
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol., 08 October 2020
Sec. Vaccines and Molecular Therapeutics
Volume 11 - 2020 | https://doi.org/10.3389/fimmu.2020.577751
This article is part of the Research TopicRecent Advances in Precision Vaccine Discovery & DevelopmentView all 26 articles
 Olubukola T. Idoko1,2*
Olubukola T. Idoko1,2* Cristina Domingo3
Cristina Domingo3 Milagritos D. Tapia4
Milagritos D. Tapia4 Samba O. Sow4
Samba O. Sow4 Christof Geldmacher5,6
Christof Geldmacher5,6 Elmar Saathoff5,6
Elmar Saathoff5,6 Beate Kampmann1,7
Beate Kampmann1,7Introduction: Although effective live attenuated yellow fever (YF) vaccines have been available for over 9 decades sporadic outbreaks continue to occur in endemic regions. These may be linked to several factors including epidemiological factors such as vector and intermediate host distribution or vaccine coverage and efficacy. The World Health Organization's research priorities include gathering systematic evidence around the potential need for booster vaccination with YF vaccine whether this follows full or fractional doses in children. Knowledge on the longevity of response to YF vaccine and the implications of this response needs to be consolidated to guide future vaccination policy.
Methods: We measured anti-YF IgG by microneutralization assay in a group of 481 African infants who had received YF vaccine as part of routine EPI programmes, to explore serological protection from YF 5–6 years post YF vaccination, as well as the effect of co variates.
Findings: Notably, 22.2% of the cohort had undetectable antibody concentrations, with another 7.5% revealing concentrations below the threshold of seropositivity of 0.5 IU/mL. Sex, season, country and time since vaccination did not affect the longevity of antibody concentration or having antibody concentrations above a defined threshold.
Conclusion: Roughly 30% of children in this cohort did not demonstrate anti-yellow fever antibody concentrations above the defined threshold of protection, with 20% having no demonstrable antibody. Knowledge on the longevity of response to YF vaccine and the implications needs to be consolidated to guide future vaccination policy.
Effective vaccines against yellow fever (YF) virus have been available for over nine decades (1). In endemic regions of the world, these vaccines are generally used as part of the routine Expanded Programme on Immunization (EPI) vaccines given to children in infancy (2). The currently available YF vaccines are live attenuated vaccines shown to be highly immunogenic and to provide long term protection after a single dose (1).The primary correlate of protection is neutralizing antibody, though cell mediated and innate immune responses have also been proposed to play a role (3).
In 2014 the World Health Organization (WHO) changed its recommendation of 10-yearly vaccination against YF to a single dose for life. Fractional doses are also in use during epidemics when vaccine supplies are limited. However, WHO has recognized the need for studies that establish the longevity of response to a single YF vaccine dose as a priority, particularly in special groups such as infants, immunocompromised individuals and those who received fractional doses of the vaccine (4–6). Establishing the longevity of response following single dose YF vaccination is key to guide future policy on the use of the vaccine, particularly in endemic settings. If the longevity of response is found to be sub optimal in these groups, millions may be vulnerable to infection in these endemic areas. The current projections of population coverage under the 2017–2026 Eliminating YF Epidemics (EYE) strategy, which implements YF vaccination in infants as part of the EPI in endemic countries (7), could be off-target if serological and possibly clinical protection from disease is short-lived.
The proportion of individuals with protective anti-yellow fever antibody reported in the literature ranges from 69 to 98% up to 11 years post vaccination (8–12). Data from children remains limited but are of particular importance, given that in endemic settings a single dose for life given in infancy would be the only YF vaccine administration. It is also possible that immune responses to YF differ between adults and children and data from children showing both production and longevity of protective antibody need to be generated, especially since it has already been demonstrated that infants show poorer and sometimes varied seroconversion rates following YF vaccination (13–16) in different settings. This finding is likely to impact on the longevity of antibody response.
To our knowledge there are limited data on longevity of YF antibody available from the African continent. One previous study by Domingo et al. involving children from Ghana and Mali examined longevity of antibody response to YF vaccine in African infants who had received the vaccines according to EPI schedules but as part of large randomized controlled trials (ideal settings). In these circumstances, “real life” factors such as cold chain maintenance and other programmatic limitations are less likely to affect overall outcome given the influence of full time study teams (17). Generating data on the antibody concentrations to YF several years post vaccination under routine EPI program conditions therefore remains important to inform the WHO guidelines with a view to assess need for booster doses if the only dose of YF vaccine is administered in infancy.
We measured anti-YF IgG by microneutralization assay in a group of African infants who had received YF vaccine as part of routine EPI programmes, to explore serological protection from YF 5–6 years post YF vaccination.
We also explored the impact of cofactors such as sex, season of vaccination, country and time since vaccination on the concentration of anti-YF IgG neutralizing antibodies.
The funders had no role in the study design, data collection, data analysis, data interpretation, or manuscript write up.
Written informed consent according Good Clinical Practice guidelines and the Declaration of Helsinki was obtained from a parent of each participant. The initial and current studies were approved by the ethics committees at the host institutions and Programme for Appropriate Technology in Health (PATH).
We assayed YF antibody responses in a cohort of African children from The Gambia (N = 243) and Mali (N = 238) using banked serum samples which were originally collected as part of an antibody persistence study to assess persistence of antibodies to MenAfriVac 4–5 years after the children had originally received MenAfriVac at 12–23 months of age. With these 481 samples we would have a power of 1.0 to detect a 10% difference in the proportion of individuals attaining protective titres assuming that one vaccine dose would result in 98% of children having protective anti-YF antibody titres 5–6 years post vaccination at a 5% alpha.
These blood samples were collected between October 2011 and April 2012 and included samples from all children who could be traced 4–5 years post MenAfriVac vaccination. Left over banked serum was accessed for this analysis (18) and current assays were conducted between January and March 2019.
As a prerequisite to enrolment into the MenAfrivac trial at age 12–23 months, the infants were required to have documentation showing that they received all recommended vaccinations for age which included one dose of YF vaccine received up to 1-year pre enrolment. The EPI program in Mali utilized yellow fever vaccines from Institute Pasteur, SANOFI Pasteur and BIOMANGUINHOS. The Gambia EPI utilized vaccines from vaccines from Institute Pasteur only. All blood samples assayed in this study were collected between 5- and 6-years post YF vaccination (corresponding to 4–5 years post study enrolment) and remaining aliquots had been stored at −70°C at the University of Siena Sera Bank in Italy. Samples were maintained at this temperature while stored at and during transport form the clinical sites and during transport to the assay lab.
The concentrations of neutralizing antibodies to YF virus were tested at the Robert Koch Institute in Berlin using a microneutralization assay (17). Briefly, 100 TCDI50 infectious doses of a YF virus 17-D (Stamaril, Sanofi Pasteur, Val de Reuil, France) were incubated with serial 2-fold dilutions of sera before inoculation into Vero cells cultured in 96-well plates. The cells were then microscopically examined for cytopathic effect 7 days later. Reference serum samples were run with each plate to minimize batch effects and ensure suitability of assay.
Although neutralizing titres of ≥1:5 or 1:10 (17, 19) are considered a surrogate of protection (seroprotection), difference in in assay methods may limit comparability of results from different laboratories. The titres were thus standardized by conversion into antibody concentrations in IU/mL using a WHO international standard (WHO International Standard, NISBC 99/616 reconstituted at 143 IU/ml) to allow for comparability with other available data. This was done by comparison with two positive controls for yellow fever neutralizing antibodies included with every assay. These controls were calibrated at 426.82 and 106.7 IU/ml, respectively. Based on earlier studies (20–22), we applied a concentration threshold of ≥0·5 IU/ml to discriminate seropositivity.
Where applicable, antibody concentrations were normalized by log 2 transformation. Uni- and multivariable mixed effects models were applied, adjusting for sex, season of vaccination and time since vaccination. Separate models were run for seropositivity (binary outcome) and raw post-vaccination antibody titres (continuous). Means, median, proportions and odds ratios were calculated along with their corresponding 95% confidence intervals. An alpha error level of 5% was used to judge significance. All analyses were performed using Stata version 14·2 (23).
Sera from 481 children (238 from Mali and 243 from The Gambia) were available of which 224 were male and 256 were female. Sex was missing for one participant.
The median antibody concentration was 1.2 [interquartile range (0.4–2.4)]. Notably, 22.2% of the cohort had undetectable antibody concentrations, with another 7.5% revealing concentrations below the threshold of seropositivity of 0.5 IU/mL. Table 1 summarizes antibody concentrations by country.
The antibody concentrations did not differ significantly by country (p = 0.13) with a trend to higher concentrations in the Malian cohort.
The median antibody titer was 1:12.0 [interquartile range (1:4.0–1:22.0)]. Of the cohort, 21.8% had undetectable antibody titres at this time point, with another 5% revealing concentrations below the defined titer for protection of 1:5.
Antibody concentration distribution was non- significantly higher concentrations in Mali (t = 1.51; p = 0.13) and showing generally low antibody tires 5–6 years post vaccination following a single dose of YF vaccine (Supplementary Figure 1).
Sex, season, country and time since vaccination did not affect the longevity of antibody concentration in uni- or multivariable regression models for antibody concentration as a continuous variable (Table 2).
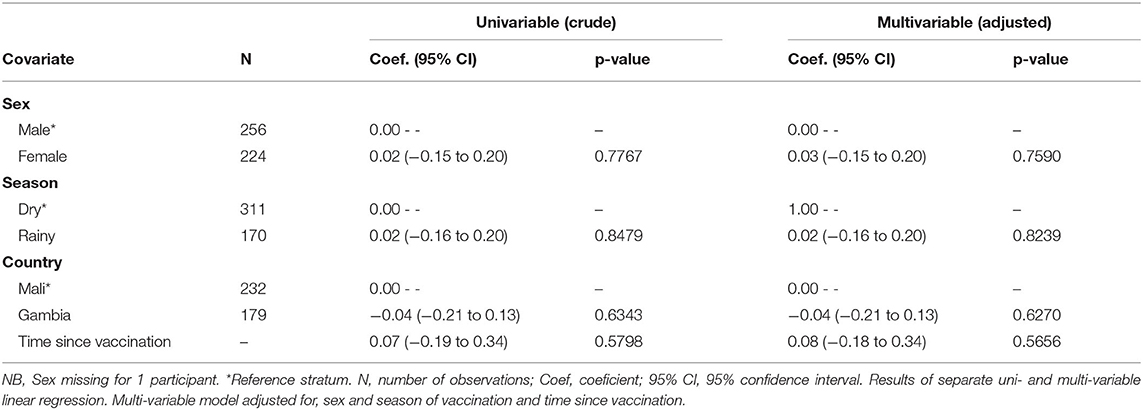
Table 2. Association of covariates at time of vaccination with log2 anti-YF antibody concentrations.
The odds of maintaining an antibody concentration above 0.5 UI/mL was also unaffected by the sex, season of vaccination, country or time since vaccination (Table 3).
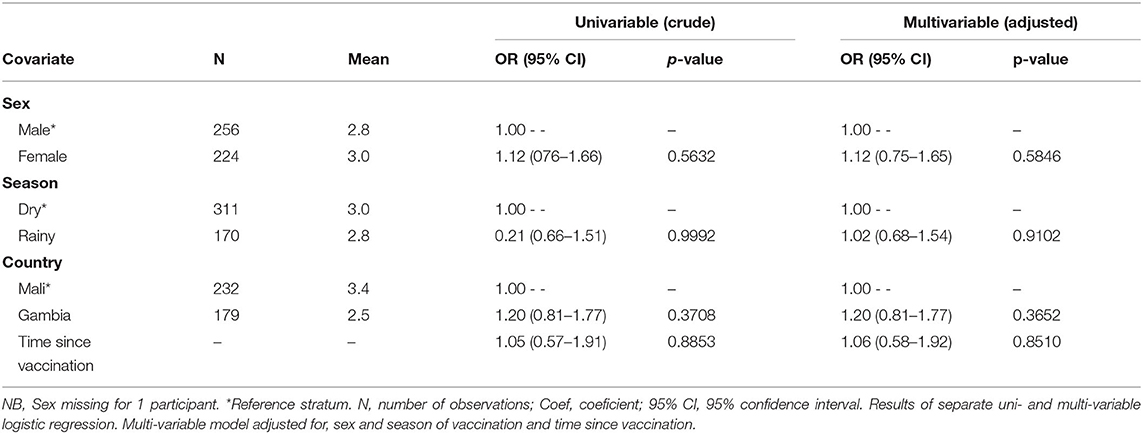
Table 3. Association of covariates at time of vaccination with recording a threshold of anti-YF antibody concentrations of 0.5 or more.
Similar findings were noted when the cut off for titres of ≥1:5 or 1:10 was applied.
The analysis of serum samples from 481 children vaccinated with one dose of YF vaccine in infancy showed that roughly 30% did not have protective antibody concentrations of 0.5 UI/ml (17) 5–6 years after vaccination. Most of this group had undetectable antibody concentrations (seronegative) and even where detectable, concentrations were below 0.1 IU/mL. This finding is concerning and may have significant implications for long term protection from YF in individuals who have received the YF vaccine as a single dose in the first year of life within EPI programs and in line with the current WHO recommendations.
By comparison, a small study in adults from the Netherlands who had received a fractional dose of YF vaccine demonstrated that 98% of the individuals vaccinated were still protected 10 years later (10), but the sample size of 40 individuals was small and represented only 48% of the original recipients of the vaccine (10). A more recent report followed up 349 Chinese adults who had received YF vaccine prior to deployment to Africa and reported negligible concentrations 11 years post vaccination (12). Several other studies have demonstrated similar decline in antibody in adults at varied time points post vaccination (9, 11, 24–26). The findings following a fractional dose of YF vaccine in The Netherlands differ from ours with a larger proportion retaining protective concentration but are in keeping with other reports of declining antibody from adults. All of these data are generated from studies in adults, unlike the pediatric data presented here. In addition, the different geographical settings (Europe, Asia vs. YF endemic Africa) have no or different prior exposure and other environmental differences which could also play a role in acquisition and persistence of antibody. Natural exposure differs between non-endemic and endemic settings and potentially also between age groups.
Following a full dose of YF vaccine in US adults all individuals who received multiple doses of YF vaccine had protective concentrations, irrespective of the time since vaccination. When only one dose had been received however, 94% had protective concentrations if that dose was <10 years from testing. When tested 10 or more years later however, only 82% had protective concentrations (11). These findings would suggest that even in adults, booster vaccinations may be warranted in certain populations. This view was published in a recent opinion piece (8).
Only 69% of children in Brazil maintained protective concentrations 10 years after receiving a full vaccine dose (8, 9). To our knowledge there has only been one study on the African continent assessing antibody longevity in children. This recent study assessed longevity in children who had received YF vaccine as part of 2 randomized controlled clinical trials. The study similarly demonstrated a drastic decline of YF immunity in children vaccinated as infants even within the ideal clinical trial settings with 50.4% (4.5 years post vaccination) and 43.1% (6 years post vaccination) retaining seropositivity in Mali and Ghana, respectively (17). These findings may be compounded by several factors including natural disease exposure but in keeping with ours suggest a population of children vulnerable to YF infection several years post vaccination irrespective of setting where vaccine was received.
Given the limited data available on persistence of antibody to YF in children and previous similar findings, our findings are timely and have the potential to inform future discussions regarding policy for YF vaccine use. The data from Brazil however does not state the vaccine used and this information may be an important consideration for the longevity of vaccine response. Our cohorts received similar vaccines although we do not have data per individual for vaccines received.
The sex, season of vaccination and time since vaccination did not impact the longevity of anti-YF antibody concentration 5–6 years post vaccination. This is similar to findings from a US cohort of adult travelers 3–4 years post vaccination with YF vaccine prior to travel to endemic regions. The only factors associated with higher antibody concentrations 3–4 years post vaccination were early onset of detection and higher antibody concentration 1-month post vaccination (27). These were not variables available for our analysis. In African studies sex was also not found to contribute to seropositivity (17).
The precise age at vaccination of our cohort (no date of birth records available) and anthropometric measures at time of vaccination were unknown and could have impact on the longevity of antibody. There may also be limitations within the EPI system ranging from cold chain maintenance to vaccine delivery methods that could have affected our results. Given the similarity of our findings with those from children vaccinated in ideal clinical trial settings however (17), this is unlikely.
As identified by WHO there is a need to gather evidence around the potential need for booster vaccination with YF vaccine whether this follows full or fractional doses in children and adults (4, 5, 28). This knowledge needs to be consolidated to guide future vaccination policy. Our findings suggest that children in this region of sub-Saharan Africa may require booster doses due at least by school entry age. Additional studies that explore antibody functionality and not just quantity would also be warranted as the function and not just the quantity of antibody is likely to mediate protection.
All datasets generated for this study are included in the article/Supplementary Material.
The initial and current studies involving human participants were reviewed and approved by Medical Research Council Unit The Gambia/Gambian Government Joint ethics Committee and Comité d'éthique de la Faculté de médecine de pharmacie et d‘odonto-stomatologie (FMPOS), Bamako, Mali. Written informed consent to participate in the the original study as well as consent for future use of banked samples was provided by the participants' legal guardian/parent.
OI conceived the study and performed the statistical analysis and drafted the manuscript. CD led the performance of the laboratory assays and critically reviewed the manuscript content. OI, BK, SS, and MT led recruitment of participants for the original studies and reviewed the manuscript. ES provided support for statistical analysis and critically reviewed manuscript content. CG and BK supported analysis and critically reviewed the manuscript. All authors contributed to the article and approved the submitted version.
Funding for the analysis of YF titres in the stored sera was received from the Wellcome Trust Institutional Strategic Support Fund to OI under the Imperial College Global Health Clinical Fellowship Scheme (Grant Ref PS2874_WMNP). The original MenAfriVac clinical trials in which these samples were collected were funded by grants to Program for Appropriate Technology in Health (PATH) from the Bill and Melinda Gates Foundation. The funders played no role in study design, data collection, data analysis, data interpretation or manuscript preparation. This MRCUG at LSHTM is jointly funded by the UK Medical Research Council (MRC) and the UK Department for International Development (DFID) under the MRC/DFID Concordat agreement and is also part of the EDCTP2 programme supported by the European Union. BK was supported by grants from UKRI (MC_UP_A900/1122, MC_UP_A900/115) and the IMmunising PRegnant women and INfants neTwork (IMPRINT) funded by the GCRF Networks in Vaccines Research and Development which was co-funded by the MRC and BBSRC.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors acknowledge the study participants and communities as well as all staff of the original trials. The authors are grateful to the Meningitis Vaccine Project for its guidance and administrative support in facilitating access to the biological samples and accompanying data in agreement with PATH. The samples and previous data were collected in the course of the MenAfriVac trials supported by grants from the Bill and Melinda Gates Foundation (to PATH). We are also grateful to the Wellcome Trust Institutional Strategic Support fund to OI for funding this analysis.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2020.577751/full#supplementary-material
Supplementary Figure 1. Distribution if anti-YF antibody concentration by Country. Kernel density: non-parametric estimation of probability density function of anti-YF concentration. NB: P-value for difference in antibody concentration between countries = 0.1348.
1. SAGE Working Group. Background Paper on Yellow Fever Vaccine (2013). Available online at: https://www.mesvaccins.net/textes/1_Background_Paper_Yellow_Fever_Vaccines.pdf
2. WHO. Recommended Routine Immunizations for Children (2020). Available online at: https://www.who.int/immunization/policy/immunization_tables/en/
3. Kollmann TR, Marchant A. Towards predicting protective vaccine responses in the very young. Trends Immunol. (2016) 37:523–34. doi: 10.1016/j.it.2016.05.005
4. WHO. Vaccines and vaccination against yellow fever: WHO Position Paper, June 2013–recommendations. Vaccine. (2015) 33:76–7. doi: 10.1016/j.vaccine.2014.05.040
5. World Health O. WHO position on the use of fractional doses - June 2017, addendum to vaccines and vaccination against yellow fever WHO: Position paper - June 2013. Vaccine. (2017) 35:5751–2. doi: 10.1016/j.vaccine.2017.06.087
7. WHO. Eliminate Yellow fever Epidemics (EYE): a global strategy, 2017-2026. Wkly Epidemiol Rec. (2017) 92:193–204.
8. Plotkin SA. Ten yearly yellow fever booster vaccinations may still be justified. J Travel Med. (2018) 25:1–2. doi: 10.1093/jtm/tay130
9. de Melo AB, da Silva Mda P, Magalhaes MC, Gonzales Gil LH, Freese de Carvalho EM, Braga-Neto UM, et al. Description of a prospective 17DD yellow fever vaccine cohort in Recife, Brazil. Am J Trop Med Hyg. (2011) 85:739–47. doi: 10.4269/ajtmh.2011.10-0496
10. Roukens AHE, van Halem K, de Visser AW, Visser LG. Long-term protection after fractional-dose yellow fever vaccination: follow-up study of a randomized, controlled, noninferiority trial. Ann Intern Med. (2018) 169:761–5. doi: 10.7326/M18-1529
11. Lindsey NP, Horiuchi KA, Fulton C, Panella AJ, Kosoy OI, Velez JO, et al. Persistence of yellow fever virus-specific neutralizing antibodies after vaccination among US travellers. J Travel Med. (2018) 25:1–12. doi: 10.1093/jtm/tay108
12. Jia Q, Jia C, Liu Y, Yang Y, Qi J, Tong L, et al. Clinical evidence for the immunogenicity and immune persistence of vaccination with yellow fever virus strain 17D in Chinese peacekeepers deployed to Africa. Antiviral Res. (2019) 162:1–4. doi: 10.1016/j.antiviral.2018.12.001
13. Idoko OT, Mohammed N, Ansah P, Hodgson A, Tapia MD, Sow SO, et al. Antibody responses to yellow fever vaccine in 9 to 11-month-old Malian and Ghanaian children. Exp Rev Vaccines. (2019) 18:867–75. doi: 10.1080/14760584.2019.1640118
14. Collaborative Group for Studies of Yellow Fever Vaccines. A randomised double-blind clinical trial of two yellow fever vaccines prepared with substrains 17DD and 17D-213/77 in children nine-23 months old. Mem Inst Oswaldo Cruz. (2015) 110:771–80. doi: 10.1590/0074-02760150176
15. Nascimento Silva JR, Camacho LA, Siqueira MM, Freire Mde S, Castro YP, Maia Mde L, et al. Mutual interference on the immune response to yellow fever vaccine and a combined vaccine against measles, mumps and rubella. Vaccine. (2011) 29:6327–34. doi: 10.1016/j.vaccine.2011.05.019
16. Roy Chowdhury P, Meier C, Laraway H, Tang Y, Hodgson A, Sow SO, et al. Immunogenicity of yellow fever vaccine coadministered with menafrivac in healthy infants in Ghana and Mali. Clin Infect Dis. (2015) 61 (Suppl. 5):S586–93. doi: 10.1093/cid/civ603
17. Domingo C, Fraissinet J, Ansah PO, Kelly C, Bhat N, Sow SO, et al. Long-term immunity against yellow fever in children vaccinated during infancy: a longitudinal cohort study. Lancet Infect Dis. (2019) 19:1363–70. doi: 10.2139/ssrn.3346514
18. Sow SO, Okoko BJ, Diallo A, Viviani S, Borrow R, Carlone G, et al. Immunogenicity and safety of a meningococcal A conjugate vaccine in Africans. N Engl J Med. (2011) 364:2293–304. doi: 10.1056/NEJMoa1003812
19. Plotkin SA. Correlates of protection induced by vaccination. Clin Vaccine Immunol. (2010) 17:1055–65. doi: 10.1128/CVI.00131-10
20. Wieten RW, Jonker EF, van Leeuwen EM, Remmerswaal EB, Ten Berge IJ, de Visser AW, et al. A single 17D yellow fever vaccination provides lifelong immunity; characterization of yellow-fever-specific neutralizing antibody and T-cell responses after vaccination. PLoS ONE. (2016) 11:e0149871. doi: 10.1371/journal.pone.0149871
21. de Menezes Martins R, Maia MLS, de Lima SMB, de Noronha TG, Xavier JR, Camacho LAB, et al. Duration of post-vaccination immunity to yellow fever in volunteers eight years after a dose-response study. Vaccine. (2018) 36:4112–7. doi: 10.1016/j.vaccine.2018.05.041
22. Campi-Azevedo AC, de Almeida Estevam P, Coelho-Dos-Reis JG, Peruhype-Magalhaes V, Villela-Rezende G, Quaresma PF, et al. Subdoses of 17DD yellow fever vaccine elicit equivalent virological/immunological kinetics timeline. BMC Infect Dis. (2014) 14:391. doi: 10.1186/1471-2334-14-391
24. Niedrig M, Lademann M, Emmerich P, Lafrenz M. Assessment of IgG antibodies against yellow fever virus after vaccination with 17D by different assays: neutralization test, haemagglutination inhibition test, immunofluorescence assay and ELISA. Trop Med Int Health. (1999) 4:867–71. doi: 10.1046/j.1365-3156.1999.00496.x
25. Groot H, Riberiro RB. Neutralizing and haemagglutination-inhibiting antibodies to yellow fever 17 years after vaccination with 17D vaccine. Bull World Health Organ. (1962) 27:699–707.
26. Collaborative Group for Studies on Yellow Fever Vaccines. Duration of post-vaccination immunity against yellow fever in adults. Vaccine. (2014) 32:4977–84. doi: 10.1016/j.vaccine.2014.07.021
27. Gibney KB, Edupuganti S, Panella AJ, Kosoy OI, Delorey MJ, Lanciotti RS, et al. Detection of anti-yellow fever virus immunoglobulin m antibodies at 3-4 years following yellow fever vaccination. Am J Trop Med Hyg. (2012) 87:1112–5. doi: 10.4269/ajtmh.2012.12-0182
Keywords: serologic, protection, 5-6 years post vaccination, yellow fever, routine immunizations
Citation: Idoko OT, Domingo C, Tapia MD, Sow SO, Geldmacher C, Saathoff E and Kampmann B (2020) Serological Protection 5–6 Years Post Vaccination Against Yellow Fever in African Infants Vaccinated in Routine Programmes. Front. Immunol. 11:577751. doi: 10.3389/fimmu.2020.577751
Received: 29 June 2020; Accepted: 03 September 2020;
Published: 08 October 2020.
Edited by:
Jay Evans, University of Montana, United StatesReviewed by:
Christine Wong, Charité – Universitätsmedizin Berlin, GermanyCopyright © 2020 Idoko, Domingo, Tapia, Sow, Geldmacher, Saathoff and Kampmann. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Olubukola T. Idoko, T2x1YnVrb2xhLklkb2tvQGxzaHRtLmFjLnVr; YnVra3lpZG9rb0BnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.