- 1Department of Oncology, Jinan Central Hospital Affiliated to Shandong University, Jinan, China
- 2Department of Radiotherapy Oncology, Shandong Provincial Qianfoshan Hospital, Shandong University, Jinan, China
- 3Department of Radiotherapy Oncology, Shandong Provincial Qianfoshan Hospital, The First Hospital Affiliated With Shandong First Medical University, Jinan, China
- 4Jinan Clinical Research Center of Shandong First Medical University, Jinan, China
- 5Department of General Surgery, Peking University Third Hospital, Beijing, China
- 6Department of Hepatobiliary Intervention, Beijing Tsinghua Changgung Hospital, School of Clinical Medicine, Tsinghua University, Beijing, China
- 7Department of Cardiovascular Diseases, Jinan Central Hospital Affiliated to Shandong University, Jinan, China
- 8Department of Oncology, Jinan Central Hospital affiliated to Shandong First Medical University, Jinan, China
Background: Systematic assessment of PD-1/PD-L1 inhibitor-related neurological toxicities is important for guiding anti-PD-1 and anti-PD-L1 immunotherapy. Therefore, we conducted this meta-analysis to reveal the relationship between PD-1/PD-L1 inhibitors and neurological toxicities among cancer patients.
Methods: Clinical trials investigating PD-1/PD-L1 inhibitors in cancer patients were identified by a systematic search of PubMed. The random-effect model was used to synthesize individual studies. Neurological toxicities, including all-grades and grades 3–5, were taken into account for the final comprehensive meta-analysis. The Newcastle Ottawa Scale (NOS) was used to assess the quality of included trials.
Results: Thirty-one clinical trials containing data of neurological toxicities were included. Compared with chemotherapy, the risk of all-grade neurological toxicities caused by PD-1/PD-L1 inhibitors was much lower in terms of peripheral neuropathy [OR = 0.07, 95%CI:(0.04, 0.13)], peripheral sensory neuropathy [OR = 0.07, 95%CI(0.04, 0.12)], dysgeusia [OR = 0.26, 95%CI:(0.19, 0.35)], paraesthesia [OR = 0.23, 95%CI:(0.14, 0.36)], and polyneuropathy [OR = 0.12, 95%CI:(0.01, 0.94)]. However, for grades 3–5, the statistically significant results were only seen in peripheral neuropathy [OR = 0.15, 95%CI:(0.07, 0.34)] and peripheral sensory neuropathy [OR = 0.13, 95%CI:(0.04, 0.40)]. No statistically significant difference regarding the risk of headache, dizziness, and Guillain–Barré syndrome was found between PD-1/PD-L1 inhibitors and chemotherapy. For PD-1/PD-L1 inhibitors plus chemotherapy, the risk trends of the above-mentioned neurological toxicities, especially grades 3–5 peripheral neuropathy [OR = 1.76, 95%CI:(1.10, 2.82)] was increased compared to chemotherapy alone.
Conclusion: Our comprehensive analysis showed that PD-1/PD-L1 inhibitors alone exhibited lower neurological toxicities than chemotherapy. However, the risk of headache, dizziness, and Guillain–Barré syndrome was similar between PD-1/PD-L1 and chemotherapy. For PD-1/PD-L1 inhibitors plus chemotherapy, the incidence trend of neurological toxicities would be increased, especially for peripheral neuropathy of grades 3–5.
Introduction
Cancer immunotherapies, developed to overcome the immune escape mechanisms of cancer progression and metastatic dissemination, are becoming familiar to oncologists (1), especially for programmed cell death protein 1 (PD-1) and its ligand (PD-L1) inhibitors. PD-1/PD-L1 inhibitors belong to immune checkpoint blocking drugs (1); they can block the binding of tumor cells to PD-1 of T cells by means of PD-L1, restore the ability to recognize tumor cells, and further restore the cell recognition and killing ability of T cells (1). Immunotherapies, including cytotoxic T lymphocyte antigen-4 (CTLA-4) and PD-1/PD-L1 had changed the treatment landscape for plenty of solid tumors but conferred unique toxicity profiles owing to their unique mechanism of actions (1–3).
Most of those toxic reactions had aroused sufficient attention from clinicians and researchers, and guidelines for related treatment had been developed for reference (2, 4). Neurological toxicities, including peripheral neuropathy, peripheral sensory neuropathy, peripheral motor neuropathy, dysgeusia, paraesthesia, headache, dizziness, Guillain–Barré syndrome, neurotoxicity, myasthenia gravis, noninfectious encephalitis/myelitis, and polyneuropathy, were mostly reported in the form of case reports or reviews and were considered to be rare immune-related adverse events (1, 5–14). The appearance of neurological toxicities might be diverse, involving any aspect of the central or peripheral nervous system accompanied by different diagnostic signs and symptoms (1).
As more and more clinical trials investigating the clinical efficacy and safety of PD-1/PD-L1 in cancer patients are being conducted, various treatment induced adverse events had been gradually reported (1, 2). However, regarding the neurological toxicities of PD-1/PD-L1, no systematic reviews and meta-analysis have been conducted in this regard (1–14). Therefore, in order to clarify the relationship between PD-1/PD-L1 inhibitors and the risk of neurological toxicities, this systematic review and meta-analysis was conducted.
Method
This research was conducted and reported according to the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) (15).
Types of Enrolled Studies
Randomized, open-label, controlled clinical trials investigating the efficacy and safety of PD-1/PD-L1 inhibitors in cancer patients were included. Phase III clinical trials, limited to solid tumors, were given a priority. Then, clinical trials of other phases would be checked for eligibility and placed in an alternative location. Clinical trials investigating hematological malignancies were beyond our consideration. In order to collect as many articles as possible, the control group was not restricted to a certain therapeutic agent or intervention. For inclusion, the study must report the data of at least one type of neurological toxicities related to immunotherapy. Articles must be published in English.
Search Strategy
Keywords, including neoplasm, cancer, precancer, malignant, premalignant, tumor, PD-1, PD-L1, and clinical trial, were used for the PubMed search with reference to participants, interventions, comparisons, outcomes, and study design (PICOS) (15). The published date was limited to the last 10 years (July 9, 2010 to July 9, 2020). Of note, some data regarding peripheral neuropathy was also collected from a former systematic review and meta-analysis (16). Four authors were designated to check the eligibility of all retrieved reports. They were also responsible for the extraction of relevant data from finally included trials. In the case of duplicated clinical trials, only one was included in the final analysis step. The corresponding authors (YS and GS) were responsible for resolving all disagreements.
Evaluation of Study Quality and Publication Bias
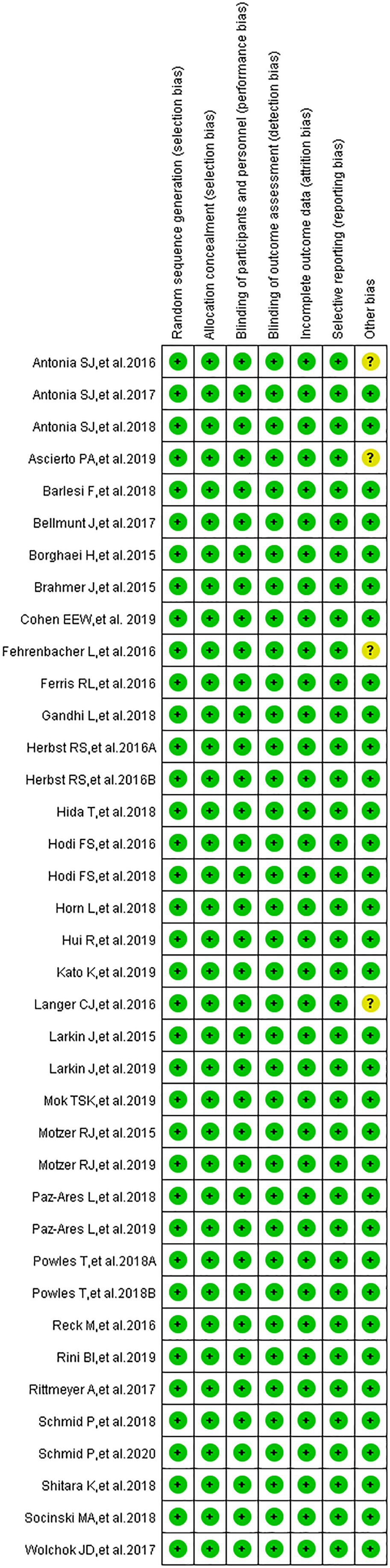
Funnel plots, Egger’s test, and the Newcastle-Ottawa scale (NOS) were used to check publication bias and risk of bias of individual trials, respectively (15, 17–20). The quality assessment included the appraisal of random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, and selective outcome reporting (shown in a single figure). Harbord’s test was used to check the risk of publication bias of enrolled clinical trials (21). A P-value of <0.05 was used as the cut-off value for statistical significance.
Outcome and Exposure of Interest
Any data of neurological toxicities, including peripheral neuropathy, peripheral sensory neuropathy, peripheral motor neuropathy, dysgeusia, paraesthesia, headache, dizziness, Guillain-Barré syndrome, neurotoxicity, and polyneuropathy, were collected and further analyzed. Baseline characteristics of included articles are summarized in (Table 1). The risk of neurological toxicities relating to all grades was our primary outcome of interest in the final meta-analysis. Grading of neurological toxicities ranged from one (mild symptoms that do not interfere with activities of daily living) to five (fatal neurological toxicities).
Assessment of Heterogeneity and Statistical Analysis
Heterogeneity of all enrolled clinical trials was identified by Cochrane’s Q statistic test (21). The grade of heterogeneity was estimated by the DerSimonian–Laird method and I2 values together, which was suggested by Higgins and colleagues (15, 21). Heterogeneity was deemed to be low, moderate, or high according to I2 values < 25, 25–50, and > 50%, respectively (16). All data analyses were completed by the software Review Manager 5.3. Owing to the existence of inherent heterogeneity among included trials, the random effect (RE) was used for the evaluation of odds ratio (OR) and their corresponding 95% confidence interval (CI) (58). Sometimes, the fixed effects (FE) model was used as a supplement. All reported P values are two-sided, and P<0.05 was deemed to be statistically significant. Subgroup analysis was made according to tumor types, treatment regimens, and PD-1/PD-L1 inhibitors.
Results
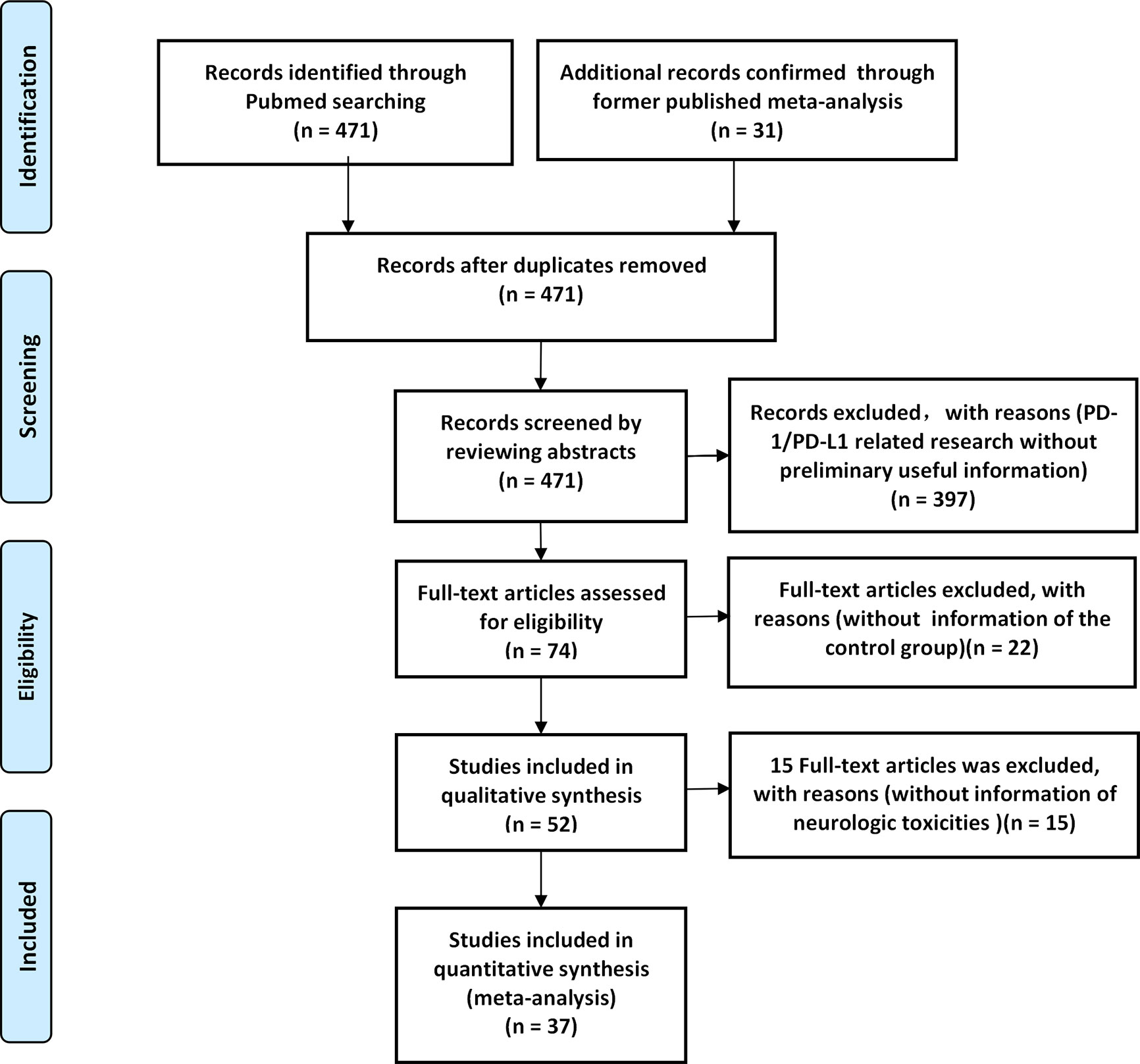
Literature Search Results
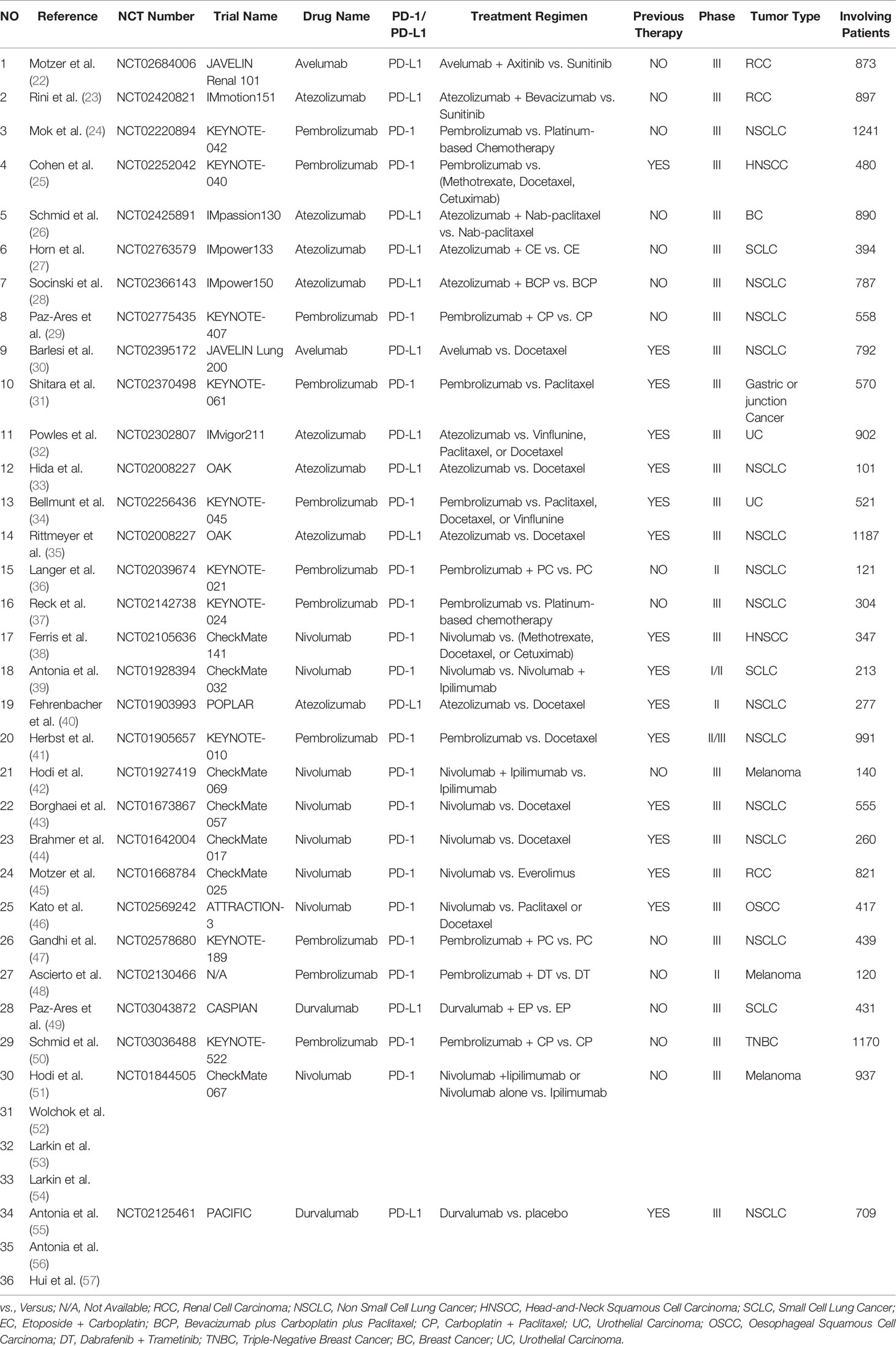
A total of 471 PD-1/PD-L1 inhibitor-related clinical trials were identified through PubMed, while 31 related studies were collected from the former published meta-analysis (16). Fifty-two articles met our preliminary screening criteria, of which 36 articles (reporting the data of neurological toxicities of 31 clinical trials involving 9960 patients) were included in the final analysis phase (22–57). Results of different periods of the same clinical trial ‘CheckMate 067’ (NCT01844505) were reported by four articles (51–54), while the results of the clinical trial ‘PACIFIC’ (NCT02125461) was reported by three articles (55–57). The baseline characteristics of the 36 enrolled articles are displayed in (Table 1) (22–57). The PRISMA flow diagram of the screening process of our review was provided in (Figure 1), while the quality of included studies is shown in (Figure 2) (22–57). After reviewing the full-texts of all included trials, 10 types of neurological toxicities were reported, including peripheral neuropathy (24–32, 34, 35, 38–41, 43, 44, 46, 50), peripheral sensory neuropathy (24–26, 29–34, 41, 42, 46, 50), dysgeusia (22, 23, 25, 26, 32–37, 41–43, 45, 47, 50), paraesthesia (25, 28, 32, 41–44, 49), headache (22, 23, 25, 26, 34, 41, 43, 47, 48, 51–57), dizziness (22, 25, 34, 36, 38, 41–44, 47, 51, 52), peripheral motor neuropathy (51), Guillain–Barré syndrome (25, 27, 33, 42, 51), neurotoxicity (25), and polyneuropathy (10, 25, 51).
Characteristics of Identified Trials
Twenty-five studies were phase III clinical trials (22–35, 37, 38, 47–49, 49–57), three were phase II trials (36, 40, 48), one was phase I/II trial (39), and one was phase II/III trial (41). Twelve clinical trials (reported in 14 articles) investigated PD-L1 (22, 23, 26–28, 30, 32, 33, 35, 40, 49, 55–57), while the remaining 18 clinical trials (reported in 22 articles) investigated PD-1 (24, 25, 29, 31, 34, 36–39, 41–48, 50–53). Among included clinical trials, nine types of tumors were reported, including non-small cell lung cancer (NSCLC) (N = 14) (24, 28–30, 33, 35–37, 40, 41, 43, 44, 47, 55–57), small cell lung cancer (SCLC) (N = 3) (27, 39, 49), renal cell carcinoma (RCC) (N = 3) (22, 23, 45), esophageal squamous cell carcinoma (OSCC) (N = 1) (46), head and neck squamous cell carcinoma (HNSCC) (N = 2) (25, 38), urothelial cancer (UC) (N = 2) (32, 34), breast cancer (BC) (N = 2) (26, 50), melanoma (N = 3) (42, 48, 51–53, 56), and gastric or junction cancer (N = 1) (31). Previous therapies were reported in 16 clinical trials (25, 30–35, 38–41, 43–46, 55–56), while PD-1/PD-L1 inhibitors were administered as a first-line therapy in the remaining 15 clinical trials (22–24, 26–29, 36, 37, 42, 47–54).
Risk of Bias
The results of the publication bias assessment, in the form of funnel plots, are provided in the supplement (Supplementary Figures 1–3, 5, 7, 9) (15, 17–20, 22–57). Low risk of bias was identified in all clinical trials regarding selection bias, performance bias, detection bias, attrition bias, and reporting bias (Figure 2) (22–57). An unclear risk relating to other biases was identified in four clinical trials (36, 39, 40, 48). None of the included trials had a high risk of bias.
Risk of Peripheral Neuropathy
Peripheral neuropathy was reported in 20 clinical trials (24–32, 34, 35, 38–41, 43, 44, 46, 50), 19 of which were included in the final meta-analysis (24–32, 34, 35, 38, 40, 41, 43, 44, 46, 50). When PD-1/PD-L1 inhibitors were compared with chemotherapy, the risk of peripheral neuropathy of all grades was noticeably lower [OR = 0.07, 95%CI:(0.04, 0.13), I2 = 62%, Z = 8.48 (P < 0.00001); Figure 3A1], even for every subgroup relating to different tumor types (24–26, 30–32, 34, 38, 40, 41, 43, 44, 46). High heterogeneity was found (I2 = 62%), which was caused mainly by the NSCLC subgroup involving PD-L1 inhibitors (I2 = 75%, Figure 3A1) (26, 30, 40). The corresponding funnel plot is provided in the supplement (S Figure 1A1). Similarly, reduced risk of peripheral neuropathy of grades 3–5 was also noted [OR = 0.15, 95%CI:(0.07, 0.340, I2 = 0%, Z = 8.48 (P <0.00001); Figure 3A2]. The corresponding funnel plot is provided in the supplement (S Figure 1A2) (24, 26, 30–32, 34, 41, 43, 44, 46).
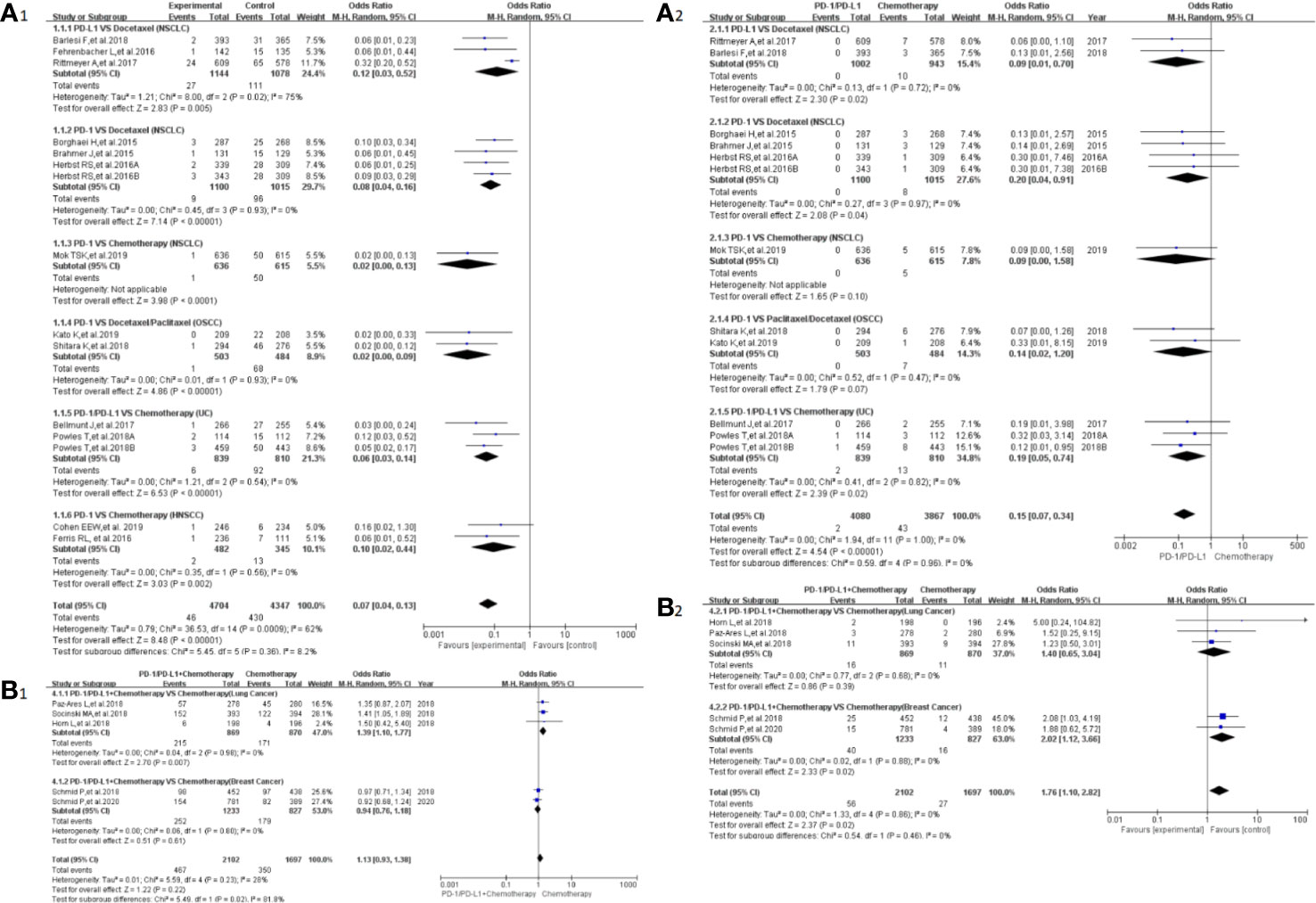
Figure 3 Forest plots of the risk of peripheral neuropathy. (A1) The risk of all-grade peripheral neuropathy calculated by the random effect (RE) model (PD-1/PD-L1 vs chemotherapy): subgroup analysis was put into practice based on PD-1/PD-L1 and tumor types in both groups. (A2) The risk of peripheral neuropathy of grades 3–5 calculated by the random effect (RE) model (PD-1/PD-L1 vs chemotherapy): subgroup analysis was put into practice based on PD-1/PD-L1 and tumor types in both groups. (B1) The risk of all grade peripheral neuropathy calculated by the random effect (RE) model (PD-1/PD-L1 + chemotherapy vs chemotherapy): subgroup analysis was put into practice based on tumor types in both groups. (B2) The risk of peripheral neuropathy of grades 3–5 calculated by the random effect (RE) model (PD-1/PD-L1 + chemotherapy vs chemotherapy): subgroup analysis was put into practice based on tumor types in both groups.
When PD-1/PD-L1 inhibitors plus chemotherapy were compared with chemotherapy (Figures 3B1, B2) (26–29, 50), a significant increase in the risk of peripheral neuropathy could only be seen in grades 3–5 [OR = 1.76, 95%CI:(1.10, 2.82), I2 = 0%, Z = 2.37 (P = 0.02); Figure 3B2] (26–29, 50). The corresponding funnel plots are provided in the supplement (S Figure 1B1, B2) (26–29, 50).
Risk of Peripheral Sensory Neuropathy
Peripheral sensory neuropathy was reported in 13 clinical trials (24–26, 29–34, 41, 42, 46, 50), 12 of which were included in the final meta-analysis (24–26, 29–34, 41, 46, 50). When PD-1/PD-L1 inhibitors were compared with chemotherapy, the risk of peripheral sensory neuropathy of all grades was obviously lower [OR = 0.07, 95%CI:(0.04, 0.12), I2 = 13%, Z = 9.50(P < 0.00001); Figure 4A1] (24, 25, 30–34, 41, 46), while similar risk trends of grades 3–5 were seen between both arms [OR = 0.13, 95%CI:(0.04, 0.40), I2 = 0%, Z=3.57 (P = 0.0004); Figure 4A2] (24, 30–32, 34, 46). The corresponding funnel plots are provided in the supplement (S Figure 2A1, A2) (24–26, 29–34, 41, 46, 50).
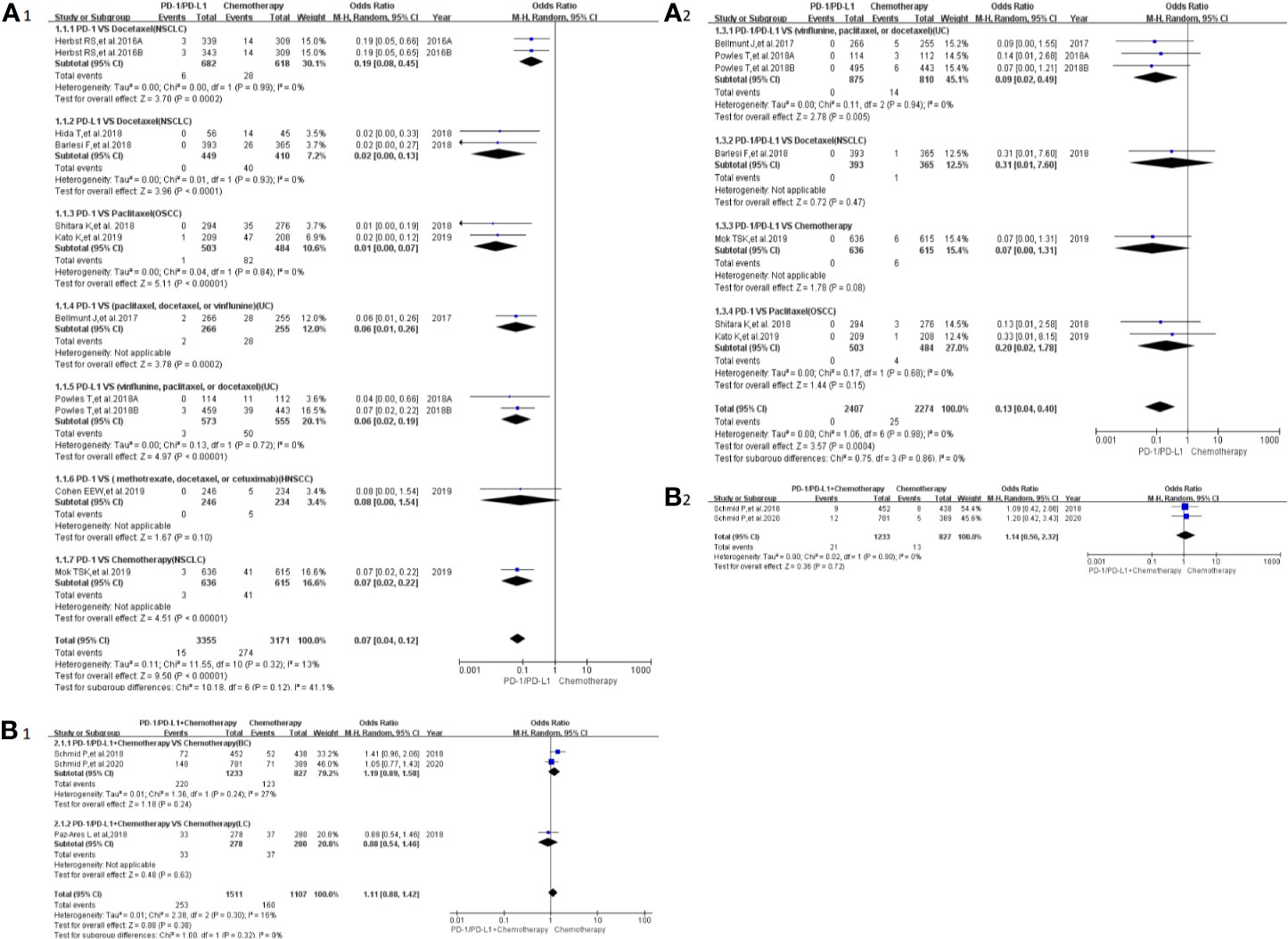
Figure 4 Forest plots of the risk of peripheral sensory neuropathy (A1) The risk of all-grade peripheral sensory neuropathy calculated by the random effect (RE) model (PD-1/PD-L1 vs chemotherapy): subgroup analysis was put into practice based on PD-1/PD-L1 and tumor types in both groups. (A2) The risk of peripheral sensory neuropathy of grades 3–5 calculated by the random effect (RE) model (PD-1/PD-L1 vs chemotherapy): subgroup analysis was put into practice based on PD-1/PD-L1 and tumor types in both groups. (B1) The risk of all-grade peripheral sensory neuropathy calculated by the random effect (RE) model (PD-1/PD-L1 + chemotherapy vs chemotherapy): subgroup analysis was put into practice based on tumor types in both groups. (B2) The risk of peripheral sensory neuropathy of grades 3–5 calculated by the random effect (RE) model (PD-1/PD-L1+ chemotherapy vs. chemotherapy): subgroup analysis was put into practice based on tumor types in both groups.
When PD-1/PD-L1 inhibitors plus chemotherapy were compared with chemotherapy (Figures 4B1, B2) (26–29, 50), no statistically significant difference was found (26, 29, 50). The corresponding funnel plots are provided in the supplement (S Figure 2B1, B2) (26, 29, 50).
Risk of Dysgeusia
Dysgeusia was reported in 16 clinical trials (22, 23, 25, 26, 32–37, 41–43, 45, 47, 50), 14 of which were included in the final meta-analysis (22, 23, 25, 26, 32–37, 41, 43, 47, 50). When PD-1/PD-L1 inhibitors were compared with chemotherapy, the risk of dysgeusia of all grades was obviously lower [OR=0.26, 95%CI:(0.19, 0.35), I2 = 0%, Z = 8.44 (P < 0.00001); Figure 5A] (25, 32–35, 37, 41, 43), especially for subgroups relating to NSCLC and UC (32–35, 37, 41, 43). The corresponding funnel plot is provided in the supplement (S Figure 3A1) (25, 32–35, 37, 41, 43).
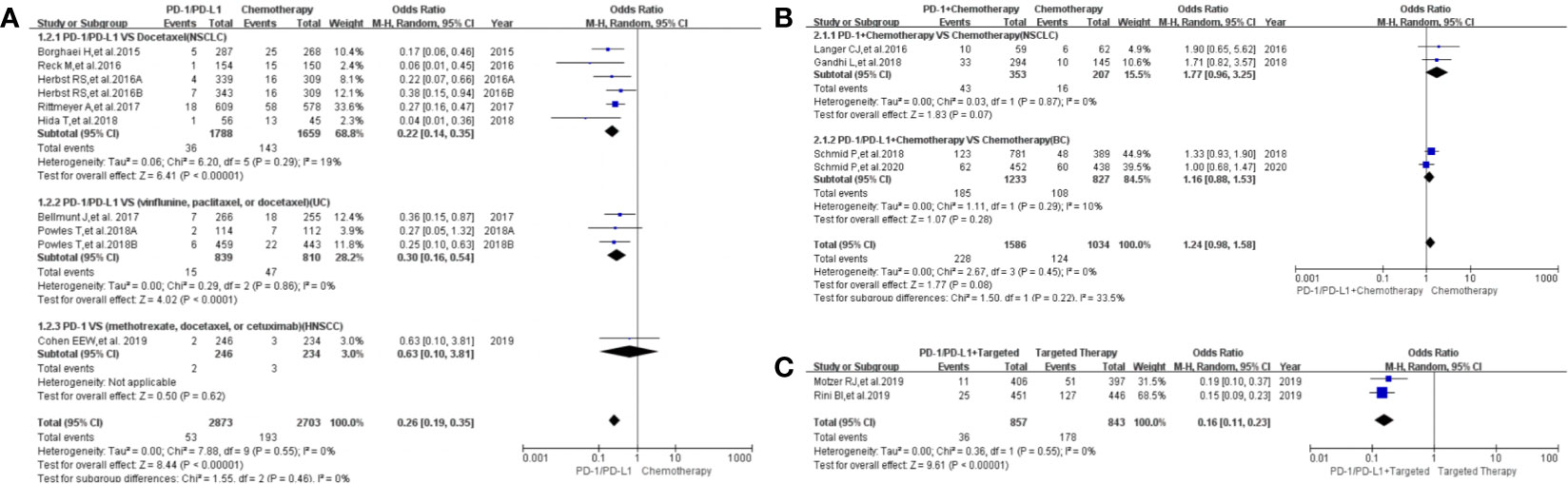
Figure 5 Forest plots of the risk of dysgeusia. (A) The risk of all-grade dysgeusia calculated by the random effect (RE) model (PD-1/PD-L1 vs chemotherapy): subgroup analysis was put into practice based on PD-1/PD-L1 and tumor types in both groups. (B) The risk of all-grade dysgeusia calculated by the random effect (RE) model (PD-1/PD-L1+ chemotherapy vs. chemotherapy): subgroup analysis was put into practice based on PD-1/PD-L1 and tumor types in both groups. (C) The risk of all-grade dysgeusia calculated by the random effect (RE) model (PD-1/PD-L1+ targeted vs. targeted therapy): subgroup analysis was put into practice based on tumor types in both groups.
When PD-1/PD-L1 inhibitors plus chemotherapy were compared with chemotherapy (Figure 5B), no statistically significant difference was noted [OR = 1.24, 95%CI:(0.98, 1.58), I2 = 0%, Z = 1.77 (P = 0.08); Figure 5B] (26, 36, 47, 50). The corresponding funnel plot is provided in the supplement (S Figure 3A2) (26, 36, 47, 50).
When PD-1/PD-L1 inhibitors plus targeted therapy were compared with targeted therapy (Figure 5C), the risk of dysgeusia of all grades was obviously lower [OR = 0.16, 95%CI:(0.11, 0.23), I2 = 0%, Z = 9.61 (P < 0.00001); Figure 5C] (22, 23). The corresponding funnel plot is provided in the supplement (S Figure 3A3) (22, 23).
The risk of dysgeusia grades 3–5 could not be analyzed in the meta-analysis due to the limited data available in the included trials (23, 47).
Risk of Paraesthesia
Paraesthesia was reported in eight clinical trials (25, 28, 32, 41–44, 49), seven of which were included in the final meta-analysis (25, 28, 32, 41, 43, 44, 49). When PD-1/PD-L1 inhibitors were compared with chemotherapy, the risk of paraesthesia of all grades was obviously lower [OR = 0.23, 95%CI:(0.14, 0.36), I2 = 0%, Z = 6.40 (P < 0.00001); Figure 6A] (25, 28, 32, 41, 43, 44, 49), especially for subgroups relating to NSCLC and UC (32, 41, 43, 44). No heterogeneity was found (Figure 6A, I2 = 0%) (25, 28, 32, 41, 43, 44, 49). The corresponding funnel plot is provided in the supplement (S Figure 3B1) (25, 28, 32, 41, 43, 44, 49).
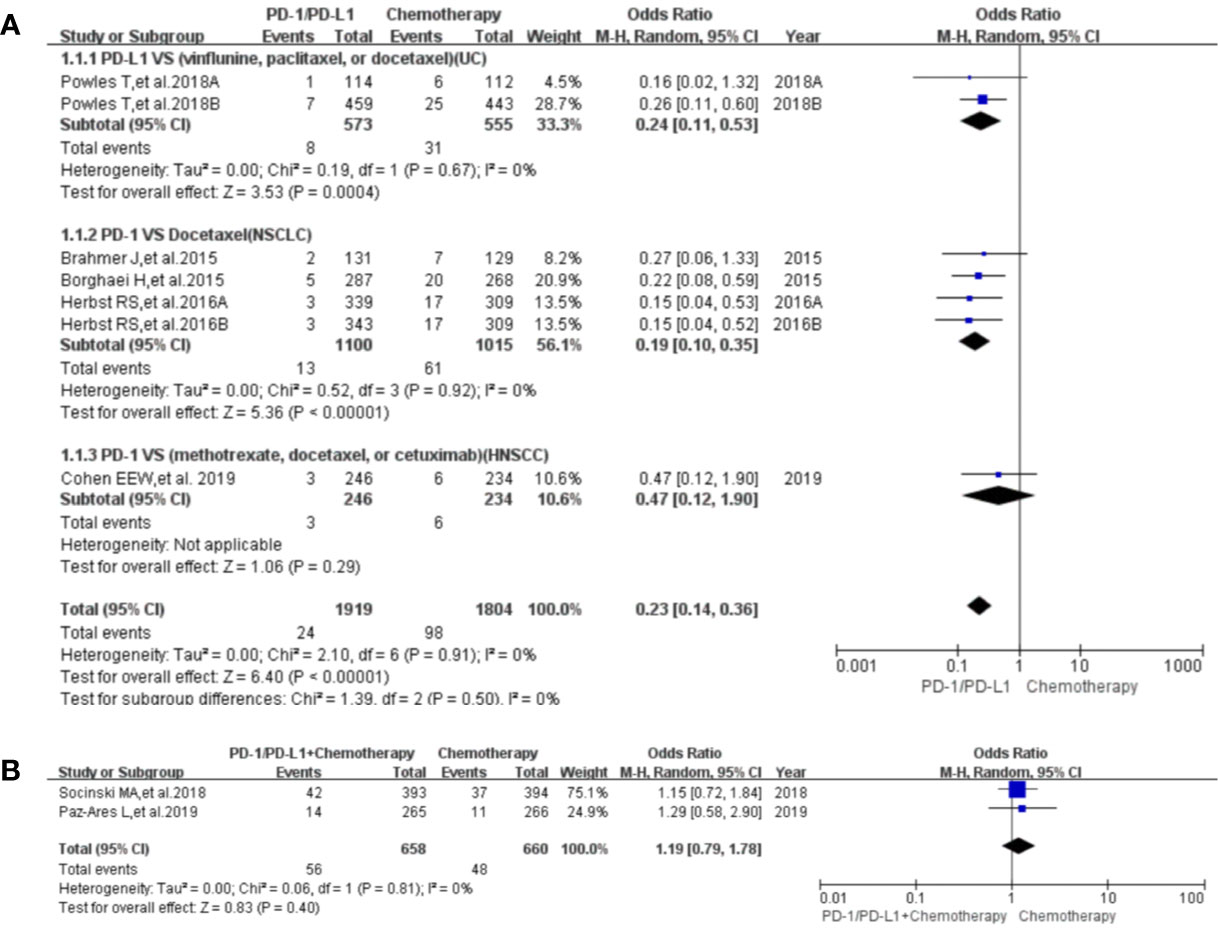
Figure 6 Forest plots of the risk of paraesthesia. (A) The risk of all-grade paraesthesia calculated by the random effect (RE) model (PD-1/PD-L1 vs. chemotherapy): subgroup analysis was put into practice based on PD-1/PD-L1 and tumor types in both groups. (B) The risk of all-grade dysgeusia calculated by the random effect (RE) model (PD-1/PD-L1 + chemotherapy vs. chemotherapy).
When PD-1/PD-L1 inhibitors plus chemotherapy were compared with chemotherapy, no statistically significant difference was found for paraesthesia of all grades [OR = 1.19, 95%CI:(0.79, 1.78), I2 = 0%, Z = 0.83 (P = 0.40); Figure 6B) (28, 49). The corresponding funnel plot is provided in the supplement (S Figure 3B2) (28, 49).
Risk of Headache
Headache was reported in 17 articles, involving 12 clinical trials (22, 23, 25, 26, 34, 41, 43, 47, 48, 51–57). When PD-1/PD-L1 inhibitors were compared with chemotherapy, no statistically significant differences were found in terms of all grade and grades 3–5 headache (S Figure 4A1, A2) (25, 34, 41, 43). A similar risk trend was also noted when PD-1/PD-L1 inhibitors plus others were compared with the control groups (S Figure 4B, C2, D1, D2) (22, 26, 47, 48, 51, 54).
When PD-1/PD-L1 inhibitors plus targeted therapy were compared with targeted therapy, the risk of headache of all grades was obviously higher [OR = 1.43, 95%CI:(1.09, 1.86), I2 = 0%, Z=2.62 (P = 0.0009); Supplementary Figure 4C1) (22, 23, 48). The corresponding funnel plots are provided in the supplement (S Figure 5) (22, 23, 25, 26, 34, 41, 43, 47, 48, 51, 54).
Risk of Dizziness
Dizziness was reported in 12 articles, involving 11 clinical trials (22, 25, 34, 36, 38, 41–44, 47, 51, 52). According to different treatment regimens, we divided all included clinical trials into four groups to investigate the risk of dizziness of all grades and grades 3–5. However, no statistically significant differences were noted (Supplementary Figure 6) (25, 34, 36, 38, 41–44, 47, 51). The corresponding funnel plots are provided in the supplement (S Figure 7) (25, 34, 36, 38, 41–44, 47, 51).
Risk of Rarely Reported Neurologic Toxicities
Other types of neurological toxicities were reported in a limited number of studies, including peripheral motor neuropathy (51), Guillain–Barré syndrome (Supplementary Figure 8A,B) (25, 27, 33, 42, 51), polyneuropathy (Supplementary Figure 8C) (10, 25, 51), neurotoxicity (25). For Guillain–Barré syndrome and polyneuropathy, compared with chemotherapy, a statistically significant reduction in their associated risk was only observed in polyneuropathy [OR = 0.12, 95%CI:(0.01, 0.940, I2 = 0%, Z = 2.02 (P = 0.04); Supplementary Figure 8C) (10, 25, 51). The corresponding funnel plots are provided in the supplement (Supplementary Figure 9) (10, 25, 27, 33, 42, 51). Due to the unavailability of relevant data regarding the other two neurological toxicities (neurotoxicity and peripheral motor neuropathy), they could not be included in the meta-analysis (25, 51).
Discussion
Most of the neurological toxicities caused by PD-1/PD-L1 inhibitors might be presented as low-grade appearances, with the potential to involve any aspect of the central or peripheral nervous system (7, 8). As more and more clinical trials reporting the efficacy and safety of PD-1/PD-L1 in cancer patients are being conducted, the reporting of drug-induced neurological toxicities has gradually increased (1, 2, 22–57). In order to clarify the relationship between PD-1/PD-L1 inhibitors and the risk of neurological toxicities in cancer patients, this meta-analysis was designed. It was the first time that neurological toxicities were comprehensively investigated through a meta-analytic approach instead of case reports and reviews (1, 5–14). It would be helpful in guiding anti-PD-1 and anti-PD-L1 immunotherapy.
Thirty-six articles, including 31 clinical trials with available data regarding neurological toxicities, were included in our study (22–57). Among the included clinical trials, lung cancer-related clinical trials accounted for the largest proportion (N = 17) (24, 27–30, 33, 35–37, 39–41, 43, 44, 47, 49, 55–57). Of note, the majority of the included clinical trials were of high quality (low risk of bias) (22–57). Therefore, the conclusion drawn from those data would be of higher credibility.
In our meta-analysis, we noted that the risk of all-grade neurological toxicities in the PD-1/PD-L1 inhibitors group was lower compared to the chemotherapy arm. These neurological toxicities included peripheral neuropathy, peripheral sensory neuropathy, dysgeusia, paraesthesia, and polyneuropathy (Figure 3A1, 4A1, 5A1, 6A1, S Figure 4A1, 8C). A similar observation was noted regarding peripheral neuropathy and peripheral sensory neuropathy of grades 3–5 (Figure 3A2, 4A2) (10, 22–47, 49–51). These findings highlight the need to pay more attention to the risk of neurological toxicities associated with chemotherapy in clinical practice, especially for docetaxel (26, 30–32, 34, 40, 41, 43, 44, 46). The subgroup analyses suggested that the encountered high heterogeneity in our analyses (I2=62%) might be related to the NSCLC subgroup (I2 = 75%, Figure 3A1) (26, 30, 40). In addition, the treatment plans involved in the three NSCLC clinical trials included in the comprehensive analysis belonged to different treatment lines (first, second, or third line); this probably might be a potential contributor to the heterogeneity of the result (I2 = 75%, Figure 3A1) (26, 30, 40). That being said, no obvious risk of publication bias was found from the corresponding funnel plots (Supplementary Figure 1A1, 2A1, 3A1, B1, 5A1, 9C). Interestingly, for headache, dizziness, and Guillain-Barré syndrome, the risk was found to be of no significance (Supplementary Figure 4A, 6A, 8A) (22, 23, 25–27, 33, 34, 36, 38, 41–44, 47, 48, 51–57), which meant that the risk trend of the aforementioned three neurological toxicities caused by PD-1/PD-L1 inhibitors was similar to that of the chemotherapy group. This finding is novel and has not been reported nor investigated by other studies in the literature.
Furthermore, Guillain–Barré syndrome was reported in five PD-1/PD-L1 groups (all cases were reported in the PD-1/PD-L1 group), while the incidence rate of the control groups was 0 (25, 27, 33, 42, 51). No statistically significant difference was noted and this could be attributed to the small number of included trials and the sensitivity of the analysis method (25, 27, 33, 42, 51). That being said, we cannot rule out the possibility that Guillain–Barré syndrome is a unique neurological toxicity of PD-1/PD-L1 inhibitors. Despite the fact that our analyses revealed some statistically insignificant results; however, the reported risks should not be ignored in clinical practice, and more attention should be paid to those fatal and rare reported neurological toxicities (25, 27, 33, 42, 51). These results might be of significant value in clinical practice. Once Guillain-Barré syndrome happened, we should first consider its associations with PD-1/PD-L1 inhibitors (25, 27, 33, 42, 51).
When PD-1/PD-L1 inhibitors plus chemotherapy were compared with chemotherapy, the trends in the risk of all-grade neurological toxicities increased without statistically significant differences (Figure 3B1, 4B1, 5B, 6B, Supplementary Figure 4B, 6B) (26–29, 36, 47, 49, 50). Statistically significant results were only found in terms of peripheral neuropathy of grades 3–5, especially for the breast cancer subgroup [OR = 1.76, 95%CI:(1.10, 2.82), I2 = 0%, Z = 2.37 (P = 0.02); Figure 3B2] (26–29, 50). In order to draw a definite conclusion, more relevant clinical trials are still warranted to be conducted, and sufficient subgroup analyses still need to be carried out.
When PD-1/PD-L1 inhibitors plus targeted therapy were compared with targeted therapy (Figure 5C), the risk of all-grade dysgeusia was notably lower than that of the control group [OR = 0.16, 95%CI:(0.11, 0.23), I2 = 0%, Z = 9.61 (P < 0.00001); Figure 5C) (22, 23). On the contrary, the risk of all-grade headache was increased compared to the targeted therapy group [OR = 1.43, 95%CI:(1.09, 1.86), I2 = 0%, Z = 2.62 (P = 0.0009); Supplementary Figure 4C1] (22, 23, 48). However, the number of analyzed studies was low, and thus, a definite conclusion could not be reached (22, 23, 48). This was also observed when PD-1/PD-L1 inhibitors plus CTLA-4 were compared with CTLA-4 analog Supplementary Figure 4D1, D2, 6C, 8B). Eventually, based on the low number of analyzed studies and the minimal data reported in these studies, our findings should be interpreted with caution, and no clinical recommendations should be implemented from these data.
Strengths and Limitations
Strengths
This article was designed according to the PRISMA guidelines. The literature searching process was carried out in accordance with the PICOS principle. We strictly limited the selection criteria to clinical trials and checked the accuracy of the extracted data carefully. The quality of the majority of the included trials was high. Subgroup analyses were put into practice as much as possible. Therefore, our meta-analysis provided a much more reliable evaluation of the relationship between PD-1/PD-L1 inhibitors and the associated risk of neurological toxicities in cancer patients compared to available evidence in the literature.
Limitations
First, compared with the control group, all the analysis results just showed the relative risk of neurological toxicities in cancer patients. Even when the associated risk of neurological toxicity was lower than that of the control group, it did not mean that PD-1/PD-L1 would not cause neurological toxicity in the experimental group. Second, the low number of studies that reported the data of certain neurological toxicities, along with the unavailability of relevant data, made it difficult to conduct a meta-analysis in this regard. Therefore, a definite conclusion could not be reached.
Conclusion
Our comprehensive review showed that PD-1/PD-L1 inhibitors alone exhibited lower neurological toxicities than chemotherapy. However, in terms of headache, dizziness, and Guillain–Barré syndrome, the risk trends were similar between both interventions. Regarding PD-1/PD-L1 inhibitors plus chemotherapy, the risk of neurological toxicities would be increased, especially for peripheral neuropathy of grades 3–5.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author Contributions
The corresponding authors (YS and GS) had the right to deal with all the data and were responsible for the decision to submit this manuscript for publication. YT, AG, SW, SZ, and XY had the full data of the manuscript. YT, AG, SW, and SZ were responsible for checking and evaluating the quality of the data and included studies. YT was assigned to write the text of this manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by the Academic Promotion Program of Shandong First Medical University (2019QL025; YS), Natural Science Foundation of Shandong Province (ZR2019MH042; YS), Jinan Science and Technology Program (201805064; YS), and the National Science and Technology Major Project of the Ministry of Science and Technology of China (2020ZX09201025; GS).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2020.595655/full#supplementary-material
Supplementary Figure 1 | Funnel plots of the risk of peripheral neuropathy. (A1) The risk of all-grade peripheral neuropathy calculated by the fixed effect (FE) model (PD-1/PD-L1 vs. chemotherapy): subgroup analysis was put into practice based on PD-1/PD-L1 and tumor types in both groups. (A2) The risk of peripheral neuropathy of grades 3–5 calculated by the fixed effect (FE) model (PD-1/PD-L1 vs. chemotherapy): subgroup analysis was put into practice based on PD-1/PD-L1 and tumor types in both groups. (B1) The risk of all-grade peripheral neuropathy calculated by the fixed effect (FE) model (PD-1/PD-L1 + chemotherapy vs. chemotherapy): subgroup analysis was put into practice based on tumor types in both groups. (B2) The risk of peripheral neuropathy of grades 3–5 calculated by the fixed effect (FE) model (PD-1/PD-L1 + chemotherapy vs. chemotherapy): subgroup analysis was put into practice based on tumor types in both groups.
Supplementary Figure 2 | Funnel plots of the risk of peripheral sensory neuropathy. (A1) The risk of all-grade peripheral sensory neuropathy calculated by the fixed effect (FE) model (PD-1/PD-L1 vs. chemotherapy): subgroup analysis was put into practice based on PD-1/PD-L1 and tumor types in both groups. (A2) The risk of peripheral sensory neuropathy of grades 3–5 calculated by the fixed effect (FE) model (PD-1/PD-L1 vs. chemotherapy): subgroup analysis was put into practice based on PD-1/PD-L1 and tumor types in both groups. (B1) The risk of all-grade peripheral sensory neuropathy calculated by the fixed effect (FE) model (PD-1/PD-L1 + chemotherapy vs. chemotherapy): subgroup analysis was put into practice based on tumor types in both groups. (B2) The risk of peripheral sensory neuropathy of grades 3–5 calculated by the fixed effect (FE) model (PD-1/PD-L1 + chemotherapy vs. chemotherapy): subgroup analysis was put into practice based on tumor types in both groups.
Supplementary Figure 3 | (A) Funnel plots of the risk of dysgeusia. (A1) The risk of all-grade dysgeusia calculated by the fixed effect (FE) model (PD-1/PD-L1 vs. chemotherapy): subgroup analysis was put into practice based on PD-1/PD-L1 and tumor types in both groups. (A2) The risk of all-grade dysgeusia calculated by the fixed effect (FE) model. (PD-1/PD-L1 + chemotherapy vs. chemotherapy): subgroup analysis was put into practice based on PD-1/PD-L1 and tumor types in both groups. (A3) The risk of all-grade dysgeusia calculated by the fixed effect (FE) model. (PD-1/PD-L1 + targeted vs. targeted therapy): subgroup analysis was put into practice based on tumor types in both groups. (B) Funnel plots of the risk of paraesthesia. (B1) The risk of all-grade paraesthesia calculated by the fixed effect (FE) model (PD-1/PD-L1 vs. chemotherapy): subgroup analysis was put into practice based on PD-1/PD-L1 and tumor types in both groups. (B2) The risk of all-grade paraesthesia calculated by the fixed effect (FE) model (PD-1/PD-L1 + chemotherapy vs. chemotherapy).
Supplementary Figure 4 | Forest plots of the risk of headache. (A1) The risk of all-grade headache calculated by the random effect (RE) model (PD-1/PD-L1 vs. chemotherapy): subgroup analysis was put into practice based on tumor types in both groups. (A2) The risk of headache of grades 3–5 calculated by the random effect (RE) model (PD-1/PD-L1 vs. chemotherapy). (B) The risk of all-grade headache calculated by the random effect (RE) model (PD-1/PD-L1 + targeted vs. targeted chemotherapy): subgroup analysis was put into practice based on PD-1 or PD-L1. (C1) The risk of all-grade headache calculated by the random effect (RE) model (PD-1/PD-L1 + targeted vs. targeted therapy): subgroup analysis was put into practice based on PD-1/PD-L1 and tumor types in both groups. (C2) The risk of headache of grades 3–5 calculated by the random effect (RE) model (PD-1/PD-L1 + targeted vs. targeted therapy). (D1) The risk of all-grade headache calculated by the random effect (RE) model (PD-1/PD-L1 + CTLA-4 vs. CTLA-4). (D2) The risk of headache of grades 3–5 calculated by the random effect (RE) model (PD-1/PD-L1 + CTLA-4 vs. CTLA-4).
Supplementary Figure 5 | Funnel plots of the risk of headache. (A1) The risk of all-grade headache calculated by the fixed effect (FE) model (PD-1/PD-L1 vs. chemotherapy): subgroup analysis was put into practice based on tumor types in both groups. (A2) The incidence risk of headache of grades 3–5 calculated by the fixed effect (FE) model (PD-1/PD-L1 vs. chemotherapy). (B) The risk of all-grade headache calculated by the fixed effect (FE) model (PD-1/PD-L1 + targeted vs. targeted therapy): subgroup analysis was put into practice based on PD-1 or PD-L1. (C1) The risk of all-grade headache calculated by the fixed effect (FE) model (PD-1/PD-L1 + targeted vs. targeted therapy): subgroup analysis was put into practice based on PD-1/PD-L1 and tumor types in both groups. (C2) The risk of headache of grades 3–5 calculated by the fixed effect (FE) model (PD-1/PD-L1 + targeted vs. targeted therapy). (D1) The risk of all-grade headache calculated by the fixed effect (FE) model (PD-1/PD-L1 + CTLA-4 vs. CTLA-4). (D2) The risk of headache of grades 3–5 calculated by the fixed effect (FE) model (PD-1/PD-L1 + CTLA-4 vs. CTLA-4).
Supplementary Figure 6 | Forest plots of the risk of dizziness. (A1) The risk of all-grade dizziness calculated by the random effect (RE) model (PD-1/PD-L1 vs. chemotherapy): subgroup analysis was put into practice based on PD-1/PD-L1 and tumor types in both groups. (A2) The risk of dizziness of grades 3–5 calculated by random effect (RE) model (PD-1/PD-L1 vs. chemotherapy). (B) The risk of all-grade dizziness calculated by the random effect (RE) model (PD-1/PD-L1 +c hemotherapy vs. chemotherapy). (C) The risk of all-grade dizziness calculated by the random effect (RE) model (PD-1/PD-L1 + CTLA-4 vs. CTLA-4).
Supplementary Figure 7 | Funnel plots of the risk of dizziness. (A1) The risk of all-grade dizziness calculated by the fixed effect (FE) model (PD-1/PD-L1 vs. chemotherapy): subgroup analysis was put into practice based on PD-1/PD-L1 and tumor types in both groups. (A2) The risk of dizziness of grades 3–5 calculated by the fixed effect (FE) model (PD-1/PD-L1 vs. chemotherapy). (B) The risk of all-grade dizziness calculated by the fixed effect (FE) model (PD-1/PD-L1 + chemotherapy vs. chemotherapy). (C) The risk of all-grade dizziness calculated by the fixed effect (FE) model (PD-1/PD-L1 + CTLA-4 vs. CTLA-4).
Supplementary Figure 8 | Forest plots of the risk of rarely reported neurological toxicities. (A1) The risk of all-grade Guillain–Barré Syndrome calculated by the random effect (RE) model (PD-1/PD-L1 vs. chemotherapy): subgroup analysis was put into practice based on PD-1/PD-L1 and tumor types in both groups. (A2) The risk of Guillain–Barré Syndrome of grades 3–5 calculated by the random effect (RE) model (PD-1/PD-L1 vs. chemotherapy). (B) The risk of all-grade Guillain–Barré Syndrome calculated by the random effect (RE) model (PD-1/PD-L1 + CTLA-4 vs. CTLA-4). (C) The risk of all-grade polyneuropathy calculated by the random effect (RE) model (PD-1/PD-L1 + CTLA-4 vs. CTLA-4).
Supplementary Figure 9 | Funnel plots of the risk of rarely reported neurological toxicities. (A1) The risk of all-grade Guillain–Barré Syndrome calculated by the fixed effect (FE) model (PD-1/PD-L1 vs. chemotherapy): subgroup analysis was put into practice based on PD-1/PD-L1 and tumor types in both groups. (A2) The risk of Guillain–Barré Syndrome of grades 3–5 calculated by the fixed effect (FE) model (PD-1/PD-L1 vs. chemotherapy). (B) The risk of all-grade Guillain–Barré Syndrome calculated by the fixed effect (FE) model (PD-1/PD-L1 + CTLA-4 vs. CTLA-4). (C) The risk of all-grade polyneuropathy calculated by the fixed effect (FE) model (PD-1/PD-L1 + CTLA-4 vs. CTLA-4).
Abbreviations
PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; PICOS, Participants, Interventions, Comparisons, Outcomes, and Study design; PD-1, Programmed Cell Death-1; PD-L1, Programmed Cell Death Ligand 1; HR, Hazard Ratios; OR. Odds Ratio; RD, Risk Difference; CI, Confidence Interval; RE, Random Effect; NSCLC, Non-Small Cell Lung Cancer; SCLC, Small Cell Lung Cancer; OSCC, Esophageal Squamous Cell Carcinoma; HNSCC, Head and Neck Squamous Cell Carcinoma; UC, Urothelial Cancer; BC, Breast Cancer; RCC, Renal Cell Carcinoma; NOS, Newcastle-Ottawa scale.
References
1. Kennedy LB. Salama AKS. A review of cancer immunotherapy toxicity. CA Cancer J Clin (2020) 70(2):86–104. doi: 10.3322/caac.21596
2. Thompson JA. New NCCN guidelines: recognition and management of immunotherapy-related toxicity. J Natl Compr Canc Netw (2018) 16(suppl 5):594–6. doi: 10.6004/jnccn.2018.0047
3. Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med (2018) 378:158–68. doi: 10.1056/NEJMra1703481
4. Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol (2018) 36:1714–68. doi: 10.1200/JCO.2017.77.6385
5. Larkin J, Chmielowski B, Lao CD, Hodi FS, Sharfman W, Weber J, et al. Neurologic serious adverse events associated with nivolumab plus ipilimumab or nivolumab alone in advanced melanoma, including a case series of encephalitis. Oncologist (2017) 22(6):709–18. doi: 10.1634/theoncologist.2016-0487
6. Johnson DB, Manouchehri A, Haugh AM, et al. Neurologic toxicity associated with immune checkpoint inhibitors: a pharmacovigilance study. J Immunother Cancer (2019) 7:134. doi: 10.1186/s40425-019-0617-x
7. Cuzzubbo S, Javeri F, Tissier M, Roumi A, Barlog C, Doridam J, et al. Neurological adverse events associated with immune checkpoint inhibitors: review of the literature. Eur J Cancer (2017) 73:1–8. doi: 10.1016/j.ejca.2016.12.001
8. Touat M, Talmasov D, Ricard D, Psimaras D. Neurological toxicities associated with immune-checkpoint inhibitors. Curr Opin Neurol (2017) 30:659–68. doi: 10.1097/WCO.0000000000000503
9. Liao B, Shroff S, Kamiya-Matsuoka C, Tummala S. Atypical neurological complications of ipilimumab therapy in patients with metastatic melanoma. Neuro Oncol (2014) 16:589–93. doi: 10.1093/neuonc/nou001
10. Vallet H, Gaillet A, Weiss N, Vanhaecke C, Saheb S, Touitou V, et al. Pembrolizumab-induced necrotic myositis in a patient with metastatic melanoma. Ann Oncol (2016) 27(7):1352–3. doi: 10.1093/annonc/mdw126
11. Kim A, Keam B, Cheun H, Lee ST, Gook HS, Han MK. Immune-checkpoint-inhibitor-induced severe autoimmune encephalitis treated by steroid and intravenous immunoglobulin. J Clin Neurol (2019) 15:259–61. doi: 10.3988/jcn.2019.15.2.259
12. Hottinger AF, de Micheli R, Guido V, Karampera A, Hagmann P, Du Pasquier R. Natalizumab may control immune checkpoint inhibitor-induced limbic encephalitis. Neurol Neuroimmunol Neuroinflamm (2018) 5:e439. doi: 10.1212/NXI.0000000000000439
13. Puzanov I, Diab A, Abdallah K, Bingham CO 3rd, Brogdon C, Dadu R, et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer (2017) 5(1):95. doi: 10.1186/s40425-017-0300-z
14. Mirabile A, Brioschi E, Ducceschi M, Piva S, Lazzari C, Bulotta A, et al. PD-1 Inhibitors-Related Neurological Toxicities in Patients with Non-Small-Cell Lung Cancer: A Literature Review. Cancers (Basel) (2019) 11(3):296. doi: 10.3390/cancers11030296
15. Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med (2009) 151:264–69. W64. doi: 10.7326/0003-4819-151-4-200908180-00135
16. Si Z, Zhang S, Yang X, Ding N, Xiang M, Zhu Q, et al. The Association Between the Incidence Risk of Peripheral Neuropathy and PD-1/PD-L1 Inhibitors in the Treatment for Solid Tumor Patients: A Systematic Review and Meta-Analysis. Front Oncol (2019) 9:866. doi: 10.3389/fonc.2019.00866
17. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. Cochrane Bias Methods Group and Cochrane Statistical Methods Group.. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ (2011) 343:d5928. doi: 10.1136/bmj.d5928
18. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics (1994) 50(4):1088–101. doi: 10.2307/2533446
19. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (1997) 315(7109):629–34. doi: 10.1136/bmj.315.7109.629
20. Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle–Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses(2009). Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (Accessed July 6, 2012).
21. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
22. Motzer RJ, Penkov K, Haanen J, et al. Avelumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med (2019) 380(12):1103–15. doi: 10.1056/NEJMoa1816047
23. Rini BI, Powles T, Atkins MB, et al. Atezolizumab plus bevacizumab versus sunitinib in patients with previously untreated metastatic renal cell carcinoma (IMmotion151): a multicentre, open-label, phase 3, randomised controlled trial. Lancet (2019) 393(10189):2404–15. doi: 10.1016/S0140-6736(19)30723-8
24. Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho BC, Turna HZ, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet (2019) 393(10183):1819–30. doi: 10.1016/S0140-6736(18)32409-7
25. Cohen EEW, Soulières D, Le Tourneau C, Dinis J, Licitra L, Ahn MJ, et al. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomised, open-label, phase 3 study. Lancet (2019) 393(10167):156–67. doi: 10.1016/S0140-6736(18)31999-8
26. Schmid P, Adams S, Rugo HS, et al. Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N Engl J Med (2018) 379(22):2108–21. doi: 10.1056/NEJMoa1809615
27. Horn L, Mansfield AS, Szczęsna A, et al. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N Engl J Med (2018) 379(23):2220–9. doi: 10.1056/NEJMoa1809064
28. Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N Engl J Med (2018) 378(24):2288–301. doi: 10.1056/NEJMoa1716948
29. Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus Chemotherapy for Squamous Non-Small-Cell Lung Cancer. N Engl J Med (2018) 379(21):2040–51. doi: 10.1056/NEJMoa1810865
30. Barlesi F, Vansteenkiste J, Spigel D, et al. Avelumab versus docetaxel in patients with platinum-treated advanced non-small-cell lung cancer (JAVELIN Lung 200): an open-label, randomised, phase 3 study [published correction appears in Lancet Oncol. 2018 Nov;19(11):e581]. Lancet Oncol (2018) 19(11):1468–79. doi: 10.1016/S1470-2045(18)30673-9
31. Shitara K, Özgüroğlu M, Bang YJ, Di Bartolomeo M, Mandalà M, Ryu MH, et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet (2018) 392(10142):123–33. doi: 10.1016/S0140-6736(18)31257-1
32. Powles T, Durán I, van der Heijden MS, et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open-label, phase 3 randomised controlled trial [published correction appears in Lancet. 2018 Oct 20;392(10156):1402]. Lancet (2018) 391(10122):748–57. doi: 10.1016/S0140-6736(17)33297-X
33. Hida T, Kaji R, Satouchi M, et al. Atezolizumab in Japanese Patients With Previously Treated Advanced Non-Small-Cell Lung Cancer: A Subgroup Analysis of the Phase 3 OAK Study. Clin Lung Cancer (2018) 19(4):e405–15. doi: 10.1016/j.cllc.2018.01.004
34. Bellmunt J, de Wit R, Vaughn DJ, et al. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N Engl J Med (2017) 376(11):1015–26. doi: 10.1056/NEJMoa1613683
35. Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial [published correction appears in Lancet. 2017 Apr 8;389(10077):e5]. Lancet (2017) 389(10066):255–65. doi: 10.1016/S0140-6736(16)32517-X
36. Langer CJ, Gadgeel SM, Borghaei H, et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol (2016) 17(11):1497–508. doi: 10.1016/S1470-2045(16)30498-3
37. Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med (2016) 375(19):1823–33. doi: 10.1056/NEJMoa1606774
38. Ferris RL, Blumenschein G Jr, Fayette J, et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N Engl J Med (2016) 375(19):1856–67. doi: 10.1056/NEJMoa1602252
39. Antonia SJ, López-Martin JA, Bendell J, et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial [published correction appears in Lancet Oncol. 2016 Jul;17 (7):e270] [published correction appears in Lancet Oncol. 2019 Feb;20(2):e70]. Lancet Oncol (2016) 17(7):883–95. doi: 10.1016/S1470-2045(19)30018-X
40. Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet (2016) 387(10030):1837–46. doi: 10.1016/S0140-6736(16)00587-0
41. Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet (2016) 387(10027):1540–50. doi: 10.1016/S0140-6736(15)01281-7
42. Hodi FS, Chesney J, Pavlick AC, et al. Combined nivolumab and ipilimumab versus ipilimumab alone in patients with advanced melanoma: 2-year overall survival outcomes in a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol (2016) 17(11):1558–68. doi: 10.1016/S1470-2045(16)30366-7
43. Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med (2015) 373(17):1627–39. doi: 10.1056/NEJMoa1507643
44. Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med (2015) 373(2):123–35. doi: 10.1056/NEJMoa1504627
45. Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med (2015) 373(19):1803–13. doi: 10.1056/NEJMoa1510665
46. Kato K, Cho BC, Takahashi M, et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): a multicentre, randomised, open-label, phase 3 trial [published correction appears in Lancet Oncol. 2019 Nov;20(11):e613]. Lancet Oncol (2019) 20(11):1506–17. doi: 10.1016/S1470-2045(19)30626-6
47. Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med (2018) 378(22):2078–92. doi: 10.1056/NEJMoa1801005
48. Ascierto PA, Ferrucci PF, Fisher R, et al. Dabrafenib, trametinib and pembrolizumab or placebo in BRAF-mutant melanoma. Nat Med (2019) 25(6):941–6. doi: 10.1038/s41591-019-0448-9
49. Paz-Ares L, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet (2019) 394(10212):1929–39. doi: 10.1016/S0140-6736(19)32222-6
50. Schmid P, Cortes J, Pusztai L, et al. Pembrolizumab for Early Triple-Negative Breast Cancer. N Engl J Med (2020) 382(9):810–21. doi: 10.1056/NEJMoa1910549
51. Hodi FS, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Cowey CL, et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol (2018) 19(11):1480–92. doi: 10.1016/S1470-2045(18)30700-9
52. Wolchok JD, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob JJ, Cowey CL, et al. Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med (2017) 377(14):1345–56. doi: 10.1056/NEJMoa1709684
53. Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med (2015) 373(1):23–34. doi: 10.1056/NEJMoa1504030
54. Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Lao CD, et al. Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med (2019) 381(16):1535–46. doi: 10.1056/NEJMoa1910836
55. Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med (2017) 377(20):1919–29. doi: 10.1056/NEJMoa1709937
56. Antonia SJ, Villegas A, Daniel D, et al. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N Engl J Med (2018) 379(24):2342–50. doi: 10.1056/NEJMoa1809697
57. Hui R, Özgüroğlu M, Villegas A, et al. Patient-reported outcomes with durvalumab after chemoradiotherapy in stage III, unresectable non-small-cell lung cancer (PACIFIC): a randomised, controlled, phase 3 study. Lancet Oncol (2019) 20(12):1670–80. doi: 10.1016/S1470-2045(19)30519-4
Keywords: neurological toxicities, cancer, meta-analysis, PD-1, PD-L1
Citation: Tian Y, Gao A, Wen Q, Wang S, Zhang S, Yang X, Su G and Sun Y (2020) Immune-Related Neurological Toxicities of PD-1/PD-L1 Inhibitors in Cancer Patients: A Systematic Review and Meta-Analysis. Front. Immunol. 11:595655. doi: 10.3389/fimmu.2020.595655
Received: 17 August 2020; Accepted: 16 November 2020;
Published: 18 December 2020.
Edited by:
Xuelei Ma, Sichuan University, ChinaReviewed by:
Patrick Dillon, University of Virginia, United StatesGuido Cavaletti, University of Milano-Bicocca, Italy
Copyright © 2020 Tian, Gao, Wen, Wang, Zhang, Yang, Su and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guohai Su, Z3VvaGFpc3UwNTMxQDEyNi5jb20=; Yuping Sun, c3VueXVwaW5nQGxpdmUuY24=
 Yuan Tian
Yuan Tian Aiqin Gao1
Aiqin Gao1 Shuisheng Zhang
Shuisheng Zhang Xiaowei Yang
Xiaowei Yang Yuping Sun
Yuping Sun