- Key Laboratory of Molecular Biology for Infectious Diseases (Ministry of Education), Department of Infectious Diseases, Institute for Viral Hepatitis, The Second Affiliated Hospital, Chongqing Medical University, Chongqing, China
Chronic hepatitis B virus (HBV) infection is one of the main causes of liver diseases, of which the natural history and clinical outcomes are associated with the role of B cells. As humoral immune cells, B cells play a critical role in the process of anti-HBV antibody production. In addition, some studies have also characterized other B cell subsets involved in antigen presentation and regulating the immune response beyond antibody secretion. However, not all B cell subsets play a positive role in the immune response to chronic HBV infection, and various B cell subsets jointly mediate persistent HBV infection, tolerance, and liver damage. Thus, we further sought to elucidate the multiple functions of B cells to gain novel insight into the understanding of chronic hepatitis B (CHB) pathogenesis. We also reviewed the current immunotherapies targeting B cells to explore novel therapeutic interventions for the treatment of chronic HBV infection.
Introduction
Hepatitis B virus (HBV) is a serious global public health problem and major cause of chronic hepatitis, cirrhosis, and hepatocellular carcinoma (HCC). It is estimated that more than 250 million people worldwide are chronically infected with HBV (1), and there are approximately one million deaths attributed to HBV-related complications [e.g., cirrhosis and hepatocellular carcinoma (HCC)] each year (2).
Following HBV infection, the risk of progression to chronicity is age-dependent. Approximately 90% of those who acquire HBV perinatally or in early childhood will develop chronic infection, whereas only 2–6% of people who are infected with hepatitis B as adults become chronically infected (3). In contrast, HCV infection progresses to a chronic persistent infection in 60–80% of infected adults (3). Generally, the natural course of chronic HBV infection can be divided into four chronological phases based on the virus–host interactions (4). The immune-tolerant phase is characterized by the active replication of HBV, HBV e antigen (HBeAg) positivity, and normal alanine aminotransferase (ALT) levels. In the immune clearance phase, HBeAg-positive patients have elevated serum ALT levels and fluctuating HBV-DNA levels. The third stage represents the inactive carrier state, in which patients clear HBeAg and develop the corresponding antibody to HBeAg (HBeAg seroconversion), with the remission of liver disease. In addition, approximately 20−30% of individuals in the inactive carrier state may experience a viral relapse and enter the reactivation phase during follow-up (4).
The pathology of hepatitis B is diverse and reflects the natural course of the disease. Acute hepatitis B is characterized by lobular disarray with swollen hepatocytes, numerous apoptotic bodies, hyperplastic Kupffer cells, and lymphocyte-predominant lobular and portal inflammation (5). In chronic hepatitis B, there is a varying degree of predominantly lymphocytic portal inflammation with interface hepatitis and spotty lobular inflammation. Inflammation is less pronounced in the immune-tolerant and inactive carrier phases, but is prominent in the immunoreactive phase. Unlike hepatitis C, chronic hepatitis B is usually not associated with lymphoid aggregates, duct (Poulsen) lesions, or steatosis (5). A typical histological feature of chronic hepatitis B is the ‘ground-glass hepatocyte’, which is due to accumulation of hepatitis B surface antigen (HBsAg) within the endoplasmic reticulum (ER) (3).
The complex interactions between HBV and the host immune system drive the process of chronic HBV infection, in which the adaptive immune system plays an important role in inducing an HBV-specific immune response. Because CHB patients are typically characterized by the dysfunction and exhaustion of HBV-specific CD4+ and CD8+ T cells (6, 7), specific T cell-mediated immune responses have become the focus of attention in HBV infection and clearance. At present, there are a substantial number of studies that have described T cell defects during a persistent HBV infection, which are mainly characterized by the sustained expression of multiple inhibitory receptors, poor effector cytotoxic activity, and impaired cytokine production (7–9). However, the role of B cells in HBV infection is often overlooked. In studies related to chronic HBV infection, there has been a reported increase in the percentage of B lymphocytes in the peripheral blood (10). In addition, B lymphocytes had infiltrated the liver (approximately 15% of the inflammatory infiltration) (11) and were clustered in the portal areas or were single cells within the lobule, which are related to liver inflammation and fibrosis (12). Therefore, it is interesting to determine whether, and how, B cells are involved in chronic HBV infection.
Knowledge of the role of B cells has consistently been primarily focused on antibody secretion during chronic HBV infection; however, as an important part of the adaptive immune response, various immune functions of B cells (e.g., antibody secretion, antigen presentation, and immune regulation) jointly mediate persistent HBV infection, tolerance, and liver damage. Therefore, attention should be paid to comprehensively understand the role of B cells in HBV infection, which is conducive to the prevention, monitoring, and treatment of HBV infection. This review describes these three aspects of B cell function during chronic HBV infection and explores the role of B cells in liver injury and immune tolerance during chronic HBV infection (Table 1). Finally, we discuss the directions of future development of B cell immunotherapy in clinical practice.
Antibody Production Function of B Cells
The early knowledge of HBV-specific B cells is primarily derived from the detection of serum antibodies that have important clinical implications. Antibodies against different HBV protein components, especially the envelope antigens (HBsAg) and nucleocapsid antigens (HBeAg and HBcAg), could be applied to the diagnosis and prediction of HBV infection (35). Anti-HBc IgM only appears during an acute HBV infection and severe exacerbation of chronic infection, whereas anti-HBc IgG is found throughout the prior, ongoing, and even occult HBV infection period (36). Quantitative serum anti-HBc levels may reflect the strength of the host adaptive anti-HBV immune activity (37, 38), and thus may serve as a predictor of HBeAg reversal following treatment with peg interferon or nucleos(t)ide analogs (NUCs) in CHB patients (39–41). Anti-HBe appears later than anti-HBc, and a high level of anti-HBe antibodies often predicts a better outcome. Immunity to HBV infection is associated with the secretion of protective anti-HBs antibodies, which represent recovery from an acute HBV infection or acquired immunity through HBV vaccination (36). In general, clinical significance exists between the various antibodies produced by HBV-specific B cells, which suggests that the function of HBV-specific antibody secretion by B cells is an important humoral immune response in HBV infection.
In order to research the humoral immune response of HBsAg-specific B cells in CHB patients, two studies have used recombinant fluorochrome-labeled HBsAg as “bait” to analyze the frequency, phenotype, and function of such specific B cells in the blood (32, 42). It was found that HBsAg-specific B cells existed at a low frequency in blood of CHB patients and contained antiviral potential. However, the cellular phenotype was similar to CD21− CD27− atypical memory B cells (atMBCs), which express high levels of inhibitory receptors, such as programmed cell death receptor-1 (PD-1). Moreover, HBsAg-specific B cells isolated from HBV-infected patients could not efficiently expand and mature into antibody-secreting cells in vitro, whereas a PD-1 blockade or the addition of IL-2, IL-21, and CD40L may partially restore the function of HBsAg-specific B cells (32, 42, 43). Since anti-HBs antibodies can function as protective neutralizing antibodies to block HBV entry or replication, it can be inferred that the dysfunction of anti-HBs secretion by B cells is beneficial for the maintenance of high levels of HBsAg and hinders HBV clearance. Indeed, apart from antigen neutralization (20, 21), there are other potential effector functions of anti-HBs antibodies during chronic HBV infection. Based on the discovery of cytoplasmic and membranous HBsAg, anti-HBs IgG might bind HBsAg and induce antibody-dependent cellular cytotoxicity (ADCC) to deplete HBV-infected hepatocytes (17, 22). In theory, anti-HBs antibodies can also bind HBsAg and exert antibody-dependent cellular phagocytosis (ADCP) to consume HBV (23). During the HBV vaccine design process, anti-HBs antibodies are utilized to participate in the formation of immune complexes and bind to dendritic cells (DC), which further induce a T cell response (24). Therefore, there are multiple pathways mediated by anti-HBs antibodies against HBV infection (Figure 1). However, defects in HBsAg-specific B cells in the secretion of anti-HBs antibodies might promote a persistent HBV infection.
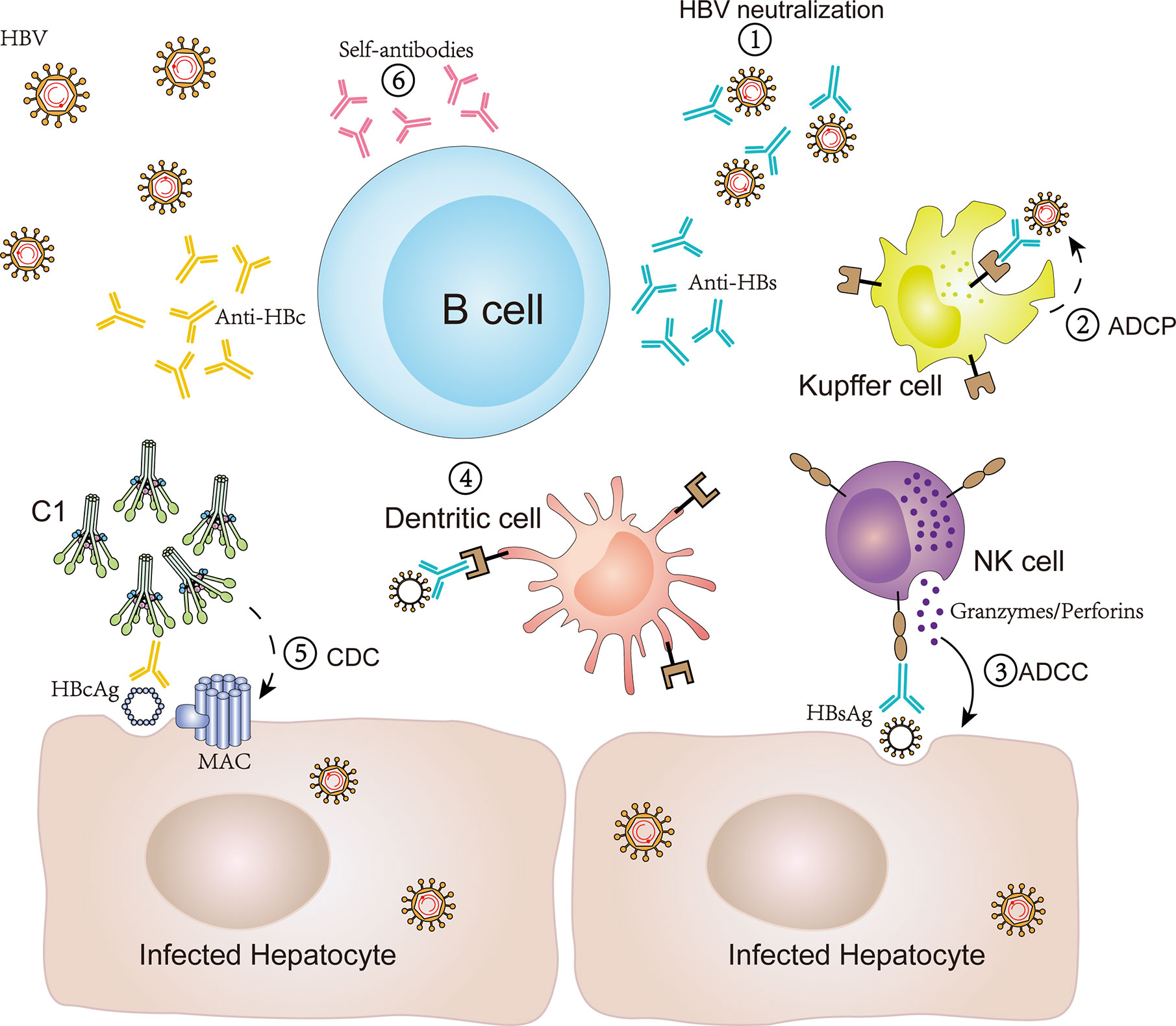
Figure 1 Antibody production by B cells and antibody-mediated immune response in CHB infection. B cells can produce antibodies, such as anti-HBs, anti-HBc and self-antibodies, and subsequently mediate an antiviral immune response or self-reaction via various potential mechanisms in CHB infection. (1) Anti-HBs antibodies bind to HBsAg to block viral entry and replication; (2) anti-HBs antibodies bind HBsAg and induce cellular phagocytosis of Kupffer cells to consume HBV (ADCP); (3) anti-HBs antibodies bind HBsAg and induce the release of perforin/granzyme in NK cells to eliminate HBV-infected hepatocytes (ADCC); (4) anti-HBs antibodies participate in forming immune complexes and bind to dendritic cells to induce a T cell response; (5) anti-HBc IgG binds HBcAg to induce hepatocyte lysis via the classical complement activation pathway initiating from C1 (CDC); (6) self-antibodies participate in an autoimmune reaction to aggravate liver inflammation. ADCC, antibody-dependent cellular cytotoxicity; ADCP, antibody-dependent cellular phagocytosis; CDC, compliment-dependent cytotoxicity.
In subsequent studies that have compared HBsAg-specific B cells, the frequency of HBcAg-specific B cells was higher, with primarily an IgG+ classical memory B cells (cMBCs) phenotype in the peripheral blood (44). In contrast to HBsAg-specific B cells, HBcAg-specific B cells were able to expand and mature into antibody-secreting B cells in vitro, which is beneficial to sustaining a large amount of anti-HBc antibodies in CHB patients (44). However, the presence of anti-HBc antibodies has been shown to play a destructive role in hepatocyte activity and HBV clearance (45–47). Specifically, anti-HBc antibodies can promote the formation of immune complexes in the liver to initiate complement activation and induce massive liver necrosis, which have been confirmed in HBV-associated acute liver failure (ALF) (18, 19).
B cell-mediated humoral immune responses are regulated by T follicular helper (TFH) cells (48). It has been demonstrated that the frequency and phenotype of cTFH cells in CHB patients were altered following infection, and were relevant to clinical parameters, which indicates that cTFH cells may be involved in the immune response against HBV (48). In follow-up studies, there was further evidence that cTFH cells regulate the humoral immune response against envelope proteins and nucleocapsids. During chronic HBV infection, cTFH cells play an important role in the seroconversion of HBeAg (49), in which the secretion of IL-21 may be critical (50, 51). Similarly, HBsAg is a strict T cell-dependent antigen, and the production of anti-HBs antibodies or the seroconversion of HBsAg also requires the aid of TFH cells (52). However, during chronic HBV infection, there are two factors that inhibit the role of TFH cells in promoting B cells to produce anti-HBs antibodies. Specifically, it is plausible that the expression of HBcAg alone or excessive CD40L from activated TFH cells results in the expression of inhibitory receptors (FcRLs and PD-1) on B cells and the accumulation of more atypical memory B cells (53). Moreover, the suppression of HBsAg-specific TFH cells mediated by T regulatory (Treg) cells and follicular regulatory T (TFR) cells is associated with an impairment of HBsAg-specific B cell responses (54–56). Wang et al. found that the response of cTFH cells against HBsAg was blocked by Treg cells in mice with persistent HBV infection, whereas the depletion of Treg cells could restore the response (54). As a subset of Treg cells, CD4+Foxp3− type 1 regulatory T (Tr1)-like cells in the liver migrated to the draining lymph node (DLN) and also suppressed germinal center (GC) formation and anti-HBs antibody production (55). Furthermore, Foxp3+ TFR cells derived from natural Treg cells have many of the same properties as Tregs (57, 58), in which the increased circulating TFR (cTFR)-like cells might similarly impair cTFH cells and participate in chronic HBV infection (56). However, in contrast to HBsAg, HBcAg with high immunogenicity could induce both T cell-dependent and T cell-independent immune responses (59). According to the detected antigenic determinants of T cells, HBcAg-specific helper T (Th) cells were found to help B cells produce anti-HBc antibodies (60). At the same time, without the help of T cells, a population of human naive B cells were found to directly bind HBcAg and were subsequently activated to secrete HBcAg-binding IgM (61).
Apart from the abnormal regulation of TFH cells, the poor differentiation of specific memory B cells into anti-HBs-secreting plasma cells also further affected the humoral immune response of B cells in CHB patients. In humoral immune responses, B cells can differentiate through two distinct pathways. On the one hand, B cells can differentiate to form extrafollicular plasmablasts for rapid antibody production and early protective immune responses. On the other hand, activated B cells can differentiate into plasma cells in germinal centers and subsequently secrete high-affinity antibodies, which confer long-lasting protection from re-infection (62, 63). However, the differentiation of plasmablasts and anti-IgG antibody production may be partially hindered in CHB infection (64). In addition, according to the initial ELISpot examination of plasma cell formation, there were fewer global B cells and plasma cells isolated from the peripheral blood of CHB patients compared with the vaccinated controls (65, 66). One reason for this observation may be the enrichment of atMBCs in the liver and functional impairment of atMBCs’ differentiation into antibody-secreting plasma cells (32, 43). Another reason might be the enrichment of these virus-specific B cells in the liver of patients, which is not conducive to migration to the bone marrow and formation of long-lived plasma cells (32).
As with many chronic diseases, racial/ethnic disparities are seen in HBV infection. The overall prevalence of chronic HBV infection in the US was 2.74% in Asians, 0.6% in African-Americans, 0.06% in Hispanics, and 0.15% in Caucasians (67, 68). Ethnicity influences the natural history and immune responses during a chronic HBV infection. According to the Hepatitis B Research Network (HBRN) database, Asians with chronic HBV infection were more likely to have longer duration of infection and higher HBV DNA level compared with African-Americans and Caucasians, and proportionally more Caucasians had an increased ALT level more than two times the upper limit of normal (ULN) compared with Asians and African-Americans (69). Asian ethnicity was associated with lower rates of anti-HBe seroconversion in children born in endemic countries compared to other ethnicities (70, 71). Caucasian ethnicity was associated with an increased chance of HBsAg loss following nucleos(t)ide analogue-induced HBeAg seroconversion (72). Production of anti-HBs or anti-HBe antibody is helper T cell dependent. CD4+T cells recognize different epitopes within the HBsAg or HBeAg molecule that can be presented by certain major histocompatibility complex (MHC) class II antigens. Polymorphisms of MHC class II genes have been shown to be associated with HBV persistence, seroclearance, seroconversion, and disease progression, but only in patients with a certain ethnic background. For example, the HLA-DR polymorphism rs 9277535 (550 A/G) associated most significantly with chronic hepatitis B and its outcomes in Asian, but not in African-American or Caucasian patients (73). Differences in antibody production may contribute to the racial/ethnic differences observed in HBV prevalence and more work needs to be done to clarify the genetic and immunologic basis of the development of HBV infection stratified by race/ethnicity.
In summary, HBV-specific B cells undergo phenotypic changes during chronic HBV infection, the dysfunction of specific antibody secretion and differentiation into plasma cells promotes the persistence of HBV infection and liver damage. TFH plays a key role in the development of antibody-secreting B cells. Unfortunately, both Treg and TFR cells can inhibit the effect of TFH cells on B cells in CHB patients, which consequently hinder the clearance of HBsAg and a resulting functional cure. In addition, although TFH can promote the production of protective neutralizing anti-HBs antibodies by B cells, an excessive number of activated TFH cells can also promote B cells to secret autoantibodies and induce liver inflammation during chronic HBV infection (16). To date, the interaction between TFH cells and B cells in patients with HBV infection remain incompletely understood. It is believed that further in-depth research regarding its molecular role (e.g., the role CD40L, IL-4, and IL-21) will add to the understanding HBV-specific B cell dysfunction.
Antigen Presentation Function of B Cells
During HBV infection and clearance, HBV-specific T cells are considered to be the main effector cells. The T cell-mediated cellular immune response requires the participation of antigen presenting cells (APCs). B cells, in addition to their function in antibody production, may play a potential role as professional APC during chronic HBV infection (74). According to an early study, HBcAg-specific B cells are roughly 105-fold more efficient than classical non-B cell APCs at presenting HBcAg to both naive Th cells in vivo and to T cells in vitro (15). This result is consistent with the transcriptional analysis that HBV-specific B cells, in addition to producing antibodies, may present HBV antigen to T cells in an obscure manner (44). Clinically, an increased number of studies have supported the role for B cells as APCs in HBV infection by evaluating the risk of HBV reactivation following rituximab (anti-CD20 antibody) treatment in B cell lymphoma patients (75–77).
In general, professional APCs, including B cells, are able to recognize and bind exogenous antigens, then present these antigens to HBV-specific CD4+ T cells via MHC-II to initiate a CD4+ T immune response. Notably, apart from MHC-II molecules, B cells were also found to express relatively high levels of major histocompatibility complex class I (MHC-I) molecules (78). Through the cross-presentation of HBcAg on MHC-I to specific CD8+ T cells, B cells are able to induce an HBcAg-specific cytotoxic T lymphocytes (CTLs) response and further prevent immune tolerance (13). At the same time, HBsAg, as a special exogenous antigen, is also related to the MHC-I molecules expressed on B cells. The study by Barnaba et al. demonstrated that HBsAg-specific B cells can cross-present HBsAg fragments to CTLs through MHC-I molecules (14); however, this process of inducing CTL cytotoxicity can cause the death of HBsAg-specific B cells and hinder the production of protective anti-HBs antibodies, which promote persistent HBV infection and long-term transmission.
During the presentation of the HBV antigen to activate T cells, HBV peptide-MHC complexes on APCs specifically bind the T-cell receptor (TCR) and then initiate the interaction between costimulatory molecules and receptors. Among them, the costimulatory molecules CD80 and CD86 expressed on APCs are required for the differentiation of T cells, and the interaction between CD80/86 and CD28 mediates critical T cell stimulatory signals (79–82). Therefore, phenotypic analysis of the cell surface molecules (especially MHC and CD80/CD86) is used as an effective surrogate readout for the antigen presentation function of B cells (83, 84). However, the frequency of circulating B cells expressing CD86 does not significantly change in CHB patients (85, 86). Notably, the expression of the costimulatory molecule CD80 and the frequency of HBsAg-specific B cells are significantly decreased in patients with immune tolerance, immune activation and immune clearance, but this decrease is reversed in patients after resolution, thus indicating a potentially impaired HBsAg peptide-presenting function of B cells in CHB patients (66). Another costimulatory molecule CD40 expression on B cells is decreased, which might influence the interaction between CD40 and CD40L and the secondary activation signal of B cells in CHB infection (87).
Other APCs such as dendritic cells (DCs), monocytes and macrophages may be also involved in the HBV immune response. DCs, the most efficient professional APCs, have the strongest antigen presenting ability and can stimulate initial T cell activation and proliferation (88). Although most studies have described defects in DCs that can hinder the T cell-mediated response (89–93), some reported functional molecules (e.g., CD83, CD86 and HLA-DR) of ex vivo-tested DCs remain largely intact in CHB patients (94, 95). Monocytes (MNs) compose about 10–15% of both the peripheral blood and intrasinusoidal mononuclear cell content. It was reported that MNs were the only professional antigen presenting cell in the peripheral blood of CHB patients to retain an HBsAg depot and in vitro differentiation of HBsAg+MNs to DCs stimulated expansion of autologous HBV-specific T cells (96). However, whether MNs from CHB patients might present the HBsAg depot to T cells in vivo remains to be determined. Liver macrophages, Kupffer cells (KCs), are the largest macrophage population in the human body. KCs have some credentials as APC, but the balance of data suggests they commonly promote T cell tolerance (97). KCs express MHC-I and MHC-II molecules, as well as co-stimulatory molecules CD80 and CD86 at low density (97). It was reported using a hydrodynamic HBV transfection model that HBcAg triggered TLR-2 on KCs, inducing IL-10 production that suppressed the HBV-specific CD8+T cell response (98). In addition, KCs might induce T cell exhaustion by upregulating galectin-9 expression in CHB patients (99).
Interestingly, in contrast to other APCs, B cells are characterized by the expression of B cell antigen receptors (BCRs), which ensure specificity during the recognition and binding of HBV antigens that mediate primary activation signal of B cells. Second, high affinity BCRs enable B cells to present specific antigens with high efficiency, even at extremely low antigen concentrations (100); in particular, when the host immune response is reactivated, activated, or there are memory B cells expressing MHCII and BCRs exhibiting strong antigen-presenting activity. Specifically, the specific molecular basis by which HBcAg binds to a high frequency of naive B cells might involve a linear motif expressed by some heavy or light BCR chains (101).
Of note, DCs, MNs, and KCs might affect B cells through secreting cytokines or presenting HBV antigen during CHB infection (33, 102–104). Follicular B cells can recognize the HBV antigens that not only exist alone but also are present on the surfaces of DCs or macrophages (102). B cell-activating factor (BAFF), an important cytokine for B lymphocyte activation, is elevated in CHB patients (105, 106). Moreover, HBeAg itself can stimulate monocytes to release BAFF, a response that might be associated with B-cell hyper-activation in CHB patients (104). KCs have been shown to promote the development of Tr1-like cells, which inactivated both Tfh cells and GC B cells via secreting IL-10, resulting in impaired GC formation and anti-HBs antibody production (55, 107).
In summary, B cells play an important role in presenting HBcAg to activate a T cell-mediated immune response, which contributes to achieving HBV clearance in chronic HBV infection (15). However, B cells may be attacked by HBsAg-specific CD8+ T cells that are activated by HBsAg presentation, which conversely play a deleterious role in HBV clearance. Regardless, the above immune responses are triggered by the BCR-antigen interaction required for efficient B cell activation (Figure 2). Thus, paying attention to the early events in this interaction would help obtain a deeper understanding of the B cell-mediated immune response in CHB patients. Additionally, it is important to note that, compared with healthy populations, a significant decrease or unchanged expression of co-stimulatory molecules, CD80 and CD86, among circulating B cells might be unfavorable for the interaction between B cells and effector T cells, which could not further amplify the T cell response during chronic HBV infection (66, 86). Therefore, there are some limitations associated with B cell presentation of HBV antigen to T cells, which should be also considered during chronic HBV infection.
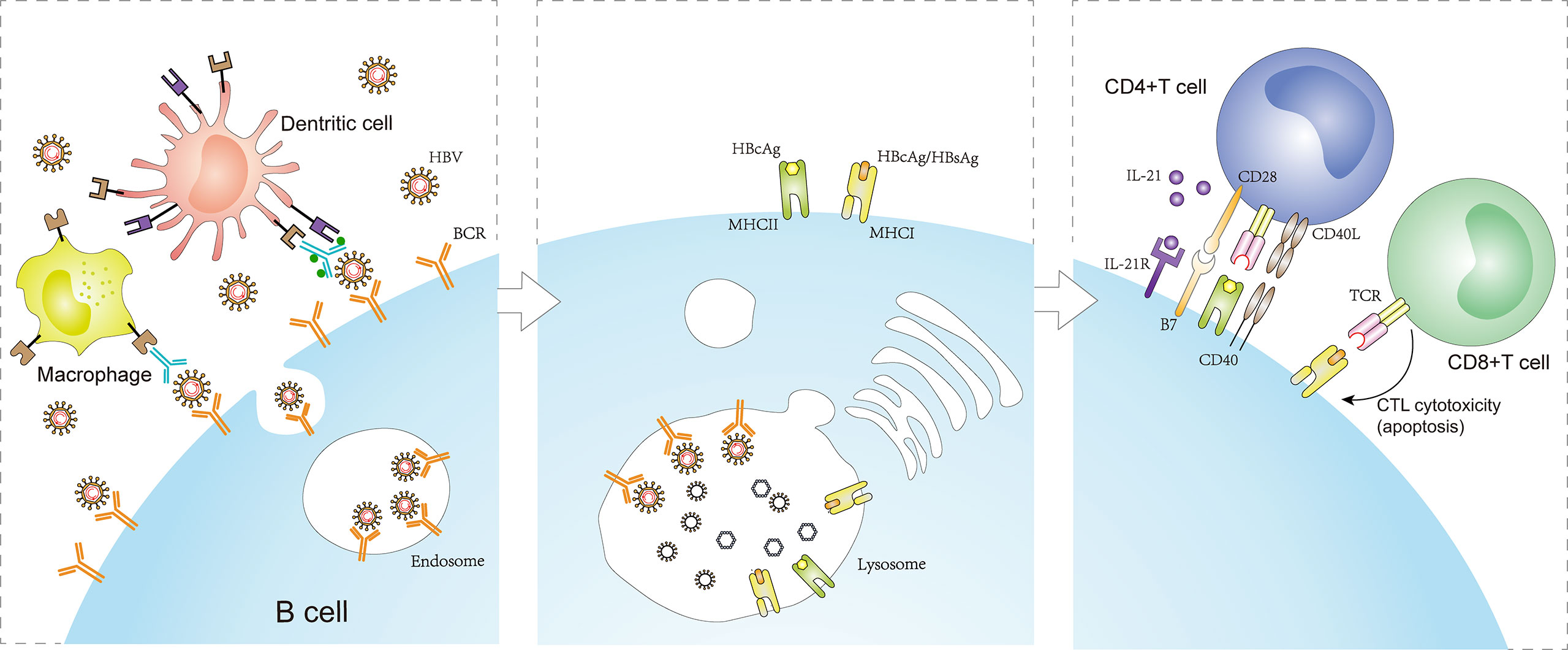
Figure 2 Antigen presentation function of B cells in CHB infection. The BCR specifically recognizes and binds HBV antigens that exist alone or are presented on the surface of macrophages or dendritic cells. The engagement enables BCR-antigen internalization into endosomes. Concomitant with receptor-mediated endocytosis, MHC-I/MHC-II molecules produced by B cells converge to form complexes with the HBcAg/HBsAg peptides processed in lysosomes and transferred to the plasma membrane. Naive CD4+ T cells are activated by B cells presenting HBcAg-MHC-II complexes, inducing a CD4+ T cell immune response. In addition, CD8+ T cells are activated by B cells presenting HBcAg/HBsAg-MHC-I complexes, inducing CTL cytotoxic reaction. However, the process promotes the apoptosis of HBsAg-infected cells (including HBsAg-infected B cells) and persistent HBV infection. BCRs, B cell antigen receptors; MHC-I, major histocompatibility complex class I; MHC-II, major histocompatibility complex class II.
Immune Regulatory Function of B Cells
In addition to antibody production and antigen presentation, B cells also regulate the immune response by secreting cytokines. B cells can be divided into effector B (Beff) cells and regulatory B (Breg) cells according to their specific cytokine secretion profile (108). Among them, Beff cells produce pro-inflammatory cytokines, such as interleukin (IL)-6, interferon (IFN)-γ and tumor necrosis factor (TNF)-α, to promote a pro-inflammatory immune response and influence effector and memory CD4+ T cell responses (33, 109). At the same time, these cytokines may play a non-cytolytic antiviral role for infected hepatocytes via inducing cccDNA decay or reducing HBV transcription (29, 30, 34). Moreover, two studies have demonstrated that IL-6 can also inhibit HBV entry via regulating the expression of an HBV-specific receptor, human liver bile acid transporter Na(+)/taurocholate co-transporting polypeptide (NTCP) (31, 110) (Figure 3). However, both the protection of hepatocytes and the tissue injury caused by IL-6 remain controversial and the reduction of these effector cytokines from atMBCs might limit their direct antiviral activity in CHB infection (32).
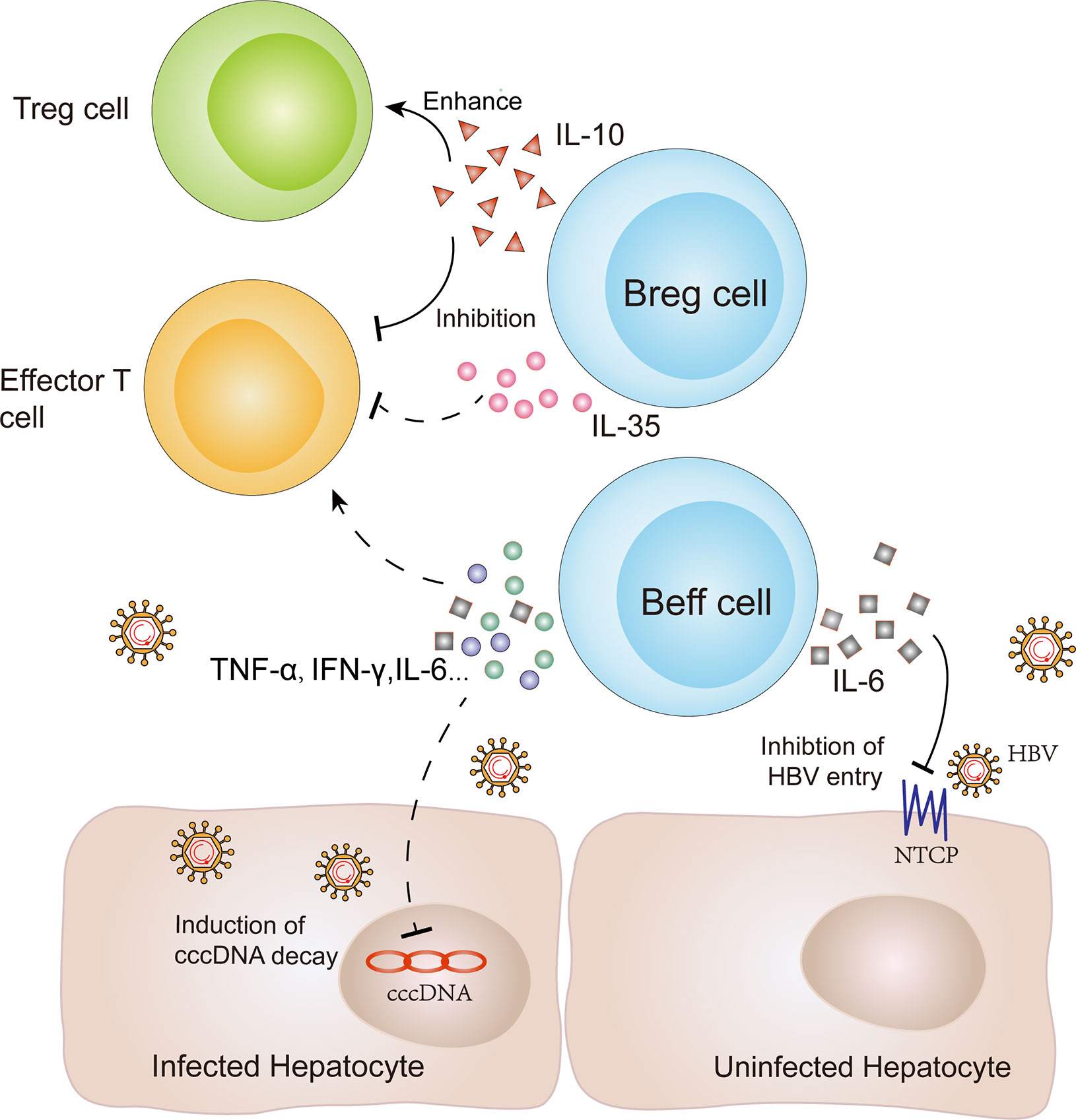
Figure 3 Immune regulation and antiviral function of B cells in CHB infection. B cells play potential immune regulatory roles to induce or inhibit the immune response via secreting different cytokines. Breg cells can produce IL-10 to inhibit effector T cell function and enhance Treg cell function. Furthermore, IL-35 secretion by Breg cells can inhibit the proliferation of naive effector T cells. In contrast, Beff cells produce proinflammatory cytokines, including interleukin (IL)-6, interferon (IFN)-γ, and tumor necrosis factor (TNF)-α, to promote effector and memory CD4+ T cell responses. At the same time, these cytokines might play a non-cytolytic antiviral role for infected hepatocytes via inducing cccDNA decay or reducing HBV transcription. In addition, IL-6 secretion by Beff cells can also inhibit HBV entry by regulating the expression of an HBV-specific receptor, known as NTCP. NTCP: Na(+)/taurocholate co-transporting polypeptide; Breg cells: regulatory B cells; Beff cells: effector B cells.
In contrast to Beff cells, Breg cells are suppressor cells, which secrete IL-10 as a crucial mediator for B cell-mediated regulation of other immune cells and the maintenance of immune tolerance (111). In the human phenotype, IL-10-producing B cells are enriched among CD19+ CD24hi CD38hi transitional B cells (112), CD19+ CD24hi CD27+ B10 cells (113), or both CD27+ memory and CD38hi transitional B cell subsets (114). In addition, Breg cell subsets expressing different surface markers have been identified, including CD19+CD25+ B cells (33), CD5+CD19+CD1dhi IL-10+ B cells (115), CD19+CD5+Foxp3+ B cells (116), and CD25+CD71+CD73− B cells (117). However, a lack of specific markers on Breg cells remains an unresolved challenge and may restrict Breg-related research in HBV infection. In addition, a study by Das et al. found that B cells producing IL-10 primarily exhibited immature/transitional CD19+ CD24hi CD38hi expression in CHB patients (118), which was utilized as a universal marker of Breg cells in related research. In subsequent horizontal and longitudinal studies, it was discovered that the proportion of Breg cells and the level of serum IL-10 were significantly increased and positively correlated with transaminase (ALT and AST) levels, viral load (HBV-DNA), and hepatic flares (118–120). These results suggest that both Breg cells and IL-10 regulate the anti-HBV immune response.
Specifically, IL-10-producing Breg cells play an important role in suppressing the HBV-specific CD8+ T cell response, whereas the depletion of Breg cells might restore the specific CD8+ T cell response (118). Since CD8+ T cells predominantly mediate the anti-HBV immune response, it can be inferred that both Breg cells and IL-10 are involved in HBV immune tolerance through suppressing CD8+ T cells during chronic HBV infection. However, the immune regulation of Breg cells is reflected by both the inhibition of CD8+ T cells, as well as the regulation of the CD4+ T cell response. Moreover, IL-10+ Breg cells can promote the inflammatory response of CD4+ CD25− effector T cells and the conversion to CD4+ CD25+ Treg cells, which suppress CTLs (25). Thus, Breg cells play a crucial role in inducing HBV immune tolerance through inhibiting effector T cells (effector CD4+ and CD8+ T cells) and enhancing Treg cells.
Although Breg cells regulate the Treg cell response, Treg cells have fewer effects on Breg cells during chronic HBV infection (26). However, TFR cells, which exhibit a similar inhibitory capacity as Treg cells, might influence the function of Breg cells. Wang et al. demonstrated that cTFR cells were enriched in the peripheral blood and elevated the inhibitory effect of Breg cells on CD8+ T cells in vitro (64). Furthermore, the signaling molecules associated with the regulatory function of Breg cells remain obscure during chronic HBV infection, in which Toll-like receptors (TLRs), B cell receptor (BCR), CD40, and costimulatory molecules CD80-CD86 on Breg cells may be considered to be relevant factors (121).
As described above, Beff cells produce proinflammatory cytokines to promote an HBV-specific immune response, whereas Breg cells play an immunosuppressive role with the help of cTFR cells in chronic HBV infection. Among them, IL-10 produced by Breg cells is a crucial cytokine associated with immune regulation. In addition, it is important to note that IL-35 is produced by Breg and Treg cells, as a novel member of the IL-12 family, which may represent another suppressive cytokine (27). Li et al. found that high levels of IL-35 expression in CD4+ T cells could inhibit the proliferation of HBV-specific CTL cells and the production of IFN-gamma (IFN-γ) in vitro (28). However, there remains a lack of research on IL-35 derived from Breg cells in HBV infection.
Immunotherapeutic Prospects of B Cells in Chronic HBV Infection
In lymphoma patients with HBV infection, treatment with rituximab (a therapeutic monoclonal antibody against CD20 that induces a profound depletion of B cells) significantly increases HBV reactivation (77, 122). This indicates that causing an effective B cell response (e.g., proliferation, cytokine secretion, and specific antibody response) and maintaining a stable B cell state are essential for the prevention of HBV reactivation and clearance of an HBV infection. Currently, pegylated interferon (Peg-IFN) and nucleoside analogs (NAs) have been approved and recommended as common first-line antiviral therapies for chronic HBV infection (123). In addition, these antiviral treatments are commonly used in two of the four clinical stages of chronic HBV infection: 1) immune activity (HBeAg-positive hepatitis); and 2) HBeAg negative hepatitis. In almost all patients with chronic HBV infection, these therapies display high efficacy, including disturbed B cell homeostasis, can be partially recovered (124); however, difficulties remain in achieving a loss of HBsAg and functional cure (persistently undetectable HBsAg) (125). A high load of HBsAg could further inhibit adaptive immune function and ultimately leads to specific tolerance that prevents patients from eradicating HBV infection. In addition, lifelong requirements for HBV antiviral therapy may increase the risk of drug toxicity. Under such circumstances, the recovery of B cell hyperactivation and functional impairment can contribute to HBsAg seroconversion of CHB patients (66). Thus, the development of long-term successful immunotherapy to further inhibit HBsAg via utilizing the multiple functions of B cells is required (Table 2).
Therapeutic HBV Vaccines
In animal experiments and early clinical studies, therapeutic vaccinations which trigger a novel immune response have substantial application prospects. To date, several categories of immunogens have been developed, including protein- or polypeptide-based, DNA- and viral vector-based vaccines. A vaccination with long peptide of preS1 domain was explored to clear HBV virions. Anti-preS1 antibody produced through sequential administration of preS1/HBsAg vaccines could finally induce HBsAg seroconversion in HBV carrier mice (126). In C57BL/6 mice, a therapeutic vaccine consisting of HBsAg/HBcAg and the CpG adjuvant elicited forceful humoral responses (147). When combined with saponin-based adjuvants, HBcAg/HBsAg compound vaccines could also induce cellular and humoral immune responses in HBV transgenic mice (148). The HBsAg-HBcAg nasal vaccine candidate (NASVAC) has shown safety and high immunogenicity in a phase I clinical trial (127, 149). Even in an open phase III trial, the efficacy of NASVAC in anti-HBV and HBeAg clearance is comparable to Peg-IFN in CHB patients (128). The recombinant yeast-based vaccine, GS-4774, contains HBV-specific antigens such as HBx protein and large HBsAg. Its safety, tolerability and immunogenicity have been verified in healthy participants (129). However, GS-4774 showed no benefit in significant HBsAg reduction in virally suppressed CHB patients (130).
Some DNA vaccines, such as PreS2 +S or PreS1-PreS2-S, also have been developed and found to induce an HBV-specific immune response in clinical trials (131, 134). In two randomized-controlled studies, PreS2 +S had limited therapeutic efficacy in immunotolerant CHB patients or inactive HBsAg carriers (132, 133). In contrast, the PreS1-PreS2-S vaccine induced anti-HBs antibodies in approximately half of the HBeAg+ CHB patients (134). Recently, novel DNA vaccine candidates comprising the S, PreS1, and Core antigens has been shown to induce specific immune response in rhesus macaques (135).
However, most therapeutic vaccinations depend on inducing an effective T cell response instead of B cell and antibody responses (130, 131). Moreover, there are limitations associated with completely restoring T cell functions and removing HBsAg with vaccinations (130, 132–134). Recently, based on the specific antibody-recognized HBsAg epitope (HBsAg-aa119−125), Zhang et al. developed a vaccine candidate (CR-T3-SEQ13) which induced a pivotal and long-term anti-HBs antibody response (136). Although studies related to the CR-T3-SEQ13 vaccine candidate are only in preliminary animal models, the therapeutic efficacy for improving the B cell-targeting vaccines might be expected.
Notably, the distributions of HBV genotypes and HLA alleles differ worldwide (150, 151). Developing therapeutic vaccines targeting B cells requires a sufficiently broad B cell repertoire covering the conserved regions of the HBV virus to ensure efficacy for different HLA populations and to prevent viral escape. In addition, most therapeutic vaccines are not developed primarily to restore the B cell response, and the detection of their functions are often neglected. Further exploring the changes in B cell function after treatment with vaccines might contribute to optimization of vaccine therapeutic approaches. Ultimately, clinical trials using HBsAg-based therapeutic vaccination have not shown lasting therapeutic effects in patients after cessation of nucleos(t)ide analogue treatment (152). Therefore, a combination of different immunotherapeutic interventions might be necessary to induce the B cell antibody response and HBV elimination.
TLR Agonists and Checkpoint Inhibitors
In contrast to therapeutic vaccinations, treatment with TLR agonists and checkpoint inhibitors is designed to reinvigorate the function of pre-existing anti-HBV immunity. Suthers et al. reported that crosstalk between the TLR7/TLR9 signaling pathway and the B cell receptor (BCR) may regulate the B cell response to antigens (153). Compared to antigen alone, mice that received both the antigen and TLR7/TLR9 ligand induced a more robust antibody response (154, 155).The TLR7 agonist GS-9620 has been shown to induce sustained humoral responses in woodchucks and chimps with CHB infection (156, 157). In addition, its safety and tolerance have been demonstrated in some clinical studies (138, 139). The TLR9 agonists CPG 7909 or 1018 ISS, co-administrated with HBsAg, have been found to induce a high antibody response in CHB patients (141–145). These results indicate that TLR7/TLR9 agonists may serve as adjuvants to improve the efficacy of B cell-targeting vaccines. The PD-1 checkpoint inhibitor, nivolumab, has been found to be safe and to lead to a decline in HBsAg in most virally suppressed CHB patients in a phase I clinical trial (137). Of note, the dysfunction of anti-HBs antibody-secreting B cells can be partially recovered by blocking PD-1, supporting the use of a PD-1 inhibitor to restore the normal function of B cells outside of T cells (42).
Furthermore, the expression of not only PD-1, but also the inhibitory receptors FcRL5, FcyRIIB, BTLA and CD22 is consistently enriched on HBsAg-specific B cells (32). Given the inhibition of BCR signaling, FcRL5 and FcγRIIB are likely to represent novel B cell therapeutic targets (158–160).
Activated B Cell Adjuvants
Currently, it is difficult to completely recover T cell function through clinical treatment, which also hinders the eradication of HBV infection. Therefore, in addition to B cell secretion of anti-HBs antibodies, attention should also be paid to the effect of B cells on T cells. To induce an HBV-specific T cell response, HBsAg/HBcAg-pulsed DC-vaccines have been designed to play the role of APCs and have exhibited immunogenicity in a phase I clinical trial (161). However, the high cost and associated technical requirements restrict the clinical application of DC adjuvants. At this point, utilizing B cells as APCs to present antigens may represent an alternative strategy to induce T cell-mediated immunity. Wu et al. demonstrated that the expression of MHC-I, MHC-II, CD80, and CD86 on B cells was increased following stimulation with human soluble CD40L (sCD40L) in vitro, and activated B cells may represent an adjuvant to present HBcAg to CTLs (146).
Target Breg Cells
In contrast to antigen presentation, B cells play an adverse role through the secretion of IL-10 during chronic HBV infection. As previously mentioned, Breg cells can both inhibit the effector T cell response, as well as enhance Treg cell suppression to further promote T cell-mediated immune tolerance. Therefore, it is possible to induce an effective immune response via effective Breg cell-targeted therapy in the future (162). However, treatment approaches that target Breg cells in chronic HBV infection remain lacking, one of the major limiting factors is the lack of surface-specific Breg cell markers (162). In HCC studies, enhanced expression of PD-1 has been found in a unique Breg cell subset (163); PD-1 blockade can be utilized to target Breg cells (164), indicating the importance of exploring specific Breg cell markers in CHB patients. In studies investigating the treatment of HCV infection and HCC, researchers have constructed the recombinant plasmid pcCD19scFv-IL10R to target mouse B10 cells and enhance the anti-HCV immune response or inhibit HCC growth in vivo (165, 166). These findings might also provide some clues for targeting Breg cells during CHB infection. Furthermore, it is important to note that blocking the inhibitory cytokines produced by Breg cells (e.g., IL-10 and IL-35) may also be useful to suppress the harmful effects of Breg cells and restore the effector T cell response in CHB patients (167).
In summary, targeting Breg cells is a promising immune therapy for CHB infection. However, some issues should be considered when developing approaches to target Breg cells. Although excessive Breg cells may lead to HBV immune tolerance, suitable Breg cells may contribute to suppressing HBV inflammation. The therapeutic degree of targeting B cells must be further investigated.
Conclusions and Future Research Agenda
HBV infection disrupts B cell homeostasis, leading to the dysregulation of B cell function (Table 3). Abnormal B cells play a significant role in a persistent HBV infection, as well as T cell-mediated immune tolerance and antibody-associated liver damage. Among these, antibody secretion, as well as immune regulation and antigen presentation of B cells are important in the pathogenesis of HBV infection.
It is important to note that B cell functionality is multi-dimensional. Specifically, in a highly immunogenic HBcAg infection, B cell-mediated cross-presentation may induce CTLs (13, 15), while Breg cells suppress the CTL response (118). In persistent HBsAg infection, B cells may secrete neutralizing antibodies to play a beneficial role (20, 21); however, B cells may also cross-present HBsAg to CTL cells to play an adverse role (14). Therefore, it would meaningful to elucidate how to restore B cell homeostasis and what measures should be taken in consideration of the advantages and disadvantages of B cells in HBV treatment.
In addition, particular attention should be paid to the effect of T cells on B cells, in which the dysfunction of TFH cells caused by Tregs and TFRs hinders the ability of B cells to secrete anti-HBs antibodies (52, 54–56). The development of HBV vaccines to restore the role of TFH cells may also facilitate B cell-mediated humoral immunity and the production of anti-HBs antibodies to functionally cure CHB patients.
Finally, in this paper, we primarily discuss the B cell function in adults with chronic HBV infection. In newborns, many components of the immune system are underdeveloped, and defects in the humoral and cellular immune response contribute to a predisposition to infection (168, 169). Based on B cell phenotype detection, HBsAg-positive infants had a higher level of immature transitional B cells at birth, which returned to normal one year after receiving the hepatitis B vaccine (170). These findings highlight the defects of B cells in neonatal HBV infection and the importance in developing vaccines to induce B cell maturation. In children with chronic hepatitis B infection, ANA(antinuclear antibodies) formation and a lower percentage of CD19+ B lymphocytes is part of the natural course of chronic infection, which may reflect a tendency towards autoimmune disease (171). Therefore, the functional characteristics of B cells in infants should be explored to optimize vaccine formulations for the early prevention and treatment of HBV infection in infants.
Author Contributions
WY designed and supervised the manuscript. YC and WY prepared the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by the National Natural Science Foundation of China (81770570, 81801990) and the Program for Outstanding Young Talent of Chongqing Kuanren Hospital and Natural Science Foundation of Chongqing, China (cstc2019jcyj-msxmX0007).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank International Science Editing (http://www.internationalscienceediting.com) for editing this manuscript.
References
1. Polaris Observatory C. Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study. Lancet Gastroenterol Hepatol (2018) 3:383–403. doi: 10.1016/S2468-1253(18)30056-6
2. Revill PA, Chisari FV, Block JM, Dandri M, Gehring AJ, Guo H, et al. A global scientific strategy to cure hepatitis B. Lancet Gastroenterol Hepatol (2019) 4:545–58. doi: 10.1016/S2468-1253(19)30119-0
3. Rehermann B, Nascimbeni M. Immunology of hepatitis B virus and hepatitis C virus infection. Nat Rev Immunol (2005) 5:215–29. doi: 10.1038/nri1573
4. Tseng TC, Huang LR. Immunopathogenesis of Hepatitis B Virus. J Infect Dis (2017) 216:S765–S70. doi: 10.1093/infdis/jix356
5. Mani H, Kleiner DE. Liver biopsy findings in chronic hepatitis B. Hepatology (2009) 49:S61–71. doi: 10.1002/hep.22930
6. Schurich A, Pallett LJ, Jajbhay D, Wijngaarden J, Otano I, Gill US, et al. Distinct Metabolic Requirements of Exhausted and Functional Virus-Specific CD8 T Cells in the Same Host. Cell Rep (2016) 16(5):1243–52. doi: 10.1016/j.celrep.2016.06.078
7. Ye B, Liu X, Li X, Kong H, Tian L. Chen Y. T-cell exhaustion in chronic hepatitis B infection: current knowledge and clinical significance. Cell Death Dis (2015) 6:e1694. doi: 10.1038/cddis.2015.42
8. Zhu W, Liu H, Zhang X. Toward Curative Immunomodulation Strategies for Chronic Hepatitis B Virus Infection. ACS Infect Dis (2019) 5:703–12. doi: 10.1021/acsinfecdis.8b00297
9. Fisicaro P, Barili V, Rossi M, Montali I, Vecchi A, Acerbi G, et al. Pathogenetic Mechanisms of T Cell Dysfunction in Chronic HBV Infection and Related Therapeutic Approaches. Front Immunol (2020) 11:849. doi: 10.3389/fimmu.2020.00849
10. Kandilarova SM, Georgieva AI, Mihaylova AP, Baleva MP, Atanasova VK, Petrova DV, et al. Immune Cell Subsets Evaluation as a Predictive Tool for Hepatitis B Infection Outcome and Treatment Responsiveness. Folia Med (Plovdiv) (2017) 59:53–62. doi: 10.1515/folmed-2017-0008
11. Walewska-Zielecka B, Madalinski K, Jablonska J, Godzik P, Cielecka-Kuszyk J, Litwinska B. Composition of inflammatory infiltrate and its correlation with HBV/HCV antigen expression. World J Gastroenterol (2008) 14(25):4040–6. doi: 10.3748/wjg.14.4040
12. Mohamadkhani A, Naderi E, Sotoudeh M, Katoonizadeh A, Montazeri G, Poustchi H. Clinical feature of intrahepatic B-lymphocytes in chronic hepatitis B. Int J Inflam (2014) 2014:896864. doi: 10.1155/2014/896864
13. Lazdina U, Alheim M, Nystrom J, Hultgren C, Borisova G, Sominskaya I, et al. Priming of cytotoxic T cell responses to exogenous hepatitis B virus core antigen is B cell dependent. J Gen Virol (2003) 84:139–46. doi: 10.1099/vir.0.18678-0
14. Barnaba V, Franco A, Alberti A, Benvenuto R, Balsano F. Selective killing of hepatitis B envelope antigen-specific B cells by class I-restricted, exogenous antigen-specific T lymphocytes. Nature (1990) 345(6272):258–60. doi: 10.1038/345258a0
15. Milich DR, Chen M, Schodel F, Peterson DL, Jones JE, Hughes JL. Role of B cells in antigen presentation of the hepatitis B core. Proc Natl Acad Sci U S A (1997) 94(26):14648–53. doi: 10.1073/pnas.94.26.14648
16. Lei Y, Hu T, Song X, Nie H, Chen M, Chen W, et al. Production of Autoantibodies in Chronic Hepatitis B Virus Infection Is Associated with the Augmented Function of Blood CXCR5+CD4+ T Cells. PLoS One (2016) 11:e0162241. doi: 10.1371/journal.pone.0162241
17. Chu CM, Liaw YF. Intrahepatic distribution of hepatitis B surface and core antigens in chronic hepatitis B virus infection. Hepatocyte with cytoplasmic/membranous hepatitis B core antigen as a possible target for immune hepatocytolysis. Gastroenterology (1987) 92:220–5. doi: 10.1016/0016-5085(87)90863-8
18. Chen Z, Diaz G, Pollicino T, Zhao H, Engle RE, Schuck P, et al. Role of humoral immunity against hepatitis B virus core antigen in the pathogenesis of acute liver failure. Proc Natl Acad Sci U S A (2018) 115(48):E11369–E78. doi: 10.1073/pnas.1809028115
19. Farci P, Diaz G, Chen Z, Govindarajan S, Tice A, Agulto L, et al. B cell gene signature with massive intrahepatic production of antibodies to hepatitis B core antigen in hepatitis B virus-associated acute liver failure. Proc Natl Acad Sci U States America (2010) 107(19):8766–71. doi: 10.1073/pnas.1003854107
20. Cerino A, Bremer CM, Glebe D, Mondelli MU. A Human Monoclonal Antibody against Hepatitis B Surface Antigen with Potent Neutralizing Activity. PLoS One (2015) 10:e0125704. doi: 10.1371/journal.pone.0125704
21. Petersen J, Dandri M, Mier W, Lütgehetmann M, Volz T, von Weizsäcker F, et al. Prevention of hepatitis B virus infection in vivo by entry inhibitors derived from the large envelope protein. Nat Biotechnol (2008) 26:335–41. doi: 10.1038/nbt1389
22. Ray MB, Desmet VJ, Fevery J, Groote JDE, Bradburne AF, Desmyter J. Distribution patterns of hepatitis B surface antigen (HBsAg) in the liver of hepatitis patients. J Clin Pathol (1976) 29:94–100. doi: 10.1136/jcp.29.2.94
23. Lu LL, Suscovich TJ, Fortune SM, Alter G. Beyond binding: antibody effector functions in infectious diseases. Nat Rev Immunol (2018) 18:46–61. doi: 10.1038/nri.2017.106
24. Yang YG, Liu YW, Hua HY, Li LJ. [Effect of Sanhuangyinchi decoction on liver damage and caspase-3 in rats with acute hepatic failure]. Nan Fang Yi Ke Da Xue Xue Bao J South Med Univ (2010) 30(11):2443–5. doi: 10.3724/SP.J.1238.2010.00512
25. Liu Y, Cheng LS, Wu SD, Wang SQ, Li L, She WM, et al. IL-10-producing regulatory B-cells suppressed effector T-cells but enhanced regulatory T-cells in chronic HBV infection. Clin Sci (Lond) (2016) 130(11):907–19. doi: 10.1042/CS20160069
26. Gong Y, Zhao C, Zhao P, Wang M, Zhou G, Han F, et al. Role of IL-10-Producing Regulatory B Cells in Chronic Hepatitis B Virus Infection. Dig Dis Sci (2015) 60:1308–14. doi: 10.1007/s10620-014-3358-1
27. Xiang XG, Xie Q. IL-35: a potential therapeutic target for controlling hepatitis B virus infection. J Dig Dis (2015) 16:1–6. doi: 10.1111/1751-2980.12218
28. Li X, Tian L, Dong Y, Zhu Q, Wang Y, Han W, et al. IL-35 inhibits HBV antigen-specific IFN-gamma-producing CTLs in vitro. Clin Sci (Lond) (2015) 129:395–404. doi: 10.1042/CS20140511
29. Palumbo GA, Scisciani C, Pediconi N, Lupacchini L, Alfalate D, Guerrieri F, et al. IL6 Inhibits HBV Transcription by Targeting the Epigenetic Control of the Nuclear cccDNA Minichromosome. PLoS One (2015) 10:e0142599. doi: 10.1371/journal.pone.0142599
30. Hösel M, Quasdorff M, Wiegmann K, Webb D, Zedler U, Broxtermann M, et al. Not interferon, but interleukin-6 controls early gene expression in hepatitis B virus infection. Hepatology (2009) 50:1773–82. doi: 10.1002/hep.23226
31. Bouezzedine F, Fardel O, Gripon P. Interleukin 6 inhibits HBV entry through NTCP down regulation. Virology (2015) 481:34–42. doi: 10.1016/j.virol.2015.02.026
32. Burton AR, Pallett LJ, McCoy LE, Suveizdyte K, Amin OE, Swadling L, et al. Circulating and intrahepatic antiviral B cells are defective in hepatitis B. J Clin Invest (2018) 128(10):4588–603. doi: 10.1172/JCI121960
33. Shen P, Fillatreau S. Antibody-independent functions of B cells: a focus on cytokines. Nat Rev Immunol (2015) 15:441–51. doi: 10.1038/nri3857
34. Xia Y, Stadler D, Lucifora J, Reisinger F, Webb D, Hösel M, et al. Interferon-γ and Tumor Necrosis Factor-α Produced by T Cells Reduce the HBV Persistence Form, cccDNA, Without Cytolysis. Gastroenterology (2016) 150:194–205. doi: 10.1053/j.gastro.2015.09.026
35. Gerlich WH. Medical virology of hepatitis B: how it began and where we are now. Virol J (2013) 10:239. doi: 10.1186/1743-422X-10-239
36. Trepo C, Chan HL, Lok A. Hepatitis B virus infection. Lancet (2014) 384:2053–63. doi: 10.1016/S0140-6736(14)60220-8
37. Zhou J, Song L, Zhao H, Yan L, Ma A, Xie S, et al. Serum hepatitis B core antibody as a biomarker of hepatic inflammation in chronic hepatitis B patients with normal alanine aminotransferase. Sci Rep (2017) 7:2747. doi: 10.1038/s41598-017-03102-3
38. Yuan Q, Song LW, Cavallone D, Moriconi F, Cherubini B, Colombatto P, et al. Total Hepatitis B Core Antigen Antibody, a Quantitative Non-Invasive Marker of Hepatitis B Virus Induced Liver Disease. PLoS One (2015) 10:e0130209. doi: 10.1371/journal.pone.0130209
39. Cai S, Li Z, Yu T, Xia M, Peng J. Serum hepatitis B core antibody levels predict HBeAg seroconversion in chronic hepatitis B patients with high viral load treated with nucleos(t)ide analogs. Infect Drug Resist (2018) 11:469–77. doi: 10.2147/IDR.S163038
40. Fan R, Sun J, Yuan Q, Xie Q, Bai X, Ning Q, et al. Baseline quantitative hepatitis B core antibody titre alone strongly predicts HBeAg seroconversion across chronic hepatitis B patients treated with peginterferon or nucleos(t)ide analogues. Gut (2016) 65:313–20. doi: 10.1136/gutjnl-2014-308546
41. Xu JH, Song LW, Li N, Wang S, Zeng Z, Si CW, et al. Baseline hepatitis B core antibody predicts treatment response in chronic hepatitis B patients receiving long-term entecavir. J Viral Hepat (2017) 24:148–54. doi: 10.1111/jvh.12626
42. Salimzadeh L, Le Bert N, Dutertre CA, Gill US, Newell EW, Frey C, et al. PD-1 blockade partially recovers dysfunctional virus-specific B cells in chronic hepatitis B infection. J Clin Invest (2018) 128(10):4573–87. doi: 10.1172/JCI121957
43. Neumann-Haefelin C, Thimme R. Entering the spotlight: hepatitis B surface antigen-specific B cells. J Clin Invest (2018) 128:4257–9. doi: 10.1172/JCI124098
44. Le Bert N, Salimzadeh L, Gill US, Dutertre CA, Facchetti F, Tan A, et al. Comparative characterization of B cells specific for HBV nucleocapsid and envelope proteins in patients with chronic hepatitis B. J Hepatol (2020) 72:34–44. doi: 10.1016/j.jhep.2019.07.015
45. Zgair AK, Ghafil JA, Al-Sayidi RH. Direct role of antibody-secreting B cells in the severity of chronic hepatitis B. J Med Virol (2015) 87(3):407–16. doi: 10.1002/jmv.24067
46. Li J, Zhang TY, Song LW, Qi X, Yu XP, Li FH, et al. Role of quantitative hepatitis B core antibody levels in predicting significant liver inflammation in chronic hepatitis B patients with normal or near-normal alanine aminotransferase levels. Hepatol Res (2018) 48:E133–E45. doi: 10.1111/hepr.12937
47. Pignatelli M, Waters J, Lever A, Iwarson S, Gerety R, Thomas HC. Cytotoxic T-cell responses to the nucleocapsid proteins of HBV in chronic hepatitis. Evidence that antibody modulation may cause protracted infection. J Hepatol (1987) 4:15–21. doi: 10.1016/s0168-8278(87)80004-1
48. Hu TT, Song XF, Lei Y, Hu HD, Ren H, Hu P. Expansion of circulating TFH cells and their associated molecules: involvement in the immune landscape in patients with chronic HBV infection. Virol J (2014) 11:54. doi: 10.1186/1743-422X-11-54
49. Zhang L, Zhang M, Li H, Chen Z, Luo A, Liu B, et al. Tfh cell-mediated humoral immune response and HBsAg level can predict HBeAg seroconversion in chronic hepatitis B patients receiving peginterferon-alpha therapy. Mol Immunol (2016) 73:37–45. doi: 10.1016/j.molimm.2016.03.011
50. Huang S, Wu J, Gao X, Zou S, Chen L, Yang X, et al. LSECs express functional NOD1 receptors: A role for NOD1 in LSEC maturation-induced T cell immunity in vitro. Mol Immunol (2018) 101:167–75. doi: 10.1016/j.molimm.2018.06.002
51. Ma SW, Huang X, Li YY, Tang LB, Sun XF, Jiang XT, et al. High serum IL-21 levels after 12 weeks of antiviral therapy predict HBeAg seroconversion in chronic hepatitis B. J Hepatol (2012) 56:775–81. doi: 10.1016/j.jhep.2011.10.020
52. Vyas AK, Sharma BC, Sarin S, Trehanpati N. Immune correlates of hepatitis B surface antigen spontaneous seroconversion in hepatitis B e antigen negative chronic hepatitis B patients. Liver Int (2018) 38:38–49. doi: 10.1111/liv.13475
53. Poonia B, Ayithan N, Nandi M, Masur H, Kottilil S. HBV induces inhibitory FcRL receptor on B cells and dysregulates B cell-T follicular helper cell axis. Sci Rep (2018) 8(1):15296. doi: 10.1038/s41598-018-33719-x
54. Chen Z, Liu Y, Yang L, Liu P, Zhang Y, Wang X. MiR-149 attenuates endoplasmic reticulum stress-induced inflammation and apoptosis in nonalcoholic fatty liver disease by negatively targeting ATF6 pathway. Immunol Lett (2020) 222:40–8. doi: 10.1016/j.imlet.2020.03.003
55. Xu L, Yin W, Sun R, Wei H, Tian Z. Liver type I regulatory T cells suppress germinal center formation in HBV-tolerant mice. Proc Natl Acad Sci U S A (2013) 110:16993–8. doi: 10.1073/pnas.1306437110
56. Wu X, Su Z, Cai B, Yan L, Li Y, Feng W, et al. Increased Circulating Follicular Regulatory T-Like Cells May Play a Critical Role in Chronic Hepatitis B Virus Infection and Disease Progression. Viral Immunol (2018) 31:379–88. doi: 10.1089/vim.2017.0171
57. Linterman MA, Pierson W, Lee SK, Kallies A, Kawamoto S, Rayner TF, et al. Foxp3+ follicular regulatory T cells control the germinal center response. Nat Med (2011) 17:975–82. doi: 10.1038/nm.2425
58. Wollenberg I, Agua-Doce A, Hernandez A, Almeida C, Oliveira VG, Faro J, et al. Regulation of the germinal center reaction by Foxp3+ follicular regulatory T cells. J Immunol (2011) 187:4553–60. doi: 10.4049/jimmunol.1101328
59. Milich DR, McLachlan A. The nucleocapsid of hepatitis B virus is both a T-cell-independent and a T-cell-dependent antigen. Science (1986) 234:1398–401. doi: 10.1126/science.3491425
60. Milich DR, McLachlan A, Thornton GB, Hughes JL. Antibody production to the nucleocapsid and envelope of the hepatitis B virus primed by a single synthetic T cell site. Nature (1987) 329(6139):547–9. doi: 10.1038/329547a0
61. Cao T, Lazdina U, Desombere I, Vanlandschoot P, Milich DR, Sallberg M, et al. Hepatitis B virus core antigen binds and activates naive human B cells in vivo: studies with a human PBL-NOD/SCID mouse model. J Virol (2001) 75(14):6359–66. doi: 10.1128/JVI.75.14.6359-6366.2001
62. MacLennan IC. Germinal centers. Annu Rev Immunol (1994) 12:117–39. doi: 10.1146/annurev.iy.12.040194.001001
63. Rajewsky K. Clonal selection and learning in the antibody system. Nature (1996) 381:751–8. doi: 10.1038/381751a0
64. Wang R, Xie R, Song Z. Circulating regulatory Tfh cells are enriched in patients with chronic hepatitis B infection and induce the differentiation of regulatory B cells. Exp Cell Res (2018) 365(2):171–6. doi: 10.1016/j.yexcr.2018.02.031
65. Tian C, Chen Y, Liu Y, Wang S, Li Y, Wang G, et al. Use of ELISpot assay to study HBs-specific B cell responses in vaccinated and HBV infected humans. Emerg Microbes Infect (2018) 7:16. doi: 10.1038/s41426-018-0034-0
66. Xu X, Shang Q, Chen X, Nie W, Zou Z, Huang A, et al. Reversal of B-cell hyperactivation and functional impairment is associated with HBsAg seroconversion in chronic hepatitis B patients. Cell Mol Immunol (2015) 12:309–16. doi: 10.1038/cmi.2015.25
67. Forde KA. Ethnic Disparities in Chronic Hepatitis B Infection: African Americans and Hispanic Americans. Curr Hepatol Rep (2017) 16:105–12. doi: 10.1007/s11901-017-0348-8
68. Kim H, Rotundo L, Yang JD, Kim D, Kothari N, Feurdean M, et al. Racial/ethnic disparities in the prevalence and awareness of Hepatitis B virus infection and immunity in the United States. J Viral Hepat (2017) 24:1052–66. doi: 10.1111/jvh.12735
69. Ghany MG, Perrillo R, Li R, Belle SH, Janssen HL, Terrault NA, et al. Characteristics of adults in the hepatitis B research network in North America reflect their country of origin and hepatitis B virus genotype. Clin Gastroenterol Hepatol (2015) 13:183–92. doi: 10.1016/j.cgh.2014.06.028
70. Marx G, Martin SR, Chicoine JF, Alvarez F. Long-term follow-up of chronic hepatitis B virus infection in children of different ethnic origins. J Infect Dis (2002) 186:295–301. doi: 10.1086/341508
71. Nicastro E, Mangili B, Giacomet V, Benincaso AR, Di Giorgio A, Sansotta N, et al. Longitudinal Immune Phenotype Assessment and Serological Outcome in Foreign-born Children With Chronic Hepatitis B. J Pediatr Gastroenterol Nutr (2020) 71:381–7. doi: 10.1097/MPG.0000000000002804
72. Van Hees S, Chi H, Hansen B, Bourgeois S, Van Vlierberghe H, Sersté T, et al. Caucasian Ethnicity, but Not Treatment Cessation is Associated with HBsAg Loss Following Nucleos(t)ide Analogue-Induced HBeAg Seroconversion. Viruses (2019) 11:687. doi: 10.3390/v11080687
73. Thomas R, Thio CL, Apps R, Qi Y, Gao X, Marti D, et al. A novel variant marking HLA-DP expression levels predicts recovery from hepatitis B virus infection. J Virol (2012) 86:6979–85. doi: 10.1128/JVI.00406-12
74. Su K, Watanabe A, Yeh C, Kelsoe G, Kuraoka M. Efficient Culture of Human Naive and Memory B Cells for Use as APCs. J Immunol (2016) 197:4163–76. doi: 10.4049/jimmunol.1502193
75. Seto WK, Chan TS, Hwang YY, Wong DK, Fung J, Liu KS, et al. Hepatitis B reactivation in patients with previous hepatitis B virus exposure undergoing rituximab-containing chemotherapy for lymphoma: a prospective study. J Clin Oncol Off J Am Soc Clin Oncol (2014) 32:3736–43. doi: 10.1200/JCO.2014.56.7081
76. Cho Y, Yu SJ, Cho EJ, Lee JH, Kim TM, Heo DS, et al. High titers of anti-HBs prevent rituximab-related viral reactivation in resolved hepatitis B patient with non-Hodgkin’s lymphoma. J Med Virol (2016) 88:1010–7. doi: 10.1002/jmv.24423
77. Paul S, Dickstein A, Saxena A, Terrin N, Viveiros K, Balk EM, et al. Role of surface antibody in hepatitis B reactivation in patients with resolved infection and hematologic malignancy: A meta-analysis. Hepatology (2017) 66:379–88. doi: 10.1002/hep.29082
78. Bennett SR, Carbone FR, Toy T, Miller JF, Heath WR. B cells directly tolerize CD8(+) T cells. J Exp Med (1998) 188(11):1977–83. doi: 10.1084/jem.188.11.1977
79. Chakraborty NG, Li L, Sporn JR, Kurtzman SH, Ergin MT, Mukherji B. Emergence of regulatory CD4+ T cell response to repetitive stimulation with antigen-presenting cells in vitro: implications in designing antigen-presenting cell-based tumor vaccines. J Immunol (1999) 162:5576–83.
80. Freeman GJ, Gribben JG, Boussiotis VA, Ng JW, Restivo VA, Lombard LA, et al. Cloning of B7-2: a CTLA-4 counter-receptor that costimulates human T cell proliferation. Science (1993) 262:909–11. doi: 10.1126/science.7694363
81. Azuma M, Ito D, Yagita H, Okumura K, Phillips JH, Lanier LL, et al. B70 antigen is a second ligand for CTLA-4 and CD28. Nature (1993) 366:76–9. doi: 10.1038/366076a0
82. Linsley PS, Brady W, Grosmaire L, Aruffo A, Damle NK, Ledbetter JA. Binding of the B cell activation antigen B7 to CD28 costimulates T cell proliferation and interleukin 2 mRNA accumulation. J Exp Med (1991) 173:721–30. doi: 10.1084/jem.173.3.721
83. Noorchashm H, Reed AJ, Rostami SY, Mozaffari R, Zekavat G, Koeberlein B, et al. B cell-mediated antigen presentation is required for the pathogenesis of acute cardiac allograft rejection. J Immunol (2006) 177:7715–22. doi: 10.4049/jimmunol.177.11.7715
84. Zhong B, Huang MP, Yin GQ, Gao X. Effects of costimulation on intrahepatic immunopathogenesis in patients with chronic HBV infection. Inflammation Res (2014) 63:217–29. doi: 10.1007/s00011-013-0691-3
85. Wongjitrat C, Sukwit S, Chuenchitra T, Seangjaruk P, Rojanasang P, Romputtan P, et al. CTLA-4 and its ligands on the surface of T- and B-lymphocyte subsets in chronic hepatitis B virus infection. J Med Assoc Thai (2013) 1:S54–9. doi: 10.1017/S001667230002379X
86. Zhao PW, Ma L, Ji HF, Yu L, Feng JY, Wang J, et al. The expression of TLR-9, CD86, and CD95 phenotypes in circulating B cells of patients with chronic viral hepatitis B or C before and after antiviral therapy. Mediators Inflamm (2015) 2015:762709. doi: 10.1155/2015/762709
87. Xing T, Xu H, Yu W. Role of T follicular helper cells and their associated molecules in the pathogenesis of chronic hepatitis B virus infection. Exp Ther Med (2013) 5:885–9. doi: 10.3892/etm.2012.864
88. Sun HH, Zhou DF, Zhou JY. The role of DCs in the immunopathogenesis of chronic HBV infection and the methods of inducing DCs maturation. J Med Virol (2016) 88:13–20. doi: 10.1002/jmv.24306
89. Beckebaum S, Cicinnati VR, Dworacki G, Muller-Berghaus J, Stolz D, Harnaha J, et al. Reduction in the circulating pDC1/pDC2 ratio and impaired function of ex vivo-generated DC1 in chronic hepatitis B infection. Clin Immunol (2002) 104(2):138–50. doi: 10.1006/clim.2002.5245
90. van der Molen RG, Sprengers D, Binda RS, de Jong EC, Niesters HG, Kusters JG, et al. Functional impairment of myeloid and plasmacytoid dendritic cells of patients with chronic hepatitis B. Hepatology (2004) 40(3):738–46. doi: 10.1002/hep.20366
91. Beckebaum S, Cicinnati VR, Zhang X, Ferencik S, Frilling A, Grosse-Wilde H, et al. Hepatitis B virus-induced defect of monocyte-derived dendritic cells leads to impaired T helper type 1 response in vitro: mechanisms for viral immune escape. Immunology (2003) 109(4):487–95. doi: 10.1046/j.1365-2567.2003.01699.x
92. Op den Brouw ML, Binda RS, van Roosmalen MH, Protzer U, Janssen HLA, van der Molen RG, et al. Hepatitis B virus surface antigen impairs myeloid dendritic cell function: a possible immune escape mechanism of hepatitis B virus. Immunology (2009) 126:280–9. doi: 10.1111/j.1365-2567.2008.02896.x
93. Wang FS, Xing LH, Liu MX, Zhu CL, Liu HG, Wang HF, et al. Dysfunction of peripheral blood dendritic cells from patients with chronic hepatitis B virus infection. World J Gastroenterol (2001) 7:537–41. doi: 10.3748/wjg.v7.i4.537
94. Tavakoli S, Mederacke I, Herzog-Hauff S, Glebe D, Grün S, Strand D, et al. Peripheral blood dendritic cells are phenotypically and functionally intact in chronic hepatitis B virus (HBV) infection. Clin Exp Immunol (2008) 151:61–70. doi: 10.1111/j.1365-2249.2007.03547.x
95. Tavakoli S, Schwerin W, Rohwer A, Hoffmann S, Weyer S, Weth R, et al. Phenotype and function of monocyte derived dendritic cells in chronic hepatitis B virus infection. J Gen Virol (2004) 85:2829–36. doi: 10.1099/vir.0.80143-0
96. Gehring AJ, Haniffa M, Kennedy PT, Ho ZZ, Boni C, Shin A, et al. Mobilizing monocytes to cross-present circulating viral antigen in chronic infection. J Clin Invest (2013) 123:3766–76. doi: 10.1172/JCI66043
97. Crispe IN. Liver antigen-presenting cells. J Hepatol (2011) 54(2):357–65. doi: 10.1016/j.jhep.2010.10.005
98. Li M, Sun R, Xu L, Yin W, Chen Y, Zheng X, et al. Kupffer Cells Support Hepatitis B Virus-Mediated CD8+ T Cell Exhaustion via Hepatitis B Core Antigen-TLR2 Interactions in Mice. J Immunol (2015) 195(7):3100–9. doi: 10.4049/jimmunol.1500839
99. Nebbia G, Peppa D, Schurich A, Khanna P, Singh HD, Cheng Y, et al. Upregulation of the Tim-3/galectin-9 pathway of T cell exhaustion in chronic hepatitis B virus infection. PLoS One (2012) 7(10):e47648. doi: 10.1371/journal.pone.0047648
100. Pape KA, Catron DM, Itano AA, Jenkins MK. The humoral immune response is initiated in lymph nodes by B cells that acquire soluble antigen directly in the follicles. Immunity (2007) 26:491–502. doi: 10.1016/j.immuni.2007.02.011
101. Lazdina U, Cao T, Steinbergs J, Alheim M, Pumpens P, Peterson DL, et al. Molecular basis for the interaction of the hepatitis B virus core antigen with the surface immunoglobulin receptor on naive B cells. J Virol (2001) 75:6367–74. doi: 10.1128/JVI.75.14.6367-6374.2001
102. Batista FD, Harwood NE. The who, how and where of antigen presentation to B cells. Nat Rev Immunol (2009) 9:15–27. doi: 10.1038/nri2454
103. Jego G, Pascual V, Palucka AK, Banchereau J. Dendritic cells control B cell growth and differentiation. Curr Dir Autoimmun (2005) 8:124–39. doi: 10.1159/000082101
104. Lu B, Zhang B, Wang L, Ma C, Liu X, Zhao Y, et al. Hepatitis B Virus e Antigen Regulates Monocyte Function and Promotes B Lymphocyte Activation. Viral Immunol (2017) 30:35–44. doi: 10.1089/vim.2016.0113
105. Yang C, Li N, Wang Y, Zhang P, Zhu Q, Li F, et al. Serum levels of B-cell activating factor in chronic hepatitis B virus infection: association with clinical diseases. J Interferon Cytokine Res (2014) 34:787–94. doi: 10.1089/jir.2014.0032
106. Khlaiphuengsin A, Chuaypen N, Hirankarn N, Avihingsanon A, Crane M, Lewin SR, et al. Circulating BAFF and CXCL10 levels predict response to pegylated interferon in patients with HBeAg-positive chronic hepatitis B. Asian Pac J Allergy Immunol (2019). doi: 10.12932/AP-050718-0365
107. Xu L, Yin W, Sun R, Wei H, Tian Z. Kupffer cell-derived IL-10 plays a key role in maintaining humoral immune tolerance in hepatitis B virus-persistent mice. Hepatology (2014) 59(2):443–52. doi: 10.1002/hep.26668
108. Hamze M, Desmetz C, Guglielmi P. B cell-derived cytokines in disease. Eur Cytokine Netw (2013) 24(1):20–6. doi: 10.1684/ecn.2013.0327
109. Duddy ME, Alter A, Bar-Or A. Distinct profiles of human B cell effector cytokines: a role in immune regulation? J Immunol (2004) 172:3422–7. doi: 10.4049/jimmunol.172.6.3422
110. Yan H, Zhong G, Xu G, He W, Jing Z, Gao Z, et al. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. Elife (2012) 1:e00049. doi: 10.7554/eLife.00049
111. Mauri C. Regulation of immunity and autoimmunity by B cells. Curr Opin Immunol (2010) 22(6):761–7. doi: 10.1016/j.coi.2010.10.009
112. Blair PA, Norena LY, Flores-Borja F, Rawlings DJ, Isenberg DA, Ehrenstein MR, et al. CD19(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic Lupus Erythematosus patients. Immunity (2010) 32(1):129–40. doi: 10.1016/j.immuni.2009.11.009
113. Iwata Y, Matsushita T, Horikawa M, Dilillo DJ, Yanaba K, Venturi GM, et al. Characterization of a rare IL-10-competent B-cell subset in humans that parallels mouse regulatory B10 cells. Blood (2011) 117:530–41. doi: 10.1182/blood-2010-07-294249
114. Bouaziz JD, Calbo S, Maho-Vaillant M, Saussine A, Bagot M, Bensussan A, et al. IL-10 produced by activated human B cells regulates CD4(+) T-cell activation in vitro. Eur J Immunol (2010) 40:2686–91. doi: 10.1002/eji.201040673
115. Kessel A, Haj T, Peri R, Snir A, Melamed D, Sabo E, et al. Human CD19(+)CD25(high) B regulatory cells suppress proliferation of CD4(+) T cells and enhance Foxp3 and CTLA-4 expression in T-regulatory cells. Autoimmun Rev (2012) 11:670–7. doi: 10.1016/j.autrev.2011.11.018
116. Noh J, Choi WS, Noh G, Lee JH. Presence of Foxp3-expressing CD19(+)CD5(+) B Cells in Human Peripheral Blood Mononuclear Cells: Human CD19(+)CD5(+)Foxp3(+) Regulatory B Cell (Breg). Immune Netw (2010) 10:247–9. doi: 10.4110/in.2010.10.6.247
117. van de Veen W, Stanic B, Yaman G, Wawrzyniak M, Sollner S, Akdis DG, et al. IgG4 production is confined to human IL-10-producing regulatory B cells that suppress antigen-specific immune responses. J Allergy Clin Immunol (2013) 131:1204–12. doi: 10.1016/j.jaci.2013.01.014
118. Das A, Ellis G, Pallant C, Lopes AR, Khanna P, Peppa D, et al. IL-10-producing regulatory B cells in the pathogenesis of chronic hepatitis B virus infection. J Immunol (2012) 189:3925–35. doi: 10.4049/jimmunol.1103139
119. Wang L, Qiu J, Yu L, Hu X, Zhao P, Jiang Y. Increased numbers of CD5+CD19+CD1dhighIL-10+ Bregs, CD4+Foxp3+ Tregs, CD4+CXCR5+Foxp3+ follicular regulatory T (TFR) cells in CHB or CHC patients. J Transl Med (2014) 12:251. doi: 10.1186/s12967-014-0251-9
120. Wang G, Liu Y, Huang R, Jia B, Su R, Sun Z, et al. Characteristics of regulatory B cells in patients with chronic hepatitis B virus infection in different immune phases. Discov Med (2017) 23:295–304.
121. Mauri C, Bosma A. Immune regulatory function of B cells. Annu Rev Immunol (2012) 30:221–41. doi: 10.1146/annurev-immunol-020711-074934
122. Loomba R, Liang TJ. Hepatitis B Reactivation Associated With Immune Suppressive and Biological Modifier Therapies: Current Concepts, Management Strategies, and Future Directions. Gastroenterology (2017) 152:1297–309. doi: 10.1053/j.gastro.2017.02.009
123. Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology (2018) 67(4):1560–99. doi: 10.1002/hep.29800
124. Sun H, Lv J, Tu Z, Hu X, Yan H, Pan Y, et al. Antiviral treatment improves disrupted peripheral B lymphocyte homeostasis in chronic hepatitis B virus-infected patients. Exp Biol Med (Maywood) (2013) 238(11):1275–83. doi: 10.1177/1535370213502626
125. Tout I, Gomes M, Ainouze M, Marotel M, Pecoul T, Durantel D, et al. Hepatitis B Virus Blocks the CRE/CREB Complex and Prevents TLR9 Transcription and Function in Human B Cells. J Immunol (2018) 201(8):2331–44. doi: 10.4049/jimmunol.1701726
126. Bian Y, Zhang Z, Sun Z, Zhao J, Zhu D, Wang Y, et al. Vaccines targeting preS1 domain overcome immune tolerance in hepatitis B virus carrier mice. Hepatology (2017) 66:1067–82. doi: 10.1002/hep.29239
127. Al-Mahtab M, Akbar SM, Aguilar JC, Uddin MH, Khan MS, Rahman S. Therapeutic potential of a combined hepatitis B virus surface and core antigen vaccine in patients with chronic hepatitis B. Hepatol Int (2013) 7:981–9. doi: 10.1007/s12072-013-9486-4
128. Al Mahtab M, Akbar S, Aguilar JC, Guillen G, Penton E, Tuero A, et al. Treatment of chronic hepatitis B naive patients with a therapeutic vaccine containing HBs and HBc antigens (a randomized, open and treatment controlled phase III clinical trial). PLoS One (2018) 13:e0201236. doi: 10.1371/journal.pone.0201236
129. Gaggar A, Coeshott C, Apelian D, Rodell T, Armstrong BR, Shen G, et al. Safety, tolerability and immunogenicity of GS-4774, a hepatitis B virus-specific therapeutic vaccine, in healthy subjects: a randomized study. Vaccine (2014) 32:4925–31. doi: 10.1016/j.vaccine.2014.07.027
130. Lok AS, Pan CQ, Han SH, Trinh HN, Fessel WJ, Rodell T, et al. Randomized phase II study of GS-4774 as a therapeutic vaccine in virally suppressed patients with chronic hepatitis B. J Hepatol (2016) 65:509–16. doi: 10.1016/j.jhep.2016.05.016
131. Mancini-Bourgine M, Fontaine H, Bréchot C, Pol S, Michel ML. Immunogenicity of a hepatitis B DNA vaccine administered to chronic HBV carriers. Vaccine (2006) 24:4482–9. doi: 10.1016/j.vaccine.2005.08.013
132. Yalcin K, Acar M, Degertekin H. Specific hepatitis B vaccine therapy in inactive HBsAg carriers: a randomized controlled trial. Infection (2003) 31:221–5. doi: 10.1007/s15010-003-3187-1
133. Yalcin K, Danis R, Degertekin H, Alp MN, Tekes S, Budak T. The lack of effect of therapeutic vaccination with a pre-S2/S HBV vaccine in the immune tolerant phase of chronic HBV infection. J Clin Gastroenterol (2003) 37:330–5. doi: 10.1097/00004836-200310000-00012
134. Hoa P, Huy NT, Thu LT, Nga CN, Nakao K, Eguchi K, et al. Randomized controlled study investigating viral suppression and serological response following pre-S1/pre-S2/S vaccine therapy combined with lamivudine treatment in HBeAg-positive patients with chronic hepatitis B. Antimicrob Agents Chemother (2009) 53:5134–40. doi: 10.1128/AAC.00276-09
135. Chuai X, Xie B, Chen H, Tan X, Wang W, Huang B, et al. The immune response of rhesus macaques to novel vaccines comprising hepatitis B virus S, PreS1, and Core antigens. Vaccine (2018) 36:3740–6. doi: 10.1016/j.vaccine.2018.05.061
136. Zhang TY, Guo XR, Wu YT, Kang XZ, Zheng QB, Qi RY, et al. A unique B cell epitope-based particulate vaccine shows effective suppression of hepatitis B surface antigen in mice. Gut (2020) 69:343–54. doi: 10.1136/gutjnl-2018-317725
137. Gane E, Verdon DJ, Brooks AE, Gaggar A, Nguyen AH, Subramanian GM, et al. Anti-PD-1 blockade with nivolumab with and without therapeutic vaccination for virally suppressed chronic hepatitis B: A pilot study. J Hepatol (2019) 71:900–7. doi: 10.1016/j.jhep.2019.06.028
138. Janssen HLA, Brunetto MR, Kim YJ, Ferrari C, Massetto B, Nguyen AH, et al. Safety, efficacy and pharmacodynamics of vesatolimod (GS-9620) in virally suppressed patients with chronic hepatitis B. J Hepatol (2018) 68:431–40. doi: 10.1016/j.jhep.2017.10.027
139. Agarwal K, Ahn SH, Elkhashab M, Lau AH, Gaggar A, Bulusu A, et al. Safety and efficacy of vesatolimod (GS-9620) in patients with chronic hepatitis B who are not currently on antiviral treatment. J Viral Hepat (2018) 25:1331–40. doi: 10.1111/jvh.12942
140. Boni C, Vecchi A, Rossi M, Laccabue D, Giuberti T, Alfieri A, et al. TLR7 Agonist Increases Responses of Hepatitis B Virus-Specific T Cells and Natural Killer Cells in Patients With Chronic Hepatitis B Treated With Nucleos(T)Ide Analogues. Gastroenterology (2018) 154:1764–77. doi: 10.1053/j.gastro.2018.01.030
141. Siegrist CA, Pihlgren M, Tougne C, Efler SM, Morris ML, AlAdhami MJ, et al. Co-administration of CpG oligonucleotides enhances the late affinity maturation process of human anti-hepatitis B vaccine response. Vaccine (2004) 23:615–22. doi: 10.1016/j.vaccine.2004.07.014
142. Jegerlehner A, Maurer P, Bessa J, Hinton HJ, Kopf M, Bachmann MF. TLR9 signaling in B cells determines class switch recombination to IgG2a. J Immunol (2007) 178:2415–20. doi: 10.4049/jimmunol.178.4.2415
143. Cooper CL, Davis HL, Morris ML, Efler SM, Adhami MA, Krieg AM, et al. CPG 7909, an immunostimulatory TLR9 agonist oligodeoxynucleotide, as adjuvant to Engerix-B HBV vaccine in healthy adults: a double-blind phase I/II study. J Clin Immunol (2004) 24:693–701. doi: 10.1007/s10875-004-6244-3
144. Halperin SA, Dobson S, McNeil S, Langley JM, Smith B, McCall-Sani R, et al. Comparison of the safety and immunogenicity of hepatitis B virus surface antigen co-administered with an immunostimulatory phosphorothioate oligonucleotide and a licensed hepatitis B vaccine in healthy young adults. Vaccine (2006) 24:20–6. doi: 10.1016/j.vaccine.2005.08.095
145. Halperin SA, Ward B, Cooper C, Predy G, Diaz-Mitoma F, Dionne M, et al. Comparison of safety and immunogenicity of two doses of investigational hepatitis B virus surface antigen co-administered with an immunostimulatory phosphorothioate oligodeoxyribonucleotide and three doses of a licensed hepatitis B vaccine in healthy adults 18-55 years of age. Vaccine (2012) 30:2556–63. doi: 10.1016/j.vaccine.2012.01.087
146. Wu C, Liu Y, Zhao Q, Chen G, Chen J, Yan X, et al. Soluble CD40 ligand-activated human peripheral B cells as surrogated antigen presenting cells: A preliminary approach for anti-HBV immunotherapy. Virol J (2010) 7:370. doi: 10.1186/1743-422X-7-370
147. Li J, Ge J, Ren S, Zhou T, Sun Y, Sun H, et al. Hepatitis B surface antigen (HBsAg) and core antigen (HBcAg) combine CpG oligodeoxynucletides as a novel therapeutic vaccine for chronic hepatitis B infection. Vaccine (2015) 33:4247–54. doi: 10.1016/j.vaccine.2015.03.079
148. Buchmann P, Dembek C, Kuklick L, Jäger C, Tedjokusumo R, von Freyend MJ, et al. A novel therapeutic hepatitis B vaccine induces cellular and humoral immune responses and breaks tolerance in hepatitis B virus (HBV) transgenic mice. Vaccine (2013) 31:1197–203. doi: 10.1016/j.vaccine.2012.12.074
149. Betancourt AA, Delgado CA, Estevez ZC, Martinez JC, Rios GV, Aureoles-Rosello SR, et al. Phase I clinical trial in healthy adults of a nasal vaccine candidate containing recombinant hepatitis B surface and core antigens. Int J Infect Dis (2007) 11(5):394–401. doi: 10.1016/j.ijid.2006.09.010
150. Lin CL, Kao JH. Hepatitis B virus genotypes and variants. Cold Spring Harb Perspect Med (2015) 5:a021436. doi: 10.1101/cshperspect.a021436
151. Singh R, Kaul R, Kaul A, Khan K. A comparative review of HLA associations with hepatitis B and C viral infections across global populations. World J Gastroenterol (2007) 13:1770–87. doi: 10.3748/wjg.v13.i12.1770
152. Lee YB, Lee JH, Kim YJ, Yoon JH, Lee HS. The effect of therapeutic vaccination for the treatment of chronic hepatitis B virus infection. J Med Virol (2015) 87:575–82. doi: 10.1002/jmv.24091
153. Suthers AN, Sarantopoulos S. TLR7/TLR9- and B Cell Receptor-Signaling Crosstalk: Promotion of Potentially Dangerous B Cells. Front Immunol (2017) 8:775. doi: 10.3389/fimmu.2017.00775
154. Castiblanco DP, Maul RW, Russell Knode LM, Gearhart PJ. Co-Stimulation of BCR and Toll-Like Receptor 7 Increases Somatic Hypermutation, Memory B Cell Formation, and Secondary Antibody Response to Protein Antigen. Front Immunol (2017) 8:1833. doi: 10.3389/fimmu.2017.01833
155. Hou B, Saudan P, Ott G, Wheeler ML, Ji M, Kuzmich L, et al. Selective utilization of Toll-like receptor and MyD88 signaling in B cells for enhancement of the antiviral germinal center response. Immunity (2011) 34:375–84. doi: 10.1016/j.immuni.2011.01.011
156. Menne S, Tumas DB, Liu KH, Thampi L, AlDeghaither D, Baldwin BH, et al. Sustained efficacy and seroconversion with the Toll-like receptor 7 agonist GS-9620 in the Woodchuck model of chronic hepatitis B. J Hepatol (2015) 62:1237–45. doi: 10.1016/j.jhep.2014.12.026
157. Lanford RE, Guerra B, Chavez D, Giavedoni L, Hodara VL, Brasky KM, et al. GS-9620, an oral agonist of Toll-like receptor-7, induces prolonged suppression of hepatitis B virus in chronically infected chimpanzees. Gastroenterology (2013) 144:1508–17. doi: 10.1053/j.gastro.2013.02.003
158. Wilson TJ, Fuchs A, Colonna M. Cutting edge: human FcRL4 and FcRL5 are receptors for IgA and IgG. J Immunol (2012) 188:4741–5. doi: 10.4049/jimmunol.1102651
159. Franco A, Damdinsuren B, Ise T, Dement-Brown J, Li H, Nagata S, et al. Human Fc receptor-like 5 binds intact IgG via mechanisms distinct from those of Fc receptors. J Immunol (2013) 190:5739–46. doi: 10.4049/jimmunol.1202860
160. Nimmerjahn F, Ravetch JV. Fcgamma receptors as regulators of immune responses. Nat Rev Immunol (2008) 8:34–47. doi: 10.1038/nri2206
161. Akbar SM, Furukawa S, Horiike N, Abe M, Hiasa Y, Onji M. Safety and immunogenicity of hepatitis B surface antigen-pulsed dendritic cells in patients with chronic hepatitis B. J Viral Hepat (2011) 18:408–14. doi: 10.1111/j.1365-2893.2010.01320.x
162. Mauri C, Menon M. Human regulatory B cells in health and disease: therapeutic potential. J Clin Invest (2017) 127:772–9. doi: 10.1172/JCI85113
163. Xiao X, Lao XM, Chen MM, Liu RX, Wei Y, Ouyang FZ, et al. PD-1hi Identifies a Novel Regulatory B-cell Population in Human Hepatoma That Promotes Disease Progression. Cancer Discov (2016) 6:546–59. doi: 10.1158/2159-8290.CD-15-1408
164. Sarvaria A, Madrigal JA, Saudemont A. B cell regulation in cancer and anti-tumor immunity. Cell Mol Immunol (2017) 14:662–74. doi: 10.1038/cmi.2017.35
165. Zhao R, Liu M, Li X, Chen H, Deng J, Huang C, et al. A strategy of targeting B10 cell by CD19scFv-IL10R for tumor therapy. Biochem Biophys Res Commun (2018) 506:990–6. doi: 10.1016/j.bbrc.2018.10.191
166. Liu M, Chen HY, Luo L, Wang Y, Zhang D, Song N, et al. Neutralization of IL-10 produced by B cells promotes protective immunity during persistent HCV infection in humanized mice. Eur J Immunol (2020) 50(9):1350-–61. doi: 10.1002/eji.201948488
167. Tavakolpour S. Inhibition of regulatory cells as a possible cure of chronically hepatitis B virus infected patients. Immunol Lett (2016) 171:70–1. doi: 10.1016/j.imlet.2015.12.007
168. Schelonka RL, Infante AJ. Neonatal immunology. Semin Perinatol (1998) 22:2–14. doi: 10.1016/s0146-0005(98)80003-7
169. Trehanpati N, Hissar S, Shrivastav S, Sarin SK. Immunological mechanisms of hepatitis B virus persistence in newborns. Indian J Med Res (2013) 138:700–10.
170. Shrivastava S, TrehanPati N, Kottilil S, Sarin SK. Decline in immature transitional B cells after hepatitis B vaccination in hepatitis B positive newborns. Pediatr Infect Dis J (2013) 32:792–4. doi: 10.1097/INF.0b013e31828df344
Keywords: B cell, hepatitis B virus, antibody production, antigen presentation, immune regulation, immunotherapy
Citation: Cai Y and Yin W (2020) The Multiple Functions of B Cells in Chronic HBV Infection. Front. Immunol. 11:582292. doi: 10.3389/fimmu.2020.582292
Received: 04 August 2020; Accepted: 16 November 2020;
Published: 14 December 2020.
Edited by:
Julia Kzhyshkowska, Heidelberg University, GermanyReviewed by:
Thorsten Demberg, Marker Therapeutics, United StatesGalileo Escobedo, General Hospital of Mexico, Mexico
Copyright © 2020 Cai and Yin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenwei Yin, eXd3QGNxbXUuZWR1LmNu; orcid.org/0000-0003-2878-9149
 Ying Cai
Ying Cai Wenwei Yin
Wenwei Yin