
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 04 December 2020
Sec. Autoimmune and Autoinflammatory Disorders
Volume 11 - 2020 | https://doi.org/10.3389/fimmu.2020.577523
This article is part of the Research Topic Recent Advances in Potential Biomarkers for Rheumatic Diseases and in Cell-based Therapies in the Management of Inflammatory Rheumatic Diseases View all 28 articles
 Johannes A. Bezuidenhout1
Johannes A. Bezuidenhout1 Chantelle Venter1
Chantelle Venter1 Timothy J. Roberts1,2,3
Timothy J. Roberts1,2,3 Gareth Tarr4
Gareth Tarr4 Douglas B. Kell1,2,5*
Douglas B. Kell1,2,5* Etheresia Pretorius1*†
Etheresia Pretorius1*†Aims: The risk of cardiovascular events in patients with Rheumatoid Arthritis (RA) is disproportionately heightened as a result of systemic inflammation. The relative effect of autoimmune-associated citrullination on the structure and thrombotic potential of fibrin(ogen) remains unknown. We therefore compared indices of vascular function, inflammation, coagulation and fibrin clot composition in RA patients with healthy controls and evaluated parameter association with disease presence.
Methods: Blood samples were collected from 30 RA patients and 30 age- and gender-matched healthy volunteers. Levels of serum amyloid A (SAA), c-reactive protein (CRP), soluble intercellular adhesion molecule 1 (sICAM-1) and soluble vascular cell adhesion molecule 1 (sVCAM-1) was measured using a sandwich immunoassay. Whole blood coagulation was assessed using Thromboelastography (TEG®). Fibrin clot networks and fiber structure was investigated using Scanning Electron Microscopy. The detection and quantification of citrullination in formed fibrin clots was performed using a fluorescently labeled Citrulline monoclonal antibody with Fluorescence Wide Field Microscopy.
Results: Concentrations of SAA, CRP and ICAM-1 were significantly elevated in RA patients compared to controls. TEG parameters relating to coagulation initiation, rate of fibrin cross-linking, and time to reach maximum thrombus generation were attenuated in RA patients. Microscopic analysis revealed denser networks of thicker fibrin fibers in RA patients compared to controls and multiple citrullinated regions within fibrin clot structures in RA patients were present.
Conclusion: Our findings provide novel evidence for the citrullination of fibrin within vasculature is more prominent in RA plasma compared to control plasma and plasma is more accessible than synovial fluid. Citrullinated fibrinogen could play a role as a determinant of thrombotic risk in RA patients.
Rheumatoid Arthritis (RA) is a chronic, systemic autoimmune disease characterized by both peripheral joint and extra-articular site inflammation, with an increased predisposition to a higher incidence of cardiovascular disease (CVD) (1). CVD is almost 50% more common in RA patients than the general population and is the most frequent cause of early mortality (2). Traditional risk factors for CVD (age, hypertension, obesity, etc.), do not fully account for the elevated occurrence of CVD events, and thus RA (genetics and disease characteristics) has been identified as a strong independent risk factor (3). The interdependence of inflammatory and hemostatic pathways is well established and observable in multiple types of tissue, organs and pathologies (4). Disruption of the tightly regulated homeostatic control of immune and hemostatic systems could result in a rapid progression towards a prothrombotic tendency, a central cause of ischemic stroke and myocardial infarction (5). This circumstance holds true for RA, with elevated levels of both pro-inflammatory and prothrombotic markers (e.g. D-dimer Fibrinogen Tissue Factor (TF) and von Willebrand factor (vWF)), which is associated with one another and with the risk of future cardiovascular complications (6).
Key intermediaries of this manifestation are the structural components of formed thrombi. Soluble fibrinogen is cleaved by thrombin in order to form dense matrices of thin fibrous protein known as fibrin (7). Polymerized fibrin networks are essential for wound healing and other occlusive physiological processes (7). However, exposure to inflammatory biomarker stimuli (such as CRP (8), SAA (9), and pro-inflammatory cytokines) can result in the alteration of mechanical and viscoelastic properties of fibrin clots into a prothrombotic phenotype. This phenomenon has previously been observed in RA plasma clots (10, 11). Various immunopathogenic processes related to RA development can exert upstream amplification of the coagulation cascade as well as impairing fibrin clot dissolution (12).
Fibrin(ogen) is also a potent pro-inflammatory signaling entity itself, mainly through ligand-receptor interactions with immune cells that further propagates pro-inflammatory effects (13). The deimination of arginine residues in fibrin(ogen) to yield non-charged citrulline residues is a distinctive RA posttranslational modification. Citrullination alters normal protein structure and function that confers antigenicity to modified proteins in RA (14). The functional relationship between citrullination and the presence of a prothrombotic fibrin clot phenotype is still poorly understood. Some studies have shown that citrullination of fibrinogen prevents thrombin digestion and subsequent fibrinogenesis (15, 16). However, the experimental conditions upon which these findings are based do not reflect physiological coagulation and is inconsistent with a predominantly hypercoagulable state seen in RA (17). Inflammation-induced fibrin formation is equally present in RA synovial spaces as it is in circulation (refer to overview of processes in Figure 1).
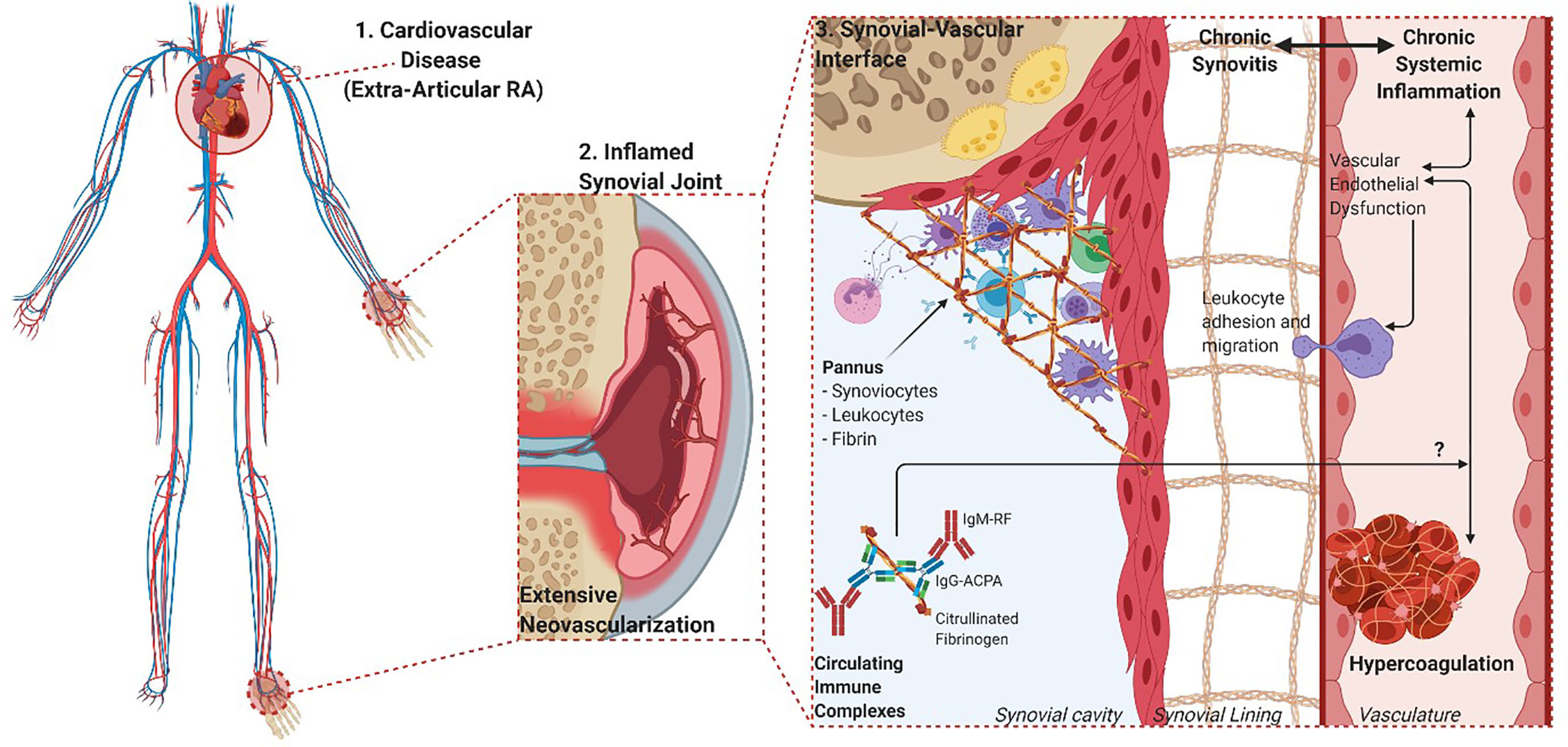
Figure 1 Overview of the overlapping processes of inflammation and coagulation in both synovial and vascular compartments. 1. The chronic and systemic nature of the inflammatory response in RA characterizes the disease as an independent risk factor for CVD. 2. The movement of leukocytes, inflammatory cytokines, procoagulant factors and immune complexes are aided by vascular endothelial dysfunction and neovascularization of hyperproliferative joint tissues. 3. The role of fibrin(ogen) is integral to the formation of hyperplastic and destructive synovial tissue (pannus) and vascular thrombosis, while being a prominent self-protein target of aberrant citrullination and autoimmunogenicity in RA (Created with BioRender.com).
Endothelial tissue dysfunction is a key process that facilitates this ubiquitous distribution of aberrant fibrin deposition in both synovia and vasculature. This pathophysiological state is characterized by the expression of cell adhesion molecules [intercellular cell adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1)], pro-inflammatory cytokines and pro-thrombotic markers (18, 19). This allows for the recruitment, translocation and propagation of inflammatory and thrombotic mediators across the synovial barrier (20).
There is significant overlap in inflammatory pathways responsible for joint damage in RA and hypercoagulation, coupled with the fact that disease severity has been correlated to more adverse cardiovascular complications (21). It is therefore important that these processes be examined systemically in RA, and not isolated to either vascular or synovial compartment. The aim of this study was to examine the extent to which the coagulation profiles and fibrin network architecture of RA patients are influenced by acute phase inflammation, endothelial dysfunction an autoimmune-related protein modification.
Ethical approval for this study was given by the Health Research Ethics Committee (HREC) of Stellenbosch University (reference number: 6983). This study was carried out in strict adherence to the International Declaration of Helsinki, South African Guidelines for Good Clinical Practice and the South African Medical Research Council (SAMRC) Ethical Guidelines for research. Written consent was obtained from all participants (RA patients and healthy participants) prior to any sample collection.
The RA sample group consisted of 30 patients (24 female and 6 male) that visited the Winelands Rheumatology Clinic (Stellenbosch, South Africa) for routine check-ups. All patients fulfilled the 2010 American College of Rheumatism/European League against Rheumatism (ACR/EULAR) classification criteria for RA diagnosis (22). The median age of RA group was 53.5 years (range 22–75 years) with a mean disease duration of 10.5 years (range 1–39 years). RA participants were excluded from the study if they presented with other severe comorbidities (such as cancer or diabetes), existing cardiovascular disease or taking anticoagulant medication. RA participants were not excluded on the basis of any antirheumatic drug treatment or the use of glucocorticosteroids. The majority of RA patients (87%) were on a schedule of non-biologic disease modifying antirheumatic drugs (DMARDS, such as methotrexate, hydroxychloroquine, sulfasalazine, or leflunomide), while a lower proportion of patients were on biologic DMARDs (60%) and cortisone (14%, 5–10 mg dosage). The control group consisted of 30 age- (median 50 years, range 28–79 years) and gender- (22 female and 8 male) matched volunteer blood donors. The inclusion criteria for healthy controls were: (i) no history of thrombotic disease or inflammatory conditions (ii) no use of any chronic medication (ii) no use of anticoagulant therapy (iii) non-smokers (iv) females not taking contraceptive medication or hormone replacement therapy (v) females that are not pregnant or lactating. All demographic information is summarized in Table 1.
Whole blood (WB) samples were collected in BD Vacutainer®blood collection tubes using 3.8% sodium citrate as anticoagulant (369714, Becton, Dickinson and Company Franklin Lakes, NJ, USA). Blood drawing on all participants was performed by a qualified nurse, or phlebotomist by sterile puncture of the antecubital vein. Blood tubes were incubated at room temperature for a minimum duration of 30 min prior to the commencement of any whole blood analysis. In order to obtain platelet poor plasma (PPP), sodium citrated blood tubes were centrifuged at 3000 × g for 15 min, aliquoted and stored at −80°C until further analysis.
Analysis of dynamic coagulation kinetics were performed on RA and control WB by means of Thromboelastograph® (TEG®) 5000 Haemostasis Analyzer System (07-033, Haemonetics®, Niles, IL, USA). In brief, coagulation is initiated by recalcification of 340 µl WB with 20 µl of 0.2 mM Calcium chloride (CaCl2) (Haemonetics®, 7003) in a disposable TEG® cup (Haemonetics®, 6211). Various kinetic clotting parameters are determined by assessing the resistance that the forming thrombus provides against the oscillating pin of the instrument (measuring at 37°C). Parameters derived from the thromboelastograph tracing consist of: reaction time (R, time from test start to initial fibrin formation in minutes), kinetics [K, time required to reach an amplitude (clot thickness) of 20 mm, in minutes], alpha angle (α, rate of fibrin accumulation indicated by degrees), maximal amplitude (MA, maximum strength of formed clot in millimeters) (24). Additionally, parameters of clot formation determined by the changes in the elastic modulus (G, in dynes.cm−2) were also included: maximum rate of thrombus generation (MRTG, in dynes.cm−2.s−1), time to maximum rate of thrombus generation (TMRTG, in minutes) and total thrombus generation (TTG, in dynes.cm−2) (25).
The ultrastructure of fibrin networks and individual fibrin fibers were examined using scanning electron microscopy (SEM). In summary, clots were prepared from thawed PPP samples of RA patients (n=10) and controls (n=10) by addition of 5 µl human thrombin (provided by South African National Blood Service, final concentration 7 IU/ml) to 10 µl PPP on a glass coverslip and transferred to a 24-well plate. Preparation consisted of washing with 1× Gibco® phosphate-buffered saline (PBS, pH 7.4) (10010015, ThermoFisher Scientific, Waltham, MA, USA), chemical fixation with 4% formaldehyde (FA) (P6418, Sigma-Aldrich, St. Louis, MO, USA) and then 1% Osmium Tetrahydroxide (OsO4) (Sigma-Aldrich, 75632), followed by dehydration with increasing grades of ethanol and 99.9% Hexamethyldisilizane (HMDS) (Sigma-Aldrich, 37921) [for detailed protocols please refer to (24)]. Samples were carbon coated using a Quorom Q150T E carbon coater (Quorom Technologies, Lewes, UK). Plasma fibrinogen concentrations of samples used to conduct SEM analysis were determined by an independent pathology laboratory. Images were captured at an electron high tension (EHT) of 1 kV using a high resolution InLens detector of the Zeiss Merlin™ (Gemini II) FE SEM (Carl Zeiss Microscopy, Munich, Germany). Fibrin fiber diameters representative of each respective sample group (RA and control) was determined by means of image analysis software ImageJ (Version 1.52p). Three representative micrographs (78,98 µm2 image size, 10,000× magnification) were calibrated to scale and overlaid with a non-destructive grid (2 µm2 tile size). Single representative fibrin fibers were measured in 28 tiles per image, producing 84 fiber diameter measurements per sample.
Plasma concentrations of soluble ICAM-1, VCAM-1, CRP, and SAA were measured by sandwich immunoassay (K15198D, Meso Scale Diagnostics, Rockville, MD, USA). RA (n=30) and control (n=30) PPP samples and reagents were prepared as per manufacturer’s protocol. Samples were run in duplicate and measurements read on an MSD Discovery Workbench 4. Analyte concentrations were calculated from the calibration curve generated by absorbance measurements of manufacturer supplied calibrator standards.
In order to determine the extent of protein deamination in fibrin networks, PPP aliquots of RA samples (n=10) and control samples (n=10) were thawed and fibrin clots prepared (refer to SEM method) on glass microscope slides in a dark room. Samples were fixed with 4% FA, washed 3× with PBS, and blocked with 5% Goat serum solution (ab7481, Abcam, Cambridge, UK) for 30 min. Clots were then stained with a 1:50 dilution Citrulline Monoclonal Antibody (2D3.1) (MA5-27573, ThermoFisher Scientific) and incubated for 1 h. Following another 3× PBS wash to remove unbound antibodies, samples were then stained with 1:500 dilution Goat Anti-Mouse IgG Secondary antibody conjugated to AlexaFluor 488 (A327273, ThermoFisher Scientific) and incubated for 1 h. Slides were washed 3× with PBS to remove unbound antibody, allowed to dry, and mounted with a glass coverslip. Samples were viewed with a Zeiss Axio Observer 7 Microscope with a Plan-Apochromat 63x/1.4 oil DIC M27 objective (Carl Zeiss Microscopy, Munich, Germany). The excitation wavelength for AlexaFluor488 was set at 450 to 480 nm and the emission wavelength at 499 to 529 nm. Three representative micrographs per sample were analyzed for relative mean fluorescent intensity (MFI) using ImageJ (Version 1.52p). Images were calibrated to scale, and a global threshold (20 pixel cut-off) applied to all analyzed micrographs.
Statistical analysis was performed using R version 4.0. Specifically, univariate logistic regression was performed to determine odds ratios (OR) for experimental variables using the logistic model in the rstanarm package (with default priors). ORs and 95% confidence intervals were extracted in the corresponding unit system (i.e. not z-scaled) for all variables except Fibrin fiber diameter and citrulline MFI shown in Figure 2 which are z-scaled to aid interpretation. Table 2 shows the ORs after adjustment for age and gender with unadjusted analysis identifying the same significance and effect sizes. In addition, results for classical statistical tests are reported as follows. The distribution of sample datasets for each variable experimental was determined using the Shapiro-Wilk test. Accordingly, p-values for each variable comparing RA to healthy controls were calculated using either a Mann-Whitney U test for nonparametric data or a Student t-test for parametric data. Statistical significance was set at p<0.05. One can see close alignment between all these and the OR results.
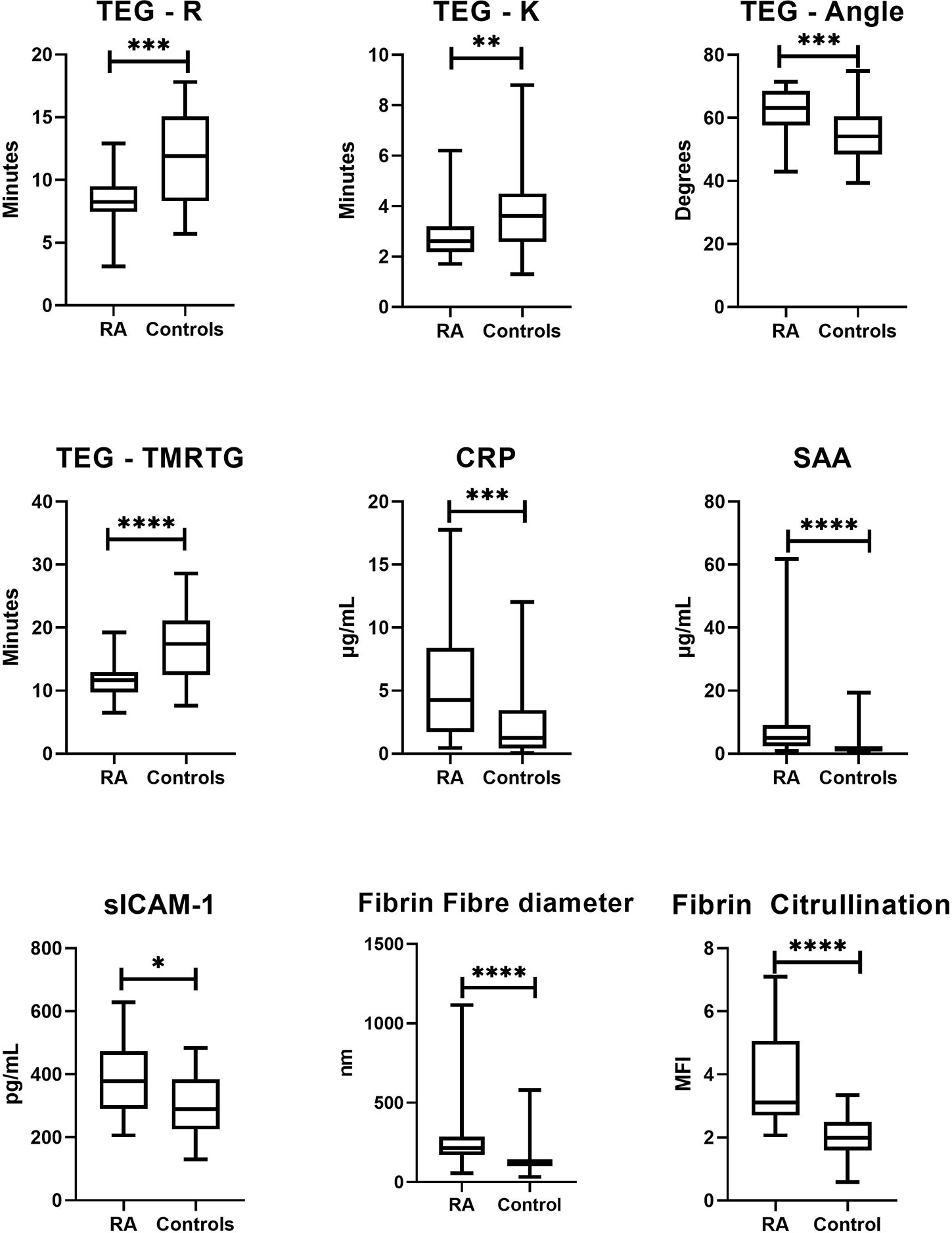
Figure 2 Box-and-whisker plots comparing sample distributions for each significantly altered study variable compared between RA patients and healthy controls. The level of statistical significance is indicated by the bars above each plot (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001). Viscoelastic coagulation analysis by TEG® indicated that RA patients had shortened phases of clot formation initiation (R: p = 0.0005; K: p = 0.01), overall clot formation (TMRTG: p < 0.0001) and rate of fibrin deposition (α-Angle: p = 0.0003). Microscopic analysis of fibrin networks revealed that fibrin fiber diameter (p < 0.0001) and fibrin citrullination (p < 0.0001) were drastically elevated in plasma clots RA patients. Plasma levels of CRP (p = 0.001), SAA (p < 0.0001) and sICAM-1 (p = 0.01) were also significantly increased in RA patients.
Demographic information of all study participants is listed in Table 1. The RA sample group closely resembles the general population distribution for age (median: 54 years) and sex (80% female) of the disease (26). The control group of healthy volunteers was closely matched to the RA group with regards to age (median: 50 years) and sex (73% female). The RA sample group was heterogeneous with respect to clinical presentation, with most patients on an anti-rheumatic drug therapy regime. The majority of RA patients also presented with positive titers for anti-cyclic citrullinated peptide (CCP) (77%) and rheumatoid factor (97%) autoantibodies.
Circulating concentrations of endothelial function and acute phase markers are shown in Table 2, with significantly altered markers graphically presented in Figure 2. As expected, all markers were elevated in RA compared to controls [CRP (median 4.25 µg/ml vs 1.26, p=0.001), SAA (4.98 µg/ml vs 1.52, p<0.0001), sICAM-1 (378 ng/ml vs 290, p=0.01), sVCAM-1 (360 ng/ml vs 326, p=0.9)]. Univariate logistic regression also indicated that levels of CRP, SAA and ICAM-1 were significantly predictive of disease status (the nominal variable defined as the presence of absence of RA, represented by healthy controls), as shown by both the ORs listed in Table 2.
Whole blood coagulation parameters as measured by TEG® are listed in Table 2, with significantly altered parameters illustrated as box-and-whisker plots in Figure 2. RA patients showed significantly altered rates of clot formation compared to healthy controls. This included shortened clot initiation (R; OR=0.675, p=0.0005 and K; OR=0.539, p=0.01), augmented fibrin cross-linking (α; OR=1.15, p=0.0003) and shortened time to maximal thrombus formation (TMRTG; OR=0.737, p<0.0001). Measures of overall clot strength (MA) and growth (TTG) were attenuated in RA but did not statistically differ from those of controls.
Further investigation into the apparent modification of the clot structure in RA was carried out by means of SEM. Results (refer to Figure 2) indicate that fibrin fiber diameters in representative areas were significantly increased in RA versus controls (median diameter 214 nm vs 120 nm, p<0.0001). Examining the networks qualitatively, it is evident that ex vivo formed clots from RA samples have denser, less porous fibrin networks. Figures 3B to D, representative of the RA sample group, illustrates the amalgamation of fibrin monomers that contribute to increased fibrin fiber diameter and overall network density. This contrasts sharply with the ultrastructural attributes of Figure 3A (Healthy control sample), which demonstrates thinner protein strands and a more permeable fibrin network. Mean levels of fibrinogen did not significantly differ between RA patients and healthy controls used for SEM imaging (median 3.15 vs 2.95 g/L, p=0.547). This may suggest that differences observed in the fibrin network structure and thromboelastic profiles of RA patients could be attributed to factors independent from direct influences of clot formation such as fibrinogen and thrombin concentration.
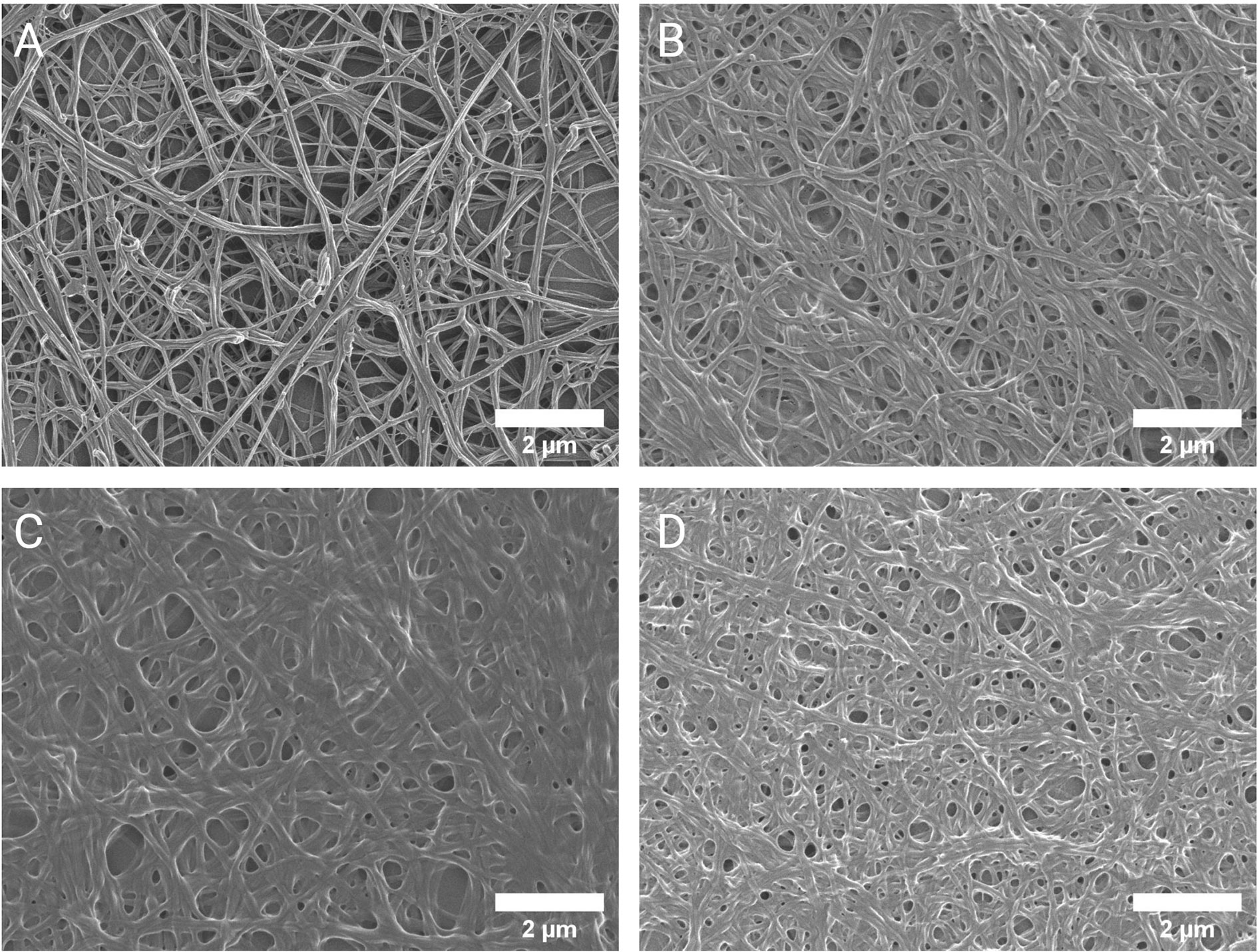
Figure 3 Scanning electron micrographs of the fibrin network ultrastructure. Representative micrographs of the fibrin network in healthy controls (A) and RA patients (B–D). The altered clot ultrastructure in RA, consisting of less permeable networks of thicker fibrin fibers, represents a prothrombotic phenotype.
To investigate the presence and extent of potential citrullination in RA (n=10) and Control (n=10) PPP thrombi, fluorescence analysis using a Citrulline-identifying monoclonal antibody with immunofluorescence microscopy was performed (Figure 4). Acquired image data (Figure 2) suggests a higher degree of citrullinated fibrin fluorescence in RA fibrin networks versus controls (median MFI 3.88 vs 2.02, p<0.0001), with logistic regression analysis (Table 2) showing that fibrinogen citrullination is associated with RA to a substantial degree (Adjusted OR=46.1, 95% CI: 7.81–419).
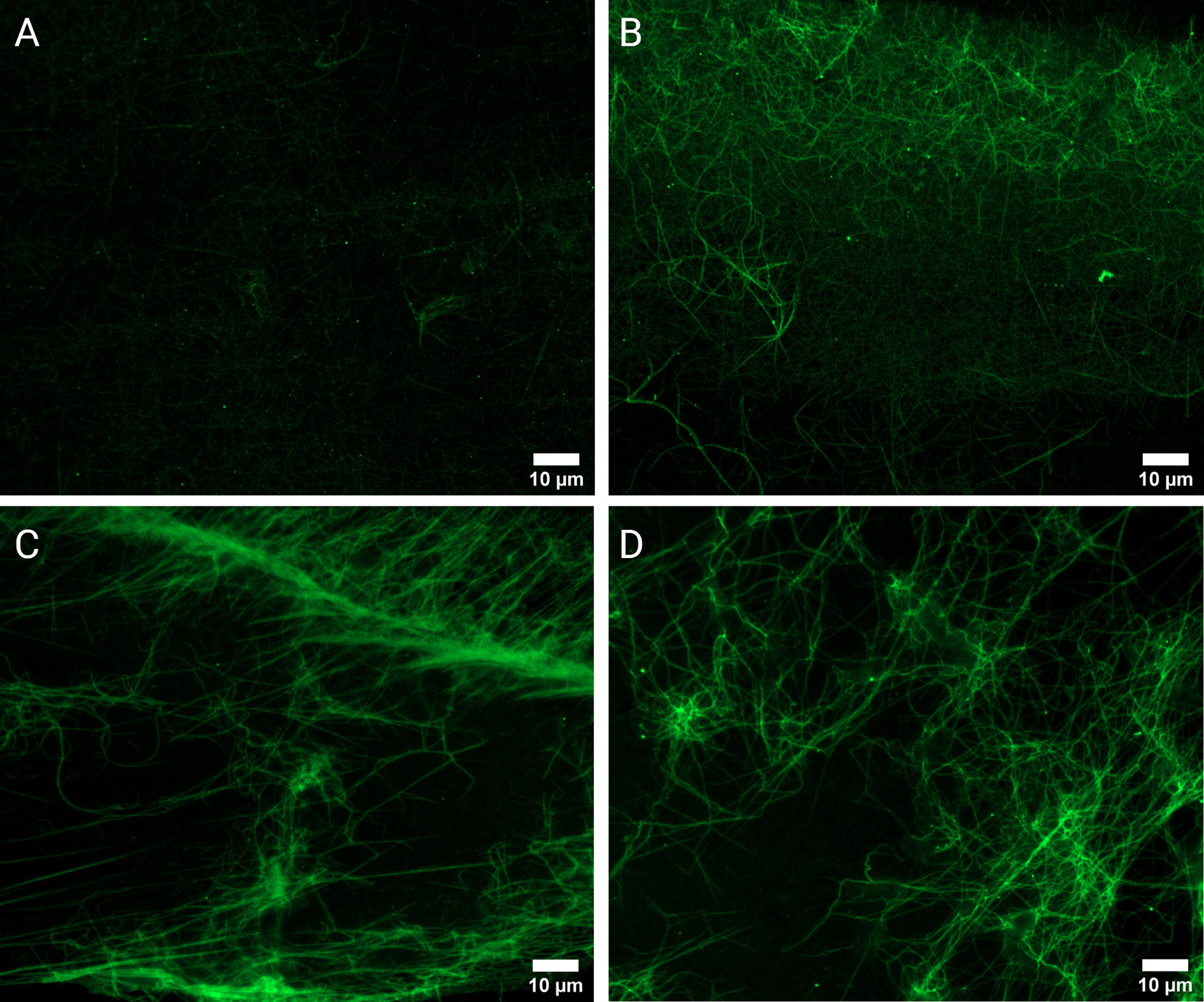
Figure 4 Fluorescence microscopy of PPP clots stained with a Citrulline monoclonal antibody (green). Micrographs of representative control (A) and RA samples (B–D). Analysis confirmed the observable presence of enhanced fluorescent signal in RA samples (n=10) versus healthy controls (n=10).
There is a need to bridge translational gaps between RA immunopathogenesis and systemic vascular and hemostatic irregularities. The link between RA autoimmune patterns and its possible role in exacerbating thrombosis is still poorly understood.
Crosstalk between immune and hemostatic systems with the endothelium represents a critical interface in which both arthritic and cardiovascular pathologies are initiated and propagated. We therefore analyzed a panel of biomarkers that are representative of this dynamic milieu and is associated with RA disease severity and CVD. Levels of both acute phase reactants (CRP and SAA) were significantly elevated in RA patients (Figure 2) and showed a strong association with the disease (Table 2). This was expected as acute phase reactant concentrations rise dramatically under acute inflammatory states, with both CRP and SAA shown to reliably predict disease severity and CVD risk in RA (27). Both molecules also have demonstratable prothrombotic cellular effects by influencing endothelial and peripheral blood mononuclear cell expression of coagulation factors. Increased levels of soluble cell adhesion molecules (sICAM-1 and sVCAM-1) indicate endothelial dysfunction that facilitates pro-inflammatory and prothrombotic conditions (28). sICAM-1 and sVCAM-1 concentrations were elevated in RA (Table 2) but were not as strongly associated with disease presence as CRP and SAA.
We also investigated the coagulation profiles of study participants. Türk et al (29). is the only recent study that has assessed thrombotic tendency in RA patient with thromboelastographic assessment (29), using the rotational thromboelastometry (ROTEM). Our findings show that coagulation initiation was amplified in RA patients with shortened velocity parameters of clot formation (R, K, α, TMRTG) (Figure 2, Table 2). Parameters relating to clot strength (MA, TTG) were attenuated in the RA sample group, but did not statistically differ from healthy controls (Table 2, Figure 2). Thus, although the blood clots form rapidly it leads to a weak clot.
Excessive hepatic production of fibrinogen is highly prevalent in RA (30), and increased plasma fibrinogen concentration is a strong contributing factor to hypercoagulation. Fibrin(ogen) is susceptible to structural and functional modifications by certain inflammatory molecules, including CRP and SAA (9). Fibrin(ogen) is also prone to post-translational modification that relates to the generation of auto-immunogenicity in RA – the relevance of this process was investigated and is discussed below.
Evaluating fibrin gel matrices visually can reveal much about thrombotic potential under inflammatory conditions. Denser fibrin fiber networks are accompanied with increased resistance to fibrinolysis and is associated with the risk for thrombotic events[reviewed in ref (31)]. Our analysis revealed denser fibrin networks in RA prepared ex vivo PPP clots compared to controls (Figure 3). This is consistent with a prothrombotic phenotype observed in previous studies that have inspected the fibrin network in RA (10, 11). The average diameter of fibrin fibers was also larger in RA clots compared to controls. Some studies have indicated that thin fibrin fibers have higher tensile strength than thicker fibers, concluding that dense networks consisting of predominantly thin fibers are more resistant to degradation (32). Fibrin networks of this nature in RA were observed by Vrancic et al. (33). However, study by Buclay et al. (34) concluded that thicker fibers are more resistant to plasmin degradation than thinner fibers, owing to their ability to elongate during lysis. It is apparent that our investigation into the structural properties of fibrin networks in RA and its relation to hemostatic function has a rather deceptive appearance.
Distinct protein modifications related to the generation of autoimmunity in RA could present an additional complication when attempting to expound underlying mechanisms responsible for excessive thrombotic risk. Citrullination is a post-translational modification in which positively charged arginine are deiminated by peptidylarginine deiminase (PAD) enzymes to form neutrally charged citrulline (35). Fibrinogen and fibrin are prominent substrates for PAD enzymes and autoantibodies targeting citrullinated fibrin(ogen) have been identified (36–41). The pathogenicity of citrullinated fibrin(ogen) immune complexes have been demonstrated both in vitro (13) and in vivo (42, 43). Citrullinated fibrin deposits are also common manifestations within synovial cavities, where it contributes to self-perpetuating inflammatory processes (44, 45). Our findings provide novel evidence for the citrullination of fibrin within vasculature which is more prominent in RA plasma compared to control plasma (Figure 4). Previously the presence of citrullinated fibrinogen could only be detected in RA synovial fluid (46). Later research by Zhao et al. (39) confirmed the presence of citrullinated fibrinogen containing immune complexes in RA plasma. The insolubility of fibrin may increase the likelihood of it being citrullinated in circulation. The binding of ACPAs to fibrin could then render it less degradable, by decreasing available binding surface to plasmin (47). There remains conjecture as to the effect of citrullination on hemostatic outcome. Citrullination of proteins results in structural unfolding (48) and loss of function (49), which increases its antigenic shelf-life. It has been demonstrated that citrullinated fibrinogen is resistant to thrombin digestion, as preferential epitopes for PADs overlap with thrombin binding sites (15, 16, 50). Despite this, fibrinogenesis in RA is by no means impaired, as evidenced by this study and others. It is plausible that high levels of fibrinogen (30) and thrombin activity (51, 52) in RA has much stronger influence on the fate of fibrinogen than PAD enzymes. There is also evidence that upstream coagulation factors and fibrinolytic components are susceptible to citrullination (53, 54). It is therefore difficult to predict a hemostatic endpoint based on overall citrullination and the effect of citrullination on thrombosis cannot be postulated on singular reactions. The implications that citrullination could have on fibrin, being the end-product of coagulation and a major determinant of thrombotic risk, remains intriguing and should be further investigated.
This study did present some limitations and challenges. The demographic and clinical presentation of the RA patient group was diverse with regards to age and gender distribution, treatment, disease duration and disease severity at the date of sample collection (Table 1). The possible confounding effect of antirheumatic drug use on the investigated parameters can be considered less pronounced with a relatively small study sample size as presented here. The strong immunosuppressant effect of both conventional and biologic DMARDs may carry the additional benefit of having cardioprotective properties. However the risk of adverse cardiovascular events have also been related to all currently available RA therapeutics (55). Corticosteroid administration in RA treatment have also been associated with a dose-dependent increase in risk for cardiovascular mortality (56).
Inflammatory and thrombotic processes are highly pertinent to the development of joint disease and cardiovascular complications. There is a need to study changes that occur within synovial environments in unison with simultaneously occurring changes within circulatory tracts. Further investigation into overlapping processes that are crucially involved in the concurrent development of both RA and CVD could reveal improved global disease markers and novel targets for therapeutic intervention. The formation and structure of fibrin clots in RA shows an atypical pattern compared to conventional observations of hypercoagulation under inflammatory conditions. We propose determining if citrullination causes a structural and functional shift in the nature of fibrin to represent an amyloid-like state. This protein modification could potentially contribute to the formation of aberrant fibrin clots in RA patients that confer a higher degree of thrombotic risk.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found at: https://1drv.ms/u/s!Aj29oJ2y_jViqW04QSVHfnjt1YNq?e=qLy8zR.
The studies involving human participants were reviewed and approved by Stellenbosch University HREC committee. The patients/participants provided their written informed consent to participate in this study.
JB: Collected, prepared and analyzed the samples and wrote the paper. CV: Technical assistance with fluorescence microscopy. TR: Statistical analysis. GT: Rheumatologist who identified patients, clinical advice. DK: edited the paper. EP: Study leader, funding, edited, and co-wrote the paper. All authors contributed to the article and approved the submitted version.
We thank the Medical Research Council of South Africa (MRC) (Self-Initiated Research Program, grant number: A0X331) and the Novo Nordisk Foundation (grant number: NNF10CC1016517) for supporting this collaboration.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Avina-Zubieta JA, Thomas J, Sadatsafavi M, Lehman AJ, Lacaille D. Risk of incident cardiovascular events in patients with rheumatoid arthritis: a meta-analysis of observational studies. Ann Rheum Dis (2012) 71(9):1524–9. doi: 10.1136/annrheumdis-2011-200726
2. Lopez-Mejias R, Castaneda S, Gonzalez-Juanatey C, Corrales A, Ferraz-Amaro I, Genre F, et al. Cardiovascular risk assessment in patients with rheumatoid arthritis: The relevance of clinical, genetic and serological markers. Autoimmun Rev (2016) 15(11):1013–30. doi: 10.1016/j.autrev.2016.07.026
3. del Rincon ID, Williams K, Stern MP, Freeman GL, Escalante A. High incidence of cardiovascular events in a rheumatoid arthritis cohort not explained by traditional cardiac risk factors. Arthritis Rheum (2001) 44(12):2737–45. doi: 10.1002/1529-0131(200112)44:12<2737::AID-ART460>3.0.CO;2-#
4. Foley JH, Conway EM. Cross Talk Pathways Between Coagulation and Inflammation. Circ Res (2016) 118(9):1392–408. doi: 10.1161/CIRCRESAHA.116.306853
5. Maino A, Rosendaal FR, Algra A, Peyvandi F, Siegerink B. Hypercoagulability Is a Stronger Risk Factor for Ischaemic Stroke than for Myocardial Infarction: A Systematic Review. PloS One (2015) 10(8):e0133523–e. doi: 10.1371/journal.pone.0133523
6. Södergren A, Karp K, Bengtsson C, Möller B, Rantapää-Dahlqvist S, Wållberg-Jonsson S. Biomarkers associated with cardiovascular disease in patients with early rheumatoid arthritis. PloS One (2019) 14(8):1–12. doi: 10.1371/journal.pone.0220531
7. Litvinov RI, Weisel JW. What Is the Biological and Clinical Relevance of Fibrin? Semin Thromb Hemost (2016) 42: (4):333–43. doi: 10.1055/s-0036-1571342
8. Salonen EM, Vartio T, Hedman K, Vaheri A. Binding of fibronectin by the acute phase reactant C-reactive protein. J Biol Chem (1984) 259(3):1496–501.
9. Page MJ, Thomson GJ, Nunes JM, Engelbrecht A-M, Nell TA, de Villiers WJ, et al. Serum amyloid A binds to fibrin (ogen), promoting fibrin amyloid formation. Sci Rep (2019) 9(1):1–14. doi: 10.1038/s41598-019-39056-x
10. Kwasny-Krochin B, Gluszko P, Undas A. Unfavorably altered fibrin clot properties in patients with active rheumatoid arthritis. Thromb Res (2010) 126(1):e11–6. doi: 10.1016/j.thromres.2010.04.007
11. Pretorius E, Oberholzer HM, van der Spuy WJ, Swanepoel AC, Soma P. Scanning electron microscopy of fibrin networks in rheumatoid arthritis: a qualitative analysis. Rheumatol Int (2012) 32(6):1611–5. doi: 10.1007/s00296-011-1805-2
12. Beinsberger J, Heemskerk JW, Cosemans JM. Chronic arthritis and cardiovascular disease: altered blood parameters give rise to a prothrombotic propensity. Semin Arthritis Rheum (2014) 44(3):345–52. doi: 10.1016/j.semarthrit.2014.06.006
13. Sokolove J, Zhao X, Chandra PE, Robinson WH. Immune complexes containing citrullinated fibrinogen costimulate macrophages via Toll-like receptor 4 and Fcgamma receptor. Arthritis Rheum (2011) 63(1):53–62. doi: 10.1002/art.30081
14. Blachere NE, Parveen S, Frank MO, Dill BD, Molina H, Orange DE. High-Titer Rheumatoid Arthritis Antibodies Preferentially Bind Fibrinogen Citrullinated by Peptidylarginine Deiminase 4. Arthritis Rheumatol (Hoboken NJ) (2017) 69(5):986–95. doi: 10.1002/art.40035
15. Damiana T, Damgaard D, Sidelmann JJ, Nielsen CH, de Maat MP, Münster A-MB, et al. Citrullination of fibrinogen by peptidylarginine deiminase 2 impairs fibrin clot structure. Clin Chim Acta (2020) 501:6–11. doi: 10.1016/j.cca.2019.10.033
16. Okumura N, Haneishi A, Terasawa F. Citrullinated fibrinogen shows defects in FPA and FPB release and fibrin polymerization catalyzed by thrombin. Clin Chim Acta Int J Clin Chem (2009) 401(1-2):119–23. doi: 10.1016/j.cca.2008.12.002
17. Bisoendial RJ, Levi M, Tak PP, Stroes ES. The prothrombotic state in rheumatoid arthritis: an additive risk factor for adverse cardiovascular events. Semin Thromb Hemost (2010) 36(4):452–7. doi: 10.1055/s-0030-1254054
18. Murdaca G, Colombo BM, Cagnati P, Gulli R, Spanò F, Puppo F. Endothelial dysfunction in rheumatic autoimmune diseases. Atherosclerosis (2012) 224(2):309–17. doi: 10.1016/j.atherosclerosis.2012.05.013
19. Hoppe B, Dörner T. Coagulation and the fibrin network in rheumatic disease: a role beyond haemostasis. Nat Rev Rheumatol (2012) 8(12):738–46. doi: 10.1038/nrrheum.2012.184
20. Elshabrawy HA, Chen Z, Volin MV, Ravella S, Virupannavar S, Shahrara S. The pathogenic role of angiogenesis in rheumatoid arthritis. Angiogenesis (2015) 18(4):433–48. doi: 10.1007/s10456-015-9477-2
21. Myasoedova E, Chandran A, Ilhan B, Major BT, Michet CJ, Matteson EL, et al. The role of rheumatoid arthritis (RA) flare and cumulative burden of RA severity in the risk of cardiovascular disease. Ann Rheum Dis (2016) 75(3):560–5. doi: 10.1136/annrheumdis-2014-206411
22. Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO 3rd, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis (2010) 69(9):1580–8. doi: 10.1136/ard.2010.138461
23. Aletaha D, Ward MM, Machold KP, Nell VP, Stamm T, Smolen JS. Remission and active disease in rheumatoid arthritis: defining criteria for disease activity states. Arthritis Rheum (2005) 52(9):2625–36. doi: 10.1002/art.21235
24. Pretorius E, Swanepoel AC, DeVilliers S, Bester J. Blood clot parameters: Thromboelastography and scanning electron microscopy in research and clinical practice. Thromb Res (2017) 154:59–63. doi: 10.1016/j.thromres.2017.04.005
25. Nielsen VG, Cohen BM, Cohen E. Elastic modulus-based thrombelastographic quantification of plasma clot fibrinolysis with progressive plasminogen activation. Blood Coagul Fibrinolysis an Int J Haemost Thromb (2006) 17(1):75–81. doi: 10.1097/01.mbc.0000198047.35010.77
26. Alamanos Y, Drosos AA. Epidemiology of adult rheumatoid arthritis. Autoimmun Rev (2005) 4(3):130–6. doi: 10.1016/j.autrev.2004.09.002
27. Shen C, Sun XG, Liu N, Mu Y, Hong CC, Wei W, et al. Increased serum amyloid A and its association with autoantibodies, acute phase reactants and disease activity in patients with rheumatoid arthritis. Mol Med Rep (2015) 11(2):1528–34. doi: 10.3892/mmr.2014.2804
28. Dessein PH, Joffe BI, Singh S. Biomarkers of endothelial dysfunction, cardiovascular risk factors and atherosclerosis in rheumatoid arthritis. Arthritis Res Ther (2005) 7(3):R634. doi: 10.1186/ar1717
29. Turk SM, Cansu DU, Teke HU, Kasifoglu T, Meltem Akay O, Bilgin M, et al. Can we predict thrombotic tendency in rheumatoid arthritis? A thromboelastographic analysis (with ROTEM). Clin Rheumatol (2018) 37(9):2341–9. doi: 10.1007/s10067-018-4134-y
30. Rooney T, Scherzer R, Shigenaga JK, Graf J, Imboden JB, Grunfeld C. Levels of plasma fibrinogen are elevated in well-controlled rheumatoid arthritis. Rheumatol (Oxford England) (2011) 50(8):1458–65. doi: 10.1093/rheumatology/ker011
31. Undas A, Ariens RA. Fibrin clot structure and function: a role in the pathophysiology of arterial and venous thromboembolic diseases. Arterioscler Thromb Vasc Biol (2011) 31(12):e88–99. doi: 10.1161/ATVBAHA.111.230631
32. Undas A. Fibrin clot properties and their modulation in thrombotic disorders. Thromb Haemost (2014) 112(1):32–42. doi: 10.1160/TH14-01-0032
33. Vranic A, Pruner I, Veselinovic M, Soutari N, Petkovic A, Jakovljevic V, et al. Assessment of hemostatic disturbances in women with established rheumatoid arthritis. Clin Rheumatol (2019) 38(11):3005–14. doi: 10.1007/s10067-019-04629-8
34. Bucay I, O’Brien ET,3, Wulfe SD, Superfine R, Wolberg AS, Falvo MR, et al. Physical determinants of fibrinolysis in single fibrin fibers. PloS One (2015) 10(2):e0116350. doi: 10.1371/journal.pone.0116350
35. Gyorgy B, Toth E, Tarcsa E, Falus A, Buzas EI. Citrullination: a posttranslational modification in health and disease. Int J Biochem Cell Biol (2006) 38(10):1662–77. doi: 10.1016/j.biocel.2006.03.008
36. Damgaard D, Bawadekar M, Senolt L, Stensballe A, Shelef MA, Nielsen CH. Relative efficiencies of peptidylarginine deiminase 2 and 4 in generating target sites for anti-citrullinated protein antibodies in fibrinogen, alpha-enolase and histone H3. PloS One (2018) 13(8):e0203214. doi: 10.1371/journal.pone.0203214
37. Robinson WH, Sokolove J. Citrullination of fibrinogen: generation of neoepitopes and enhancement of immunostimulatory properties. Arthritis Res Ther (2012) 14(Suppl 1):O30–O. doi: 10.1186/ar3585
38. Blachère NE, Parveen S, Frank MO, Dill BD, Molina H, Orange DE. High-Titer Rheumatoid Arthritis Antibodies Preferentially Bind Fibrinogen Citrullinated by Peptidylarginine Deiminase 4. Arthritis Rheumatol (Hoboken NJ) (2017) 69(5):986–95. doi: 10.1002/art.40035
39. Zhao X, Okeke NL, Sharpe O, Batliwalla FM, Lee AT, Ho PP, et al. Circulating immune complexes contain citrullinated fibrinogen in rheumatoid arthritis. Arthritis Res Ther (2008) 10(4):R94–R. doi: 10.1186/ar2478
40. Masson-Bessiere C, Sebbag M, Girbal-Neuhauser E, Nogueira L, Vincent C, Senshu T, et al. The major synovial targets of the rheumatoid arthritis-specific antifilaggrin autoantibodies are deiminated forms of the alpha- and beta-chains of fibrin. J Immunol (Baltimore Md 1950) (2001) 166(6):4177–84. doi: 10.4049/jimmunol.166.6.4177
41. Hill JA, Al-Bishri J, Gladman DD, Cairns E, Bell DA. Serum autoantibodies that bind citrullinated fibrinogen are frequently found in patients with rheumatoid arthritis. J Rheumatol (2006) 33(11):2115–9.
42. Yue D, Brintnell W, Mannik LA, Christie DA, Haeryfar SM, Madrenas J, et al. CTLA-4Ig blocks the development and progression of citrullinated fibrinogen-induced arthritis in DR4-transgenic mice. Arthritis Rheum (2010) 62(10):2941–52. doi: 10.1002/art.27597
43. Cordova KN, Willis VC, Haskins K, Holers VM. A citrullinated fibrinogen-specific T cell line enhances autoimmune arthritis in a mouse model of rheumatoid arthritis. J Immunol (Baltimore Md 1950) (2013) 190(4):1457–65. doi: 10.4049/jimmunol.1201517
44. Sanchez-Pernaute O, Largo R, Calvo E, Alvarez-Soria MA, Egido J, Herrero-Beaumont G. A fibrin based model for rheumatoid synovitis. Ann Rheum Dis (2003) 62(12):1135–8. doi: 10.1136/ard.2003.011767
45. Sanchez-Pernaute O, Filkova M, Gabucio A, Klein M, Maciejewska-Rodrigues H, Ospelt C, et al. Citrullination enhances the pro-inflammatory response to fibrin in rheumatoid arthritis synovial fibroblasts. Ann Rheum Dis (2013) 72(8):1400–6. doi: 10.1136/annrheumdis-2012-201906
46. Takizawa Y, Suzuki A, Sawada T, Ohsaka M, Inoue T, Yamada R, et al. Citrullinated fibrinogen detected as a soluble citrullinated autoantigen in rheumatoid arthritis synovial fluids. Ann Rheum Dis (2006) 65(8):1013–20. doi: 10.1136/ard.2005.044743
47. Sebbag M, Moinard N, Auger I, Clavel C, Arnaud J, Nogueira L, et al. Epitopes of human fibrin recognized by the rheumatoid arthritis-specific autoantibodies to citrullinated proteins. Eur J Immunol (2006) 36(8):2250–63. doi: 10.1002/eji.200535790
48. Tarcsa E, Marekov LN, Mei G, Melino G, Lee SC, Steinert PM. Protein unfolding by peptidylarginine deiminase. Substrate specificity and structural relationships of the natural substrates trichohyalin and filaggrin. J Biol Chem (1996) 271(48):30709–16. doi: 10.1074/jbc.271.48.30709
49. Travers TS, Harlow L, Rosas IO, Gochuico BR, Mikuls TR, Bhattacharya SK, et al. Extensive Citrullination Promotes Immunogenicity of HSP90 through Protein Unfolding and Exposure of Cryptic Epitopes. J Immunol (Baltimore Md 1950) (2016) 197(5):1926–36. doi: 10.4049/jimmunol.1600162
50. Nakayama-Hamada M, Suzuki A, Furukawa H, Yamada R, Yamamoto K. Citrullinated fibrinogen inhibits thrombin-catalysed fibrin polymerization. J Biochem (2008) 144(3):393–8. doi: 10.1093/jb/mvn079
51. Kern A, Balog A, Dulic S, Barabas E, Kiszelak M, Vasarhelyi B. Alterations of the thrombin generation profile in rheumatoid arthritis. J Thromb Thrombolysis (2016) 41(3):359–64. doi: 10.1007/s11239-015-1251-1
52. Undas A, Gissel M, Kwasny-Krochin B, Gluszko P, Mann KG, Brummel-Ziedins KE. Thrombin generation in rheumatoid arthritis: dependence on plasma factor composition. Thromb Haemost (2010) 104(2):224–30. doi: 10.1160/TH10-02-0091
53. Chang X, Yamada R, Sawada T, Suzuki A, Kochi Y, Yamamoto K. The inhibition of antithrombin by peptidylarginine deiminase 4 may contribute to pathogenesis of rheumatoid arthritis. Rheumatol (Oxford England) (2005) 44(3):293–8. doi: 10.1093/rheumatology/keh473
54. Tilvawala R, Nguyen SH, Maurais AJ, Nemmara VV, Nagar M, Salinger AJ, et al. The Rheumatoid Arthritis-Associated Citrullinome. Cell Chem Biol (2018) 25(6):691–704.e6. doi: 10.1016/j.chembiol.2018.03.002
55. Yuri Gasparyan A, Ayvazyan L, Cocco G, D Kitas G. Adverse cardiovascular effects of antirheumatic drugs: implications for clinical practice and research. Curr Pharm Des (2012) 18(11):1543–55. doi: 10.2174/138161212799504759
Keywords: rheumatoid arthritis, fibrinogen, coagulation, citrullination, cardiovascular risk
Citation: Bezuidenhout JA, Venter C, Roberts TJ, Tarr G, Kell DB and Pretorius E (2020) Detection of Citrullinated Fibrin in Plasma Clots of Rheumatoid Arthritis Patients and Its Relation to Altered Structural Clot Properties, Disease-Related Inflammation and Prothrombotic Tendency. Front. Immunol. 11:577523. doi: 10.3389/fimmu.2020.577523
Received: 29 June 2020; Accepted: 05 November 2020;
Published: 04 December 2020.
Edited by:
Katarzyna Bogunia-Kubik, Hirszfeld Institute of Immunology and Experimental Therapy (PAS), PolandReviewed by:
Aleksandra Antovic, Karolinska Institutet, SwedenCopyright © 2020 Bezuidenhout, Venter, Roberts, Tarr, Kell and Pretorius. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Douglas B. Kell, ZGJrQGxpdi5hYy51aw==; Etheresia Pretorius, cmVzaWFwQHN1bi5hYy56YQ==
†ORCID: Etheresia Pretorius, orcid.org/0000-0002-9108-2384
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.