- 1Division of Internal Medicine, University Hospital Basel, Basel, Switzerland
- 2Laboratory Medicine, Division of Medical Immunology, University Hospital Basel, Basel, Switzerland
- 3Clinic of Radiology and Nuclear Medicine, University Hospital Basel, Basel, Switzerland
- 4Pharming Group, Leiden, Netherlands
- 5Department of Clinical Research and Department of Biomedicine, University Hospital Basel, Basel, Switzerland
- 6Department of Infectious Diseases and Hospital Epidemiology, University Hospital Basel, Basel, Switzerland
A dysregulated immune response with hyperinflammation is observed in patients with severe coronavirus disease 2019 (COVID-19). The aim of the present study was to assess the safety and potential benefits of human recombinant C1 esterase inhibitor (conestat alfa), a complement, contact activation and kallikrein-kinin system regulator, in severe COVID-19. Patients with evidence of progressive disease after 24 h including an oxygen saturation <93% at rest in ambient air were included at the University Hospital Basel, Switzerland in April 2020. Conestat alfa was administered by intravenous injections of 8400 IU followed by 3 additional doses of 4200 IU in 12-h intervals. Five patients (age range, 53–85 years; one woman) with severe COVID-19 pneumonia (11–39% lung involvement on computed tomography scan of the chest) were treated a median of 1 day (range 1–7 days) after admission. Treatment was well-tolerated. Immediate defervescence occurred, and inflammatory markers and oxygen supplementation decreased or stabilized in 4 patients (e.g., median C-reactive protein 203 (range 31–235) mg/L before vs. 32 (12–72) mg/L on day 5). Only one patient required mechanical ventilation. All patients recovered. C1INH concentrations were elevated before conestat alfa treatment. Levels of complement activation products declined after treatment. Viral loads in nasopharyngeal swabs declined in 4 patients. In this uncontrolled case series, targeting multiple inflammatory cascades by conestat alfa was safe and associated with clinical improvements in the majority of severe COVID-19 patients. Controlled clinical trials are needed to assess its safety and efficacy in preventing disease progression.
Introduction
The current pandemia of coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) remains a major global health challenge. The clinical spectrum of COVID-19 ranges from asymptomatic carriers to respiratory failure. A dysregulated immune response with evidence of hyperinflammation is observed in patients with severe COVID-19 (1). Siddiqi et al. suggested a disease stage model, where early disease is charaterized by infection and replication of the virus. Subsequently, pulmonary involvement and marked hyperinflammation occur during the next stage (2). The exact factors promoting disease progression are not yet known. However, sustained activation of the complement system (CS) induced by coronaviruses (CoV) seems to play a crucial role in this context.
The complement system (CS) is an integral part of the innate immune system and consists of a number of distinct plasma proteins that act as a first line of defense inducing an inflammatory response after opsonisation of pathogens and dying cells (3, 4). Inflammatory responses include the activation of macrophages, neutrophils, platelets and endothelial cells, and interactions with other plasmatic cascades. While the CS does not seem to be critical for controlling CoV replication, unregulated complement activation - induced by viruses including influenza and CoV - plays a crucial role in the pathogenesis of acute lung injury (ALI). Indeed, an animal model suggests that the CS mediates SARS-CoV-induced lung disease and regulates the proinflammatory response. Complement deficient mice infected with SARS-CoV were affected less severely and showed a reduced lung involvement and lower local and systemic cytokine levels compared to control mice (5). In line, inhibition of complement C5a signaling alleviated lung damage in animal infection models using MERS-CoV (6) and influenza H7N9 (7). Similar results were reported with inhibition of complement cascade C3a in an animal infection model using avian influenza (8). Gao et al. investigated the interaction of MERS-CoV, SARS-CoV, and SARS-CoV-2 with the lectin pathway of complement in more detail (9). They demonstrate an interaction of these highly pathogenic CoV with mannose-binding lectin associated serine protease-2 (MASP-2), the key activator of the lectin pathway of complement (10), leading to uncontrolled activation of the complement cascade. In line, MASP-2 knock-out mice and mice treated with MASP-2 inhibitors showed significantly milder symptoms in a virus protein mouse pneumonia model. Mannose-binding lectin, the pattern recognition molecule of the lectin pathway, that activates MASP-2 upon binding to pathogens, was found to bind to SARS-CoV spike glycoprotein (11). In COVID-19 patients, increased plasma levels of complement activation products C5a and soluble C5b-9 have been observed (12). Furthermore, autopsy findings from 5 patients with severe COVID-19 revealed excessive complement activation in lung tissue associated with complement-mediated microthrombotic disease (13).
Beside CS activation, involvement of the contact activation (CAS) and kallikrein-kinin system (KKS) in thromboinflammation, capillary leakage and subsequent pulmonary angioedema has been suspected. Angiotensin-converting enzyme 2 (ACE2), a cell membrane bound protein used by SARS-CoV-2 to enter cells, also inactivates bradykinin degradation products, and expression of ACE2 as well as its enzymatic activity is decreased by SARS-CoV (14). Hence, the interaction of SARS-CoV-2 with ACE2 may impair its function, leading to a relative abundance of bradykinin degradation products and local pulmonary edema.
The role of the CAS has not been elucidated in COVID-19. However, dysregulated coagulation is commonly observed in critically-ill patients with COVID-19 and thromboembolism has been reported more frequently compared to other diseases causing severe sepsis (15, 16). This may be a consequence of over-activation of CAS since sepsis and ARDS are prototypic states that strongly activate the CAS (17, 18). Consistent with thromboinflammation, microthrombi have been observed in autopsy series in COVID-19 (19).
C1 esterase inhibitor (C1INH) is a strong inhibitor of the CS, CAS, and KKS and interacts with stressed endothelial cells. C1INH treatment was associated with reduced capillary leakage after stem cell transplantation and organ damage in human sepsis (20–22). Recently, C1INH was identified among other inflammatory proteins to be upregulated in severe vs. non-severe COVID-19 in a proteomic and metabolomic characterization (23). In a mouse model of highly pathogenic CoV, C1INH treatment was able to block MASP-2 mediated overactivation of the complement system and subsequent lung injury and death (9). Lastly, when examining published interactomes of SARS-CoV-1 and−2, a significant interaction of C1INH with CoV-1 proteins was documented including proteins that are highly similar to their orthologous CoV-2 proteins (24).
Investigating a regulator of the CS, CAS, and KKS for the first time in severe COVID-19, we report the experience of early administration of conestat alfa, a recombinant human C1INH, in non-critically ill patients to prevent deterioration.
Materials and Methods
The treatments occurred at the University Hospital Basel from April 2, 2020, to April 28, 2020 and were approved by the Ethics Committee of Northwest and Central Switzerland (EKNZ 2020-1013). All participants consented to the compassionate use of conestat alfa and the acquisition of health-related personal data and biological material.
Study Drug
Conestat alfa is a recombinant human C1INH that shares an identical protein structure with plasma-derived C1INH but has a different glycosylation pattern. Comparable inhibition of most target proteases has been demonstrated (25). Despite the broad interference with several cascades and targets, major adverse events or unique toxicities have not been demonstrated in previous studies with the exception of a potential risk of allergic reactions in patients with rabbit dander allergy.
Patients and Intervention
Patients with polymerase chain reaction-confirmed and severe COVID-19 (26) were included if they were at least 18 years old, showed evidence of progressive disease after 24 h, and had a C-reactive protein level of at least 30 mg/L and oxygen saturation of <93% at rest in ambient air. Exclusion criteria included admission to ICU, immunosuppression, and known allergy to rabbit dander. Conestat alfa was administered by 4 intravenous injections in 12-h intervals over 48 h. The dosage was identical for all patients irrespective of body weight (8400 IU as initial dosage followed by 4200 IU).
Data Collection
Clinical information was collected from the electronic hospital information system. Affected lung tissue on a computed tomography (CT) scan was quantified semi-automatically using software for lung density analysis in Chest CT available at our radiology department [CT Pulmo 3D included in Syngo.Via VB30A, Siemens Healthineers, Forchheim, Germany; method similar as described recently (27)]. The processing included semi-automatic segmentation of the lungs followed by a threshold-analysis of Hounsfield units (HU), where pulmonary involvement was defined as the percentage of lung parenchyma with a CT-density between −600 and 0 HU. Concentration of complement proteins and C1INH antigen were analyzed in serum or plasma by standardized nephelometric assays (C3, C4, C1INH) or ELISA assays in duplicate according to the instructions of the manufacturer (C4d, C5a; Quidel, San Diego, U.S.A.). A control group was established from the database of all COVID-19 patients admitted during the same period (March and April 2020) at the University Hospital Basel (n = 165). Propensity score-matching (1:3 matching of cases and controls) was performed to adjust for relevant confounders (nearest-neighborhood method; caliper width of 0.25 times the standard deviation of the logit of propensity scores). The following variables were included in the matching model: gender (exact), age, lung involvement on CT scan of the chest and Charlson Comorbidity index. The maximum standardized difference of the mean was 0.05 and the area under the curve was 0.85. Characteristics of the group were compared with the use of the Fisher's exact test for categorical variables and the Mann-Whitney U-test for continuous variables.
Results
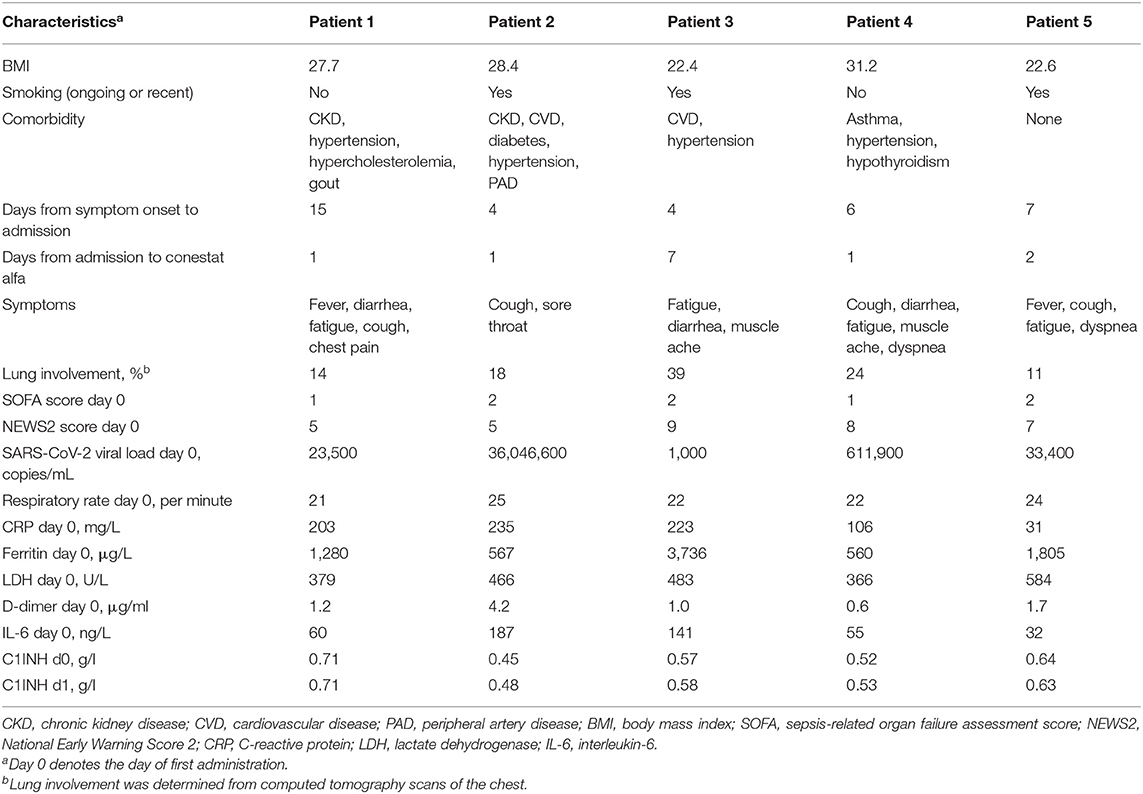
Five patients (4 males, median age 60 years, range 53–85 years) were treated with conestat alfa. Four had preexisting medical conditions (Table 1). A CT scan of the chest demonstrated moderate to severe pneumonia with involvement of lung parenchyma ranging from 11 to 39% (Figure 1A). All patients received hydroxychloroquine and lopinavir/ritonavir (LPV/r) as per local treatment guideline at the time. Conestat alfa was administered a median of 1 day (range 1–7 days) after admission for 48 h and was well-tolerated. No treatment associated serious adverse events were recorded.
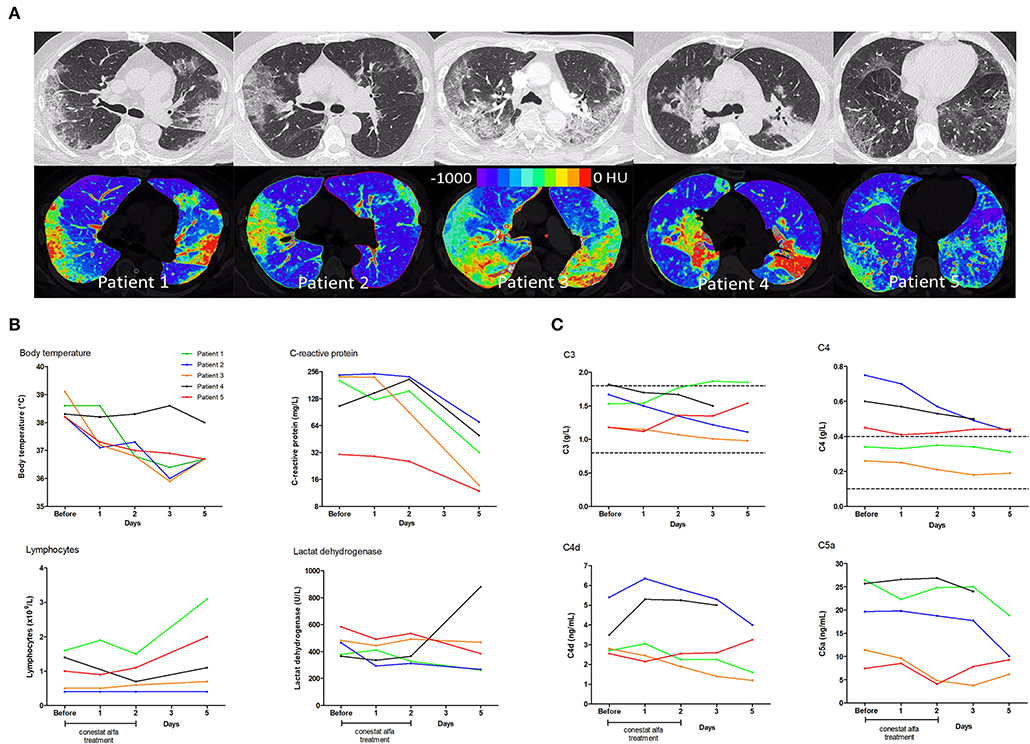
Figure 1. Chest computed tomography scans and temporal changes of laboratory parameters in five patients treated with conestat alfa. (A) Upper row: Original images in lung kernel reconstruction showing ground-glass opacities and consolidations involving multiple segments of both lungs with subpleural predominance. Lower row: color-coded maps from lung density analysis used to quantify the percentage of affected lung volume. (B) Temporal changes of body temperature, plasma C-reactive protein levels, lymphocyte counts and lactate dehydrogenase levels. (C) Temporal changes in concentration of serum complement component C3 and C4, and plasma activation products complement component C4d and C5a. Reference ranges are depicted by dashed lines.
Outcome
Immediate defervescence occurred in all but one patient (patient 4) within 48 h (Figure 1B). Three patients were weaned off oxygen supplementation and discharged early. In patient 3, conestat alfa was administered late during admission (day 7) after rapid progression (pulmonary involvement increased from 7 to 39%). Hence, only 12 h after the start of conestat alfa tocilizumab was also administered. Only 1 patient deteriorated after 48 h of treatment and required mechanical ventilation. Tocilizumab and amoxicillin/clavulanic acid were administered. The patient was extubated after 10 days. All patients were discharged within 3 weeks (range 6–20 days).
Outcome was compared to a matched control population of 15 patients. Baseline characteristics, admission laboratory parameters and treatments administered were similar in both groups (Table 2), except for present smoking [3/5 (60%) of the conestat alfa group and 0/15 (0%) for the matched control population]. However, 8/15 (53%) patients in the control population required mechanical ventilation or died, compared to only 1 (20%) in the conestat alfa group.
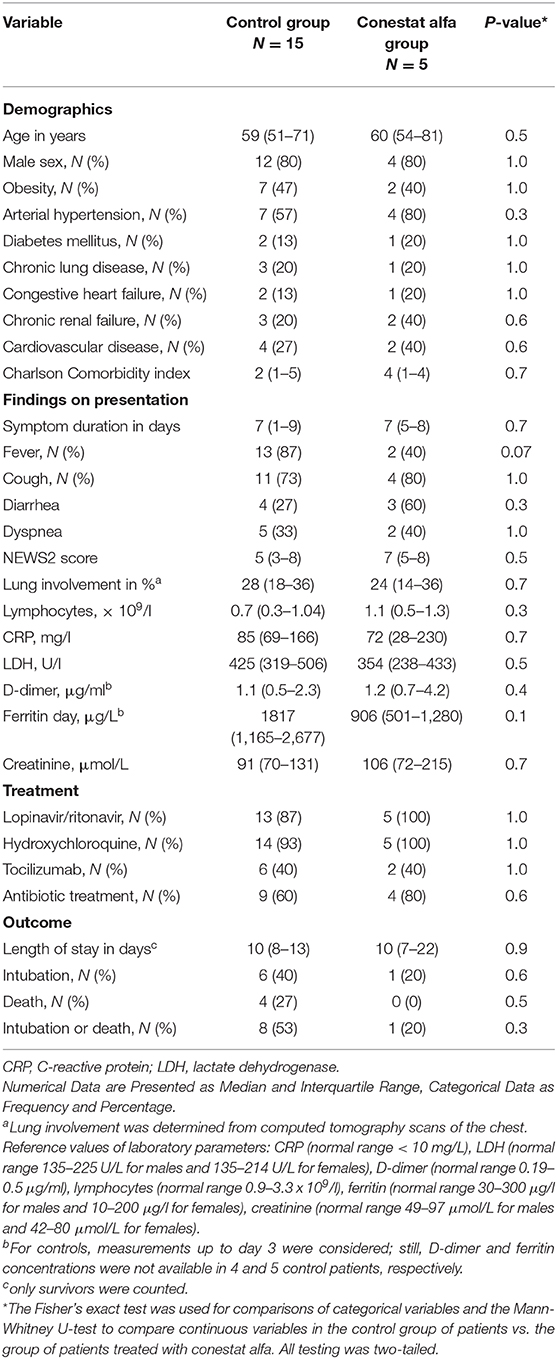
Table 2. Comparison of demographics, clinical features and outcome of 5 SARS-CoV-2 infected patients treated with conestat alfa and a matched control group.
Course of Laboratory Parameters
Inflammatory markers decreased or stabilized in 4/5 patients (Figure 1B). The individual course of inflammatory markers, which were measured inconsistently in the control population is depicted in Figure S1. C1INH concentrations were elevated in all patients before conestat alfa treatment (range, 0.45–0.71 g/L, normal range 0.21–0.39, for individual values see Table 1). Concentration of plasma complement activation products C4d and C5a decreased within 5 days in most patients (Figure 1C). SARS-CoV-2 viral loads in nasopharyngeal swabs declined in 4/5 patients.
Safety Events After Administration of Conestat Alfa
Patient 2 developed acute on chronic renal failure on day 2 attributed to severe diarrhea secondary to COVID-19 infection and LPV/r treatment. After hydration with Ringer's lactate and sodium bicarbonate solution and cessation of LPV/r, renal function returned to almost baseline before discharge. Grade 2 liver injury (alanine transaminase elevation >2.5–5.0x upper limit of normal) was documented in 3 patients, which was attributed to treatment with LPV/r, amoxicillin/clavulanic acid, tocilizumab or paracetamol. Incident asymptomatic deep venous thrombosis was diagnosed on routine ultrasound in one patient (patient 4) after admission to the ICU, and the patient was anticoagulated. Clot resolution was documented on a subsequent ultrasound before ICU discharge.
Discussion
A range of strategies have been proposed and are being evaluated to dampen hyper-inflammation in COVID-19 such as corticosteroid therapies, antibodies against IL-1 or IL-6, treatments interfering with the interferon pathway and anti-tumor necrosis factor therapies (28). In particular, the CS has gained attention as a potential therapeutic target in COVID-19 patients (29, 30), and first experiences targeting C3 and C5 of the complement system have been published (31, 32). However, in this case series, we investigated – to the best of our knowledge – for the first time a strategy of interfering with 3 plasmatic cascades including the CS (i.e., CS, CAS, and KKS) in 5 non-critically ill patients with severe COVID-19. Treatment with conestat alfa over 48 h was well-tolerated. No signal of impaired viral clearance emerged from our case series.
The clinical condition improved in all but 1 patient, reflected by immediate defervescence, improvement in oxygen requirements, and a decline in systemic inflammation and markers of complement activation. Notably, 4/5 patients treated early did not require mechanical ventilation despite the presence of risk factors such as markedly elevated inflammatory proteins. In the majority of patients (4/5) IL-6 levels were markedly elevated (>80 ng/ml) during the disease course, a finding shown to predict respiratory failure and mechanical ventilation, previously (33).
Similar to previous studies in sepsis (20), C1INH protein levels were elevated in COVID-19 patients consistent with an acute phase response. However, elevated C1INH levels may not be sufficient to block ongoing extensive CS, CAS, and KKS activation in COVID-19. An increased amount of modified (cleaved) inactive C1INH in patients with severe sepsis was documented previously, which may indicate a relative C1INH-deficient state (34). Of note, concentration of complement activation products C4d and C5a decreased following conestat alfa administration. Early supplementation of C1INH with conestat alfa is thus a plausible treatment option in selected patients with COVID-19, thereby inhibiting upstream complement proteases and its associated over-activation of the CS in COVID-19 (13, 29).
Limitations
The present study has a number of limitations. First, this was a small case series with a heterogeneous patient population and no controls. Patients may have improved without conestat alfa treatment, although the immediate defervesence and decrease in inflammatory markers is reassuring. Second, sustained complement inhibition may not have been achieved with this current regimen, given the short half-life of conestat alfa and limited treatment duration. Third, all patients were treated with multiple other agents used off-label for the treatment of COVID-19 including LPV/r and hydroxychloroquine.
Conclusion
In this uncontrolled case series of 5 non-critically ill patients with severe COVID-19 pneumonia, administration of conestat alfa over 48 h to inhibit the CS, CAS and KKS was well-tolerated and associated with improvement in the clinical condition of 4 patients. Consequently, we have initiated a randomized controlled trial to investigate this promising approach (ClinicalTrials.gov, number NCT04414631).
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics Committee of Northwest and Central Switzerland (EKNZ 2020-1013). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
SM, BG, MT, and MO designed the study. MO, PU, SM, PC, IH, MR, PS, GS, MT, and SB performed the study, collected, analyzed, and interpreted the data. PU and MO drafted the manuscript. All authors critically revised the manuscript.
Funding
Funded by personal and departmental funds of the project leader (MO); Conestat alfa (Ruconest®, Pharming Biotechnologies B.V., Leiden, The Netherlands) was provided free of charge by the manufacturer.
Conflict of Interest
BG reports being employed by Pharming Group NV. MT reports receiving grants from the Swiss National Science Foundation, Roche, Novartis, and Idorsia outside of the submitted work. MO reports receiving consulting fees from Pharming Biotechnologies B.V. during the conduct of the study and grants from Pharming Biotechnologies B.V. outside the submitted work.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are devoted to the nurses and physicians who cared for the COVID-19 patients during these stressful times.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2020.02072/full#supplementary-material
Figure S1. Temporal changes of body temperature, plasma C-reactive protein levels, lymphocyte counts and lactate dehydrogenase levels in 15 control patients. For patient 9, no C-reactive protein and lactate dehydrogenase levels are available during the admission period. Day 0 denotes the day of admission.
References
1. Giamarellos-Bourboulis EJ, Netea MG, Rovina N, Akinosoglou K, Antoniadou A, Antonakos N, et al. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe. (2020) 27:992–1000.e3. doi: 10.1016/j.chom.2020.04.009
2. Siddiqi HK, Mehra MR. COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal. J Heart Lung Transplant. (2020) 39:405–7. doi: 10.1016/j.healun.2020.03.012
3. Walport MJ. Complement. Second of two parts. N Engl J Med. (2001) 344:1140–4. doi: 10.1056/NEJM200104123441506
4. Walport MJ. Complement. First of two parts. N Engl J Med. (2001) 344:1058–66. doi: 10.1056/NEJM200104053441406
5. Gralinski LE, Sheahan TP, Morrison TE, Menachery VD, Jensen K, Leist SR, et al. Complement activation contributes to severe acute respiratory syndrome coronavirus pathogenesis. mBio. (2018) 9:e01753-18. doi: 10.1128/mBio.01753-18
6. Jiang Y, Zhao G, Song N, Li P, Chen Y, Guo Y, et al. Blockade of the C5a-C5aR axis alleviates lung damage in hDPP4-transgenic mice infected with MERS-CoV. Emerg Microbes Infect. (2018) 7:77. doi: 10.1038/s41426-018-0063-8
7. Sun S, Zhao G, Liu C, Fan W, Zhou X, Zeng L, et al. Treatment with anti-C5a antibody improves the outcome of H7N9 virus infection in African green monkeys. Clin Infect Dis. (2015) 60:586–95. doi: 10.1093/cid/ciu887
8. Sun S, Zhao G, Liu C, Wu X, Guo Y, Yu H, et al. Inhibition of complement activation alleviates acute lung injury induced by highly pathogenic avian influenza H5N1 virus infection. Am J Respir Cell Mol Biol. (2013) 49:221–30. doi: 10.1165/rcmb.2012-0428OC
9. Gao T, Hu M, Zhang X. Highly pathogenic coronavirus N protein aggravates lung injury by MASP-2-mediated complement over-activation. medRxi [Preprint]. (2020). doi: 10.1101/2020.03.29.20041962
10. Heja D, Kocsis A, Dobo J, Szilagyi K, Szasz R, Zavodszky P, et al. Revised mechanism of complement lectin-pathway activation revealing the role of serine protease MASP-1 as the exclusive activator of MASP-2. Proc Natl Acad Sci USA. (2012) 109:10498–503. doi: 10.1073/pnas.1202588109
11. Zhou Y, Lu K, Pfefferle S, Bertram S, Glowacka I, Drosten C, et al. A single asparagine-linked glycosylation site of the severe acute respiratory syndrome coronavirus spike glycoprotein facilitates inhibition by mannose-binding lectin through multiple mechanisms. J Virol. (2010) 84:8753–64. doi: 10.1128/JVI.00554-10
12. Cugno M, Meroni PL, Gualtierotti R, Griffini S, Grovetti E, Torri A, et al. Complement activation in patients with Covid-19: A novel therapeutic target. J Allergy Clin Immunol. (2020)146:215–7. doi: 10.1016/j.jaci.2020.05.006
13. Magro C, Mulvey JJ, Berlin D, Nuovo G, Salvatore S, Harp J, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. (2020) 220:1–13. doi: 10.1016/j.trsl.2020.04.007
14. Sodhi CP, Wohlford-Lenane C, Yamaguchi Y, Prindle T, Fulton WB, Wang S, et al. Attenuation of pulmonary ACE2 activity impairs inactivation of des-Arg(9) bradykinin/BKB1R axis and facilitates LPS-induced neutrophil infiltration. Am J Physiol Lung Cell Mol Physiol. (2018) 314:L17–31. doi: 10.1152/ajplung.00498.2016
15. Helms J, Tacquard C, Severac F, Leonard-Lorant I, Ohana M, Delabranche X, et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Inten Care Med. (2020) 4:1–10. doi: 10.1007/s00134-020-06062-x
16. Poissy J, Goutay J, Caplan M, Parmentier E, Duburcq T, Lassalle F, et al. Pulmonary embolism in COVID-19 patients: awareness of an increased prevalence. Circulation. (2020) 142:184–6. doi: 10.1161/CIRCULATIONAHA.120.047430
17. Carvalho AC, DeMarinis S, Scott CF, Silver LD, Schmaier AH, Colman RW. Activation of the contact system of plasma proteolysis in the adult respiratory distress syndrome. J Lab Clin Med. (1988) 112:270–7.
18. Schmaier AH. The contact activation and kallikrein/kinin systems: pathophysiologic and physiologic activities. J Thromb Haemost. (2016) 14:28–39. doi: 10.1111/jth.13194
19. Menter T, Haslbauer JD, Nienhold R, Savic S, Hopfer H, Deigendesch N, et al. Post-mortem examination of COVID19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings of lungs and other organs suggesting vascular dysfunction. Histopathology. (2020). doi: 10.1111/his.14134. [Epub ahead of print].
20. Igonin AA, Protsenko DN, Galstyan GM, Vlasenko AV, Khachatryan NN, Nekhaev IV, et al. C1-esterase inhibitor infusion increases survival rates for patients with sepsis*. Crit Care Med. (2012) 40:770–7. doi: 10.1097/CCM.0b013e318236edb8
21. Hack CE, Ogilvie AC, Eisele B, Eerenberg AJ, Wagstaff J, Thijs LG. C1-inhibitor substitution therapy in septic shock and in the vascular leak syndrome induced by high doses of interleukin-2. Inten Care Med. (1993) 19(Suppl. 1):S19–28. doi: 10.1007/BF01738946
22. Caliezi C, Zeerleder S, Redondo M, Regli B, Rothen HU, Zurcher-Zenklusen R, et al. C1-inhibitor in patients with severe sepsis and septic shock: beneficial effect on renal dysfunction. Criti Care Med. (2002) 30:1722–8. doi: 10.1097/00003246-200208000-00008
23. Shen B, Yi X, Sun Y, Bi X, Du J, Zhang C, et al. Proteomic and metabolomic characterization of COVID-19 patient sera. Cell. (2020) 182:59–72.e15. doi: 10.1016/j.cell.2020.05.032
24. Thomson TM, Toscano E, Casis E, Paciucci R. C1-INH and the contact system in COVID-19. Br J Haematol. (2020). doi: 10.20944/preprints202005.0262.v1. [Epub ahead of print].
25. van Veen HA, Koiter J, Vogelezang CJ, van Wessel N, van Dam T, Velterop I, et al. Characterization of recombinant human C1 inhibitor secreted in milk of transgenic rabbits. J Biotechnol. (2012) 162:319–26. doi: 10.1016/j.jbiotec.2012.09.005
26. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese center for disease control and prevention. JAMA. (2020). doi: 10.1001/jama.2020.2648. [Epub ahead of print].
27. Colombi D, Bodini FC, Petrini M, Maffi G, Morelli N, Milanese G, et al. Well-aerated lung on admitting chest CT to predict adverse outcome in COVID-19 pneumonia. Radiology. (2020) 2020:201433. doi: 10.1148/radiol.2020201433
28. Ye Q, Wang B, Mao J. The pathogenesis and treatment of the ‘cytokine Storm' in COVID-19. J Infect. (2020) 80:607–13. doi: 10.1016/j.jinf.2020.03.037
29. Risitano AM, Mastellos DC, Huber-Lang M, Yancopoulou D, Garlanda C, Ciceri F, et al. Complement as a target in COVID-19? Nat Rev Immunol. (2020) 20:343–4. doi: 10.1038/s41577-020-0320-7
30. Campbell CM, Kahwash R. Will complement inhibition be the new target in treating COVID-19 related systemic thrombosis? Circulation. (2020) 141:1739–41. doi: 10.1161/CIRCULATIONAHA.120.047419
31. Mastaglio S, Ruggeri A, Risitano AM, Angelillo P, Yancopoulou D, Mastellos DC, et al. The first case of COVID-19 treated with the complement C3 inhibitor AMY-101. Clin Immunol. (2020) 215:108450. doi: 10.1016/j.clim.2020.108450
32. Diurno F, Numis FG, Porta G, Cirillo F, Maddaluno S, Ragozzino A, et al. Eculizumab treatment in patients with COVID-19: preliminary results from real life ASL Napoli 2 Nord experience. Eur Rev Med Pharmacol Sci. (2020) 24:4040–7. doi: 10.26355/eurrev_202004_20875
33. Herold T, Jurinovic V, Arnreich C, Lipworth BJ, Hellmuth JC, Bergwelt-Baildon MV, et al. Elevated levels of IL-6 and CRP predict the need for mechanical ventilation in COVID-19. J Allergy Clin Immunol. (2020) 146:128–36.e4. doi: 10.1016/j.jaci.2020.05.008
Keywords: COVID-19, C1 esterase inhibitor, SARS-CoV-2, inflammation, complement system, kallikrein-kinin system, contact activation system
Citation: Urwyler P, Moser S, Charitos P, Heijnen IAFM, Rudin M, Sommer G, Giannetti BM, Bassetti S, Sendi P, Trendelenburg M and Osthoff M (2020) Treatment of COVID-19 With Conestat Alfa, a Regulator of the Complement, Contact Activation and Kallikrein-Kinin System. Front. Immunol. 11:2072. doi: 10.3389/fimmu.2020.02072
Received: 17 June 2020; Accepted: 30 July 2020;
Published: 14 August 2020.
Edited by:
Marcin Okrój, Intercollegiate Faculty of Biotechnology of University of Gdaǹsk and Medical University of Gdaǹsk, PolandReviewed by:
Timothy M. Thomson, Consejo Superior de Investigaciones Científicas (CSIC), SpainZoltan Prohaszka, Semmelweis University, Hungary
Copyright © 2020 Urwyler, Moser, Charitos, Heijnen, Rudin, Sommer, Giannetti, Bassetti, Sendi, Trendelenburg and Osthoff. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michael Osthoff, bWljaGFlbC5vc3Rob2ZmQHVzYi5jaA==
†These authors have contributed equally to this work
‡ORCID: Michael Osthoff orcid.org/0000-0001-5439-957X
 Pascal Urwyler
Pascal Urwyler Stephan Moser
Stephan Moser Panteleimon Charitos
Panteleimon Charitos Ingmar A. F. M. Heijnen2
Ingmar A. F. M. Heijnen2 Parham Sendi
Parham Sendi Marten Trendelenburg
Marten Trendelenburg Michael Osthoff
Michael Osthoff