
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Immunol., 17 November 2020
Sec. Cytokines and Soluble Mediators in Immunity
Volume 11 - 2020 | https://doi.org/10.3389/fimmu.2020.594735
This article is part of the Research TopicRole of the IL-23/IL-17 Pathway in Chronic Immune-Mediated Inflammatory Diseases: Mechanisms and Targeted TherapiesView all 21 articles
Interleukin-17 (IL-17) is an essential proinflammatory cytokine, which is mainly secreted by the CD4+ helper T cells (Th17 cells) and subsets of innate lymphoid cells. IL-17A is associated with the pathogenesis of inflammatory diseases, including psoriasis, atopic dermatitis, hidradenitis suppurativa, alopecia areata, pityriasis rubra pilaris, pemphigus, and systemic sclerosis. Interleukin-23 (IL-23) plays a pivotal role in stimulating the production of IL-17 by activating the Th17 cells. The IL-23/IL-17 axis is an important pathway for targeted therapy for inflammatory diseases. Emerging evidence from clinical trials has shown that monoclonal antibodies against IL-23, IL-17, and tumor necrosis factor are effective in the treatment of patients with psoriasis, atopic dermatitis, hidradenitis suppurativa, pityriasis rubra pilaris, pemphigus, and systemic sclerosis. Here, we summarize the latest knowledge about the biology, signaling, and pathophysiological functions of the IL-23/IL-17 axis in inflammatory skin diseases. The currently available biologics targeting the axis is also discussed.
Interleukin-17A (IL-17A) is cloned from a T cell hybridoma activated in rodents (1) and is related to several immune-mediated disorders, such as autoimmune (2), oncogenic (3), and infectious (4) diseases. The T helper 17 (Th17) cells constitute a unique subset of CD4+ T cells and are the major source of IL-17 (5). IL-17A triggers cellular reactions not only in the keratinocytes, but also in some other cells, including neutrophils, endothelial cells, fibroblasts, and osteoclasts (6–10). In keratinocytes, the binding of IL-17A to IL-17 receptor (IL-17R) A, IL-17C, or IL-17RD stimulates keratinocyte proliferation. Subsequently, the release of inflammatory mediators and chemokines leads to inflammatory reaction (11, 12).
The cytokines, interleukin-23 (IL-23) and IL-17, have been confirmed to markedly affect chronic inflammation (10, 13–16). In addition, the discovery of the IL-23/IL-17 pathway has contributed to a clearer understanding of the underlying mechanism of inflammatory diseases. At present, therapies for inflammatory diseases have advanced from general immunosuppression to biologics against the IL-23/IL-17 signaling pathway, such as IL-17, IL-12/23 and IL-23 inhibitors. In this review, we highlight the potential implications of dysregulation of the IL-23/IL-17 axis in chronic inflammatory skin diseases, including psoriasis, hidradenitis suppurativa (HS), atopic dermatitis (AD), alopecia areata (AA), pityriasis rubra pilaris (PRP), pemphigus, and systemic sclerosis (SSc).
In this review, we are not intended to comprehensively review all pathways identified through human and murine laboratory studies or all clinical trials and case series in various inflammatory skin diseases. Nevertheless, we intent to focus on those targets of IL-23/IL-17 pathway demonstrated to be effective or potentially effective for treating human inflammatory skin diseases. We searched the published literature from PubMed and ClinicalTrial.gov with the search terms including ‘IL-17,’ ‘psoriasis,’ ‘atopic dermatitis,’ ‘hidradenitis suppurativa,’ ‘alopecia areata,’ ‘pityriasis rubra pilaris,’ ‘pemphigus,’ ‘systemic sclerosis,’ ‘secukinumab,’ ‘ixekizumab,’ ‘brodalumab,’ ‘bimekizumab,’ ‘ustekinumab,’ ‘tildrakizumab,’ ‘guselkumab,’ and ‘risankizumab’. We mainly focused on publications written in English between September 1, 2010, and September 15, 2020. We chose the references depending on the basis of their originality and relevance to the topic.
Th17 cells are known to play an important role in inflammatory and autoimmune diseases (17–19). In general, IL-23 is involved in the activation of Th17 cells to induce the production of IL-17A, IL-17F, tumor necrosis factor (TNF), and IL-6 (20). Binding to its receptors, IL-23 contributes to the phosphorylation of receptor-associated JAKs and specific Tyr residues, and this is followed by activating the transcription of IL-17 and other genes. The participation of IL-23 is crucial in the differentiation of IL-17-expressing phenotypes, via activating the transcription factor retinoid-related orphan receptor-γt (ROR-γt) and signal transducer and activator of transcription 3 (STAT3) (21–23).
IL-17 induces expression of downstream genes by stimulating activation of pathways, including canonical nuclear factor-κB (NF-κB), CCAAT/enhancer-binding protein (C/EBP) family, and mitogen-activated protein kinase (MAPK) (Figure 1). The key complex, which is consisted of IL-17A/A, IL-17A/F, or IL-17F/F cytokine and IL-17RA or IL-17RC, is the start hallmark of IL-17 signaling transduction (24, 25). Moreover, IL-17RD is also found to be a functional receptor for IL-17A groups. Together with IL-17RC, IL-17RD acts on the downstream of proinflammatory gene expression of IL-17 signaling (12). IL-17R is characterized by a unique structure in its cytoplasmic tail, termed SEF/IL-17R (SEFIR) domain (26). IL-17 signaling recruits Act1 to IL-17R through interaction platform of SEFIR domain (27). Then Act1 (also known as an E3 ligase) promotes activation of distinct downstream signaling cascades by tumor-necrosis factor receptor–associated factor (TRAF) 6 (28). TRAF6 then recruits and stimulates the transforming growth factor β-activated kinase 1 and the inhibitor of kappa B kinase complex, resulting in activation of NF-κB, C/EBPβ, C/EBPδ, and MAPK pathway (29–31). IL-17R-Act1 complex binds with MEKK3 and MEK5, leading to keratinocyte proliferation (32). Act1 binds with TRAF2-TRAF5 to maintain the mRNA stability targeting IL-17 gene (33). In contrast, TRAF3 triggers a negative reaction in activation of NF-κB and MAPK pathway, resulting in suppressing the formation of IL-17R-Act1-TRAF6 (34). TRAF6, in combination with A20 (an anti-inflammatory protein) when presented, blocks the activation of NF-κB and MAPK to negatively regulate IL-17 signaling (35).
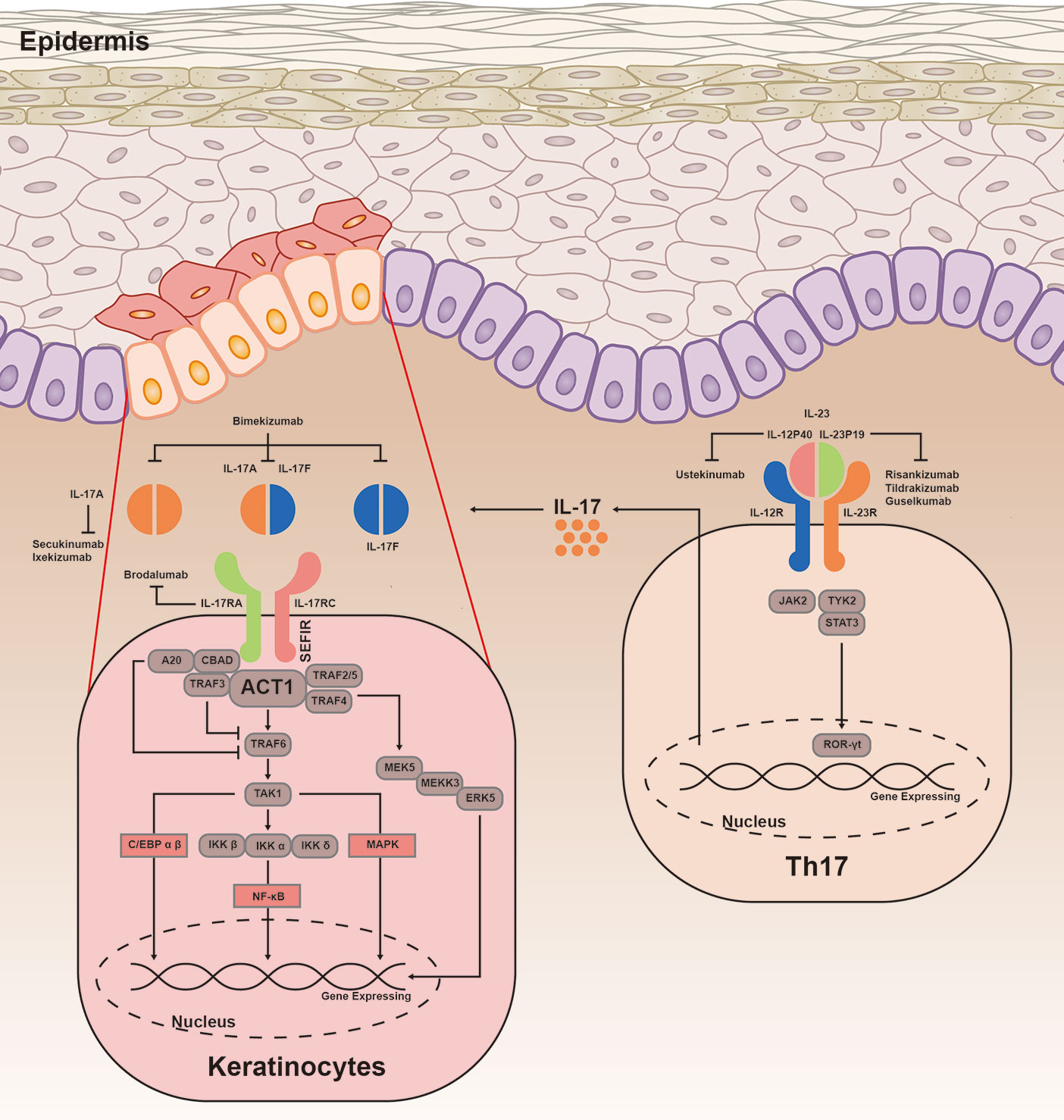
Figure 1 IL-23/IL-17 signaling transduction. IL-23 is important in differentiation of Th17 cells, by promoting the production of IL-17A, IL-17F, TNF, and IL-6. IL-23 is heterodimeric and composed of IL-12p40 and IL-23p19. Binding to its receptors, IL-23 involves in phosphorylation of JAKs and TYK, as well as phosphorylation and dimerization of STAT3. Subsequently, STAT3 homodimers regulates the expression of ROR-γt to promote the gene expression. The combination of IL-17A/A, IL-17A/F, or IL-17F/F cytokine with IL-17RA and IL-17RC is found to be a crucial complex of immune response. IL-17R acts on Act1 through interaction platform of the SEFIR domain. Upon ligand binding, Act1 activates NF-κB, C/EBP family, and MAPK pathway by inducing various TRAF proteins. Act1 is essential for mediating ubiquitination of TRAF6, then TRAF6 triggers a positive reaction in multiple different pathways. TRAF6 recruits and stimulates the TAK1 and IKK complex, leading to activation of NF-κB pathway. IL-17R-Act1 complex together with TRAF4, MEKK3, and MEK5 to promote activation of ERK5. In addition, ACT1-TRAF2-TRAF5 complex is capable to maintain the mRNA stability targeting the IL-17 gene. The inhibitors A20 and TRAF3 are linked with IL-17RA, dependent on the CBAD. C/EBP, CCAAT/enhancer-binding proteins; NF-κB, canonical nuclear factor-κB; MAPK, mitogen-activated protein kinase; TRAF, tumor necrosis factor receptor associated factor; TAK1, transforming growth factor-β activated kinase 1; IKK, inhibitor of kappa B kinase; ERK5, extracellular signal-regulated kinase 5; RORγt, retinoid-related orphan receptor-γt; STAT3, signal transducer and activator of transcription 3; JAK2, Janus activated kinase 2; TYK2, tyrosine kinase 2.
In patients with psoriasis, the IL-17 concentrations increase not only in the skin lesions and peripheral blood, but also in the nonlesional and uninvolved skin (36–40). There is evidence indicating that the main sources of IL-17A in patients with psoriasis are the neutrophils (41), Th17 cells (42), mast cells (43, 44), CD8+ T cells (45), αβ T (46), γδ T cells (47), and innate lymphoid cells (48, 49) in the skin lesions.
Psoriasis autoantigens, such as LL37 (50), NFKBIZ (51), ADAMTSL5 (52), and CARMA2 (53), play a crucial role in the production of IL-17A and are involved in the pathogenesis of psoriasis. In psoriasis, the combination of LL37 with the patient’s own DNA leads to the activation of the Toll-like receptor 9 (54). The self-DNA-LL37 complex acts on Toll-like receptor 7 in the plasmacytoid dendritic cells (DCs) and triggers the activation of the classical myeloid DCs (55) (Figure 2). Subsequently, the myeloid DCs produce IL-12 and IL-23. IL-23 induces the differentiation of the CD4+ T cells into the Th1 cells and Th17 cells by stimulating the transcription factor ROR-γt and STAT3 (56, 57). Thereafter, the activated Th17 cells secrete Th17 cytokines (IL-17A, IL-22, and TNF-α), leading to the development of a positive feedback loop. IL-17 plays a key role in stimulating the NF-κB and MAPK signaling pathways (53, 58), contributing to a high expression of proinflammatory factors (CC-chemokine ligand 20 and CC-chemokine receptor 6) (14, 38). These proinflammatory cytokines and chemokines recruit the inflammatory cells and stimulate keratinocyte proliferation (59).
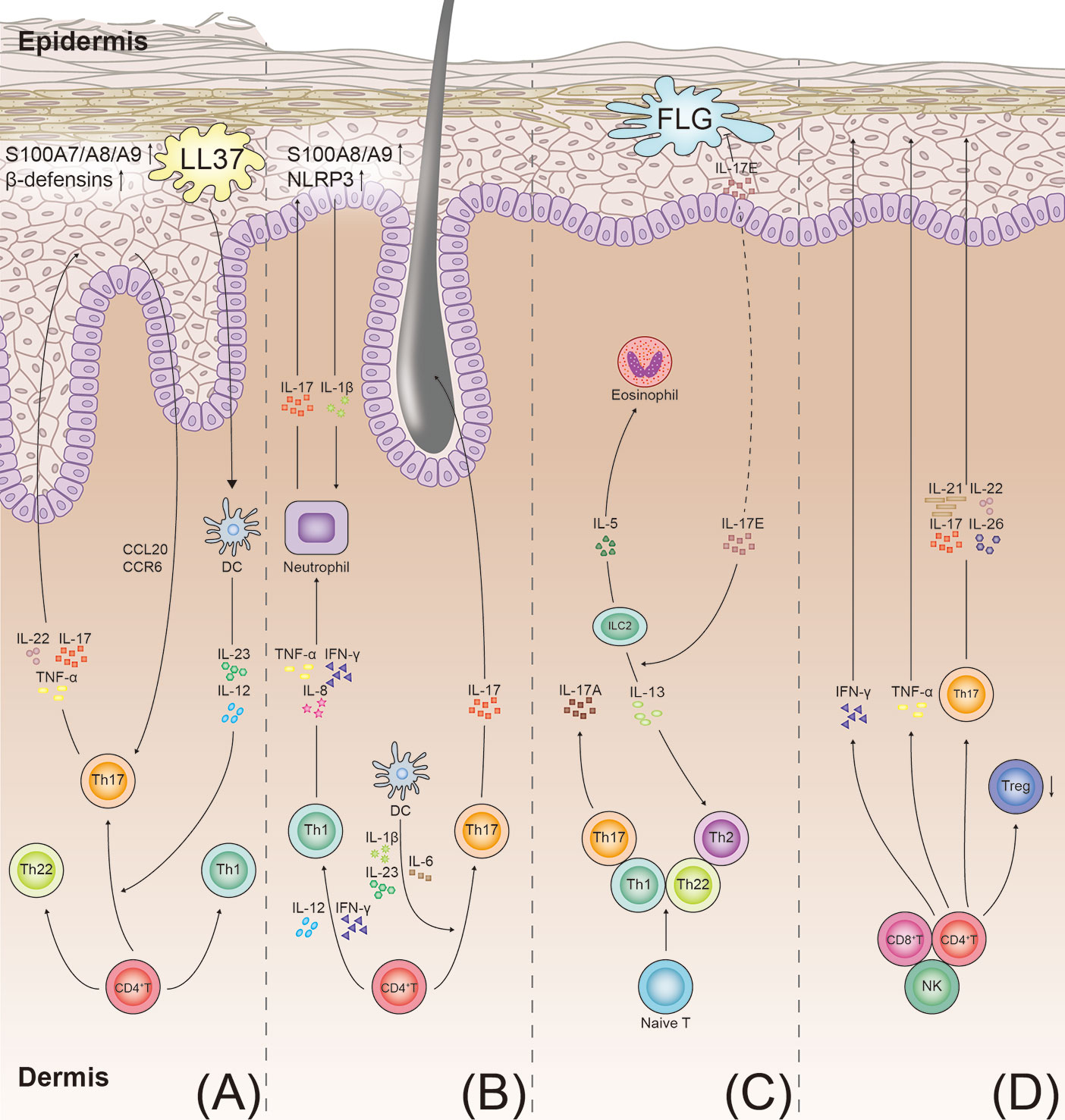
Figure 2 T-cell immune axis and associated cytokines in the pathogenesis of psoriasis, hidradenitis suppurativa, atopic dermatitis, and alopecia areata. (A) Psoriasis develops through the aberrant activation of the dendritic cells producing IL-12 and IL-23. The dendritic cells induce the differentiation of the Th 17 cells and Th1 cells. IL-23 promotes the Th17 cells to secrete IL-17, IL-22, and TNF-α. In keratinocytes, IL-17 also stimulates production of antimicrobial peptides (S100A7/A8/A9 proteins and beta defensins). These cytokines promote keratinocyte proliferation and neutrophil recruitment, resulting in the formation of psoriatic plaques. (B) In hidradenitis suppurativa, the T cells involved in the pathogenesis of hidradenitis suppurativa include the Th1 and Th17 cells. IL-23 induces the differentiation of the Th17 cells and overexpression of IL-17. IL-17 induces the expression of the proinflammatory proteins (S100A8/A9) and NLRP3 in the keratinocytes. More inflammatory cytokines are recruited to the follicular unit and perilesional skin. (C) In atopic dermatitis, with impairment of the skin barrier in patients with atopic dermatitis, the damaged keratinocytes produce inflammatory cytokines (IL-17E). The cytokines stimulate the ILC2s to secrete type 2 cytokines (IL-5 and IL-13). IL-17E also inhibits the synthesis of FLG. IL-4, IL-13, and IL-31 directly stimulate the sensory nerves to promote pruritus. (D) In alopecia areata, the elevated IFN-γ levels in the perifollicular area activate the differentiation of the CD4+ T cells into various types of T cells, as shown. Th17 cells act on the hair follicle by producing proinflammatory mediators (IL-17, IL-21, IL-22, and IL-26), ultimately leading to the disruption of hair growth. TNF, tumor necrosis factor; NLRP3, NACHT, LRR, and NACHT, LRR, and PYD domains-containing protein 3; FLG, filaggrin; ILC2s, type 2 innate lymphoid cells; DC, dendritic cell.
IL-17A plays an essential role in inflammation, metabolism, and bone/joint damage (8, 10, 15, 60–62). The IL-17 levels and Th17 cell frequencies are high in the skin, synovial fluid, and synovium tissue of the patients with psoriatic arthritis (PsA) (18, 63–65). In the synovium of patients with PsA, the mast cells and CD8+ T cells are the main sources of IL-17A (66). The aberrant expression of IL-17A directly affects the osteoclast precursors, leading to bone destruction in PsA (67). In addition, IL-17A interacts with the mediators of the Wnt signaling pathway in the osteoblasts and osteocytes, thus, preventing bone formation (10). Psoriatic inflammation is not restricted to the skin or joints. Metabolic disorders, such as hyperglycemia (68) and cardiovascular risks (69), are also associated with psoriasis.
Apart from IL-17A, the other IL-17 family members (IL-17C, E, and F) may also be involved in the pathogenesis of psoriasis (70–72). In contrast to that in the nonlesional psoriatic skin, mRNA expression of IL-17A, IL-17C, IL-17E, and IL-17F is increased in the lesional psoriatic skin (39, 70, 73). The IL-17C and IL-17E levels are higher than the IL-17A levels in the skin lesions in the psoriatic animal models (7, 73). Through the STAT3 pathway, the binding of IL-17E to IL-17RB induces keratinocyte proliferation to amplify skin inflammation in the psoriatic animal models (70). However, the effects of IL17-C on the mechanisms of psoriasis are not clear. Both IL-17F and IL-17A share homomeric and heterodimeric proteins with 50% sequence identity. The molecular structure and function of IL-17F are highly similar to those of IL-17A (74). Bimekizumab (an inhibitor of both IL-17A and IL-17F) is more effective than a blockade of IL-17A or IL-17F alone, especially for suppressing neutrophil chemotaxis and activating the synoviocytes or human dermal fibroblasts in vitro (71).
The targeted biologics in the treatment of psoriasis are listed in Supplementary Table. Biologics targeting IL-23 or IL-17A have shown remarkable effects in the treatment of psoriasis. The anti-IL-17 agents approved by the FDA include secukinumab (anti-IL-17A), ixekizumab (anti-IL-17A), brodalumab (anti-IL-17RA), and bimekizumab (anti-IL-17A and -17F). Biologics against IL-23 include ustekinumab (anti-IL-12/23p40), tildrakizumab (anti-IL-23p19), guselkumab (anti-IL-23p19), and risankizumab (anti-IL-23p19).
Secukinumab, a human immunoglobulin G1 monoclonal antibody against IL-17A, is an effective and safe biologic for psoriasis, involving skin, nails (75), and PsA (76). The data from the phase III randomized trials (ERASURE and FIXTURE) showed that secukinumab at doses of 300 or 150 mg is effective and safe for the treatment of moderate-to-severe psoriasis up to week 52 (Supplementary Table) (77). Secukinumab maintains significant powerful and long-lasting effects on the patients receiving the 300 mg secukinumab treatment every 4 weeks. The Psoriasis Area and Severity Index (PASI) 90/100 was 66.4%/41% at 156 weeks (78).
In the FUTURE 2 study, patients with PsA receiving secukinumab therapy achieved excellent and sustained improvement in the PASI90 and American College of Rheumatology 50 (ACR50) response at week 24 (Supplementary Table) (79). The results for patients who continued the study showed that, at week 104, the ACR50 response rates were 50.6 and 36% with the 300 and 150 mg doses of secukinumab, respectively (80). Secukinumab is important in suppressing synovitis and structural bone changes in patients with PsA at week 24, and low rates of radiographic progression are maintained at week 52 with secukinumab (81, 82).
Secukinumab has significant and long-term efficacy for the treatment of nail psoriasis. Therefore, the improvement in the Nail Psoriasis Severity Index (NAPSI) in the secukinumab 300 and secukinumab 150 groups was 73 and 63.6% at week 128, respectively (75). In summary, secukinumab shows excellent and sustained efficacy for the treatment of patients with moderate-to-severe plaque psoriasis and patients with psoriasis with or without arthritis and nail involvement.
Ixekizumab, a humanized immunoglobulin G4 monoclonal antibody, selectively blocks IL-17A. A multicenter trial (UNCOVER-3) reported that ixekizumab shows long-term efficacy for treating moderate-to-severe plaque psoriasis and that the treatment effects are strongly sustained for up to 156 weeks (Supplementary Table) (83, 84). Similarly, ixekizumab maintains promising clinical improvements in the scalp, nails, and palm (83).
According to the data from phase III studies (SPIRIT-P1 and SPIRIT-P2), ixekizumab is associated with improvements in disease prognosis and physical function in patients with active PsA, particularly, in those who are refractory to therapies or have an inadequate response to the anti-TNF therapies (Supplementary Table) (85, 86). To date, blockade of IL-17A is being advocated as first-line for treatment of PsA by the European League Against Rheumatism in 2019 (87). In general, the aforementioned studies suggest that ixekizumab is effective in controlling psoriasis and PsA, particularly in patients with lesions in hard-to-treat areas or in those who are refractory to treatments.
Brodalumab, a fully human immunoglobulin G2 IL-17RA antagonist, leads to a rapid improvement in the molecular, histological, and clinical features of psoriasis at week 12 (88). The AMAAGINE-1 study showed that brodalumab shows sustained efficacy (120 weeks) in the treatment of moderate-to-severe plaque psoriasis (Supplementary Table) (89, 90). Brodalumab inhibits a broader range of targets, namely, IL-17AA, IL-17AF, IL-17FF, IL-17C, and IL-17E via IL-17RA, compared with secukinumab and ixekizumab. An open-label study involving 39 patients with moderate-to-severe psoriasis revealed that brodalumab treatment may be effective for the patients who did not respond to secukinumab, ixekizumab, or ustekinumab (91, 92).
Moreover, the data from an open-label study have indicated significant beneficial effect of brodalumab on psoriatic erythroderma (n = 18) and generalized pustular psoriasis (n = 12; Supplementary Table) (93). However, the sample size of this study was small; hence, multicenter trials with large sample sizes of patients with psoriatic erythroderma or generalized pustular psoriasis should be conducted.
Bimekizumab, a humanized monoclonal IgG1 antagonist neutralizing both IL-17A and IL-17F, is effective for PsA and moderate-to-severe plaque psoriasis (71, 94). Two phase II trials (BE ABLE 1 and BE ABLE 2) reported the safety and efficacy of bimekizumab for the treatment of moderate-to-severe plaque psoriasis (Supplementary Table) (94, 95). Patients with active PsA, who were administered bimekizumab, showed marked improvements in their condition at week 48 (Supplementary Table) (96).
The most commonly noted treatment-emergent adverse events (TEAEs) are infections, nasopharyngitis, headache, and diarrhea in patients treated with IL-17 inhibitors compared with those treated with a placebo (76, 97–99). A systematic review speculated that it was safe to use IL-17 antagonists (secukinumab, ixekizumab, and brodalumab) for patients with psoriasis with latent tuberculosis infection (100). However, eczematous eruptions were reported in some patients after treatment with biologics against IL-17A (secukinumab or ixekizumab) for 3–4 months (101). To date, the mechanism underlying the onset of eczematous adverse events after anti-IL-17A treatment is not clear. Both the Th1 and Th2 responses are involved in the pathogenesis of eczema. This may be due to the anti-IL-17 biologics mainly inhibit the Th17 cytokines and mediate an imbalance in the Th2/Th17 immune response, thus leading to eczematous eruptions (102–104). The deficiency of Th17 cells, IL-17RA, and IL-17F are essential for host defense against fungal pathogens in mucocutaneous and oral epithelial cells (105–109). The risk of chronic mucocutaneous candidiasis increases in patients received IL-17 blockades (secukinumab, ixekizumab, brodalumab, or bimekizumab) (94, 96, 110).
Ustekinumab, a humanized IgG1 monoclonal antibody against the p40 subunit of IL-12 and IL-23, is approved for treating adult and pediatric patients with moderate-to-severe plaque psoriasis (111). Two phase III trials (PHOENIX 1 and PHOENIX 2) reported rapid and sustained efficacy of ustekinumab when administered at doses of 45 mg or 90 mg every 12 weeks for patients with moderate-to-severe plaque psoriasis (Supplementary Table) (112, 113).
According to the phase IV trial (VIP-U), ustekinumab may reduce aortic vascular inflammation transiently (at week 12) and downregulate the expression of the inflammatory cytokines (TNF-α, IL-1β, IL-17A, IL-18, and IL-6) sustainably (at week 52) (114). In 25 patients with psoriasis, inflammation in the liver, spleen, and artery decreased after treatment with ustekinumab, as indicated by the radiography findings (115). A summary analysis indicated that ustekinumab has long-term (5 years) safety with respect to patients with moderate-to-severe psoriasis (116).
Risankizumab, a humanized IgG1 monoclonal antibody inhibits the p19 subunit of IL-23 (117). In the landmark UltIMMa-1 and UltIMMa-2 studies, 150 mg risankizumab proved beneficial in the treatment of moderate-to-severe psoriasis compared with a placebo and ustekinumab (Supplementary Table) (118).
Tildrakizumab is a humanized IgG1 monoclonal antagonist, targeting IL-23p19. The data from reSURFACE 1 and reSURFACE 2 have indicated significant safety and efficacy of tildrakizumab for the treatment of chronic plaque psoriasis; moreover, tildrakizumab was well-tolerated by the patients (Supplementary Table) (119). A pooled analysis of three trials showed that tildrakizumab maintains beneficial impact and low rates of serious TEAEs. The PASI75 scores of patients continuously treated with 100 mg and 200 mg tildrakizumab at 64 weeks were 86 and 83%, respectively (120).
Guselkumab, a human monoclonal anti-IL-23p19 antagonist, is used for the treatment of PsA (121). Two phase III trials (DISCOVER-1 and DISCOVER-2) showed that patients with PsA treated with guselkumab showed excellent and rapid, improvements in their condition; moreover, guselkumab treatment was safe for these patients. The results of the trials revealed that, at week 24, the ACR20 response rates were 52 and 64% for guselkumab administered every 4 weeks and 8 weeks, respectively (Supplementary Table) (122, 123).
In summary, the aforementioned clinical trials reported that anti-IL-23 antibodies can successfully control psoriasis and PsA. However, the long-term follow-up data on anti-IL-23p19 biologics are limited. Multicenter studies with large sample sizes should be conducted in the future to evaluate the long-term efficacy and safety of these antagonists.
HS is a Th1/Th17-driven inflammatory skin disease (Figure 2) (124). The histopathological analysis of skin biopsy samples has revealed that the frequencies of the Th17 cells and regulatory T (Treg) cells are elevated in the lesional HS skin (19). The levels of inflammatory cytokines (IL-17, IL-23, IL-1β, TNF-α, and IL-12) are high in the lesional, perilesional, and uninvolved skin of patients with HS (125, 126). The serum levels of IL-17 and S100A8/A9 are higher in the patients with HS than in healthy individuals (127, 128).
The neutrophils and Th17 cells are the major sources of IL-17 in HS; in contrast, the keratinocytes are a key source of proinflammatory molecules, including S100A8/A9, NACHT, LRR, and PYD domains-containing protein 3, and caspase-1 (19, 129). Notably, HS showed histopathological changes characteristic of epidermal psoriasiform hyperplasia, follicular plugging, and infiltration of low-grade leucocytes in the uninvolved skin of perilesional HS (130). In such microenvironments, the IL-17-stimulated keratocytes show upregulation of the expression of the proinflammatory proteins (S100A8/A9) and promotion of the release of IL-1β by activation of the NACHT, LRR, and PYD domains-containing protein 3. Consequently, large amounts of proinflammatory molecules are recruited to promote the influx of the neutrophils, which, in turn, upregulate the release of IL-17 and S100A8/A9; thus, a positive-feedback loop of the inflammatory response is maintained (Figure 2) (129, 131).
An open-label and single-site exploratory trial has reported the efficacy of targeting IL-17A with secukinumab in the treatment of HS. The nine patients administrated of 300 mg secukinumab once a week from baseline for 5 weeks and then every 4 weeks. At 24 weeks, 67% patients with HS achieved Hidradenitis Suppurativa Clinical Response (HiSCR) score (132). Very recently, an open-label pilot cohort study on 10 patients assessed the well tolerability and clinical response of brodalumab in the treatment of moderate to severe HS. It demonstrated that patients received 210 mg brodalumab achieving HiSCR at week 12, and HiSCR improvement occurred as early as week 2 (133). An open-label study indicates that, at week 40, a moderate-to-marked improvement of the modified Sartorius score was achieved in 82% (14/17) of patients with HS receiving IL-12/23 biologic ustekinumab therapy (134). The data on the efficacy and safety of biologics for treating HS are limited, and further studies with adequate sample sizes are required to establish the effective and long-term impact of treatment.
Traditionally, AD was considered a Th2 immune response with elevated levels of IgE. Studies have revealed that the Th1, Th2, Th22, and Th17 cells are involved in the pathogenesis of AD (Figure 2) (135, 136). It has been demonstrated that Th22 and Th17 immune responses contribute to chronic skin lesions of AD, especially in pediatric, intrinsic, and Asian patients (137–140).
IL-17E (also called IL-25) level increases in the epidermis in patients with AD (141). In keratinocytes, the null mutation of filaggrin gene (FLG) is associated with the skin barrier dysfunction, increasing the risk of AD (142, 143). FLG synthesis is suppressed by IL-17E in the keratinocytes (144). Moreover, the data from mouse models indicated that IL-17E induces the type 2 innate lymphoid cells to produce type 2 cytokines (IL-5 and IL-13) (145).
To investigate the efficacy of anti-IL-17A biologics in AD, a randomized phase II trial was conducted involving 41 patients who were administered secukinumab. However, the trial results showed that at week 16, both clinical assessments (the Scoring Atopic Dermatitis index and Eczema Area and Severity Index) and lesional skin immunohistochemical analysis of patients receiving secukinumab revealed no significant improvement compared with those receiving a placebo (146). This trial demonstrated that IL17 is not a pivotal contributor to the pathogenesis of AD, even in the subsets of patients with higher Th17 activation.
Although ustekinumab showed promising efficacy in a review which included published case reports and case series (147), no efficacy was observed in randomized controlled trials of targeting IL-12/23 for treating patients with AD (148, 149). Additional studies with large sample sizes and may show the efficacy of ustekinumab in treating AD.
AA is a common inflammatory skin disorder, which is characterized by nonscarring hair loss via infiltration of the CD8+ T cells and increase in the levels of cytokines (IFN-γ, TNF-α, IL-17 and IL-4; Figure 2) (150). IL-2, IFN-γ, IL-10, IL-13, and IL-17A are expressed at high levels in the serum of patients with AA, while the level of transforming growth factor -β1 is decreased (151, 152). The Th17 cell frequencies and IL-17 levels significantly increased both in the peripheral blood and scalp lesions in patients with AA; however, the frequency of Treg cells decreased (153, 154). Studies have reported that patients with AA do not show any response to the administration of anti-IL-23/IL-12 ustekinumab (n = 4) or anti-IL-17A secukinumab (n = 7) (155, 156). Therefore, it cannot be concluded the contribution of Th17/IL-17 in the pathogenesis of AA. Further clinical trials with large sample size may reveal the value of IL-17 as a target of AA.
PRP is a rare acquired inflammatory skin disease. The levels of Th17 and Th1 cytokines increase in the lesional skin of the patients with PRP, including IL-17A, IL-17F, IL-22, TNF, IL-6, IL-12, IL-23, and IL-1β (157). The IL-23/Th17 axis seems to be important in the pathogenesis of PRP due to the clinical and histopathologic improvement in the targeting IL-12/23 and IL-17A (ustekinumab, secukinumab, and ixekizumab) treatment of patients with PRP (157–160). In a single-armed trial, analyzing changes in the clinical signs and symptoms (using PASI scores) showed that PASI50, PASI75 and PASI90 response rates were 58, 42, and 17% respectively during ixekizumab treatment of PRP at week 24 (160). For those 5 patients who failed to conventional therapies, all of them have achieved clinical improvement from ustekinumab, particularly, changes in decreased erythema, follicular hyperkeratosis, and scaling during a 15-month follow-up period (161).
In the serum and lesional skin of patients with pemphigus vulgaris, the levels of IL-23 and IL-17 increase, both are significantly correlated with diseases severity (162, 163). The frequency of CD4+IL-17+cells and the level of IL-23R mRNA show increases in the serum of patients with pemphigus foliaceus (164), in contrast to showing decreases in newly diagnosed patients with pemphigus vulgaris (165). This may be a result of the Th17 cells have plasticity and converting to Th1-like Th17 cells (165–167). There are some reports that the frequency of Th17 cells and level of IL-17 show decreases in other autoimmune and inflammatory diseases, such as lipopolysaccharides responsive beige-like anchor protein deficiency (165, 167).
The imbalance and dysfunction of Th17/Treg cells are crucial to the generation of SSc (168). Quantitative analysis of Th17 cytokines in lesional skin of SSc showed that the expression of IL-17A, IL-13, IL-22, and IL-26 mRNA are higher compared with healthy control (169, 170). The levels of circulating Th17 cells and IL-17 elevated in serum of patients with SSc. They are in correlation with disease severity and collagen overproduction (171, 172). The elevated levels of IL-17A act on dermal vascular smooth muscle cells to promote vascular fibrosis in the patients with SSc, via activating extracellular signal-regulated kinase 1/2 signaling pathway (173).
In summary, the Th17/IL-17 axis has been identified as a key factor in skin inflammatory diseases, such as psoriasis, HS, AD, PRP, pemphigus, and SSc. Neutralizing IL-17 or IL-23 in psoriasis, HS and PRP has shown promising clinical improvements. Additional studies are required to identify whether IL-17 is involved in the pathogenesis of AA, PRP, pemphigus, and SSc, which may lead to the development of targeted strategies for efficiently ameliorating or specifically eliminating these debilitating diseases.
TL, YL, JQ, and HF contributed to conception, literature search, and manuscript writing. SL, SY, ST, and YD created graphical illustrations. TL, JQ, and HF contributed to manuscript revision and read the submitted version. All authors contributed to the article and approved the submitted version.
This work was supported by the National Natural Science Foundation of China (81972931 to HF), the Medical and Health Science and Technology Project of Health Commission of Zhejiang Province (2020KY558 to JQ), and the Zhejiang Provincial Natural Science Foundation (LY20H110001 to YL).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2020.594735/full#supplementary-material
1. Rouvier E, Luciani MF, Mattéi MG, Denizot F, Golstein P. CTLA-8, cloned from an activated T cell, bearing AU-rich messenger RNA instability sequences, and homologous to a herpesvirus saimiri gene. J Immunol (1993) 150(12):5445–56.
2. Zhou J, An X, Dong J, Wang Y, Zhong H, Duan L, et al. IL-17 induces cellular stress microenvironment of melanocytes to promote autophagic cell apoptosis in vitiligo. FASEB J (2018) 32(9):4899–916. doi: 10.1096/fj.201701242RR
3. Zepp JA, Zhao J, Liu C, Bulek K, Wu L, Chen X, et al. IL-17A-Induced PLET1 Expression Contributes to Tissue Repair and Colon Tumorigenesis. J Immunol (2017) 199(11):3849–57. doi: 10.4049/jimmunol.1601540
4. Burstein VL, Guasconi L, Beccacece I, Theumer MG, Mena C, Prinz I, et al. IL-17-Mediated Immunity Controls Skin Infection and T Helper 1 Response during Experimental Microsporum canis Dermatophytosis. J Invest Dermatol (2018) 138(8):1744–53. doi: 10.1016/j.jid.2018.02.042
5. Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol (2005) 6(11):1133–41. doi: 10.1038/ni1261
6. Park MJ, Moon SJ, Lee EJ, Jung KA, Kim EK, Kim DS, et al. IL-1-IL-17 Signaling Axis Contributes to Fibrosis and Inflammation in Two Different Murine Models of Systemic Sclerosis. Front Immunol (2018) 9:1611. doi: 10.3389/fimmu.2018.01611
7. Johnston A, Fritz Y, Dawes SM, Diaconu D, Al-Attar PM, Guzman AM, et al. Keratinocyte overexpression of IL-17C promotes psoriasiform skin inflammation. J Immunol (2013) 190(5):2252–62. doi: 10.4049/jimmunol.1201505
8. Karbach S, Croxford AL, Oelze M, Schüler R, Minwegen D, Wegner J, et al. Interleukin 17 drives vascular inflammation, endothelial dysfunction, and arterial hypertension in psoriasis-like skin disease. Arterioscler Thromb Vasc Biol (2014) 34(12):2658–68. doi: 10.1161/atvbaha.114.304108
9. Senra L, Mylonas A, Kavanagh RD, Fallon PG, Conrad C, Borowczyk-Michalowska J, et al. IL-17E (IL-25) Enhances Innate Immune Responses during Skin Inflammation. J Invest Dermatol (2019) 139(8):1732–42.e17. doi: 10.1016/j.jid.2019.01.021
10. Uluçkan Ö, Jimenez M, Karbach S, Jeschke A, Graña O, Keller J, et al. Chronic skin inflammation leads to bone loss by IL-17-mediated inhibition of Wnt signaling in osteoblasts. Sci Transl Med (2016) 8(330):330ra37. doi: 10.1126/scitranslmed.aad8996
11. Moos S, Mohebiany AN, Waisman A, Kurschus FC. Imiquimod-Induced Psoriasis in Mice Depends on the IL-17 Signaling of Keratinocytes. J Invest Dermatol (2019) 139(5):1110–7. doi: 10.1016/j.jid.2019.01.006
12. Su Y, Huang J, Zhao X, Lu H, Wang W, Yang XO, et al. Interleukin-17 receptor D constitutes an alternative receptor for interleukin-17A important in psoriasis-like skin inflammation. Sci Immunol (2019) 4(36). doi: 10.1126/sciimmunol.aau9657
13. Fujino S, Andoh A, Bamba S, Ogawa A, Hata K, Araki Y, et al. Increased expression of interleukin 17 in inflammatory bowel disease. Gut (2003) 52(1):65–70. doi: 10.1136/gut.52.1.65
14. Wu NL, Huang DY, Tsou HN, Lin YC, Lin WW. Syk mediates IL-17-induced CCL20 expression by targeting Act1-dependent K63-linked ubiquitination of TRAF6. J Invest Dermatol (2015) 135(2):490–8. doi: 10.1038/jid.2014.383
15. Nesmond S, Muller C, Le Naour R, Viguier M, Bernard P, Antonicelli F, et al. Characteristic Pattern of IL-17RA, IL-17RB, and IL-17RC in Monocytes/Macrophages and Mast Cells From Patients With Bullous Pemphigoid. Front Immunol (2019) 10:2107. doi: 10.3389/fimmu.2019.02107
16. Koenders MI, Kolls JK, Oppers-Walgreen B, van den Bersselaar L, Joosten LA, Schurr JR, et al. Interleukin-17 receptor deficiency results in impaired synovial expression of interleukin-1 and matrix metalloproteinases 3, 9, and 13 and prevents cartilage destruction during chronic reactivated streptococcal cell wall-induced arthritis. Arthritis Rheum (2005) 52(10):3239–47. doi: 10.1002/art.21342
17. Xiao X, Shi X, Fan Y, Wu C, Zhang X, Minze L, et al. The Costimulatory Receptor OX40 Inhibits Interleukin-17 Expression through Activation of Repressive Chromatin Remodeling Pathways. Immunity (2016) 44(6):1271–83. doi: 10.1016/j.immuni.2016.05.013
18. Jandus C, Bioley G, Rivals JP, Dudler J, Speiser D, Romero P. Increased numbers of circulating polyfunctional Th17 memory cells in patients with seronegative spondylarthritides. Arthritis Rheum (2008) 58(8):2307–17. doi: 10.1002/art.23655
19. Moran B, Sweeney CM, Hughes R, Malara A, Kirthi S, Tobin AM, et al. Hidradenitis Suppurativa Is Characterized by Dysregulation of the Th17:Treg Cell Axis, Which Is Corrected by Anti-TNF Therapy. J Invest Dermatol (2017) 137(11):2389–95. doi: 10.1016/j.jid.2017.05.033
20. Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med (2005) 201(2):233–40. doi: 10.1084/jem.20041257
21. Venken K, Jacques P, Mortier C, Labadia ME, Decruy T, Coudenys J, et al. RORgammat inhibition selectively targets IL-17 producing iNKT and gammadelta-T cells enriched in Spondyloarthritis patients. Nat Commun (2019) 10(1):9. doi: 10.1038/s41467-018-07911-6
22. Kurdi AT, Bassil R, Olah M, Wu C, Xiao S, Taga M, et al. Tiam1/Rac1 complex controls Il17a transcription and autoimmunity. Nat Commun (2016) 7:13048. doi: 10.1038/ncomms13048
23. Cho JJ, Xu Z, Parthasarathy U, Drashansky TT, Helm EY, Zuniga AN, et al. Hectd3 promotes pathogenic Th17 lineage through Stat3 activation and Malt1 signaling in neuroinflammation. Nat Commun (2019) 10(1):701. doi: 10.1038/s41467-019-08605-3
24. Goepfert A, Lehmann S, Blank J, Kolbinger F, Rondeau J-M. Structural Analysis Reveals that the Cytokine IL-17F Forms a Homodimeric Complex with Receptor IL-17RC to Drive IL-17RA-Independent Signaling. Immunity (2020) 52(3):499–512. doi: 10.1016/j.immuni.2020.02.004
25. Ho AW, Shen F, Conti HR, Patel N, Childs EE, Peterson AC, et al. IL-17RC is required for immune signaling via an extended SEF/IL-17R signaling domain in the cytoplasmic tail. J Immunol (2010) 185(2):1063–70. doi: 10.4049/jimmunol.0903739
26. Onishi RM, Park SJ, Hanel W, Ho AW, Maitra A, Gaffen SL. SEF/IL-17R (SEFIR) is not enough: an extended SEFIR domain is required for il-17RA-mediated signal transduction. J Biol Chem (2010) 285(43):32751–9. doi: 10.1074/jbc.M110.121418
27. Zhang B, Liu C, Qian W, Han Y, Li X, Deng J. Structure of the unique SEFIR domain from human interleukin 17 receptor A reveals a composite ligand-binding site containing a conserved alpha-helix for Act1 binding and IL-17 signaling. Acta Crystallogr D Biol Crystallogr (2014) 70(Pt 5):1476–83. doi: 10.1107/S1399004714005227
28. Qian Y, Liu C, Hartupee J, Altuntas CZ, Gulen MF, Jane-Wit D, et al. The adaptor Act1 is required for interleukin 17-dependent signaling associated with autoimmune and inflammatory disease. Nat Immunol (2007) 8(3):247–56. doi: 10.1038/ni1439
29. Patel DN, King CA, Bailey SR, Holt JW, Venkatachalam K, Agrawal A, et al. Interleukin-17 stimulates C-reactive protein expression in hepatocytes and smooth muscle cells via p38 MAPK and ERK1/2-dependent NF-kappaB and C/EBPbeta activation. J Biol Chem (2007) 282(37):27229–38. doi: 10.1074/jbc.M703250200
30. Chang SH, Park H, Dong C. Act1 adaptor protein is an immediate and essential signaling component of interleukin-17 receptor. J Biol Chem (2006) 281(47):35603–7. doi: 10.1074/jbc.C600256200
31. Bulek K, Liu C, Swaidani S, Wang L, Page RC, Gulen MF, et al. The inducible kinase IKKi is required for IL-17-dependent signaling associated with neutrophilia and pulmonary inflammation. Nat Immunol (2011) 12(9):844–52. doi: 10.1038/ni.2080
32. Wu L, Chen X, Zhao J, Martin B, Zepp JA, Ko JS, et al. A novel IL-17 signaling pathway controlling keratinocyte proliferation and tumorigenesis via the TRAF4-ERK5 axis. J Exp Med (2015) 212(10):1571–87. doi: 10.1084/jem.20150204
33. Sun D, Novotny M, Bulek K, Liu C, Li X, Hamilton T. Treatment with IL-17 prolongs the half-life of chemokine CXCL1 mRNA via the adaptor TRAF5 and the splicing-regulatory factor SF2 (ASF). Nat Immunol (2011) 12(9):853–60. doi: 10.1038/ni.2081
34. Zhu S, Pan W, Shi P, Gao H, Zhao F, Song X, et al. Modulation of experimental autoimmune encephalomyelitis through TRAF3-mediated suppression of interleukin 17 receptor signaling. J Exp Med (2010) 207(12):2647–62. doi: 10.1084/jem.20100703
35. Garg AV, Ahmed M, Vallejo AN, Ma A, Gaffen SL. The deubiquitinase A20 mediates feedback inhibition of interleukin-17 receptor signaling. Sci Signal (2013) 6(278):ra44. doi: 10.1126/scisignal.2003699
36. Chiricozzi A, Suarez-Farinas M, Fuentes-Duculan J, Cueto I, Li K, Tian S, et al. Increased expression of interleukin-17 pathway genes in nonlesional skin of moderate-to-severe psoriasis vulgaris. Br J Dermatol (2016) 174(1):136–45. doi: 10.1111/bjd.14034
37. Takahashi H, Tsuji H, Hashimoto Y, Ishida-Yamamoto A, Iizuka H. Serum cytokines and growth factor levels in Japanese patients with psoriasis. Clin Exp Dermatol (2010) 35(6):645–9. doi: 10.1111/j.1365-2230.2009.03704.x
38. Harper EG, Guo C, Rizzo H, Lillis JV, Kurtz SE, Skorcheva I, et al. Th17 cytokines stimulate CCL20 expression in keratinocytes in vitro and in vivo: implications for psoriasis pathogenesis. J Invest Dermatol (2009) 129(9):2175–83. doi: 10.1038/jid.2009.65
39. Johansen C, Usher PA, Kjellerup RB, Lundsgaard D, Iversen L, Kragballe K. Characterization of the interleukin-17 isoforms and receptors in lesional psoriatic skin. Br J Dermatol (2009) 160(2):319–24. doi: 10.1111/j.1365-2133.2008.08902.x
40. Kolbinger F, Loesche C, Valentin MA, Jiang X, Cheng Y, Jarvis P, et al. β-Defensin 2 is a responsive biomarker of IL-17A-driven skin pathology in patients with psoriasis. J Allergy Clin Immunol (2017) 139(3):923–32.e8. doi: 10.1016/j.jaci.2016.06.038
41. Dyring-Andersen B, Honoré TV, Madelung A, Bzorek M, Simonsen S, Clemmensen SN, et al. Interleukin (IL)-17A and IL-22-producing neutrophils in psoriatic skin. Br J Dermatol (2017) 177(6):e321–e2. doi: 10.1111/bjd.15533
42. Cua DJ, Tato CM. Innate IL-17-producing cells: the sentinels of the immune system. Nat Rev Immunol (2010) 10(7):479–89. doi: 10.1038/nri2800
43. Mashiko S, Bouguermouh S, Rubio M, Baba N, Bissonnette R, Sarfati M. Human mast cells are major IL-22 producers in patients with psoriasis and atopic dermatitis. J Allergy Clin Immunol (2015) 136(2):351–9.e1. doi: 10.1016/j.jaci.2015.01.033
44. Brembilla NC, Stalder R, Senra L, Boehncke WH. IL-17A localizes in the exocytic compartment of mast cells in psoriatic skin. Br J Dermatol (2017) 177(5):1458–60. doi: 10.1111/bjd.15358
45. Res PC, Piskin G, de Boer OJ, van der Loos CM, Teeling P, Bos JD, et al. Overrepresentation of IL-17A and IL-22 producing CD8 T cells in lesional skin suggests their involvement in the pathogenesis of psoriasis. PLoS One (2010) 5(11):e14108. doi: 10.1371/journal.pone.0014108
46. Ueyama A, Imura C, Fusamae Y, Tsujii K, Furue Y, Aoki M, et al. Potential role of IL-17-producing CD4/CD8 double negative alphabeta T cells in psoriatic skin inflammation in a TPA-induced STAT3C transgenic mouse model. J Dermatol Sci (2017) 85(1):27–35. doi: 10.1016/j.jdermsci.2016.10.007
47. Cai Y, Shen X, Ding C, Qi C, Li K, Li X, et al. Pivotal role of dermal IL-17-producing gammadelta T cells in skin inflammation. Immunity (2011) 35(4):596–610. doi: 10.1016/j.immuni.2011.08.001
48. Bernink JH, Ohne Y, Teunissen MBM, Wang J, Wu J, Krabbendam L, et al. c-Kit-positive ILC2s exhibit an ILC3-like signature that may contribute to IL-17-mediated pathologies. Nat Immunol (2019) 20(8):992–1003. doi: 10.1038/s41590-019-0423-0
49. Teunissen MBM, Munneke JM, Bernink JH, Spuls PI, Res PCM, Te Velde A, et al. Composition of innate lymphoid cell subsets in the human skin: enrichment of NCR(+) ILC3 in lesional skin and blood of psoriasis patients. J Invest Dermatol (2014) 134(9):2351–60. doi: 10.1038/jid.2014.146
50. Lande R, Botti E, Jandus C, Dojcinovic D, Fanelli G, Conrad C, et al. The antimicrobial peptide LL37 is a T-cell autoantigen in psoriasis. Nat Commun (2014) 5:5621. doi: 10.1038/ncomms6621
51. Tsoi LC, Spain SL, Ellinghaus E, Stuart PE, Capon F, Knight J, et al. Enhanced meta-analysis and replication studies identify five new psoriasis susceptibility loci. Nat Commun (2015) 6:7001. doi: 10.1038/ncomms8001
52. Arakawa A, Siewert K, Stohr J, Besgen P, Kim SM, Ruhl G, et al. Melanocyte antigen triggers autoimmunity in human psoriasis. J Exp Med (2015) 212(13):2203–12. doi: 10.1084/jem.20151093
53. Wang M, Zhang S, Zheng G, Huang J, Songyang Z, Zhao X, et al. Gain-of-Function Mutation of Card14 Leads to Spontaneous Psoriasis-like Skin Inflammation through Enhanced Keratinocyte Response to IL-17A. Immunity (2018) 49(1):66–79.e5. doi: 10.1016/j.immuni.2018.05.012
54. Lande R, Gregorio J, Facchinetti V, Chatterjee B, Wang YH, Homey B, et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature (2007) 449(7162):564–9. doi: 10.1038/nature06116
55. Ganguly D, Chamilos G, Lande R, Gregorio J, Meller S, Facchinetti V, et al. Self-RNA-antimicrobial peptide complexes activate human dendritic cells through TLR7 and TLR8. J Exp Med (2009) 206(9):1983–94. doi: 10.1084/jem.20090480
56. Venken K, Jacques P, Mortier C, Labadia ME, Decruy T, Coudenys J, et al. RORγt inhibition selectively targets IL-17 producing iNKT and γδ-T cells enriched in Spondyloarthritis patients. Nat Commun (2019) 10(1):9. doi: 10.1038/s41467-018-07911-6
57. Zheng Y, Danilenko DM, Valdez P, Kasman I, Eastham-Anderson J, Wu J, et al. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature (2007) 445(7128):648–51. doi: 10.1038/nature05505
58. Shimizu T, Kamata M, Fukaya S, Hayashi K, Fukuyasu A, Tanaka T, et al. Anti-IL-17A and IL-23p19 antibodies but not anti-TNFα antibody induce expansion of regulatory T cells and restoration of their suppressive function in imiquimod-induced psoriasiform dermatitis. J Dermatol Sci (2019) 95(3):90–8. doi: 10.1016/j.jdermsci.2019.07.006
59. Frischknecht L, Vecellio M, Selmi C. The role of epigenetics and immunological imbalance in the etiopathogenesis of psoriasis and psoriatic arthritis. Ther Adv Musculoskelet Dis (2019) 11:1759720x19886505. doi: 10.1177/1759720x19886505
60. Song X, Dai D, He X, Zhu S, Yao Y, Gao H, et al. Growth Factor FGF2 Cooperates with Interleukin-17 to Repair Intestinal Epithelial Damage. Immunity (2015) 43(3):488–501. doi: 10.1016/j.immuni.2015.06.024
61. Ono T, Okamoto K, Nakashima T, Nitta T, Hori S, Iwakura Y, et al. IL-17-producing γδ T cells enhance bone regeneration. Nat Commun (2016) 7:10928. doi: 10.1038/ncomms10928
62. Lou F, Sun Y, Xu Z, Niu L, Wang Z, Deng S, et al. Excessive Polyamine Generation in Keratinocytes Promotes Self-RNA Sensing by Dendritic Cells in Psoriasis. Immunity (2020) 53(1):204–16.e10. doi: 10.1016/j.immuni.2020.06.004
63. van Baarsen LG, Lebre MC, van der Coelen D, Aarrass S, Tang MW, Ramwadhdoebe TH, et al. Heterogeneous expression pattern of interleukin 17A (IL-17A), IL-17F and their receptors in synovium of rheumatoid arthritis, psoriatic arthritis and osteoarthritis: possible explanation for nonresponse to anti-IL-17 therapy? Arthritis Res Ther (2014) 16(4):426. doi: 10.1186/s13075-014-0426-z
64. Raychaudhuri SP, Raychaudhuri SK, Genovese MC. IL-17 receptor and its functional significance in psoriatic arthritis. Mol Cell Biochem (2012) 359(1-2):419–29. doi: 10.1007/s11010-011-1036-6
65. Belasco J, Louie JS, Gulati N, Wei N, Nograles K, Fuentes-Duculan J, et al. Comparative genomic profiling of synovium versus skin lesions in psoriatic arthritis. Arthritis Rheumatol (2015) 67(4):934–44. doi: 10.1002/art.38995
66. Noordenbos T, Yeremenko N, Gofita I, van de Sande M, Tak PP, Caňete JD, et al. Interleukin-17-positive mast cells contribute to synovial inflammation in spondylarthritis. Arthritis Rheum (2012) 64(1):99–109. doi: 10.1002/art.33396
67. Adamopoulos IE, Suzuki E, Chao CC, Gorman D, Adda S, Maverakis E, et al. IL-17A gene transfer induces bone loss and epidermal hyperplasia associated with psoriatic arthritis. Ann Rheum Dis (2015) 74(6):1284–92. doi: 10.1136/annrheumdis-2013-204782
68. Wan MT, Shin DB, Hubbard RA, Noe MH, Mehta NN, Gelfand JM. Psoriasis and the risk of diabetes: A prospective population-based cohort study. J Am Acad Dermatol (2018) 78(2):315–22.e1. doi: 10.1016/j.jaad.2017.10.050
69. Egeberg A, Gisondi P, Carrascosa JM, Warren RB, Mrowietz U. The role of the interleukin-23/Th17 pathway in cardiometabolic comorbidity associated with psoriasis. J Eur Acad Dermatol Venereol (2020) 34(8):1695–706. doi: 10.1111/jdv.16273
70. Xu M, Lu H, Lee YH, Wu Y, Liu K, Shi Y, et al. An Interleukin-25-Mediated Autoregulatory Circuit in Keratinocytes Plays a Pivotal Role in Psoriatic Skin Inflammation. Immunity (2018) 48(4):787–98.e4. doi: 10.1016/j.immuni.2018.03.019
71. Glatt S, Baeten D, Baker T, Griffiths M, Ionescu L, Lawson ADG, et al. Dual IL-17A and IL-17F neutralisation by bimekizumab in psoriatic arthritis: evidence from preclinical experiments and a randomised placebo-controlled clinical trial that IL-17F contributes to human chronic tissue inflammation. Ann Rheum Dis (2018) 77(4):523–32. doi: 10.1136/annrheumdis-2017-212127
72. Vandeghinste N, Klattig J, Jagerschmidt C, Lavazais S, Marsais F, Haas JD, et al. Neutralization of IL-17C Reduces Skin Inflammation in Mouse Models of Psoriasis and Atopic Dermatitis. J Invest Dermatol (2018) 138(7):1555–63. doi: 10.1016/j.jid.2018.01.036
73. Senra L, Stalder R, Alvarez Martinez D, Chizzolini C, Boehncke WH, Brembilla NC. Keratinocyte-Derived IL-17E Contributes to Inflammation in Psoriasis. J Invest Dermatol (2016) 136(10):1970–80. doi: 10.1016/j.jid.2016.06.009
74. Wright JF, Guo Y, Quazi A, Luxenberg DP, Bennett F, Ross JF, et al. Identification of an interleukin 17F/17A heterodimer in activated human CD4+ T cells. J Biol Chem (2007) 282(18):13447–55. doi: 10.1074/jbc.M700499200
75. Reich K, Sullivan J, Arenberger P, Jazayeri S, Mrowietz U, Augustin M, et al. Secukinumab shows high and sustained efficacy in nail psoriasis: 2.5-year results from the randomized placebo-controlled TRANSFIGURE study. Br J Dermatol (2020). doi: 10.1111/bjd.19262
76. Mease PJ, McInnes IB, Kirkham B, Kavanaugh A, Rahman P, van der Heijde D, et al. Secukinumab Inhibition of Interleukin-17A in Patients with Psoriatic Arthritis. N Engl J Med (2015) 373(14):1329–39. doi: 10.1056/NEJMoa1412679
77. Langley RG, Elewski BE, Lebwohl M, Reich K, Griffiths CE, Papp K, et al. Secukinumab in plaque psoriasis–results of two phase 3 trials. N Engl J Med (2014) 371(4):326–38. doi: 10.1056/NEJMoa1314258
78. Bissonnette R, Luger T, Thaçi D, Toth D, Lacombe A, Xia S, et al. Secukinumab demonstrates high sustained efficacy and a favourable safety profile in patients with moderate-to-severe psoriasis through 5 years of treatment (SCULPTURE Extension Study). J Eur Acad Dermatol Venereol (2018) 32(9):1507–14. doi: 10.1111/jdv.14878
79. McInnes IB, Mease PJ, Kirkham B, Kavanaugh A, Ritchlin CT, Rahman P, et al. Secukinumab, a human anti-interleukin-17A monoclonal antibody, in patients with psoriatic arthritis (FUTURE 2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet (2015) 386(9999):1137–46. doi: 10.1016/s0140-6736(15)61134-5
80. McInnes IB, Mease PJ, Ritchlin CT, Rahman P, Gottlieb AB, Kirkham B, et al. Secukinumab sustains improvement in signs and symptoms of psoriatic arthritis: 2 year results from the phase 3 FUTURE 2 study. Rheumatol (Oxford) (2017) 56(11):1993–2003. doi: 10.1093/rheumatology/kex301
81. Kampylafka E, d’Oliveira I, Linz C, Lerchen V, Stemmler F, Simon D, et al. Resolution of synovitis and arrest of catabolic and anabolic bone changes in patients with psoriatic arthritis by IL-17A blockade with secukinumab: results from the prospective PSARTROS study. Arthritis Res Ther (2018) 20(1):153. doi: 10.1186/s13075-018-1653-5
82. van der Heijde D, Mease PJ, Landewé RBM, Rahman P, Tahir H, Singhal A, et al. Secukinumab provides sustained low rates of radiographic progression in psoriatic arthritis: 52-week results from a phase 3 study, FUTURE 5. Rheumatol (Oxford) (2020) 59(6):1325–34. doi: 10.1093/rheumatology/kez420
83. Leonardi C, Maari C, Philipp S, Goldblum O, Zhang L, Burkhardt N, et al. Maintenance of skin clearance with ixekizumab treatment of psoriasis: Three-year results from the UNCOVER-3 study. J Am Acad Dermatol (2018) 79(5):824–30. doi: 10.1016/j.jaad.2018.05.032
84. Blauvelt A, Gooderham M, Iversen L, Ball S, Zhang L, Agada NO, et al. Efficacy and safety of ixekizumab for the treatment of moderate-to-severe plaque psoriasis: Results through 108 weeks of a randomized, controlled phase 3 clinical trial (UNCOVER-3). J Am Acad Dermatol (2017) 77(5):855–62. doi: 10.1016/j.jaad.2017.06.153
85. Mease PJ, van der Heijde D, Ritchlin CT, Okada M, Cuchacovich RS, Shuler CL, et al. Ixekizumab, an interleukin-17A specific monoclonal antibody, for the treatment of biologic-naive patients with active psoriatic arthritis: results from the 24-week randomised, double-blind, placebo-controlled and active (adalimumab)-controlled period of the phase III trial SPIRIT-P1. Ann Rheum Dis (2017) 76(1):79–87. doi: 10.1136/annrheumdis-2016-209709
86. Nash P, Kirkham B, Okada M, Rahman P, Combe B, Burmester G-R, et al. Ixekizumab for the treatment of patients with active psoriatic arthritis and an inadequate response to tumour necrosis factor inhibitors: results from the 24-week randomised, double-blind, placebo-controlled period of the SPIRIT-P2 phase 3 trial. Lancet (2017) 389(10086):2317–27. doi: 10.1016/s0140-6736(17)31429-0
87. Gossec L, Baraliakos X, Kerschbaumer A, de Wit M, McInnes I, Dougados M, et al. EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2019 update. Ann Rheum Dis (2020) 79(6):700–12. doi: 10.1136/annrheumdis-2020-217159
88. Tomalin LE, Russell CB, Garcet S, Ewald DA, Klekotka P, Nirula A, et al. Short-term transcriptional response to IL-17 receptor-A antagonism in the treatment of psoriasis. J Allergy Clin Immunol (2020) 145(3):922–32. doi: 10.1016/j.jaci.2019.10.041
89. Papp K, Menter A, Leonardi C, Soung J, Weiss S, Pillai R, et al. Long-term efficacy and safety of brodalumab in psoriasis through 120 weeks and after withdrawal and retreatment: subgroup analysis of a randomized phase III trial (AMAGINE-1). Br J Dermatol (2020). doi: 10.1111/bjd.19132
90. Papp KA, Reich K, Paul C, Blauvelt A, Baran W, Bolduc C, et al. A prospective phase III, randomized, double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis. Br J Dermatol (2016) 175(2):273–86. doi: 10.1111/bjd.14493
91. Kimmel G, Chima M, Kim HJ, Bares J, Yao CJ, Singer G, et al. Brodalumab in the treatment of moderate to severe psoriasis in patients when previous anti-interleukin 17A therapies have failed. J Am Acad Dermatol (2019) 81(3):857–9. doi: 10.1016/j.jaad.2019.05.007
92. Langley RG, Armstrong AW, Lebwohl MG, Blauvelt A, Hsu S, Tyring S, et al. Efficacy and safety of brodalumab in patients with psoriasis who had inadequate responses to ustekinumab: subgroup analysis of two randomized phase III trials. Br J Dermatol (2019) 180(2):306–14. doi: 10.1111/bjd.17318
93. Yamasaki K, Nakagawa H, Kubo Y, Ootaki K. Efficacy and safety of brodalumab in patients with generalized pustular psoriasis and psoriatic erythroderma: results from a 52-week, open-label study. Br J Dermatol (2017) 176(3):741–51. doi: 10.1111/bjd.14702
94. Blauvelt A, Papp KA, Merola JF, Gottlieb AB, Cross N, Madden C, et al. Bimekizumab for patients with moderate-to-severe plaque psoriasis: 60-week results from BE ABLE 2, a randomized, double-blinded, placebo-controlled phase 2b extension study. J Am Acad Dermatol (2020). doi: 10.1016/j.jaad.2020.05.105
95. Papp KA, Merola JF, Gottlieb AB, Griffiths CEM, Cross N, Peterson L, et al. Dual neutralization of both interleukin 17A and interleukin 17F with bimekizumab in patients with psoriasis: Results from BE ABLE 1, a 12-week randomized, double-blinded, placebo-controlled phase 2b trial. J Am Acad Dermatol (2018) 79(2):277–86. doi: 10.1016/j.jaad.2018.03.037
96. Ritchlin CT, Kavanaugh A, Merola JF, Schett G, Scher JU, Warren RB, et al. Bimekizumab in patients with active psoriatic arthritis: results from a 48-week, randomised, double-blind, placebo-controlled, dose-ranging phase 2b trial. Lancet (2020) 395(10222):427–40. doi: 10.1016/s0140-6736(19)33161-7
97. Genovese MC, Mysler E, Tomita T, Papp KA, Salvarani C, Schwartzman S, et al. Safety of ixekizumab in adult patients with plaque psoriasis, psoriatic arthritis and axial spondyloarthritis: data from 21 clinical trials. Rheumatol (Oxford) (2020). doi: 10.1093/rheumatology/keaa189
98. Puig L, Lebwohl M, Bachelez H, Sobell J, Jacobson AA. Long-term efficacy and safety of brodalumab in the treatment of psoriasis: 120-week results from the randomized, double-blind, placebo- and active comparator-controlled phase 3 AMAGINE-2 trial. J Am Acad Dermatol (2020) 82(2):352–9. doi: 10.1016/j.jaad.2019.05.095
99. Strober B, Leonardi C, Papp KA, Mrowietz U, Ohtsuki M, Bissonnette R, et al. Short- and long-term safety outcomes with ixekizumab from 7 clinical trials in psoriasis: Etanercept comparisons and integrated data. J Am Acad Dermatol (2017) 76(3):432–40.e17. doi: 10.1016/j.jaad.2016.09.026
100. Fowler E, Ghamrawi RI, Ghiam N, Liao W, Wu JJ. Risk of tuberculosis reactivation during interleukin-17 inhibitor therapy for psoriasis: a systematic review. J Eur Acad Dermatol Venereol (2020) 34(7):1449–56. doi: 10.1111/jdv.16254
101. Caldarola G, Pirro F, Di Stefani A, Talamonti M, Galluzzo M, D’Adamio S, et al. Clinical and histopathological characterization of eczematous eruptions occurring in course of anti IL-17 treatment: a case series and review of the literature. Expert Opin Biol Ther (2020) 20(6):665–72. doi: 10.1080/14712598.2020.1727439
102. Krueger JG, Fretzin S, Suárez-Fariñas M, Haslett PA, Phipps KM, Cameron GS, et al. IL-17A is essential for cell activation and inflammatory gene circuits in subjects with psoriasis. J Allergy Clin Immunol (2012) 130(1):145–54.e9. doi: 10.1016/j.jaci.2012.04.024
103. Napolitano M, Megna M, Fabbrocini G, Nisticò SP, Balato N, Dastoli S, et al. Eczematous eruption during anti-interleukin 17 treatment of psoriasis: an emerging condition. Br J Dermatol (2019) 181(3):604–6. doi: 10.1111/bjd.17779
104. Al-Janabi A, Foulkes AC, Mason K, Smith CH, Griffiths CEM, Warren RB. Phenotypic switch to eczema in patients receiving biologics for plaque psoriasis: a systematic review. J Eur Acad Dermatol Venereol (2020) 34(7):1440–8. doi: 10.1111/jdv.16246
105. Puel A, Cypowyj S, Bustamante J, Wright JF, Liu L, Lim HK, et al. Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity. Science (2011) 332(6025):65–8. doi: 10.1126/science.1200439
106. Kisand K, Bøe Wolff AS, Podkrajsek KT, Tserel L, Link M, Kisand KV, et al. Chronic mucocutaneous candidiasis in APECED or thymoma patients correlates with autoimmunity to Th17-associated cytokines. J Exp Med (2010) 207(2):299–308. doi: 10.1084/jem.20091669
107. Conti HR, Bruno VM, Childs EE, Daugherty S, Hunter JP, Mengesha BG, et al. IL-17 Receptor Signaling in Oral Epithelial Cells Is Critical for Protection against Oropharyngeal Candidiasis. Cell Host Microbe (2016) 20(5):606–17. doi: 10.1016/j.chom.2016.10.001
108. Meller S, Di Domizio J, Voo KS, Friedrich HC, Chamilos G, Ganguly D, et al. T(H)17 cells promote microbial killing and innate immune sensing of DNA via interleukin 26. Nat Immunol (2015) 16(9):970–9. doi: 10.1038/ni.3211
109. Li J, Ritelli M, Ma CS, Rao G, Habib T, Corvilain E, et al. Chronic mucocutaneous candidiasis and connective tissue disorder in humans with impaired JNK1-dependent responses to IL-17A/F and TGF-β. Sci Immunol (2019) 4(41). doi: 10.1126/sciimmunol.aax7965
110. Saunte DM, Mrowietz U, Puig L, Zachariae C. Candida infections in patients with psoriasis and psoriatic arthritis treated with interleukin-17 inhibitors and their practical management. Br J Dermatol (2017) 177(1):47–62. doi: 10.1111/bjd.15015
111. Philipp S, Menter A, Nikkels AF, Barber K, Landells I, Eichenfield LF, et al. Ustekinumab for the treatment of moderate-to-severe plaque psoriasis in paediatric patients (≥ 6 to < 12 years of age): efficacy, safety, pharmacokinetic and biomarker results from the open-label CADMUS Jr study. Br J Dermatol (2020) 183(4):664–72. doi: 10.1111/bjd.19018
112. Papp KA, Langley RG, Lebwohl M, Krueger GG, Szapary P, Yeilding N, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet (2008) 371(9625):1675–84. doi: 10.1016/s0140-6736(08)60726-6
113. Leonardi CL, Kimball AB, Papp KA, Yeilding N, Guzzo C, Wang Y, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1). Lancet (2008) 371(9625):1665–74. doi: 10.1016/s0140-6736(08)60725-4
114. Gelfand JM, Shin DB, Alavi A, Torigian DA, Werner T, Papadopoulos M, et al. Randomized, Double-Blind, Placebo-Controlled Crossover Study of the Effects of Ustekinumab on Vascular Inflammation in Psoriasis (the VIP-U Trial). J Invest Dermatol (2020) 140(1):85–93.e2. doi: 10.1016/j.jid.2019.07.679
115. Kim BS, Lee WK, Pak K, Han J, Kim GW, Kim HS, et al. Ustekinumab treatment is associated with decreased systemic and vascular inflammation in patients with moderate-to-severe psoriasis: Feasibility study using (18)F-fluorodeoxyglucose PET/CT. J Am Acad Dermatol (2019) 80(5):1322–31. doi: 10.1016/j.jaad.2018.03.011
116. Papp KA, Griffiths CE, Gordon K, Lebwohl M, Szapary PO, Wasfi Y, et al. Long-term safety of ustekinumab in patients with moderate-to-severe psoriasis: final results from 5 years of follow-up. Br J Dermatol (2013) 168(4):844–54. doi: 10.1111/bjd.12214
117. Papp KA, Blauvelt A, Bukhalo M, Gooderham M, Krueger JG, Lacour JP, et al. Risankizumab versus Ustekinumab for Moderate-to-Severe Plaque Psoriasis. N Engl J Med (2017) 376(16):1551–60. doi: 10.1056/NEJMoa1607017
118. Gordon KB, Strober B, Lebwohl M, Augustin M, Blauvelt A, Poulin Y, et al. Efficacy and safety of risankizumab in moderate-to-severe plaque psoriasis (UltIMMa-1 and UltIMMa-2): results from two double-blind, randomised, placebo-controlled and ustekinumab-controlled phase 3 trials. Lancet (2018) 392(10148):650–61. doi: 10.1016/s0140-6736(18)31713-6
119. Reich K, Papp KA, Blauvelt A, Tyring SK, Sinclair R, Thaçi D, et al. Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (reSURFACE 1 and reSURFACE 2): results from two randomised controlled, phase 3 trials. Lancet (2017) 390(10091):276–88. doi: 10.1016/s0140-6736(17)31279-5
120. Kimball AB, Papp KA, Reich K, Gooderham M, Li Q, Cichanowitz N, et al. Efficacy and safety of tildrakizumab for plaque psoriasis with continuous dosing, treatment interruption, dose adjustments and switching from etanercept: results from phase III studies. Br J Dermatol (2020) 182(6):1359–68. doi: 10.1111/bjd.18484
121. Deodhar A, Gottlieb AB, Boehncke WH, Dong B, Wang Y, Zhuang Y, et al. Efficacy and safety of guselkumab in patients with active psoriatic arthritis: a randomised, double-blind, placebo-controlled, phase 2 study. Lancet (2018) 391(10136):2213–24. doi: 10.1016/s0140-6736(18)30952-8
122. Mease PJ, Rahman P, Gottlieb AB, Kollmeier AP, Hsia EC, Xu XL, et al. Guselkumab in biologic-naive patients with active psoriatic arthritis (DISCOVER-2): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet (2020) 395(10230):1126–36. doi: 10.1016/s0140-6736(20)30263-4
123. Deodhar A, Helliwell PS, Boehncke W-H, Kollmeier AP, Hsia EC, Subramanian RA, et al. Guselkumab in patients with active psoriatic arthritis who were biologic-naive or had previously received TNFα inhibitor treatment (DISCOVER-1): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet (2020) 395(10230):1115–25. doi: 10.1016/s0140-6736(20)30265-8
124. Thomi R, Cazzaniga S, Seyed Jafari SM, Schlapbach C, Hunger RE. Association of Hidradenitis Suppurativa With T Helper 1/T Helper 17 Phenotypes: A Semantic Map Analysis. JAMA Dermatol (2018) 154(5):592–5. doi: 10.1001/jamadermatol.2018.0141
125. Kelly G, Hughes R, McGarry T, van den Born M, Adamzik K, Fitzgerald R, et al. Dysregulated cytokine expression in lesional and nonlesional skin in hidradenitis suppurativa. Br J Dermatol (2015) 173(6):1431–9. doi: 10.1111/bjd.14075
126. Schlapbach C, Hanni T, Yawalkar N, Hunger RE. Expression of the IL-23/Th17 pathway in lesions of hidradenitis suppurativa. J Am Acad Dermatol (2011) 65(4):790–8. doi: 10.1016/j.jaad.2010.07.010
127. Matusiak L, Szczech J, Bieniek A, Nowicka-Suszko D, Szepietowski JC. Increased interleukin (IL)-17 serum levels in patients with hidradenitis suppurativa: Implications for treatment with anti-IL-17 agents. J Am Acad Dermatol (2017) 76(4):670–5. doi: 10.1016/j.jaad.2016.10.042
128. Wieland CW, Vogl T, Ordelman A, Vloedgraven HG, Verwoolde LH, Rensen JM, et al. Myeloid marker S100A8/A9 and lymphocyte marker, soluble interleukin 2 receptor: biomarkers of hidradenitis suppurativa disease activity? Br J Dermatol (2013) 168(6):1252–8. doi: 10.1111/bjd.12234
129. Lima AL, Karl I, Giner T, Poppe H, Schmidt M, Presser D, et al. Keratinocytes and neutrophils are important sources of proinflammatory molecules in hidradenitis suppurativa. Br J Dermatol (2016) 174(3):514–21. doi: 10.1111/bjd.14214
130. van der Zee HH, de Ruiter L, Boer J, van den Broecke DG, den Hollander JC, Laman JD, et al. Alterations in leucocyte subsets and histomorphology in normal-appearing perilesional skin and early and chronic hidradenitis suppurativa lesions. Br J Dermatol (2012) 166(1):98–106. doi: 10.1111/j.1365-2133.2011.10643.x
131. Le Jan S, Plée J, Vallerand D, Dupont A, Delanez E, Durlach A, et al. Innate immune cell-produced IL-17 sustains inflammation in bullous pemphigoid. J Invest Dermatol (2014) 134(12):2908–17. doi: 10.1038/jid.2014.263
132. Prussick L, Rothstein B, Joshipura D, Saraiya A, Turkowski Y, Abdat R, et al. Open-label, investigator-initiated, single-site exploratory trial evaluating secukinumab, an anti-interleukin-17A monoclonal antibody, for patients with moderate-to-severe hidradenitis suppurativa. Br J Dermatol (2019) 181(3):609–11. doi: 10.1111/bjd.17822
133. Frew JW, Navrazhina K, Grand D, Sullivan-Whalen M, Gilleaudeau P, Garcet S, et al. The effect of subcutaneous brodalumab on clinical disease activity in hidradenitis suppurativa: An open-label cohort study. J Am Acad Dermatol (2020) 83(5):1341–8. doi: 10.1016/j.jaad.2020.05.007
134. Blok JL, Li K, Brodmerkel C, Horvátovich P, Jonkman MF, Horváth B. Ustekinumab in hidradenitis suppurativa: clinical results and a search for potential biomarkers in serum. Br J Dermatol (2016) 174(4):839–46. doi: 10.1111/bjd.14338
135. Nograles KE, Zaba LC, Shemer A, Fuentes-Duculan J, Cardinale I, Kikuchi T, et al. IL-22-producing “T22” T cells account for upregulated IL-22 in atopic dermatitis despite reduced IL-17-producing TH17 T cells. J Allergy Clin Immunol (2009) 123(6):1244–52.e2. doi: 10.1016/j.jaci.2009.03.041
136. Guttman-Yassky E, Suárez-Fariñas M, Chiricozzi A, Nograles KE, Shemer A, Fuentes-Duculan J, et al. Broad defects in epidermal cornification in atopic dermatitis identified through genomic analysis. J Allergy Clin Immunol (2009) 124(6):1235–44.e58. doi: 10.1016/j.jaci.2009.09.031
137. Noda S, Suárez-Fariñas M, Ungar B, Kim SJ, de Guzman Strong C, Xu H, et al. The Asian atopic dermatitis phenotype combines features of atopic dermatitis and psoriasis with increased TH17 polarization. J Allergy Clin Immunol (2015) 136(5):1254–64. doi: 10.1016/j.jaci.2015.08.015
138. Czarnowicki T, He H, Canter T, Han J, Lefferdink R, Erickson T, et al. Evolution of pathologic T-cell subsets in patients with atopic dermatitis from infancy to adulthood. J Allergy Clin Immunol (2020) 145(1):215–28. doi: 10.1016/j.jaci.2019.09.031
139. Gittler JK, Shemer A, Suárez-Fariñas M, Fuentes-Duculan J, Gulewicz KJ, Wang CQ, et al. Progressive activation of T(H)2/T(H)22 cytokines and selective epidermal proteins characterizes acute and chronic atopic dermatitis. J Allergy Clin Immunol (2012) 130(6):1344–54. doi: 10.1016/j.jaci.2012.07.012
140. Suárez-Fariñas M, Dhingra N, Gittler J, Shemer A, Cardinale I, de Guzman Strong C, et al. Intrinsic atopic dermatitis shows similar TH2 and higher TH17 immune activation compared with extrinsic atopic dermatitis. J Allergy Clin Immunol (2013) 132(2):361–70. doi: 10.1016/j.jaci.2013.04.046
141. Aktar MK, Kido-Nakahara M, Furue M, Nakahara T. Mutual upregulation of endothelin-1 and IL-25 in atopic dermatitis. Allergy (2015) 70(7):846–54. doi: 10.1111/all.12633
142. Oyoshi MK, Murphy GF, Geha RS. Filaggrin-deficient mice exhibit TH17-dominated skin inflammation and permissiveness to epicutaneous sensitization with protein antigen. J Allergy Clin Immunol (2009) 124(3):485–93, 93 e1. doi: 10.1016/j.jaci.2009.05.042
143. Gutowska-Owsiak D, Schaupp AL, Salimi M, Selvakumar TA, McPherson T, Taylor S, et al. IL-17 downregulates filaggrin and affects keratinocyte expression of genes associated with cellular adhesion. Exp Dermatol (2012) 21(2):104–10. doi: 10.1111/j.1600-0625.2011.01412.x
144. Hvid M, Vestergaard C, Kemp K, Christensen GB, Deleuran B, Deleuran M. IL-25 in atopic dermatitis: a possible link between inflammation and skin barrier dysfunction? J Invest Dermatol (2011) 131(1):150–7. doi: 10.1038/jid.2010.277
145. Salimi M, Barlow JL, Saunders SP, Xue L, Gutowska-Owsiak D, Wang X, et al. A role for IL-25 and IL-33-driven type-2 innate lymphoid cells in atopic dermatitis. J Exp Med (2013) 210(13):2939–50. doi: 10.1084/jem.20130351
146. Ungar B, Pavel AB, Li R, Kimmel G, Nia J, Hashim P, et al. Phase 2 randomized, double-blind study of IL-17 targeting with secukinumab in atopic dermatitis. J Allergy Clin Immunol (2020). doi: 10.1016/j.jaci.2020.04.055
147. Pan Y, Xu L, Qiao J, Fang H. A systematic review of ustekinumab in the treatment of atopic dermatitis. J Dermatolog Treat (2018) 29(6):539–41. doi: 10.1080/09546634.2017.1406894
148. Khattri S, Brunner PM, Garcet S, Finney R, Cohen SR, Oliva M, et al. Efficacy and safety of ustekinumab treatment in adults with moderate-to-severe atopic dermatitis. Exp Dermatol (2017) 26(1):28–35. doi: 10.1111/exd.13112
149. Saeki H, Kabashima K, Tokura Y, Murata Y, Shiraishi A, Tamamura R, et al. Efficacy and safety of ustekinumab in Japanese patients with severe atopic dermatitis: a randomized, double-blind, placebo-controlled, phase II study. Br J Dermatol (2017) 177(2):419–27. doi: 10.1111/bjd.15493
150. Garzorz N, Alsisi M, Todorova A, Atenhan A, Thomas J, Lauffer F, et al. Dissecting susceptibility from exogenous triggers: the model of alopecia areata and associated inflammatory skin diseases. J Eur Acad Dermatol Venereol (2015) 29(12):2429–35. doi: 10.1111/jdv.13325
151. Atwa MA, Youssef N. Bayoumy NM. T-helper 17 cytokines (interleukins 17, 21, 22, and 6, and tumor necrosis factor-α) in patients with alopecia areata: association with clinical type and severity. Int J Dermatol (2016) 55(6):666–72. doi: 10.1111/ijd.12808
152. Tembhre MK, Sharma VK. T-helper and regulatory T-cell cytokines in the peripheral blood of patients with active alopecia areata. Br J Dermatol (2013) 169(3):543–8. doi: 10.1111/bjd.12396
153. Han YM, Sheng YY, Xu F, Qi SS, Liu XJ, Hu RM, et al. Imbalance of T-helper 17 and regulatory T cells in patients with alopecia areata. J Dermatol (2015) 42(10):981–8. doi: 10.1111/1346-8138.12978
154. Loh SH, Moon HN, Lew BL, Sim WY. Role of T helper 17 cells and T regulatory cells in alopecia areata: comparison of lesion and serum cytokine between controls and patients. J Eur Acad Dermatol Venereol (2018) 32(6):1028–33. doi: 10.1111/jdv.14775
155. Ortolan LS, Kim SR, Crotts S, Liu LY, Craiglow BG, Wambier C, et al. IL-12/IL-23 neutralization is ineffective for alopecia areata in mice and humans. J Allergy Clin Immunol (2019) 144(6):1731–4.e1. doi: 10.1016/j.jaci.2019.08.014
156. Guttman-Yassky E, Nia JK, Hashim PW, Mansouri Y, Alia E, Taliercio M, et al. Efficacy and safety of secukinumab treatment in adults with extensive alopecia areata. Arch Dermatol Res (2018) 310(8):607–14. doi: 10.1007/s00403-018-1853-5
157. Feldmeyer L, Mylonas A, Demaria O, Mennella A, Yawalkar N, Laffitte E, et al. Interleukin 23-Helper T Cell 17 Axis as a Treatment Target for Pityriasis Rubra Pilaris. JAMA Dermatol (2017) 153(4):304–8. doi: 10.1001/jamadermatol.2016.5384
158. Napolitano M, Abeni D, Didona B. Biologics for pityriasis rubra pilaris treatment: A review of the literature. J Am Acad Dermatol (2018) 79(2):353–9.e11. doi: 10.1016/j.jaad.2018.03.036
159. Schuster D, Pfister-Wartha A, Bruckner-Tuderman L, Schempp CM. Successful Treatment of Refractory Pityriasis Rubra Pilaris With Secukinumab. JAMA Dermatol (2016) 152(11):1278–80. doi: 10.1001/jamadermatol.2016.3885
160. Haynes D, Strunck JL, Topham CA, Ortega-Loayza AG, Kent G, Cassidy PB, et al. Evaluation of Ixekizumab Treatment for Patients With Pityriasis Rubra Pilaris: A Single-Arm Trial. JAMA Dermatol (2020) 156(6):668–75. doi: 10.1001/jamadermatol.2020.0932
161. Napolitano M, Lembo L, Fania L, Abeni D, Didona D, Didona B. Ustekinumab treatment of pityriasis rubra pilaris: A report of five cases. J Dermatol (2018) 45(2):202–6. doi: 10.1111/1346-8138.14114
162. Xue J, Su W, Chen Z, Ke Y, Du X, Zhou Q. Overexpression of interleukin-23 and interleukin-17 in the lesion of pemphigus vulgaris: a preliminary study. Mediators Inflamm (2014) 2014:463928. doi: 10.1155/2014/463928
163. Timoteo RP, da Silva MV, Miguel CB, Silva DA, Catarino JD, Rodrigues Junior V, et al. Th1/Th17-Related Cytokines and Chemokines and Their Implications in the Pathogenesis of Pemphigus Vulgaris. Mediators Inflamm (2017) 2017:7151285. doi: 10.1155/2017/7151285
164. Ben Jmaa M, Abida O, Fakhfakh R, Bahloul E, Sellami K, Gaddour L, et al. Involvement of the IL23/Th17 Pathway in the Pathogenesis of Tunisian Pemphigus Foliaceus. Mediators Inflamm (2018) 2018:8206983. doi: 10.1155/2018/8206983
165. Gholibeigian Z, Izad M, Daneshpazhooh M, Mortazavi H, Salehi Z, Behruzifar S, et al. Decreased serum levels of interleukin-17, interleukin-23, TGF-β in pemphigus vulgaris patients, and their association with disease phase. Dermatol Ther (2020) e14071. doi: 10.1111/dth.14071
166. Kamali AN, Noorbakhsh SM, Hamedifar H, Jadidi-Niaragh F, Yazdani R, Bautista JM, et al. A role for Th1-like Th17 cells in the pathogenesis of inflammatory and autoimmune disorders. Mol Immunol (2019) 105:107–15. doi: 10.1016/j.molimm.2018.11.015
167. Azizi G, Mirshafiey A, Abolhassani H, Yazdani R, Ghanavatinejad A, Noorbakhsh F, et al. The imbalance of circulating T helper subsets and regulatory T cells in patients with LRBA deficiency: Correlation with disease severity. J Cell Physiol (2018) 233(11):8767–77. doi: 10.1002/jcp.26772
168. Mo C, Zeng Z, Deng Q, Ding Y, Xiao R. Imbalance between T helper 17 and regulatory T cell subsets plays a significant role in the pathogenesis of systemic sclerosis. BioMed Pharmacother (2018) 108:177–83. doi: 10.1016/j.biopha.2018.09.037
169. Zhou Y, Hou W, Xu K, Han D, Jiang C, Mou K, et al. The elevated expression of Th17-related cytokines and receptors is associated with skin lesion severity in early systemic sclerosis. Hum Immunol (2015) 76(1):22–9. doi: 10.1016/j.humimm.2014.12.008
170. Gonçalves RSG, Pereira MC, Dantas AT, Almeida AR, Rego M, Lima EA, et al. CCL3, IL-7, IL-13 and IFNγ transcripts are increased in skin’s biopsy of systemic sclerosis. Exp Dermatol (2019) 28(10):1172–5. doi: 10.1111/exd.13982
171. Robak E, Gerlicz-Kowalczuk Z, Dziankowska-Bartkowiak B, Wozniacka A, Bogaczewicz J. Serum concentrations of IL-17A, IL-17B, IL-17E and IL-17F in patients with systemic sclerosis. Arch Med Sci (2019) 15(3):706–12. doi: 10.5114/aoms.2019.84738
172. Yang X, Yang J, Xing X, Wan L, Li M. Increased frequency of Th17 cells in systemic sclerosis is related to disease activity and collagen overproduction. Arthritis Res Ther (2014) 16(1):R4. doi: 10.1186/ar4430
Keywords: IL-17 family, IL-23, IL-23/IL-17 axis, psoriasis, targeted therapy
Citation: Liu T, Li S, Ying S, Tang S, Ding Y, Li Y, Qiao J and Fang H (2020) The IL-23/IL-17 Pathway in Inflammatory Skin Diseases: From Bench to Bedside. Front. Immunol. 11:594735. doi: 10.3389/fimmu.2020.594735
Received: 14 August 2020; Accepted: 20 October 2020;
Published: 17 November 2020.
Edited by:
Matteo Vecellio, University of Oxford, United KingdomReviewed by:
Maddalena Napolitano, University of Molise, ItalyCopyright © 2020 Liu, Li, Ying, Tang, Ding, Li, Qiao and Fang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianjun Qiao, cWlhb2ppYW5qdW5Aemp1LmVkdS5jbg==; Hong Fang, ZmFuZ2hvbmd6eUB6anUuZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.