
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 26 January 2021
Sec. Autoimmune and Autoinflammatory Disorders
Volume 11 - 2020 | https://doi.org/10.3389/fimmu.2020.609198
During COVID-19 pandemic the care of onco-hematologic and autoimmune patients has raised the question whether they are at higher risk of infection and/or worse outcome. Here, we describe the clinical course of COVID-19 pneumonia in patients with autoimmune cytopenias (AIC) regularly followed at a reference center in Northern Italy. The study period started from COVID-19 outbreak (February 22, 2020) until the time of writing. Moreover, we provide a review of the literature, showing that most cases reported so far are AIC developed during or secondary to COVID-19 infection. At variance, data about AIC pre-existing to COVID infection are scanty. The 4 patients here described (2 autoimmune hemolytic anemias, AIHA, 1 Evans syndrome, and 1 immune thrombocytopenia) with COVID-19 pneumonia belong to a large cohort of 500 AIC patients, making this study nearly population-based. The observed frequency (4/501; 0.7%) is only slightly superior to that of the general population admitted to hospital/intensive care unit (0.28/0.03%, respectively) in Lombardy in the same period of observation. All cases occurred between March 21 and 25, whilst no more AIC were recorded later on. Although different in intensity of care needed, all patients recovered from COVID-19 pneumonia, with apparently no detrimental effect of previous/current immunomodulatory treatments. AIHA relapse occurred in two patients, but promptly responded to therapy. With limitations due to sample size, these results suggest a favorable outcome and a lower-than-expected incidence of COVID-19 pneumonia in patients with previously diagnosed AIC, and allow speculating that immunomodulatory drugs used for AIC may play a beneficial rather than a harmful effect on COVID-19 infection.
COVID-19 pandemic has raised several concerns regarding patients with oncologic, onco-hematologic, and immune-mediated inflammatory/rheumatologic diseases (1–8). On one hand, both disease-related and therapy-induced immunosuppression may increase the risk to contract COVID-19 infection and complicate its management. On the other hand, most of the therapies that have shown some efficacy in COVID-19 infection include drugs with documented immunomodulatory activities, such as steroids, hydroxychloroquine (HCQ), tocilizumab (anti-interleukin 6 receptor), anakinra (anti-interleukin 1 receptor) (9, 10), and complement inhibitors (11), several of them currently employed in various autoimmune diseases. The rationale of their use in COVID-19 infection resides in reducing the exaggerated immune activation and consequent thrombo-inflammation that plays a major role in the unfavorable course of the disease (12). Several case reports described the occurrence of autoimmune hemolytic anemia (AIHA) or immune thrombocytopenia (ITP) developing after or concomitantly to COVID-19 infection (13, 14). At variance, data are lacking about the prevalence and clinical course of COVID-19 infection (asymptomatic or clinically overt) in patients already diagnosed with autoimmune cytopenias (AIC), including AIHA, ITP, chronic idiopathic neutropenia (CIN), and aplastic anemia (AA). Here we describe 4 patients with COVID-19 pneumonia identified among a large cohort of AIC regularly followed at a tertiary university center in Milan, Lombardy, from the outbreak (February 22, 2020) until the time of writing. Furthermore, we critically analyzed the reports of AIC in COVID-19 infection available in literature.
Patients with AIC and COVID-19 pneumonia included one warm AIHA (wAIHA), one cold agglutinin disease (CAD), one ITP and one Evans syndrome (wAIHA plus ITP). They belong to a cohort of 501 AIC actively followed at Hematology Unit of IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan, Italy. Clinical, laboratory and radiologic data of patients were collected retrospectively from clinical charts; patients with AIC who developed COVID-19 infection were prospectively followed from February 22, 2020 (time of the first SARS-CoV-2 infection in Lombardy, Italy) until the time of writing. Diagnoses of AIC were made according to international guidelines (15–18). Diagnosis of COVID-19 pneumonia was based on positive nasopharyngeal swab (RT-PCR) and typical radiologic findings of bilateral interstitial pneumonia (19). The study was approved by the local Ethical Committee and patients gave informed consent. The study was conducted in accordance with the Declaration of Helsinki. A review of literature about AIC and COVID-19 was performed by searching for indexed articles from February to September 2020 in MEDLINE via PubMed and the National Library of Medicine. The research included cases of COVID-19 infection occurring in previously diagnosed AIC and AIC developing at the onset of COVID-19 infection.
The clinical features of the AIC cohort (wAIHA, CAD, ITP, CIN, and AA) are summarized in Table 1. Patients were mostly middle-aged (with a wide range), and females, as already known for AIC. The follow-up preceding COVID-19 infection was quite long, with a median of about 2 years, and some cases have been followed for more than 15–20 years. Almost all patients with wAIHA, CAD, ITP, and AA had been already treated, with some of them up to five therapy lines; at variance, only one patient with CIN received immunosuppression. Previous infections were particularly frequent in AA, although their rate was not negligible in the other conditions; thrombotic events occurred more often in wAIHA.
Table 2 shows the most relevant features of COVID-19 infection of our 4 patients. More in detail, patient #1 is a 59-year-old male diagnosed with primary wAIHA in 2018 and previously treated with steroids and rituximab. At the time of pneumonia, he was receiving an experimental phosphoinositide 3-kinase inhibitor, obtaining a complete response (CR). On March 22, he was admitted to a local hospital because of fever and dyspnoea and was diagnosed with bilateral COVID-19 pneumonia, confirmed by a typical CT scan. The experimental drug was stopped, prednisone increased, and HCQ, tocilizumab, and darunavir administered. After one week, due to worsening respiratory distress, he was transferred to ICU and intubated; low molecular weight heparin (LMWH) prophylaxis was instituted, and several antibiotics administered (ampicillin, meropenem, imipenem, and linezolid) due to likely superimposed bacterial infection. Patient’s general conditions ameliorated and on April 8, he was extubated and transferred to sub-intensive care unit (SICU) and shifted to low-flow oxygen support. Gentamicin and vancomycin were necessary because of Enterococcus faecalis hospital-acquired septicemia. After the resolution of pneumonia, the patient developed an acute bilateral flaccid paraparesis of lower limbs. Brain MRI showed oedema involving posterior parasagittal parietal cortex, with aspects of cerebritis and/or vasculitis. Blood tests for infective causes and autoantibodies for vasculitides resulted negative. These findings were interpreted as dysimmune encephalitis secondary to COVID-19 infection. High-dose intravenous immunoglobulins (IvIg, 0.4 g/kg/day for 5 days) were administered with a slow amelioration of symptoms and disappearance of MRI alterations, allowing discharge after 122 days of hospitalization. During all this period, wAIHA has been in remission, without transfusion requirement.
Patient #2 is a 90-year-old male diagnosed with ITP in 2011, successfully treated with steroids +/- IvIg and in persistent remission since 2013. On March 5, he was admitted for ITP relapse and treated with steroids and IvIg obtaining complete response. After discharge he was re-admitted to SICU (March 24) for a moderate COVID-19 pneumonia (probably contracted during previous admission). Treatment included continuous positive airway pressure ventilation, HCQ, full-dose LMWH, and continuing full-dose steroids. COVID-associated pneumonia resolved, and the patient continued steroid tapering with persistent ITP remission. Subsequently, the patient was transferred to the general medicine ward and contracted hospital-acquired bacterial pneumonia that required several antibiotics. However, on July 15, a methicillin-sensible Staphilococcus aureus septicemia developed and the patient died from multi organ failure.
Patient #3 is a 78-year-old male with relapsed/refractory Evans’ syndrome diagnosed in 2010. Previous treatments included various cycles of steroids and IvIg, cyclophosphamide, 2 cycles of rituximab. In August 2019, he has been enrolled in a clinical trial with an experimental spleen tyrosine kinase inhibitor for AIHA relapse. His past medical history consisted of arterial hypertension, previous myocardial infarction with ventricular fibrillation, stroke, two septic shocks, and osteonecrosis of the femoral head. On March 25, 2020, he presented at the outpatient clinic with typical symptoms of COVID-19 pneumonia (fever, dyspnoea, desaturation to 80%). He was intercepted by the preventive measures adopted at our hematology department (anamnesis, temperature measurement, swabs) (20). The experimental drug was interrupted, and he was admitted to the internal medicine ward. Pneumonia required low-flow oxygen support, steroids, HCQ, azithromycine, full-dose LMWH, and empirical antibiotic therapy for superimposed bacterial infection. The patient rapidly recovered from pneumonia but experienced two complications: paroxysmal atrial fibrillation treated with amiodarone, and wAIHA relapse that required IvIg and full-dose steroid (prednisone 1 mg/kg/day for 3 weeks followed by slow tapering, still ongoing). After discharge, the study drug was resumed on June 22, and AIHA was in remission at the time of writing.
Patient #4 is a 71-year-old female diagnosed with CAD in 2015 previously treated with steroids and rituximab with complete response. Comorbidities included arterial hypertension and osteoporosis. On March 21, 2020, she was admitted to the internal medicine ward of a local hospital with typical features of SARS-CoV-2 pneumonia and CAD relapse (Hb 5.5 g/dL) requiring transfusions and full-dose steroids. COVID-19 infection was treated with high-flow oxygen, HCQ, azithromycine, lopinavir/ritonavir, and ceftriaxone for superimposed bacterial pneumonia. The patient was discharged after 4 weeks with Hb 9.6 g/dL. Subsequently, hemoglobin rapidly improved, allowing quick tapering of steroids. At the time of writing, blood counts showed normal Hb levels.
The abrupt outbreak of COVID-19 infection began in Lombardy, Northern Italy, on February 22, 2020, with the first patient developing severe pneumonia and ICU admission. In the following weeks, an exponential increase of cases was recorded with a peak at the end of March 2020, and a subsequent progressive decline at the end of May 2020. This curve is quite different for that observed in other countries, where the pandemic developed thereafter and is still ongoing (Figure 1). Moreover, about 40% of all Italian COVID-19 cases (42,161/101,739) at the pandemic peak have been recorded in Lombardy. Thereafter this percentage showed no modification, as other regions of Italy maintained a low/absent burden of COVID-19 infection. Among the 42,161 cases, 11,815 and 1,330 were admitted to hospital and ICU, respectively, with an overall mortality around 15% (21, 22). The peculiar setting of our tertiary center in Lombardy, which is a reference center for benign hematology with dedicated Unit and staff, allows some epidemiologic speculations. The size and the characteristics of the population with AIC are representative of disease epidemiology/clinical features, and patients have been regularly followed in the months preceding and during the pandemic, suggesting that our findings may be nearly population-based. The frequency of COVID-19 pneumonia cases within our cohort of AIC (4/501; 0.7%) was only slightly superior to that of the general population admitted to hospital/ICU in Lombardy at the date of March 30 (11,815/1,330 out of 42,161; 0.28/0.03%, respectively for wards and ICU) (21). After this date and until the time of writing no further cases of COVID-19 pneumonia requiring hospital admission were observed among the cohort of AIC patients.
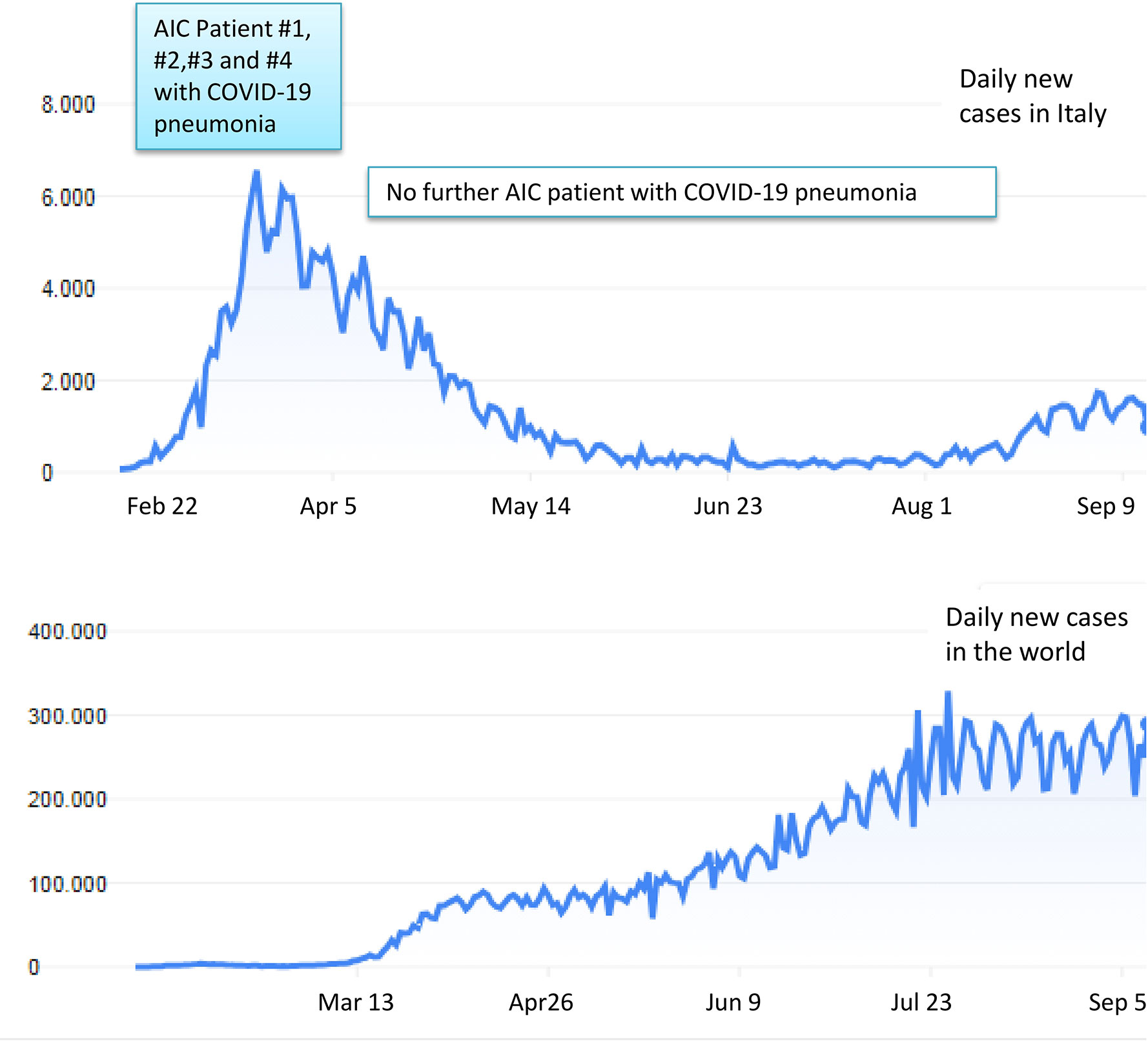
Figure 1 Trends of COVID-19 contagions in Italy (upper panel) and worldwide (lower panel). The four patients with AIC developing COVID-19 pneumonia at our Center were all registered between the end of February and the beginning of April 2020, in coincidence with contagions’ peak in our Country.
We considered two settings: COVID19 infection occurring in previously diagnosed AIC (Table 3), that is more similar to our study, and AIC developing after or concomitant to COVID19 infection (Table 4). Regarding the first, there are few cases reported: 2 Evans syndrome (23, 24) and 2 AA patients (25, 26) who experienced COVID-19 infection, with variable clinical course and outcome (2 resolved, 1 died, and 1 not reported). Other patients with various autoimmune disorders (systemic lupus erythematosus, rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, anti-phospholipid syndrome, inflammatory bowel disease) experienced COVID-19 infection, generally with a mild course (5–7, 44). In particular, Haberman et al. reported a prospective case series of 86 patients with immune-mediated inflammatory disease who had either confirmed (59 patients) or highly suspected (27 patients) symptomatic COVID-19 infection, 72% on therapy with biologics or Janus kinase inhibitors. Patients requiring hospitalization (16%, comparable to general COVID-19 infected population), were older and more comorbid than outpatients, and mainly suffered from rheumatoid arthritis. Two patients suffered from more severe disease (1 died) and neither of them was receiving biologic therapies. A recent Italian survey (44) identified 148 symptomatic cases among a series of 916 patients with autoimmune diseases. Symptoms were mainly mild and similar to the general population (cough, sore throat, fever, arthromyalgias, diarrhoea, conjunctivitis, and ageusia/hyposmia) and no deaths were observed. Importantly, only 3% of patients withdrew a medication, mainly immunosuppressants or biologics, due to COVID-19 infection, and >90% of patients declared the adoption of social distancing (36.6% adopted it before official lockdown). In both studies, the Authors concluded that the incidence and severity of COVID-19 were similar to those of the general population, and that the baseline use of biologics was not associated with worse outcomes.
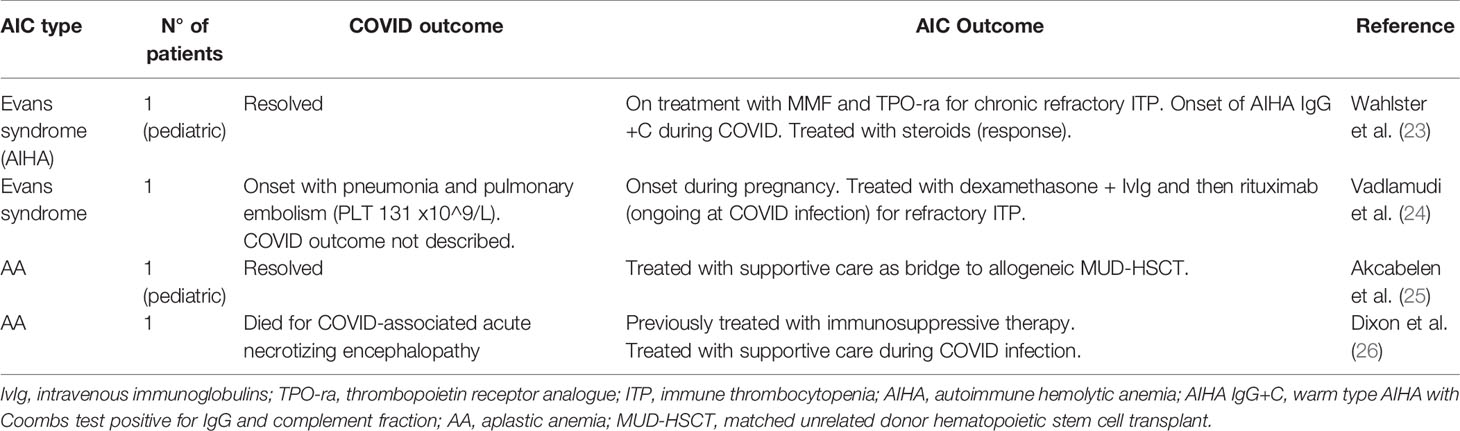
Table 3 Literature review of COVID-19 infection occurring in previously diagnosed autoimmune cytopenias (AIC).
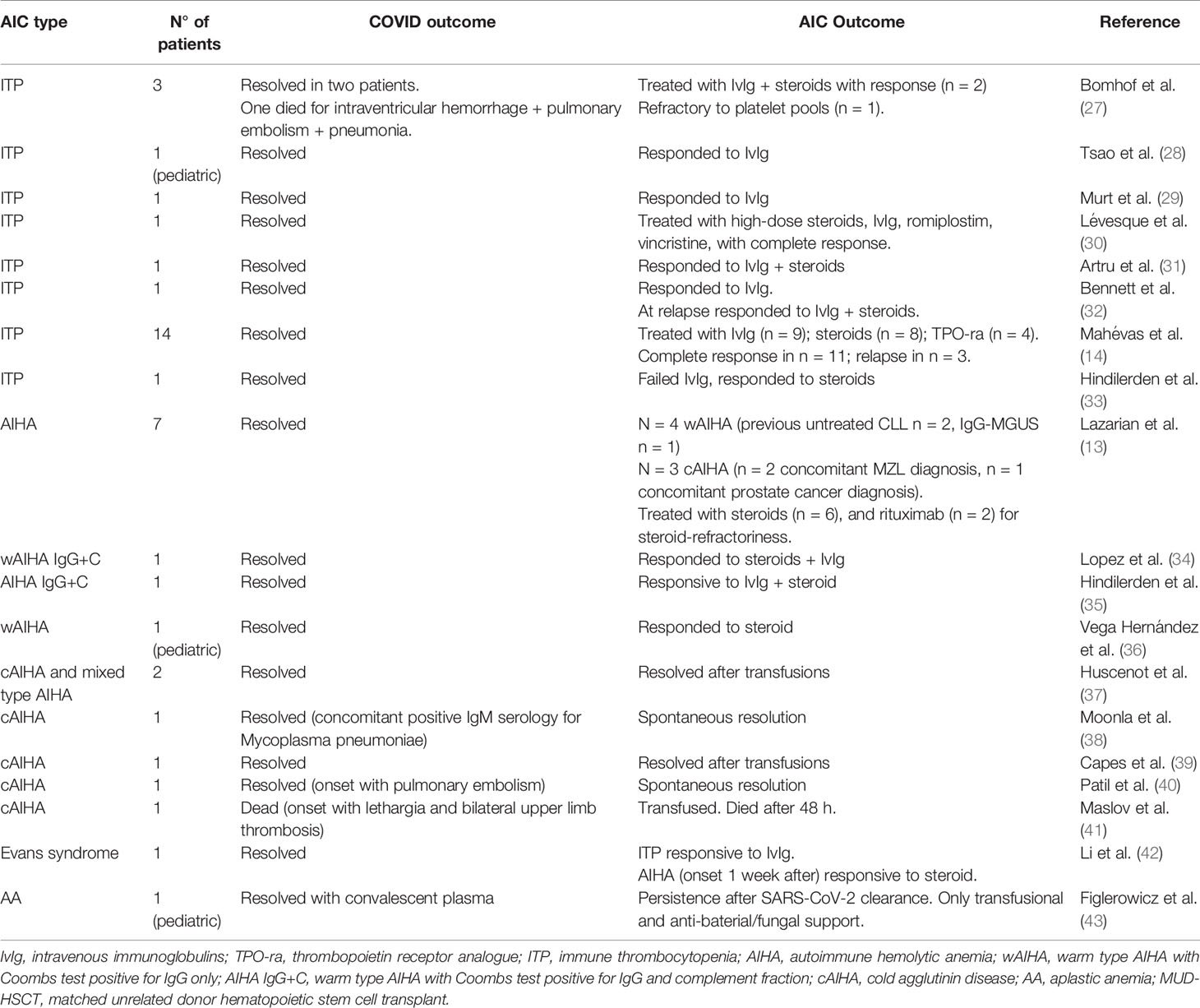
Table 4 Literature review of autoimmune cytopenias (AIC) developing after or concomitant to COVID-19 infection.
Regarding AIC cases reported after or concomitant to COVID-19 infection/pneumonia (Table 4), the most represented is ITP, with 23 patients (mostly case reports) that developed COVID-19-associated acute/severe thrombocytopenia. This cytopenia was not present before infection and was different from the frequently observed mild thrombocytopenia accompanying COVID-19 (14, 27–33). All cases resolved with steroids and/or IvIg with the exception of one patient who died for intra-ventricular hemorrhage and pulmonary embolism/pneumonia (27). Few cases required other treatments (TPO-RA in 5 patients, of whom 1 also received vincristine). Sixteen AIHA patients were reported, mostly cold forms, of whom one fatal (13, 34–41). Four cases were associated with previous lymphoproliferative disorders (chronic lymphocytic leukemia and mantle zone lymphoma). Treatments included steroids+/-IvIg in half of cases, and rituximab in two patients. Some cases required only transfusion support and two resolved without specific therapy. Finally, a patient with Evans syndrome (concomitant ITP and AIHA) and a pediatric patient with AA have been described (42, 43), the first resolved with steroids and IvIg, the second recovered from COVID-infection but still required AA treatment.
Here, we describe four patients with AIC who favorably recovered from COVID-19 pneumonia. Many concerns have been raised about COVID-19 incidence and clinical course in patients with autoimmune diseases, especially those on immunomodulatory/immunosuppressant therapy. Haberman et al. first described the characteristics of COVID-19 infection in a large series of patients with systemic autoimmune diseases (5), reporting an incidence comparable to the general population and a favorable outcome in both hospitalized and outpatients. Importantly, baseline use of biologics was not associated with worse COVID-19 outcomes. Contrarily, other reports on rheumatologic patients indicated an increased incidence of COVID-19 in this patients’ population, and pointed out that this frequency may be underestimated due to the known “diagnostic gap” of asymptomatic patients and to some clinical features shared by the two conditions (7). Our experience is more similar to that of Haberman et al., with less than 1% incidence of symptomatic COVID-19 infection and a favorable outcome in the majority of them. However, under-detection of asymptomatic cases cannot be excluded, despite hematologic patients are educated to refer to the Hematology Unit for almost all clinical problems, and we provided active and close follow-up through telemedicine means. Moreover, the preventive measures adopted at our Unit may have contributed to reduce contagions (20). Additionally, autoimmune cytopenias may imply a less severe immune disruption as compared to systemic autoimmune conditions.
A plethora of immunosuppressant drugs have been tested in COVID-19 pneumonia itself, with only dexamethazone showing evidence for better survival (45, 46). Consistently, in our experience the use of immunomodulatory/immunosuppressant drugs, including biologics, did not seem to either increase the incidence or adversely impact on the outcome. In fact, patient #1 experienced a favorable COVID-19 outcome without concurrent wAIHA relapse. This may be also related to aggressive COVID-19 specific therapy, although the effect of the recent AIHA-directed immunomodulation cannot be excluded. Patient #3 had a heavily pre-treated relapsed/refractory Evans syndrome, and a high burden of comorbidities. The clinical course of COVID-19 infection was even more favorable than in the previous case, although wAIHA relapse required treatment with resuming of the experimental drug after recovery from pneumonia. Also, in patient #4, COVID-19 infection was concomitant to severe CAD relapse, possibly triggered by virus-induced hyperactivation of the complement cascade. However, the latter 2 AIC reactivations were not catastrophic and promptly managed. Finally, patient #2, who experienced a milder COVID-19 disease, had a fatal outcome probably more related to older age and comorbidities. Importantly, ITP was in remission and previous immunosuppressive therapy was very limited (steroids only). Notably, no COVID-19 pneumonia was observed among patients with CIN and AA, who are classically considered at risk of infection.
Our COVID-19 pneumonia cases occurred in patients previously diagnosed with AIC. This setting is poorly described in literature for hematologic diseases, at variance with systemic autoimmune/rheumatologic disorders. Most of COVID-related cytopenias described in literature developed at the time of COVID-19 infection (generally within the first month). It is largely known that autoimmune cytopenias may be triggered by viral and bacterial infections, due to mechanisms of molecular mimicry, neo-antigen generation and hidden epitope spreading (47, 48). Interestingly, the RBC membrane protein ankyrin-1 was found to share a 100% identity with the SARS-CoV-2 surface glycoprotein Spike (49), suggesting a molecular mimicry mechanism for AIHA. Regarding ITP, anti-GP IIb/IIIa, GP-Ib/IX, or GP-V antibodies have been identified in several cases (50), although a sequence homology between SARS-CoV-2 and platelet components still needs to be documented. Furthermore, during infections, platelets and viruses interact in a sialic acid–dependent manner, leading to increased hepatic clearance of platelets. Sialic acids may act as additional receptors for SARS-COV-2 spike protein, possibly accounting for thrombocytopenia in COVID-19, as observed for influenza virus (51–53). Finally, AIC secondary to infections are thought to be often transient and/or promptly responsive to first-line therapy. Accordingly, the literature review showed that some cases of AIC secondary to COVID-19 resolved spontaneously and most responded to frontline steroid therapy. In the acute setting, the differential diagnosis of AIC may be challenging, since COVID infection itself is characterized by a variable degree of cytopenia as well as immunoregulatory cytokine storm (54). This is particularly true for thrombocytopenia, whose autoimmune origin is difficult to establish (14). Although our Unit is a reference center for autoimmune cytopenias and Lombardy was heavily hit by COVID-19 infection, we did not observe nor were notified of new-onset AIC secondary to SARS-CoV-2 infection. Finally, evidences are accumulating on the immune-disruptive potential of COVID-19 infection. In particular, a very severe onset of systemic lupus erythematous plus anti-phospholipid syndrome (55) and various immune-mediated neurologic sequelae (56) are increasingly described. In our series, the only immune complication secondary to COVID infection was the dysimmune encephalitis in patient #1.
In conclusion, although limited in sample size, our experience shows a lower-than-expected prevalence and favorable outcome of COVID-19 pneumonia in patient with AIC. We may hypothesize that previous/concurrent immunosuppressive treatments, rather than increasing the risk of contagion, may smolder the clinical course of the disease.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Comitato Etico Milano Area 2, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, via F. Sforza 28, 20100, Milano, Italy. The patients/participants provided their written informed consent to participate in this study.
All authors (WB, JAG, and BF) followed patients, wrote and revised the manuscript for important intellectual content. All authors contributed to the article and approved the submitted version.
This work was supported by research funding from Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico (RC 2019).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. van de Haar J, Hoes LR, Coles CE, Seamon K, Fröhling S, Jäger D, et al. Caring for patients with cancer in the COVID-19 era. Nat Med (2020) 26:665–71. doi: 10.1038/s41591-020-0874-8
2. He W, Chen L, Chen L, Yuan G, Fang Y, Chen W, et al. COVID-19 in persons with haematological cancers. Leukemia (2020) 34:1637–45. doi: 10.1038/s41375-020-0836-7
3. El-Sharkawi D, Iyengar S. Haematological cancers and the risk of severe COVID-19: Exploration and critical evaluation of the evidence to date. Br J Haematol (2020) 190:336–45. doi: 10.1111/bjh.16956
4. Valenza F, Papagni G, Marchianò A, Daidone MG, DeBraud F, Colombo MP, et al. Response of a comprehensive cancer center to the COVID-19 pandemic: the experience of the Fondazione IRCCS-Istituto Nazionale dei Tumori di Milano. Tumori (2020) 106:193–202. doi: 10.1177/0300891620923790
5. Haberman R, Axelrad J, Chen A, Castillo R, Yan D, Izmirly P, et al. Covid-19 in Immune-Mediated Inflammatory Diseases - Case Series from New York. N Engl J Med (2020) 383:85–8. doi: 10.1056/NEJMc2009567
6. Tomelleri A, Sartorelli S, Campochiaro C, Baldissera EM, Dagna L. Impact of COVID-19 pandemic on patients with large-vessel vasculitis in Italy: a monocentric survey. Ann Rheum Dis (2020) 79:1252–3. doi: 10.1136/annrheumdis-2020-217600
7. Ferri C, Giuggioli D, Raimondo V, L’Andolina M, Tavoni A, Cecchetti R, et al. COVID-19 and rheumatic autoimmune systemic diseases: report of a large Italian patients series. Clin Rheumatol (2020) 39:1–10. doi: 10.1007/s10067-020-05334-7
8. Hasseli R, Mueller-Ladner U, Schmeiser T, Hoyer BF, Krause A, Lorenz HM, et al. National registry for patients with inflammatory rheumatic diseases (IRD) infected with SARS-CoV-2 in Germany (ReCoVery): a valuable mean to gain rapid and reliable knowledge of the clinical course of SARS-CoV-2 infections in patients with IRD. RMD Open (2020) 6:e001332. doi: 10.1136/rmdopen-2020-001332
9. Alijotas-Reig J, Esteve-Valverde E, Belizna C, Selva-O’Callaghan A, Pardos-Gea J, Quintana A, et al. Immunomodulatory therapy for the management of severe COVID-19. Beyond the anti-viral therapy: A comprehensive review. Autoimmun Rev (2020) 19:102569. doi: 10.1016/j.autrev.2020.102569
10. Burrage DR, Koushesh S, Sofat N. Immunomodulatory Drugs in the Management of SARS-CoV-2. Front Immunol (2020) 11:1844. doi: 10.3389/fimmu.2020.01844
11. Polycarpou A, Howard M, Farrar CA, Greenlaw R, Fanelli G, Wallis R, et al. Rationale for targeting complement in COVID-19. EMBO Mol Med (2020) 12:e12642. doi: 10.15252/emmm.202012642
12. Connors JM, Levy JH. Thromboinflammation and the hypercoagulability of COVID-19. J Thromb Haemost (2020) 18:1559–61. doi: 10.1111/jth.14849
13. Lazarian G, Quinquenel A, Bellal M, Siavellis J, Jacquy C, Re D, et al. Autoimmune haemolytic anaemia associated with COVID-19 infection. Br J Haematol (2020) 190:29–31. doi: 10.1111/bjh.16794
14. Mahévas M, Moulis G, Andres E, Riviere E, Garzaro M, Crickx E, et al. Clinical characteristics, management and outcome of Covid-19-associated immune thrombocytopenia. A French multicenter series. Br J Haematol (2020) 190:e224–9. doi: 10.1111/bjh.17024
15. Jäger U, Barcellini W, Broome CM, Gertz MA, Hill A, Hill QA, et al. Diagnosis and treatment of autoimmune hemolytic anemia in adults: Recommendations from the First International Consensus Meeting. Blood Rev (2020) 41:100648. doi: 10.1016/j.blre.2019.100648
16. Neunert C, Terrell DR, Arnold DM, Buchanan G, Cines DB, Cooper N, et al. American Society of Hematology 2019 guidelines for immune thrombocytopenia. Blood Adv (2019) 3:3829–66. doi: 10.1182/bloodadvances.2019000966
17. Newburger PE. Autoimmune and other acquired neutropenias. Hematol Am Soc Hematol Educ Program (2016) 2016:38–42. doi: 10.1182/asheducation-2016.1.38
18. Killick SB, Bown N, Cavenagh J, Dokal I, Foukaneli T, Hill A, et al. Guidelines for the diagnosis and management of adult aplastic anaemia. Br J Haematol (2016) 172:187–207. doi: 10.1111/bjh.13853
19. Blažić I, Brkljačić B, Frija G. The use of imaging in COVID-19-results of a global survey by the International Society of Radiology. Eur Radiol (2020). doi: 10.1007/s00330-020-07252-3
20. Fattizzo B, Giannotta JA, Barcellini W, Barbanti MC, Bucelli C, Cassin R, et al. Ensuring continuity of care of hematologic patients during COVID-19 pandemic in a tertiary hospital in Lombardy (Italy). Blood Adv (2020) 4:2996–9. doi: 10.1182/bloodadvances.2020002120
21. Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, et al. Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA (2020) 323:1574–81. doi: 10.1001/jama.2020.5394
22. COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). Available at: https://www.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6 (Accessed May 2, 2020).
23. Wahlster L, Weichert-Leahey N, Trissal M, Grace RF, Sankaran VG. COVID-19 presenting with autoimmune hemolytic anemia in the setting of underlying immune dysregulation. Pediatr Blood Cancer (2020) 3:e28382. doi: 10.1002/pbc.28382
24. Vadlamudi G, Hong L, Keerthy M. Evans Syndrome Associated with Pregnancy and COVID-19 Infection. Case Rep Obstet Gynecol (2020) 2020:8862545. doi: 10.1155/2020/8862545
25. Akcabelen YM, Koca Yozgat A, Parlakay AN, Yarali N. COVID-19 in a child with severe aplastic anemia. Pediatr Blood Cancer (2020) 67:e28443. doi: 10.1002/pbc.28443
26. Dixon L, Varley J, Gontsarova A, Mallon D, Tona F, Muir D, et al. COVID-19-related acute necrotizing encephalopathy with brain stem involvement in a patient with aplastic anemia. Neurol Neuroimmunol Neuroinflamm (2020) 7:e789. doi: 10.1212/NXI.0000000000000789
27. Bomhof G, Mutsaers PGNJ, Leebeek FWG, Te Boekhorst PAW, Hofland J, Croles FN, et al. COVID-19-associated immune thrombocytopenia. Br J Haematol (2020) 190:e61–4. doi: 10.1111/bjh.16850
28. Tsao HS, Chason HM, Fearon DM. Immune Thrombocytopenia (ITP) in a Pediatric Patient Positive for SARS-CoV-2. Pediatrics (2020) 146:e20201419. doi: 10.1542/peds.2020-1419
29. Murt A, Eskazan AE, Yılmaz U, Ozkan T, Ar MC. COVID-19 presenting with immune thrombocytopenia: A case report and review of the literature. J Med Virol (2020) 4:2048–58. doi: 10.1002/jmv.26138
30. Lévesque V, Millaire É, Corsilli D, Rioux-Massé B, Carrier FM. Severe immune thrombocytopenic purpura in critical COVID-19. Int J Hematol (2020) 1:1–5. doi: 10.21203/rs.3.rs-32479/v1
31. Artru F, Alberio L, Moradpour D, Stalder G. Acute immune thrombocytopaenic purpura in a patient with COVID-19 and decompensated cirrhosis. BMJ Case Rep (2020) 13:e236815. doi: 10.1136/bcr-2020-236815
32. Bennett J, Brown C, Rouse M, Hoffmann M, Ye Z. Immune Thrombocytopenia Purpura Secondary to COVID-19. Cureus (2020) 12:e9083. doi: 10.7759/cureus.9083
33. Hindilerden F, Yonal-Hindilerden I, Sevtap S, Kart-Yasar K. Immune Thrombocytopenia in a Very Elderly Patient With Covid-19. Front Med (Lausanne) (2020) 7:404 a. doi: 10.3389/fmed.2020.00404
34. Lopez C, Kim J, Pandey A, Huang T, DeLoughery TG. Simultaneous onset of COVID-19 and autoimmune haemolytic anaemia. Br J Haematol (2020) 190:31–2. doi: 10.1111/bjh.16786
35. Hindilerden F, Yonal-Hindilerden I, Akar E, Yesilbag Z, Kart-Yasar K. Severe Autoimmune Hemolytic Anemia in COVID-19 İnfection, Safely Treated with Steroids. Mediterr J Hematol Infect Dis (2020) 12:e2020053 b. doi: 10.4084/mjhid.2020.053
36. Vega Hernández P, Borges Rivas Y, Ortega Sánchez E, Marqués Cabrero A, Remedios Mateo L, Silvera Roig P, et al. Autoimmune Hemolytic Anemia in a Pediatric Patient With Severe Acute Respiratory Syndrome Coronavirus 2 Infection. Pediatr Infect Dis J (2020) 39:e288. doi: 10.1097/INF.0000000000002809
37. Huscenot T, Galland J, Ouvrat M, Rossignol M, Mouly S, Sène D, et al. SARS-CoV-2-associated cold agglutinin disease: a report of two cases. Ann Hematol (2020) 99:1943–4. doi: 10.1007/s00277-020-04129-9
38. Moonla C, Watanaboonyongcharoen P, Suwanpimolkul G, Paitoonpong L, Jantarabenjakul W, Chanswangphuwana C, et al. Cold agglutinin disease following SARS-CoV-2 and Mycoplasma pneumoniae co-infections. Clin Case Rep (2020) 20:723–4. doi: 10.1002/ccr3.3152
39. Capes A, Bailly S, Hantson P, Gerard L, Laterre PF. COVID-19 infection associated with autoimmune hemolytic anemia. Ann Hematol (2020) 99:1679–80. doi: 10.1007/s00277-020-04137-9
40. Patil NR, Herc ES, Girgis M. Cold agglutinin disease and autoimmune hemolytic anemia with pulmonary embolism as a presentation of COVID-19 infection. Hematol Oncol Stem Cell Ther (2020) S1658-3876:30116–3. doi: 10.1016/j.hemonc.2020.06.005
41. Maslov DV, Simenson V, Jain S, Badari A. COVID-19 and Cold Agglutinin Hemolytic Anemia. TH Open (2020) 4:e175–7. doi: 10.1055/s-0040-1715791
42. Li M, Nguyen CB, Yeung Z, Sanchez K, Rosen D, Bushan S. Evans syndrome in a patient with COVID-19. Br J Haematol (2020) 190:e59–61. doi: 10.1111/bjh.16846
43. Figlerowicz M, Mania A, Lubarski K, Lewandowska Z, Służewski W, Derwich K, et al. First case of convalescent plasma transfusion in a child with COVID-19-associated severe aplastic anemia. Transfus Apher Sci (2020) 1:102866. doi: 10.1016/j.transci.2020.102866
44. Zen M, Fuzzi E, Astorri D, Saccon F, Padoan R, Ienna L, et al. SARS-CoV-2 infection in patients with autoimmune rheumatic diseases in northeast Italy: A cross-sectional study on 916 patients. J Autoimmun (2020) 112:102502. doi: 10.1016/j.jaut.2020.102502
45. Favalli EG, Ingegnoli F, De Lucia O, Cincinelli G, Cimaz R, Caporali R. COVID-19 infection and rheumatoid arthritis: Faraway, so close! Autoimmun Rev (2020) 19:102523. doi: 10.1016/j.autrev.2020.102523
46. Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, et al. Dexamethasone in Hospitalized Patients with Covid-19 - Preliminary Report. N Engl J Med (2020). doi: 10.1056/NEJMoa2021436
47. Barcellini W, Giannotta J, Fattizzo B. Autoimmune hemolytic anemia in adults: primary risk factors and diagnostic procedures. Expert Rev Hematol (2020) 13:585–97. doi: 10.1080/17474086.2020.1754791
48. Barcellini W, Fattizzo B. The Changing Landscape of Autoimmune Hemolytic Anemia. Front Immunol (2020) 11:946. doi: 10.3389/fimmu.2020.00946
49. Angileri F, Légaré S, Marino Gammazza A, Conway de Macario E, Macario AJL, Cappello F. Is molecular mimicry the culprit in the autoimmune haemolytic anaemia affecting patients with COVID-19? Br J Haematol (2020) 190:e92–3. doi: 10.1111/bjh.16883
50. Bhattacharjee S, Banerjee M. Immune Thrombocytopenia Secondary to COVID-19: a Systematic Review. SN Compr Clin Med (2020) 2:1–11. doi: 10.1007/s42399-020-00521-8
51. Baker AN, Richards SJ, Guy CS, Congdon TR, Hasan M, Zwetsloot AJ, et al. The SARS-COV-2 Spike Protein Binds Sialic Acids and Enables Rapid Detection in a Lateral Flow Point of Care Diagnostic Device. ACS Cent Sci (2020) 5:acscentsci.0c00855. doi: 10.1021/acscentsci.0c00855
52. Morniroli D, Giannì ML, Consales A, Pietrasanta C, Mosca F. Human Sialome and Coronavirus Disease-2019 (COVID-19) Pandemic: An Understated Correlation? Front Immunol (2020) 11:1480. doi: 10.3389/fimmu.2020.01480
53. Jansen AJG, Spaan T, Low HZ, Di Iorio D, van den Brand J, Tieke M, et al. Influenza-induced thrombocytopenia is dependent on the subtype and sialoglycan receptor and increases with virus pathogenicity. Blood Adv (2020) 4:2967–78. doi: 10.1182/bloodadvances.2020001640
54. Tang Y, Liu J, Zhang D, Xu Z, Ji J, Wen C. Cytokine Storm in COVID-19: The Current Evidence and Treatment Strategies. Front Immunol (2020) 11:1708. doi: 10.3389/fimmu.2020.01708
55. Mantovani Cardoso E, Hundal J, Feterman D, Magaldi J. Concomitant new diagnosis of systemic lupus erythematosus and COVID-19 with possible antiphospholipid syndrome. Just a coincidence? A case report and review of intertwining pathophysiology. Clin Rheumatol (2020) 39:2811–5. doi: 10.1007/s10067-020-05310-1
Keywords: COVID-19, warm autoimmune hemolytic anemia, cold agglutinin disease, immune thrombocytopenia, aplastic anemia, chronic idiopathic neutropenia
Citation: Barcellini W, Giannotta JA and Fattizzo B (2021) Are Patients With Autoimmune Cytopenias at Higher Risk of COVID-19 Pneumonia? The Experience of a Reference Center in Northern Italy and Review of the Literature. Front. Immunol. 11:609198. doi: 10.3389/fimmu.2020.609198
Received: 22 September 2020; Accepted: 14 December 2020;
Published: 26 January 2021.
Edited by:
Stefania Gallucci, Temple University, United StatesReviewed by:
Maria I. Bokarewa, University of Gothenburg, SwedenCopyright © 2021 Barcellini, Giannotta and Fattizzo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bruno Fattizzo, YnJ1bm8uZmF0dGl6em9AdW5pbWkuaXQ=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.