
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Immunol., 17 December 2020
Sec. Molecular Innate Immunity
Volume 11 - 2020 | https://doi.org/10.3389/fimmu.2020.614402
This article is part of the Research TopicInnate Cells in the Pathogenesis of Food AllergyView all 17 articles
Despite attempts to halt it, the prevalence of food allergy is increasing, and there is an unmet need for strategies to prevent morbidity and mortality from food-induced allergic reactions. There are no known medications that can prevent anaphylaxis, but several novel therapies show promise for the prevention of food-induced anaphylaxis through targeting of the high-affinity IgE receptor (FcϵRI) pathway. This pathway includes multiple candidate targets, including tyrosine kinases and the receptor itself. Small molecule inhibitors of essential kinases have rapid onset of action and transient efficacy, which may be beneficial for short-term use for immunotherapy buildup or desensitizations. Short courses of FDA-approved inhibitors of Bruton’s tyrosine kinase can eliminate IgE-mediated basophil activation and reduce food skin test size in allergic adults, and prevent IgE-mediated anaphylaxis in humanized mice. In contrast, biologics may provide longer-lasting protection, albeit with slower onset. Omalizumab is an anti-IgE antibody that sequesters IgE, thereby reducing FcϵRI expression on mast cells and basophils. As a monotherapy, it can increase the clinical threshold dose of food allergen, and when used as an adjunct for food immunotherapy, it decreases severe reactions during buildup phase. Finally, lirentelimab, an anti-Siglec-8 antibody currently in clinical trials, can prevent IgE-mediated anaphylaxis in mice through mast cell inhibition. This review discusses these and other emerging therapies as potential strategies for preventing food-induced anaphylaxis. In contrast to other food allergy treatments which largely focus on individual allergens, blockade of the FcϵRI pathway has the advantage of preventing clinical reactivity from any food.
Approximately 15 million people in the United States have food allergy and are at risk for anaphylaxis, a potentially life-threatening systemic allergic reaction (1). There is no cure for food allergy, and no known therapies are capable of preventing anaphylaxis, so food allergy is primarily managed by avoiding triggering foods. Unfortunately, accidental exposures still occur. In contrast, there are scenarios in which patients are intentionally exposed to known allergens such as during food oral immunotherapy (OIT). Despite its efficacy in inducing desensitization to protect against food-induced anaphylaxis, many patients stop OIT prior to reaching maintenance due to frequent and/or severe adverse reactions from the OIT doses themselves (2, 3). Therefore, there is an unmet need for novel strategies of preventing food-induced anaphylaxis from both accidental exposures and during therapeutic procedures such as OIT buildup.
Food allergy is mediated by food-specific IgE found in circulation and bound to the high affinity receptor FcϵRI on the surface of mast cells and basophils. When food protein binds to its specific IgE and cross-links the receptor, downstream activation of various kinases induces degranulation, leukotriene and prostaglandin production, and de novo cytokine production, which collectively cause clinical symptoms (4). Because all IgE-mediated reactions involve signaling through FcϵRI, targeting it or its pathway components would be an ideal strategy for preventing food-induced reactions. This review discusses components of the IgE pathway as potential therapeutic targets for preventing food-induced anaphylaxis (Table 1).
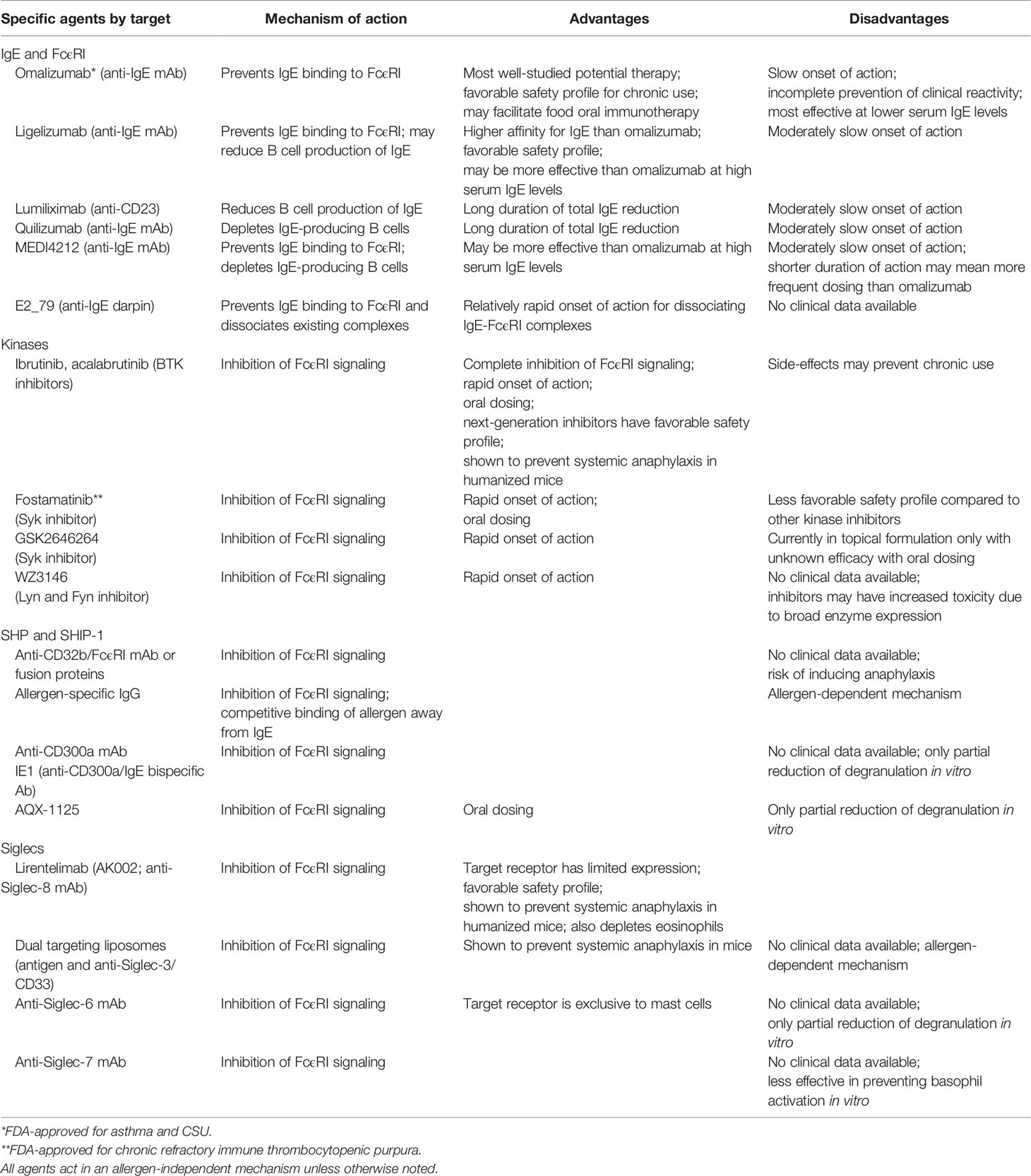
Table 1 Investigational therapies for food-induced anaphylaxis that target IgE or its pathway components.
IgE binding to FcϵRI stabilizes the receptor’s expression on the surface of mast cells and basophils; thus, FcϵRI expression is regulated by the level of total serum IgE (5, 6). Because the binding affinity of IgE to FcϵRI is extremely high (< 1 nmol/L), disruption of this interaction has proven difficult therapeutically. Strategies which focus on reducing the levels of total IgE or disrupting its binding to FcϵRI show promise for the prevention of IgE-mediated reactions to foods. The first demonstration that anti-IgE therapy could raise food threshold doses during oral challenge came from a trial with the anti-IgE monoclonal antibody (mAb) talizumab (developed by Tanox) in peanut-allergic subjects (7). Talizumab never progressed further in clinical trials, but other anti-IgE therapies show promise for this indication.
Omalizumab (Xolair®; manufacturers Genentech and Novartis) is a humanized antibody that binds to the C3 domain of IgE, thereby sequestering free IgE away from FcϵRI (8). The resulting downregulation of surface FcϵRI expression on mast cells and basophils renders them less sensitive to allergen-mediated activation. Already FDA-approved for asthma and chronic spontaneous urticaria (CSU), omalizumab has also demonstrated modest effect as a monotherapy for food allergy. Omalizumab treatment for 6–8 weeks increased patients’ threshold dose of peanut protein from a median of 80 to 6,500 mg in one open-label study in allergic adults (9), and another trial showed an 81-fold increase in peanut threshold dose in allergic subjects after 24 weeks of omalizumab compared to a 4.1-fold increase in the placebo-treated group (10). In food-allergic children receiving omalizumab for their severe asthma, omalizumab increased threshold doses for milk, egg, wheat, and hazelnut from an average of 1,013 to 8,727 mg of food protein after 16 weeks of treatment (11). Unfortunately, not all subjects in these trials displayed a significant increase in their food threshold dose on omalizumab. More research is needed to determine its efficacy in preventing reactivity from food exposures and to identify which patients would benefit most from omalizumab monotherapy.
Omalizumab has also been recently studied as an adjuvant therapy for food OIT. Early open-label studies suggested that 9 to 12 weeks of omalizumab therapy could facilitate rapid oral desensitization to cow’s milk and peanut in high-risk patients (12, 13). Subsequently, Wood et al. demonstrated in a double-blind, placebo-controlled (DBPC) trial that milk-allergic subjects treated with omalizumab experienced fewer adverse reactions during OIT build-up (with 2.1% of doses provoking reactions) compared to those treated with placebo (16.1% of doses) (14). None of the reactions experienced during omalizumab treatment required medical treatment, compared to 3.8% of reactions in subjects on placebo. However, omalizumab had no effect on subjects’ ability to pass an exit oral food challenge, nor did it affect rates of sustained unresponsiveness to milk 4 months after cessation of OIT. Another DBPC trial using omalizumab during multi-food OIT showed a significant reduction in the median per-participant percentage of OIT doses causing adverse reactions in those receiving omalizumab (27%) compared to placebo (68%), especially for gastrointestinal and respiratory symptoms (15). Additional trials are ongoing to evaluate omalizumab’s ability to facilitate multi-food OIT (NCT03881696).
Ligelizumab (Novartis) is another humanized anti-IgE mAb which binds to free IgE with higher affinity than omalizumab. Ligelizumab effectively prevents passive systemic anaphylaxis (PSA) in human FcϵRIα transgenic mice (16). Interestingly, though ligelizumab also binds to the C3 domain of IgE, it can bind to CD23-bound IgE on B cells as well, unlike omalizumab (16). Though the mechanism is unclear, this property allows ligelizumab to prevent new IgE production. Ligelizumab has demonstrated superior and more durable suppression of total IgE levels, skin prick test responses to allergens, and basophil surface FcϵRI expression in humans compared to omalizumab (17). This may make it more effective than omalizumab in preventing IgE-mediated reactions in patients who have especially high serum IgE levels. It has not yet been tested in food allergy, though clinical trials have shown efficacy in CSU (NCT02477332).
The low-affinity IgE receptor, FcϵRII or CD23, appears to regulate IgE homeostasis (18). Anti-CD23 treatment of B cells has been shown to reduce IgE production (19). In a phase I trial, a single dose of lumiliximab (Biogen Idec Inc.), an anti-CD23 antibody, significantly reduced circulating serum IgE in asthma patients (20).
Quilizumab (Genentech) is a humanized afucosylated anti-IgE antibody in development that binds to M1’, a domain specific to membrane-bound IgE in B cells. In this way, it reduces circulating IgE via apoptotic depletion of IgE-producing B cells, but does not bind to circulating IgE or IgE already bound to FcϵRI. In early clinical trials, a single dose of quilizumab reduced serum IgE levels in patients with allergic rhinitis or asthma for approximately 6 months (21). Unfortunately, despite reducing IgE levels, it failed to improve symptoms in a trial for antihistamine-refractory CSU (22). The future of quilizumab is unknown, but it may have a potential application in treating food allergy given its ability to prevent further IgE production.
MEDI4212 is a high-affinity antibody for IgE which can both bind to circulating IgE as well as membrane-bound IgE, thus depleting IgE-producing B cells through antibody-dependent cell-mediated cytotoxicity (23). Because of its dual action in reducing IgE, it may be more effective than omalizumab in patients with very high IgE levels. Its phase I trial demonstrated superior reduction of total IgE levels in atopic subjects after a single MEDI4212 dose compared to subjects treated with a dose of omalizumab (24).
Eggel et al. created a non-immunoglobulin ankyrin repeat protein (DARPin) inhibitor of IgE, E2_79, which not only blocks IgE from binding to FcϵRI, but is also able to rapidly dissociate preformed IgE-FcϵRI complexes (25). Intravenous infusion of E2_79 just 6 hours prior to antigen challenge was shown to prevent passive cutaneous anaphylaxis (PCA) in human FcϵRIα transgenic mice (26). These data suggest that E2_79 could rapidly dissociate food-specific IgE from allergic cells in patients in vivo, though it has not yet been tested in clinical trials.
Numerous kinases are involved in FcϵRI pathway signaling, including spleen tyrosine kinase (Syk), Bruton’s tyrosine kinase (BTK), Lyn, Fyn, phospholipase Cγ (PLCγ), PI3 kinase (PI3K), and others (4). All of these enzymes are potential targets for inhibition of IgE-mediated activation of mast cells and basophils. Only recently, with the use of next-generation kinase inhibitors that are more specific for their target enzymes, have we begun to elucidate precisely which kinases are necessary to inhibit in order to prevent IgE-mediated reactions.
BTK is largely expressed in leukocytes including mast cells, basophils, B cells, neutrophils, monocytes, and NK cells and is thought to be essential for IgE-dependent activation of human cells (27–29). As an essential enzyme for B cell receptor signaling, it has been pharmacologically targeted for the treatment of B cell malignancies, and the relatively recent FDA-approval of selective BTK inhibitors has created the opportunity to repurpose these medications for the prevention of IgE-mediated anaphylaxis. Dispenza et al. showed that just two clinically-relevant oral doses of acalabrutinib, a second-generation irreversible BTK inhibitor, completely prevented moderate-severity PSA in humanized NSG-SGM3 mice which have mature human mast cells and basophils (27). Even more remarkably, it significantly protected against mortality during fatal anaphylaxis in this mouse model. Data in vivo in humans is still preliminary, but ibrutinib has been shown to suppress IgE-mediated ex vivo basophil activation and skin prick testing to both aeroallergens and foods in allergic subjects (30–32). Clinical trials using BTK inhibitors to prevent clinically reactivity to foods in food allergic adults are currently ongoing in the authors’ laboratory.
In addition to mast cells and basophils, Syk is expressed in numerous organ systems. Multiple studies have demonstrated Syk inhibition as a potential strategy for preventing anaphylaxis. The active metabolite of fostamatinib (Tavalisse®; Rigel Pharmaceuticals), which was approved in 2018 for chronic refractory immune thrombocytopenic purpura, prevented ex vivo basophil activation after a single dose in humans (33), as well as anaphylaxis to peanut in a mouse model of peanut allergy (34). GSK2646264 is a Syk inhibitor in a cream formulation in clinical trials for CSU (NCT02424799) which has been shown to attenuate IgE-mediated histamine release from human mast cells, though it is unclear if this compound would be safe and effective as an oral medication for the prevention of anaphylaxis (35). The Syk inhibitor NVP-QAB205 demonstrated excellent activity in preventing human mast cell and basophil activation (36, 37), but it did not progress to clinical trials due to potential toxicities like several other Syk inhibitors (38–41). Like BTK inhibitors, clinical trials are needed to demonstrate safety and efficacy for using Syk inhibitors to prevent food-induced anaphylaxis.
Lyn and Fyn are kinases upstream of Syk in the FcϵRI pathway and may each play both positive and negative regulatory roles on IgE-mediated activation of human cells. Evidence that their inhibition can prevent IgE-mediated reactions largely arises from studies using non-specific compounds. The EGFR inhibitor WZ3146 effectively blocked mast cell and basophil activation in vitro through off-target antagonist activity on Lyn and Fyn (42). AZD7762, and inhibitor of Chk1 with Lyn/Fyn activity, and had similar efficacy in preventing LAD2 mast cell activation (43), but cardiac toxicity in Phase I trials prevented further development. Ultimately, Lyn and Fyn may be useful targets for preventing food allergy reactions if they can be specifically targeted, but even specific inhibitors may have an unfavorable toxicities given the broad expression profile of these kinases.
Upon activation, various mast cell and basophil inhibitory receptors with immunoreceptor tyrosine inhibitory motifs (ITIMs) recruit phosphotyrosine phosphatases (SHP‐1 and SHP‐2) and inositol phosphatases (SHIP‐1), which then provide direct inhibitory feedback on the FcϵRI pathway.
Expressed on basophils and some tissue mast cells as well as other cells, the low-affinity IgG receptor FcγRIIb (CD32b) is a potential target for the prevention of allergen-mediated anaphylaxis (44). Co-aggregation of CD32b with FcϵRI has been shown to inhibit IgE-mediated mast cell and basophil activation (45). Cross-linking these receptors can be achieved in an allergen-independent manner with bispecific antibodies to FcϵRI and CD32b or Fcϵ-Fcγ fusion proteins to prevent allergic reactivity (46–48), though these strategies have yet to move forward to clinical trials. In contrast, specific IgG antibodies can induce allergen-specific IgE-FcϵRI-CD32b cross-linking in the presence of allergen, as well as competitively block allergen binding to its specific IgE (49). Clinical trials using cat-specific IgG cocktails for treatment of respiratory allergies are ongoing (NCT03838731) (50), but no trials are currently investigating this approach in food allergy.
CD300a is an inhibitory receptor expressed on numerous human immune cells including mast cells and basophils. Cross-linking of CD300a recruits SHP-1 and SHIP-1 to elicit strong inhibitory signals on the FcϵRI pathway (51). Monoclonal antibodies to CD300a partially prevented IgE-mediated CD63 upregulation in human basophils (52, 53), and IE1, a bispecific antibody recognizing IgE and CD300a, was shown to inhibit FcϵRI signaling and IgE-mediated degranulation in human mast cells in a dose-dependent manner (54).
As a SHIP-1 activator, AQX-1125 reduced IgE-mediated mast cell degranulation in vitro and showed efficacy in murine models of allergic asthma (55). It is currently in clinical trials for atopic dermatitis (NCT02324972).
Sialic acid-binding immunoglobulin-type lectins (Siglecs) are transmembrane receptors found on the surface of immune cells, so-called because they bind to sialic acid-containing ligands. Most Siglecs have ITIMs or ITIM-like motifs, which upon ligand binding, enable inhibitory signaling to counteract the actions of tyrosine kinases in the FcϵRI pathway (56, 57). Their differing ligand specificity and relatively restricted expression profiles make Siglecs good candidate targets for the suppression IgE-mediated activation of mast cells and/or basophils.
Siglec-8 is the best studied and most promising siglec target for the treatment of allergic diseases, with expression limited to human mast cells, basophils, and eosinophils (58, 59). In vitro, pre-incubation of human CD34-derived mast cells with anti-Siglec-8 mAb markedly shifted the anti-FcϵRI antibody-induced secretion dose response curve for histamine and PGD2 secretion (60). Siglec-8’s ITIM domain was found to be essential for this function, suggesting that its mechanism of action may involve phosphatase recruitment as discussed above. Furthermore, pretreatment with the humanized non-fucosylated IgG1 anti-Siglec-8 antibody lirentelimab (Allakos, Inc.) completely prevented human IgE-mediated PSA in NSG-SGM3 BLT humanized mice (61), suggesting that it may be a useful therapy for preventing anaphylaxis in humans. Lirentelimab has shown efficacy in other mast cell-driven diseases in early clinical trials, including CSU (NCT03436797), atopic keratoconjunctivitis (NCT03379311), and indolent systemic mastocytosis (NCT02808793). The most advanced efforts with lirentelimab involve a phase III study in eosinophilic gastritis and duodenitis (NCT04322604), a disorder characterized by increased numbers of tissue eosinophils and mast cells, based on positive phase II results (62).
CD33 (also known as Siglec-3) is expressed on most human myeloid cells, including mast cells and basophils. Duan et al. showed that dual targeting of CD33 and specific IgE on mast cells with liposomes expressing CD33L and a synthetic antigen (TNP) could prevent IgE-mediated anaphylaxis (63). These liposomes prevented TNP-induced degranulation in human mast cells and prevented clinical response during PSA in transgenic mice expressing human CD33. Intriguingly, the liposomes’ inhibitory effects were sustained for at least 2–3 days after infusion, potentially due to endocytosis of the TNP-IgE-FcϵRI complex in mast cells and/or liposomes’ interference of TNP binding to its specific IgE. One limitation of this approach is that these liposomes act in an antigen-specific manner, which may necessitate the use of several different types of liposomes to treat patients with multiple food allergies.
Siglec-7 is expressed on human mast cells, monocytes, eosinophils, NK cells, and, to a lesser extent, basophils (64). Pre-incubation of CD34-derived mast cells with the combination of an activating anti-Siglec-7 antibody, an anti-IgE antibody, and a cross-linking anti-mouse IgG F(ab’)2 completely prevented degranulation and partially prevented PGD2 release and GM-CSF production (64). This inhibition was dependent on Siglec-7 directly crosslinking with FcϵRI, which has not always been found to be the case for other Siglecs that inhibit FcϵRI-mediated signaling such as Siglec-6 or -8 (60, 65). Interestingly, Siglec-7 engagement on human basophils only partially reduces IgE-mediated degranulation, a discrepancy thought to be due to the relatively low expression of Siglec-7 on basophils. Siglec-6 is a geographically unique target, given that its expression is primarily limited to mast cells (66), but its engagement on mast cells has shown only modest (approximately 30%) reduction in IgE-mediated degranulation (65). Further studies are needed to determine the efficacy of targeting either Siglec-6 or -7 for the prevention of IgE-mediated anaphylaxis.
Despite attempts at its prevention, the prevalence of IgE-mediated food allergy is increasing. Therapies targeting IgE and the FcϵRI pathway may fill the need for preventing food-induced anaphylaxis. One major benefit to this approach is that it is not allergen specific, unlike food OIT. Additionally, FcϵRI pathway inhibition would prevent the release of all mast cell and basophil mediators, unlike most current allergy treatments (such as antihistamines and leukotriene receptor antagonists) which only counteract the effects of a few of many mediators that participate in causing allergic reactions.
Both short-term and long-term protection strategies to prevent anaphylaxis are needed to improve the quality of life of food allergic patients. Short-term therapies which can facilitate food OIT build-up to prevent adverse reactions would allow more patients to reach maintenance dose. Alternatively, short-term treatments which could protect against accidental exposures during isolated high risk situations (e.g. a family vacation abroad or birthday parties) would reduce morbidity and mortality and help alleviate anxiety for those suffering from food allergies. Perhaps more importantly, therapies are needed which could be used chronically to reliably prevent reactions from accidental food exposures.
Many questions remain regarding the utility and safety of the above therapies, most of which are still experimental or in clinical development. Each therapy’s risk-benefit ratio and mechanism of action will determine its specific indication (Table 1). BTK inhibitors have a rapid onset of action and are highly effective at preventing IgE-mediated anaphylaxis, making them good candidates for short-term episodic use to prevent reactivity to foods, but they may not be suitable for long-term use based on the safety profile of the currently FDA-approved BTK inhibitors. Omalizumab has a favorable safety profile when used chronically, but it has slower onset of efficacy, and its ability to reliably prevent anaphylaxis (especially fatal anaphylaxis) is unknown. More studies are needed to determine the safety and efficacy of targeting the FcϵRI pathway as a protective measure against food-induced anaphylaxis.
MD conceived of the concept and wrote the manuscript. BB and DM contributed sections and provided feedback on the manuscript. All authors contributed to the article and approved the submitted version.
This work was funded in part by a K23 mentored career award from NIH to MD (grant AI143965), a U19 grant from NIH to BB (AI136443), the Ludwig Family Foundation, and the Johns Hopkins School of Medicine Clinician Scientist Career Development Award.
MD has received research funding from Acerta Pharma/AstraZeneca in addition to consultant fees from AlphaSights, BVF Partners, and DAVA Oncology. BB has also received research funding from Acerta Pharma/AstraZeneca, as well as consultant fees from Regeneron, Sanofi, and GlaxoSmithkline. Finally, BB receives remuneration for serving on the scientific advisory board of Allakos, Inc. and owns stock in Allakos. He is a co-inventor on existing Siglec-8–related patents and thus may be entitled to a share of royalties received by Johns Hopkins University during development and potential sales of such products. BB is also a co-founder of Allakos; the terms of this arrangement are being managed by Johns Hopkins University and Northwestern University in accordance with their conflict of interest policies. DM consults for Boehringer-Ingelheim.
1. Shaker MS, Wallace DV, Golden DBK, Oppenheimer J, Bernstein JA, Campbell RL, et al. Anaphylaxis-a 2020 practice parameter update, systematic review, and Grading of Recommendations, Assessment, Development and Evaluation (GRADE) analysis. J Allergy Clin Immunol (2020) 145(4):1082–123. doi: 10.1016/j.jaci.2020.01.017
2. Wood RA. Oral Immunotherapy for Food Allergy. J Invest Allergol Clin Immunol (2017) 27(3):151–9. doi: 10.18176/jiaci.0143
3. Vazquez-Cortes S, Jaqueti P, Arasi S, Machinena A, Alvaro-Lozano M, Fernandez-Rivas M. Safety of Food Oral Immunotherapy: What We Know, and What We Need to Learn. Immunol Allergy Clinics North America (2020) 40(1):111–33. doi: 10.1016/j.iac.2019.09.013
4. Peavy RD, Metcalfe DD. Understanding the mechanisms of anaphylaxis. Curr Opin Allergy Clin Immunol (2008) 8(4):310–5. doi: 10.1097/ACI.0b013e3283036a90
5. Hsu C. and MacGlashan D, Jr. IgE antibody up-regulates high affinity IgE binding on murine bone marrow-derived mast cells. Immunol Lett (1996) 52(2-3):129–34. doi: 10.1016/0165-2478(96)02599-0
6. Lantz CS, Yamaguchi M, Oettgen HC, Katona IM, Miyajima I, Kinet JP, et al. IgE regulates mouse basophil Fc epsilon RI expression in vivo. J Immunol (1997) 158(6):2517–21.
7. Leung DY, Sampson HA, Yunginger JW, Burks AW Jr., Schneider LC, Wortel CH, et al. Effect of anti-IgE therapy in patients with peanut allergy. N Engl J Med (2003) 348(11):986–93. doi: 10.1056/NEJMoa022613
8. Liu J, Lester P, Builder S, Shire SJ. Characterization of complex formation by humanized anti-IgE monoclonal antibody and monoclonal human IgE. Biochemistry (1995) 34(33):10474–82. doi: 10.1021/bi00033a020
9. Savage JH, Courneya JP, Sterba PM, Macglashan DW, Saini SS, Wood RA. Kinetics of mast cell, basophil, and oral food challenge responses in omalizumab-treated adults with peanut allergy. J Allergy Clin Immunol (2012) 130(5):1123–9. doi: 10.1016/j.jaci.2012.05.039
10. Sampson HA, Leung DY, Burks AW, Lack G, Bahna SL, Jones SM, et al. randomized, doubleblind, parallelgroup, placebocontrolled oral food challenge trial of Xolair (omalizumab) in peanut allergy. J Allergy Clin Immunol (2011) 127(5):1309–10 e1. doi: 10.1016/j.jaci.2011.01.051
11. Fiocchi A, Artesani MC, Riccardi C, Mennini M, Pecora V, Fierro V, et al. Impact of Omalizumab on Food Allergy in Patients Treated for Asthma: A Real-Life Study. J Allergy Clin Immunol In Pract (2019) 7(6):1901–9 e5. doi: 10.1016/j.jaip.2019.01.023
12. Nadeau KC, Schneider LC, Hoyte L, Borras I, Umetsu DT. Rapid oral desensitization in combination with omalizumab therapy in patients with cow’s milk allergy. J Allergy Clin Immunol (2011) 127(6):1622–4. doi: 10.1016/j.jaci.2011.04.009
13. Schneider LC, Rachid R, LeBovidge J, Blood E, Mittal M, Umetsu DT. A pilot study of omalizumab to facilitate rapid oral desensitization in high-risk peanut-allergic patients. J Allergy Clin Immunol (2013) 132(6):1368–74. doi: 10.1016/j.jaci.2013.09.046
14. Wood RA, Kim JS, Lindblad R, Nadeau K, Henning AK, Dawson P, et al. A randomized, double-blind, placebo-controlled study of omalizumab combined with oral immunotherapy for the treatment of cow’s milk allergy. J Allergy Clin Immunol (2016) 137(4):1103–10 e11. doi: 10.1016/j.jaci.2015.10.005
15. Andorf S, Purington N, Block WM, Long AJ, Tupa D, Brittain E, et al. Anti-IgE treatment with oral immunotherapy in multifood allergic participants: a double-blind, randomised, controlled trial. Lancet Gastroenterol Hepatol (2018) 3(2):85–94. doi: 10.1016/S2468-1253(17)30392-8
16. Gasser P, Tarchevskaya SS, Guntern P, Brigger D, Ruppli R, Zbaren N, et al. The mechanistic and functional profile of the therapeutic anti-IgE antibody ligelizumab differs from omalizumab. Nat Commun (2020) 11(1):165. doi: 10.1038/s41467-019-13815-w
17. Arm JP, Bottoli I, Skerjanec A, Floch D, Groenewegen A, Maahs S, et al. Pharmacokinetics, pharmacodynamics and safety of QGE031 (ligelizumab), a novel high-affinity anti-IgE antibody, in atopic subjects. Clin Exp Allergy (2014) 44(11):1371–85. doi: 10.1111/cea.12400
18. Cooper AM, Hobson PS, Jutton MR, Kao MW, Drung B, Schmidt B, et al. Soluble CD23 controls IgE synthesis and homeostasis in human B cells. J Immunol (2012) 188(7):3199–207. doi: 10.4049/jimmunol.1102689
19. Nakamura T, Kloetzer WS, Brams P, Hariharan K, Chamat S, Cao X, et al. In vitro IgE inhibition in B cells by anti-CD23 monoclonal antibodies is functionally dependent on the immunoglobulin Fc domain. Int J Immunopharmacol (2000) 22(2):131–41. doi: 10.1016/S0192-0561(99)00068-5
20. Rosenwasser LJ, Busse WW, Lizambri RG, Olejnik TA, Totoritis MC. Allergic asthma and an anti-CD23 mAb (IDEC-152): results of a phase I, single-dose, dose-escalating clinical trial. J Allergy Clin Immunol (2003) 112(3):563–70. doi: 10.1016/S0091-6749(03)01861-X
21. Gauvreau GM, Harris JM, Boulet LP, Scheerens H, Fitzgerald JM, Putnam WS, et al. Targeting membrane-expressed IgE B cell receptor with an antibody to the M1 prime epitope reduces IgE production. Sci Transl Med (2014) 6(243):243ra85. doi: 10.1126/scitranslmed.3008961
22. Harris JM, Cabanski CR, Scheerens H, Samineni D, Bradley MS, Cochran C, et al. A randomized trial of quilizumab in adults with refractory chronic spontaneous urticaria. J Allergy Clin Immunol (2016) 138(6):1730–2. doi: 10.1016/j.jaci.2016.06.023
23. Nyborg AC, Zacco A, Ettinger R, Jack Borrok M, Zhu J, Martin T, et al. Development of an antibody that neutralizes soluble IgE and eliminates IgE expressing B cells. Cell Mol Immunol (2016) 13(3):391–400. doi: 10.1038/cmi.2015.19
24. Sheldon E, Schwickart M, Li J, Kim K, Crouch S, Parveen S, et al. Pharmacokinetics, Pharmacodynamics, and Safety of MEDI4212, an Anti-IgE Monoclonal Antibody, in Subjects with Atopy: A Phase I Study. Adv Ther (2016) 33(2):225–51. doi: 10.1007/s12325-016-0287-8
25. Kim B, Eggel A, Tarchevskaya SS, Vogel M, Prinz H, Jardetzky TS. Accelerated disassembly of IgE-receptor complexes by a disruptive macromolecular inhibitor. Nature (2012) 491(7425):613–7. doi: 10.1038/nature11546
26. Eggel A, Baravalle G, Hobi G, Kim B, Buschor P, Forrer P, et al. Accelerated dissociation of IgE-FcepsilonRI complexes by disruptive inhibitors actively desensitizes allergic effector cells. J Allergy Clin Immunol (2014) 133(6):1709–19 e8. doi: 10.1016/j.jaci.2014.02.005
27. Dispenza MC, Krier-Burris RA, Chhiba KD, Undem BJ, Robida PA, Bochner BS. Bruton’s tyrosine kinase inhibition effectively protects against human IgE-mediated anaphylaxis. J Clin Invest (2020) 130(9):4759–70. doi: 10.1172/JCI138448
28. Smiljkovic D, Blatt K, Stefanzl G, Dorofeeva Y, Skrabs C, Focke-Tejkl M, et al. BTK inhibition is a potent approach to block IgE-mediated histamine release in human basophils. Allergy (2017) 72(11):1666–76. doi: 10.1111/all.13166
29. MacGlashan D Jr., Honigberg LA, Smith A, Buggy J, Schroeder JT. Inhibition of IgE-mediated secretion from human basophils with a highly selective Bruton’s tyrosine kinase, Btk, inhibitor. Int Immunopharmacol (2011) 11(4):475–9. doi: 10.1016/j.intimp.2010.12.018
30. Regan JA, Cao Y, Dispenza MC, Ma S, Gordon LI, Petrich AM, et al. Ibrutinib, a Bruton’s tyrosine kinase inhibitor used for treatment of lymphoproliferative disorders, eliminates both aeroallergen skin test and basophil activation test reactivity. J Allergy Clin Immunol (2017) 140(3):875–9.e1. doi: 10.1016/j.jaci.2017.03.013
31. Advani RH, Buggy JJ, Sharman JP, Smith SM, Boyd TE, Grant B, et al. Bruton tyrosine kinase inhibitor ibrutinib (PCI-32765) has significant activity in patients with relapsed/refractory B-cell malignancies. J Clin Oncol (2013) 31(1):88–94. doi: 10.1200/JCO.2012.42.7906
32. Dispenza MC, Pongracic JA, Singh AM, Bochner BS. Short-term ibrutinib therapy suppresses skin test responses and eliminates IgE-mediated basophil activation in adults with peanut or tree nut allergy. J Allergy Clin Immunol (2018) 141(5):1914–6. doi: 10.1016/j.jaci.2017.12.987
33. Braselmann S, Taylor V, Zhao H, Wang S, Sylvain C, Baluom M, et al. R406, an orally available spleen tyrosine kinase inhibitor blocks fc receptor signaling and reduces immune complex-mediated inflammation. J Pharmacol Exp Ther (2006) 319(3):998–1008. doi: 10.1124/jpet.106.109058
34. Burton OT, Noval Rivas M, Zhou JS, Logsdon SL, Darling AR, Koleoglou KJ, et al. Immunoglobulin E signal inhibition during allergen ingestion leads to reversal of established food allergy and induction of regulatory T cells. Immunity (2014) 41(1):141–51. doi: 10.1016/j.immuni.2014.05.017
35. Ramirez Molina C, Falkencrone S, Skov PS, Hooper-Greenhill E, Barker M, Dickson MC. GSK2646264, a spleen tyrosine kinase inhibitor, attenuates the release of histamine in ex vivo human skin. Br J Pharmacol (2019) 176(8):1135–42. doi: 10.1111/bph.14610
36. MacGlashan D Jr., Undem BJ. Inducing an anergic state in mast cells and basophils without secretion. J Allergy Clin Immunol (2008) 121(6):1500–6, 6 e1-4. doi: 10.1016/j.jaci.2008.04.019
37. Patou J, Holtappels G, Affleck K, van Cauwenberge P, Bachert C. Syk-kinase inhibition prevents mast cell activation in nasal polyps. Rhinology (2011) 49(1):100–6. doi: 10.4193/Rhino09.147
38. Nam ST, Park YH, Kim HW, Kim HS, Lee D, Lee MB, et al. Suppression of IgE-mediated mast cell activation and mouse anaphylaxis via inhibition of Syk activation by 8-formyl-7-hydroxy-4-methylcoumarin, 4mu8C. Toxicol Appl Pharmacol (2017) 332:25–31. doi: 10.1016/j.taap.2017.07.015
39. Li X, Kwon O, Kim DY, Taketomi Y, Murakami M, Chang HW. NecroX-5 suppresses IgE/Ag-stimulated anaphylaxis and mast cell activation by regulating the SHP-1-Syk signaling module. Allergy (2016) 71(2):198–209. doi: 10.1111/all.12786
40. Kato T, Iwasaki H, Kobayashi H, Miyagawa N, Matsuo A, Hata T, et al. JTE-852, a novel spleen tyrosine kinase inhibitor, blocks mediator secretion from mast cells with immunoglobulin E crosslinking. Eur J Pharmacol (2017) 801:1–8. doi: 10.1016/j.ejphar.2017.02.048
41. Deng Y, Jin F, Li X, Park SJ, Chang JH, Kim DY, et al. Sauchinone suppresses FcepsilonRI-mediated mast cell signaling and anaphylaxis through regulation of LKB1/AMPK axis and SHP-1-Syk signaling module. Int Immunopharmacol (2019) 74:105702. doi: 10.1016/j.intimp.2019.105702
42. Park YH, Kim DK, Kim HS, Lee D, Lee MB, Min KY, et al. WZ3146 inhibits mast cell Lyn and Fyn to reduce IgE-mediated allergic responses in vitro and in vivo. Toxicol Appl Pharmacol (2019) 383:114763. doi: 10.1016/j.taap.2019.114763
43. Park YH, Kim DK, Kim HW, Kim HS, Lee D, Lee MB, et al. Repositioning of anti-cancer drug candidate, AZD7762, to an anti-allergic drug suppressing IgE-mediated mast cells and allergic responses via the inhibition of Lyn and Fyn. Biochem Pharmacol (2018) 154:270–7. doi: 10.1016/j.bcp.2018.05.012
44. Gomez G. Current Strategies to Inhibit High Affinity FcepsilonRI-Mediated Signaling for the Treatment of Allergic Disease. Front Immunol (2019) 10:175. doi: 10.3389/fimmu.2019.00175
45. Daeron M, Malbec O, Latour S, Arock M, Fridman WH. Regulation of high-affinity IgE receptor-mediated mast cell activation by murine low-affinity IgG receptors. J Clin Invest (1995) 95(2):577–85. doi: 10.1172/JCI117701
46. Tam SW, Demissie S, Thomas D, Daeron M. A bispecific antibody against human IgE and human FcgammaRII that inhibits antigen-induced histamine release by human mast cells and basophils. Allergy (2004) 59(7):772–80. doi: 10.1111/j.1398-9995.2004.00332.x
47. Zhu D, Kepley CL, Zhang M, Zhang K, Saxon A. A novel human immunoglobulin Fc gamma Fc epsilon bifunctional fusion protein inhibits Fc epsilon RI-mediated degranulation. Nat Med (2002) 8(5):518–21. doi: 10.1038/nm0502-518
48. Allen LC, Kepley CL, Saxon A, Zhang K. Modifications to an Fcgamma-Fcvarepsilon fusion protein alter its effectiveness in the inhibition of FcvarepsilonRI-mediated functions. J Allergy Clin Immunol (2007) 120(2):462–8. doi: 10.1016/j.jaci.2007.04.019
49. Strait RT, Morris SC, Finkelman FD. IgG-blocking antibodies inhibit IgE-mediated anaphylaxis in vivo through both antigen interception and Fc gamma RIIb cross-linking. J Clin Invest (2006) 116(3):833–41. doi: 10.1172/JCI25575
50. Orengo JM, Radin AR, Kamat V, Badithe A, Ben LH, Bennett BL, et al. Treating cat allergy with monoclonal IgG antibodies that bind allergen and prevent IgE engagement. Nat Commun (2018) 9(1):1421. doi: 10.1038/s41467-018-03636-8
51. Stenton GR, Mackenzie LF, Tam P, Cross JL, Harwig C, Raymond J, et al. Characterization of AQX-1125, a small-molecule SHIP1 activator: Part 2. Efficacy studies in allergic and pulmonary inflammation models in vivo. Br J Pharmacol (2013) 168(6):1519–29. doi: 10.1111/bph.12038
52. Gibbs BF, Sabato V, Bridts CH, Ebo DG, Ben-Zimra M, Levi-Schaffer F. Expressions and inhibitory functions of CD300a receptors on purified human basophils. Exp Dermatol (2012) 21(11):884–6. doi: 10.1111/exd.12018
53. Sabato V, Verweij MM, Bridts CH, Levi-Schaffer F, Gibbs BF, De Clerck LS, et al. CD300a is expressed on human basophils and seems to inhibit IgE/FcepsilonRI-dependent anaphylactic degranulation. Cytometry B Clin Cytom (2012) 82(3):132–8. doi: 10.1002/cyto.b.21003
54. Bachelet I, Munitz A, Levi-Schaffer F. Abrogation of allergic reactions by a bispecific antibody fragment linking IgE to CD300a. J Allergy Clin Immunol (2006) 117(6):1314–20. doi: 10.1016/j.jaci.2006.04.031
55. Stenton GR, Mackenzie LF, Tam P, Cross JL, Harwig C, Raymond J, et al. Characterization of AQX-1125, a small-molecule SHIP1 activator: Part 1. Effects on inflammatory cell activation and chemotaxis in vitro and pharmacokinetic characterization in vivo. Br J Pharmacol (2013) 168(6):1506–18. doi: 10.1111/bph.12039
56. Bornhofft KF, Goldammer T, Rebl A, Galuska SP. Siglecs: A journey through the evolution of sialic acid-binding immunoglobulin-type lectins. Dev Comp Immunol (2018) 86:219–31. doi: 10.1016/j.dci.2018.05.008
57. Karra L, Levi-Schaffer F. Down-Regulation of Mast Cell Responses through ITIM Containing Inhibitory Receptors. Boston, MA: Springer (2011). doi: 10.1007/978-1-4419-9533-9_9
58. Kikly KK, Bochner BS, Freeman SD, Tan KB, Gallagher KT, D’Alessio KJ, et al. Identification of SAF-2, a novel siglec expressed on eosinophils, mast cells, and basophils. J Allergy Clin Immunol (2000) 105(6 Pt 1):1093–100. doi: 10.1067/mai.2000.107127
59. Floyd H, Ni J, Cornish AL, Zeng Z, Liu D, Carter KC, et al. Siglec-8. A novel eosinophil-specific member of the immunoglobulin superfamily. J Biol Chem (2000) 275(2):861–6. doi: 10.1074/jbc.275.2.861
60. Yokoi H, Choi OH, Hubbard W, Lee HS, Canning BJ, Lee HH, et al. Inhibition of FcepsilonRI-dependent mediator release and calcium flux from human mast cells by sialic acid-binding immunoglobulin-like lectin 8 engagement. J Allergy Clin Immunol (2008) 121(2):499–505.e1. doi: 10.1016/j.jaci.2007.10.004
61. Youngblood BA, Brock EC, Leung J, Falahati R, Bryce PJ, Bright J, et al. AK002, a Humanized Sialic Acid-Binding Immunoglobulin-Like Lectin-8 Antibody that Induces Antibody-Dependent Cell-Mediated Cytotoxicity against Human Eosinophils and Inhibits Mast Cell-Mediated Anaphylaxis in Mice. Int Arch Allergy Immunol (2019) 180(2):91–102. doi: 10.1159/000501637
62. Dellon ES, Peterson KA, Murray JA, Falk GW, Gonsalves N, Chehade M, et al. Anti-Siglec-8 Antibody for Eosinophilic Gastritis and Duodenitis. N Engl J Med (2020) 383(17):1624–34. doi: 10.1056/NEJMoa2012047
63. Duan S, Koziol-White CJ, Jester WF Jr., Nycholat CM, Macauley MS, Panettieri RA Jr., et al. CD33 recruitment inhibits IgE-mediated anaphylaxis and desensitizes mast cells to allergen. J Clin Invest (2019) 129(3):1387–401. doi: 10.1172/JCI125456
64. Mizrahi S, Gibbs BF, Karra L, Ben-Zimra M, Levi-Schaffer F. Siglec-7 is an inhibitory receptor on human mast cells and basophils. J Allergy Clin Immunol (2014) 134(1):230–3. doi: 10.1016/j.jaci.2014.03.031
65. Yu Y, Blokhuis BRJ, Diks MAP, Keshavarzian A, Garssen J, Redegeld FA. Functional Inhibitory Siglec-6 Is Upregulated in Human Colorectal Cancer-Associated Mast Cells. Front Immunol (2018) 9:2138. doi: 10.3389/fimmu.2018.02138
Keywords: anaphylaxis, Bruton’s tyrosine kinase, Siglec, omalizumab, IgE, FcϵRI signaling, food allergy
Citation: Dispenza MC, Bochner BS and MacGlashan DW Jr (2020) Targeting the FcεRI Pathway as a Potential Strategy to Prevent Food-Induced Anaphylaxis. Front. Immunol. 11:614402. doi: 10.3389/fimmu.2020.614402
Received: 06 October 2020; Accepted: 16 November 2020;
Published: 17 December 2020.
Edited by:
Ana Olivera, National Institutes of Health (NIH), United StatesReviewed by:
Hans Oettgen, Boston Children’s Hospital and Harvard Medical School, United StatesCopyright © 2020 Dispenza, Bochner and MacGlashan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Melanie C. Dispenza, bWRpc3BlbjFAamhtaS5lZHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.