- 1Department of Rheumatology, The Third Affiliated Hospital of Sun Yat-Sen University, Guangzhou, China
- 2Medical Examination Center, The Third Affiliated Hospital of Sun Yat-Sen University, Guangzhou, China
- 3VIP Medical Center, The Third Affiliated Hospital of Sun Yat-Sen University, Guangzhou, China
Objective: Gout is a chronic disease characterized by the deposition of monosodium urate (MSU) crystals in tissue. Study with a focus on adaptive immune response remains to be understood although innate immune response has been reported extensively in gout etiology. Our study attempted to investigate the association of gout-related immune cell imbalance with clinical features and comorbidity with renal impairment and the implicated pathogenesis via the assessment of T and B cell subsets in different activity phases or with immune effects combined with the analyses of clinical parameters.
Methods: Fifty-eight gout patients and 56 age- and sex-matched healthy individuals were enrolled. To learn the roles of circulating T cells, a lymphocyte profile incorporating 32 T cell subsets was tested from isolated freshly peripheral blood monocyte cells (PBMCs) with multiple-color flow cytometry. Furthermore, the collected clinical features of participants were used to analyze the characteristics of these differential cell subsets. Stratified on the basis of the level of creatinine (Cr, enzymatic method), all patients were categorized into Crlow (Cr ≤ 116 μmol/L) and Crhi (Cr > 116 μmol/L) groups to exploit whether these gout-associated T cell subsets were functional in gout-targeted kidney dysfunction. The differentiation of B cells was investigated in gout patients.
Results: Our results show that CD 4+ T cells, Th2 cells, and Tc2 cells were upregulated, whereas Tc17 cells were downregulated. Tfh cells skewed toward the polarization of Tfh2 cells. Specifically, Tfh2 cells increased, but Tfh1 cells decreased, accompanied with aging for gout patients, suggesting that age might trigger the skewing of Tfh1/Tfh2 cell subsets to influence gout development. Moreover, Tfh2 cells were connected to renal dysfunction as well. No alterations of B cell subsets were observed in patients when compared to controls.
Conclusions: Our data demonstrate age-specific dysfunctions of Tfh1/2 cells in gout occurrence, and Tfh2 cell upregulation is associated with gout-targeted renal dysfunction. However, Tfh2 cells may function in auto-inflammatory gout independent of helping B differentiation, and an in-depth study remains to be conducted.
Introduction
Gout is a systemic disease caused by the deposition of monosodium urate (MSU) crystals in tissue (1), characterized most commonly by episodes of acute and extremely painful arthritis, normally impairing the big toe but involving other joints in some patients as well (2). Previous studies indicate that an MSU crystal–mediated cytoplasmic NOD-like receptor family pyrin domain containing 3 (NALP3) inflammasome pathway in the innate immune system exists in the progression of gouty inflammation (3, 4). Recently, some specific T cell populations were also reported to participate in adaptive immune responses in gouty arthritis. Lee et al. found that the receptor activator of nuclear factor-κ B ligand (RANKL)-expressed T cells destroy the bone to some degree in gout arthritis (5). The differentiation and activation of Th17 cells was induced by MSU crystals in the presence of IL-1α/β and IL-18 (6). However, to our knowledge, only a few investigations have exploited the roles of different T lymphocyte subsets in the predisposition to gout.
Gout attacks usually begin, ranging from the fourth to the sixth decade. A large epidemiological study in 2005 shows that the prevalence is about 1% in men aged within a range of 35–44 years and increases to more than 7% in those over 65 years (7). This evidence suggests that gout prevalence depends highly on age. Compelling proof identifies that aging causes the decline of the innate and adaptive immune systems, which is also called immunosenescence. Age-associated defects on T cell function and phenotypes are reported (8, 9). However, the association of aging with T cell function remains be delineated in gout.
In this study, we attempt to investigate gout-related immunophenotyping alterations and their associations with age and the comorbidity with renal dysfunction with flow cytometry in extensive lymphocyte profiles.
Methods
Ethics Statement
Ethics approval for this study was permitted by the medical ethics committee of the Third Affiliated Hospital of Sun Yat-sen University in compliance with the Declaration of Helsinki. The written informed consents were obtained from all participants for research use.
Study Subjects
Cases enrolled were outpatients and inpatients with gout registered in the rheumatology division of the Third Affiliated Hospital of Sun Yat-sen University. Healthy participants were enrolled from the medical examination center of the hospital. Gout patients were identified according to the American College of Rheumatology 1977 diagnosis criteria for gout. Participants aged ≤44 years were defined as young, and those >44 years old were defined as middle-aged and elderly according to World Health Organization criteria. Controls had no inflammatory or rheumatic diseases or cancers and no antibiotic or immune-suppressing medicine use. Gout participants were excluded if they had other rheumatic disorders, inflammatory diseases, or cancers. Clinical features were collected, including demographics, creatinine (Cr), and estimated glomerular filtration rate (eGFR).
Lymphocyte Immunophenotyping by Flow Cytometry
Immunophenotyping of peripheral blood lymphocytes was quantified with flow cytometry (BD) according to the manufacturer’s instructions. Peripheral blood mononuclear cells (PBMCs) from freshly collected EDTA-anticoagulated whole blood were isolated, followed by incubation and tests with a panel of the following individual or combinations of labeled antibodies encompassing CD3-PerCP-Cy5.5, CD4-APC-H7, CD8-BV510, CD127-BV421, CD25 L-PE, CD45RAL-FITC, CCR7-AF647, and CD28-PE-Cy7 for T cell subsets; CD3-APC-H7, CD4-PE-Cy7, CD8-PerCP-Cy5.5, CD183-AF488, CD196 (CCR6)-BV510, CXCR5 (CD185)-AF647, CD194-BV421, and CD279L-PE for T cell subsets; and CD45-APC-H7, CD19-PerCP-Cy5.5, CD27-BV421, IgD-BB515, IgM-BV510, CD38L-APC, CD24L-PE, and CD21-PE-Cy7 for B cell subsets or isotype-matched controls for 30 min. More detailed information about T/B cell labels for categorization are described in Table 1. For intracellular markers, fixed and targeted cells were fixed, permeabilized, and then stained using Alexa Fluor 488-anti-PD-1 (BD) to analyze. Finally, the cells were suspended with phosphate-buffered saline and then analyzed with BD flow cytometry.
Statistical Analysis
SPSS 23.0 software was used for statistical analysis. The Shapiro–Wilk test was used for distribution. Reference ranges were calculated using mean ± standard deviation for parametric data and median (P25, P75) for nonparametric data. The distinction between two groups was compared using 2-sided tests for parametric data and nonparametric Wilcoxon rank sum tests. Gender ratio was compared between controls and patients using a chi-square test. Multiple group comparisons were conducted by one-way ANOVA, followed by LSD analysis of variance. Association of variables with age and renal function parameters were tested using a parametric Pearson test or nonparametric Spearman’s rank correlation test. P<0.05 was considered as statistical significance.
Results
Some Specifically Changeable T Lymphocytes Were Observed in Gout Patients
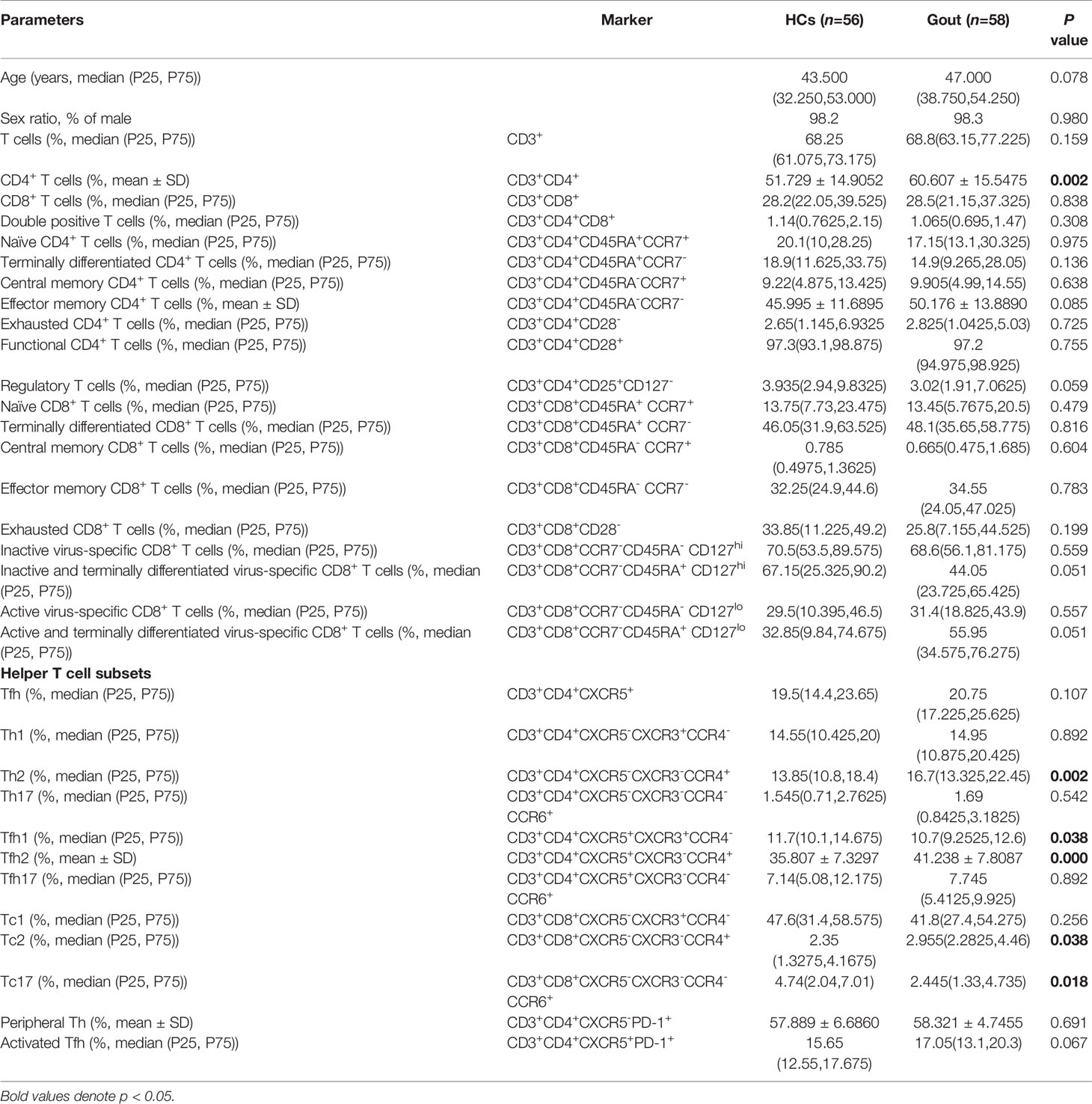
In the study, 58 gout cases and 56 healthy gender- and age-matched participants were enrolled for the assessment of human immune cell compartmentalization. The Chi-square test showed no distinctions for gender ratio between the case and control groups. To evaluate comprehensively the functional subpopulations of T lymphocytes in gout status, 32 T cell subsets were measured (Table 1). Median (P25, P75) used for mean age was 43.50 (32.25, 53.00) years in the healthy control (HC) group and 47.00 (38.75, 54.25) years in gout patients. When compared to the healthy cohort, five parameters containing CD3+CD4+ T cell percentage (p=0.002), and CD3+CD4+CXCR5-CXCR3-CCR4+ Th2 cell percentage (p=0.002), CD3+CD4+CXCR5+CXCR3-CCR4+ Tfh2 cell percentage (p=0.000) and CD3+CD8+CXCR5-CXCR3-CCR4+ Tc2 (p=0.038) were overexpressed and CD3+CD4+CXCR5+CXCR3+CCR4- Tfh1 (P=0.038) cells and CD3+CD8+CXCR5-CXCR3-CCR4-CCR6+ Tc17 (P=0.018) were underexpressed significantly in gout populations. Other T lymphocytes had no significant alterations. This evidence implies that the frequencies of some lymphocyte subsets were dysregulated in a gout attack.
The Dysregulation of Tfh1/Tfh2 Cell Subsets Is Age-Specific
To identify whether age was associated with the dysbiosis of the aforementioned gout-associated lymphocytes, individuals from both controls and cases were classified into young (age ≤44 years) and middle-aged and elderly subpopulations (age >44 years). Mean age (median (P25, P75)) was 32.00 (28.00, 38.00) years for 29 young subjects with a range of 26–44 years and was 53.00 (47.00, 57.00) for 26 middle-aged and elderly subjects with a range of 45–69 years in the healthy cohort. Mean age (median (P25, P75)) was 34.00 (28.50, 41.50) years for 21 young subjects with a range 22–44 years and was 52.00 (48.50, 61.50) for 37 middle-aged and elderly subjects with a range of 45–72 years in the gouty cohort. The ratio of young subjects and middle-aged and elderly subjects between the two cohorts was similar in statistics (P=0.094). Significant increased frequency of CD4+ T cells existed within healthy populations although there were no alterations of CD4+ T cells in the middle-aged and elderly populations (Supplementary Figure 1A). Dampened Tfh1 cells and enhanced Tfh2 cells were observed in middle-aged and elderly subjects within gout cases (Figures 1A, B). Tc2 cells were higher in middle-aged and elderly patients than young patients but did not change in healthy individuals (Supplementary Figure 1C). Other gout-related lymphocytes were found to be insignificant between the young and middle-aged and elderly groups (Supplementary Figures 1B, D). The association between age and Tfh1/Tfh2 cell percentage was analyzed using the Pearson or Spearman correlation test and showed a negative age association of Tfh1 cell percentage (r=−0.457, p<0.001, Figures 1C, D) but a positive age association of Tfh2 cell percentage in gout (r=0.452, p<0.001, Figures 1E, F). No significant age associations were found in the control cohort (P=0.399 for Tfh1, P=0.347 for Tfh2).
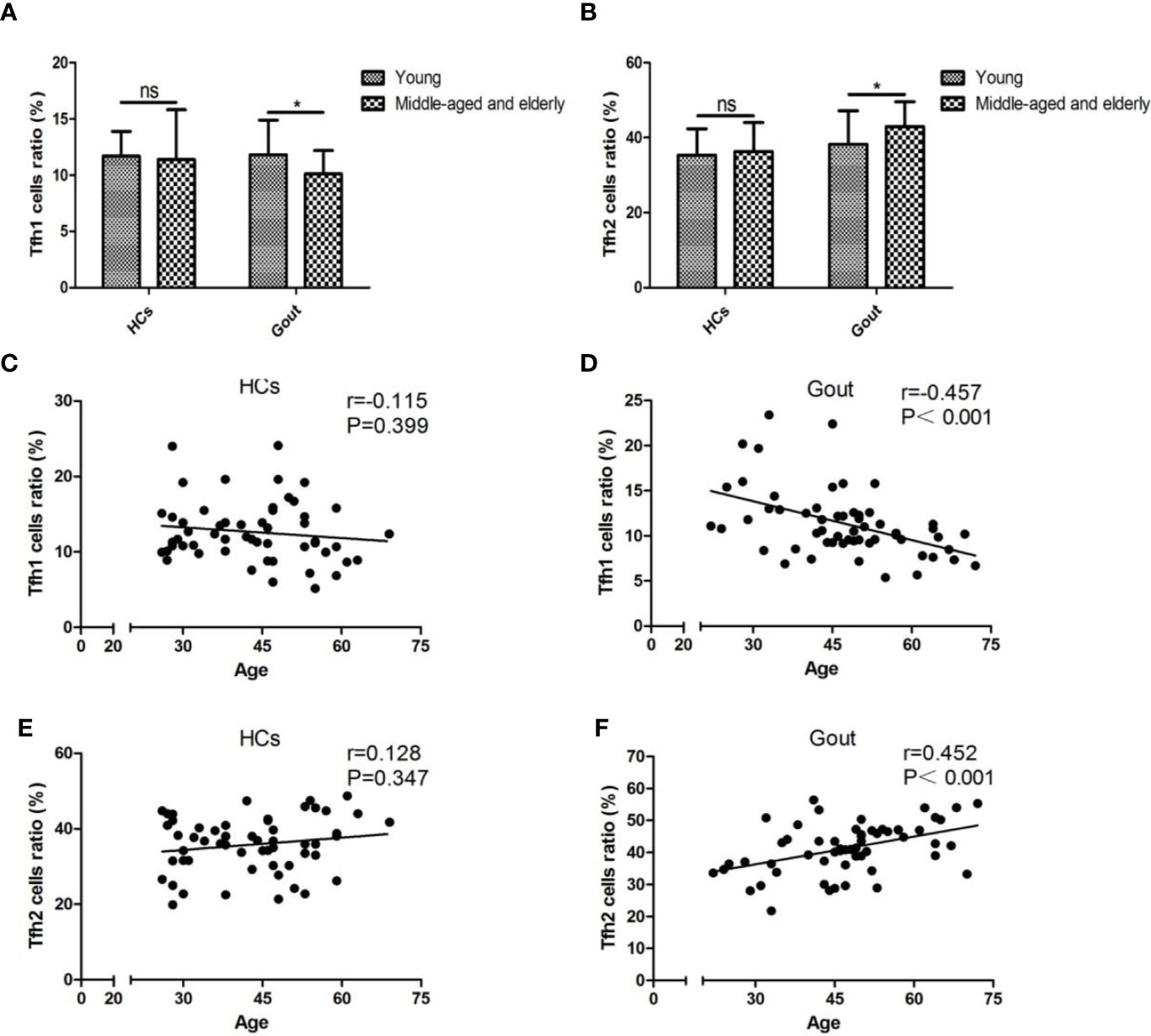
Figure 1 Tfh1/Tfh2 cell subsets are age-specific in gout patients. (A, B) The abundances of Tfh1 and Tfh2 cells are compared by t test for parametric data and the nonparametric Wilcoxon rank sum test between young and middle-aged and elderly populations in the HC and gout groups. Pearson or Spearman correlation analysis shows that unassociated with HCs expressing Tfh1 cells (C), age inversely correlated with Tfh1 cells of gout patients with a pronounced significance, P < 0.001 (D). Age has no significant association with Tfh2 cells of HCs, P=0.347 (E), whereas they are positively interrelated in the gout group, P < 0.001 (F). HCs, healthy controls; ns, not significant. *P < 0.05.
Age-Specific Tfh2 Cell Augmentation Is Linked With Kidney Dysfunction in Gout
According to the level of serum Cr, 32 gout patients were stratified into Crlow (Cr ≤ 116umol/L) and Crhi (Cr >116 umol/L) groups. More detailed results of clinical parameters, including Cr for the evaluation of kidney function, are described in Supplementary Table 1. Tfh2 cells were more abundant in gout patients with low Cr (P=0.019, Figure 2A) than controls and most abundant in patients with high Cr, which suggests that gout-aggravated kidney is associated with Tfh2 cell upregulation. We also observe that Tfh2 cells are positively associated with Cr (r=0.450, P< 0.05, Figure 2B) and inversely correlated with eGFR (r=0.453, P< 0.01, Figure 2C) in gout cases. To test whether age influenced kidney involvement related to Tfh2 cells in gout patients, a Pearson correlation coefficient analyzed the relation between age and Tfh2 cells in Crlow and Crhi gout populations. A positive association between age and Tfh2 cells was found exclusively in Crhi gout populations (r=0.794, P<0.05, Figure 2D). Collectively, age-specific Tfh2 cells might have a marked impact on targeted kidney impairment.
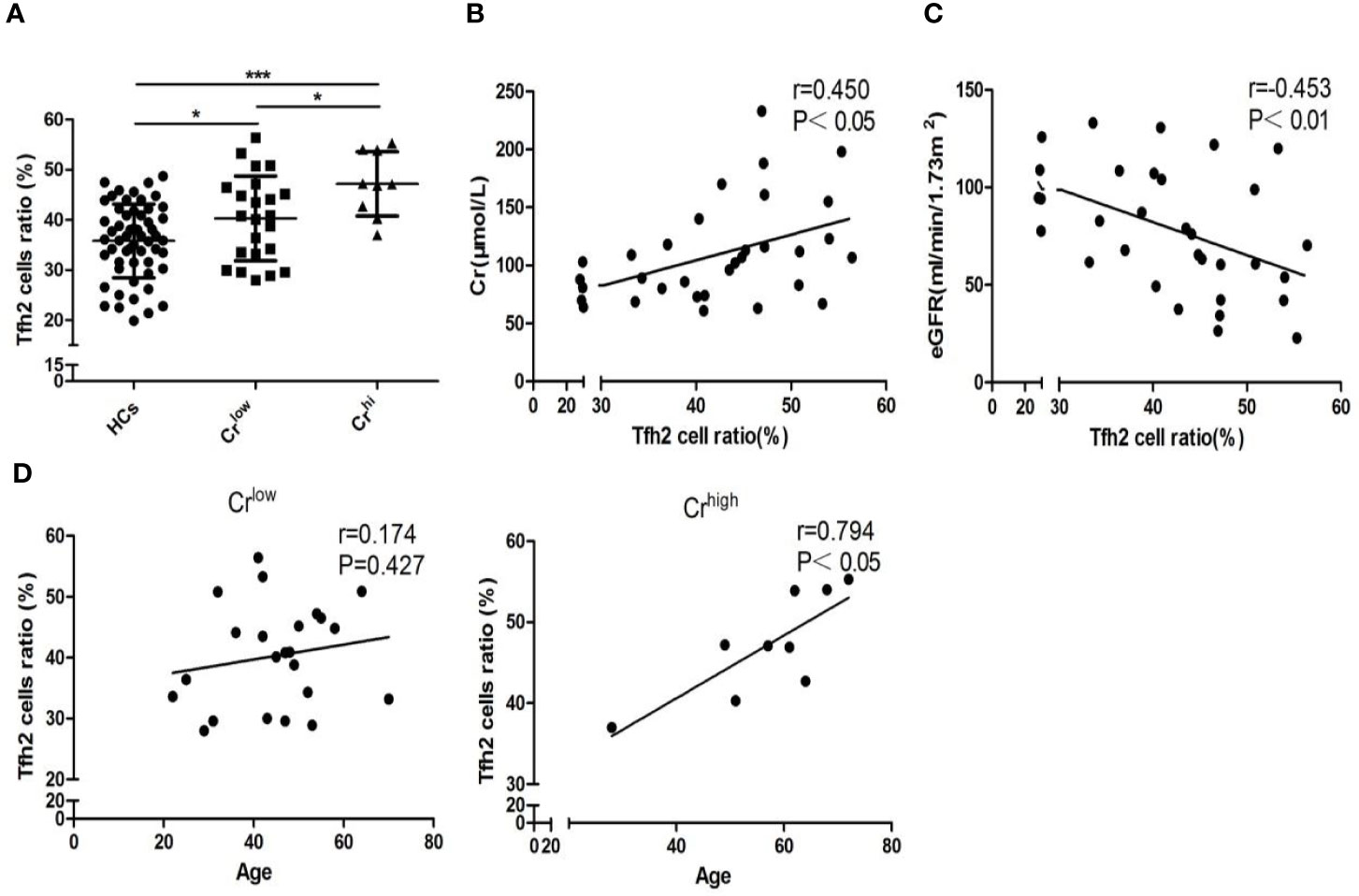
Figure 2 Comorbid kidney impairment linked with augmented age-specific Tfh2 cells. (A) Tfh2 cells of HCs, Crlow, and Crhi were compared based on one-ANOVO analysis followed by LSD analysis of variance. Patients have the most abundant Tfh2 cells in the Crhi group (P<0.05), and patients of the low Cr group are more abundant (P<0.05) when compared with controls. Furthermore, Pearson correlation analysis shows Tfh2 cells positively associated with Cr (B) and negatively associated with eGFR (C) of gout patients. (D) In the Crlow group, age is not relevant to Tfh2 cell expression, but it has a highly positive correlation in the Crhi group. HCs, healthy controls; Cr, creatinine; eGFR, estimated glomerular filtration rate. Crlow indicates those patients with Cr ≤ 116 umol/L (enzymic method). Crhi indicates those patients with Cr>116 umol/L (enzymic method) *P < 0.05. ***P < 0.001.
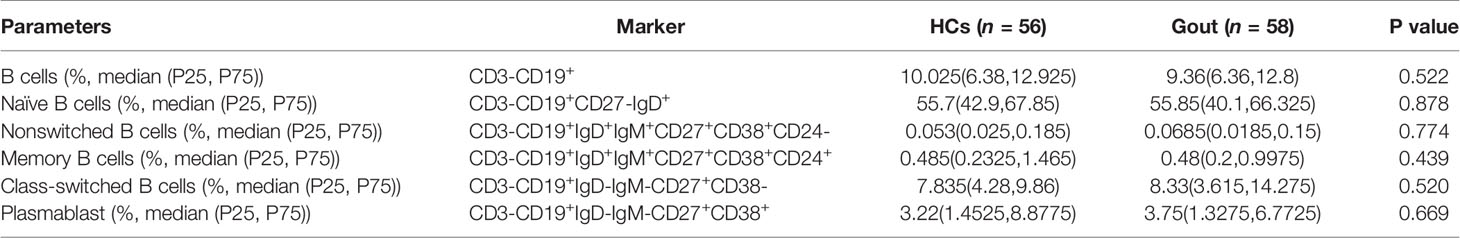
No Differential Distinctions Appeared in B Cell Development of Gout Patients
Mechanically, to interrogate the further pathway of Tfh cell subsets, we detected the different phases of B cells in the development process, including CD3-CD19+ total B cells, CD3-CD19+CD27-IgD+ naïve B cells, CD3-CD19+IgD+CD27- CD38lowCD21low cells, CD3-CD19+IgD+IgM+CD27+CD38+CD24- nonswitched B cells, CD3-CD19+IgD+IgM+CD27+CD38+CD24+ memory B cells, CD3-CD19+IgD-IgM-CD27+CD38- class-switched memory B cells, and CD3-CD19+IgD-IgM-CD27+CD38+ plasmablasts in circulation. As a consequence, there were no novel findings in the differential distinctions between gout patients and healthy controls (Table 2).
Discussion
Overall, our study shows gout patients exhibit an overrepresentation of CD4+ T cells, Th2 cells, Tfh2 cells, and Tc2 cells and an underrepresentation of Tfh1 cells and Tc17 cells. Initiatively, the skewing of circulating Tfh2 cell polarization was found to be age-specific in gout patients. Furthermore, the outgrowth of age-associated Tfh2 cells contributes to gouty comorbidity with renal impairment. To the best of our knowledge, this is a novel finding to report the association of Tfh cell subgroups with gout and organ deterioration.
MSU-triggered NLRP3 activation and derived cascades of innate immune responses have been expansively confirmed in gout etiology. Meanwhile, several studies focus on specific acquired immune cells, such as RANKL-expressing T cells (5) and Th17 cells (6). However, the role of different T lymphocyte subpopulations in circulation of gout patients remains obscure. Our study shows that some specific T lymphocytes were changed in gout, including CD4+ T, Th2, Tfh1, Tfh2, Tc2, and Tc17, which suggests gout is associated with out-of-control growth of T lymphocytes. Follicular helper T cells, a subset of CD4+ T cells, are specialized regulators of T cell help to B cells and are required for induction of germinal center (GC) B cell responses (10, 11). A disproportion dominated by Tfh2 cells and Tfh17 cells has been reported in several autoimmune and allergenic diseases such as juvenile dermatomyositis (12), allergic rhinitis (13), and systemic lupus (14).
In addition, our study found the balanced skewing of gout-perturbed Tfh1/Tfh2 cells was linked with aging. Since the 1990s, accumulated human and animal experiments were conducted devoted to the impact of aging on Th1 and Th2 cells, which share the same cytokine secretions with respective Tfh1 and Tfh2 cells (6) based on total T cells or CD4+ T cell–produced serum cytokine detection as well as analysis at the gene or protein level. Supporting us compellingly, Abb J et al. found the production of Th1-like interferon (IFN) gamma by human PBMCs in defending against virus infection was lower in elderly subjects (greater than 50 years) than those young populations (less than 50 years) (15). Aging is accompanied by progressive decline in immune function, termed immunosenescence (16), comprising the alterations of immune cell counts and functions. Gupta S noted that molecular signaling of extrinsic and intrinsic pathways of apoptosis or programmed cell death has been proposed to explain immunosenescence (17). Cell death occurs due to apoptosis, necrosis, and autophagy. Uric acid, one of these damage-associated molecular patterns (DAMPs) (18), released by dying cells can trigger an acute inflammatory response with the stimulation of tissue-resident macrophages to produce proinflammatory mediators (19). Dying cells stimulate DC activation as an adjuvant and promote T and B cell responses when injected into animals admixed with an antigen (20, 21). In addition, immunologically active MSU crystals have been shown to stimulate the activation of DCs and augment immune responses as well (18). Mirjam Kool reported that UA crystals activated dendritic cells to promote Th2 cell immunity through spleen tyrosine kinase and PI3-kinase d signaling but independent of the inflammasome (Nlrp3, Pycard) or the interleukin-1 (Myd88, IL-1r) axis (22). From this, we postulate that the release of uric acid from dying cells that accompanies aging may contribute to the skewing polarization of Tfh2 cells in Tfh cells; the possible mechanism remains to be investigated in depth.
Gouty patients are reported to have more renal impairment than those with osteoarthritis (7). Both allopurinol and febuxostat treatments support the hypothesis that hyperuricemia per se can have an adverse impact on kidney function (23). However, to date, this conundrum regarding the cause of the strong association between impaired nephron function and gout remain to be disentangled (24). In addition, we find that the presence of kidney comorbidity is merely associated with Tfh2 cell augmentation, which provides another perspective, to some extent, that age-induced Tfh2 cells might link gout and renal comorbidity.
Prior studies validate that Tfh cells produce IL-21 to promote GC B cell proliferation, class-switch recombination, memory B cell formation, and plasma cell differentiation (25, 26). To uncover the association between Tfh cells and antigen-dependent differentiation of B cells in gout, the distribution of the circulating B cell subpopulations were observed, containing total B cells, naïve B cells, nonswitched class B cells, switched class B cells, memory B cells, and plasmablasts. However, the pronounced disproportion of these subpopulations were not observed, suggesting that Tfh2 cells might function in the pathogenesis of gout in a B cell–independent manner. As documented by Zhang et al. (27) in inflammatory bowel disease, IRF8-regulated Tfh cells can function as B cell–independent and pathogenic mediators of colitis.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by Medical Ethics Committee of the Third Affiliated Hospital of Sun Yat-sen University. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author Contributions
Study conceptualization: YH, XW, LG, and JG. Data curation: YH, QW, QL, BJ, YW, and SC. Formal analysis: YH, XL, JO, YJ, and YX. Methodology: YH, XW, LG, XZ, JO, QW, ZC, LF, and MQ. Project administration and resources: JG. Software: YH, JO, ZC, XZ, YJ, and LT. Supervision: JG. Writing original draft: YH, XW, LG, and JG. Writing—review and editing: YH, XW, LG, YJ, LT, ZhL, ZeL, MQ, and JG. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor declared a past co-authorship with one of the authors JG.
Acknowledgments
Special thanks to the support from the National Key R&D Program of China (2016YFC0903500); Guangdong Science and Technology Plan Project (2016A020216013); and the “Five and five” Project of the Third Affiliated Hospital of Sun Yat-Sen University (k00004). The authors appreciate Prof. Zhinan Yin (The First Affiliated Hospital, Biomedical Translational Research Institute, Jinan University, Guangzhou 510632, China) for guiding this study design.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2020.625458/full#supplementary-material
References
1. Dalbeth N, Merriman TR, Stamp LK. Gout. Lancet (2016) 388(10055):2039–52. doi: 10.1016/S0140-6736(16)00346-9
3. Martin WJ, Walton M, Harper J. Resident macrophages initiating and driving inflammation in a monosodium urate monohydrate crystal-induced murine peritoneal model of acute gout. Arthritis Rheum (2009) 60(1):281–9. doi: 10.1002/art.24185
4. Martinon F, Pétrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature (2006) 440(7081):237–41. doi: 10.1038/nature04516
5. Lee SJ, Nam K, Jin HM, Cho YN, Lee SE, Kim TJ, et al. Bone destruction by receptor activator of nuclear factor B ligand-expressing T cells in chronic gouty arthritis. Arthritis Res Ther (2011) 13(5):R164. doi: 10.1186/ar3483
6. Conforti-Andreoni C, Spreafico R, Qian H. Uric acid-driven Th17 differentiation requires inflammasome-derived IL-1 and IL-18. J Immunol (2011) 187(11):5842–50. doi: 10.4049/jimmunol.1101408
7. Mikuls TR. Gout epidemiology: results from the UK General Practice Research Database, 1990-1999. Ann Rheum Dis (2005) 64(2):267–72. doi: 10.1136/ard.2004.024091
8. Fülöp T, Larbi A, Pawelec G. Human T Cell Aging and the Impact of Persistent Viral Infections. Front Immunol (2013) 4:271. doi: 10.3389/fimmu.2013.00271
9. Haynes L, Maue AC. Effects of aging on T cell function. Curr Opin Immunol (2009) 21(4):414–7. doi: 10.1016/j.coi.2009.05.009
10. Liu-Bryan R, Pritzker K, Firestein GS, Terkeltaub R. TLR2 signaling in chondrocytes drives calcium pyrophosphate dihydrate and monosodium urate crystal-induced nitric oxide generation. J Immunol (2005) 174(8):5016–23. doi: 10.4049/jimmunol.174.8.5016
11. Liu-Bryan R, Scott P, Sydlaske A, Rose DM, Terkeltaub R. Innate immunity conferred by toll-like receptors 2 and 4 and myeloid differentiation factor 88 expression is pivotal to monosodium urate monohydrate crystal-induced inflammation. Arthritis Rheum (2005) 52(9):2936–46. doi: 10.1002/art.21238
12. Morita R, Schmitt N, Bentebibel S, Ranganathan R, Bourdery L, Zurawski G, et al. Human Blood CXCR5+CD4+ T Cells Are Counterparts of T Follicular Cells and Contain Specific Subsets that Differentially Support Antibody Secretion. Immunity (2011) 34(1):108–21. doi: 10.1016/j.immuni.2010.12.012
13. Kamekura R, Shigehara K, Miyajima S, Jitsukawa S, Kawata K, Yamashita K, et al. Alteration of circulating type 2 follicular helper T cells and regulatory B cells underlies the comorbid association of allergic rhinitis with bronchial asthma. Clin Immunol (2015) 158(2):204–11. doi: 10.1016/j.clim.2015.02.016
14. Kim CJ, Lee C, Jung J, Ghosh A, Hasan SN, Hwang S, et al. The Transcription Factor Ets1 Suppresses T Follicular Helper Type 2 Cell Differentiation to Halt the Onset of Systemic Lupus Erythematosus. Immunity (2018) 49(6):1034–48. doi: 10.1016/j.immuni.2018.10.012
15. Abb J, Abb H, Deinhardt F. Age-related decline of human interferon alpha and interferon gamma production. Blut: Z für die Gesamte Blutforschung (1984) 48(5):285–9. doi: 10.1007/BF00320399
16. Pawelec G, Solana R. lmmunosenescence. Immunol Today (1997) 18(11):514–6. doi: 10.1016/S0167-5699(97)01145-6
17. Gupta S. A Matter of Life and Death of T-Lymphocytes in Immunosenescence. In: Immunosenescence. New York, NY: Springer New York (2007). p. 44–56. doi: 10.1007/978-0-387-76842-7_5
18. Shi Y, Evans JE, Rock KL. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature (2003) 425(6957):516–21. doi: 10.1038/nature01991
19. Kono H, Chen C, Ontiveros F, Rock KL. Uric acid promotes an acute inflammatory response to sterile cell death in mice. J Clin Invest (2010) 120(6):1939–49. doi: 10.1172/JCI40124
20. Gallucci S, Lolkema M, Matzinger P. Natural adjuvants: endogenous activators of dendritic cells. Nat Med (1999) 5(11):1249–55. doi: 10.1038/15200
21. Shi Y, Zheng W, Rock KL. Cell Injury Releases Endogenous Adjuvants that Stimulate Cytotoxic T Cell Responses. Proc Natl Acad Sci USA (2000) 97(26):14590–5. doi: 10.1073/pnas.260497597
22. Kool M, Willart MA, van Nimwegen M, Bergen I, Pouliot P, Virchow JC, et al. An unexpected role for uric acid as an inducer of T helper 2 cell immunity to inhaled antigens and inflammatory mediator of allergic asthma. Immunity (2011) 34(4):527–40. doi: 10.1016/j.immuni.2011.03.015
23. Gibson T. Hyperuricemia, gout and the kidney. Curr Opin Rheumatol (2012) 24(2):127–31. doi: 10.1097/BOR.0b013e32834f049f
24. Gibson T, Highton J, Potter C. Renal impairment and gout. Ann Rheum Dis (1980) 39(5):417–23. doi: 10.1136/ard.39.5.417
25. Johnston J, Hambly K, Foster D, Schrader S, Presnell SR, Parrish-Novak J, et al. Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature (2000) 408(6808):57–63. doi: 10.1038/35040504
26. Pene J, Gauchat JF, Lecart S, Drouet E, Guglielmi P, Boulay V, et al. Cutting edge: IL-21 is a switch factor for the production of IgG1 and IgG3 by human B cells. J Immunol (2004) 172(9):5154–7. doi: 10.4049/jimmunol.172.9.5154
Keywords: gout, Tfh1/2 cells, aging, B cells, kidney impairment
Citation: Huang Y, Wu X, Gui L, Jiang Y, Tu L, Li X, Jiang B, Wang Y, Zheng X, Wei Q, Li Q, Ou J, Chen Z, Xie Y, Lin Z, Liao Z, Fang L, Qiu M, Cao S and Gu J (2021) Age-Specific Imbalance of Circulating Tfh Cell Subsets and Its Association With Gout-Targeted Kidney Impairment. Front. Immunol. 11:625458. doi: 10.3389/fimmu.2020.625458
Received: 03 November 2020; Accepted: 16 November 2020;
Published: 11 January 2021.
Edited by:
James Cheng-Chung Wei, Chung Shan Medical University Hospital, TaiwanReviewed by:
Ming-Shiou Jan, Chung Shan Medical University, TaiwanXuewu Zhang, Peking University People’s Hospital, China
Copyright © 2021 Huang, Wu, Gui, Jiang, Tu, Li, Jiang, Wang, Zheng, Wei, Li, Ou, Chen, Xie, Lin, Liao, Fang, Qiu, Cao and Gu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jieruo Gu, Z3VqaWVydW9AMTYzLmNvbQ==
 Yefei Huang
Yefei Huang Xinyu Wu
Xinyu Wu Lian Gui1
Lian Gui1 Liudan Tu
Liudan Tu Xiaomin Li
Xiaomin Li Xuqi Zheng
Xuqi Zheng Qiujing Wei
Qiujing Wei Jiayong Ou
Jiayong Ou Zena Chen
Zena Chen Ya Xie
Ya Xie Zhiming Lin
Zhiming Lin Zetao Liao
Zetao Liao Shuangyan Cao
Shuangyan Cao Jieruo Gu
Jieruo Gu