
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Immunol. , 09 November 2020
Sec. Primary Immunodeficiencies
Volume 11 - 2020 | https://doi.org/10.3389/fimmu.2020.577442
COVID-19 has become a worldwide pandemic caused by the novel coronavirus named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Severe cases of COVID-19 have accounted for 10–20% of all infections, leading to more than 500,000 deaths. Increasing evidence has suggested that the inflammatory cytokine storm originating from the anti-SARS-CoV-2 immune response plays an important role in the pathogenesis of critically ill patients with COVID-19, which leads to mixed antagonistic response syndrome (MARS). In the early stage of severe COVID-19, systemic inflammatory response syndrome causes acute respiratory distress syndrome, multiple organ dysfunction syndrome, and even multiple organ failure. In the late stage of severe disease, increased production of anti-inflammatory cytokines drives the immune response to become dominated by compensatory anti-inflammatory response syndrome, which leads to immune exhaustion and susceptibility to secondary infections. Therefore, precise immunomodulation will be beneficial for patients with severe COVID-19, and immunosuppressive or immune enhancement therapy will depend on the disease course and immune status. This review summarizes the current understanding of the immunopathogenesis of severe COVID-19, especially the role of the inflammatory cytokine storm in disease progression. Immune indicators and immunotherapy strategies for severe COVID-19 are reviewed and the potential implications discussed.
Coronavirus disease 2019 (COVID-19) is spreading rapidly worldwide. As of June 19, 2020, more than 8 million confirmed cases have been reported in more than 200 countries (1), and the number of cases continues to increase rapidly. On February 11, 2020, based on gene sequence homology, the Coronaviridae Study Group of the International Committee on Taxonomy of Viruses concluded that the new coronavirus is a sister virus of severe acute respiratory syndrome coronavirus (SARS-CoV) (2), which is also a member of the Betacoronavirus genus, and officially named it SARS-CoV-2. At the same time, the World Health Organization renamed “novel coronavirus pneumonia” to “COVID-19.” In general, coronaviruses infecting humans can be divided into low-pathogenic human coronaviruses (HCoVs), including HCoV-229E, HCoV-OC43, HCoV-NL63, and HCoV-HKU, and highly pathogenic coronaviruses, such as SARS-CoV and Middle East respiratory syndrome coronavirus (MERS-CoV) (3). Low-pathogenic HCoVs infect the upper respiratory tract and cause seasonal mild to moderate cold-like respiratory diseases in healthy people. In contrast, highly pathogenic HCoVs infect the lower respiratory tract, causing severe pneumonia, which can lead to fatal acute lung injury (ALI) and acute respiratory distress syndrome (ARDS), resulting in high morbidity and mortality (4, 5). At the time of this writing, the incidence of ARDS in COVID-19 patients has been 14.8%, severe cases have accounted for 18.1% of all infections, and the number of deaths has been rising (6). Immune dysregulation induced by highly pathogenic HCoVs, characterized by a large amount of inflammatory cell infiltration and the production of large amounts of proinflammatory cytokines/chemokines, leading to systemic inflammatory response syndrome (SIRS) and ARDS, plays a critical pathogenic role in disease deterioration and even leads to the death of patients. In this review, we focus on immunological assessment and immunotherapy for patients with severe COVID-19 pneumonia.
SARS-CoV-2 is homologous to SARS-CoV at both the nucleotide and amino acid levels (2). Findings from immunohistochemical and in situ hybridization analyses on autopsied patients with SARS-CoV pneumonia (hereinafter referred to as SARS) confirmed that the SARS-CoV spike protein is expressed only in angiotensin-converting enzyme II (ACE2)+ cells (7). Moreover, SARS-CoV-2 enters cells via ACE2 receptors (8). It is speculated that SARS-CoV-2 may share similar pathogenic mechanisms and pathological changes with SARS-CoV. Compared with the SARS-CoV receptor binding domain (RBD), the SARS-CoV-2 RBD exhibits a tighter conformation in its human ACE2 (hACE2) binding ridge; moreover, several residue changes in the SARS-CoV-2 RBD stabilize the two binding hot spots in the RBD/hACE2 interface (9). These structural features of the SARS-CoV-2 RBD enhance its binding affinity for hACE2, suggesting that SARS-CoV-2 is more likely than SARS-CoV to infect humans (10).
COVID-19 patients can be clinically characterized into two types: those with mild cases, which can be quickly controlled or self-limiting, and those with severe cases, which often progress to severe or critical illness and can be life-threatening. Clinical manifestations suggest that old age, the presence of comorbidities, a high respiratory viral load, and detection of viral RNA in the blood are high-risk factors for severe disease. A prospective study demonstrated that the clinical course of severe SARS has three unique stages. In the first stage, patients experience fever, coughing, and other symptoms, and the fever then improves. In the second stage (after an average of 8–9 days), patients again develop high fever, accompanied by hypoxemia and pneumonia-like symptoms. In the third stage, patients progress to ARDS, which is life-threatening. Plots of the viral load in the respiratory tract exhibit an inverted V-shape throughout the course of the disease, with the viral load peaking on day 10. The continued deterioration of the disease in the later stage is considered to be unrelated to viral replication; rather, it is suspected to be associated with the immune response.
The pathological changes in SARS have been summarized as lung lesions, immune organ damage, systemic vasculitis, and changes in systemic toxicity (11). Pulmonary lesions include extensive bilateral consolidation; localized hemorrhagic necrosis; desquamative alveolitis; bronchitis; alveolar epithelial cell hyperplasia and desquamation; exudation of alveolar proteins, monocytes, lymphocytes, plasma cells, and alveolar epithelial cells; formation of an inner hyaline membrane; and the presence of viral inclusion bodies. Massive necrosis of splenic lymphatic tissue and local necrosis of lymph nodes are observed. Systemic vasculitis includes interstitial edema in the heart, lungs, liver, kidneys, adrenal glands, and striated muscles; localized fibrinoid necrosis; and infiltration of monocytes, lymphocytes, and plasma cells into the vascular wall. The small veins exhibit thrombosis. Changes in systemic toxicity include degeneration and necrosis of parenchymal cells in the lungs, liver, kidneys, heart, and adrenal glands. The lungs, immune organs, and systemic small blood vessels are believed to be the main targets of viral attack. Extensive lung consolidation, diffuse alveolar injury and hyaline membrane formation, respiratory distress, and decreased immune function are the main causes of death. Autopsy of another eight patients with SARS confirmed the detection of SARS-CoV particles and genomic sequences in many circulating lymphocytes, monocytes, and lymphoid tissues. In addition, SARS-CoV particles and genomic sequences have been detected in respiratory epithelial cells, intestinal mucosa, distal renal tubular epithelial cells, brain neurons, and macrophages in various organs. Therefore, the comprehensive pathogenesis of SARS is characterized by aberrant immune responses and lung injury. ACE2 expression in humans has been detected via immunohistochemistry in 15 different organs and tissues across 93 patients (oral and nasal mucosa, nasopharynx, lungs, stomach, small intestine, colon, skin, lymph nodes, thymus, bone marrow, spleen, liver, kidneys, and brain) (12). ACE2 proteins are most significantly expressed on the surface of alveolar epithelial cells and small intestinal epithelial cells. In addition, ACE2 was present on arterial and venous endothelial cells and arterial smooth muscle cells of all organs studied. ACE2 has also been detected in monocytes/macrophages (13) and in CD68+CD169+ macrophages in the spleens and lymph nodes of patients with COVID-19 (14). TMPRSS2 has been identified as an alternative receptor for SARS-COV-2 and has been found on lymphocytes and macrophages (15). Furthermore, SARS-COV-2 viral particles or proteins have been found in the spleens and hilar lymph nodes of patients who died of COVID-19 (16). These results suggest that immune cells/organs may be attacked by SARS-COV-2 infection in addition to the systemic effect of the abnormal immune response to the virus. The results from 4 SARS patients showed high expression levels of proinflammatory cytokines in SARS-COV-infected ACE2+ cells but not in uninfected ACE2+ cells (7), suggesting that proinflammatory cytokines play an important role in disease development. These results are consistent with the increase in neutrophils and monocytes and the decrease in CD4 and CD8 T cells in the peripheral blood of patients with fatal SARS.
Similar pathological changes have been found in the lungs of COVID-19 patients. Pathological examination in patients who died of COVID-19 showed extensive lung consolidation, diffuse alveolar injury, robust macrophage infiltration, alveolar hyaline membrane formation, and respiratory distress (17). A large amount of clinical data has shown that COVID-19 patients exhibit significantly reduced peripheral blood lymphocyte counts, which is associated with disease severity, suggesting a key role of this parameter in predicting severe COVID-19 disease (10, 18). The early decrease in peripheral blood lymphocytes may be attributed to changes in lymphocyte distribution due to their infiltration into the lungs to participate in antiviral inflammatory responses. However, late in the disease course, the peripheral blood lymphocyte count in patients with severe COVID-19 continues to decline, and atrophy of lymphatic organs and reductions in bone marrow hyperplasia have been found in patients who died of COVID-19. How do these effects arise? Do they occur due to an excessive anti-inflammatory response, or does SARS-CoV-2 directly infect lymphocytes and cause immune cell destruction? ACE2 expression has not been detected in immune cells or organs (12), and no evidence indicates that SARS-CoV-2 infects immune cells via ACE2.
The inflammatory cytokine storm, also known as cytokine storm or cytokine release syndrome (CRS), is a severe excessive immune response caused by a positive feedback loop between cytokines and immune cells. The symptoms of CRS include high fever, erythema, edema, extreme fatigue, and nausea, and it is an important cause of sepsis and multiple organ failure (19).
The innate immune response is the first line of defense against viral infections. Cytokines and chemokines play important roles in the immune response and immunopathology of viral infections. Dysregulated, excessive immune responses lead to SARS immunopathology (20). Macrophages are the key sentinel cells in the respiratory system. The poor ability of SARS-CoV-infected macrophages to produce chemokines and interferon β (IFN-β), a key component of innate immunity, may be an early pathogenic mechanism of SARS (21). In vitro assays have demonstrated that dendritic cells (DCs) infected with SARS-CoV produce low levels of the antiviral cytokines IFN-α and IFN-β; moderate levels of the proinflammatory cytokines tumor necrosis factor (TNF) and interleukin-6 (IL-6); and high levels of the inflammatory chemokines CCL3, CCL5, CCL2, and CXCL10 (22). Another in vitro study showed that, upon infection with SARS-CoV, alveolar epithelial cells (A549) produce large amounts of CCL3, CCL5, CCL2, and CXCL10, while mononuclear cells (THP-1) produce large amounts of CCL2, CXCL8/IL-8, CCL3, CXCL10, CCL4, and CCL5 (23). These cytokines/chemokines are the key factors for the chemotaxis of neutrophils, monocytes, and activated T cells, and their excessive production may lead to dysregulation of the immune response to SARS-CoV infection. These studies showed that the dysregulation, delay, and/or exaggeration of the responses of SARS-CoV-infected macrophages, DCs, and alveolar epithelial cells to cytokines and chemokines may play an important role in the deterioration of patients with SARS.
Young BALB/c mice infected with the MA15 virus, a type of SARS-CoV to which mice are susceptible, exhibit the pathological features of diffuse alveolar injury, increased aggregation of monocytes/macrophages and neutrophils, pulmonary edema, and hyaline membrane formation, consistent with the pathology of lethal SARS in humans (24). Early use of type I IFN could ameliorate the immunopathology of SARS in mice, whereas delayed overexpression of type I IFN, aggregation of mononuclear macrophages, and dramatic increases in cytokines/chemokines are lethal factors (25). Another study in the same mouse model showed that the levels of ARDS-related cytokines were significantly increased in mice that experienced lethal lung pathological changes, suggesting that disproportionate intensity and kinetics of the host innate immune response result in severe respiratory stress and even death independent of viral kinetics (26).
Clinically, the serum levels of proinflammatory cytokines (IFN-γ, IL-1, IL-6, IL-12, and transforming growth factor β) and chemokines (CCL2, CXCL10, and IL-8) were found to be significantly higher in patients with severe SARS than in patients with nonsevere SARS (27). In addition, the levels of IL-18, IFN-γ-inducible protein-10 (IP-10), monokines induced by IFN-γ, and monocyte chemotactic protein-1 (MCP-1) were significantly higher in patients who died than in those who survived, suggesting that IFN-γ-related cytokine storms are involved in immunopathological injury in SARS patients (28). These studies showed that, in the early stage, SARS is characterized by high serum levels of proinflammatory cytokines (IL-6, IFN-α, IFN-γ) and chemokines as well as high expression levels of IFN-stimulated genes (ISGs). An uncontrolled interferon response may lead to the failure of the transition from innate immunity to acquired immunity, which is closely correlated with poor disease prognosis (28, 29).
The incidence of ARDS in COVID-19 patients is as high as 14.8–17% (6, 30) but as high as 61.1% in patients in the intensive care unit (ICU) (10). Evidence from COVID-19 patients in the ICU suggests that high serum levels of proinflammatory cytokines (IL-2, IL-7, granulocyte colony-stimulating factor, IP-10, MCP-1, MIP1A, and TNF-α) play an important role in the pathogenesis of COVID-19 (31). Patients with severe disease exhibited higher serum levels of IL-2R and IL-6 than patients with mild or moderate disease (32). Similar results were found in two other clinical studies: the levels of IL-2R, IL-6, and TNF-α were significantly higher in patients with severe COVID-19 than in those with moderate disease, suggesting that the cytokine storm is a key factor in the deterioration of patients with COVID-19 (33, 34). Additionally, patients with severe COVID-19 exhibited significant reductions in the peripheral blood lymphocyte, T lymphocyte, CD4+ T cell, and CD8+ T cell counts. These findings confirmed that SARS-CoV-2-related SIRS and ARDS exist in patients with severe COVID-19 and that acute kidney injury and myocardial injury are present in patients who die of COVID-19. Another study found that CD4+ T lymphocytes in COVID-19 patients are rapidly activated to become pathogenic T-helper (Th) 1 cells and produce granulocyte macrophage colony-stimulating factor and other cytokines, further inducing the generation of CD14+CD16+ monocytes that produce high levels of IL-6 and consequently accelerating the inflammatory response. Intrapulmonary accumulation of abnormally activated immune cells can induce immune damage, leading to pulmonary dysfunction and rapid death. The excessive, ineffective host immune response of pathogenic T cells and inflammatory monocytes may be associated with severe pulmonary pathology (35).
The results of the above studies suggest that the severity of COVID-19 may be attributed to the decreased function of sentinel macrophages, the excessively delayed response of DC and alveolar epithelial cells to cytokines and chemokines, the formation of an inflammatory cytokine storm, monocyte infiltration, and lymphocyte dysfunction. In the early stage of severe COVID-19, the inflammatory cytokine storm leads to mixed antagonistic response syndrome (MARS), which is dominated by SIRS and results in ARDS, coagulation dysfunction, acute renal injury, myocardial injury, multiple organ dysfunction syndrome (MODS), and even multiple organ failure (MOF). In the late stage of disease, increasing levels of anti-inflammatory cytokines (IL-4, IL-10) inhibit the activation of immune cells, and the immune response of MARS shifts to domination by compensatory anti-inflammatory response syndrome (CARS), which leads to immune exhaustion and susceptibility to secondary infections.
Significant decreases in circulating T lymphocyte, CD4+ T cell, and CD8+ T cell counts, which are associated with disease severity, have been found in both SARS and COVID-19 (10, 36, 37). The absolute numbers of T lymphocytes, CD4+ T cells, and CD8+ T cells in patients with severe disease (294.0, 177.5, and 89.0 × 106/L, respectively) were found to be significantly lower than those in patients with moderate disease (640.5, 381.5, and 254.0 × 106/L), indicating that total lymphocyte counts and T cell subset counts can be used to evaluate disease severity (33). A study showed that patients in the early stage of COVID-19 who were ≥50 years of age and had a peripheral blood neutrophil-to-lymphocyte ratio (NLR) of ≥3.13 were likely to develop severe disease (38). Another study showed that the neutrophil count was significantly increased in COVID-19 patients not only at onset but also 13–15 days after onset, while the lymphocyte count continued to decline; in parallel, levels of both inflammatory (IL-6, IL-2) and anti-inflammatory (IL-4, IL-10) cytokines increased (39). IL-6 peaked within 3 days and then decreased briefly at 4–6 days. IL-10 peaked at 4–6 days; thereafter, the levels of IL-6 and IL-10 remained high but began to decline after 16 days. IL-6 can also be used to predict severe sepsis (40). The results of that study suggest that, in patients with severe COVID-19, SIRS may occur very soon (≤3 days), and a mixed response with CARS—i.e., MARS—subsequently begins on days 4–6. Lymphocytes were found to be gradually depleted as the disease progressed, resulting in immunosuppression (on approximately day 16), manifesting as atrophy of the immune organs, secondary infection, and MODS. A similar clinical process was confirmed in another clinical study: the median time from onset to dyspnea was 8 days in patients with severe COVID-19, and rapid progression within 3 days usually predicted a poor prognosis (31). Other studies showed elevated levels of IL-6 in COVID-19 patients, and the serum IL-6 level was significantly higher in patients with severe disease than in those without severe disease (108 ± 12 ng/L vs 34 ± 7 ng/L) (32–34). These findings suggest that the serum IL-6 level can be used as a key indicator of disease severity. The IL-6/IL-10 ratio may also be an important indicator for assessing the immune status of COVID-19 patients (27), although no established cutoff value is available to identify SIRS and/or MARS. Considering the aforementioned studies collectively, we believe that disease stage should be considered when serum IL-6 levels are used to evaluate the immune status of patients with COVID-19 and that the predictive value of this parameter is relatively low 16 days after disease onset. In addition, the serum IL-2R level was found to be significantly higher in patients with severe COVID-19 than in patients without severe disease (1185 ± 80 U/ml vs 631 ± 37 U/ml) (32, 33), suggesting that serum IL-2R may also play a role in predicting the severity and prognosis of COVID-19. Since both SARS and COVID-19 patients exhibit intrapulmonary infiltration primarily of macrophages, the serum MCP-1 level may be an alternative indicator of disease severity (7, 41). IFN-γ is a key factor causing cytokine storms (28); thus, serum IP-10 and ISG levels are suitable for the assessment of disease severity (27, 29, 31, 41). The level of the neutrophil chemokine IL-8 can also be used to assess the disease condition (41, 42).
In summary, an increase in the IL-6 level to five times the upper limit of normal; an NLR of >3.13; a peripheral blood lymphocyte count of <500 × 106/L; and significantly increased serum levels of IL-2R, IL-8, IFN-γ, and IP-10 may be indicators of SIRS. Early and timely immunosuppression may attenuate or even block the development of ARDS and/or MODS caused by SIRS. On the other hand, significantly elevated IL-4 and IL-10 levels and a progressively reduced peripheral blood lymphocyte count may suggest an immune status dominated by CARS, for which immune enhancement therapy is needed.
In addition to vaccine development and approaches that directly target the virus or block viral entry, treatments addressing the immunopathology of the infection have become a major focus. Critically ill patients with COVID-19 are immunopathologically characterized by SIRS-CARS-MARS and ARDS; SIRS predominates, leading to ARDS in the early stage and ultimately evolving to domination by CARS, resulting in the suppression of immune function in the later stage. Therefore, immunosuppressive or immune enhancing therapy should be applied as appropriate according to the disease course and as determined by monitoring the immune status (Figure 1).
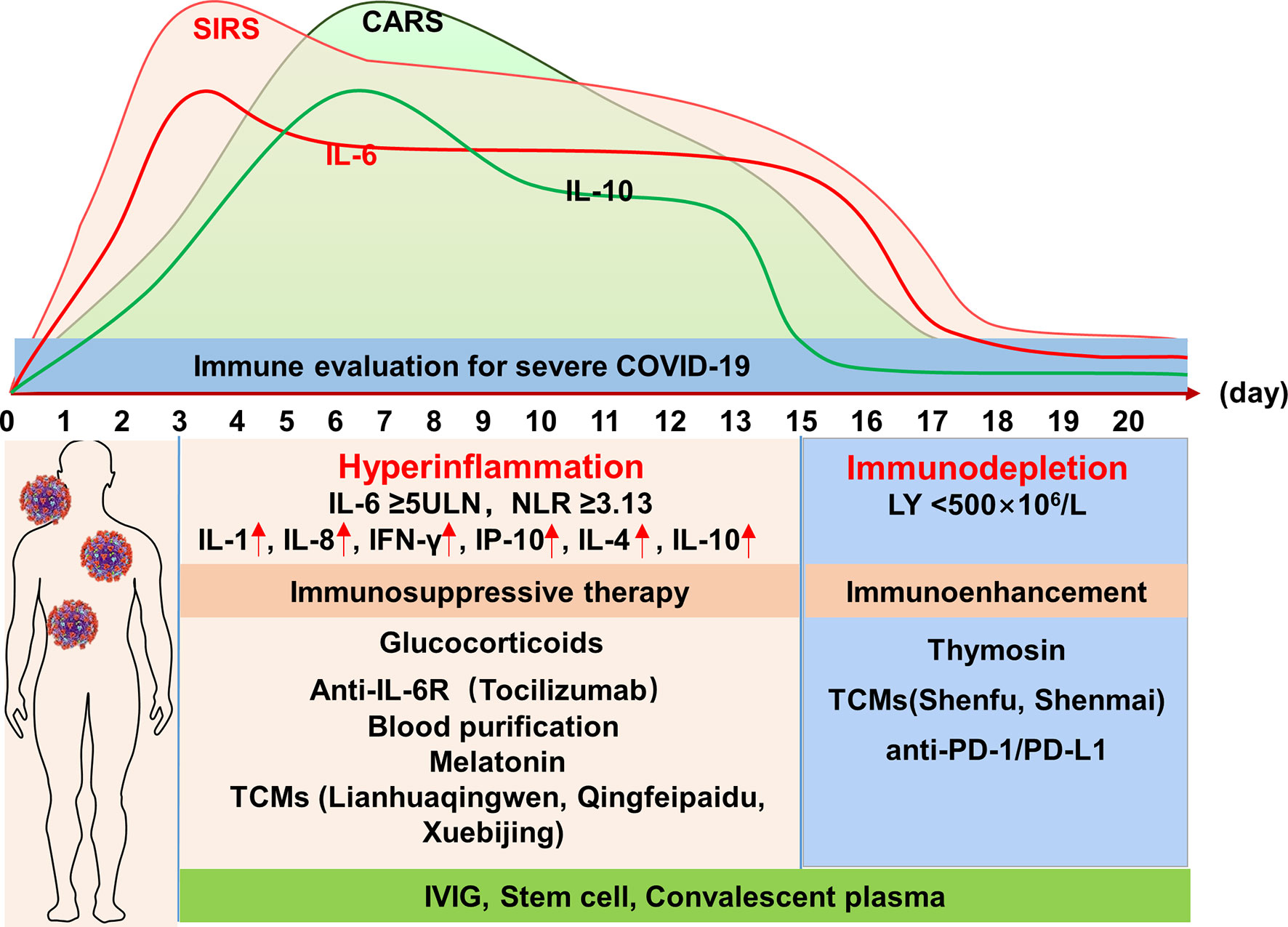
Figure 1 Profile of the immune response of severe COVID-19 and potential immunotherapeutic approaches. IL, interleukin; NLR, neutrophil-to-lymphocyte ratio; LY, lymphocyte; IFN-γ, interferon-γ; IP-10, IFN-γ-inducible protein-10; TCMs, traditional Chinese medicines; IVIG, intravenous immunoglobulin; PD-1, programmed cell death-1; PD-L1, programmed death ligand 1.
Because SIRS and ARDS are characterized by cytokine storms, immunosuppressive strategies that can be adopted include glucocorticoids to suppress the intensity of the immune response, monoclonal antibodies specific for proinflammatory cytokines, blood purification to remove inflammatory mediators, traditional Chinese medicines (TCMs), and stem cell therapy.
Glucocorticoids are the most common anti-inflammatory drugs; they can inhibit inflammation, and they can also inhibit the immune response and pathogen clearance, leading to potent side effects. During the SARS epidemic, excessive use of glucocorticoids was suspected to cause femoral head necrosis or diabetes. The steroid toxicity of glucocorticoids also leads to affective mental disorders in combination with personal vulnerability and possible psychosocial stressors. Moreover, early use of glucocorticoids increases the plasma viral load, with increased peak lactate dehydrogenase levels. The application of glucocorticoids in critically ill patients with COVID-19 has been controversial. Some studies have shown that the use of glucocorticoids delays the negative conversion of viral RNA in oropharyngeal swabs and feces (43) and that systemic application of glucocorticoids does not confer significant benefits (44). Excessive doses, long-term treatment, and improper selection of indications may contribute to the clinical ineffectiveness and even severe side effects of glucocorticoids. The clinical benefits of glucocorticoids may depend on the indication for glucocorticoid treatment (disease severity) and the timing, dose, and duration of intervention. In a large-sample study on SARS treatment, the crude mortality rate of the 1188 patients receiving steroid treatment was lower than that of the 99 patients in the control group (17.0% vs 28.3%); the patients who received low-dose oral prednisolone and those that received high-dose methylprednisolone had the lowest mortality rates (45). These findings suggest that the application of corticosteroids with high anti-inflammatory activity at the appropriate time and dose corresponding to disease severity may help to control immunopathological lung injury, thereby improving the prognosis of SARS. In another study, patients receiving the early methylprednisolone shock treatment regimen exhibited lower oxygen demand and better imaging improvement, without increased side effects, than patients receiving other corticosteroid regimens (46). In a study on COVID-19 pneumonia, patients with severe disease were administered glucocorticoid treatment (median hydrocortisone equivalent of 400.0 mg/day) for an average of 9.5 days. The blood oxygen saturation (SaO2) and arterial partial pressure of oxygen (PaO2) increased 3–5 days after treatment, and the cytokine storm was effectively inhibited in the ARDS stage, which allowed time for disease control and did not increase mortality (47). Recently, a randomized controlled clinical trial (RECOVERY trial) conducted in the UK reported for the first time that dexamethasone could reduce the mortality of patients with COVID-19. Compared with 4300 patients receiving standard care, low-to-medium dose (6 mg) dexamethasone daily for 10 consecutive days reduced the mortality rate by one-third in COVID-19 patients who were on a ventilator and by 20% for those who needed oxygen but had not been on a ventilator; no significant adverse events were found (48). Recently, WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group published the results of a prospective meta-analysis evaluating the efficacy of corticosteroids in critically ill patients with COVID-19, which pooled data of 1703 cases of critically ill patients from 7 RCTs conducted in 12 countries. Compared with usual care or placebo, administration of systemic corticosteroids was associated with lower 28-day all-cause mortality (49). An important observation from the RECOVERY trial and the above prospective meta-analysis showed that corticosteroids provided benefit only for severely ill patients with COVID-19 immunopathologically dominated by SARS-CoV-2-associated hyper inflammation. In patients with mild COVID-19, the immune response is under the control of the immune system, which, preferable for eliminating the virus, and glucocorticoids are unnecessary and may lead to delayed virus clearance (48–50). Thus, glucocorticoid therapy is recommended for patients with severe COVID-19 at the appropriate time of the early stage (SIRS stage) of disease, with the severity-dependent dosage, and for the limited treatment period in which initial impact therapy is applicable. A regimen with short-term (3–5 days), low-dose methylprednisolone (1–2 mg/kg/day) was recommended by the Chinese guidelines for COVID-19 (51).
IL-6 is a key factor in the inflammatory cytokine storm. Tocilizumab, a humanized anti-IL-6R antibody approved for the treatment of rheumatoid arthritis, CRS, and idiopathic multicentric Castleman disease (IMCD) (52), is recommended for the treatment of COVID-19-induced CRS (53). A study showed that tocilizumab had significant therapeutic efficacy in patients with severe COVID-19; 19 of the 20 patients with severe disease who received the treatment recovered and were discharged, and the rest of the patients recovered well (54). Experimental intravenous administration of tocilizumab for the treatment of COVID-19 was carried out in China and Italy and showed encouraging results (55). In addition, a clinical trial (ChiCTR2000029765) of tocilizumab for the treatment of COVID-19 is currently underway. Other monoclonal antibodies specific for proinflammatory cytokines also show prospects for treating COVID-19, which include Canakinumab, a monoclonal antibody targeting IL-1β (56); Eculizumab, C5-targeting monoclonal antibody (57); Narsoplimab, a monoclonal antibody against mannose-binding protein-associated serine protease 2 (58); and Dupilumab, a monoclonal antibody of the immunoglobulin G4 subclass (59).
Blood purification treatment can theoretically eliminate serum inflammatory cytokines. In the treatment of H7N9-induced severe pneumonia, which is also characterized by ARDS, plasma exchange (PE) combined with continuous veno-venous hemofiltration treatment can significantly reduce serum levels of cytokines and improve outcomes (60). Several studies have shown the attractive effects of blood purification treatment in relieving cytokine storms and improving the outcomes for patients critically ill with COVID-19 (61–63). Importantly, better efficacy can be achieved with earlier application of blood purification therapies (64–66). Blood purification has been recommended by consensus of Chinese experts for the treatment of patients with severe COVID-19 (51, 67).
Mesenchymal stem cells (MSCs) can regulate the proliferation, activation, and effector functions of all immune cells and play an immunosuppressive role in the activity and cytokine secretion of neutrophils and macrophages (68, 69). Thus, MSCs could reduce the occurrence of cytokine storm in acute-phase responses of COVID-19. Besides their powerful anti-inflammatory and immunoregulatory abilities, MSCs can also inhibit cell apoptosis and promote endogenous tissue regeneration, which will contribute to the tissue repair from SARS-CoV-2 induced lung injury. Furthermore, MSCs can release antimicrobial molecules that will benefit the clearance of SARS-CoV-2 (70). Bone marrow MSCs have shown therapeutic efficacy in patients with ALI, ARDS, asthma, chronic obstructive pulmonary disease, or idiopathic pulmonary fibrosis (71). Seven COVID-19 patients who received ACE2-expressing bone marrow MSCs showed significant improvement in lung function and symptoms within 2 days after treatment. After 3–6 days of treatment, the peripheral blood lymphocyte counts increased; C-reactive protein (CRP) levels decreased; and excessively activated immune cells that secrete cytokines, including CXCR3+CD4+ T cells, CXCR3+CD8+ T cells, and CXCR3+ NK cells, disappeared. Significantly lower serum TNF-α and higher serum IL-10 levels were observed in patients who received bone marrow MSC therapy than in patients in the placebo group (72). These results show that MSCs have promising therapeutic prospects for severe COVID-19. More than 10 registered clinical trials evaluating stem cell therapy for COVID-19 are currently underway, and the results of these randomized controlled trial (RCTs) are expected to further validate the effects of MSCs.
Hydroxychloroquine sulfate and chloroquine can block internal TLR signaling and reduce the production of proinflammatory cytokines, and they have been widely used to treat autoimmune diseases like arthritis (73). Hydroxychloroquine can also inhibit proinflammatory Ca2+-activated K+ channels, leading to impaired inflammasome activation (74). On the other hand, chloroquine has been shown to have significant anti-SARS-CoV-2 efficacy both in vivo and in vitro (75, 76). This efficacy is especially observed in combination with azithromycin. Therefore, the dual antiviral and anti-inflammatory effects of hydroxychloroquine are worthy of further evaluation in patients with COVID-19 pneumonia. However, the application of hydroxychloroquine failed to reduce the risk of mechanical ventilation and all-cause death in hospitalized patients with COVID-19. On the contrary, hydroxychloroquine has been reportedly associated with an increased risk of death, especially when combined with macrolide (77–79).
Convalescent plasma has been used to treat critically ill patients with COVID-19 pneumonia. On the one hand, anti-SARS-CoV-2 antibodies in convalescent plasma can neutralize the virus and facilitate control of the virus to reduce related tissue damage. On the other hand, the plasma of patients in the convalescent phase also contains antibodies specific for inflammatory cytokines, which can neutralize inflammatory cytokines and play an important role in suppressing the inflammatory cytokine storm. In a pilot study, treatment with convalescent plasma achieved good clinical efficacy in five patients with severe COVID-19 and ARDS, and all recovered (80). In another study, 10 patients with severe COVID-19 received convalescent plasma therapy. The clinical symptoms were significantly relieved within 3 days and were accompanied by increases in oxygenated hemoglobin saturation and peripheral blood lymphocyte count and a decrease in CRP levels. Moreover, obvious absorption of intrapulmonary lesions was observed within 7 days. Interestingly, the viral RNA test results became negative in seven of the 10 patients who received convalescent plasma therapy, and no adverse effects occurred in any patient (81). The efficacy and safety of convalescent plasma therapy for patients with severe COVID-19 have been verified by many additional studies (82–84), including an international investigation of a convalescent plasma trial for COVID-19 infection (85).
Melatonin is a well-known anti-inflammatory and antioxidant molecule. It has protective effects on ALI/ARDS caused by viruses and other pathogens and exhibits potential efficacy against severe COVID-19 (86).
TCM has been widely used in China in patients with COVID-19 pneumonia (Table 1). In addition to its antiviral activity against SARS-CoV-2, Lianhuaqingwen capsule exhibits robust anti-inflammatory effects in vitro, leading to significant reductions in the levels of proinflammatory cytokines, including TNF-α, IL-6, CCl-2/MCP-1, and CXCL-10/I P-10 (87). Lianhuaqingwen has been shown to be effective in the treatment of COVID-19 in many clinic trials, including RCTs (87, 93). Another widely used TCM in China for COVID-19, Qingfeipaidu, a multicomponent herbal formula, has been shown to have antiviral, anti-inflammatory, and metabolic programming activities (88). Xuebijing injection, a patented TCM developed for the treatment of SARS in China, is indicated for infection-associated SIRS. The results of a meta-analysis of 16 papers, including 1335 cases of sepsis in a Chinese population, showed that, compared with ulinastatin alone, the combination of Xuebijing with ulinastatin led to a shorter mechanical ventilation time, shorter ICU stay, improved 28-day survival rate, reduced incidence of MODS and mortality, decreased procalcitonin concentration, ameliorated acute physiology and chronic health evaluation (APACHE) II score, and reduced levels of TNF-α and IL-6 (94). Xuebijing also exhibited antithrombotic activity, which could prevent vascular embolism caused by COVID-19 (91). Xuebijing has been recommended for the treatment of severe COVID-19 in China (51).
The immunopathology in the late stage of COVID-19 is dominated by CARS. Increasing levels of anti-inflammatory cytokines (mainly IL-4 and IL-10) and numbers of Treg cells inhibit the production and activation of immune cells, resulting in a sustained reduction in the numbers and functions of immune cells. Consequently, patients become susceptible to various secondary opportunistic infections that in turn exacerbate disease progression. Timely immune enhancement therapy can rectify the immune deficiency and augment the anti-infection capacity. Available agents include thymosin, human gamma globulin, and TCMs. In addition, blood purification can remove anti-inflammatory cytokines, and administration of fresh plasma can replenish antibodies, complement, and coagulation factors.
A meta-analysis of 19 RCTs suggested that thymosin-α1 may be beneficial in reducing mortality and regulating inflammation in sepsis patients (95). Animal experiments have shown that thymosin-α1 can reduce lung injury in septic rats through the Notch signaling pathway (96). ARDS caused by pneumonia after kidney transplantation is often accompanied by profound immunosuppression and high mortality, while thymosin can significantly improve patient outcomes (97). Thymosin-α1 is recommended as an immune enhancement therapy for severe COVID-19 pneumonia in China (51).
Human gamma globulin has been shown to be effective in the treatment of SARS patients (98). It is speculated that during the pathogenesis of COVID-19, SARS-CoV-2 causes respiratory tract injury and enters the bloodstream to cause systemic damage to ACE2+ cells. Human gamma globulin is believed to enhance immune function through supplementation with diverse antibodies, which are active in the prevention of secondary infection and in blocking the inflammatory cytokine storm (99, 100).
Intravenous immunoglobulin (IVIG) can also help control inflammation and inhibit T cell activation through negatively regulating TCR signaling, affecting the number and function of regulatory T cells (101). Thus, with the dual immunomodulatory properties of both anti-inflammation and anti-infection activities, IVIG is suitable for severe COVID-19 for the whole course of disease.
In rats with severe sepsis, Shenmai injection combined with recombinant IL-12 (rIL-12) was found to increase survival rate (102). In patients with sepsis, emodin can significantly reduce serum IL-10 levels (103); in addition, Shenfu injection has been shown to be effective and is routinely used in the treatment of septic shock in China (104). The above TCMs show immunomodulatory effects, mainly manifested as an enhanced immune response, which may be beneficial in patients in the late CARS stage of severe COVID-19 (51).
The immune checkpoint mechanism is an endogenous component of the immune system responsible for coordinating the physiological immune response, maintaining self-tolerance and protecting tissues from damage. High levels of programmed cell death-1 (PD-1) and T cell immunoglobulin and mucin domain-3 (Tim-3) have been found in T cells of patients with severe COVID-19 (105), and T cell depletion and dysfunction are common in severe COVID-19 patients (106, 107). Blocking of PD-1 or programmed death ligand 1 (PD-L1) can prevent T cell death, regulate cytokine production, and protect organs from dysfunction (106, 107). A recent phase Ib trial reported that, in patients with systemic sepsis, the application of anti-PD-1 monoclonal antibody nivolumab led to restoration of the counts and functions of lymphocytes without affecting the levels of IL-6, IL-8, and TNF-α (108). Therefore, immune checkpoint inhibitors combined with IL-6/IL-6R-specific antibodies are expected to be a new hope for COVID-19 patients (109, 110). It is cautious in the application of checkpoint inhibitors in patients with COVID-19 associated ARDS, since PD-1/PD-L1 antibodies may lead to over-enhanced immune responses or even cytokine storm.
Critically ill patients with COVID-19 pneumonia are pathophysiologically characterized by the presence of an inflammatory cytokine storm/SIRS, which leads to ARDS and septic shock that in turn can result in tissue damage (MODS/MOF) and even be life threatening. In the late stage of disease, CARS-dominated MARS inhibits the production and activation of immune cells, leading to severe defects in immune function and rendering the patient susceptible to secondary opportunistic infections, thereby accelerating disease progression and causing high mortality. Therefore, critically ill patients with COVID-19 pneumonia require precise stage-dependent immunomodulation, i.e., early immunosuppression in the SIRS/ARDS stage and immune enhancement to combat late-stage immunodepression. Thus, the SIRS/CARS/ARDS immune status should be accurately assessed with proper indicators and/or applicable models. In-depth study of the pathogenesis in critically ill patients with COVID-19 pneumonia will further verify the efficacy of these immunomodulatory therapeutics and improve the outcomes of COVID-19 pneumonia.
YZ searched the literature, performed review design, and wrote the manuscript. YC revised the manuscript and designed the figure and table. YZ and YC contributed equally to the manuscript. ZM contributed to the conception and revision of the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by the National Science and Technology Major Project (Grant No. 2018ZX10302-206 and 2018ZX10723203-005) and the Project of Hubei University of Medicine (FDFR201902 and 2020XGFYZR05).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. World Health Organization. Coronavirus disease 2019 (COVID-19), Situation report. Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports/ (Accessed Jun 19, 2020).
2. Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med (2020) 382:727–33. doi: 10.1056/NEJMoa2001017
3. Channappanavar R, Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol (2017) 39:529–39. doi: 10.1007/s00281-017-0629-x
4. van Boheemen S, de Graaf M, Lauber C, Bestebroer TM, Raj VS, Zaki AM, et al. Genomic characterization of a newly discovered coronavirus associated with acute respiratory distress syndrome in humans. mBio (2012) 3(6):e00473–12. doi: 10.1128/mBio.00473-12
5. Kuiken T, Fouchier RAM, Schutten M, Rimmelzwaan GF, van Amerongen G, van Riel D, et al. Newly discovered coronavirus as the primary cause of severe acute respiratory syndrome. Lancet (2003) 362:263–70. doi: 10.1016/s0140-6736(03)13967-0
6. Sun P, Qie S, Liu Z, Ren J, Li K, Xi J. Clinical characteristics of hospitalized patients with SARS-CoV-2 infection: A single arm meta-analysis. J Med Virol (2020) 92:612–7. doi: 10.1002/jmv.25735
7. He L, Ding Y, Zhang Q, Che X, He Y, Shen H, et al. Expression of elevated levels of pro-inflammatory cytokines in SARS-CoV-infected ACE2+ cells in SARS patients: relation to the acute lung injury and pathogenesis of SARS. J Pathol (2006) 210:288–97. doi: 10.1002/path.2067
8. Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature (2020) 579:270–3. doi: 10.1038/s41586-020-2012-7
9. Shang J, Ye G, Shi K, Wan Y, Luo C, Aihara H, et al. Structural basis of receptor recognition by SARS-CoV-2. Nature (2020) 581:221–4. doi: 10.1038/s41586-020-2179-y
10. Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA (2020) 323:1061–9. doi: 10.1001/jama.2020.1585
11. Nicholls JM, Poon LL, Lee KC, Ng WF, Lai ST, Leung CY, et al. Lung pathology of fatal severe acute respiratory syndrome. Lancet (2003) 361:1773–8. doi: 10.1016/s0140-6736(03)13413-7
12. Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol (2004) 203:631–7. doi: 10.1002/path.1570
13. Yang X, Dai T, Zhou X, Qian H, Guo R, Lei L, et al. Analysis of adaptive immune cell populations and phenotypes in the patients infected by SARSCoV-2. medRxiv (2020) 2020.03.23.20040675. doi: 10.1101/2020.03.23.20040675
14. Chen Y, Feng Z, Diao B, Wang R, Wang G, Wang C, et al. The novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) directly decimates human spleens and lymph nodes. medRxiv (2020) 2020.03.27.20045427. doi: 10.1101/2020.03.27.20045427
15. Bertram S, Heurich A, Lavender H, Gierer S, Danisch S, Perin P, et al. Influenza and SARS-coronavirus activating proteases TMPRSS2 and HAT are expressed at multiple sites in human respiratory and gastrointestinal tracts. PLoS One (2012) 7:e35876. doi: 10.1371/journal.pone.0035876
16. Wang J, Jiang M, Chen X, Montaner LJ. Cytokine storm and leukocyte changes in mild versus severe SARS-CoV-2 infection: Review of 3939 COVID-19 patients in China and emerging pathogenesis and therapy concepts. J Leukoc Biol (2020) 108:17–41. doi: 10.1002/JLB.3COVR0520-272R
17. Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med (2020) 8:420–2. doi: 10.1016/S2213-2600(20)30076-X
18. Chan KW, Wong VT, Tang SCW. COVID-19: An Update on the Epidemiological, Clinical, Preventive and Therapeutic Evidence and Guidelines of Integrative Chinese-Western Medicine for the Management of 2019 Novel Coronavirus Disease. Am J Chin Med (2020) 48:737–62. doi: 10.1142/S0192415X20500378
19. Chousterman BG, Swirski FK, Weber GF. Cytokine storm and sepsis disease pathogenesis. Semin Immunopathol (2017) 39:517–28. doi: 10.1007/s00281-017-0639-8
20. Davidson S, Maini MK, Wack A. Disease-promoting effects of type I interferons in viral, bacterial, and coinfections. J Interferon Cytokine Res (2015) 35:252–64. doi: 10.1089/jir.2014.0227
21. Cheung CY, Poon LL, Ng IH, Luk W, Sia SF, Wu MH, et al. Cytokine responses in severe acute respiratory syndrome coronavirus-infected macrophages in vitro: possible relevance to pathogenesis. J Virol (2005) 79:7819–26. doi: 10.1128/JVI.79.12.7819-7826.2005
22. Law HK, Cheung CY, Ng HY, Sia SF, Chan YO, Luk W, et al. Chemokine up-regulation in SARS-coronavirus-infected, monocyte-derived human dendritic cells. Blood (2005) 106:2366–74. doi: 10.1182/blood-2004-10-4166
23. Yen YT, Liao F, Hsiao CH, Kao CL, Chen YC, Wu-Hsieh BA. Modeling the early events of severe acute respiratory syndrome coronavirus infection in vitro. J Virol (2006) 80:2684–93. doi: 10.1128/JVI.80.6.2684-2693.2006
24. Roberts A, Deming D, Paddock CD, Cheng A, Yount B, Vogel L, et al. A mouse-adapted SARS-coronavirus causes disease and mortality in BALB/c mice. PLoS Pathog (2007) 3:e5. doi: 10.1371/journal.ppat.0030005
25. Channappanavar R, Fehr AR, Vijay R, Mack M, Zhao J, Meyerholz DK, et al. Dysregulated Type I Interferon and Inflammatory Monocyte-Macrophage Responses Cause Lethal Pneumonia in SARS-CoV-Infected Mice. Cell Host Microbe (2016) 19:181–93. doi: 10.1016/j.chom.2016.01.007
26. Rockx B, Baas T, Zornetzer GA, Haagmans B, Sheahan T, Frieman M, et al. Early upregulation of acute respiratory distress syndrome-associated cytokines promotes lethal disease in an aged-mouse model of severe acute respiratory syndrome coronavirus infection. J Virol (2009) 83:7062–74. doi: 10.1128/JVI.00127-09
27. Chien JY, Hsueh PR, Cheng WC, Yu CJ, Yang PC. Temporal changes in cytokine/chemokine profiles and pulmonary involvement in severe acute respiratory syndrome. Respirology (2006) 11:715–22. doi: 10.1111/j.1440-1843.2006.00942.x
28. Huang KJ, Su IJ, Theron M, Wu YC, Lai SK, Liu CC, et al. An interferon-gamma-related cytokine storm in SARS patients. J Med Virol (2005) 75:185–94. doi: 10.1002/jmv.20255
29. Cameron MJ, Ran L, Xu L, Danesh A, Bermejo-Martin JF, Cameron CM, et al. Interferon-mediated immunopathological events are associated with atypical innate and adaptive immune responses in patients with severe acute respiratory syndrome. J Virol (2007) 81:8692–706. doi: 10.1128/JVI.00527-07
30. Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet (2020) 395:507–13. doi: 10.1016/S0140-6736(20)30211-7
31. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet (2020) 395:497–506. doi: 10.1016/S0140-6736(20)30183-5
32. Chen L, Liu HG, Liu W, Liu J, Liu K, Shang J, et al. [Analysis of clinical features of 29 patients with 2019 novel coronavirus pneumonia]. Zhonghua Jie He He Hu Xi Za Zhi (2020) 43:203–8. doi: 10.3760/cma.j.issn.1001-0939.2020.03.013
33. Chen G, Wu D, Guo W, Cao Y, Huang D, Wang H, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest (2020) 130:2620–9. doi: 10.1172/JCI137244
34. Pedersen SF, Ho YC. SARS-CoV-2: a storm is raging. J Clin Invest (2020) 130:2202–5. doi: 10.1172/JCI137647
35. Zhou Y, Fu B, Zheng X. Aberrant pathogenic GM-CSF+ T cells and inflammatory CD14+CD16+ monocytes in severe pulmonary syndrome patients of a new coronavirus. bioRxiv (2020) 2020.02.12.945576. doi: 10.1101/2020.02.12.945576%\2020-04-0416:28:00
36. Wan S, Xiang Y, Fang W, Zheng Y, Li B, Hu Y, et al. Clinical features and treatment of COVID-19 patients in northeast Chongqing. J Med Virol (2020) 92:797–806. doi: 10.1002/jmv.25783
37. Cui W, Fan Y, Wu W, Zhang F, Wang JY, Ni AP. Expression of lymphocytes and lymphocyte subsets in patients with severe acute respiratory syndrome. Clin Infect Dis (2003) 37:857–9. doi: 10.1086/378587
38. Liu J, Liu Y, Xiang P, Pu L, Xiong H, Li C, et al. Neutrophil-to-lymphocyte ratio predicts critical illness patients with 2019 coronavirus disease in the early stage. J Transl Med (2020) 18:206. doi: 10.1186/s12967-020-02374-0
39. Liu J, Li S, Liu J, Liang B, Wang X, Wang H, et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine (2020) 55:102763. doi: 10.1016/j.ebiom.2020.102763
40. Oberholzer A, Souza SM, Tschoeke SK, Oberholzer C, Abouhamze A, Pribble JP, et al. Plasma cytokine measurements augment prognostic scores as indicators of outcome in patients with severe sepsis. Shock (Augusta Ga) (2005) 23:488–93.
41. Wong CK, Lam CW, Wu AK, Ip WK, Lee NL, Chan IH, et al. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin Exp Immunol (2004) 136:95–103. doi: 10.1111/j.1365-2249.2004.02415.x
42. Zhang Y, Li J, Zhan Y, Wu L, Yu X, Zhang W, et al. Analysis of serum cytokines in patients with severe acute respiratory syndrome. Infect Immun (2004) 72:4410–5. doi: 10.1128/IAI.72.8.4410-4415.2004
43. Ling Y, Xu SB, Lin YX, Tian D, Zhu ZQ, Dai FH, et al. Persistence and clearance of viral RNA in 2019 novel coronavirus disease rehabilitation patients. Chin Med J (Engl) (2020) 133:1039–43. doi: 10.1097/CM9.0000000000000774
44. Liu K, Fang YY, Deng Y, Liu W, Wang MF, Ma JP, et al. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin Med J (Engl) (2020) 133:1025–31. doi: 10.1097/CM9.0000000000000744
45. Yam LY, Lau AC, Lai FY, Shung E, Chan J, Wong V, et al. Corticosteroid treatment of severe acute respiratory syndrome in Hong Kong. J Infect (2007) 54:28–39. doi: 10.1016/j.jinf.2006.01.005
46. Ho JC, Ooi GC, Mok TY, Chan JW, Hung I, Lam B, et al. High-dose pulse versus nonpulse corticosteroid regimens in severe acute respiratory syndrome. Am J Respir Crit Care Med (2003) 168:1449–56. doi: 10.1164/rccm.200306-766OC
47. Zhou W, Liu Y, Tian D, Wang C, Wang S, Cheng J, et al. Potential benefits of precise corticosteroids therapy for severe 2019-nCoV pneumonia. Signal Transduct Target Ther (2020) 5:18. doi: 10.1038/s41392-020-0127-9
48. Group RC, Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, et al. Dexamethasone in Hospitalized Patients with Covid-19 - Preliminary Report. N Engl J Med (2020) NEJMoa2021436. doi: 10.1056/NEJMoa2021436
49. WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group SJ, Murthy S, Diaz JV, Slutsky AS, Villar J, Angus DC, et al. Association Between Administration of Systemic Corticosteroids and Mortality Among Critically Ill Patients With COVID-19: A Meta-analysis. JAMA (2020) 324(13):1330–41. doi: 10.1001/jama.2020.17023 Sep 2.
50. Cain DW, Cidlowski JA. After 62 years of regulating immunity, dexamethasone meets COVID-19. Nat Rev Immunol (2020) 20:587–8. doi: 10.1038/s41577-020-00421-x
51. China NHCotpsRo. Guidance for Corona Virus Disease. People’s Medical Publishing House (2019). Available at: http://www.pmph.com/.
52. Uciechowski P, Dempke WCM. Interleukin-6: A Masterplayer in the Cytokine Network. Oncology (2020) 98:131–7. doi: 10.1159/000505099
53. Liu B, Li M, Zhou Z, Guan X, Xiang Y. Can we use interleukin-6 (IL-6) blockade for coronavirus disease 2019 (COVID-19)-induced cytokine release syndrome (CRS)? J Autoimmun (2020) 111:102452. doi: 10.1016/j.jaut.2020.102452
54. Xu X, Han M, Li T, Sun W, Wang D, Fu B, et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci U S A (2020) 117:10970–5. doi: 10.1073/pnas.2005615117
55. Harrison C. Coronavirus puts drug repurposing on the fast track. Nat Biotechnol (2020) 38:379–81. doi: 10.1038/d41587-020-00003-1
56. Caracciolo M, Macheda S, Labate D, Tescione M, La Scala S, Vadala E, et al. Case Report: Canakinumab for the Treatment of a Patient With COVID-19 Acute Respiratory Distress Syndrome. Front Immunol (2020) 11:1942. doi: 10.3389/fimmu.2020.01942
57. Mastellos DC, Pires da Silva BGP, Fonseca BAL, Fonseca NP, Auxiliadora-Martins M, Mastaglio S, et al. Complement C3 vs C5 inhibition in severe COVID-19: Early clinical findings reveal differential biological efficacy. Clin Immunol (2020) 220:108598. doi: 10.1016/j.clim.2020.108598
58. Rambaldi A, Gritti G, Mico MC, Frigeni M, Borleri G, Salvi A, et al. Endothelial injury and thrombotic microangiopathy in COVID-19: Treatment with the lectin-pathway inhibitor narsoplimab. Immunobiology (2020) 152001. doi: 10.1016/j.imbio.2020.152001
59. Thangaraju P, Venkatesan N, Sudha TYS, Venkatesan S, Thangaraju E. Role of Dupilumab in Approved Indications of COVID-19 Patient: an Efficacy-Based Nonsystematic Critical Analysis. SN Compr Clin Med (2020) 1–5. doi: 10.1007/s42399-020-00510-x
60. Liu X, Zhang Y, Xu X, Du W, Su K, Zhu C, et al. Evaluation of plasma exchange and continuous veno-venous hemofiltration for the treatment of severe avian influenza A (H7N9): a cohort study. Ther Apher Dial (2015) 19:178–84. doi: 10.1111/1744-9987.12240
61. Ma J, Xia P, Zhou Y, Liu Z, Zhou X, Wang J, et al. Potential effect of blood purification therapy in reducing cytokine storm as a late complication of critically ill COVID-19. Clin Immunol (2020) 214:108408. doi: 10.1016/j.clim.2020.108408
62. Wang Q, Hu Z. Successful recovery of severe COVID-19 with cytokine storm treating with extracorporeal blood purification. Int J Infect Dis (2020) 96:618–20. doi: 10.1016/j.ijid.2020.05.065
63. Padala SA, Vakiti A, White JJ, Mulloy L, Mohammed A. First Reported Use of Highly Adsorptive Hemofilter in Critically Ill COVID-19 Patients in the USA. J Clin Med Res (2020) 12:454–7. doi: 10.14740/jocmr4228
64. Chen G, Zhou Y, Ma J, Xia P, Qin Y, Li X. Is there a role for blood purification therapies targeting cytokine storm syndrome in critically severe COVID-19 patients? Ren Fail (2020) 42:483–8. doi: 10.1080/0886022X.2020.1764369
65. Yigenoglu TN, Ulas T, Dal MS, Korkmaz S, Erkurt MA, Altuntas F. Extracorporeal blood purification treatment options for COVID-19: The role of immunoadsorption. Transfus Apher Sci (2020) 59:102855. doi: 10.1016/j.transci.2020.102855
66. Ronco C, Bagshaw SM, Bellomo R, Clark WR, Husain-Syed F, Kellum JA, et al. Extracorporeal Blood Purification and Organ Support in the Critically Ill Patient during COVID-19 Pandemic: Expert Review and Recommendation. Blood Purif (2020) 1–11. doi: 10.1159/000508125
67. Xu K, Cai H, Shen Y, Ni Q, Chen Y, Hu S, et al. Management of corona virus disease-19 (COVID-19): the Zhejiang experience. J Zhejiang Univ Med Sci (2020) 49(1):147–57. doi: 10.3785/j.issn.1008-9292.2020.02.02
68. Harrell CR, Jovicic N, Djonov V, Arsenijevic N, Volarevic V. Mesenchymal Stem Cell-Derived Exosomes and Other Extracellular Vesicles as New Remedies in the Therapy of Inflammatory Diseases. Cells (2019) 8(12):1605. doi: 10.3390/cells8121605
69. Harrell CR, Jankovic MG, Fellabaum C, Volarevic A, Djonov V, Arsenijevic A, et al. Molecular Mechanisms Responsible for Anti-inflammatory and Immunosuppressive Effects of Mesenchymal Stem Cell-Derived Factors. Adv Exp Med Biol (2019) 1084:187–206. doi: 10.1007/5584_2018_306
70. Sadeghi S, Soudi S, Shafiee A, Hashemi SM. Mesenchymal stem cell therapies for COVID-19: Current status and mechanism of action. Life Sci (2020) 262:118493. doi: 10.1016/j.lfs.2020.118493
71. Harrell CR, Sadikot R, Pascual J, Fellabaum C, Jankovic MG, Jovicic N, et al. Mesenchymal Stem Cell-Based Therapy of Inflammatory Lung Diseases: Current Understanding and Future Perspectives. Stem Cells Int (2019) 2019:4236973. doi: 10.1155/2019/4236973
72. Leng Z, Zhu R, Hou W, Feng Y, Yang Y, Han Q, et al. Transplantation of ACE2(-) Mesenchymal Stem Cells Improves the Outcome of Patients with COVID-19 Pneumonia. Aging Dis (2020) 11:216–28. doi: 10.14336/AD.2020.0228
73. Gao W, Xiong Y, Li Q, Yang H. Inhibition of Toll-Like Receptor Signaling as a Promising Therapy for Inflammatory Diseases: A Journey from Molecular to Nano Therapeutics. Front Physiol (2017) 8:508. doi: 10.3389/fphys.2017.00508
74. Eugenia Schroeder M, Russo S, Costa C, Hori J, Tiscornia I, Bollati-Fogolin M, et al. Pro-inflammatory Ca(++)-activated K(+) channels are inhibited by hydroxychloroquine. Sci Rep (2017) 7:1892. doi: 10.1038/s41598-017-01836-8
75. Liu J, Cao R, Xu M, Wang X, Zhang H, Hu H, et al. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov (2020) 6:16. doi: 10.1038/s41421-020-0156-0
76. Gautret P, Lagier JC, Parola P, Hoang VT, Meddeb L, Mailhe M, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents (2020) 56:105949. doi: 10.1016/j.ijantimicag.2020.105949
77. Magagnoli J, Narendran S, Pereira F, Cummings TH, Hardin JW, Sutton SS, et al. Outcomes of Hydroxychloroquine Usage in United States Veterans Hospitalized with COVID-19. Med (N Y) (2020). doi: 10.1016/j.medj.2020.06.001
78. Geleris J, Sun Y, Platt J, Zucker J, Baldwin M, Hripcsak G, et al. Observational Study of Hydroxychloroquine in Hospitalized Patients with Covid-19. N Engl J Med (2020) 382:2411–8. doi: 10.1056/NEJMoa2012410
79. Mehra MR, Desai SS, Ruschitzka F, Patel AN. RETRACTED: Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis. Lancet (2020) S0140-6736(20)31180-6. doi: 10.1016/S0140-6736(20)31180-6
80. Shen C, Wang Z, Zhao F, Yang Y, Li J, Yuan J, et al. Treatment of 5 Critically Ill Patients With COVID-19 With Convalescent Plasma. JAMA (2020) 323:1582–9. doi: 10.1001/jama.2020.4783
81. Duan K, Liu B, Li C, Zhang H, Yu T, Qu J, et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci U S A (2020) 117:9490–6. doi: 10.1073/pnas.2004168117
82. Ye M, Fu D, Ren Y, Wang F, Wang D, Zhang F, et al. Treatment with convalescent plasma for COVID-19 patients in Wuhan, China. J Med Virol (2020) 10.1002/jmv.25882. doi: 10.1002/jmv.25882
83. Zhang B, Liu S, Tan T, Huang W, Dong Y, Chen L, et al. Treatment With Convalescent Plasma for Critically Ill Patients With Severe Acute Respiratory Syndrome Coronavirus 2 Infection. Chest (2020) 158:e9–13. doi: 10.1016/j.chest.2020.03.039
84. Ahn JY, Sohn Y, Lee SH, Cho Y, Hyun JH, Baek YJ, et al. Use of Convalescent Plasma Therapy in Two COVID-19 Patients with Acute Respiratory Distress Syndrome in Korea. J Korean Med Sci (2020) 35:e149. doi: 10.3346/jkms.2020.35.e149
85. Murphy M, Estcourt L, Grant-Casey J, Dzik S. International Survey of Trials of Convalescent Plasma to Treat COVID-19 Infection. Transfus Med Rev (2020) 34:151–7. doi: 10.1016/j.tmrv.2020.06.003
86. Zhang R, Wang X, Ni L, Di X, Ma B, Niu S, et al. COVID-19: Melatonin as a potential adjuvant treatment. Life Sci (2020) 250:117583. doi: 10.1016/j.lfs.2020.117583
87. Runfeng L, Yunlong H, Jicheng H, Weiqi P, Qinhai M, Yongxia S, et al. Lianhuaqingwen exerts anti-viral and anti-inflammatory activity against novel coronavirus (SARS-CoV-2). Pharmacol Res (2020) 156:104761. doi: 10.1016/j.phrs.2020.104761
88. Chen J, Wang YK, Gao Y, Hu LS, Yang JW, Wang JR, et al. Protection against COVID-19 injury by qingfei paidu decoction via anti-viral, anti-inflammatory activity and metabolic programming. BioMed Pharmacother (2020) 129:110281. doi: 10.1016/j.biopha.2020.110281
89. Cao P, Wu S, Wu T, Deng Y, Zhang Q, Wang K, et al. The important role of polysaccharides from a traditional Chinese medicine-Lung Cleansing and Detoxifying Decoction against the COVID-19 pandemic. Carbohydr Polym (2020) 240:116346. doi: 10.1016/j.carbpol.2020.116346
90. Yang R, Liu H, Bai C, Wang Y, Zhang X, Guo R, et al. Chemical composition and pharmacological mechanism of Qingfei Paidu Decoction and Ma Xing Shi Gan Decoction against Coronavirus Disease 2019 (COVID-19): In silico and experimental study. Pharmacol Res (2020) 157:104820. doi: 10.1016/j.phrs.2020.104820
91. Li Q, Wang H, Li X, Zheng Y, Wei Y, Zhang P, et al. The role played by traditional Chinese medicine in preventing and treating COVID-19 in China. Front Med (2020) 14(5):681–8. doi: 10.1007/s11684-020-0801-x
92. Zhang D, Zhang B, Lv JT, Sa RN, Zhang XM, Lin ZJ. The clinical benefits of Chinese patent medicines against COVID-19 based on current evidence. Pharmacol Res (2020) 157:104882. doi: 10.1016/j.phrs.2020.104882
93. Zhang X, Cao D, Liu J, Zhang Q, Liu M. Efficacy and safety of Lianhua Qingwen combined with conventional antiviral Western Medicine in the treatment of coronavirus disease (covid-19) in 2019: Protocol for a systematic review and meta-analysis. Med (Baltimore) (2020) 99:e21404. doi: 10.1097/MD.0000000000021404
94. Xiao SH, Luo L, Liu XH, Zhou YM, Liu HM, Huang ZF. Curative efficacy and safety of traditional Chinese medicine xuebijing injections combined with ulinastatin for treating sepsis in the Chinese population: A meta-analysis. Med (Baltimore) (2018) 97:e10971. doi: 10.1097/MD.0000000000010971
95. Liu F, Wang HM, Wang T, Zhang YM, Zhu X. The efficacy of thymosin alpha1 as immunomodulatory treatment for sepsis: a systematic review of randomized controlled trials. BMC Infect Dis (2016) 16:488. doi: 10.1186/s12879-016-1823-5
96. Zhang Y, Xia D, Li L, Gu Y, Shi L, Ma C, et al. Thymosin alpha1 alleviates lung injury in sepsis rats via notch signaling pathway. Panminerva Med (2020). doi: 10.23736/S0031-0808.20.03856-2
97. Sun Q, Liu ZH, Chen J, Ji S, Tang Z, Cheng Z, et al. An aggressive systematic strategy for acute respiratory distress syndrome caused by severe pneumonia after renal transplantation. Transpl Int (2006) 19:110–6. doi: 10.1111/j.1432-2277.2005.00245.x
98. Ho JC, Wu AY, Lam B, Ooi GC, Khong PL, Ho PL, et al. Pentaglobin in steroid-resistant severe acute respiratory syndrome. Int J Tuberc Lung Dis Off J Int Union Against Tuberc Lung Dis (2004) 8(10):1173–9.
99. Lin L, Lu L, Cao W, Li T. Hypothesis for potential pathogenesis of SARS-CoV-2 infection-a review of immune changes in patients with viral pneumonia. Emerg Microbes Infect (2020) 9:727–32. doi: 10.1080/22221751.2020.1746199
100. Bergsten H, Madsen MB, Bergey F, Hyldegaard O, Skrede S, Arnell P, et al. Correlation between immunoglobulin dose administered and plasma neutralization of streptococcal superantigens in patients with necrotizing soft tissue infections. Clin Infect Dis (2020) 71(7):1772–5. doi: 10.1093/cid/ciaa022
101. Hori A, Fujimura T, Murakami M, Park J, Kawamoto S. Intravenous immunoglobulin (IVIg) acts directly on conventional T cells to suppress T cell receptor signaling. Biochem Biophys Res Commun (2020) 522:792–8. doi: 10.1016/j.bbrc.2019.11.169
102. Yu YH, Cui NQ, Fu Q, Li J. Change of TH1/TH2 cytokine equilibrium in rats with severe sepsis and therapeutic effect of recombinant interleukin-12 and Shenmai injection. Chin J Integr Med (2005) 11:136–41. doi: 10.1007/BF02836471
103. Yu Y-h, Cui N-q, Wang G-l. [Monocyte response and regulatory effect of emodin and shenmai injection on it in patients with severe sepsis]. Chin J Integr Tradit Western Med (2006) 26 Suppl:98–101.
104. Huang P, Guo Y, Feng S, Zhao G, Li B, Liu Q. Efficacy and safety of Shenfu injection for septic shock: A systematic review and meta-analysis of randomized controlled trials. Am J Emerg Med (2019) 37:2197–204. doi: 10.1016/j.ajem.2019.03.032
105. Diao B, Wang C, Tan Y, Chen X, Liu Y, Ning L, et al. Reduction and Functional Exhaustion of T Cells in Patients With Coronavirus Disease 2019 (COVID-19). Front Immunol (2020) 11:827. doi: 10.3389/fimmu.2020.00827
106. Zheng HY, Zhang M, Yang CX, Zhang N, Wang XC, Yang XP, et al. Elevated exhaustion levels and reduced functional diversity of T cells in peripheral blood may predict severe progression in COVID-19 patients. Cell Mol Immunol (2020) 17:541–3. doi: 10.1038/s41423-020-0401-3
107. Zheng M, Gao Y, Wang G, Song G, Liu S, Sun D, et al. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell Mol Immunol (2020) 17:533–5. doi: 10.1038/s41423-020-0402-2
108. Hotchkiss RS, Colston E, Yende S, Crouser ED, Martin GS, Albertson T, et al. Immune checkpoint inhibition in sepsis: a Phase 1b randomized study to evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics of nivolumab. Intensive Care Med (2019) 45:1360–71. doi: 10.1007/s00134-019-05704-z
109. Di Cosimo S, Malfettone A, Perez-Garcia JM, Llombart-Cussac A, Miceli R, Curigliano G, et al. Immune checkpoint inhibitors: a physiology-driven approach to the treatment of coronavirus disease 2019. Eur J Cancer (2020) 135:62–5. doi: 10.1016/j.ejca.2020.05.026
Keywords: coronavirus disease 2019, severe acute respiratory syndrome coronavirus 2, pneumonia, critical illness, immunomodulation
Citation: Zhang Y, Chen Y and Meng Z (2020) Immunomodulation for Severe COVID-19 Pneumonia: The State of the Art. Front. Immunol. 11:577442. doi: 10.3389/fimmu.2020.577442
Received: 21 July 2020; Accepted: 06 October 2020;
Published: 09 November 2020.
Edited by:
Antonio Condino-Neto, University of São Paulo, BrazilReviewed by:
Mark Ballow, University of South Florida, United StatesCopyright © 2020 Zhang, Chen and Meng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhongji Meng, emhvbmdqaS5tZW5nQDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.