
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 08 October 2020
Sec. Immunological Tolerance and Regulation
Volume 11 - 2020 | https://doi.org/10.3389/fimmu.2020.589380
Severe COVID-19 patients show various immunological abnormalities including T-cell reduction and cytokine release syndrome, which can be fatal and is a major concern of the pandemic. However, it is poorly understood how T-cell dysregulation can contribute to the pathogenesis of severe COVID-19. Here we show single cell-level mechanisms for T-cell dysregulation in severe COVID-19, demonstrating new pathogenetic mechanisms of T-cell activation and differentiation underlying severe COVID-19. By in silico sorting CD4+ T-cells from a single cell RNA-seq dataset, we found that CD4+ T-cells were highly activated and showed unique differentiation pathways in the lung of severe COVID-19 patients. Notably, those T-cells in severe COVID-19 patients highly expressed immunoregulatory receptors and CD25, whilst repressing the expression of FOXP3. Furthermore, we show that CD25+ hyperactivated T-cells differentiate into multiple helper T-cell lineages, showing multifaceted effector T-cells with Th1 and Th2 characteristics. Lastly, we show that CD25-expressing hyperactivated T-cells produce the protease Furin, which facilitates the viral entry of SARS-CoV-2. Collectively, CD4+ T-cells from severe COVID-19 patients are hyperactivated and FOXP3-mediated negative feedback mechanisms are impaired in the lung, which may promote immunopathology. Therefore, our study proposes a new model of T-cell hyperactivation and paralysis that drives immunopathology in severe COVID-19.
By analyzing single cell transcriptomes, we demonstrate that CD4+ T-cells in severe COVID-19 have an impaired FOXP3-mediated negative feedback, generating CD25+ hyperactivated T-cells, which show multifaceted effector T-cell differentiation and uniquely produce the protease Furin.
Negative regulatory mechanisms of T-cell activation control inflammation in cancer, autoimmunity, and infections thus preventing excessive and prolonged inflammation which can induce tissue destruction, or immunopathology. Immune checkpoints such as CTLA-4 and PD-1 are well known examples of T-cell intrinsic regulators. Upon recognizing antigens, T-cells are activated and start to express PD-1 and CTLA-4, which in turn suppresses the two major signaling pathways for T-cells: T-cell receptor (TCR) signaling and CD28 co-stimulation (1). In addition, the transcription factor Foxp3 can be induced in activated T-cells, especially in humans, and plays a key role as an inducible negative regulator during inflammation (2).
COVID-19 is caused by the coronavirus SARS-CoV-2, which is closely related to the severe acute respiratory syndrome coronavirus (SARS-CoV). The major symptoms such as cough and diarrhea in mild to moderate patients can be understood through the type of tissues that can be infected by SARS-CoV-2. SARS-CoV-2 binds to angiotensin-converting enzyme 2 (ACE2) on the surface of human cells through its spike (S) protein. Viral entry is enhanced by the type II transmembrane serine protease TMPRSS2, which cleaves a part of S protein and thereby exposes the fusion domain of the S-protein (3, 4). SARS-CoV-2 establishes infections through epithelial cells in the upper and lower airways, which express ACE2 and TMPRSS2 (5). In addition, the pro-protein convertase Furin activates the S-protein of SARS and SARS-CoV-2 (6, 7). Intriguingly, T-cell specific deletion of Furin results in the impairment of effector T-cells and regulatory T-cells (Tregs) and leads to the development of age-related autoimmunity, which is accompanied by increased serum IFN-γ, IL-4, IL-6, IL-13, and IgE (8). In addition, Furin is preferentially expressed by Th1 cells and is critical for their IFN-γ production (9). As evidenced in a parasite infection model, Furin-deficient CD4+ T cells are skewed towards a Th2 phenotype (10).
It is poorly understood how SARS-CoV-2 induces severe infection in a minority of patients, who develop respiratory distress and multiorgan failure. These severe patients show elevated serum cytokines, respiratory failure, hemophagocytosis, elevated ferritin, D-dimer, and soluble CD25 (IL-2R α chain, sCD25), which are characteristic features of secondary hemophagocytic lymphohistiocytosis (sHLH)-like conditions or cytokine release syndrome (CRS). In fact, severe COVID-19 patients have elevated levels of prototypic CRS cytokines from innate immune cells including IL-6, TNF-α, and IL-10 (11, 12). Recently McGonagle et al. proposed that activated macrophages drive immune reactions that induce diffuse pulmonary intravascular coagulopathy, or immunothrombosis, in severe COVID-19 patients (13). While this may explain the unique pulmonary pathology of severe COVID-19 patients, the underlying molecular mechanisms are poorly understood.
Importantly, CRS in severe COVID-19 patients may be more systemic and involve a wide range of T-cells. Firstly, circulating T-cells are severely reduced in severe SARS-CoV-2 infections for unknown reasons (12, 14). Intriguingly, severe COVID-19 patients show elevated serum IL-2 and soluble CD25 (IL-2 receptor α chain) (11, 12). Since IL-2 is a potent growth factor for CD25-expressing activated T-cells (15), the elevation of both IL-2 and CD25 indicates that a positive feedback loop for T-cell activation is established and overrunning in severe patients. These collectively highlight the roles of T-cells in the pathogenesis of severe SARS-CoV-2 infection, although the pathogenetic mechanisms are largely unknown.
In this study, we analyzed the transcriptomes of CD4+ T-cells from a single cell RNA-seq dataset from a recent study (16) and thereby investigated the gene regulation dynamics during SARS-CoV-2 infection. We show that SARS-CoV-2 induces multiple activation and differentiation processes in CD4+ T-cells in a unique manner. We identify defects in Foxp3-mediated negative regulation, which accelerates T-cell activation and death. In addition, by analyzing multiple transcriptome datasets, we propose the possibility that those abnormally activated T-cells enhance viral entry through the production of Furin in severe COVID-19 patients.
The single-cell-RNA-seq data from COVID-19 patients and healthy individuals was obtained from GSE145926 (16). The microarray data of murine T-cell subpopulation were from the Immunological Genome Project [GSE15907(17)]. The RNA-seq data in GSE73213 (18) was used for time course analysis of naïve and memory CD4+ T-cells.
We used h5 files of the scRNA-seq dataset [GSE145926(16)] which were aligned to the human genome (GRCh38) using Cell Ranger, by importing them into the CRAN package Seurat 3.0 (19).
Single cells with high mitochondrial gene expression (higher than 5%) were excluded from further analyses. In silico sorting of CD4+ T-cells was performed by identifying them as the single cells CD4 and CD3E, while excluding cells positive for the lineage markers ITGAX, ITGAM, PAX5 and CD19, because no other methods, including the Bioconductor package singleR, reliably identified CD4+ T-cells. The TCR-seq data of GSE145926 (16) was used to validate the in silico sorting and also for analyzing gene expression in expanded clones. Expanded TCR clones in Figure 2G are defined as T-cells that have more than one single cell with the same TCR clonotype in the TCR-seq data. Note that the scRNA-seq data and the TCR-seq data are integrated and comparable. Macrophages were similarly identified by the ITGAM expression and lack of PAX5, CD19 and CD3E expressions.
PCA was applied on the scaled data followed by a K-nearest neighbor clustering in the PCA space. UMAP was performed on clustered data using the first PCA axes. Differentially expressed genes were identified by adjusted p-values < 0.05 using the function FindMarkers of Seurat. Th1, Th2, and IL-10 signature were defined as the sum of the normalized gene expression of IFNG, TBX21, IL21A (Th1); GATA3, IL4, IL6 (Th2); and RORC, IL17A, IL17F (Th17), respectively.
The enrichment of biological pathways in the gene lists was tested by the Bioconductor package clusterProfiler, (20) using the Reactome database through the Bioconductor package ReactomePA, and pathways with false discovery rate < 0.01 and q-value < 0.1 were considered significant.
Trajectories were identified using the Bioconductor package slingshot, assuming that the cluster that shows the highest expression of IL7R and CCR7 is the origin. The CRAN package ggplot2 was used to apply a generalized additive model of the CRAN package gam to each gene expression data. Genes that were differentially expressed across pseudotime were obtained by applying the generalized additive model to the dataset using gam, performing ANOVA for nonparametric effects and thereby testing if each gene expression is significantly changed across each pseudotime (p-value < 0.05).
The enrichment of cytokine-expressing single cell T-cells was tested using a chi-square test. The time course data of FURIN expression was analyzed by one-way ANOVA with Tukey’s honest significant difference test.
We recently showed that, using scRNA-seq analysis, melanoma-infiltrating T-cells are activated in situ and differentiate into either FOXP3high activated Tregs or PD-1high T follicular helper (Tfh)-like effector T-cells (21). We hypothesized that those mechanisms for T-cell activation and differentiation in inflammatory sites are altered in COVID-19 patients. To address this issue, we analyzed the scRNA-seq data from bronchoalveolar lavage (BAL) fluids of moderate and severe COVID-19 patients (16).
Firstly, we performed in silico sorting of CD4+ T-cells and analyzed their transcriptomes (Figure 1A). We applied a dimensional reduction to the CD4+ T-cell data using Uniform Manifold Approximation and Projection (UMAP) and Principal Component Analysis (Supplementary Figure 1A). As expected, most T-cells were from either moderate or severe COVID-19 patients. Notably, clusters 1, 2, 3, 5, 6, and 7 did not contain any cells from healthy controls (HC) (Figures 1B, C), indicating that these cells uniquely differentiated during the infection, regardless of whether it was moderate or severe disease.
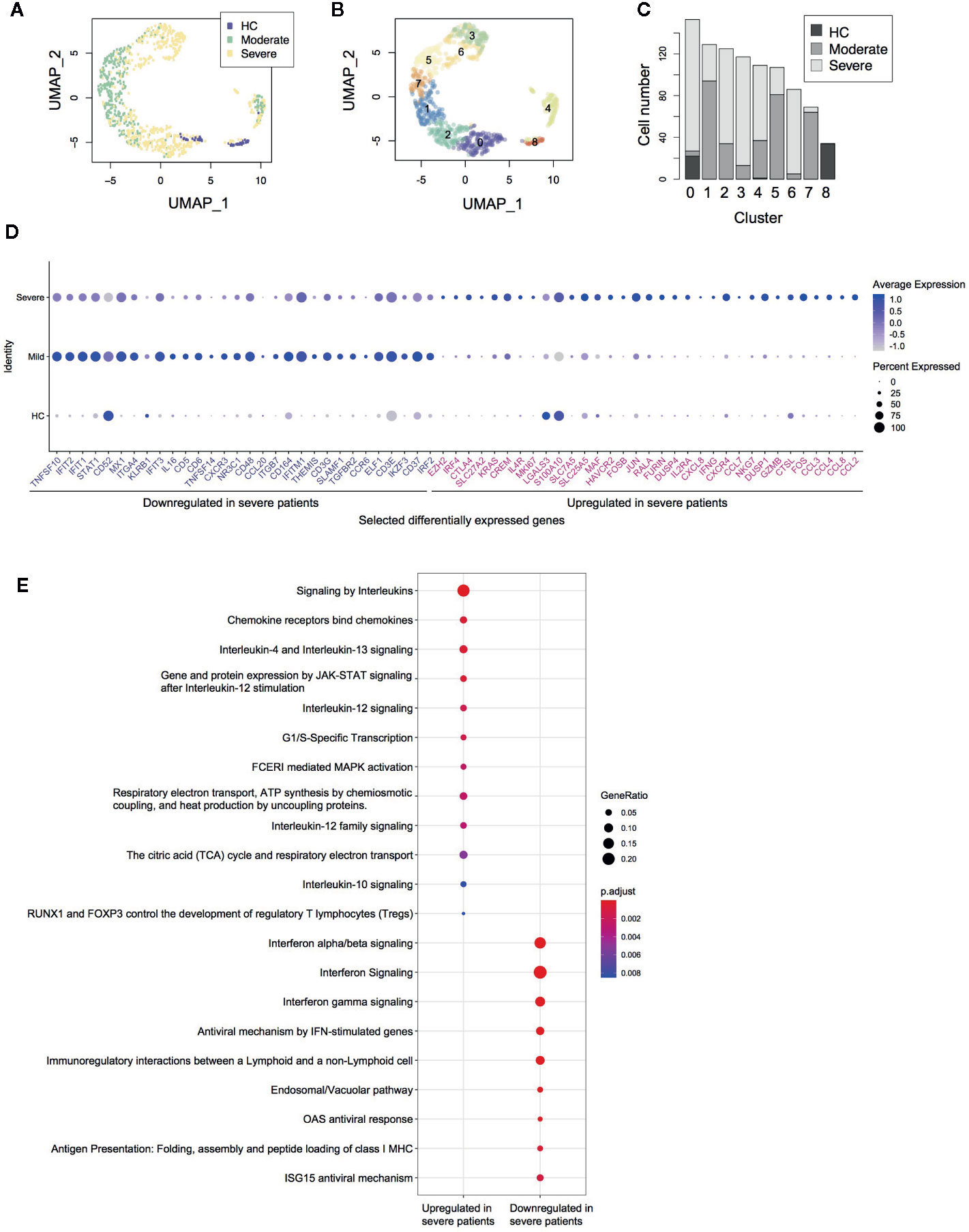
Figure 1 Single cell transcriptomes of CD4+ T-cells from COVID-19 patients. (A) UMAP analysis of in silico sorted CD4+ T-cells from COVID-19 patients. The colour code indicates the groups of patients: healthy control (HC), moderate and severe COVID-19 patients. (B) Clustering of single cells in the UMAP space, showing 9 Clusters (Clusters 0–8). (C) The proportion of single cells from each group in each cluster. (D) Dot plots showing the expression of selected differentially expressed genes between severe and moderate patients. (E) Pathway analysis of the differentially expressed genes.
Differential gene expression analysis showed that in comparison to moderate patients, CD4+ T-cells from severe COVID-19 patients expressed higher levels of the AP-1 genes FOS, FOSB, and JUN, the activation marker MKI67 (Ki67), Th2-related genes IL4R and MAF, and chemokines including CCL2, CCL3, CCL4, CCL7, CCL8, and CXCL8 (Figure 1D, Supplementary Figure 1B). These suggest that CD4+ T-cells in severe COVID-19 patients are highly activated in the lung, recruiting macrophages, T-cells, and other immune cells. Notably, CD4+ T-cells in severe patients expressed higher levels of immunoregulatory genes including immune checkpoints (CTLA4, HAVCR2 [TIM-3], and LGALS3 [Galectin-3]) as well as the Tregs and T-cell activation marker IL2RA (CD25) (Figure 1D, Supplementary Figure 1B). These were further confirmed by pathway analysis, which identified interleukin, JAK-STAT, and MAPK signaling pathways as significantly enriched pathways (Figure 1E).
On the other hand, CD4+ T-cells from severe patients showed decreased expression of interferon-induced genes including IFIT1, IFIT2, IFIT3, and IFITM1 (Figure 1D). Pathway analysis also showed that CD4+ T-cells from severe patients expressed lower levels of the genes related to interferon downstream pathways (Figure 1E), suggesting that type-I interferons are suppressed in severe patients. Notably, CD4+ T-cells in severe patients showed lower expression of the TNF superfamily ligands TNFSF10 (TRAIL) and TNFSF14 (LIGHT) and the surface protein SLAMF1 and KLRB1, all of which have roles in viral infections (22–25).
Next, we performed a pseudotime analysis in the UMAP space, identifying two major trajectories of T-cells, originating in Cluster 0 (Figures 1B, 2A). Pseudotime 1 (t1) involved Clusters 0, 2, 1, 7, 5, 6, and 3, showing a longer trajectory, while pseudotime 2 (t2) involved Clusters 0, 8, and 4. Interestingly, the cells in the origin showed high expression of IL-7 receptor (IL7R), which is a marker of naïve-like T-cells in tissues (21). The expression of IL7R was gradually downregulated across the two pseudotime trajectories (t1 and t2, Figure 2B). Intriguingly, T-cells at the ends of both trajectories included MKI67 (Ki-67)+ T-cells and some cells were NR4A1+ or NR4A3+ (Figure 2C, Supplementary Figure S1C), which indicated activation and cognate antigen signalling (26). These analyses support that the trajectories successfully captured two major pathways for T-cells to be activated during the infection.
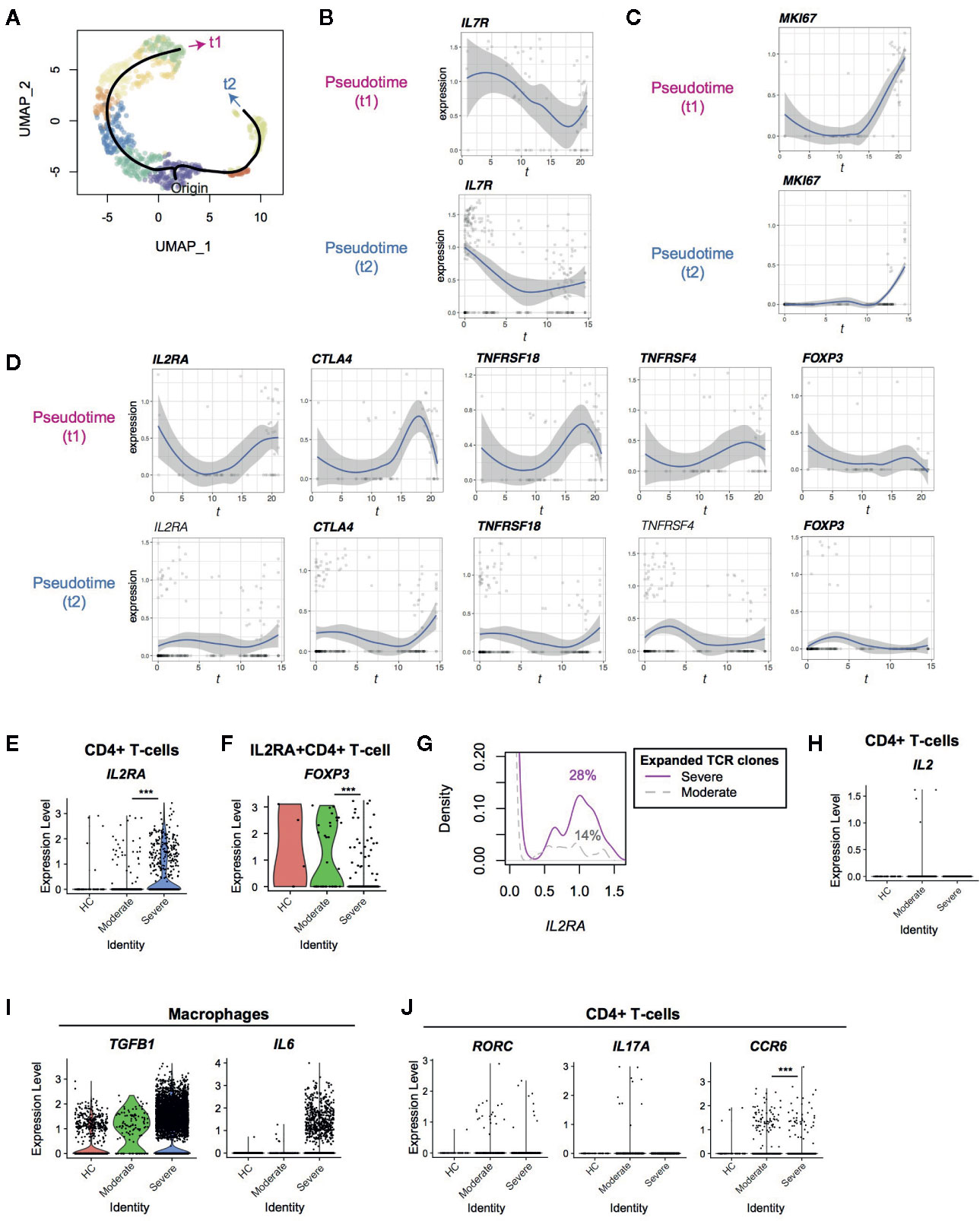
Figure 2 Pseudotime analysis of CD4+ T-cells from Covid-19 patients for Treg-associated genes. (A) Two pseudotime trajectories were identified in the UMAP space. (B, C) The expression of (B) IL7R and (C) MKI67 in the pseudotime trajectories. (D) Gene expression dynamics of Treg-associated genes in the pseudotime trajectories. Genes with significant changes across pseudotime are highlighted by bold text. (E, F) The expression of IL2RA in (E) CD4+ T-cells and (F) IL2RA+CD4+ T-cells from the three groups. (G) IL2RA expression in CD4+T-cells with expanded TCR clones (n ≥ 2) in severe patients (magenta, solid line) and moderate patients (grey, broken line). Numbers indicate the percentage of IL2RA+ cells in each group. (H) The expression of IL2 in CD4+ T-cells from the three groups. (I) The expression of TGFB1 and IL6 in macrophages from the three groups. (J) The expression of the Th17 genes including RORC, IL17A, and CCR6 in CD4+ T-cells from the three groups. *** means p < .001.
Interestingly, well-known immunoregulatory genes including IL2RA, CTLA4, TNFRSF4 (OX-40) and TNFRSF18 (GITR) were more expressed in T-cells across pseudotime 1 than pseudotime 2 (Figure 2D, Supplementary Figure 1B). Although these genes are often associated with Tregs, FOXP3 was not induced in both of these trajectories, and thus most of the T-cells did not become Tregs. T-cells in pseudotime 2 showed modest increase of CTLA4 and TNFRSF18 only towards the end of the trajectory (Figure 2D).
Furthermore, IL2RA was significantly upregulated in CD4+ T-cells from severe COVID-19 patients compared to moderately affected patients (Figure 2E). Since CD25 (IL2RA) is a key marker for Tregs and activated T-cells (27), we asked if CD25-expressing T-cells in COVID-19 patients were Tregs. Intriguingly, the percentage of FOXP3+ cells in IL2RA+ CD4+ T-cells was significantly reduced in severe patients compared to moderate patients (15.3 vs 48.6%, Figure 2F). This indicates that FOXP3 transcription is repressed in CD25+ T-cells further suggesting that IL2RA+ T-cells are activated T-cells or “ex-Tregs” (i.e. effector T-cells that were used to express FOXP3 but more recently downregulated FOXP3 expression (28, 29), rather than functional Tregs.
Since T-cells in the late phase of pseudotime 1 upregulated IL2RA and MKI67, we asked if expanded TCR clones expressed more IL2RA specifically in severe patients. Expanded TCR clones in severe patients had more IL2RA+ T-cells than those in moderate patients (14% and 28% cells in moderate and severe patients expressed IL2RA, respectively; p < 0.001, Figure 2G), confirming that IL2RA+ T-cells are associated with the severe phenotype. However, no significant difference was observed between expanded and non-expanded TCR clones in severe patients. Notably, IL2 transcription was not induced in CD4+ T-cells in severe patients (Figure 2H), suggesting that CD25+ activated T-cells in severe patients die, at least partly, by cytokine deprivation.
Given that T-cells from severe patients dominated in the last part of pseudotime 1 (i.e. Clusters 3 and 6), these findings indicate that T-cells become more activated and vigorously proliferate in severe COVID-19 patients than moderate patients. These CD25+ activated T-cells are likely to be short-lived and do not initiate FOXP3 transcription in severe COVID-19 patients, while they can differentiate into Tregs in moderate infections.
Firstly, we hypothesized that FOXP3 transcription was actively repressed by cytokines in the microenvironments in severe COVID-19 patients. FOXP3 transcription is activated by IL-2 and TGF-β signaling and is repressed by IL-6 and IL-12 signaling (30). In fact, some macrophages from severe COVID-19 patients expressed TGFB1 and IL6 (Figure 2I), as shown by Liao et al. (16) However, CD4+ T-cells did not increase Th17-associated genes including RORC, IL17A, and IL17F, and the expression of CCR6, a marker for Th17 cells, was significantly reduced in severe COVID-19 patients (Figure 2J and Supplementary Figure 1D). This suggests that the differentiation of both Tregs and Th17 is inhibited.
Th17 differentiating T-cells express both Foxp3 and RORγ-t before they mature (31). In addition, FOXP3intermediate CD45RA - T-cells express RORγ-t and Th17 cytokines (32). Together with the scRNA-seq analysis results above, these support the model that activated T-cells show differentiation arrest or preferentially die before becoming Tregs or Th17 cells in severe COVID-19 patients. Alternatively, given that IL-6 and TGF-β seem to be available in severe COVID-19 patients, the differentiation of the common precursor T-cells to Tregs and Th17 cells may be blocked by unidentified factors. Importantly, IL2RA expression was significantly increased in severe COVID-19 patients in comparison to moderate patients, whilst very few T-cells expressed IL2 (Figure 2H).
PD-1 is another key immunoregulatory molecule for suppressing immune responses during viral infection (33). However, PD-1 may play multiple roles in CD4+ T-cells, as PD-1 is a marker for Tfh. In fact, PD-1high BCL6high Tfh-like T-cells are a major effector population in melanoma tissues (21). Thus we asked if PD-1-expressing T-cells show Tfh differentiation and/or if PD-1-expressing T-cells can succumb to PD-1 ligand-mediated inhibition in COVID-19 patients. However, in SARS-CoV-2 infection, BCL6 was not induced in the major activation and differentiation pathway, pseudotime 1, indicating that those activated T-cells did not differentiate into Tfh. Comparatively, T-cells in pseudotime 2 showed some upregulation, although this was statistically not significant (Figure 3A). This suggests that Tfh differentiation was suppressed in COVID-19 patients. PDCD1 was highly upregulated in both pseudotime 1 and 2, suggesting that these cells are vulnerable to PD-1 ligand-mediated suppression. Interestingly, macrophages from severe COVID-19 patients expressed higher levels of CD274 (PD-L1) yet the expression of PDCD1LG2 (PD-L2) was not significantly different between moderate and severe patients (Figure 3B). These results indicate that PD-1-mediated T-cell regulation was at least partially operating in severe COVID-19 patients. Meanwhile, TBX21 and GATA3 expression is induced in T-cells across pseudotime 1, suggesting that these T-cells may differentiate into Th1 and Th2 cells (Figure 3C).
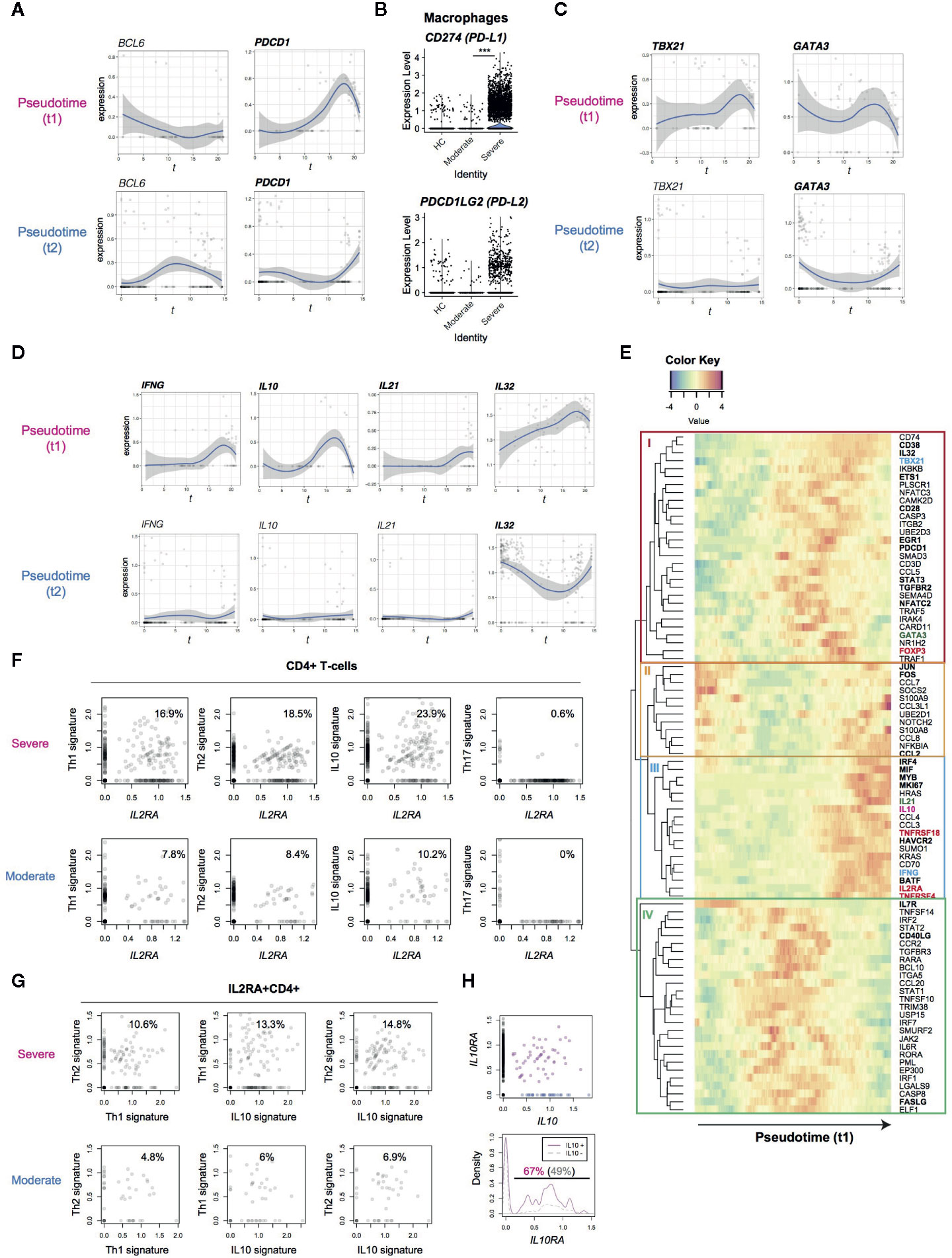
Figure 3 Analysis of the expression dynamics of effector T-cell genes in CD4+ T-cells from COVID-19 patients. (A) The expression of BCL6 and PDCD1 in the pseudotime trajectories. (B) The expression of PD1 ligand genes, PD-L1 (CD274) and PD-L2 (PDCDLG2), in macrophages from the three groups. (C) The expression of Th1 transcription factor, TBX21, and Th2 transcription factor, GATA3, in the pseudotime trajectories. (D) Gene expression dynamics of IFNG, IL10, IL21 and IL32 in the pseudotime trajectories. (E) Heatmap analysis of selected genes in pseudotime 1 (t1). Here the rows are key genes that are differentially expressed across pseudotime 1, and the columns are single cells in the order of pseudotime 1. Th1, Th2, and Treg-associated genes are highlighted by cyan, green, and red. In addition, genes with significant changes across pseudotime are highlighted by bold text. (F, G) The expression of signature genes in (F) CD4+ T-cells and (G) IL2RA+CD4+ T-cells. (H) (upper panel) The expression of IL10 and IL10RA in CD4+ T-cells from COVID-19 patients. IL10+IL10RA+ double positive cells are highlighted by purple, while IL10+ single positive cells are shown by blue. (lower panel) Density plots of IL10RA expression in IL10+CD4+ T-cells and IL10-CD4+ T-cells.
T-cells in the late phase of pseudotime 1 upregulated the expression of cytokines including IFNG and IL10, which are Th1 and Th2 cytokines, respectively (Figure 3D). In addition, IL21 (a Th2 and Th17 cytokine) was upregulated in some cells in pseudotime 1 whereas IL-32 was highly sustained in both of the pseudotime trajectories. These indicate that differentiation processes for T-cell lineages are simultaneously induced in activated T-cells from the lung of COVID-19 patients.
Accordingly, we hypothesized that CD25-expressing activated T-cells preferentially differentiate into effector T-cells in severe COVID-19 patients, instead of their most frequent fate as Tregs in a normal setting (27). In order to test this hypothesis and reveal dynamics of each T-cell differentiation pathway, we analyzed co-regulated genes across pseudotime 1, obtaining 4 gene modules by a hierarchical clustering (Figure 3E).
Heatmap analysis of pseudotime 1 successfully captured the pseudo-temporal order of gene expression: genes in modules II and IL7R are firstly activated, followed by genes in module IV (apart from IL7R), subsequently by genes in module I, and lastly genes in module III alongside module II genes again (Figure 3E). Reasonably, module II contained the AP-1 transcription factors FOS and JUN, suggesting that T-cells that highly express these genes have been recently activated. In pseudotime 1, these FOS+JUN+ T-cells were followed by T-cells with high expression of genes in module IV, which contained CD40LG and FASLG (Figure 3E). These CD40LG+FASLG+ T-cells are considered to activate CD40-expressing macrophages and dendritic cells as well as inducing apoptosis of FAS-expressing cells by providing CD40 signaling and Fas signaling upon contact.
CD40LG+FASLG+ T-cells are followed by T-cells that highly expressed genes in module I, which include the Th1 transcription factor TBX21, the Th2 transcription factor GATA3, and FOXP3. In addition, these T-cells upregulated the immediate early genes EGR1 and NFATC2 and the activation markers CD38 and PDCD1 (Figure 3E). These collectively suggest that those CD38+ PDCD1+ T-cells received sustained TCR signals and became activated, expressing TBX21, GATA3, and FOXP3 as TCR signal downstream genes.
Lastly, T-cells in the final part of pseudotime 1 upregulated the expression of genes in modules II and III. Module III contained the cytokines IL10 and IL21, immune checkpoints TNFRSF4 and HAVCR2 (TIM-3), Tregs and activation marker IL2RA and TNFRSF18, all of which were found to be upregulated (Figure 3E). On the other hand, FOXP3, TBX21 and GATA3 were largely repressed in the T-cells. Intriguingly, those T-cells highly expressed the transcription factors for the differentiation of effector Tregs (i.e. activated Tregs with enhanced immunoregulatory activities) including IRF4, MYB, and BATF. Therefore, those activated T-cells partially show an effector Treg phenotype but lack their cardinal immunoregulatory features because FOXP3 expression is repressed and the effector cytokines are transcribed. Notably, those activated T-cells also upregulated AP-1 (FOS/JUN) and other genes in module II, suggesting that these CD25+ activated T-cells had received TCR and/or costimulatory signals such as TNFRSF signals. Given that this last phase of pseudotime 1 (i.e. UMAP clusters 3 and 6) is dominated by CD4+ T-cells from severe patients, these results collectively support that FOXP3-mediated negative feedback on T-cell activation is defective in COVID-19 patients.
In order to further understand why CD25-expressing T-cells failed to differentiate into effector Tregs, we hypothesized that CD25-expressing activated T-cells are more likely to differentiate into multiple effector T-cell subsets in severe COVID-19 patients than moderately affected individuals. In fact, IL2RA+CD4+ T-cells from severe COVID-19 patients expressed Th1, Th2, and IL-10 signature genes more frequently (Figure 3F) whereas Th17 differentiation was suppressed in IL2RA+ T-cells from both groups.
Although IL-10 has been classically regarded as a Th2 cytokine, Th1 cells can produce IL-10 (34). In fact, IL4, IL5, IL12A, and IL13, were not detected in any T-cells analyzed in the dataset (data not shown). Thus, we asked if Th2 differentiation was diverted into IL-10 producing immunoregulatory T-cells (Tr1), which differentiate by IL-10 signaling and produce IL-10 and thereby suppress immune responses particularly in mucosal tissues (35). Surprisingly, however, higher frequencies of IL2RA+ T-cells expressed Th1 and Th2 signature genes, concomitantly expressing IL10 signature genes (Figure 3G). This strongly supports that IL-10 producing T-cells are not immunoregulatory but Th1-Th2 multifaceted effector T-cells.
IL10RA and IL10RB were expressed in activated T-cells in both of the trajectories (Supplementary Figure 1C), and the frequency of IL10RA+ cells was significantly higher in IL10+ cells than IL10- cells (67 vs 49%, p = 0.003, Figure 3H). This suggests that a positive feedback loop for IL-10 expression promoted the differentiation of the multifaceted effector T-cells in an autocrine manner (36, 37).
Intriguingly, FURIN expression was increased in CD4+ T-cells from severe COVID-19 patients (Figure 1D). Furin was previously associated with Treg functions in a knockout study (8), although the underlying mechanisms were not clear. In addition, it was not known if Furin was specifically expressed in Tregs amongst CD4+ T-cells. Recently we showed that the majority of Treg-type genes are in fact regulated by TCR signaling (21, 38), since Tregs receive infrequent-yet-regular TCR signals in vivo (26). We hypothesise that T-cells produce Furin upon activation, which can enhance the viral entry of SARS-CoV-2 into lung epithelial cells during inflammation.
Firstly, we analyzed the microarray data of various CD4+ T-cell populations from mice (17). In line with our previous observations (21), all the antigen-experienced and activated T-cell populations including Tregs, memory-phenotype T-cells, and tissue-infiltrating effector T-cells showed higher expression of FURIN than naïve T-cell populations (Figure 4A).
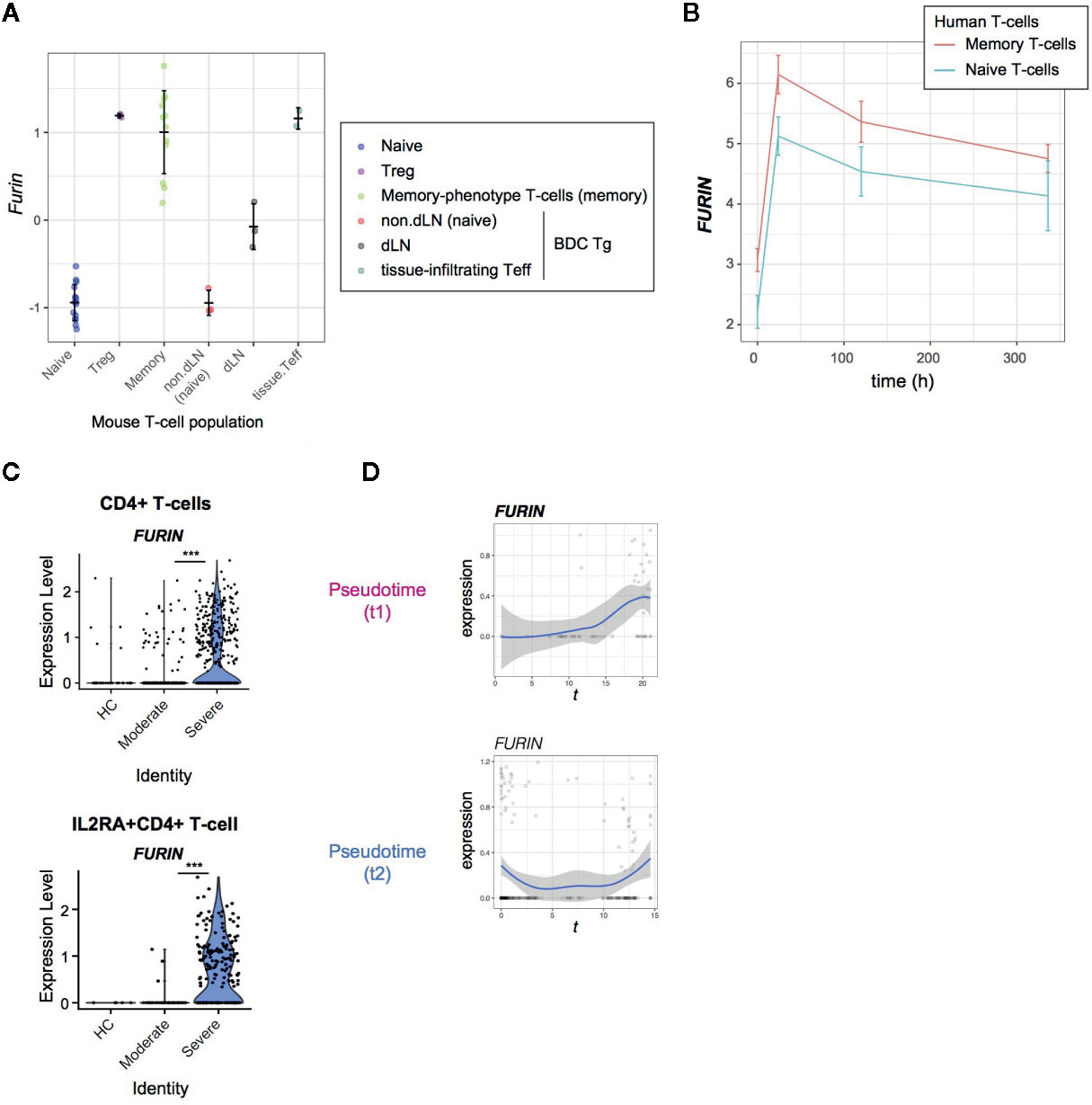
Figure 4 FURIN expression in activated CD4+ T-cells in normal conditions and COVID-19 infection. (A) FURIN expression in CD4+ T-cell subpopulations from normal mice: naïve T-cells (naïve), Tregs, memory-phenotype T-cells (memory) from WT mice; non-draining lymph nodes (non-dLN, naïve), draining lymph nodes (dLN) of the pancreas, and pancreas-infiltrating effector T-cells (tissue-infiltrating Teff) of diabetes-prone BDC transgenic (Tg) mice. (B) Time course analysis of FURIN expression in human memory T-cells and naïve T-cells. (C) The expression of FURIN in CD4+ T-cells (upper) and IL2RA+CD4+ T-cells (lower) from the groups of patients and HC. (D) Gene expression dynamics of FURIN in the pseudotime trajectories 1 and 2.
Next, we asked if human naïve and memory CD4+ T cells can express FURIN upon receiving TCR signals. We addressed this question using the time course RNA-seq analysis of CD45RA+CD45RO−CD4+ naïve T-cells and CD45RA−CD45RO+ CD4+ memory T-cells which were obtained from 4 individuals and activated by anti-CD3 and anti-CD28 antibodies (18). FURIN expression was markedly induced within 24 h after stimulation and was sustained over 2 weeks in the culture (Figure 4B). Memory T-cells also showed higher expression of FURIN over the time course (p = 0.059), with a significant difference at its peak (24 h, p = 0.004). Thus, FURIN expression is induced by TCR signals in human and mouse T-cells.
In SARS-CoV-2 infection, 36% of CD4+ T-cells and 56% of IL2RA+CD4+ T-cells from severe patients expressed FURIN, while in moderate patients only 11% and 30% of those cells, respectively, expressed FURIN (Figure 4C). Importantly, FURIN was significantly induced in CD4+ T-cells in pseudotime 1, particularly when T-cells upregulated CD25, CTLA-4, and TNFRSF molecules, but not in pseudotime 2 (Figure 4D). These collectively support that FURIN expression is induced in highly activated non-regulatory CD25+CD4+ T-cells in severe COVID-19 patients.
Our study has shown that CD4+ T-cells in severe COVID-19 patients have dysregulated activation and differentiation mechanisms. The most remarkable defect was the decoupling of Treg-type activation and FOXP3 expression, which normally occurs simultaneously to sustain the effector Treg population while inflammation is resolved (38).
FOXP3 is induced in activated T-cells by TCR signals and its transcription is further enhanced by IL-2 and TGF-β signaling. Once expressed in T-cells, FOXP3 proteins bind to pre-existing transcription factors, particularly RUNX1 and ETS1, and thereby convert RUNX1-ETS1-containing transcriptional machineries for T-cell activation and effector function into immunoregulatory ones (38, 39). The Treg-type transcriptional regulation is characterized by the activation of immunoregulatory proteins, including many of the surface immune checkpoints such as CTLA-4, GITR, and OX-40, while cytokine genes are generally repressed. This Treg-type setting is further enhanced upon activation, when Tregs begin to show the effector Treg phenotype, further upregulating the expression of the immune checkpoint molecules. Importantly, Tregs need to sustain FOXP3 transcription in a persistent manner across time, (40) otherwise they can downregulate FOXP3 expression and become effector T-cells (28, 29).
Since IL-2 signaling enhances FOXP3 transcription, CD25+ T-cells are likely to differentiate into FOXP3+ Tregs in normal situations (27). However, in severe COVID-19 patients, those CD25+ T-cells are considered to be vigorously proliferating, whilst becoming multifaceted effector T-cells or dying, instead of maturing into FOXP3+ Tregs. Accordingly, we propose to define the unique activation status of CD25+FOXP3- T-cells as hyperactivated T-cells (Figure 5). CD25 expression occurs mostly in CD4+ T-cells, and therefore, these CD25+ hyperactivated T-cells are likely to be the source of the elevated serum soluble CD25 in severe COVID-19 patients. These hyperactivated CD25+ T-cells produce Furin, which can further enhance SARS-CoV-2 viral entry into pulmonary epithelial cells. Since hyperactivated CD25+ T-cells constitute a rare population, the role of their Furin production, if any, is likely to be limited to their microenvironments. Still, it is possible that, when hyperactivated CD25+ T-cells closely interact with antigen-presenting cells that present viral antigens, their Furin expression may make both antigen presenting cells and themselves more susceptible to the viral infection, which can further dysregulate the T-cell system.
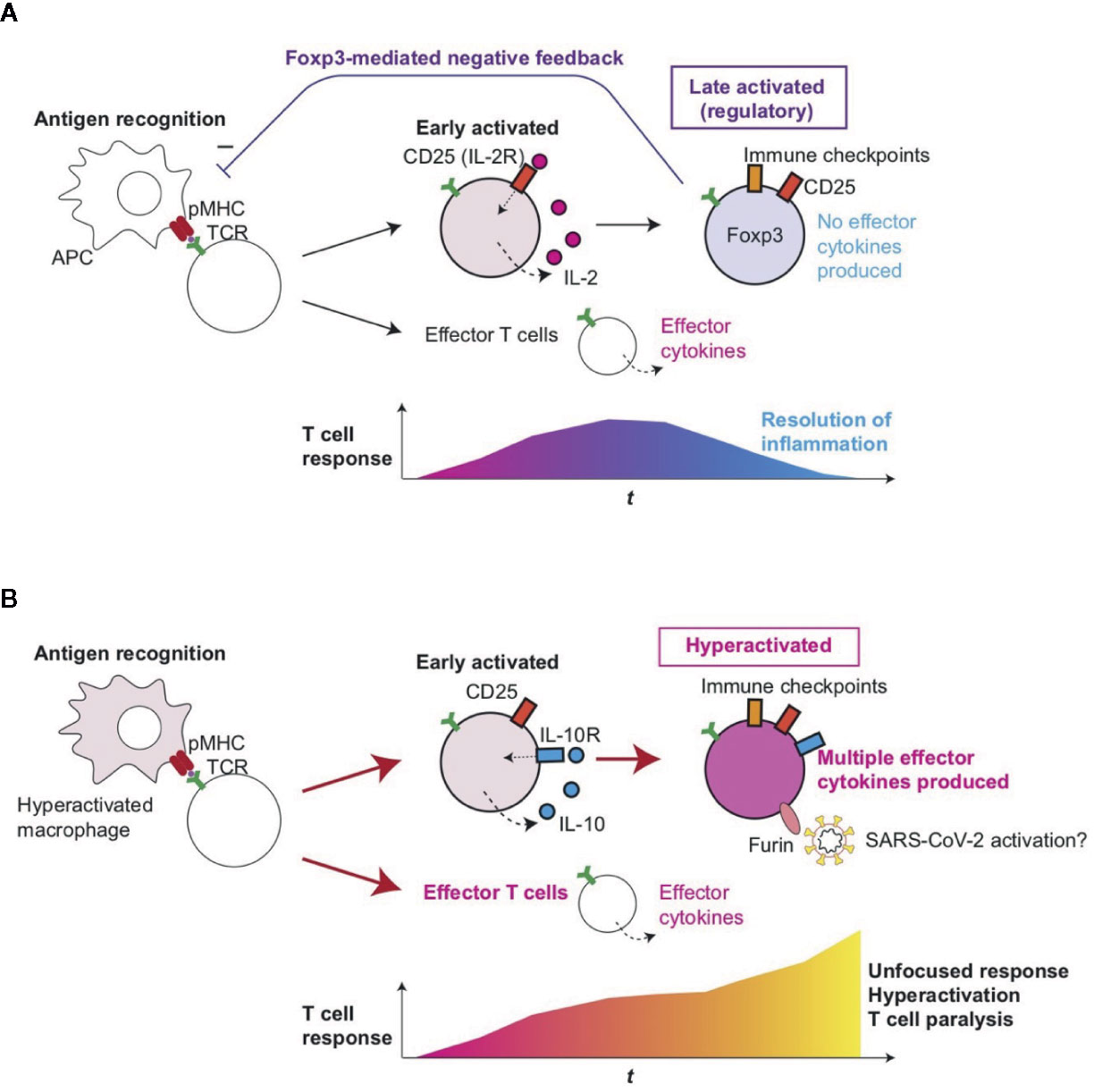
Figure 5 Roles of T-cell hyperactivation in the lung of severe COVID-19 patients. (A) Peripheral Treg differentiation in normal conditions. Antigen-presenting cells (APC) present antigens as peptide-MHC complex (pMHC) to CD4+ T-cells, which triggers TCR signaling and subsequent activation and differentiation processes. Initially, early activated T-cells start to produce CD25 and IL-2, establishing a positive feedback loop for T-cell activation and proliferation. Some T-cells can differentiate into effector T-cells such as Th1 and Th2. Since IL-2 signaling enhances FOXP3 transcription, prolonged activation results in the expression of immune checkpoints such as CTLA-4 and FOXP3, which represses the transcription of effector cytokine genes. CD25+CTLA-4+FOXP3+ T-cells can consume and occupy immunological resources including IL-2 and CD28 signaling, and thereby mediate a negative feedback loop on the initial T-cell activation (27). This leads to the suppression of T-cell responses and the resolution of inflammation. (B) In severe COVID-19 patients, hyperactivated macrophages (13) may present antigens to CD4+ T-cells, which are activated and differentiate into CD25+ IL10R+ early activated T-cells which produce IL-10 rather than IL-2. FOXP3 transcription remains to be suppressed due to this and other unidentified mechanisms such as metabolism, while cytokines such as IL-10 further enhance the activation of CD25+ T-cells, resulting in the generation of CD25+ hyperactivated T-cells that express immune checkpoints, multiple effector T-cell cytokines, and Furin. The multifaceted Th differentiation may lead to unfocused T-cell responses and thereby paralyze the T-cell system. In addition, Furin can activate the S-protein of SARS-CoV-2 and thereby enhance viral entry into lung epithelial cells.
The risk factors for the development of severe COVID-19 include age, obesity, cardiovascular diseases, diabetes, and the use of corticosteroids (41, 42). These diseases are associated with dysregulated hormonal and metabolic environments that can dysregulate the homeostasis of CD25+ T-cells and FOXP3-expressing Tregs. Thus, it is imperative to investigate if genes and metabolites associated with the disease conditions have any roles in promoting the differentiation of hyperactivated T-cells. Previous reports showed that Tregs accumulated in atherosclerotic lesions, (43) and FOXP3 expression was reduced in CD25+CD4+ T-cells from patients with prior myocardial infarctions (44). In addition, T-cells in patients with obesity may show different responses to T-cell activation. Intriguingly, leptin a key hormone produced by adipose tissue, is thought to prevent CD25+CD4+ T-cell proliferation but is relatively deficient in obese patients (45, 46). Furthermore, the function of Tregs is impaired in type-1 diabetes patients (47). In addition, FOXP3 transcription is transiently activated in T-cells of severe COVID-19 patients but may be repressed due to their unique metabolic states. T-cell activation is dependent on glycolysis, which converts glucose to pyruvate, and the tricarboxylic acid (TCA) cycle, which activates oxidative phosphorylation (OXPHOS) and generates ATP in mitochondria (48). Treg differentiation is more dependent on OXPHOS and can be inhibited by glycolysis (49). Importantly, our pathway analysis suggested that these metabolic pathways were altered in severe COVID-19 patients, although further studies on metabolism are required. Furthermore, the hypoxic environment in the lung of severe COVID-19 patients may activate HIF-1α, which mediates aerobic glycolysis, and thereby promotes the degradation of FOXP3 proteins (50). The reduction of FOXP3 proteins may result in the abrogation of the FOXP3 autoregulatory transcriptional loop thus blocking Treg differentiation (40).
Notably, all the moderate patients in fact developed bilateral pneumonia, whereas five out of six severe patients were in critical condition and received invasive mechanical ventilation. Although the sample number is small, this suggests that the deterioration of the respiratory function, not pneumonia itself, is associated with the T-cell hyperactivation. Alternatively and not exclusively, it is noted that there were significant differences in the age of the moderate vs severe groups: all the three moderate patients were in the 30’s (precisely, 35, 36, and 37 years old), while four out of six critical patients were in the 60’s and the other two were 46 and 57 (16). Thus, it is yet to be determined if hyperactivated T-cells are associated with ageing and if they are differentiated in young severe patients as well.
CD25+ hyperactivated T-cells also expressed PD-1, and PD-L1 expression in macrophages was increased in severe COVID-19 patients. This clearly shows that the PD-1 system is not able to control hyperactivated T-cells. This may be due to the status of macrophages and other antigen-presenting cells because CD80 on these cells disrupts the PD-1 - PD-L1 interaction and thereby abrogates PD-1-mediated suppression (51). In addition, PD-L1 expression on lung epithelial cells may play a role in regulating PD-1-expressing T-cells, as shown in other viruses including Influenza Virus and Respiratory Syncytial Virus (52, 53).
Hyperactivated T-cells differentiated into multifaceted Th1-Th2 cells with IL-10 expression. While IL-10 may serve as a growth factor for these cells through their IL-10 receptors, other cytokines in the microenvironment may drive the expression of both Th1 and Th2 transcription factors. Prototypic cytokines for Th1 and Th2 were not differentially expressed in all single cells between moderate and severe patients in the current dataset. Importantly, although T-bet and Gata3 are usually considered as Th1 and Th2 transcription factors, respectively, the expression of both T-bet and Gata3 is induced in CD4+ T-cells by TCR signals at the early stage of T-cell activation (54, 55). This suggests that the multifaceted Th1-Th2 T-cells are in fact still at the early stage of differentiation. Importantly, Foxp3-deficient T-cells show a similar phenotype with multifaceted Th1-Th2 differentiation. Using bacterial artificial chromosome (BAC) Foxp3-GFP reporter and Foxp3-deficient Scurfy mice, Kuczma et al. showed that Foxp3-transcribing T-cells without functional Foxp3 proteins produced both IFN-γ and IL-4 (56). Although the biological significance of the multifaceted Th differentiation in severe SARS-CoV-2 infection is not yet clear, we suggest that such unfocused T-cell responses will lead to the activation of broad-range of immune cells in an unorganized manner contributing to the hyperactivation as well as paralysis of the immune system in severe COVID-19 patients (Figure 5).
Intriguingly, severe COVID-19 patients show elevated levels of serum cytokines such as IL-6, TNF-α, and IL-10, and also increased sCD25 in the serum (11, 12). Given that CD25 expression is predominantly found in T-cells, particularly in activated T-cells and Tregs, (38) it is likely that significant proportions of T-cells are activated in severe COVID-19 patients and contribute to their ‘cytokine storm’ or CRS. Firstly, since cell surface CD25 can be shed from CD25+CD4+ T-cells, (57) CD25+ hyperactivated T-cells may be a significant source of sCD25 in COVID-19 CRS. Secondly, CD25+ hyperactivated T-cells may contribute to the elevation of some of the serum cytokines such as IL-10 in severe COVID-19 patients (11, 12). Notably, CD25+ hyperactivated T-cells are activated and proliferative, they do not increase in number and also fail to differentiate into Treg as their FOXP3 expression is repressed. These indicate the unique dynamics of activated T-cells in severe COVID-19: a significant proportion of T-cells become activated, proliferate and rapidly die before differentiating into Treg. Such high turn-over of CD25+ hyperactivated T-cells may contribute to immunothrombosis, which is immune-mediated acceleration of thrombosis and is another feature of severe COVID-19 (13). Intriguingly, the cancer immunotherapy chimeric antigen receptor T-cell (CAR-T) therapy uses gene-modified activated T-cells as effectors and can induce disseminated intravascular coagulation and immunothrombosis (58).
In conclusion, our study demonstrates that SARS-CoV-2 drives hyperactivation of CD4+ T-cells and immune paralysis to promote the pathogenesis of disease and thus life-threatening symptoms in severely affected individuals. Therefore, therapeutic approaches to inhibit T-cell hyperactivation and paralysis may need to be developed for severe COVID-19 patients.
All datasets presented in this study are cited in the article.
MO conceived and designed the study. BK and MO analyzed data and performed experiments. JA-S and CT analyzed data in the literature. YS and MO developed the methodologies. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
MO is supported by MRC project grant (MR/S000208/1) and is currently a Visiting Associate Professor in IRCMS, Kumamoto University to conduct the Kakenhi project 19H05426. This research was supported in part by grants from the Japan Agency for Medical Research and Development (grant numbers JP20jm0210074 to YS and MO; JP20fk0410023 and JP19fm0208012 to YS). The authors declare that no conflicts of interest exist in relation to this manuscript. This manuscript has been released as a pre-print at bioRxiv: Kalfaoglu B, et al. T-cell hyperactivation and paralysis in severe COVID-19 infection revealed by single-cell analysis. bioRxiv 2020:2020.05.26.115923. doi: 10.1101/2020.05.26.115923.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2020.589380/full#supplementary-material
1. Okazaki T, Okazaki IM. Stimulatory and Inhibitory Co-signals in Autoimmunity. Adv Exp Med Biol (2019) 1189:213–32. doi: 10.1007/978-981-32-9717-3_8
2. Bending D, Ono M. From stability to dynamics: understanding molecular mechanisms of regulatory T cells through Foxp3 transcriptional dynamics. Clin Exp Immunol (2018) 197(1):14–23. doi: 10.1111/cei.13194
3. Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell (2020) 181:271–280.e278. doi: 10.1016/j.cell.2020.02.052
4. Glowacka I, Bertram S, Muller M, Allen P, Soilleux E, Pfefferle S, et al. Evidence that TMPRSS2 Activates the Severe Acute Respiratory Syndrome Coronavirus Spike Protein for Membrane Fusion and Reduces Viral Control by the Humoral Immune Response. J Virol (2011) 85:4122–34. doi: 10.1128/jvi.02232-10
5. Sungnak W, Huang N, Bécavin C, Berg M, Queen R, Litvinukova M, et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med (2020) 26(5):681–7. doi: 10.1038/s41591-020-0868-6
6. Coutard B, Valle C, de Lamballerie X, Canard B, Seidah N, Decroly E. The spike glycoprotein of the new coronavirus 2019-nCoV contains a furin-like cleavage site absent in CoV of the same clade. Antiviral Res (2020) 176:104742. doi: 10.1016/j.antiviral.2020.104742
7. Hoffmann M, Kleine-Weber H, Pöhlmann S. A Multibasic Cleavage Site in the Spike Protein of SARS-CoV-2 Is Essential for Infection of Human Lung Cells. Mol Cell (2020) 78:779–784.e775. doi: 10.1016/j.molcel.2020.04.022
8. Pesu M, Watford W, Wei L, Xu L, Fuss I, Strober W, et al. T-cell-expressed proprotein convertase furin is essential for maintenance of peripheral immune tolerance. Nature (2008) 455:246–50. doi: 10.1038/nature07210
9. Pesu M, Muul L, Kanno Y, O’Shea JJ. Proprotein convertase furin is preferentially expressed in T helper 1 cells and regulates interferon gamma. Blood (2006) 108:983–5. doi: 10.1182/blood-2005-09-3824
10. Oksanen A, Aittomäki S, Jankovic D, Ortutay Z, Pulkkinen K, Hämäläinen S, et al. Proprotein convertase FURIN constrains Th2 differentiation and is critical for host resistance against Toxoplasma gondii. J Immunol (2014) 193:5470–9. doi: 10.4049/jimmunol.1401629
11. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet (2020) 395:497–506. doi: 10.1016/S0140-6736(20)30183-5
12. Chen G, Wu D, Guo W, Cao Y, Huang D, Wang H, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest (2020) 130:2620–9. doi: 10.1172/JCI137244
13. McGonagle D, O’Donnell JS, Sharif K, Emery P, Bridgewood C. Immune mechanisms of pulmonary intravascular coagulopathy in COVID-19 pneumonia. Lancet Rheumatol (2020) 2:e437–45. doi: 10.1016/S2665-9913(20)30121-1
14. Diao B, Wang C, Tan Y, Chen X, Liu Y, Ning L, et al. Reduction and Functional Exhaustion of T Cells in Patients with Coronavirus Disease 2019 (COVID-19). medRxiv (2020) 11:827. doi: 10.1101/2020.02.18.20024364
15. Shimizu A, Kondo S, Sabe H, Ishida N, Honjo T. Structure and function of the interleukin 2 receptor: affinity conversion model. Immunol Rev (1986) 92:103–20. doi: 10.1111/j.1600-065X.1986.tb01496.x
16. Liao M, Liu Y, Yuan J, Wen Y, Xu G, Zhao J. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat Med (2020) 26(6):842–4. doi: 10.1038/s41591-020-0901-9
17. Heng TS, Painter MW, Immunological Genome Project, C. The Immunological Genome Project: networks of gene expression in immune cells. Nat Immunol (2008) 9:1091–4. doi: 10.1038/ni1008-1091
18. LaMere SA, Thompson RC, Komori HK, Mark A, Salomon DR. Promoter H3K4 methylation dynamically reinforces activation-induced pathways in human CD4 T cells. Genes Immun (2016) 17:283–97. doi: 10.1038/gene.2016.19
19. Stuart T, Butler A, Hoffman P, Hafemeister C, Papalexi E, Mauck W, et al. Comprehensive Integration of Single-Cell Data. Cell (2019) 177:1888–1902.e1821. doi: 10.1016/j.cell.2019.05.031
20. Yu G, Wang L-G, Han Y, He Q-Y. clusterProfiler: an R Package for Comparing Biological Themes Among Gene Clusters. OMICS: A J Integr Biol (2012) 16:284–7. doi: 10.1089/omi.2011.0118
21. Bradley A, Hashimoto T, Ono M. Elucidating T Cell Activation-Dependent Mechanisms for Bifurcation of Regulatory and Effector T Cell Differentiation by Multidimensional and Single-Cell Analysis. Front Immunol (2018) 9:1444. doi: 10.3389/fimmu.2018.01444
22. Ishikawa E, Nakazawa M, Yoshinari M, Minami M. Role of Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand in Immune Response to Influenza Virus Infection in Mice. J Virol (2005) 79:7658–63. doi: 10.1128/jvi.79.12.7658-7663.2005
23. Ware CF, Šedý JR. TNF Superfamily Networks: bidirectional and interference pathways of the herpesvirus entry mediator (TNFSF14). Curr Opin Immunol (2011) 23:627–31. doi: 10.1016/j.coi.2011.08.008
24. Gonçalves-Carneiro D, McKeating JA, Bailey D. The Measles Virus Receptor SLAMF1 Can Mediate Particle Endocytosis. J Virol (2017) 91:e02255–02216. doi: 10.1128/jvi.02255-16
25. Fergusson J, Hühn M, Swadling L, Walker L, Kurioka A, Llibre A, et al. CD161intCD8+ T cells: a novel population of highly functional, memory CD8+ T cells enriched within the gut. Mucosal Immunol (2016) 9:401–13. doi: 10.1038/mi.2015.69
26. Bending D, Martín P, Paduraru A, Ducker C, Marzaganov E, Laviron M, et al. A timer for analyzing temporally dynamic changes in transcription during differentiation in vivo. J Cell Biol (2018) 217(8):2931–50. doi: 10.1083/jcb.201711048
27. Ono M, Tanaka RJ. Controversies concerning thymus-derived regulatory T cells: fundamental issues and a new perspective. Immunol Cell Biol (2016) 94:3–10. doi: 10.1038/icb.2015.65
28. Bailey-Bucktrout SL, Martinez-Llordella M, Zhou X, Anthony B, Rosenthal W, Luche H, et al. Self-antigen-driven activation induces instability of regulatory T cells during an inflammatory autoimmune response. Immunity (2013) 39:949–62. doi: 10.1016/j.immuni.2013.10.016
29. Miyao T, Floess S, Setoguchi R, Luche H, Fehling H, Waldmann H, et al. Plasticity of Foxp3(+) T cells reflects promiscuous Foxp3 expression in conventional T cells but not reprogramming of regulatory T cells. Immunity (2012) 36:262–75. doi: 10.1016/j.immuni.2011.12.012
30. O’Malley JT, Sehra S, Thieu V, Yu Q, Chang H, Stritesky G, et al. Signal transducer and activator of transcription 4 limits the development of adaptive regulatory T cells. Immunology (2009) 127:587–95. doi: 10.1111/j.1365-2567.2008.03037.x
31. Zhou L, Lopes J, Chong M, Ivanov I, Min R. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature (2008) 453:236–40. doi: 10.1038/nature06878
32. Miyara M, Yoshioka Y, Kitoh A, Shima T, Wing K, Niwa A, et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity (2009) 30:899–911. doi: 10.1016/j.immuni.2009.03.019
33. Schönrich G, Raftery MJ. The PD-1/PD-L1 Axis and Virus Infections: A Delicate Balance. Front Cell Infect Microbiol (2019) 9:207. doi: 10.3389/fcimb.2019.00207
34. Trinchieri G. Interleukin-10 production by effector T cells: Th1 cells show self control. J Exp Med (2007) 204:239–43. doi: 10.1084/jem.20070104
35. Roncarolo MG, Gregori S, Bacchetta R, Battaglia M, Gagliani N. The Biology of T Regulatory Type 1 Cells and Their Therapeutic Application in Immune-Mediated Diseases. Immunity (2018) 49:1004–19. doi: 10.1016/j.immuni.2018.12.001
36. Brockmann L, Gagliani N, Steglich B, Giannou A, Kempski J, Pelczar P, et al. IL-10 Receptor Signaling Is Essential for TR1 Cell Function In Vivo. J Immunol (2017) 198:1130–41. doi: 10.4049/jimmunol.1601045
37. Couper KN, Blount DG, Riley EM. IL-10: The Master Regulator of Immunity to Infection. J Immunol (2008) 180:5771–7. doi: 10.4049/jimmunol.180.9.5771
38. Ono M. Control of regulatory T-cell differentiation and function by T-cell receptor signalling and Foxp3 transcription factor complexes. Immunology (2020) 160:24–37. doi: 10.1111/imm.13178
39. Ono M, Yaguchi H, Ohkura N, Kitabayashi I, Nagamura Y, Nomura T, et al. Foxp3 controls regulatory T-cell function by interacting with AML1/Runx1. Nature (2007) 446:685–9. doi: 10.1038/nature05673
40. Bending D, Paduraru A, Ducker C, Prieto Martín P, Crompton T, Ono M. A temporally dynamic Foxp3 autoregulatory transcriptional circuit controls the effector Treg programme. EMBO J (2018) 37(16). doi: 10.15252/embj.201899013
41. Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, et al. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Internal Med (2020) 180(7):934. doi: 10.1001/jamainternmed.2020.0994
42. Zheng K, Gao F, Wang X, Sun Q, Pan K, Wang T, et al. Letter to the Editor: Obesity as a risk factor for greater severity of COVID-19 in patients with metabolic associated fatty liver disease. Metabolism (2020) 108:154244–4. doi: 10.1016/j.metabol.2020.154244
43. Meng X, Zhang K, Li J, Dong M, Yang J, An G. Statins induce the accumulation of regulatory T cells in atherosclerotic plaque. Mol Med (Cambridge Mass.) (2012) 18:598–605. doi: 10.2119/molmed.2011.00471
44. George J, Schwartzenberg S, Medvedovsky D, Jonas M, Charach G, Afek A, et al. Regulatory T cells and IL-10 levels are reduced in patients with vulnerable coronary plaques. Atherosclerosis (2012) 222:519–23. doi: 10.1016/j.atherosclerosis.2012.03.016
45. Brennan AM, Mantzoros CS. Drug Insight: the role of leptin in human physiology and pathophysiology—emerging clinical applications. Nat Clin Pract Endocrinol Metab (2006) 2:318–27. doi: 10.1038/ncpendmet0196
46. De Rosa V, Procaccini C, Calì G, Pirozzi G, Fontana S, Zappacosta S, et al. A Key Role of Leptin in the Control of Regulatory T Cell Proliferation. Immunity (2007) 26:241–55. doi: 10.1016/j.immuni.2007.01.011
47. Visperas A, Vignali DAA. Are Regulatory T Cells Defective in Type 1 Diabetes and Can We Fix Them? J Immunol (Baltimore Md 1950) (2016) 197:3762–70. doi: 10.4049/jimmunol.1601118
48. Pearce EL, Pearce EJ. Metabolic Pathways in Immune Cell Activation and Quiescence. Immunity (2013) 38:633–43. doi: 10.1016/j.immuni.2013.04.005
49. Gabriel SS, Kallies A. Sugars and fat – A healthy way to generate functional regulatory T cells. Eur J Immunol (2016) 46:2705–9. doi: 10.1002/eji.201646663
50. Dang EV, Barbi J, Yang H, Jinasena D, Yu H, Zheng Y, et al. Control of T(H)17/T(reg) balance by hypoxia-inducible factor 1. Cell (2011) 146:772–84. doi: 10.1016/j.cell.2011.07.033
51. Sugiura D, Maruhashi T, Okazaki I, Shimizu K, Maeda T, Takemoto T, et al. Restriction of PD-1 function by cis-PD-L1/CD80 interactions is required for optimal T cell responses. Science (2019) 364:558–66. doi: 10.1126/science.aav7062
52. McNally B, Ye F, Willette M, Flaño E. Local Blockade of Epithelial PDL-1 in the Airways Enhances T Cell Function and Viral Clearance during Influenza Virus Infection. J Virol (2013) 87:12916–24. doi: 10.1128/jvi.02423-13
53. Stanciu LA, Bellettato C, Laza‐Stanca V, Coyle A, Papi A, Johnston S. Expression of Programmed Death–1 Ligand (PD-L) 1, PD-L2, B7-H3, and Inducible Costimulator Ligand on Human Respiratory Tract Epithelial Cells and Regulation by Respiratory Syncytial Virus and Type 1 and 2 Cytokines. J Infect Dis (2006) 193:404–12. doi: 10.1086/499275
54. Pipkin ME, Rao A. SnapShot: effector and memory T cell differentiation. Cell (2009) 138:606.e601–606.e6062. doi: 10.1016/j.cell.2009.07.020
55. Furmanski AL, Saldana J, Ono M, Sahni H, Paschalidis N, D’Acquisto F, et al. Tissue-Derived Hedgehog Proteins Modulate Th Differentiation and Disease. J Immunol (2013) 190:2641–9. doi: 10.4049/jimmunol.1202541
56. Kuczma M, Podolsky R, Garge N, Daniely D, Pacholczyk R, Ignatowicz L, et al. Foxp3-Deficient Regulatory T Cells Do Not Revert into Conventional Effector CD4+ T Cells but Constitute a Unique Cell Subset. J Immunol (2009) 183:3731–41. doi: 10.4049/jimmunol.0800601
57. Pedersen AE, Lauritsen JP. CD25 Shedding by Human Natural Occurring CD4+CD25+ Regulatory T Cells does not Inhibit the Action of IL-2. Scand J Immunol (2009) 70:40–3. doi: 10.1111/j.1365-3083.2009.02268.x
Keywords: T-cells, CD25, FOXP3, regulatory T-cells (Tregs), single cell RNA-seq, Furin, COVID-19, SARS-CoV-2
Citation: Kalfaoglu B, Almeida-Santos J, Tye CA, Satou Y and Ono M (2020) T-Cell Hyperactivation and Paralysis in Severe COVID-19 Infection Revealed by Single-Cell Analysis. Front. Immunol. 11:589380. doi: 10.3389/fimmu.2020.589380
Received: 30 July 2020; Accepted: 16 September 2020;
Published: 08 October 2020.
Edited by:
Anne Cooke, University of Cambridge, United KingdomReviewed by:
Nick Holmes, University of Cambridge, United KingdomCopyright © 2020 Kalfaoglu, Almeida-Santos, Tye, Satou and Ono. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Masahiro Ono, bS5vbm9AaW1wZXJpYWwuYWMudWs=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.