
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Immunol. , 21 October 2020
Sec. Nutritional Immunology
Volume 11 - 2020 | https://doi.org/10.3389/fimmu.2020.02141
This article is part of the Research Topic Impact of Early Life Nutrition on Immune System Development and Related Health Outcomes in Later Life View all 18 articles
 Betty C. A. M. van Esch1,2
Betty C. A. M. van Esch1,2 Mojtaba Porbahaie3
Mojtaba Porbahaie3 Suzanne Abbring1
Suzanne Abbring1 Johan Garssen1,2
Johan Garssen1,2 Daniel P. Potaczek4,5
Daniel P. Potaczek4,5 Huub F. J. Savelkoul3
Huub F. J. Savelkoul3 R. J. Joost van Neerven3,6*
R. J. Joost van Neerven3,6*Specific and adequate nutrition during pregnancy and early life is an important factor in avoiding non-communicable diseases such as obesity, type 2 diabetes, cardiovascular disease, cancers, and chronic allergic diseases. Although epidemiologic and experimental studies have shown that nutrition is important at all stages of life, it is especially important in prenatal and the first few years of life. During the last decade, there has been a growing interest in the potential role of epigenetic mechanisms in the increasing health problems associated with allergic disease. Epigenetics involves several mechanisms including DNA methylation, histone modifications, and microRNAs which can modify the expression of genes. In this study, we focus on the effects of maternal nutrition during pregnancy, the effects of the bioactive components in human and bovine milk, and the environmental factors that can affect early life (i.e., farming, milk processing, and bacterial exposure), and which contribute to the epigenetic mechanisms underlying the persistent programming of immune functions and allergic diseases. This knowledge will help to improve approaches to nutrition in early life and help prevent allergies in the future.
There is increasing evidence to suggest that maternal diet during pregnancy, breastfeeding, early life nutrition, and early life malnutrition can have sustained effects on immunological outcomes, such as respiratory allergies, and metabolic outcomes such as type 2 diabetes and obesity. Nutritional programming during gestation might permanently affect the immunological competence and nutritional status in early life Figure 1. This is exemplified by the thrifty phenotype, where the metabolic response to undernutrition during the fetal period is inappropriate during overnutrition later in life, leading to disease manifestations (1). Several studies have since shown that prenatal exposure to famine is associated with the development of type 2 diabetes later in life (2–4), and an epigenetic link was demonstrated in relation to the Dutch hunger winter where epigenetic modification of the IGF2 gene was shown to be linked to famine during prenatal development (5).
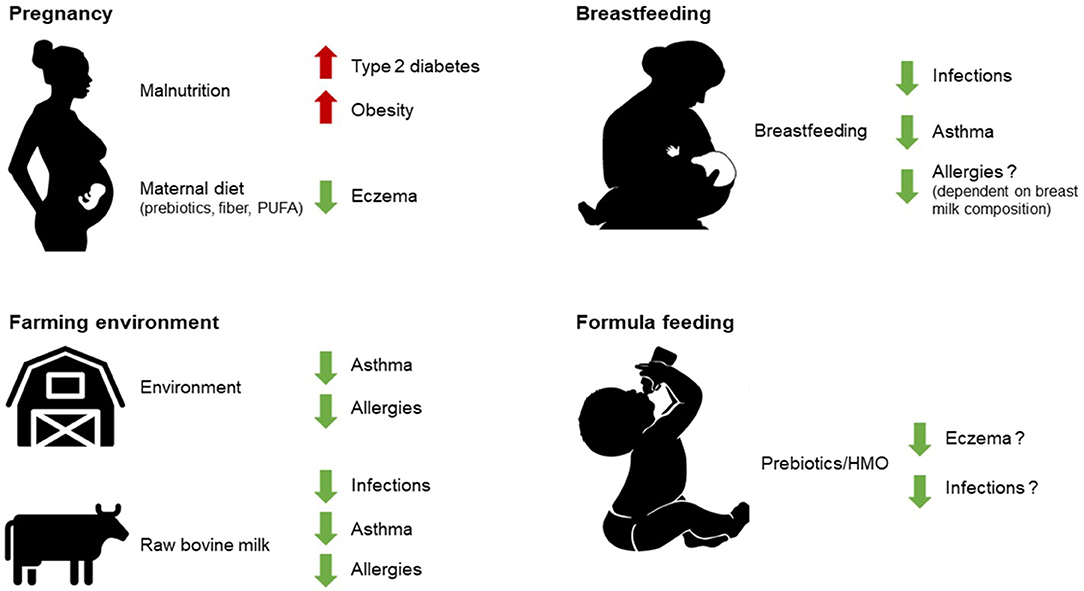
Figure 1. As described in this review, early life nutrition (breastfeeding, raw milk consumption, and some infant formula components), early life environmental exposures (such as farming environment), as well as prenatal development under the influence of maternal diet can all have sustained effects on health outcomes later in life. PUFA, polyunsaturated fatty acids; HMO, human milk oligosaccharides.
Epigenetic mechanisms may play an important role in these effects. It has even been suggested that early life nutrition forms the basis for susceptibility to a plethora of chronic age-related non-communicable diseases (NCD), like respiratory allergies (6–9). Thus, specific and adequate nutrition during pregnancy and early life are considered important factors that could reduce instances of allergic diseases. Epidemiologic and experimental studies show that nutrition is important for (immunological) health, especially when we are very young and during prenatal development, which may influence health and disease throughout our lives (6, 10). The structures of the mucosal immune system in the gastrointestinal (GI) tract are fully developed in utero by gestational week 28 (11). Increasing evidence suggests that maternal diet and other prenatal exposures can influence this development by crossing the placenta (12–14). In the first year of life, the mucosal immune system is further shaped by microbial colonization and oral feeding (15). Breastfeeding is the normal way of providing newborns with nutrients for healthy growth and development and a diet exclusively comprised of breastfeeding has various beneficial outcomes, such as reducing the risk of GI diseases, allergies, colitis, and respiratory infections (16). Besides conferring protection against these short-term outcomes, breastfeeding also reduces the long-term risks of developing diseases like type 2 diabetes and obesity (17). In analogy to breast milk, raw, unprocessed, bovine milk is a rich source of immunomodulatory components (18–20). Studies have indicated that it may protect against common respiratory infections in infants that consume unprocessed bovine milk (21). In addition, epidemiological evidence shows a clear association between the consumption of raw cow's milk and the prevention of allergy development (22–29). Epigenetic mechanisms that are regulated by many immune processes can thereby influence the course of allergic diseases.
Epigenetic mechanisms (Box 1) and transcription regulatory factors allow a flexible adaptation in the fetus. They neonate to a fluctuating external environment whereby heritable, non-DNA encoded, alterations in gene expression patterns occur. Especially relevant in early life, several factors drive the epigenetic changes that occur throughout life: environment (e.g., exposure to microbial components in inhaled dust), diet (e.g., components present in breast milk and bovine milk), and the GI microbiota and its metabolites (e.g., through the production of short-chain fatty acids [SCFA] after fermentation of dietary non-digestible oligosaccharides). Thus, environmental, dietary, and microbiota-derived epigenetic modifications during gestation and early life can shape future immunity to the development of diseases like obesity, type 2 diabetes, allergy, asthma, and infections. Most of our current knowledge on the environmental and dietary effects on epigenetics and early life immune function comes from epidemiological findings which indicate that children growing up on farms have a decreased risk of developing allergies, especially asthma. For this reason, we will focus this review on the effects of maternal nutrition during pregnancy, the effects of bioactive components in human and bovine milk, and the environmental factors in early life that can contribute to the epigenetic mechanisms involved in the course of allergic diseases.
Box 1. Epigenetic mechanisms.
Epigenetics refers to systems that control gene expression in a heritable fashion without changing the genomic sequences. The epigenome is much more flexible than the genome and shows different phenotype variations that are influenced by environmental factors and dietary habits. Epigenetic mechanisms include DNA methylation, histone modifications, and RNA interference by microRNAs (miRNAs) (See in this Box figure). Epigenetic mechanisms thus contribute to the regulation of gene expression at the level of transcription by DNA methylation and by modifying chromatin accessibility through posttranslational modifications of histones, and after transcription by mRNA silencing. These epigenetic mechanisms can regulate gene expression by modifying the accessibility of the DNA to transcription enzymes without altering the DNA nucleotide sequence, influencing stability of mRNA or translation efficiency, and others (30–32). The transfer of a methyl group onto DNA, performed by DNA methyltransferases (DNMTs), can directly regulate the rate of gene transcription. DNA demethylation is catalyzed by several enzymes serving as controllers for the equilibrium of DNA methylation (33). For example, methylation of DNA in the promoter regions of cytokines can influence immune responsiveness by steering Th cell differentiation into Th1, Th2, Th17, or Treg (34, 35). For more details see Box 2. In addition, histone modifications like acetylation, methylation, phosphorylation and others can also modulate the development and activity of immune cells. Histone acetylation is an important remodeling activity that is catalyzed by a series of enzymes called histone acetyltransferases (HATs). Acetylation is generally considered as a permissive activity that facilitates gene transcription. On the contrary, histone deacetylases (HDACs) reverse HAT activity and tighten up the folding of DNA around the histones and make them less accessible for transcription factors (31, 36). The interplay between HATs and HDACs determines the histone acetylation balance and regulates the gene expression (37, 38) and production of pro-inflammatory (IL-1β, IL-5, IL-6, IL-8, IL-12, and TNFα) and anti-inflammatory mediators (IL-10). Histone methyltransferases (HMTs) and demethylases (HDMs) serve as controller enzymes for the equilibrium of histone methylation (31). Finally, RNA interference can occur by small noncoding RNAs, most notably miRNAs that are found in biological fluids as well as in extracellular vesicles (e.g., in milk). MiRNAs represent short noncoding RNA molecules of 18 to 23 nucleotides that control gene expression by inducing mRNA degradation and/or inhibit post-transcriptional translation. As a result, specific miRNA can silence selective gene expression (32). For example, milk contains extracellular vesicles or exosomes that contain a wide range of microRNAs, including miR-21, miR-29b, miR-148a, and miR-155 that is known to influence Foxp3 expression and Treg development (39).
Epigenetic changes have been strongly associated with allergies and asthma and might thereby serve as biomarkers. The role of epigenetic mechanisms, particularly DNA methylation, in allergic diseases is at the interface of gene regulation, environmental stimuli, and developmental processes, thereby determining the pathogenesis of asthma and allergy. Alterations of the DNA methylation status in the genes specific for a different subset of T helper (Th) cells that are considered to be a good example of how epigenetic modulation can influence the development of asthma and other allergic diseases.
The differentiation of naïve CD4+ T cells into Th subpopulations is strictly regulated, with changes in epigenetic marks at main lineage-determining loci encoding transcription factors like GATA3, RORγt, TBX21, and Foxp3 playing a pivotal role. These changes affect the differentiation into mature Th subpopulations, such as Th1, Th2 (and Th9), regulatory T cells (Treg cells), and Th17 (30, 35, 47, 48). In naïve CD4+ T cells, which express a moderate level of GATA3 mRNA after receiving signals via the T cell receptors (TCRs) in the presence of IL-4, activated STAT6 proteins bind to the GATA3 gene locus, driving Th2 differentiation, which is a characteristic in the development of allergy. Differentiation of human CD4+ cells into the Th2 subtype is accompanied by the induction of DNase I hypersensitive (DHS) sites and CpG demethylation around these (DHS) regions within the IL-4 and IL-13 promoters. Extensive studies of the Th2 cytokine locus control region have shown that specific sites undergo rapid demethylation during Th2 differentiation (49).
In addition to DNA methylation, histone modifications are also important in guiding T-cell differentiation. T-bet and GATA3 transcription factors control lineage-specific histone acetylation of IFN-γ and IL-4 loci during Th1/Th2 differentiation. Rapid methylation of H3K9 and H3K27 residues (repressive marks) at the IFN-γ locus was associated with differentiating toward Th1 cells, while demethylation of H3K9 and methylation of H3K27 was associated with Th2 differentiation (49). Epithelial alarmins (IL-25, IL-33, thymic stromal lymphopoietin [TSLP]) induce an inflammatory response in the respiratory mucosal membrane. IL-33 binds to its receptor ST2 on memory Th2 cells and induces epigenetic changes of the IL-5 gene, resulting in the generation of IL-5-producing Th2 cells (47). Thus, Th2 differentiation, which is characteristic of allergy, is triggered by phosphorylation of STAT6 signal transducers and expression of GATA3 and Th2 cytokines, including IL-4 (47).
Demethylation of the IL-4 promoter leads to allergic sensitization (48). Th1 differentiation is in turn triggered by phosphorylation of STAT4 signaling, and expression of the transcription factor T-bet and cytokine. For a more detailed description of epigenetics and T cell development, see Box 2. Asthmatic individuals show a lower histone deacetylase (HDAC): histone acetylase (HAT) ratio, i.e., a relative decrease of HDAC enzymes, which is corrected by proper anti-asthma treatment (50). The DNA methylation status of Foxp3 is regulated within a highly conserved region within the CpG-rich Treg-specific demethylated region with a differential Foxp3 demethylation status in children with an active cows milk allergy (CMA) and acquisition of immune tolerance (51).
Box 2. Epigenetics and T-cell subset development.
The differentiation of naïve CD4+ T cells upon antigen exposure into effector T helper (Th) subsets (Th1, Th2, and Th17) or induced regulatory T (iTreg) cells relies on epigenetic regulation and the establishment of cell-fate programs (40, 41). DNA methylation and chromatin modifications at pivotal loci in Th cells such as IFN-γ, IL-4 and, Foxp3 contribute to the formation of stable, heritable gene expression patterns. Methylation of CpG dinucleotides specially at promoter or other regulatory regions of genes is generally considered a repressive feature causing silenced genes what mostly seen in (embryonic) stem cells. Targeted loci DNA demethylation is required during early or late hematopoietic cell differentiation (41, 42). For instance, DNA demethylation plays a role in the expression of Th2 cell-related cytokine, IL-4 (43) and, Treg cell-related regulators (44, 45). Besides DNA methylation, histone modifications including acetylation and methylation have a role in the development of Th cell lineage. Histone acetylation, associated with the control of gene expression by condensing or relaxing the chromatin structure to repress or activate transcription, respectively, regulates the expression of several inflammatory mediators of the immune system. In this regard, modifications of histones occur in the enhancer and promoter regions of the STAT4 and STAT1 transcription factor binding sites upstream of the IFN-γ and TBX21 (T-bet) gene to direct Th1 differentiation. In contrast, activation of STAT6 in response to IL-4 occurs leading to the expression of IL-4 and GATA3 transcription factor genes in Th2 differentiating cells. Driving naïve CD4+ T cells toward Th17 phenotype requires STAT3 activation followed by expression of RORC gene encoding RORγt transcription factor and subsequently the production of IL-17 cytokines. Alternatively, upon naïve CD4+ T cells exposure to TGF-β, STAT5 transcription factor engages leading to changes in Foxp3 gene promoter site and commitment of cells into Treg fate. These specific histone modifications lead to engagement of lineage-specific key transcription factors which ensures Th phenotype stabilization and prevents the cells from skewing toward alternative commitments (35, 42, 46).
The WHO recommends exclusive breastfeeding for infants during the first 6 months of life, and that it should be given alongside complementary feeding up until children are 2 years old (52). If mothers are unable to breastfeed, many children receive early life nutrition alternatives that are based on bovine milk. Therefore, this section of the study is focused on breast milk, bovine milk, and their components.
There is increasing evidence to suggest that the maternal diet during pregnancy and breastfeeding can have sustained effects on immunological outcomes in the infant and even have ramifications for their health later in life. The maternal diet can modify some immune supporting micronutrients in breast milk, such as the fat-soluble vitamins A and D, as well as the water-soluble B vitamins, and polyunsaturated fatty acids (PUFA), but maternal diet does not influence other components such as iron and zinc (53). Although there is some conflicting data, supplementation of maternal diet with vitamins and micronutrients during pregnancy and breastfeeding does not seem to prevent infections and allergies in offspring (54, 55).
Long-chain PUFA (LCPUFA) induce inflammation by modulating inflammatory mediators like prostaglandins and immunomodulatory factors like IL-10 and TSLP (56). Consumption of omega-3 PUFA correlates with the inhibition of TLR4 signaling and thereby the production of inflammatory cytokines (IL-1, IL-6, and TNFα), which is reflected by a lower risk of allergies, whereas consumption of saturated fats and omega-6 PUFA, a potential trigger for TLR4-induced inflammation, has been associated with a higher risk of allergies. In addition, PUFA supplementation during pregnancy was associated with a reduction in allergic outcomes after birth (57, 58), but not when it was supplemented to infants (8, 59–61), suggesting that pregnancy is an important time that influences the development of the immune system.
Probiotics are living microorganisms which, when administered in adequate amounts, confer a health benefit to the host. They generally exist of Lactobacillus, Bifidobacterium, or Escherichia species, which are commonly found in a normal microbiota. Prebiotics are mostly dietary fibers that are non-digestible food ingredients and beneficially affect the host's health by selectively stimulating the growth and/or activity of some genera of microorganisms in the colon, generally lactobacilli and bifidobacteria.
Intestinal microbiota strongly influence the maturation of the immune system (62) and particularly the development of immune tolerance, because they affect the Th1/Th2/Th17/Treg balance. The microbiota composition is modulated by dietary components that help shaping and timing of the composition of the early microbiome (63, 64). In addition, microbiota can be transmitted directly into the uterus during fetal development, passage through the birth canal or during cesarean-section, breastfeeding, and when providing care to the offspring (65, 66).
Food supplements, which are often termed functional foods, have been used to alter, modify, and reinstate pre-existing intestinal microbiota (67). Supplementation of prebiotics, probiotics, and synbiotics (68–74), as well as PUFA (58, 69, 75–77) during pregnancy and breastfeeding, may reduce eczema in infants. This is further supported by preclinical studies, which indicated that supplementing the maternal diet with specific pre- or probiotics affects milk composition (78) and that supplementing non-digestible oligosaccharides diminished allergic disease in offspring (79–81). This may, in part, be linked to the production of SCFA by the intestinal microbiota (82–86). Even though maternal diet during pregnancy and breastfeeding can modulate the prevalence of allergy in the offspring, the potential role of breastfeeding in allergy prevention is still under discussion, as it seems to be linked to variations in breast milk composition rather than to breastfeeding per se (53, 87).
Most of our current knowledge on the effects of environment and diet on epigenetics and early life immune function is based on epidemiological findings, which indicate that children who grow up on farms have a decreased risk of developing allergies, especially asthma. Allergies are multifactorial, Th2-driven diseases that are triggered by gene-environment interactions. Environmental factors can interact with genes involved in asthma and allergy development via epigenetic mechanisms, such as DNA methylation and histone modifications. These epigenetic mechanisms can regulate gene expression by modifying the accessibility of the DNA to transcription enzymes without altering the DNA nucleotide sequence (30, 33). In addition to the consumption of raw cow's milk (22–29), contact with livestock and animal feed along with other farm-related exposures have shown independent protective effects, indicating that a farm/country lifestyle can contributes to a reduced risk of asthma and allergies in children (25, 27, 88–90). Interestingly, the timing of these exposures seems to be crucial, with the strongest effects observed for exposures that occurred in utero and during the first year of life (23, 91, 92). Since the protective “farm effect” was demonstrated to sustain into adult life (25), effects might be mediated via epigenetic inheritance/regulation.
Several epigenome wide-association studies concerning allergies have been performed and reviewed (30). These studies showed that allergic disease is accompanied by changing DNA methylation patterns in Th2, Th1, Th17, Th9, and Treg subsets in the affected tissues. DNA methylation changes by demethylation and increased FoxP3+ regulatory T cell numbers in peripheral blood mononuclear cells were shown in 4.5-year-old farm children (93). These regulatory T cell numbers were negatively associated with doctor-diagnosed asthma. It remains to be seen if these changes also precede the onset of allergic disease and can be predictive for allergy development, but questions remain as to how are these epigenetic changes induced. It has been suggested that the epigenome is affected by the farm environment. The first indication for a potential role of epigenetic regulation in the protective “farm effect” was provided by Slaats et al. who demonstrated that DNA methylation of the promoter region of CD14 in placentas of mothers living on farms was lower compared to mothers not living on a farm (94). These lower DNA methylation levels were reflected in higher CD14 mRNA expression levels (95). Interestingly, a higher expression of the CD14 gene was also observed in farmers' children (96). Prenatal farm exposure was also associated with increased gene expression of other innate immune receptors, such as TLR5, TLR7, TLR8, and TLR9, at birth (97, 98) and TLR2 and TLR4 in farm-raised children at school age (95, 96). Maternal exposure to farm environments increases the number of T regulatory (Treg) cells in the cord blood of infants, which is associated with decreased Th2 cytokines and may be linked to demethylation at the FOXp3 promoter (99). Whether epigenetic inheritance is underlying these effects requires further investigation. Further evidence that the farm environment affects the epigenome was provided by a pilot study which showed hypermethylation of genes related to IgE regulation and Th2 differentiation in cord blood from farmers' as compared to non-farmers' children (100). Interestingly, at least part of the protective effect triggered by those factors has been ascribed to the farm bacteria, for instance, Acinetobacter lwoffii (101, 102), with a pivotal contribution of downstream epigenetic mechanisms, specifically histone modifications (103).
Human milk contains a unique combination of lipids, proteins, carbohydrates, vitamins, and minerals and thereby provides an ideal source of nutrition for the healthy growth and development of a newborn (104). However, human milk is more than nutrition as it also contains bioactive components that can modulate the immune system, such as immunoglobulins, lactoferrin, human milk oligosaccharides (HMO), long-chain fatty acids, and anti-inflammatory cytokines (18, 105, 106). Most of the immunologically relevant components in breast milk are also found in bovine milk (18). Several key components of breast milk that are not present at high enough levels in bovine milk are added to infant formula to provide the crucial nutrients needed. These include prebiotics or even single HMO like 2'-fucosyllactose (as an alternative to the complex mixture of HMO in breast milk), lactoferrin, PUFA, vitamins, and minerals.
One of the major differences between human breast milk and bovine milk is the amount and diversity of the HMO, i.e., complex, non-digestible oligosaccharides (107, 108). The HMO in breast milk constitutes about 20% of the milk saccharides next to the major carbohydrate in milk, lactose. Human breast milk contains ~5–15 mg/ml of these non-digestible HMO, consisting of up to 200 or more unique structures. In contrast, bovine milk only contains a few of these oligosaccharides, at much lower levels. One injected, HMO survive passage and digestion through the stomach and small intestine and reach the colon, where they are fermented into SCFA like acetate, butyrate, and propionate (107, 108). In addition, they shape the microbiota by selectively enhancing the growth of bifidobacteria and lactobacilli. These SCFAs serve as an energy source for colonic intestinal tissue and shape the interactions between the host and its gut microbiota. Furthermore, SCFA reduces intestinal pH, limit outgrowth of Enterobacteriaceae, and support intestinal barrier function. HMO is the key factor in shaping the development of immunity and early microbiota after birth. HMO have effects on microbiota and infections (107, 108). Of these, 2'-fucosyllactose is the HMO that is most abundantly present in breast milk and has therefore been chosen as the first HMO that was introduced in infant nutrition in 2018.
Prebiotics are non-digestible oligosaccharides like galacto-oligosaccharides (GOS) and fructo-oligosaccharides (FOS), and have widely been used in infant nutrition to mimic the bifidogenic- and SCFA-inducing effect of HMO. There is some evidence that prebiotic oligosaccharides in infant nutrition may prevent eczema in infants (109–112). It is not clear if these effects also extend to the prevention of other allergic diseases, as only one study to date has reported the effects of prebiotics on asthma and food allergy (113). For probiotics, effects are also seen when they are added in infant nutrition (68). As can be seen in detail in Lomax and Calder (114), several studies have reported that infant formula supplemented with prebiotics have a trend toward or even a significant preventive effect on the occurrence of gastrointestinal infections. Trends toward decreased fever episodes, antibiotic use, and upper respiratory tract infections (URTI) have been described. Two studies, by Bruzzese et al. and Arslanoglu et al. and performed with scGOS/lcFOS, supplemented very young infants from early after birth for 6–12 months (115, 116). Both studies showed a significant reduction in gastroenteritis (115) and a reduction in the total number of infections (116). A study from Westerbeek et al., in which scGOS/lcFOS were combined with acidic oligosaccharides (pAOS) showed a non-significant tendency toward fewer serious infections (117). This study was, however, conducted over a shorter time period, and the infants were preterm. In two other studies infants were older than 6 months (118, 119) were supplemented with oligofructose, one did not show an effect on diarrhea, whilst the latter observed a protective effect against diarrhea. Since these components and their effects have been reviewed in detail previously, we will not address them in detail here, and will instead, only focus on their potential epigenetic and long-lasting immune health effects.
Both human milk and bovine milk contain many other bioactive components that can modulate immune function [reviewed in (18, 19, 105–107)]. The components in human and in bovine milk that can be isolated in large quantities have largely been studied as separate entities, because they are potential infant nutrition ingredients. Several of these components, such as transforming growth factor-β (TGF-β) (120), bovine lactoferrin (121–124), bovine alkaline phosphatase (19, 125), bovine osteopontin (126, 127), and the milk fat globular membrane (MFGM) (128), as well as milk exosomes (39), have been linked to immunological outcomes with varying levels of evidence (infection, allergy). Another milk component that may have more sustained immunological effects are bovine IgG antibodies. Where IgA is the predominant immunoglobulin isotype in breast milk, bovine milk has a larger amount of IgG (129). Bovine milk IgG (bIgG) has been shown to bind to aeroallergens (130) as well as to respiratory pathogens such as respiratory syncytial virus (RSV), and can inhibit infection of human cells with human RSV (131). Through the formation of immune complexes, bIgG can enhance RSV-specific T cell responses (132). Similarly, bovine colostrum, which is a rich source of IgG can prevent the infection of mice with RSV (133). Different from adaptive immunity, innate immunity was until recently believed to lead to immune memory. However, vaccination studies have shown that after vaccination—that is associated with cross-protection to other pathogens—the innate immune response is increased to the vaccine, but also other pathogens (134, 135). The mechanism of this was elucidated in several mechanistic studies and was shown to be dependent on epigenetic modification of monocytes and macrophages (136–139). Even though epigenetic modification was not directly shown, bovine IgG can induce trained immunity in monocytes (140). In addition to possibly preventing some of the epigenetic modifications induced by infection with respiratory viruses, which would be the result of the lower prevalence of respiratory tract infections (21), bovine IgG may also directly modify subsequent innate immune responses in infants.
Several epigenome wide-association studies on allergies have been performed, as reviewed elsewhere (30). These studies have shown that allergic disease is accompanied by changing DNA methylation patterns in Th2, Th1, Th17, Th9, and Treg subsets in affected tissues. The epigenetic mechanism behind T cell subset differentiation is strongly affected by essential micronutrients (folate, vitamins B2, B6, and B12, methionine choline, and betaine) (141), bioactive food components (tea polyphenols, genistein from soybean, isothiocyanates from plant foods, curcumin, and curcumin-derived synthetic analogs) (142), total diet (fiber, protein, fat, and hormones) (143), ethanol, and carbohydrates (144). Dietary compounds, especially vitamin D, folate, and zinc, also have the potency to interfere with DNA methylation and thereby steer the Th1-Th2 balance. In addition to these effects on DNA methylation, prenatal supplementation with PUFA or maternal levels of folate, and microbiota-derived SCFA have been associated with changes in histone acetylation patterns at important T cell differentiation regulating genes (Box 2). After birth, these immunomodulatory dietary components are also transferred to the newborn via breast milk.
As already mentioned, the mechanisms underlying the anti-allergic effects of human milk are most probably complex, as human milk contains not only nutritional substances but also functional molecules including polysaccharides, cytokines, proteins, and other components forming a real biological system which can modulate and shape the innate and adaptive immune responses of the infant in very early life (104, 145). If and how those components affect the epigenetic status of the growing child and what consequences this has for allergy development need to be addressed in future studies. Considering the observations made about farm milk (see below), as well as indications that breastfeeding may be capable of changing DNA methylation patterns in the offspring (146), such studies are justified.
Epigenetic modulation of the Foxp3 gene by farm milk was demonstrated in an animal model. In this study, exposure to raw, unprocessed, cow's milk for 8 days, increased histone acetylation of Foxp3 in splenocyte-derived CD4+ T cells compared to processed milk exposure (147). In the same study, mice were subjected to an ovalbumin-induced food allergy model after milk exposure and, interestingly, histone acetylation of Th2 genes was lower in raw milk-pretreated mice compared to processed milk-pretreated mice. These mice also showed a reduction in food allergic symptoms (147). As for farm exposure, exposure to raw milk in the first year of life was also associated with changes in gene expression of the innate immune receptors (98). Moreover, it was demonstrated that a polymorphism in the CD14 gene influenced the protective effect of raw cow milk consumption on allergic diseases (148). DNA demethylation and increased Foxp3+ in the regulatory T cell numbers in the peripheral blood mononuclear cells of 4.5 year-old children were also shown in farm children (93). These regulatory T cell numbers were negatively associated with doctor-diagnosed asthma. It remains to be seen if these changes also precede the onset of allergic disease and can be predictive of allergy development.
There is evidence that the epigenome is affected by the farming environment. The first indication for a potential role of epigenetic regulation in the protective “farm effect” was provided by Slaats et al. who demonstrated that DNA methylation of the promoter region of CD14 in placentas of mothers living on a farm was lower compared to mothers not living on a farm (94). These lower DNA methylation levels were reflected in higher CD14 mRNA expression levels (95). Interestingly, a higher expression of the CD14 gene was also observed in the children of farmers (96). Prenatal farm exposure was also associated with increased gene expression of other innate immune receptors, such as TLR5, TLR7, TLR8, and TLR9, at birth (97, 98) and TLR2 and TLR4 in farm-raised children at school age (91, 96). Maternal exposure to farming environments increased the number of Treg cells in the cord blood of infants, which is associated with decreased Th2 cytokines and may be linked to demethylation at the Foxp3 promoter (50). Whether epigenetic inheritance is the underlying cause of these effects requires further research. Additional evidence that the farm environment affects the epigenome was provided by a pilot study that showed DNA hypermethylation of genes related to IgE regulation and Th2 differentiation in cord blood from the children of farmers as compared to the children of non-farmers (100).
Interestingly, both human and cow's milk contain extracellular vesicles, or exosomes, that are resistant to the acidic environment in the stomach and RNAses in the GI tract. These exosomes contain a variety of especially immune function-related microRNAs (miRNAs). miRNAs represent short noncoding RNA molecules that control 40–60% of the total gene expression by inducing mRNA degradation and/or post-transcriptional inhibition of translation. As a result, specific miRNA can silence selective gene expression. The expression of a single gene can be regulated by several miRNAs, and likewise, a single miRNA can regulate over 100 genes (32, 149). This activity thereby constitutes an epigenetic mechanism by which nutritional factors can influence immune activity or the induction of tolerance by affecting the Th1-Th2 balance. Bovine milk exosomes are taken up by human macrophages (150) and epithelial cells (151, 152), exosomes become systemically available in the body of laboratory animals upon oral delivery (153), and bovine miRNA are detectible in the blood after drinking pasteurized milk (154). However, systemic availability could not be demonstrated for breast milk derived exosomes (155) or vegetable derived miRNA (156). Breast milk-derived exosomes were described in 2007 to enhance Treg development in vitro (157). Based on miRNA content, bovine milk exosomes contain immunoregulatory miRNAs, like miRNA155, that are involved in the development of Tregs and are thought to play a role in the effect of raw milk consumption on asthma (39). In addition to allergy, orally delivered bovine milk exosomes ameliorated arthritis in a murine model (158), and recent evidence also links milk exosomes to the prevention of necrotizing enterocolitis and intestinal damage in in vitro and in vivo investigations (159, 160). These studies suggest that miRNAs in human and raw bovine milk exosomes may have epigenetic effects in infants.
Several studies have implicated the SCFA butyrate, propionate, and acetate as epigenetic modifiers of early life immunity, especially in the development of asthma (161). In addition to regulating Treg differentiation and histone acetylation, SCFAs can induce effector T cell differentiation in secondary lymphoid organs by inhibiting endogenous HDAC activity independent of activation of G-protein-coupled receptor (GPCR). In more detail, SCFA can modulate diverse cell processes by two mechanisms, either via interacting with the GPCR (GPR43, GPR41, GPR109A) on the plasma membrane or following a receptor-independent entrance to the cells (162). SCFA entry occurs through passive diffusion or actively by the involvement of two transporters, namely, monocarboxylate transporter 1 (MCT1/SLa16a1) and sodium-coupled monocarboxylate transporter 1 (SMCT1/SLc5a8). These receptors and transporter molecules are widely present in immune and non-immune cells (162, 163). This effect is highly pronounced for butyrate and to a lesser extent for propionate and acetate (164–166). HDAC inhibition allows HATs activity leading to histone hyperacetylation and subsequently an altered gene expression (37) which might, for instance, result in the proliferation of Treg cells (167–169). The significance of this mechanism is illustrated by the fact that bovine, but not human, milk triglycerides contain a relatively high concentration of the SCFA butyrate (18). Altogether, present evidence implies that HDAC inhibitory activity of SCFA might be cell and tissue dependent, and the gene expression pattern is related to the cellular stage and other environmental signals. If bovine milk consumption is associated with decreased allergy prevalence, does this also mean that milk components can affect epigenetic mechanisms? There is no in vivo evidence that the induction of SCFA by sialyllactose when ingested in bovine milk, but sialyllactose has been reported to induce SCFA production in in vitro fecal microbiota cultures (170) and may thus affect histone acetylation in infants. A high fiber diet (resulting in SCFA production in the colon) or direct feeding of SCFA has been shown to prevent airway inflammation in animal models (84, 85), and SCFA levels in fecal samples of children associated inversely with sensitization to aeroallergens (171, 172).
In addition to allergies, intestinal immunity can also be influenced by microbiota-derived metabolites. For example, tryptophan metabolites can act as aryl hydrocarbon receptor (AhR) ligands, inducing IL-22 and antibacterial peptide production (173), SCFA can directly support the intestinal epithelial barrier, and bile acids can also be metabolized by the microbiota and influence intestinal barrier function and immunity (174). Two studies reported a decreased risk of wheezing in infants because of high maternal dairy intake (175, 176). Taken together, alterations in the local cellular microenvironment and the microbiome (56) allow milk to induce epigenetic changes in both maternal and neonatal nutrition-mediated genes, which can ultimately affect immune programming in the offspring (177).
This review summarizes current knowledge on the potential effects of human and bovine milk on neonatal immunity and epigenetic programming and its possible consequences on the development of allergies in early childhood and beyond (see Figure 1).
Breast milk is the food of choice for newborns and infants. When breast milk is not sufficiently available, cow's milk based formula is the best alternative, and thus cow's milk has become an integral part of early life diet.
Several epidemiological studies that have shown that exposure to a farm environment as well as to raw/unprocessed cow's milk in the prenatal period and early childhood is associated with protection against the development of asthma and other allergies later in life. Many cow's milk components have been shown to have similar effects on human immune cells as their breast milk counterparts.
Some of the molecular pathways that may explain the association between the consumption of raw milk asthma and allergy may be linked to epigenetics. Epigenetic mechanisms like DNA methylation, but also histone modifications, and non-classical epigenetics represented by miRNA may all contribute to the effects induced by raw cow's milk.
However, milk and dairy products are subject to industrial processing to ensure microbiological safety. As a result, milk proteins can be denatured, and lose their functional activity. In addition, glycation of milk proteins is thought to increase the risk of developing cow's milk allergy, illustrating that preserving milk proteins and preventing glycation may be important innovations to help prevent allergies.
Based on what is currently known on immunological and epigenetic effects that can be exerted by human and different types of bovine milk, future research should focus on enhancing the functional (immunological as well as epigenetic) activity of milk components in early life nutrition, and on establishing epigenetic markers of immunological responses to milk. These could be especially important for diagnostic purposes and assessing the risk of developing CMA. Knowledge gathered during studies on the epigenetic effects of milk can be used in the future to drive the development of preventive or therapeutic anti-allergic strategies based on components that affect epigenetic mechanisms.
Finally, the continuation of epidemiologic and mechanistic studies on the effects of the components of breast and bovine milk on human immune function and health will increase our knowledge and help in finding potential applications that may help prevent allergies in the neonatal period.
All authors contributed to the writing of the manuscript.
BE and JG are partly employed by Nutricia Research. RN is employed by FrieslandCampina.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Hales CN, Barker DJ. Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis. Diabetologia. (1992) 35:595–601. doi: 10.1007/BF00400248
2. de Rooij SR, Painter RC, Phillips DI, Osmond C, Michels RP, Godsland IF, et al. Impaired insulin secretion after prenatal exposure to the dutch famine. Diabetes Care. (2006) 29:1897–901. doi: 10.2337/dc06-0460
3. Lumey LH, Khalangot MD, Vaiserman AM. Association between type 2 diabetes and prenatal exposure to the ukraine famine of 1932-33: a retrospective cohort study. Lancet Diabetes Endocrinol. (2015) 3:787–94. doi: 10.1016/S2213-8587(15)00279-X
4. Li C, Lumey LH. Exposure to the chinese famine of 1959-61 in early life and long-term health conditions: a systematic review and meta-analysis. Int J Epidemiol. (2017) 46:1157–70. doi: 10.1093/ije/dyx013
5. Heijmans BT, Tobi EW, Stein AD, Putter H, Blauw GJ, Susser ES, et al. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc Natl Acad Sci USA. (2008) 105:17046–9. doi: 10.1073/pnas.0806560105
6. Prescott SL. Early nutrition as a major determinant of 'immune health': implications for allergy, obesity and other noncommunicable diseases. Nestle Nutr Inst Workshop Ser. (2016) 85:1–17. doi: 10.1159/000439477
7. Harb H, Alashkar Alhamwe B, Acevedo N, Frumento P, Johansson C, Eick L, et al. Epigenetic modifications in placenta are associated with the child's sensitization to allergens. Biomed Res Int. (2019) 2019:1315257. doi: 10.1155/2019/1315257
8. Acevedo N, Frumento P, Harb H, Alashkar Alhamwe B, Johansson C, Eick L, et al. Histone acetylation of immune regulatory genes in human placenta in association with maternal intake of olive oil and fish consumption. Int J Mol Sci. (2019) 20:1060. doi: 10.3390/ijms20051060
9. Prescott SL. Early-life environmental determinants of allergic diseases and the wider pandemic of inflammatory noncommunicable diseases. J Allergy Clin Immunol. (2013) 131:23–30. doi: 10.1016/j.jaci.2012.11.019
10. Koletzko B, Brands B, Grote V, Kirchberg FF, Prell C, Rzehak P, et al. Long-term health impact of early nutrition: the power of programming. Ann Nutr Metab. (2017) 70:161–9. doi: 10.1159/000477781
11. Georgountzou A, Papadopoulos NG. Postnatal innate immune development: from birth to adulthood. Front Immunol. (2017) 8:957. doi: 10.3389/fimmu.2017.00957
12. West CE, D'Vaz N, Prescott SL. Dietary immunomodulatory factors in the development of immune tolerance. Curr Allergy Asthma Rep. (2011) 11:325–33. doi: 10.1007/s11882-011-0200-0
13. Torow N, Marsland BJ, Hornef MW, Gollwitzer ES. Neonatal mucosal immunology. Mucosal Immunol. (2017) 10:5–17. doi: 10.1038/mi.2016.81
14. McDade TW. Early environments and the ecology of inflammation. Proc Natl Acad Sci USA. (2012) 109(Suppl. 2):17281–8. doi: 10.1073/pnas.1202244109
15. Brugman S, Perdijk O, van Neerven RJ, Savelkoul HF. Mucosal immune development in early life: setting the stage. Arch Immunol Ther Exp. (2015) 63:251–68. doi: 10.1007/s00005-015-0329-y
16. Agostoni C, Braegger C, Decsi T, Kolacek S, Koletzko B, Michaelsen KF, et al. Breast-feeding: a commentary by the espghan committee on nutrition. J Pediatr Gastroenterol Nutr. (2009) 49:112–25. doi: 10.1097/MPG.0b013e31819f1e05
17. Victora CG, Bahl R, Barros AJ, Franca GV, Horton S, Krasevec J, et al. Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet. (2016) 387:475–90. doi: 10.1016/S0140-6736(15)01024-7
18. van Neerven RJ, Knol EF, Heck JM, Savelkoul HF. Which factors in raw cow's milk contribute to protection against allergies? J Allergy Clin Immunol. (2012) 130:853–8. doi: 10.1016/j.jaci.2012.06.050
19. Abbring S, Hols G, Garssen J, van Esch BCAM. Raw cow's milk consumption and allergic diseases - the potential role of bioactive whey proteins. Eur J Pharmacol. (2019) 843:55–65. doi: 10.1016/j.ejphar.2018.11.013
20. Perdijk O, van Splunter M, Savelkoul HFJ, Brugman S, van Neerven RJJ. Cow's milk and immune function in the respiratory tract: potential mechanisms. Front Immunol. (2018) 9:143. doi: 10.3389/fimmu.2018.00143
21. Loss G, Depner M, Ulfman LH, van Neerven RJ, Hose AJ, Genuneit J, et al. Consumption of unprocessed cow's milk protects infants from common respiratory infections. J Allergy Clin Immunol. (2015) 135:56–62. doi: 10.1016/j.jaci.2014.08.044
22. Loss G, Apprich S, Waser M, Kneifel W, Genuneit J, Buchele G, et al. The protective effect of farm milk consumption on childhood asthma and atopy: the gabriela study. J Allergy Clin Immunol. (2011) 128:766–73 e4. doi: 10.1016/j.jaci.2011.07.048
23. Riedler J, Braun-Fahrlander C, Eder W, Schreuer M, Waser M, Maisch S, et al. Exposure to farming in early life and development of asthma and allergy: a cross-sectional survey. Lancet. (2001) 358:1129–33. doi: 10.1016/S0140-6736(01)06252-3
24. Brick T, Hettinga K, Kirchner B, Pfaffl MW, Ege MJ. The beneficial effect of farm milk consumption on asthma, allergies, and infections: from meta-analysis of evidence to clinical trial. J Allergy Clin Immunol Pract. (2020) 8:878–89 e3. doi: 10.1016/j.jaip.2019.11.017
25. von Mutius E, Vercelli D. Farm living: effects on childhood asthma and allergy. Nat Rev Immunol. (2010) 10:861–8. doi: 10.1038/nri2871
26. Sozanska B, Pearce N, Dudek K, Cullinan P. Consumption of unpasteurized milk and its effects on atopy and asthma in children and adult inhabitants in rural poland. Allergy. (2013) 68:644–50. doi: 10.1111/all.12147
27. Ege MJ, Frei R, Bieli C, Schram-Bijkerk D, Waser M, Benz MR, et al. Not all farming environments protect against the development of asthma and wheeze in children. J Allergy Clin Immunol. (2007) 119:1140–7. doi: 10.1016/j.jaci.2007.01.037
28. Waser M, Michels KB, Bieli C, Floistrup H, Pershagen G, von Mutius E, et al. Inverse association of farm milk consumption with asthma and allergy in rural and suburban populations across europe. Clin Exp Allergy. (2007) 37:661–70. doi: 10.1111/j.1365-2222.2006.02640.x
29. Perkin MR, Strachan DP. Which aspects of the farming lifestyle explain the inverse association with childhood allergy? J Allergy Clin Immunol. (2006) 117:1374–81. doi: 10.1016/j.jaci.2006.03.008
30. Potaczek DP, Harb H, Michel S, Alhamwe BA, Renz H, Tost J. Epigenetics and allergy: from basic mechanisms to clinical applications. Epigenomics. (2017) 9:539–71. doi: 10.2217/epi-2016-0162
31. Alaskhar Alhamwe B, Khalaila R, Wolf J, von Bulow V, Harb H, Alhamdan F, et al. Histone modifications and their role in epigenetics of atopy and allergic diseases. Allergy Asthma Clin Immunol. (2018) 14:39. doi: 10.1186/s13223-018-0259-4
32. Baskara-Yhuellou I, Tost J. The impact of micrornas on alterations of gene regulatory networks in allergic diseases. Adv Protein Chem Struct Biol. (2020) 120:237–312. doi: 10.1016/bs.apcsb.2019.11.006
33. Alashkar Alhamwe B, Alhamdan F, Ruhl A, Potaczek DP, Renz H. The role of epigenetics in allergy and asthma development. Curr Opin Allergy Clin Immunol. (2020) 20:48–55. doi: 10.1097/ACI.0000000000000598
34. Martino DJ, Prescott SL. Silent mysteries: epigenetic paradigms could hold the key to conquering the epidemic of allergy and immune disease. Allergy. (2010) 65:7–15. doi: 10.1111/j.1398-9995.2009.02186.x
35. Suarez-Alvarez B, Rodriguez RM, Fraga MF, Lopez-Larrea C. DNA methylation: a promising landscape for immune system-related diseases. Trends Genet. (2012) 28:506–14. doi: 10.1016/j.tig.2012.06.005
36. Grozinger CM, Schreiber SL. Deacetylase enzymes: biological functions and the use of small-molecule inhibitors. Chem Biol. (2002) 9:3–16. doi: 10.1016/S1074-5521(02)00092-3
37. Eberharter A, Becker PB. Histone acetylation: a switch between repressive and permissive chromatin. Second in review series on chromatin dynamics. EMBO Rep. (2002) 3:224–9. doi: 10.1093/embo-reports/kvf053
38. Verdone L, Caserta M, Di Mauro E. Role of histone acetylation in the control of gene expression. Biochem Cell Biol. (2005) 83:344–53. doi: 10.1139/o05-041
39. Melnik BC, John SM, Carrera-Bastos P, Schmitz G. Milk: a postnatal imprinting system stabilizing foxp3 expression and regulatory t cell differentiation. Clin Transl Allergy. (2016) 6:18. doi: 10.1186/s13601-016-0108-9
40. Janson PC, Winerdal ME, Winqvist O. At the crossroads of t helper lineage commitment-epigenetics points the way. Biochim Biophys Acta. (2009) 1790:906–19. doi: 10.1016/j.bbagen.2008.12.003
41. Wilson CB, Rowell E, Sekimata M. Epigenetic control of t-helper-cell differentiation. Nat Rev Immunol. (2009) 9:91–105. doi: 10.1038/nri2487
42. Tripathi SK, Lahesmaa R. Transcriptional and epigenetic regulation of t-helper lineage specification. Immunol Rev. (2014) 261:62–83. doi: 10.1111/imr.12204
43. Makar KW, Perez-Melgosa M, Shnyreva M, Weaver WM, Fitzpatrick DR, Wilson CB. Active recruitment of DNA methyltransferases regulates interleukin 4 in thymocytes and t cells. Nat Immunol. (2003) 4:1183–90. doi: 10.1038/ni1004
44. Lal G, Zhang N, van der Touw W, Ding Y, Ju W, Bottinger EP, et al. Epigenetic regulation of foxp3 expression in regulatory t cells by DNA methylation. J Immunol. (2009) 182:259–73. doi: 10.4049/jimmunol.182.1.259
45. Baron U, Floess S, Wieczorek G, Baumann K, Grutzkau A, Dong J, et al. DNA demethylation in the human foxp3 locus discriminates regulatory t cells from activated foxp3(+) conventional t cells. Eur J Immunol. (2007) 37:2378–89. doi: 10.1002/eji.200737594
46. Hirahara K, Vahedi G, Ghoreschi K, Yang XP, Nakayamada S, Kanno Y, et al. Helper t-cell differentiation and plasticity: insights from epigenetics. Immunology. (2011) 134:235–45. doi: 10.1111/j.1365-2567.2011.03483.x
47. Onodera A, Kokubo K, Nakayama T. Epigenetic and transcriptional regulation in the induction, maintenance, heterogeneity, and recall-response of effector and memory th2 cells. Front Immunol. (2018) 9:2929. doi: 10.3389/fimmu.2018.02929
48. Oestreich KJ, Weinmann AS. Transcriptional mechanisms that regulate t helper 1 cell differentiation. Curr Opin Immunol. (2012) 24:191–5. doi: 10.1016/j.coi.2011.12.004
49. Kim LK, Esplugues E, Zorca CE, Parisi F, Kluger Y, Kim TH, et al. Oct-1 regulates il-17 expression by directing interchromosomal associations in conjunction with ctcf in t cells. Mol Cell. (2014) 54:56–66. doi: 10.1016/j.molcel.2014.02.004
50. Begin P, Nadeau KC. Epigenetic regulation of asthma and allergic disease. Allergy Asthma Clin Immunol. (2014) 10:27. doi: 10.1186/1710-1492-10-27
51. Paparo L, Nocerino R, Bruno C, Di Scala C, Cosenza L, Bedogni G, et al. Randomized controlled trial on the influence of dietary intervention on epigenetic mechanisms in children with cow's milk allergy: the epicma study. Sci Rep. (2019) 9:2828. doi: 10.1038/s41598-019-45226-8
52. World Health Organization. Infant and Young Child Nutrition. Global Strategy on Infant and Young Child Feeding. (2002). Available online at: https://www.who.int/nutrition/topics/infantfeeding_recommendation/en/ (accessed April 6, 2020).
53. Munblit D, Peroni DG, Boix-Amoros A, Hsu PS, Van't Land B, Gay MCL, et al. Human milk and allergic diseases: an unsolved puzzle. Nutrients. (2017) 9:894. doi: 10.3390/nu9080894
54. Prentice S. They are what you eat: can nutritional factors during gestation and early infancy modulate the neonatal immune response? Front Immunol. (2017) 8:1641. doi: 10.3389/fimmu.2017.01641
55. Abrams EM, Chan ES. It's not mom's fault: prenatal and early life exposures that do and do not contribute to food allergy development. Immunol Allergy Clin North Am. (2019) 39:447–57. doi: 10.1016/j.iac.2019.06.001
56. Amarasekera M, Prescott SL, Palmer DJ. Nutrition in early life, immune-programming and allergies: the role of epigenetics. Asian Pac J Allergy Immunol. (2013) 31:175–82.
57. Dunstan JA, Mori TA, Barden A, Beilin LJ, Taylor AL, Holt PG, et al. Fish oil supplementation in pregnancy modifies neonatal allergen-specific immune responses and clinical outcomes in infants at high risk of atopy: a randomized, controlled trial. J Allergy Clin Immunol. (2003) 112:1178–84. doi: 10.1016/j.jaci.2003.09.009
58. Gunaratne AW, Makrides M, Collins CT. Maternal prenatal and/or postnatal n-3 long chain polyunsaturated fatty acids (lcpufa) supplementation for preventing allergies in early childhood. Cochrane Database Syst Rev. (2015) 22:CD010085. doi: 10.1002/14651858.CD010085.pub2
59. D'Vaz N, Meldrum SJ, Dunstan JA, Lee-Pullen TF, Metcalfe J, Holt BJ, et al. Fish oil supplementation in early infancy modulates developing infant immune responses. Clin Exp Allergy. (2012) 42:1206–16. doi: 10.1111/j.1365-2222.2012.04031.x
60. Schindler T, Sinn JK, Osborn DA. Polyunsaturated fatty acid supplementation in infancy for the prevention of allergy. Cochrane Database Syst Rev. (2016) 10:CD010112. doi: 10.1002/14651858.CD010112.pub2
61. Harb H, Irvine J, Amarasekera M, Hii CS, Kesper DA, Ma Y, et al. The role of pkczeta in cord blood t-cell maturation towards th1 cytokine profile and its epigenetic regulation by fish oil. Biosci Rep. (2017) 37:BSR20160485. doi: 10.1042/BSR20160485
62. Nauta AJ, Ben Amor K, Knol J, Garssen J, van der Beek EM. Relevance of pre- and postnatal nutrition to development and interplay between the microbiota and metabolic and immune systems. Am J Clin Nutr. (2013) 98:586–93S. doi: 10.3945/ajcn.112.039644
63. Lynch SV, Boushey HA. The microbiome and development of allergic disease. Curr Opin Allergy Clin Immunol. (2016) 16:165–71. doi: 10.1097/ACI.0000000000000255
64. Palmer DJ, Huang RC, Craig JM, Prescott SL. Nutritional influences on epigenetic programming: asthma, allergy, and obesity. Immunol Allergy Clin North Am. (2014) 34:825–37. doi: 10.1016/j.iac.2014.07.003
65. Hollingsworth JW, Maruoka S, Boon K, Garantziotis S, Li Z, Tomfohr J, et al. In utero supplementation with methyl donors enhances allergic airway disease in mice. J Clin Invest. (2008) 118:3462–9. doi: 10.1172/JCI34378
66. Riiser A. The human microbiome, asthma, and allergy. Allergy Asthma Clin Immunol. (2015) 11:35. doi: 10.1186/s13223-015-0102-0
67. Pandey KR, Naik SR, Vakil BV. Probiotics, prebiotics and synbiotics- a review. J Food Sci Technol. (2015) 52:7577–87. doi: 10.1007/s13197-015-1921-1
68. Cuello-Garcia CA, Brozek JL, Fiocchi A, Pawankar R, Yepes-Nunez JJ, Terracciano L, et al. Probiotics for the prevention of allergy: a systematic review and meta-analysis of randomized controlled trials. J Allergy Clin Immunol. (2015) 136:952–61. doi: 10.1016/j.jaci.2015.04.031
69. Garcia-Larsen V, Ierodiakonou D, Jarrold K, Cunha S, Chivinge J, Robinson Z, et al. Diet during pregnancy and infancy and risk of allergic or autoimmune disease: a systematic review and meta-analysis. PLoS Med. (2018) 15:e1002507. doi: 10.1371/journal.pmed.1002507
70. West CE. Probiotics for allergy prevention. Benef Microbes. (2016) 7:171–9. doi: 10.3920/BM2015.0073
71. Enomoto T, Sowa M, Nishimori K, Shimazu S, Yoshida A, Yamada K, et al. Effects of bifidobacterial supplementation to pregnant women and infants in the prevention of allergy development in infants and on fecal microbiota. Allergol Int. (2014) 63:575–85. doi: 10.2332/allergolint.13-OA-0683
72. Rautava S, Isolauri E. The development of gut immune responses and gut microbiota: effects of probiotics in prevention and treatment of allergic disease. Curr Issues Intest Microbiol. (2002) 3:15–22.
73. Rautava S, Luoto R, Salminen S, Isolauri E. Microbial contact during pregnancy, intestinal colonization and human disease. Nat Rev Gastroenterol Hepatol. (2012) 9:565–76. doi: 10.1038/nrgastro.2012.144
74. Wickens K, Black PN, Stanley TV, Mitchell E, Fitzharris P, Tannock GW, et al. A differential effect of 2 probiotics in the prevention of eczema and atopy: a double-blind, randomized, placebo-controlled trial. J Allergy Clin Immunol. (2008) 122:788–94. doi: 10.1016/j.jaci.2008.07.011
75. Miles EA, Calder PC. Can early omega-3 fatty acid exposure reduce risk of childhood allergic disease? Nutrients. (2017) 9:784. doi: 10.3390/nu9070784
76. Willemsen LEM. Dietary n-3 long chain polyunsaturated fatty acids in allergy prevention and asthma treatment. Eur J Pharmacol. (2016) 785:174–86. doi: 10.1016/j.ejphar.2016.03.062
77. Sausenthaler S, Koletzko S, Schaaf B, Lehmann I, Borte M, Herbarth O, et al. Maternal diet during pregnancy in relation to eczema and allergic sensitization in the offspring at 2 y of age. Am J Clin Nutr. (2007) 85:530–7. doi: 10.1093/ajcn/85.2.530
78. Azagra-Boronat I, Tres A, Massot-Cladera M, Franch A, Castell M, Guardiola F, et al. Lactobacillus fermentum cect5716 supplementation in rats during pregnancy and lactation affects mammary milk composition. J Dairy Sci. (2020) 103:2982–92. doi: 10.3168/jds.2019-17384
79. Hogenkamp A, Knippels LM, Garssen J, van Esch BCAM. Supplementation of mice with specific nondigestible oligosaccharides during pregnancy or lactation leads to diminished sensitization and allergy in the female offspring. J Nutr. (2015) 145:996–1002. doi: 10.3945/jn.115.210401
80. Hogenkamp A, Thijssen S, van Vlies N, Garssen J. Supplementing pregnant mice with a specific mixture of nondigestible oligosaccharides reduces symptoms of allergic asthma in male offspring. J Nutr. (2015) 145:640–6. doi: 10.3945/jn.114.197707
81. van Vlies N, Hogenkamp A, Thijssen S, Dingjan GM, Knipping K, Garssen J, et al. Effects of short-chain galacto- and long-chain fructo-oligosaccharides on systemic and local immune status during pregnancy. J Reprod Immunol. (2012) 94:161–8. doi: 10.1016/j.jri.2012.02.007
82. Mischke M, Plosch T. More than just a gut instinct-the potential interplay between a baby's nutrition, its gut microbiome, and the epigenome. Am J Physiol Regul Integr Comp Physiol. (2013) 304:R1065–9. doi: 10.1152/ajpregu.00551.2012
83. Gray LE, O'Hely M, Ranganathan S, Sly PD, Vuillermin P. The maternal diet, gut bacteria, and bacterial metabolites during pregnancy influence offspring asthma. Front Immunol. (2017) 8:365. doi: 10.3389/fimmu.2017.00365
84. Trompette A, Gollwitzer ES, Yadava K, Sichelstiel AK, Sprenger N, Ngom-Bru C, et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med. (2014) 20:159–66. doi: 10.1038/nm.3444
85. Thorburn AN, McKenzie CI, Shen S, Stanley D, Macia L, Mason LJ, et al. Evidence that asthma is a developmental origin disease influenced by maternal diet and bacterial metabolites. Nat Commun. (2015) 6:7320. doi: 10.1038/ncomms8320
86. Torow N, Hornef MW. The neonatal window of opportunity: setting the stage for life-long host-microbial interaction and immune homeostasis. J Immunol. (2017) 198:557–63. doi: 10.4049/jimmunol.1601253
87. Fujimura T, Lum SZC, Nagata Y, Kawamoto S, Oyoshi MK. Influences of maternal factors over offspring allergies and the application for food allergy. Front Immunol. (2019) 10:1933. doi: 10.3389/fimmu.2019.01933
88. Remes ST, Iivanainen K, Koskela H, Pekkanen J. Which factors explain the lower prevalence of atopy amongst farmers' children? Clin Exp Allergy. (2003) 33:427–34. doi: 10.1046/j.1365-2222.2003.01566.x
89. Riedler J, Eder W, Oberfeld G, Schreuer M. Austrian children living on a farm have less hay fever, asthma and allergic sensitization. Clin Exp Allergy. (2000) 30:194–200. doi: 10.1046/j.1365-2222.2000.00799.x
90. von Ehrenstein OS, von Mutius E, Illi S, Baumann L, Bohm O, von Kries R. Reduced risk of hay fever and asthma among children of farmers. Clin Exp Allergy. (2000) 30:187–93. doi: 10.1046/j.1365-2222.2000.00801.x
91. Ege MJ, Bieli C, Frei R, van Strien RT, Riedler J, Ublagger E, et al. Prenatal farm exposure is related to the expression of receptors of the innate immunity and to atopic sensitization in school-age children. J Allergy Clin Immunol. (2006) 117:817–23. doi: 10.1016/j.jaci.2005.12.1307
92. Douwes J, Cheng S, Travier N, Cohet C, Niesink A, McKenzie J, et al. Farm exposure in utero may protect against asthma, hay fever and eczema. Eur Respir J. (2008) 32:603–11. doi: 10.1183/09031936.00033707
93. Lluis A, Depner M, Gaugler B, Saas P, Casaca VI, Raedler D, et al. Increased regulatory t-cell numbers are associated with farm milk exposure and lower atopic sensitization and asthma in childhood. J Allergy Clin Immunol. (2014) 133:551–9. doi: 10.1016/j.jaci.2013.06.034
94. Slaats GG, Reinius LE, Alm J, Kere J, Scheynius A, Joerink M. DNA methylation levels within the cd14 promoter region are lower in placentas of mothers living on a farm. Allergy. (2012) 67:895–903. doi: 10.1111/j.1398-9995.2012.02831.x
95. Joerink M, Oortveld MA, Stenius F, Rindsjo E, Alm J, Scheynius A. Lifestyle and parental allergen sensitization are reflected in the intrauterine environment at gene expression level. Allergy. (2010) 65:1282–9. doi: 10.1111/j.1398-9995.2010.02328.x
96. Lauener RP, Birchler T, Adamski J, Braun-Fahrlander C, Bufe A, Herz U, et al. Expression of cd14 and toll-like receptor 2 in farmers' and non-farmers' children. Lancet. (2002) 360:465–6. doi: 10.1016/S0140-6736(02)09641-1
97. Roduit C, Wohlgensinger J, Frei R, Bitter S, Bieli C, Loeliger S, et al. Prenatal animal contact and gene expression of innate immunity receptors at birth are associated with atopic dermatitis. J Allergy Clin Immunol. (2011) 127:179–85.e1. doi: 10.1016/j.jaci.2010.10.010
98. Loss G, Bitter S, Wohlgensinger J, Frei R, Roduit C, Genuneit J, et al. Prenatal and early-life exposures alter expression of innate immunity genes: the pasture cohort study. J Allergy Clin Immunol. (2012) 130:523–30 e9. doi: 10.1016/j.jaci.2012.05.049
99. Schaub B, Liu J, Höppler S, Schleich I, Huehn J, Olek S, et al. Maternal farm exposure modulates neonatal immune mechanisms through regulatory T cells. J Allergy Clin Immunol. (2009) 123:774–82.e5. doi: 10.1016/j.jaci.2009.01.056
100. Michel S, Busato F, Genuneit J, Pekkanen J, Dalphin JC, Riedler J, et al. Farm exposure and time trends in early childhood may influence DNA methylation in genes related to asthma and allergy. Allergy. (2013) 68:355–64. doi: 10.1111/all.12097
101. Conrad ML, Ferstl R, Teich R, Brand S, Blumer N, Yildirim AO, et al. Maternal tlr signaling is required for prenatal asthma protection by the nonpathogenic microbe acinetobacter lwoffii f78. J Exp Med. (2009) 206:2869–77. doi: 10.1084/jem.20090845
102. Hagner S, Harb H, Zhao M, Stein K, Holst O, Ege MJ, et al. Farm-derived gram-positive bacterium staphylococcus sciuri w620 prevents asthma phenotype in hdm- and ova-exposed mice. Allergy. (2013) 68:322–9. doi: 10.1111/all.12094
103. Brand S, Teich R, Dicke T, Harb H, Yildirim AO, Tost J, et al. Epigenetic regulation in murine offspring as a novel mechanism for transmaternal asthma protection induced by microbes. J Allergy Clin Immunol. (2011) 128:618–25 e1–7. doi: 10.1016/j.jaci.2011.04.035
104. Ballard O, Morrow AL. Human milk composition: nutrients and bioactive factors. Pediatr Clin North Am. (2013) 60:49–74. doi: 10.1016/j.pcl.2012.10.002
105. Verhasselt V. Oral tolerance in neonates: from basics to potential prevention of allergic disease. Mucosal Immunol. (2010) 3:326–33. doi: 10.1038/mi.2010.25
106. Boix-Amoros A, Collado MC, Van't Land B, Calvert A, Le Doare K, Garssen J, et al. Reviewing the evidence on breast milk composition and immunological outcomes. Nutr Rev. (2019) 77:541–56. doi: 10.1093/nutrit/nuz019
107. Triantis V, Bode L, van Neerven RJJ. Immunological effects of human milk oligosaccharides. Front Pediatr. (2018) 6:190. doi: 10.3389/fped.2018.00190
108. Bode L. Human milk oligosaccharides: every baby needs a sugar mama. Glycobiology. (2012) 22:1147–62. doi: 10.1093/glycob/cws074
109. Osborn DA, Sinn JK. Prebiotics in infants for prevention of allergy. Cochrane Database Syst Rev. (2013) 28:CD006474. doi: 10.1002/14651858.CD006474.pub3
110. Cuello-Garcia C, Fiocchi A, Pawankar R, Yepes-Nunez JJ, Morgano GP, Zhang Y, et al. Prebiotics for the prevention of allergies: a systematic review and meta-analysis of randomized controlled trials. Clin Exp Allergy. (2017) 47:1468–77. doi: 10.1111/cea.13042
111. Eigenmann PA. Evidence of preventive effect of probiotics and prebiotics for infantile eczema. Curr Opin Allergy Clin Immunol. (2013) 13:426–31. doi: 10.1097/ACI.0b013e3283630bad
112. de Moura PN, Rosario Filho NA. The use of prebiotics during the first year of life for atopy prevention and treatment. Immun Inflamm Dis. (2013) 1:63–9. doi: 10.1002/iid3.8
113. Arslanoglu S, Moro GE, Schmitt J, Tandoi L, Rizzardi S, Boehm G. Early dietary intervention with a mixture of prebiotic oligosaccharides reduces the incidence of allergic manifestations and infections during the first two years of life. J Nutr. (2008) 138:1091–5. doi: 10.1093/jn/138.6.1091
114. Lomax AR, Calder PC. Prebiotics, immune function, infection and inflammation: a review of the evidence. Br J Nutr. (2009) 101:633–58. doi: 10.1017/S0007114508055608
115. Bruzzese E, Volpicelli M, Squeglia V, Bruzzese D, Salvini F, Bisceglia M, et al. A formula containing galacto- and fructo-oligosaccharides prevents intestinal and extra-intestinal infections: an observational study. Clin Nutr. (2009) 28:156–61. doi: 10.1016/j.clnu.2009.01.008
116. Arslanoglu S, Moro GE, Boehm G. Early supplementation of prebiotic oligosaccharides protects formula-fed infants against infections during the first 6 months of life. J Nutr. (2007) 137:2420–4. doi: 10.1093/jn/137.11.2420
117. Westerbeek EA, van den Berg JP, Lafeber HN, Fetter WP, Boehm G, Twisk JW, et al. Neutral and acidic oligosaccharides in preterm infants: a randomized, double-blind, placebo-controlled trial. Am J Clin Nutr. (2010) 91:679–86. doi: 10.3945/ajcn.2009.28625
118. Duggan C, Penny ME, Hibberd P, Gil A, Huapaya A, Cooper A, et al. Oligofructose-supplemented infant cereal: 2 randomized, blinded, community-based trials in peruvian infants. Am J Clin Nutr. (2003) 77:937–42. doi: 10.1093/ajcn/77.4.937
119. Waligora-Dupriet AJ, Campeotto F, Nicolis I, Bonet A, Soulaines P, Dupont C, et al. Effect of oligofructose supplementation on gut microflora and well-being in young children attending a day care centre. Int J Food Microbiol. (2007) 113:108–13. doi: 10.1016/j.ijfoodmicro.2006.07.009
120. Khaleva E, Gridneva Z, Geddes DT, Oddy WH, Colicino S, Blyuss O, et al. Transforming growth factor beta in human milk and allergic outcomes in children: a systematic review. Clin Exp Allergy. (2019) 49:1201–13. doi: 10.1111/cea.13409
121. Manzoni P, Rinaldi M, Cattani S, Pugni L, Romeo MG, Messner H, et al. Bovine lactoferrin supplementation for prevention of late-onset sepsis in very low-birth-weight neonates: a randomized trial. JAMA. (2009) 302:1421–8. doi: 10.1001/jama.2009.1403
122. Manzoni P, Stolfi I, Messner H, Cattani S, Laforgia N, Romeo MG, et al. Bovine lactoferrin prevents invasive fungal infections in very low birth weight infants: a randomized controlled trial. Pediatrics. (2012) 129:116–23. doi: 10.1542/peds.2011-0279
123. King JC Jr., Cummings GE, Guo N, Trivedi L, Readmond BX, et al. A double-blind, placebo-controlled, pilot study of bovine lactoferrin supplementation in bottle-fed infants. J Pediatr Gastroenterol Nutr. (2007) 44:245–51. doi: 10.1097/01.mpg.0000243435.54958.68
124. Chen K, Chai L, Li H, Zhang Y, Xie HM, Shang J, et al. Effect of bovine lactoferrin from iron-fortified formulas on diarrhea and respiratory tract infections of weaned infants in a randomized controlled trial. Nutrition. (2016) 32:222–7. doi: 10.1016/j.nut.2015.08.010
125. Abbring S, Ryan JT, Diks MAP, Hols G, Garssen J, van Esch BCAM. Suppression of food allergic symptoms by raw cow's milk in mice is retained after skimming but abolished after heating the milk - a promising contribution of alkaline phosphatase. Nutrients. (2019) 11:1499. doi: 10.3390/nu11071499
126. Lonnerdal B, Kvistgaard AS, Peerson JM, Donovan SM, Peng YM. Growth, nutrition, and cytokine response of breast-fed infants and infants fed formula with added bovine osteopontin. J Pediatr Gastroenterol Nutr. (2016) 62:650–7. doi: 10.1097/MPG.0000000000001005
127. West CE, Kvistgaard AS, Peerson JM, Donovan SM, Peng YM, Lonnerdal B. Effects of osteopontin-enriched formula on lymphocyte subsets in the first 6 months of life: a randomized controlled trial. Pediatr Res. (2017) 82:63–71. doi: 10.1038/pr.2017.77
128. Timby N, Hernell O, Vaarala O, Melin M, Lonnerdal B, Domellof M. Infections in infants fed formula supplemented with bovine milk fat globule membranes. J Pediatr Gastroenterol Nutr. (2015) 60:384–9. doi: 10.1097/MPG.0000000000000624
129. Ulfman LH, Leusen JHW, Savelkoul HFJ, Warner JO, van Neerven RJJ. Effects of bovine immunoglobulins on immune function, allergy, and infection. Front Nutr. (2018) 5:52. doi: 10.3389/fnut.2018.00052
130. Collins AM, Roberton DM, Hosking CS, Flannery GR. Bovine milk, including pasteurised milk, contains antibodies directed against allergens of clinical importance to man. Int Arch Allergy Appl Immunol. (1991) 96:362–7. doi: 10.1159/000235523
131. den Hartog G, Jacobino S, Bont L, Cox L, Ulfman LH, Leusen JH, et al. Specificity and effector functions of human rsv-specific igg from bovine milk. PLoS ONE. (2014) 9:e112047. doi: 10.1371/journal.pone.0112047
132. Nederend M, van Stigt AH, Jansen JHM, Jacobino SR, Brugman S, de Haan CAM, et al. Bovine igg prevents experimental infection with rsv and facilitates human t cell responses to RSV. Front Immunol. (2020) 11:1701. doi: 10.3389/fimmu.2020.01701
133. Xu ML, Kim HJ, Wi GR, Kim HJ. The effect of dietary bovine colostrum on respiratory syncytial virus infection and immune responses following the infection in the mouse. J Microbiol. (2015) 53:661–6. doi: 10.1007/s12275-015-5353-4
134. Aaby P, Roth A, Ravn H, Napirna BM, Rodrigues A, Lisse IM, et al. Randomized trial of bcg vaccination at birth to low-birth-weight children: beneficial nonspecific effects in the neonatal period? J Infect Dis. (2011) 204:245–52. doi: 10.1093/infdis/jir240
135. Benn CS, Netea MG, Selin LK, Aaby P. A small jab - a big effect: nonspecific immunomodulation by vaccines. Trends Immunol. (2013) 34:431–9. doi: 10.1016/j.it.2013.04.004
136. Kleinnijenhuis J, Quintin J, Preijers F, Joosten LA, Ifrim DC, Saeed S, et al. Bacille calmette-guerin induces nod2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc Natl Acad Sci USA. (2012) 109:17537–42. doi: 10.1073/pnas.1202870109
137. Quintin J, Saeed S, Martens JHA, Giamarellos-Bourboulis EJ, Ifrim DC, Logie C, et al. Candida albicans infection affords protection against reinfection via functional reprogramming of monocytes. Cell Host Microbe. (2012) 12:223–32. doi: 10.1016/j.chom.2012.06.006
138. Cheng SC, Quintin J, Cramer RA, Shepardson KM, Saeed S, Kumar V, et al. Mtor- and hif-1alpha-mediated aerobic glycolysis as metabolic basis for trained immunity. Science. (2014) 345:1250684. doi: 10.1126/science.1250684
139. Saeed S, Quintin J, Kerstens HH, Rao NA, Aghajanirefah A, Matarese F, et al. Epigenetic programming of monocyte-to-macrophage differentiation and trained innate immunity. Science. (2014) 345:1251086. doi: 10.1126/science.1251086
140. van Splunter M, van Osch TLJ, Brugman S, Savelkoul HFJ, Joosten LAB, Netea MG, et al. Induction of trained innate immunity in human monocytes by bovine milk and milk-derived immunoglobulin g. Nutrients. (2018) 10:1378. doi: 10.3390/nu10101378
141. Marques AH, O'Connor TG, Roth C, Susser E, Bjorke-Monsen AL. The influence of maternal prenatal and early childhood nutrition and maternal prenatal stress on offspring immune system development and neurodevelopmental disorders. Front Neurosci. (2013) 7:120. doi: 10.3389/fnins.2013.00120
142. Claycombe KJ, Brissette CA, Ghribi O. Epigenetics of inflammation, maternal infection, and nutrition. J Nutr. (2015) 145:1109–15S. doi: 10.3945/jn.114.194639
143. Choi SW, Friso S. Epigenetics: a new bridge between nutrition and health. Adv Nutr. (2010) 1:8–16. doi: 10.3945/an.110.1004
144. Paparo L, di Costanzo M, di Scala C, Cosenza L, Leone L, Nocerino R, et al. The influence of early life nutrition on epigenetic regulatory mechanisms of the immune system. Nutrients. (2014) 6:4706–19. doi: 10.3390/nu6114706
145. Rajani PS, Seppo AE, Jarvinen KM. Immunologically active components in human milk and development of atopic disease, with emphasis on food allergy, in the pediatric population. Front Pediatr. (2018) 6:218. doi: 10.3389/fped.2018.00218
146. Hartwig FP, Loret de Mola C, Davies NM, Victora CG, Relton CL. Breastfeeding effects on DNA methylation in the offspring: a systematic literature review. PLoS ONE. (2017) 12:e0173070. doi: 10.1371/journal.pone.0173070
147. Abbring S, Wolf J, Ayechu-Muruzabal V, Diks MAP, Alashkar Alhamwe B, Alhamdan F, et al. Raw cow's milk reduces allergic symptoms in a murine model for food allergy - a potential role for epigenetic modifications. Nutrients. (2019) 11:1721. doi: 10.3390/nu11081721
148. Bieli C, Eder W, Frei R, Braun-Fahrlander C, Klimecki W, Waser M, et al. A polymorphism in cd14 modifies the effect of farm milk consumption on allergic diseases and cd14 gene expression. J Allergy Clin Immunol. (2007) 120:1308–15. doi: 10.1016/j.jaci.2007.07.034
149. Cui J, Zhou B, Ross SA, Zempleni J. Nutrition, micrornas, and human health. Adv Nutr. (2017) 8:105–12. doi: 10.3945/an.116.013839
150. Izumi H, Tsuda M, Sato Y, Kosaka N, Ochiya T, Iwamoto H, et al. Bovine milk exosomes contain microrna and mrna and are taken up by human macrophages. J Dairy Sci. (2015) 98:2920–33. doi: 10.3168/jds.2014-9076
151. Wolf T, Baier SR, Zempleni J. The intestinal transport of bovine milk exosomes is mediated by endocytosis in human colon carcinoma caco-2 cells and rat small intestinal iec-6 cells. J Nutr. (2015) 145:2201–6. doi: 10.3945/jn.115.218586
152. Zempleni J, Sukreet S, Zhou F, Wu D, Mutai E. Milk-derived exosomes and metabolic regulation. Annu Rev Anim Biosci. (2019) 7:245–62. doi: 10.1146/annurev-animal-020518-115300
153. Munagala R, Aqil F, Jeyabalan J, Gupta RC. Bovine milk-derived exosomes for drug delivery. Cancer Lett. (2016) 371:48–61. doi: 10.1016/j.canlet.2015.10.020
154. Baier SR, Nguyen C, Xie F, Wood JR, Zempleni J. Micrornas are absorbed in biologically meaningful amounts from nutritionally relevant doses of cow milk and affect gene expression in peripheral blood mononuclear cells, hek-293 kidney cell cultures, and mouse livers. J Nutr. (2014) 144:1495–500. doi: 10.3945/jn.114.196436
155. Title AC, Denzler R, Stoffel M. Uptake and function studies of maternal milk-derived micrornas. J Biol Chem. (2015) 290:23680–91. doi: 10.1074/jbc.M115.676734
156. Link J, Thon C, Schanze D, Steponaitiene R, Kupcinskas J, Zenker M, et al. Food-derived xeno-micrornas: influence of diet and detectability in gastrointestinal tract-proof-of-principle study. Mol Nutr Food Res. (2019) 63:e1800076. doi: 10.1002/mnfr.201800076
157. Admyre C, Johansson SM, Qazi KR, Filen JJ, Lahesmaa R, Norman M, et al. Exosomes with immune modulatory features are present in human breast milk. J Immunol. (2007) 179:1969–78. doi: 10.4049/jimmunol.179.3.1969
158. Arntz OJ, Pieters BC, Oliveira MC, Broeren MG, Bennink MB, de Vries M, et al. Oral administration of bovine milk derived extracellular vesicles attenuates arthritis in two mouse models. Mol Nutr Food Res. (2015) 59:1701–12. doi: 10.1002/mnfr.201500222
159. Li B, Hock A, Wu RY, Minich A, Botts SR, Lee C, et al. Bovine milk-derived exosomes enhance goblet cell activity and prevent the development of experimental necrotizing enterocolitis. PLoS ONE. (2019) 14:e0211431. doi: 10.1371/journal.pone.0211431
160. Gao R, Zhang R, Qian T, Peng X, He W, Zheng S, et al. A comparison of exosomes derived from different periods breast milk on protecting against intestinal organoid injury. Pediatr Surg Int. (2019) 35:1363–8. doi: 10.1007/s00383-019-04562-6
161. Woo V, Alenghat T. Host-microbiota interactions: epigenomic regulation. Curr Opin Immunol. (2017) 44:52–60. doi: 10.1016/j.coi.2016.12.001
162. Koh A, De Vadder F, Kovatcheva-Datchary P, Backhed F. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell. (2016) 165:1332–45. doi: 10.1016/j.cell.2016.05.041
163. Kim CH, Park J, Kim M. Gut microbiota-derived short-chain fatty acids, t cells, and inflammation. Immune Netw. (2014) 14:277–88. doi: 10.4110/in.2014.14.6.277
164. Kendrick SF, O'Boyle G, Mann J, Zeybel M, Palmer J, Jones DE, et al. Acetate, the key modulator of inflammatory responses in acute alcoholic hepatitis. Hepatology. (2010) 51:1988–97. doi: 10.1002/hep.23572
165. Kiefer J, Beyer-Sehlmeyer G, Pool-Zobel BL. Mixtures of scfa, composed according to physiologically available concentrations in the gut lumen, modulate histone acetylation in human ht29 colon cancer cells. Br J Nutr. (2006) 96:803–10. doi: 10.1017/BJN20061948
166. Sealy L, Chalkley R. The effect of sodium butyrate on histone modification. Cell. (1978) 14:115–21. doi: 10.1016/0092-8674(78)90306-9
167. Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, et al. Metabolites produced by commensal bacteria promote peripheral regulatory t-cell generation. Nature. (2013) 504:451–5. doi: 10.1038/nature12726
168. Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory t cells. Nature. (2013) 504:446–50. doi: 10.1038/nature12721
169. Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly YM, et al. The microbial metabolites, short-chain fatty acids, regulate colonic treg cell homeostasis. Science. (2013) 341:569–73. doi: 10.1126/science.1241165
170. Perdijk O, van Baarlen P, Fernandez-Gutierrez MM, van den Brink E, Schuren FHJ, Brugman S, et al. Sialyllactose and galactooligosaccharides promote epithelial barrier functioning and distinctly modulate microbiota composition and short chain fatty acid production in vitro. Front Immunol. (2019) 10:94. doi: 10.3389/fimmu.2019.00762
171. Roduit C, Frei R, Ferstl R, Loeliger S, Westermann P, Rhyner C, et al. High levels of butyrate and propionate in early life are associated with protection against atopy. Allergy. (2019) 74:799–809. doi: 10.1111/all.13660
172. Arrieta MC, Stiemsma LT, Dimitriu PA, Thorson L, Russell S, Yurist-Doutsch S, et al. Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci Transl Med. (2015) 7:307ra152. doi: 10.1126/scitranslmed.aab2271
173. Gao J, Xu K, Liu H, Liu G, Bai M, Peng C, et al. Impact of the gut microbiota on intestinal immunity mediated by tryptophan metabolism. Front Cell Infect Microbiol. (2018) 8:13. doi: 10.3389/fcimb.2018.00013
174. Lee-Sarwar KA, Lasky-Su J, Kelly RS, Litonjua AA, Weiss ST. Gut microbial-derived metabolomics of asthma. Metabolites. (2020) 10:97. doi: 10.3390/metabo10030097
175. Chatzi L, Garcia R, Roumeliotaki T, Basterrechea M, Begiristain H, Iñiguez C, et al. Mediterranean diet adherence during pregnancy and risk of wheeze and eczema in the first year of life: inma (spain) and rhea (greece) mother-child cohort studies. Br J Nutr. (2013) 110:2058–68. doi: 10.1017/S0007114513001426
176. Miyake Y, Sasaki S, Tanaka K, Hirota Y. Dairy food, calcium and vitamin d intake in preg nancy, and wheeze and eczema in infants. Eur Respir J. (2010) 35:1228–34. doi: 10.1183/09031936.00100609
Keywords: epigenetics, epigenetic imprinting, environmental factors, unprocessed (raw) milk, breastfeeding, allergy, nutritional programming, bioactive milk components
Citation: Esch BCAMv, Porbahaie M, Abbring S, Garssen J, Potaczek DP, Savelkoul HFJ and Neerven RJJv (2020) The Impact of Milk and Its Components on Epigenetic Programming of Immune Function in Early Life and Beyond: Implications for Allergy and Asthma. Front. Immunol. 11:2141. doi: 10.3389/fimmu.2020.02141
Received: 27 May 2020; Accepted: 06 August 2020;
Published: 21 October 2020.
Edited by:
Daniel Munblit, I.M. Sechenov First Moscow State Medical University, RussiaReviewed by:
David Jim Martino, University of Western Australia, AustraliaCopyright © 2020 Esch, Porbahaie, Abbring, Garssen, Potaczek, Savelkoul and Neerven. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: R. J. Joost van Neerven, am9vc3QudmFubmVlcnZlbkB3dXIubmw=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.