- 1Department of Rheumatology and Clinical Immunology, The First Affiliated Hospital of Xiamen University, Xiamen, China
- 2Department of Science & Technology, Xiamen Key Laboratory of Rheumatology and Clinical Immunology, Xiamen, China
Background: Exposure to Epstein-Barr virus (EBV) infection has been hypothesized to be an important risk factor for multiple rheumatic diseases, but the serological evidence so far for its role in Sjögren’s syndrome (SjS) is not clearly established yet. This study aimed to assess the seroepidemiological associations of antibodies to EBV with SjS.
Methods: A seroepidemiological study containing 119 patients with SjS and 65 healthy controls was first performed, in which the associations of SjS with four commonly studied EBV antibodies including IgM-anti-viral capsid antigen (anti-VCA) antibody, IgG-anti-VCA antibody, IgG-anti-early antigen (anti-EA) antibody, and IgG-anti-EBV nuclear antigen 1 (anti-EBNA1) antibody were evaluated. A systematic review and meta-analysis of eligible seroepidemiological studies was also carried out, and data syntheses were performed using random-effect meta-analysis.
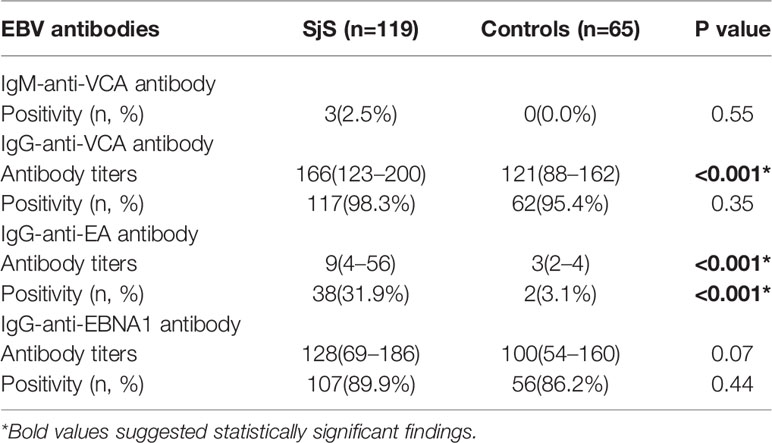
Results: In the case-control study, the patients with SjS had both a significantly higher prevalence of IgG-anti-EA antibody positivity (31.9% vs. 3.1%, P < 0.001) and high titers of IgG-anti-EA antibody (P < 0.001) than healthy controls. The titer of IgG-anti-VCA antibody was significantly increased in the patients with SjS compared with healthy controls (P < 0.001). IgG-anti-EA antibody seropositive patients with SjS had lower levels of both C3 (P = 0.002) and C4 (P = 0.02), and the titer of IgG-anti-EA antibody was inversely related to the levels of both C3 (r = -0.31, P < 0.001) and C4 (r = -0.20, P = 0.03). A total of 14 eligible studies on the serological associations between EBV infection and SjS were finally included into the meta-analysis, which suggested obvious associations of SjS with IgM-anti-VCA antibody [Odds ratio (OR) = 5.77, 95%CI 1.73–19.25, P = 0.004] and IgG-anti-EA antibody (OR = 9.97, 95%CI 4.58-21.67, P < 0.00001).
Conclusions: The findings from this study provide strong serological evidence for the association between EBV infection and SjS. SjS has obvious associations with IgM-anti-VCA antibody and IgG-anti-EA antibody. IgG-anti-EA antibody is linked to low levels of C3 and C4 in the patients with SjS, the significance of which needs to be addressed in further studies.
Introduction
Sjögren’s syndrome (SjS) is a complex and heterogeneous rheumatic disease (1). SjS is characterized by autoantibody production and lymphocyte infiltration in exocrine glands such as salivary and lacrimal glands (2, 3). Exocrine glandular injuries induced by autoimmune attacks can gradually cause dryness of eyes and mouth (1). Like systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA), the pathogenesis of SjS involves a complex interplay between genetic, immune and environmental factors, and the underlying molecular mechanisms remain to be defined (4–7). Though many studies have explored possible treatments of SjS, effective targeted therapies for SjS are still lacking (8, 9). Further studies are needed to elucidate SjS pathogenesis and find possible therapeutic targets.
Infections such as Epstein-Barr virus (EBV) infection have been proposed as one of the environmental triggers of SjS (10, 11). EBV is a common herpes virus affecting more than 90% of the total population worldwide (12). The possible roles of EBV infection in rheumatic diseases have been postulated for many years, and it has proven to be an important environmental trigger of several autoimmune diseases such as SLE and multiple sclerosis (MS) (13–16). Like SLE, a possible link between EBV infection and SjS has also been proposed for many years (17, 18). Increased prevalence of EBV infection and elevated viral loads in salivary glands had been reported in patients with SjS (19–21). There were also some studies exploring the serological evidence for the pathogenic role of EBV infection in SjS, and associations of SjS with EBV antibodies such as IgM-anti-viral capsid antigen (anti-VCA) antibody, IgG-anti-VCA antibody, IgG-anti-early antigen (anti-EA) antibody and IgG-anti-EBV nuclear antigen 1 (anti-EBNA1) antibody had been studied. However, the findings from these various studies were inconsistent (22–28). Therefore, the serological evidence for the contribution of EBV infection to the pathophysiology of SjS is not firmly established. To further explore the link between EBV antibodies and SjS, we performed an original seroepidemiological case-control study at first. We then carried out a systematic review and meta-analysis of available seroepidemiological studies on the associations between antibodies to EBV and SjS.
Methods
Participants
A seroepidemiological study using case-control design was performed to evaluate the association between EBV infection and SjS, in which the associations of SjS with EBV antibodies were analyzed. 119 patients with SjS were recruited in The First Affiliated Hospital of Xiamen University from June 2018 to December 2019. SjS was diagnosed according to the 2016 American College of Rheumatology (ACR) and the European League Against Rheumatism (EULAR) classification criteria for SjS (29). 65 healthy controls without autoimmune diseases were randomly recruited from individuals receiving routine medical examination in our hospital, whose age and gender matched SjS patients. The mean age of the SjS patients was 51.2 ± 15.7 years, and the mean age of the healthy controls was 50.7 ± 11.1 years (Supplementary Table 1). This study was approved by the Medical Ethics Committee at The First Affiliated Hospital of Xiamen University (KY202015-034). Written informed consent was obtained from all participants.
Clinical Assessment
The disease activity of SjS patients was evaluated by the EULAR Sjögren’s syndrome disease activity index (ESSDAI) (30). Clinical data such as age, gender, disease history, clinical manifestations and treatment drugs were collected. Outcomes of laboratory tests such as anti-SSA/Ro, anti-SSB/La, C3, and C4 were also recorded.
Detection of EBV Antibodies
Sera from SjS patients and healthy controls were stored at -80°C. Four commonly used antibodies to EBV including IgM-anti-VCA antibody, IgG-anti-VCA antibody, IgG-anti-EA antibody and IgG-anti-EBNA1 antibody were analyzed. EBV antibodies were detected by using commercial enzyme-linked immunosorbent assay (ELISA) kits according to the manufacturer’s instructions (EUROIMMUN, Germany). The cut-off values for positivity of IgG-anti-VCA antibody, IgG-anti-EA antibody and IgG-anti-EBNA1 antibody were defined as 22 RU/ml, and the cut-off value for IgM-anti-VCA antibody positivity was defined as a ratio of ODPatient/ODStandard or ODControl/ODStandard more than 1.1.
Statistical Analysis
Quantitative variables were shown as mean ± standard deviation (SD) or median with interquartile range (IQR). Categorical variables were shown as counts with percentages. Differences in quantitative data between groups were compared by student’s t-test or Mann-Whitney U test when necessary. Differences in categorical variables between groups were compared by Chi-square or Fischer’s exact tests. Odds ratios (OR) with 95% confidence intervals (95%CI) were calculated to evaluate the associations between EBV antibodies and SjS. Data analyses were performed using GraphPad (Version 7.0, GraphPad Software, California, USA) and STATA (Version 12.0, StataCorp, Texas, USA). All tests were two-sided, and outcomes were considered statistically significant at P < 0.05.
Systematic Review and Meta-Analysis
A systematic review and meta-analysis was performed to evaluate the relationship between antibodies to EBV and patients with SjS. We searched Pubmed and China National Knowledge Infrastructure (CNKI) from inception to May 16, 2020, to identify epidemiological studies on the serological associations between antibodies to EBV and SjS. We also searched the reference citations of those included studies to identify more possible articles. The following terms were used in the literature search: (Epstein-Barr virus OR EBV OR human herpesvirus 4 OR HHV-4) AND (Sjogren’s syndrome OR Sjögren’s syndrome OR Sjogren syndrome OR Sjögren syndrome). No language restriction was applied. Articles concerning the epidemiological associations of anti-EBV antibodies with SjS were reviewed. Studies eligible into the systematic review met the following criteria: 1) Clinical observational studies such as cohort studies, cross-sectional studies or case-control studies; 2) Participants contained at least 10 SjS patients; 3) Reporting data on the serological associations of EBV antibodies with SjS; 4) EBV antibodies were examined using recommended clinical laboratory methods such as immunofluorescence (IF) or ELISA; 5) Data did not overlap with other included studies. Studies not meeting the above eligible criteria were all excluded. Studies that did not specify the type of anti-EBV antibodies or did not provide usable data were excluded.
Two authors independently extracted data from eligible studies such as authors, country, sample size, types of EBV antibodies, laboratory methods, diagnostic criteria of SjS, cut-off values of seropositivity, and risk estimates with 95%CIs. Discrepancies in the data extracted by those two authors were resolved via group discussions among all authors.
Heterogeneity among included studies was assessed using the I2-statistic, and I2 more than 50% suggested high heterogeneity (31). To reduce the impact of heterogeneity on the pooled risk estimates, meta-analysis was performed by DerSimonian and Laird’s random-effect model (32). Publication bias was assessed by funnel plot. Review Manager (Version 5.2; Cochrane, London, United Kingdom) was used in statistical analyses, and P values less than 0.05 were considered statistically significant.
Results
Case-Control Study
The clinical characteristics of SjS patients and healthy controls in the case-control study are shown in Supplementary Table 1. There was no obvious difference in age and gender between SjS patients and healthy controls (P > 0.05). The mean ESSDAI of those 119 SjS patients was 2.1 ± 1.5.
Compared with healthy subjects, SjS patients had both a significantly higher prevalence of IgG-anti-EA antibody positivity (31.9% vs. 3.1%, P < 0.001) and higher titers of IgG-anti-EA antibody (P < 0.001; Table 1). There was no obvious difference in the prevalence of IgM-anti-VCA antibody, IgG-anti-VCA antibody and IgG-anti-EBNA1 antibody between SjS patients and controls (P > 0.05; Table 1). However, the titer of IgG-anti-VCA antibody was significantly increased in SjS patients compared to healthy controls (P < 0.001; Table 1, Figures 1A–C). The titer of anti-EBNA1 IgG antibody was marginally increased in SjS patients than healthy controls (P = 0.07; Table 1).
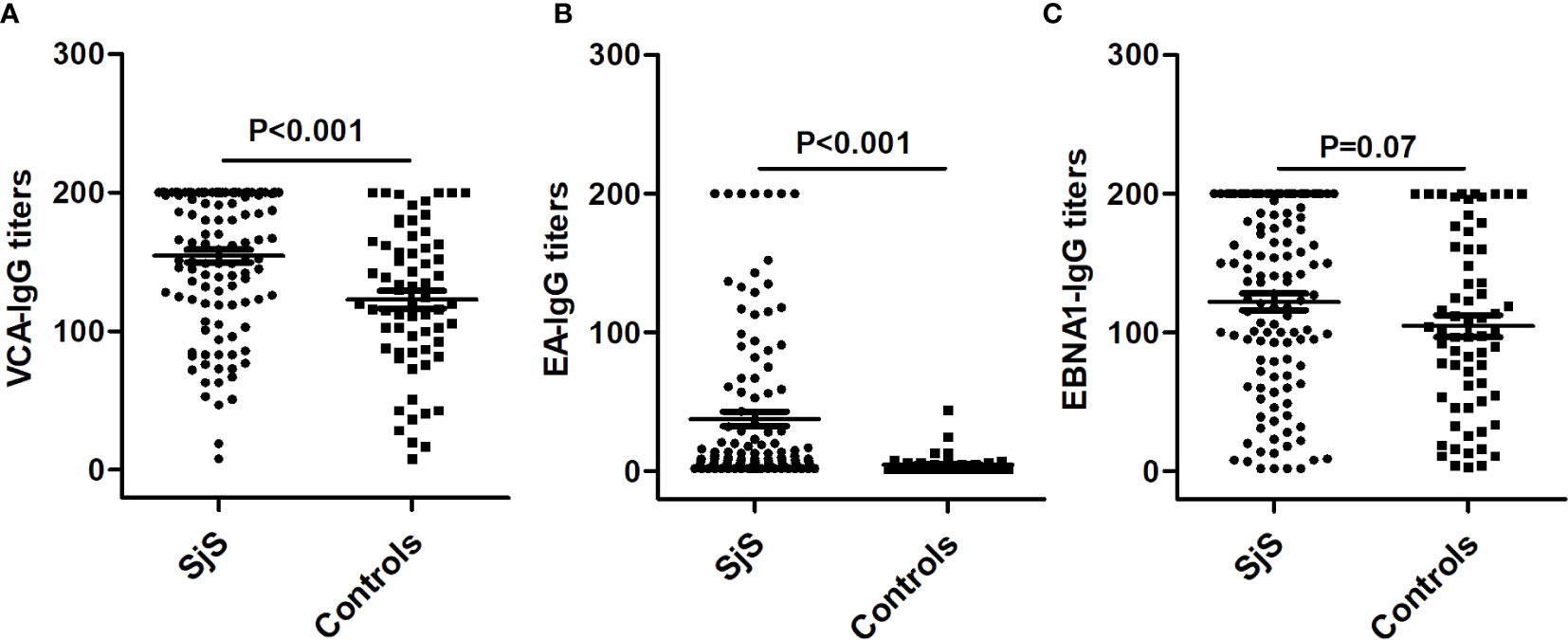
Figure 1 Differences in the titers of IgG-anti-VCA antibody, IgG-anti-EA antibody and IgG-anti-EBNA1 antibody between SjS patients and healthy controls. (A) The difference in the titers of IgG-anti-VCA antibody between SjS patients and healthy controls. (B) The difference in the titers of IgG-anti-EA antibody between SjS patients and healthy controls. (C) The difference in the titers of IgG-anti-EBNA1 antibody between SjS patients and healthy controls.
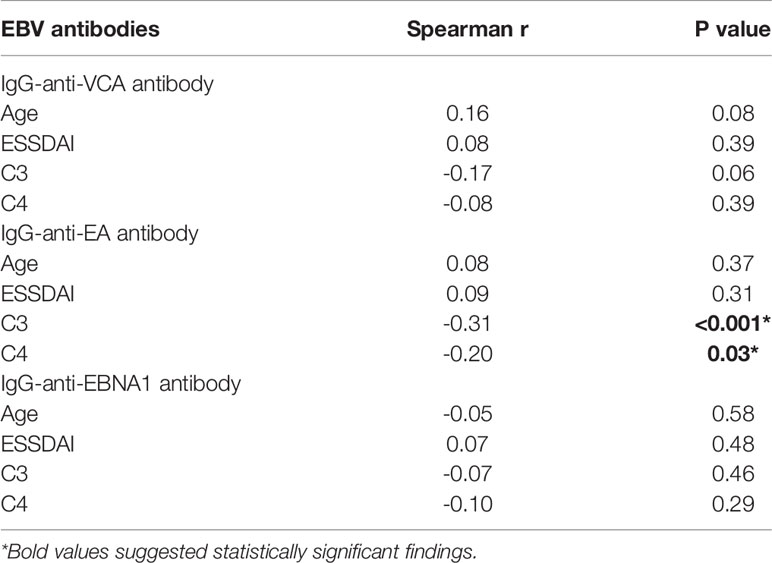
The clinical characteristics of SjS patients stratified by seropositivity status of EBV antibodies were then analyzed. As shown in the supplementary tables (Supplementary Tables 2–5), IgG-anti-EA antibody seropositive patients with SjS had lower levels of both C3 (P = 0.002) and C4 (P = 0.02). Among those 119 SjS patients, the titer of IgG-anti-EA antibody was inversely related to the levels of C3 (r = -0.31, P < 0.001) and C4 (r = -0.20, P = 0.03), and a marginally significant inverse correlation between IgG-anti-VCA antibody titer and C3 level was also observed (r = -0.17, P = 0.06) (Table 2). The titers of other EBV antibodies were not significantly related to the levels of C3 and C4 except for the marginally significant correlation between IgG-anti-VCA antibody and C3 level (P = 0.06; Table 2). The disease activity of SjS patients evaluated by ESSDAI was not significantly correlated with EBV antibodies (P > 0.05; Table 2).
Systematic Review and Meta-Analysis
A total of 261 publications were found in the literature search. 224 studies were excluded after the initial evaluation by reading titles and abstracts. The rest of 37 studies were evaluated in details by reading full-texts, and 24 studies were further excluded as they did not analyze the epidemiological associations of EBV antibodies with SjS. Finally, 13 published studies focusing on the serological association between EBV infection and SjS were identified (22–28, 33–38). Among those 13 published studies, nine studies reported outcomes on IgG-anti-VCA antibody, seven studies reported outcomes on IgM-anti-VCA antibody, 6 studies reported outcomes on IgG-anti-EA antibody, and nine studies reported outcomes on IgG-anti-EBNA1 antibody (Table 3). Together with the present case-control study, a total of 14 available studies on the serological association between EBV infection and SjS were finally included into the meta-analysis (Table 3).
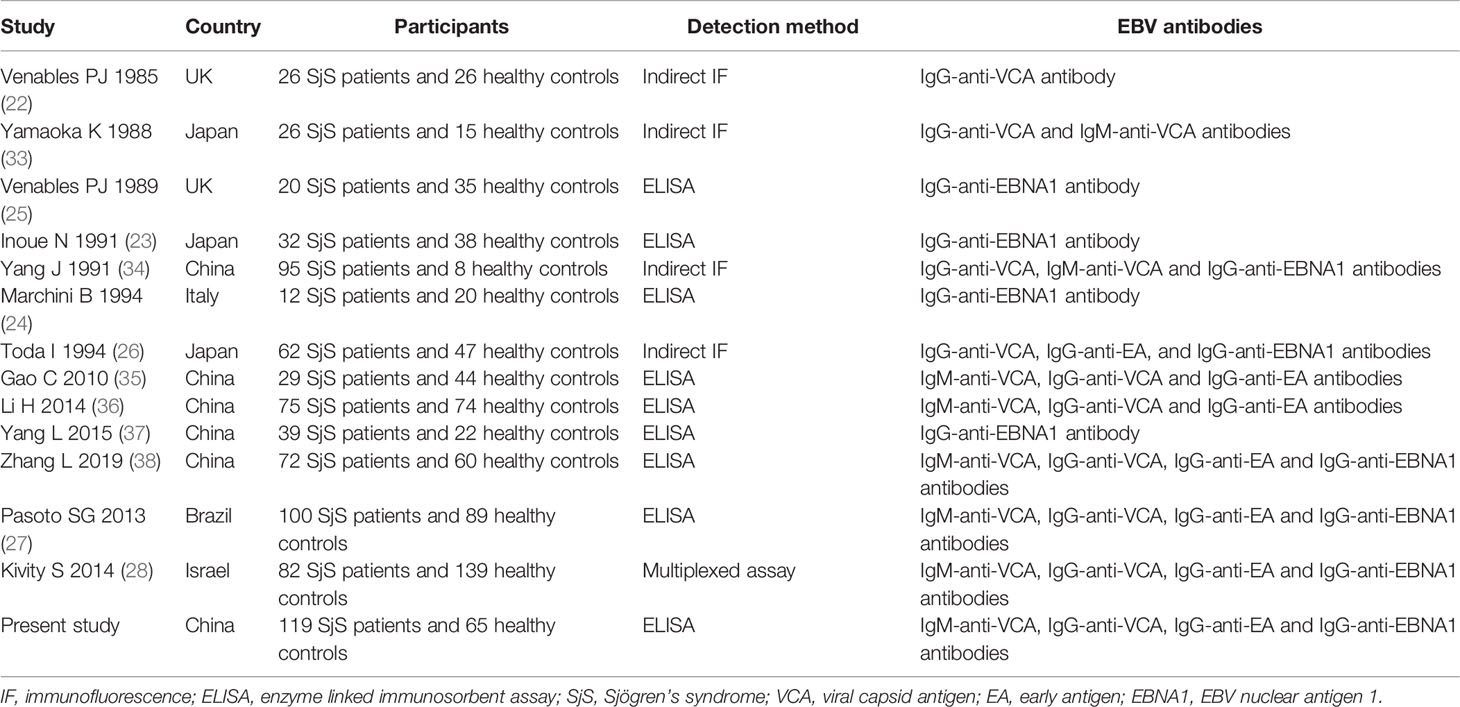
Table 3 Summarization of 14 studies focusing on the serological association between EBV infection and SjS.
Obvious heterogeneity was found among those eight studies on the association between IgM-anti-VCA antibody and SjS (I2 = 59%; Figures 2A–D). Meta-analysis of those eight studies suggested that IgM-anti-VCA antibody was significantly associated with SjS (Pooled OR = 5.77, 95%CI 1.73-19.25, P = 0.004) (Figure 2A). Obvious heterogeneity was also found among those seven studies on the association between IgG-anti-EA antibody and SjS (I2 = 62%), and meta-analysis of those seven studies revealed an obvious association between IgG-anti-EA antibody and SjS (Pooled OR = 9.97, 95%CI 4.58-21.67, P < 0.00001) (Figure 2C). However, obvious associations of SjS with IgG-anti-EBNA1 antibody and IgG-anti-VCA antibody were not found (P > 0.05; Figures 2B, D).
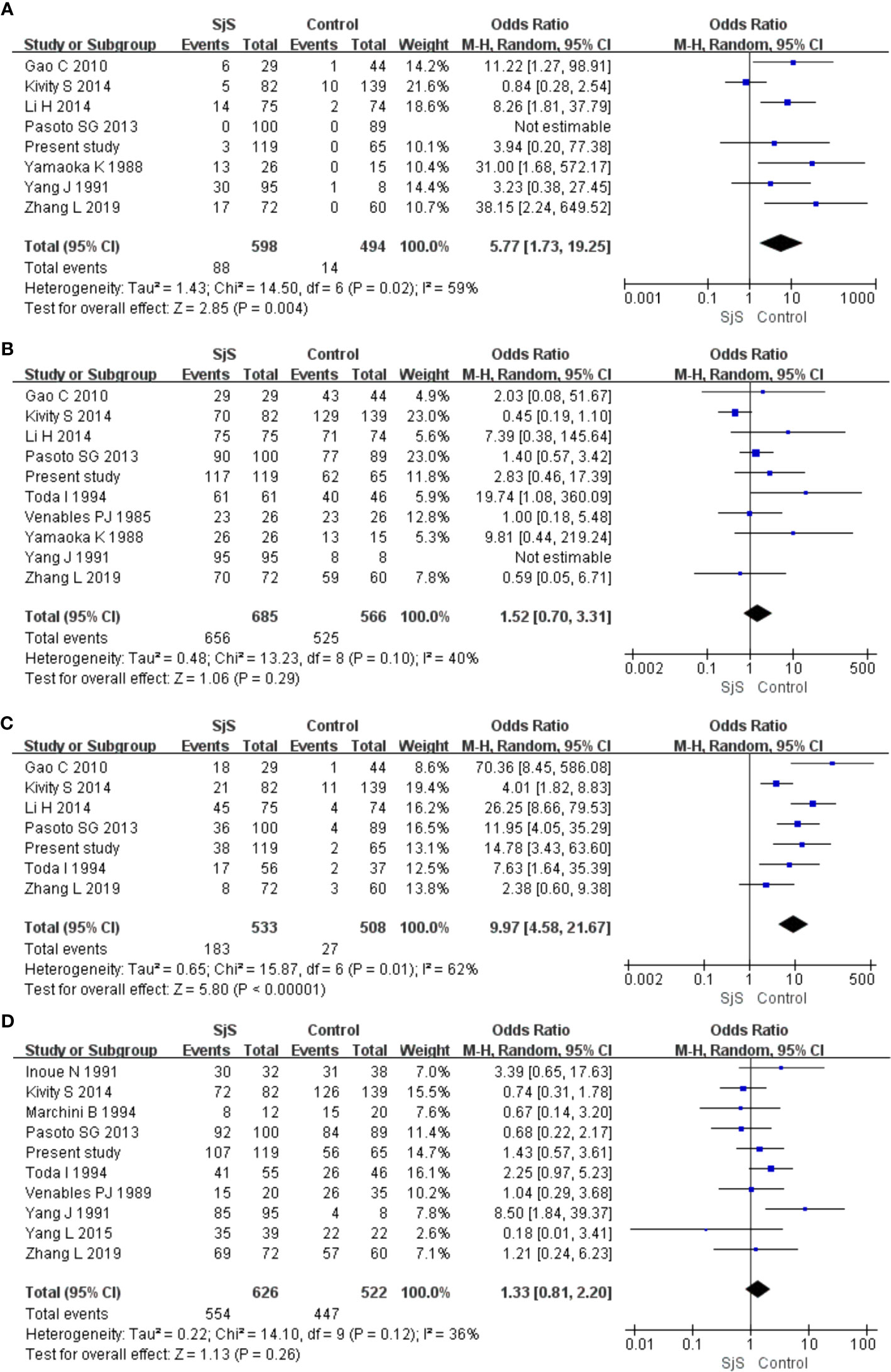
Figure 2 Forest plots in the meta-analysis of the associations between EBV antibodies and SjS. (A) Forest plot in the meta-analysis of the association between IgM-anti-VCA antibody and SjS. (B) Forest plot in the meta-analysis of the association between IgG-anti-VCA antibody and SjS. (C) Forest plot in the meta-analysis of the association between IgG-anti-EA antibody and SjS. (D) Forest plot in the meta-analysis of the association between IgG-anti-EBNA1 antibody and SjS.
Funnel plots in this meta-analysis suggested low risk of publication bias, and all those 4 funnel plots were nearly symmetric (Figure 3).
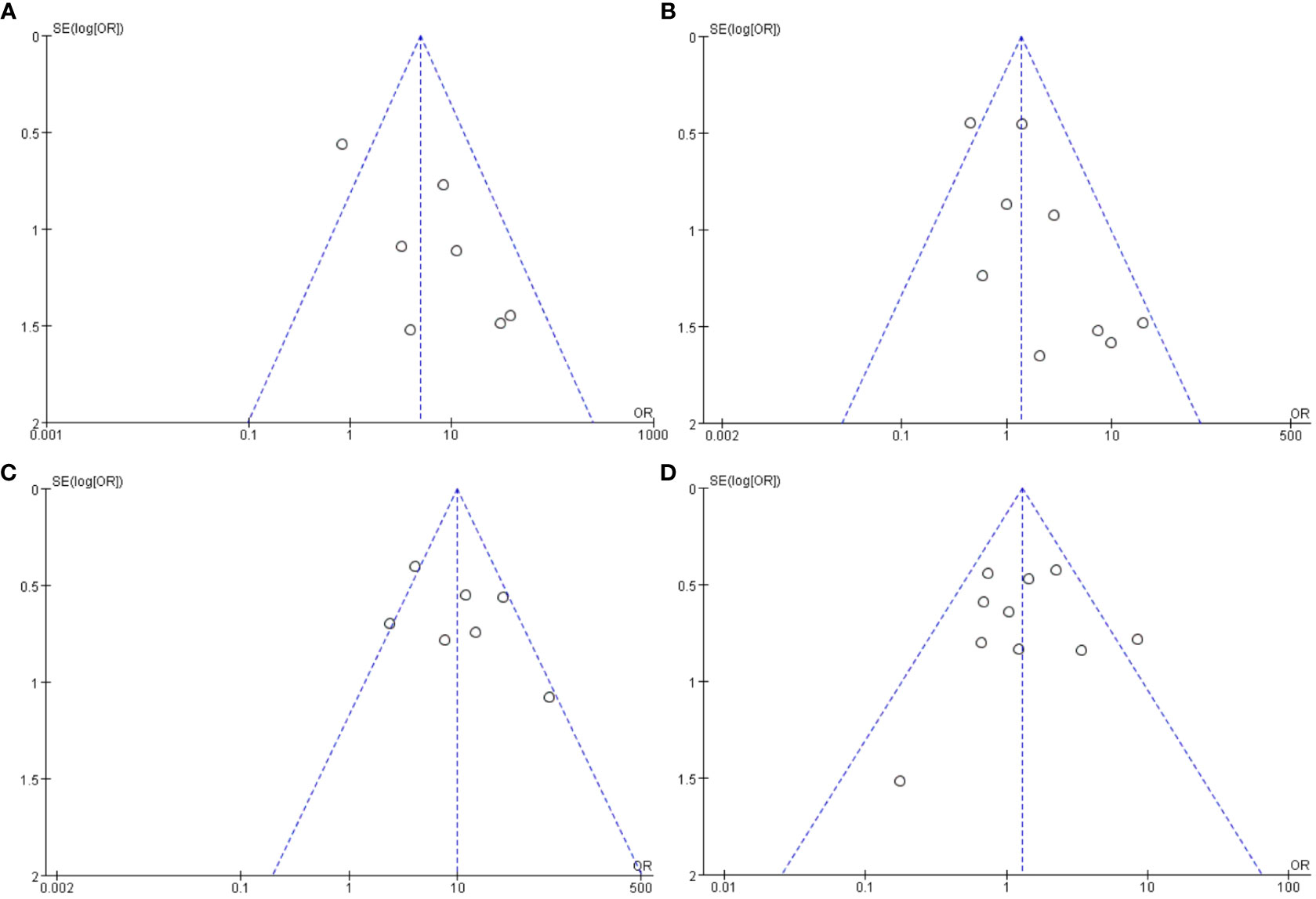
Figure 3 Funnel plots in the meta-analysis of the associations between EBV antibodies and SjS. (A) Funnel plot in the meta-analysis on IgM-anti-VCA antibody. (B) Funnel plot in the meta-analysis on IgG-anti-VCA antibody. (C) Funnel plot in the meta-analysis on IgG-anti-EA antibody. (D) Funnel plot in the meta-analysis on IgG-anti-EBNA1 antibody.
Discussion
Though EBV infection has been hypothesized to be a possible risk factor of SjS in a long term, prior studies have revealed conflicting results and the serological evidence is not yet definitely established (Table 3). For instance, half of those published studies on the association between IgM-anti-VCA antibody and SjS did not detect an obvious correlation, which may result from the low statistical power caused by the limited sample size. In the present study, we assessed the serological association between antibodies to EBV and SjS through both an original case-control study and a meta-analysis. The meta-analysis included a total of 14 studies, and could provide a more robust evaluation of the associations between antibodies to EBV and SjS by increasing sample size and statistical power. An obvious association of SjS with IgG-anti-EA antibody was observed in both our case-control study and the meta-analysis, and an obvious association of SjS with IgM-anti-VCA antibody was confirmed in the meta-analysis. However, IgG-anti-VCA antibody and IgG-anti-EBNA1 antibody were not associated with SjS in either the case-control study or the meta-analysis. Therefore, the above findings provide serological evidence for the association between EBV infection and SjS, and also scrutinize the different associations of SjS with different antibodies to EBV.
Multiple factors such as genetic factors, immunological abnormality and environmental risk factors are involved in the development of rheumatic diseases, and EBV infection has been proposed as an important environmental risk factor (39, 40). A meta-analysis by Hanlon et al. found that patients with SLE had significantly higher rates of IgG-anti-VCA antibody, IgA-anti-VCA antibody and IgG-anti-EA antibody than controls, but not IgG-anti-EBNA1 antibody (41). A recent study by Jog et al. revealed that EBV serological reactivation was associated with the probability of transitioning to SLE in at-risk individuals (42). A meta-analysis by Kudaeva et al. reported that the patients with RA had obviously higher rates of IgG-anti-VCA and IgG-anti-EA antibodies than controls, but not for IgG-anti-EBNA1 antibody (43). Therefore, there is compelling serological evidence for EBV infection as an important risk factor of SLE and RA. Our study revealed the obvious associations of SjS with IgM-anti-VCA antibody and IgG-anti-EA antibody via a systematic review and meta-analysis of 14 available studies, which added the evidence of EBV infection as an important environmental risk factor for SjS development.
There are some possible explanations for the discrepancy in the associations of SjS with different EBV antibodies. Molecular mimicry has been suggested to be a key mechanism underlying the role of EBV infection in triggering autoimmune diseases (44, 45). The sequence similarities or homologous peptides between human proteins and EBV viral proteins are different across those EBV viral antigens, and immune responses to different EBV viral antigens are distinct (46, 47). Different EBV viral antigens may have distinct cross reactivity with different autoantigens, and thus trigger the immune system to produce specific antibodies with distinct pathogenicity in SjS etiology, which may lead to the discrepancy in the associations of SjS with different EBV antibodies (48, 49). In addition, the non-significant associations of SjS with other EBV antibodies may result from the low statistical power caused by the relatively small sample size. For instance, though our study and one prior study indicated that the titer of IgG-anti-VCA antibody was significantly increased in patients with SjS compared with healthy controls (50), IgG-anti-VCA antibody positivity was not associated with SjS in both the original case-control study and the meta-analysis. Both patients with SjS and healthy controls have been found to have a high prevalence of IgG-anti-VCA antibody positivity (over 90%), and the sample size required to detect a significant association between IgG-anti-VCA antibody positivity and SjS in an epidemiological study is undoubtedly increased. To provide a more accurate estimate of the associations of SjS with EBV antibodies, further epidemiological studies on a large-scale are recommended.
An intriguing finding in our study is the inverse association between the titer of IgG-anti-EA antibody and the levels of C3 (r = -0.31, P < 0.001) and C4 (r = -0.20, P = 0.02) (Table 2). Low C3 or C4 levels can reflect disease severity of the patients with SjS, and are related to disease progression and poor outcomes (51–53). The lower levels of C3 and C4 in IgG-anti-EA antibody seropositive patients with SjS suggest that IgG-anti-EA antibody may have the possibility of promoting the progression of SjS by exhausting C3 and C4. Wilson et al. found that the serum cryoprecipitates from SjS patients contained autoantibodies to La and rheumatoid factor (RF) as well as complement proteins C3 and C4, which could activate classical complement pathway or alternative complement pathway (54). A key possible mechanism for the decreased C3 and C4 is the activation of complement system during EBV infection. In absence of antibodies, complement system may be activated with viral surface molecules and polysaccharides via alternative and mannose-lectin pathways during innate immunity (55, 56). In the presence of antibodies, antigen-antibody complexes formed by EBV antigens bound by antibodies can further activate complement system (57–59). Both C3 and C4 are the key components of the complement system, and can be exhausted by EBV-induced activation of the complement system. Furthermore, higher levels of IgG-anti-VCA antibody and IgG-anti-EA antibody have been proposed as indicators of chronic or frequent EBV reactivation, and EBV reactivation is intensively involved in the progression of EBV-related diseases (60–63). Frequent EBV reactivation can promote the production of EBV-related antibodies, which can further exhaust circulating complement proteins and result in decreased C3 and C4.
EBV can infect a variety of cell types such as B cells, epithelial cells, T cells and dendritic cells (64, 65). Published studies have revealed that high EBV viral loads and EBV-related antibodies exist in salivary glandular epithelial cells (SGECs) and saliva of SjS patients, and EBV antigens such as the lytic cycle antigen EA/D also exist in the SGECs of SjS patients (19, 66–68). Additionally, MHC-II molecules and viral early antigen have been found to be inappropriately expressed in SGECs of SjS patients, which may lead to vicious immune responses and promote the chronic inflammation in SGECs during SjS development (69). These studies imply that EBV can infect SGECs and trigger immune damages to salivary epithelium, which may promote the autoimmune processes in SjS pathogenesis.
EBV infection may be involved in SjS pathogenesis by promoting the activation of autoreactive B and T cells. EBV can infect B cells via envelope gp350/220 binding to the complement receptor type 2 (CR2) on B cells and via gp42 interacting with HLA class II molecules on B cells (64, 70, 71). The binding of the complex of C3d and EBV or antigen-antibody complexes to B cells brings CD19 into proximity of BCR-associated kinases, and the cytoplasmic tail of CD19 rapidly becomes tyrosine-phosphorylated, which can further promote the proliferation and activation of B cells (72–75). Some EBV proteins such as LMP2A and LMP1 have proven to prevent infected B cells from apoptosis and thus promote the progression of autoimmunity (76–79). Some studies have suggested that EBV can alter the differentiation and interrupt the normal function of T cells through different mechanisms, which may contribute to autoimmunity development (80–83). Therefore, EBV infection can cause the abnormal activation of B cells and/or T cells, which can further result in loss of immune tolerance and the development of autoimmunity (84–87). In SjS, active EBV infection has been found to be related to ectopic lymphoid structures (ELS) in the glandular tissues of SjS patients, suggesting that it may drive local autoimmune response and the activation of autoreactive B cells during the progression of SjS (88, 89).
There are other explanations for the roles of EBV infection in the pathogenesis of SjS (11). One explanation is molecular mimicry between EBV proteins and self antigens, which may promote the development of SjS through inducing the formation of cross-reactive autoantibodies to both pathogens and self-antigens (90–93). For instance, a study by Navone et al. found that antibodies to EBV could recognize autoantigens such as alpha-fodrin and tear lipocalin (94). Moreover, EBV can directly regulate innate immune cells such as dendritic cells (95, 96). An activated interferon-α (IFN-α) signature pathway is involved in the autoimmune process of SjS, and EBV DNA and RNA have been reported to activate plasmacytoid dendritic cells (pDC) through engagement of Toll-like receptor 9 (TLR-9) and TLR-7, and then increase IFN-α production (95, 96). To date, there is no confirming evidence to verify the pathogenic role of EBV in SjS pathogenesis, and the link remains to be further elaborated.
Several limitations exist in the present study. A major limitation is the retrospective case-control design in those included studies, which was unable to adequately evaluate the causality between antibodies to EBV and SjS. Further studies using a prospective design are recommended. Besides, the sample size of some included studies was limited, and owing to the limited sample size of recruited participants, the influence of other confounding factors such as treatment drugs on the associations of anti-EBV antibodies with SjS was not excluded. Finally, the cut-off values determining seropositivity of anti-EBV antibodies were different among the included studies, which may result in obvious heterogeneity in the meta-analysis and impair the strength of the findings.
In summary, the findings from this study provide strong serological evidence for the association between EBV infection and SjS. Moreover, anti-EA-IgG antibody is likely linked to low levels of C3 and C4 in patients with SjS. More efforts in addressing the roles of EBV infection in SjS and the underlying molecular mechanisms are needed.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Medical Ethics Committee at The First Affiliated Hospital of Xiamen University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
GS and JX designed the study and wrote the manuscript. ZJ and BW analyzed data and wrote the manuscript. XZ, RC, PR, YH, and PW collected samples and data. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by grants from the National Natural Science Foundation of China (Grant No. 81971536 and No. U1605223) to GS, the First Affiliated Hospital of Xiamen University Projects for Young Scholar, China Funding (No. XYY2016013) to ZJ, and the National Natural Science Foundation of China (No.81701556) to YH.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
All authors would like to thank the researchers for their data used in meta-analysis in this work.
Abbreviations
anti-EA, anti-early antigen; anti-EBNA1, anti-EBV nuclear antigen 1; anti-VCA, anti-viral capsid antigen; CR2, complement receptor type 2; CNKI, China National Knowledge Infrastructure; EBV, Epstein-Barr virus; ELISA, enzyme-linked immunosorbent assay; ELS, ectopic lymphoid structures; ESSDAI, European League Against Rheumatism Sjögren’s syndrome disease activity index; IF, immunofluorescence; IFN-α, interferon-α; IQR, interquartile range; MS, multiple sclerosis; OR, Odds ratios; pDC, plasmacytoid dendritic cells; RA, rheumatoid arthritis; RF, rheumatoid factor; SD, standard deviation; SGECs, salivary glandular epithelial cells; SjS, Sjögren’s syndrome; SLE, systemic lupus erythematosus; TLR-9, Toll-like receptor 9; 95%CI, 95% confidence interval.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2020.590444/full#supplementary-material
References
1. Mariette X, Criswell LA. Primary Sjogren’s Syndrome. N Engl J Med (2018) 378(10):931–9. doi: 10.1056/NEJMcp1702514
2. Martin-Nares E, Hernandez-Molina G. Novel autoantibodies in Sjogren’s syndrome: A comprehensive review. Autoimmun Rev (2019) 18(2):192–8. doi: 10.1016/j.autrev.2018.09.003
3. Mavragani CP, Moutsopoulos HM. Sjogren’s syndrome. Annu Rev Pathol (2014) 9:273–85. doi: 10.1146/annurev-pathol-012513-104728
4. Psianou K, Panagoulias I, Papanastasiou AD, de Lastic AL, Rodi M, Spantidea PI, et al. Clinical and immunological parameters of Sjogren’s syndrome. Autoimmun Rev (2018) 17(10):1053–64. doi: 10.1016/j.autrev.2018.05.005
5. Fessler J, Fasching P, Raicht A, Hammerl S, Weber J, Lackner A, et al. Lymphopenia in primary Sjogren’s syndrome is associated with premature aging of naive CD4+ T cells. Rheumatology (2020). doi: 10.1093/rheumatology/keaa105
6. Bjork A, Mofors J, Wahren-Herlenius M. Environmental factors in the pathogenesis of primary Sjogren’s syndrome. J Internal Med (2020) 287(5):475–92. doi: 10.1111/joim.13032
7. Lucchesi D, Coleby R, Pontarini E, Prediletto E, Rivellese F, Hill DG, et al. Impaired IL-27 Mediated Control of CD4+ T Cell Function Impacts on Ectopic Lymphoid Structure Formation in Patients with Sjogren’s Syndrome. Arthritis Rheumatol (2020). doi: 10.1002/art.41289
8. Felten R, Scher F, Sibilia J, Gottenberg JE, Arnaud L. The pipeline of targeted therapies under clinical development for primary Sjogren’s syndrome: A systematic review of trials. Autoimmun Rev (2019) 18(6):576–82. doi: 10.1016/j.autrev.2018.12.008
9. Ramos-Casals M, Brito-Zeron P, Bombardieri S, Bootsma H, De Vita S, Dorner T, et al. EULAR recommendations for the management of Sjogren’s syndrome with topical and systemic therapies. Ann Rheum Dis (2020) 79(1):3–18. doi: 10.1136/annrheumdis-2019-216114
10. Bjork A, Thorlacius GE, Mofors J, Richardsdotter Andersson E, Ivanchenko M, Tingstrom J, et al. Viral antigens elicit augmented immune responses in primary Sjogren’s syndrome. Rheumatology (2020) 59(7):1651–61. doi: 10.1093/rheumatology/kez509
11. Maslinska M. The role of Epstein-Barr virus infection in primary Sjogren’s syndrome. Curr Opin Rheumatol (2019) 31(5):475–83. doi: 10.1097/BOR.0000000000000622
12. Smatti MK, Al-Sadeq DW, Ali NH, Pintus G, Abou-Saleh H, Nasrallah GK. Epstein-Barr Virus Epidemiology, Serology, and Genetic Variability of LMP-1 Oncogene Among Healthy Population: An Update. Front Oncol (2018) 8:211. doi: 10.3389/fonc.2018.00211
13. Belbasis L, Bellou V, Evangelou E, Ioannidis JP, Tzoulaki I. Environmental risk factors and multiple sclerosis: an umbrella review of systematic reviews and meta-analyses. Lancet Neurol (2015) 14(3):263–73. doi: 10.1016/S1474-4422(14)70267-4
14. Draborg AH, Jorgensen JM, Muller H, Nielsen CT, Jacobsen S, Iversen LV, et al. Epstein-Barr virus early antigen diffuse (EBV-EA/D)-directed immunoglobulin A antibodies in systemic lupus erythematosus patients. Scand J Rheumatol (2012) 41(4):280–9. doi: 10.3109/03009742.2012.665944
15. Toussirot E, Roudier J. Epstein-Barr virus in autoimmune diseases. Best Pract Res Clin Rheumatol (2008) 22(5):883–96. doi: 10.1016/j.berh.2008.09.007
16. Almohmeed YH, Avenell A, Aucott L, Vickers MA. Systematic review and meta-analysis of the sero-epidemiological association between Epstein Barr virus and multiple sclerosis. PloS One (2013) 8(4):e61110. doi: 10.1371/journal.pone.0061110
17. Harley JB, Zoller EE. Editorial: What caused all these troubles, anyway? Epstein-Barr virus in Sjogren’s syndrome reevaluated. Arthritis Rheumatol (2014) 66(9):2328–30. doi: 10.1002/art.38725
18. Miyasaka N, Saito I, Haruta J. Possible involvement of Epstein-Barr virus in the pathogenesis of Sjogren’s syndrome. Clin Immunol Immunopathol (1994) 72(2):166–70. doi: 10.1006/clin.1994.1124
19. Karameris A, Gorgoulis V, Iliopoulos A, Frangia C, Kontomerkos T, Ioakeimidis D, et al. Detection of the Epstein Barr viral genome by an in situ hybridization method in salivary gland biopsies from patients with secondary Sjogren’s syndrome. Clin Exp Rheumatol (1992) 10(4):327–32.
20. Tateishi M, Saito I, Yamamoto K, Miyasaka N. Spontaneous production of Epstein-Barr virus by B lymphoblastoid cell lines obtained from patients with Sjogren’s syndrome. Possible involvement of a novel strain of Epstein-Barr virus in disease pathogenesis. Arthritis Rheum (1993) 36(6):827–35. doi: 10.1002/art.1780360614
21. Mariette X, Gozlan J, Clerc D, Bisson M, Morinet F. Detection of Epstein-Barr virus DNA by in situ hybridization and polymerase chain reaction in salivary gland biopsy specimens from patients with Sjogren’s syndrome. Am J Med (1991) 90(3):286–94. doi: 10.1016/0002-9343(91)80007-9
22. Venables PJ, Ross MG, Charles PJ, Melsom RD, Griffiths PD, Maini RN. A seroepidemiological study of cytomegalovirus and Epstein-Barr virus in rheumatoid arthritis and sicca syndrome. Ann Rheum Dis (1985) 44(11):742–6. doi: 10.1136/ard.44.11.742
23. Inoue N, Harada S, Miyasaka N, Oya A, Yanagi K. Analysis of antibody titers to Epstein-Barr virus nuclear antigens in sera of patients with Sjogren’s syndrome and with rheumatoid arthritis. J Infect Dis (1991) 164(1):22–8. doi: 10.1093/infdis/164.1.22
24. Marchini B, Dolcher MP, Sabbatini A, Klein G, Migliorini P. Immune response to different sequences of the EBNA I molecule in Epstein-Barr virus-related disorders and in autoimmune diseases. J Autoimmun (1994) 7(2):179–91. doi: 10.1006/jaut.1994.1014
25. Venables PJ, Baboonian C, Horsfall AC, Halliday D, Maini RN, Teo CG, et al. The response to Epstein-Barr virus infection in Sjogren’s syndrome. J Autoimmun (1989) 2(4):439–48. doi: 10.1016/0896-8411(89)90173-x
26. Toda I, Ono M, Fujishima H, Tsubota K. Sjogren’s syndrome (SS) and Epstein-Barr virus (EBV) reactivation. Ocul Immunol Inflammation (1994) 2(2):101–9. doi: 10.3109/09273949409057066
27. Pasoto SG, Natalino RR, Chakkour HP, Viana Vdos S, Bueno C, Leon EP, et al. EBV reactivation serological profile in primary Sjogren’s syndrome: an underlying trigger of active articular involvement? Rheumatol Int (2013) 33(5):1149–57. doi: 10.1007/s00296-012-2504-3
28. Kivity S, Arango MT, Ehrenfeld M, Tehori O, Shoenfeld Y, Anaya JM, et al. Infection and autoimmunity in Sjogren’s syndrome: a clinical study and comprehensive review. J Autoimmun (2014) 51:17–22. doi: 10.1016/j.jaut.2014.02.008
29. Shiboski CH, Shiboski SC, Seror R, Criswell LA, Labetoulle M, Lietman TM, et al. American College of Rheumatology/European League Against Rheumatism classification criteria for primary Sjogren’s syndrome: A consensus and data-driven methodology involving three international patient cohorts. Ann Rheum Dis (2017) 76(1):9–16. doi: 10.1136/annrheumdis-2016-210571
30. Seror R, Ravaud P, Bowman SJ, Baron G, Tzioufas A, Theander E, et al. EULAR Sjogren’s syndrome disease activity index: development of a consensus systemic disease activity index for primary Sjogren’s syndrome. Ann Rheum Dis (2010) 69(6):1103–9. doi: 10.1136/ard.2009.110619
31. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (2003) 327(7414):557–60. doi: 10.1136/bmj.327.7414.557
32. DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clin Trials (1986) 7(3):177–88. doi: 10.1016/0197-2456(86)90046-2
33. Yamaoka K, Miyasaka N, Yamamoto K. Possible involvement of Epstein-Barr virus in polyclonal B cell activation in Sjogren’s syndrome. Arthritis Rheum (1988) 31(8):1014–21. doi: 10.1002/art.1780310812
34. Yang J, Zhang N, Zeng Y, Dong Y, Li H, Han R, et al. [Possible etiological relations between Sjogren’s syndrome and Epstein-Barr virus]. Zhonghua Yi Xue Za Zhi (1991) 71(3):131–5. 10.
35. Gao C, Xin M, Liu X, Chao Y, Luo B. Detection and Significance of Epstein Barr Virus Markers in Peripheral Blood of Patients with Primary Sjogren’s Syndrome. Prog Modern Biomed (2010) 10(20):3900–2.
36. Li H, Wang J, Wang L. Analysis of the situation of EB virus infection in patients with Sjögren’s syndrome. Chin J Nosocomiol (2014) 24(24):6154–5.
37. Yang L, Fu P. Expression of EBNA1 and EBNA2 in the labial salivary gland of patients with sicca syndrome. J Clin Dermatol (2015) 44(2):73–7.
38. Zhang L, Wang R, Zhou Y, Zhang G. Correlation between Epstein-Barr virus and Sjögren’s syndrome. Chin Remedies Clinics (2019) 19(4):556–9.
39. Bo M, Erre GL, Niegowska M, Piras M, Taras L, Longu MG, et al. Interferon regulatory factor 5 is a potential target of autoimmune response triggered by Epstein-barr virus and Mycobacterium avium subsp. paratuberculosis in rheumatoid arthritis: investigating a mechanism of molecular mimicry. Clin Exp Rheumatol (2018) 36(3):376–81.
40. Berti A, Felicetti M, Peccatori S, Bortolotti R, Guella A, Vivaldi P, et al. EBV-induced lymphoproliferative disorders in rheumatic patients: A systematic review of the literature. Joint Bone Spine (2018) 85(1):35–40. doi: 10.1016/j.jbspin.2017.01.006
41. Hanlon P, Avenell A, Aucott L, Vickers MA. Systematic review and meta-analysis of the sero-epidemiological association between Epstein-Barr virus and systemic lupus erythematosus. Arthritis Res Ther (2014) 16(1):R3. doi: 10.1186/ar4429
42. Jog NR, Young KA, Munroe ME, Harmon MT, Guthridge JM, Kelly JA, et al. Association of Epstein-Barr virus serological reactivation with transitioning to systemic lupus erythematosus in at-risk individuals. Ann Rheum Dis (2019) 78(9):1235–41. doi: 10.1136/annrheumdis-2019-215361
43. Kudaeva FM, Speechley MR, Pope JE. A systematic review of viral exposures as a risk for rheumatoid arthritis. Semin Arthritis Rheum (2019) 48(4):587–96. doi: 10.1016/j.semarthrit.2018.03.011
44. Tengvall K, Huang J, Hellström C, Kammer P, Biström M, Ayoglu B, et al. Molecular mimicry between Anoctamin 2 and Epstein-Barr virus nuclear antigen 1 associates with multiple sclerosis risk. Proc Natl Acad Sci U S A (2019) 116(34):16955–60. doi: 10.1073/pnas.1902623116
45. Csorba K, Schirmbeck LA, Tuncer E, Ribi C, Roux-Lombard P, Chizzolini C, et al. Anti-C1q Antibodies as Occurring in Systemic Lupus Erythematosus Could Be Induced by an Epstein-Barr Virus-Derived Antigenic Site. Front Immunol (2019) 10:2619. doi: 10.3389/fimmu.2019.02619
46. Capone G, Calabro M, Lucchese G, Fasano C, Girardi B, Polimeno L, et al. Peptide matching between Epstein-Barr virus and human proteins. Pathog Dis (2013) 69(3):205–12. doi: 10.1111/2049-632X.12066
47. Bo M, Niegowska M, Eames HL, Almuttaqi H, Arru G, Erre GL, et al. Antibody response to homologous epitopes of Epstein-Barr virus, Mycobacterium avium subsp. paratuberculosis and IRF5 in patients with different connective tissue diseases and in mouse model of antigen-induced arthritis. J Transl Autoimmun (2020) 3:100048. doi: 10.1016/j.jtauto.2020.100048
48. Agmon-Levin N, Dagan A, Peri Y, Anaya JM, Selmi C, Tincani A, et al. The interaction between anti-Ro/SSA and anti-La/SSB autoantibodies and anti-infectious antibodies in a wide spectrum of auto-immune diseases: another angle of the autoimmune mosaic. Clin Exp Rheumatol (2017) 35(6):929–35.
49. Sanosyan A, Daien C, Nutz A, Bollore K, Bedin AS, Morel J, et al. Discrepancy of Serological and Molecular Patterns of Circulating Epstein-Barr Virus Reactivation in Primary Sjogren’s Syndrome. Front Immunol (2019) 10:1153. doi: 10.3389/fimmu.2019.01153
50. Suzuki M, Nagata S, Hiramatsu K, Takagi I, Ito H, Kitao S, et al. Elevated levels of soluble Fc epsilon RII/CD23 and antibodies to Epstein-Barr virus in patients with Sjogren’s syndrome. Acta Otolaryngol Suppl (1996) 525:108–12.
51. Flores-Chavez A, Kostov B, Solans R, Fraile G, Maure B, Feijoo-Masso C, et al. Severe, life-threatening phenotype of primary Sjogren’s syndrome: clinical characterisation and outcomes in 1580 patients (GEAS-SS Registry). Clin Exp Rheumatol (2018) 36 Suppl 112(3):121–9.
52. Nocturne G, Virone A, Ng WF, Le Guern V, Hachulla E, Cornec D, et al. Rheumatoid Factor and Disease Activity Are Independent Predictors of Lymphoma in Primary Sjogren’s Syndrome. Arthritis Rheumatol (2016) 68(4):977–85. doi: 10.1002/art.39518
53. Brito-Zeron P, Kostov B, Fraile G, Caravia-Duran D, Maure B, Rascon FJ, et al. Characterization and risk estimate of cancer in patients with primary Sjogren syndrome. J Hematol Oncol (2017) 10(1):90. doi: 10.1186/s13045-017-0464-5
54. Wilson M, Arroyave C, Miles L, Tan E. Immune reactants in cryoproteins. Relationship to complement activation. Ann Rheum Dis (1977) 36(6):540–8. doi: 10.1136/ard.36.6.540
55. Christensen T, Petersen T, Thiel S, Brudek T, Ellermann-Eriksen S, Møller-Larsen A. Gene-environment interactions in multiple sclerosis: innate and adaptive immune responses to human endogenous retrovirus and herpesvirus antigens and the lectin complement activation pathway. J Neuroimmunol (2007) 183(1-2):175–88. doi: 10.1016/j.jneuroim.2006.09.014
56. Mold C, Bradt BM, Nemerow GR, Cooper NR. Epstein-Barr virus regulates activation and processing of the third component of complement. J Exp Med (1988) 168(3):949–69. doi: 10.1084/jem.168.3.949
57. Weis JH, Morton CC, Bruns GA, Weis JJ, Klickstein LB, Wong WW, et al. A complement receptor locus: genes encoding C3b/C4b receptor and C3d/Epstein-Barr virus receptor map to 1q32. J Immunol (1987) 138(1):312–5.
58. Wands JR, Perrotto JL, Isselbacher KJ. Circulating immune complexes and complement sequence activation in infectious mononucleosis. Am J Med (1976) 60(2):269–72. doi: 10.1016/0002-9343(76)90436-8
59. Vivanco F, Muñoz E, Vidarte L, Pastor C. The covalent interaction of C3 with IgG immune complexes. Mol Immunol (1999) 36(13-14):843–52. doi: 10.1016/s0161-5890(99)00105-4
60. Gulley ML. Molecular diagnosis of Epstein-Barr virus-related diseases. J Mol Diagn (2001) 3(1):1–10. doi: 10.1016/S1525-1578(10)60642-3
61. Cardenas-Mondragon MG, Torres J, Sanchez-Zauco N, Gomez-Delgado A, Camorlinga-Ponce M, Maldonado-Bernal C, et al. Elevated Levels of Interferon-gamma Are Associated with High Levels of Epstein-Barr Virus Reactivation in Patients with the Intestinal Type of Gastric Cancer. J Immunol Res (2017) 2017:7069242. doi: 10.1155/2017/7069242
62. Christian LM, Iams JD, Porter K, Glaser R. Epstein-Barr virus reactivation during pregnancy and postpartum: effects of race and racial discrimination. Brain Behav Immun (2012) 26(8):1280–7. doi: 10.1016/j.bbi.2012.08.006
63. Masuda S, Mori M, Arai K, Uzawa A, Muto M, Uchida T, et al. Epstein-Barr virus persistence and reactivation in neuromyelitis optica. J Neurol Neurosurg Psychiatry (2015) 86(10):1137–42. doi: 10.1136/jnnp-2014-308095
64. Hutt-Fletcher L. Epstein-Barr virus entryl. J Virol (2007) 81(15):7825–32. doi: 10.1128/JVI.00445-07
65. Crawford D. Biology and disease associations of Epstein-Barr virus. Philos Trans R Soc Lond B Biol Sci (2001) 356(1408):461–73. doi: 10.1098/rstb.2000.0783
66. Wen S, Shimizu N, Yoshiyama H, Mizugaki Y, Shinozaki F. Takada K.Association of Epstein-Barr virus (EBV) with Sjögren’s syndrome: differential EBV expression between epithelial cells and lymphocytes in salivary glands. Am J Pathol (1996) 149(5):1511–7.
67. Tonoyan L, Vincent-Bugnas S, Olivieri CV, Doglio A. New Viral Facets in Oral Diseases: The EBV Paradox. Int J Mol Sci (2019) 20(23):5861. doi: 10.3390/ijms20235861
68. Fox RI, Pearson G, Vaughan JH. Detection of Epstein-Barr virus-associated antigens and DNA in salivary gland biopsies from patients with Sjogren’s syndrome. J Immunol (1986) 137(10):3162–8.
69. Venables PJ, Teo CG, Baboonian C, Griffin BE, Hughes RA. Persistence of Epstein-Barr virus in salivary gland biopsies from healthy individuals and patients with Sjögren’s syndrome. Clin Exp Immunol (1989) 75(3):359–64.
70. Fingeroth JD, Weis JJ, Tedder TF, Strominger JL, Biro PA, Fearon DT. Epstein-Barr virus receptor of human B lymphocytes is the C3d receptor CR2. Proc Natl Acad Sci U S A (1984) 81(14):4510–4. doi: 10.1073/pnas.81.14.4510
71. Li Q, Turk SM, Hutt-Fletcher LM. The Epstein-Barr virus (EBV) BZLF2 gene product associates with the gH and gL homologs of EBV and carries an epitope critical to infection of B cells but not of epithelial cells. J Virol (1995) 69(7):3987–94. doi: 10.1128/JVI.69.7.3987-3994.1995
72. Kudo T, Tachibana T. Phenotype characteristics of human B cells studied by Epstein-Barr virus infection. II. C3 receptor switching during human B cell differentiation. Immunol Lett (1984) 8(6):343–8. doi: 10.1016/0165-2478(84)90022-1
73. Hutt-Fletcher LM. Synergistic activation of cells by Epstein-Barr virus and B-cell growth factor. J Virol (1987) 61(3):774–81. doi: 10.1128/JVI.61.3.774-781.1987
74. Masucci MG, Szigeti R, Ernberg I, Hu CP, Torsteinsdottir S, Frade R, et al. Activation of B lymphocytes by Epstein-Barr virus/CR2 receptor interaction. Eur J Immunol (1987) 17(6):815–20. doi: 10.1002/eji.1830170613
75. van Noesel CJ, Lankester AC, van Schijndel GM, van Lier RA. The CR2/CD19 complex on human B cells contains the src-family kinase Lyn. Int Immunol (1993) 5(7):699–705. doi: 10.1093/intimm/5.7.699
76. Portis T, Longnecker R. Epstein-Barr virus (EBV) LMP2A mediates B-lymphocyte survival through constitutive activation of the Ras/PI3K/Akt pathway. Oncogene (2004) 23(53):8619–28. doi: 10.1038/sj.onc.1207905
77. Price AM, Dai J, Bazot Q, Patel L, Nikitin PA, Djavadian R, et al. Epstein-Barr virus ensures B cell survival by uniquely modulating apoptosis at early and late times after infection. Elife (2017) 6:e22509. doi: 10.7554/eLife.22509
78. Pender MP. Infection of autoreactive B lymphocytes with EBV, causing chronic autoimmune diseases. Trends Immunol (2003) 11):584–8. doi: 10.1016/j.it.2003.09.005
79. Croia C, Serafini B, Bombardieri M, Kelly S, Humby F, Severa M, et al. Epstein-Barr virus persistence and infection of autoreactive plasma cells in synovial lymphoid structures in rheumatoid arthritis. Ann Rheum Dis (2013) 72(9):1559–68. doi: 10.1136/annrheumdis-2012-202352
80. Paterson RL, Kelleher C, Amankonah TD, Streib JE, Xu JW, Jones JF, et al. Model of Epstein-Barr virus infection of human thymocytes: expression of viral genome and impact on cellular receptor expression in the T-lymphoblastic cell line, HPB-ALL. Blood (1995) 85(2):456–64. doi: 10.1182/blood.V85.2.456.bloodjournal852456
81. Long Y, Zhao X, Xia C, Liu X, Fan C, Liu C. Infection of Epstein-Barr Virus is Associated with the Decrease of Helios(+)FoxP3(+)Regulatory T Cells in Active Ulcerative Colitis Patients. Immunol Invest (2020). doi: 10.1080/08820139.2020.1723021
82. Salloum N, Hussein HM, Jammaz R, Jiche S, Uthman IW, Abdelnoor AM, et al. Epstein-Barr virus DNA modulates regulatory T-cell programming in addition to enhancing interleukin-17A production via Toll-like receptor 9. PloS One (2018) 13(7):e0200546. doi: 10.1371/journal.pone.0200546
83. Tirotta E, Duncker P, Oak J, Klaus S, Tsukamoto MR, Gov L, et al. Epstein-Barr virus-induced gene 3 negatively regulates neuroinflammation and T cell activation following coronavirus-induced encephalomyelitis. J Neuroimmunol (2013) 254(1-2):110–6. doi: 10.1016/j.jneuroim.2012.10.005
84. Babcock GJ, Hochberg D, Thorley-Lawson AD. The expression pattern of Epstein-Barr virus latent genes in vivo is dependent upon the differentiation stage of the infected B cell. Immunity (2000) 13(4):497–506. doi: 10.1016/s1074-7613(00)00049-2
85. Adler B, Schaadt E, Kempkes B, Zimber-Strobl U, Baier B, Bornkamm GW. Control of Epstein-Barr virus reactivation by activated CD40 and viral latent membrane protein 1. Proc Natl Acad Sci U S A (2002) 99(1):437–42. doi: 10.1073/pnas.221439999
86. Nagata K, Kumata K, Nakayama Y, Satoh Y, Sugihara H, Hara S, et al. Epstein-Barr Virus Lytic Reactivation Activates B Cells Polyclonally and Induces Activation-Induced Cytidine Deaminase Expression: A Mechanism Underlying Autoimmunity and Its Contribution to Graves’ Disease. Viral Immunol (2017) 30(3):240–49. doi: 10.1089/vim.2016.0179
87. Wang H, Nicholas MW, Conway KL, Sen P, Diz R, Tisch RM, et al. EBV latent membrane protein 2A induces autoreactive B cell activation and TLR hypersensitivity. J Immunol (2006) 177(5):2793–802. doi: 10.4049/jimmunol.177.5.2793
88. Croia C, Astorri E, Murray-Brown W, Willis A, Brokstad KA, Sutcliffe N, et al. Implication of Epstein-Barr virus infection in disease-specific autoreactive B cell activation in ectopic lymphoid structures of Sjogren’s syndrome. Arthritis Rheumatol (2014) 66(9):2545–57. doi: 10.1002/art.38726
89. Onuora S. Connective tissue diseases: Epstein-Barr virus in Sjogren’s syndrome salivary glands drives local autoimmunity. Nat Rev Rheumatol (2014) 10(7):384. doi: 10.1038/nrrheum.2014.97
90. Vaughan JH, Nguyen MD, Valbracht JR, Patrick K, Rhodes GH. Epstein-Barr virus-induced autoimmune responses. II. Immunoglobulin G autoantibodies to mimicking and nonmimicking epitopes. Presence in autoimmune disease. J Clin Invest (1995) 95(3):1316–27. doi: 10.1172/JCI117782
91. Vaughan JH, Valbracht JR, Nguyen MD, Handley HH, Smith RS, Patrick K, et al. Epstein-Barr virus-induced autoimmune responses. I. Immunoglobulin M autoantibodies to proteins mimicking and not mimicking Epstein-Barr virus nuclear antigen-1. J Clin Invest (1995) 95(3):1306–15. doi: 10.1172/JCI117781
92. Hung T, Pratt GA, Sundararaman B, Townsend MJ, Chaivorapol C, Bhangale T, et al. The Ro60 autoantigen binds endogenous retroelements and regulates inflammatory gene expression. Science (2015) 350(6259):455–9. doi: 10.1126/science.aac7442
93. Cusick MF, Libbey JE, Fujinami RS. Molecular mimicry as a mechanism of autoimmune disease. Clin Rev Allergy Immunol (2012) 42(1):102–11. doi: 10.1007/s12016-011-8293-8
94. Navone R, Lunardi C, Gerli R, Tinazzi E, Peterlana D, Bason C, et al. Identification of tear lipocalin as a novel autoantigen target in Sjogren’s syndrome. J Autoimmun (2005) 25(3):229–34. doi: 10.1016/j.jaut.2005.09.021
95. Quan TE, Roman RM, Rudenga BJ, Holers VM, Craft JE. Epstein-Barr virus promotes interferon-alpha production by plasmacytoid dendritic cells. Arthritis Rheum (2010) 62(6):1693–701. doi: 10.1002/art.27408
Keywords: Epstein-Barr virus, Sjögren’s syndrome, systematic review, associations, meta-analysis
Citation: Xuan J, Ji Z, Wang B, Zeng X, Chen R, He Y, Rao P, Wu P and Shi G (2020) Serological Evidence for the Association Between Epstein-Barr Virus Infection and Sjögren’s Syndrome. Front. Immunol. 11:590444. doi: 10.3389/fimmu.2020.590444
Received: 01 August 2020; Accepted: 09 October 2020;
Published: 30 October 2020.
Edited by:
Allen Jay Rosenspire, Wayne State University, United StatesReviewed by:
Gunnar Houen, Statens Serum Institut (SSI), DenmarkWilliam Lee, Wadsworth Center, United States
Copyright © 2020 Xuan, Ji, Wang, Zeng, Chen, He, Rao, Wu and Shi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guixiu Shi, Z3NoaUB4bXUuZWR1LmNu
†These authors have contributed equally to this work
 Jingxiu Xuan
Jingxiu Xuan Zhiqian Ji1†
Zhiqian Ji1† Bin Wang
Bin Wang Yan He
Yan He Puqi Wu
Puqi Wu Guixiu Shi
Guixiu Shi