
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Immunol., 30 September 2020
Sec. Molecular Innate Immunity
Volume 11 - 2020 | https://doi.org/10.3389/fimmu.2020.559166
This article is part of the Research TopicNomenclature: Avoiding Babylonian Speech Confusion in Present Day ImmunologyView all 13 articles
 Lukas Heger1†
Lukas Heger1† Thomas P. Hofer2†
Thomas P. Hofer2† Venetia Bigley3
Venetia Bigley3 I. Jolanda M. de Vries4,5
I. Jolanda M. de Vries4,5 Marc Dalod6
Marc Dalod6 Diana Dudziak1,7,8,9*†
Diana Dudziak1,7,8,9*† Loems Ziegler-Heitbrock10*†
Loems Ziegler-Heitbrock10*†Currently three bona fide dendritic cell (DC) types are distinguished in human blood. Herein we focus on type 2 DCs (DC2s) and compare the three defining markers CD1c, CD172, and CD301. When using CD1c to define DC2s, a CD14+ and a CD14− subset can be detected. The CD14+ subset shares features with monocytes, and this includes substantially higher expression levels for CD64, CD115, CD163, and S100A8/9. We review the current knowledge of these CD1c+CD14+ cells as compared to the CD1c+CD14− cells with respect to phenotype, function, transcriptomics, and ontogeny. Here, we discuss informative mutations, which suggest that two populations have different developmental requirements. In addition, we cover subsets of CD11c+CD8− DC2s in the mouse, where CLEC12A+ESAMlow cells, as compared to the CLEC12A−ESAMhigh subset, also express higher levels of monocyte-associated markers CD14, CD3, and CD115. Finally, we summarize, for both man and mouse, the data on lower antigen presentation and higher cytokine production in the monocyte-marker expressing DC2 subset, which demonstrate that the DC2 subsets are also functionally distinct.
In human blood, cells with dendritic cell (DC) properties have been classified as plasmacytoid DCs (pDCs), as CD141+ DCs and as CD1c+ DCs (1–3). CD141+ DCs are also termed DC1s or cDC1s, while CD1c+ DCs are defined as DC2s or cDC2s, with “c” standing variously for conventional or classical (4).
The pDCs express CD123 and CD303 and are characterized by their unique ability to produce high amounts of type I Interferon (5).
The CD141+ DCs co-express CD370 (CLEC9A) (6–8) and they are described to activate CD8+ T cells via the MHC class I pathway including cross-presentation of exogenous antigen to CD8+ T cells (9–11). Their high ability to cross-present antigen from necrotic cells may be due to the expression of CLEC9A, since this receptor was shown to efficiently bind necrotic cells (12) via binding to actin filaments (13).
CD1c+ DCs can present antigen to both CD4+ and to CD8+ T cells (9, 14), however, when cultured with necrotic cells then they are inferior to CD141+ DCs in cross-presentation of necrotic cell derived antigen (9). The CD1c+ DCs form the largest DC subset in human lympho-hematopoietic tissues (8). Due to their efficacy in antigen presentation and T cell activation, CD1c+ as well as CD141+ DCs are attractive cell populations for vaccination studies with primary blood DCs (15, 16).
For all of these three DC types, at least two subsets have been described: for the pDCs a CD2− and a CD2+ subset has been reported (17), for CD141+ DC there is a XCR1− and a XCR1+ subset with the XCR1− cells being the putative precursors of the XCR1+ DCs (18). Finally, within the CD1c+ DC population a differential expression of CD5 and of the monocyte-associated CD14 molecule has been reported. The CD14+ subset shows higher expression levels for several additional monocyte associated markers. This prompts the question whether the CD14+ and CD14− subsets have a different ontogeny and specifically whether the CD1c+ CD14+ cells are linked to the monocyte lineage. With a focus on man and mouse, these questions will be addressed herein.
The initial question is, whether there are reliable markers in man and mouse to define DC2s as compared to CD141+ DCs and to monocytes/macrophages. There are three markers used for DC2s and these are i) CD1c, ii) SIRPα (CD172a) and iii) CLEC10A (MGL or CD301). For the purpose of this review, we will preferentially use the CD nomenclature.
CD1c is a frequently employed marker for DCs in man (1). CD1c is part of the MHC-like CD1 family of genes and it is involved in the presentation of lipid-based antigens to T cells (19). Importantly, while CD1c is found in many species including horses and panda bears, no murine homologue could be identified.
In human blood, CD1c was consistently found to label a population distinct from CD141+ DCs and from classical monocytes (20). In addition, CD1c expression is strongly expressed on almost all B cells (21), making it important to exclude CD19+CD20+ B cells when defining CD1c+ DCs. Moreover, it had been noted early on that CD1c, even after exclusion of B cells, is not restricted to DCs since it can be induced readily on monocytes by culture with GM-CSF within one day (22). Also, CD1c can be found on CD141+ DCs after FLT3L injection into apparently healthy volunteers (23). Of note, even CD141+ cells isolated from human skin appeared to co-express CD1c (24). Taken together, although the marker CD1c is widely used for the description of the DC2 subset, one should be aware of the fact that the molecule is not uniquely expressed on the DC2s, when performing flow cytometry or immunohistological analyses.
CD172a (SIRP-α) is another marker frequently used to define DC2s. CD172a is a transmembrane glycoprotein, consisting of three extracellular Ig-domains and two intracellular ITIM motifs that mediate negative signals after binding of CD47 to the N-terminal Ig-domain (25).
In man, CD172a is expressed by blood and tissue CD1c+ cells but it is low on CD141+ DC1s in various tissues (26). However, CD172a is also expressed by granulocytes and by monocytes (27) and this is also the case in pigs (28). Therefore, several additional markers are needed for unequivocal identification of DC2 cells in blood and tissue when using CD172a.
Similar to man, CD172a also selectively stains mouse DC2s but not DC1s. In the mouse spleen, CD172a is strongly expressed by lineage-negative CD11c+CD4+ but not by CD11c+CD8+ DCs (29), which is consistent with a selective staining of DC2s. Also, in mouse thymus a CD11c+CD8+SIRPα− and a CD11c+ CD11b+CD8−SIRPα+ (=CD172a+) cell population was described, and these represent the DC1 and DC2 subsets, respectively (30, 31). Others found, however, that CD172a is not completely absent from CD103+ DC1 cells, since in ocular mucosa it is expressed at a low level by these cells (32). Still, it was suggested that mouse CD172a+ DC2s can be clearly separated from CD172a−/low DC1s when the latter are defined via XCR1 (33). In conclusion, CD172a is a suitable marker for the definition of DC2s, but as several other cell types express this marker, these cells need to be carefully excluded in flow cytometry analyses.
CD301 (CLEC10A, MGL) has been suggested more recently as a defining marker for human CD1c+ cells (8, 34). CD301 is identical to MGL (macrophage C-type galactose/N-acetylgalactosamine-specific lectin) and it acts as an endocytic receptor. In the mouse, it was cloned from elicited peritoneal cells (35) and the human gene was cloned from monocytes after 7-day culture with IL-2 (36). CD301 is expressed by monocytes cultured with GM-CSF and IL-4 for 7d (37). Also, very low levels of CD301 mRNA and protein were reported for intermediate monocytes (38). Hence, there is an apparent association of CD301 with monocytes/macrophages.
In this context, Heger et al. (34) have assessed CD301/CLEC10A for its suitability as a marker for DC2s. In these studies, CD301 was highly selective for CD1c+ DCs. Only a small fraction of thymic B cells and a subset of monocytes/macrophages in the spleen was found positive for CD301 under steady-state conditions (34). Therefore, CD301 appears to have great potential as a unique marker for DC2s in man, with only some expression on monocytes/macrophages to be considered.
In the mouse, CD301 exists in two forms with different carbohydrate specificities, namely MGL1 and MGL2 (39). Based on structure, expression pattern, and carbohydrate specificity, mouse MGL2 (also termed CD301b) appears to be the homolog of human MGL (CLEC10A, CD301) (40). Staining of bone marrow cells with anti-MGL antibodies identifies a population of cells that is solely positive for MGL1 and another population positive for both MGL1 and MGL2. Hence, antibodies against MGL1 stain more cells, and this includes pDCs and macrophages. Also, anti-MGL2 stains peritoneal macrophages (40). Additional studies using a Mgl2-DTR/GFP DTR-cell-depleting mouse model suggest a role of MHC class II+ CD11c+CD301b+ cells in resistance against HSV2, and these cells were suggested to be DCs (41). Further studies reported on CD11c+CD301b+ cells, which were addressed as DCs (42), while F4/80+CD11b+CD206+CD301b+ were defined as macrophages (43). Also, CD301b+CD11c+CD11b+MHCclassIIhighF4/80intCD206+ mononuclear phagocytes were described in various tissues including fat, liver, and muscle with very few cells seen in blood (44). In addition, Langerhans cells in the mouse skin show a strong signal for MGL (45) but it still needs to be determined whether this is CD301a or CD301b. Taken together, CD301 in the human system and CD301b in the mouse system are promising identifiers of DC2s, but additional markers and a careful approach are required for correct identification of these cells.
While in the past, CD1c has been the main marker to define human DC2s, it may well be that CD172a and CD301 might serve a similar function. Comparative analysis may be helpful to define, which of these markers or which combination thereof is most appropriate to define DC2 cells.
In summary, the three markers that can be used to define DC2 cells (CD1c, CD172a/SIRPα, and CD301/CLEC10A/MGL) are not exclusively expressed by these cells. Therefore, they need to be combined with additional markers to exclude B cells, pDCs, and monocytes/macrophages, as appropriate.
While the expression profiles of CD1c, CD172a, and CD301 apply to homeostasis, additional markers may have to be added in inflammatory disease where cytokines can induce DC2-associated markers on other cell types. For example, as mentioned earlier CD1c can be found on CD141+ DCs after FLT3L injection into apparently healthy volunteers (23). With the singular use of CD1c as a DC2 marker, such FLT3L-induced cells would be wrongly assigned to the DC2 lineage.
Since monocytes are CD172a-positive in the steady state and since in vitro culture of monocytes with GM-CSF can induce expression of CD1c (22) and of CD301 (37) it is important to exclude monocytes/macrophages, when defining DC2 cells in blood and more importantly in tissue. This is particularly relevant in the context of inflammatory diseases when cytokines like GM-CSF are increased (46). An informative example is sickle cell disease, which goes along with increased blood GM-CSF levels (47), with increased numbers of CD16+ monocytes (48) and with expression of CD1c on monocytes (49). It remains to be determined whether these monocytes in the blood of sickle cell disease patients are akin to the CD14+ subset of CD1c+ DCs. In any event, these cells may contribute to the pathophysiology of the disease via production of inflammatory cytokines.
Overall, these deliberations show that determination of DC2s in inflammatory conditions requires additional steps in order to unequivocally define these cells.
In the 2010 nomenclature proposal it had been noted for the CD1c+ myeloid DCs in human blood that these DCs can be separated into CD14− and CD14low cells (1). This was based on the original studies by Thomas and Lipsky (50) demonstrating antigen presenting activity in a population of CD33++CD16−CD14+ cells, cells that later were shown to be CD1c+ (51). Of note, the typical approach to DC analysis in human blood starts with the exclusion of lymphocytes and of monocytes. Monocytes are excluded using a CD14 antibody, but depending on the reagent and the conditions used, this may or may not remove CD14low cells.
An example of the CD14 expression pattern of CD1c+ DCs is given in Figure 1. Compared to the isotype control, there is strong CD1c-staining among the CD14− cells and a gradually decreasing expression of CD1c among the CD14low cells (Figure 1A). The isotype control shows a few events within the CD1c+ DC2 gate (Figure 1B).
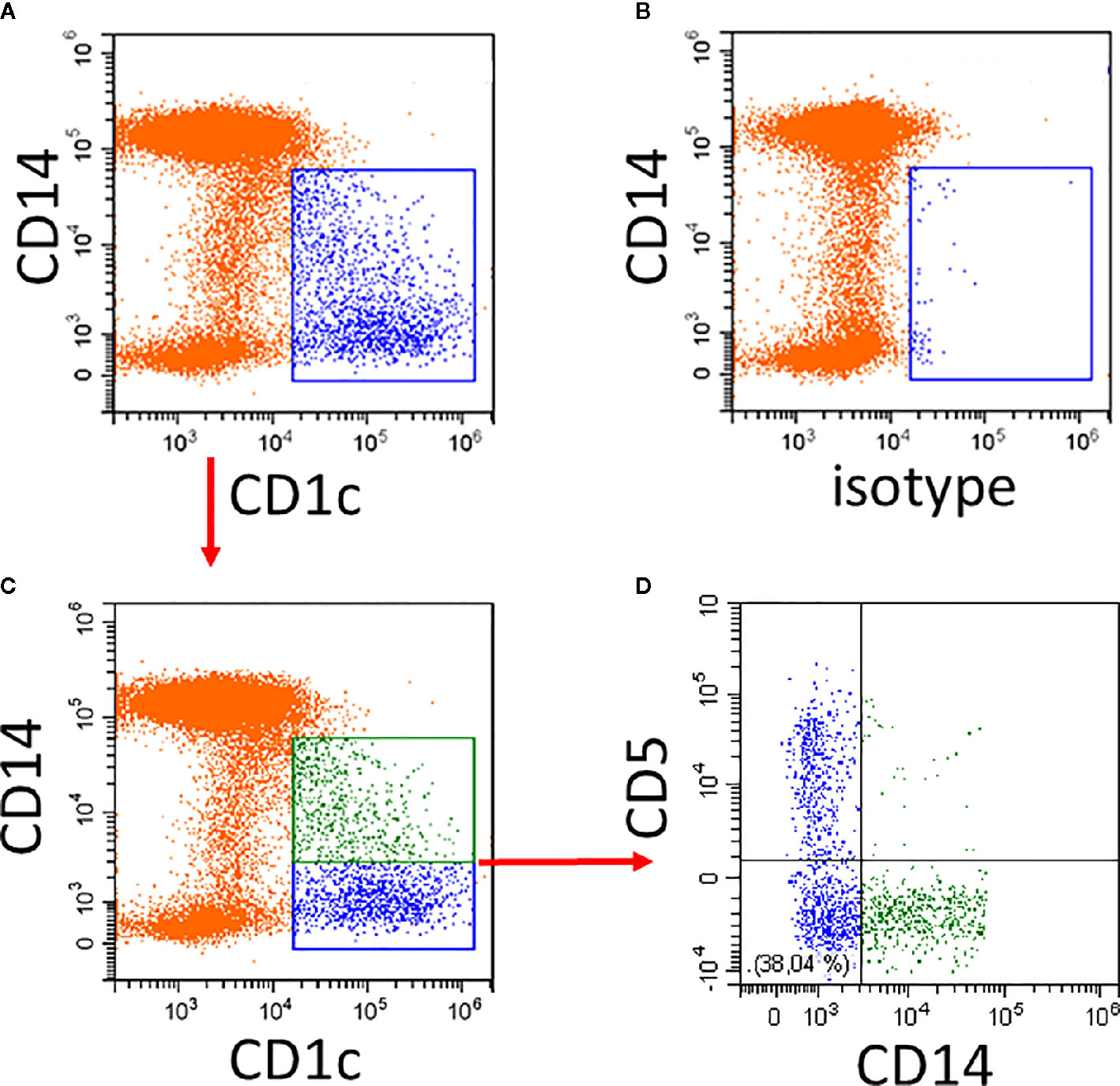
Figure 1 Illustration of CD1c+ DCs and its subsets in human blood. Whole blood was stained with CD14, CD16, CD19, HLA-DR, and CD1c antibodies and the expression of CD1c (A) compared to isotype control (B) was analyzed on HLA-DR+ non-B cells. Of note, the CD14low CD1c− cells in (A) represent the CD16+ monocytes. (C, D) show additional staining for CD5. In the example in (C), the CD1c+ cells are divided into a CD14+ subset (green) and a CD14− subset (blue). As shown in (D), the CD14− subset in blue can be further subdivided into CD5+ and CD5− cells. In the lower left there is a population of CD14− CD5− cells. Red arrows indicate the gating sequence.
The cells within the CD1c gate can then be separated into CD1c+CD14+ and CD1c+CD14− cells (Figure 1C) with the CD14+ cells (green in Figure 1A) accounting for about 40% of all CD1c+ DCs. The CD5+ cells are distinct from the CD14+ cells.
Recently, in a study not excluding CD14+ cells, it was reported that in apparently healthy donors about one third of the CD19−CD1c+ cells are CD14+ (15). These CD1c+CD14+ cells, compared to the CD1c+CD14− subset, were shown to express similar levels of HLA-DR and CD33 but higher levels of CD11b and clearly higher levels of PD-L1 (CD274) (15). Upon LPS stimulation, these cells showed a trend to produce higher amounts of TNF and IL-10, but they were less efficient in inducing T cell proliferation induced by allogeneic leukocytes. The T cell proliferation could be improved by addition of an anti-PD-L1 antibody (15). Furthermore, while the CD1c+CD14− subset readily induced IFNγ production in CD4+ T cells, the CD1c+CD14+ subset completely failed to do so. Only when CD1c+CD14+ DCs were stimulated with GM-CSF or LPS then a low level of IFNγ production could be induced. This suggests that the CD1c+CD14+ do not induce but rather impede T cell proliferation and differentiation toward the TH1 lineage.
In blood of melanoma patients with metastatic disease, the frequency of the CD14+ subset of CD1c+ cells in blood was found to be increased more than three-fold. Upon vaccination with antigen-loaded CD1c+ DCs, patients with a high proportion of the CD1c+CD14+ subset showed lower T cell proliferation to control antigen (15). This underscores the notion that CD1c+CD14+ cells in cancer patients are not potent T cell stimulators but rather show suppressive activity. Together, these findings were taken to design an optimized cellular vaccine, in which the CD14+ subset is removed from the CD1c+ DC product for vaccination of patients with melanoma and other malignancies (15, 52).
Transcriptome analyses comparing the CD1c+CD14+ cells to CD1c+CD14− cells showed the CD1c+CD14+ cells to express higher mRNA levels for MafB and the CSF1-receptor (CD115) and lower levels for FLT3 and IRF4. In addition, higher levels for TLR7, TLR8, CLEC7A (CD369), CLEC12A (CD371), and CLEC12B were found for the CD1c+CD14+ DCs (15).
Hierarchical clustering using these transcriptome data suggested that the CD1c+CD14+ DC2 subset to be in between classical monocytes and the CD1c+CD14− cells but closer to the classical monocytes (15). However, a comparison to a comprehensive set of blood DCs and monocytes is still required in order to assign them to either monocytes or DCs, when using transcriptomics as a tool. The central features of DC2 subsets in this study by Bakdash et al. are summarized in Figure 2.
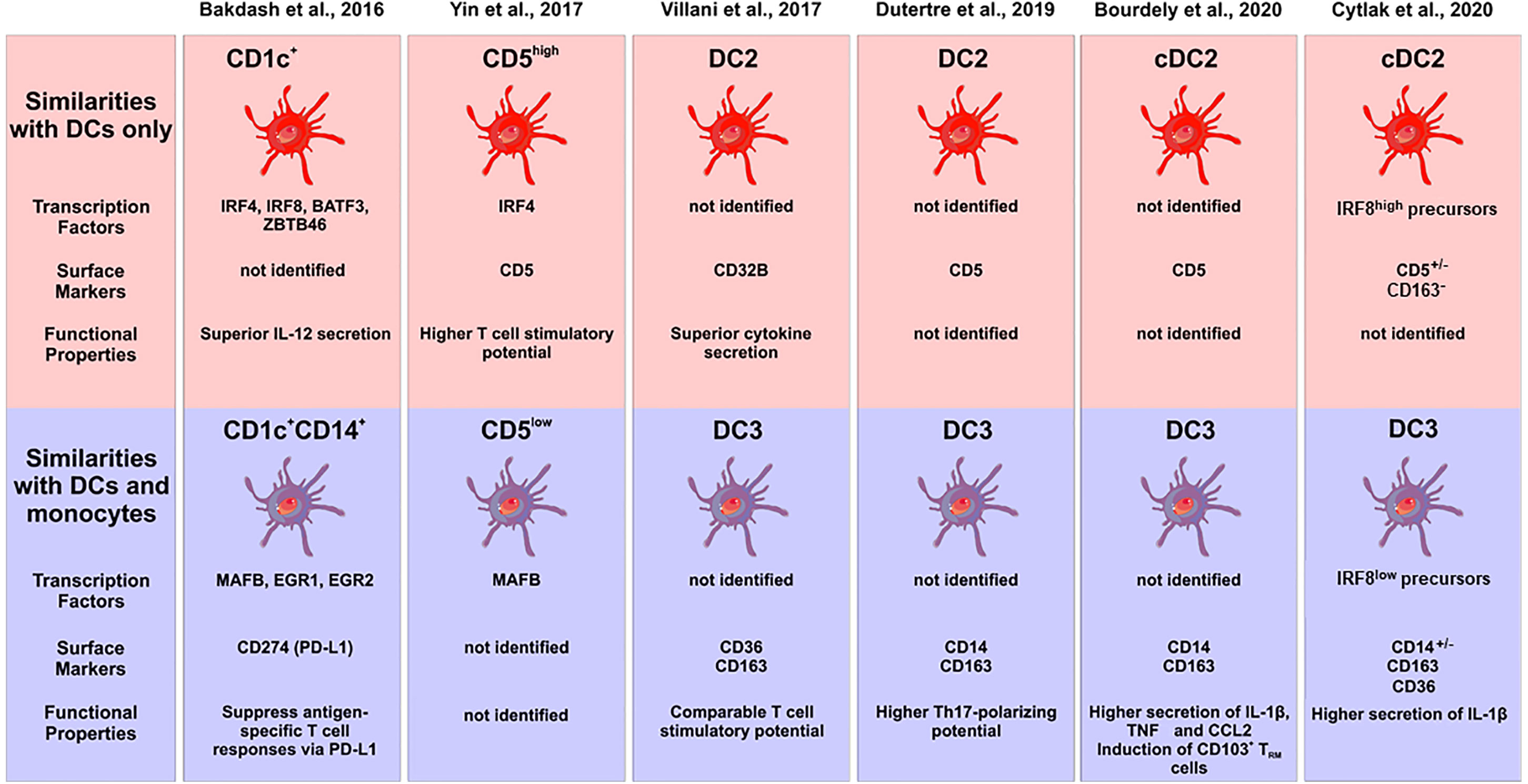
Figure 2 Characterization of subsets of human DC2s in the recent literature. The different studies are listed at the top, the upper panel gives the subsets with pure DC features, the lower panel shows the subsets with monocyte features. Characteristic transcription factors, cell surface markers and functional properties are given when available. The cellular images are provided and adapted from Servier Medical Art (smart.servier.com).
The existence of two subsets of DC2 in man was confirmed recently in single cell sequencing studies on peripheral blood mononuclear cells (53). Here, both subsets were positive for CD1c, CLEC10A and FcϵR1A. One subset expressed higher transcript levels for MHC class II molecules and CD1c, while the other was higher for S100A8/9, ANXA1, F13A, VCAN (versican), FCN1 (ficolin 1), RNase2, CD163, and CD14. Many of the latter molecules are associated with monocytes but both CD1c+ subsets clustered separately from monocytes in this study. This work by Villani et al. is also listed in Figure 2.
Furthermore, Schroder et al. have studied the properties of cells isolated with CD1c-magnetic beads from human blood mononuclear cells, and they noted a CD1c+CD14− population and a CD1c+CD14+ population, with the latter showing low level CD1c (54). Here, a higher expression of CD135 (FLT3) on the CD1c+CD14− cells and a higher expression of CD115 (M-CSFR) on the CD1c+CD14+ cells was observed, and the CD1c+CD14+ subset was interpreted to represent monocytes.
In early studies, a differential expression of CD5 had been reported on human blood DCs (55, 56). More recently in a 2017 study, the lineage-negative HLA-DR+CD123−CD11c+CD1c+ cells have been subdivided into CD5low and CD5high cells (57). Gene expression analysis showed higher SIGLEC6 and IRF4 transcripts in the CD1c+CD5high cells, while the CD1c+CD5low cells expressed higher levels of CD14, MAFB, S100A8/9, RNAse2, CD163, and Ficolin1. A few of these transcripts were tested at the protein level and here the higher expression of CD14 and S100A9 was confirmed for the CD1c+CD5low cells (57). This work by Yin et al. is listed in Figure 2.
The reciprocal gene expression pattern for CD1c+CD5high cells (57) and the CD1c+CD14low cells (15) suggests that these two subsets might be mutually exclusive and that CD1c+ cells might consist of CD14−CD5high and CD14+CD5low cells. As illustrated in Figure 1, CD5 and CD14 are expressed on distinct cell subsets (see Figure 1D).
This pattern is consistent with what has been described by Meyerson et al. (58). The latter study and our illustrative figure demonstrate a population of CD1c+CD5−CD14− cells and the question is, whether this subset represents a distinct population. In this context, Dutertre et al. (59) analyzed DCs and their subsets with an extensive panel of cell surface markers. In this study, more than 300 protein markers were employed and markers HLA-DQ and FcϵRIA on DCs and CD88 and CD89 on monocytes were identified as best discriminating markers. On this basis, the DC2s including the CD14+ subset could be phenotypically separated from classical monocytes, albeit there is low level expression of both CD88 and CD89 on DC2s.
DCs are thought to be specifically governed by FLT3 (Fms-Like Tyrosine Kinase 3) and injection of this growth factor into patients was shown to result in a shift of the proportions of DC2 versus classical monocytes. Here, both CD1c+CD14+ and CD1c+CD14− DC2s increased relative to the classical monocytes arguing for a DC nature of the CD1c+CD14+ cells (59). A more detailed analysis in the same report then revealed four distinct subsets of CD1c+ cells, which are one CD5+ subset and three CD5− subsets, the latter consisting of CD14−CD163−, CD14−CD163+ and CD14+CD163+ cells. Some salient features of the typical DC2 and the subset with monocyte features is given in Figure 2 (see Dutertre et al).
Three different phenotypes of DC2 cells in human blood were also described in a 38-marker CYTOF analysis (60). The DC2s showed differential expression levels for CD172a and CD163 and the authors concluded that there were CD172highCD163low and CD172lowCD163med and CD172highCD163high DCs. The relationship of these three phenotypes to the CD14+ und CD5+ subsets remains to be determined.
Taken together, among the CD1c+ DCs there is higher expression for several monocyte-associated genes (CD14, CD115, MAFB, S100A8/9, CD163, and Ficolin1) in cells defined either as CD14+ cells or as CD5−.
In order to appropriately address the question of lineage assignment of the subsets of CD1c+ DCs, approaches using a broad panel of different monocytes, macrophages, and dendritic cells are required.
In a recent report on human CD1c+ DC2 subsets, the CD5+ cells were defined as cDC2, while cells positive for CD14 and CD163 were termed DC3 (61). The CD14+ subset of CD1c+ cells, when compared to the CD1c+CD5+ DCs, was shown to express higher levels of TNF and CCL2 and to induce features of tissue-resident memory cells in CD8+ T cells (see summary in Figure 2). Furthermore, it was demonstrated that GM-CSF but not FLT3L is able to support development of the CD1c+CD14+ DCs in a humanized mouse model. In in vitro studies, GM-CSF was able to induce these cells from a granulocyte-monocyte-dendritic cells precursor (GMDP) but not from common dendritic cell precursor or cMoP, indicating that this subset may have a distinct developmental pathway (61).
In addition, functional studies might be able to address the question of lineage assignment. To this end, recently the ability of DCs to activate the inflammasome and induce the release of IL-1β has been revisited (62) and it was shown that among the GM-CSF induced mouse bone marrow-derived cells only the macrophages but not the DCs were efficient producers of IL-1β. If a relevant IL-1β production by the CD14+ subset of DC2 can be demonstrated then this would add another monocyte characteristic to these cells. Cytlak et al. addressed this question by comparing human CD1c+CD5+/−CD163− DC2, and CD1c+CD163+ DCs (termed DC3) (63). Here, it was noted that the CD1c+CD163+ cells, when stimulated with a mixture of TLR ligands followed by intracellular staining and flow cytometry, showed IL-1β production as high as monocytes, while the CD1c+CD163- cells showed a low level production of this cytokine (see summary in Figure 2). Similar results were obtained for IL-10, while the two DC subsets produced comparable amounts of IL-12 (63).
Moreover, DC2s can be generated in vitro from CD34+ hematopoietic stem cells (64) and more specifically from cells with the phenotype of MLPs, CMPs, and GMPs (65). The generation of subsets of DC2 was only studied recently (63). Here, CMPs and CD33+ GMPs were found to have CD1c+CD14+ DC2 potential and the CD14+ subset was shown to segregate with monocytes. In contrast, CD1c+CD14- DC2s could be generated from CD123+ GMPs and segregated with pDC and cDC1 potential.
Mutations of genes involved in development of DCs can be informative as to lineage assignment. Homozygous and heterozygous loss of function and dominant negative mutations of the IRF8 gene have been described. It was shown that bi-allelic loss of function mutations of the IRF8 gene led to a complete absence of DCs and monocytes (66, 67).
For two independent cases with recurrent disseminated BCG infection and heterozygous dominant negative mutation in the IRF8 gene, normal numbers of monocyte subsets and no decrease of pDCs and DC1s were initially reported, while there was an apparent reduction in CD1c expression on CD11c+ cells (66). In a later study by the same researchers, in a more detailed analysis using CLEC9A in addition to CD141 for DC1 definition and CD1c for DC2 identification, it was noted that DC1s are, in fact, decreased and the decrease of CD1c expression was confirmed (68).
Cytlak et al. looked at CD1c+ DCs in a kindred with a heterozygous dominant-negative mutation of IRF8, which led to a moderate deficiency of this transcription factor (63). This went along with depletion of cDC1 and pDC but increased blood monocytes and normal numbers of CD1c+ DC2s. Analysis of subsets, however, revealed an almost complete absence of the CD1c+CD5+ cells, while the CD1c+CD5− subset was expanded (63). Whereas the moderate level activity of IRF8 is apparently sufficient to allow for development of the CD14+ monocyte subsets and of the CD1c+CD14+ DC subset, it is not sufficient to allow for generation of CD1c+CD14−CD5+ DCs. This indicates that the CD14+ and CD5+ subsets of CD1c+DCs have distinct developmental requirements and that the CD1c+CD14+ DC2 subset is associated with monocytes. Analysis of additional mutations of genes involved in DC development in man may help to further support this point.
When looking at IRF8 CRISPR/Cas9 knock-out mutation in human in vitro induced pluripotent stem cells (iPS), it was noted that the generation of pDC and DC1 cells driven by FLT3L, SCF, GM-CSF, and IL-4 (FSG4) was ablated but the generation of CD1c+ DC2s was unaffected (69). These data indicate that this intriguing in vitro system more closely mimics partial IRF8 deficiency in vivo. Still, manipulation of the IRF8 gene penetrance may help in unravelling the in vitro development of DC2s and their subsets. On the other hand, the generation of DC2s in the absence of IRF8 in this in vitro system may be due to the strong effects of the exogenous cytokines, since without lineage specifying cytokines, DC2 cells are absent in these IRF8−/− cultures similar to what is seen in immuno-deficient patients (69). Again, it will be important to analyze DC2 subsets in this system.
Moreover, Borriello et al. reported on the expression of the receptor for thymic stromal lymphopoietin (TSLPR), which is induced by stimulation via TLR4 in CD1c+CD14low cells among human blood mononuclear cells (70). TSLP directs type-2 immune reactions and it acts on a broad range of leukocytes including T cells, macrophages, DCs, mast cells, and ILCs indicating that its receptor is widely expressed (71). Borriello et al. noted that induction is specific to the CD1c+CD14low cells and that there is little induction in CD16+ monocytes. It will be important to assess the induction of the TSLPR in the CD14− subsets of CD1c+ DC2s, in order to determine whether expression might be specific to the monocyte-related subset.
Taken together, while in the studies by Borriello et al. and Sontag et al. (69, 70) the analysis of DC2 subsets is still outstanding, the data on development of DC2 subsets show evidence of co-segregation of the monocyte-marker expressing DC2 subset with monocytes.
Because of the ease of accessibility, many human studies are performed on blood samples, but there are also a number of studies that look at DCs in human lymphoid and non-lymphoid tissues (8, 24, 60, 72–75) but only a few studies address subsets of DC2s in tissue.
Of note, interpretation of data in human tissue has to be done with caution: While in blood the monocyte subsets can be clearly defined and dissected from DCs, it is more difficult in tissues to exclude macrophages that may have up-regulated DC2-associated markers. Furthermore, such DC2-marker-positive macrophages may or may not co-exist with bona fide monocyte-lineage derived cells, and this can make lineage-assignment very demanding.
With respect to subsets of DC2s Yin et al. noted CD5+ and CD5− subsets of CD1c+ DCs in human tonsils and similar to blood the CD5+ cells formed the minor subset (57).
Looking at single cell suspensions isolated from the nasal mucosa, all of the CD1c+ cells expressed low levels of CD14 (76). Another study on nasal, bronchial, and intestinal mucosa showed low-level CD1c expression on the CD11chigh subset of CD14+ cells (54).
In the human lung, CD14+ and CD14− subsets of CD1c were observed in lavage samples and transcriptome analysis demonstrated higher ZBTB46, FLT3, CD83, and CCR7 mRNA levels in the CD14− subset, while the CD1c+CD14+ subset showed higher CD36, CD163, CD369 (=CLEC7A=Dectin-1), and S100A8/9 (77). Hence, the CD14+ subset of CD1c+ cells in the lung alveoli enriched for monocyte-associated genes. Also, in an analysis of bronchoalveolar lavage samples from healthy volunteers, a BTLA+ and a BTLA− subset of CD1c+CD11c+ cells were noted and here a higher expression of monocyte/macrophage-associated genes CD14, CD163, S100A8, and CD115 was noted at the transcript level for the BTLA− cells (78). For the human intestine, Watchmaker et al. identified among the lineage-negative, HLA-DR+CD11c+ cells two subsets of CD172a+ cells, one CD103+ and one CD103−. Hierarchical clustering using transcriptome data demonstrated that the CD103−CD172+ cells clustered with blood monocytes and the authors suggested that they might be monocyte-derived (72).
In summary, there is evidence for a monocyte-marker positive DC2 subset in various human tissues like tonsil, lung, and intestine.
For the mouse similar to man, pDCs, DC1s, and DC2s have been described (79–82). However, data on DCs in mouse blood are scarce, and most studies are done on tissue samples. Regarding subsets of DC2, CD11c+CD8− cells in mouse spleen and mesenteric lymphnodes Kasahara and Clark described subsets of DCIR2+DCAL2− and DCIR2−DCAL2+, i.e. CLEC4A4+CLEC12A− and CLEC4A4−CLEC12A+, respectively (83). Here, the CLEC4A4−CLEC12A+ cells exclusively produced TNF and IL12 in response to the TLR9 ligand CpG. Separate studies by Lewis et al. on cells from mouse spleen and intestine revealed that DC2-type cells with the phenotype CD8−CD11c++CD11b+ cells can be subdivided into an ESAMhigh and an ESAMlow subset (ESAM = Endothelial cell–selective adhesion molecule) (84). Here, the ESAMhigh cells expressed higher levels of MHC class II molecules, while the ESAMlow cells showed higher transcript levels for M-CSFR, CCR2, Lysozyme, CD14 and CD36, markers which are typical of the monocyte lineage. Furthermore, the ESAMlow subset was able to produce higher levels of TNF and IL-12, when stimulated with the TLR-9 ligand CpG DNA. Also, activation via TLR-2, using heat-killed Listeria monocytogenes, led to superior TNF production by ESAMlow cells. Phagocytosis of latex beads was similar for both subsets as was the capacity to present in vitro OVA peptide using transgenic responder T cells expressing OVA-specific TCR (OT-II cells) (84). However, when testing antigen presentation in vivo a robust response of OT-II cells was noted in wild type animals, while in knock-out mice, lacking ESAMhigh cells, only a minimal response of OT-II cells was observed. This suggests that the ESAMhigh cells are required for an efficient antigen presentation in vivo (84).
Importantly, the ESAMlow cells were noted to be CLEC12Ahigh (84) thereby linking the studies by Lewis et al. (84) and Kasahara et al. (83). The conclusion from both studies is that the ESAMlowCLEC12Ahigh subset of CD11c+CD8− mouse DC2s expresses monocyte-associated markers and is more potent in producing cytokines TNF and IL-12.
Also, Lewis et al. (84) demonstrated that blockade of Notch2 signalling led to selective ablation of the ESAMhigh subset. This ESAMhigh subset was shown to derive from DC precursors, while the ESAMlow cells were suggested to be myeloid progenitor-derived. In other words, Notch2 signalling is required for the development of ESAMhigh DC2s but not for ESAMlow DC2s (84). In an in vitro study, DCs were generated from progenitor cell lines by culture with a FLT3L and Notch ligand expressing cell line. Here, transcriptome analysis revealed that with Notch2 activation the CD11b+CD24−CD8− DC2s were similar to the splenic ESAMhigh DC2 subset (85).
Mutations of human Notch2 have been described in two families with the patients showing multiple abnormalities of liver, lung, heart, and kidneys and typical facial features (86). At this point, no immunological workup has been published on such patients, and it is therefore not known whether these patients have abnormalities of the immune system.
As mentioned above, a bi-allelic mutation of the IRF8 gene leads to a depletion of DC2 cells in man (66). By contrast, in the mouse it was shown that Icsbp (=Irf8)−/− animals lack pDC and CD8+ DCs (DC1), while CD4+ DCs (DC2) were readily detected (87, 88). No information on subsets of DC2s is available, and it remains to be determined whether there is a differential effect on ESAMhigh versus ESAMlow subsets in in Irf8−/− animals.
Taken together, in the mouse the ESAM expression level can be used to define subsets of DC2 with ESAMlow subsets showing features of monocytes.
Another recent study by Brown et al. defined in mouse lymphoid organs two subsets of lin−CD90−CD64−Ly6C−CD11c+ MHCII+XCR1−CD11b+cDC2s based on their differential expression of the transcription factor T-bet (89). The T-bet+ DC2s, dubbed DC2A, were found to overlap with the ESAM+ DC2s previously described by Lewis et al. (84). The T-bet− cDC2s, called DC2B, showed higher expression of monocyte-associated genes. However, only a subset of these cells expressed M-CSF-R (CD115) and lysozyme mRNA, reminiscent of monocyte-related DC2 subset, which we discussed earlier in man and mouse. The authors also noted that the T-bet− population was heterogeneous with respect to expression of C-type lectin receptors and consisted of a CLEC10A− CLEC12A−, of a CLEC10A− CLEC12A+ and of a CLEC10A+CLEC12A+ population.
Interestingly the T-bet+ cDC2 gene expression signature subset was neither detected in mouse nor in human blood (89). However, gene expression analysis of two samples from melanoma patients identified a cluster of myeloid cells that expressed T-bet target genes and additional signature genes like AREG and NR4A3. Also, in human spleen CD301− DCs with transcriptomic similarities to murine T-bet+ DC2A cells were described.
Brown et al. (89) suggest that the human blood CD1c+ cDC2s might be analogous to the mouse T-bet− CLEC10A+ DC2B, while in human blood there is no homologue of the mouse T-bet+ cDC2s. Clearly more studies, including functional tests, are required in order to collate these recent findings into a unified scheme and to align subsets across species.
For the lung of wild type mice, it was shown that there are CD14+ cells among a population of CD11b+CD24+CD64− DC2s (90). Also, the DC2 cells were either Irf4+ or Irf4− and in Irf4−/−animals the CD24+ cells were decreased with a residual population remaining. Hence, one might speculate that the CD14+ cells represent an IRF4-independent population of mouse DC2 cells.
Recently, Bosteels et al. have shown that in the mouse lung CD26+CD172+ DC2s in response to type I interferon can up-regulate IRF8 and CD64 and acquire features of both DC1s and monocytes (91). This suggests that there is further complexity of dendritic cells in inflammation, and it stresses the necessity to carefully delineate DC subsets in disease settings, where such inflammatory DC2s increase. Analysis of ESAM and CLEC12A (CD371) in these cells, and their progenitors may help to elucidate the relationship to the two DC2 subsets described for the mouse (83, 84).
Taken together, also in the mouse lung, DC2s and their subsets can be readily detected.
There is a long-standing effort to dissect monocytes/macrophages and dendritic cells (3). This task becomes easier when the focus is set on bona fide DCs, i.e. the pDCs, CD1c+ DCs, and the CD141+ DCs in human blood. Still, there is concern that there might be overlap between the monocyte and DC lineage in some areas.
Regarding the CD1c+ DCs in blood, the open question then remains whether the CD14+ subset represents bona fide DCs or whether they belong to the monocyte lineage. The expression of markers like CD115 and S100A by these cells as well as their low antigen presenting capacity would support a monocyte nature.
A comparison between man and mouse of the DC2 subsets without and with monocyte features is given in Figure 3. In both species the monocyte-related subset (green in Figure 3) is characterized by higher expression of cell surface molecules like CD115 and cytokines like TNF.
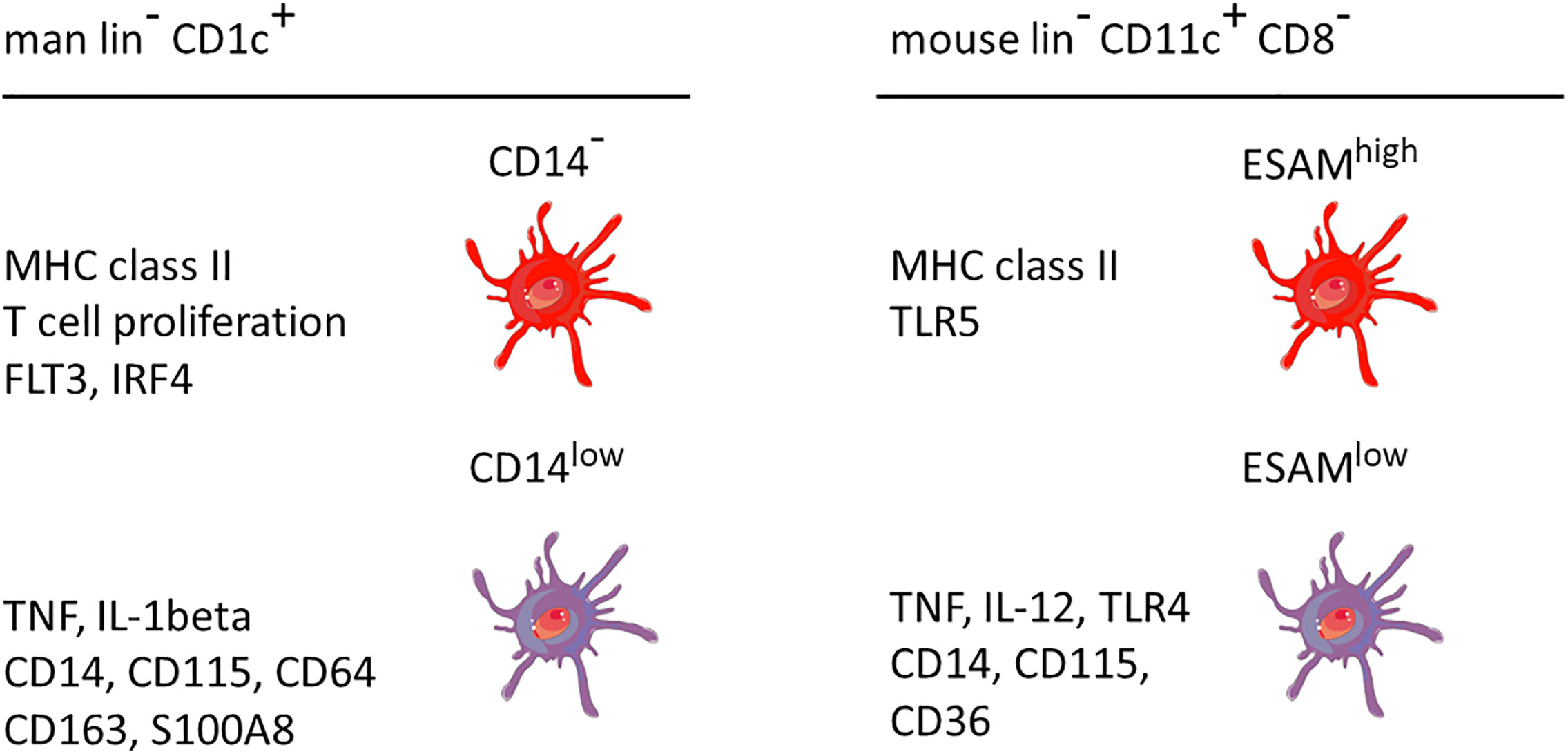
Figure 3 Properties of two main DC2 subsets in man and mouse. The markers are listed based on a higher expression in the respective subset compared to the other subset, i.e. the other subset can also be positive but at a lower level. This cartoon is restricted to the subset with monocyte features as compared to a subset covering the remaining DC2 cells. The latter has been reported to include up to three distinct populations as detailed in the text. lin− = lineage negative. The human data are a summary of a series of studies compiled in Figure 2. The mouse data refer to Lewis et al., 2011, and Kasahara et al., 2012. The cellular images are provided and adapted from Servier Medical Art (smart.servier.com).
It remains to be resolved, whether these monocyte features may be explained by i) DC-lineage cells developing monocyte features, by ii) the cells being derived from mature classical monocytes similar to monocyte-derived DCs generated in GM-CSF cultures in vitro or iii) whether these cells are derived from a bone marrow precursor in common with monocytes.
Steps to be taken to resolve this issue are
a. comparative single cell transcriptomics including CITE-seq approaches with a large set of prototypic monocytes/macrophages and dendritic cells from the same donor across different tissues,
b. analysis of informative immune-deficiencies including knock-outs to study the mouse CD8−CD11c+CD11b+ ESAMlow DCs,
c. development from hematopoietic progenitor cells with or without bar coding, and
d. analysis of informative mutations in clonal hematopoiesis.
As mentioned earlier, a recent study has addressed some of these points and has looked into IRF8 immunodeficiencies and into in vitro development of DCs from progenitors. Here, differential transcription factor requirements for the CD14+ and the CD5+ subset of DC2s were apparent, and the CD1c+CD14+ DC2s showed co-segregation of with monocytes (63). While the issue of lineage assignment of the CD1c+CD14+ cells in human blood still needs to be resolved, it has become clear that there may be more than two subsets of DC2. Therefore, future work will have to address, in man and in the mouse, all subsets of DC2s and their role in homeostasis and in disease in order to arrive at an adequate nomenclature.
LZ-H conceived the project. LZ-H, MD, and DD drafted the paper. All authors contributed to the article and approved the submitted version.
DD was partly supported by grants from the German Research Foundation [Deutsche Forschungsgemeinschaft (DFG)] (CRC1181-TPA7 and DU548/5-1), Agency national research (ANR)/German Research Foundation program (DU548/6-1) Bayresq.Net (Bavarian Ministry of Sciences), and Interdisziplinäres Zentrum für Klinische Forschung (IZKF-A80). LH was supported by Erlanger Leistungsbezogene Anschubfinanzierung und Nachwuchsförderung (ELAN) (DE-17-09-15-1-Heger). VB was supported by the Wellcome Trust grant 101155/Z13/Z.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank the members of Dudziak team for their critical comments.
1. Ziegler-Heitbrock L, Ancuta P, Crowe S, Dalod M, Grau V, Hart DN, et al. Nomenclature of monocytes and dendritic cells in blood. Blood (2010) 116(16):e74–80. doi: 10.1182/blood-2010-02-258558
2. Heidkamp GF, Lehmann CHK, Heger L, Baranska A, Hoffmann A, Lühr J, et al. Functional Specialization of Dendritic Cell Subsets. Encyclopedia Cell Biol (2016) 3:588–604. doi: 10.1016/B978-0-12-394447-4.30076-1
3. Amon L, Lehmann CHK, Baranska A, Schoen J, Heger L, Dudziak D. Transcriptional control of dendritic cell development and functions. Int Rev Cell Mol Biol (2019) 349:55–151. doi: 10.1016/bs.ircmb.2019.10.001
4. Guilliams M, Ginhoux F, Jakubzick C, Naik SH, Onai N, Schraml BU, et al. Dendritic cells, monocytes and macrophages: a unified nomenclature based on ontogeny. Nat Rev Immunol (2014) 14(8):571–8. doi: 10.1038/nri3712
5. Swiecki M, Colonna M. The multifaceted biology of plasmacytoid dendritic cells. Nat Rev Immunol (2015) 15(8):471–85. doi: 10.1038/nri3865
6. Caminschi I, Proietto A II, Ahmet F, Kitsoulis S, Shin Teh J, Lo JC, et al. The dendritic cell subtype-restricted C-type lectin Clec9A is a target for vaccine enhancement. Blood (2008) 112(8):3264–73. doi: 10.1182/blood-2008-05-155176
7. Poulin LF, Salio M, Griessinger E, Anjos-Afonso F, Craciun L, Chen JL, et al. Characterization of human DNGR-1+ BDCA3+ leukocytes as putative equivalents of mouse CD8alpha+ dendritic cells. J Exp Med (2010) 207(6):1261–71. doi: 10.1084/jem.20092618
8. Heidkamp GF, Sander J, Lehmann CHK, Heger L, Eissing N, Baranska A, et al. Human lymphoid organ dendritic cell identity is predominantly dictated by ontogeny, not tissue microenvironment. Sci Immunol (2016) 1(6). doi: 10.1126/sciimmunol.aai7677
9. Jongbloed SL, Kassianos AJ, McDonald KJ, Clark GJ, Ju X, Angel CE, et al. Human CD141+ (BDCA-3)+ dendritic cells (DCs) represent a unique myeloid DC subset that cross-presents necrotic cell antigens. J Exp Med (2010) 207(6):1247–60. doi: 10.1084/jem.20092140
10. Bachem A, Guttler S, Hartung E, Ebstein F, Schaefer M, Tannert A, et al. Superior antigen cross-presentation and XCR1 expression define human CD11c+CD141+ cells as homologues of mouse CD8+ dendritic cells. J Exp Med (2010) 207(6):1273–81. doi: 10.1084/jem.20100348
11. Crozat K, Guiton R, Contreras V, Feuillet V, Dutertre CA, Ventre E, et al. The XC chemokine receptor 1 is a conserved selective marker of mammalian cells homologous to mouse CD8alpha+ dendritic cells. J Exp Med (2010) 207(6):1283–92. doi: 10.1084/jem.20100223
12. Sancho D, Joffre OP, Keller AM, Rogers NC, Martinez D, Hernanz-Falcon P, et al. Identification of a dendritic cell receptor that couples sensing of necrosis to immunity. Nature (2009) 458(7240):899–903. doi: 10.1038/nature07750
13. Zhang JG, Czabotar PE, Policheni AN, Caminschi I, Wan SS, Kitsoulis S, et al. The dendritic cell receptor Clec9A binds damaged cells via exposed actin filaments. Immunity (2012) 36(4):646–57. doi: 10.1016/j.immuni.2012.03.009
14. Nizzoli G, Krietsch J, Weick A, Steinfelder S, Facciotti F, Gruarin P, et al. Human CD1c+ dendritic cells secrete high levels of IL-12 and potently prime cytotoxic T-cell responses. Blood (2013) 122(6):932–42. doi: 10.1182/blood-2013-04-495424
15. Bakdash G, Buschow S II, Gorris MA, Halilovic A, Hato SV, Skold AE, et al. Expansion of a BDCA1+CD14+ Myeloid Cell Population in Melanoma Patients May Attenuate the Efficacy of Dendritic Cell Vaccines. Cancer Res (2016) 76(15):4332–46. doi: 10.1158/0008-5472.CAN-15-1695
16. Bol KF, Schreibelt G, Rabold K, Wculek SK, Schwarze JK, Dzionek A, et al. The clinical application of cancer immunotherapy based on naturally circulating dendritic cells. J Immunother Cancer (2019) 7(1):109. doi: 10.1186/s40425-019-0580-6
17. Matsui T, Connolly JE, Michnevitz M, Chaussabel D, Yu C II, Glaser C, et al. CD2 distinguishes two subsets of human plasmacytoid dendritic cells with distinct phenotype and functions. J Immunol (2009) 182(11):6815–23. doi: 10.4049/jimmunol.0802008
18. Balan S, Arnold-Schrauf C, Abbas A, Couespel N, Savoret J, Imperatore F, et al. Large-Scale Human Dendritic Cell Differentiation Revealing Notch-Dependent Lineage Bifurcation and Heterogeneity. Cell Rep (2018) 24(7):1902–15 e6. doi: 10.1016/j.celrep.2018.07.033
19. Moody DB, Ulrichs T, Muhlecker W, Young DC, Gurcha SS, Grant E, et al. CD1c-mediated T-cell recognition of isoprenoid glycolipids in Mycobacterium tuberculosis infection. Nature (2000) 404(6780):884–8. doi: 10.1038/35009119
20. Collin M, Bigley V. Monocyte, Macrophage, and Dendritic Cell Development: the Human Perspective. Microbiol Spectr (2016) 4(5):79–97. doi: 10.1128/microbiolspec.MCHD-0015-2015
21. Small TN, Knowles RW, Keever C, Kernan NA, Collins N, O’Reilly RJ, et al. M241 (CD1) expression on B lymphocytes. J Immunol (1987) 138(9):2864–8.
22. Kasinrerk W, Baumruker T, Majdic O, Knapp W, Stockinger H. CD1 molecule expression on human monocytes induced by granulocyte-macrophage colony-stimulating factor. J Immunol (1993) 150(2):579–84.
23. Breton G, Lee J, Zhou YJ, Schreiber JJ, Keler T, Puhr S, et al. Circulating precursors of human CD1c+ and CD141+ dendritic cells. J Exp Med (2015) 212(3):401–13. doi: 10.1084/jem.20141441
24. Haniffa M, Shin A, Bigley V, McGovern N, Teo P, See P, et al. Human tissues contain CD141hi cross-presenting dendritic cells with functional homology to mouse CD103+ nonlymphoid dendritic cells. Immunity (2012) 37(1):60–73. doi: 10.1016/j.immuni.2012.04.012
25. Cant CA, Ullrich A. Signal regulation by family conspiracy. Cell Mol Life Sci (2001) 58(1):117–24. doi: 10.1007/PL00000771
26. Granot T, Senda T, Carpenter DJ, Matsuoka N, Weiner J, Gordon CL, et al. Dendritic Cells Display Subset and Tissue-Specific Maturation Dynamics over Human Life. Immunity (2017) 46(3):504–15. doi: 10.1016/j.immuni.2017.02.019
27. Seiffert M, Cant C, Chen Z, Rappold I, Brugger W, Kanz L, et al. Human signal-regulatory protein is expressed on normal, but not on subsets of leukemic myeloid cells and mediates cellular adhesion involving its counterreceptor CD47. Blood (1999) 94(11):3633–43. doi: 10.1182/blood.V94.11.3633.423k01_3633_3643
28. Ezquerra A, Revilla C, Alvarez B, Perez C, Alonso F, Dominguez J. Porcine myelomonocytic markers and cell populations. Dev Comp Immunol (2009) 33(3):284–98. doi: 10.1016/j.dci.2008.06.002
29. Lahoud MH, Proietto A II, Gartlan KH, Kitsoulis S, Curtis J, Wettenhall J, et al. Signal regulatory protein molecules are differentially expressed by CD8- dendritic cells. J Immunol (2006) 177(1):372–82. doi: 10.4049/jimmunol.177.1.372
30. Baba T, Nakamoto Y, Mukaida N. Crucial contribution of thymic Sirp alpha+ conventional dendritic cells to central tolerance against blood-borne antigens in a CCR2-dependent manner. J Immunol (2009) 183(5):3053–63. doi: 10.4049/jimmunol.0900438
31. Ardouin L, Luche H, Chelbi R, Carpentier S, Shawket A, Montanana Sanchis F, et al. Broad and Largely Concordant Molecular Changes Characterize Tolerogenic and Immunogenic Dendritic Cell Maturation in Thymus and Periphery. Immunity (2016) 45(2):305–18. doi: 10.1016/j.immuni.2016.07.019
32. Khandelwal P, Blanco-Mezquita T, Emami P, Lee HS, Reyes NJ, Mathew R, et al. Ocular mucosal CD11b+ and CD103+ mouse dendritic cells under normal conditions and in allergic immune responses. PloS One (2013) 8(5):e64193. doi: 10.1371/journal.pone.0064193
33. Gurka S, Hartung E, Becker M, Kroczek RA. Mouse Conventional Dendritic Cells Can be Universally Classified Based on the Mutually Exclusive Expression of XCR1 and SIRPalpha. Front Immunol (2015) 6:35. doi: 10.3389/fimmu.2015.00035
34. Heger L, Balk S, Luhr JJ, Heidkamp GF, Lehmann CHK, Hatscher L, et al. CLEC10A Is a Specific Marker for Human CD1c(+) Dendritic Cells and Enhances Their Toll-Like Receptor 7/8-Induced Cytokine Secretion. Front Immunol (2018) 9:744. doi: 10.3389/fimmu.2018.00744
35. Sato M, Kawakami K, Osawa T, Toyoshima S. Molecular cloning and expression of cDNA encoding a galactose/N-acetylgalactosamine-specific lectin on mouse tumoricidal macrophages. J Biochem (1992) 111(3):331–6. doi: 10.1093/oxfordjournals.jbchem.a123758
36. Suzuki N, Yamamoto K, Toyoshima S, Osawa T, Irimura T. Molecular cloning and expression of cDNA encoding human macrophage C-type lectin. Its unique carbohydrate binding specificity for Tn antigen. J Immunol (1996) 156(1):128–35.
37. Higashi N, Fujioka K, Denda-Nagai K, Hashimoto S, Nagai S, Sato T, et al. The macrophage C-type lectin specific for galactose/N-acetylgalactosamine is an endocytic receptor expressed on monocyte-derived immature dendritic cells. J Biol Chem (2002) 277(23):20686–93. doi: 10.1074/jbc.M202104200
38. Wong KL, Tai JJ, Wong WC, Han H, Sem X, Yeap WH, et al. Gene expression profiling reveals the defining features of the classical, intermediate, and nonclassical human monocyte subsets. Blood (2011) 118(5):e16–31. doi: 10.1182/blood-2010-12-326355
39. van Vliet SJ, Saeland E, van Kooyk Y. Sweet preferences of MGL: carbohydrate specificity and function. Trends Immunol (2008) 29(2):83–90. doi: 10.1016/j.it.2007.10.010
40. Denda-Nagai K, Aida S, Saba K, Suzuki K, Moriyama S, Oo-Puthinan S, et al. Distribution and function of macrophage galactose-type C-type lectin 2 (MGL2/CD301b): efficient uptake and presentation of glycosylated antigens by dendritic cells. J Biol Chem (2010) 285(25):19193–204. doi: 10.1074/jbc.M110.113613
41. Shin H, Kumamoto Y, Gopinath S, Iwasaki A. CD301b+ dendritic cells stimulate tissue-resident memory CD8+ T cells to protect against genital HSV-2. Nat Commun (2016) 7:13346. doi: 10.1038/ncomms13346
42. Linehan JL, Dileepan T, Kashem SW, Kaplan DH, Cleary P, Jenkins MK. Generation of Th17 cells in response to intranasal infection requires TGF-beta1 from dendritic cells and IL-6 from CD301b+ dendritic cells. Proc Natl Acad Sci U.S.A. (2015) 112(41):12782–7. doi: 10.1073/pnas.1513532112
43. Shook B, Xiao E, Kumamoto Y, Iwasaki A, Horsley V. CD301b+ Macrophages Are Essential for Effective Skin Wound Healing. J Invest Dermatol (2016) 136(9):1885–91. doi: 10.1016/j.jid.2016.05.107
44. Kumamoto Y, Camporez JPG, Jurczak MJ, Shanabrough M, Horvath T, Shulman G II, et al. CD301b(+) Mononuclear Phagocytes Maintain Positive Energy Balance through Secretion of Resistin-like Molecule Alpha. Immunity (2016) 45(3):583–96. doi: 10.1016/j.immuni.2016.08.002
45. Dupasquier M, Stoitzner P, Wan H, Cerqueira D, van Oudenaren A, Voerman JS, et al. The dermal microenvironment induces the expression of the alternative activation marker CD301/mMGL in mononuclear phagocytes, independent of IL-4/IL-13 signaling. J Leukoc Biol (2006) 80(4):838–49. doi: 10.1189/jlb.1005564
46. Xu WD, Firestein GS, Taetle R, Kaushansky K, Zvaifler NJ. Cytokines in chronic inflammatory arthritis. II. Granulocyte-macrophage colony-stimulating factor in rheumatoid synovial effusions. J Clin Invest (1989) 83(3):876–82. doi: 10.1172/JCI113971
47. Croizat H. Circulating cytokines in sickle cell patients during steady state. Br J Haematol (1994) 87(3):592–7. doi: 10.1111/j.1365-2141.1994.tb08318.x
48. Singhal R, Chawla S, Rathore DK, Bhasym A, Annarapu GK, Sharma V, et al. Development of pro-inflammatory phenotype in monocytes after engulfing Hb-activated platelets in hemolytic disorders. Clin Immunol (2017) 175:133–42. doi: 10.1016/j.clim.2016.12.007
49. Sloma I, Zilber MT, Charron D, Girot R, Tamouza R, Gelin C. Upregulation and atypical expression of the CD1 molecules on monocytes in sickle cell disease. Hum Immunol (2004) 65(11):1370–6. doi: 10.1016/j.humimm.2004.09.009
50. Thomas R, Lipsky PE. Human peripheral blood dendritic cell subsets. Isolation and characterization of precursor and mature antigen-presenting cells. J Immunol (1994) 153(9):4016–28.
51. MacDonald KP, Munster DJ, Clark GJ, Dzionek A, Schmitz J, Hart DN. Characterization of human blood dendritic cell subsets. Blood (2002) 100(13):4512–20. doi: 10.1182/blood-2001-11-0097
52. van Ee TJ, Van Acker HH, van Oorschot TG, Van Tendeloo VF, Smits EL, Bakdash G, et al. BDCA1+CD14+ Immunosuppressive Cells in Cancer, a Potential Target? Vaccines (Basel) (2018) 6(3). doi: 10.3390/vaccines6030065
53. Villani AC, Satija R, Reynolds G, Sarkizova S, Shekhar K, Fletcher J, et al. Single-cell RNA-seq reveals new types of human blood dendritic cells, monocytes, and progenitors. Science (2017) 356(6335). doi: 10.1126/science.aah4573
54. Schroder M, Melum GR, Landsverk OJ, Bujko A, Yaqub S, Gran E, et al. CD1c-Expression by Monocytes - Implications for the Use of Commercial CD1c+ Dendritic Cell Isolation Kits. PloS One (2016) 11(6):e0157387. doi: 10.1371/journal.pone.0157387
55. Wood GS, Freudenthal PS. CD5 monoclonal antibodies react with human peripheral blood dendritic cells. Am J Pathol (1992) 141(4):789–95.
56. Strobl H, Scheinecker C, Riedl E, Csmarits B, Bello-Fernandez C, Pickl WF, et al. Identification of CD68+lin- peripheral blood cells with dendritic precursor characteristics. J Immunol (1998) 161(2):740–8.
57. Yin X, Yu H, Jin X, Li J, Guo H, Shi Q, et al. Human Blood CD1c+ Dendritic Cells Encompass CD5high and CD5low Subsets That Differ Significantly in Phenotype, Gene Expression, and Functions. J Immunol (2017) 198(4):1553–64. doi: 10.4049/jimmunol.1600193
58. Meyerson HJ, Osei E, Schweitzer K, Blidaru G, Edinger A, Schlegelmilch J, et al. CD1c(+) myeloid dendritic cells in myeloid neoplasia. Cytometry B Clin Cytom (2016) 90(4):337–48. doi: 10.1002/cyto.b.21332
59. Dutertre CA, Becht E, Irac SE, Khalilnezhad A, Narang V, Khalilnezhad S, et al. Single-Cell Analysis of Human Mononuclear Phagocytes Reveals Subset-Defining Markers and Identifies Circulating Inflammatory Dendritic Cells. Immunity (2019) 51(3):573–89 e8. doi: 10.1016/j.immuni.2019.08.008
60. Alcantara-Hernandez M, Leylek R, Wagar LE, Engleman EG, Keler T, Marinkovich MP, et al. High-Dimensional Phenotypic Mapping of Human Dendritic Cells Reveals Interindividual Variation and Tissue Specialization. Immunity (2017) 47(6):1037–50.e6. doi: 10.1016/j.immuni.2017.11.001
61. Bourdely P, Anselmi G, Vaivode K, Ramos RN, Missolo-Koussou Y, Hidalgo S, et al. Transcriptional and Functional Analysis of CD1c(+) Human Dendritic Cells Identifies a CD163(+) Subset Priming CD8(+)CD103(+) T Cells. Immunity (2020) 53(2):335–52. doi: 10.1016/j.immuni.2020.06.002
62. Erlich Z, Shlomovitz I, Edry-Botzer L, Cohen H, Frank D, Wang H, et al. Macrophages, rather than DCs, are responsible for inflammasome activity in the GM-CSF BMDC model. Nat Immunol (2019) 20(4):397–406. doi: 10.1038/s41590-019-0313-5
63. Cytlak U, Resteu A, Pagan S, Green K, Milne P, Maisuria S, et al. Differential IRF8 Transcription Factor Requirement Defines Two Pathways of Dendritic Cell Development in Humans. Immunity (2020) 53(2):353–70.e8. doi: 10.1016/j.immuni.2020.07.003
64. Proietto A II, Mittag D, Roberts AW, Sprigg N, Wu L. The equivalents of human blood and spleen dendritic cell subtypes can be generated in vitro from human CD34(+) stem cells in the presence of fms-like tyrosine kinase 3 ligand and thrombopoietin. Cell Mol Immunol (2012) 9(6):446–54. doi: 10.1038/cmi.2012.48
65. Helft J, Anjos-Afonso F, van der Veen AG, Chakravarty P, Bonnet D, Reis e Sousa C. Dendritic Cell Lineage Potential in Human Early Hematopoietic Progenitors. Cell Rep (2017) 20(3):529–37. doi: 10.1016/j.celrep.2017.06.075
66. Hambleton S, Salem S, Bustamante J, Bigley V, Boisson-Dupuis S, Azevedo J, et al. IRF8 mutations and human dendritic-cell immunodeficiency. N Engl J Med (2011) 365(2):127–38. doi: 10.1056/NEJMoa1100066
67. Bigley V, Maisuria S, Cytlak U, Jardine L, Care MA, Green K, et al. Biallelic interferon regulatory factor 8 mutation: A complex immunodeficiency syndrome with dendritic cell deficiency, monocytopenia, and immune dysregulation. J Allergy Clin Immunol (2018) 141(6):2234–48. doi: 10.1016/j.jaci.2017.08.044
68. Kong XF, Martinez-Barricarte R, Kennedy J, Mele F, Lazarov T, Deenick EK, et al. Disruption of an antimycobacterial circuit between dendritic and helper T cells in human SPPL2a deficiency. Nat Immunol (2018) 19(9):973–85. doi: 10.1038/s41590-018-0178-z
69. Sontag S, Forster M, Qin J, Wanek P, Mitzka S, Schuler HM, et al. Modelling IRF8 Deficient Human Hematopoiesis and Dendritic Cell Development with Engineered iPS Cells. Stem Cells (2017) 35(4):898–908. doi: 10.1002/stem.2565
70. Borriello F, Iannone R, Di Somma S, Vastolo V, Petrosino G, Visconte F, et al. Lipopolysaccharide-Elicited TSLPR Expression Enriches a Functionally Discrete Subset of Human CD14(+) CD1c(+) Monocytes. J Immunol (2017) 198(9):3426–35. doi: 10.4049/jimmunol.1601497
71. Corren J, Ziegler SF. TSLP: from allergy to cancer. Nat Immunol (2019) 20(12):1603–9. doi: 10.1038/s41590-019-0524-9
72. Watchmaker PB, Lahl K, Lee M, Baumjohann D, Morton J, Kim SJ, et al. Comparative transcriptional and functional profiling defines conserved programs of intestinal DC differentiation in humans and mice. Nat Immunol (2014) 15(1):98–108. doi: 10.1038/ni.2768
73. Lindstedt M, Lundberg K, Borrebaeck CA. Gene family clustering identifies functionally associated subsets of human in vivo blood and tonsillar dendritic cells. J Immunol (2005) 175(8):4839–46. doi: 10.4049/jimmunol.175.8.4839
74. Segura E, Valladeau-Guilemond J, Donnadieu MH, Sastre-Garau X, Soumelis V, Amigorena S. Characterization of resident and migratory dendritic cells in human lymph nodes. J Exp Med (2012) 209(4):653–60. doi: 10.1084/jem.20111457
75. Segura E, Durand M, Amigorena S. Similar antigen cross-presentation capacity and phagocytic functions in all freshly isolated human lymphoid organ-resident dendritic cells. J Exp Med (2013) 210(5):1035–47. doi: 10.1084/jem.20121103
76. Melum GR, Farkas L, Scheel C, Van Dieren B, Gran E, Liu YJ, et al. A thymic stromal lymphopoietin-responsive dendritic cell subset mediates allergic responses in the upper airway mucosa. J Allergy Clin Immunol (2014) 134(3):613–621 e7. doi: 10.1016/j.jaci.2014.05.010
77. Patel V II, Booth JL, Duggan ES, Cate S, White VL, Hutchings D, et al. Transcriptional Classification and Functional Characterization of Human Airway Macrophage and Dendritic Cell Subsets. J Immunol (2017) 198(3):1183–201. doi: 10.4049/jimmunol.1600777
78. Jardine L, Wiscombe S, Reynolds G, McDonald D, Fuller A, Green K, et al. Lipopolysaccharide inhalation recruits monocytes and dendritic cell subsets to the alveolar airspace. Nat Commun (2019) 10(1):1999. doi: 10.1038/s41467-019-09913-4
79. Vremec D, Shortman K. What’s in a Name? Some Early and Current Issues in Dendritic Cell Nomenclature. Front Immunol (2015) 6:267. doi: 10.3389/fimmu.2015.00267
80. Durai V, Murphy KM. Functions of Murine Dendritic Cells. Immunity (2016) 45(4):719–36. doi: 10.1016/j.immuni.2016.10.010
81. Ginhoux F, Guilliams M, Naik SH. Editorial: Dendritic Cell and Macrophage Nomenclature and Classification. Front Immunol (2016) 7:168. doi: 10.3389/fimmu.2016.00168
82. Schraml BU, Reis e Sousa C. Defining dendritic cells. Curr Opin Immunol (2015) 32:13–20. doi: 10.1016/j.coi.2014.11.001
83. Kasahara S, Clark EA. Dendritic cell-associated lectin 2 (DCAL2) defines a distinct CD8alpha- dendritic cell subset. J Leukoc Biol (2012) 91(3):437–48. doi: 10.1189/jlb.0711384
84. Lewis KL, Caton ML, Bogunovic M, Greter M, Grajkowska LT, Ng D, et al. Notch2 receptor signaling controls functional differentiation of dendritic cells in the spleen and intestine. Immunity (2011) 35(5):780–91. doi: 10.1016/j.immuni.2011.08.013
85. Kirkling ME, Cytlak U, Lau CM, Lewis KL, Resteu A, Khodadadi-Jamayran A, et al. Notch Signaling Facilitates In Vitro Generation of Cross-Presenting Classical Dendritic Cells. Cell Rep (2018) 23(12):3658–3672 e6. doi: 10.1016/j.celrep.2018.05.068
86. McDaniell R, Warthen DM, Sanchez-Lara PA, Pai A, Krantz ID, Piccoli DA, et al. NOTCH2 mutations cause Alagille syndrome, a heterogeneous disorder of the notch signaling pathway. Am J Hum Genet (2006) 79(1):169–73. doi: 10.1086/505332
87. Schiavoni G, Mattei F, Sestili P, Borghi P, Venditti M, Morse HC, et al. ICSBP is essential for the development of mouse type I interferon-producing cells and for the generation and activation of CD8alpha(+) dendritic cells. J Exp Med (2002) 196(11):1415–25. doi: 10.1084/jem.20021263
88. Aliberti J, Schulz O, Pennington DJ, Tsujimura H, Reis e Sousa C, Ozato K, et al. Essential role for ICSBP in the in vivo development of murine CD8alpha + dendritic cells. Blood (2003) 101(1):305–10. doi: 10.1182/blood-2002-04-1088
89. Brown CC, Gudjonson H, Pritykin Y, Deep D, Lavallee VP, Mendoza A, et al. Transcriptional Basis of Mouse and Human Dendritic Cell Heterogeneity. Cell (2019) 179(4):846–63.e24. doi: 10.1016/j.cell.2019.09.035
90. Schlitzer A, McGovern N, Teo P, Zelante T, Atarashi K, Low D, et al. IRF4 transcription factor-dependent CD11b+ dendritic cells in human and mouse control mucosal IL-17 cytokine responses. Immunity (2013) 38(5):970–83. doi: 10.1016/j.immuni.2013.04.011
Keywords: DC2, CD1c, CD172, CD301, CD14, dendritic cells, DC subsets
Citation: Heger L, Hofer TP, Bigley V, de Vries IJM, Dalod M, Dudziak D and Ziegler-Heitbrock L (2020) Subsets of CD1c+ DCs: Dendritic Cell Versus Monocyte Lineage. Front. Immunol. 11:559166. doi: 10.3389/fimmu.2020.559166
Received: 05 May 2020; Accepted: 14 September 2020;
Published: 30 September 2020.
Edited by:
Francesca Granucci, University of Milano-Bicocca, ItalyReviewed by:
Francesco Borriello, Boston Children’s Hospital and Harvard Medical School, United StatesCopyright © 2020 Heger, Hofer, Bigley, de Vries, Dalod, Dudziak and Ziegler-Heitbrock. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Diana Dudziak, ZGlhbmEuZHVkemlha0B1ay1lcmxhbmdlbi5kZQ==; Loems Ziegler-Heitbrock, TFpIQG1vbm9jeXRlLmV1
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.