
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CLINICAL TRIAL article
Front. Immunol. , 24 March 2021
Sec. Microbial Immunology
Volume 12 - 2021 | https://doi.org/10.3389/fimmu.2021.618150
This article is part of the Research Topic The Role of Dietary Interventions in The Regulation of Host-Microbe Interactions View all 24 articles
 Chaofei Xia1†
Chaofei Xia1† Chunling Jiang2,3†
Chunling Jiang2,3† Wenyu Li1
Wenyu Li1 Jing Wei1
Jing Wei1 Hu Hong1
Hu Hong1 Jingao Li2,3
Jingao Li2,3 Liu Feng2,3
Liu Feng2,3 Hong Wei4*
Hong Wei4* Hongbo Xin1*
Hongbo Xin1* Tingtao Chen1*
Tingtao Chen1*Earlier evidence has proven that probiotic supplements can reduce concurrent chemoradiotherapy (CCRT)-induced oral mucositis (OM) in nasopharyngeal cancer (NPC). The incidence of severe OM (grade 3 or higher) was the primary endpoint in this study. We first enrolled 85 patients with locally advanced NPC who were undergoing CCRT. Of them, 77 patients were finally selected and randomized (1:1) to receive either a probiotic cocktail or placebo. To investigate the protective effects and the mechanism of probiotic cocktail treatment on OM induced by radiotherapy and chemotherapy, we randomly divided the rats into the control (C) group, the model (M) group, and the probiotic (P) group. After treatment, samples from the tongue, blood, and fecal and proximal colon tissues on various days (7th, 14th, and 21st days) were collected and tested for the inflammatory response, cell apoptosis, intestinal permeability, and intestinal microbial changes. We found that patients taking the probiotic cocktail showed significantly lower OM. The values of the incidence of 0, 1, 2, 3, and 4 grades of OM in the placebo group and in the probiotic cocktail group were reported to be 0, 14.7, 38.2, 32.4, and 14.7% and 13.9, 36.1, 25, 22.2, and 2.8%, respectively. Furthermore, patients in the probiotic cocktail group showed a decrease in the reduction rate of CD3+ T cells (75.5% vs. 81%, p < 0.01), CD4+ T cells (64.53% vs. 79.53%, p < 0.01), and CD8+ T cells (75.59 vs. 62.36%, p < 0.01) compared to the placebo group. In the rat model, the probiotic cocktail could ameliorate the severity of OM, decrease the inflammatory response, cause cell apoptosis and intestinal permeability, and restore the structure of gut microbiota to normalcy. In conclusion, the modified probiotic cocktail significantly reduces the severity of OM by enhancing the immune response of patients with NPC and modifying the structure of gut microbiota.
Clinical Trial Registration: The Clinical Trial Registration should be the NCT03112837.
Nasopharyngeal carcinoma (NPC) is a prevalent malignant neoplasm in southern China, and concurrent chemoradiotherapy (CCRT) is the standard treatment for locally advanced NPC worldwide (1). Toxic side effects caused by CCRT occur during and after the treatment, and oral mucositis (OM) is probably the most common complication in patients with head-and-neck cancer (accounting for ~80%) (2). OM not only interferes with the quality of the life of the patient but also gives rise to a 19% interruption rate in radiotherapy or CCRT (3). Though topical agents, such as palifermin, chlorhexidine, actovegin, kangfuxin, royal jelly, zinc supplement, benzydamine, cryotherapy, laser therapy, and professional oral hygiene, are used for CCRT-induced OM, there is still no accepted standard therapy for the prevention and treatment of OM (4). Therefore, a feasible and effective method to prevent OM during cancer treatment is urgently required.
The intestinal microbiota has become an important regulator of host immunity and may affect the outcome of cancer immunotherapy (5–7). Cancer treatments, such as radiotherapy and chemotherapy, might cause a decrease in the immunity of patients with cancer, eventually aggravating immunotherapy-induced mucosal toxicity (8). Evidence from studies on humans and experimental animals suggested that intestinal microbiota, such as probiotics, could modulate the anti-cancer immune response and attenuate cancer treatment-related toxic side effects (9–11). Oral supplementation of Bifidobacterium, alone or with anti-programed cell death of protein 1 ligand 1 (PDL1), in mice had promoted CD8+T cell-induced anti-tumor immunity (12). In line with this study, Vetizou et al. (10) also found that Bacteroidales played an important role in the immunostimulatory effects of cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) blockade by promoting the maturation of intratumoral dendritic cells and with a TH1 response detected in the lymph nodes of the draining tumor.
In a previous study, the probiotic drugs, such as Bifidobacterium longum, Lactobacillus lactis and Enterococcus faecium, exerted a therapeutic effect and could reduce the severity of OM in patients with NPC, who underwent CCRT, and greatly increased the number of immune cells (13). However, all of the probiotic drugs in China were approved 10–20 years ago, and some problems, such as the misidentified bacteria on the label or the use of potential pathogens, such as E. faecium and Bacillus cereus, in drugs, hindered their further development. For example, E. faecium has been considered as a probiotic and is used in more than 75% of probiotic drugs, while the latter strain is considered as an opportunistic pathogen other than as a probiotic due to its multi-drug resistance and virulent factors (14).
Therefore, in the present study, we first isolated Lactobacillus plantarum from the feces of a healthy crowd living in the cancer-free village through high-throughput sequencing analysis. Then, the above L. plantarum and Bifidobacterium animalis, which were isolated from Bama Changshou Village 30 years ago, were mixed with Lactobacillus rhamnosus and Lactobacillus acidophilusto form a probiotic cocktail. Next, the effectiveness of the probiotic cocktail on OM in patients with NPC was investigated via the randomized, double-blind, placebo-controlled trial. Finally, the possible protective mechanism of the probiotic cocktail on OM was further investigated through the OM rat model.
Fecal samples from healthy people (HP) (n = 5, healthy employees from the Jiangxi Cancer Hospital), tumor patients (TP) (n = 5, tumor patients from the Jiangxi Cancer Hospital), and non-cancer people (NT) (n = 5, healthy residents from the cancer-free village, Wuyuan, Jiangxi, Nanchang, PR, China) were collected in June 2016, and high-throughput sequencing analysis was used to compare the microbial diversity among these samples. A viable counting method was used to isolate bacteria with the de Man–Rogosa–Sharpe (MRS) medium by mainly screening for Lactobacillus spp. from the feces of cancer-free village residents, and the isolates were identified using a gene sequencing technology (15).
The L. plantarum MH-301 was isolated from cancer-free village residents, and B. animalis subsp. Lactis LPL-RH (Harbin Meihua Biotechnology Co., Ltd., Harbin, Heilongjiang, PR China and Isolated from Changshou Village, Bama Town in Guangxi Province, China), L. rhamnosus LGG-18 (Harbin Meihua Biotechnology Co., Ltd., Harbin, Heilongjiang, PR China), and L. acidophilus (Harbin Meihua Biotechnology Co., Ltd., Harbin, Heilongjiang, PR China) were selected to make a probiotic cocktail, and the acid tolerance test (16), the anti-oxidative test (17), the antimicrobial test (18), the adherence assay test (19), and the adherence assay test (19) were conducted to evaluate the probiotic characteristics of the selected strains.
Male and female patients (18–70 years old) diagnosed with locally advanced NPC were enrolled for the randomized, double-blind, placebo-controlled trial at the Jiangxi Cancer Hospital in China. The clinical stage of the patient was determined according to the 8th edition of the International Union Against Cancer/American Joint Committee on Cancer TNM staging system, and patients diagnosed with NPC without distant metastasis and who had a Karnofsky score were enrolled. Patients with a previous history of cancer or co-existing tumors, who were unable to take oral medicine and/or absorb drugs in the digestive tract, who had a high risk for antimicrobial agents, who had OM or recurrent OM before CCRT, and who had serious and/or uncontrollable infections, or other diseases were excluded.
We first enrolled 85 patients with locally advanced NPC who were undergoing CCRT. About 77 patients were finally selected and randomized (1:1) to receive either a probiotic mixture or a placebo. The process of random allocation is as follows: the random allocation sequence was performed at a ratio of 1:1 using nQueryAdvisor®v7.0 software, which uses a pseudo-random number generator. The randomization sequence was produced before the first enrollment. The executor performed inclusion following the inclusion and exclusion criteria. Then, patients with NPC were assigned to the probiotic cocktail group or the control group by the clinical research technician who was also blinded. According to the double-blind method, those who participated in this study did not know the type of treatment that each patient with NPC received.
According to the guidelines of the National Comprehensive Cancer Network (NCCN), all patients underwent cisplatin chemotherapy [32 fractions of 70 Gy radiotherapy (2.19 Gy/d, 5 d/wk) with a total tumor volume and a clinical target volume of 60 Gy] and intensity-modulated radiation therapy (IMRT) [32 fractions for 45 days (6–7 weeks in total) and intravenously infused with cisplatin (100 mg/m2) on days 1, 22, and 43].
Oral probiotic cocktail (containing L. plantarum MH-301109 CFU, B. animalis subsp. Lactis LPL-RH109 CFU, L. rhamnosus LGG-18109 CFU, and L. acidophilus 109 CFU), or placebo were supplied to patients for 7 weeks (one capsule, 2 times a day) from the first day of chemoradiotherapy to the end. The severity, occurrences, and symptoms of OM were evaluated by at least two advanced radiation oncologists [the common terminology of National Cancer Institute for adverse events (version 4.0)]. The short-term efficacy response rates (the Response Evaluation Criteria in Solid Tumors based on MRI) were evaluated for patients with complete and partial radiotherapy responses after the completion of CCRT (20). Patient weights were recorded weekly; their biochemical parameter analysis, determination of lymphocyte immunity, and routine blood analysis were measured.
This study was approved by the local clinical research Ethics Committee and was conducted by following the Declaration of Helsinki (Clinical Trials number, NCT03112837). All patients gave their informed consent before the trial.
Microbial DNA was obtained from the fecal samples of the HP group (from the employees of Jiangxi Cancer Hospital, n = 10), before the treatment of radiotherapy plus chemotherapy plus a placebo (BRCP) group (n = 10), before the treatment of radiotherapy plus chemotherapy plus the probiotic combination (BRCPM) group (n = 10), after treatment with radiotherapy plus chemotherapy plus a placebo (ARCP) group (n = 10), and after treatment with radiotherapy plus chemotherapy plus the probiotic combination ARCPM group (n = 10). Samples were kept at −80°C until DNA extraction. Bacterial genomic DNA was extracted from fecal samples using the DNA magnetics and extract kit (Tiangen Biotech, Beijing, China), according to the instruction of the manufacturer. Total genomic DNA was amplified with a forward primer, F341 5′-ACT CCT ACG GGR SGC AGC AG-3′, and a reverse primer, R806 5′- GGA CTA CVV GGG TAT CTA ATC-3′ that amplified the regions from V3 to V4 of the 16S ribosomal DNA gene for high-throughput sequencing analysis (PRJNA579226) (21).
Male Sprague-Dawley rats, aging 8–10 weeks, were purchased from Hunan Si Lake King of Experimental Animal Co., Ltd. (Changsha, Hunan, China). The rats were habituated to the animal facility for 2 weeks before beginning the experiment and kept under a 12 h light/dark cycle, with a temperature of 21 ± 1°C and humidity of 55 ± 10%. Food and water were given ad libitum. Animal care and procedures were followed under the guidelines of the National Institutes of Health and the Care and Use of Laboratory Animals. All experiments were approved by the Ethical Committee of the Nanchang University.
For the mucositis model, both radiation and chemotherapy were used for the induction of OM with busulfan (Sigma-Aldrich, MO, USA) at a dose of 6 mg/kg for 4 days of chemotherapy (22). The rats were anesthetized with an intraperitoneal injection of ketamine (Rotex, Trittau, Germany) before irradiation. Then, the rats were irradiated one at a time in the head region with 20 Gy, using Clinac 600C, a 4-MV therapeutic linear accelerator (Varian Medical Systems Inc., Palo Alto, CA, USA) at a dose rate of 2 Gy/min, to expose the oral mucosa to radiation, where a 1.5 cm bolus was used for the radiation dose buildup (23). The rats were divided into three groups: the control (C) group (n = 13) received an identical volume of gelatine physiological saline (i.g.) for 21 days without radiotherapy and chemotherapy; the model (M) group (n = 13) was treated with an identical volume of i.g. for 21 days with radiotherapy and chemotherapy; and the probiotic (T) group (n = 13) was pretreated with a probiotic combination containing L. plantarum MH-301109 CFU, B. animalis subsp. Lactis LPL-RH109 CFU, L. rhamnosusLGG-18109 CFU, and L. acidophilus 109 CFU, for 7 days (1 ml, 1 time a day) before radiotherapy and chemotherapy. All animals were monitored daily to examine the status of the oral cavity, the amount of oral intake, weight, and survival.
To evaluate the histopathologic changes in the tongue, five rats in each group were sacrificed on the 7th and 14th days, and three rats in each group were sacrificed on the 21st day. The mucosa from the tongue was collected at the end of the experiment. The tongue was exposed and photographed, tongue samples (collected on 7th, 14th, and the 21st days) were divided into two parts separately, and one part was fixed in 10% buffered formalin for 48 h and embedded in paraffin. Then, multiple sections (4 μm thick) were deparaffinized with xylene and stained with H&E. The remaining part of the tongue samples was used for quantitative real-time PCR (qRT-PCR) and Western blot. Samples of blood and feces were obtained for biochemical assays and high-throughput sequencing (PRJNA579226), respectively.
Total RNA was extracted from the tongue tissue using the Tri Reagent Kit (Sigma Aldrich, MO, USA) according to the instructions of the manufacturer. Equal amounts of RNA were used to synthesize complementary DNA (cDNA) using the Fast Quant RT Kit (Tiangen, Beijing, China). The cDNA was used for qRT-PCR with the KAPA SYBR FAST Universal 2× qPCR Master Mix (Kapa Biosystems, MA, USA). The expression of IL-1β, IL-6, tumor necrosis factor α (TNF-α), and the housekeeping gene, such as glyceraldehyde 3-phosphate dehydrogenase (GAPDH), was assessed by qRT-PCR. The relative amount of transcripts for target genes was determined for each cDNA sample after normalization against GAPDH. The data were analyzed using the 2−ΔΔCT method. The following primers were used: GAPDH, 5′-AGC CAA AAG GGT CAT CAT CT-3′ (forward) and 5′-GGG GCC ATC CAC AGT CTT CT-3′ (reverse); IL-6, 5′-GAA ATC GTG GAA ATG AG-3′ (forward) and 5′-GCT TAG GCA TAA CGC ACT-3′ (reverse); IL-1β, 5′-GTG TCT TTC CCG TGG ACC TTC-3′ (forward) and 5′-TCA TCT CGG AGC CTG TAG TGC-3′ (reverse); TNF-α, 5′-GTG GAA CTG GCA GAA GAG GCA-3′ (forward) and 5′-AGA GGG AGG CCA TTT GGG AAC-3′ (reverse).
The protein from the tongue and colon tissues was prepared with the RIPA lysis buffer containing protease and phosphatase inhibitors. Protein (25–30 μg) was loaded on 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto a polyvinylidene fluoride (PVDF) membrane. After blocking with 5% bovine serum albumin (BSA) (in TBS-T buffer), the membrane was incubated with a primary antibody, followed by incubation with the horseradish peroxidase (HRP)-conjugated IgG (1:5000, CST, USA). Protein bands were detected by an electro chemical luminescence (ECL) reagent and analyzed by the Flurochem System (FluorChemE, Cell Biosciences Inc., CA, USA). The primary antibody includes rabbit anti-GAPDH (1:5000, CST, Cat#5174), rabbit anti-NF-κB (1:1000, CST, Cat# 8242S), rabbit anti-phosphorylated-NF-κB (p-NF-κB; 1:1000, Abcam, Cat# ab86299), mouse anti-toll-like receptor 4 (TLR4; 1:1000, Santa Cruz, Cat# sc-293072), rabbit anti-B-cell lymphoma-2 (BCl-2; 1:1000, CST, Cat# 3498S), rabbit anti-BCl-2-associated-x-protein (Bax; 1:1000, CST, Cat# 14796S), rabbit anti-Claudin-1(1:1000, CST, Cat# 4933S), and rabbit anti-zonula occludens-1 (ZO-1; 1:1000, Cat# 5406S).
Deoxyribonucleic acid extraction and high-throughput sequencing of animal fecal bacteria were consistent with the above human experiments. The fecal samples from C group, M group, and the T group were collected before sacrifice and kept at −80°C until DNA extraction. The DNA magnetics and extract kit (Tiangen, Biotech, Beijing, China) was used to extract the fecal bacterial genomic DNA, according to the instruction of the manufacturer. The total genomic DNA was amplified with the forward primer, F341 5′-ACT CCT ACG GGR SGC AGC AG-3′, and the reverse primer, R806 5′- GGA CTA CVV GGG TAT CTA ATC-3′ that amplified the regions from V3 to V4 of the 16S ribosomal DNA gene for high-throughput sequencing analysis (PRJNA579226).
The reported incidence of severe OM after receiving chemoradiotherapy in NPC is at 70–80% (24). Assuming that an average incidence of OM in the placebo and probiotics groups was at 74% and 34%, respectively, 70 patients were enrolled to ensure statistical significance (two-sided α = 0.05, 1–β = 0.9 and 1:1 ratio). We analyzed data from all randomized patients who received at least one dose of the drug. The most recent observations were used to estimate the missing values of the primary efficacy point, while the primary comparable analysis, secondary efficacy points, and the missing values for safety were not retained, and those values were analyzed to obtain the actual data.
Paired-end reads from the original DNA fragments were joined using FLASH Software (25). The joined pairs were quality filtered with the UPARSE software package, and the UPARSE pipeline was used to cluster the remaining sequences into operational taxonomic units (OTUs) at a minimum pair-wise identity of 97% (26). The annotated taxonomic information for each representative sequence, selected from each OTU, was determined using the ribosomal database project (RDP) classifier (27). The data from the OTUs were then used to calculate the Alpha-diversity (α-diversity) metrics by using QIIME (28). Distances between microbial communities obtained from different samples were calculated with the weighted UniFrac beta-diversity metric via QIIME (29). Non-metric multidimensional scaling (NMDS) analysis and principal coordinate analysis (PCoA) were used to visualize the pairwise UniFrac distances among the samples.
All data were reported as means and SD, and the results were analyzed with SPSS 23.0 software (SPSS, Inc., Chicago, Illinois) by the Student's t-test and the one-way ANOVA. The p < 0.05 was regarded as statistically significant.
First, a high-throughput sequencing analysis was used to compare the intestinal-microbial diversity among HP, patients with cancer, and cancer-free village residents. The NMDS analysis showed that samples in HP and NT groups clustered together, while they deviated from the samples of patients with tumor (TP group) (Figure 1A). The Venn diagram indicated that 375 common OTUs were observed from HP, TP, and NT groups (Figure 1B), and the decreased relative abundance of probiotics, such as Lactobacillus (HP: TP: NT = 8: 2: 13%), Bifidobacterium, and Akkermansia, was obtained at the genus level (Figure 1C). The results of qRT-PCR further confirmed that the abundance of probiotics, such as Lactobacillus and Bifidobacterium, in the NT group was higher than that in the HP and TP groups (p < 0.001), while the abundance of harmful bacteria, such as Clostridium, Enterococcus, and Enterobacter, in the NT group was reduced, compared with HP and TP groups (p < 0.001; Figure 1D).
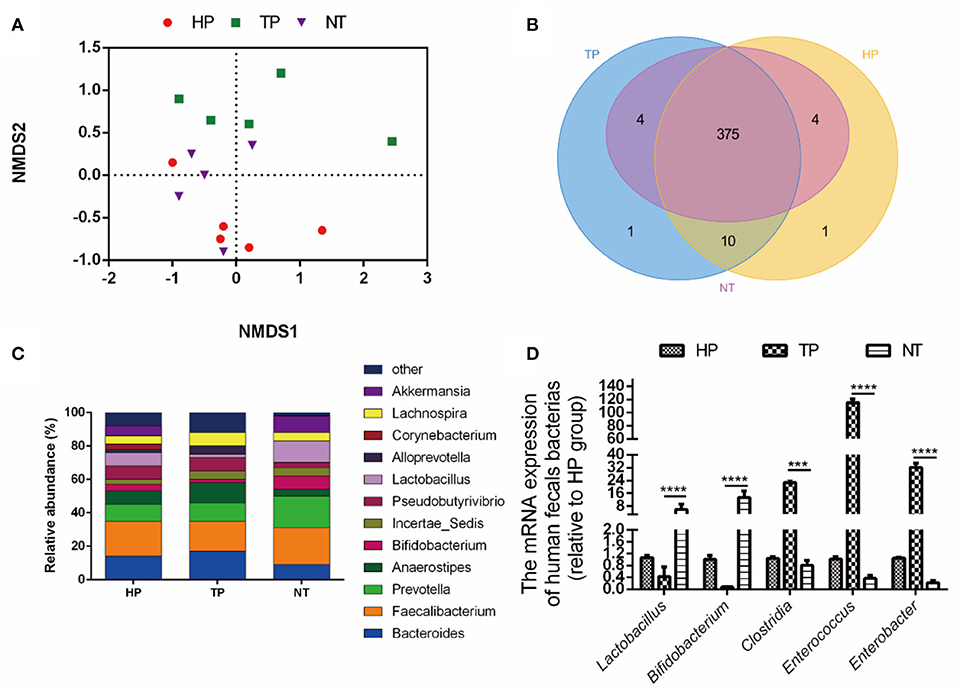
Figure 1. Selection of probiotic strains. (A) The non-metric multidimensional scaling (NMDS) analysis of healthy people (HP) from employees of the Jiangxi Cancer Hospital, tumor patients (TP) from the Jiangxi Cancer Hospital, and non-cancer people (NT) (healthy residents at the cancer-free village). (B) The Venn diagram of the intestinal microbiota among HP, TP, and NT. (C) The relative abundance of the bacteria among groups HP, TP, and NT. (D) The relative abundance of bacteria among the HP, TP, and NT groups with quantitative real-time PCR (qRT-PCR). The data are presented as means ± SD, where ***p < 0.001 and **** p < 0.0001.
Then, the selective MRS medium for Lactobacillus was used to selectively isolate Lactobacilli and 10 strains, namely Clostridium tertium MH282454.1, Weissellacibaria KU555931.1, L. curvatus LC129556.1, W. confusa KC416985.1, L. plantarum KJ779102.1, L. reuteri KX881777.1, L. paracasei MG822869.1, Enterococcus faecium KX267939.1, L. mucosae FJ751778.1, and Pediococcuspentosaceus KJ806297.1, were identified from feces of cancer-free village residents (Supplementary Table 1). Finally, the L. plantarum KJ779102.1 was chosen for further study according to the standard of the China Food and Drug Administration. This strain has been stored as a patent bacterium in the Institute of Microbiology, Chinese Academy of Sciences (L. plantarum MH-301).
To prepare the probiotic cocktail in clinic trial, L. plantarum MH-301, B. animalis subsp. Lactis LPL-RH, L. rhamnosusnosus LGG-18, and L. acidophilus were finally chosen for their acid resistance, high resistance to bile salts, strong oxidation resistance, broad spectrum of antibacterial ability, and high cell adhesion (Supplementary Figure 1).
A total of 85 patients were assessed for eligibility evaluation, and eight patients were excluded for their failure to meet the inclusion criteria. The remaining 77 patients were randomly assigned to the probiotics (39 patients) or the placebo group (38 patients) in the ratio of 1:1 patients, three patients in the probiotics group were further excluded due to complication, and four patients in the placebo group were excluded for complication (two patients) or withdrawal of consent (two patients). In the end, 34 patients were finally designated as the placebo group, and 36 patients were finally designated as the probiotic group (Figure 2). There was no marked difference of baseline characteristics in patients between the placebo group and the probiotic group, and the details of gender, age, tumor stage, and node stage of patients who completed the treatment were summarized in Supplementary Table 2.
As shown in Figure 3A, probiotic cocktail significantly reduced the severity of OM in patients with NPC who underwent CCRT. The rate of incidence of 0, 1, 2, 3, and 4 grades of OM were 0, 14.7, 38.2, 32.4, and 14.7%, respectively, in the ARCP group, while they were 13.9, 36.1, 25, 22.2, and 2.8%, respectively, in the ARCPM group (p < 0.01). Moreover, probiotic cocktail significantly attenuated the negative impact of CCRT on immunity. Oral administration of probiotic cocktail greatly enhanced the reduction rate of CD3+ T cells (75.5 vs. 81%, p < 0.01), CD4+ T cells (64.53%vs. 79.53%, p < 0.01), and CD8+ T cells (75.59 vs. 62.36%, p < 0.01) compared to patients in the ARCP group (Figures 3B–D). No significant differences of reduction rate of lymphocyte (80.81 vs. 84.44%, p > 0.05), hemoglobin (10.94 vs. 12%, p > 0.05), and body weight (6.53 vs. 6.7%, p > 0.05) were observed between the ARCPM and ARCP groups (Figures 3E–G).
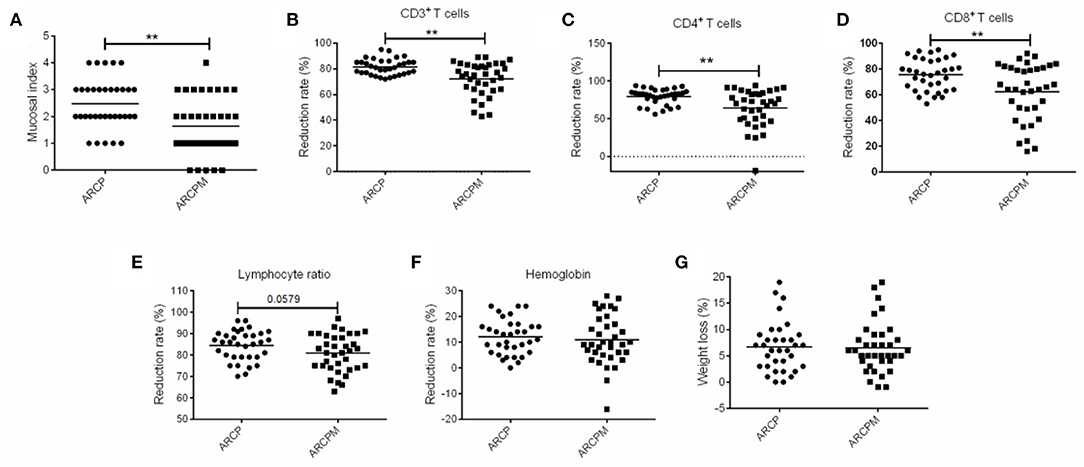
Figure 3. The combination of probiotics reduced oral mucositis (OM) by improving the immunity of patients with nasopharyngeal cancer (NPC). (A) Mucosal index. (B) Reduction rate of CD3+T cells. (C) Reduction rate of CD4+T cells. (D) Reduction rate of CD8+T cells. (E) Reduction rate of lymphocyte. (F) Reduction rate of hemoglobin. (G) Weight loss. The data are presented as means ± SD, where **p < 0.01.
In total, 2,936,897 clean tags and 9,941 OTUs were obtained with an average of 196.4 OTUs in each group (Supplementary Table 3). The Venn diagram reflected the difference of OTUs in all groups. In total, 325 common OTUs were identified in all groups and 47 OTUs were identified specifically in the HP group. Notably, eight OTUs belonged only to the RCP groups (BRCP and ARCP groups), and 16 OTUs were identified exclusively in RCPM groups (BRCPM and ARCPM groups) (Figure 4A).
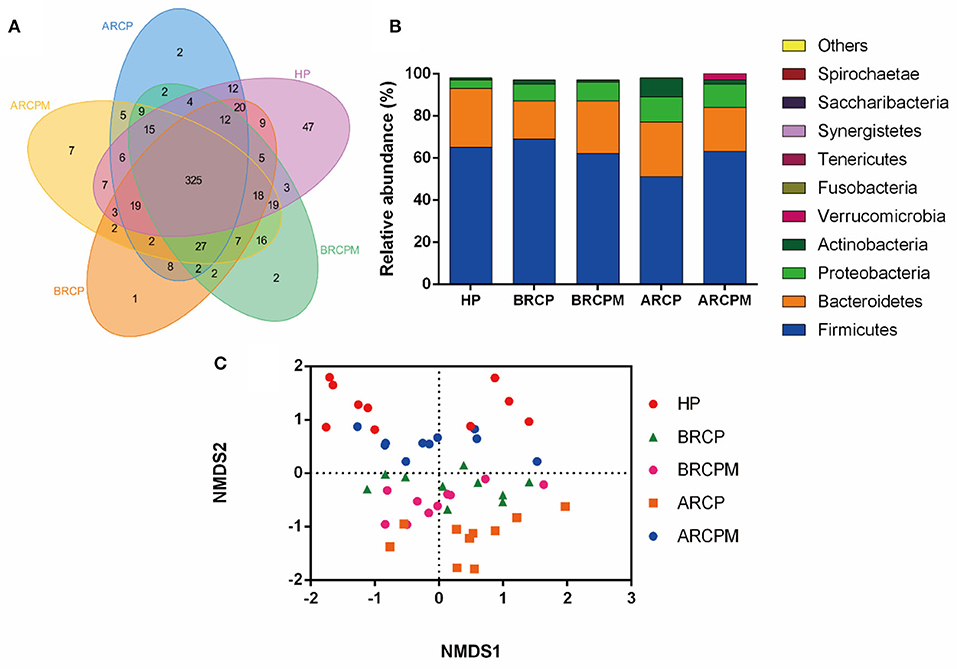
Figure 4. Effects of the combination of probiotics on the composition of bacterial communities in patients with NPC. (A) The relative abundance of bacteria among HP from the employees of the Jiangxi Cancer Hospital, before the treatment of radiotherapy plus chemotherapy plus a placebo (BRCP), after treatment with radiotherapy plus chemotherapy plus a placebo (ARCP), before the treatment of radiotherapy plus chemotherapy plus the probiotic combination (BRCPM), and after treatment with radiotherapy plus chemotherapy plus the probiotic combination (ARCPM). (B) The Venn diagram of the intestinal microbiota among HP, BRCP, ARCP, BRCPM, and ARCPM groups. (C) The NMDS analysis of groups HP, BRCP, ARCP, BRCPM, and ARCPM.
At the phylum level, the data of the top 10 populations of microorganisms were analyzed. Firmicute, Bacteroidetes, Proteobacteria, and Actinobacteria were predominated in HP, ARCP, BRCP, ARCPM, and BRCPM groups (Firmicutes: 66.03, 52.10, 69.41, 63.30, and 63.10%, respectively; Bacteroidetes: 28.02, 27.22, 18.82, 19.01, and 25.30%, respectively; Proteobacteria: 4.45, 11.89, 8.942, 11.13, and 9.45%, respectively; and Actinobacteria: 1.49, 8.18, 2.20, 3.00, and 1.72%, respectively). The abundance of Bacteroidetes and Actinobacteria were increased and the abundance of Firmicutes was decreased in the ARCP group, but probiotic cocktail enriched the abundance of Firmicutes and reduced the abundance of Bacteroidetes and Actinobacteria to the normal level (Figure 4B).
The NMDS analysis found that BRCPM, ARCPM, BRCP, and HP groups were clustered together, while the ARCP group diverged from other groups and showed a scattered distribution, indicating that the probiotic cocktail restored the gut dysbiosis in patients with NPC who underwent CCRT (Figure 4C).
As shown in Figure 5A, the mucosal index was evaluated on the 7th, 14th, and 21st days. The incidence of grade 3 or over was significantly increased in the M group than those in the C group on the 7th, 14th, and 21st days (76.9 vs. 0%, 100 vs. 0%, and 66.7 vs. 0%; p < 0.01; respectively). Otherwise, the incidence of grade 3 or over was significantly decreased in the T group than those in the M group on the 7th, 14th, and 21st day (23.1 vs. 76.9%, 50 vs. 100%, and 0 vs. 66.7%; p < 0.01; respectively). It is worth mentioning that the severity of OM was alleviated on the 21st day both in the M and T groups compared with that on the 7th and 14th days.
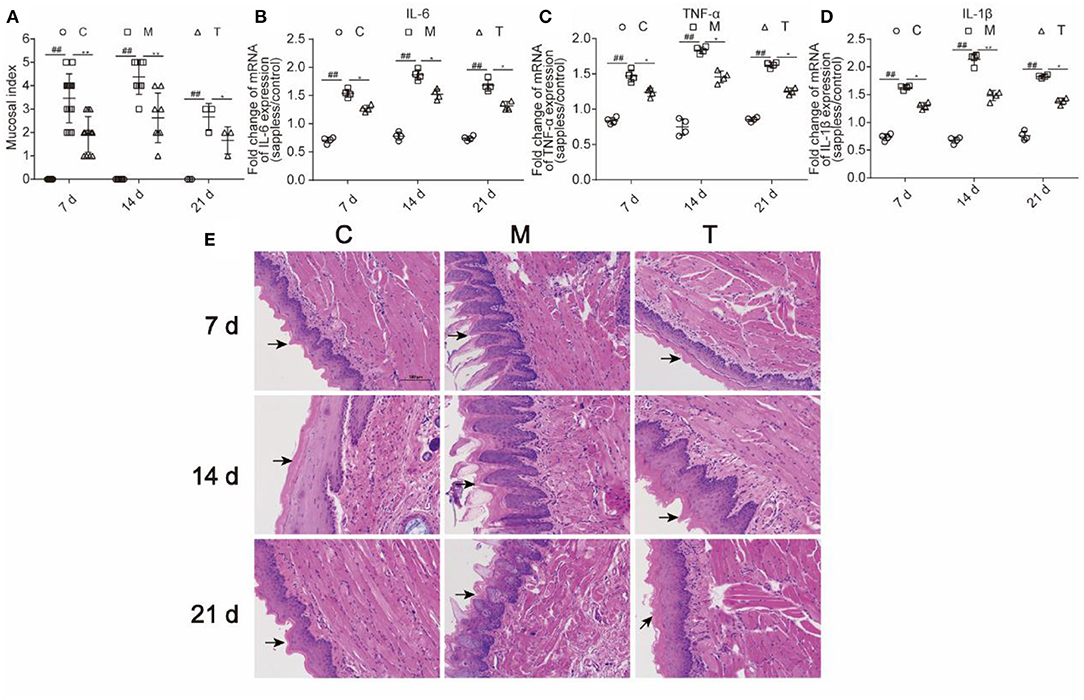
Figure 5. The probiotic cocktail relieved tongue tissue inflammatory response in rats with OM caused by radiotherapy and chemotherapy. (A) Mucosal index. (B) The expression of IL-6 in the mRNA level. (C) The expression level of TNF-α in the mRNA level. (D) The expression of IL-1β in the mRNA level. (E) H&E staining of the tongue tissue among the C, M, and T groups on the 7th, 14th, and 21st days. The data are presented as means ± SD, where *p < 0.05, **p < 0.01, and ##p < 0.01.
The hemogram of rats was measured on the 7th, 14th, and 21st days. As shown in Table 1, the concentration of leukocyte was higher in the M group than in the C group on the 7th, 14th, and 21st days (18.39 ± 4.06 vs. 12.38 ± 2.53, p < 0.01; 20.65 ± 3.35 vs. 15.06 ± 2.00, p < 0.01; 16.63 ± 2.05 vs. 13.70 ± 2.45; p < 0.05). However, the concentration of leukocyte was lower in the T group than in the M group (16.32 ± 2.57 vs. 18.39 ± 4.06, p < 0.05; 16.80 ± 3.02 vs. 20.65 ± 3.35, p < 0.05; 15.90 ± 1.90 vs. 16.63 ± 2.05, p < 0.05). This result suggested that probiotic cocktail suppressed peripheral immune response by reducing the level of blood leukocyte in rats that were treated with radiotherapy and chemotherapy.
To evaluate the inflammation of oral mucosa, expressions of IL-6, IL-1β, and TNF-α in the mRNA level of the tongue tissue were measured (Figures 5B–D). The M group demonstrated significantly higher expression level than the C group in IL-6 (1.54 vs. 0.71, p < 0.01; 1.87 vs. 0.78, p < 0.01; 1.68 vs. 0.74, p < 0.01, respectively), IL-1β (1.63 vs. 0.73, p < 0.01; 2.14 vs. 0.68, p < 0.001; 1.83 vs. 0.76, p < 0.01, respectively), and TNF-α (1.47 vs. 0.84, p < 0.01; 1.84 vs. 0.75, p < 0.01; 1.63 vs. 0.86, p < 0.01, respectively), on the 7th, 14th, and 21st days. The T group showed remarkably lower expression level than the M group in IL-6 (1.27 vs. 1.54, p < 0.05; 1.52 vs. 1.87, p < 0.05;1.32 vs. 1.68, p < 0.05, respectively), IL-1β (1.30 vs. 1.63, p < 0.05; 1.48 vs. 2.14, p < 0.05; 1.38 vs. 1.83, p < 0.05, respectively) and TNF-α (1.24 vs. 1.47, p < 0.05; 1.46 vs. 1.84, p < 0.05; 1.26 vs. 1.63, p < 0.05, respectively) on the 7th, 14th, and 21st days. The result indicated that probiotic cocktail remarkably attenuated oral inflammation in rats that were treated with radiotherapy and chemotherapy.
The histology samples of the tongue tissue of each group were collected on the 7th, 14th, and 21st days. The H&E staining visually reflected the severity of the tongue and its mucosal thickening in the C, M, and T groups. Severe destruction of tongue epithelium, the erosion of the corneum, and the proliferation of basal cells were found in the M group compared with the C group. However, the damage of the tongue tissue in the T group was observed to be lighter than the M group, which indicated that the probiotic cocktail could relieve the inflammatory response. The structural damage of the tongue tissue was observed on the 7th day, deteriorated on the 14th day, and was partly repaired on the 21st day both in the M and T groups (Figure 5E).
These results indicated that the probiotic cocktail inhibited the peripheral immune response, inflammation, and pathological damage, further to alleviate the severity of OM in rats induced by radiotherapy and chemotherapy.
Inflammation-related proteins, cell apoptosis, and intestinal TJ proteins were measured in the tongue and colon tissue, respectively (Figure 6). Compared with the C group, a higher expression of TLR4 (7th, 14th, and 21st days) and P-NF-κB/NF-κB (7th, 14th, and 21st days) in the M group were observed (p < 0.001). However, the probiotic cocktail markedly downregulated the expression of TLR4 (7th, 14th, and 21st days) and P-NF-κB/NF-κB since the 7th day (p < 0.001) in rats with OM. Also, the probiotic cocktail significantly inhibited the apoptosis caused by radiotherapy and chemotherapy via reducing the ratio of Bax/Bcl-2 (Figure 6B).
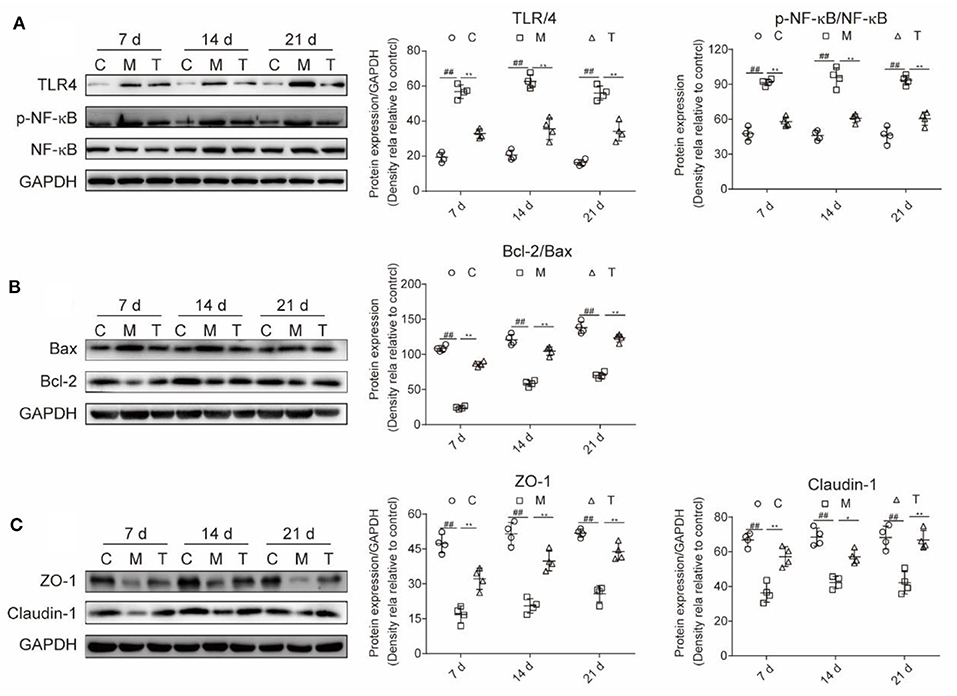
Figure 6. The combination of probiotics ameliorated the upregulation of TLR4/NF-κB, tongue tissue apoptosis and improved the expression of intestinal tight junction (TJ) in rats with OM caused by radiotherapy and chemotherapy. (A) Protein expression level of TLR4, P-NF-κB, and NF-κB. (B) Protein expression level of apoptosis-associated factors, Bcl-2 and Bax. (C) The expression level of intestinal TJ proteins, ZO-1 and claudin-1. The data are presented as means ± SD, where *p < 0.05, **p < 0.01, #p < 0.05, and ##P < 0.01.
The integrity of the intestinal barrier was measured with intestinal TJ proteins. The results indicated that the expression of ZO-1 (7th, 14th, and 21st days) and Claudin-1 (7th, 14th, and 21st days) were greatly reduced in rats with OM, and the probiotic cocktail markedly restored the expression of ZO-1 (7th, 14th, and 21st days) and Claudin-1 (7th, 14th, and 21st days) to normal levels (Figure 6C).
In summary, the probiotic cocktail attenuates the severity of OM possibly by downregulating the TLR4/NF-κB signaling pathway, reducing cell apoptosis, and downregulating the intestinal TJ proteins.
To test whether the probiotic cocktail could reverse the gut dysbiosis induced by radiotherapy and chemotherapy in rats, we analyzed the composition and community structure of bacteria in feces through 16S rRNA gene sequencing. As illustrated in Figures 7A,B, the probiotic cocktail improved the microbial α-diversity, the Shannon index, and the Simpson index, on the 7th and 14th days, which were decreased in rats with OM, although there was no significant statistical difference. The PCoA analysis showed that fecal microbial populations of animals in the C and T groups clustered together and diverged from that of the rats with OM over the study period, indicating that the probiotic cocktail partially shaped the alterations of the microbial community in rats with OM caused by radiotherapy and chemotherapy (Figure 7C).
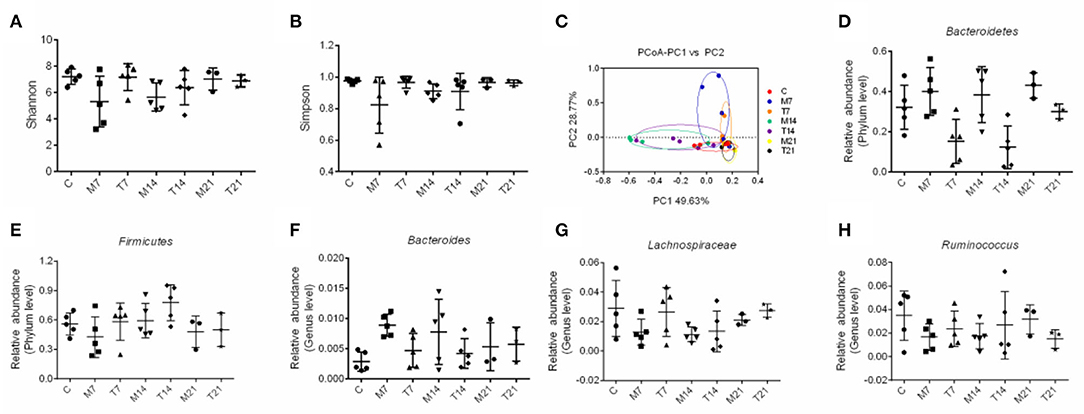
Figure 7. Effects of probiotic cocktail on the composition of bacterial communities in rats with OM caused by radiotherapy and chemotherapy. (A) Shannon index of intestinal bacterial communities among C, M, and T groups at days 7, 14, and 21. (B) Simpson index of intestinal bacterial communities among C, M, and T groups on the 7th, 14th, and 21st days. (C) Principle coordinate analysis (PCoA) of intestinal bacterial communities among C, M, and T groups on the 7th, 14th, and 21st days. (D) Relative abundance of Bacteroidetes of intestinal bacterial communities at the phylum level among C, M, and T groups on the 7th, 14th, and 21st days. (E) Relative abundance of Firmicutes of intestinal bacterial communities at the phylum level among C, M, and T groups on the 7th, 14th, and 21st days. (F) Relative abundance of Bacteroidetes of intestinal bacterial communities at the genus level among C, M, and T groups on the 7th, 14th, and 21st days. (G) Relative abundance of Lachnospiraceae of intestinal bacterial communities at the genus level among C, M, and T groups on the 7th, 14th, and 21st days. (H) Relative abundance of Ruminococcus of intestinal bacterial communities at the genus level among the C, M, and T groups on the 7th, 14th, and 21st days.
The abundance of Firmicutes was decreased and the abundance of Bacteroidetes showed an increasing trend in the M group on the 7th, 14th, and 21st days. Moreover, anti-inflammation-related bacteria, such as Lachnospiraceae and Ruminococcus, were reduced and inflammation-related bacteria, such as Bacteroides, were enriched in the M group on the 7th, 14th, and 21st days. However, the abundance of Firmicutes, Lachnospiraceae, and Ruminococcus was increased and the abundance of Bacteroidetes and Bacteroides was decreased in the T group than that of the M group, though there was no significant statistical difference (Figures 7D–H). These results suggested that the probiotic cocktail shaped gut dysbiosis and prevented inflammation in radiotherapy and chemotherapy-induced rats.
In this study, we investigated the impact of the combination of L. plantarum MH-301, B. animalis subsp. Lactis LPL-RH, L. rhamnosus LGG-18, and L. acidophilus on OM for the first time. As expected, the results showed that 47.1% of the patients in the ARCP group developed ≥grade 3 OM, whereas OM was only 25% developed in the ARCPM group, and this supported the protective effect of the probiotic cocktail against OM. Consistent with the clinical test, the probiotic cocktail also reduced the severity of grade 3 OM (23.1 vs. 76.9%, 50 vs. 100%, and 0 vs. 66.7%) on the 7th, 14th, and 21st days in radiotherapy and chemotherapy-induced rats. In addition, the probiotic cocktail also improved the immunity of patients with NPC and restored gut dysbiosis to normal both in the patients with NPC and rats with OM induced by radiotherapy and chemotherapy.
Recently, a series of studies have indicated that gut microbiota might modulate the response to immunotherapy in cancer treatment (30, 31). It was found that treating mice with probiotic L. plantarum (KC836552.1) significantly reduced tumor volume and activated immune responses, such as enhanced levels of CD8+ T and NK cells in patients with cancer (32). Recently, it has become evident that the ability of gut microbiota to regulate immunity in cancer therapy modulates the susceptibility to toxic side effects (8, 33). Bifidobacterium ameliorated chemotherapy-induced mucositis via promoting the expression of CD4+T cell immunity in rats with cancer (34). Moreover, evidence has suggested that probiotics initiate memory in the T and B cells, trigger adaptive immunity, and activate the immune system which may stimulate the production of salivary glycoproteins and antimicrobial peptides, finally protecting the oral mucosa from damage (35). As reported in an earlier study (13), this study also showed that RCPM significantly enhanced the number of T-cells (CD3+ T, CD8+ T, and CD4+ T cells) and decreased the severity of OM in comparison with those of patients with RCP, which further confirmed that probiotics modulate human immune responses to cancer treatment, eventually reducing the related side effects (36). Besides, it seemed that the effect of this probiotic cocktail on patients with OM and NPC was better than the combination of the previous probiotics (B. longum, L. lactis, and E. faecium).
We used an animal model to verify the assumption that the probiotic cocktail might have beneficial effects on OM induced by radiotherapy and chemotherapy and to make a primary exploration of the mechanisms. In our study, we observed the severity of the oral damage caused by radiotherapy and chemotherapy in rats. The pathogenesis of oral inflammation induced by chemotherapy and irradiation is complex. It had been proposed that radiotherapy and chemotherapy cause DNA and non-DNA damage to the epithelium of cells, tissues, and blood vessels (37). It could also cause reactive oxygen species, followed by the activation of TLR4/NF-κB pathway, which could promote the production of pro-inflammation factors (TNF-α, IL-1β, and IL-6), and accelerate apoptosis (Bax/Bcl-2), and ultimately aggravated tissue damage and lead to bacterial, viral, and fungal infections (38, 39). TNF-α, IL-6, IL-1β, and cell apoptosis played a critical role in the development of mucositis (40). Moreover, evidence has indicated that the IL-6 level positively correlated with the severity of mucositis both in radiation-induced OM mice and in the head and neck of patients with cancer who underwent radiotherapy or radiochemotherapy (41, 42). The results demonstrated that probiotic cocktail administration diminished the upregulation of TLR4/NF-κB and elevated the levels of pro-inflammatory cytokines and cell apoptosis caused by chemotherapy and irradiation.
The intestinal epithelial barrier prevents the entry of exterior antigens from the gut lumen into the host, which may exacerbate both local and systemic immune responses (43). The front line of this barrier is composed of epithelial cells and apical junctional complexes encompassing TJ proteins between the adjacent epithelial cells (44). Previous studies had found that CCRT treatment for cancer also aggravated the dysfunction of the intestinal barrier and caused peripheral immune activation and inflammation (45–47). As expected, the results also showed that TJ proteins (ZO-1 and Claudin-1) were reduced and neutrophils were increased in the M group. Besides, the expression of proteins forming TJ was also influenced by gut microbiota. Probiotics have been shown to increase the expression of TJ protein and restore intestinal permeability, eventually suppressing peripheral neutrophils (48, 49). In line with this study, ZO-1 and Claudin-1 were found to increase in the T group, suggesting that probiotics prevented system-immune activation and inflammation, including an increase in TNF-α, IL-1β, and IL-6 in the oral cavity, which eventually could ameliorate OM.
Radiotherapy and chemotherapy also change intestinal microbiota, which leads to altered colonic epithelial cell homeostasis, impaired barrier function, and increased susceptibility to OM (46, 50, 51). The NMDS analysis was conducted, and our clinical experiments suggested that CCRT had obviously disturbed the diversity of gut microbiota, and the samples from the ARCP group were scattered far from the samples from the HP group, whereas the administration of the mixture of probiotics markedly restored the microbial diversity in the ARCP group than that of the HP and ARCPM groups. Similar to the results of the clinical trial, our rat model also indicated that the bacterial communities had recovered back to normal after being treated with the probiotic cocktail for 21 days with the PCoA. This suggested that the probiotic cocktail had significantly reduced the side effects of CCRT by sustaining the bacterial homeostasis of the intestines.
The gut microbiome of T group rats also reflected enriched species-richness as well as a significant shift in the overall microbial diversity at the phylum and genera level compared to the M group. Recent studies have also revealed that some disadvantage of bacterial strains were increased, while the bacterial strains which were beneficial for health were reduced in patients with cancer who underwent CCRT (52, 53). Accordingly, compared to control, we observed a lower relative abundance of Firmicutes and a higher relative abundance of Bacteroidetes at the phylum level both in patients with NPC and in mice with OM. We also found that a higher abundance of Actinobacillus was observed in patients with NPC than those in HP. In addition, higher Bacteroides, lower Lachnospiraceae, and lower Ruminococcus at the genus level were observed in mice with OM. Based on the previous study, Actinobacillus were usually found to be both an oral symbiotic and an opportunistic pathogen, which were associated with the pathogenesis of meningitis, sinusitis, pleural empyema, and bronchopneumonia (54, 55). Besides, Actinobacillus might seriously affect the homeostasis of oropharyngeal microorganisms, and was one of the susceptible factors for patients with NPC who had severe mucositis (55). Bacteroides have been observed to be a prominent feature in patients with inflammatory bowel disease (56) and are associated with mucus degradation and a pro-inflammatory phenotype (57). Other bacteria in the Firmicutes phylum, such as Lachnospiraceae and Ruminococcaceae, demonstrated an anti-inflammatory process by reducing pro-inflammatory cytokines (IL-12 and IFN-γ) and increasing the anti-inflammatory cytokines (IL-10) (58). In addition to the anti-inflammatory activity, Lachnospiraceae and Ruminococcaceae were also considered to be associated with the butyrate-producing process (59). Butyrate was suggested to be important in ameliorating mucosal inflammation and maintaining the intestinal barrier (60). It is important to emphasize that the intestinal ecosystem is partially important to maintain human health. Specific changes, such as the decrease of Firmicutes and the increase of Bacteroidetes in this ecosystem, may contribute to the development of inflammatory-related disease (61). Taken together, the increase in the abundance of inflammation-related Bacteroidetes and Actinobacillus and a decrease in the abundance of anti-inflammation-related Firmicutes in patients with NPC who were treated with CCRT may have affected the severity of OM to some extent.
Probiotics, such as L. lactis and B. longum, were expected to be useful for intestinal inflammation and OM (62, 63). A recent study also demonstrated that a mix of Bacillus subtilis (2.9 × 108 CFU/g), B. bifidum (2.0 × 108 CFU/g), E. faecium (2.1 × 108CFU/g), and L. acidophilus (1.0 × 108 CFU/g) reduced the histological severity of intestinal mucositis and OM in rats treated with chemotherapy (64). Probiotic-based treatments have proven to be beneficial for chemotherapy- or radiotherapy-induced mucositis, possibly by regulating the microbiome and inhibiting the pro-inflammatory cytokines (65, 66). In the same way, B. bifidum G9-1(BBG9-1) eliminates 5-FU-induced mucositis by inhibiting secondary inflammation through a reduction in the abundance of Bacteroidetes and the corresponding increase in the abundance of Firmicutes (67). In addition, Lactobacillus significantly reversed the chemotherapy- or radiation-disturbed composition of Firmicutes and Bacteroidetes, thereby reducing pro-inflammatory reactions and mucositis (46, 68, 69). Thus, the changed abundance of Firmicutes and Bacteroidetes induced by radiotherapy and chemotherapy were associated with intestinal inflammation, to further induce or increase the incidence of OM, and the probiotic cocktail decreased the severity of OM by regulating the homeostasis of intestinal bacteria.
There are some strengths in our study. First, we isolated L. plantarum from free village residents. Secondly, according to human clinical and rat trials, probiotic combinations, namely L. plantarum, B. animalis subsp. Lactis LPL-RH, L. rhamnosusnosus LGG-18, and L. acidophilus, might reduce the severity of OM in patients with NPC who were treated with radiotherapy and chemotherapy. Finally, our results indicated that the probiotic cocktail might alleviate the severity of OM in patients with NPC who were treated with radiotherapy and chemotherapy by regulating gut microbiota dysbiosis and enhancing immunity. There are some limitations in this study. One disadvantage is that the number of patients with NPC was not large enough and more patients with NPC are needed in the future to confirm the results; another limitation is the need for fecal microbiota transplantation to further identify the role of gut microbiota on the effectiveness of probiotic cocktail on OM of patients with NP.
These results indicated that the probiotic cocktail could significantly reduce the severity of OM in patients with NPC, which might be related to improving the immunity of patients with NPC and regulating gut microbiota homeostasis. The results of the probiotic cocktail on rats with OM induced by radiotherapy and chemotherapy further confirmed that the probiotic cocktail could ameliorate the severity of OM by modulating the gut dysbiosis related to inflammatory responses.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
This study was approved by the local clinical research Ethics Committee and was conducted following the Helsinki declaration (No. 2017ky023). All the patients gave their informed consent before the trail. The patients/participants provided their written informed consent to participate in this study. The animal study was reviewed and approved by Animal care and procedures were under the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals, and all experiments were approved by the Ethical Committee of the of Nanchang University.
TC, HX, and HW conceived and designed the study. CX, TC, and CJ did the data processing and wrote the first draft of the paper. TC, CX, CJ, JW, WL, HH, JL, LF, HW, and HX checked and revised the first draft of the paper. All authors contributed to the article and approved the submitted version.
This study was supported by the National Natural Science Foundation of China (Grant no. 82060638), Academic and technical leaders of major disciplines in Jiangxi Province (Grant no. 20194BCJ22032), and Double thousand plan of Jiangxi Province (high end Talents Project of scientific and technological innovation).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2021.618150/full#supplementary-material
1. Chua MLK, Wee JTS, Hui EP, Chan ATC. Nasopharyngeal carcinoma. Lancet. (2016) 387:1012–24. doi: 10.1016/S0140-6736(15)00055-0
2. Nishimura N, Nakano K, Ueda K, Kodaira M, Yamada S, Mishima Y, et al. Prospective evaluation of incidence and severity of oral mucositis induced by conventional chemotherapy in solid tumors and malignant lymphomas. Support Care Cancer. (2012) 20:2053–9. doi: 10.1007/s00520-011-1314-6
3. Villa S, Sonis T. Mucositis: pathobiology and management. Curr Opin Oncol. (2015) 27:159–64. doi: 10.1097/CCO.0000000000000180
4. Daugelaite G, Uzkuraityte K, Jagelaviciene E, Filipauskas A. Prevention and treatment of chemotherapy and radiotherapy induced oral mucositis. Medicina. (2019) 55:25. doi: 10.3390/medicina55020025
5. Honda K, Littman DR. The microbiota in adaptive immune homeostasis and disease. Nature. (2016) 535:75–84. doi: 10.1038/nature18848
6. Rooks MG, Garrett WS. Gut microbiota, metabolites and host immunity. Nat Rev Immunol. (2016) 16:341–52. doi: 10.1038/nri.2016.42
7. Cui M, Xiao H, Li Y, Zhou L, Zhao S, Luo D, et al. Faecal microbiota transplantation protects against radiation-induced toxicity. EMBO Mol Med. (2017) 9:448–61. doi: 10.15252/emmm.201606932
8. Roy S, Trinchieri G. Microbiota: a key orchestrator of cancer therapy. Nat Rev Cancer. (2017) 17:271–85. doi: 10.1038/nrc.2017.13
9. Lakritz JR, Poutahidis T, Levkovich T, Varian BJ, Ibrahim YM, Chatzigiagkos A, et al. Beneficial bacteria stimulate host immune cells to counteract dietary and genetic predisposition to mammary cancer in mice. Int J Cancer. (2014) 135:529–40. doi: 10.1002/ijc.28702
10. Vetizou M, Pitt JM, Daillere R, Lepage P, Waldschmitt N, Flament C, et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. (2015) 350:1079–84. doi: 10.1126/science.aad1329
11. Daillere R, Vetizou M, Waldschmitt N, Yamazaki T, Isnard C, Poirier-Colame V, et al. Enterococcus hirae and barnesiella intestinihominis facilitate cyclophosphamide-induced therapeutic immunomodulatory effects. Immunity. (2016) 45:931–43. doi: 10.1016/j.immuni.2016.09.009
12. Sivan, Corrales L, Hubert N, Williams JB, Aquino-Michaels K, Earley ZM, et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. (2015) 350:1084–9. doi: 10.1126/science.aac4255
13. Jiang C, Wang H, Xia C, Dong Q, Chen E, Qiu Y, et al. A randomized, double-blind, placebo-controlled trial of probiotics to reduce the severity of oral mucositis induced by chemoradiotherapy for patients with nasopharyngeal carcinoma. Cancer. (2019) 125:1081–90. doi: 10.1002/cncr.31907
14. Gao W, Howden BP, Stinear TP. Evolution of virulence in Enterococcus faecium, a hospital-adapted opportunistic pathogen. Curr Opin Microbiol. (2018) 41:76–82. doi: 10.1016/j.mib.2017.11.030
15. Liu Y, Gibson GR, Walton GE. An in vitro approach to study effects of prebiotics and probiotics on the faecal microbiota and selected immune parameters relevant to the elderly. PLoS ONE. (2016) 11:e0162604. doi: 10.1371/journal.pone.0162604
16. Munoz-Quezada S, Chenoll E, Vieites JM, Genoves S, Maldonado J, Bermudez-Brito M, et al. Isolation, identification and characterisation of three novel probiotic strains (Lactobacillus paracasei CNCM I-4034, Bifidobacterium breve CNCM I-4035 and Lactobacillus rhamnosus CNCM I-4036) from the faeces of exclusively breast-fed infants. Br J Nutr. (2013) 109 (Suppl. 2):S51–62. doi: 10.1017/S0007114512005211
17. Jiang M, Deng K, Jiang C, Fu M, Guo C, Wang X, et al. Evaluation of the antioxidative, antibacterial, and anti-inflammatory effects of the aloe fermentation supernatant containing Lactobacillus plantarum HM218749.1. Mediators Inflamm. (2016) 2016:2945650. doi: 10.1155/2016/2945650
18. Charteris WP, Kelly PM, Morelli L, Collins JK. Antibiotic susceptibility of potentially probiotic Lactobacillus species. J Food Prot. (1998) 61:1636–43. doi: 10.4315/0362-028X-61.12.1636
19. do Carmo MS, Noronha FM, Arruda MO, Costa EP, Bomfim MR, Monteiro AS, et al. Lactobacillus fermentum ATCC 23271 displays in vitro inhibitory activities against Candida spp. Front Microbiol. (2016) 7:1722. doi: 10.3389/fmicb.2016.01722
20. Nishino M, Jagannathan JP, Ramaiya NH, Van den Abbeele AD. Revised RECIST guideline version 1.1: what oncologists want to know and what radiologists need to know. AJR Am J Roentgenol. (2010) 195:281–9. doi: 10.2214/AJR.09.4110
21. Velasquez-Mejia EP, de la Cuesta-Zuluaga J, Escobar JS. Impact of DNA extraction, sample dilution, and reagent contamination on 16S rRNA gene sequencing of human feces. Appl Microbiol Biotechnol. (2018) 102:403–11. doi: 10.1007/s00253-017-8583-z
22. Patel A, Biswas S, Shoja MH, Ramalingayya GV, Nandakumar K. Protective effects of aqueous extract of Solanum nigrum Linn. leaves in rat models of oral mucositis. ScientificWorldJournal. (2014) 2014:345939. doi: 10.1155/2014/345939
23. Lee SW, Jung KI, Kim YW, Jung HD, Kim HS, Hong JP. Effect of epidermal growth factor against radiotherapy-induced oral mucositis in rats. Int J Radiat Oncol Biol Phys. (2007) 67:1172–8. doi: 10.1016/j.ijrobp.2006.10.038
24. Zheng B, Zhu X, Liu M, Yang Z, Yang L, Lang J, et al. Randomized, double-blind, placebo-controlled trial of shuanghua baihe tablets to prevent oral mucositis in patients with nasopharyngeal cancer undergoing chemoradiation therapy. Int J Radiat Oncol Biol Phys. (2018) 100:418–26. doi: 10.1016/j.ijrobp.2017.10.013
25. Magoc T, Salzberg SL. FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics. (2011) 27:2957–63. doi: 10.1093/bioinformatics/btr507
26. Edgar RC. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Methods. (2013) 10:996–8. doi: 10.1038/nmeth.2604
27. Wang Q, Garrity GM, Tiedje JM, Cole JR. Naive bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol. (2007) 73:5261–7. doi: 10.1128/AEM.00062-07
28. Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. (2010) 7:335–6. doi: 10.1038/nmeth.f.303
29. Lozupone C, Lladser ME, Knights D, Stombaugh J, Knight R. UniFrac: an effective distance metric for microbial community comparison. Isme J. (2011) 5:169–72. doi: 10.1038/ismej.2010.133
30. Iida N, Dzutsev A, Stewart CA, Smith L, Bouladoux N, Weingarten RA, et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science. (2013) 342:967–70. doi: 10.1126/science.1240527
31. Couzin-Frankel J. Breakthrough of the year 2013. Cancer immunotherapy. Science. (2013) 342:1432–3. doi: 10.1126/science.342.6165.1432
32. Hu J, Wang C, Ye L, Yang W, Huang H, Meng F, et al. Anti-tumour immune effect of oral administration of Lactobacillus plantarum to CT26 tumour-bearing mice. J Biosci. (2015) 40:269–279. doi: 10.1007/s12038-015-9518-4
33. Perez-Chanona E, Trinchieri G. The role of microbiota in cancer therapy. Curr Opin Immunol. (2016) 39:75–81. doi: 10.1016/j.coi.2016.01.003
34. Mi H, Dong Y, Zhang B, Wang H, Peter CCK, Gao P, et al. Bifidobacterium infantis ameliorates chemotherapy-induced intestinal mucositis via regulating T cell immunity in colorectal cancer rats. Cell Physiol Biochem. (2017) 42:2330–41. doi: 10.1159/000480005
35. Thomas S, Izard J, Walsh E, Batich K, Chongsathidkiet P, Clarke G, et al. The host microbiome regulates and maintains human health: a primer and perspective for non-microbiologists. Cancer Res. (2017) 77:1783–812. doi: 10.1158/0008-5472.CAN-16-2929
36. Zitvogel L, Ayyoub M, Routy B, Kroemer G. Microbiome and anticancer immunosurveillance. Cell. (2016) 165:276–87. doi: 10.1016/j.cell.2016.03.001
37. Clemente M, Rizzetto L, Castronovo G, Perissi E, Tanturli M, Cozzolino F, et al. Effects of near-infrared laser radiation on the survival and inflammatory potential of Candida spp. involved in the pathogenesis of chemotherapy-induced oral mucositis. Eur J Clin Microbiol Infect Dis. (2015) 34:1999–2007. doi: 10.1007/s10096-015-2443-5
38. Tancharoen S, Shakya P, Narkpinit S, Dararat P, Kikuchi K. Anthocyanins Extracted from Oryza sativa L. Prevent fluorouracil-induced nuclear factor-kappab activation in oral mucositis: in vitro and in vivo studies. Int J Mol Sci. (2018) 19:2981. doi: 10.3390/ijms19102981
39. Luo J, Bian L, Blevins MA, Wang D, Liang C, Du D, et al. Smad7 promotes healing of radiotherapy-induced oral mucositis without compromising oral cancer therapy in a xenograft mouse model. Clin Cancer Res. (2019) 25:808–18. doi: 10.1158/1078-0432.CCR-18-1081
40. Viet CT, Corby PM, Akinwande A, Schmidt BL. Review of preclinical studies on treatment of mucositis and associated pain. J Dent Res. (2014) 93:868–75. doi: 10.1177/0022034514540174
41. Meirovitz A, Kuten M, Billan S, Abdah-Bortnyak R, Sharon A, Peretz T, et al. Cytokines levels, severity of acute mucositis and the need of PEG tube installation during chemo-radiation for head and neck cancer–a prospective pilot study. Radiat Oncol. (2010) 5:16. doi: 10.1186/1748-717X-5-16
42. Mangoni M, Sottili M, Gerini C, Desideri I, Bastida C, Pallotta S, et al. A PPAR gamma agonist protects against oral mucositis induced by irradiation in a murine model. Oral Oncol. (2017) 64:52–8. doi: 10.1016/j.oraloncology.2016.11.018
43. Mu Q, Kirby J, Reilly CM, Luo XM. Leaky gut as a danger signal for autoimmune diseases. Front Immunol. (2017) 8:598. doi: 10.3389/fimmu.2017.00598
44. Suzuki T. Regulation of intestinal epithelial permeability by tight junctions. Cell Mol Life Sci. (2013) 70:631–59. doi: 10.1007/s00018-012-1070-x
45. van Vliet MJ, Harmsen HJ, de Bont ES, Tissing WJ. The role of intestinal microbiota in the development and severity of chemotherapy-induced mucositis. PLoS Pathog. (2010) 6:e1000879. doi: 10.1371/journal.ppat.1000879
46. Touchefeu Y, Montassier E, Nieman K, Gastinne T, Potel G, Bruley des Varannes S, et al. Systematic review: the role of the gut microbiota in chemotherapy- or radiation-induced gastrointestinal mucositis - current evidence and potential clinical applications. Aliment Pharmacol Ther. (2014) 40:409–21. doi: 10.1111/apt.12878
47. Li Y, Dong J, Xiao H, Zhang S, Wang B, Cui M, et al. Gut commensal derived-valeric acid protects against radiation injuries. Gut Microbes. (2020) 11:789–806. doi: 10.1080/19490976.2019.1709387
48. Laval L, Martin R, Natividad JN, Chain F, Miquel S, Desclee de Maredsous C, et al. Lactobacillus rhamnosus CNCM I-3690 and the commensal bacterium Faecalibacterium prausnitzii A2-165 exhibit similar protective effects to induced barrier hyper-permeability in mice. Gut Microbes. (2015) 6:1–9. doi: 10.4161/19490976.2014.990784
49. Celiberto LS, Pinto RA, Rossi EA, Vallance BA, Cavallini DCU. Isolation and characterization of potentially probiotic bacterial strains from mice: proof of concept for personalized probiotics. Nutrients. (2018) 10:1684. doi: 10.3390/nu10111684
50. Hong BY, Sobue T, Choquette L, Dupuy AK, Thompson A, Burleson JA, et al. Chemotherapy-induced oral mucositis is associated with detrimental bacterial dysbiosis. Microbiome. (2019) 7:66. doi: 10.1186/s40168-019-0679-5
51. Al-Qadami G, Van Sebille Y, Le H, Bowen J. Gut microbiota: implications for radiotherapy response and radiotherapy-induced mucositis. Expert Rev Gastroenterol Hepatol. (2019) 13:485–96. doi: 10.1080/17474124.2019.1595586
52. Okubo R, Kinoshita T, Katsumata N, Uezono Y, Xiao J, Matsuoka YJ. Impact of chemotherapy on the association between fear of cancer recurrence and the gut microbiota in breast cancer survivors. Brain Behav Immun. (2019) 85:186–91. doi: 10.1016/j.bbi.2019.02.025
53. Montassier E, Gastinne T, Vangay P, Al-Ghalith GA, Bruley des Varannes S, Massart S, et al. Chemotherapy-driven dysbiosis in the intestinal microbiome. Aliment Pharmacol Ther. (2015) 42:515–28. doi: 10.1111/apt.13302
54. Simon-Soro A, Tomas I, Cabrera-Rubio R, Catalan MD, Nyvad B, Mira A. Microbial geography of the oral cavity. J Dent Res. (2013) 92:616–21. doi: 10.1177/0022034513488119
55. Zhu XX, Yang XJ, Chao YL, Zheng HM, Sheng HF, Liu HY, et al. The potential effect of oral microbiota in the prediction of mucositis during radiotherapy for nasopharyngeal carcinoma. EBioMedicine. (2017) 18:23–31. doi: 10.1016/j.ebiom.2017.02.002
56. Swidsinski A, Weber J, Loening-Baucke V, Hale LP, Lochs H. Spatial organization and composition of the mucosal flora in patients with inflammatory bowel disease. J Clin Microbiol. (2005) 43:3380–9. doi: 10.1128/JCM.43.7.3380-3389.2005
57. Le Chatelier E, Nielsen T, Qin J, Prifti E, Hildebrand F, Falony G, et al. Richness of human gut microbiome correlates with metabolic markers. Nature. (2013) 500:541–6. doi: 10.1038/nature12506
58. Sokol H, Pigneur B, Watterlot L, Lakhdari O, Bermudez-Humaran LG, Gratadoux JJ, et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci USA. (2008) 105:16731–6. doi: 10.1073/pnas.0804812105
59. Chakraborti CK. New-found link between microbiota and obesity. World J Gastrointest Pathophysiol. (2015) 6:110–9. doi: 10.4291/wjgp.v6.i4.110
60. Canani RB, Costanzo MD, Leone L, Pedata M, Meli R, Calignano A. Potential beneficial effects of butyrate in intestinal and extraintestinal diseases. World J Gastroenterol. (2011) 17:1519–28. doi: 10.3748/wjg.v17.i12.1519
61. Uchiyama K, Naito Y, Takagi T. Intestinal microbiome as a novel therapeutic target for local and systemic inflammation. Pharmacol Ther. (2019) 199:164–72. doi: 10.1016/j.pharmthera.2019.03.006
62. Joossens M, De Preter V, Ballet V, Verbeke K, Rutgeerts P, Vermeire S. Effect of oligofructose-enriched inulin (OF-IN) on bacterial composition and disease activity of patients with Crohn's disease: results from a double-blinded randomised controlled trial. Gut. (2012) 61:958. doi: 10.1136/gutjnl-2011-300413
63. Braat H, Rottiers P, Hommes DW, Huyghebaert N, Remaut E, Remon JP, et al. A phase I trial with transgenic bacteria expressing interleukin-10 in Crohn's disease. Clin Gastroenterol Hepatol. (2006) 4:754–9. doi: 10.1016/j.cgh.2006.03.028
64. Gerhard D, Sousa F, Andraus RAC, Pardo PE, Nai GA, Neto HB, et al. Probiotic therapy reduces inflammation and improves intestinal morphology in rats with induced oral mucositis. Braz Oral Res. (2017) 31:e71. doi: 10.1590/1807-3107bor-2017.vol31.0071
65. Prisciandaro LD, Geier MS, Butler RN, Cummins AG, Howarth GS. Evidence supporting the use of probiotics for the prevention and treatment of chemotherapy-induced intestinal mucositis. Crit Rev Food Sci Nutr. (2011) 51:239–47. doi: 10.1080/10408390903551747
66. Pico-Monllor JA, Mingot-Ascencao JM. Search and selection of probiotics that improve mucositis symptoms in oncologic patients. A systematic review. Nutrients. (2019) 11:2322. doi: 10.3390/nu11102322
67. Kato S, Hamouda N, Kano Y, Oikawa Y, Tanaka Y, Matsumoto K, et al. Probiotic Bifidobacterium bifidum G9-1 attenuates 5-fluorouracil-induced intestinal mucositis in mice via suppression of dysbiosis-related secondary inflammatory responses. Clin Exp Pharmacol Physiol. (2017) 44:1017–25. doi: 10.1111/1440-1681.12792
68. Chang CW, Liu CY, Lee HC, Huang YH, Li LH, Chiau JC, et al. Lactobacillus casei variety rhamnosus probiotic preventively attenuates 5-fluorouracil/oxaliplatin-induced intestinal injury in a syngeneic colorectal cancer model. Front Microbiol. (2018) 9:983. doi: 10.3389/fmicb.2018.00983
Keywords: probiotics, oral mucositis, intestinal microbiota, nasopharyngeal cancer, radiotherapy and chemotherapy
Citation: Xia C, Jiang C, Li W, Wei J, Hong H, Li J, Feng L, Wei H, Xin H and Chen T (2021) A Phase II Randomized Clinical Trial and Mechanistic Studies Using Improved Probiotics to Prevent Oral Mucositis Induced by Concurrent Radiotherapy and Chemotherapy in Nasopharyngeal Carcinoma. Front. Immunol. 12:618150. doi: 10.3389/fimmu.2021.618150
Received: 16 October 2020; Accepted: 10 February 2021;
Published: 24 March 2021.
Edited by:
Ian Marriott, University of North Carolina at Charlotte, United StatesReviewed by:
Degang Song, Janssen Pharmaceuticals, Inc., United StatesCopyright © 2021 Xia, Jiang, Li, Wei, Hong, Li, Feng, Wei, Xin and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tingtao Chen, Y2hlbnRpbmd0YW8xOTg0QDE2My5jb20=; Y2hlbnRpbmd0YW9AbmN1LmVkdS5jbg==; Hongbo Xin, eGluaGJAbmN1LmVkdS5jbg==; aG9uZ2JveGluQHlhaG9vLmNvbQ==; Hong Wei, d2VpaG9uZzYzNTI4QDE2My5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.