
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 25 March 2021
Sec. Autoimmune and Autoinflammatory Disorders
Volume 12 - 2021 | https://doi.org/10.3389/fimmu.2021.589639
This article is part of the Research Topic Uveitis: Immunity, Genes and Microbes View all 23 articles
Interleukin (IL)33, a member of the IL1 superfamily, functions as a nuclear factor and mediates biological effects by interacting with the ST2 receptor. Recent studies have described IL33 as an emerging pro-inflammatory cytokine in the immune system, and IL33/ST2 gene polymorphisms have been implicated in the pathogenesis of various immune diseases. However, the underlying mechanisms of IL33/ST2 in Behcet’s disease (BD) remain to be defined. Here, we investigated the association between IL33/ST2 gene polymorphisms and BD in 585 BD uveitis (BDU) patients and 834 healthy controls using Agena MassARRAY iPLEX platform. We found that rs3821204 was associated with the development of BDU. Moreover, the frequency of rs2210463 G allele was lower in patients with genital involvement. Association analysis revealed a much greater genetic difference between complete-type and incomplete-type BD groups, including three SNPs (rs7044343, rs1048274, and rs2210463). Our findings suggest that IL33/ST2 gene polymorphisms are involved in the pathogenesis of BDU. Different genetic backgrounds may exist in complete-type and incomplete-type BD patients.
Behçet’s disease (BD) is a chronic, relapsing, systemic vasculitis involving complex pathologic processes characterized by recurrent episodes of uveitis, oral and genital ulcers, and skin lesions (1). It may also affect the digestive, cardiovascular, and nervous systems and involve joints and large vessels (1). Eye involvement is one of the most serious manifestations of BD with high incidence of disability. It affects about 70% of patients and is characterized by uveitis (BDU) (2). The onset of BD usually occurs in the third or fourth decade of life and is rare in children or individuals over the age of 40 years. The etiology of BD is still unclear, although, an inflammatory response triggered by immune and unknown environmental factors in a genetically susceptible individual has been proposed as its cause. Among all genetic factors, HLA-B51 has been confirmed as the strongest risk factor for BD. However, the presence of HLA-B51 alone is not sufficient to explain all the symptoms of BD patients (3).
Interleukin (IL)33, a member of the IL-1 family, is evolutionarily conserved in mammals and a ligand for the orphan receptor ST2. It is constitutively expressed by tissue barrier cells, such as epithelial and endothelial cells, and various innate immune cells, such as macrophages and dendritic cells. Both enhanced innate immunity and neutrophil hyperactivity with endothelial damage, which are part of BD pathogenesis (4), are related to disrupted IL33 levels. Therefore, we hypothesized that BD may be associated with IL33/ST2 genetic abnormalities.
In the present study, we aimed to establish the role of IL33 and ST2 genes in the risk of developing BDU in a well-characterized Chinese cohort by genotyping and association analysis. We analyzed single nucleotide polymorphisms (SNPs) of IL33 (rs1891385, rs2210463, rs11792633, rs7044343, rs1048274) and ST2 (rs3821204, rs12712142, rs2058660) with respect to BDU pathogenesis and characteristic BDU phenotypes in these patients.
This study was approved by the Ethics Committee of Peking Union Medical College Hospital. A total of 585 patients with BDU were recruited from the Peking Union Medical College Hospital (PUMCH, 190 patients) and the First Affiliated Hospital of Chongqing Medical University (395 patients) between January 2018 and June 2019. All patients with BD were diagnosed according to the International BD Study Group criteria. Eight hundred and thirty-four healthy controls with no previous history of immune-related diseases were enrolled from the health examination center of PUMCH. All participants were self-reported as Han Chinese and were unrelated.
Of the 585 BD patients included, detailed clinical data for 553 patients was recorded. Based on their clinical characteristics, patients were divided into the following subgroups: complete-type and incomplete-type BD groups (1); severe and non-severe BD groups; early-onset and late-onset BD groups.
In case of complete-type BD, all four main symptoms (ocular lesion, recurrent aphthous ulcers on the oral mucosa, genital ulcers, and skin lesion) appeared during the clinical course. In case of incomplete-type BD, either of the following conditions was met: 1. Three of the four main symptoms, or two main symptoms and two additional symptoms (arthritis without deformity or sclerosis, epididymitis, gastrointestinal lesion represented by ileocecal ulceration, vascular lesions, and central nervous system lesions including and above middle class of severity) appeared during the clinical course; 2. Typical ocular lesions and other main symptoms, or two additional symptoms appeared during the clinical course.
Severe and non-severe BD were classified based on clinical severity score ≥7 or <7 points, respectively. Clinical severity score was determined as previously described (5, 6): 1 point for each of the mild symptoms (oral ulcers, genital ulcers, arthralgia, typical skin lesions such as erythema, nodosum-like lesions, papulopustular lesions, and folliculitis), 2 points for each of the moderate symptoms (arthritis, deep vein thrombosis of the legs, anterior uveitis, gastrointestinal involvement), and 3 points for each of the severe symptoms (posterior/panuveitis, retinal vasculitis, arterial thrombosis, neuro Behcet’s disease, bowel perforation).
In case of early-onset BD, the age of first BD attack was <40 years, while in late-onset BD group, the age of first BD attack was ≥40 years.
Considering the vital effects of IL33 and ST2 in immune-mediated diseases, eight SNPs (rs1891385, rs2210463, rs11792633, rs7044343, rs1048274, rs2058660, rs3821204, rs12712142), which were reported to show a positive association with systemic lupus erythematosus, ankylosing spondylitis (AS), or other immune-related diseases, based on GWAS and candidate gene association studies, were selected for further analysis.
A total of 2 ml blood sample was collected in an ethylenediaminetetraacetic acid (EDTA) anticoagulant tube from each subject. Genomic DNA was extracted from peripheral blood cells using a kit from Thermo Fisher Scientific Inc. according to the manufacturer’s instructions. DNA concentration was determined by using a Nanodrop spectrophotometer (Thermo, Wilmington, DE, USA) using the 260/280 nm ratio and diluted to a working concentration of 15–20 ng/μl. The SNPs of IL33 and ST2 were genotyped using Agena MassArray system (Agena iPLEX assay, San Diego, CA, USA) following the manufacturer’s instructions. Both polymerase chain reaction (PCR) and single-base extension primers for IL33 (rs1891385, rs2210463, rs11792633, rs7044343, rs1048274) and ST2 (rs3821204, rs12712142, rs2058660) were designed by the Assay Design Suite (ADS, Agena). After multiplex PCR amplifications, the products were used for locus specific single base extension reactions and the final products were desalted and transferred on to a 384-element SpectroCHIP array. Allele detection was conducted by matrix-assisted laser desorption ionization time of flight mass spectrometry (MALDI-TOF MS). The mass spectrogram data were analyzed using MassArray Typer 4.0 software (Agena).
The current SNP assay method is based on primer extension and offers two levels of specificity. First, a locus-specific PCR reaction takes place, followed by a locus-specific primer extension reaction (iPLEX assay) in which an oligonucleotide primer anneals immediately upstream of the polymorphic site being genotyped. In the iPLEX assay, the primer and amplified target DNA are incubated with mass-modified dideoxynucleotide terminators. The primer extension is made according to the sequence of the variant site, and is a single complementary mass-modified base. Through the use of MALDI-TOF mass spectrometry, the mass of the extended primer is determined. The primer’s mass indicates the sequence and, therefore, the alleles present at the polymorphic site of interest (7).
Duplicate samples, negative controls, and blank controls were included on the plates to ensure the accuracy of genotyping.
Statistical analyses were performed using PLINK 1.07 software (Shaun Purcell, Boston, MA, USA). The Hardy-Weinberg equilibrium (HWE) of each SNP was examined by using the Chi-square (χ2) test in healthy controls. Any SNPs that deviated from the HWE (P < 0.05 in the control groups) would be excluded from subsequent analysis. Differences in genotype and allele frequencies between cases and controls, and between different BD subgroups and phenotypes were analyzed using χ2 test. The odds ratio (OR) and 95% confidence interval (95% CI) of associations were calculated. For multiple comparisons, Pc values (corrected for multiple comparisons by the Bonferroni adjustment test) <0.05 were deemed to be statistically significant. For genetic model testing (additive, dominant, and recessive model), logistic regression analyses were used.
The characteristics of the controls and patients with BD are shown in Table 1. The gender distributions in both controls and cases were similar (p = 0.127). The median age was 36.0 (31.0–42.0) years and 32.0 (26.0–39.0) years for controls and cases respectively. The success rate for sequencing of each SNP was more than 99%. The eight SNPs of IL33 and ST2 selected in the present study did not show any deviation from HWE (all P > 0.05) in controls, and thus, the data were suitable for further comparisons.
ST2 SNPs rs3821204, rs12712142, and rs2058660 were analyzed in both cases and controls and rs3821204 G allele was found to be associated with BDU development (X2 = 7.94, Pc = 0.039; Table 2). Although the frequency of GG genotype of ST2 rs3821204 was higher in the control group than that in the BDU group (10.71 vs 6.16%), genotype frequency analysis failed to reach the statistical significance threshold as the trend was not statistically significant after Bonferroni correction (Pc = 0.051; Table 2). Logistic analysis with three models (i.e., additive, dominant, and recessive) revealed significant differences in the genotypic distributions of rs3821204 in both additive (Pc = 0.036) and recessive (Pc = 0.027) models (Table 3).
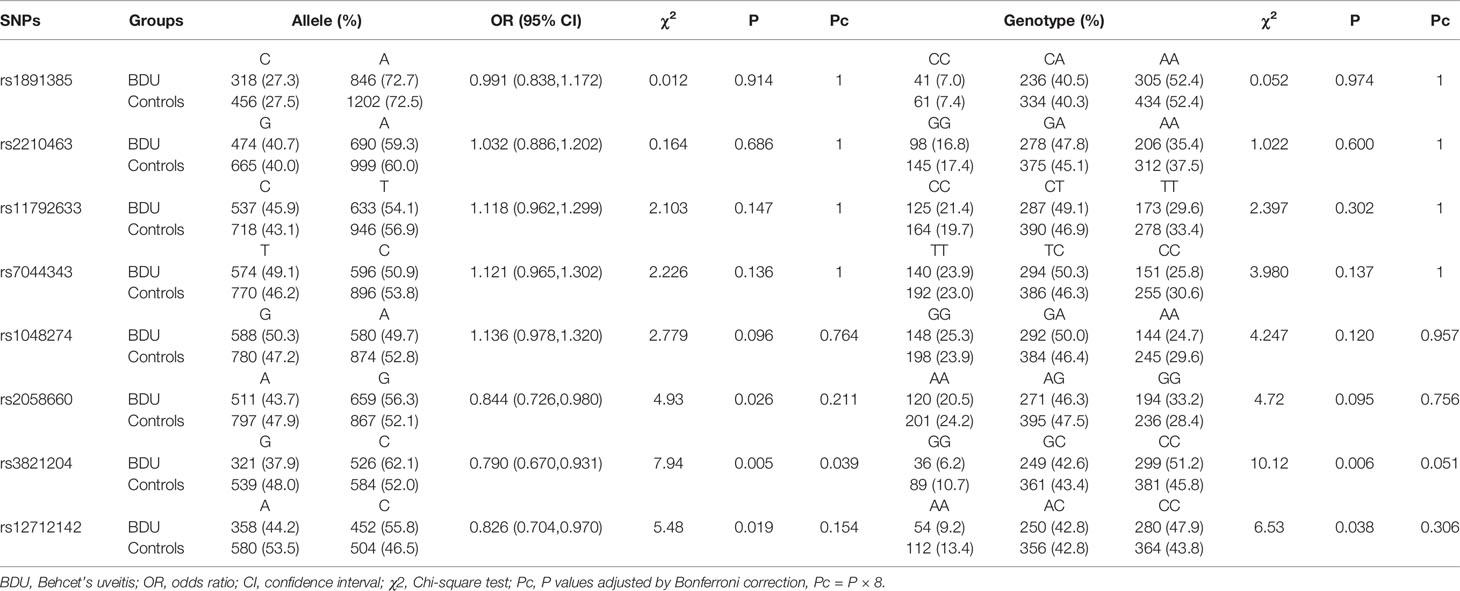
Table 2 Allele and genotype distribution of the IL-33 and ST2 gene markers in BDU patients and controls.
We then detected IL33 SNPs (rs11792633, rs1048274, rs7044343, rs2210463, and rs1891385) in the study population. Our study showed that neither genotype nor allele frequencies of these five SNPs were statistically significant between patients and healthy controls (Pc > 0.05; Table 2). Moreover, logistic regression analysis with three genetic models (i.e., additive, dominant, and recessive) also did not reveal any statistical difference between patient and control groups (Pc > 0.05; Table 3).
Association analyses of ST2 and IL33 SNPs with different BD subgroups and phenotypes were also performed (Tables 4A and 4B). The allele frequencies of IL33 SNPs rs2210463 (P = 0.0028, Pc = 0.022), rs7044343 (P = 0.0034, Pc = 0.027), and rs1048274 (P = 0.0050, Pc = 0.040) were all significantly different between the complete-type and incomplete-type BDU groups. The allele frequency of IL33 SNP rs2210463 (P = 0.006, Pc = 0.048) was significantly different between patients with and without genital ulcers. No allele frequency differences were found between the early- and late-onset or severe and non-severe BDU groups in any ST2 or IL33 SNP. Moreover, no association was found between erythema nodosum, or arthritis and IL33 or ST2 SNPs.
Although the etiopathogenesis of BD remains uncertain, microbial agent triggers, environmental factors, genetic predisposition, and immunological abnormalities have been implicated (8–10). Complex disorders implicating innate and adaptive immunity in humoral and cellular immunity settings are observed in BD. IL33, a member of the IL1 family that potently drives the production of T helper (Th)-2-associated cytokines and promotes responses by cytotoxic NK cells, Th1 cells, and CD8+ T cells (11), has been associated with various immune-related diseases (12, 13). ST2, the receptor of IL33, is highly expressed in many immunocytes (14).
In the present study, we demonstrated significant association between the IL33/ST2 pathway SNP rs3821204 and the development of BDU. The rs3821204 polymorphism occurs in the 3′ untranslated region of ST2, which was found to be functional with the rs3821204 gene mutation by disrupting the binding site of miR-202-3p, resulting in plasma level changes of sST2 (15, 16). sST2 is the soluble form of ST2, which together with the membrane-bound form (ST2L), comprise the two main ST2 existing forms (17). ST2L functions as a transmembrane signaling receptor of IL33, mediating the effect of IL33 on inflammatory processes, while sST2 acts as a decoy receptor that prevents the interaction of ST2L with IL33 (18, 19).
Several studies have reported associations between IL33 rs7044343 polymorphism and autoimmune diseases, including rheumatoid arthritis, systemic sclerosis, and BD in Turkey (20–22). Previous case-control study revealed a significant association between the rs7044343 TT genotype and Turkish BD patients in the discovery population (22). However, in the present study, rs7044343 SNP was not found to be associated with the development of BD in patients belonging to Chinese Han ethnicity. Ethnic differences may be a reason for this discrepancy, as genetic heterogeneity between races has been reported to be 58% in a previous research (23).
The association of IL33/ST2 SNPs with different BD phenotype subgroups were also investigated. No associations were found except for genital ulcer and the BD of the complete-type. As compared with the other phenotype-based subgroups, greater genetic differences were found between the complete-type and incomplete-type BD groups, which indicated that different genetic backgrounds may exist in these groups of BD patients. Although previous studies reported that similar gene mechanisms underlie the pathogenesis of different immune diseases (24, 25), in the present study, rs2058660 [inflammatory bowel disease-associated (26)], rs1891385 [AS-associated (27)], and rs11792633 [systemic sclerosis-associated (28)] SNPs were not associated with either onset or phenotypes of BD. Compared with the classical immune diseases, BD presents more extensive pathological changes in tissues and organs with featured spontaneous exacerbation and remission clinical courses (29), which, together with the genetic findings, indicate that different molecular mechanisms may exist in BD pathology as compared to classical immune diseases.
Our study had several limitations. First, genetic variants may have different effects in diverse ethnicities. In this study, only Chinese Han individuals were recruited, thus, our results may not be generalizable to other ethnicities. Second, all the study subjects were enrolled from two tertiary ophthalmologic centers, therefore, selection bias may exist. Third, the mRNA expressions of BD patients with different SNP genotypes were not analyzed in this study, further studies are needed to validate our findings.
In conclusion, we reported the association of rs3821204, rs2210463, rs7044343, rs1048274 with the development or phenotypes of BDU, and for the first time revealed significant genetic differences between patients with complete-type and incomplete-type BD. Our findings indicate that IL33/ST2 polymorphisms have a close and probable causal association with BDU, and different genetic backgrounds may exist in complete-type and incomplete-type BD patients.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by The Ethics committee of Peking Union Medical College Hospital. Written informed consent to participate in this study was provided by the participants/participants’ legal guardian/next of kin.
MP participated in the study design, patient enrollment, data collection and analysis and manuscript drafting. MZ participated in the study design, patient enrollment, data collection and analysis, manuscript drafting, and coordination of experimental work. XL participated in the data interpretation and analysis. PY, CZ, and FG participated in the patient enrollment. MP, YQ, AL, and JX carried out the experimental work. All authors contributed to the article and approved the submitted version.
This work was supported by the National Natural Science Foundation of China [81770917] (fees for patient enrollment, carrying out the experimental work and open access publication) and Beijing Municipal Science and Technology Commission No. Z171100001017217 (fees for patient enrollment).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We would like to thank all the patients and controls who participated in this study and thank Prof. Zhi Zheng and Jun Zhou for help in carrying out the experimental work.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2021.589639/full#supplementary-material
1. Kurokawa MS, Yoshikawa H, Suzuki N. Behcet’s disease. Semin Respir Crit Care Med (2004) 25:557–68. doi: 10.1055/s-2004-836147
2. Deuter CM, Kotter I, Wallace GR, Murray PI, Stubiger N, Zierhut M. Behcet’s disease: ocular effects and treatment. Prog Retin Eye Res (2008) 27:111–36. doi: 10.1016/j.preteyeres.2007.09.002
3. Akman A, Sallakci N, Coskun M, Bacanli A, Yavuzer U, Alpsoy E, et al. TNF-alpha gene 1031 T/C polymorphism in Turkish patients with Behcet’s disease. Br J Dermatol (2006) 155:350–6. doi: 10.1111/j.1365-2133.2006.07348.x
4. Hisanaga K. [The etiology, diagnosis, and treatment of neurological complications in Behcet disease and its related disorder Sweet disease]. Rinsho Shinkeigaku (2019) 59:1–12. doi: 10.5692/clinicalneurol.cn-001238
5. Krause I, Rosen Y, Kaplan I, Milo G, Guedj D, Molad Y, et al. Recurrent aphthous stomatitis in Behcet’s disease: clinical features and correlation with systemic disease expression and severity. J Oral Pathol Med (1999) 28:193–6. doi: 10.1111/j.1600-0714.1999.tb02023.x
6. Akman A, Ekinci NC, Kacaroglu H, Yavuzer U, Alpsoy E, Yegin O. Relationship between periodontal findings and specific polymorphisms of interleukin-1alpha and -1beta in Turkish patients with Behcet’s disease. Arch Dermatol Res (2008) 300:19–26. doi: 10.1007/s00403-007-0794-1
7. Gabriel S, Ziaugra L, Tabbaa D. SNP genotyping using the Sequenom MassARRAY iPLEX platform. Curr Protoc Hum Genet (2009) Chapter 2: 2–12. doi: 10.1002/0471142905.hg0212s60
8. Alpsoy E, Kodelja V, Goerdt S, Orfanos CE, Zouboulis C. Serum of patients with Behcet’s disease induces classical (pro-inflammatory) activation of human macrophages in vitro. Dermatology (2003) 206:225–32. doi: 10.1159/000068888
9. Zhou ZY, Chen SL, Shen N, Lu Y. Cytokines and Behcet’s disease. AUTOIMMUN Rev (2012) 11:699–704. doi: 10.1016/j.autrev.2011.12.005
10. Mochizuki M. Immunotherapy for Behcet’s disease. Int Rev Immunol (1997) 14:49–66. doi: 10.3109/08830189709116844
11. Molofsky AB, Savage AK, Locksley RM. Interleukin-33 in Tissue Homeostasis, Injury, and Inflammation. Immunity (2015) 42:1005–19. doi: 10.1016/j.immuni.2015.06.006
12. Pei C, Barbour M, Fairlie-Clarke KJ, Allan D, Mu R, Jiang HR. Emerging role of interleukin-33 in autoimmune diseases. Immunology (2014) 141:9–17. doi: 10.1111/imm.12174
13. Yang Z, Liang Y, Xi W, Li C, Zhong R. Association of increased serum IL-33 levels with clinical and laboratory characteristics of systemic lupus erythematosus in Chinese population. Clin Exp Med (2011) 11:75–80. doi: 10.1007/s10238-010-0115-4
14. Yagami A, Orihara K, Morita H, Futamura K, Hashimoto N, Matsumoto K, et al. IL-33 mediates inflammatory responses in human lung tissue cells. J Immunol (2010) 185:5743–50. doi: 10.4049/jimmunol.0903818
15. Wei ZH, Li YY, Huang SQ, Tan ZQ. Genetic variants in IL-33/ST2 pathway with the susceptibility to hepatocellular carcinoma in a Chinese population. Cytokine (2019) 118:124–9. doi: 10.1016/j.cyto.2018.03.036
16. Wu F, Li L, Wen Q, Yang J, Chen Z, Wu P, et al. A functional variant in ST2 gene is associated with risk of hypertension via interfering MiR-202-3p. J Cell Mol Med (2017) 21:1292–9. doi: 10.1111/jcmm.13058
17. Tu X, Nie S, Liao Y, Zhang H, Fan Q, Xu C, et al. The IL-33-ST2L pathway is associated with coronary artery disease in a Chinese Han population. Am J Hum Genet (2013) 93:652–60. doi: 10.1016/j.ajhg.2013.08.009
18. Hong YS, Moon SJ, Joo YB, Jeon CH, Cho ML, Ju JH, et al. Measurement of interleukin-33 (IL-33) and IL-33 receptors (sST2 and ST2L) in patients with rheumatoid arthritis. J Korean Med Sci (2011) 26:1132–9. doi: 10.3346/jkms.2011.26.9.1132
19. Kim DJ, Baek SY, Park MK, Park KS, Lee JH, Park SH, et al. Serum level of interleukin-33 and soluble ST2 and their association with disease activity in patients with Behcet’s disease. J Korean Med Sci (2013) 28:1145–53. doi: 10.3346/jkms.2013.28.8.1145
20. Li C, Mu R, Guo J, Wu X, Tu X, Liu X, et al. Genetic variant in IL33 is associated with susceptibility to rheumatoid arthritis. Arthritis Res Ther (2014) 16:R105. doi: 10.1186/ar4554
21. Koca SS, Pehlivan Y, Kara M, Alibaz-Oner F, Oztuzcu S, Yilmaz N, et al. The IL-33 gene is related to increased susceptibility to systemic sclerosis. RHEUMATOL Int (2016) 36:579–84. doi: 10.1007/s00296-015-3417-8
22. Koca SS, Kara M, Deniz F, Ozgen M, Demir CF, Ilhan N, et al. Serum IL-33 level and IL-33 gene polymorphisms in Behcet’s disease. Rheumatol Int (2015) 35:471–7. doi: 10.1007/s00296-014-3111-2
23. Ioannidis JP, Ntzani EE, Trikalinos TA. ‘Racial’ differences in genetic effects for complex diseases. Nat Genet (2004) 36:1312–8. doi: 10.1038/ng1474
24. Ermann J, Fathman CG. Autoimmune diseases: genes, bugs and failed regulation. Nat Immunol (2001) 2:759–61. doi: 10.1038/ni0901-759
25. Anaya JM. Common mechanisms of autoimmune diseases (the autoimmune tautology). Autoimmun Rev (2012) 11:781–4. doi: 10.1016/j.autrev.2012.02.002
26. Latiano A, Palmieri O, Pastorelli L, Vecchi M, Pizarro TT, Bossa F, et al. Associations between genetic polymorphisms in IL-33, IL1R1 and risk for inflammatory bowel disease. PloS One (2013) 8:e62144. doi: 10.1371/journal.pone.0062144
27. Fan D, Ding N, Yang T, Wu S, Liu S, Liu L, et al. Single nucleotide polymorphisms of the interleukin-33 (IL-33) gene are associated with ankylosing spondylitis in Chinese individuals: a case-control pilot study. Scand J Rheumatol (2014) 43:374–9. doi: 10.3109/03009742.2014.882408
28. Koca SS, Pehlivan Y, Kara M, Oner FA, Yilmaz N, Cetin GY, et al. AB0152 The rs11792633-t allele of il-33 gene is associated with systemic sclerosis. Ann RHEUM Dis (2013) 72:A832. doi: 10.1136/annrheumdis-2013-eular.2475
Keywords: Behcet’s disease, Behcet’s uveitis, uveitis, gene polymorphism, single nucleotide polymorphism, interleukin 33, ST2
Citation: Pei M, Liu X, Yang P, Zhao C, Gao F, Qu Y, Liang A, Xiao J and Zhang M (2021) Genetic Association of Interleukin 33/ST2 Polymorphisms With Behcet’s Uveitis. Front. Immunol. 12:589639. doi: 10.3389/fimmu.2021.589639
Received: 31 July 2020; Accepted: 26 February 2021;
Published: 25 March 2021.
Edited by:
Betty Diamond, Feinstein Institute for Medical Research, United StatesReviewed by:
Hongsong Yu, Zunyi Medical University, ChinaCopyright © 2021 Pei, Liu, Yang, Zhao, Gao, Qu, Liang, Xiao and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Meifen Zhang, bWVpZmVuX3poYW5nQGhvdG1haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.