- 1Centre for Rheumatology/Division of Medicine, University College London, London, United Kingdom
- 2Global Clinical Development, EMD Serono Research and Development Institute, Inc., Billerica, MA, United States (an affiliate of Merck KGaA, Darmstadt, Germany
- 3Global Biostatistics, EMD Serono Research and Development Institute, Inc., Billerica, MA, United States (an affiliate of Merck KGaA, Darmstadt, Germany
- 4Arthritis and Clinical Immunology Program, Oklahoma Medical Research Foundation, Oklahoma City, OK, United States
A Commentary on
Systematic Review of Safety and Efficacy of Atacicept in Treating Immune-Mediated Disorders
By Kaegi C, Steiner UC, Wuest B, et al. (2020). Front Immunol. 11:433. doi: 10.3389/fimmu.2020.00433
Introduction
We read with interest the systematic review article published in Frontiers in Immunology by Kaegi and colleagues, which analyzed information from studies of atacicept across several immune-mediated disorders. Whilst we welcome the effort the authors have made in collating studies of atacicept in different therapy areas, especially the benefit for clinicians and researchers in the field, we have identified several inconsistencies, errors, omissions, and critical flaws in the reporting and interpretation of efficacy and safety. Here, we have highlighted some of the methodological and factual errors in the review (summarized in detail in Table 1) to provide essential balance and context. This response was supported by Merck KGaA, Darmstadt, Germany, who are developing atacicept.
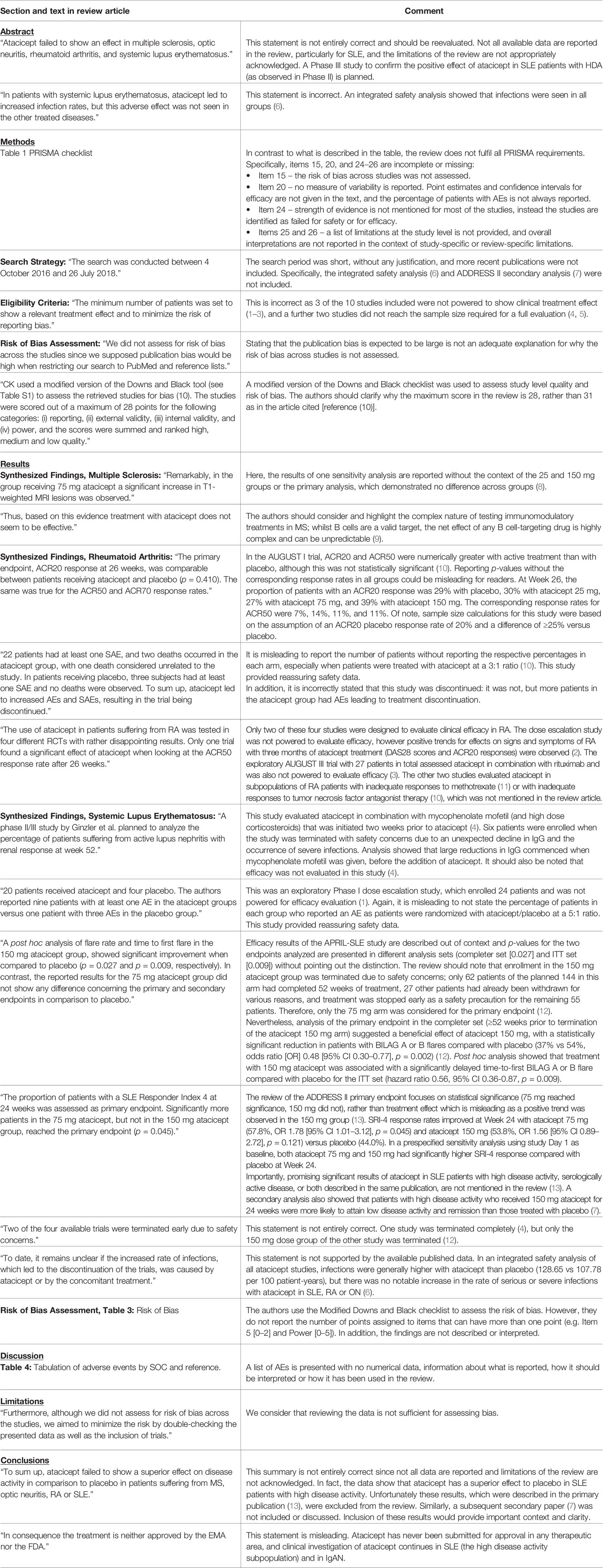
Table 1 Details of missing, misleading and incorrect information in the Kaegi et al. systematic review article.
Scope of the Original Review
The authors identified 10 studies of atacicept in multiple sclerosis (MS), optic neuritis (ON), rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE) suitable for inclusion in their systematic review. The search period was short, from October 2016 to July 2018, and key publications from 2019 were not included. It was claimed that only studies with a minimum number of patients to show a relevant treatment effect were eligible, however, 3 of the 10 studies included were not powered to show clinical treatment effect (1–3) and a further 2 studies did not reach the sample size required for a full evaluation (4, 5). The review was said to be guided by the PRISMA checklist, but there is incomplete or incorrect information provided to meet PRISMA requirements (Table 1). For example, the risk of bias across studies is not assessed and treatment effect measures are not reported in the text. These details are essential for readers to interpret the results correctly.
Efficacy Data Reporting
Four SLE studies were included without discussion of the challenging nature of using clinical outcome composite endpoints in this setting, which can result in apparently conflicting results. For example, the primary endpoint in the TULIP-1 trial of anifrolumab using SLE Responder Index-4 was not met (14), whereas the TULIP-2 trial which used the British Isles Lupus Assessment Group-based Combined Lupus Assessment did meet its primary endpoint (15). The atacicept flare prevention trial (APRIL-SLE) provided a novel approach including patients who had recently had a lupus flare that was controlled by a relatively short course of glucocorticoids, but this was not mentioned in the review (12).
The analysis of the ADDRESS II primary endpoint focuses on statistical significance, which is misleading as a trend was observed in the 150 mg group (13). SLE is a clinically heterogenous disease and so it is important to identify specific cohorts of patients who may respond to a treatment; the beneficial effect of atacicept in a predefined subpopulation of ADDRESS II patients with high disease activity (HDA, SLEDAI-2K ≥10) (7, 13) was not discussed in the review article.
Inaccuracies are also evident in the reporting of efficacy data relating to MS and RA trials for atacicept, as summarized in Table 1.
Safety Data Reporting
Safety data are reported out of context or with insufficient detail (Table 1).
A large safety analysis of atacicept, comprising 17 clinical studies of 1568 subjects and including 761 SLE patients, was not included or discussed (6). The safety profile and number of reported deaths in atacicept studies were found to be comparable with that of other biologic therapies, including belimumab and blisibimod for SLE, but this context was not given in the review article (6, 16–18). Data for atacicept across all studies show that infections and infestations are the most commonly reported treatment-emergent adverse event (45.6%) (6). This is not unexpected since atacicept reduces immunoglobulin levels and B and plasma cell numbers, and is consistent with other biologic agents used to treat autoimmune diseases (6, 19). Overall, atacicept is associated with increased infection rates compared with placebo, however, serious and severe infections are not higher with atacicept in patients with SLE, RA or ON (6).
The authors correctly report that two infection-related deaths occurred in the 150 mg arm of the APRIL-SLE trial, but this led to discontinuation of the 150 mg arm only, not the whole trial as stated (12). Unfortunately, many trials in SLE record a small number of deaths. The APRIL-LN study in SLE was stopped with six patients enrolled due to a decline in serum IgG and the occurrence of serious infections (4). On further analysis the decline in IgG levels was linked to the mycophenolate prescribed prior to the addition of atacicept. A risk mitigation strategy was implemented for subsequent studies; in the Phase II ADDRESS II study of over 300 SLE patients, infection rates were lower and no deaths associated with atacicept were reported (13). Therefore, with the implementation of effective mitigation measures to reduce the risk of infection, the benefit of atacicept for SLE patients with HDA may outweigh the risks (6). It is imperative that this is highlighted in the review article.
Conclusion
Kaegi et al. conclude that atacicept failed to show superior effect on disease activity in comparison to placebo in MS, ON, RA and SLE without inclusion of all relevant data, especially in the case of SLE, or full acknowledgement of the limitations of the review. In fact, in all studies, atacicept did show an effect on disease activity (as indicated by a reduction in biomarkers) but this was not always translated to measurable clinical efficacy over placebo (standard of care).
In the Phase II trials in RA, while the efficacy endpoint was not met, the safety profile was acceptable. MS and ON studies were discontinued due to increased disease activity. However, SLE published data indicate that atacicept is beneficial for SLE patients with HDA, which is the target population for future SLE trials with atacicept. This offers some hope of positive clinical outcome in a field notorious for the number of failed trials. Future studies will further assess and confirm clinical efficacy of atacicept in SLE.
It is therefore misleading to state that on this basis atacicept was not approved in these therapeutic areas when the drug has never been submitted for approval. Clinical investigation of atacicept continues in SLE and IgA nephropathy.
Author Contributions
All authors contributed to the article and approved the submitted version. All authors agree to be accountable for this article.
Funding
The development of this article included medical writing support, provided by Bioscript Science, Macclesfield, UK and funded by Merck KGaA, Darmstadt, Germany.
Conflict of Interest
DAI has received consultant fees from EMD Serono Research and Development Institute, Inc. (an affiliate of Merck KGaA, Darmstadt, Germany), Celgene, AstraZeneca and Servier; consulting fees have been passed to a local arthritis charity. JTM has received grants/research support from GSK and BMS (investigator-initiated studies); consultant or data quality management fees from EMD Serono Research and Development Institute, Inc. (an affiliate of Merck KGaA, Darmstadt, Germany), Eli Lilly, Remegen, GSK, UCB, Celgene, Abbvie, Amgen, Daitchi Sankyo, Astellas, Pfizer, Genentech, AstraZeneca, Jannsen, Servier, ILTOO and Xencor. AK and AA are employees of EMD Serono Research and Development Institute, Inc. (an affiliate of Merck KGaA, Darmstadt, Germany). Merck KGaA sponsored the clinical development of atacicept and were involved in study design, collection, analysis, interpretation of data, the writing of this article and the decision to submit it for publication. Bioscript Science, Macclesfield, UK provided medical writing support, funded by Merck KGaA, Darmstadt, Germany.
Acknowledgments
We thank the patients and study teams involved in the clinical trials of atacicept. Medical writing assistance was provided by Bioscript Science, Macclesfield, UK.
References
1. Pena-Rossi C, Nasonov E, Stanislav M, Yakusevich V, Ershova O, Lomareva N, et al. An exploratory dose-escalating study investigating the safety, tolerability, pharmacokinetics and pharmacodynamics of intravenous atacicept in patients with systemic lupus erythematosus. Lupus (2009) 18(6):547–55. doi: 10.1177/0961203309102803
2. Tak PP, Thurlings RM, Rossier C, Nestorov I, Dimic A, Mircetic V, et al. Atacicept in patients with rheumatoid arthritis: Results of a multicenter, phase ib, double-blind, placebo-controlled, dose-escalating, single- and repeated-dose study. Arthritis Rheumatol (2008) 58(1):61–72. doi: 10.1002/art.23178
3. van Vollenhoven RF, Wax S, Li Y, Tak PP. Safety and efficacy of atacicept in combination with rituximab for reducing the signs and symptoms of rheumatoid arthritis: a phase II, randomized, double-blind, placebo-controlled pilot trial. Arthritis Rheumatol (2015) 67(11):2828–36. doi: 10.1002/art.39262
4. Ginzler EM, Wax S, Rajeswaran A, Copt S, Hillson J, Ramos E, et al. Atacicept in combination with MMF and corticosteroids in lupus nephritis: results of a prematurely terminated trial. Arthritis Res Ther (2012) 14(1):R33. doi: 10.1186/ar3738
5. Sergott RC, Bennett JL, Rieckmann P, Montalban X, Mikol D, Freudensprung U, et al. ATON: Results from a Phase II randomized trial of the B-cell-targeting agent atacicept in patients with optic neuritis. J Neurol Sci (2015) 351(1):174–8. doi: 10.1016/j.jns.2015.02.019
6. Gordon C, Bassi R, Chang P, Kao A, Jayne D, Wofsy D, et al. Integrated safety profile of atacicept: an analysis of pooled data from the atacicept clinical trial programme. Rheumatol Adv Pract (2019) 3(2):rkz021. doi: 10.1093/rap/rkz021
7. Morand EF, Isenberg DA, Wallace DJ, Kao AH, Vazquez-Mateo C, Chang P, et al. Attainment of treat-to-target endpoints in SLE patients with high disease activity in the atacicept phase 2b ADDRESS II study. Rheumatology (2020) 56(10):2930–8. doi: 10.1093/rheumatology/keaa029
8. Kappos L, Hartung H-P, Freedman MS, Boyko A, Radü EW, Mikol DD, et al. Atacicept in multiple sclerosis (ATAMS): a randomised, placebo-controlled, double-blind, phase 2 trial. Lancet Neurol (2014) 13(4):353–63. doi: 10.1016/S1474-4422(14)70028-6
9. Lühder F, Gold R. Trial and error in clinical studies: lessons from ATAMS. Lancet Neurol (2014) 13(4):340–1. doi: 10.1016/S1474-4422(14)70050-X
10. Genovese MC, Kinnman N, de La Bourdonnaye G, Pena Rossi C, Tak PP. Atacicept in patients with rheumatoid arthritis and an inadequate response to tumor necrosis factor antagonist therapy: Results of a phase II, randomized, placebo-controlled, dose-finding trial. Arthritis Rheumatol (2011) 63(7):1793–803. doi: 10.1002/art.30373
11. van Vollenhoven RF, Kinnman N, Vincent E, Wax S, Bathon J. Atacicept in patients with rheumatoid arthritis and an inadequate response to methotrexate: Results of a phase II, randomized, placebo-controlled trial. Arthritis Rheumatol (2011) 63(7):1782–92. doi: 10.1002/art.30372
12. Isenberg D, Gordon C, Licu D, Copt S, Rossi CP, Wofsy D. Efficacy and safety of atacicept for prevention of flares in patients with moderate-to-severe systemic lupus erythematosus (SLE): 52-week data (APRIL-SLE randomised trial). Ann Rheum Dis (2015) 74(11):2006–15. doi: 10.1136/annrheumdis-2013-205067
13. Merrill JT, Wallace DJ, Wax S, Kao A, Fraser PA, Chang P, et al. Efficacy and safety of atacicept in patients with systemic lupus erythematosus. Arthritis Rheumatol (2018) 70(2):266–76. doi: 10.1002/art.40360
14. Furie RA, Morand EF, Bruce IN, Manzi S, Kalunian KC, Vital EM, et al. Type I interferon inhibitor anifrolumab in active systemic lupus erythematosus (TULIP-1): a randomised, controlled, phase 3 trial. Lancet Rheumatol (2019) 1(4):e208–19. doi: 10.1016/S2665-9913(19)30076-1
15. Morand EF, Furie R, Tanaka Y, Bruce IN, Askanase AD, Richez C, et al. Trial of anifrolumab in active systemic lupus erythematosus. N Engl J Med (2019) 382(3):211–21. doi: 10.1056/NEJMoa1912196
16. Furie R, Petri M, Zamani O, Cervera R, Wallace DJ, Tegzova D, et al. A phase III, randomized, placebo-controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis Rheumatol (2011) 63(12):3918–30. doi: 10.1002/art.30613
17. Furie RA, Leon G, Thomas M, Petri MA, Chu AD, Hislop C, et al. A phase 2, randomised, placebo-controlled clinical trial of blisibimod, an inhibitor of B cell activating factor, in patients with moderate-to-severe systemic lupus erythematosus, the PEARL-SC study. Ann Rheum Dis (2015) 74(9):1667–75. doi: 10.1136/annrheumdis-2013-205144
18. Wallace DJ, Stohl W, Furie RA, Lisse JR, McKay JD, Merrill JT, et al. A phase II, randomized, double-blind, placebo-controlled, dose-ranging study of belimumab in patients with active systemic lupus erythematosus. Arthritis Rheumatol (2009) 61(9):1168–78. doi: 10.1002/art.24699
Keywords: BAFF, APRIL, TACI, B cell, monoclonal antibody, multiple sclerosis, rheumatoid arthritis, systemic lupus erythematosus
Citation: Isenberg DA, Kao AH, Aydemir A and Merrill JT (2020) Commentary: Systematic Review of Safety and Efficacy of Atacicept in Treating Immune-Mediated Disorders. Front. Immunol. 11:592639. doi: 10.3389/fimmu.2020.592639
Received: 07 August 2020; Accepted: 07 October 2020;
Published: 11 November 2020.
Edited by:
Rolando Cimaz, University of Milan, ItalyReviewed by:
Vasileios Kyttaris, Beth Israel Deaconess Medical Center and Harvard Medical School, United StatesCopyright © 2020 Isenberg, Kao, Aydemir and Merrill. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: David A. Isenberg, ZC5pc2VuYmVyZ0B1Y2wuYWMudWs=
 David A. Isenberg
David A. Isenberg Amy H. Kao
Amy H. Kao Aida Aydemir
Aida Aydemir Joan T. Merrill
Joan T. Merrill