- 1Department of Endocrinology and Metabolism, Laboratory of Endocrinology and Metabolism, National Clinical Research Center for Geriatrics, West China Hospital, Sichuan University, Chengdu, China
- 2Department of Integrated Traditional Chinese and Western Medicine, Laboratory of Endocrinology and Metabolism, West China Hospital, Sichuan University, Chengdu, China
The complex crosstalk between the immune and the skeletal systems plays an indispensable role in the maintenance of skeletal homeostasis. Various cytokines are involved, including interleukin (IL)-17A. A variety of immune and inflammatory cells produces IL-17A, especially Th17 cells, a subtype of CD4+ T cells. IL-17A orchestrates diverse inflammatory and immune processes. IL-17A induces direct and indirect effects on osteoclasts. The dual role of IL-17A on osteoclasts partly depends on its concentrations and interactions with other factors. Interestingly, IL-17A exerts a dual role in osteoblasts in vitro. IL-17A is a bone-destroying cytokine in numerous immune-mediated bone diseases including postmenopausal osteoporosis (PMOP), rheumatoid arthritis (RA), psoriatic arthritis (PsA) and axial spondylarthritis (axSpA). This review will summarize and discuss the pathophysiological roles of IL-17A on the skeletal system and its potential strategies for application in immune-mediated bone diseases.
Introduction
Over the last 20 years, a growing body of research has focused on the relationship between the skeletal and immune systems. Subsequently, the term “osteoimmunology” was defined for this field of study. Accumulating evidence has shown that multiple components of immune systems including immune organs, multiple immune cells, and immune factors, participate in bone metabolism. In turn, bone cells, including osteoclasts, osteoblasts, bone lining cells, and osteocytes, are indispensable for the regulation of immune systems. The interaction between the skeletal and immune systems constitutes a complex network and is involved in the pathological process of many immune-mediated bone diseases. Recent studies have shown that IL-17A as one of the immune-derived cytokines participates in the regulation of bone metabolism. Understanding the effect of IL-17A on bone metabolism is more conducive to develop new-targeted drugs for immune-related bone diseases. This review will summarize the current knowledge of IL-17A in the skeletal system and will discuss the potential clinical value of IL-17A in immune-mediated bone diseases.
IL-17A Signaling Pathway and Function
The IL-17 family includes six major isoforms: IL-17A, IL-17B, IL-17C, IL-17D, IL-17E, and IL-17F. Six of these isoforms interact with the five receptors (IL-17RA-E), respectively (1). IL-17A was the first member discovered and the most studied of the IL-17 family. Thereafter, following large-scale sequencing of the human and other vertebrate genomes, additional isoforms homologous to IL-17A were found (2). In 1993, Rouvier et al. cloned IL-17 for the first time. IL-17 was initially called the murine cytotoxic T lymphocyte-associated antigen-8 (mCTLA8) and was found to share 57% homology with the open reading frame 13 (ORF13) of Herpesvirus saimiri (HVS) (3). Subsequently, Yao et al. and Fossiez et al. cloned IL-17A in 1995 and 1996, respectively. Humans and mice share 25% amino acid sequence homology in IL-17A (4). IL-17A has been reported to be involved in inflammation and hematopoiesis and its secretion might be restricted to activated memory CD4 + T cells (4, 5). Current studies indicate that IL-17A is mainly produced by a special CD4+ T cell subtype, Th17 cells (6). In addition, other types of lymphocytes including IL-17+ CD8+ T cells (Tc17 cells) (7), invariant natural killer T (8), Foxp3+ Treg cells (9), γδ T cells (10), lymphoid−tissue inducer (LTi)−like cells (11), innate lymphoid cell (ILC3) (12), and NK cells can produce IL-17A. Besides, lymphocytes, myeloid cells including macrophages/monocytes (13), neutrophils (14), mast cells (15), Paneth cells (16) can secret IL-17A. Moreover, fibroblasts can also produce IL-17A (17). Multiple cytokines affect the expression of IL-17A, IL-1β, tumor necrosis factor (TNF)-β, IL-21, and IL-23 stimulate the expression of IL-17A in T cells (18), while interferon (IFN)-α inhibits the expression of IL-17A in T cells (19). Thus, IL-17A is derived from a variety of immune and inflammatory cells and its expression is regulated by a variety of immune factors.
IL-17A interacts with its receptors to activate downstream regulators and trigger cellular responses. Receptors for IL-17A are ubiquitously expressed on the cellular surface including synoviocytes, chondrocytes, fibroblasts, monocytes/macrophages, mast cells (20, 21). Bone cells including osteoclasts and osteoblasts also express IL-17RA (22). The interaction between IL-17RA and IL-17RC forms a complex to mediate the functions of IL-17A. The binding of IL-17A to the related receptor sites of IL-17RA alters the affinity and specificity of the symmetry receptor site. This response promotes the form of IL-17RA/RC heterodimer and makes an optimal response to mediate the functions of IL-17A homodimers (23, 24). Both IL-17RA and IL-17RC are type I transmembrane proteins. IL-17RA includes two extracellular fibronectin II-like domains and two intracellular “SEFIR” domains (25, 26). The SEFIR is homologous to Toll-IL-1R (TIR) domains found in the TLR/IL-1R family and is crucial for triggering downstream signaling events. IL-17A binds to its heterodimeric receptors complex and then recruits Act1 to activate classic IL-17A signaling cascades through receptor-associated factor 6 (TRAF6) proteins. TRAF6 binding subsequently triggers the mitogen-activated protein kinase (MAPK) pathway, extracellular signal–regulated kinase 1/2 (ERK1/2) pathway, and nuclear factor-κB (NF-κB) pathway. Among the non-classical signaling pathways, IL-17A integrates with epidermal growth factor receptor (EGFR), Notch 1, homolog translocation-associated (NOTCH1), C-type lectin receptor components, and interacts with fibroblast growth factor (FGF) signaling to initiate downstream biological responses (27).
In physiological conditions, IL-17A, as an immune and inflammatory-related factor, plays a protective role in host defenses against many bacterial and fungal pathogens (28). IL-17A activates neutrophils to promote neutrophil recruitment and accumulation (29). Meanwhile, IL-17A also affects the activity of B and T cells to act as a bridge between innate and acquired immune responses. Many studies have suggested that IL-17A is involved in the pathophysiological process of multiple diseases, including inflammatory bowel disease, breast cancer (30), lung cancer (31), cardiovascular system (32), uveitis (33), rheumatoid arthritis (RA), and psoriasis.
Effects of IL-17A on the Skeletal System
Osteoclasts
The skeleton maintains physiological function through a dynamic balance of bone formation and resorption. Osteoclasts derive from the monocyte/macrophage lineage and are key players in bone resorption. IL-17A acts directly on osteoclast precursors. Exposure to IL-17A (0.1–1 ng/ml) induces the expression of colony-stimulating factor-1 receptor (c-Fms) and receptor activator of nuclear factor-κB (RANK) on human peripheral blood mononuclear cells (hPBMCs), thereby promoting more hPBMCs to differentiate into functional osteoclasts. The effect is not dose-dependent, but 1 ng/ml of IL-17A shows the best induction (34). The direct effect of IL-17A on osteoclast precursors seems to be dependent on its concentration. A low concentration of IL-17A (0.5ng/ml) promotes autophagy of osteoclast precursors by activating the RANKL-JNK signaling pathway, thereby enhancing RANKL-induced osteoclast differentiation. However, treatment with a high concentration of IL-17A (5–50 ng/ml) inhibits autophagy and decreases osteoclast formation (35). In addition, a low level of IL-17A can reduce the apoptosis of osteoclasts and thus increases the number of osteoclasts by targeting the RANKL-Beclin1-autophagy-TRAF3 pathway (36). In turn, high levels of IL-17A increase apoptosis of osteoclasts and ultimately reduce pro-osteoclast mediators including cathepsin K, tartrate-resistant acid phosphatase (TRAP), and matrix metalloproteinase (MMP)-9 (36, 37). Interestingly, a higher concentration of IL-17A (100 ng/ml) promotes RANKL-induced polynuclear osteoclast formation and increases the expression of RANK and TRAP (38) (Figure 1).
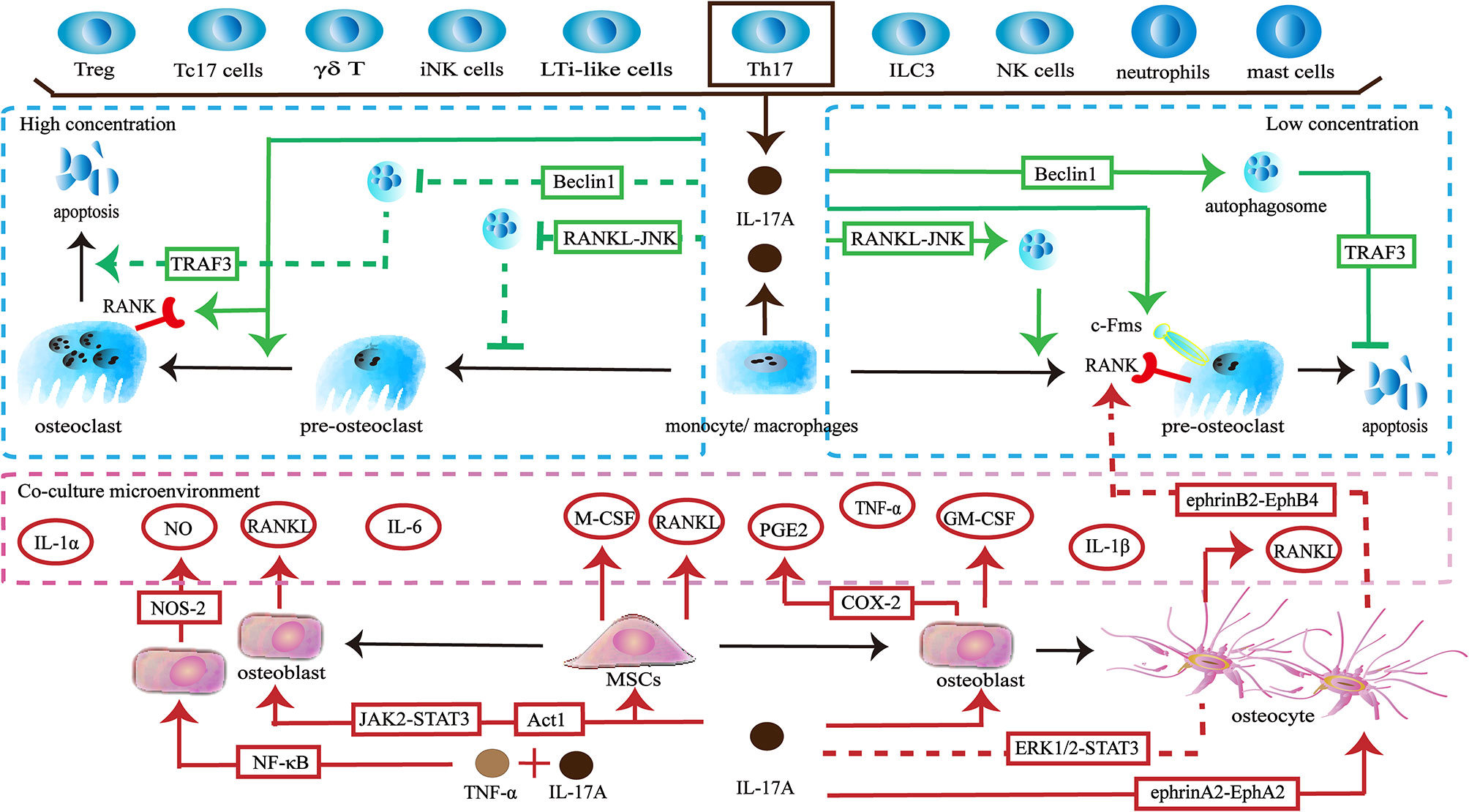
Figure 1 Effects of IL-17A on osteoclasts. ① IL-17A is produced by multiple lymphocytes including Treg cells, IL-17+ CD8+ T cells (Tc17 cells), γδ T cells, invariant natural killer T (iNK cells), and Th17 cells. Myeloid cells including macrophages/monocytes, neutrophils and mast cells can also secrete IL-17A. Th17 cells are the main source of IL-17A. ② Low concentrations of IL-17A promote osteoclastogenesis through the RANKL-JNK signaling pathway and reduces the apoptosis of osteoclasts through the RANKL-Beclin1-autophagy-TRAF3 pathway. IL-17A increases the expression of c-Fms in osteoclast precursors to promote proliferation and differentiation. IL-17A increases the number of RANK+ osteoclast precursors to influence subsequent RANKL-dependent osteoclastogenesis. ③ High concentration of IL-17A (5–50 ng/ml) inhibits osteoclastogenesis through the RANKL-JNK signaling pathway and promotes the apoptosis of osteoclasts through the RANKL-Beclin1-autophagy-TRAF3 pathway. However, a higher concentration of IL-17A (100 ng/ml) increases the number of RANK+ osteoclast precursors and induces polynuclear osteoclast formation. ④ IL-17A acts on osteoclast-supporting cells including mesenchymal stem cells, osteoblasts and osteocytes to produce various cytokines and molecules to regulate osteoclastogenesis indirectly.
Conversely, IL-17A can regulate osteoclast formation by targeting osteoclast-supporting cells. When activated by IL-17A, human bone marrow-derived mesenchymal stem cells (hBM-MSCs) secrete M-CSF and RANKL, thereby supporting osteoclastogenesis (39). When IL-17A binds to its receptors IL-17RA SEFIR/TILL domain on pre-osteoclasts, they trigger Act1 adaptor protein and may activate downstream JAK2-STAT3 signaling to promote the expression of RANKL (40–43). The upregulation of RANKL and the increase the ratio of RANKL/osteoprotegerin (OPG) promotes osteoclastogenesis (44, 45). Moreover, IL-17A stimulates osteoblast precursors to produce cyclooxygenase-2 (COX-2) related-prostaglandin E2 (PGE2), which is a facilitated factor in osteoclasts formation (46). The synergistic effects of IL-17A and TNF-α activate NF-κB-dependent pathways to promote the production of nitric oxide synthase-2 (NOS-2) and nitric oxide (NO). NO triggers the RANKL-RANK pathway to increase osteoclastic bone resorption (47). In addition, IL-17A and TNF-α synergistically induce osteoblast precursors to produce inflammatory factors including IL-1α, IL-1β and IL-6. These cytokines can up-regulate osteoclast activity (48). When activated by IL-17A, osteocytes inhibit the ERK1/2-STAT3 pathway and increase the RANKL/OPG ratio and TNF-α, thereby enhancing osteoclast formation. Furthermore, due to the activation of reversed ephrinA2-EphA2 signaling and suppression of ephrinB2-EphB4 signaling between osteocytes and osteoclast precursors, RANK+ bone marrow macrophages (BMMs) are increased, which influences subsequent RANKL-dependent osteoclastogenesis (49). In addition to providing osteoclastic activating factors, IL-17A can promote the expression of inhibitory factors. IL-17A promotes osteoblasts to produce granulocyte-macrophage colony-stimulating factor (GM-CSF), which in turn reduces the expression of RANK in osteoclast precursors and thus may weaken RANKL-RANK signaling to inhibit osteoclastogenesis (50). Moreover, GM-CSF maintains monocytes in an undifferentiated state by downregulating c-Fos, Fra-1, and nuclear factor of activated T cells 1 (Nfatc1) (51) (Figure 1).
The in vitro effects of IL-17A on osteoclasts are dual. Recent findings indicate that the direct effects of IL-17A on osteoclastogenesis are related to its concentration, but are not dose-dependent. Low concentration of IL-17A promotes osteoclastogenesis, while IL-17A begins to inhibit the formation of osteoclasts as the concentration increases. Strangely, further increases in the concentrations of IL-17A promote osteoclastogenesis. The precise relationship requires further exploration. In addition, IL-17A is involved in osteoclastogenesis via other types of cells and factors. The integrated network of cells and the factors they produce makes the specific effects attributable to IL-17A difficult to determine. The dominant effect may vary in different states. Thus, the role of IL-17A needs to be explored in more complex environments in vivo.
Osteoblasts
The osteoblast is another important player involved in maintaining bone homeostasis. When IL-17A binds receptors on pre-osteoblasts, it promotes their proliferation in a dose-dependent manner (49, 52, 53). When IL-17A activates TRAF6 and Act1 to initiate Ras-related C3 botulinum toxin substrate 1 guanosine triphosphatase (Rac1 GTPase) and NADPH oxidase 1 (Nox1), the expression of reactive oxygen species (ROS) is upregulated to promote pre-osteoblasts proliferation (39).
Slightly confusingly, the effects of IL-17A on osteoblastic differentiation in vitro are fraught with contradictions. A study showed that IL−17A promoted the differentiation of murine pre-osteoblastic MC3T3−E1 through the phosphoinositide 3-kinase-serine/threonine kinases (PI3K-AKT) pathway, whereas another study showed that IL−17A of the same concentration inhibited osteoblastic differentiation of MC3T3‐E1 (54, 55). IL-17A can cause an increase in the osteoblastic differentiation of murine calvarial osteoblasts by up-regulating the expression of genes involved in osteoblastic differentiation including Runx2, ALP, osterix, osteocalcin and type I collagen (Colla1), osteoprotegerin (OPG), bone sialoprotein (Ibsp), and osteopontin (Spp1) (43, 44). However, IL-17A inhibits osteogenic differentiation of rat calvarial osteoblast cells by down-regulating expression of genes involved in osteoblastic differentiation including Runx2, ALP, osterix, osteocalcin and type I collagen (56, 57). Different species lead to the expressed differential of IL-17R, which might partly explain this opposite effect (56). When activated by IL-17A, mice bone marrow mesenchymal stem cells (BM-MSCs) secrete IL‐6 and IL‐1β, thereby activating the AKT, STAT3, and ERK1/2 pathways to promote osteoblastic differentiation (55). However, IL-17A inhibits the Wnt signaling, resulting in reduced levels of osteoblast differentiation markers (osterix and osteocalcin) and early osteocyte markers (Dmp1 and Phex), thereby inhibiting osteoblastic differentiation of BM-MSCs (52). Moreover, IL-17A increases the expression of N-cadherin to inhibit PTHR1-LRP-6 interaction in osteoblasts, which also can inhibit the Wnt-signaling pathway (58) (Figure 2).
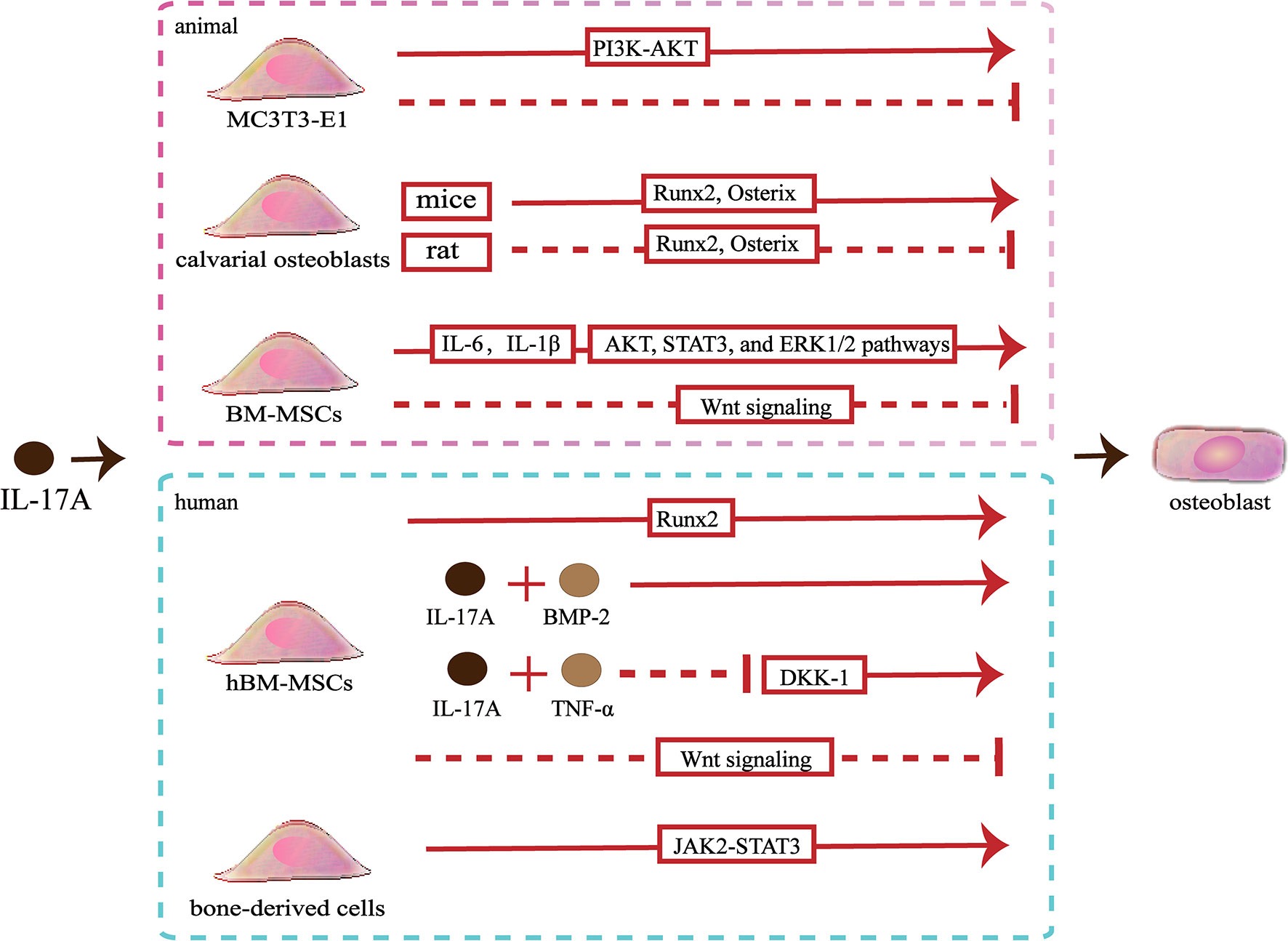
Figure 2 Effects of IL-17A on osteoblasts. ①IL−17A promotes MC3T3-E1 to differentiate into osteoblasts by activating the PI3K-AKT signaling pathways, whereas another study indicated that IL-17A inhibited osteoblastic differentiation of MC3T3-E1. ② IL-17A upregulates Runx2 and osterix expression to promote mice calvarial osteoblast to differentiate into mature osteoblast, whereas downregulates Runx2 and osterix expression to inhibit rat calvarial osteoblast to differentiate into mature osteoblast. ③ IL-17A promotes the secrete of IL‐6 and IL‐1β to activate the AKT, STAT3, and ERK1/2 pathways and promotes osteoblastic differentiation of bone marrow mesenchymal stem cells (BM-MSCs), whereas IL-17A inhibits osteoblastic differentiation through inhibiting the Wnt signaling pathway.④ IL-17A promotes human BM-MSCs (hBM-MSCs) to differentiate into osteoblasts by upregulating Runx2 expression. IL-17A with bone morphogenetic protein-2 (BMP-2) or with tumor necrosis factor (TNF)-α synergistically enhance osteogenic differentiation. However, IL-17A inhibits osteoblastic differentiation of hBM-MSCs by inhibiting the Wnt signaling pathway. ⑤ IL-17A promotes bone-derived cells to differentiate into osteoblasts by activating the JAK2-STAT3 signaling pathways.
Studies involving human pre-osteoblasts have indicated that IL-17A could promote bone-derived cells to differentiate into osteoblasts through JAK2/STAT3 signaling (59). In addition, IL-17A promotes the differentiation of hBM-MSCs into osteoblasts and promotes the mineralization of osteoblasts by upregulating bone formation-related gene ALP and Runx2 (39). The synergistic effects of IL-17A and bone morphogenetic protein-2 (BMP-2) promote the osteogenic differentiation of hBM-MSCs (60). Besides, the synergistic effects of IL-17A and TNF-α enhance osteogenic differentiation and mineralization of hBM-MSCs by down-regulating Dickkopf-1 (DKK-1), an inhibitor of the Wnt-signaling pathway (61). Osteoblasts and adipocytes are differentiated from a common pluripotent precursor, the mesenchymal stem cell (MSC). Many studies have suggested the differentiation decision of osteoblasts and adipocytes is delicately balanced and may even have a competitive relationship. IL-17A may steer mesenchymal stem cells into an osteogenic fate. IL-17A activates COX-2-induced prostaglandin E2 to inhibit lipid-related proteins include PPAR-γ, FABP4, and adiponectin. Therefore, the differentiation of hBM-MSCs into adipocytes is reduced (62). However, one study indicated that IL-17A inhibited osteogenic differentiation with up-regulated expression of the Wnt antagonist secreted frizzled-related protein 1 (sFRP1) and down-regulated expression of Wnt3 and Wnt6 in hBM-MSCs (63) (Figure 2).
The in vitro effects of IL-17A on osteoblasts are difficult to be defined. The effects of IL-17A on osteoblasts may not depend on the concentrations. IL-17A probably exerts distinct roles depending on the in vitro model used to assess osteoblast development. In addition, different species may also be partly responsible for the controversial results. It is not excluded that the different experimental methods also influence results. To achieve the precise effects of IL-17A on osteoblasts, the type of in vitro model, the correspondence between in vitro or in vivo effects, and the similarity of the effects between animal models and humans should be considered.
Effects of IL-17A on Bone Disease
The knockout of IL-17A or its receptors in animal models does not affect bone mass, osteoclast numbers, or osteoblast numbers (34, 40, 64–66). Moreover, neutralizing antibodies directed against IL-17A in wild-type mice also do not influence bone mass (65). These results indicate that IL-17A might not have any effect on bone under normal physiological conditions, and it only plays a role in inflammatory conditions or injury. The involvement of IL-17A in immune-mediated bone disease is worthy of exploration.
Postmenopausal Osteoporosis
Women undergoing natural menopause often experience postmenopausal osteoporosis (PMOP) with a decrease in bone mineral density (BMD) and an increased risk of fractures (67). Estrogen deficiency is the pivotal reason for PMOP. Estrogen deficiency increases osteoclast formation by increasing the number of hematopoietic progenitors and recruiting osteoclast progenitors. Likewise, estrogen deficiency allows prolonged survival of osteoclasts, and the net increase in bone resorption leads to bone loss (68, 69). Recent studies show that osteoimmunology is involved in the pathogenesis of PMOP. Furthermore, T-cell activity is increased while B-cell activity is decreased in postmenopausal women (70, 71). Estrogen deficiency can activate T cells and promotes the production of a variety of immune factors. These factors include IL-6 (72), TNF-α (73), IFN-γ (74), IL-1β, and TNF-β (75), all of which enhance bone loss.
Despite one study showing that the level of serum IL-17A in postmenopausal women with low BMD is not significantly different from that in women with normal BMD (76), other studies have indicated that postmenopausal women with osteoporosis have a higher concentration of serum IL-17A, and have more peripheral blood IL-17-producing CD4+ T-cells (58, 77–80). In postmenopausal women with osteoporosis, the concentration of serum IL-17A is negatively correlated with BMD, but is positively correlated with sRANKL level (78, 79).
In animal studies, ovariectomy (OVX) causes estrogen deficiency and bone loss. The drastic reduction of estrogen increases expression of the differentiation factors of Th17 including STAT3, ROR-α, and ROR-γt, which indicates that more peripheral blood mononuclear cells can differentiate into Th17 and produce IL-17A (80). The level of IL-17A in the bone marrow and blood are increased after OVX (38). IL-17RA knockdown and anti-IL17 antibody injection both protect against bone loss caused by estrogen deficiency (40). Anti-IL-17 antibodies and parathyroid hormone (PTH) can be used in combination to further protect OVX-induced bone loss (58, 81). Anti-IL17 antibodies exert a bone protective effect by inhibiting osteoclast formation, decreasing the apoptosis of osteoblasts and promoting the formation of mineralized nodules. Moreover, the blocking of IL-17A may inhibit osteoblasts to produce osteoclastogenic factors including TNF-α, IL-6, and RANKL in OVX mice (38, 40). Interestingly, anti-IL-17A antibodies have also been reported to reverse the higher frequency of CD4+ T cells and the proliferation of B220+ cells in bone marrow caused by estrogen deficiency. Anti-IL-17A antibodies exert an immuno-protective effect and translate to superior skeletal outcomes (81).
Rheumatoid Arthritis
RA is an autoimmune disease characterized by the upregulation of various immune factors that recruit and activate various immune cells, especially T and B cells to destroy cartilage and bone (82). RA patients have higher levels of IL-17A in synovial tissue and fluid compared with normal subjects (83–85). In a 2-year prospective study, the expression of IL-17A in synovial tissues was associated with increased joint damage progression in RA (86). Except for synovial tissue, RA patients have a higher concentration of serum IL-17A, which is proportional to the severity of RA (87–90). Moreover, the PBMCs of patients with RA produce more IL-17A (91). The increased levels of IL-17A in synovial fluid, serum, PBMCs are associated with the Disease Activity Score of 28 joints (DAS28), and levels of C-reactive protein (CRP), the erythrocyte sedimentation rate (ESR), and rheumatoid factor (RF) expression (92, 93). In addition, evidence suggests that IL-17A is not only related to the progression of the disease but is also associates with the occurrence of the disease. Studies indicate that IL-17A plays an important role in the pre-onset, early, and chronic stages of RA (94, 95).
Collagen-induced arthritis (CIA) is the most common animal model for studies involving RA (96). High levels of IL-17A are detected in CD4+ T cells and γδT cells located in joints of CIA mice (97). Th17 cells are localized adjacent to osteoclasts in the subarticular cartilage and express IL-17A, indicating the involvement of IL-17A in bone destruction of CIA (97). Local injection of IL-17A in the joint increases the morbidity of CIA and joint damage, while local injection of an adenoviral vector expressing murine IL-17A in the joint also accelerates the initiation of CIA and inflammation (98). Treatment with a soluble IL-17R fusion protein or anti-IL-17A antibody prevents bone erosion and the initiation of CIA (99, 100). In the progression of CIA, the local injection of IL-17A in knee-joint promotes arthritis and exacerbates joint damage (101). Anti-IL-17A antibodies ameliorate the severity of arthritis, cartilage damage, and bone loss (97, 102). Combinations that neutralize both TNF-α and IL-17A can also alleviate CIA progression (103). The combination of anti-IL-1β and anti-IL-17A antibodies significantly reduce the severity of arthritis, alleviates bone and cartilage damage, and down-regulates IL-1β, IL-6, IL-17A, IFN-γ, RANKL, and MMP-3 (104, 105). IL-17A plays an important role not only in the pathogenesis but also in the progression of the disease. Moreover, IL-17A is involved in the pathological process of bone erosion and bone loss.
The pathological mechanism of IL-17A may involve the immune activation and an immune cascade reaction in RA. In addition, the activation of osteoclasts promotes bone erosion in RA. Collagen-specific T cells and collagen-specific IgG2a are involved in the development of CIA. IL-17A is responsible for the priming of collagen-specific T cells and collagen-specific IgG2a production (106). Anti-IL-17A significantly reduces splenocytes proliferation and reduces leukocyte recruitment in CIA (105, 107). Anti-IL-17A also down-regulates IL-1β, IL-1, IL-6, IL-17A, and IFN-γ in the joint (104, 105). Increased osteoclast activity in the subchondral, trabecular, and cortical bone erosion areas is observed after local IL-17A overexpression in joint (98, 101, 102).
Several drugs targeting IL-17A are currently being evaluated in clinical trials, but the benefit seems to be not satisfactory for RA. Brodalumab, a human anti-IL-17 receptor A (IL-17RA) monoclonal antibody, did not demonstrate clinical efficacy in active RA patients (108). The humanized anti-IL-17A monoclonal antibody ixekizumab improved the signs and symptoms of RA patients in a phase II study, but the efficacy was not considered robust sufficient to support continued development (109). Bimekizumab is a monoclonal antibody that selectively neutralizes IL-17A and IL-17F. Bimekizumab plus certolizumab pegol further reduced disease activity score 28-joint count C-reactive protein (DAS28(CRP)) for RA patient in a phase II study, but more messages about the efficacy and safety is lack (110). Secukinumab, a fully human monoclonal antibody directed against IL-17A, has advanced in phase III studies. Secukinumab achieved 20% improvement in the American College of Rheumatology criteria (ACR20) at week 24 among patients with active RA, although, studies have suggested that secukinumab may not provide additional benefit beyond the currently approved therapies to such patients and further development was not pursued due to lack well-pleasing efficacy (111–114).
Psoriatic Arthritis
PsA is an immune-mediated chronic inflammatory arthritis associated with psoriasis. PsA presents synovial inflammation, bone destruction, and juxta-articular new bone formation (115, 116). Aberrant cytokine expression of TNF-α, IL-23, IL-22, IL-9, IL-15 is involved in the pathological mechanisms of PsA (117). Serum IL-17A levels are higher in psoriasis patients (118). IL-17+ CD4+ T cells and IL-17A secretion increase in peripheral blood and synovial fluid of PsA (119, 120). Besides CD4+ T cells, IL-17A-producing ILCs are present in the synovial fluid of PsA (121). IL-17A+CD8+ T cells are enriched in the joints of patients with PsA and have been correlated with disease activity and bone erosion (7).
In the animal model of PsA, increased serum IL-17A is associated with bone loss. The imbalance between osteoblasts and osteoclasts is the main cause for the appearance of PsA in the bone. Skin-resident cells such as keratinocytes, γδT cells, and innate lymphoid cells express IL-17A, which inhibits osteoblasts and osteocytes function through the Wnt signaling (52). In addition, IL-17A may also promote epidermal sheet, keratinocytes and skin resident T cells to produce RANKL (122).
Clinical trials of antagonizing IL-17A in PsA are underway. Secukinumab improves the signs and symptoms of active PsA (123). At the same time, secukinumab inhibits the progression of bone erosions and maintains bone stability (124–127). In 2016, secukinumab became the first targeting IL-17A drug approved by the FDA for the treatment of active PsA. Ixekizumab, an IL-17A specific monoclonal antibody, improved the signs and symptoms of patients with active PsA and inhibited bone damage progression in PsA (128, 129). In 2017, ixekizumab was approved by the FDA for the treatment of PsA. Brodalumab, a fully human monoclonal antibody targeting the IL-17 RA, achieved ACR20 at week 16 among patients with PsA in a phase III study (130). However, the trials were terminated early due to a possible safety concern about suicidal ideation and behavior (131). Bimekizumab, which inhibits both IL-17A and IL-17F, improved ACR50 in patients with active PsA in a phase II trial and phase III trials that are currently underway (132).
Axial Spondyloarthritis
Axial spondyloarthritis (axSpA) is chronic inflammatory bone diseases including non-radiographic axial spondyloarthritis (nr-axSpA) and radiographic axial spondyloarthritis (ankylosing spondylitis [AS]). Bone destruction and new bone formation may occur simultaneously in axSpA. Various types of cytokines including IL-17A, TNF-α and IL-23 are involved in the pathological processes (133, 134). Many studies have indicated that IL-17 is involved in immunopathogenesis of axSpA (135). IL-17+ CD4+ T cells increase in peripheral blood of axSpA and IL-17A synthesis also increases (120, 136–138). Levels of IL-17A in the synovial fluid are elevated in patients with AS (59). Serum IL-17A levels are also higher in AS and elevated IL-17 serum levels may associate with the development of AS (139, 140). A few studies have focused on the role of IL-17 in the processes of axSpA bone damage. IL-17A promotes local mesenchymal stem cell populations to osteoblast differentiation and increases mineralization in AS by JAK2/STAT3 signaling, which may be a mechanism of ankyloses progression (59). Anti-IL-17A treatment prevented bone loss and induced new bone formation in an animal model of pathogenic SpA, mycobacterium tuberculosis-induced disease in B27/hβ2m-transgenic rats (141).
Several IL-17A targeted drugs are currently in clinical trials. Secukinumab and Ixekizumab are both anti-interleukin-17A monoclonal antibodies and have been reported to improve the signs and symptoms of axSpA (142–148). To date, the FDA has approved both antibodies for the treatment of adults with active AS and nr-axSpA with objective signs of inflammation. Netakimab, a humanized monoclonal antibody targeting IL-17A, significantly achieved 20% improvement in Assessment of Spondyloarthritis International Society (ASAS20) response among patients with AS in a phase II study (149). Bimekizumab, a monoclonal antibody that selectively neutralizes IL-17A and IL-17F, achieved ASAS40 response at week 12 in a phase II trial (150). Phase III trials that aim to assess the efficacy and safety of netakimab and bimekizumab in AS patients are currently underway.
Conclusion and Perspectives
IL-17A is involved in innate immune responses and adaptive immunity. Meanwhile, IL-17A plays an important role in bone homeostasis via activation of complex cellular and molecular interactions. IL-17A may exert direct positive or negative effects on osteoclastogenesis depending on its concentration in vitro. Osteoblasts are most closely associated with osteoclasts, which both are involved in bone metabolism. IL-17A indirectly regulates osteoclastogenesis by inducing multiple factors derived from the osteoclast-supporting cells. The effects of IL-17A on osteoblasts may depend on the different experimental models of osteoblast development and species tested in vitro. These aforementioned cell studies provide evidence supporting the skeletal-regulatory properties of IL-17A and support the concept that IL-17A acts as the link between the skeletal and the immune systems. Future research should focus on the molecular pathways involved and explore the precise reasons for the dual effects of IL-17A in bone cells.
Mechanistic studies have hinted that IL-17A is a bone-destroying cytokine involved in immune-mediated bone diseases, such as PMOP, RA, PsA, and axSpA. IL-17A exerts a negative effect on bone by promoting osteoclastogenesis, excessively activates bone formation, and initiates an immunologic cascade. Indeed anti-IL-17A therapy has produced promising results in clinical trials of RA, PsA, and axSpA, although, few studies have focused on bone damage. A deeper understanding of the molecular mechanisms of IL-17A involved in bone disease may supply novel therapeutic interventions and provide a new thought to prevent bone loss and osteoporosis associated with immune-mediated bone diseases.
Author Contributions
XY provided the conception of the manuscript. MT and LL were contributed to perform the literature search and drafted the work. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by grants from the National Natural Science Foundation of China [No. 81770875]; the Sichuan University [No. 2018SCUH0093]; the Post-Doctor Research Project, West China Hospital, Sichuan University [No.19HXBH053]; the Health and Family Planning Commission of Sichuan Province [No. 19PJ096]; and the 1.3.5 project for discipline of excellence, West China Hospital, Sichuan University [No. 2020HXFH008, No. ZYJC18003]; the National Clinical Research Center for Geriatrics of West China Hospital (No. Z2018B05).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Liu S. Structural Insights into the Interleukin-17 Family Cytokines and Their Receptors. Adv Exp Med Biol (2019) 1172:97–117. doi: 10.1007/978-981-13-9367-9_5
2. Aggarwal S, Gurney AL. IL-17: prototype member of an emerging cytokine family. J leukocyte Biol (2002) 71(1):1–8. doi: 10.1189/jlb.71.1.1
3. Rouvier E, Luciani MF, Mattéi MG, Denizot F, Golstein P. CTLA-8, cloned from an activated T cell, bearing AU-rich messenger RNA instability sequences, and homologous to a herpesvirus saimiri gene. J Immunol (1993) 150(12):5445–56.
4. Yao Z, Painter SL, Fanslow WC, Ulrich D, Macduff BM, Spriggs MK, et al. Human IL-17: a novel cytokine derived from T cells. J Immunol (1995) 155(12):5483–6.
5. Fossiez F, Djossou O, Chomarat P, Flores-Romo L, Ait-Yahia S, Maat C, et al. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J Exp Med (1996) 183(6):2593–603. doi: 10.1084/jem.183.6.2593
6. Miossec P. IL-17 and Th17 cells in human inflammatory diseases. Microbes Infect (2009) 11(5):625–30. doi: 10.1016/j.micinf.2009.04.003
7. Menon B, Gullick NJ, Walter GJ, Rajasekhar M, Garrood T, Evans HG, et al. Interleukin-17+CD8+ T Cells Are Enriched in the Joints of Patients With Psoriatic Arthritis and Correlate With Disease Activity and Joint Damage Progression. Arthritis Rheumatol (2014) 66(5):1272–81. doi: 10.1002/art.38376
8. Venken K, Jacques P, Mortier C, Labadia ME, Decruy T, Coudenys J, et al. RORgammat inhibition selectively targets IL-17 producing iNKT and gammadelta-T cells enriched in Spondyloarthritis patients. Nat Commun (2019) 10(1):9. doi: 10.1038/s41467-018-07911-6
9. Zhu L, Song H, Zhang L, Meng H. Characterization of IL-17-producing Treg cells in type 2 diabetes patients. Immunologic Res (2019) 67(4-5):443–9. doi: 10.1007/s12026-019-09095-7
10. Coffelt SB, Kersten K, Doornebal CW, Weiden J, Vrijland K, Hau C-S, et al. IL-17-producing γδ T cells and neutrophils conspire to promote breast cancer metastasis. Nature (2015) 522(7556):345–8. doi: 10.1038/nature14282
11. Crellin NK, Trifari S, Kaplan CD, Cupedo T, Spits H. Human NKp44+IL-22+ cells and LTi-like cells constitute a stable RORC+ lineage distinct from conventional natural killer cells. J Exp Med (2010) 207(2):281–90. doi: 10.1084/jem.20091509
12. Triggianese P, Conigliaro P, Chimenti MS, Biancone L, Monteleone G, Perricone R, et al. Evidence of IL-17 producing innate lymphoid cells in peripheral blood from patients with enteropathic spondyloarthritis. Clin Exp Rheumatol (2016) 34(6):1085–93.
13. Okamoto N, Homma M, Kawaguchi Y, Kabasawa N, Uto Y, Hattori N, et al. Increased expression of interleukin-17 is associated with macrophages in chronic immune thrombocytopenia. Int J Clin Exp Pathol (2018) 11(5):2419–29.
14. Li L, Huang L, Vergis AL, Ye H, Bajwa A, Narayan V, et al. IL-17 produced by neutrophils regulates IFN-gamma-mediated neutrophil migration in mouse kidney ischemia-reperfusion injury. J Clin Invest (2010) 120(1):331–42. doi: 10.1172/JCI38702
15. Hueber AJ, Asquith DL, Miller AM, Reilly J, Kerr S, Leipe J, et al. Mast cells express IL-17A in rheumatoid arthritis synovium. J Immunol (Baltimore Md 1950) (2010) 184(7):3336–40. doi: 10.4049/jimmunol.0903566
16. Takahashi N, Vanlaere I, de Rycke R, Cauwels A, Joosten LAB, Lubberts E, et al. IL-17 produced by Paneth cells drives TNF-induced shock. J Exp Med (2008) 205(8):1755–61. doi: 10.1084/jem.20080588
17. Sheibanie AF, Khayrullina T, Safadi FF, Ganea D. Prostaglandin E2 exacerbates collagen-induced arthritis in mice through the inflammatory interleukin-23/interleukin-17 axis. Arthritis Rheum (2007) 56(8):2608–19. doi: 10.1002/art.22794
18. Golebski K, Ros XR, Nagasawa M, van Tol S, Heesters BA, Aglmous H, et al. IL-1β, IL-23, and TGF-β drive plasticity of human ILC2s towards IL-17-producing ILCs in nasal inflammation. Nat Commun (2019) 10(1):2162. doi: 10.1038/s41467-019-09883-7
19. Chalise J, Narendra S, Paudyal B, Magnusson M. Interferon alpha inhibits antigen-specific production of proinflammatory cytokines and enhances antigen-specific transforming growth factor beta production in antigen-induced arthritis. Arthritis Res Ther (2013) 15(5):R143. doi: 10.1186/ar4326
20. Nesmond S, Muller C, Le Naour R, Viguier M, Bernard P, Antonicelli F, et al. Characteristic Pattern of IL-17RA, IL-17RB, and IL-17RC in Monocytes/Macrophages and Mast Cells From Patients With Bullous Pemphigoid. Front Immunol (2019) 10:2107:2107. doi: 10.3389/fimmu.2019.02107
21. Zrioual S, Toh ML, Tournadre A, Zhou Y, Cazalis MA, Pachot A, et al. IL-17RA and IL-17RC receptors are essential for IL-17A-induced ELR+ CXC chemokine expression in synoviocytes and are overexpressed in rheumatoid blood. J Immunol (Baltimore Md 1950) (2008) 180(1):655–63. doi: 10.4049/jimmunol.180.1.655
22. Shen F, Gaffen SL. Structure-function relationships in the IL-17 receptor: implications for signal transduction and therapy. Cytokine (2008) 41(2):92–104. doi: 10.1016/j.cyto.2007.11.013
23. Krstic J, Obradovic H, Kukolj T, Mojsilovic S, Okic-Dordevic I, Bugarski D, et al. An Overview of Interleukin-17A and Interleukin-17 Receptor A Structure, Interaction and Signaling. Protein Pept Lett (2015) 22(7):570–8. doi: 10.2174/0929866522666150520145554
24. Liu S, Song X, Chrunyk BA, Shanker S, Hoth LR, Marr ES, et al. Crystal structures of interleukin 17A and its complex with IL-17 receptor A. Nat Commun (2013) 4:1888. doi: 10.1038/ncomms2880
25. Gaffen SL. Structure and signalling in the IL-17 receptor family. Nat Rev Immunol (2009) 9(8):556–67. doi: 10.1038/nri2586
27. Li X, Bechara R, Zhao J, McGeachy MJ, Gaffen SL. IL-17 receptor-based signaling and implications for disease. Nat Immunol (2019) 20(12):1594–602. doi: 10.1038/s41590-019-0514-y
28. Cypowyj S, Picard C, Maródi L, Casanova JL, Puel A. Immunity to infection in IL-17-deficient mice and humans. Eur J Immunol (2012) 42(9):2246–54. doi: 10.1002/eji.201242605
29. Zenobia C, Hajishengallis G. Basic biology and role of interleukin-17 in immunity and inflammation. Periodontol 2000 (2015) 69(1):142–59. doi: 10.1111/prd.12083
30. Fabre JAS, Giustinniani J, Garbar C, Merrouche Y, Antonicelli F, Bensussan A. The Interleukin-17 Family of Cytokines in Breast Cancer. Int J Mol Sci (2018) 19(12):3880. doi: 10.3390/ijms19123880
31. Wu F, Xu J, Huang Q, Han J, Duan L, Fan J, et al. The Role of Interleukin-17 in Lung Cancer. Mediators Inflammation (2016) 2016:8494079. doi: 10.1155/2016/8494079
32. Robert M, Miossec P. Effects of Interleukin 17 on the cardiovascular system. Autoimmun Rev (2017) 16(9):984–91. doi: 10.1016/j.autrev.2017.07.009
33. Guedes MC, Borrego LM, Proença RD. Roles of interleukin-17 in uveitis. Indian J Ophthalmol (2016) 64(9):628–34. doi: 10.4103/0301-4738.194339
34. Adamopoulos IE, Chao CC, Geissler R, Laface D, Blumenschein W, Iwakura Y, et al. Interleukin-17A upregulates receptor activator of NF-kappaB on osteoclast precursors. Arthritis Res Ther (2010) 12(1):R29. doi: 10.1186/ar2936
35. Ke D, Fu X, Xue Y, Wu H, Zhang Y, Chen X, et al. IL-17A regulates the autophagic activity of osteoclast precursors through RANKL-JNK1 signaling during osteoclastogenesis in vitro. Biochem Biophys Res Commun (2018) 497(3):890–6. doi: 10.1016/j.bbrc.2018.02.164
36. Xue Y, Liang Z, Fu X, Wang T, Xie Q, Ke D. IL-17A modulates osteoclast precursors’ apoptosis through autophagy-TRAF3 signaling during osteoclastogenesis. Biochem Biophys Res Commun (2019) 508(4):1088–92. doi: 10.1016/j.bbrc.2018.12.029
37. Kitami S, Tanaka H, Kawato T, Tanabe N, Katono-Tani T, Zhang F, et al. IL-17A suppresses the expression of bone resorption-related proteinases and osteoclast differentiation via IL-17RA or IL-17RC receptors in RAW264.7 cells. Biochimie (2010) 92(4):398–404. doi: 10.1016/j.biochi.2009.12.011
38. Tyagi AM, Srivastava K, Mansoori MN, Trivedi R, Chattopadhyay N, Singh D. Estrogen deficiency induces the differentiation of IL-17 secreting Th17 cells: a new candidate in the pathogenesis of osteoporosis. PloS One (2012) 7(9):e44552. doi: 10.1371/journal.pone.0044552
39. Huang H, Kim HJ, Chang EJ, Lee ZH, Hwang SJ, Kim HM, et al. IL-17 stimulates the proliferation and differentiation of human mesenchymal stem cells: implications for bone remodeling. Cell Death Differ (2009) 16(10):1332–43. doi: 10.1038/cdd.2009.74
40. DeSelm CJ, Takahata Y, Warren J, Chappel JC, Khan T, Li X, et al. IL-17 mediates estrogen-deficient osteoporosis in an Act1-dependent manner. J Cell Biochem (2012) 113(9):2895–902. doi: 10.1002/jcb.24165
41. Zhang F, Wang CL, Koyama Y, Mitsui N, Shionome C, Sanuki R, et al. Compressive force stimulates the gene expression of IL-17s and their receptors in MC3T3-E1 cells. Connective Tissue Res (2010) 51(5):359–69. doi: 10.3109/03008200903456942
42. Funaki Y, Hasegawa Y, Okazaki R, Yamasaki A, Sueda Y, Yamamoto A, et al. Resolvin E1 Inhibits Osteoclastogenesis and Bone Resorption by Suppressing IL-17-induced RANKL Expression in Osteoblasts and RANKL-induced Osteoclast Differentiation. Yonago Acta Med (2018) 61(1):8–18. doi: 10.33160/yam.2018.03.002
43. Wang Z, Tan J, Lei L, Sun W, Wu Y, Ding P, et al. The positive effects of secreting cytokines IL-17 and IFN-γ on the early-stage differentiation and negative effects on the calcification of primary osteoblasts in vitro. Int Immunopharmacol (2018) 57:1–10. doi: 10.1016/j.intimp.2018.02.002
44. Kim HJ, Seo SJ, Kim J-Y, Kim Y-G, Lee Y. IL-17 promotes osteoblast differentiation, bone regeneration, and remodeling in mice. Biochem Biophys Res Commun (2020) 55(4):1044–50. doi: 10.1016/j.bbrc.2020.02.054
45. Li JY, Yu M, Tyagi AM, Vaccaro C, Hsu E, Adams J, et al. IL-17 Receptor Signaling in Osteoblasts/Osteocytes Mediates PTH-Induced Bone Loss and Enhances Osteocytic RANKL Production. J Bone mineral Res Off J Am Soc Bone Mineral Res (2019) 34(2):349–60. doi: 10.1002/jbmr.3600
46. Zhang F, Tanaka H, Kawato T, Kitami S, Nakai K, Motohashi M, et al. Interleukin-17A induces cathepsin K and MMP-9 expression in osteoclasts via celecoxib-blocked prostaglandin E2 in osteoblasts. Biochimie (2011) 93(2):296–305. doi: 10.1016/j.biochi.2010.10.001
47. Van Bezooijen RL, Papapoulos SE, Lowik CW. Effect of interleukin-17 on nitric oxide production and osteoclastic bone resorption: is there dependency on nuclear factor-kappaB and receptor activator of nuclear factor kappaB (RANK)/RANK ligand signaling? Bone (2001) 28(4):378–86. doi: 10.1016/s8756-3282(00)00457-9
48. Van bezooijen RL, Farih-Sips HC, Papapoulos SE, Lowik CW. Interleukin-17: A new bone acting cytokine in vitro. J Bone mineral Res Off J Am Soc Bone Mineral Res (1999) 14(9):1513–21. doi: 10.1359/jbmr.1999.14.9.1513
49. Liao C, Cheng T, Wang S, Zhang C, Jin L, Yang Y. Shear stress inhibits IL-17A-mediated induction of osteoclastogenesis via osteocyte pathways. Bone (2017) 101:10–20. doi: 10.1016/j.bone.2017.04.003
50. Balani D, Aeberli D, Hofstetter W, Seitz M. Interleukin-17A stimulates granulocyte-macrophage colony-stimulating factor release by murine osteoblasts in the presence of 1,25-dihydroxyvitamin D(3) and inhibits murine osteoclast development in vitro. Arthritis Rheum (2013) 65(2):436–46. doi: 10.1002/art.37762
51. Atanga E, Dolder S, Dauwalder T, Wetterwald A, Hofstetter W. TNFalpha inhibits the development of osteoclasts through osteoblast-derived GM-CSF. Bone (2011) 49(5):1090–100. doi: 10.1016/j.bone.2011.08.003
52. Uluçkan Ö, Jimenez M, Karbach S, Jeschke A, Graña O, Keller J, et al. Chronic skin inflammation leads to bone loss by IL-17-mediated inhibition of Wnt signaling in osteoblasts. Sci Transl Med (2016) 8(330):330ra37. doi: 10.1126/scitranslmed.aad8996
53. Huang W, La Russa V, Alzoubi A, Schwarzenberger P. Interleukin-17A: a T-cell-derived growth factor for murine and human mesenchymal stem cells. Stem Cells (Dayton Ohio) (2006) 24(6):1512–8. doi: 10.1634/stemcells.2005-0156
54. Tan J-Y, Lei L-H, Chen X-T, Ding P-H, Wu Y-M, Chen L-L. AKT2 is involved in the IL−17A−mediated promotion of differentiation and calcification of murine preosteoblastic MC3T3−E1 cells. Mol Med Rep (2017) 16(5):5833–40. doi: 10.3892/mmr.2017.7315
55. Liao C, Zhang C, Jin L, Yang Y. IL-17 alters the mesenchymal stem cell niche towards osteogenesis in cooperation with osteocytes. J Cell Physiol (2020) 235(5):4466–80. doi: 10.1002/jcp.29323
56. Kim YG, Park JW, Lee JM, Suh JY, Lee JK, Chang BS, et al. IL-17 inhibits osteoblast differentiation and bone regeneration in rat. Arch Oral Biol (2014) 59(9):897–905. doi: 10.1016/j.archoralbio.2014.05.009
57. Zhang J-R, Pang D-D, Tong Q, Liu X, Su D-F, Dai S-M. Different Modulatory Effects of IL-17, IL-22, and IL-23 on Osteoblast Differentiation. Mediators Inflammation (2017) 2017:5950395–. doi: 10.1155/2017/5950395
58. Mansoori MN, Shukla P, Singh D. Combination of PTH (1-34) with anti-IL17 prevents bone loss by inhibiting IL-17/N-cadherin mediated disruption of PTHR1/LRP-6 interaction. Bone (2017) 105:226–36. doi: 10.1016/j.bone.2017.09.010
59. Jo S, Wang SE, Lee YL, Kang S, Lee B, Han J, et al. IL-17A induces osteoblast differentiation by activating JAK2/STAT3 in ankylosing spondylitis. Arthritis Res Ther (2018) 20(1):115–. doi: 10.1186/s13075-018-1582-3
60. Croes M, Öner FC, van Neerven D, Sabir E, Kruyt MC, Blokhuis TJ, et al. Proinflammatory T cells and IL-17 stimulate osteoblast differentiation. Bone (2016) 84:262–70. doi: 10.1016/j.bone.2016.01.010
61. Osta B, Lavocat F, Eljaafari A, Miossec P. Effects of Interleukin-17A on Osteogenic Differentiation of Isolated Human Mesenchymal Stem Cells. Front Immunol (2014) 425. doi: 10.3389/fimmu.2014.00425
62. Shin JH, Shin DW, Noh M. Interleukin-17A inhibits adipocyte differentiation in human mesenchymal stem cells and regulates pro-inflammatory responses in adipocytes. Biochem Pharmacol (2009) 77(12):1835–44. doi: 10.1016/j.bcp.2009.03.008
63. Wang Z, Jia Y, Du F, Chen M, Dong X, Chen Y, et al. IL-17A Inhibits Osteogenic Differentiation of Bone Mesenchymal Stem Cells via Wnt Signaling Pathway. Med Sci Monit Int Med J Exp Clin Res (2017) 23:4095–101. doi: 10.12659/msm.903027
64. Goswami J, Hernandez-Santos N, Zuniga LA, Gaffen SL. A bone-protective role for IL-17 receptor signaling in ovariectomy-induced bone loss. Eur J Immunol (2009) 39(10):2831–9. doi: 10.1002/eji.200939670
65. Li JY, D’Amelio P, Robinson J, Walker LD, Vaccaro C, Luo T, et al. IL-17A Is Increased in Humans with Primary Hyperparathyroidism and Mediates PTH-Induced Bone Loss in Mice. Cell Metab (2015) 22(5):799–810. doi: 10.1016/j.cmet.2015.09.012
66. Sato K, Suematsu A, Okamoto K, Yamaguchi A, Morishita Y, Kadono Y, et al. Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J Exp Med (2006) 203(12):2673–82. doi: 10.1084/jem.20061775
67. Compston JE, McClung MR, Leslie WD. Osteoporosis. Lancet (2019) 393(10169):364–76. doi: 10.1016/S0140-6736(18)32112-3
68. Black DM, Rosen CJ. Clinical Practice. Postmenopausal Osteoporosis. New Engl J Med (2016) 374(3):254–62. doi: 10.1056/NEJMcp1513724
69. Zhao R. Immune regulation of osteoclast function in postmenopausal osteoporosis: a critical interdisciplinary perspective. Int J Med Sci (2012) 9(9):825–32. doi: 10.7150/ijms.5180
70. Faienza MF, Ventura A, Marzano F, Cavallo L. Postmenopausal osteoporosis: the role of immune system cells. Clin Dev Immunol (2013) 2013:575936. doi: 10.1155/2013/575936
71. D’Amelio P, Grimaldi A, Di Bella S, Brianza SZM, Cristofaro MA, Tamone C, et al. Estrogen deficiency increases osteoclastogenesis up-regulating T cells activity: a key mechanism in osteoporosis. Bone (2008) 43(1):92–100. doi: 10.1016/j.bone.2008.02.017
72. Zhong Z, Qian Z, Zhang X, Chen F, Ni S, Kang Z, et al. Tetrandrine Prevents Bone Loss in Ovariectomized Mice by Inhibiting RANKL-Induced Osteoclastogenesis. Front Pharmacol (2020) 10:1530. doi: 10.3389/fphar.2019.01530
73. Kim B-J, Bae SJ, Lee S-Y, Lee Y-S, Baek J-E, Park S-Y, et al. TNF-α mediates the stimulation of sclerostin expression in an estrogen-deficient condition. Biochem Biophys Res Commun (2012) 424(1):170–5. doi: 10.1016/j.bbrc.2012.06.100
74. Xiong Q, Zhang L, Ge W, Tang P. The roles of interferons in osteoclasts and osteoclastogenesis. Joint bone Spine Rev du rhumatisme (2016) 83(3):276–81. doi: 10.1016/j.jbspin.2015.07.010
75. Gao Y, Qian WP, Dark K, Toraldo G, Lin AS, Guldberg RE, et al. Estrogen prevents bone loss through transforming growth factor beta signaling in T cells. Proc Natl Acad Sci USA (2004) 101(47):16618–23. doi: 10.1073/pnas.0404888101
76. Mannucci C, Calapai G, Gangemi S. Commentary: Circulatory pattern of cytokines, adipokines and bone markers in postmenopausal women with low BMD. Front Immunol (2019) 10:2666. doi: 10.3389/fimmu.2019.02666
77. Zhao R, Wang X, Feng F. Upregulated Cellular Expression of IL-17 by CD4+ T-Cells in Osteoporotic Postmenopausal Women. Ann Nutr Metab (2016) 68(2):113–8. doi: 10.1159/000443531
78. Zhang J, Fu Q, Ren Z, Wang Y, Wang C, Shen T, et al. Changes of serum cytokines-related Th1/Th2/Th17 concentration in patients with postmenopausal osteoporosis. Gynecol Endocrinol Off J Int Soc Gynecol Endocrinol (2015) 31(3):183–90. doi: 10.3109/09513590.2014.975683
79. Molnár I, Bohaty I, Somogyiné-Vári É. IL-17A-mediated sRANK ligand elevation involved in postmenopausal osteoporosis. Osteoporosis Int (2014) 25(2):783–6. doi: 10.1007/s00198-013-2548-6
80. Shukla P, Mansoori MN, Singh D. Efficacy of anti-IL-23 monotherapy versus combination therapy with anti-IL-17 in estrogen deficiency induced bone loss conditions. Bone (2018) 110:84–95. doi: 10.1016/j.bone.2018.01.027
81. Tyagi AM, Mansoori MN, Srivastava K, Khan MP, Kureel J, Dixit M, et al. Enhanced immunoprotective effects by anti-IL-17 antibody translates to improved skeletal parameters under estrogen deficiency compared with anti-RANKL and anti-TNF-alpha antibodies. J Bone mineral Res Off J Am Soc Bone Mineral Res (2014) 29(9):1981–92. doi: 10.1002/jbmr.2228
82. Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet (London England) (2016) 388(10055):2023–38. doi: 10.1016/s0140-6736(16)30173-8
83. Chabaud M, Durand JM, Buchs N, Fossiez F, Page G, Frappart L, et al. Human interleukin-17: A T cell-derived proinflammatory cytokine produced by the rheumatoid synovium. Arthritis Rheum (1999) 42(5):963–70. doi: 10.1002/1529-0131(199905)42:5<963::aid-anr15>3.0.co;2-e
84. Li N, Wang JC, Liang TH, Zhu MH, Wang JY, Fu XL, et al. Pathologic finding of increased expression of interleukin-17 in the synovial tissue of rheumatoid arthritis patients. Int J Clin Exp Pathol (2013) 6(7):1375–9.
85. Ziolkowska M, Koc A, Luszczykiewicz G, Ksiezopolska-Pietrzak K, Klimczak E, Chwalinska-Sadowska H, et al. High levels of IL-17 in rheumatoid arthritis patients: IL-15 triggers in vitro IL-17 production via cyclosporin A-sensitive mechanism. J Immunol (Baltimore Md 1950) (2000) 164(5):2832–8. doi: 10.4049/jimmunol.164.5.2832
86. Kirkham BW, Lassere MN, Edmonds JP, Juhasz KM, Bird PA, Lee CS, et al. Synovial membrane cytokine expression is predictive of joint damage progression in rheumatoid arthritis: a two-year prospective study (the DAMAGE study cohort). Arthritis Rheum (2006) 54(4):1122–31. doi: 10.1002/art.21749
87. Siloşi I, Boldeanu MV, Cojocaru M, Biciuşcă V, Pădureanu V, Bogdan M, et al. The Relationship of Cytokines IL-13 and IL-17 with Autoantibodies Profile in Early Rheumatoid Arthritis. J Immunol Res (2016) 2016:3109135. doi: 10.1155/2016/3109135
88. Costa CM, Santos M, Pernambuco AP. Elevated levels of inflammatory markers in women with rheumatoid arthritis. J Immunoassay Immunochem (2019) 40(5):540–54. doi: 10.1080/15321819.2019.1649695
89. Schofield C, Fischer SK, Townsend MJ, Mosesova S, Peng K, Setiadi AF, et al. Characterization of IL-17AA and IL-17FF in rheumatoid arthritis and multiple sclerosis. Bioanalysis (2016) 8(22):2317–27. doi: 10.4155/bio-2016-0207
90. Lee YH, Bae SC. Associations between circulating IL-17 levels and rheumatoid arthritis and between IL-17 gene polymorphisms and disease susceptibility: a meta-analysis. Postgraduate Med J (1102) 2017) 93:465–71. doi: 10.1136/postgradmedj-2016-134637
91. Kim KW, Cho ML, Park MK, Yoon CH, Park SH, Lee SH, et al. Increased interleukin-17 production via a phosphoinositide 3-kinase/Akt and nuclear factor kappaB-dependent pathway in patients with rheumatoid arthritis. Arthritis Res Ther (2005) 7(1):R139–48. doi: 10.1186/ar1470
92. Roşu A, Mărgăritescu C, Stepan A, Muşetescu A, Ene M. IL-17 patterns in synovium, serum and synovial fluid from treatment-naïve, early rheumatoid arthritis patients. Romanian J Morphol Embryol = Rev roumaine morphologie embryologie (2012) 53(1):73–80.
93. El-Maghraby HM, Rabie RA, Makram WK. Correlation between Relative Expression of IL 17 and PERP in Rheumatoid Arthritis Patients and Disease Activity. Egyptian J Immunol (2019) 26(2):19–29.
94. Kokkonen H, Söderström I, Rocklöv J, Hallmans G, Lejon K, Rantapää Dahlqvist S. Up-regulation of cytokines and chemokines predates the onset of rheumatoid arthritis. Arthritis Rheum (2010) 62(2):383–91. doi: 10.1002/art.27186
95. Raza K, Falciani F, Curnow SJ, Ross EJ, Lee CY, Akbar AN, et al. Early rheumatoid arthritis is characterized by a distinct and transient synovial fluid cytokine profile of T cell and stromal cell origin. Arthritis Res Ther (2005) 7(4):R784–95. doi: 10.1186/ar1733
96. Wu S, Meng Z, Zhang Y. Correlation between rheumatoid arthritis and immunological changes in a rheumatoid arthritis rat model. J Biol Regul Homeostatic Agents (2018) 32(6):1461–6.
97. Pollinger B, Junt T, Metzler B, Walker UA, Tyndall A, Allard C, et al. Th17 cells, not IL-17+ gammadelta T cells, drive arthritic bone destruction in mice and humans. J Immunol (Baltimore Md 1950) (2011) 186(4):2602–12. doi: 10.4049/jimmunol.1003370
98. Lubberts E, Joosten LA, van de Loo FA, Schwarzenberger P, Kolls J, van den Berg WB. Overexpression of IL-17 in the knee joint of collagen type II immunized mice promotes collagen arthritis and aggravates joint destruction. Inflammation Res Off J Eur Histamine Res Soc [et al] (2002) 51(2):102–4. doi: 10.1007/bf02684010
99. Bush KA, Farmer KM, Walker JS, Kirkham BW. Reduction of joint inflammation and bone erosion in rat adjuvant arthritis by treatment with interleukin-17 receptor IgG1 Fc fusion protein. Arthritis Rheum (2002) 46(3):802–5. doi: 10.1002/art.10173
100. Lubberts E, Joosten LA, Oppers B, van den Bersselaar L, Coenen-de Roo CJ, Kolls JK, et al. IL-1-independent role of IL-17 in synovial inflammation and joint destruction during collagen-induced arthritis. J Immunol (Baltimore Md 1950) (2001) 167(2):1004–13. doi: 10.4049/jimmunol.167.2.1004
101. Lubberts E, van den Bersselaar L, Oppers-Walgreen B, Schwarzenberger P, Coenen-de Roo CJ, Kolls JK, et al. IL-17 promotes bone erosion in murine collagen-induced arthritis through loss of the receptor activator of NF-kappa B ligand/osteoprotegerin balance. J Immunol (Baltimore Md 1950) (2003) 170(5):2655–62. doi: 10.4049/jimmunol.170.5.2655
102. Lubberts E, Koenders MI, Oppers-Walgreen B, van den Bersselaar L, Coenen-de Roo CJJ, Joosten LAB, et al. Treatment with a neutralizing anti-murine interleukin-17 antibody after the onset of collagen-induced arthritis reduces joint inflammation, cartilage destruction, and bone erosion. Arthritis Rheumatic (2004) 50(2):650–9. doi: 10.1002/art.20001
103. Shen F, Verma AH, Volk A, Jones B, Coleman BM, Loza MJ, et al. Combined Blockade of TNF-α and IL-17A Alleviates Progression of Collagen-Induced Arthritis without Causing Serious Infections in Mice. J Immunol (2019) 202(7):2017–26. doi: 10.4049/jimmunol.1801436
104. Zhang Y, Ren G, Guo M, Ye X, Zhao J, Xu L, et al. Synergistic effects of interleukin-1β and interleukin-17A antibodies on collagen-induced arthritis mouse model. Int Immunopharmacol (2013) 15(2):199–205. doi: 10.1016/j.intimp.2012.12.010
105. Li Q, Ren G, Xu L, Wang Q, Qi J, Wang W, et al. Therapeutic efficacy of three bispecific antibodies on collagen-induced arthritis mouse model. Int Immunopharmacol (2014) 21(1):119–27. doi: 10.1016/j.intimp.2014.04.018
106. Nakae S, Nambu A, Sudo K, Iwakura Y. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J Immunol (Baltimore Md 1950) (2003) 171(11):6173–7. doi: 10.4049/jimmunol.171.11.6173
107. Chao CC, Chen SJ, Adamopoulos IE, Davis N, Hong K, Vu A, et al. Anti-IL-17A therapy protects against bone erosion in experimental models of rheumatoid arthritis. Autoimmunity (2011) 44(3):243–52. doi: 10.3109/08916934.2010.517815
108. Martin DA, Churchill M, Flores-Suarez L, Cardiel MH, Wallace D, Martin R, et al. A phase Ib multiple ascending dose study evaluating safety, pharmacokinetics, and early clinical response of brodalumab, a human anti-IL-17R antibody, in methotrexate-resistant rheumatoid arthritis. Arthritis Res Ther (2013) 15(5):R164. doi: 10.1186/ar4347
109. Genovese MC, Greenwald M, Cho CS, Berman A, Jin L, Cameron GS, et al. A phase II randomized study of subcutaneous ixekizumab, an anti-interleukin-17 monoclonal antibody, in rheumatoid arthritis patients who were naive to biologic agents or had an inadequate response to tumor necrosis factor inhibitors. Arthritis Rheumatol (2014) 66(7):1693–704. doi: 10.1002/art.38617
110. Glatt S, Taylor PC, McInnes IB, Schett G, Landewé R, Baeten D, et al. Efficacy and safety of bimekizumab as add-on therapy for rheumatoid arthritis in patients with inadequate response to certolizumab pegol: a proof-of-concept study. Ann Rheumatic Dis (2019) 78(8):1033–40. doi: 10.1136/annrheumdis-2018-214943
111. Genovese MC, Durez P, Richards HB, Supronik J, Dokoupilova E, Aelion JA, et al. One-year Efficacy and Safety Results of Secukinumab in Patients With Rheumatoid Arthritis: Phase II, Dose-finding, Double-blind, Randomized, Placebo-controlled Study. J Rheumatol (2014) 41(3):414–21. doi: 10.3899/jrheum.130637
112. Blanco FJ, Möricke R, Dokoupilova E, Codding C, Neal J, Andersson M, et al. Secukinumab in Active Rheumatoid Arthritis: A Phase III Randomized, Double-Blind, Active Comparator- and Placebo-Controlled Study. Arthritis Rheumatol (2017) 69(6):1144–53. doi: 10.1002/art.40070
113. Huang Y, Fan Y, Liu Y, Xie W, Zhang Z. Efficacy and safety of secukinumab in active rheumatoid arthritis with an inadequate response to tumor necrosis factor inhibitors: a meta-analysis of phase III randomized controlled trials. Clin Rheumatol (2019) 38(10):2765–76. doi: 10.1007/s10067-019-04595-1
114. Dokoupilová E, Aelion J, Takeuchi T, Malavolta N, Sfikakis PP, Wang Y, et al. Secukinumab after anti-tumour necrosis factor-α therapy: a phase III study in active rheumatoid arthritis. Scand J Rheumatol (2018) 47(4):276–81. doi: 10.1080/03009742.2017.1390605
115. de Vlam K, Gottlieb AB, Mease PJ. Current concepts in psoriatic arthritis: pathogenesis and management. Acta Dermato Venereologica (2014) 94(6):627–34. doi: 10.2340/00015555-1833
116. Ritchlin CT, Colbert RA, Gladman DD. Psoriatic Arthritis. New Engl J Med (2017) 376(10):957–70. doi: 10.1056/NEJMra1505557
117. Veale DJ, Fearon U. The pathogenesis of psoriatic arthritis. Lancet (London England) (2018) 391(10136):2273–84. doi: 10.1016/s0140-6736(18)30830-4
118. Coimbra S, Oliveira H, Reis F, Belo L, Rocha S, Quintanilha A, et al. Interleukin (IL)-22, IL-17, IL-23, IL-8, vascular endothelial growth factor and tumour necrosis factor-α levels in patients with psoriasis before, during and after psoralen-ultraviolet A and narrowband ultraviolet B therapy. Br J Dermatol (2010) 163(6):1282–90. doi: 10.1111/j.1365-2133.2010.09992.x
119. Benham H, Norris P, Goodall J, Wechalekar MD, FitzGerald O, Szentpetery A, et al. Th17 and Th22 cells in psoriatic arthritis and psoriasis. Arthritis Res Ther (2013) 15(5):R136. doi: 10.1186/ar4317
120. Jandus C, Bioley G, Rivals JP, Dudler J, Speiser D, Romero P. Increased numbers of circulating polyfunctional Th17 memory cells in patients with seronegative spondylarthritides. Arthritis Rheum (2008) 58(8):2307–17. doi: 10.1002/art.23655
121. Leijten EF, van Kempen TS, Boes M, Michels-van Amelsfort JM, Hijnen D, Hartgring SA, et al. Brief report: enrichment of activated group 3 innate lymphoid cells in psoriatic arthritis synovial fluid. Arthritis Rheumatol (2015) 67(10):2673–8. doi: 10.1002/art.39261
122. Raimondo A, Lembo S, Di Caprio R, Donnarumma G, Monfrecola G, Balato N, et al. Psoriatic cutaneous inflammation promotes human monocyte differentiation into active osteoclasts, facilitating bone damage. Eur J Immunol (2017) 47(6):1062–74. doi: 10.1002/eji.201646774
123. McInnes IB, Mease PJ, Kirkham B, Kavanaugh A, Ritchlin CT, Rahman P, et al. Secukinumab, a human anti-interleukin-17A monoclonal antibody, in patients with psoriatic arthritis (FUTURE 2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet (London England) (2015) 386(9999):1137–46. doi: 10.1016/s0140-6736(15)61134-5
124. Mease P, van der Heijde D, Landewé R, Mpofu S, Rahman P, Tahir H, et al. Secukinumab improves active psoriatic arthritis symptoms and inhibits radiographic progression: primary results from the randomised, double-blind, phase III FUTURE 5 study. Ann Rheumatic Dis (2018) 77(6):890–7. doi: 10.1136/annrheumdis-2017-212687
125. Wu D, Li C, Zhang S, Wong P, Cao Y, Griffith JF, et al. Effect of biologics on radiographic progression of peripheral joint in patients with psoriatic arthritis: meta-analysis. Rheumatol (Oxford England) (2020) 59(11):3172–80. doi: 10.1093/rheumatology/keaa313
126. Kampylafka E, d’Oliveira I, Linz C, Lerchen V, Stemmler F, Simon D, et al. Resolution of synovitis and arrest of catabolic and anabolic bone changes in patients with psoriatic arthritis by IL-17A blockade with secukinumab: results from the prospective PSARTROS study. Arthritis Res Ther (2018) 20(1):153. doi: 10.1186/s13075-018-1653-5
127. Mease PJ, McInnes IB, Kirkham B, Kavanaugh A, Rahman P, van der Heijde D, et al. Secukinumab Inhibition of Interleukin-17A in Patients with Psoriatic Arthritis. New Engl J Med (2015) 373(14):1329–39. doi: 10.1056/NEJMoa1412679
128. Nash P, Kirkham B, Okada M, Rahman P, Combe B, Burmester GR, et al. Ixekizumab for the treatment of patients with active psoriatic arthritis and an inadequate response to tumour necrosis factor inhibitors: results from the 24-week randomised, double-blind, placebo-controlled period of the SPIRIT-P2 phase 3 trial. Lancet (London England) (2017) 389(10086):2317–27. doi: 10.1016/s0140-6736(17)31429-0
129. Mease PJ, van der Heijde D, Ritchlin CT, Okada M, Cuchacovich RS, Shuler CL, et al. Ixekizumab, an interleukin-17A specific monoclonal antibody, for the treatment of biologic-naive patients with active psoriatic arthritis: results from the 24-week randomised, double-blind, placebo-controlled and active (adalimumab)-controlled period of the phase III trial SPIRIT-P1. Ann Rheumatic Dis (2017) 76(1):79–87. doi: 10.1136/annrheumdis-2016-209709
130. Mease PJ, Helliwell PS, Hjuler KF, Raymond K, McInnes I. Brodalumab in psoriatic arthritis: results from the randomised phase III AMVISION-1 and AMVISION-2 trials. Ann Rheumatic Dis (2020) 80(2):185–93. doi: 10.1136/annrheumdis-2019-216835
131. Foulkes AC, Warren RB. Brodalumab in psoriasis: evidence to date and clinical potential. Drugs context (2019) 8:212570. doi: 10.7573/dic.212570
132. Ritchlin CT, Kavanaugh A, Merola JF, Schett G, Scher JU, Warren RB, et al. Bimekizumab in patients with active psoriatic arthritis: results from a 48-week, randomised, double-blind, placebo-controlled, dose-ranging phase 2b trial. Lancet (London England) (2020) 395(10222):427–40. doi: 10.1016/s0140-6736(19)33161-7
133. Sieper J, Braun J, Dougados M, Baeten D. Axial spondyloarthritis. Nat Rev Dis Primers (2015) 1:15013. doi: 10.1038/nrdp.2015.13
134. Ranganathan V, Gracey E, Brown MA, Inman RD, Haroon N. Pathogenesis of ankylosing spondylitis - recent advances and future directions. Nat Rev Rheumatol (2017) 13(6):359–67. doi: 10.1038/nrrheum.2017.56
135. Taams LS, Steel KJA, Srenathan U, Burns LA, Kirkham BW. IL-17 in the immunopathogenesis of spondyloarthritis. Nat Rev Rheumatol (2018) 14(8):453–66. doi: 10.1038/s41584-018-0044-2
136. Jansen DT, Hameetman M, van Bergen J, Huizinga TW, van der Heijde D, Toes RE, et al. IL-17-producing CD4+ T cells are increased in early, active axial spondyloarthritis including patients without imaging abnormalities. Rheumatol (Oxford England) (2015) 54(4):728–35. doi: 10.1093/rheumatology/keu382
137. Shen H, Goodall JC, Hill Gaston JS. Frequency and phenotype of peripheral blood Th17 cells in ankylosing spondylitis and rheumatoid arthritis. Arthritis Rheum (2009) 60(6):1647–56. doi: 10.1002/art.24568
138. Kenna TJ, Davidson SI, Duan R, Bradbury LA, McFarlane J, Smith M, et al. Enrichment of circulating interleukin-17-secreting interleukin-23 receptor-positive γ/δ T cells in patients with active ankylosing spondylitis. Arthritis Rheum (2012) 64(5):1420–9. doi: 10.1002/art.33507
139. Mei Y, Pan F, Gao J, Ge R, Duan Z, Zeng Z, et al. Increased serum IL-17 and IL-23 in the patient with ankylosing spondylitis. Clin Rheumatol (2011) 30(2):269–73. doi: 10.1007/s10067-010-1647-4
140. Liu W, Wu YH, Zhang L, Liu XY, Xue B, Wang Y, et al. Elevated serum levels of IL-6 and IL-17 may associate with the development of ankylosing spondylitis. Int J Clin Exp Med (2015) 8(10):17362–76.
141. van Tok MN, van Duivenvoorde LM, Kramer I, Ingold P, Pfister S, Roth L, et al. Interleukin-17A Inhibition Diminishes Inflammation and New Bone Formation in Experimental Spondyloarthritis. Arthritis Rheumatol (2019) 71(4):612–25. doi: 10.1002/art.40770
142. Pavelka K, Kivitz A, Dokoupilova E, Blanco R, Maradiaga M, Tahir H, et al. Efficacy, safety, and tolerability of secukinumab in patients with active ankylosing spondylitis: a randomized, double-blind phase 3 study, MEASURE 3. Arthritis Res Ther (2017) 19(1):285. doi: 10.1186/s13075-017-1490-y
143. Baraliakos X, Kivitz AJ, Deodhar AA, Braun J, Wei JC, Delicha EM, et al. Long-term effects of interleukin-17A inhibition with secukinumab in active ankylosing spondylitis: 3-year efficacy and safety results from an extension of the Phase 3 MEASURE 1 trial. Clin Exp Rheumatol (2018) 36(1):50–5.
144. Ashany D, Stein EM, Goto R, Goodman SM. The Effect of TNF Inhibition on Bone Density and Fracture Risk and of IL17 Inhibition on Radiographic Progression and Bone Density in Patients with Axial Spondyloarthritis: a Systematic Literature Review. Curr Rheumatol Rep (2019) 21(5):20. doi: 10.1007/s11926-019-0818-9
145. Deodhar A, Blanco R, Dokoupilová E, Hall S, Kameda H, Kivitz AJ, et al. Secukinumab improves signs and symptoms of non-radiographic axial spondyloarthritis: primary results of a randomized controlled phase III study. Arthritis Rheumatol (2020) 73(1):110–20. doi: 10.1002/art.41477
146. van der Heijde D, Cheng-Chung Wei J, Dougados M, Mease P, Deodhar A, Maksymowych WP, et al. Ixekizumab, an interleukin-17A antagonist in the treatment of ankylosing spondylitis or radiographic axial spondyloarthritis in patients previously untreated with biological disease-modifying anti-rheumatic drugs (COAST-V): 16 week results of a phase 3 randomised, double-blind, active-controlled and placebo-controlled trial. Lancet (London England) (2018) 392(10163):2441–51. doi: 10.1016/s0140-6736(18)31946-9
147. Dougados M, Wei JC, Landewé R, Sieper J, Baraliakos X, Van den Bosch F, et al. Efficacy and safety of ixekizumab through 52 weeks in two phase 3, randomised, controlled clinical trials in patients with active radiographic axial spondyloarthritis (COAST-V and COAST-W). Ann Rheumatic Dis (2020) 79(2):176–85. doi: 10.1136/annrheumdis-2019-216118
148. Deodhar A, van der Heijde D, Gensler LS, Kim TH, Maksymowych WP, Østergaard M, et al. Ixekizumab for patients with non-radiographic axial spondyloarthritis (COAST-X): a randomised, placebo-controlled trial. Lancet (London England) (2020) 395(10217):53–64. doi: 10.1016/s0140-6736(19)32971-x
149. Erdes S, Nasonov E, Kunder E, Pristrom A, Soroka N, Shesternya P, et al. Primary efficacy of netakimab, a novel interleukin-17 inhibitor, in the treatment of active ankylosing spondylitis in adults. Clin Exp Rheumatol (2020) 38(1):27–34.
150. van der Heijde D, Gensler LS, Deodhar A, Baraliakos X, Poddubnyy D, Kivitz A, et al. Dual neutralisation of interleukin-17A and interleukin-17F with bimekizumab in patients with active ankylosing spondylitis: results from a 48-week phase IIb, randomised, double-blind, placebo-controlled, dose-ranging study. Ann Rheumatic Dis (2020) 79(5):595–604. doi: 10.1136/annrheumdis-2020-216980
Keywords: osteoimmunology, interleukin-17A, osteoclasts, osteoblasts, postmenopausal osteoporosis, rheumatoid arthritis, psoriatic arthritis, axial spondyloarthritis
Citation: Tang MJ, Lu LY and Yu XJ (2021) Interleukin-17A Interweaves the Skeletal and Immune Systems. Front. Immunol. 11:625034. doi: 10.3389/fimmu.2020.625034
Received: 02 November 2020; Accepted: 23 December 2020;
Published: 04 February 2021.
Edited by:
Rupesh K. Srivastava, All India Institute of Medical Sciences, IndiaReviewed by:
Arthur Kavanaugh, University of California, San Diego, United StatesIneke Jansen, VU University Amsterdam, Netherlands
Copyright © 2021 Tang, Lu and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xijie Yu, xijieyu@scu.edu.cn; xijieyu@hotmail.com
†These authors have contributed equally to this work
 Mengjia Tang
Mengjia Tang