- 1Department of Medical Microbiology and Immunology, University of Alberta, Edmonton, AB, Canada
- 2School of Public Health, University of Alberta, Edmonton, AB, Canada
Pregnancy-associated malaria (PAM) caused by Plasmodium falciparum can result in detrimental outcomes for both mother and infant, including low infant birth weight, preterm birth, maternal anemia, spontaneous abortion, and maternal and/or infant mortality. Maternal anemia is a particularly complex outcome, as the body must both maintain erythropoiesis and tolerance of the growing fetus, while directing a Th1 response against the parasite. Underlying the pathogenesis of PAM is the expression of variant surface antigens (VSAPAM) on the surface of infected red blood cells (iRBC) that mediate sequestration of the iRBC in the placenta. Naturally acquired antibodies to VSAPAM can block sequestration and activate opsonic phagocytosis, both associated with improved pregnancy outcomes. In this review, we ask whether VSAPAM antibodies can also protect mothers against malarial anemia. Studies were identified where VSAPAM antibody titres and/or function were associated with higher maternal hemoglobin levels, thus supporting additional protective mechanisms for these antibodies against PAM. Yet these associations were not widely observed, and many studies reported no association between protection from maternal anemia and VSAPAM antibodies. We discuss the epidemiological, biological and technical factors that may explain some of the variability among these studies. We appraise the current evidence of these complex interactions between PAM-specific immunity and maternal anemia, propose potential mechanisms, and discuss knowledge gaps.
Introduction
Pregnant women are especially vulnerable to malaria compared to non-pregnant adults in Africa (1) and 11 million pregnancies are at risk for complications from pregnancy-associated malaria (PAM) on this continent (2) in addition to those in lower transmission regions elsewhere (3). PAM is attributed to poor fetal outcomes including stillbirths (4), low birth weight infants (2) and premature births (5). PAM also significantly contributes to severe maternal anemia (6).
During a Plasmodium falciparum infection in high transmission settings, primigravid women have an especially high risk of poor pregnancy outcomes, and this risk decreases with gravidity [reviewed in (7)]. Pregnancy outcomes can affect both mother (maternal anemia, maternal morbidity and mortality) and child (low infant birth weight, pre-term birth, and spontaneous abortion). After several instances of PAM, protection may be conferred by acquired immunity (8). Specifically, antibodies to pregnancy-specific variant surface antigens (VSAPAM) expressed on the surface of the infected red blood cell (iRBC) are known to disrupt the adherence of iRBCs to chondroitin sulfate A (CSA) in the placenta and prevent parasite sequestration here (9, 10). One of the key targets of these maternal antibodies is VAR2CSA, a VSA expressed on the iRBC surface that directly binds to CSA (11). VAR2CSA antibodies are associated with improved pregnancy outcomes (12) and as a result, are the focus of vaccines to prevent PAM (13, 14).
While antibody-mediated immune mechanisms are associated with improved birth outcomes, particularly increased infant birth weight, we do not know whether these humoral mechanisms could protect women from developing anemia. By preventing sequestration of iRBCs in the placenta or opsonizing VSAs on the surface of iRBCs, VSAPAM antibodies may promote iRBC phagocytosis in the intervillous spaces (IVS) of the placenta or parasite killing in the spleen, lowering parasitemia and reducing the risk of maternal anemia. However, the development of anemia in PAM and its connection to humoral immunity is not well defined and potential underlying mechanisms of protection warrant investigation (15). Here, we first consider the causes of maternal anemia in PAM, and then examine the studies that investigated associations between anemia and VSAPAM antibodies.
Mechanisms of Anemia in The Context of PAM
Maternal anemia is a common, but particularly complex consequence of PAM, typically defined as a hemoglobin level less than 11 g/dL, with mild and severe anemia for pregnant women defined at 10–10.9 g/dL and less than 7 g/dL, respectively (16). In a healthy pregnancy, the maternal vasculature must vasodilate approximately 5 weeks into pregnancy, with total blood volume and red cell mass significantly increased (17). An increase in plasma volume compared to red cell production results in physiological anemia during pregnancy that usually resolves by the third trimester (reviewed in (18, 19)). However, anemia is exacerbated by poor nutrition, iron deficiency, and maternal hypertension (reviewed in (20)).
If anemia develops due to malaria infection, this can result in maternal morbidity and mortality as the red cell population is compromised. Some mechanisms of anemia are shared between uncomplicated malaria and PAM, such as obligate hemolysis as the schizont ruptures, which triggers a pro-inflammatory response. In uncomplicated malaria, a profound loss of nRBCs contributes to anemia (21), as red cell deformability is reduced, which has been correlated with a lower hemoglobin concentration (22, 23) but whether this also occurs in the placenta is not known. This loss of non-infected RBCs (nRBCs) is yet to be observed in the PAM setting. Dysregulated erythropoiesis is also reported in uncomplicated malaria, often due to production of pro-inflammatory mediators including cytokines, nitric oxide, and lipoperoxides that can lead to bone marrow dysfunction [reviewed in (24, 25)].
In pregnancy, tolerance to the fetus must be maintained primarily with a T helper (Th) 2 response, through secretions of TGF-β, IL-4, and IL-6 (26–28). However, a P. falciparum infection triggers a Th1 response, increasing pro-inflammatory cytokine secretions including IL-1β, TNFα, and IFNγ. Moore et al. observed that intervillous blood mononuclear cells from multigravid women negative for placental malaria had the highest secretions of IFNγ, while cells from primigravid and secundigravid women positive for placental malaria had low IFNγ secretions (29). Thus, an increased production of IFNγ may help control a placental infection. However, pro-inflammatory mediators have also been associated with detrimental placental inflammation. Increased TNFα in primigravid women was associated with placental lesions, while increased IL-1β was associated with congenital malaria (30). This pro-inflammatory response was associated with severe maternal anemia (27). The increase in pro-inflammatory cytokines may also inhibit erythropoiesis in the bone marrow, leading to bone marrow dysfunction and contributing to overall maternal anemia (reviewed in (31), though this has not been shown specifically for PAM. In response to the pro-inflammatory state in the placenta, IL-10 is frequently elevated along with pro-inflammatory mediators in PAM (32, 33) in attempts to maintain a healthy pregnancy (reviewed in (34). Thus, IL-10 can be considered a marker for dangerous placental inflammation (29, 32, 35, 36) and levels of IL-10 correlated with maternal anemia (32, 33).
A source of these cytokines in the placenta are monocytes and macrophages (34, 37, 38), which often accumulate in the IVS during infection. Antibodies may fine-tune this response by directly targeting the parasite, blocking iRBC sequestration in the placenta and/or increasing opsonic phagocytosis by targeting iRBCs for engulfment. These potential mechanisms, as illustrated in Figure 1, may lower parasitemia in the placenta so that a prolonged inflammatory response is not required.
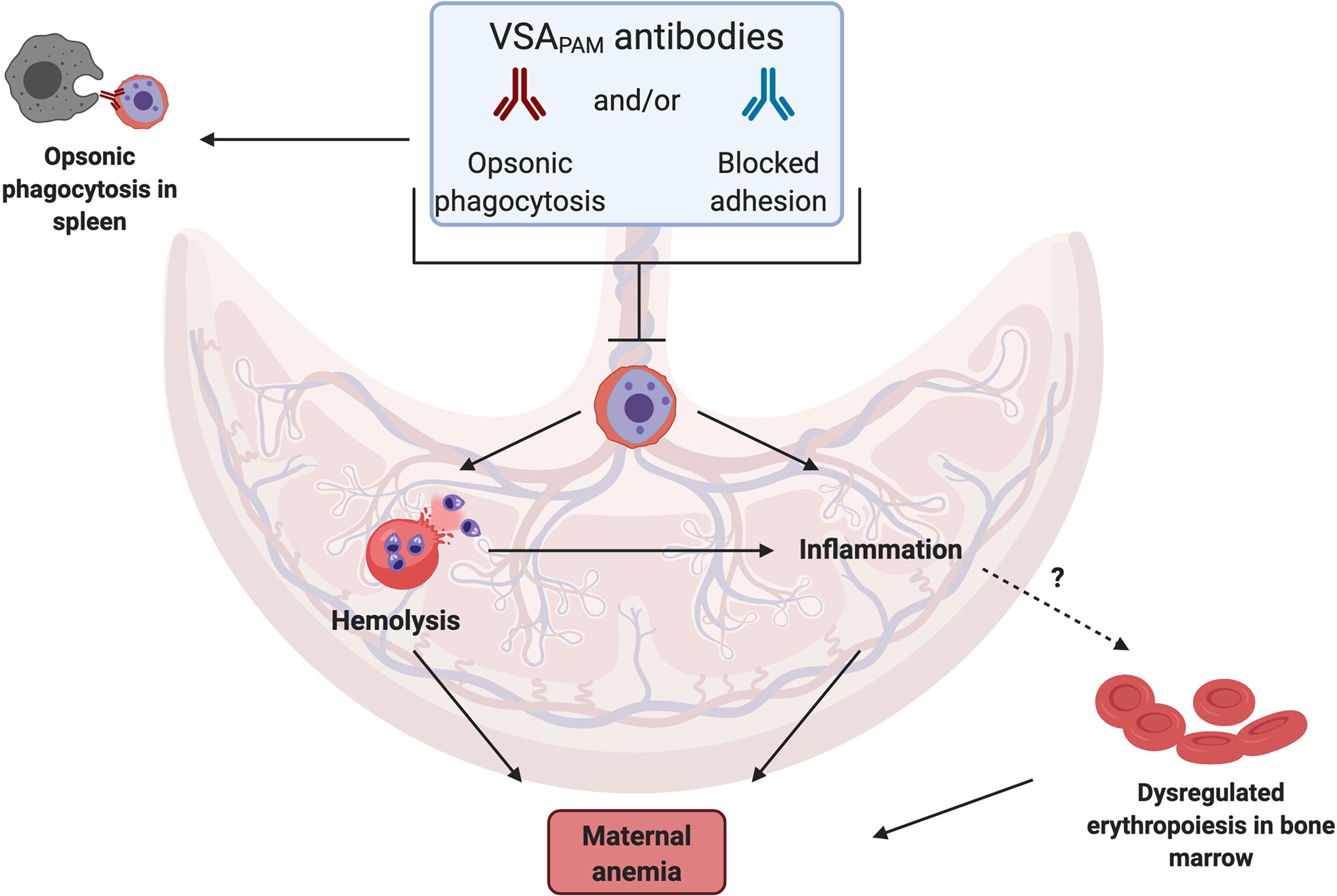
Figure 1 Mechanisms by which VSAPAM antibodies could protect against anemia in PAM. VSAPAM antibodies that bind to the surface of iRBCs can promote iRBC clearance from the placenta by preventing sequestration and/or promoting opsonic phagocytosis in the IVS of the placenta or in the spleen. Both of these mechanisms would reduce placental parasitemia and could prevent several of the downstream mechanisms that lead to maternal anemia; one of these is iRBC hemolysis, where parasite material is released and detected by toll-like receptors present on cells in the IVS, activating a pro-inflammatory response. Reduced inflammation may in turn prevent dysregulated erythropoiesis in the bone marrow, further protecting against maternal anemia. (Created with BioRender.com).
Further investigation is required to elucidate the mechanisms of anemia in PAM compared with uncomplicated malaria. Far less is published on anemia in PAM, and yet there may be significant differences due to changes in immune regulation that occur in pregnancy. For example, a Th1 response to combat a malaria infection could pose a greater risk in pregnancy compared to uncomplicated malaria as maintenance of a Th2 response is important for the health of the fetus. The investigation of the role of VSAPAM antibodies is further complicated by the multifactorial causes of anemia during PAM, which may alter the relationship between antibody titre/function and maternal anemia. Thus, a connection between VSAPAM antibodies and maternal anemia may be difficult to elucidate.
Studies of VSAPAM Antibodies and Maternal Anemia
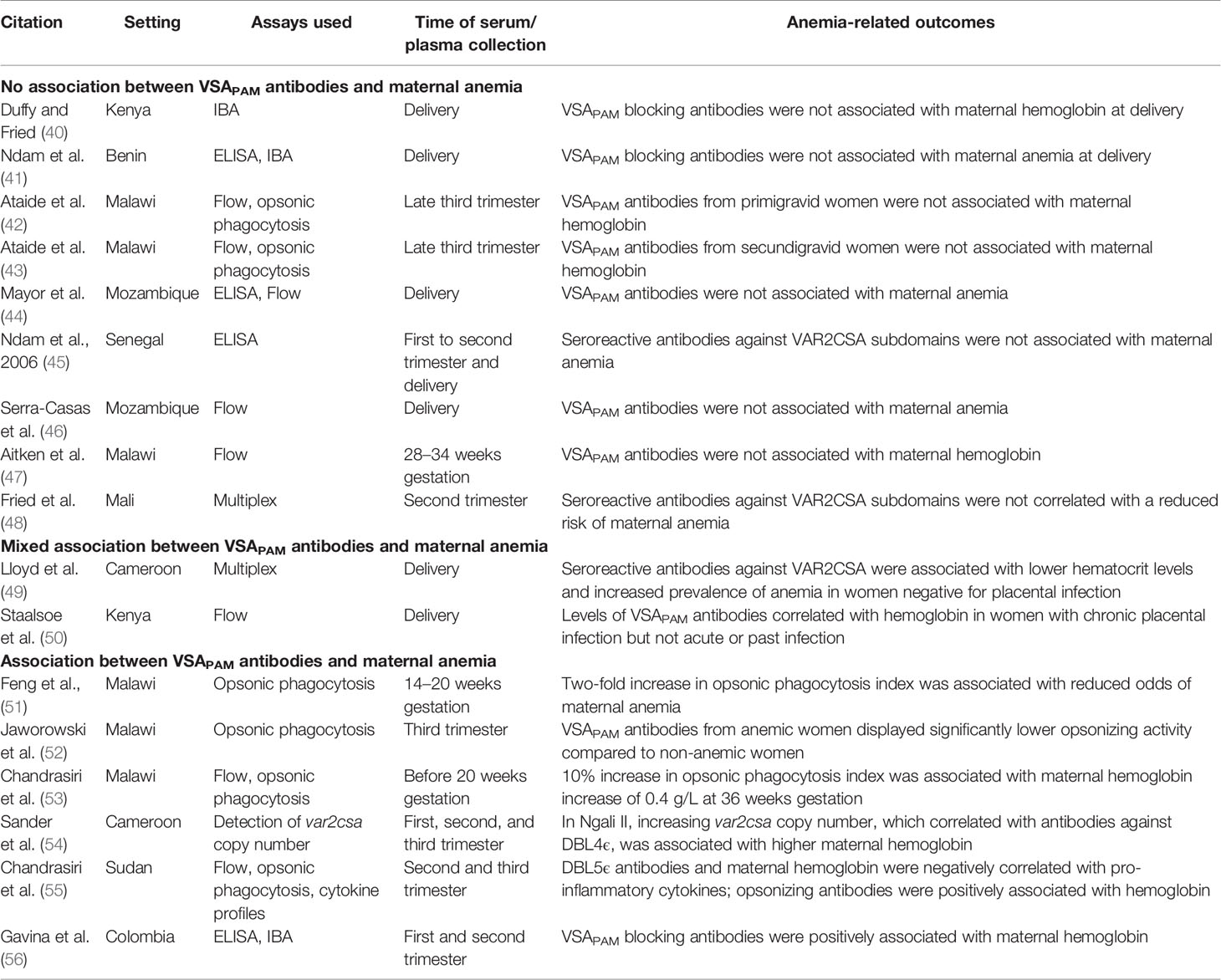
Women without prior exposure to PAM lack antibodies to the unique pregnancy-specific VSA that mediate sequestration of iRBCs to the placenta. Pregnant women can eventually acquire antibodies against these VSA and the levels of these antibodies increase with gravidity (8, 39). There is evidence for an association between VSAPAM antibodies and improved birth weight (40), but less is known whether these antibodies can also protect women from maternal anemia. Here, we discuss the limited studies on VSAPAM antibodies and anemia (Table 1).
Studies With No Correlation Between VSAPAM Antibodies and Maternal Anemia
In the first study to relate measures of VSAPAM antibodies to birth outcomes, plasma from Kenyan pregnant mothers was tested in the inhibition of binding assay (IBA) with placental isolates and correlated to infant birth weight, gestational age, and maternal hemoglobin at delivery (40). Plasma that blocked >35% of parasite binding to CSA was considered positive for blocking antibodies. Such antibodies were only observed in 1 of 47 primigravid women, while in secondigravid women, anti-adhesion activity was greater and associated with both increased infant birth weight and gestational age. However, there was no relationship between anti-adhesion antibodies and maternal hemoglobin levels (40).
This association was also observed in a study in Benin, where antibody titres were measured against the recombinant proteins DBL1-DBL2X, DBL5ϵ, and DBL6ϵ by ELISA, and functional activity was assessed by IBA (41). Increased anti-adhesion activity was associated with a decreased risk of placental infection at delivery and low birth weight, but there was no association between antibody titres or anti-adhesion activity and maternal anemia at delivery (41).
Opsonic phagocytosis of iRBCs is an important measure of antibody function. In primigravid women from Malawi, serum samples were collected in the late third trimester and tested for reactivity to CS2 parasites by flow cytometry and opsonic phagocytosis (42). No association was observed with maternal anemia or infant birth weight and VSAPAM antibodies. The study was continued with third trimester samples from secundigravid women (43). Again, no association was observed between maternal anemia and VSAPAM IgG or opsonic activity, but a positive correlation between opsonic phagocytosis and infant birth weight was observed.
Other studies compared hemoglobin measurements to IgG titres but not to antibody function. In Mozambique, serum samples collected at delivery were tested by ELISA for reactivity against a number of malarial antigens expressed in uncomplicated malaria and during PAM, including DBL5ϵ and DBL6ϵ, along with reactivity to two placental isolates (44). VSAPAM IgG against one isolate was associated with increased birth weight and gestational age of infants, but no association was found with maternal anemia. In Senegal, IgG was measured against the VAR2CSA subdomains DBL1X, DBL5ϵ, and DBL6ϵ by ELISA and compared with maternal anemia at enrollment and delivery in samples from primigravid, secondigravid, and multigravid women (45). IgG levels against DBL5ϵ and DBL6ϵ at enrollment and delivery were parity-dependent, and high IgG titres correlated with past malaria infection rather than acute PAM. In this study, there was no association between the levels of antibodies against these VAR2CSA domains and maternal anemia or birth weight at any measured time point. Similarly, in a study from Mozambique, the levels of antibody that stained the native VSA on CS2 parasites in flow cytometry did not associate with maternal anemia at delivery (46). Surprisingly, these antibodies did not correlate with other birth outcomes either, including preterm birth or low birth weight.
Two longitudinal studies reported VSAPAM antibody and hemoglobin levels throughout pregnancy, and both failed to identify a correlation between antibody levels at enrollment and pregnancy outcomes. The first study reported IgG titres in pregnant Malawi women from serum collected at various points in pregnancy including study enrollment, 28–34 gestational weeks, and 1–6 months postpartum (47). There was a non-significant downward trend in antibody levels against VSA-expressing parasites CS2 and HCS3-VSA from enrollment to one month postpartum, but IgG remained detectable 6 months after delivery in 72% of women. Despite the persistence of the VSAPAM antibodies, they were not associated with an increase in maternal hemoglobin levels. Fried et al. published findings from Mali with plasma samples collected at enrollment, 30–32 gestational weeks, and at delivery (48). A multiplex bead assay was used to determine seropositivity to a large number of VAR2CSA subdomains, including DBL2X, DBL4ϵ, and ID1-ID2a, but notably not the full-length protein. Increased antibody titres against either of the DBL domains were not significantly associated with any type of protection.
Mixed Association Between VSAPAM Antibodies and Maternal Anemia
In Cameroon, two groups of women, one positive and one negative for placental malaria, both had overall low antibody levels to full-length VAR2CSA at delivery (49). Among women positive for placental malaria, higher seropositivity against VAR2CSA was associated with a reduced risk of placental parasitemia and low birth weight infants, but not maternal anemia at delivery. Interestingly, in women negative for placental malaria, the presence of VAR2CSA antibodies was associated with lower hematocrit at delivery and increased prevalence of maternal anemia. This is hypothesized to be the product of a malaria infection that was cleared early in pregnancy, with the presence of antibodies marking this past infection. But, the maternal hematocrit and prevalence of anemia are only slightly different between women with and without VAR2CSA antibodies, thus this effect may not be biologically relevant.
With samples from a large cohort of pregnant women from Kilifi, VSAPAM IgG levels were measured by flow cytometry against various P. falciparum placental isolates and compared to hemoglobin levels (57). Increased severity of maternal anemia was correlated with low IgG levels in chronic cases of PAM (placentas infected with parasites and hemozoin pigment present) and the level of VSAPAM antibodies in these women was a strong predictor of maternal hemoglobin levels. However, no correlation was observed in women with acute or past placental infection.
Studies That Inversely Correlate VSAPAM Antibodies to Maternal Anemia
The findings from six studies suggest that VSAPAM antibodies can reduce the severity of maternal anemia. In Malawi, two studies investigated the function of VSAPAM antibodies by testing for opsonic phagocytosis. The first study revealed that a two-fold increase in the phagocytosis index was associated with a significant, 70% reduction in the odds of developing maternal anemia at delivery (51). Another study investigated third trimester serum samples and found that VSAPAM IgG reactivity in flow correlated with activity in the opsonic phagocytosis assay (r = 0.60), but serum from anemic women displayed significantly lower opsonic activity (52). Notably, coinfection with HIV reduced opsonic activity, but did not affect inhibition of binding levels or total IgG against VSAPAM (52).
In a later study from Malawi, antibody titres and function correlated to maternal hemoglobin at 36 weeks gestation, infant birth weight, gestational age, infant length, and placental histology at delivery (53). There was a significant positive association between IgG reactivity against CS2 parasites and maternal hemoglobin levels; a 10% increase in reactivity in flow corresponded to a hemoglobin increase of 0.5 g/L. Similarly, a 10% increase in the phagocytosis index corresponded to a hemoglobin increase of 0.4 g/L. Collectively, these studies support the hypothesis that opsonic phagocytosis could be an important effector mechanism of protection from maternal anemia.
VSAPAM antibody-mediated protection from anemia may relate to the number of var2csa genes per P. falciparum genome in the parasites that infect pregnant women (54). Parasites with more than one var2csa gene are more common in PAM, compared to the non-pregnant population, and may provide a selective advantage over parasites with only one var2csa gene. In the Cameroonian village of Ngali II, increasing var2csa copy numbers in the parasites infecting pregnant women were associated with higher hemoglobin levels at delivery, but not with birth weight. However, in another region, Yaoundé, this relationship was reversed, with a negative correlation observed between var2csa copy number and birth weight. In Ngali II, IgG against DBL4ϵ, but not the full-length VAR2CSA, correlated positively with gene copy number. The authors hypothesized that parasites with multiple copies of var2csa may elicit immunity to specific DBL domains of VAR2CSA, and the protection observed might be due to a strong immune response that develops in women infected with these parasites.
Along with titre and functional assays, Chandrasiri et al. examined cytokine profiles of pregnant women from Sudan (55). Cytokine profiles were measured with a multiplex bead array that detected IL-1β, IL-6, IL-8, IL-10, IL-12, and TNFα. IFNγ was also measured by ELISA. Distinct cytokine profiles were observed in women with severe PAM compared to uninfected controls, with IFNγ, IL-6, and IL-10 significantly elevated. Parasite density was positively associated with levels of pro-inflammatory cytokines IL-6 and IL-8. Women with severe cases had low levels of IgG against VSAPAM tested in flow, compared to uninfected controls. However, antibodies against DBL5ϵ were significantly greater than controls and negatively correlated with IL-1β, IL-6, and IL-8. Hemoglobin levels in these women were also negatively associated with IL-1β, IL-6, IL-8, and TNFα. Consistent with the studies from Malawi, opsonizing activity was positively associated with maternal hemoglobin at study enrollment. This study revealed associations between cytokine levels and VSAPAM antibodies. An influx of pro-inflammatory cytokines could increase the risk of maternal anemia, but the presence of antibodies may have an effect on cytokine profiles.
Only one study from outside Africa identified a positive correlation between VSAPAM antibodies and hemoglobin levels. This study was based in Colombia and examined a cohort of women with submicroscopic PAM (56). While VAR2CSA-specific antibody levels did not correlate with hemoglobin levels, the activity of these sera in the IBA was significantly associated with higher maternal hemoglobin levels at delivery. Interestingly, an association between antibody titre or function was not seen with birth weight, which is more commonly observed than associations with maternal anemia.
Trends and Caveats
The results summarized above are divided on whether VSAPAM antibodies contribute to protection from maternal anemia induced by PAM. These conclusions are consistent with a recent meta-analysis that examined associations between VSAPAM antibodies and poor pregnancy outcomes, and included many of the same studies (58). Overall, the meta-analysis suggested that VSAPAM antibodies are more likely to be markers of infection, instead of a source of protection against adverse outcomes, including anemia (58). However, additional analyses of the primary data provided by the authors revealed heterogeneous associations of VSAPAM antibodies with anemia depending on a number of variables, such as gravidity, study design and the type of assay used to characterize antibodies. Considering these data and the results from the studies described previously, we identified various factors that may explain the discrepancies between these study findings.
Study Setting
The level of malaria transmission in the study region influences the frequency of exposure to parasites and the diversity of strains. In turn, this could impact the magnitude and breadth of antibodies to VSA. Lloyd et al. collected samples from Yaoundé, a low transmission area with an entomological inoculation rate of approximately one infectious bite per person per month (49). In this study, just over half of women with a placental infection had antibodies to VAR2CSA, and this frequency was only 26.9% among multigravid women. Without sufficient exposure during pregnancy, a correlation between antibodies and pregnancy outcomes may not be detected. However, the study site of Fried et al. had intense seasonal transmission but there was no association between VSAPAM antibodies and anemia, despite a positive correlation between infection history and antibody titres against several VAR2CSA subdomains (48). In this setting submicroscopic infections (SMI) were common, with a frequency between 19.8% and 25.4% in women who were blood smear negative at enrollment, and these infections were associated with an increase in VSAPAM antibody levels, particularly in multigravid women. SMI may boost antibody responses without contributing to poor pregnancy outcomes, thus an association between VSAPAM antibody titres and outcomes may not be observed. A similar finding was observed in Colombia, a low transmission setting, where history of SMI did not correlate directly with maternal hemoglobin measurements (56). Based on these few studies, it is unclear how transmission intensity impacts associations between VSAPAM antibodies and anemia.
In certain settings, the fraction of anemia attributable to malaria may be low, masking a protective effect of VSAPAM antibodies. In one of the cohorts from Malawi, 62% of women were anemic and the mean hemoglobin level was 10.6 g/dL, likely reflecting non-malarial causes of anemia (43). In Kenya, Staalsoe et al. also reported overall low hemoglobin values, with 15.6% of women having hemoglobin concentrations under 7 g/dL (57). Ndam et al. reported 62% of women were anemic at enrollment and 45% at delivery in a cohort from Benin, with only 16% and 12% of women testing positive for malaria infection at enrollment and delivery, respectively (41). Several factors besides malaria could influence maternal anemia, including micronutrient deficiency, coinfections, and quality of prenatal care [reviewed in (24, 25)]. Interestingly, in the cohort investigated by Chandrasiri et al. where there was a protective association between VSAPAM antibodies and maternal anemia, women were given supplements (including iron) and the median hemoglobin was 11 g/dL (53). Perhaps this supplementation controlled for malaria-independent causes of anemia, revealing an association between the VSAPAM antibodies and malaria-attributable anemia.
Study Design
Depending on the study design, samples are collected at various times during pregnancy and/or at delivery and this could affect the interpretation of study findings. For example, VSAPAM antibodies detected early in pregnancy may indicate a prior exposure to VSAPAM in a previous pregnancy; thus, a pregnant woman may begin pregnancy with some immunity that may be boosted during subsequent pregnancies. Staalsoe et al. found that by six months postpartum, titres of VSAPAM IgG decreased, but levels were boosted in women by the second trimester of their next pregnancy (59). Modeling studies estimate that VAR2CSA antibodies can persist over many years (60). Of those studies that reported an association between VSAPAM antibodies and maternal hemoglobin levels, 4 out of 6 assessed antibody titre and/or function measured at first to second trimester (51, 53, 55, 56). In contrast, most of the studies that did not find a protective association with maternal anemia collected samples late in pregnancy, in the third trimester and at delivery (40, 41, 44, 46, 49, 45). If these VSAPAM antibodies developed as a result of infection late in pregnancy, increased antibody levels may reflect recent boosting from infection and obscure a relationship between antibody and protection from anemia. Thus, VSAPAM antibodies detected later in pregnancy may be markers of infection, rather than a source of protection, as noted by Cutts et al. (58), and others.
The gravidity of women included in the study can also greatly impact the titre, persistence and quality of antibodies investigated. Antibodies against VSA expressed in PAM develop with each successive infection in pregnancy; in high transmission settings, multigravid women are more likely to have antibodies that correlate with better pregnancy outcomes than primigravid women, supporting that these antibodies are maintained into the next pregnancy. Fried et al. reported that VSAPAM antibodies from primigravid women are short-lived, compared to those from secundigravid and multigravid women (48). In the 2010 Ataide et al. study, only primigravid women were included and samples were collected in the late third trimester, with no protective association between VSAPAM antibodies and anemia observed (42). The same cohort was followed into the second pregnancy, and again there was no association between maternal hemoglobin and VSAPAM IgG or opsonic phagocytosis activity (43). Thus, protective qualities of VSAPAM antibodies might not fully develop until infections in multiple pregnancies have occurred. In Malawi, recognition of iRBCs by VSAPAM antibodies from secundigravid and multigravid women, but not primigravid women, was associated with a reduced odds of anemia (47, 58). In contrast to the related studies discussed above, this association was observed in samples collected in the third trimester of pregnancy.
Another important consideration is treatment history. IPTp with 1500/75 mg of sulphadoxine and pyrimethamine (SP) during antenatal care visits is recommended by WHO in most areas in sub-Saharan Africa to reduce placental malaria (61). This treatment has the potential to affect the acquisition of VSAPAM antibodies, as parasitemia is prevented or cleared to the point that plasma cells will not produce antibodies against VSAPAM targets. In Ndam et al. where no association was observed between VSAPAM antibodies and maternal anemia at delivery, women received two doses of SP during pregnancy (41). Aitken et al. reported lower levels of VSAPAM antibodies associated with IPTp treatment (47). Similar observations were made in other studies from Kenya (50) and Ghana (62), where reductions in antibody titres were associated with IPTp treatment (though pregnancy outcomes were not reported). In contrast, Serra-Casas et al. failed to observe an effect of IPTp on VSAPAM antibody levels (46). Therefore, the effects of IPTp on VSAPAM antibodies in relation to anemia are unclear. But, it is unlikely that IPTp treatment contributes to the severity of maternal anemia itself, as a controlled or lowered parasitemia should benefit the health of the mother.
Finally, HIV is known to affect the humoral response in PAM, which was observed by a number of studies examined in this review (42–44, 46, 52). In the two studies by Ataide et al. VSAPAM phagocytic antibodies were reduced in HIV positive women compared to women who were HIV negative but this was only observed when antibody activity was measured using a functional assay; there was no change in the antibody reactivity to iRBCs by flow cytometry (42, 43). The presence of HIV in a cohort may therefore reduce VSAPAM antibody levels and/or function to a level too low to improve outcomes, particularly in lower transmission settings (49) or when women are treated with IPTp (46).
Assay Selection
It is necessary to assess antibody titre and particularly function to make comparisons with pregnancy outcomes. From the group of studies that did not find an association with maternal anemia, IBA was the primary functional assay used, with only two studies in this group that measured opsonic phagocytosis activity (42, 43). Inhibition of adhesion by VSAPAM antibodies is more commonly associated with higher infant birth weight due to P. falciparum infections during pregnancy (40). In only one study, from Colombia, adhesion-blocking activity was associated with increased maternal hemoglobin at delivery (56). Several of the studies point to opsonic phagocytic activity as a particularly important correlate of protection against maternal anemia. Chandrasiri et al. showed that only antibodies with opsonic phagocytic activity were associated with reduced odds of severe malaria, rather than antibody titre measured by ELISA (55). Similarly, Feng et al. observed a correlation between a reduced odds ratio of maternal anemia and VSAPAM antibody activity both by flow cytometry and opsonic phagocytosis, but the relationship between phagocytic activity was stronger than VSAPAM seroreactivity in flow (51). Interestingly, in one study, VSAPAM IgG measured by flow correlated more strongly with opsonic activity than anti-adhesion activity in the IBA (52).
Functional assays can also focus on specific IgG subclasses, rather than measuring total IgG by flow cytometry or ELISA. IgG1 and IgG3 are cytophilic subclasses that interact with Fc receptors on other immune cells, including monocytes and macrophages, and promote opsonic phagocytosis (63). These are the dominant subclasses in PAM, while IgG2 and IgG4 levels do not significantly change (64). Opsonic phagocytosis assays specifically measure these cytophilic subclasses, whereas testing for total IgG may not reveal a relationship with pregnancy outcomes. Future studies should characterize antibody populations using functional assays, along with assessments of titre and subclass, to test for associations with anemia.
Overall, there are many factors that can affect the relationship between maternal anemia and VSAPAM antibodies. Based on our interpretation of the studies described above, the discordance between the study findings may be attributed in part to the timing of sample collection and assay selection.
Potential Mechanisms of Antibody-Mediated Protection from Maternal Anemia in PAM
Though evidence for a specific role for VSAPAM antibodies in protection against maternal anemia is currently inconclusive, we can speculate on putative mechanisms of protection to help inform future research (Figure 1). We hypothesize that protective antibodies could directly target the iRBCs in the placental environment, either through opsonization or by physically blocking adhesion to CSA. Based on the group of studies that found an association with improved outcomes, VSAPAM antibodies that mediate opsonic phagocytosis of iRBCs may be more likely to be protective against maternal anemia than antibodies that inhibit parasite binding to the placenta, suggesting these antibodies may have distinct effector mechanisms. Antibodies with dual functions may also exist.
Antibody binding to iRBCs can lead to different downstream effects. Clearing the iRBC from the placenta either by preventing sequestration and/or recruiting immune cells would prevent the hemolysis associated with anemia and the associated inflammatory response. Erythropoiesis is also susceptible to a dysregulated pro-inflammatory response, a common cause of anemia in uncomplicated malaria and PAM [reviewed in (24)]. VSAPAM antibodies can potentially contribute to these responses by reducing cytokine dysregulation and targeting immune cells to iRBCs. As observed by Chandrasiri et al. pro-inflammatory cytokines IL-1β, IL-6, and IL-8 negatively correlated with antibody titres against DBL5ϵ in pregnant women with severe malaria (55). In this study, opsonizing antibody activity and cytokine profiles were not compared directly, but maternal hemoglobin levels increased with increasing levels of opsonizing antibodies (53). In another study, cytokine secretions were measured after opsonic phagocytosis of CS2 parasites had occurred, using serum from Malawian pregnant women. These results were compared to antibody-independent phagocytosis and a shift in the cytokine profile was observed, with an increase in production of IL-1β and TNFα when antibodies were present (65). Opsonized iRBCs activated the inflammasome and production of IL-1β by macrophages, but this was also observed with unopsonized iRBCs. Thus, the secretion of pro-inflammatory mediators likely occurs in the placenta with and without sufficient titres of VSAPAM antibodies. Perhaps the labeling of iRBCs with VSAPAM antibodies leads to Th1-like immune responses to appropriately respond to the parasite without leading to dysregulated inflammation and inhibition of erythropoiesis.
Future Directions
As the pathways leading to anemia in PAM are complicated and multifactorial, further research is critical to better understand any potential underlying immune mechanisms in response to malaria infection that contribute to or protect women from anemia. Longitudinal studies should be continued in and outside of Africa to increase the body of knowledge available on infection, VSAPAM antibody levels, and pregnancy outcomes. Studies should be conducted in many diverse settings to reveal context-specific effects on the acquisition of PAM immunity and the prevalence of maternal anemia. Treatment practices and circulation of other pathogens in a particular area can also affect humoral immunity.
In those studies where VSAPAM antibodies are associated with higher hemoglobin levels in mothers, it will be exciting to unravel the underlying mechanisms of protection. We propose a basic model for how these antibodies that target placental iRBCs could block downstream pathogenic effects that lead to anemia, yet the mechanisms that directly contribute to this process need to be delineated. While the data support a role for these antibodies in blocking sequestration in the placenta and promoting opsonic phagocytosis, emerging evidence linking the innate and adaptive immune systems merit exploration. For example, data is emerging on the role of the complement system in uncomplicated malaria. The antibody-mediated activation of the classical complement pathway was observed in samples from children and was an indicator of protective immunity (66). In addition, NK cells may participate in the response to PAM. Long et al. showed that antibodies from VAR2CSA-immunized rabbits induced iRBC lysis in vitro (67). Further, human IgG pooled from plasma of multigravid women and incubated with parasites and NK cells inhibited parasite growth by approximately 50%. No connection to anemia was examined, but this is an interesting component of the PAM response that should be further investigated with respect to pregnancy outcomes.
This review highlights the complexity of the body’s response to PAM and the large gaps in the current knowledge on immune mechanisms underlying protection. Pregnant women and young children remain at great risk of morbidity and mortality from malaria. Continued research in this area will add to our understanding of PAM and potentially reveal novel therapy and vaccine strategies to combat this disease.
Author Contributions
MCW conducted the literature review. MCW and SKY jointly wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
MCW holds a CIHR Frederick Banting and Charles Best Canada Graduate Scholarship.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor declared a past co-authorship with one of the authors, SY.
Acknowledgement
We thank Michael Good and Sedami Gnidehou for their valuable feedback during the preparation of this review.
References
1. McGregor IA. Epidemiology, malaria and pregnancy. Am J Trop Med Hyg (1984) 33(4):517–25. doi: 10.4269/ajtmh.1984.33.517
3. Dellicour S, Tatem AJ, Guerra CA, Snow RW, ter Kuile FO. Quantifying the number of pregnancies at risk of malaria in 2007: a demographic study. PLoS Med (2010) 7(1):e1000221. doi: 10.1371/journal.pmed.1000221
4. Moore KA, Simpson JA, Scoullar MJL, McGready R, Fowkes FJI. Quantification of the association between malaria in pregnancy and stillbirth: a systematic review and meta-analysis. Lancet Glob Health (2017) 5(11):e1101–e12. doi: 10.1016/S2214-109X(17)30340-6
5. Laelago T, Yohannes T, Tsige G. Determinants of preterm birth among mothers who gave birth in East Africa: systematic review and meta-analysis. Ital J Pediatr (2020) 46(1):10. doi: 10.1186/s13052-020-0772-1
6. Brabin B, Rogerson S. The epidemiology and outcomes of maternal malaria. In: Patrick E, Duffy MF, editors. Malaria in Pregnancy: Deadly Parasite, Susceptible Host. London: CRC Press (2001). p. 28–55.
7. Ataide R, Mayor A, Rogerson SJ. Malaria, primigravidae, and antibodies: knowledge gained and future perspectives. Trends Parasitol (2014) 30(2):85–94. doi: 10.1016/j.pt.2013.12.007
8. Fried M, Nosten F, Brockman A, Brabin BJ, Duffy PE. Maternal antibodies block malaria. Nature (1998) 395(6705):851–2. doi: 10.1038/27570
9. Fried M, Duffy PE. Adherence of Plasmodium falciparum to chondroitin sulfate A in the human placenta. Science (1996) 272(5267):1502–4. doi: 10.1126/science.272.5267.1502
10. Ricke CH, Staalsoe T, Koram K, Akanmori BD, Riley EM, Theander TG, et al. Plasma antibodies from malaria-exposed pregnant women recognize variant surface antigens on Plasmodium falciparum-infected erythrocytes in a parity-dependent manner and block parasite adhesion to chondroitin sulfate A. J Immunol (2000) 165(6):3309–16. doi: 10.4049/jimmunol.165.6.3309
11. Salanti A, Staalsoe T, Lavstsen T, Jensen AT, Sowa MP, Arnot DE, et al. Selective upregulation of a single distinctly structured var gene in chondroitin sulphate A-adhering Plasmodium falciparum involved in pregnancy-associated malaria. Mol Microbiol (2003) 49(1):179–91. doi: 10.1046/j.1365-2958.2003.03570.x
12. Salanti A, Dahlback M, Turner L, Nielsen MA, Barfod L, Magistrado P, et al. Evidence for the involvement of VAR2CSA in pregnancy-associated malaria. J Exp Med (2004) 200(9):1197–203. doi: 10.1084/jem.20041579
13. Sirima SB, Richert L, Chene A, Konate AT, Campion C, Dechavanne S, et al. PRIMVAC vaccine adjuvanted with Alhydrogel or GLA-SE to prevent placental malaria: a first-in-human, randomised, double-blind, placebo-controlled study. Lancet Infect Dis (2020) 20(5):585–97. doi: 10.1016/S1473-3099(19)30739-X
14. Mordmuller B, Sulyok M, Egger-Adam D, Resende M, de Jongh WA, Jensen MH, et al. First-in-human, Randomized, Double-blind Clinical Trial of Differentially Adjuvanted PAMVAC, A Vaccine Candidate to Prevent Pregnancy-associated Malaria. Clin Infect Dis (2019) 69(9):1509–16. doi: 10.1093/cid/ciy1140
15. Rogerson SJ, Mwapasa V, Meshnick SR. Malaria in pregnancy: linking immunity and pathogenesis to prevention. Am J Trop Med Hyg (2007) 77(6 Suppl):14–22.
16. WHO. Haemoglobin concentrations for the diagnosis of anaemia and assesment of severity. Geneva: World Health Organizaiton (2011). Available at: http://www.who.int/vmnis/indicators/haemoglobin.
17. Chapman AB, Abraham WT, Zamudio S, Coffin C, Merouani A, Young D, et al. Temporal relationships between hormonal and hemodynamic changes in early human pregnancy. Kidney Int (1998) 54(6):2056–63. doi: 10.1046/j.1523-1755.1998.00217.x
18. Sanghavi M, Rutherford JD. Cardiovascular physiology of pregnancy. Circulation (2014) 130(12):1003–8. doi: 10.1161/CIRCULATIONAHA.114.009029
19. Vricella LK. Emerging understanding and measurement of plasma volume expansion in pregnancy. Am J Clin Nutr (2017) 106(Suppl 6):1620S–5S. doi: 10.3945/ajcn.117.155903
20. Cao C, O’Brien KO. Pregnancy and iron homeostasis: an update. Nutr Rev (2013) 71(1):35–51. doi: 10.1111/j.1753-4887.2012.00550.x
21. Jakeman GN, Saul A, Hogarth WL, Collins WE. Anaemia of acute malaria infections in non-immune patients primarily results from destruction of uninfected erythrocytes. Parasitology (1999) 119(Pt 2):127–33. doi: 10.1017/s0031182099004564
22. Dondorp AM, Angus BJ, Chotivanich K, Silamut K, Ruangveerayuth R, Hardeman MR, et al. Red blood cell deformability as a predictor of anemia in severe falciparum malaria. Am J Trop Med Hyg (1999) 60(5):733–7. doi: 10.4269/ajtmh.1999.60.733
23. Looareesuwan S, Davis TM, Pukrittayakamee S, Supanaranond W, Desakorn V, Silamut K, et al. Erythrocyte survival in severe falciparum malaria. Acta Trop (1991) 48(4):263–70. doi: 10.1016/0001-706x(91)90014-b
24. Ghosh K, Ghosh K. Pathogenesis of anemia in malaria: a concise review. Parasitol Res (2007) 101(6):1463–9. doi: 10.1007/s00436-007-0742-1
26. de Moraes-Pinto MI, Vince GS, Flanagan BF, Hart CA, Johnson PM. Localization of IL-4 and IL-4 receptors in the human term placenta, decidua and amniochorionic membranes. Immunology (1997) 90(1):87–94. doi: 10.1046/j.1365-2567.1997.00139.x
27. Fried M, Muga RO, Misore AO, Duffy PE. Malaria elicits type 1 cytokines in the human placenta: IFN-gamma and TNF-alpha associated with pregnancy outcomes. J Immunol (1998) 160(5):2523–30.
28. Achidi EA, Apinjoh TO, Titanji VP. Malaria parasitemia and systemic cytokine bias in pregnancy. Int J Gynaecol Obstet (2007) 97(1):15–20. doi: 10.1016/j.ijgo.2006.12.015
29. Moore JM, Nahlen BL, Misore A, Lal AA, Udhayakumar V. Immunity to placental malaria. I. Elevated production of interferon-gamma by placental blood mononuclear cells is associated with protection in an area with high transmission of malaria. J Infect Dis (1999) 179(5):1218–25. doi: 10.1086/314737
30. Dobano C, Berthoud T, Manaca MN, Nhabomba A, Guinovart C, Aguilar R, et al. High production of pro-inflammatory cytokines by maternal blood mononuclear cells is associated with reduced maternal malaria but increased cord blood infection. Malar J (2018) 17(1):177. doi: 10.1186/s12936-018-2317-2
31. Perkins DJ, Were T, Davenport GC, Kempaiah P, Hittner JB, Ong’echa JM. Severe malarial anemia: innate immunity and pathogenesis. Int J Biol Sci (2011) 7(9):1427–42. doi: 10.7150/ijbs.7.1427
32. Kabyemela ER, Muehlenbachs A, Fried M, Kurtis JD, Mutabingwa TK, Duffy PE. Maternal peripheral blood level of IL-10 as a marker for inflammatory placental malaria. Malar J (2008) 7:26. doi: 10.1186/1475-2875-7-26
33. Hountohotegbe T, Gbedande K, Agbota G, Ibitokou S, Massougbodji A, Deloron P, et al. Circulating Cytokines Associated with Poor Pregnancy Outcomes in Beninese Exposed to Infection with Plasmodium falciparum. Infect Immun (2020) 88(8). doi: 10.1128/IAI.00042-20
34. Thaxton JE, Sharma S. Interleukin-10: a multi-faceted agent of pregnancy. Am J Reprod Immunol (2010) 63(6):482–91. doi: 10.1111/j.1600-0897.2010.00810.x
35. Suguitan AL Jr., Leke RG, Fouda G, Zhou A, Thuita L, Metenou S, et al. Changes in the levels of chemokines and cytokines in the placentas of women with Plasmodium falciparum malaria. J Infect Dis (2003) 188(7):1074–82. doi: 10.1086/378500
36. Suguitan AL Jr., Cadigan TJ, Nguyen TA, Zhou A, Leke RJ, Metenou S, et al. Malaria-associated cytokine changes in the placenta of women with pre-term deliveries in Yaounde, Cameroon. Am J Trop Med Hyg (2003) 69(6):574–81. doi: 10.4269/ajtmh.2003.69.574
37. Walter PR, Garin Y, Blot P. Placental pathologic changes in malaria. A histologic and ultrastructural study. Am J Pathol (1982) 109(3):330–42.
38. Ismail MR, Ordi J, Menendez C, Ventura PJ, Aponte JJ, Kahigwa E, et al. Placental pathology in malaria: a histological, immunohistochemical, and quantitative study. Hum Pathol (2000) 31(1):85–93. doi: 10.1016/s0046-8177(00)80203-8
39. O’Neil-Dunne I, Achur RN, Agbor-Enoh ST, Valiyaveettil M, Naik RS, Ockenhouse CF, et al. Gravidity-dependent production of antibodies that inhibit binding of Plasmodium falciparum-infected erythrocytes to placental chondroitin sulfate proteoglycan during pregnancy. Infect Immun (2001) 69(12):7487–92. doi: 10.1128/IAI.69.12.7487-7492.2001
40. Duffy PE, Fried M. Antibodies that inhibit Plasmodium falciparum adhesion to chondroitin sulfate A are associated with increased birth weight and the gestational age of newborns. Infect Immun (2003) 71(11):6620–3. doi: 10.1128/IAI.71.11.6620-6623.2003
41. Ndam NT, Denoeud-Ndam L, Doritchamou J, Viwami F, Salanti A, Nielsen MA, et al. Protective Antibodies against Placental Malaria and Poor Outcomes during Pregnancy, Benin. Emerg Infect Dis (2015) 21(5):813–23. doi: 10.3201/eid2105.141626
42. Ataide R, Hasang W, Wilson DW, Beeson JG, Mwapasa V, Molyneux ME, et al. Using an improved phagocytosis assay to evaluate the effect of HIV on specific antibodies to pregnancy-associated malaria. PLoS One (2010) 5(5):e10807. doi: 10.1371/journal.pone.0010807
43. Ataide R, Mwapasa V, Molyneux ME, Meshnick SR, Rogerson SJ. Antibodies that induce phagocytosis of malaria infected erythrocytes: effect of HIV infection and correlation with clinical outcomes. PLoS One (2011) 6(7):e22491. doi: 10.1371/journal.pone.0022491
44. Mayor A, Kumar U, Bardaji A, Gupta P, Jimenez A, Hamad A, et al. Improved pregnancy outcomes in women exposed to malaria with high antibody levels against Plasmodium falciparum. J Infect Dis (2013) 207(11):1664–74. doi: 10.1093/infdis/jit083
45. Tuikue Ndam NG, Salanti A, Le-Hesran JY, Cottrell G, Fievet N, Turner L, et al. Dynamics of anti-VAR2CSA immunoglobulin G response in a cohort of senegalese pregnant women. J Infect Dis (2006) 193(5):713–20. doi: 10.1086/500146
46. Serra-Casas E, Menendez C, Bardaji A, Quinto L, Dobano C, Sigauque B, et al. The effect of intermittent preventive treatment during pregnancy on malarial antibodies depends on HIV status and is not associated with poor delivery outcomes. J Infect Dis (2010) 201(1):123–31. doi: 10.1086/648595
47. Aitken EH, Mbewe B, Luntamo M, Maleta K, Kulmala T, Friso MJ, et al. Antibodies to chondroitin sulfate A-binding infected erythrocytes: dynamics and protection during pregnancy in women receiving intermittent preventive treatment. J Infect Dis (2010) 201(9):1316–25. doi: 10.1086/651578
48. Fried M, Kurtis JD, Swihart B, Morrison R, Pond-Tor S, Barry A, et al. Antibody levels to recombinant VAR2CSA domains vary with Plasmodium falciparum parasitaemia, gestational age, and gravidity, but do not predict pregnancy outcomes. Malar J (2018) 17(1):106. doi: 10.1186/s12936-018-2258-9
49. Lloyd YM, Fang R, Bobbili N, Vanda K, Ngati E, Sanchez-Quintero MJ, et al. Association of Antibodies to VAR2CSA and Merozoite Antigens with Pregnancy Outcomes in Women Living in Yaounde, Cameroon. Infect Immun (2018) 86(9):e00166-18. doi: 10.1128/IAI.00166-18
50. Staalsoe T, Shulman CE, Dorman EK, Kawuondo K, Marsh K, Hviid L. Intermittent preventive sulfadoxine-pyrimethamine treatment of primigravidae reduces levels of plasma immunoglobulin G, which protects against pregnancy-associated Plasmodium falciparum malaria. Infect Immun (2004) 72(9):5027–30. doi: 10.1128/IAI.72.9.5027-5030.2004
51. Feng G, Aitken E, Yosaatmadja F, Kalilani L, Meshnick SR, Jaworowski A, et al. Antibodies to variant surface antigens of Plasmodium falciparum-infected erythrocytes are associated with protection from treatment failure and the development of anemia in pregnancy. J Infect Dis (2009) 200(2):299–306. doi: 10.1086/599841
52. Jaworowski A, Fernandes LA, Yosaatmadja F, Feng G, Mwapasa V, Molyneux ME, et al. Relationship between human immunodeficiency virus type 1 coinfection, anemia, and levels and function of antibodies to variant surface antigens in pregnancy-associated malaria. Clin Vaccine Immunol (2009) 16(3):312–9. doi: 10.1128/CVI.00356-08
53. Chandrasiri UP, Fowkes FJ, Beeson JG, Richards JS, Kamiza S, Maleta K, et al. Association between malaria immunity and pregnancy outcomes among Malawian pregnant women receiving nutrient supplementation. Malar J (2016) 15(1):547. doi: 10.1186/s12936-016-1597-7
54. Sander AF, Salanti A, Lavstsen T, Nielsen MA, Theander TG, Leke RG, et al. Positive selection of Plasmodium falciparum parasites with multiple var2csa-type PfEMP1 genes during the course of infection in pregnant women. J Infect Dis (2011) 203(11):1679–85. doi: 10.1093/infdis/jir168
55. Chandrasiri UP, Randall LM, Saad AA, Bashir AM, Rogerson SJ, Adam I. Low antibody levels to pregnancy-specific malaria antigens and heightened cytokine responses associated with severe malaria in pregnancy. J Infect Dis (2014) 209(9):1408–17. doi: 10.1093/infdis/jit646
56. Gavina K, Gnidehou S, Arango E, Hamel-Martineau C, Mitran C, Agudelo O, et al. Clinical Outcomes of Submicroscopic Infections and Correlates of Protection of VAR2CSA Antibodies in a Longitudinal Study of Pregnant Women in Colombia. Infect Immun (2018) 86(4):e00797-17. doi: 10.1128/IAI.00797-17
57. Staalsoe T, Shulman CE, Bulmer JN, Kawuondo K, Marsh K, Hviid L. Variant surface antigen-specific IgG and protection against clinical consequences of pregnancy-associated Plasmodium falciparum malaria. Lancet (2004) 363(9405):283–9. doi: 10.1016/S0140-6736(03)15386-X
58. Cutts JC, Agius PA, Zaw L, Powell R, Moore K, Draper B, et al. Pregnancy-specific malarial immunity and risk of malaria in pregnancy and adverse birth outcomes: a systematic review. BMC Med (2020) 18(1):14. doi: 10.1186/s12916-019-1467-6
59. Staalsoe T, Megnekou R, Fievet N, Ricke CH, Zornig HD, Leke R, et al. Acquisition and decay of antibodies to pregnancy-associated variant antigens on the surface of Plasmodium falciparum-infected erythrocytes that protect against placental parasitemia. J Infect Dis (2001) 184(5):618–26. doi: 10.1086/322809
60. Fowkes FJ, McGready R, Cross NJ, Hommel M, Simpson JA, Elliott SR, et al. New insights into acquisition, boosting, and longevity of immunity to malaria in pregnant women. J Infect Dis (2012) 206(10):1612–21. doi: 10.1093/infdis/jis566
61. WHO. A Strategic Framework for Malaaria Prevention and Control During Pregnancy in the African Region. Brazzaville: WHO Regional Office for Africa (2004). Contract No.: AFR/MAL/04/01.
62. Stephens JK, Kyei-Baafour E, Dickson EK, Ofori JK, Ofori MF, Wilson ML, et al. Effect of IPTp on Plasmodium falciparum antibody levels among pregnant women and their babies in a sub-urban coastal area in Ghana. Malar J (2017) 16(1):224. doi: 10.1186/s12936-017-1857-1
63. Tebo AE, Kremsner PG, Luty AJ. Fcgamma receptor-mediated phagocytosis of Plasmodium falciparum-infected erythrocytes in vitro. Clin Exp Immunol (2002) 130(2):300–6. doi: 10.1046/j.1365-2249.2002.01972.x
64. Elliott SR, Brennan AK, Beeson JG, Tadesse E, Molyneux ME, Brown GV, et al. Placental malaria induces variant-specific antibodies of the cytophilic subtypes immunoglobulin G1 (IgG1) and IgG3 that correlate with adhesion inhibitory activity. Infect Immun (2005) 73(9):5903–7. doi: 10.1128/IAI.73.9.5903-5907.2005
65. Zhou J, Ludlow LE, Hasang W, Rogerson SJ, Jaworowski A. Opsonization of malaria-infected erythrocytes activates the inflammasome and enhances inflammatory cytokine secretion by human macrophages. Malar J (2012) 11:343. doi: 10.1186/1475-2875-11-343
66. Reiling L, Boyle MJ, White MT, Wilson DW, Feng G, Weaver R, et al. Targets of complement-fixing antibodies in protective immunity against malaria in children. Nat Commun (2019) 10(1):610. doi: 10.1038/s41467-019-08528-z
Keywords: pregnancy-associated malaria, anemia, inflammation, placenta, VAR2CSA antibodies, VSAPAM antibodies
Citation: Wiebe MC and Yanow SK (2020) Do Antibodies to Malaria Surface Antigens Play a Role in Protecting Mothers From Maternal Anemia? Front. Immunol. 11:609957. doi: 10.3389/fimmu.2020.609957
Received: 24 September 2020; Accepted: 17 November 2020;
Published: 18 December 2020.
Edited by:
Justin Yai Alamou Doritchamou, National Institute of Allergy and Infectious Diseases (NIAID), United StatesReviewed by:
Stephen Rogerson, The University of Melbourne, AustraliaDiane Wallace Taylor, University of Hawaii, United States
Copyright © 2020 Wiebe and Yanow. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stephanie K. Yanow, eWFub3dAdWFsYmVydGEuY2E=
 Madeleine C. Wiebe
Madeleine C. Wiebe Stephanie K. Yanow
Stephanie K. Yanow