.01.004
86. Warren TK, Mitchell KA, Lawrence BP. Exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) suppresses the humoral and cell-mediated immune responses to influenza A virus without affecting cytolytic activity in the lung. Toxicol Sci. (2000) 56:114–23. doi: 10.1093/toxsci/56.1.114
87. Vorderstrasse BA, Bohn AA, Lawrence BP. Examining the relationship between impaired host resistance and altered immune function in mice treated with TCDD. Toxicology. (2003) 188:15–28. doi: 10.1016/S0300-483X(02)00749-7
88. Neff-LaFord HD, Vorderstrasse BA, Lawrence BP. Fewer CTL, not enhanced NK cells, are sufficient for viral clearance from the lungs of immunocompromised mice. Cell Immunol. (2003) 226:54–64. doi: 10.1016/j.cellimm.2003.11.005
89. Lawrence BP, Vorderstrasse BA. Activation of the aryl hydrocarbon receptor diminishes the memory response to homotypic influenza virus infection but does not impair host resistance. Toxicol Sci. (2004) 79:304–14. doi: 10.1093/toxsci/kfh094
90. Neff-LaFord H, Teske S, Bushnell TP, Lawrence BP. Aryl hydrocarbon receptor activation during influenza virus infection unveils a novel pathway of IFN-γ production by phagocytic cells. J Immunol. (2007) 179:247–55. doi: 10.4049/jimmunol.179.1.247
91. Wheeler JLH, Martin KC, Lawrence BP. Novel cellular targets of ahr underlie alterations in neutrophilic inflammation and inducible nitric oxide synthase expression during influenza virus infection. J Immunol. (2013) 190:659–68. doi: 10.4049/jimmunol.1201341
92. Teske S, Bohn AA, Regal JF, Neumiller JJ, Lawrence BP. Activation of the aryl hydrocarbon receptor increases pulmonary neutrophilia and diminishes host resistance to influenza A virus. Am J Physiol Lung Cell Mol Physiol. (2005) 289:L111-24. doi: 10.1152/ajplung.00318.2004
93. Teske S, Bohn AA, Hogaboam JP, Lawrence BP. Aryl hydrocarbon receptor targets pathways extrinsic to bone marrow cells to enhance neutrophil recruitment during influenza virus infection. Toxicol Sci. (2008) 102:89–99. doi: 10.1093/toxsci/kfm282
94. Lawrence BP, Roberts AD, Neumiller JJ, Cundiff JA, Woodland DL. Aryl hydrocarbon receptor activation impairs the priming but not the recall of influenza virus-specific CD8 + T cells in the lung. J Immunol. (2006) 177:5819–28. doi: 10.4049/jimmunol.177.9.5819
95. Gordon DE, Jang GM, Bouhaddou M, Xu J, Obernier K, White KM, et al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature. (2020) 583:459–68. doi: 10.1038/s41586-020-2286-9
96. Grunewald ME, Shaban MG, Mackin SR, Fehr AR, Perlman S. Murine coronavirus infection activates the aryl hydrocarbon receptor in an indoleamine 2,3-dioxygenase-independent manner, contributing to cytokine modulation and proviral TCDD-inducible-PARP expression. J Virol. (2019) 94:319–35. doi: 10.1128/JVI.01743-19
97. Thomas T, Stefanoni D, Reisz JA, Nemkov T, Bertolone L, Francis RO, et al. COVID-19 infection alters kynurenine and fatty acid metabolism, correlating with IL-6 levels and renal status. JCI Insight. (2020) 5:e140327. doi: 10.1172/jci.insight.140327
98. Turski WA, Wnorowski A, Turski GN, Turski CA, Turski L. AhR and IDO1 in pathogenesis of Covid-19 and the “Systemic AhR activation syndrome:” a translational review and therapeutic perspectives. Restor Neurol Neurosci. (2020) 38:343–54. doi: 10.3233/RNN-201042
99. Simmonds P, Becher P, Bukh J, Gould EA, Meyers G, Monath T, et al. ICTV virus taxonomy profile: Flaviviridae. J Gen Virol. (2017) 98:2–3. doi: 10.1099/jgv.0.000672
100. Posen HJ, Keystone JS, Gubbay JB, Morris SK. Epidemiology of Zika virus, 1947-2007. BMJ Glob Heal. (2016) 1:e000087. doi: 10.1136/bmjgh-2016-000087
101. Faria NR, Quick J, Claro IM, Thézé J, de Jesus JG, Giovanetti M, et al. Establishment and cryptic transmission of Zika virus in Brazil and the Americas. Nature. (2017) 546:406–10. doi: 10.1038/nature22401
102. Barbeito-Andrés J, Schuler-Faccini L, Garcez PP. Why is congenital Zika syndrome asymmetrically distributed among human populations? PLoS Biol. (2018) 16:1–11. doi: 10.1371/journal.pbio.2006592
103. Tang H, Hammack C, Ogden SC, Wen Z, Qian X, Li Y, et al. Zika virus infects human cortical neural progenitors and attenuates their growth. Cell Stem Cell. (2016) 18:587–90. doi: 10.1016/j.stem.2016.02.016
104. Militão de Albuquerque M de FP, de Souza W V, Mendes A da CG, Lyra TM, Ximenes RAA, Araújo TVB, et al. Pyriproxyfen and the microcephaly epidemic in Brazil - An ecological approach to explore the hypothesis of their association. Mem Inst Oswaldo Cruz. (2016) 111:774–6. doi: 10.1590/0074-02760160291
105. Fan H, Su X, Yang B, Zhao A. Aryl hydrocarbon receptor and unexplained miscarriage. J Obstet Gynaecol Res. (2017) 43:1029–36. doi: 10.1111/jog.13309
106. Giovannoni F, Bosch I, Polonio CM, Torti MF, Wheeler MA, Li Z, et al. AHR is a Zika virus host factor and a candidate target for antiviral therapy. Nat Neurosci. (2020) 23:939–51. doi: 10.1038/s41593-020-0664-0
107. de Souza Anselmo C, Sardela VF, de Sousa VP, Pereira HMG. Zebrafish (Danio rerio): A valuable tool for predicting the metabolism of xenobiotics in humans? Comp Biochem Physiol Part - C Toxicol Pharmacol. (2018) 212:34–46. doi: 10.1016/j.cbpc.2018.06.005
108. Hallet E, Flamand C, Rousset D, Bonifay T, Fritzell C, Matheus S, et al. Zika virus infection in pregnant women in French Guiana: more precarious-more at risk. PLoS Negl Trop Dis. (2020) 14:1–10. doi: 10.1371/journal.pntd.0008193
109. Vota D, Torti M, Paparini D, Giovannoni F, Merech F, Hauk V, et al. Zika virus infection of first trimester trophoblast cells affects cell migration, metabolism, and immune homeostasis control. J Cell Physiol. (2020) 1–13. doi: 10.1002/jcp.30203
110. Katzelnick LC, Narvaez C, Arguello S, Lopez Mercado B, Collado D, Ampie O, et al. Zika virus infection enhances future risk of severe dengue disease. Science. (2020) 369:1123–8. doi: 10.1126/science.abb6143
111. Bekerman E, Einav S. Infectious disease. Combating emerging viral threats. Science. (2015) 348:282–3. doi: 10.1126/science.aaa3778
112. Nelson DR. The immunopathogenesis of hepatitis C virus infection. Clin Liver Dis. (2001) 5:931–53. doi: 10.1016/S1089-3261(05)70202-6
113. Ohashi H, Nishioka K, Nakajima S, Kim S, Suzuki R, Aizaki H, et al. The aryl hydrocarbon receptor-cytochrome P450 1A1 pathway controls lipid accumulation and enhances the permissiveness for hepatitis C virus assembly. J Biol Chem. (2018) 293:19559–71. doi: 10.1074/jbc.RA118.005033
114. Adams MJ, Lefkowitz EJ, King AMQ, Harrach B, Harrison RL, Knowles NJ, et al. Changes to taxonomy and the International Code of Virus Classification and Nomenclature ratified by the International Committee on Taxonomy of Viruses (2017). Arch Virol. (2017) 162:2505–38. doi: 10.1007/s00705-017-3358-5
115. Zhou YH, Sun L, Chen J, Sun WW, Ma L, Han Y, et al. Tryptophan metabolism activates aryl hydrocarbon receptor-mediated pathway to promote HIV-1 infection and reactivation. MBio. (2019) 10:1–16. doi: 10.1128/mBio.02591-19
116. Kueck T, Cassella E, Holler J, Kim B, Bieniasz PD. The aryl hydrocarbon receptor and interferon gamma generate antiviral states via transcriptional repression. Elife. (2018) 7:1–22. doi: 10.7554/eLife.38867
117. Yao Y, Hoffer A, Chang -y. C, Puga A. Dioxin activates HIV-1 gene expression by an oxidative stress pathway requiring a functional cytochrome P450 CYP1A1 enzyme. Environ Health Perspect. (1995) 103:366–71. doi: 10.1289/ehp.95103366
118. Gollapudi S, Kim CH, Patel A, Sindhu R, Gupta S. Dioxin activates human immunodeficiency virus-1 expression in chronically infected promonocytic U1 cells by enhancing NF-κB activity and production of tumor necrosis factor-α. Biochem Biophys Res Commun. (1996) 226:889–94. doi: 10.1006/bbrc.1996.1445
119. Ohata H, Tetsuka T, Hayashi H, Onozaki K, Okamoto T. 3-Methylcholanthrene activates human immunodeficiency virus type 1 replication via aryl hydrocarbon receptor. Microbiol Immunol. (2003) 47:363–70. doi: 10.1111/j.1348-0421.2003.tb03408.x
120. Tsyrlov IB, Pokrovsky A. Stimulatory effect of the CYPlal inducer 2,3,7,8-tetrachlorodibenzo-pdioxin on the reproduction of HIV-1 in human lymphoid cell culture. Xenobiotica. (1993) 23:457–67. doi: 10.3109/00498259309057034
121. James C, Harfouche M, Welton NJ, Turner KME, Abu-Raddad LJ, Gottlieb SL, et al. Herpes simplex virus: global infection prevalence and incidence estimates, 2016. Bull World Health Organ. (2020) 98:315–29. doi: 10.2471/BLT.19.237149
122. Zuhair M, Smit GSA, Wallis G, Jabbar F, Smith C, Devleesschauwer B, et al. Estimation of the worldwide seroprevalence of cytomegalovirus: a systematic review and meta-analysis. Rev Med Virol. (2019) 29:e2034. doi: 10.1002/rmv.2034
123. Nowalk A, Green M. Epstein-Barr virus. Microbiol Spectr. (2016) 4:3. doi: 10.1128/microbiolspec.DMIH2-0011-2015
124. Prober CG. Human herpesvirus 6. Adv Exp Med Biol. (2011) 697:87–90. doi: 10.1007/978-1-4419-7185-2_7
125. Minhas V, Wood C. Epidemiology and transmission of Kaposi's sarcoma-associated herpesvirus. Viruses. (2014) 6:4178–94. doi: 10.3390/v6114178
126. Koshy E, Mengting L, Kumar H, Jianbo W. Epidemiology, treatment and prevention of herpes zoster: a comprehensive review. Indian J Dermatol Venereol Leprol. (2018) 84:251–62. doi: 10.4103/ijdvl.IJDVL_1021_16
127. Veiga-Parga T, Suryawanshi A, Rouse BT. Controlling viral immuno-inflammatory lesions by modulating aryl hydrocarbon receptor signaling. PLoS Pathog. (2011) 7:1–16. doi: 10.1371/journal.ppat.1002427
128. Cannon MJ, Westbrook K, Levis D, Schleiss MR, Thackeray R, Pass RF. Awareness of and behaviors related to child-to-mother transmission of cytomegalovirus. Prev Med (Baltim). (2012) 54:351–7. doi: 10.1016/j.ypmed.2012.03.009
129. Murayama T, Inoue M, Nomura T, Mori S, Eizuru Y. 2,3,7,8-Tetrachlorodibenzo-p-dioxin is a possible activator of human cytomegalovirus replication in a human fibroblast cell line. Biochem Biophys Res Commun. (2002) 296:651–6. doi: 10.1016/S0006-291X(02)00921-X
130. Hardell L, Carlberg M, Hardell L, Carlberg M, Lindström G, van Bavel B, et al. Adipose tissue concentrations of dioxins and dibenzofurans, titers of antibodies to Epstein-Barr virus early antigen and the risk for non-Hodgkin lymphoma. Environ Res. (2001) 87:99–107. doi: 10.1006/enrs.2001.4295
131. Stanček D, Košecká G, Oltman M, Keleová A, Jahnová E. Links between prolonged exposure to xenobiotics, increased incidence of hepatopathies, immunological disturbances and exacerbation of latent epstein-barr virus infections. Int J Immunopharmacol. (1995) 17:321–8. doi: 10.1016/0192-0561(95)00006-N
132. Küppers R. B cells under influence: Transformation of B cells by Epstein-Barr virus. Nat Rev Immunol. (2003) 3:801–12. doi: 10.1038/nri1201
133. Kashuba E V, Gradin K, Isaguliants M, Szekely L, Poellinger L, Klein G, et al. Regulation of transactivation function of the aryl hydrocarbon receptor by the Epstein-Barr virus-encoded EBNA-3 protein. J Biol Chem. (2006) 281:1215–23. doi: 10.1074/jbc.M509036200
134. Kashuba E, Kashuba V, Pokrovskaja K, Klein G, Szekely L. Epstein-Barr virus encoded nuclear protein EBNA-3 binds XAP-2, a protein associated with Hepatitis B virus X antigen. Oncogene. (2000) 19:1801–6. doi: 10.1038/sj.onc.1203501
135. Yamada T, Horimoto H, Kameyama T, Hayakawa S, Yamato H, Dazai M, et al. Constitutive aryl hydrocarbon receptor signaling constrains type I interferon-mediated antiviral innate defense. Nat Immunol. (2016) 17:687–94. doi: 10.1038/ni.3422
136. Liu Y, Lv J, Liu J, Li M, Xie J, Lv Q, et al. Mucus production stimulated by IFN-AhR signaling triggers hypoxia of COVID-19. Cell Res. (2020) 30:1078–87. doi: 10.1038/s41422-020-00435-z
137. Cheong JE, Sun L. Targeting the IDO1/TDO2–KYN–AhR pathway for cancer immunotherapy – challenges and opportunities. Trends Pharmacol Sci. (2018) 39:307–25. doi: 10.1016/j.tips.2017.11.007
138. Yeste A, Nadeau M, Burns EJ, Weiner HL, Quintana FJ. Nanoparticle-mediated codelivery of myelin antigen and a tolerogenic small molecule suppresses experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA. (2012) 109:11270–5. doi: 10.1073/pnas.1120611109
139. Kenison JE, Jhaveri A, Li Z, Khadse N, Tjon E, Tezza S, et al. Tolerogenic nanoparticles suppress central nervous system inflammation. Proc Natl Acad Sci USA. (2020) 117:32017–28. doi: 10.1073/pnas.2016451117
140. Dabbu Kumar J, Jian L, Rong H, Hua Z. Emerging and reemerging human viral diseases. Ann Microbiol Res. (2018) 2:31–44. doi: 10.36959/958/567
Keywords: aryl hydrocarbon receptor, viral infections, RNA viruses, DNA viruses, host response
Citation: Torti MF, Giovannoni F, Quintana FJ and García CC (2021) The Aryl Hydrocarbon Receptor as a Modulator of Anti-viral Immunity. Front. Immunol. 12:624293. doi: 10.3389/fimmu.2021.624293
Received: 31 October 2020; Accepted: 03 February 2021;
Published: 05 March 2021.
Edited by:
David H. Sherr, Boston University, United StatesReviewed by:
Zoltan Janos Vereb, University of Szeged, HungaryCopyright © 2021 Torti, Giovannoni, Quintana and García. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cybele Carina García, Y3lnYXJjaWFAcWIuZmNlbi51YmEuYXI=; Francisco Javier Quintana, ZnF1aW50YW5hQHJpY3MuYndoLmhhcnZhcmQuZWR1
†These authors have contributed equally to this work
‡These authors have contributed equally to this work and share senior authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.