
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 22 February 2021
Sec. Autoimmune and Autoinflammatory Disorders
Volume 12 - 2021 | https://doi.org/10.3389/fimmu.2021.587393
This article is part of the Research Topic Uveitis: Immunity, Genes and Microbes View all 23 articles
 Jing Xu1
Jing Xu1 Guannan Su1
Guannan Su1 Xinyue Huang1
Xinyue Huang1 Rui Chang1
Rui Chang1 Zhijun Chen1
Zhijun Chen1 Zi Ye1
Zi Ye1 Qingfeng Cao1
Qingfeng Cao1 Aize Kijlstra2
Aize Kijlstra2 Peizeng Yang1*
Peizeng Yang1*To investigate aqueous metabolic profiles in Vogt-Koyanagi-Harada (VKH) and Behcet’s disease (BD), we applied ultra-high-performance liquid chromatography equipped with quadrupole time-of flight mass spectrometry in aqueous humor samples collected from these patients and controls. Metabolite levels in these three groups were analyzed by univariate logistic regression. The differential metabolites were subjected to subsequent pathway analysis by MetaboAnalyst. The results showed that both partial-least squares discrimination analysis and hierarchical clustering analysis showed specific aqueous metabolite profiles when comparing VKH, BD, and controls. There were 28 differential metabolites in VKH compared to controls and 29 differential metabolites in BD compared to controls. Amino acids and fatty acids were the two most abundant categories of differential metabolites. Furthermore, pathway enrichment analysis identified several perturbed pathways, including pantothenate and CoA biosynthesis when comparing VKH with the control group, and D-arginine and D-ornithine metabolism and phenylalanine metabolism when comparing BD with the control group. Aminoacyl-tRNA biosynthesis was altered in both VKH and BD when compared to controls. Our findings suggest that amino acids metabolism as well as two fatty acids, palmitic acid and oleic acid, may be involved in the pathogenesis of BD and VKH.
Uveitis known as intraocular inflammation is one of the leading causes of visual impairment and blindness in the world (1, 2). Vogt-Koyanagi-Harada (VKH) disease and Behcet’s disease (BD) are two of the most commonly seen uveitis entities in China (3, 4). The incidence rate of uveitis in China is 111.3 persons per 100,000 person-years (5) and a frequency of 12.7% and 8.7% has been reported for VKH and BD in Chinese uveitis patients, respectively (4). VKH disease is a multisystemic autoimmune disease characterized by recurrent bilateral granulomatous panuveitis accompanied with systemic involvements including poliosis, alopecia, vitiligo, central nervous system, and auditory abnormalities (6). BD is recognized as an autoinflammatory disorder accompanied with recurrent uveitis, oral aphthae, genital ulcers, and typical skin lesions (7). The exact pathogenesis of both VKH disease and BD remains unclear and omics analysis may advance the understanding of these diseases.
Metabolomics is a rapidly expanding field in life science which leads to the growing interest in metabolism (8). Metabolites, serving as downstream products of transcription, translation, and post-translational protein modification, can reflect and be influenced by local physiological events (9). Therefore, a study of metabolomics could contribute to a better understanding of the pathophysiology of intraocular inflammation. Recent metabolomic studies in VKH disease or BD, using sweat or urine samples have revealed a specific metabolite profile (10, 11). Aqueous humor fills the anterior chamber of the eye and contributes to a normal intraocular homeostatic environment (12). Its composition may probably be a better reflection of local physiological changes associated with intraocular diseases than the analysis of samples obtained from peripheral sites. As far as we know, no aqueous metabolomic study has been reported for VKH or BD and this was therefore the subject of the study described here.
Liquid chromatography/mass spectrometry (LC/MS) is a commonly applied technique for untargeted metabolomics detection with robust reliability and reproducibility (13–15). In view of these features, we performed an untargeted metabolomic analysis of aqueous humor samples from VKH disease and BD patients taken during cataract surgery and compared it with samples from senile cataract controls, applying ultra-high-performance liquid chromatography equipped with quadrupole time-of flight mass spectrometry (UPLC-Q-TOF/MS).
Individuals selected for this study included 15 VKH patients, 15 BD patients, and 15 senile cataract controls. The VKH and BD patients included, had developed cataract as a complication of their uveitis. The demographic data of the cohorts were collected (16) and shown in Table 1. Criteria of an international committee and those developed by our group were used to make the diagnosis of VKH disease (17, 18). Diagnosis of BD was based on the International Study Group’s criteria (19). Patients with any unclear diagnosis were excluded from the study. We also excluded patients with other ocular condition such as optic nerve disease, macular abnormalities or diabetic retinopathy. As for controls, the exclusion criteria were subjects with uveitis and a history of any other autoimmune disease.
All participants underwent cataract surgery at the First Affiliated Hospital of Chongqing Medical University (Chongqing, China) from August, 2017 to May, 2019. Our method for cataract removal in uveitis patients has been reported elsewhere (20). Signed informed consents were obtained from all enrolled participants. The study received the approval of the Ethics Committee of the First Affiliated Hospital of Chongqing Medical University and followed the tenets of the Declaration of Helsinki.
Anterior chamber paracentesis was performed to collect approximately 200 μl of aqueous humor samples at the beginning of the cataract surgery under the sterile condition. Care was taken to avoid contamination of samples with blood or touching intraocular tissues. The specimens were stored at −80°C until UPLC-Q-TOF/MS analysis. All specimens were thawed at 4°C. To remove protein, 80 μl aqueous humor aliquots were mixed with 320 μl of cold methanol/acetonitrile (1:1, v/v) which was then centrifuged for 20 min (14,000 g, 4°C). The supernatant was dried in a vacuum centrifuge. Quality control (QC) specimens consisting of mixing with 10 μl of each sample were analyzed with other samples to monitor the repeatability and stability of instrument. The QC specimens were inserted regularly in the cohort of tested samples, which could monitor the reliability of the whole procedure.
Aqueous metabolomic analyses were applied using an Agilent 1290 Infinity LC system (Agilent Technologies, Santa-Clara, California, USA) coupled with an AB SCIEX Triple TOF 6600 System (AB SCIEX, Framingham, MA, USA). For chromatographic separation, ACQUITY HSS T3 1.8 mm (2.1 mm × 100 mm) columns were used for sample analyses in both positive and negative modes. The column temperature was set at 25°C. The mobile phase was set as follows: A = 25 mM ammonium acetate and 25 mM ammonium hydroxide in water, and B = acetonitrile. The elution procedure was set as follows. After keeping for 0.5 min, the gradient of 95% B was linearly reduced to 65% in 6.5 min. Then it was decreased to 40% in 1 min. After keeping 40% B for 1 min, it was increased to 95% in 10 s. A 3-min re-equilibration period was employed in the procedure. The delivery flow rate was set at 300 μl/min, and 2 μl aqueous specimen was injected into the column.
UPLC-Q-TOF/MS was conducted in both positive and negative ion modes. Electrospray ionization source conditions were set as Ion Source Gas1 = 60 psi, Ion Source Gas2 = 60 psi, curtain gas = 30 psi, source temperature = 600°C, and IonSpray Voltage Floating = 5500 V (+) and −5500 V (−). The MS/MS spectra were obtained by using information dependent acquisition (IDA) coupled with the selected high sensitivity mode. The parameters were set as the collision energy = 50 eV (+) and −20 eV (−), declustering potential = 60 V (+), and −60 V (−), excluding isotopes within 4 Da, and candidate ions to monitor per cycle = 6.
The raw UPLC-Q-TOF/MS data were converted to MzXML files using Proteo Wizard MSconverter, and then imported with XCMS online software for data processing (https://xcmsonline.scripps.edu/landing_page.php?pgcontent=mainPage). The parameters including peak picking, retention time, and peak grouping were aligned by XCMS. We completed compound identification of metabolites by comparing the accuracy m/z value (<25 ppm) and MS/MS spectra. After being normalized, the processed data were uploaded into SIMCA-P (version 14.1, Umetrics, Umea, Sweden) for multivariate data analysis. Then we conducted principal component analysis (PCA) and partial least square discriminant analysis (PLS-DA) to acquire an overview of the metabolomics data in both positive and negative models. The contribution of each metabolite was calculated from the PLS-DA model and expressed as variable importance in the projection (VIP) score. To evaluate the significance of metabolites, those metabolites with a VIP score >1 were analyzed statistically with Student’s t-test. We performed the Benjamini–Hochberg procedure for multiple testing adjustments, whereby the critical false discovery rate (FDR) was set to 0.05. The Human Metabolome Database (HMDB) (https://hmdb.ca/) was used to define the classes of metabolites.
Venny software version 2.1.0 (https://bioinfogp.cnb.csic.es/tools/venny/) was used to generate a Venn diagram which could directly show the common and unique differential metabolites among the three groups. Multi Experiment Viewer (MeV) software version 4.9.0 was applied for the heat plot and hierarchical cluster analysis of metabolites. A Volcano plot was made with Graphpad Prism 7.0. MetaboAnalyst (https://www.metaboanalyst.ca/), an open database source, was used to identify metabolic pathways and to perform pathway enrichment analysis.
Statistical analysis of the data was performed using SPSS 22.0. Results are presented as the mean ± standard deviation (SD) for continuous variables. Normality was assessed by the Shapiro-Wilk test and p > 0.05 referred to normally distributed data. Student’s t-test, Variance (ANOVA), Fisher’s exact test and the Pearson chi-square test were used where appropriate. Age was adjusted by univariate logistic regression analysis to reduce bias. A p value less than 0.05 was considered as statistically significant.
A total of 45 participants were enrolled in the study, including 15 VKH subjects (male/female: 8/7), 15 BD subjects (male/female: 9/6), and 15 senile cataract controls (male/female: 6/9) (Table 1). No significant differences were present with regards to sex and IOP among the groups (p = 0.54 and p = 0.13, respectively). All the 30 uveitis patients were on treatment with systemic corticosteroids, without additional biologic agents. The number of VKH patients receiving immunosuppressants (15/15, 100%) was similar to that in the BD group (13/15, 86.57%). Overall, the treatment regimens did not vary between the BD and VKH groups.
To identify the aqueous metabolic profiles among the three groups tested, aqueous humor specimens were applied for untargeted metabolomics analysis. The total ion chromatograms are shown in Figure S1. Only slight changes of the spectral peaks of the QC samples were found, indicating that the method showed a satisfactory reproducibility. A total of 14,592 positive-model signals and 10,985 negative-model signals were detected in this study after signal filtering and peak alignment. After pareto scaling of the data, PCA models showed that the QC samples were tightly clustered in both positive and negative modes (Figure S2), which also suggests the high reproducibility of the method and credibility of the data. PLS-DA plots were performed to characterize the metabolic profiles for both positive and negative modes, and revealed clear differences when comparing VKH vs. control, BD vs. control, and VKH vs. BD (Figure 1).
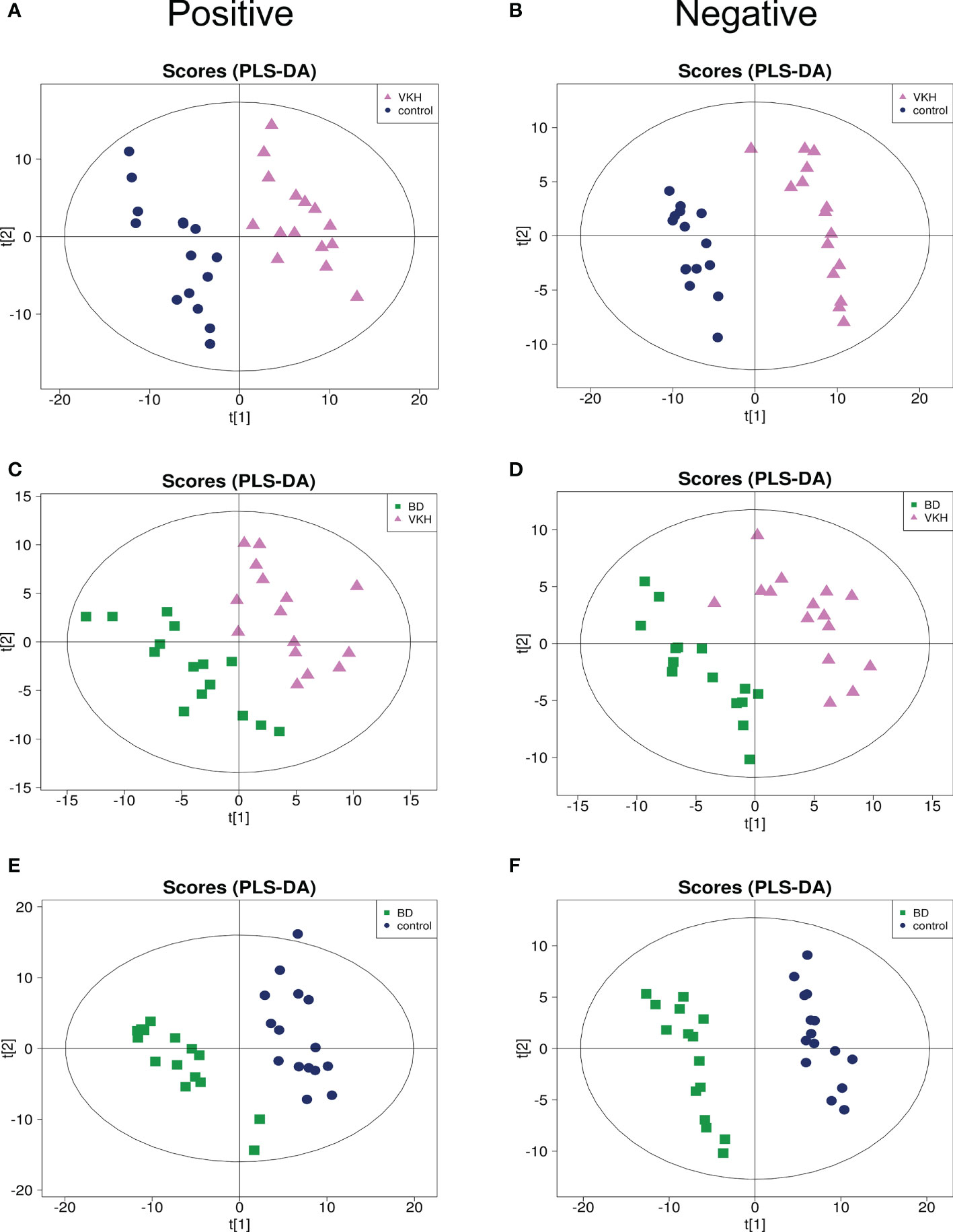
Figure 1 Partial least square discriminant analysis (PLS-DA) for any two of the three groups. PLS-DA plots for Vogt-Koyanagi-Harada disease (VKH) versus senile cataract controls separation in positive mode (A) and negative mode (B); PLS-DA plots for VKH disease and Behcet’s disease (BD) separation in positive mode (C) and negative mode (D); PLS-DA plots for BD and senile cataract controls separation in positive mode (E) and negative mode (F).
Metabolites with a VIP > 1 were filtered to perform Student’s t-test. The results were adjusted with FDR < 0.05 for multiple comparisons. To identify differentially expressed metabolites for subsequent analysis, we further raised the criteria to a VIP > 1.5. In total, 84 metabolites with a VIP > 1.5 and p < 0.05 (FDR corrected) were considered as differentially expressed between the groups (Table S1). HMDB was used to gain the classes of the 84 metabolites. It was found that amino acids and fatty acids were the two categories with the most abundant differential metabolites. As shown in Figure S3, 64 metabolites for VKH vs. control and 65 metabolites for BD vs. control showed significant differences. We found 27 differentially expressed metabolites between VKH and BD (data not shown). However, the significance was lost after FDR correction. The volcano plots were made to describe the variation tendencies of metabolites for VKH vs. control and BD vs. control (Figure S4).
To further investigate metabolites that could differentiate a group of patients from the other participants, we conducted univariate logistic regression analysis (Table 2). There were 41 differential metabolites between the groups after adjustment (Figure 2). There were 28 and 29 significantly differential metabolites for VKH vs. control and BD vs. control, respectively (Figure 3). Among them, nine amino acids and two fatty acids were overlapping differential metabolites. Palmitic acid and oleic acid levels, the two long-chain fatty acids, were significantly elevated in both VKH and BD compared to controls. Except for N-acetylhistidine, the other eight amino acids were significantly increased in both VKH and BD compared to controls, including asymmetric dimethylarginine, L-lysine, D-ornithine, D-pipecolic acid, L-methionine, creatinine, L-pipecolic acid, and gamma-Glutamyllysine. L-histidine, ornithine, and L-valine were differential amino acids when VKH patients were compared with controls. L-phenylalanine, D-arginine, N-acetyl-L-aspartic acid, L-alanine, arginyl-cysteine, and L-arginine were differential amino acids for BD vs. controls.

Table 2 Results of univariate logistic regression analysis comparing the differential metabolites levels of patients to those of controls.
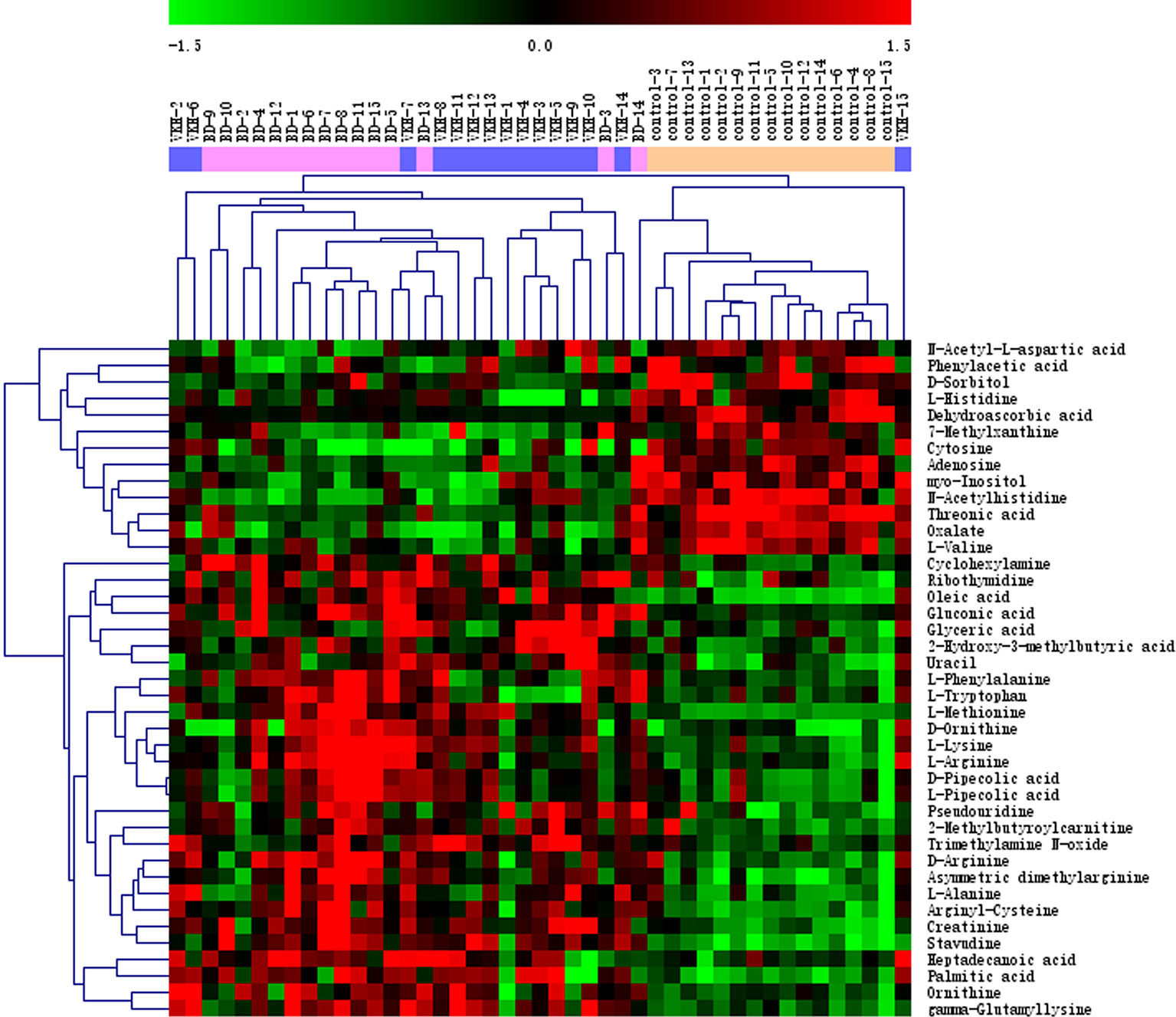
Figure 2 Heat plot of the significantly differential metabolites among three groups. VKH, Vogt-Koyanagi-Harada disease; BD, Behcet’s disease.
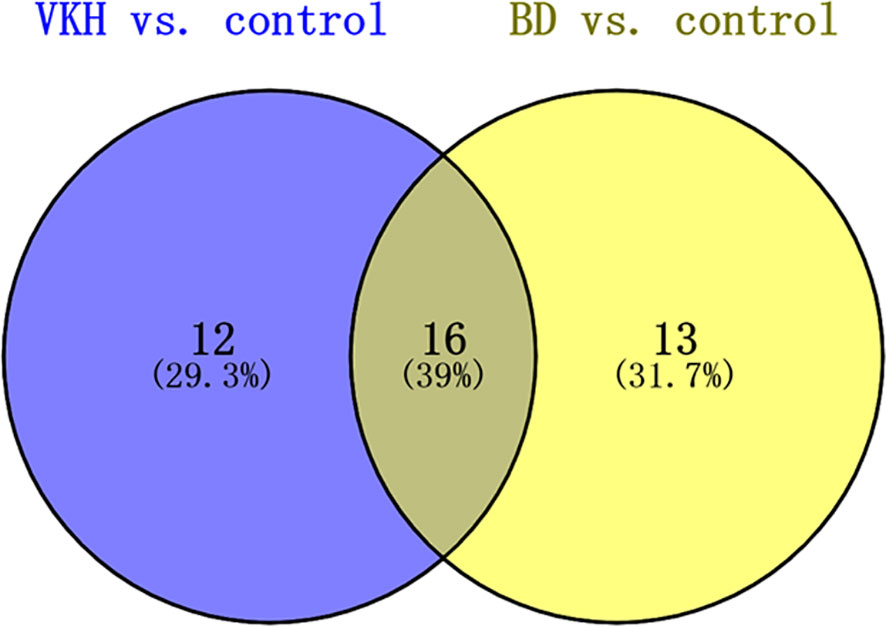
Figure 3 Venn diagram of metabolites after logistic regression analyses adjusting which significantly differed in comparisons between VKH disease, BD, and control groups. VKH, Vogt-Koyanagi-Harada disease; BD, Behcet’s disease.
Subsequently, MetaboAnalyst was used to compare metabolic disturbances between VKH, BD, and the control groups (Figure 4). There were two metabolic pathways that were significantly perturbed when comparing VKH patients with controls, and three pathways when comparing BD patients with controls (Table 3). The aminoacyl-tRNA biosynthesis pathway was significantly altered in both VKH and BD as compared to controls. In addition, we found significant differences in pantothenate and coenzyme A (CoA) biosynthesis for VKH vs. control, and in D-arginine and D-ornithine metabolism and phenylalanine metabolism for BD vs. control.
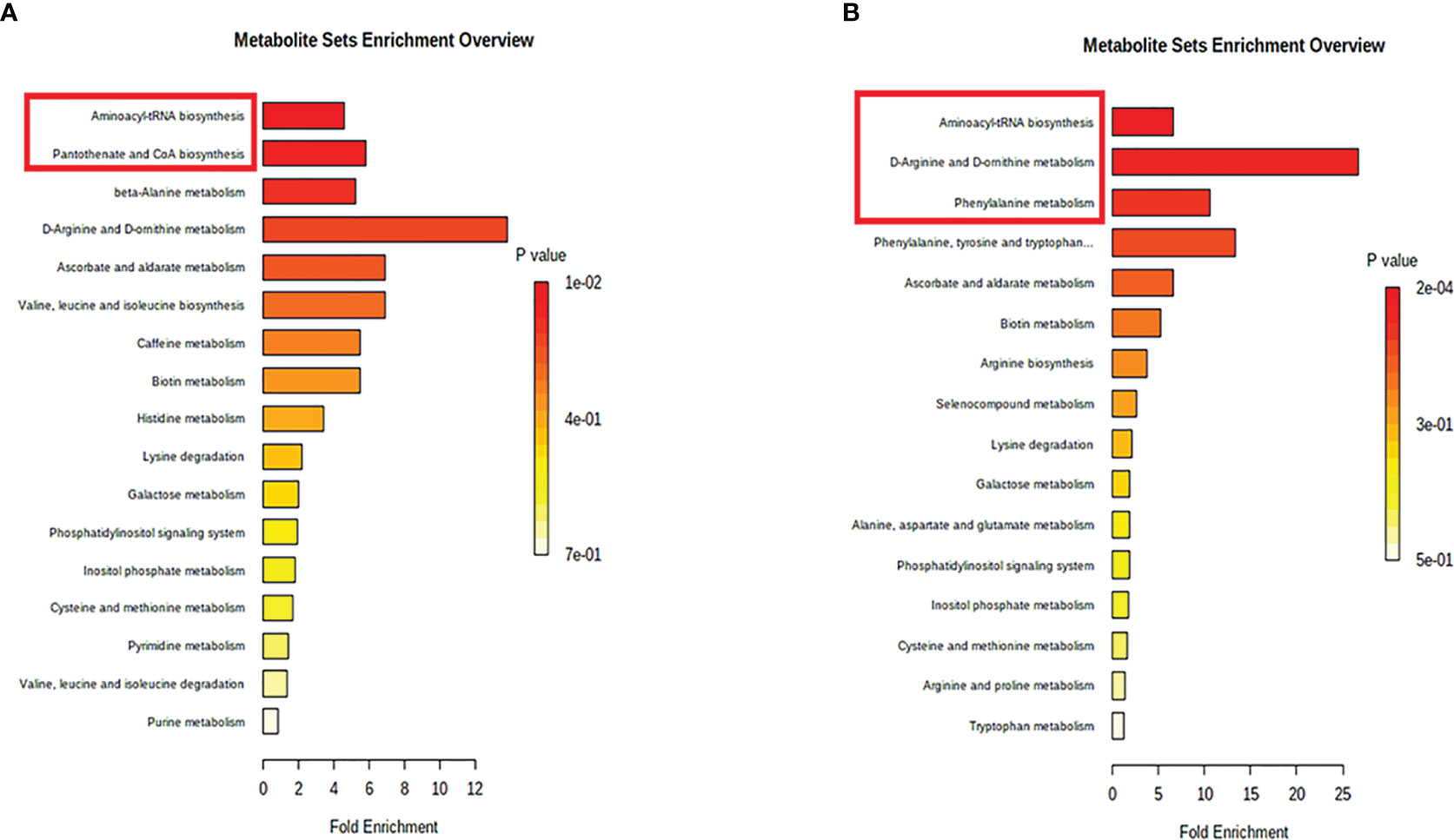
Figure 4 Pathway analysis of the differential metabolites in Vogt-Koyanagi-Harada disease group (A) and Behcet’s disease group (B). The significantly perturbed pathways were in red rectangle.
In the present study, we found that aqueous humor from VKH and BD patients showed distinct metabolic profiles compared to senile cataract controls. After correction, 28 and 29 metabolites were differentially expressed for VKH vs. control and BD vs. control, respectively. Amino acids and fatty acids were the two most abundant differential metabolite categories. Also, significant alterations were found in several metabolic pathways, including aminoacyl-tRNA biosynthesis, pantothenate and CoA biosynthesis, D-arginine and D-ornithine metabolism and phenylalanine metabolism. Experimental verification is needed to validate the role of the pathways identified in this clinical study in the pathogenesis of uveitis. There are very good animal models of autoimmune uveitis (21–23) and further studies are planned to confirm our clinical findings in these models.
Fatty acids are of great importance for immune regulation and may contribute to the development of some autoimmune diseases (24–27). Previous studies have shown that serum oleic acid level and urine palmitic acid and oleic acid levels were significantly higher in BD (11, 28). Moreover, our recent studies in VKH disease identified that sweat palmitic acid level (10) and serum oleic acid level (unpublished data) were increased in VKH. In the current study aqueous palmitic acid and oleic acid levels were significantly higher in VKH and BD compared to controls. The elevated intraocular and peripheral levels of both palmitic acid and oleic acid levels observed in VKH and BD indicates that they may play an important role in the pathophysiology of uveitis. Further studies are needed to investigate the functions of these two fatty acids and their association in the pathogenesis of uveitis.
Amino acids are important protein building stones but can also play a role in the functioning of the immune system (29). It was reported that glutamine metabolism had a distinct role to promote Th17 cells but constrained Th1 cell differentiation (30). Moreover, alanine was recently found to be essential for early T cell activation and deprivation of it might impair T cell effector functions (31). Previous metabolic profiling of aqueous humor obtained from acute anterior uveitis and Posner–Schlossman syndrome patients showed differences concerning amino acid metabolism (32, 33). Our previous metabolomics study of sweat also revealed an important role for amino acid metabolism in the pathogenesis of VKH disease (10). In our present study, we found 12 and 15 amino acids were differentially expressed in VKH vs. control and BD vs. control, respectively. When compared to controls, D-arginine, D-ornithine, and L-phenylalanine, as well as its catabolite phenylacetic acid were significantly increased in BD patients, suggesting a possible role for the pathways regulating these specific amino acids in the pathogenesis of BD. Several studies have reported that the pantothenate and CoA biosynthesis pathway is associated with amino acid metabolism, whereby branched-chain amino acids provide CoA derivatives to enter the tricarboxylic acid cycle (29, 34, 35). The involvement of pantothenate and CoA biosynthesis was evident from a decrease in L-valine and increase in uracil that we observed in VKH compared to controls.
Another interesting finding from our current study came from the analysis of Aminoacyl-tRNAs, which are the essential substrates that transport specific amino acids to incorporate them into the polypeptide chain produced during translation (36, 37). The aminoacyl-tRNA biosynthesis pathway is associated with various diseases, including several autoimmune diseases (38–40). Of interest is our finding that the most significant pathway in our study was aminoacyl-tRNA biosynthesis which involved L-Histidine, L-Methionine, L-Lysine and L-Valine for VKH, and L-Phenylalanine, L-Arginine, L-Methionine, L-Alanine, L-Lysine and L-Tryptophan for BD. Both VKH disease and BD showed an increase of L-Methionine and L-Lysine and the involvement of the associated aminoacyl-tRNA biosynthesis pathway (Figure 3). Therefore, we speculated that these two uveitis entities might share metabolic regulation pathways. Although these two uveitis entities show very specific features, they could be driven by common immunopathological mechanisms (41, 42).
There are several limitations in the current study. First, for ethical reasons, we used aqueous humor samples obtained during cataract surgery. Prior to cataract surgery for uveitis, the inflammation in these eyes has to be controlled and patients enrolled in our study are therefore in an inactive state. Despite this limitation, we do believe that the data are interesting because the composition of the aqueous humor can reflect the status of the basic intraocular environment in a uveitis patient. Second, the sample size used in our study may seem small, but aqueous humor is not easily accessible like serum or urine. In view of ethical considerations and current practice in our hospital, we only collected aqueous humor when the patient was undergoing an operation to remove a cataract from their eyes. These operations are not very frequently done and it therefore took us quite some time to collect the desired number of samples from these two well-defined uveitis entities. Most aqueous metabolomics studies in the field of ophthalmology consist of less than 30 samples in each group (32, 33). The relatively small sample size might limit the finding of other differentially expressed metabolites. This may explain why the significance of differential metabolites was lost when comparing VKH with BD. Both entities are very different from each other, both from a clinical and a pathological perspective (6). A larger population is therefore needed to validate the results in future. Third, patients enrolled in our study were still on treatment with a low dose of systemic corticosteroids and immunosuppressants and we cannot exclude an effect of treatment on our findings. It is not ethical to terminate therapy in these patients for the mere purpose of research. On the other hand our data are different from those reported in an earlier study on glucocorticoid-induced metabolome changes (43), and are in agreement with metabolic studies in uveitis (10, 11). Our control group consisted of senile cataract patients whose age is inevitable older than our uveitis patients, but again, it is not ethically justified to collect aqueous samples from age matched healthy controls. In view of this limitation, we used a univariate logistic regression analysis to adjust for age. Using an age-stratified analysis we found no significant differences for the 41 differential metabolites between the different age groups (Table S2). In our study we set the accuracy m/z value at 25 ppm, although a more strict setting of 10 ppm might also have been used. Many previous reports in this field use a setting of 25 ppm (10, 44). We believe that several metabolites might have been missed if one would use a smaller accuracy m/z value.
In summary, we found specific aqueous metabolic profiles for VKH disease and BD. Differential amino acid and fatty acid expression in aqueous humor could play important roles in the pathogenesis of the intraocular inflammation in these two diseases. We further provide evidence for a possible role of the aminoacyl-tRNA biosynthesis pathway in uveitis. Further research is needed to investigate the exact role of these metabolites and relevant metabolic pathways in the diagnosis and treatment of VKH disease and BD.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by the Ethics Committee of the First Affiliated Hospital of Chongqing Medical University. The patients/participants provided their written informed consent to participate in this study.
PY and JX conceived the idea and designed the study. PY, JX, and QC contributed to collecting the aqueous humor and clinical data. JX, XH, RC, ZC, ZY, and GS performed the experiments and analyzed the data. JX wrote the manuscript. PY and AK reviewed data interpretation and edited the manuscript. All authors contributed to the article and approved the submitted version.
This study was supported by National Natural Science Foundation Key Program (81930023), Natural Science Foundation Major International (Regional) Joint Research Project (81720108009), Chongqing Outstanding Scientists Project (2019), Chongqing Key Laboratory of Ophthalmology (CSTC, 2008CA5003), Chongqing Science & Technology Platform and Base Construction Program (cstc2014pt-sy10002), and the Chongqing Chief Medical Scientist Project (2018).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2021.587393/full#supplementary-material
1. Rothova A, Suttorp-van Schulten MS, Frits Treffers W, Kijlstra A. Causes and frequency of blindness in patients with intraocular inflammatory disease. Br J Ophthalmol (1996) 80:332–6. doi: 10.1136/bjo.80.4.332
2. McCluskey PJ, Towler HM, Lightman S. Management of chronic uveitis. BMJ (2000) 320:555–8. doi: 10.1136/bmj.320.7234.555
3. Yang P, Zhang Z, Zhou H, Li B, Huang X, Gao Y, et al. Clinical patterns and characteristics of uveitis in a tertiary center for uveitis in China. Curr Eye Res (2005) 30:943–8. doi: 10.1080/02713680500263606
4. Yang P, Zhong Z, Du L, Li F, Chen Z, Zhu Y, et al. Prevalence and clinical features of systemic diseases in Chinese patients with uveitis. Br J Ophthalmol (2021) 105:75–82. doi: 10.1136/bjophthalmol-2020-315960
5. Hwang DK, Chou YJ, Pu CY, Chou P. Epidemiology of uveitis among the Chinese population in Taiwan: a population-based study. Ophthalmology (2012) 119:2371–6. doi: 10.1016/j.ophtha.2012.05.026
6. Yang P, Ren Y, Li B, Fang W, Meng Q, Kijlstra A. Clinical characteristics of Vogt-Koyanagi-Harada syndrome in Chinese patients. Ophthalmology (2007) 114:606–14. doi: 10.1016/j.ophtha.2006.07.040
7. Yang P, Fang W, Meng Q, Ren Y, Xing L, Kijlstra A. Clinical features of chinese patients with Behçet’s disease. Ophthalmology (2008) 115:312–8.e4. doi: 10.1016/j.ophtha.2007.04.056
8. Wishart DS. Metabolomics for Investigating Physiological and Pathophysiological Processes. Physiol Rev (2019) 99:1819–75. doi: 10.1152/physrev.00035.2018
9. Oldiges M, Lütz S, Pflug S, Schroer K, Stein N, Wiendahl C. Metabolomics: current state and evolving methodologies and tools. Appl Microbiol Biotechnol (2007) 76:495–511. doi: 10.1007/s00253-007-1029-2
10. Cui X, Su G, Zhang L, Yi S, Cao Q, Zhou C, et al. Integrated omics analysis of sweat reveals an aberrant amino acid metabolism pathway in Vogt-Koyanagi-Harada disease. Clin Exp Immunol (2020) 200:250–9. doi: 10.1111/cei.13435
11. Ahn JK, Kim J, Hwang J, Song J, Kim KH, Cha HS. Urinary Metabolomic Profiling to Identify Potential Biomarkers for the Diagnosis of Behcet’s Disease by Gas Chromatography/Time-of-Flight-Mass Spectrometry. Int J Mol Sci (2017) 18:2039. doi: 10.3390/ijms18112309
12. Streilein JW. Ocular immune privilege: therapeutic opportunities from an experiment of nature. Nat Rev Immunol (2003) 3:879–89. doi: 10.1038/nri1224
13. Sindelar M, Patti GJ. Chemical Discovery in the Era of Metabolomics. J Am Chem Soc (2020) 142:9097–105. doi: 10.1021/jacs.9b13198
14. Fan Y, Li Y, Chen Y, Zhao YJ, Liu LW, Li J, et al. Comprehensive Metabolomic Characterization of Coronary Artery Diseases. J Am Coll Cardiol (2016) 68:1281–93. doi: 10.1016/j.jacc.2016.06.044
15. Gika HG, Theodoridis GA, Wingate JE, Wilson ID. Within-day reproducibility of an HPLC-MS-based method for metabonomic analysis: application to human urine. J Proteome Res (2007) 6:3291–303. doi: 10.1021/pr070183p
16. Yang P, Du L, Ye Z. How To Deal With Uveitis Patients? Curr Mol Med (2018) 17:468–70. doi: 10.2174/1566524018666180207153342
17. Read RW, Holland GN, Rao NA, Tabbara KF, Ohno S, Arellanes-Garcia L, et al. Revised diagnostic criteria for Vogt-Koyanagi-Harada disease: report of an international committee on nomenclature. Am J Ophthalmol (2001) 131:647–52. doi: 10.1016/s0002-9394(01)00925-4
18. Yang P, Zhong Y, Du L, Chi W, Chen L, Zhang R, et al. Development and Evaluation of Diagnostic Criteria for Vogt-Koyanagi-Harada Disease. JAMA Ophthalmol (2018) 136:1025–31. doi: 10.1001/jamaophthalmol.2018.2664
19. Criteria for diagnosis of Behçet’s disease. International Study Group for Behçet’s Disease. Lancet (1990) 335:1078–80.
20. Ji Y, Hu K, Li C, Li P, Kijlstra A, Eghrari AO, et al. Outcome and Prognostic Factors of Phacoemulsification Cataract Surgery in Vogt-Koyanagi-Harada Uveitis. Am J Ophthalmol (2018) 196:121–8. doi: 10.1016/j.ajo.2018.08.041
21. Luger D, Caspi RR. New perspectives on effector mechanisms in uveitis. Semin Immunopathol (2008) 30:135–43. doi: 10.1007/s00281-008-0108-5
22. Bing SJ, Shemesh I, Chong WP, Horai R, Jittayasothorn Y, Silver PB, et al. AS101 ameliorates experimental autoimmune uveitis by regulating Th1 and Th17 responses and inducing Treg cells. J Autoimmun (2019) 100:52–61. doi: 10.1016/j.jaut.2019.02.006
23. Qiu Y, Zhu Y, Yu H, Zhou C, Kijlstra A, Yang P. Dynamic DNA Methylation Changes of Tbx21 and Rorc during Experimental Autoimmune Uveitis in Mice. Mediators Inflamm (2018) 2018:9129163. doi: 10.1155/2018/9129163
24. Yaqoob P. Fatty acids as gatekeepers of immune cell regulation. Trends Immunol (2003) 24:639–45. doi: 10.1016/j.it.2003.10.002
25. Batista-Gonzalez A, Vidal R, Criollo A, Carreño LJ. New Insights on the Role of Lipid Metabolism in the Metabolic Reprogramming of Macrophages. Front Immunol (2019) 10:2993. doi: 10.3389/fimmu.2019.02993
26. Frommer KW, Schäffler A, Rehart S, Lehr A, Müller-Ladner U, Neumann E. Free fatty acids: potential proinflammatory mediators in rheumatic diseases. Ann Rheum Dis (2015) 74:303–10. doi: 10.1136/annrheumdis-2013-203755
27. Tsoukalas D, Fragoulakis V, Sarandi E, Docea AO, Papakonstaninou E, Tsilimidos G, et al. Targeted Metabolomic Analysis of Serum Fatty Acids for the Prediction of Autoimmune Diseases. Front Mol Biosci (2019) 6:120. doi: 10.3389/fmolb.2019.00120
28. Ahn JK, Kim J, Hwang J, Song J, Kim KH, Cha HS. Potential metabolomic biomarkers for reliable diagnosis of Behcet’s disease using gas chromatography/ time-of-flight-mass spectrometry. Joint Bone Spine (2018) 85:337–43. doi: 10.1016/j.jbspin.2017.05.019
29. Kelly B, Pearce EL. Amino Assets: How Amino Acids Support Immunity. Cell Metab (2020) 32:154–75. doi: 10.1016/j.cmet.2020.06.010
30. Johnson MO, Wolf MM, Madden MZ, Andrejeva G, Sugiura A, Contreras DC, et al. Distinct Regulation of Th17 and Th1 Cell Differentiation by Glutaminase-Dependent Metabolism. Cell (2018) 175:1780–95.e19. doi: 10.1016/j.cell.2018.10.001
31. Ron-Harel N, Ghergurovich JM, Notarangelo G, LaFleur MW, Tsubosaka Y, Sharpe AH, et al. T Cell Activation Depends on Extracellular Alanine. Cell Rep (2019) 28:3011–21.e4. doi: 10.1016/j.celrep.2019.08.034
32. Verhagen FH, Stigter ECA, Pras-Raves ML, Burgering BMT, Imhof SM, Radstake T, et al. Aqueous Humor Analysis Identifies Higher Branched Chain Amino Acid Metabolism as a Marker for Human Leukocyte Antigen-B27 Acute Anterior Uveitis and Disease Activity. Am J Ophthalmol (2019) 198:97–110. doi: 10.1016/j.ajo.2018.10.004
33. Wang H, Zhai R, Sun Q, Wu Y, Wang Z, Fang J, et al. Metabolomic Profile of Posner-Schlossman Syndrome: A Gas Chromatography Time-of-Flight Mass Spectrometry-Based Approach Using Aqueous Humor. Front Pharmacol (2019) 10:1322. doi: 10.3389/fphar.2019.01322
34. Neinast MD, Jang C, Hui S, Murashige DS, Chu Q, Morscher RJ, et al. Quantitative Analysis of the Whole-Body Metabolic Fate of Branched-Chain Amino Acids. Cell Metab (2019) 29:417–29.e4. doi: 10.1016/j.cmet.2018.10.013
35. Zhou J, Tang L, Wang JS. Assessment of the adverse impacts of aflatoxin B(1) on gut-microbiota dependent metabolism in F344 rats. Chemosphere (2019) 217:618–28. doi: 10.1016/j.chemosphere.2018.11.044
36. Sheppard K, Yuan J, Hohn MJ, Jester B, Devine KM, Söll D. From one amino acid to another: tRNA-dependent amino acid biosynthesis. Nucleic Acids Res (2008) 36:1813–25. doi: 10.1093/nar/gkn015
37. Yao P, Fox PL. Aminoacyl-tRNA synthetases in medicine and disease. EMBO Mol Med (2013) 5:332–43. doi: 10.1002/emmm.201100626
38. Santos ALM, Vitório JG, de Paiva MJN, Porto BLS, Guimarães HC, Canuto GAB, et al. Frontotemporal dementia: Plasma metabolomic signature using gas chromatography-mass spectrometry. J Pharm BioMed Anal (2020) 189:113424. doi: 10.1016/j.jpba.2020.113424
39. Zhang Q, Yin X, Wang H, Wu X, Li X, Li Y, et al. Fecal Metabolomics and Potential Biomarkers for Systemic Lupus Erythematosus. Front Immunol (2019) 10:976. doi: 10.3389/fimmu.2019.00976
40. Koch MW, Ilnytskyy Y, Golubov A, Metz LM, Yong VW, Kovalchuk O. Global transcriptome profiling of mild relapsing-remitting versus primary progressive multiple sclerosis. Eur J Neurol (2018) 25:651–8. doi: 10.1111/ene.13565
41. Geri G, Terrier B, Rosenzwajg M, Wechsler B, Touzot M, Seilhean D, et al. Critical role of IL-21 in modulating TH17 and regulatory T cells in Behçet disease. J Allergy Clin Immunol (2011) 128:655–64. doi: 10.1016/j.jaci.2011.05.029
42. Li F, Yang P, Liu X, Wang C, Hou S, Kijlstra A. Upregulation of interleukin 21 and promotion of interleukin 17 production in chronic or recurrent Vogt-Koyanagi-Harada disease. Arch Ophthalmol (2010) 128:1449–54. doi: 10.1001/archophthalmol.2010.265
43. Bordag N, Klie S, Jürchott K, Vierheller J, Schiewe H, Albrecht V, et al. Glucocorticoid (dexamethasone)-induced metabolome changes in healthy males suggest prediction of response and side effects. Sci Rep (2015) 5:15954. doi: 10.1038/srep15954
44. Zhou X, Liu L, Lan X, Cohen D, Zhang Y, Ravindran AV, et al. Polyunsaturated fatty acids metabolism, purine metabolism and inosine as potential independent diagnostic biomarkers for major depressive disorder in children and adolescents. Mol Psychiatry (2019) 24:1478–88. doi: 10.1038/s41380-018-0047-z
Keywords: Vogt-Koyanagi-Harada disease, Behcet’s disease, metabolomics, pathway, amino acids, fatty acids
Citation: Xu J, Su G, Huang X, Chang R, Chen Z, Ye Z, Cao Q, Kijlstra A and Yang P (2021) Metabolomic Analysis of Aqueous Humor Identifies Aberrant Amino Acid and Fatty Acid Metabolism in Vogt-Koyanagi-Harada and Behcet’s Disease. Front. Immunol. 12:587393. doi: 10.3389/fimmu.2021.587393
Received: 26 July 2020; Accepted: 08 January 2021;
Published: 22 February 2021.
Edited by:
Antonio Vitale, University of Siena, ItalyReviewed by:
Ouyang Hui, Jiangxi University of Traditional Chinese Medicine, ChinaCopyright © 2021 Xu, Su, Huang, Chang, Chen, Ye, Cao, Kijlstra and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peizeng Yang, cGVpemVuZ3ljbXVAMTI2LmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.