
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol., 04 May 2021
Sec. Inflammation
Volume 12 - 2021 | https://doi.org/10.3389/fimmu.2021.591154
This article is part of the Research TopicMachine Learning, AI and Data Integration Approaches to ImmunologyView all 15 articles
 Ali Mubin Aral1‡
Ali Mubin Aral1‡ Ruben Zamora1,2‡
Ruben Zamora1,2‡ Derek Barclay1
Derek Barclay1 Jinling Yin1
Jinling Yin1 Fayten El-Dehaibi1
Fayten El-Dehaibi1 Vasil E. Erbas3
Vasil E. Erbas3 Liwei Dong4
Liwei Dong4 Zhaoxiang Zhang4
Zhaoxiang Zhang4 Huseyin Sahin5†
Huseyin Sahin5† Vijay S. Gorantla6*
Vijay S. Gorantla6* Yoram Vodovotz1,2*
Yoram Vodovotz1,2*Systems-level insights into inflammatory events after vascularized composite allotransplantation (VCA) are critical to the success of immunomodulatory strategies of these complex procedures. To date, the effects of tacrolimus (TAC) immunosuppression on inflammatory networks in VCA, such as in acute rejection (AR), have not been investigated. We used a systems biology approach to elucidate the effects of tacrolimus on dynamic networks and principal drivers of systemic inflammation in the context of dynamic tissue-specific immune responses following VCA. Lewis (LEW) rat recipients received orthotopic hind limb VCA from fully major histocompatibility complex-mismatched Brown Norway (BN) donors or matched LEW donors. Group 1 (syngeneic controls) received LEW limbs without TAC, and Group 2 (treatment group) received BN limbs with TAC. Time-dependent changes in 27 inflammatory mediators were analyzed in skin, muscle, and peripheral blood using Principal Component Analysis (PCA), Dynamic Bayesian Network (DyBN) inference, and Dynamic Network Analysis (DyNA) to define principal characteristics, central nodes, and putative feedback structures of systemic inflammation. Analyses were repeated on skin + muscle data to construct a “Virtual VCA”, and in skin + muscle + peripheral blood data to construct a “Virtual Animal.” PCA, DyBN, and DyNA results from individual tissues suggested important roles for leptin, VEGF, various chemokines, the NLRP3 inflammasome (IL-1β, IL-18), and IL-6 after TAC treatment. The chemokines MCP-1, MIP-1α; and IP-10 were associated with AR in controls. Statistical analysis suggested that 24/27 inflammatory mediators were altered significantly between control and TAC-treated rats in peripheral blood, skin, and/or muscle over time. “Virtual VCA” and “Virtual Animal” analyses implicated the skin as a key control point of dynamic inflammatory networks, whose connectivity/complexity over time exhibited a U-shaped trajectory and was mirrored in the systemic circulation. Our study defines the effects of TAC on complex spatiotemporal evolution of dynamic inflammation networks in VCA. We also demonstrate the potential utility of computational analyses to elucidate nonlinear, cross-tissue interactions. These approaches may help define precision medicine approaches to better personalize TAC immunosuppression in VCA recipients.
The potential for vascularized composite allotransplantation (VCA) as a reconstructive or restorative option has been established in over 200 procedures performed worldwide, including 145 upper extremity (hand), 42 face, and 26 uterus transplants (with over 10 live births). Nevertheless, the risks of lifelong, high-dose or multi-drug systemic immunosuppression remain the key challenge limiting life enhancing benefits of VCA (1–3).
Tacrolimus (TAC; FK506, Prograf ®), is an FDA-approved immunosuppressant which is the keystone agent in VCA protocols (4, 5). Although TAC is mostly effective in controlling acute rejection (AR), it does not prevent chronic rejection (CR). Multiple hand transplant (6, 7) and face transplant recipients have succumbed to CR (8, 9). The main limitation of TAC is its narrow therapeutic range, which underlies its toxic side effects (10–12). Excessive immunosuppression with TAC can lead to nephrotoxicity, malignancy, or opportunistic infection); inadequate immunosuppression can increase risks of AR or CR.
VCA tissues are primary triggers and targets of the host adaptive and effector immune responses, with draining lymph nodes serving as the primary sites of allorecognition (13). Conventional/standard immunosuppression regimens have prolonged VCA graft survival; however, in most cases, standard baseline immunosuppression (such as TAC) fails to fully prevent long-term skin rejection/deterioration. This is because skin in VCA is highly immunogenic due to unique cellular, cytokine, and chemokine networks (14, 15).
Notwithstanding the antigenicity of the skin, inflammation is the primary determinant of rejection post-VCA (16). Computational modeling suggests that this could be caused by cytokine- or chemokine-mediated immune events such as ischemia-reperfusion injury (IRI), surgical inflammation (SI), and AR after transplantation in general, including VCA (17). Though properly-regulated inflammation allows for timely recognition and repair of traumatic injury and subsequent VCA, insufficient (18) or self-sustaining (19) inflammation can lead to long-lasting immune dysregulation (20). This prolonged immune dysregulation and ongoing tissue damage, can, in turn, predispose to transplant rejection in various contexts (21, 22), including VCA (16, 23, 24).
The success of systemic immunosuppressive therapies or other immunomodulatory strategies is hampered by our lack of understanding of the key dynamic networks and principal drivers of such local/systemic inflammation after VCA. We and others have previously utilized systems and computational biology approaches to help decipher the complexity of inflammation in the context of trauma, sepsis, and wound healing, under the rubric of translational systems biology of inflammation (25, 26). Here, we implement a unique translational systems biology approach, extending initial work characterizing dynamic inflammatory changes in experimental VCA (27, 28). Our overall objective was to model protein-level inflammation networks after limb transplantation, focusing on the early and ongoing local (skin, muscle) and systemic (peripheral blood) processes and their cross-interactions, as well as investigating the effects of TAC on these complex dynamics.
This study utilized the following workflow, which is detailed below. Briefly, rats were subjected to syngeneic or allogeneic hind limb transplantation with TAC, followed by analysis of multiple inflammatory mediators at various time points and integration of the data via various machine learning methods.
The study was approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Pittsburgh and by the Department of Defense Animal Care and Utilization Review Office (ACURO). Full MHC mismatched, male Lewis (LEW) and Brown Norway (BN) rats (Charles River Laboratories, OH), 10-12 weeks age and weighing 300-320 grams, were used in this study. Rats were anesthetized using isoflurane. A circumferential incision was made in a LEW or BN rat donor, at the level of inguinal ligament to expose femoral artery and vein. Epigastric vessels were ligated, and dissected femoral vessels were transected at the level of inguinal ligament. The two limbs were amputated at the level of middle femur. The recipient LEW rat was prepared similarly. Bone fixation was performed with 18-gauge needle. Muscle groups were repaired with 4/0 Vicryl (Ethicon Inc., Somerville, NJ). Femoral artery and vein anastomosis were done with 10-0 Nylon stitches (Aros Surgical Inc, CA). Skin was closed with 4-0 Nylon suture (Aros Surgical Inc, CA) (29).
Due to limitations associated with the animal protocol, a maximum of 3-4 samples were obtained from any given experimental animal, for a total of 7 time-points across multiple animals (d0-d20) as shown in detail in the Supplementary Material. Both the rats and time points were randomized. Hierarchical clustering analysis of all the data broken down by rats showed no clear clustering based on rats (as shown in Supplementary Figure 1 for the plasma data), suggesting the absence of any batch effects. Group 1 (syngeneic controls, n=84 samples across all time-points): LEW recipients received full MHC-matched LEW hindlimbs without TAC. Group 2 (treatment group, n=84 samples across all time-points): LEW recipients received full MHC-mismatched BN limbs with TAC (1 mg/kg/day, a dose used in prior studies utilizing this animal model (30)) administered intraperitoneally until postoperative day (POD) 20 followed by drug withdrawal. Group 3 (rejection controls, n=8): LEW recipients received full MHC-mismatched BN hindlimbs without TAC. The primary comparison in this study was between Groups 1 and 2, with Group 3 serving as a comparator to demonstrate the timeline and intensity of AR in the absence of immunosuppression.
Skin and muscle tissue samples were collected from hind limbs at 0, 3, 5, 7, 9, 11, and 20 days in RNAlater solution (Sigma-Aldrich, St. Louis, MO) in addition to peripheral blood samples, and all were stored at -80°C until analysis. Total protein isolation and determination were carried out using the BCA protein assay kit from Pierce (Rockford, IL) with bovine serum albumin as standard as previously described (31). Rat inflammatory mediators were measured using a Luminex™ MagPix apparatus (Luminex, Austin, TX) and antibody kit (EMD Millipore Kit, Billerica, MA) as per manufacturer’ s specifications. The antibody bead kit included: Eotaxin (CCL11), Granulocyte Colony-Stimulating Factor (G-CSF), Granulocyte Macrophage Colony-Stimulating factor (GM-CSF), Keratinocyte-derived Cytokine (Gro-α/KC/CXCL1), Interferon-γ (IFN-γ), Interleukin (IL)- 1α, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12p70, IL-13, IL-17A, IFN-γ– inducible Protein 10 (IP-10/CXCL10), Leptin, Monocyte Chemoattractant Protein (MCP-1/CCL2), Macrophage Inflammatory Protein-1α (MIP-1α/CCL3), MIP-2/Gro-β, Tumor Necrosis Factor-α (TNF-α), Regulated on Activation, Normal T cell Expressed and Secreted (RANTES/CCL5), and Vascular Endothelial Growth Factor (VEGF). The final mediator concentrations are expressed in pg/mg protein (skin and muscle) or pg/ml (peripheral blood) as indicated. Experimental data are presented as mean ± SEM.
Rat limb allografts were assessed daily for evidence of rejection by inspection. Skin rejection was classified per appearance according to a 5‐point Banff grading scale: grade 0—no signs of rejection; grade I—erythema; grade II—erythema and edema; grade III—epidermolysis; grade IV—mummification and necrosis (32, 33). Skin and muscle tissue samples (4 mm punch) were collected from hind limbs at 0, 3, 5, 7, 9, 11, 20, 23, 25, 27 and 31 days (with time points past 20 days being obtained only in order to define the degree of protection from AR afforded by TAC histologically, but not used in computational analyses). Animals were sacrificed at specific time points until the 31-day study end point or upon Grade III Banff AR – whichever occurred earlier. Specimens of allograft skin and muscle were formalin‐fixed and paraffin‐embedded or snap‐frozen in liquid nitrogen. Sections were H&E stained and evaluated for lymphocytic infiltration, dermal/epidermal interphase reaction and necrosis by a pathologist in a blinded fashion.
Time-dependent changes of inflammatory mediator levels were assessed for significance using One-Way Analysis of Variance (ANOVA). Comparison between experimental groups (syngeneic-control vs. allogeneic-TAC) was carried out by Two-Way ANOVA followed by Holm-Sidak post hoc test (significance set at P<0.05) using Sigma Plot (Systat Software, San Jose, CA) as indicated.
Dynamic Bayesian Network (DyBN) inference (34) is a technique to define the most likely single network structure that best characterizes the dynamic interactions among systemic inflammatory mediators across all time points, in the process suggesting likely feedback structures that define central nodes. The networks may also suggest possible mechanisms by which the progression of the inflammatory response differs within a given experimental subgroup. In this analysis, time courses of unprocessed inflammatory mediator measurements were used as input for a DyBN inference algorithm (34), implemented in MATLAB® essentially as described previously (34) and modified by our group for the study of systemic acute inflammation (35–37). In brief, given time-series data, DyBN analysis provides a way of inferring causal relationships among variables (e.g. inflammatory mediators) based on probabilistic measure. Unlike standard correlative approaches, DyBNs consider the joint distribution of the entire dataset when making inferences about the dependencies between variables or nodes in the network. The values of each node are assumed to be distributed according to a chosen model (e.g. Gaussian) and the relationships among nodes are defined by the structure of the directed network and the corresponding conditional probability distributions of the interacting nodes. Network structure is inferred by a sampling technique that iteratively proposes candidate structures and evaluates them based on how well they fit the observed data using a specified scoring criterion, until reaching convergence on a network structure with the highest score. The algorithm uses an inhomogeneous dynamic changepoint model, with a Bayesian Gaussian with score equivalence (BGe) scoring criterion. The output of the aforementioned algorithm is a final graph structure indicating the interactions. Central/high-feedback nodes are those that exhibit self-feedback in addition to being connected to other nodes. Feedback loops can be important in systemic inflammation for control/resolution.
Dynamic Network Analysis (DyNA) (38) was aimed to define the central inflammatory network nodes as a function of both time and treatment in a granular fashion over time intervals; and unlike DyBN, DyNA is deterministic and cannot represent feedback structures but is useful for defining hub nodes at distinct time intervals. Rats underwent hind-limb VCA along hind-limb VCA along with syngeneic and allogeneic groups as described and peripheral blood samples were obtained at postoperative 0, 3, 5, 7, 9, 11, and 20 days. Using inflammatory mediator measurements of three time points for each experimental group, networks were created over two consecutive periods (day 3-5, day 5-7, day 9-11, and day 11-20) using MATLAB® software; data were not binned. Connections ([edges], or number of trajectories of inflammatory mediators that move in parallel [black edges = positive correlations] or in an anti-parallel [red edges = negative correlations] fashion) were created if the Pearson correlation coefficient between any two nodes (inflammatory mediators) at the same time interval was greater or equal to a threshold of an absolute value of 0.7, indicating a strong correlation. The network connectivity/complexity for each time interval was calculated using the following formula: Sum (N1 + N2 +… + Nn)/(n−1), where N represents the number of connections for each mediator, and n is the total number of mediators analyzed.
Principal component analysis (PCA) (39) was carried out to identify the inflammatory mediators that contributed the most to the overall variance of the response in peripheral blood of rats that underwent hind-limb VCA along with syngeneic and allogeneic groups (0-20 days) using normalized data, as described (38). To represent the 3D-PCA results for the first three main components, we employed MetaboAnalyst, a web-based tool suite developed for comprehensive metabolomic data analysis that also supports a wide array of functions for statistical, functional, as well as data visualization tasks (https://www.metaboanalyst.ca). To allow for comparison of experimental groups and tissue sources with similar variance, we calculated the contribution of each mediator to the overall variance (99.99%) and then selected those (highlighted in colored boxes) contributing to the top 25% variance of the inflammatory response in each tissue in each group as reported previously (40), using MATLAB® software (The MathWorks, Inc., Natick, MA). Data were not binned.
Skin and muscle biopsies at POD 31 from Group 1 (syngeneic transplants) (Figure 1B) revealed no inflammatory infiltrates in epidermis or dermis [Figure 1B (1)] as well as in the muscle [Figure 1B (2)]. Animals in Group 2 (allogeneic transplants with TAC) were followed until POD 31 following withdrawal of TAC at POD 20 (Figure 1C). Despite absence of clinical signs of rejection, the epidermis showed mild perivascular infiltration without epidermal dyskeratosis or apoptosis (Figure 1C (3), white arrow). The deep dermis showed mild to moderate peri-adnexal lymphocyte infiltration with involvement of hair follicles (Figure 1C (3) as well as muscle (Figure 1C (4); Grade I to II Banff AR [indicated as “I” and “II”]). Figure 1A (Group 3) shown here for comparison only is a skin (1) and muscle (2) biopsy from an allogeneic transplant without TAC, maintained only out to 11 days due to animal care regulations. These transplants in Group 3 undergo complete rejection by 7 +/- 2 days after surgery with epidermolysis and hair loss (white arrow), and necrosis of epidermis with severe, dense inflammation in upper and deep dermis and muscle, respectively (Grade II to IV Banff AR) [Figure 1A (1 and 2)].
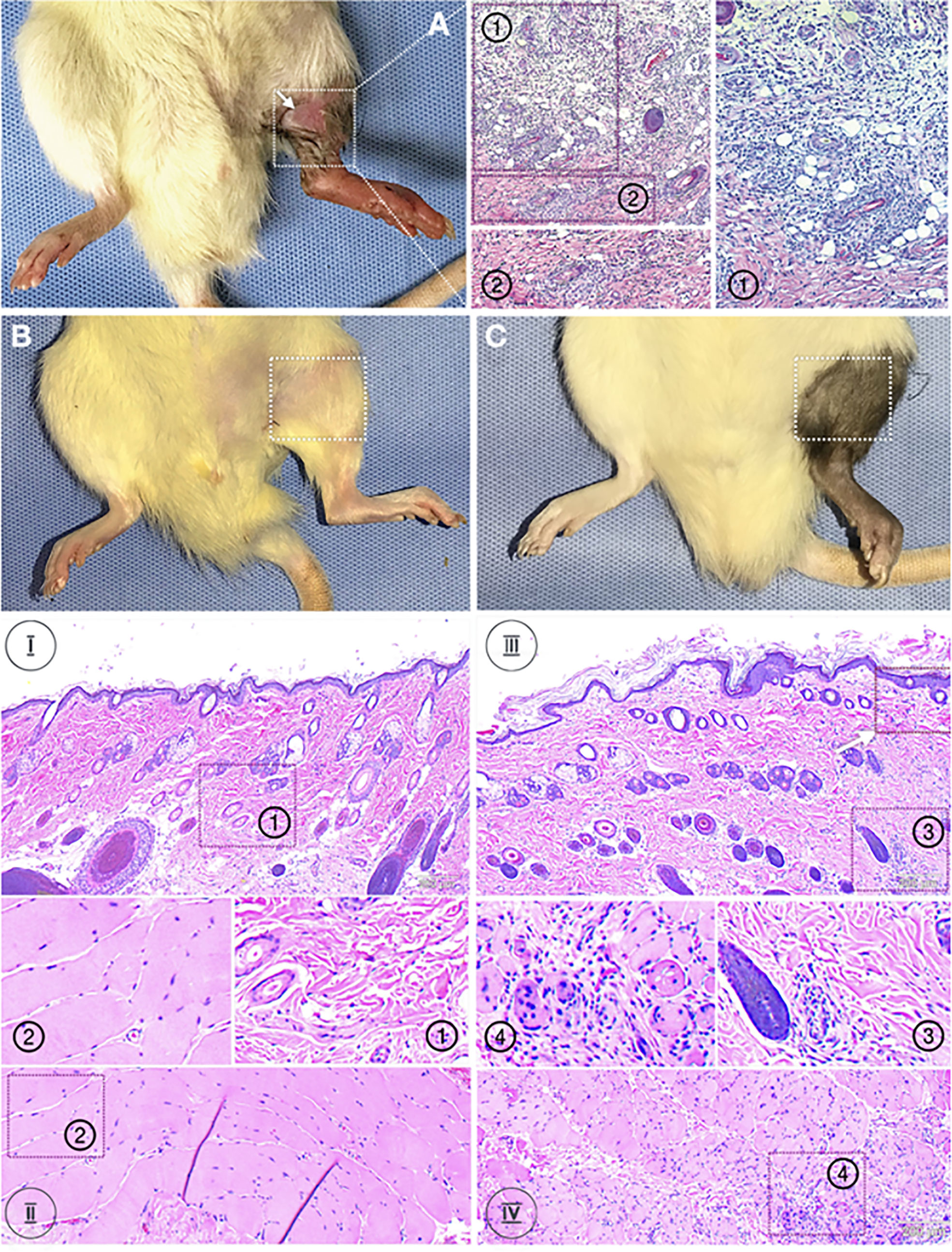
Figure 1 Histology of skin and muscle samples. Animals were randomized into two experimental groups: Group 1 (n=8, syngeneic controls; LEW recipients receiving MHC-matched LEW limbs without TAC. Group 2 (n=8, treatment group) LEW recipients received full MHC-mismatched BN limbs with TAC administered until postoperative day 20 followed by drug withdrawal. Representative animals from each group were euthanized and VCA tissues analyzed for inflammatory and other pathology. Panel (A): a skin (1) and muscle (2) biopsy from an allogeneic transplant without TAC. These transplants undergo complete rejection by 7 +/- 2 days after surgery with epidermolysis and hair loss (white arrow), and necrosis of epidermis with severe, dense inflammation in upper and deep dermis and muscle, respectively (Grade II to IV Banff AR) (1 and 2). Panel (B): Group 1 (syngeneic transplants). Skin and muscle biopsies at POD 31 revealed no inflammatory infiltrates in the epidermis, dermis, or muscle. Panel (C): Group 2 (allogeneic transplants with TAC) were followed until POD 31 following withdrawal of TAC at POD 20. Despite absence of clinical signs of rejection, the epidermis showed mild perivascular infiltration without epidermal dyskeratosis or apoptosis (white arrow). The deep dermis showed mild to moderate peri-adnexal lymphocyte infiltration with involvement of hair follicles as well as muscle (Grade I to II Banff AR).
We next sought to define dynamic changes in inflammatory mediators in the skin, muscle, and systemic circulation in Group 2 (representing the clinical scenario) versus Group 1 (syngeneic) controls. One-Way ANOVA of time-courses showed that animals receiving TAC had the greatest temporal change in inflammatory mediators in peripheral blood (17/27 [63%]), followed by skin (14/27 [52%]) and muscle (11/27 [41%]) (Supplementary Table 1). The muscle was the tissue with the greatest degree of temporal inflammatory changes in the syngeneic control group (13/27 mediators [52%]) with fewer changes in skin and peripheral blood (7/27 mediators [44%] each) (Supplementary Table 1). Furthermore, comparison by Two-Way ANOVA of Groups 2 vs. 1 revealed significant differences in multiple inflammatory mediators, as shown in Supplementary Figure 2.
We used DyBN inference to highlight putative feedbacks among variables and to identify inflammatory mediators that might act as central controllers of VCA-induced inflammation. We considered as central those nodes that exhibit self-feedback and connect to other nodes (i.e., these nodes were high-feedback nodes). Based on this definition, IL-1α was a central node in skin samples from Group 2 animals receiving TAC, upstream of IL-18, RANTES (which all exhibited mutual cross-interactions) as well as multiple additional downstream mediators (Figure 2A, upper panel). In contrast, IL-1α, IL-18, and leptin appeared as central nodes in the skin of Group 1 (syngeneic) animals (Figure 2A, lower panel). This network pattern was interconnected with multiple additional mediators, suggesting a central inflammatory role for pathways involving leptin, pyroptosis, and the NLRP3 inflammasome. Leptin and VEGF were central nodes in the muscle of Group 2, interconnected with IL-1α and IL-18 as well as multiple other inflammatory mediators (Figure 2B, upper panel). In comparison, VEGF was the sole central node in the muscle of Group 1 animals, with two-way connections to IFN-γ and MCP-1 along with various connections to multiple inflammatory mediators (Figure 2B, lower panel). These results suggest the possible role of tissue ischemia (VEGF) along with the leptin pathway.
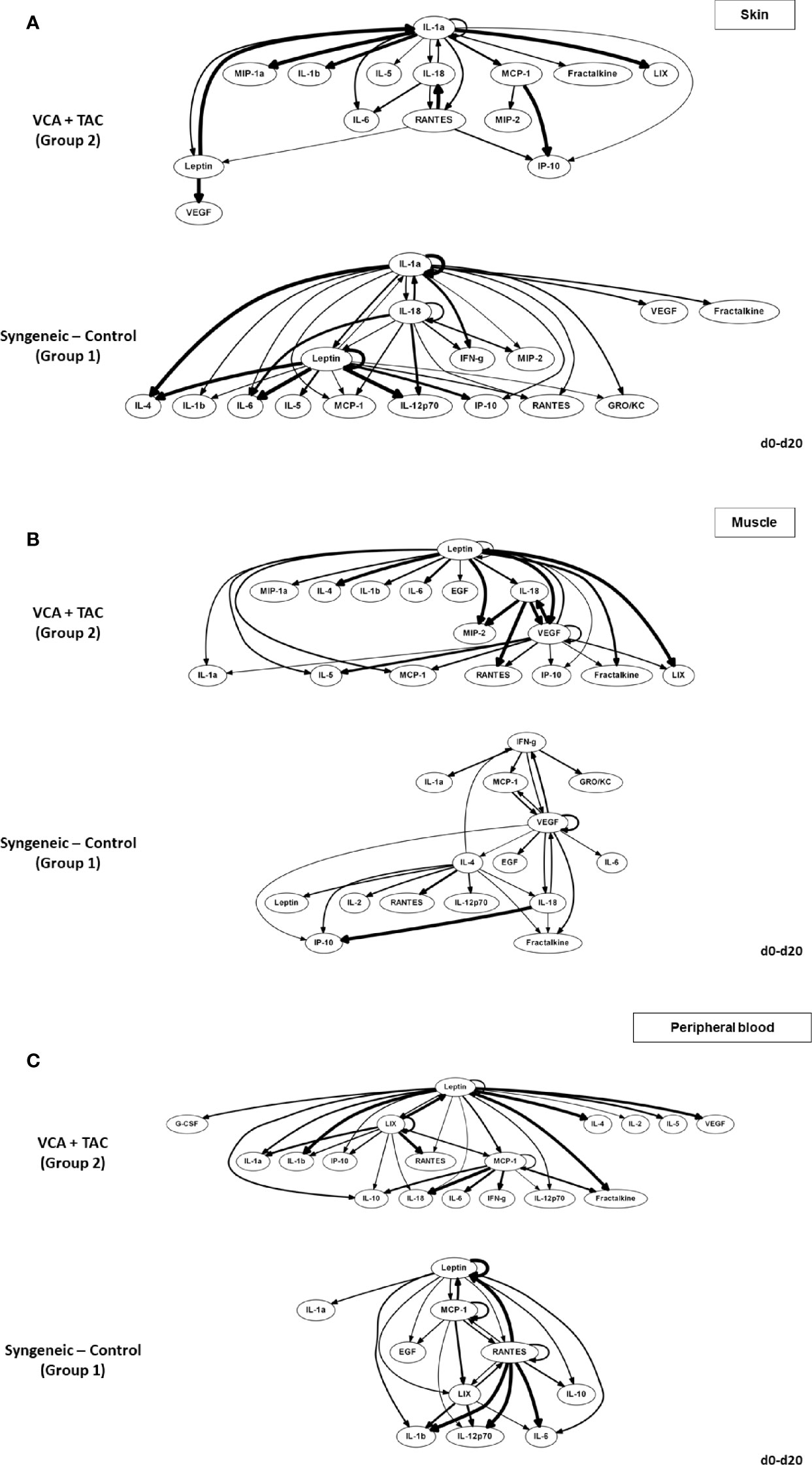
Figure 2 Differential DyBN network patterns in rats undergoing VCA + TAC vs. Syngeneic Control. LEW rat recipients received full MHC-mismatched BN limbs with TAC (1 mg/kg/day, i.p.) until postoperative day 20 followed by drug withdrawal as described in Materials and Methods. LEW rat recipients that received MHC-matched LEW limbs without TAC served as control. Peripheral blood, skin and muscle tissue samples were collected at 0, 3, 5, 7, 9, 11, and 20 days and assayed for 27 inflammatory mediators using the rat multiplex Luminex™ assay followed by DyBN analysis as described in Materials and Methods. Panels (A–C) show the inflammatory networks for skin, muscle, and peripheral blood, respectively. This analysis suggested the presence of distinct central nodes in the skin, muscle, and peripheral circulation of animals undergoing VCA + TAC as compared to syngeneic transplant controls.
The systemic (peripheral blood) inflammatory response in the presence of TAC (Group 2) was inferred to involve leptin, LIX, and MCP-1 as central nodes, upstream of a complex and highly interconnected set of inflammatory mediators (Figure 2C, upper panel). Group 1 rats exhibited a systemic inflammatory response that was less complex, with leptin, MCP-1, and RANTES as central nodes (Figure 2C, lower panel). These results suggest a relatively similar core systemic inflammatory response in Group 1 and 2, albeit with a more complex overall response with TAC treatment.
We used DyNA for the granular visualization of the temporal evolution of inflammatory networks after VCA. Dynamic Networks were analyzed at POD 3-5, POD 5-7, POD 7-9, POD 9-11, and POD 11-20. In this analysis, inflammatory mediators are depicted as network nodes (red circles in Supplementary Figure 3), and the statistical correlation among nodes is depicted as edges (black or red lines interconnecting any two nodes, with black lines representing a positive correlation and red lines representing a negative correlation).
A key metric of these networks, namely network connectivity/complexity quantifies these qualitative impressions. Analysis of the dynamic connectivity/complexity of the inflammatory networks suggested that networks under TAC treatment (Group 2) evolve quite differently over time in skin, muscle, and peripheral blood as compared to without TAC (Group 1) (Figure 3). The general dynamic pattern in Group 2 suggested a high initial network connectivity/complexity in skin and muscle that was reduced by POD 7-9, but which then rose through POD 11-20 (Figure 3A). In contrast, inflammatory network connectivity/complexity in all tissues of syngeneic transplant (Group 1) animals was trending towards resolution over the full time period studied (Figure 3B). An analysis of the total number of network connections over time suggested a greater network connectivity in peripheral blood and skin of TAC treated vs. syngeneic transplant; however, the opposite pattern was observed in the muscle (Figure 3C). It was also interesting to note that the highest network connectivity in TAC-treated animals was found in the peripheral blood, while the highest network connectivity in syngeneic transplants was observed in the muscle (Figure 3C). The results are summarized in Table 1.
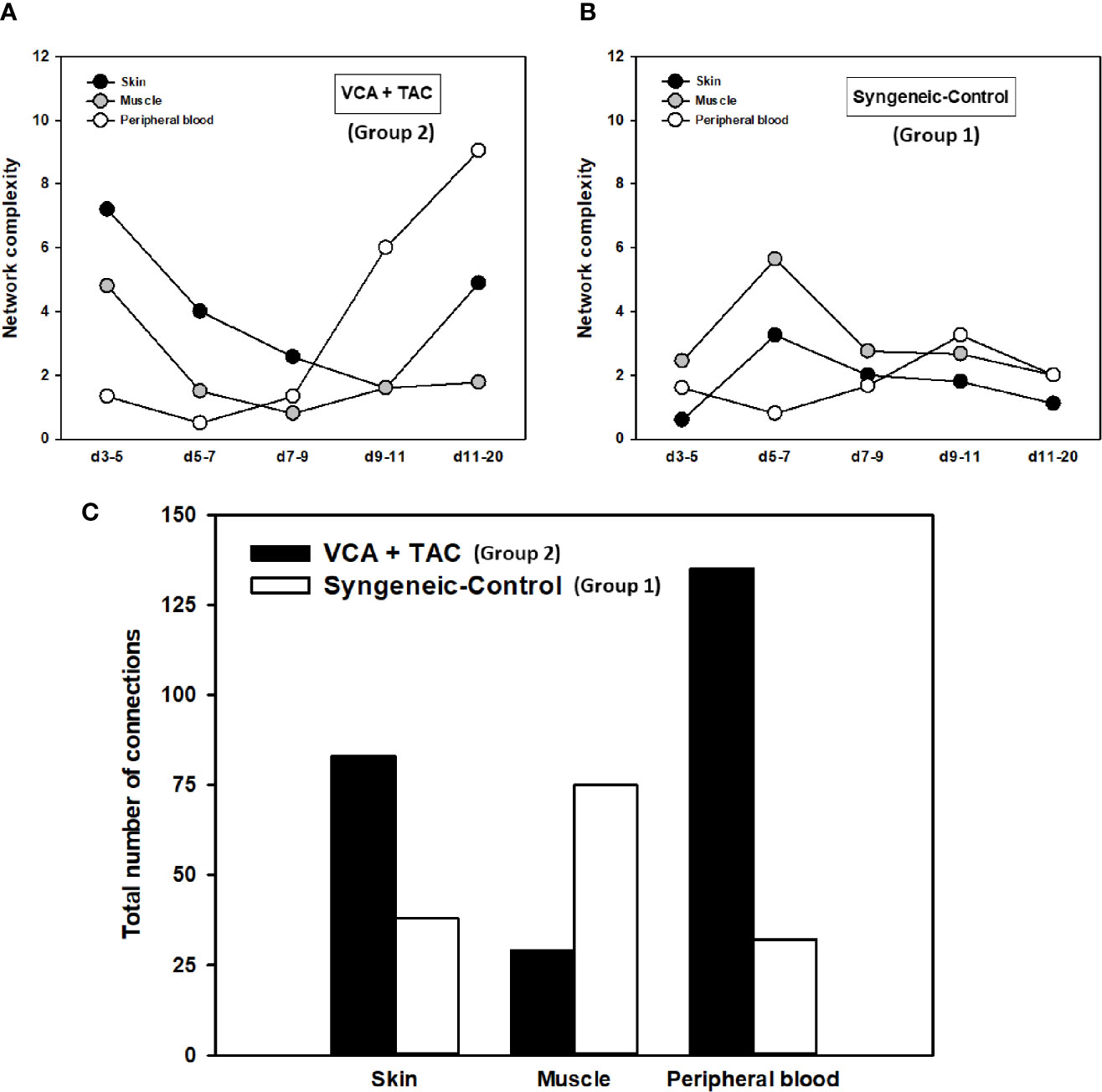
Figure 3 Dynamic inflammatory networks in skin, muscle and peripheral blood are characterized by unique time-dependent evolution after VCA. LEW rat recipients received full MHC-mismatched BN limbs with TAC (1 mg/kg/day, i.p.) until postoperative day 20 followed by drug withdrawal as described in Materials and Methods. LEW rat recipients that received MHC-matched LEW limbs without TAC served as control. Peripheral blood, skin and muscle tissue samples were collected at 0, 3, 5, 7, 9, 11, and 20 days and assayed for 27 inflammatory mediators using the rat multiplex Luminex™ assay followed by DyNA performed during five time-intervals as described in Materials and Methods. Figure shows the network complexity (stringency level 0.7) in skin, muscle, and peripheral blood in VCA+TAC (A) vs. Syngeneic-Control (B) and total number of connections (C) calculated as described in Materials and Methods. This analysis suggested the presence of distinct dynamic networks and network connectivity/complexity patterns in the skin, muscle, and peripheral circulation of animals undergoing VCA + TAC as compared to syngeneic transplant controls.
We used PCA to define the key variables contributing to the variance in a given dataset, especially a dataset which represent a dynamic process. An analysis using the first three principal components accounted for widely differing degrees of variance depending on the tissue assessed and suggested that the two experimental groups could not be separated (Supplementary Figure 4). Accordingly, we sought to account for a uniform degree of variance (99.9%) and then compare the top 25% of the mediators in each tissue in each group as reported previously (40) Based on this analysis, the skin (Figure 4A), muscle (Figure 4B), and peripheral blood (Figure 4C) in TAC-treated animals (Group 2) were inferred to involve a differential set of mediators in each tissue as compared to those observed after syngeneic transplants (Group 1). The results are summarized in Table 1.
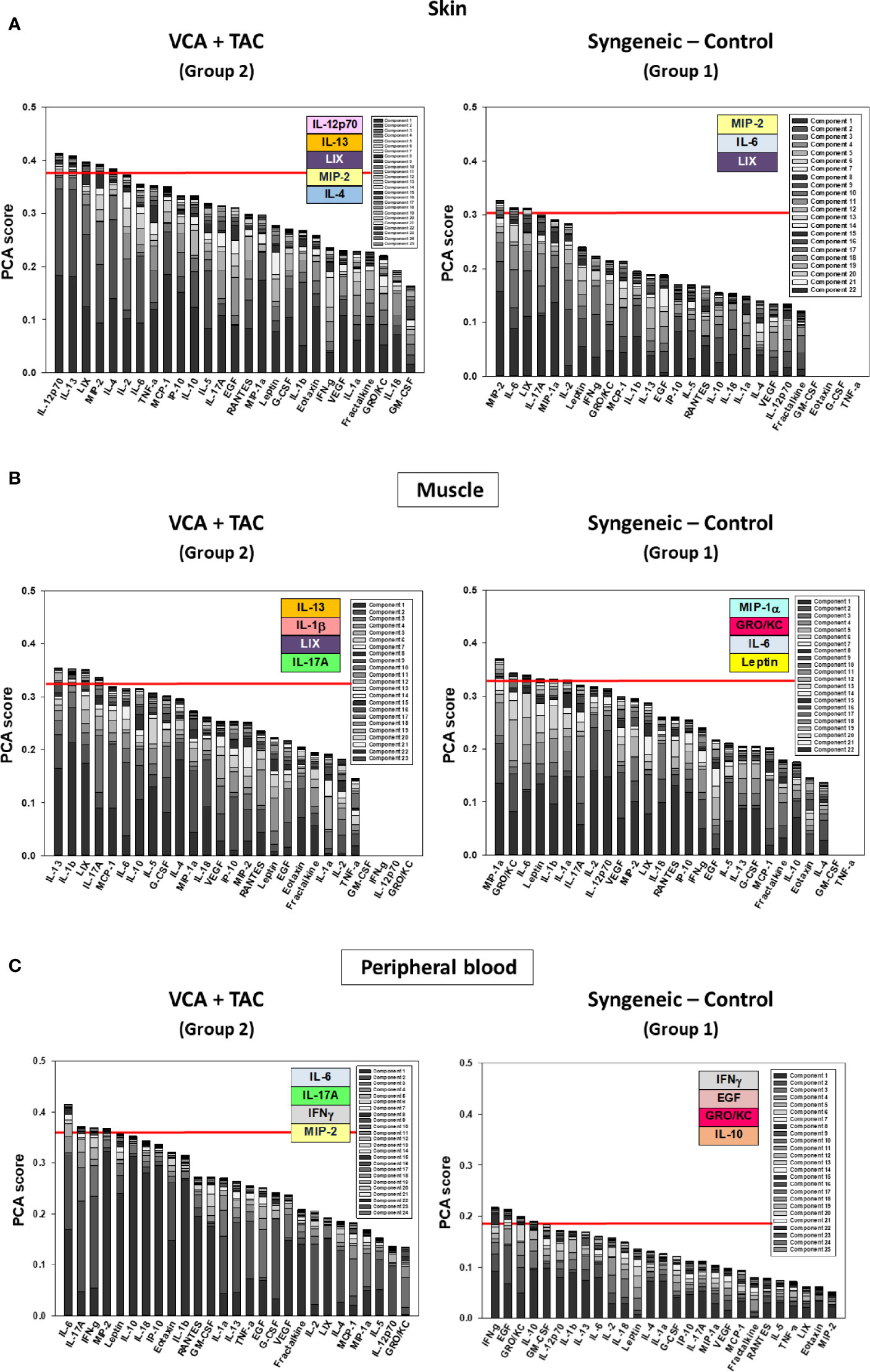
Figure 4 Principal Component Analysis (PCA) suggests differential inflammatory characteristics after VCA + TAC vs. Syngeneic-Control. LEW rat recipients received full MHC-mismatched BN limbs with TAC (1 mg/kg/day, i.p.) until postoperative day 20 followed by drug withdrawal as described in Materials and Methods. LEW rat recipients that received MHC-matched LEW limbs without TAC served as control. Peripheral blood, skin and muscle tissue samples were collected at 0, 3, 5, 7, 9, 11, and 20 days and assayed for 27 inflammatory mediators using the rat multiplex Luminex™ assay followed by PCA performed as described in Materials and Methods. The number of mediators contributing to the top 25% variance of the inflammatory response (shown above the red line) are highlighted in colored boxes in VCA + TAC vs. Syngeneic-Control in skin (A), muscle (Panel B) and peripheral blood (Panel C) as indicated.
A key aspect of VCA is the multi-tissue nature of the transplant. Previous studies (13, 28), as well our current findings thus far, have examined the inflammatory response in individual components (skin and muscle). However, it is likely that cross-tissue interactions mediate the inflammatory and immune response in the setting of VCA (41). However, it is difficult, if not impossible, to define experimentally and directly the tissue-specific inflammatory contributions of skin and muscle. We therefore next hypothesized that we could integrate the skin and muscle data on inflammatory cytokines using DyBN, DyNA, and PCA to define such cross-tissue interactions (in essence defining an “in silico VCA”).
DyBN of combined skin and muscle data supported the notion that there is complex, cross-tissue crosstalk in both TAC treated as well as syngeneic recipients. With TAC treatment, skin MCP-1, IL-1α, and IL-18, showed multiple connections to inflammatory mediators in skin and muscle combined (Figure 5A). Leptin, while not a central node, exhibited two-way feedback with IL-1α (Figure 5A). In syngeneic transplants, there were no discernible central mediators, though muscle IL-4 and skin IL-18 exhibited two-way interactions (Figure 5B). DyNA of the skin + muscle data suggested that, as inferred from the single-tissue data, inflammatory networks in VCA + TAC animals exhibited dropped to zero network connectivity/complexity between POD 5-9, and then increased to levels higher than those of syngeneic transplant animals through day 20. In contrast, inflammatory networks in syngeneic transplant animals trended towards resolution of inflammation over time (Figure 6). Detailed inflammatory networks are depicted in Supplementary Figure 5. Finally, PCA suggested a fairly similar overall degree of inflammatory activation with TAC treatment (Group 2) and without it (Group 1), but with different principal characteristics (Figure 7).
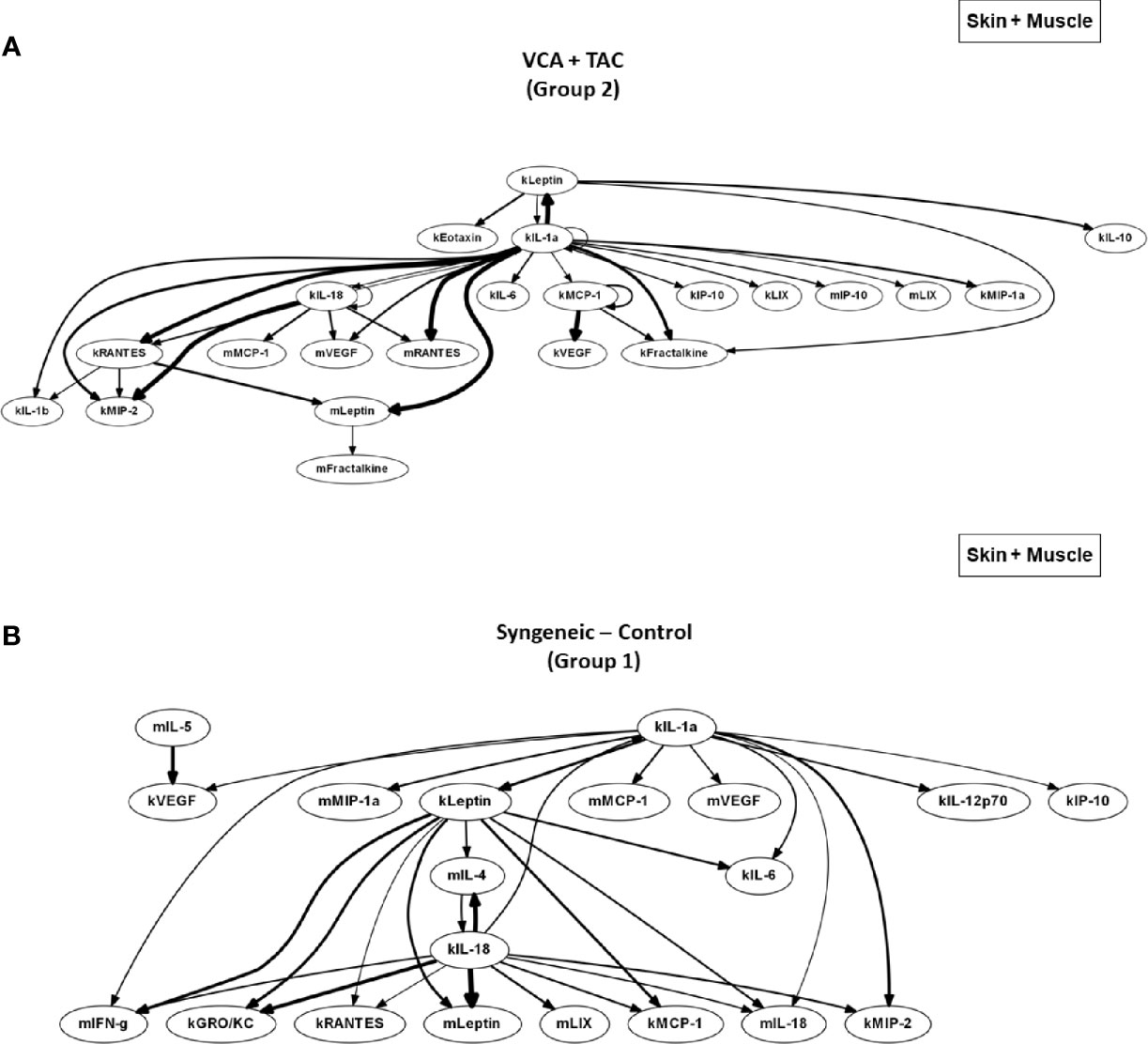
Figure 5 DyBN network patterns in rats undergoing VCA +TAC vs. Syngeneic Control. LEW rat recipients received full MHC-mismatched BN limbs with TAC (1 mg/kg/day, i.p.) until postoperative day 20 followed by drug withdrawal as described in Materials and Methods. LEW rat recipients that received MHC-matched LEW limbs without TAC served as control. Skin and muscle tissue samples were collected at 0, 3, 5, 7, 9, 11, and 20 days and assayed for 27 inflammatory mediators using the rat multiplex Luminex™ assay followed by DyBN analysis (skin and muscle data combined) as described in Materials and Methods. Panels (A, B) show the inflammatory networks for VCA + TAC and Syngeneic-Control, respectively.
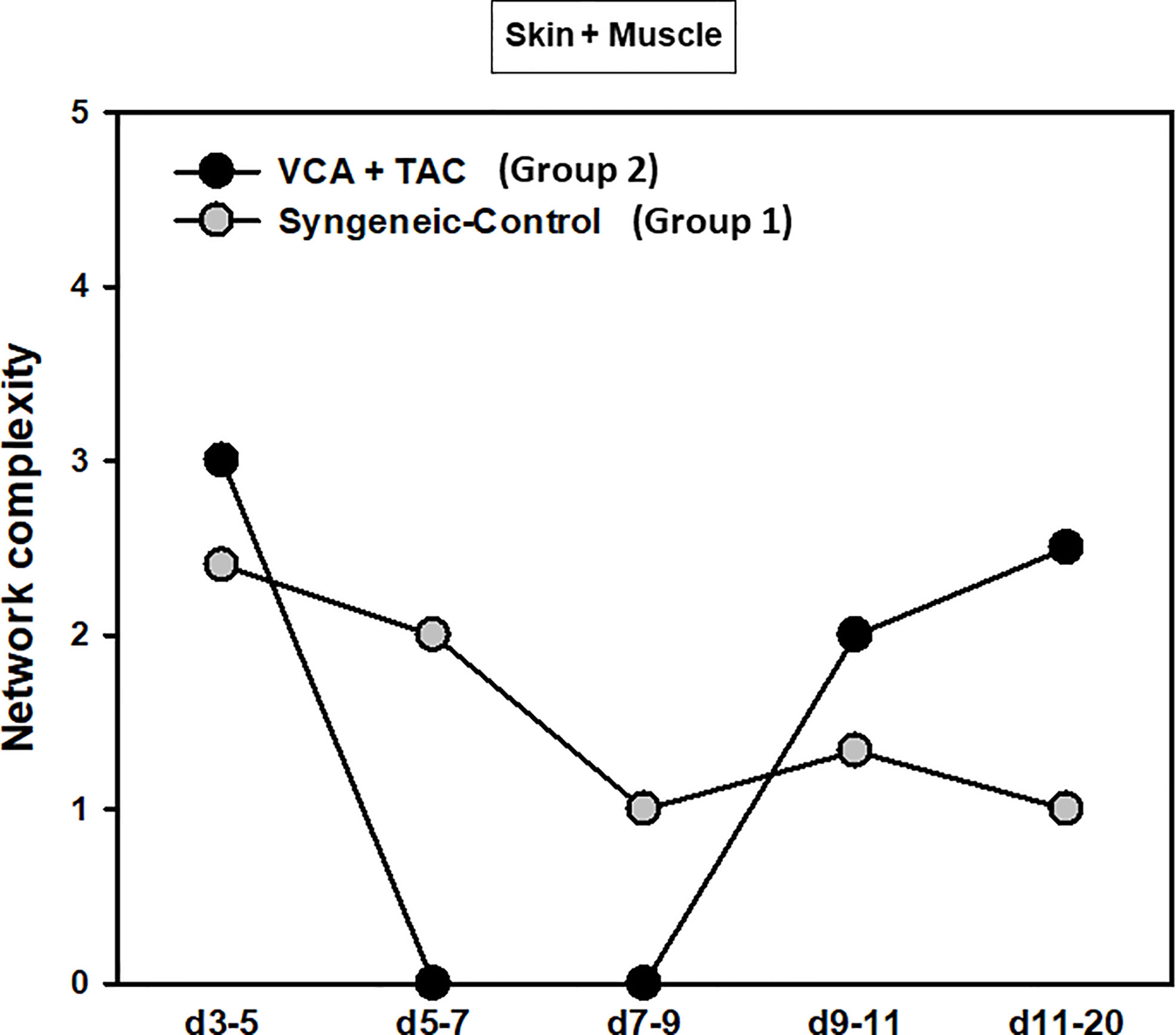
Figure 6 Dynamic inflammatory networks in skin and muscle are characterized by differential time-dependent evolution after VCA. LEW rat recipients received full MHC-mismatched BN limbs with TAC (1 mg/kg/day, i.p.) until postoperative day 20 followed by drug withdrawal as described in Materials and Methods. LEW rat recipients that received MHC-matched LEW limbs without TAC served as control. Skin and muscle tissue samples were collected at 0, 3, 5, 7, 9, 11, and 20 days and assayed for 27 inflammatory mediators using the rat multiplex Luminex™ assay followed by DyNA performed during five time-intervals as described in Materials and Methods. Figure shows the network complexity (stringency level 0.7) in skin and muscle (combined) in VCA+TAC vs. Syngeneic-Control calculated as described in Materials and Methods.
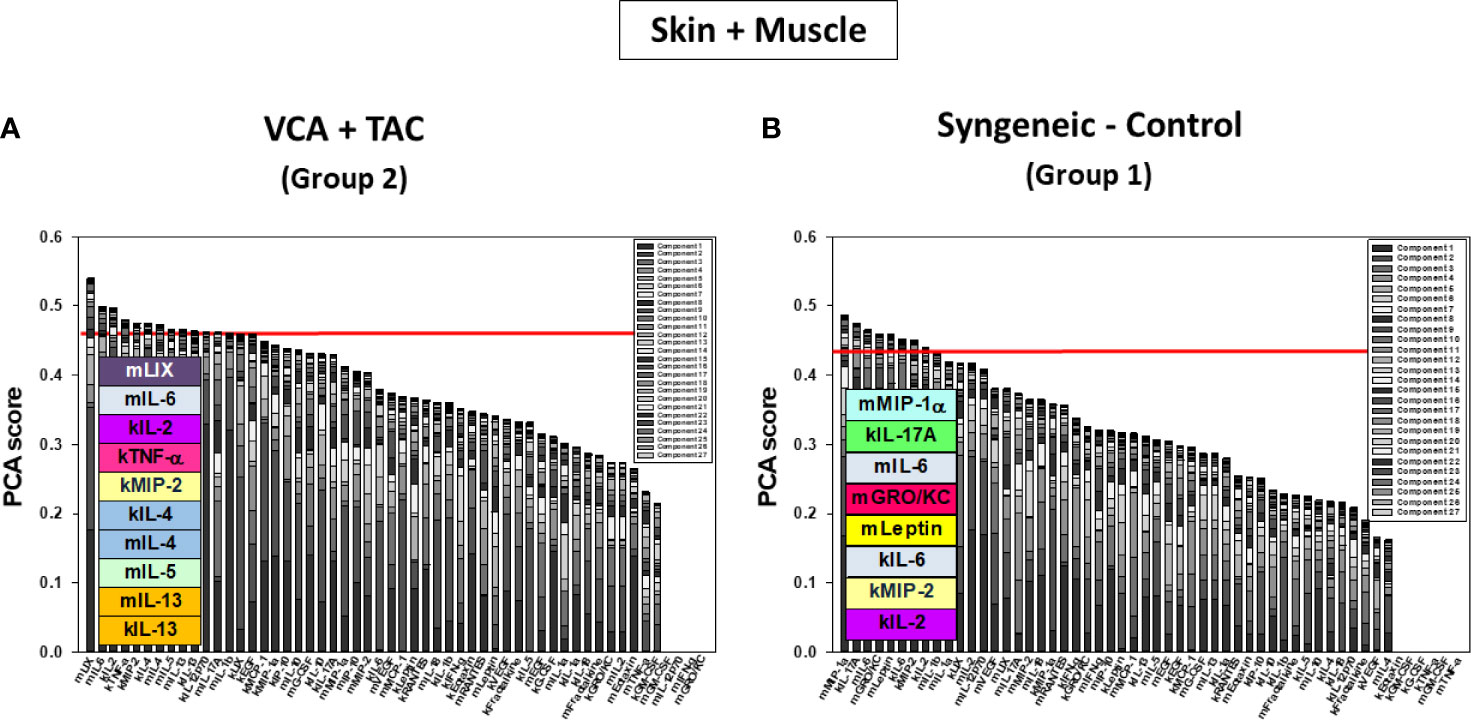
Figure 7 Principal Component Analysis (PCA) suggests differential inflammatory characteristics after VCA + TAC vs. Syngeneic-Control. LEW rat recipients received full MHC-mismatched BN limbs with TAC (1 mg/kg/day, i.p.) until postoperative day 20 followed by drug withdrawal as described in Materials and Methods. LEW rat recipients that received MHC-matched LEW limbs without TAC served as control. Skin and muscle tissue samples were collected at 0, 3, 5, 7, 9, 11, and 20 days and assayed for 27 inflammatory mediators using the rat multiplex Luminex™ assay followed by PCA performed as described in Materials and Methods. The number of mediators contributing to the top 25% variance of the inflammatory response (shown above the red line) are highlighted in colored boxes in skin and muscle (combined) in VCA + TAC (A) vs. Syngeneic-Control (B) as indicated.
The expression of CYP3A5 genes that encode monooxygenases important for TAC metabolism in vivo is negligible in VCA components such as the skin, skeletal muscle, or adipose tissue as compared to the liver or intestine, which are the major sites of TAC metabolism (42). Therefore, differences in pharmacokinetics and pharmacodynamics of TAC across different tissues, combined with differential partitioning of TAC in peripheral blood (RBC or lymphocytes) versus tissues may underlie the complex interplay of cytokines and chemokines that drive immune rejection in VCA (43).We therefore integrated the skin and muscle data with data on the systemic (peripheral blood) response of inflammatory mediators using DyBN, DyNA, and PCA (in essence defining an “in silico animal”), with the goal of inferring local and systemic cross-talk and potentially gaining insights into these complex effects of TAC.
DyBN of skin + muscle + peripheral blood (virtual animal) data suggested a complex inflammasome interaction after TAC treatment (Group 2). Peripheral blood Leptin was found as the primary central node with high influence. Peripheral blood LIX was also a central node with fewer connections (Figure 8A). However, in syngeneic control animals, fewer inflammatory mediators were involved. Central nodes were peripheral blood Leptin, LIX, and RANTES, as well as skin IL-18 (Figure 8B). DyNA of skin + muscle + peripheral blood showed high initial network connectivity/complexity in VCA, resolving partially by POD 7-9 and rising at later time points (Figure 8C). However, in the syngeneic control group, network connectivity/complexity was relatively constant over time (Figure 8C). Detailed inflammatory networks are depicted in Supplementary Figure 6. Finally, PCA suggested a similar overall degree of inflammatory activation in both VCA + TAC and syngeneic transplant, but with different principal characteristics (Figure 9).
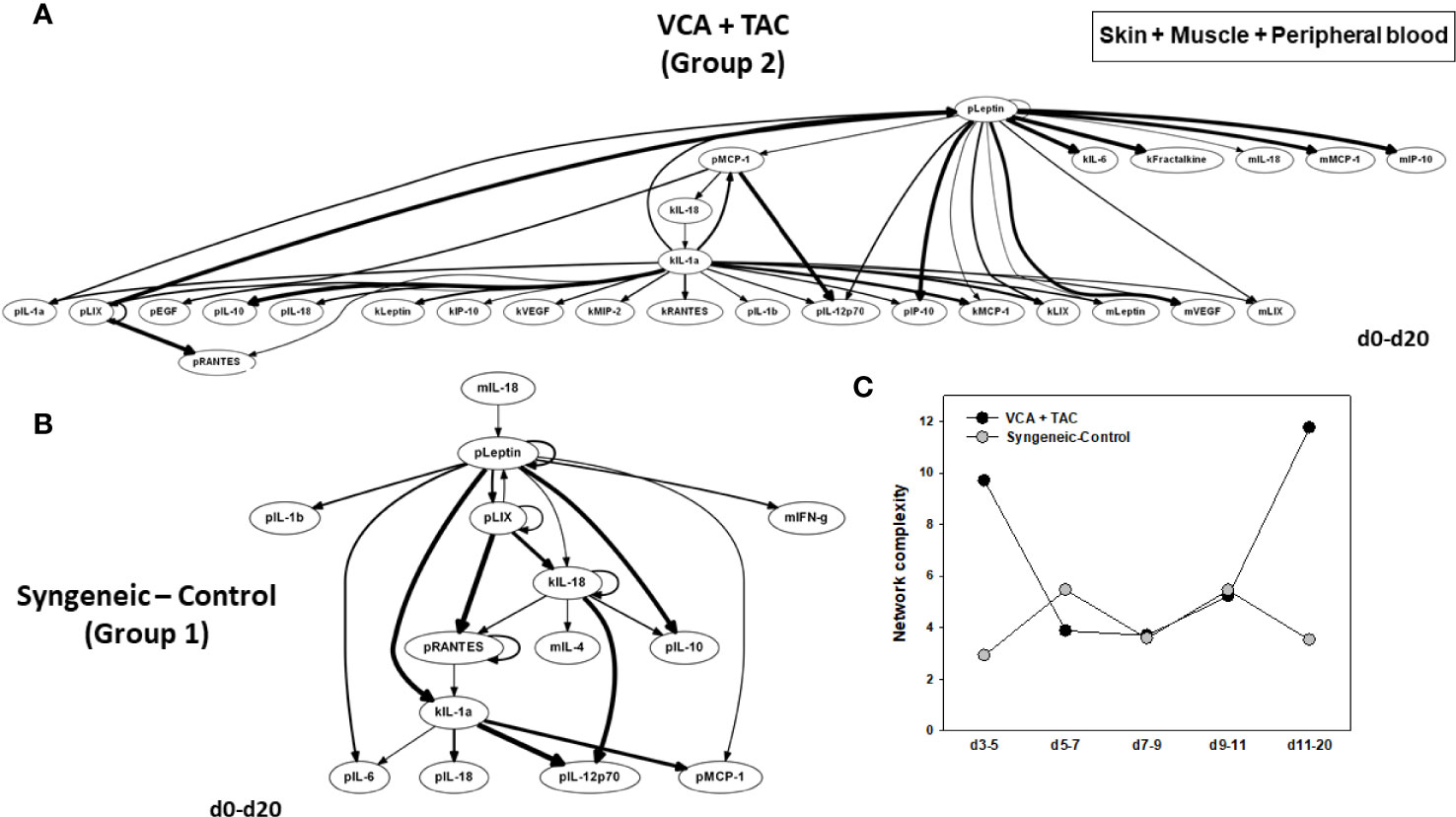
Figure 8 Differential inflammatory network patterns in rats undergoing VCA +TAC vs. Syngeneic Control. LEW rat recipients received full MHC-mismatched BN limbs with TAC (1 mg/kg/day, i.p.) until postoperative day 20 followed by drug withdrawal as described in Materials and Methods. LEW rat recipients that received MHC-matched LEW limbs without TAC served as control. Peripheral blood, skin and muscle tissue samples were collected at 0, 3, 5, 7, 9, 11, and 20 days and assayed for 27 inflammatory mediators using the rat multiplex Luminex™ assay followed by DyBN analysis and DyNA as described in Materials and Methods. Panels (A, B) show the DyBNs for skin, muscle and peripheral blood (combined) in VCA + TAC and Syngeneic-Control, respectively. Panel (C) shows the network complexity (stringency level 0.7) in skin, muscle, and peripheral blood (combined) in VCA+TAC vs. Syngeneic-Control as indicated.
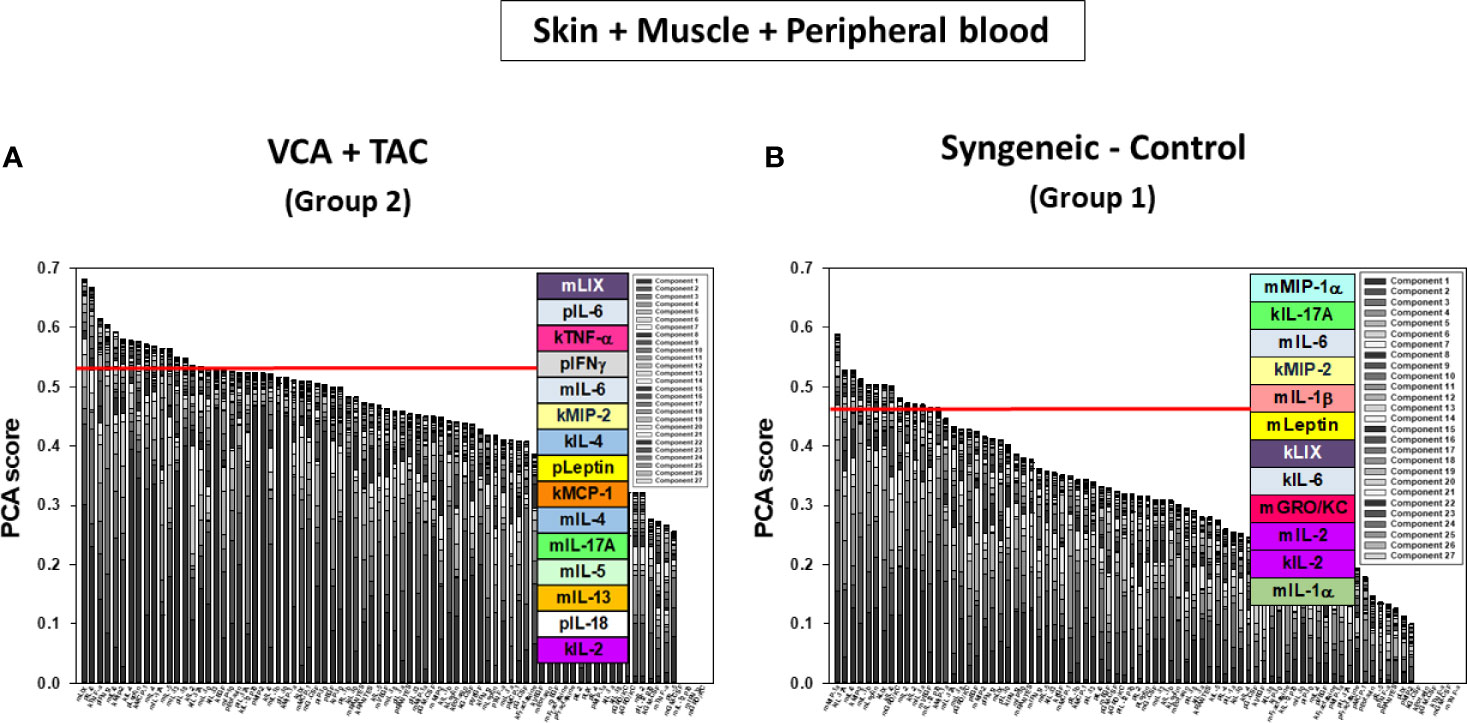
Figure 9 Principal Component Analysis (PCA) suggests differential inflammatory characteristics after VCA + TAC vs. Syngeneic-Control. LEW rat recipients received full MHC-mismatched BN limbs with TAC (1 mg/kg/day, i.p.) until postoperative day 20 followed by drug withdrawal as described in Materials and Methods. LEW rat recipients that received MHC-matched LEW limbs without TAC served as control. Peripheral blood, skin and muscle tissue samples were collected at 0, 3, 5, 7, 9, 11, and 20 days and assayed for 27 inflammatory mediators using the rat multiplex Luminex™ assay followed by PCA performed as described in Materials and Methods. Figure shows the number of mediators contributing to the top 25% variance of the inflammatory response (above the red line) for skin, muscle, and peripheral blood (combined) in VCA + TAC (A) vs. Syngeneic-Control (B) as indicated.
Our group has previously utilized various machine learning approaches to define the hallmarks and functional modules of acute inflammation in animal models of trauma, sepsis, and transplantation (25, 28, 38, 44–46). In the current study, we examined the singular characteristics of inflammation in the setting of VCA, in order to discern the key drivers of local tissue and systemic inflammation as well as the effect of TAC on such responses. Findings from the present study largely concur with our earlier published studies of protein-level mediators of inflammation in skin and muscle of small animal limb VCA (28) and, as discussed below, with a multitude of studies on various aspects of transplantation. Thus, inflammatory patterns associated with AR are specific for the individual tissue and may serve as superior surrogates for early detection and targeted treatment of AR. Furthermore, we utilized dynamic network inference to unify data across tissues and the circulation, demonstrating the capability of these machine learning tools to unify data across space and time.
We used TAC in our study as it is a key drug in the majority of VCA and solid-organ transplant regimens. Studies have shown that TAC suppresses TNF-α-induced MCP-1 and IP-10 expression via the inhibition of p38 MAP kinase activation. However, TAC does not suppress TNF-α-induced IL-6 expression (47). In the present studies, we found that inflammation evolves differently in animals treated with TAC vs. syngeneic controls, as reflected and quantified in their network complexity. In syngeneic control animals (Group 1), the inflammatory response is least complex in the peripheral blood, followed by skin and muscle. In contrast, in TAC-treated rats, the exact opposite network complexity pattern was observed. Furthermore, the connectivity/complexity of the systemic inflammatory response (i.e. in the peripheral blood) was much higher in TAC-treated rats; in contrast, dynamic network connectivity/complexity in TAC-treated rats was lower in the muscle of TAC treated rats, and initially higher and later lower in the skin. Taken together, we interpret these results to suggest that TAC has both local and systemic effects, namely that it suppresses inflammation effectively in the muscle but at the cost of increasing systemic inflammation. We hypothesize that the skin, known to be highly immunogenic in the setting of VCA, plays a modulating role, and that TAC impacts that process. These differences in network complexity may be related to the numbers of T cells in each tissue, which are the main cells targeted by TAC to control inflammation in transplant, and a key source of the inflammatory mediators assessed in the present study. This hypothesis is supported by the differential degree of inflammatory infiltrates observed in our histopathologic analyses; future studies will aim to define the cell sub-populations in each tissue.
The main achievement of this study was the in silico unification of data across skin, muscle, and the peripheral circulation. Combining skin and muscle data yielded an “in silico VCA,” while the addition of peripheral blood data arguably yielded a virtual animal (though the typical usage of the term “virtual organism” centers on the use of mechanistic computational modeling, a framework wherein it is possible to simulate behaviors outside of the data used to calibrate the model) (48, 49). We have recently published a detailed, cross-tissue examination of acute inflammation induced by Gram-negative bacterial lipopolysaccharide (LPS) in both wild type mice and mice lacking the main receptor for LPS (Toll-like receptor 4) (40). While that study focused on multiple internal organs as well as the systemic circulation, neither skin nor muscle were assessed, nor were the data integrated to define true cross-tissue inflammatory circuits. In addition, LPS challenge is a simpler experimental paradigm as compared to VCA. The results of the computational tissue integration analyses suggest extensive cross-tissue interactions between skin and muscle, with a key regulatory role for circulating inflammatory mediators such as leptin.
A literature review indicates that currently there are no in vitro or in silico assays which would comprehensively represent rejection following VCA. The rodent hind limb transplant VCA used in this study is a robust, reliable, and reproducible model that has been validated independently for over 3 decades (13, 50). The rat hind limb is comprised of multiple tissues (skin, muscle, vessel, lymphatics, lymph nodes, bone, cartilage, bone marrow, nerve and adipose tissue) and mimics a clinical VCA such as hand or face transplant. Given its composite tissue nature, this experimental model allows investigation of complex variables in local/systemic inflammation such as surgical trauma, IRI, and the immunogenic effects of multiple allogeneic tissues. Indeed, the stringency of this experimental model was required in order to study the effects of TAC comprehensively. The inflammatory manifestations of surgical trauma or immune rejection occur at the molecular, cellular, tissue and systemic levels. The initial innate immune response involves the release of cytokines, chemokines, and damage-associated molecular proteins (DAMPs) (51), which we have implicated previously as being central to AR using mathematical modeling (17). In the current study, syngeneic transplants (Group 1) were used to study the surgical trauma triggered inflammatory response in the absence of an alloresponse, and allogeneic transplants with TAC (Group 2) helped elucidate the response of surgical trauma induced inflammation and antigen-dependent adaptive immune responses to TAC immunosuppressive therapy. Rejection responses in these experimental groups were compared histopathologically to those of allogeneic transplants without TAC (Group 3, used to study the antigen-dependent immune response that lead to rejection following VCA). Our results suggest that machine learning approaches can elucidate dynamic networks of local and systemic inflammation associated with alloresponses and TAC immunosuppression, which ultimately likely impact AR and possibly also CR.
Unlike in solid organs, antigen presenting cells (APCs) such as Langerhans cell (LC) and keratinocyte components in the skin make this tissue a highly immunogenic barrier to management with conventional immunosuppression. Graft LCs and donor dendritic cells migrate to the local draining lymph nodes (DLNs) after transplantation, where they prime T cells for direct presentation of allogeneic major histocompatibility (MHC) antigens. Later, graft-infiltrating recipient DCs pick up donor major and minor histocompatibility antigens, move back to DLNs, and cross-prime T cells via the indirect pathway. The effector T cell response to such LC and DC priming is key to initiation and perpetuation of the host-versus-graft immune response. Early AR in VCA is mediated predominantly by a cross-communication between CD8+ T effector and CD4+ T helper cells. CD4+ T cells also trigger B cell-mediated alloantibody production responsible for late AR, and humoral rejection involving antibody-dependent cellular cytotoxicity or chronic allograft vasculopathy, leading to ischemic graft loss (5, 52). On the other hand, native CD4+ T helper cells can differentiate into Th1 and Th2 subsets (53). Secreted mediators from APCs, such as IL-12, trigger secretion of Th1 and Th2 cytokines such as IFN-γ, IL-2, TNFα; IL-4, IL-10, and IL-13 (54), which modulate cellular immunity and also affect macrophage and T cell proliferation and function. Results of PCA in the skin of Group support these mechanisms, with top mediators including IL-12p70, IL-13, and IL-4.
One key mediator inferred by PCA as a primary characteristic of inflammation in Group 2 muscle was IL-17A. Transplant rejection is associated with a predominance of Th1 and Th17 responses over Th2 responses (55–58). In a cohort study of face transplants, it was found that IFN-γ, IL-17A, and Th1 as well as Th17 cells were elevated significantly in rejecting grafts (59). Interestingly, a Th1/Th17 pattern was also seen in inflammatory skin conditions such as psoriasis or lupus, possibly because Leptin suppresses regulatory T cells and enhances Th17 cells (60). IL-4-secreting Th cells were found to be protective in kidney transplant recipients (61). In addition, tissue trauma causes suppression of Th1 responses and induces Th2 responses with selective cellular suppression (62).
Using DyBN inference in our experimental VCA setting, we identified IL-1α, IL-18, IL-1β, and IL-4 as principal characteristics associated with, or potential drivers of, transplant rejection. Interestingly, IL-1α is an allograft-induced DAMP (63) that has been implicated in IL-17A production in an experimental model of transplant rejection (64). Interleukins-1β and -18 implicate the NLRP3 inflammasome, known to be involved in transplant rejection (65). Another cytokine associated with transplant rejection is IL-4, possibly due to its role in promoting M2 macrophage differentiation and function (66). We also found evidence suggesting that Leptin and IL-18 were leading central nodes and potential drivers interacting with pyroptosis and NLRP inflammasome in the skin, whereas Leptin was also involved in a pro-healing/M2 program via IL-4, IL-5, and IL-13 in muscle. Leptin is secreted by adipocytes, the placenta, and the stomach (67). The cytokine receptor family includes Leptin receptors, (68). Leptin is known to increase acutely as a response to inflammatory triggers (69). Leptin levels, as well as IL-1β and IL-6, increase in burn injury, which suggests that Leptin may be related to pro-inflammatory cytokines (including a Th1 phenotype shift) (70). Indeed, Leptin injection reverses the immunosuppressive effect of burn injury (71). Leptin levels also increase after sepsis and septic shock, probably as a host mechanism to defend against bacterial infection (72). Leptin modulates nonspecific cytokine responses mediated by macrophages and is required for complement mediated activity of neutrophils (73). Leptin promotes VEGF, IL-6. and PGE2 production (74). Most importantly, and relevant to our current study, high concentrations of Leptin in serum constitute an independent risk factor for the development of AR in kidney transplants (75).
DyNA suggests that inflammatory network complexity was decreased in POD 5-9 in skin and muscle; however, peripheral blood had intermittent inflammation from POD Days 0-20. Based on DyBN inference, the surgical inflammation following syngeneic transplants (Group 1) showed a similar systemic response as with allogeneic transplants receiving TAC, but the inflammatory response was simpler. DyNA suggested that inflammatory networks in the skin and muscle were more complex at earlier time points, whereas the network in peripheral blood was more complex in the middle time points. In the VCA + TAC setting (Group 1), we found IL-1α in skin, IL-18 in muscle, and Leptin in peripheral blood as central nodes in DyBN (data not shown). DyNA showed decreasing complexity in peripheral blood, contrary to complexity in skin and muscle were increasing over time. The impact of IL12p70 that has already been shown to be a potential early diagnostic marker for AR was not as pronounced in the TAC-treated animals as compared to the syngeneic or allogeneic transplants without TAC. Interferon-γ and IL-4 had a high ranking in all groups, indicating a non-specific effect in inflammation. In contrast, IL-1α and IL-18 exhibited an expression profile which indicates a possible key role in VCA rejection and makes these cytokines interesting candidates for therapeutic interventions (28).
Our results in individual tissues are in agreement with prior studies from our group and others utilizing various statistical approaches to define predictors of AR in the context of experimental VCA. We have used multiplexed analysis technology to study protein levels of 27 inflammatory mediators in skin and muscle biopsies as well as peripheral blood samples from syngeneic and allogeneic limb transplants without immunosuppression and limb allografts treated with TAC, with the goal of creating predictive statistical models. We examined the levels of these inflammatory analytes at different postoperative days, with a focus on the early postoperative phase where no histological alterations were observed in any of the three groups (POD 3 and 5) (28). Initially, non-parametric univariate analysis of the inflammatory mediators from skin and muscle were performed. Five inflammatory mediators (GM-CSF, IL1-α, IL-4, IL-12p70, IL-5, TNF-α) were significantly different at least in one group (adjusted p<0.05; Kruskal-Wallis test [KW]) in both skin and muscle. IL-12p70 and TNF-α were highly significantly different in the allograft versus the tacrolimus-treated animals (28). For identification of inflammatory mediators with the highest predicting value, at early time points, multivariate analyses were performed, suggesting that GM-CSF, IL-4, IL-12p70, IL-5, and TNF-α could be promising biomarkers of AR. The prediction accuracy of these models within skin was 87.1% and in muscle 100%, and a pairwise multivariate logistic regression analysis between the study groups (Group 1 vs. Group 2, Group 2 vs. Group 1 and Group 1 vs. Group 1) and applying a leave-one-out cross validation strategy resulted in an area under curve (AUC) from receiver operating characteristics (ROC) for skin of 0.5, 0.69, and 0.86 and for muscle of 1.0, 1.0, and 1.0) (28). In related work, a recent study in syngeneic and allogeneic groin flap VCAs analyzed gene and protein mediators on POD 2 and 5 using quantitative real-time PCR and multiplex analyses to delineate whether injury-induced inflammation triggers allograft rejection. IL-18, IFNγ, IP-10/CXCL10, RANTES/CCL5, Gro-α/CXCL1, and IL-10 were upregulated in the allogeneic group while remaining unchanged in the syngeneic group (76).
A primary study limitation was the short duration of follow-up of animals for 20 days for multiplex collection/analyses of tissue and peripheral blood samples. However, our goal was to identify the nature, pattern, and timeline of inflammatory mediator expression and complex network interactions occurring early after VCA during the prime time window that encompasses inflammatory responses secondary to surgical trauma, ischemia/reperfusion injury and AR. Other important limitations are those inherent to the computational methods employed. For example, DyBN inference assumes a fixed network structure over the full time course; while this allows for the inference of central nodes that feedback upon themselves and affect downstream nodes, the assumption of a fixed network structure may be incorrect or insufficient. In contrast, while DyNA allows for network inference over defined time intervals, this method does not allow for the direct inference of nodes involved in self-feedback. Hence, these methods were utilized in concert.
In conclusion, this study extends initial work characterizing dynamic inflammatory changes in rats undergoing VCA (27, 28). We have defined protein-level dynamic networks of inflammation in skin, muscle, and the systemic circulation in the context of experimental VCA and gained insights into the potential impact of TAC. Together, we believe that our comprehensive, computational, and systems biology analysis of the production and dynamic interactions of key mediators during the onset, evolution, and progression of inflammation/rejection after VCA with and without the influence of TAC could define novel strategies for non-invasive surrogate monitoring, graft specific immunomodulation, and optimization of immunologic and functional graft survival outcomes.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
The animal study was reviewed and approved by Institutional Animal Care and Use Committee (IACUC) of the University of Pittsburgh and the Department of Defense Animal Care and Utilization Review Office (ACURO).
AA performed animal experiments and wrote manuscript. RZ performed computational analyses and wrote manuscript. DB performed Luminex analyses. JY performed Luminex analysis. FE-D performed Luminex analysis. VE performed animal experiments. LD performed animal experiments. ZZ performed animal experiments. HS performed animal experiments. VG conceived studies and wrote manuscript. YV conceived studies and wrote manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by Dept. of Defense grant W81 XWH-15-1-0336 (YV, PI).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2021.591154/full#supplementary-material
1. Shores JT, Brandacher G, Lee WP. Hand and Upper Extremity Transplantation: An Update of Outcomes in the Worldwide Experience. Plast Reconstr Surg (2015) 135(2):351e–60e. doi: 10.1097/PRS.0000000000000892
2. Gorantla VS, Plock JA, Davis MR. Reconstructive Transplantation: Evolution, Experience, Ethics, and Emerging Concepts. In: Anesthesia and Perioperative Care for Organ Transplantation. New York, NY: Springer (2016). doi: 10.1007/978-1-4939-6377-5_44
3. Jusko WJ, Piekoszewski W, Klintmalm GB, Shaefer MS, Hebert MF, Piergies AA, et al. Pharmacokinetics of Tacrolimus in Liver Transplant Patients. Clin Pharmacol Ther (1995) 57(3):281–90. doi: 10.1016/0009-9236(95)90153-1
4. Gorantla VS, Barker JH, Jones JW Jr., Prabhune K, Maldonado C, Granger DK. Immunosuppressive Agents in Transplantation: Mechanisms of Action and Current Anti-Rejection Strategies. Microsurgery (2000) 20(8):420–9. doi: 10.1002/1098-2752(2000)20:8<420::AID-MICR13>3.0.CO;2-O
5. Chandraker A, Arscott R, Murphy GF, Lian CG, Bueno EM, Marty FM, et al. The Management of Antibody-Mediated Rejection in the First Presensitized Recipient of a Full-Face Allotransplant. Am J Transplant (2014) 14(6):1446–52. doi: 10.1111/ajt.12715
6. Kaufman CL, Breidenbach W. World Experience After More Than a Decade of Clinical Hand Transplantation: Update From the Louisville Hand Transplant Program. Handb Clin (2011) 27(4):417–21, vii-viii. doi: 10.1016/j.hcl.2011.08.004
7. Kaufman CL, Ouseph R, Blair B, Kutz JE, Tsai TM, Scheker LR, et al. Graft Vasculopathy in Clinical Hand Transplantation. Am J Transplant (2012) 12(4):1004–16. doi: 10.1111/j.1600-6143.2011.03915.x
8. Krezdorn N, Lian CG, Wells M, Wo L, Tasigiorgos S, Xu S, et al. Chronic Rejection of Human Face Allografts. Am J Pathol (2018) 19(4):1168–77. doi: 10.1111/ajt.15143
9. Morelon E, Petruzzo P, Kanitakis J, Dakpe S, Thaunat O, Dubois V, et al. Face Transplantation: Partial Graft Loss of the First Case 10 Years Later. Am J Transplant (2017) 17(7):1935–40. doi: 10.1111/ajt.14218
10. Brandacher G, Lee WP, Schneeberger S. Minimizing Immunosuppression in Hand Transplantation. Expert Rev Clin Immunol (2012) 8(7):673–83; quiz 684. doi: 10.1586/eci.12.54
11. Schnider JT, Weinstock M, Plock JA, Solari MG, Venkataramanan R, Zheng XX, et al. Site-Specific Immunosuppression in Vascularized Composite Allotransplantation: Prospects and Potential. Clin Dev Immunol (2013) 2013:495212. doi: 10.1155/2013/495212
12. Sellares J, de Freitas DG, Mengel M, Reeve J, Einecke G, Sis B, et al. Understanding the Causes of Kidney Transplant Failure: The Dominant Role of Antibody-Mediated Rejection and Nonadherence. Am J Transplant (2012) 12(2):388–99. doi: 10.1111/j.1600-6143.2011.03840.x
13. Lee WP, Yaremchuk MJ, Pan YC, Randolph MA, Tan CM, Weiland AJ. Relative Antigenicity of Components of a Vascularized Limb Allograft. Plast Reconstr Surg (1991) 87(3):401–11. doi: 10.1097/00006534-199103000-00001
14. Ho AW, Kupper TS. T Cells and the Skin: From Protective Immunity to Inflammatory Skin Disorders. Nat Rev Immunol (2019) 19(8):490–502. doi: 10.1038/s41577-019-0162-3
15. Gorantla VS, Demetris AJ. Acute and Chronic Rejection in Upper Extremity Transplantation: What Have We Learned? Handb Clin (2011) 27(4):481–93, ix. doi: 10.1016/j.hcl.2011.08.006
16. Schneeberger S, Gorantla VS, van Riet RP, Lanzetta M, Vereecken P, van Holder C, et al. Atypical Acute Rejection After Hand Transplantation. Am J Transplant (2008) 8(3):688–96. doi: 10.1111/j.1600-6143.2007.02105.x
17. Day J, Metes D, Vodovotz Y. Mathematical Modeling of Early Cellular Innate and Adaptive Immune Responses to Ischemia-Reperfusion Injury and Solid Organ Allotransplantation. Front Immunol (2015) 6:484. doi: 10.3389/fimmu.2015.00484
18. Namas R, Ghuma A, Torres A, Polanco P, Gomez H, Barclay D, et al. An Adequately Robust Early TNF-a Response is a Hallmark of Survival Following Trauma/Hemorrhage. PloS One (2009) 4(12):e8406. doi: 10.1371/journal.pone.0008406
19. Neunaber C, Zeckey C, Andruszkow H, Frink M, Mommsen P, Krettek C, et al. Immunomodulation in Polytrauma and Polymicrobial Sepsis - Where do We Stand? Recent Pat Inflamm Allergy Drug Discovery (2011) 5(1):17–25. doi: 10.2174/187221311794474892
20. Xiao W, Mindrinos MN, Seok J, Cuschieri J, Cuenca AG, Gao H, et al. A Genomic Storm in Critically Injured Humans. J Exp Med (2011) 208(13):2581–90. doi: 10.1084/jem.20111354[doi
21. Huang X, Moore DJ, Ketchum RJ, Nunemaker CS, Kovatchev B, McCall AL, et al. Resolving the Conundrum of Islet Transplantation by Linking Metabolic Dysregulation, Inflammation, and Immune Regulation. Endocr Rev (2008) 29(5):603–30. doi: 10.1210/er.2008-0006
22. Vitalone MJ, Naesens M, Sigdel T, Li L, Hseih S, Sarwal MM. The Dual Role of Epithelial-to-Mesenchymal Transition in Chronic Allograft Injury in Pediatric Renal Transplantation. Transplantation (2011) 92(7):787–95. doi: 10.1097/TP.0b013e31822d092c
23. Antony AK, Rodby K, Tobin MK, O’Connor MI, Pearl RK, Dipietro LA, et al. Composite Tissue Allotransplantation and Dysregulation in Tissue Repair and Regeneration: A Role for Mesenchymal Stem Cells. Front Immunol (2013) 4:188. doi: 10.3389/fimmu.2013.00188
24. Messner F, Fischer AC, Runggaldier E, Sprung S, Muller J, Eiter S, et al. Mechanical Irritation in Vascularized Composite Tissue Allotransplantation Triggers Localized Skin Rejection. Transplantation (2020) 104(5):956–69. doi: 10.1097/TP.0000000000003075
25. Vodovotz Y., An G. Translational Systems Biology: Concepts and Practice for the Future of Biomedical Research. New York, NY: Elsevier (2014).
26. Vodovotz Y, Csete M, Bartels J, Chang S, An G. Translational Systems Biology of Inflammation. PLoS Comput Biol (2008) 4:1–6. doi: 10.1371/journal.pcbi.1000014
27. Zamora R, Ravuri SK, Plock JA, Vodovotz Y, Gorantla VS. Differential Inflammatory Networks Distinguish Responses to Bone Marrow-Derived Versus Adipose-Derived Mesenchymal Stem Cell Therapies in Vascularized Composite Allotransplantation. J Trauma Acute Care Surg (2017) 83(1 Suppl 1):S50–8. doi: 10.1097/ta.0000000000001489
28. Wolfram D, Starzl R, Hackl H, Barclay D, Hautz T, Zelger B, et al. Insights From Computational Modeling in Inflammation and Acute Rejection in Limb Transplantation. PloS One (2014) 9(6):e99926. doi: 10.1371/journal.pone.0099926
29. Sucher R, Oberhuber R, Margreiter C, Rumberg G, Jindal R, Lee WP, et al. Orthotopic Hind-Limb Transplantation in Rats. J Vis Exp (2010) 41):2022. doi: 10.3791/2022
30. Olariu R, Denoyelle J, Leclère FM, Dzhonova DV, Gajanayake T, Banz Y, et al. Intra-Graft Injection of Tacrolimus Promotes Survival of Vascularized Composite Allotransplantation. J Surg Res (2017) 218:49–57. doi: 10.1016/j.jss.2017.05.046
31. Metukuri MR, Beer-Stolz D, Namas RA, Dhupar R, Torres A, Loughran PA, et al. Expression and Subcellular Localization of BNIP3 in Hypoxic Hepatocytes and Liver Stress. Am J Physiol Gastrointest Liver Physiol (2009) 296(3):G499–509. doi: 10.1152/ajpgi.90526.2008[doi
32. Schneider M, Cardones AR, Selim MA, Cendales LC. Vascularized Composite Allotransplantation: A Closer Look At the Banff Working Classification. Transpl Int (2016) 29(6):663–71. doi: 10.1111/tri.12750
33. Cendales LC, Kanitakis J, Schneeberger S, Burns C, Ruiz P, Landin L, et al. The Banff 2007 Working Classification of Skin-Containing Composite Tissue Allograft Pathology. Am J Transplant (2008) 8(7):1396–400. doi: 10.1111/j.1600-6143.2008.02243.x
34. Grzegorczyk M, Husmeier D. Improvements in the Reconstruction of Time-Varying Gene Regulatory Networks: Dynamic Programming and Regularization by Information Sharing Among Genes. Bioinformatics (2011) 27(5):693–9. doi: 10.1093/bioinformatics/btq711
35. Azhar N, Ziraldo C, Barclay D, Rudnick DA, Squires RH, Vodovotz Y, et al. Analysis of Serum Inflammatory Mediators Identifies Unique Dynamic Networks Associated With Death and Spontaneous Survival in Pediatric Acute Liver Failure. PloS One (2013) 8(11):e78202. doi: 10.1371/journal.pone.0078202
36. Emr B, Sadowsky D, Azhar N, Gatto LA, An G, Nieman GF, et al. Removal of Inflammatory Ascites is Associated With Dynamic Modification of Local and Systemic Inflammation Along With Prevention of Acute Lung Injury: In Vivo and in Silico Studies. Shock (2014) 41(4):317–23. doi: 10.1097/SHK.0000000000000121
37. Zaaqoq AM, Namas R, Almahmoud K, Azhar N, Mi Q, Zamora R, et al. Inducible protein-10, a Potential Driver of Neurally Controlled interleukin-10 and Morbidity in Human Blunt Trauma. Crit Care Med (2014) 42(6):1487–97. doi: 10.1097/CCM.0000000000000248
38. Mi Q, Constantine G, Ziraldo C, Solovyev A, Torres A, Namas R, et al. A Dynamic View of Trauma/Hemorrhage-Induced Inflammation in Mice: Principal Drivers and Networks. PloS One (2011) 6:e19424. doi: 10.1371/journal.pone.0019424
39. Janes KA, Yaffe MB. Data-Driven Modelling of Signal-Transduction Networks. Nat Rev Mol Cell Biol (2006) 7(11):820–8. doi: 10.1038/nrm2041
40. Zamora R, Korff S, Mi Q, Barclay D, Schimunek L, Zucca R, et al. A Computational Analysis of Dynamic, Multi-Organ Inflammatory Crosstalk Induced by Endotoxin in Mice. PloS Comput Biol (2018) 14(11):e1006582. doi: 10.1371/journal.pcbi.1006582
41. Leonard DA, Kurtz JM, Cetrulo CL Jr. Vascularized Composite Allotransplantation: Towards Tolerance and the Importance of Skin-Specific Immunobiology. Curr Opin Organ Transplant (2013) 18(6):645–51. doi: 10.1097/mot.0000000000000022
42. Koch I, Weil R, Wolbold R, Brockmöller J, Hustert E, Burk O, et al. Interindividual Variability and Tissue-Specificity in the Expression of Cytochrome P450 3A Mrna. Drug Metab Dispos (2002) 30(10):1108–14. doi: 10.1124/dmd.30.10.1108
43. Brunet M, van Gelder T, Åsberg A, Haufroid V, Hesselink DA, Langman L, et al. Therapeutic Drug Monitoring of Tacrolimus-Personalized Therapy: Second Consensus Report. Ther Drug Monit (2019) 41(3):261–307. doi: 10.1097/ftd.0000000000000640
44. Namas R, Namas R, Lagoa C, Barclay D, Mi Q, Zamora R, et al. Hemoadsorption Reprograms Inflammation in Experimental Gram-negative Septic Fibrin Peritonitis: Insights From In Vivo and in Silico Studies. Mol Med (2012) 18:1366–74. doi: 10.2119/molmed.2012.00106
45. Nieman K, Brown D, Sarkar J, Kubiak B, Ziraldo C, Vieau C, et al. A Two-Compartment Mathematical Model of Endotoxin-Induced Inflammatory and Physiologic Alterations in Swine. Crit Care Med (2012) 40:1052–63. doi: 10.1097/CCM.0b013e31823e986a
46. Fontes PM, Vodovotz ,Y, Lopez ,R, van der Plaats ,A, Light WR, Minnervini M, et al. Liver Preservation With Machine Perfusion and a Newly Developed Cell-Free Oxygen Carrier Solution Under Subnormothermic Conditions. Am J Transplant (2015) 15(2):381–94. doi: 10.1111/ajt.12991
47. Aomatsu T, Imaeda H, Takahashi K, Fujimoto T, Kasumi E, Yoden A, et al. Tacrolimus (FK506) Suppresses TNF-α-Induced CCL2 (Mcp-1) and CXCL10 (Ip-10) Expression Via the Inhibition of P38 MAP Kinase Activation in Human Colonic Myofibroblasts. Int J Mol Med (2012) 30(5):1152–8. doi: 10.3892/ijmm.2012.1094
48. Li NY, Verdolini K, Clermont G, Mi Q, Rubinstein EN, Hebda PA, et al. A Patient-Specific in Silico Model of Inflammation and Healing Tested in Acute Vocal Fold Injury. PloS One (2008) 3(7):e2789. doi: 10.1371/journal.pone.0002789
49. Brown D, Namas RA, Almahmoud K, Zaaqoq A, Sarkar J, Barclay DA, et al. Trauma in Silico: Individual-specific Mathematical Models and Virtual Clinical Populations. Sci Transl Med (2015) 7(285):285ra61. doi: 10.1126/scitranslmed.aaa3636
50. Unadkat JV, Schneeberger S, Horibe EH, Goldbach C, Solari MG, Washington KM, et al. Composite Tissue Vasculopathy and Degeneration Following Multiple Episodes of Acute Rejection in Reconstructive Transplantation. Am J Transplant (2010) 10(2):251–61. doi: 10.1111/j.1600-6143.2009.02941.x
51. Zettel KR BT II. Disorder of Systemic Inflammation in Sepsis and Trauma: A Systems Perspective. In: Complex Systems and Computational Biology Approaches to Acute Inflammation. New York: Springer (2013). doi: 10.1007/978-1-4614-8008-2_6
52. Weissenbacher A, Hautz T, Zelger B, Zelger BG, Mayr V, Brandacher G, et al. Antibody-Mediated Rejection in Hand Transplantation. Transpl Int (2014) 27(2):e13–7. doi: 10.1111/tri.12233[doi
53. Marik PE, Flemmer M. The Immune Response to Surgery and Trauma: Implications for Treatment. J Trauma Acute Care Surg (2012) 73(4):801–8. doi: 10.1097/TA.0b013e318265cf87
54. Elenkov IJ. Systemic Stress-Induced Th2 Shift and its Clinical Implications. Int Rev Neurobiol (2002) 52:163–86. doi: 10.1016/S0074-7742(02)52009-2
55. Li XC, Zand MS, Li Y, Zheng XX, Strom TB. On Histocompatibility Barriers, Th1 to Th2 Immune Deviation, and the Nature of the Allograft Responses. J Immunol (1998) 161(5):2241–7.
56. Fiorentino DF, Bond MW, Mosmann TR. Two Types of Mouse T Helper Cell. IV. Th2 Clones Secrete a Factor That Inhibits Cytokine Production by Th1 Clones. J Exp Med (1989) 170(6):2081–95. doi: 10.1084/jem.170.6.2081
57. D’Elios MM, Josien R, Manghetti M, Amedei A, de Carli M, Cuturi MC, et al. Predominant Th1 Cell Infiltration in Acute Rejection Episodes of Human Kidney Grafts. Kidney Int (1997) 51(6):1876–84. doi: 10.1038/ki.1997.256
58. van Loosdregt J, van Oosterhout MF, Bruggink AH, van Wichen DF, van Kuik J, de Koning E, et al. The Chemokine and Chemokine Receptor Profile of Infiltrating Cells in the Wall of Arteries With Cardiac Allograft Vasculopathy is Indicative of a Memory T-helper 1 Response. Circulation (2006) 114(15):1599–607. doi: 10.1161/circulationaha.105.597526
59. Borges TJ, O’Malley JT, Wo L, Murakami N, Smith B, Azzi J, et al. Codominant Role of Interferon-gamma- and Interleukin-17-Producing T Cells During Rejection in Full Facial Transplant Recipients. Am J Transplant (2016) 16(7):2158–71. doi: 10.1111/ajt.13705
60. Fujita Y, Fujii T, Mimori T, Sato T, Nakamura T, Iwao H, et al. Deficient Leptin Signaling Ameliorates Systemic Lupus Erythematosus Lesions in MRL/Mp-Fas Lpr Mice. J Immunol (2014) 192(3):979–84. doi: 10.4049/jimmunol.1301685
61. Tsaur I, Gasser M, Aviles B, Lutz J, Lutz L, Grimm M, et al. Donor Antigen-Specific Regulatory T-cell Function Affects Outcome in Kidney Transplant Recipients. Kidney Int (2011) 79(9):1005–12. doi: 10.1038/ki.2010.533
62. Osuka A, Ogura H, Ueyama M, Shimazu T, Lederer JA. Immune Response to Traumatic Injury: Harmony and Discordance of Immune System Homeostasis. Acute Med Surg (2014) 1(2):63–9. doi: 10.1002/ams2.17
63. Rao DA, Pober JS. Endothelial Injury, Alarmins, and Allograft Rejection. Crit Rev Immunol (2008) 28(3):229–48. doi: 10.1615/critrevimmunol.v28.i3.40
64. Rao DA, Eid RE, Qin L, Yi T, Kirkiles-Smith NC, Tellides G, et al. Interleukin (IL)-1 Promotes Allogeneic T Cell Intimal Infiltration and IL-17 Production in a Model of Human Artery Rejection. J Exp Med (2008) 205(13):3145–58. doi: 10.1084/jem.20081661
65. Weigt SS, Palchevskiy V, Belperio JA. Inflammasomes and IL-1 Biology in the Pathogenesis of Allograft Dysfunction. J Clin Invest (2017) 127(6):2022–9. doi: 10.1172/jci93537
66. Tay SS, Plain KM, Bishop GA. Role of IL-4 and Th2 Responses in Allograft Rejection and Tolerance. Curr Opin Organ Transplant (2009) 14(1):16–22. doi: 10.1097/MOT.0b013e32831ebdf5
67. Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional Cloning of the Mouse Obese Gene and its Human Homologue. Nature (1994) 372(6505):425–32. doi: 10.1038/372425a0
68. Elson CO, Sartor RB, Tennyson GS, Riddell RH. Experimental Models of Inflammatory Bowel Disease. Gastroenterology (1995) 109(4):1344–67. doi: 10.1016/0016-5085(95)90599-5
69. Faggioni R, Feingold KR, Grunfeld C. Leptin Regulation of the Immune Response and the Immunodeficiency of Malnutrition. FASEB J (2001) 15(14):2565–71. doi: 10.1096/fj.01-0431rev
70. Lord GM, Matarese G, Howard JK, Baker RJ, Bloom SR, Lechler RI. Leptin Modulates the T-cell Immune Response and Reverses Starvation-Induced Immunosuppression. Nature (1998) 394(6696):897–901. doi: 10.1038/29795
71. Cakir B, Cevik H, Contuk G, Ercan F, Eksioglu-Demiralp E, Yegen BC. Leptin Ameliorates Burn-Induced Multiple Organ Damage and Modulates Postburn Immune Response in Rats. Regul Pept (2005) 125(1-3):135–44. doi: 10.1016/j.regpep.2004.08.032
72. Arnalich F, Lopez J, Codoceo R, Jim nez M, Madero R, Montiel C. Relationship of Plasma Leptin to Plasma Cytokines and Human Survivalin Sepsis and Septic Shock. J Infect Dis (1999) 180(3):908–11. doi: 10.1086/314963
73. Moore SI, Huffnagle GB, Chen GH, White ES, Mancuso P. Leptin Modulates Neutrophil Phagocytosis of Klebsiella Pneumoniae. Infect Immun (2003) 71(7):4182–5. doi: 10.1128/IAI.71.7.4182-4185.2003
74. Shen Y, Wang Q, Zhao Q, Zhou J. Leptin Promotes the Immune Escape of Lung Cancer by Inducing Proinflammatory Cytokines and Resistance to Apoptosis. Mol Med Rep (2009) 2(2):295–9. doi: 10.3892/mmr_00000099
75. Dedinska I, Mackova N, Kantarova D, Kovacikova L, Granak K, Laca L, et al. Leptin - A New Marker for Development of Post-Transplant Diabetes Mellitus? J Diabetes Complications (2018) 32(9):863–9. doi: 10.1016/j.jdiacomp.2018.07.002
Keywords: vascularized composite allotransplantation, inflammation, systems biology, network analysis, immunosuppression, acute rejection
Citation: Aral AM, Zamora R, Barclay D, Yin J, El-Dehaibi F, Erbas VE, Dong L, Zhang Z, Sahin H, Gorantla VS and Vodovotz Y (2021) The Effects of Tacrolimus on Tissue-Specific, Protein-Level Inflammatory Networks in Vascularized Composite Allotransplantation. Front. Immunol. 12:591154. doi: 10.3389/fimmu.2021.591154
Received: 03 August 2020; Accepted: 14 April 2021;
Published: 04 May 2021.
Edited by:
Antonio Cappuccio, Mount Sinai Hospital, United StatesReviewed by:
Miguel Fribourg, Icahn School of Medicine at Mount Sinai, United StatesCopyright © 2021 Aral, Zamora, Barclay, Yin, El-Dehaibi, Erbas, Dong, Zhang, Sahin, Gorantla and Vodovotz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yoram Vodovotz, dm9kb3ZvdHp5QHVwbWMuZWR1; Vijay S. Gorantla, dmdvcmFudGxAd2FrZWhlYWx0aC5lZHU=
†Present address: Huseyin Sahin, Plastic Surgery, Karadeniz Technical University, Trabzon, Turkey
‡These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.