- 1Schepens Eye Research institute/Massachusetts Eye and Ear, Department of Ophthalmology, Harvard Medical School, Boston, MA, United States
- 2Institute of Clinical Medicine, Faculty of Medicine, University of Oslo, Oslo, Norway
- 3Department of Medical Biochemistry, Oslo University Hospital, Oslo, Norway
- 4Department of Plastic and Reconstructive Surgery, University of Oslo, Oslo, Norway
- 5Center for Experimental Therapeutics and Reperfusion Injury, Department of Anesthesia, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA, United States
The amount of mucin secreted by conjunctival goblet cells is regulated to ensure the optimal level for protection of the ocular surface. Under physiological conditions lipid specialized pro-resolving mediators (SPM) are essential for maintaining tissue homeostasis including the conjunctiva. The protein Annexin A1 (AnxA1) can act as an SPM. We used cultured rat conjunctival goblet cells to determine if AnxA1 stimulates an increase in intracellular [Ca2+] ([Ca2+]i) and mucin secretion and to identify the signaling pathways. The increase in [Ca2+]i was determined using fura2/AM and mucin secretion was measured using an enzyme-linked lectin assay. AnxA1 stimulated an increase in [Ca2+]i and mucin secretion that was blocked by the cell-permeant Ca2+ chelator BAPTA/AM and the ALX/FPR2 receptor inhibitor BOC2. AnxA1 increased [Ca2+]i to a similar extent as the SPMs lipoxin A4 and Resolvin (Rv) D1 and histamine. The AnxA1 increase in [Ca2+]i and mucin secretion were inhibited by blocking the phospholipase C (PLC) pathway including PLC, the IP3 receptor, the Ca2+/ATPase that causes the intracellular Ca2+ stores to empty, and blockade of Ca2+ influx. Inhibition of protein kinase C (PKC) and Ca2+/calmodulin-dependent protein kinase also decreased the AnxA1-stimulated increase in [Ca2+]i and mucin secretion. In contrast inhibitors of ERK 1/2, phospholipase A2 (PLA2), and phospholipase D (PLD) did not alter AnxA1-stimulated increase in [Ca2+]i, but did inhibit mucin secretion. Activation of protein kinase A did not decrease either the AnxA1-stimulated rise in [Ca2+]i or secretion. We conclude that in health, AnxA1 contributes to the mucin layer of the tear film and ocular surface homeostasis by activating the PLC signaling pathway to increase [Ca2+]i and stimulate mucin secretion and ERK1/2, PLA2, and PLD to stimulate mucin secretion from conjunctival goblet cells.
Introduction
Inflammation is a component in common to both allergic conjunctivitis and dry eye disease – two frequently occurring diseases of the ocular surface (cornea and conjunctiva) (1). In allergic conjunctivitis allergens penetrate the conjunctival epithelium and initiate the production of pro-inflammatory mediators including histamine, leukotrienes, and prostaglandins. These compounds cause vasodilation, pain, edema and recruitment of neutrophils, macrophages, and mast cells into the conjunctiva (2). In acute inflammation, the infiltrating leukocytes switch from producing pro-inflammatory mediators to generate the specialized pro-resolving lipid mediators (SPMs) lipoxins, resolvins, protectins, and maresins (2–6). These SPMs actively terminate inflammation by blocking the effects of the pro-inflammatory mediators on their target tissues, including the conjunctival goblet cells. The protein annexin A1 (AnxA1) is also pro-resolving (6, 7). In general, failed endogenous resolution mechanisms lead to uncontrolled and chronic inflammation (1). In addition to resolving inflammation, the SPMs also maintain homeostasis of the conjunctival epithelium to keep the ocular surface healthy.
Conjunctival goblet cells synthesize and secrete large, high molecular weight, gel forming mucins (8). These mucins make up the innermost layer of the tear film (9). MUC5AC, the major mucin secreted by conjunctival goblet cells, can trap and remove ocular allergens and airborne pathogens (8). An optimum amount of MUC5AC secreted into the tear film is critical for ocular surface health, as a depleted number of goblet cells and mucin secretion lead to corneal and conjunctival damage (8). Conversely, MUC5AC overproduction is a symptom of allergic conjunctivitis and unhealthy for the ocular surface (8). Lipid SPMs including D-series and E-series resolvins, lipoxins, and maresins act by blocking excess mucin secretion from conjunctival goblet cells during inflammation, as well as stimulating mucin secretion under physiological conditions, thus maintaining ocular surface homeostasis (10–12).
AnxA1 is an anti-inflammatory, pro-resolving protein originally described as a mediator of glucocorticoid action (13). The synthesis of AnxA1 is a primary anti-inflammatory mechanism of glucocorticoids. AnxA1 works at least partly by suppressing phospholipase A2 activity, thus blocking the production of pro-inflammatory eicosanoids (14). During inflammation, endogenous glucocorticoids are secreted from the adrenal gland to avoid an excessively vigorous inflammatory response that could potentially damage the host (15). Glucocorticoids can stimulate both the synthesis and secretion of AnxA1 (16). After being synthesized AnxA1 is mobilized to the plasma membrane and secreted by three different mechanisms: 1) the ATP-binding (ABC) transporter, 2) direct phosphorylation of serine-27 that localizes it to the plasma membrane, and 3) exocytosis by granule fusion with the cell membrane (17). The secreted AnxA1 can then interact with the ALX/FPR2 receptor in a paracrine, autocrine, or juxtacrine fashion.
As an effector molecule of glucocorticoids, AnxA1 has potential as an endogenous anti-inflammatory drug, and the use of AnxA1 therapeutically likely has fewer side effects than the use of glucocorticoids. AnxA1 contributes to the regulation of a wide variety of cellular events, including both acute and chronic inflammation, ischemic injury, fever, pain, and the release of arachidonic acid (14). AnxA1, as well as its N-terminal peptides, have also been found to potently inhibit neutrophil trafficking in mice (7). AnxA1 could prevent development of pro-inflammatory disease in the conjunctiva.
To determine if AnxA1 plays a role in maintaining the mucous layer in ocular surface health we investigated if exogenous addition of AnxA1 can increase intracellular [Ca2+] and stimulate MUC5AC secretion in cultured primary conjunctival goblet cells from rats and determined which signaling pathways AnxA1 activates in these cells.
Materials and Methods
Materials
AnxA1 was obtained from MyBiosource (San Diego, CA). The compound was stored at -20°C. Prior to use, AnxA1 was immediately diluted in Krebs-Ringer bicarbonate buffer with HEPES (KRB-HEPES, 119 mM NaCl, 4.8 mM KCl, 1.0 mM CaCl2, 1.2 mM MgSO4, 1.2 mM KH2PO4, 25 mM NaHCO3, 10 mM HEPES, and 5.5 mM glucose [pH 7.45]) to the required concentration and added to the conjunctival goblet cells. RPMI-1640 cell culture medium, penicillin/streptomycin, trypsin, and L-glutamine were purchased from Lonza (Walkerville, IL). Fetal bovine serum (FBS) was from Atlanta Biologicals (Norcross, GA). Fura-2 was from Life Technologies (Grand Island, NY). U73122 and U73343, KN92 and KN93 were purchased from Tocris Bioscience (Ellisville, MO). The lectin Ulex Europaeus Agglutinin (UEA-1), histamine, BAPTA/AM, 2-aminoethyl diphenylborinate (2-APB), 1-butanol (1-but), t-butanol (t-but), carbachol, aristolochic acid (AA), thapsigargin and RO317549 were all purchased from Sigma-Aldrich (St Louis, MO). UO126 was obtained from R&D Systems (Minneapolis, MN). H89 and synthetic lipoxin A4 (LXA4, 5(S),6(R),15(S)-TriHETE) were from EMD Millipore (Billerica, MA). Resolvin (Rv) D1 (7S, 8R, 17S-trihydroxy-4Z, 9E 11E, 13Z, 19Z-docosahexaenoic acid) was purchased from Cayman Chemical, Ann Arbor, MI). N-Boc-Phe-Leu-Phe-Leu-Phe (BOC2) was obtained from Genscript Corp in Piscataway, NJ.
Animals
Four- to eight-week-old male Sprague-Dawley rats (Taconic Farms, Germantown, NY, USA) were anesthetized with CO2 and decapitated. The bulbar and forniceal conjunctival epithelium was surgically removed from both eyes. All experiments were in accordance with the US National Research Council’s Guide for the Care and Use of Laboratory Animals, the US Public Health Service’s Policy on Humane Care and Use of Laboratory Animals, and Guide for the Care and Use of Laboratory Animals and were approved by the Schepens Eye Research Institute Animal Care and Use of Committee.
Culture of Conjunctival Goblet Cells
Goblet cells from rat conjunctiva were grown in organ culture as described previously (3). The conjunctiva was dissected free of underlying connective tissue. The conjunctival explants were grown on six-well plates in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS), 2 mM glutamine, and 100 μg/mL penicillin-streptomycin for approximately one week. The goblet cells were then trypsinized and plated onto glass bottom petri dishes or 24 well plates, for [Ca2+]i measurements or mucin secretion measurements, respectively. First passage goblet cells were used in all experiments. The identity of the cultured cells was checked by evaluating staining with antibody to cytokeratin 7 (detects goblet cell bodies) and the lectin UEA-1 (detects goblet cell secretory products) to ensure that goblet cells predominated in the cultures.
Measurement of cDNA Expression by Semi-Quantitative PCR
Cultured goblet cells were homogenized in TRIzol and total RNA isolated according to manufacturer’s instructions. One microgram of total RNA was used for complementary DNA (cDNA) synthesis using the Superscript First-Strand Synthesis system for RT-PCR (Invitrogen, Carlsbad, CA). The cDNA was amplified by the polymerase chain reaction (PCR) using primers specific to AnxA1 using the Jumpstart REDTaq Readymix Reaction Mix (Sigma-Aldrich, St. Louis, MO) in a thermal cycler (Master Cycler, Eppendorf, Hauppauge, NY). The forward primer was GTG ATC GCT GTG AGG ATA TGA G, and the reverse primer was TAC AGA GCA GTT GGG ATG TTT AG. These primers generated 504 bp fragments. β-Actin primers, the positive control, were forward CGT CAT ACT CCT GCT TGC TGA TCC A and the reverse primer was ATC TGG CAC CAC ACC TTC TAC AAT GG CT and generated a 790 bp fragment. The conditions were as follows: 5 min at 95°C followed by 35 cycles of 1 min at 94°C, 30 seconds at annealing temperature for 1 min at 72°C with a final hold at 72°C for 10 min. Samples with no cDNA served as the negative control. Amplification products were separated by electrophoresis on a 1.5% agarose gel and visualized by ethidium bromide staining.
Measurement of Mucin Secretion
Cultured rat goblet cells were plated in 24-well plates and grown to confluence (1, 10–12, 18, 19). After serum starving cultured goblet cells for 2 h, the goblet cells were stimulated with AnxA1 in increasing concentrations in serum-free RPMI 1640 supplemented with 0.5% BSA for 2 h. The inhibitors were added 30 minutes prior to stimulation with AnxA1. An enzyme-linked lectin assay (ELLA) with the lectin UEA-1 was used to measure high molecular weight glycoprotein secretion that included MUC5AC produced by goblet cells. The supernatants were collected and analyzed for the total amount of lectin-detectable glycoconjugates, which quantifies the amount of goblet cell secretion as described previously (1, 3, 10–12, 20–22). The cells were scraped, and the cell homogenate was analyzed for the total amount of protein by using the Bradford protein assay. High molecular weight glycoconjugate secretion was normalized to total protein in the homogenate. Secretion was expressed as fold increase above basal that was set to 1.
Measurement of Intracellular [Ca2+]
Cultured rat conjunctival goblet cells were transferred to 35-mm glass bottom culture dishes and incubated overnight. The goblet cells were then incubated for 1 h at 37°C with Krebs-Ringer bicarbonate buffer with HEPES (KRB-HEPES) (119 mM NaCl, 4.8 mM KCl, 1.0 mM CaCl2, 1.2 mM MgSO4, 1.2 mM KH2PO4, 25 MM NaHCO3, 10 mM HEPES, and 5.5 mM glucose) (pH 7.45) plus 0.5% BSA containing 0.5 fura-2/AM (Invitrogen, Grand Island, NY), 8 μM pluronic acid F127 (Sigma-Aldrich, St. Louis, MO, USA) and 250 μM sulfinpyrazone (Sigma-Aldrich), followed by washing in KRB-HEPES containing sulfinpyrazone. Ca2+ measurements were made with a ratio imaging system (InCyt Im2; Intracellular Imaging, Cincinnati, OH) using excitation wavelengths of 340 and 380 nm and an emission wavelength of 505 nm. A minimum of 10 cells were selected in each experimental condition. AnxA1 was added either alone or after incubation with inhibitors for 15 or 30 min and intracellular [Ca2+] ([Ca2+]i) measured. After addition of agonist, data were collected in real time. The data are presented as [Ca2+]i over time and peak [Ca2+]i, calculated by subtracting the average value before the agonist AnxA1 was added (basal) from the peak [Ca2+]i.
Statistical Analysis
Results were expressed as the fold-increase above basal. Data are expressed as mean ± SEM. Data were analyzed by Student’s t-test. p< 0.05 was considered statistically significant.
Results
AnxA1 Is Expressed in Rat Conjunctival Goblet Cells
The protein AnxA1 was detected in a wide range of tissues, including the lung, intestine and bone marrow (14). We used PCR to investigate the expression of AnxA1 in cultured rat conjunctival goblet cells (Figure 1). A single band at the correct size of 504 bp was found in cDNA isolated from the conjunctivas of three individual animals. Expression of β-actin was used as a control. As AnxA1 is a protein, this is the first pro-resolving mediator whose presence in rat conjunctival goblet cells was verified by PCR.
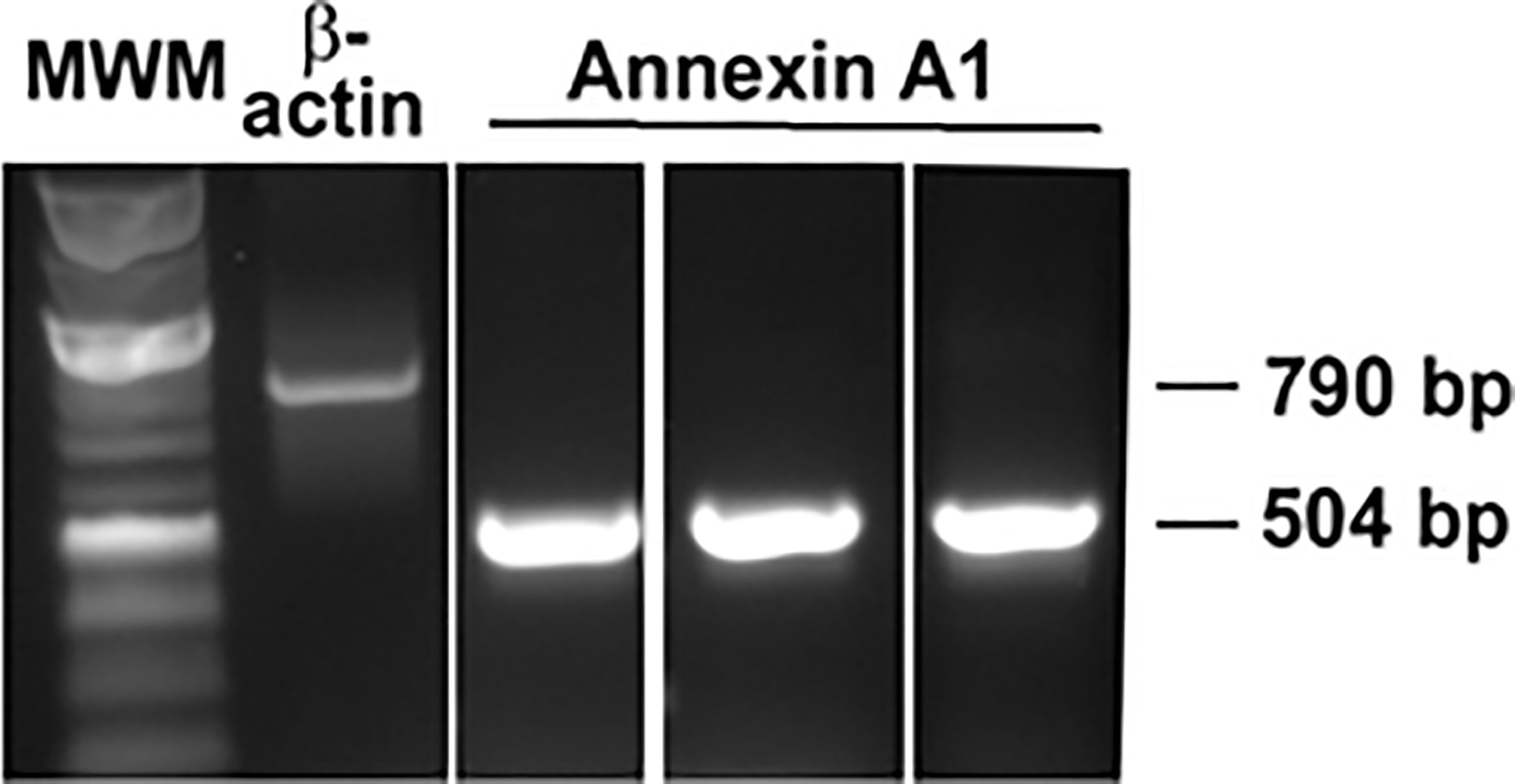
Figure 1 AnxA1 expression is detected in cultured rat conjunctival goblet cells by RT-PCR. Each lane represents cDNA from a different animal. β-Actin was used as a control.
AnxA1 Stimulates Mucin Secretion in Rat Conjunctival Goblet Cells
Lipid specialized pro-resolving mediators (SPM) such as LXA4, RvD1, and RvE1 stimulate mucin secretion from cultured rat conjunctival goblet cells (10–12). To explore if the protein AnxA1 stimulates secretion, goblet cells were stimulated for two h with AnxA1 (10-11-10-8 M) or a positive control histamine (10-5 M) (18, 23) and the amount of secretion was then measured (Figure 2). Histamine is known to stimulate glycoconjugate secretion from conjunctival goblet cells (22, 24). AnxA1 increased mucin secretion in a concentration-dependent manner with a maximum increase at 10-10 M. Significant increases occurred from 10-11 M to 10-9 M AnxA1 and were 1.7 ± 0.2, 3.2 ± 0.3 and 2.0 ± 0 fold above basal, respectively from three independent experiments. In comparison histamine significantly increased mucin secretion from basal by 2.2 ± 0.4 fold.
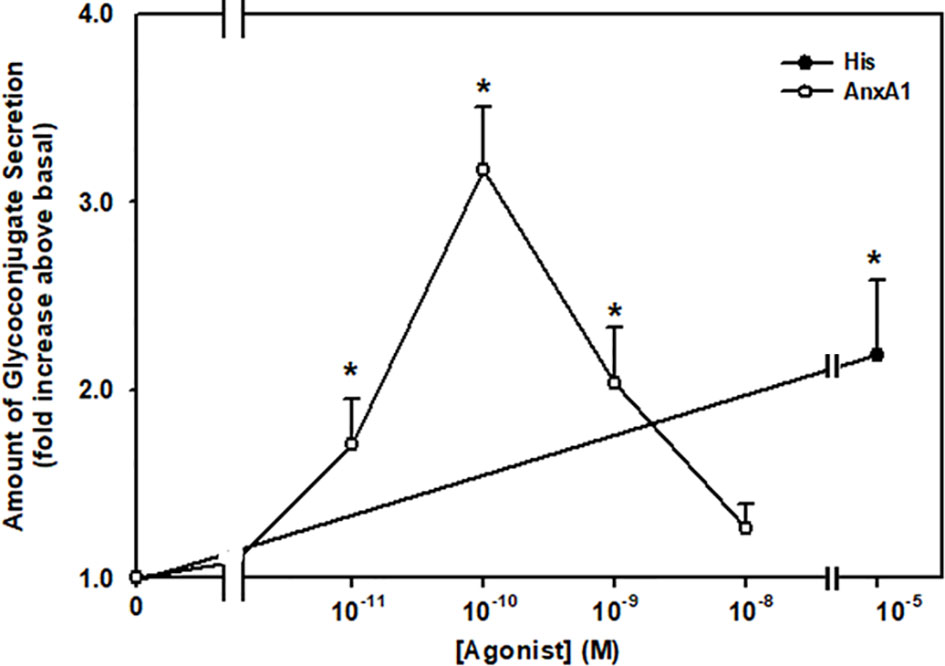
Figure 2 AnxA1 increases glycoconjugate secretion from cultured rat conjunctival goblet cells. Goblet cells were incubated with increasing concentrations of annexin A1 (AnxA1, 10-11 - 10-8 M) or histamine (His, 10-5 M). Data are presented as mean ± SEM from three independent experiments. * Indicates significant difference from basal.
AnxA1 Increases [Ca2+]i in Rat Conjunctival Goblet Cells
As AnxA1 stimulates conjunctival goblet cells to secrete mucins, we determined if AnxA1 alters the [Ca2+]i. Cultured goblet cells were incubated with fura-2/AM before stimulation with AnxA1 (10−11 -10−8 M). AnxA1 increased [Ca2+]i in a time and concentration-dependent manner (Figures 3A–C). All concentrations of AnxA1 significantly increased [Ca2+]i with the highest increase of 467.3 ± 95.1 nM occurring at 10-9 M (Figure 3C). These data are from five independent experiments. Therefore, AnxA1 increased [Ca2+]i in conjunctival goblet cells.
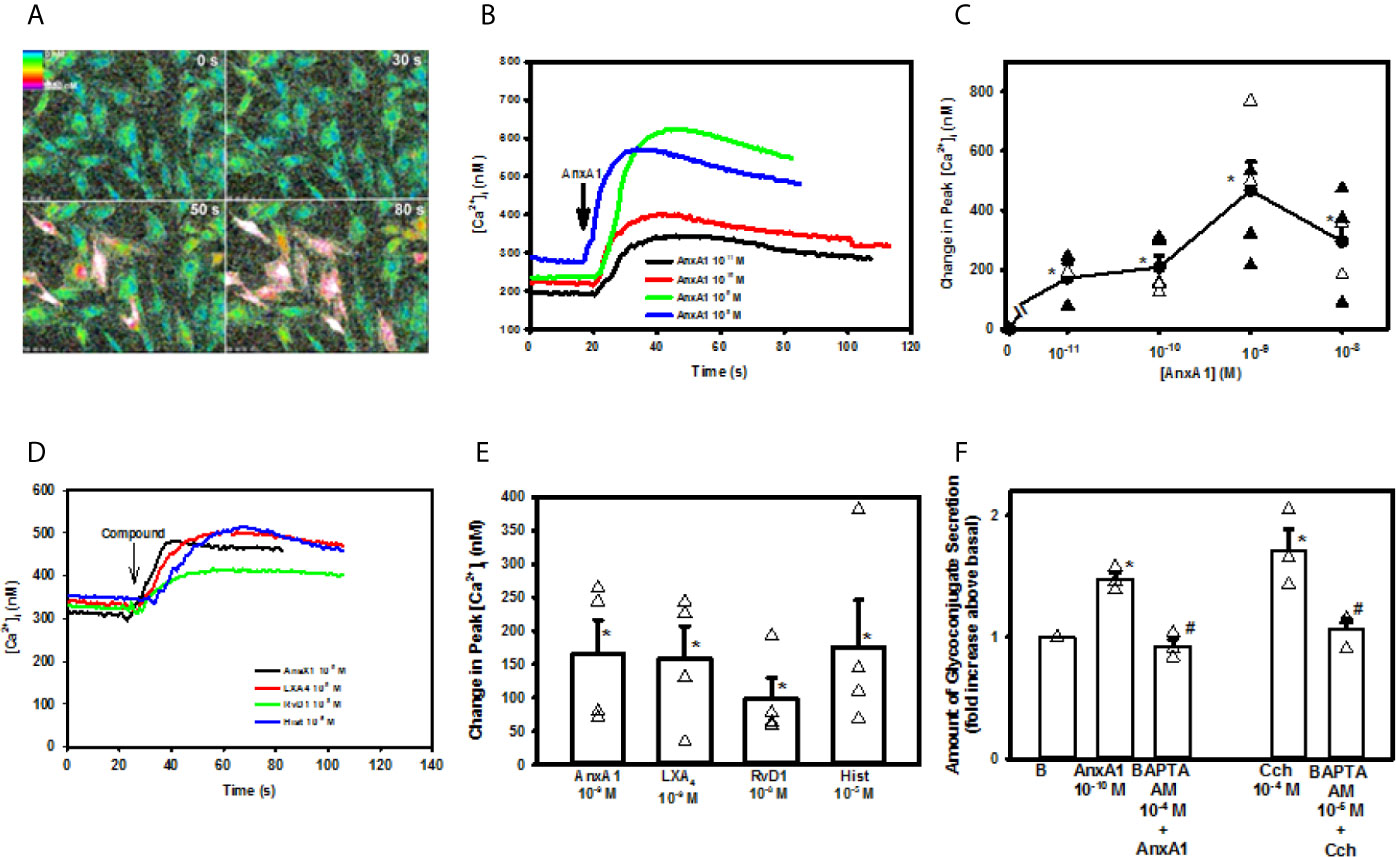
Figure 3 AnxA1 elevates [Ca2+]i to stimulate glycoconjugate secretion in cultured rat conjunctival goblet cells. Cultured goblet cells were incubated with fura-2/AM and stimulated with increasing concentrations of Annexin A1 (AnxA1, 10-11 M to 10-8 M). Pseudo color images of [Ca2+]i of goblet cells stimulated by AnxA1 10-9 M at four times are shown in (A) The increase in [Ca2+]i over time with AnxA1 (10-11 M to 10-8 M) is shown in (B) The change in peak [Ca2+]i after stimulation with AnxA1 (10-11 M to 10-8 M) is shown in (C) Increase in [Ca2+]i over time in response to AnxA1 (10-9 M), LXA4 (10-9 M), RvD1 (10-9 M) and histamine (hist, 10-5 M) is shown in (D) The change in peak [Ca2+]i with AnxA1, LXA4, RvD1, and histamine is shown in (E) Glycoconjugate secretion from goblet cells treated vehicle, AnxA1 (10-10 M), with the Ca2+ chelator BAPTA-AM (10-4 M) followed by stimulation with AnxA1, carbachol (Cch, 10-4 M) or BAPTA-AM (10-4 M) followed by carbachol is shown in (F) Data are mean ± SEM from 4 independent experiments (A–E) or 3 (F) independent experiments. *indicates significant difference from basal; #indicates significant difference from AnxA1 or Cch alone.
To compare the [Ca2+]i responses induced by other known mediators with that of AnxA1, goblet cells were stimulated with AnxA1, the lipid SPMs LXA4 and RvD1, and the allergic mediator histamine. All compounds were used at the concentrations previously found to result in the highest increase in [Ca2+]i (10, 12). Each mediator significantly increased [Ca2+]i over basal and to the same extent, as there was no significant difference between them (n=4, Figures 3D, E).
AnxA1 Uses Intracellular Ca2+ to Stimulate Secretion in Rat Conjunctival Goblet Cells
To determine if the AnxA1-stimulated increase in [Ca2+]i leads to mucin secretion, rat goblet cells were incubated with the intracellular calcium chelator BAPTA/AM (10-4 M) for 30 minutes prior to AnxA1 (10-9 M) stimulation. The cholinergic agonist carbachol (Cch) at 10-4 M was used as a control. To ensure that BAPTA chelated [Ca2+]i and prevented the increase in [Ca2+]i induced by AnxA1 and Cch, [Ca2+]i was measured (Supplemental Figure 1). AnxA1 significantly increased [Ca2+]i to a peak of 128.8 ± 11.5 nM.When goblet cells were preincubated with BAPTA/AM, the AnxA1-stimulated increase in [Ca2+]i was significantly inhibited to 30.1 ± 7.5 nM. The Ca2+ response to Cch, a positive control, was also significantly inhibited from 227.0 ± 54.8 nM to 29.3 ± 50.2 nM in the presence of BAPTA. These results were from 4 independent experiments.
We then investigated if AnxA1 uses to increase mucin secretion. Rat cultured goblet cells were treated with BAPTA/AM (10-4 M), stimulated by AnxA1 (10-10 M) and secretion measured. In three independent experiments, AnxA1 alone significantly increased glycoconjugate secretion by 1.5 ± 0.05 fold above basal (Figure 3F). BAPTA/AM significantly inhibited AnxA1-stimulated mucin secretion to 0.9 ± 0.06 fold above basal. Cch (10-4 M)-stimulated glycoconjugate secretion was also significantly inhibited from 1.7 ± 0.2 fold to 1.1 ± 0.1 fold above basal. These findings demonstrate that AnxA1 increases [Ca2+]i to stimulate mucin secretion in conjunctival goblet cells.
AnxA1 Uses Both Intracellular Ca2+ Stores and Extracellular Ca2+ to Increase [Ca2+]i in Rat Conjunctival Goblet Cells
To examine the role that intracellular Ca2+ stores play in AnxA1 stimulation, goblet cells were incubated with the Ca2+ reuptake inhibitor thapsigargin. Thapsigargin depletes the ER of Ca2+ by blocking the Ca2+/ATPase in the endoplasmic reticulum (ER) that inhibits Ca2+ from being taken up by the ER (25). The ER store of Ca2+ is depleted as Ca2+ passively leaks out of the ER and is not replaced as shown by the increase in [Ca2+]i over time (Figure 4A). AnxA1 added alone induced a peak increase in [Ca2+]i of 166.8 ± 26.7 nM (Figures 4A, B, n=6). The AnxA1-induced Ca2+ response was significantly blocked by thapsigargin pre-treatment and was 24.6 ± 9.7 nM. In the same cells, Cch was used as a control and increased [Ca2+]i to 243.4 ± 24.0 nM. Cch-stimulated increase in [Ca2+]i was also blocked by pre-treatment with thapsigargin and was 44.4 ± 21.2 nM. These experiments indicate that AnxA1 works by mobilizing Ca2+ from the ER to increase cytoplasmic [Ca2+]i.
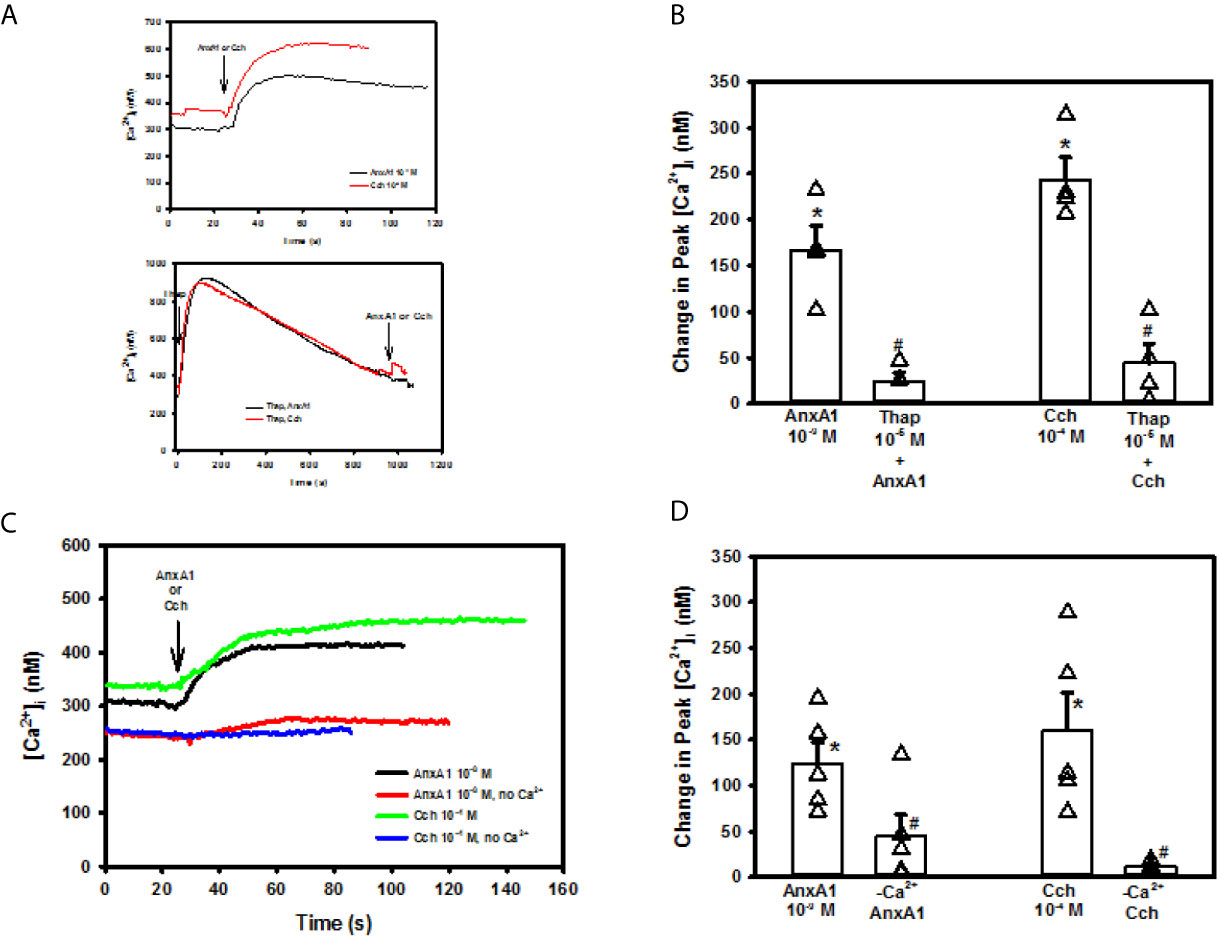
Figure 4 Annexin A1 (Anx1, 10-9 M) or carbachol (Cch, 10-4 M) (A top panel) or AnxA1 uses intracellular and extracellular Ca2+ to increase [Ca2+]i in cultured rat conjunctival goblet cells. Cultured rat goblet cells were treated with thapsigargin (10-5 M) (first arrow) for 15 minutes before stimulation indicated by second arrow with annexin A1 (AnxA1, 10-9 M) or Cch (10-4 M). (A bottom panel), [Ca2+]i over time is shown in (A) Change in peak [Ca2+]i is shown in (B) Goblet cells were incubated in KRB solution with and without CaCl2 and stimulated with AnxA1 (10−9 M) or Carbachol (Cch, 10-4 M). [Ca2+]i over time is shown in (C) Change in peak [Ca2+]i is shown in (D) Data are mean ± SEM from 4 (A, B) or 6 (C, D) independent experiments. *indicates significant difference from basal; #indicates significance from AnxA1 or Cch alone.
After an agonist depletes intracellular Ca2+ stores, this depletion activates STIM1 and Orai to induce Ca2+ influx to replace those stores. Since AnxA1 used intracellular Ca2+ stores, we investigated if AnxA1 used extracellular Ca2+. Cells were incubated in KRB with and without extracellular CaCl2. AnxA1 (10-9 M) added to the KRB containing CaCl2 significantly increased [Ca2+]i by 124.1 ± 23.0 nM (Figures 4C, D, n=4). This increase in [Ca2+]i was significantly reduced to 44.0 ± 23.7 nM when AnxA1 was added to KRB without CaCl2, indicating that AnxA1 stimulates influx of extracellular Ca2+ to increase [Ca2+]i. Cch (10-4 M), a control, elevated [Ca2+]i to 160.4 ± 40.9 nM. Cch-stimulated increase in [Ca2+]i was significantly blocked by the CaCl2-free solution and was 11.8 ± 2.1 nM. Based on these results, AnxA1 releases Ca2+ from intracellular Ca2+ stores and stimulates extracellular Ca2+ influx to increase the [Ca2+]i in conjunctival goblet cells.
AnxA1 Uses the ALX/FPR2 Receptor to Increase [Ca2+]i and Stimulate Secretion in Rat Conjunctival Goblet Cells
In tissues and cells examined, AnxA1 works through the G-protein coupled formyl peptide receptor 2 (ALX/FPR2) (14, 26). To confirm that AnxA1 uses this receptor in goblet cells, cultured rat conjunctival goblet cells were incubated with the ALX/FPR2 antagonist BOC-2 (10-4 M) for 30 minutes before stimulating the goblet cells with AnxA1 (10-9 M) or LXA4 (10-9 M), which is known to bind to ALX/FPR2 and is a positive control (12). AnxA1 added alone significantly increased [Ca2+]i with a peak of 265.4 ± 61.0 nM (Figures 5A, C, n=5). BOC-2 significantly blocked AnxA1’s increase in [Ca2+]i to 54.1 ± 23.9 nM. The LXA4 response was 180.8 ± 52.4 nM (Figures 5B, C). In the presence of BOC-2, the LXA4 response was significantly reduced to 47.7 ± 20.0 nM.
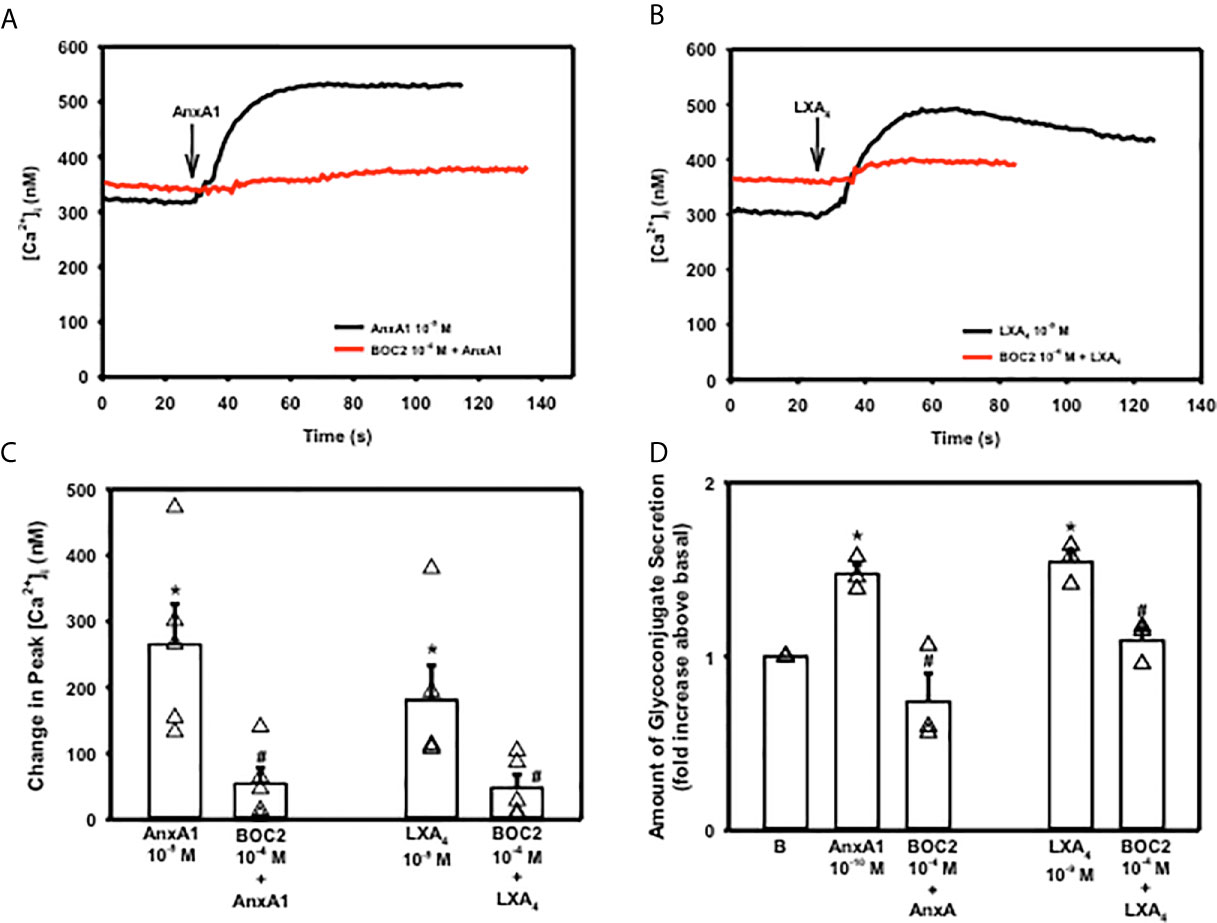
Figure 5 AnxA1 uses the ALX/FPR2 receptor to increase [Ca2+]i and stimulate secretion in cultured rat conjunctival goblet cells. Goblet cells were preincubated with BOC2 (10-4 M) for 30 minutes and stimulated with vehicle or annexin A1 (AnxA1, 10-9 M) shown in (A, C) and vehicle or the control LXA4 (10-9 M) shown in (B, C) [Ca2+]i over time is shown in (A, B) Change in peak [Ca2+]i is shown in (C) Amount of glycoconjugate secretion is shown in (D) with AnxA1 at 10-10 M. B indicates basal (vehicle) in (D) Data in are mean ± SEM from 4 (A–C) or 3 (D) independent experiments. *indicates significant difference from basal; #indicates significance from AnxA1 or LXA4 alone.
To determine if AnxA1 uses the ALX/FPR2 receptor to stimulate high molecular weight glycoprotein secretion, cells were preincubated with BOC-2 (10-4 M) followed by AnxA1 (10-10 M) (Figure 5D, n=3). LXA4 (10-9 M) was again used as a positive control. Secretion was significantly increased by 1.5 ± 0.05 fold above basal when AnxA1 (10-10 M) was added by itself. AnxA1 stimulated secretion was significantly inhibited by BOC-2 to 0.74 ± 0.16 fold above basal. LXA4 stimulated secretion was also inhibited by BOC-2. These data indicate that AnxA1 uses the ALX/FPR2 receptor to stimulate an increase in [Ca2+]i and secretion in conjunctival goblet cells.
AnxA1 Activates the PLC Pathway and Its Downstream Effectors IP3 and PKC to Increase [Ca2+]i and Stimulate Secretion in Rat Conjunctival Goblet Cells
The phospholipase C (PLC) pathway is critical for cellular Ca2+ signaling as activation of PLC generates both inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG) (27). IP3 interacts with its receptors on the ER to release Ca2+ from intracellular stores, while DAG activates protein kinase C (PKC). To explore if AnxA1 activates the PLC pathway in rat conjunctival goblet cells, these cells were preincubated with either the PLC inhibitor U73122 or its negative control U73343 at 10-7 M, before addition of AnxA1 (10-9 M) or a positive control Cch [10-4 M) (28)]. AnxA1 added alone significantly increased [Ca2+]i with a peak of 217.1 ± 34.6 nM (Figure 6A, n=3) The active inhibitor U73122 significantly decreased the AnxA1 stimulated increase in [Ca2+]i to 63 ± 10.0 nM, while the inactive inhibitor U73343 did not significantly block AnxA1. The Cch response was also inhibited by U73122, but not U73343 (28).
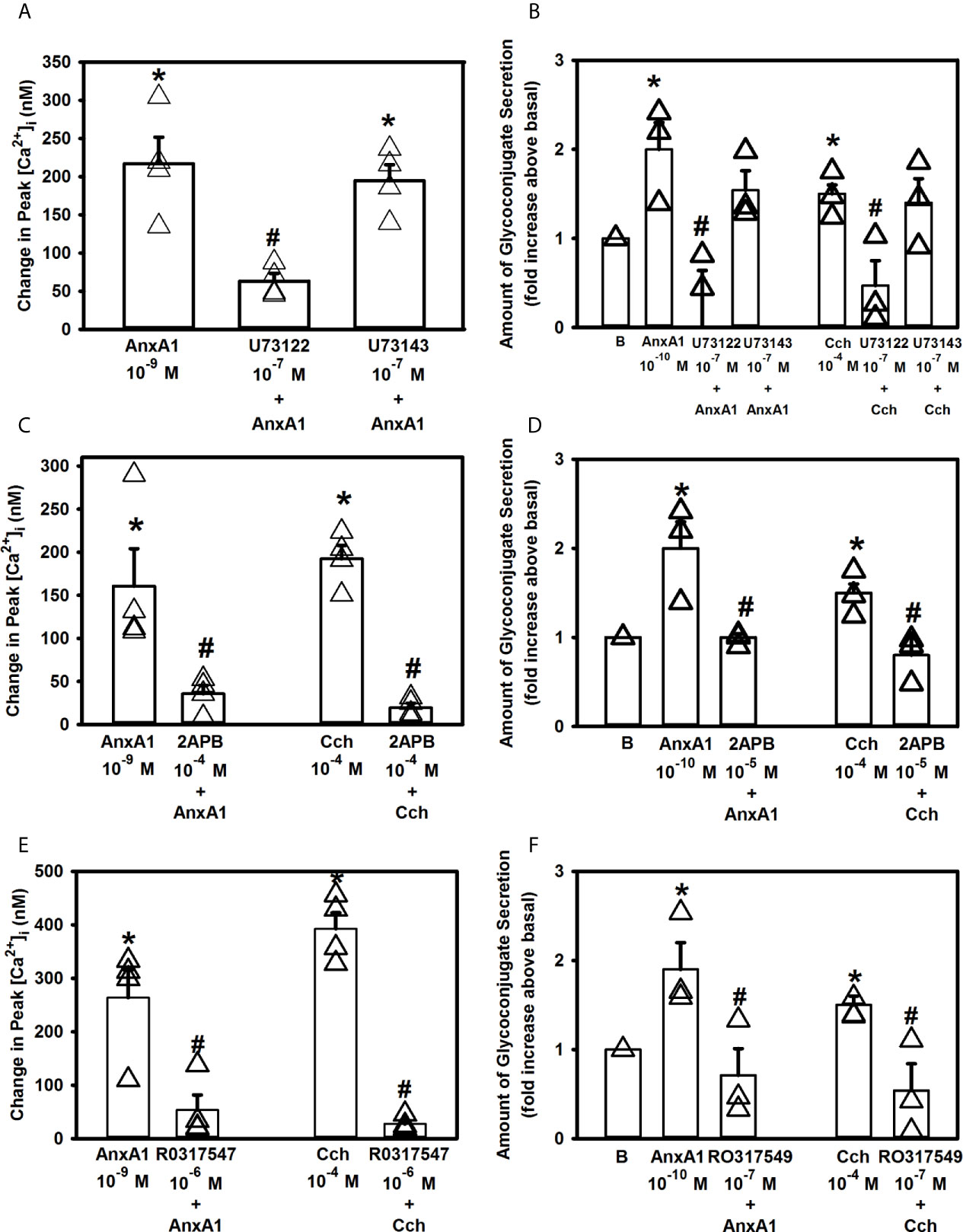
Figure 6 AnxA1 activates the Phospholipase C (PLC) pathway and its downstream effectors to increase [Ca2+]i in cultured rat conjunctival goblet cells. Cultured goblet cells were treated with vehicle, the PLC inhibitor U73122, its inactive analog U73343 (both at 10-7 M, A, B), 2APB (10-5 M, C, D), or the PKC inhibitor RO317549 (10-7 M, E, F) for 30 minutes before stimulation with annexin A1 (AnxA1, 10-9 M) in (A, C, E) or with AnxA1 at 10-10 M in (B, D, F) or the positive control carbachol (Cch, 10-4). Change in peak [Ca2+]i are shown in (A, C, E) while glycoconjugate secretion is shown in (B, D, F). B (vehicle) indicates basal in (B, D, F). Data are mean ± SEM from three (A, B, D, F), four (C, E) independent experiments. *indicates significant difference from basal; #indicates significance from AnxA1 or carbachol alone.
The effects of U73122 and U73143 on secretion were then determined. AnxA1 (10-10 M) increased secretion 2.0 ± 0.3 fold above basal (Figure 6B, n=3). AnxA1-stimulated response was completely inhibited by preincubation with U73122 (10-7 M), but not U73143 (10-7 M). U73122, but not U73143, also significantly inhibited secretion stimulated by Cch (10-4 M), which was a positive control. These data show that AnxA1 stimulates PLC to increase [Ca2+]i and stimulate secretion.
To further explore the PLC pathway, goblet cells were incubated for 30 min with 2APB (10-5 M), an inhibitor of IP3 receptors on the ER (29). AnxA1 (10-9 M) added alone significantly increased [Ca2+]i by 160.6 ± 43.4 nM (Figure 6C, n=4). AnxA1-induced [Ca2+]i increase was significantly decreased to 35.7 ± 9.6 nM after 30 minutes of incubation with 2APB. Cch (10-4 M) was used as a positive control and its stimulation of [Ca2+]i was significantly blocked by 2APB as well.
A similar effect of 2APB was found when secretion was examined. AnxA1 (10-10 M) increased secretion 2.0 ± 0.3 fold above basal (Figure 6D, n=3). AnxA1-stimulated response was significantly inhibited by incubation with 2APB, which also significantly inhibited secretion stimulated by Cch (10-4 M), the positive control.
Next, we investigated the effect of the PKC inhibitor RO317549. Goblet cells were incubated for 30 minutes with RO317549 (10-7 M) before stimulation with AnxA1 (10-9 M) or a positive control Cch (10-4 M). [Ca2+]i was significantly increased when AnxA1 was added alone (263.7 ± 51.8 nM, Figure 6E, n=4). RO317549 significantly blocked the effect of AnxA1 on [Ca2+]i and was 27.0 ± 6.3 nM)). Cch-stimulated increase in [Ca2+]i was also significantly inhibited.
Inhibition of PKC with RO317549 also blocked AnxA1-stimulated secretion. AnxA1 increased secretion by 1.9 ± 0.3 fold above basal (Figure 6F, n=3). RO317549 significantly reduced AnxA1-induced secretion to 0.7 ± 0.3 fold above basal. As a positive control, Cch stimulated secretion was also significantly inhibited
In conjunctival goblet cells inhibitors of PLC activity, IP3 interaction with its receptors, and PKC each blocked the AnxA1-stimulated function measured. Based on these findings, AnxA1 activates the PLC pathway both to increase intracellular Ca2+ and stimulate secretion through the production of IP3 and activation of PKC.
AnxA1 Increases [Ca2+]i and Secretion Through Activation of Ca2+/CaMK II in Conjunctival Goblet Cells
Free intracellular Ca2+ can bind to calmodulin, to create a calmodulin/calcium complex. This complex can bind to different proteins such as Ca2+/calmodulin dependent protein kinase II (Ca2+/CaMK) (30). We have previously shown that LXA4, through the ALX/FPR2 receptor, increases [Ca2+]i through Ca2+/CaMK (12). To investigate if AnxA1 also activates Ca2+/CaMK, goblet cells were preincubated with the Ca2+/CaMK inhibitor KN93 or the inactive analog KN92 at 10-7 M for 30 minutes. AnxA1 (10-9 M) significantly increased [Ca2+]i to a peak of 235.6 ± 50.0 nM (Figure 7A). KN93 but not KN92 significantly blocked AnxA1-stimulated increase in [Ca2+]i and was 97.6 ± 19.6 (Figure 7A, n=4). A positive control Cch (10-4 M) significantly increased [Ca2+]i by 203.5 ± 40.2 nM. KN 93, but not KN92, significantly decreased Cch-stimulated increase in [Ca2+]i.
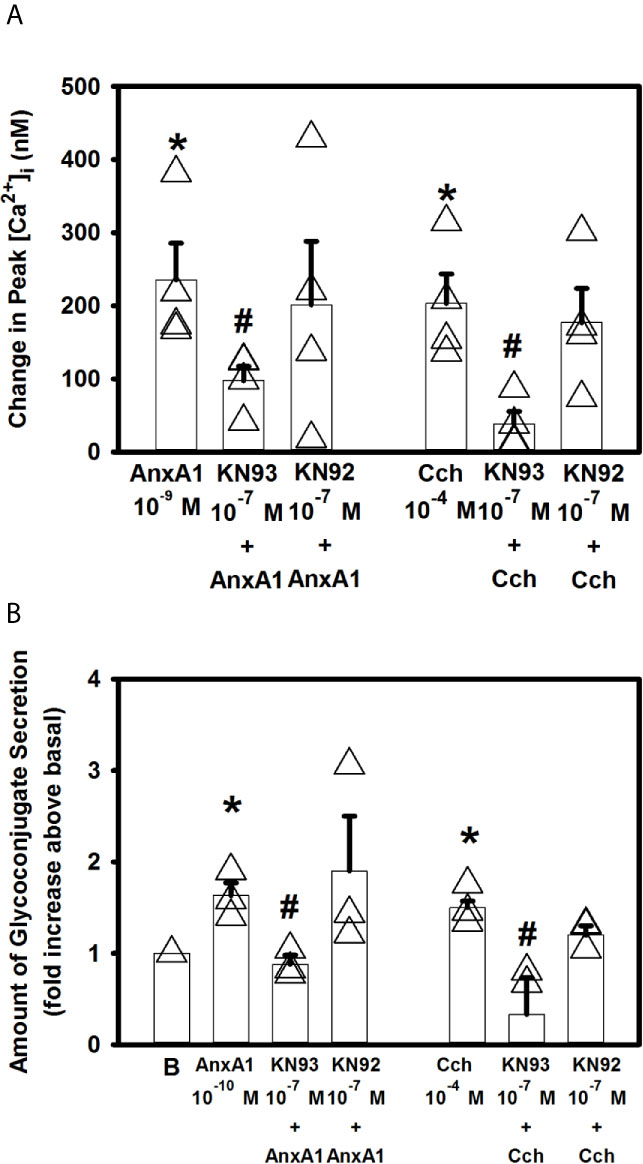
Figure 7 AnxA1 activates the calcium/calmodulin-dependent protein kinase (Ca2+/CaMK) to increase [Ca2+]i and stimulate glycoconjugate secretion in cultured rat conjunctival goblet cells. Goblet cells were treated with the Ca2+/CaMK inhibitor KN93, its inactive analog KN92,or vehicle for 30 minutes prior to stimulation with annexin A1 (AnxA1, 10-9 M) or carbachol (Cch, 10-4 M). Change in peak [Ca2+]i is shown in (A) AnxA1 (10-10 M)-stimulated glycoconjugate secretion is shown in (B) indicates basal (vehicle) in (B) Data are mean ± SEM from 4 (A) or 3 (B) independent experiments. *indicates significant difference from basal. #indicates significance from Cch alone.
KN93 also significantly decreased 10-10 M AnxA1-stimulated secretion from 1.6 ± 0.1 to 0.9 ± 0.1 fold above basal (Figure 7B, n=3). KN92 had no effect on AnxA1-stimulated secretion. KN93, but not KN92, also significantly inhibited 10-4 M Cch-stimulated secretion. These data indicate that AnaX1 uses Ca2+/CaMK to increase [Ca2+]i and stimulate glycoconjugate secretion.
AnxA1 Does Not Increase [Ca2+]i But Does Increase Secretion Through Activation of ERK 1/2 in Rat Conjunctival Goblet Cells
The ERK 1/2 pathway is a component of a signaling cascade that is activated in cells by a variety of stimuli (18, 31, 32). To determine if the ERK 1/2 pathway plays a role in AnxA1-stimualted increase in [Ca2+]i the ERK 1/2 inhibitor UO126 was used. Rat conjunctival goblet cells were incubated with UO126 (10-8–10-6 M) for 30 minutes (Figure 8A, n=3). AnxA1 (10-9 M) added alone significantly increased [Ca2+]i by 152.5 ± 37.2 nM. AnxA1-induced [Ca2+]i increase was not significantly blocked by UO126 at any concentration. Histamine (10-5 M), which is known to activate ERK 1/2 was used as a control (22) and was significantly blocked by all three concentrations of UO126.
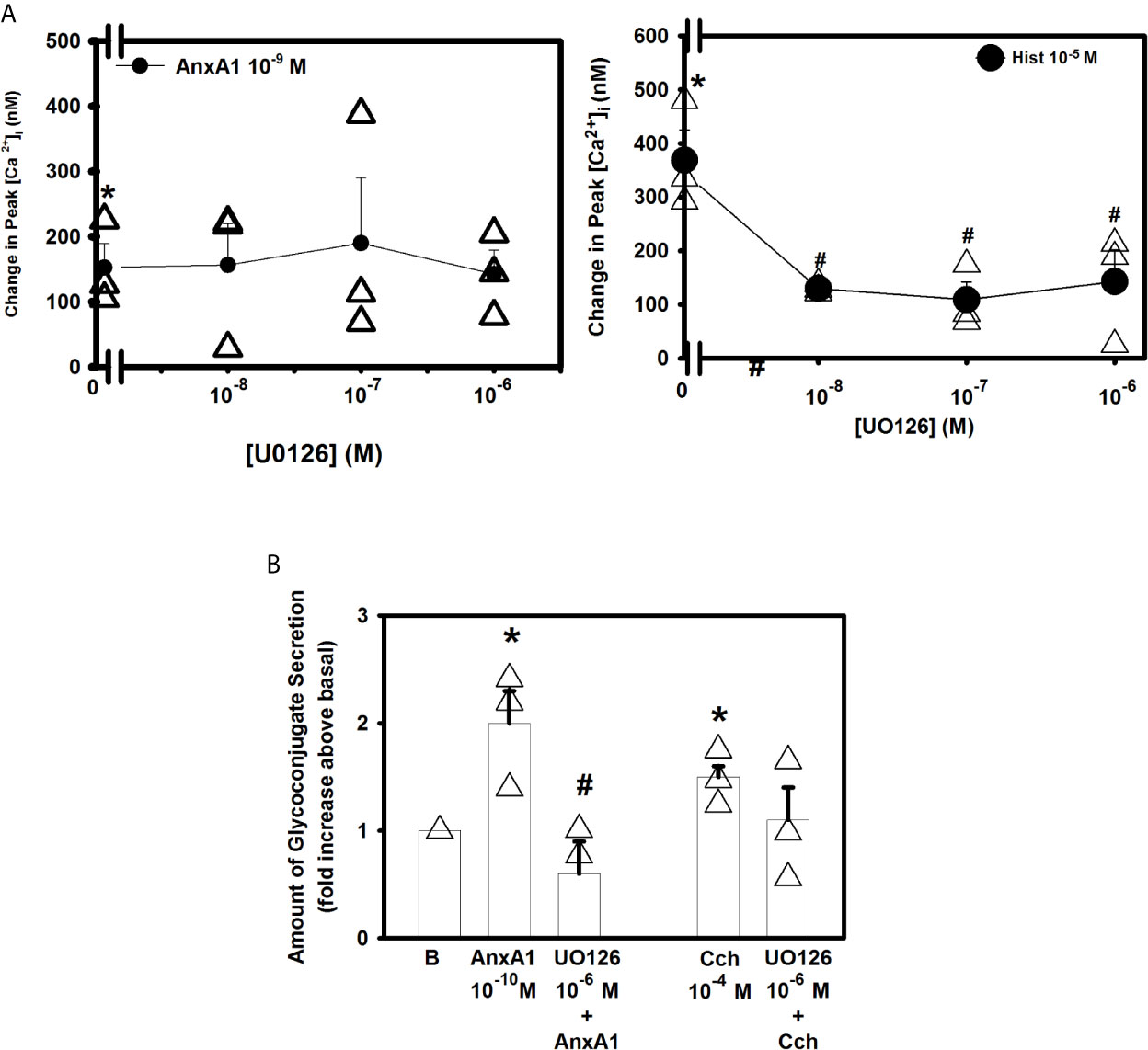
Figure 8 AnxA1 does not activate extracellular regulated protein kinase (ERK) 1/2 to increase [Ca2+]i but does activate it to stimulate glycoconjugate secretion in cultured rat conjunctival goblet cells. Rat conjunctival goblet cells were treated with vehicle or the ERK 1/2 inhibitor UO126 (10-8 - 10-6 M) for 30 minutes prior to stimulation with either annexin A1 (AnxA1, 10-9 M) in (A) or with AnxA1 at 10-10 M in (B), or the control histamine (Hist, 10-5 M) or carbachol (Cch, 10-4 M). Change in peak [Ca2+]I is shown in (A) Glycoconjugate secretion is shown in (B) B indicates basal (vehicle) in (B) Data are mean ± SEM from 4 (A) or 3 (B) independent experiments. *indicates significant difference from basal. #indicates significance from either AnxA1 or histamine alone.
To examine the effects of UO126 on glycoconjugate secretion, goblet cells were incubated with AnxA1 with and without UO126 (10-7 M). AnxA1(10-10 M) alone increased secretion by 2.0 ± 0.3 fold above basal (Figure 8B, n=3). UO126 significantly decreased AnxA1-stimulated response and was 0.6 ± 0.3 fold above basal. In contrast to previous studies (31) U0126 did not inhibit the carbachol-induced secretory response. These data indicate that AnxA1 does not activate ERK1/2 to increase [Ca2+]i but does activate it to stimulate glycoconjugate secretion.
AnxA1 Does Not Use PKA to Increase [Ca2+]i in Rat Conjunctival Goblet Cells
To determine if AnxA1 increases cAMP levels to activate protein kinase A (PKA) to increase [Ca2+]i and stimulate secretion, goblet cells were preincubated with the PKA inhibitor H89 (10-5 M) for 30 minutes before stimulating the cells with AnxA1 (10-9 M) (Supplemental Figure 2A, n=9). AnxA1 added alone significantly increased [Ca2+]i by 420 ± 111.9 nM. H89 did not significantly inhibit AnxA1 stimulated increase in [Ca2+]i. Vasoactive intestinal peptide (VIP, 10-8 M), which is known to increase cAMP (19), was used as a control. VIP-stimulated increase in [Ca2+]i was 548.1 ± 110.6 nM. H89 significantly blocked VIP-stimulated increase in [Ca2+]i and was 252.5 ± 39.6 nM.
Similar to the effects on [Ca2+]i H89 did not significantly inhibit AnxA1-stimulated glycoconjugate secretion (Supplemental Figure 2B, n=3). However, the positive control, VIP-stimulated secretion was inhibited by H89. These data indicate that activation of PKA does not play a role in AnxA1-stimulated increase in [Ca2+]i or secretion.
AnxA1 Does Not Increase [Ca2+]i But Does Stimulate Secretion Through the PLD Pathway in Rat Conjunctival Goblet Cells
Phospholipase D (PLD) is an important regulator of several critical aspects of cell physiology, and is involved in cell functions like endocytosis, exocytosis, cell migration and mitosis (33). We previously showed that the other SPMs such as RvD1, RvE1 and LXA4 work through PLD (10–12). To explore if AnxA1 uses PLD, cultured rat conjunctival goblet cells were incubated for 15 minutes with either the PLD inhibitor 1-but or the inactive inhibitor t-but, both at 0.3% (Figure 9A). AnxA1 (10-9 M) alone significantly increased [Ca2+]i to a peak of 141.2 ± 50.0 nM (n=4). Neither preincubation with 1-but or t-but altered the effect of AnxA1 on the Ca2+ response. The increase in [Ca2+]i stimulated by the positive control carbachol (10-4 M) was significantly blocked by the active inhibitor 1-but, but not the inactive inhibitor t-but.
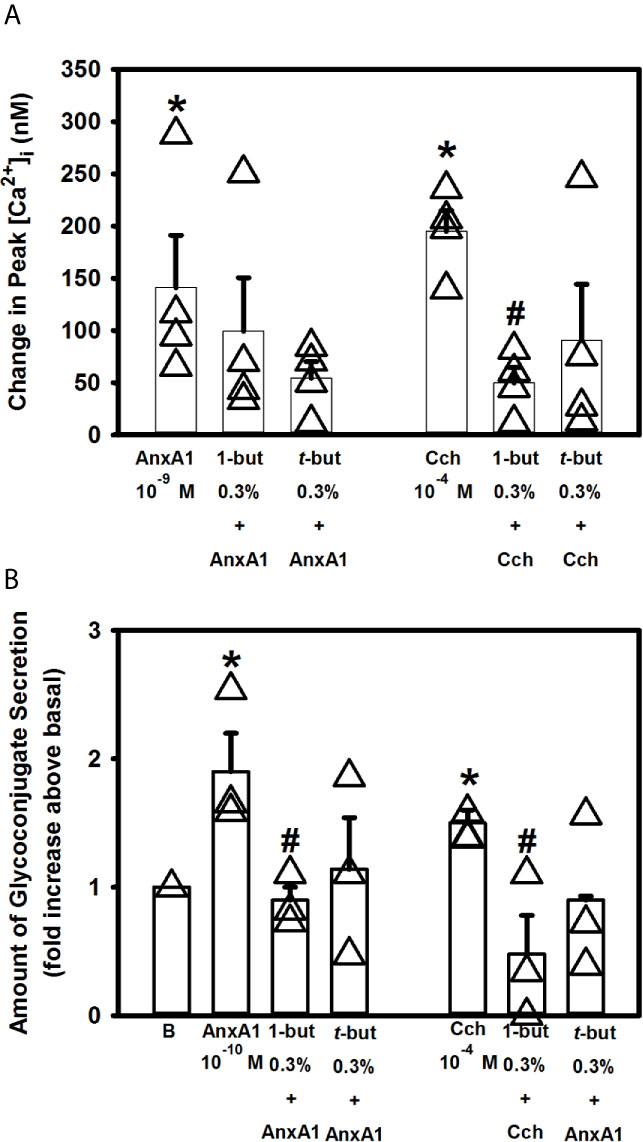
Figure 9 AnxA1 does not active the phospholipase D (PLD) pathway to increase [Ca2+]i but activates it to stimulate secretion in cultured rat conjunctival goblet cells. Rat conjunctival goblet cells were treated with vehicle, the PLD inhibitor 1-butanol (1-but) or the inactive inhibitor t-butanol (t-but) for 15 minutes prior to stimulation with annexin A1 (AnxA1, 10-9 M) in (A) or with AnxA1 at 10-10 M in (B), or carbachol (Cch, 10-4 M). Change in peak [Ca2+]I is shown in (A) Glycoconjugate secretion is shown in (B) Data are mean ± SEM from 4 (A) or 3 (B) independent experiments. *indicates significant difference from basal. #indicates significant difference from either AnxA1 or Cch alone.
When secretion was measured, AnxA1 (10-10 M) alone increased secretion 1.9 ± 0.3 fold above basal (Figure 9B, n=3)). Incubation with 1-but, but not t-but, significantly decreased this response that was 0.9 ± 0.1 fold above basal. Similarly to AnxA1, stimulation of secretion by Cch (10-4 M) was significantly decreased by 1-but, but not t-but. Based on these findings we conclude that AnxA1 does not active the PLD pathway to stimulate an increase in [Ca2+]i but does activate PLD to stimulate secretion in cultured rat conjunctival goblet cells.
AnxA1 Does Not Increase [Ca2+]i But Does Stimulate Secretion Through PLA2 Pathway in Cultured Rat Goblet Cells
Another phospholipase that is present and can be activated in goblet cells is the PLA2 pathway (10–12). To determine if AnxA1 utilizes PLA2 to increase [Ca2+]i and secretion, goblet cells were incubated with the PLA2 inhibitor aristolochic acid (AA) for 30 min prior to stimulation with AnxA1 (Figure 10A). AnxA1 (10-9 M) significantly increased peak [Ca2+]i by 530.0 ± 109.2 nM (n=4). Preincubation with AA at 10-6 M significantly altered the AnxA1-stimulated response that was 228.1 ± 60.7 nM. AA at 10-5 M did not significantly alter the AnxA1 response. The positive control Cch (10-4 M)-stimulated response was significantly decreased with AA 10-6 and 10-5 M from 542.6 ± 78.6 nM to 217.7 ± 65.4 nM and 257.1 ± 52.6 nM, respectively.
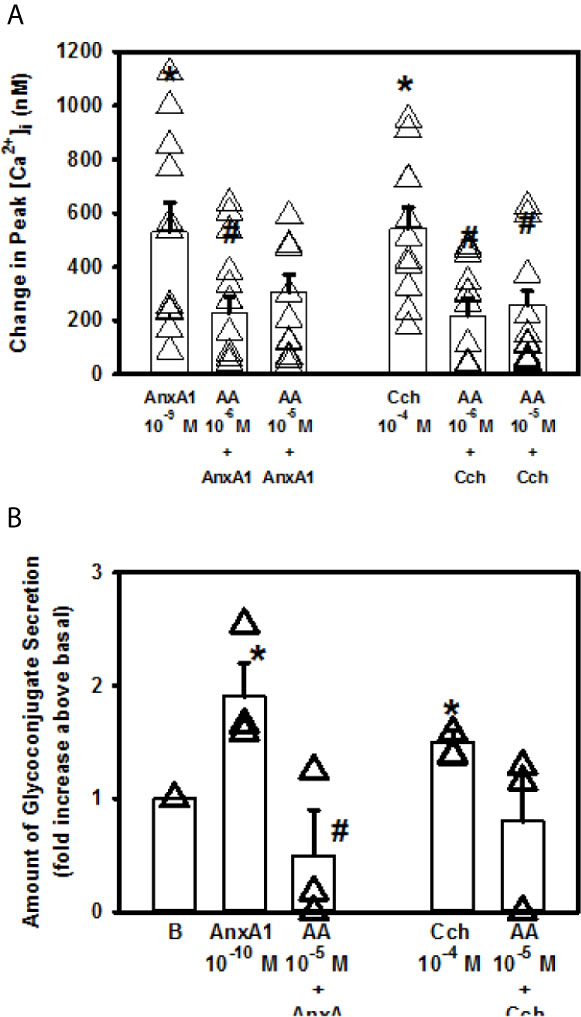
Figure 10 AnxA1 does not active the phospholipase A2 (PLA2) pathway to increase [Ca2+]i but activates it to stimulate secretion in cultured rat conjunctival goblet cells. Rat conjunctival goblet cells were preincubated with either vehicle or the PLA2 inhibitor aristolochic acid (AA, 10-5 M) for 30 minutes prior to stimulation with annexin A1 (AnxA1, 10-9 M) in (A) or with AnxA1 at 10-10 M in (B), or carbachol (Cch, 10-4 M). Change in peak [Ca2+]i is shown in (A) Glycoconjugate secretion is shown in (B) Data are mean ± SEM from 4 (A) or 3 (B) independent experiments. *indicates significant difference from basal. #indicates significant difference from AnxA1 alone.
AnxA1 (10-10 M) increased glycoconjugate secretion by 1.9 ± 0.3 fold above basal (Figure 10B, n=3). This response was significantly inhibited by AA (10-5 M) to 0.5 ± 0.4. Thus AnxA1 does not active the PLA2 pathway to stimulate an increase in [Ca2+]i but does activate PLA2 to stimulate secretion in cultured rat conjunctival goblet cells.
Discussion
In the present study, we showed that AnxA1 contributes to tear film and ocular surface homeostasis by activating the PLC pathway, including its downstream effectors IP3 and PKC, through interaction with the ALX/FPR2 receptor (Figure 11). This results agrees with findings in Hodges et al. (12) using a desensitization protocol that indicates that AnxA1 interacts with the same receptor as LXA4 and RvD1, the ALX/FPR2 receptor. This interaction increases [Ca2+]i and stimulates glycoconjugate secretion including the protective mucin MUC5AC from conjunctival goblet cells. AnxA1-stimulated increase in [Ca2+]i is dependent on both intracellular Ca2+ stores and influx of extracellular Ca2+ consistent with an interaction with Orai and STIM-1 known to regulate these processes in most tissues in which stimulation of G-protein-coupled receptors activates PLC (34).
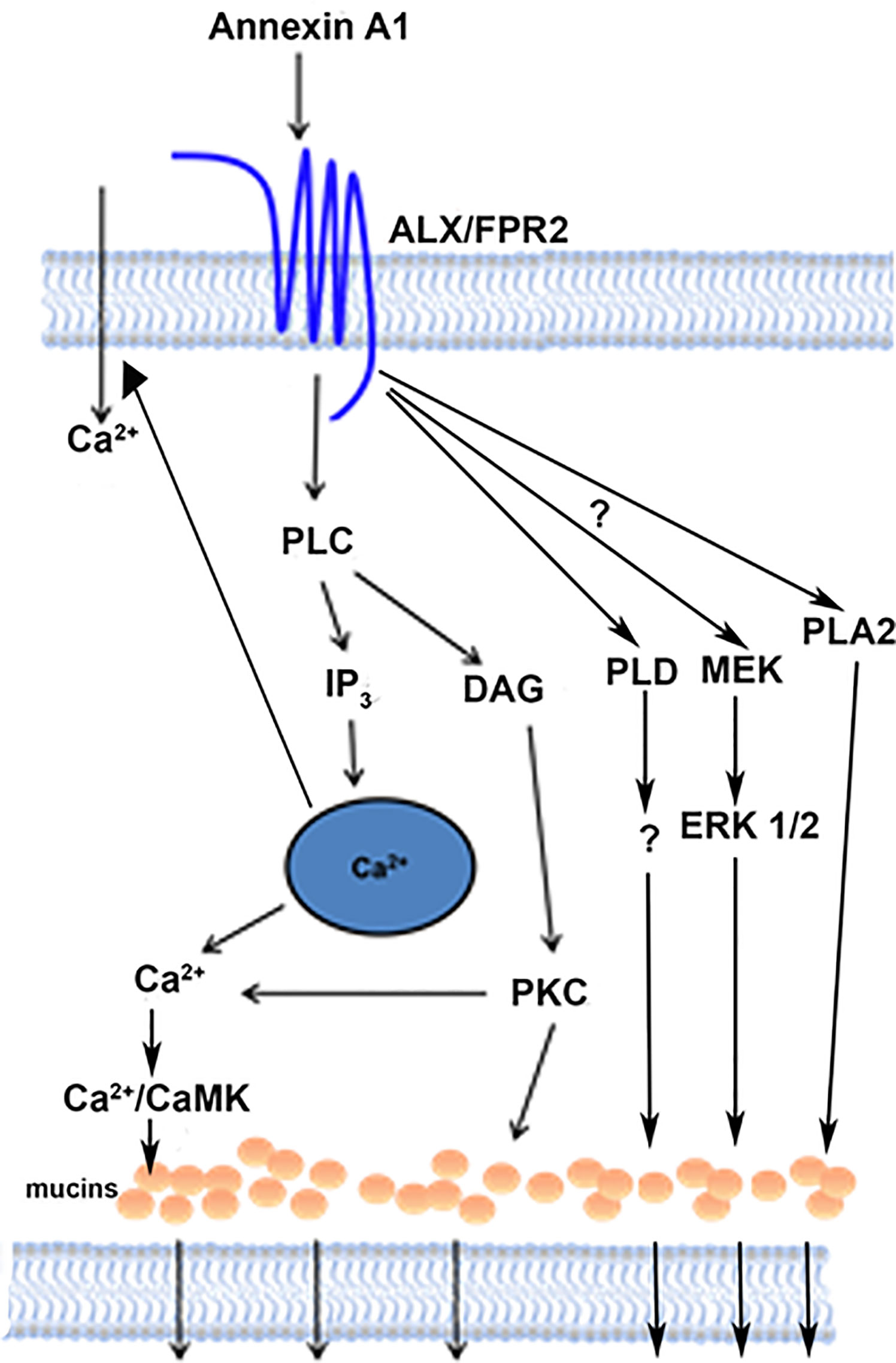
Figure 11 Schematic diagram of signaling pathways activated by AnxA1. AnxA1 binds to the ALX/FPR2 receptor to active the PLC, PLD, and PLA2 pathways, and consequently the secretion of mucins from conjunctival goblet cells. AnxA1 also stimulates influx of Ca2+. PLC, phospholipase C; DAG, diacylglycerol; IP3, inositol triphosphate; PKC, protein kinase C; Ca2+/CamK, calcium/calmodulin-dependent kinase; PLD, phospholipase D; MEK, mitogen-activated protein kinase kinase; ERK 1/2, extracellular regulated kinase; PLA2, phospholipase A2.
AnxA1 is present in a variety of other tissues like bone marrow, the intestine and lungs (14, 35). In this study, we showed by RT-PCR that AnxA1 is expressed in conjunctival goblet cells and thus is available to activate these cells. This indicates that AnxA1 not only works during ocular inflammation, but also under physiological conditions, by secreting a basal level of mucins to the tear film and thus maintaining ocular homeostasis. We have demonstrated this for other SPMs including LXA4, RvD1, RvD2, maresin 1, and RvE1 (10–12). Unlike the lipid SPMs AnxA1 is a protein, thus we were able to verify its expression by RT-PCR. In contrast, complex lipidomic measurements are needed to detect the presence of the lipid SPMs. As the lipid LXA4 is produced by the cornea, it is possible that LXA4 could diffuse via tears to goblet cells in order to stimulate glycoconjugate secretion. In contrast, AnxA1 is found in conjunctival goblet cells and could be secreted to act upon goblet cells or stratified squamous cells. As AnxA1 is both present and has a role in goblet cell function, AnxA1 likely plays a role in ocular health under physiological conditions as well as preventing or treating the multitude of ocular surface inflammatory diseases. Future study of the regulation of AnxA1 synthesis and secretion in conjunctival goblet cells is warranted.
The ALX/FPR2 receptor is a G-protein-coupled receptor and is the receptor to which AnxA1 binds (36). In addition to its presence on goblet cells (12) the ALX/FPR2 receptor has been identified in human neutrophils, epithelial cells, endothelial cells and monocytes (37, 38). Using ALX/FPR2, AnxA1 functions to resolve diverse inflammatory events, including reduction of joint injury in experimental arthritis (39), lessening of salivary gland inflammation (40), and contributes to corneal wound healing (41). AnxA1 in uterine epithelial cells also activates the ALX/FPR2 receptor to upregulate the membrane-spanning mucin MUC1, tight junction molecules claudin-1 and occludens 1 to increase adherence of trophoblast steroids, a model for embryo implantation (42). Herein we showed that AnxA1 activates the ALX/FPR2 receptor in rat conjunctival goblet cells and that this activation leads to the increase in [Ca2+]i and secretion of mucins into the tear film. We previously studied the SPMs LXA4 and RvD1, which also activate the ALX/FPR2 receptor in rats. Cooray et al. have shown that the ALX/FPR2 receptors can homodimerize or heterodimerize with FPR1 or FPR3 depending on which agonist is bound (43). In addition, ALX/FPR2 has multiple binding sites notably different lipid and protein sites and binds to multiple compounds (44). AnxA1, a protein, and RvD1 and LXA4, which are lipids, bind to different receptor regions in addition to inducing dissimilar receptor conformations (45). This differential binding and a potential difference in FPR receptor family dimerization could explain the complex desensitization we obtained with addition of AnxA1, LXA4, and RvD1 (46). These dissimilarities could make the three compounds differ in their effectiveness to treat ocular allergy and other forms of ocular inflammation.
This differential binding of AnxA1, LXA4, and RvD1 to bind to the same receptor in conjunctival goblet cells could also lead to ligand-specific activation of signaling pathways. In fact, the AnxA1, LXA4, and RvD1 activation of downstream signaling cascades are not fully similar. While AnxA1, LXA4, and RvD1 each activate the PLC pathway, only LXA4 and RvD1 activate PLD, PLA2 and ERK 1/2 (10, 12) to stimulate an increase both in [Ca2+]i and mucin secretion. This pattern of pathway activation is consistent with AnxA1 activating the peptide site on ALX/FPR2, whereas LXA4 and RvD1 activate the lipid site or overlapping sites.
In the present study, we found AnxA1 to have a peak increase in [Ca2+]i at a 10-9 M concentration, while the maximum response for secretion was at 10-10 M. Furthermore, AnxA1 at 10-8 M did not even increase secretion. These findings could be due to several factors. The increase in [Ca2+]i occurs very rapidly, within seconds, and is measured in the time frame of seconds. In contrast, secretion, although it can occur rapidly, is measured after 2 hours. The rapid increase in [Ca2+]i may not directly link to secretion. The peak [Ca2+]i measured is primarily a function of Ca2+ release from intracellular stores whereas stimulation of secretion also is regulated by the subsequent influx of Ca2+ as well as the size of the intracellular Ca2+ stores. The maximum concentration of AnxA1 used to increase the [Ca2+]i compared to that used for secretion may result from the differential dependence on the use of distinct mechanisms of Ca2+ handling in the goblet cells.
In further support of the independence of AnxA1 concentration on peak [Ca2+] and secretion is that secretion, but not peak [Ca2+] is stimulated by AnxA1 activation of PLA2, PLD, and ERK1/2. Thus the increase in peak [Ca2+] and secretion are differentially regulated by AnxA1. This is in contrast to the increase in peak [Ca2+] and secretion that when stimulated by LXA4 or RvD1 are both dependent on activation of the PLC, PLD, and PLA2 pathways. This finding is also consistent with binding of the protein and the lipids to different sites on ALX/FPR2.
In the present study we found that AnxA1 was able to significantly increase [Ca2+]i at a notably low concentration (10-12 M) in conjunctival goblet cells. In contrast, LXA4 increased [Ca2+]i at 10-8 M (10). Unfortunately the effect of RvD1 concentration on [Ca2+]i was not performed at concentrations below 10-10 M (unpublished data, DA Dartt 2020). The differential effect of AnxA1 and LXA4 concentration on [Ca2+]i is consistent with their binding to different areas on the ALX/FPR2 receptor.
Furthermore, we found that AnxA1 was not dependent on either the ERK 1/2, PLA2, or the PLD pathway to increase [Ca2+]i although AnxA1 does utilize these pathways to stimulate secretion. In conjunctival goblet cells AnxA1 did not activate ERK 1/2 pathway to increase [Ca2+]i, but rather more likely AnxA1 increased [Ca2+]i to activate ERK1/2. ERK1/2 activity can be Ca2+-dependent via activation of PLC by a G-protein-coupled receptor or can be independent of Ca2+ when activated by β-arrestin-dependent down regulation of G-protein-coupled receptors (47). Published literature showed that in multiple tissues the binding of AnxA1 to the ALX/FPR2 receptor leads to the transient phosphorylation of ERK 1/2 (7, 13, 48, 49), which is associated with a prompt increase in [Ca2+]i (7, 38, 50). This suggests that in goblet cells, the activation of ERK 1/2 occurs downstream of the rise of [Ca2+]i after activation of PLC. Similarly for AnxA1 activation of PLD, the rise in Ca2+ occurs before activation of PLD. An increase in Ca2+ and activation of PKC as could occur by PLC stimulation could activate PLD in the goblet cells as documented in a mast cell line (51). The signaling pathways activated by AnxA1 are complex, cell specific, and species dependent.
The function of AnxA1 has been studied in a variety of disease models in order to explore this agonist’s potential as a novel anti-inflammatory therapeutic agent, including models of inflammatory bowel disease (52), rheumatoid arthritis (53), chronic atherogenesis (54), myocardial reperfusion injury (55), myocardial infarction (56), and now ocular inflammation. AnxA1 is thought to have potential as a new treatment option for inflammation by being an effector molecule of glucocorticoids (57). Synthetic glucocorticoids play an extensive and crucial role in the treatment of unwanted and uncontrolled inflammation but come with potentially harmful metabolic side effects with long-term use (57). It is believed that therapeutic application of endogenous anti-inflammatory mediators like AnxA1 will be effective and cause fewer side effects by inducing natural pathways of resolution of inflammation (58–60). AnxA1 could be used therapeutically to treat the multiple inflammatory diseases of the ocular surface especially dry eye and ocular allergy as well as to prevent these diseases or to maintain ocular surface homeostasis in health.
In conclusion, our findings demonstrate that the glucocorticoid effector protein AnxA1 has potential in prevention or as a new treatment for dry eye, allergic conjunctivitis and other forms of ocular surface inflammation through its effect on conjunctival goblet cells. AnxA1 works through activation of the ALX/FPR2 receptor to stimulate multiple signaling pathways, ultimately leading to the secretion of high molecular weight glycoconjugates including mucins into the ocular tear film. These mucins are a crucial component of a healthy tear film, and thus the regulation of mucin secretion from conjunctival goblet cells by AnxA1 is an important contributor to ocular surface health, and prevention of ocular surface disease and treatment of these diseases.
Author’s Note
This manuscript is dedicated to Robin R. Hodges who passed away on March 13, 2021. For over 30 years she managed the Dartt laboratory ensuring its excellence. She was respected and beloved by all.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The animal study was reviewed and approved by Schepens Eye Research Institute Animal Care and Use of Committee.
Author Contributions
AL, MO, JB, and RH performed experiments. AL, RH, TU, CS, and DD wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by Norwegian Research Council to AL and by NIH R01 EY019470 to DD and RO1GM038765 to CS.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2021.618653/full#supplementary-material
Supplementary Figure 1 | Chelation of blocks AnxA1-stimulated increase in [Ca2+]i in cultured rat conjunctival goblet cells. Cultured rat goblet cells were preincubated with the Ca2+ chelator BAPTA/AM (10-4 M) prior to stimulation with either AnxA1 (10-9 M) or carbachol (Cch, 10-4 M) and change in peak [Ca2+]i shown. Data are mean ± SEM from 4 independent experiments. * indicates significant difference from basal; # indicates significant difference from either AnxA1 or Cch alone.
Supplementary Figure 2 | AnxA1 is not dependent on protein kinase A (PKA) to increase [Ca2+]i in cultured rat conjunctival goblet cells. Goblet cells were preincubated with the PKA inhibitor H89 for 30 minutes before stimulation with vehicle, annexin A1 (AnxA1, 10-9 M) in (A) or with AnxA1 (10-10 M) in (B), or vasoactive intestinal peptide (VIP, 10-8 M). Change in peak [Ca2+]i is shown in (A). Glycoconjugate secretion is shown in (B). B indicates basal (vehicle) in (B). Data are mean ± SEM from 9 (A) or 3 (B) independent experiments. * indicates significant difference from zero; # indicates significance from AnxA1 or VIP alone.
References
1. Li D, Hodges RR, Jiao J, Carozza RB, Shatos MA, Chiang N, et al. Resolvin D1 and aspirin-triggered resolvin D1 regulate histamine-stimulated conjunctival goblet cell secretion. Mucosal Immunol (2013) 6(6):1119–30. doi: 10.1038/mi.2013.7
2. Fredman G, Serhan CN. Specialized proresolving mediator targets for RvE1 and RvD1 in peripheral blood and mechanisms of resolution. Biochem J (2011) 437(2):185–97. doi: 10.1042/BJ20110327
3. Dartt DA, Hodges RR, Li D, Shatos MA, Lashkari K, Serhan CN. Conjunctival goblet cell secretion stimulated by leukotrienes is reduced by resolvins D1 and E1 to promote resolution of inflammation. J Immunol (2011) 186(7):4455–66. doi: 10.4049/jimmunol.1000833
4. Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol (2008) 8(5):349–61. doi: 10.1038/nri2294
5. Serhan CN, Arita M, Hong S, Gotlinger K. Resolvins, docosatrienes, and neuroprotectins, novel omega-3-derived mediators, and their endogenous aspirin-triggered epimers. Lipids (2004) 39(11):1125–32. doi: 10.1007/s11745-004-1339-7
6. Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol (2005) 6(12):1191–7. doi: 10.1038/ni1276
7. Perretti M, D’Acquisto F. Annexin A1 and glucocorticoids as effectors of the resolution of inflammation. Nat Rev Immunol (2009) 9(1):62–70. doi: 10.1038/nri2470
8. Dartt DA, Masli S. Conjunctival epithelial and goblet cell function in chronic inflammation and ocular allergic inflammation. Curr Opin Allergy Clin Immunol (2014) 14(5):464–70. doi: 10.1097/ACI.0000000000000098
9. Dartt DA. Control of mucin production by ocular surface epithelial cells. Exp eye Res (2004) 78(2):173–85. doi: 10.1016/j.exer.2003.10.005
10. Lippestad M, Hodges RR, Utheim TP, Serhan CN, Dartt DA. Resolvin D1 Increases Mucin Secretion in Cultured Rat Conjunctival Goblet Cells via Multiple Signaling Pathways. Investigative Ophthalmol Vis Sci (2017) 58: (11):4530–44. doi: 10.1167/iovs.17-21914
11. Lippestad M, Hodges RR, Utheim TP, Serhan CN, Dartt DA. Signaling pathways activated by resolvin E1 to stimulate mucin secretion and increase intracellular Ca(2+) in cultured rat conjunctival goblet cells. Exp Eye Res (2018) 173:64–72. doi: 10.1016/j.exer.2018.04.015
12. Hodges RR, Li D, Shatos MA, Bair JA, Lippestad M, Serhan CN, et al. Lipoxin A4 activates ALX/FPR2 receptor to regulate conjunctival goblet cell secretion. Mucosal Immunol (2017) 10(1):46–57. doi: 10.1038/mi.2016.33
13. Hayhoe RP, Kamal AM, Solito E, Flower RJ, Cooper D, Perretti M. Annexin 1 and its bioactive peptide inhibit neutrophil-endothelium interactions under flow: indication of distinct receptor involvement. Blood (2006) 107(5):2123–30. doi: 10.1182/blood-2005-08-3099
14. Gavins FNE, Hickey MJ. Annexin A1 and the regulation of innate and adaptive immunity. Front Immunol (2012) 3:1–11. doi: 10.3389/fimmu.2012.00354
15. Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocrine Rev (2000) 21(1):55–89. doi: 10.1210/er.21.1.55
16. Yazid S, Norling LV, Flower RJ. Anti-inflammatory drugs, eicosanoids and the annexin A1/FPR2 anti-inflammatory system. Prostaglandins Other Lipid Mediat (2012) 98(3-4):94–100. doi: 10.1016/j.prostaglandins.2011.11.005
17. Purvis GSD, Solito E, Thiemermann C. Annexin-A1: Therapeutic Potential in Microvascular Disease. Front Immunol (2019) 10:938. doi: 10.3389/fimmu.2019.00938
18. He M, Lippestad M, Li D, Hodges RR, Utheim TP, Dartt DA. Activation of the EGF Receptor by Histamine Receptor Subtypes Stimulates Mucin Secretion in Conjunctival Goblet Cells. Invest Ophthalmol Visual Sci (2018) 59(8):3543–53. doi: 10.1167/iovs.18-2476
19. Li D, Jiao J, Shatos MA, Hodges RR, Dartt DA. Effect of VIP on intracellular [Ca2+], extracellular regulated kinase 1/2, and secretion in cultured rat conjunctival goblet cells. Invest Ophthalmol Vis Sci (2013) 54(4):2872–84. doi: 10.1167/iovs.12-11264
20. Dartt DA, Rios JD, Kanno H, Rawe IM, Zieske JD, Ralda N, et al. Regulation of conjunctival goblet cell secretion by Ca(2+)and protein kinase C. Exp Eye Res (2000) 71(6):619–28. doi: 10.1006/exer.2000.0915
21. Hodges RR, Dartt DA. Tear Film Mucins: Front Line Defenders of the Ocular Surface; Comparison with Airway and Gastrointestinal Tract Mucins. Exp eye Res (2013) 117:62–78. doi: 10.1016/j.exer.2013.07.027
22. Li D, Carozza RB, Shatos MA, Hodges RR, Dartt DA. Effect of histamine on Ca(2+)-dependent signaling pathways in rat conjunctival goblet cells. Invest Ophthalmol Visual Sci (2012) 53(11):6928–38. doi: 10.1167/iovs.12-10163
23. Contreras-Ruiz L, Ghosh-Mitra A, Shatos MA, Dartt DA, Masli S. Modulation of Conjunctival Goblet Cell Function by Inflammatory Cytokines. Mediators Inflamm (2013) 2013:1–11. doi: 10.1155/2013/636812
24. Hayashi D, Li D, Hayashi C, Shatos M, Hodges RR, Dartt DA. Role of histamine and its receptor subtypes in stimulation of conjunctival goblet cell secretion. Invest Ophthalmol Visual Sci (2012) 53(6):2993–3003. doi: 10.1167/iovs.11-8748
25. Luo D, Broad LM, Bird GS, Putney JW Jr. Signaling pathways underlying muscarinic receptor-induced [Ca2+]i oscillations in HEK293 cells. J Biol Chem (2001) 276(8):5613–21. doi: 10.1074/jbc.M007524200
26. Walther A, Riehemann K, Gerke V. A novel ligand of the formyl peptide receptor: annexin I regulates neutrophil extravasation by interacting with the FPR. Mol Cell (2000) 5(5):831–40. doi: 10.1016/S1097-2765(00)80323-8
27. Putney JW, Tomita T. Phospholipase C Signaling and Calcium Influx. Adv Biol Regul (2012) 52(1):152–64. doi: 10.1016/j.advenzreg.2011.09.005
28. Olsen MV, Lyngstadaas AV, Bair JA, Hodges RR, Utheim TP, Serhan CN, et al. Maresin 1, a specialized proresolving mediator, stimulates intracellular [Ca(2+)] and secretion in conjunctival goblet cells. J Cell Physiol (2021) 236(1):340–53. doi: 10.1002/jcp.29846
29. Mikoshiba K. Role of IP3 receptor signaling in cell functions and diseases. Adv Biol Regul (2015) 57:217–27. doi: 10.1016/j.jbior.2014.10.001
30. Marcelo KL, Means AR, York B. The Ca(2+)/Calmodulin/CaMKK2 Axis: Nature’s Metabolic CaMshaft. Trends Endocrinol metabol: TEM (2016) 27(10):706–18. doi: 10.1016/j.tem.2016.06.001
31. Kanno H, Horikawa Y, Hodges RR, Zoukhri D, Shatos MA, Rios JD, et al. Cholinergic agonists transactivate EGFR and stimulate MAPK to induce goblet cell secretion. Am J Physiol Cell Physiol (2003) 284(4):C988–98. doi: 10.1152/ajpcell.00582.2001
32. Dartt DA, Kanno H, Rios J, Zoukhri D. Role of mitogen-activated protein kinase in cholinergic stimulation of conjunctival goblet cell secretion. Adv Exp Med Biol (2002) 506(Pt A):297–300. doi: 10.1007/978-1-4615-0717-8_41
33. Foster DA, Xu L. Phospholipase D in cell proliferation and cancer. Mol Cancer Research: MCR (2003) 1(11):789–800.
34. Bird GS, Putney JW. “Pharmacology of Store-Operated Calcium Entry Channels”. In: Kozak JA, Putney JW, editors. Calcium Entry Channels in Non-Excitable Cells. Boca Raton: CRC Press (2018). p. 311–24.
35. Trentin PG, Ferreira TPT, Arantes ACS, Ciambarella BT, Cordeiro RSB, Flower RJ, et al. Annexin A1 mimetic peptide controls the inflammatory and fibrotic effects of silica particles in mice. Br J Pharmacol (2015) 172(12):3058–71. doi: 10.1111/bph.13109
36. Spurr L, Nadkarni S, Pederzoli-Ribeil M, Goulding NJ, Perretti M, D’Acquisto F. Comparative analysis of Annexin A1-formyl peptide receptor 2/ALX expression in human leukocyte subsets. Int Immunopharmacol (2011) 11(1):55–66. doi: 10.1016/j.intimp.2010.10.006
37. Chiang N, Serhan CN, Dahlen SE, Drazen JM, Hay DW, Rovati GE, et al. The lipoxin receptor ALX: potent ligand-specific and stereoselective actions in vivo. Pharmacol Rev (2006) 58(3):463–87. doi: 10.1124/pr.58.3.4
38. Babbin BA, Lee WY, Parkos CA, Winfree LM, Akyildiz A, Perretti M, et al. Annexin I regulates SKCO-15 cell invasion by signaling through formyl peptide receptors. J Biol Chem (2006) 281(28):19588–99. doi: 10.1074/jbc.M513025200
39. Odobasic D, Jia Y, Kao W, Fan H, Wei X, Gu R, et al. Formyl peptide receptor activation inhibits the expansion of effector T cells and synovial fibroblasts and attenuates joint injury in models of rheumatoid arthritis. Int Immunopharmacol (2018) 61:140–9. doi: 10.1016/j.intimp.2018.05.028
40. Wang CS, Baker OJ. The G-Protein-Coupled Receptor ALX/Fpr2 Regulates Adaptive Immune Responses in Mouse Submandibular Glands. Am J Pathol (2018) 188(7):1555–62. doi: 10.1016/j.ajpath.2018.04.003
41. Leedom AJ, Sullivan AB, Dong B, Lau D, Gronert K. Endogenous LXA4 circuits are determinants of pathological angiogenesis in response to chronic injury. Am J Pathol (2010) 176(1):74–84. doi: 10.2353/ajpath.2010.090678
42. Hebeda CB, Sandri S, Benis CM, Md P-S, RA L, Reutelingsperger C, et al. Annexin A1/Formyl Peptide Receptor Pathway Controls Uterine Receptivity to the Blastocyst. Cells (2020) 9(5):1188. doi: 10.3390/cells9051188
43. Cooray SN, Gobbetti T, Montero-Melendez T, McArthur S, Thompson D, Clark AJ, et al. Ligand-specific conformational change of the G-protein-coupled receptor ALX/FPR2 determines proresolving functional responses. Proc Natl Acad Sci U S A (2013) 110(45):18232–7. doi: 10.1073/pnas.1308253110
44. Back M, Powell WS, Dahlen SE, Drazen JM, Evans JF, Serhan CN, et al. Update on leukotriene, lipoxin and oxoeicosanoid receptors: IUPHAR Review 7. Br J Pharmacol (2014) 171(15):3551–74. doi: 10.1111/bph.12665
45. Le Y, Ye RD, Gong W, Li J, Iribarren P, Wang JM. Identification of functional domains in the formyl peptide receptor-like 1 for agonist-induced cell chemotaxis. FEBS J (2005) 272(3):769–78. doi: 10.1111/j.1742-4658.2004.04514.x
46. Maderna P, Cottell DC, Toivonen T, Dufton N, Dalli J, Perretti M, et al. FPR2/ALX receptor expression and internalization are critical for lipoxin A4 and annexin-derived peptide-stimulated phagocytosis. FASEB J (2010) 24(11):4240–9. doi: 10.1096/fj.10-159913
47. Lin A, DeFea KA. beta-Arrestin-kinase scaffolds: turn them on or turn them off? Wiley Interdiscip Rev Syst Biol Med (2013) 5(2):231–41. doi: 10.1002/wsbm.1203
48. He R, Sang H, Ye RD. Serum amyloid A induces IL-8 secretion through a G protein-coupled receptor, FPRL1/LXA4R. Blood (2003) 101(4):1572–81. doi: 10.1182/blood-2002-05-1431
49. Cattaneo F, Parisi M, Ammendola R. Distinct Signaling Cascades Elicited by Different Formyl Peptide Receptor 2 (FPR2) Agonists. Int J Mol Sci (2013) 14(4):7193–230. doi: 10.3390/ijms14047193
50. Solito E, Kamal A, Russo-Marie F, Buckingham JC, Marullo S, Perretti M. A novel calcium-dependent proapoptotic effect of annexin 1 on human neutrophils. FASEB J (2003) 17(11):1544–6. doi: 10.1096/fj.02-0941fje
51. Chahdi A, Choi WS, Kim YM, Fraundorfer PF, Beaven MA. Serine/threonine protein kinases synergistically regulate phospholipase D1 and 2 and secretion in RBL-2H3 mast cells. Mol Immunol (2002) 38(16-18):1269–76. doi: 10.1016/S0161-5890(02)00074-3
52. Leoni G, Neumann PA, Kamaly N, Quiros M, Nishio H, Jones HR, et al. Annexin A1-containing extracellular vesicles and polymeric nanoparticles promote epithelial wound repair. J Clin Invest (2015) 125(3):1215–27. doi: 10.1172/JCI76693
53. Yang YH, Morand E, Leech M. Annexin A1: potential for glucocorticoid sparing in RA. Nat Rev Rheumatol (2013) 9(10):595–603. doi: 10.1038/nrrheum.2013.126
54. Kusters DH, Chatrou ML, Willems BA, De Saint-Hubert M, Bauwens M, van der Vorst E, et al. Pharmacological Treatment with Annexin A1 Reduces Atherosclerotic Plaque Burden in LDLR-/- Mice on Western Type Diet. PLoS One (2015) 10(6):e0130484. doi: 10.1371/journal.pone.0130484
55. Qin C, Buxton KD, Pepe S, Cao AH, Venardos K, Love JE, et al. Reperfusion-induced myocardial dysfunction is prevented by endogenous annexin-A1 and its N-terminal-derived peptide Ac-ANX-A1(2-26). Br J Pharmacol (2013) 168(1):238–52. doi: 10.1111/j.1476-5381.2012.02176.x
56. Qin C, Yang YH, May L, Gao X, Stewart AG, Tu Y, et al. Cardioprotective potential of annexin-A1 mimetics in myocardial infarction. Pharmacol Ther (2015) 148:47–65. doi: 10.1016/j.pharmthera.2014.11.012
57. Perretti M, Gavins FN. Annexin 1: an endogenous anti-inflammatory protein. News Physiol Sci: an Int J Physiol Produced Jointly by Int Union Physiol Sci Am Physiol Soc (2003) 18:60–4. doi: 10.1152/nips.01424.2002
58. Perretti M, Dalli J. Exploiting the Annexin A1 pathway for the development of novel anti-inflammatory therapeutics. Br J Pharmacol (2009) 158(4):936–46. doi: 10.1111/j.1476-5381.2009.00483.x
59. Sugimoto MA, Vago JP, Teixeira MM, Sousa LP. Annexin A1 and the Resolution of Inflammation: Modulation of Neutrophil Recruitment, Apoptosis, and Clearance. J Immunol Res (2016) 2016:1–13. doi: 10.1155/2016/8239258
Keywords: ocular surface, annexin A1, secretion, specialized pro resolving mediators, mucin
Citation: Lyngstadaas AV, Olsen MV, Bair JA, Hodges RR, Utheim TP, Serhan CN and Dartt DA (2021) Pro-Resolving Mediator Annexin A1 Regulates Intracellular Ca2+ and Mucin Secretion in Cultured Goblet Cells Suggesting a New Use in Inflammatory Conjunctival Diseases. Front. Immunol. 12:618653. doi: 10.3389/fimmu.2021.618653
Received: 17 October 2020; Accepted: 11 March 2021;
Published: 22 April 2021.
Edited by:
Mats Bemark, University of Gothenburg, SwedenReviewed by:
Harry D. Dawson, Agricultural Research Service (USDA), United StatesKathryn A. Knoop, Mayo Clinic, United States
Copyright © 2021 Lyngstadaas, Olsen, Bair, Hodges, Utheim, Serhan and Dartt. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Darlene A. Dartt, RGFybGVuZV9kYXJ0dEBtZWVpLmhhcnZhcmQuZWR1
†Deceased
 Anne V. Lyngstadaas
Anne V. Lyngstadaas Markus V. Olsen1,2,3
Markus V. Olsen1,2,3 Robin R. Hodges
Robin R. Hodges Charles N. Serhan
Charles N. Serhan Darlene A. Dartt
Darlene A. Dartt