- 1Department of Endocrinology, Zhejiang Provincial People's Hospital, People's Hospital of Hangzhou Medical College, Hangzhou, China
- 2Department of Cardiology, Second Affiliated Hospital, Zhejiang University, Hangzhou, China
- 3Key Laboratory of Tumour Microenvironment and Immune Therapy of Zhejiang Province, Second Affiliated Hospital, Zhejiang University, Hangzhou, China
- 4Department of Breast Surgery, Second Affiliated Hospital, Zhejiang University, Hangzhou, China
- 5School of Medicine, Chu Kochen Honors College, Zhejiang University, Hangzhou, China
- 6Department of Anatomy, School of Medicine, Zhejiang University, Hangzhou, China
Increasing evidence has revealed that the initiation and progression of breast cancer are greatly affected by the immune environment. Neutrophils are the most abundant leucocytes in circulation and act as the spearhead in inflammation, including in breast cancer. Circulating neutrophils are closely related to the prognosis of breast cancer patients, and tumor-infiltrating neutrophils have varied functions at different stages of breast cancer, such as antitumor or tumor-promoting neutrophils, which are termed N1 and N2 neutrophils, respectively. In this review, we will discuss the utility of circulating neutrophils for predicting prognosis and therapeutic efficacy and the underlying mechanisms of their chemotaxis, the dynamic regulation of their antitumor or protumor functions and their different spatial distributions in tumor microenvironment. Finally, we also discuss the possibility of targeting neutrophils as a therapeutic strategy in breast cancer.
Introduction
Breast cancer (BC) is the most common malignancy in women worldwide (1). Although BC is classified as a malignant disease with low immunogenicity, recent evidence has revealed a promising outcome of therapies with blocking immune checkpoints in both early and advanced stages (2–4). The efficacy of immunotherapy is closely related to the tumor immune microenvironment, especially to infiltrating immune cells (5). To date, macrophages and T cells are the most well-studied immune cells in BC, whereas increasing evidence has indicated that neutrophils are also key in the oncogenesis and metastasis of BC; in addition, circulating neutrophils have been reported to have great prognostic prediction value (6). Neutrophils are the most abundant leucocytes in blood and usually act as the first line of host defense against pathogens (7). However, due to their short life span (an average of 6–8 h in blood) (8), it is difficult to employ this subset of cells for experiments, which has resulted in a poor understanding of their role in solid tumors. In addition, some contradictory results reported in vitro studies or animal experiments have suggested a dual effect of neutrophils in tumor development.
Neutrophils can present both antitumorigenic (“N1”) and protumorigenic (“N2”) phenotypes in various cancers or specific circumstances. The term neutrophil in several studies also includes both mature neutrophils and myeloid-derived suppressor cells (MDSCs). MDSCs are described as a subset of neutrophils with immunosuppressive functions that express CD11b and Gr1 (9, 10) and can be divided into monocytic (M) (CD11b+/Ly6C+) MDSCs and G/PMN (CD11b+/Ly6G+) MDSCs (11), and G/PMN MDSCs usually share a common set of markers and similar morphological features with neutrophils (9).
To avoid confusion, we mainly focus on the biological function of mature neutrophils and related therapeutic strategies for targeting them in BC. We provide a comprehensive review of the prognostic value of circulating neutrophils and the mechanisms of how tumor-associated neutrophils (TANs) exert antitumor or tumor-promoting functions in BC, and in the end, we also discuss the potential of targeting neutrophils as a therapeutic strategy in cancer.
Prognostic Value of the Neutrophil-to-Lymphocyte Ratio (NLR)
Tumors can be thought of as wounds that will not heal and are characterized by chronic inflammation. Neutrophils are the most rapidly responding immune cells to inflammation, and many studies have found that the NLR is closely related to the prognosis and treatment response in patients bearing BC (12, 13). A recent meta-analysis of 39 studies, including 17,079 patients with both early and advanced BC, revealed that patients with a higher NLR before treatment had poorer disease-free survival (DFS) than those with a lower NLR before treatment, but the NLR was not related to overall survival (OS); the subgroup analysis found that the NLR was associated with prognosis only in early-stage patients but not in patients with metastasis (14). Since similar meta-analyses were not based on individual patient data, which may cause significant bias, we reviewed and compared the individual reports and found some issues worth discussing here. Widmann et al. first reported the correlation between the NLR and BC prognosis in 316 patients, and it was found that a higher NLR (≥3.3) before treatment was an adverse factor for both short- and long-term mortality (15). The majority of retrospective studies thereafter have drawn similar conclusions (16–19), and the NLR was found to be consistent among different BC subtypes at baseline (20, 21). However, a prospective substudy of GEICAM/9906, which comprised 1,246 patients, did not find any prognostic value of the NLR after adjustment for clinicopathological factors; in addition, a high NLR was independently associated with worse DFS in only high-risk patients (the hormone receptor-negative/HER2+ population and in patients with ≥3 lymph node metastases) (22). Another study with 247 early BC patients also found that the NLR before surgery was not associated with DFS (23), indicating that the presurgery NLR may be valuable only in patients with a high tumor burden.
In addition to the above studies, several studies also explored the prognostic value of the NLR posttreatment or with continuous assessment. A retrospective study comparing the absolute lymphocyte count (ALC) and the NLR eight consecutive times before and after chemotherapy found that patients who died had lower ALC and higher NLR values than patients who remained alive throughout the treatment course; additionally, among the patients who died, a steady increase in the NLR over the baseline measurement was observed at subsequent time points (24). Another retrospective study included 330 BC patients with DFS values of more than 5 years, and it interestingly found that NLR sampled during follow-up rather than before any treatment was an independent prognostic factor for late recurrence (21). However, there is still no compelling explanation for the abovementioned inconsistent results. In addition, since lymphocytes are critical in cancer immune surveillance and neutrophils have been reported to play a protumor role in most studies, low lymphocytes and high neutrophils in circulation may also suggest immunosuppression status (10), and studies focused on the relationship between neoadjuvant chemotherapy (NCT) and the NLR might support the above hypothesis. A comprehensive review of the existing reports shows that most studies have found that a low NLR indicates a higher NCT response and pathological complete response (pCR) rate (25–27); in addition, the NLR has showed predictive value not only in all molecular types of BC but also in both operable and locally advanced BC (18, 28, 29). Interestingly, although Suppan et al. did not find a significant correlation between the initial NLR and prognosis, the same cohort revealed a low NLR as a significant parameter for predicting chemotherapy response (p = 0.012) (23). A low NLR was also reported to be associated with a higher response rate to primary endocrine therapy for locally advanced or metastatic BC (30, 31).
Although increasing evidence suggests a close association between the NLR and prognosis in BC, several issues remain that make clinical application difficult. One of the most important reasons is the lack of a consensus cut-off value. As we list here (Table 1), the cut-off values for the NLR in the published studies were between 2 and 4. In addition, based on individual studies, the sensitivity of the NLR fluctuates greatly (50–94.1%), and the specificity is much lower (26.5–51.6%) (18, 29, 49). Therefore, some researchers have tried to determine a better alternative parameter. In addition to the NLR, the platelet-to-lymphocyte (PLR) ratio has also been investigated and compared with the NLR in BC. A single central retrospective study with 434 hormone receptor-negative non-metastatic BC patients reported that both elevated NLR and PLR were associated with poor OS; however, the multivariate analysis revealed that only the NLR (p < 0.001) but not the PLR (p = 0.104) was a significant indicator for both DFS and OS (50). Additionally, since the absolute lymphocyte count has also been reported as a prognostic factor, the predictive values of the PLR and NLR were evaluated after adjusting for the total lymphocyte count. The results showed that the PLR was no longer a significant predictor for 5-year mortality, and the NLR remained a significant predictor irrespective of the lymphocyte count (51). Furthermore, it was revealed that the combination of the NLR and PLR could further improve the predictive value. Two retrospective studies found that the highest rate of pCR (32%) was in the group of patients with an NLRlow/PLRlow profile, and the lowest rate (19%) was in the group with an NLRhigh/PLRhigh profile (18); in addition, when the cut-off values for the NLR and PLR were applied, the specificity of predicting a pCR increased from 38 to 52% (49).
However, the causal relationship between the NLR and poor prognosis in malignant disease has yet to be illuminated. According to an assessment with paired peripheral blood and pancreatic cancer specimens, Takakura et al. found that a high NLR was associated with increased tumor-associated macrophages (TAMs) and decreased tumor-associated lymphocytes but was not significantly related to CD66b+ infiltrating neutrophils (52). Therefore, it seems that an increase in neutrophils in peripheral blood is not necessarily related to the number of TANs. Several basic studies have suggested a unique mechanism of the pro-tumor function of circulating neutrophils: protecting circulating tumor cells (CTCs). Circulating neutrophils can cluster around tumor cells and induce tumor cell aggregation, aiding tumor cell survival by hiding them from immune surveillance (53). Neutrophil extracellular traps (NETs) are webs of decondensed chromatin fibers conjugated together with histones, myeloperoxidase (MPO), elastase, and other cytoplasmic proteins (54). Recent studies also found that neutrophils could form many NETs both in circulation and in tumor lesions and could coordinate with platelets to capture CTCs and facilitate cancer metastasis (55). In addition, neutropenia is very common in cancer patients undergoing chemotherapy, and supportive treatment with granulocyte colony-stimulating factor (G-CSF) can induce a neutrophilic response; as a consequence, neutrophils are primed toward a pro-NETotic phenotype and may suppress the cytotoxic activity of T cells as well as impair immune surveillance (24, 56, 57). On the other hand, lymphocytes have the propensity to mount an adaptive antitumor response in malignant disease (58), and decreased lymphocyte numbers are considered to be related to an insufficient immunologic reaction, which may increase the risk of tumor relapse or metastasis (59). Clearly, a general association between prognosis and the NLR exists in BC, but large prospective studies and rigorous research are still required to determine its clinical significance.
Mechanism of Neutrophil Chemotaxis to the Tumor Microenvironment
Neutrophils are considered the main immune cells that provide protection against invading pathogens, which can be induced by trauma, infection, and malignant disease (60). The recruitment of neutrophils is greatly dependent on certain chemokines, including interleukin (IL)-8 (also known as CXCL-8), CXCL-1, and CXCL-2 (61). IL-8 is a proinflammatory cytokine and acknowledged as the most important chemoattractant for neutrophils in the tumor microenvironment (62). IL-8 mainly comes from endothelial cells (ECs) and monocytes in the tumor microenvironment upon certain stimulation, such as physical injury, hypoxia, chemotherapy or radiotherapy, and other cell types, including fibroblasts and keratinocytes, can secrete IL-8 as well (63, 64). In addition to its chemotactic effect, it was revealed that IL-8 could provoke neutrophils to release NETs to assist cancer cell migration (5). By live-cell fluorescence microscopy, Gupta et al. confirmed that activated ECs could induce NETosis characterized by typical extracellular DNA lattices when cocultured with polymorphonuclear neutrophils (PMNs) and activated ECs (65). In addition, activated ECs produce other inflammatory cytokines, such as P-selectin, E-selectin, and intercellular adhesion molecule 1 (ICAM-1), to facilitate neutrophil adhesion to ECs and migration (66). Furthermore, tumor-promoting neutrophils in BC cells are also characterized by high expression of matrix metalloproteinases-9 (MMP-9) (67, 68), which was found to cleave CXCL-5, potentiating its action in neutrophil recruitment as a positive feedback function in tumors (15, 69). IL-17 was also found to control neutrophil recruitment in lung metastasis of BC in a mouse model: CD3+CD4+ and γδ T cells were the major sources of IL-17 (70, 71), and it was interesting to find that the absence of γδ T cells or neutrophils markedly reduced pulmonary and lymph node metastases without influencing primary tumor progression, which suggested a collaborative relationship between γδ T cells and neutrophils in promoting BC lung metastasis. However, in an orthotopic hepatocellular carcinoma model, Sofia et al. reported that TANs exert an overt antitumor role by suppressing γδ T17 cells via reactive oxygen species (ROS) (72), contrary to the phenomenon that within the 4T1-derived BC model, CD11b+/Ly-6G+ neutrophils that infiltrate and surround liver metastases were found to be tumor promoting (73). These controversial results suggest both promoting and suppressive roles of TANs in different circumstances.
High-mobility group box 1 (HMGB1) usually acts as a damage-associated molecular pattern that is released by dying cells or stressed cells to initiate inflammation and was later found to be an important chemoattractant for neutrophils (74). Epithelial cell-derived HMGB1 was found to recruit neutrophils to the necrotic site through its receptor RAGE (75). Enrichment of platelets has been reported in the microenvironment of multiple cancers, including BC (76), and infiltrating platelets could be activated by the large amounts of adenosine phosphate released by necrotic cells as a result of chemotherapy (77). Activated platelet-derived HMGB1, known as the major mediator of injury-induced thrombosis in vivo (74), can also stimulate NETosis through Toll-like receptor 4 (TLR4) and RAGE on neutrophils, and as a positive feedback mechanism, released NETs strongly induce a prothrombotic state and activate platelets (78). Meanwhile, tumor cell-derived exosomal HMGB1 was also found to activate neutrophils through the TLR4/NF-κB pathway, which promotes its survival by increasing the autophagic response and polarizing TANs to a protumor type (79). It is noteworthy that various reports imply the core position of the NF-κB pathway in the activation and recruitment of neutrophils (80, 81). In addition to HMGB1, tumor cells, including BC cells, have been reported to secrete other peptides, such as a2 isoform V-ATPase (a2V), to activate the NF-κB pathway in neutrophils, thereby promoting their recruitment and inhibiting their apoptosis (82, 83). Additionally, breast involution after weaning is characterized by acute inflammation and an increase in estrogen. It was found that estrogen could induce the mammary infiltration of neutrophils and upregulate the expression of protumor cytokines/chemokines, such as COX-2 and MMPs, in mammary infiltrating neutrophils (84).
In addition, similar to lymphocytes and macrophages, neutrophils are more likely to localize in tumors of triple-negative breast cancer (TNBC) than to tumors of other BC subtypes (85). Recently, Zhang et al. identified neutrophils and macrophages as the most frequent infiltrating immune cells in various BC murine models, and BC could be classified into a macrophage-enriched subtype (MES) and a neutrophil-enriched subtype (NES). It was interesting to find that there were only a few neutrophils in the MES but a large number of macrophages in the NES (57). This mutual repelling phenomenon in the MES and NES may result in spatial segregation within the same tumor. The authors speculated that a possible mechanism could be the factors derived from macrophages that inhibit the IL-8-dependent chemotaxis of neutrophils (86).
Antitumor Function of TANs in BC
The polarization of neutrophils can be differentially regulated in the tumor microenvironment. In a mouse model, Fridlender et al. found that TANs from the early tumor stage were like tumor-killing cells, which produce high levels of hydrogen peroxide (H2O2), tumor necrosis factor (TNF)-α and NO, and that TANs are more likely to obtain a protumorigenic phenotype with tumor progression (87). Although few studies have directly compared the phenotype and function of TANs between early- and late-stage tumors, there are still some clues to support this hypothesis. A phenotypical and functional analysis of TANs in early-stage lung cancer found an activated phenotype (CD62lowCD54high) that was able to stimulate T cell proliferation and IFN-γ release, which suggested a pro-inflammatory rather than immunosuppressive state of TANs in early-stage lung cancer (88). MPO is an enzyme characteristic of mature “N1” type neutrophils, which are able to convert H2O2 to cytotoxic hypochlorous acid (HOCI) (87, 89). Recently, a retrospective study of 928 BC cases revealed that MPO-positive neutrophils (defined as ≥5 cells/tissue punch) were found in 16% of evaluable cases, while the luminal (ER/PR+ and Her2-), Her2-enriched and triple-negative types had positive rates of 13, 29.7, and 26.4%, respectively, in addition, in univariate analyses, infiltration by MPO-positive neutrophils was a significant independent favorable indicator for both OS and DFS. Notably, almost all of the patients included in this study had early-stage disease (T1-2 72%, N0-1 89%), and the data suggested that MPO-positive neutrophils were much more abundant in BC cases with low T and N stages than in advanced cases (90).
In addition, a direct tumor killing function of neutrophils has also been reported. One of the classical factors working against tumor cells is ROS. Recent research in mouse BC models revealed that ROS-mediated cell lysis was dependent on Ca2+ channels and mediated by transient receptor potential cation channel, subfamily M, member 2 (TRPM2) expression on tumor cells (91). Although TCGA analysis revealed a high expression of TRPM2 in BC cells (http://gepia2.cancer-pku.cn/#index), active NOX1, catalase and SOD were also increased in the membrane of cancer cells, forming a complex mechanism by which tumor cell apoptosis induced by ROS is prevented (92). In addition, tumor cells are characterized by enhanced metabolic activity and high levels of intracellular ROS (93), which indicates that direct cytotoxic effects of neutrophil-produced ROS are not sufficient. In addition to the direct cytotoxic effect, TANs containing ROS have been found to strongly suppress IL-17-producing γδ T cells (72), which are critical for shaping the immune suppressive microenvironment in various solid tumors (94–96), and have also been reported to promote BC cell extravasation and metastasis (71). In addition, neutrophils could also express Fc receptors and exert antibody-dependent cellular cytotoxicity (ADCC) effects similar to those of T cells and macrophages, leading to a trogocytosis effect to destroy cancer cells (97). However, some studies have indicated that neutrophils are more likely to be distributed at the periphery of tumors at the initiation stage (85, 87), which may make controlling tumor growth with these cell-cell contact-dependent mechanisms ineffective.
Protumor Effects of TANs
More studies suggest that neutrophils facilitate tumor promotion and metastasis in BC than antitumor effect. Overexpression of the chemokines CCL2 and CCL17 is a recognized feature of N2 neutrophils. Richmond et al. (98) found that exogenous CCL2 enhances the killing effect of neutrophils against BC cells in vitro, while this antitumor activity was not observed in vivo. Instead, intranasal delivery of CCL2 to BALB/c mice markedly enhanced lung metastasis of BC cells and increased the recruitment of CD4+ T cells and CD8+ central memory T cells. CCL17 secretion from TANs was found to support tumor growth by recruiting CD4+ Treg cells and macrophages (99). In addition to recruiting immune-suppressive cells, TANs were reported to promote the accumulation of BC cells in the lung and directly inhibit natural killer (NK) cell-mediated clearance of tumor cells (100). Human NK cells can be divided into CD56dim (antitumor) and CD56bright (protumor) subsets, and CD56bright NK cells are enriched in the tumor microenvironment and draining lymph nodes (101, 102). Early reports revealed that ROS and arginase-1 from neutrophils impair the maturation and cytotoxic function of NK cells (103), but CD56brightCD16− NK cell are resistant to neutrophil-derived ROS, perhaps due to their high antioxidative capacity (104). Meanwhile, NK cells could be recruited by TANs via CCL2 and CCL5, which may explain the preferential accumulation of CD56bright NK cells in tumor microenvironments with high ROS levels (105).
Extracellular arginine is crucial to signal local CD8+ cells and increase their CD3ζ expression, which is key for T cells to survey antigens presented on MHC class I molecules, and it was also found to be necessary for T cell activation and survival (106). Tumor cell-derived IL-8 could lead to TAN degranulation, resulting in arginase-1 release and conversion of extracellular arginine to ornithine and urea, thereby dampening the survival and cytotoxic effect of CD8+ T cells (53, 107, 108). Neutrophil elastase (NE) is also released by TANs and can be endocytosed by tumor cells via neuropilin-1 (NRP1); this results in the cross-presentation of PR1, which is an NE-derived HLA-A2-restricted peptide that may be an immunotherapeutic target (109). Besides, upon endocytosis, NE is to bind insulin receptor substrate-1 (IRS-1), which removes the inhibitory effect of IRS-1 on phosphatidylinositol 3-kinase (PI3K) to enhance the proliferation of cancer cells (110).
Recent reports highlighted the leukocytes, especially neutrophils preferentially uptake tumor derived extracellular vesicles, or named exosomes (111). Hypercoagulability is one of the important characteristics of malignant tumors, and has been reported associated with NETs. Breast cancer cell 4T1-derived exosomes induced NETs formation in neutrophils, besides, tumor-derived exosomes also interacted with NETs to significantly accelerate venous thrombosis in vivo (112). Furthermore, several reports also indicated the cancer derived exosomes prolonged lifespan of neutrophils, and also polarized neutrophils toward pro-tumor type (79, 113).
In addition to direct modulation of the protumor microenvironment, increasing evidence has found that neutrophils promote tumor cell migration and the formation of a metastatic niche (6, 13, 114). Tumor angiogenesis is regarded as a prerequisite for tumor metastasis, and TANs have been recognized as an important source of vascular endothelial growth factor (VEGF) upon specific stimulation in the tumor microenvironment (115, 116). Neutrophils were also found to be one of the main sources of MMP-9 (117), and the link between MMP-9 and VEGF has been reported previously. The absence of MMP-9 has been reported to have a similar function as the inhibition of VEGF signaling, indicating that MMP-9 serves as an angiogenic switch during tumorigenesis by inducing VEGF release from the matrix (117–119). In addition, Gabriele et al. also found that MMP-9 was expressed by a small number of cells in close proximity to the vasculature, such as infiltrating inflammatory cells, rather than tumor cells (118). In addition, several serine proteases are also produced by TANs, such as NE, cathepsin G and proteinase-3, which have been reported to activate MMP-2 to promote tumor invasion and proliferation (120, 121). In addition, although neutrophils were reported to produce little tissue inhibitor of matrix metalloprotease (TIMP-1), Wang et al. observed that BC cells with CD90-positive expression could induce the TIMP-1 secretion by TANs, and as a reciprocal effect, TIMP-1 induced EMT and metastasis in BC (122). Other neutrophil-derived cytokines such as IL-1β, IL-6, and IL-17α have been reported to initiate EMT of cancer cells by activating JAK2/STAT3 and ERK signaling (123, 124).
In addition to modulating the primary tumor microenvironment, neutrophils can also assist the formation of the cancer premetastatic niche in distant organs. CTCs are precursors for metastatic lesion formation; intravascular NETs were found to protect CTCs from attack by circulating immune cells; and dysregulated NETs were found to induce inflammatory vascular injury, EC shrinkage and tissue damage (53, 125–127). Moreover, in vitro and in vivo experiments found that activated neutrophils promote the adherence of CTCs to ECs and facilitate their lung and liver metastasis (128). Recently, Aceto et al. provided strong evidence that neutrophils escort CTCs in BC to assist metastasis (129). With detection of cell surface markers and Wright Giemsa staining, they identified that most CTC-associated white blood cells were N2-like neutrophils. In addition, single-cell RNA sequencing revealed higher Ki-67 expression in disseminated tumor cells from CTC neutrophil clusters than in standalone CTCs. In the same study, TNF-α, oncostatin M, IL-1β, and IL-6 were frequently expressed by CTC-associated neutrophils and matched by the receptors on corresponding CTCs; on the other hand, CTCs from the CTC neutrophil clusters expressed high gene levels encoding G-CSF, transforming growth factor (TGF)-β3 and IL-15, which have been reported to activate neutrophils (130–132), illuminating a mechanism of neutrophil-CTC cluster formation.
In addition to escorting CTCs in circulation, several studies have found that neutrophil accumulation is a prerequisite for cancer metastasis. For both orthotopic transplantation and spontaneous BC models, neutrophils were suggested to accumulate in the distant organ before cancer cells infiltration (6, 133). Obesity and elevated cholesterol are risk factors for BC development and poor prognosis (134, 135). Interestingly, 27-hydroxycholesterol (27HC) increased the number of polymorphonuclear-neutrophils and γδ T cells at distal metastatic sites, and neutrophils were required for the metastatic effects of 27HC (136). Egeblad et al. (137) developed a confocal intravital lung imaging system and found that NETs were formed early in the lung and continued to form for the next few days after tail vein injection of BC cells. In addition, based on immunofluorescence staining of human primary BC and matched metastatic lung lesions, they found that the abundance of NETs was highest in TNBC, but NETs were absent or very rare in luminal BC samples, which may explain the higher metastatic ability of TNBCs than luminal BCs. In ovarian cancer, an influx of neutrophils in the omentum was also observed before metastasis, and blockade of NET formation with peptidyl arginine deiminase 4 (PAD4), an enzyme that is essential for NET formation, could decease omental colonization of cancer cells (133). In addition to supporting colonization of cancer cells, lung-infiltrating neutrophils has also been reported to directly promote cancer proliferation via release of high levels of S100A8, S100A9, Bv8, MMP-9 and the lipid leukotriene B4, which stimulate the migration and proliferation of BC cells, and activate the MAPK/Erk pathway in BC cells to potentiate their tumorigenic capacity (6, 138). Interestingly, BC can remain dormant and clinically undetectable before late recurrence decades later, and it has been reported that inflammation induced by stimuli such as lipopolysaccharide or smoking triggers neutrophils to accumulate and NET formation, which can cause tumor recurrence by activating the integrin and FAK/ERK/MLCK/YAP signaling pathways to awaken dormant tumor cells (139). Overall, evidence is mounting that neutrophils play a significant detrimental role in every step of cancer metastasis.
Spatial Distribution and Various Cluster of TANs
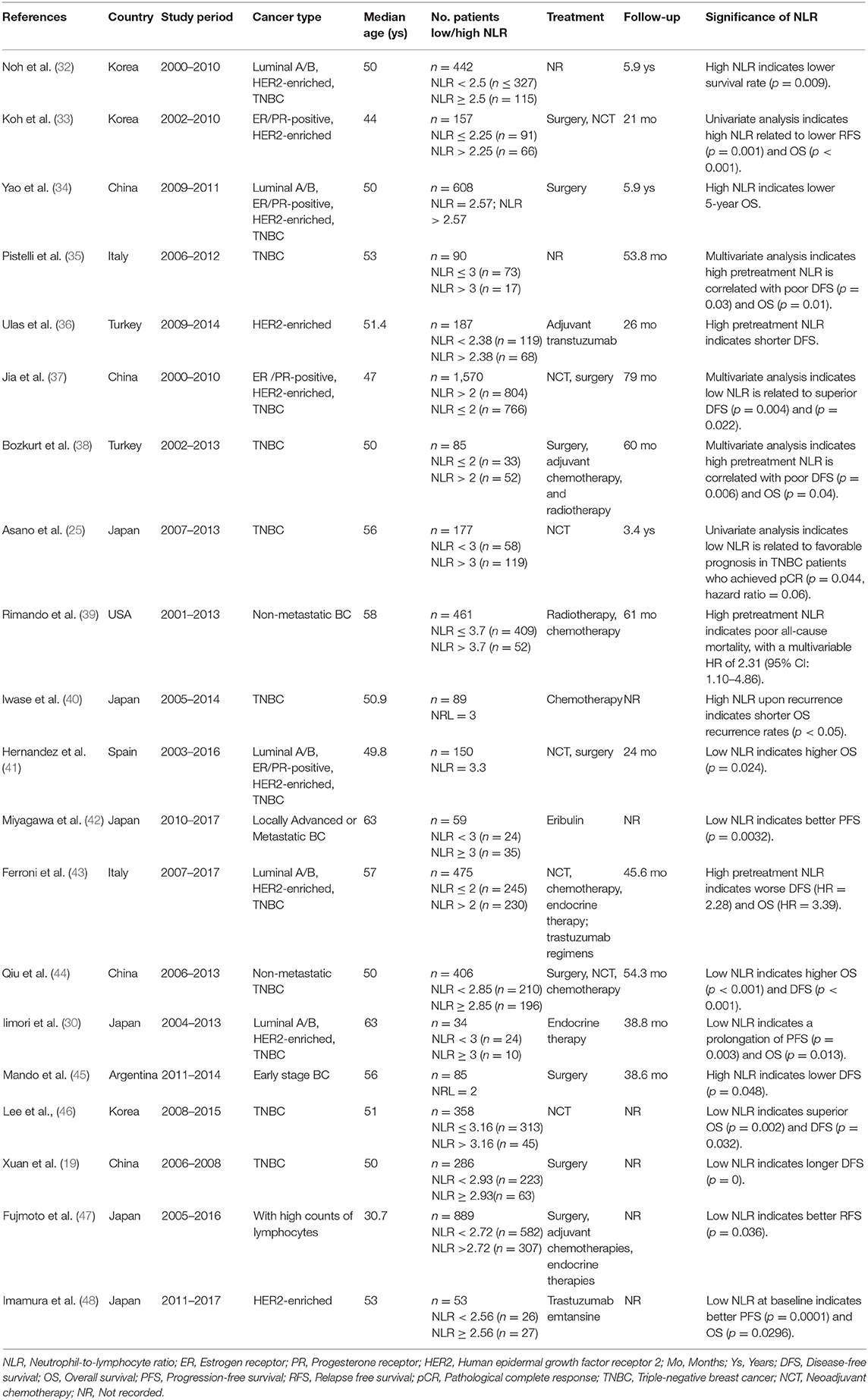
Several studies have suggested that the spatial distribution of TANs is different between early- and advanced-stage cancers, which is related to the biological function of TANs (antitumor or protumor functions) (Figure 1). A mouse model of lung carcinoma and mesothelioma revealed that TANs were scattered around the periphery of the tumor site in the early stage, while neutrophils were more distributed among the tumor cells in the late stage (87). Another retrospective study of BC defined TANs as neutrophils in direct contact with carcinoma cells and showed that 47.7% of cases were TAN positive, but the frequency of cancer cell contacting-TANs was much higher in advanced-stage cases than in early-stage cases (85), which also indicates that neutrophils are dynamically modulated by the tumor microenvironment both in phenotype and spatial position. Recently, Wang et al. evaluated the association between parenchymal and stromal neutrophil counts and clinical outcomes with their own BC datasets and found that neutrophils in the tumor parenchyma, rather than those in the stroma, were an independent poor prognostic factor (122).
Figure 1. Schematic diagram of spatial distribution and functional of TANs in early and late stage breast cancer. As a quick response, tumor associated neutrophils (TANs) are scattered around the periphery of the tumor site in early stage, and exert tumor inhibition function; with tumor progression, the TANs are more likely to distributed among and direct contact with tumor cells, and function as tumor promoting cells via shaping immune suppressive microenvironment, enhancing angiogenesis, and caner metastasis.
Because of the lack of direct information from previous publications, we tried to determine the abundance and subtype of TANs in human BC via the CIBERSORT-LM7 deconvolution algorithm based on mRNA expression datasets (GSE6532, GSE9195, GSE16446, GSE17907, GSE19615, GSE20685, GSE20713, GSE21563, GSE31448, GSE42568, GSE48390, and GSE58984) (140). Our analysis indicated that the proportion of neutrophils was significantly higher in BC cases with a higher grade and of the luminal B, TNBC and HER2+ subtypes but was not associated with tumor size or axillary lymph node metastasis (Figures 2A–D). Recently, Klein et al. (142) used single-cell RNA sequencing (scRNA-seq) to map tumor-infiltrating myeloid cells in non-small-cell lung cancer patients and revealed that tumor-infiltrating neutrophils (TINs) could be clustered into five subsets (hN1-hN5). hN1 cells were characterized by high expression of Arginase-1, MMP9/8, S100A8 and S100A9, and ADAM8. As we discussed in the previous section, almost of these genes play a tumor-promoting role in BC. Another earlier research focused on immune microenvironment also profiled BC infiltrating 45,000 immune cells with scRNA-seq, and identified neutrophils in half of the patients. However, the neutrophils and mast cells were excluded in analysis due to their great heterogeneity (143). In addition, Wagner et al. (141) performed a single-cell analysis to map the microenvironment of BC using mass cytometry, and found the abundance of neutrophils (also termed as granulocytes) significant higher in juxta-tumoral tissue than tumor, and it is noted that nearly 90% of the included patients were early stage (IA-IIB) and luminal subtype. Since the CIBERSORT and scRNA-seq analysis are both based on transcriptome level, here we extracted the original data of Wagner's study to evaluate the neutrophil distribution stratified with different pathological features again (141). The results confirmed the frequency of TANs were greater in tumor with larger size and higher grade, but not associated with lymph nodes metastasis (Figures 2E–H); besides, we also compared the relative proportion of neutrophils between juxta-tumoral and tumor tissues among different tumor size, the negative results (Figure 2H) suggested that the increase of neutrophils infiltration in tumor may be a continuous chemotactic process from para-tumoral tissue toward the tumor. More rigorous experiments are needed in the future to delineate the dynamic changes in neutrophil function during this process.
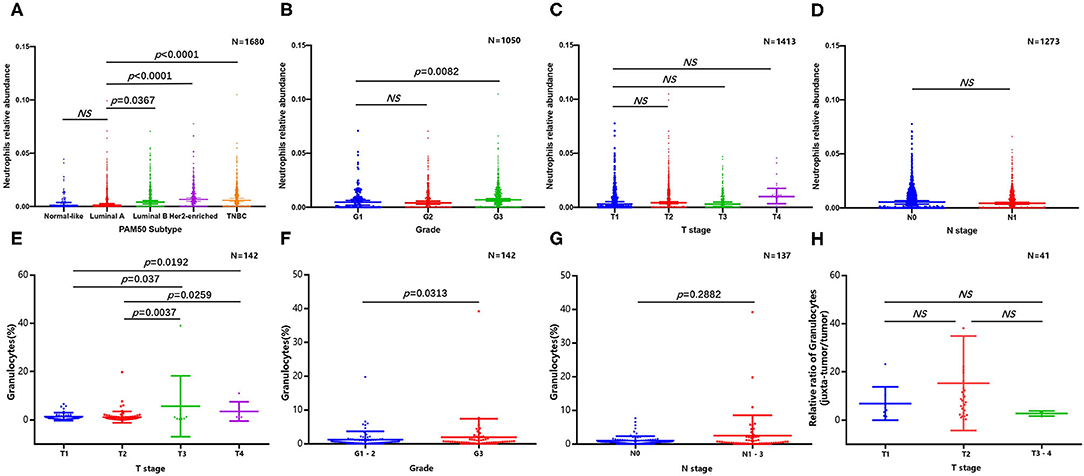
Figure 2. Abundance of tumor infiltrating neutrophils in breast cancer. CIBERSORT algorithm analysis was performed based on mRNA expression datasets (GSE6532, GSE9195, GSE16446, GSE17907, GSE19615, GSE20685, GSE20713, GSE21563, GSE31448, GSE42568, GSE48390, and GSE58984). Comparison of breast cancer infiltrating neutrophils with different molecular subtype (A), grade (B), tumor size (C), and lymph nodes metastasis (D); original data based on mass cytometry (141) was extracted and re-analyzed to evaluate the abundance of neutrophil stratified with different tumor size (E), grade (F), and lymph nodes metastasis (G), relative proportion of neutrophils between juxta-tumoral and tumor tissues among different tumor size was also presented (H).
The Impact of Chemotherapy and Radiotherapy on TANs
Chemotherapy and radiotherapy are integral parts of BC treatment that can influence the immune microenvironment. Anthracycline and cyclophosphamide-based chemotherapy regimens are still widely used in BC treatment (144, 145). It has been reported that anthracycline as well as cyclophosphamide can impair the actin polymerization of neutrophils, which results in insensitivity of neutrophils to the chemotactic effect of IL-8, therefore decreasing the infiltration of neutrophils in BC (146). In addition, the migration ability of neutrophils was also impaired by paclitaxel, a cornerstone drug in BC treatment, which could be attributed to the increased cell stiffness and decreased compliance induced by enhanced microtubule assembly (147). Platinum-based chemotherapeutic strategies have also been widely applied in various solid malignancies, such as colorectal cancer, ovarian cancer, and BC (148–150). Determination of neutrophil-specific chemokine expression by RT-PCR confirmed that oxaliplatin plus lipid A, which has been reported to exert antitumor effects against different tumor types, including colon cancer, BC and melanoma (151, 152), increased CXCL-1, CXCL-2, and IL-8 gene expression in tumors, thereby stimulating recruitment of antitumor N1-like neutrophils and impeding cancer progression (153).
The impact of radiotherapy on neutrophils has also been reported. In the EMT6.5 mammary tumor model, conventional radiotherapy (CRT) but not microbeam radiation therapy (MRT) induced a substantial increase in TAMs and TANs, and increased levels of CCL2 (which, as mentioned above, can be released by TANs to exert chemoattractant functions) were also observed in tumors subjected to CRT (154). In addition, different radiation regimens (20 Gy, 4 × 2 Gy, 2 Gy, or 0 Gy) induce different immune responses. High single doses (20 Gy) induce a delayed type of primary necrosis with characteristics of mitotic catastrophe and plasma membrane disintegration. The protein damage-associated molecular patterns (DAMPs) released by these dying cells stimulate sequential recruitment of neutrophils and monocytes in vivo (155). Furthermore, elevated infiltration of neutrophils was observed in various radiation-induced pneumonia models (156–158). However, in human BC, there is no direct evidence for how radiation affects the variations in neutrophils in the tumor microenvironment, but it has been reported that the NLR could be an independent prognostic factor in TNBC following radiotherapy (159).
Targeting Neutrophils for Cancer Treatment
Since the majority of studies have revealed a protumor function of neutrophils in BC, targeting neutrophils as a therapeutic strategy has been investigated. In mouse mammary tumor models, depletion of neutrophils with anti-Ly6G antibodies resulted in diminished tumor formation and lung metastasis (160). In addition, multiple BC xenograft models have proved that tumors enriched in neutrophils are more likely to be resistant to immune checkpoint blockade (ICB) therapy, while depleting neutrophils could restore the efficiency of ICB to reduce tumor recurrence and significantly improve progression-free survival (57). However, it is impossible to eradicate all neutrophils in cancer patients since it would cause severe immunodeficiency and infection, so it is more desirable to block the chemotaxis of neutrophils in tumor tissues or to prevent their polarization to the N2-like phenotype.
IFN-β and TGF-β are the cytokines that are most often reported to modulate the switch between N1 and N2-like neutrophil polarization. Steven et al. first revealed that TGF-β blockade significantly increased the influx of antitumor neutrophils and activated CD8+ T cells in a BC mouse model (161). Thereafter, a population of low-density neutrophils (LDNs) featuring impaired antitumor function and immunosuppressive properties accumulated continuously with cancer progression, including in BC. This LDN subpopulation consists of both immature MDSCs and mature neutrophils that are transformed from “normal,” antitumor, high-density neutrophils (HDNs) in a TGF-β-dependent mechanism (162). Mice deficient in IFN-β showed rapid tumor growth and large amounts of neutrophil infiltration with high expression of c-myc and stat3, which are known as enhancers of MMP-9, VEGF, and CXCR4 expression (163). Furthermore, nicotinamide phosphoribosyl transferase (NAMPT), an enzyme with cytokine-like features involved in the salvage pathway of nicotinamide adenine dinucleotide (NAD) biosynthesis (164), was found to be highly expressed and to modulate the tumorigenicity of TANs. Targeting NAMPT in TANs led to their antitumor conversion and antiangiogenic polarization by inhibiting SIRT1 signaling, which resulted in efficient repression of tumor growth (165). In addition, CXCR2 blockade in a K-ras mutant mouse model of lung cancer induced tumor regression, which was related to reduced neutrophil chemotaxis and polarization from N2- to N1-like cells (166). Transfusion of neutrophils (granulocyte transfusion, GTX) to cancer patients has also been tested, but due to the short life span of neutrophils and severe adverse events, such as respiratory distress and even death, it needs further investigation (167).
Conclusions
A schematic picture depicting the dual role of neutrophils in BC (Figure 3). Here, we provide a comprehensive review of circulating and TINs in BC to highlight their importance in the tumor microenvironment. Although increasing evidence suggests a close association of neutrophils with treatment outcome and prognosis in BC, as well as their utility in predicting these parameters, it is still difficult to utilize the NLR or TANs as clinical tools due to the lack of reliable markers to distinguish N1 and N2 neutrophils and the lack of a unanimous cut-off value for the NLR. In addition, existing evidence suggests an interesting phenomenon in which the spatial distribution and function of neutrophils are dynamically regulated with tumor progression, although the detailed mechanism requires further research. Overall, exploring more effective and low-toxicity strategies to inhibit protumor neutrophil polarization is a promising approach for cancer treatment.
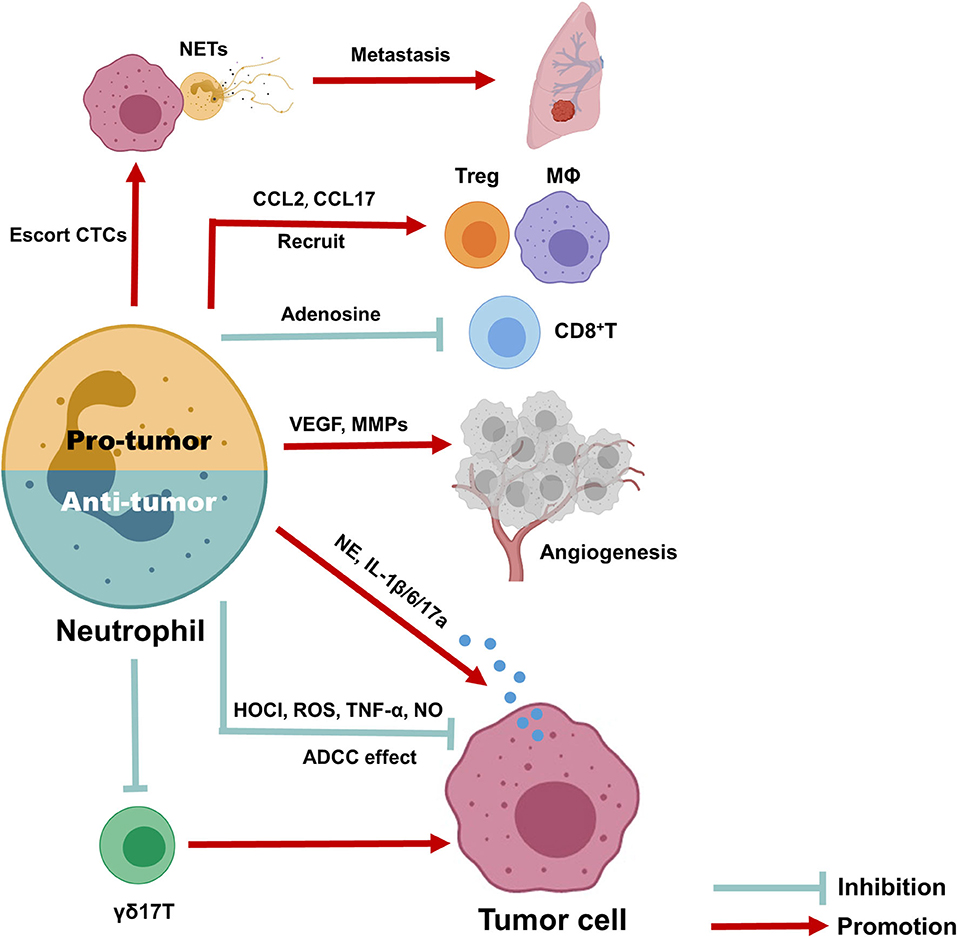
Figure 3. Anti- and pro-tumor function of neutrophils in breast cancer. Due to the dynamic regulation of neutrophils in tumor microenvironment, it can either function as inhibit or promote tumor progression. The anti-tumor neutrophils can exert anti-tumor function through antibody-dependent cellular cytotoxicity (ADCC) effect, produce HOCI, ROS, TNF-α, and NO as direct killing effect, and suppress immune suppressive cells, such as IL-17 producing γδ T cells. To the contrary, pro-tumor neutrophils can produce CCL2 and CCL17 to recruit CD4+ Treg cells and anti-inflammatory macrophages, together with release arginase-1 to inhibit the activation of CD8+ cells, therefore promote immune suppressive microenvironment; they also promote tumor angiogenesis via release MMP9 and VEGF and produce NETs to escort circulating tumor cells and promote cancer metastasis; finally, neutrophils could release elastase, IL-6, IL-1β, and IL-17 to promote tumor cells proliferation and EMT directly.
Author Contributions
YS and CN wrote the manuscript. CZ and WZ edited the manuscript. YS, WZ, and SP collected the related literatures. HH, JJ, and WC finished the tables and figures. TZ provided the feedback and guidance. All authors read and approved the final manuscript.
Funding
This work was supported by the Natural Science Foundation of Zhejiang Province (Grant No. LR19H160001); Natural Science Foundation of China (Grant Nos. 81773065, 81800024, 81502463).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
BC, Breast cancer; MDSCs, Myeloid-derived suppressor cells; TANs, Tumor-associated neutrophils; NLR, Neutrophil-to-lymphocyte ratio; DFS, Disease-free survival; OS, Overall survival; ALC, Absolute lymphocyte count; NCT, Neoadjuvant chemotherapy; pCR, Pathological complete response; PLR, Platelet-to-lymphocyte ratio; TAMs, Tumor-associated macrophages; CTCs, Circulating tumor cells; NETs, Neutrophil extracellular traps; MPO, Myeloperoxidase; G-CSF, Granulocyte colony-stimulating factor; ECs, Endothelial cells; PMNs, Polymorphonuclear neutrophils; ICAM-1, Intercellular adhesion molecule 1; MMP-9, Matrix metalloproteinases-9; ROS, Reactive oxygen species; HMGB1, High-mobility group box 1; TLR4, Toll-like receptor 4; TNBC, Triple-negative breast cancer; MES, Macrophage-enriched subtype; NES, Neutrophil-enriched subtype; H2O2, Hydrogen peroxide; TNF-α, Tumor necrosis factor-α; HOCI, Hypochlorous acid; TRPM2, Transient receptor potential cation channel, subfamily M, member 2; ADCC, Antibody-dependent cellular cytotoxicity; NK, Natural killer; NE, Neutrophil elastase; NRP1, Neuropilin-1; IRS-1, Insulin receptor substrate-1; PI3K, Phosphatidylinositol 3-kinase; VEGF, Vascular endothelial growth factor; TIMP-1, Tissue inhibitor of matrix metalloprotease; TGF-β, Transforming growth factor-β; 27HC, 27-hydroxycholesterol; PAD4, Peptidyl arginine deiminase 4; TINs, Tumor-infiltrating neutrophils; CRT, Conventional radiotherapy; MRT, Microbeam radiation therapy; DAMPs, Damage-associated molecular patterns; ICB, Immune checkpoint blockade; LDNs, Low-density neutrophils; HDNs, High-density neutrophils; NAMPT, Nicotinamide phosphoribosyl transferase; NAD, Nicotinamide adenine dinucleotide; GTX, granulocyte transfusion.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. (2020) 70:7–30. doi: 10.3322/caac.21590
2. Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH, Iwata H, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. (2018) 379:2108–21. doi: 10.1056/NEJMoa1809615
3. Schmid P, Salgado R, Park YH, Munoz-Couselo E, Kim SB, Sohn J, et al. (2020). Pembrolizumab plus chemotherapy as neoadjuvant treatment of high-risk, early-stage triple-negative breast cancer: results from the phase 1b open-label, multicohort KEYNOTE-173 study. Ann Oncol. 31, 569–81. doi: 10.1016/j.annonc.2020.01.072
4. Schmid P, Cortes J, Pusztai L, McArthur H, Kummel S, Bergh J, et al. Pembrolizumab for early triple-negative breast cancer. N Engl J Med. (2020) 382:810–21. doi: 10.1056/NEJMoa1910549
5. Martinez LM, Robila V, Clark NM, Du W, Idowu MO, Rutkowski MR, et al. Regulatory T cells control the switch from in situ to invasive breast cancer. Front Immunol. (2019) 10:1942. doi: 10.3389/fimmu.2019.01942
6. Wculek SK, Malanchi I. Neutrophils support lung colonization of metastasis-initiating breast cancer cells. Nature. (2015) 528:413–7. doi: 10.1038/nature16140
7. Zhang X, Xu W. Neutrophils diminish T-cell immunity to foster gastric cancer progression: the role of GM-CSF/PD-L1/PD-1 signalling pathway. Gut. (2017) 66:1878–80. doi: 10.1136/gutjnl-2017-313923
8. Zhang X, Zhang W, Yuan X, Fu M, Qian H, Xu W. Neutrophils in cancer development and progression: Roles, mechanisms, implications (review). Int J Oncol. (2016) 49:857–67. doi: 10.3892/ijo.2016.3616
9. Coffelt SB, Wellenstein MD, de Visser KE. Neutrophils in cancer: neutral no more. Nat Rev Cancer. (2016) 16:431–46. doi: 10.1038/nrc.2016.52
10. Shaul ME, Fridlender ZG. Tumour-associated neutrophils in patients with cancer. Nat Rev Clin Oncol. (2019) 16:601–20. doi: 10.1038/s41571-019-0222-4
11. Kumar V, Patel S, Tcyganov E, Gabrilovich DI. The nature of myeloid-derived suppressor cells in the tumor microenvironment. Trends Immunol. (2016) 37:208–20. doi: 10.1016/j.it.2016.01.004
12. Ethier JL, Desautels D, Templeton A, Shah PS, Amir E. Prognostic role of neutrophil-to-lymphocyte ratio in breast cancer: a systematic review and meta-analysis. Breast Cancer Res. (2017) 19:2. doi: 10.1186/s13058-016-0794-1
13. Mouchemore KA, Anderson RL, Hamilton JA. Neutrophils, G-CSF and their contribution to breast cancer metastasis. FEBS J. (2018) 285:665–79. doi: 10.1111/febs.14206
14. Guo W, Lu X, Liu Q, Zhang T, Li P, Qiao W, et al. Prognostic value of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio for breast cancer patients: an updated meta-analysis of 17079 individuals. Cancer Med. (2019) 8:4135–48. doi: 10.1002/cam4.2281
15. Azab B, Bhatt VR, Phookan J, Murukutla S, Kohn N, Terjanian T, et al. Usefulness of the neutrophil-to-lymphocyte ratio in predicting short- and long-term mortality in breast cancer patients. Ann Surg Oncol. (2012) 19:217–24. doi: 10.1245/s10434-011-1814-0
16. Li Y, Shao Y, Bai L, Zhou X. Increased derived neutrophil-to-lymphocyte ratio and breast imaging-reporting and data system classification predict poor survival in patients with non-distant metastatic HER2+ breast cancer treated with neoadjuvant chemotherapy. Cancer Manag Res. (2018) 10:3841–7. doi: 10.2147/CMAR.S174537
17. Ren K, Yin Y, He F, Shao Y, Wang S. Prognostic role of derived neutrophil-to-lymphocyte ratio in surgical triple-negative breast cancer. Cancer Manag Res. (2018) 10:4891–8. doi: 10.2147/CMAR.S180695
18. Graziano V, Grassadonia A, Iezzi L, Vici P, Pizzuti L, Barba M, et al. Combination of peripheral neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio is predictive of pathological complete response after neoadjuvant chemotherapy in breast cancer patients. Breast. (2019) 44:33–8. doi: 10.1016/j.breast.2018.12.014
19. Xuan Q, Yang Y, Ji H, Tang S, Zhao J, Shao J, et al. Combination of the preoperative albumin to globulin ratio and neutrophil to lymphocyte ratio as a novel prognostic factor in patients with triple negative breast cancer. Cancer Manag Res. (2019) 11:5125–31. doi: 10.2147/CMAR.S195324
20. Elyasinia F, Keramati MR, Ahmadi F, Rezaei S, Ashouri M, Parsaei R, et al. Neutrophil-lymphocyte ratio in different stages of breast cancer. Acta Med Iran. (2017) 55:228–32.
21. Yersal O, Cetinkunar S, Aktimur R, Aziret M, Ozdas S, Erdem H, et al. Neutrophil/Lymphocyte and platelet/lymphocyte ratios are not different among breast cancer subtypes. Asian Pac J Cancer Prev. (2017) 18:2227–31. doi: 10.22034/APJCP.2017.18.8.2227
22. Templeton AJ, Rodriguez-Lescure A, Ruiz A, Alba E, Calvo L, Ruiz-Borrego M, et al. Prognostic role for the derived neutrophil-to-lymphocyte ratio in early breast cancer: a GEICAM/9906 substudy. Clin Transl Oncol. (2018) 20:1548–56. doi: 10.1007/s12094-018-1885-5
23. Suppan C, Bjelic-Radisic V, La Garde M, Groselj-Strele A, Eberhard K, Samonigg H, et al. Neutrophil/Lymphocyte ratio has no predictive or prognostic value in breast cancer patients undergoing preoperative systemic therapy. BMC Cancer. (2015) 15:1027. doi: 10.1186/s12885-015-2005-3
24. Patel DA, Xi J, Luo JQ, Hassan B, Thomas S, Ma CX, et al. Neutrophil-to-lymphocyte ratio as a predictor of survival in patients with triple-negative breast cancer. Breast Cancer Res Treat. (2019) 174:443–52. doi: 10.1007/s10549-018-05106-7
25. Asano Y, Kashiwagi S, Onoda N, Noda S, Kawajiri H, Takashima T, et al. Predictive value of neutrophil/lymphocyte ratio for efficacy of preoperative chemotherapy in triple-negative breast cancer. Ann Surg Oncol. (2016) 23:1104–10. doi: 10.1245/s10434-015-4934-0
26. Chae S, Kang KM, Kim HJ, Kang E, Park SY, Kim JH, et al. Neutrophil-lymphocyte ratio predicts response to chemotherapy in triple-negative breast cancer. Curr Oncol. (2018) 25:e113–9. doi: 10.3747/co.25.3888
27. Rivas M, Acevedo F, Dominguez F, Galindo H, Camus M, Oddo D, et al. The neutrophil to lymphocyte ratio predicts the response to neoadjuvant chemotherapy in luminal B breast cancer. Asian Pac J Cancer Prev. (2019) 20:2209–12. doi: 10.31557/APJCP.2019.20.7.2209
28. Eryilmaz MK, Mutlu H, Salim DK, Musri FY, Tural D, Coskun HS. The neutrophil to lymphocyte ratio has a high negative predictive value for pathologic complete response in locally advanced breast cancer patients receiving neoadjuvant chemotherapy. Asian Pac J Cancer Prev. (2014) 15:7737–40. doi: 10.7314/APJCP.2014.15.18.7737
29. Rao JS, Hanumappa HK, Joseph EP, Chowdappa RG, Ramesh R. Elevated neutrophil-lymphocyte ratio in luminal-type locally advanced breast cancer to circumvent neo-adjuvant chemotherapy. Indian J Surg Oncol. (2019) 10:454–9. doi: 10.1007/s13193-019-00944-3
30. Iimori N, Kashiwagi S, Asano Y, Goto W, Takada K, Takahashi K, et al. Clinical significance of the neutrophil-to-lymphocyte ratio in endocrine therapy for stage IV breast cancer. In Vivo. (2018) 32:669–75. doi: 10.21873/invivo.11292
31. Takada K, Kashiwagi S, Asano Y, Goto W, Takahashi K, Shibutani M, et al. Clinical evaluation of dynamic monitoring of neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios in primary endocrine therapy for advanced breast cancer. Anticancer Res. (2019) 39:5581–8. doi: 10.21873/anticanres.13752
32. Noh H, Eomm M, Han A. Usefulness of pretreatment neutrophil to lymphocyte ratio in predicting disease-specific survival in breast cancer patients. J Breast Cancer. (2013) 16:55–9. doi: 10.4048/jbc.2013.16.1.55
33. Koh YW, Kang HJ, Park C, Yoon DH, Kim S, Suh C, et al. Prognostic significance of the ratio of absolute neutrophil count to absolute lymphocyte count in classic hodgkin lymphoma. Am J Clin Pathol. (2012) 138:846–54. doi: 10.1309/Ajcpo46gfkgnxcbr
34. Yao MY, Liu Y, Jin HL, Liu XJ, Lv KZ, Wei HY, et al. Prognostic value of preoperative inflammatory markers in Chinese patients with breast cancer. Oncotargets Ther. (2014) 7:1743–52. doi: 10.2147/Ott.S69657
35. Pistelli M, De Lisa M, Ballatore Z, Caramanti M, Pagliacci A, Battelli N, et al. (2015) Pre-treatment neutrophil to lymphocyte ratio may be a useful tool in predicting survival in early triple negative breast cancer patients. BMC Cancer 15:195. doi: 10.1186/s12885-015-1204-2
36. Ulas A Avci N Kos T Cubukcu E Olmez OF Bulut N . Are neutrophil/lymphocyte ratio and platelet/lymphocyte ratio associated with prognosis in patients with HER2-positive early breast cancer receiving adjuvant trastuzumab? J Buon. (2015) 20:714–22.
37. Jia W, Wu J, Jia H, Yang Y, Zhang X, Chen K, et al. The peripheral blood neutrophil-to-lymphocyte ratio is superior to the lymphocyte-to-monocyte ratio for predicting the long-term survival of triple-negative breast cancer patients. PLoS ONE. (2015) 10:e0143061. doi: 10.1371/journal.pone.0143061
38. Bozkurt O, Karaca H, Berk V, Inanc M, Duran AO, Ozaslan E, et al. Predicting the role of the pretreatment neutrophil to lymphocyte ratio in the survival of early triple-negative breast cancer patients. J Buon. (2015) 20:1432–9.
39. Rimando J, Campbell J, Kim JH, Tang SC, Kim S. The pretreatment neutrophil/lymphocyte ratio is associated with all-cause mortality in black and white patients with non-metastatic breast cancer. Front Oncol. (2016) 6:81. doi: 10.3389/fonc.2016.00081
40. Iwase T, Sangai T, Sakakibara M, Sakakibara J, Ishigami E, Hayama S, et al. An increased neutrophil-to-lymphocyte ratio predicts poorer survival following recurrence for patients with breast cancer. Mol Clin Oncol. (2017) 6:266–70. doi: 10.3892/mco.2016.1101
41. Hernandez CM, Madrona AP, Vazquez PJG, Fernandez PJG, Merino GR, Romero JLA, et al. Usefulness of lymphocyte-to-monocyte, neutrophil-to-monocyte and neutrophil-to-lymphocyte ratios as prognostic markers in breast cancer patients treated with neoadjuvant chemotherapy. Clin Trans Oncol. (2018) 20:476–83. doi: 10.1007/s12094-017-1732-0
42. Miyagawa Y, Araki K, Bun A, Ozawa H, Fujimoto Y, Higuchi T, et al. Significant association between low baseline neutrophil-to-lymphocyte ratio and improved progression-free survival of patients with locally advanced or metastatic breast cancer treated with eribulin but not with nab-paclitaxel. Clin Breast Cancer. (2018) 18:400–9. doi: 10.1016/j.clbc.2018.03.002
43. Ferroni P, Roselli M, Buonomo OC, Spila A, Portarena I, Laudisi A, et al. Prognostic significance of neutrophil-to-lymphocyte ratio in the framework of the 8th TNM edition for breast cancer. Anticancer Res. (2018) 38:4705–12. doi: 10.21873/anticanres.12777
44. Qiu XT, Song YC, Cui YX, Liu Y. Increased neutrophil-lymphocyte ratio independently predicts poor survival in non-metastatic triple-negative breast cancer patients. Iubmb Life. (2018) 70:529–35. doi: 10.1002/iub.1745
45. Mando P, Rizzo M, Roberti MP, Julia EP, Pampena MB, de la Puente CP, et al. High neutrophil to lymphocyte ratio and decreased CD69(+)NK cells represent a phenotype of high risk in early-stage breast cancer patients. Oncotargets Ther. (2018) 11:2901–10. doi: 10.2147/Ott.S160911
46. Lee J, Kim DM, Lee A. Prognostic role and clinical association of tumor-infiltrating lymphocyte, programmed death ligand-1 expression with neutrophil-lymphocyte ratio in locally advanced triple-negative breast cancer. Cancer Res Treat. (2019) 51:649–63. doi: 10.4143/crt.2018.270
47. Fujimoto Y, Ozawa H, Higuchi T, Miyagawa Y, Bun A, Imamura M, et al. Improved prognosis of low baseline neutrophil-to-lymphocyte ratio is significantly exclusive in breast cancer patients with high absolute counts of lymphocytes. Mol Clin Oncol. (2019) 10:275–84. doi: 10.3892/mco.2018.1783
48. Imamura M, Morimoto T, Egawa C, Fukui R, Bun A, Ozawa H, et al. Significance of baseline neutrophil-to-lymphocyte ratio for progression-free survival of patients with HER2-positive breast cancer treated with trastuzumab emtansine. Sci Rep. (2019) 9:1811. doi: 10.1038/s41598-018-37633-0
49. Kim HY, Kim TH, Yoon HK, Lee A. The role of neutrophil-lymphocyte ratio and platelet-lymphocyte ratio in predicting neoadjuvant chemotherapy response in breast cancer. J Breast Cancer. (2019) 22:425–38. doi: 10.4048/jbc.2019.22.e41
50. Liu C, Huang Z, Wang QS, Sun B, Ding LJ, Meng XY, et al. Usefulness of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in hormone-receptor-negative breast cancer. Oncotargets Ther. (2016) 9:4653–60. doi: 10.2147/OTT.S106017
51. Azab B, Shah N, Radbel J, Tan P, Bhatt V, Vonfrolio S, et al. Pretreatment neutrophil/lymphocyte ratio is superior to platelet/lymphocyte ratio as a predictor of long-term mortality in breast cancer patients. Med Oncol. (2013) 30:432. doi: 10.1007/s12032-012-0432-4
52. Takakura K, Ito Z, Suka M, Kanai T, Matsumoto Y, Odahara S, et al. Comprehensive assessment of the prognosis of pancreatic cancer: peripheral blood neutrophil-lymphocyte ratio and immunohistochemical analyses of the tumour site. Scand J Gastroenterol. (2016) 51:610–7. doi: 10.3109/00365521.2015.1121515
53. Hurt B, Schulick R, Edil B, El Kasmi KC, Barnett C. Cancer-promoting mechanisms of tumor-associated neutrophils. Am J Surgery. (2017) 214:938–44. doi: 10.1016/j.amjsurg.2017.08.003
54. Arpinati L, Shaul ME, Kaisar-Iluz N, Mali S, Mahroum S, Fridlender ZG. NETosis in cancer: a critical analysis of the impact of cancer on neutrophil extracellular trap (NET) release in lung cancer patients vs. mice. Cancer Immunol Immunother. (2020) 69:199–213. doi: 10.1007/s00262-019-02474-x
55. Snoderly HT, Boone BA, Bennewitz MF. Neutrophil extracellular traps in breast cancer and beyond: current perspectives on NET stimuli, thrombosis and metastasis, and clinical utility for diagnosis and treatment. Breast Cancer Res. (2019) 21:145. doi: 10.1186/s13058-019-1237-6
56. Faria SS, Fernandes PC, Silva MJB, Lima VC, Fontes W, Freitas R, et al. The neutrophil-to-lymphocyte ratio: a narrative review. Ecancermedicalscience. (2016) 10:702. doi: 10.3332/ecancer.2016.702
57. Kim IS Gao Y Welte T Wang H Liu J Janghorban M . Immuno-subtyping of breast cancer reveals distinct myeloid cell profiles and immunotherapy resistance mechanisms. Nat Cell Biol. (2019) 21:1113–26. doi: 10.1038/s41556-019-0373-7
58. Mohme M, Riethdorf S, Pantel K. Circulating and disseminated tumour cells - mechanisms of immune surveillance and escape. Nat Rev Clin Oncol. (2017) 14:155–67. doi: 10.1038/nrclinonc.2016.144
59. Zhu J, De Tenbossche CGP, Cane S, Colau D, Van Baren N, Lurquin C, et al. Resistance to cancer immunotherapy mediated by apoptosis of tumor-infiltrating lymphocytes. Nat Commun. (2017) 8:1404. doi: 10.1038/s41467-017-00784-1
60. Zhou J, Nefedova Y, Lei A, Gabrilovich D. Neutrophils and PMN-MDSC: their biological role and interaction with stromal cells. Semin Immunol. (2018) 35:19–28. doi: 10.1016/j.smim.2017.12.004
61. Galdiero MR, Garlanda C, Jaillon S, Marone G, Mantovani A. Tumor associated macrophages and neutrophils in tumor progression. J Cell Physiol. (2013) 228:1404–12. doi: 10.1002/jcp.24260
62. Disis ML, Stanton SE. Immunotherapy in breast cancer: an introduction. Breast. (2018) 37:196–9. doi: 10.1016/j.breast.2017.01.013
63. Desbaillets I, Diserens AC, Tribolet N, Hamou MF, van Meir EG. Upregulation of interleukin 8 by oxygen-deprived cells in glioblastoma suggests a role in leukocyte activation, chemotaxis, and angiogenesis. J Exp Med. (1997) 186:1201–12. doi: 10.1084/jem.186.8.1201
64. Palena C, Hamilton DH, Fernando RI. Influence of IL-8 on the epithelial-mesenchymal transition and the tumor microenvironment. Future Oncol. (2012) 8:713–22. doi: 10.2217/fon.12.59
65. Gupta AK, Joshi MB, Philippova M, Erne P, Hasler P, Hahn S, et al. Activated endothelial cells induce neutrophil extracellular traps and are susceptible to NETosis-mediated cell death. Febs Lett. (2010) 584:3193–7. doi: 10.1016/j.febslet.2010.06.006
66. Corre I, Paris F, Huot J. The p38 pathway, a major pleiotropic cascade that transduces stress and metastatic signals in endothelial cells. Oncotarget. (2017) 8:55684–714. doi: 10.18632/oncotarget.18264
67. Chen G, Ding XF, Pressley K, Bouamar H, Wang B, Zheng G, et al. Everolimus inhibits the progression of ductal carcinoma in situ to invasive breast cancer via downregulation of MMP9 expression. Clin Cancer Res. (2019) 26:1486–96. doi: 10.1158/1078-0432.CCR-19-2478
68. Owyong M, Chou J, van den Bijgaart RJ, Kong N, Efe G, Maynard C, et al. MMP9 modulates the metastatic cascade and immune landscape for breast cancer anti-metastatic therapy. Life Sci Alliance. (2019) 2:e201800226. doi: 10.26508/lsa.201800226
69. Song J, Wu C, Zhang X, Sorokin LM. In vivo processing of CXCL5 (LIX) by matrix metalloproteinase (MMP)-2 and MMP-9 promotes early neutrophil recruitment in IL-1beta-induced peritonitis. J Immunol. (2013) 190:401–10. doi: 10.4049/jimmunol.1202286
70. Benevides L, da Fonseca DM, Donate PB, Tiezzi DG, De Carvalho DD, de Andrade JM, et al. IL17 promotes mammary tumor progression by changing the behavior of tumor cells and eliciting tumorigenic neutrophils recruitment. Cancer Res. (2015) 75:3788–99. doi: 10.1158/0008-5472.CAN-15-0054
71. Coffelt SB, Kersten K, Doornebal CW, Weiden J, Vrijland K, Hau CS, et al. IL-17-producing gammadelta T cells and neutrophils conspire to promote breast cancer metastasis. Nature. (2015) 522:345–8. doi: 10.1038/nature14282
72. Mensurado S, Rei M, Lanca T, Ioannou M, Goncalves-Sousa N, Kubo H, et al. Tumor-associated neutrophils suppress pro-tumoral IL-17+ gammadelta T cells through induction of oxidative stress. PLoS Biol. (2018) 16:e2004990. doi: 10.1371/journal.pbio.2004990
73. Tabaries S, Ouellet V, Hsu BE, Annis MG, Rose AA, Meunier L, et al. Granulocytic immune infiltrates are essential for the efficient formation of breast cancer liver metastases. Breast Cancer Res. (2015) 17:45. doi: 10.1186/s13058-015-0558-3
74. Vogel S, Bodenstein R, Chen QW, Feil S, Feil R, Rheinlaender J, et al. Platelet-derived HMGB1 is a critical mediator of thrombosis. J Clin Invest. (2015) 125:4638–54. doi: 10.1172/JCI81660
75. Huebener P, Pradere JP, Hernandez C, Gwak GY, Caviglia JM, Mu XR, et al. The HMGB1/RAGE axis triggers neutrophil-mediated injury amplification following necrosis. J Clin Invest. (2019) 129:1802. doi: 10.1172/JCI126976
76. Fuentes E, Palomo I, Rojas A. Cross-talk between platelet and tumor microenvironment: role of multiligand/RAGE axis in platelet activation. Blood Rev. (2016) 30:213–21. doi: 10.1016/j.blre.2015.11.005
77. Pather K, Dix-Peek T, Duarte R, Chetty N, Augustine TN. Breast cancer cell-induced platelet activation is compounded by tamoxifen and anastrozole in vitro. Thromb Res. (2019) 177:51–8. doi: 10.1016/j.thromres.2019.02.027
78. Tadie JM, Bae HB, Jiang SN, Park DW, Bell CP, Yang H, et al. HMGB1 promotes neutrophil extracellular trap formation through interactions with Toll-like receptor 4. Am J Physiol Lung Cell Mol Physiol. (2013) 304:L342–9. doi: 10.1152/ajplung.00151.2012
79. Zhang X, Shi H, Yuan X, Jiang PC, Qian H, Xu WR. Tumor-derived exosomes induce N2 polarization of neutrophils to promote gastric cancer cell migration. Mol Cancer. (2018) 17:146. doi: 10.1186/s12943-018-0898-6
80. Baudiss K, de Paula Vieira R, Cicko S, Ayata K, Hossfeld M, Ehrat N, et al. C1P attenuates lipopolysaccharide-induced acute lung injury by preventing NF-kappaB activation in neutrophils. J Immunol. (2016) 196:2319–26. doi: 10.4049/jimmunol.1402681
81. Zeng J. Values of detection of NF-kappaB activation level combined with IL-6 and TNF-alpha levels in peripheral neutrophils in the prediction of multiple organ dysfunction syndrome in patients with severe multiple trauma. Exp Ther Med. (2018) 16:2478–82. doi: 10.3892/etm.2018.6472
82. Fusella F, Secli L, Busso E, Krepelova A, Moiso E, Rocca S, et al. The IKK/NF-kappa B signaling pathway requires Morgana to drive breast cancer metastasis. Nat Commun. (2017) 8:1636. doi: 10.1038/s41467-017-01829-1
83. Ibrahim SA, Kulshrestha A, Katara GK, Riehl V, Sahoo M, Beaman KD. Cancer-associated V-ATPase induces delayed apoptosis of protumorigenic neutrophils. Mol Oncol. (2020) 14:590–610. doi: 10.1002/1878-0261.12630
84. Chung HH Or YZ Shrestha S Loh JT Lim CL Ong Z . Estrogen reprograms the activity of neutrophils to foster protumoral microenvironment during mammary involution. Sci Rep. (2017) 7:46485. doi: 10.1038/srep46485
85. Soto-Perez-de-Celis E, Chavarri-Guerra Y, Leon-Rodriguez E, Gamboa-Dominguez A. Tumor-associated neutrophils in breast cancer subtypes. Asian Pac J Cancer Prev. (2017) 18:2689–93. doi: 10.22034/APJCP.2017.18.10.2689
86. Pahler J, Tazzyman S, Erez N, Chen YY, Murdoch C, Nozawa H, et al. Plasticity in tumor-promoting inflammation: impairment of macrophage recruitment evokes a compensatory neutrophil response. Neoplasia. (2008) 10:329–40. doi: 10.1593/neo.07871
87. Mishalian I, Bayuh R, Levy L, Zolotarov L, Michaeli J, Fridlender ZG. Tumor-associated neutrophils (TAN) develop pro-tumorigenic properties during tumor progression. Cancer Immunol Immunother. (2013) 62:1745–56. doi: 10.1007/s00262-013-1476-9
88. Eruslanov EB, Bhojnagarwala PS, Quatromoni JG, Stephen TL, Ranganathan A, Deshpande C, et al. Tumor-associated neutrophils stimulate T cell responses in early-stage human lung cancer. J Clin Invest. (2014) 124:5466–80. doi: 10.1172/JCI77053
89. Kato Y. Neutrophil myeloperoxidase and its substrates: formation of specific markers and reactive compounds during inflammation. J Clin Biochem Nutr. (2016) 58:99–104. doi: 10.3164/jcbn.15-104
90. Zeindler J, Angehrn F, Droeser R, Daster S, Piscuoglio S, Ng CKY, et al. Infiltration by myeloperoxidase-positive neutrophils is an independent prognostic factor in breast cancer. Breast Cancer Res Treat. (2019) 177:581–9. doi: 10.1007/s10549-019-05336-3
91. Gershkovitz M, Caspi Y, Fainsod-Levi T, Katz B, Michaeli J, Khawaled S, et al. TRPM2 mediates neutrophil killing of disseminated tumor cells. Cancer Res. (2018) 78:2680–90. doi: 10.1158/0008-5472.CAN-17-3614
92. Bauer G. Targeting extracellular ROS signaling of tumor cells. Anticancer Res. (2014) 34:1467–82.
93. Glasauer A, Chandel NS. Targeting antioxidants for cancer therapy. Biochem Pharmacol. (2014) 92:90–101. doi: 10.1016/j.bcp.2014.07.017
94. Wu P, Wu D, Ni C, Ye J, Chen W, Hu G, et al. gamma delta T17 cells promote the accumulation and expansion of myeloid-derived suppressor cells in human colorectal cancer. Immunity. (2014) 40:785–800. doi: 10.1016/j.immuni.2014.03.013
95. McKenzie DR, Kara EE, Bastow CR, Tyllis TS, Fenix KA, Gregor CE, et al. IL-17-producing gamma delta Tcells switch migratory patterns between resting and activated states. Nat Commun. (2017) 8:15632. doi: 10.1038/ncomms15632
96. Chen HC, Eling N, Martinez-Jimenez CP, O'Brien LM, Carbonaro V, Marioni JC, et al. IL-7-dependent compositional changes within the gamma delta T cell pool in lymph nodes during ageing lead to an unbalanced anti-tumour response. EMBO Rep. (2019) 20:e47379. doi: 10.15252/embr.201847379
97. Matlung HL, Babes L, Zhao XW, van Houdt M, Treffers LW, van Rees DJ, et al. Neutrophils kill antibody-opsonized cancer cells by trogoptosis. Cell Rep. (2018) 23:3946–59. doi: 10.1016/j.celrep.2018.05.082
98. Lavender N, Yang JM, Chen SC, Sai JQ, Johnson CA, Owens P, et al. The Yin/Yan of CCL2: a minor role in neutrophil anti-tumor activity in vitro but a major role on the outgrowth of metastatic breast cancer lesions in the lung in vivo. BMC Cancer. (2017) 17:88. doi: 10.1186/s12885-017-3074-2
99. Mishalian I, Bayuh R, Eruslanov E, Michaeli J, Levy L, Zolotarov L, et al. Neutrophils recruit regulatory T-cells into tumors via secretion of CCL17-A new mechanism of impaired antitumor immunity. Int J Cancer. (2014) 135:1178–86. doi: 10.1002/ijc.28770
100. Spiegel A, Brooks MW, Houshyar S, Reinhardt F, Ardolino M, Fessler E, et al. Neutrophils suppress intraluminal NK cell-mediated tumor cell clearance and enhance extravasation of disseminated carcinoma cells. Cancer Discov. (2016) 6:630–49. doi: 10.1158/2159-8290.CD-15-1157
101. Gogali F, Paterakis G, Rassidakis GZ, Liakou CI, Liapi C. CD3(-)CD16(-)CD56(bright) immunoregulatory NK cells are increased in the tumor microenvironment and inversely correlate with advanced stages in patients with papillary thyroid cancer. Thyroid. (2013) 23:1561–8. doi: 10.1089/thy.2012.0560
102. Frazao A, Messaoudene M, Nunez N, Dulphy N, Roussin F, Sedlik C, et al. CD16(+)NKG2A(high) natural killer cells infiltrate breast cancer-draining lymph nodes. Cancer Immunol Res. (2019) 7:208–18. doi: 10.1158/2326-6066.CIR-18-0085
103. Bassani B, Baci D, Gallazzi M, Poggi A, Bruno A, Mortara L. Natural killer cells as key players of tumor progression and angiogenesis: old and novel tools to divert their pro-tumor activities into potent anti-tumor effects. Cancers (Basel). (2019) 11:461. doi: 10.3390/cancers11040461
104. Harlin H, Hanson M, Johansson CC, Sakurai D, Poschke I, Norell H, et al. The CD16- CD56(bright) NK cell subset is resistant to reactive oxygen species produced by activated granulocytes and has higher antioxidative capacity than the CD16+ CD56(dim) subset. J Immunol. (2007) 179:4513–9. doi: 10.4049/jimmunol.179.7.4513
105. Araujo JM, Gomez AC, Aguilar A, Salgado R, Balko JM, Bravo L, et al. Effect of CCL5 expression in the recruitment of immune cells in triple negative breast cancer. Sci Rep. (2018) 8:4899. doi: 10.1038/s41598-018-23099-7
106. Geiger R, Rieckmann JC, Wolf T, Basso C, Feng Y, Fuhrer T, et al. L-arginine modulates T cell metabolism and enhances survival and anti-tumor activity. Cell. (2016) 167:829–42.e813. doi: 10.1016/j.cell.2016.09.031
107. Clappaert EJ, Murgaski A, Van Damme H, Kiss M, Laoui D. Diamonds in the rough: harnessing tumor-associated myeloid cells for cancer therapy. Front Immunol. (2018) 9:2250. doi: 10.3389/fimmu.2018.02250
108. Gun SY, Lee SWL, Sieow JL, Wong SC. Targeting immune cells for cancer therapy. Redox Biol. (2019) 25:101174. doi: 10.1016/j.redox.2019.101174
109. Kerros C, Tripathi SC, Zha D, Mehrens JM, Sergeeva A, Philips AV, et al. Neuropilin-1 mediates neutrophil elastase uptake and cross-presentation in breast cancer cells. J Biol Chem. (2017) 292:10295–305. doi: 10.1074/jbc.M116.773051
110. Houghton AM, Rzymkiewicz DM, Ji HB, Gregory AD, Egea EE, Metz HE, et al. Neutrophil elastase-mediated degradation of IRS-1 accelerates lung tumor growth. Nat Med. (2010) 16:219–U127. doi: 10.1038/nm.2084
111. Chennakrishnaiah S, Meehan B, D'Asti E, Montermini L, Lee TH, Karatzas N, et al. Leukocytes as a reservoir of circulating oncogenic DNA and regulatory targets of tumor-derived extracellular vesicles. J Thromb Haemost. (2018) 16:1800–13. doi: 10.1111/jth.14222
112. Leal AC, Mizurini DM, Gomes T, Rochael NC, Saraiva EM, Dias MS, et al. Tumor-derived exosomes induce the formation of neutrophil extracellular traps: implications for the establishment of cancer-associated thrombosis. Sci Rep. (2017) 7:6438. doi: 10.1038/s41598-017-06893-7
113. Hwang WL, Lan HY, Cheng WC, Huang SC, Yang MH. Tumor stem-like cell-derived exosomal RNAs prime neutrophils for facilitating tumorigenesis of colon cancer. J Hematol Oncol. (2019) 12:10. doi: 10.1186/s13045-019-0699-4
114. Zhuang XQ, Zhang H, Li XY, Li XX, Cong M, Peng FL, et al. Differential effects on lung and bone metastasis of breast cancer by Wnt signalling inhibitor DKK1. Nat Cell Biol. (2017) 19:1274–85. doi: 10.1038/ncb3613
115. Kuang DM, Zhao QY, Wu Y, Peng C, Wang JN, Xu ZQ, et al. Peritumoral neutrophils link inflammatory response to disease progression by fostering angiogenesis in hepatocellular carcinoma. J Hepatol. (2011) 54:948–55. doi: 10.1016/j.jhep.2010.08.041
116. Liang W, Ferrara N. The complex role of neutrophils in tumor angiogenesis and metastasis. Cancer Immunol Res. (2016) 4:83–91. doi: 10.1158/2326-6066.CIR-15-0313
117. Ardi VC, Kupriyanova TA, Deryugina EI, Quigley JP. Human neutrophils uniquely release TIMP-free MMP-9 to provide a potent catalytic stimulator of angiogenesis. Proc Natl Acad Sci USA. (2007) 104:20262–7. doi: 10.1073/pnas.0706438104
118. Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K, et al. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol. (2000) 2:737–44. doi: 10.1038/35036374
119. Yang L, DeBusk LM, Fukuda K, Fingleton B, Green-Jarvis B, Shyr Y, et al. Expansion of myeloid immune suppressor Gr+CD11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell. (2004) 6:409–21. doi: 10.1016/j.ccr.2004.08.031
120. Shamamian P, Schwartz JD, Pocock BJ, Monea S, Whiting D, Marcus SG, et al. Activation of progelatinase A (MMP-2) by neutrophil elastase, cathepsin G, and proteinase-3: a role for inflammatory cells in tumor invasion and angiogenesis. J Cell Physiol. (2001) 189:197–206. doi: 10.1002/jcp.10014
121. Dumitru CA, Lang S, Brandau S. Modulation of neutrophil granulocytes in the tumor microenvironment: mechanisms and consequences for tumor progression. Semin Cancer Biol. (2013) 23:141–8. doi: 10.1016/j.semcancer.2013.02.005
122. Wang Y, Chen J, Yang L, Li J, Wu W, Huang M, et al. Tumor-contacted neutrophils promote metastasis by a CD90-TIMP-1 juxtacrine-paracrine loop. Clin Cancer Res. (2019) 25:1957–69. doi: 10.1158/1078-0432.CCR-18-2544
123. Zhang W, Gu J, Chen J, Zhang P, Ji R, Qian H, et al. Interaction with neutrophils promotes gastric cancer cell migration and invasion by inducing epithelial-mesenchymal transition. Oncol Rep. (2017) 38:2959–66. doi: 10.3892/or.2017.5942
124. Li S, Cong XL, Gao HY, Lan XW, Li ZG, Wang WP, et al. Tumor-associated neutrophils induce EMT by IL-17a to promote migration and invasion in gastric cancer cells. J Exp Clin Cancer Res. (2019) 38:6. doi: 10.1186/s13046-019-1168-1
125. Cools-Lartigue J, Spicer J, McDonald B, Gowing S, Chow S, Giannias B, et al. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J Clin Invest. (2013) 123:3446–58. doi: 10.1172/JCI67484
126. Nicolas-Avila JA, Adrover JM, Hidalgo A. Neutrophils in homeostasis, immunity, and cancer. Immunity. (2017) 46:15–28. doi: 10.1016/j.immuni.2016.12.012
127. Wen L, Guo LP, Zhang W, Li YJ, Jiang WX, Di XB, et al. Cooperation between the inflammation and coagulation systems promotes the survival of circulating tumor cells in renal cell carcinoma patients. Front Oncol. (2019) 9:504. doi: 10.3389/fonc.2019.00504
128. Spicer JD, McDonald B, Cools-Lartigue JJ, Chow SC, Giannias B, Kubes P, et al. Neutrophils promote liver metastasis via Mac-1-mediated interactions with circulating tumor cells. Cancer Res. (2012) 72:3919–27. doi: 10.1158/0008-5472.CAN-11-2393
129. Szczerba BM, Castro-Giner F, Vetter M, Krol I, Gkountela S, Landin J, et al. Neutrophils escort circulating tumour cells to enable cell cycle progression. Nature. (2019) 566:553–7. doi: 10.1038/s41586-019-0915-y
130. Rothstein G, Rhondeau SM, Peters CA, Christensen RD, Lynch D, Gillis S. Stimulation of neutrophil production in CSF-1-responsive clones. Blood. (1988) 72:898–902. doi: 10.1182/blood.V72.3.898.bloodjournal723898
131. Verri WA Jr, Cunha TM, Ferreira SH, Wei X, Leung BP, Fraser A, et al. IL-15 mediates antigen-induced neutrophil migration by triggering IL-18 production. Eur J Immunol. (2007) 37:3373–80. doi: 10.1002/eji.200737488
132. He JQ, Shumansky K, Connett JE, Anthonisen NR, Pare PD, Sandford AJ. Association of genetic variations in the CSF2 and CSF3 genes with lung function in smoking-induced COPD. Eur Respir J. (2008) 32:25–34. doi: 10.1183/09031936.00040307
133. Lee W, Ko SY, Mohamed MS, Kenny HA, Lengyel E, Naora H. Neutrophils facilitate ovarian cancer premetastatic niche formation in the omentum. J Exp Med. (2019) 216:176–94. doi: 10.1084/jem.20181170
134. Jiralerspong S, Kim ES, Dong W, Feng L, Hortobagyi GN, Giordano SH. Obesity, diabetes, and survival outcomes in a large cohort of early-stage breast cancer patients. Ann Oncol. (2013) 24:2506–14. doi: 10.1093/annonc/mdt224
135. Suzuki H, Seki A, Hosaka T, Matsumoto N, Tomita M, Takahashi M, et al. Effects of a structured group intervention on obesity among breast cancer survivors. Breast Cancer. (2020) 27:236–42. doi: 10.1007/s12282-019-01013-x
136. Baek AE, Yu YRA, He SS, Wardell SE, Chang CY, Kwon S, et al. The cholesterol metabolite 27 hydroxycholesterol facilitates breast cancer metastasis through its actions on immune cells. Nat Commun. (2017) 8:864. doi: 10.1038/s41467-017-00910-z
137. Park J, Wysocki RW, Amoozgar Z, Maiorino L, Fein MR, Jorns J, et al. Cancer cells induce metastasis-supporting neutrophil extracellular DNA traps. Sci Transl Med. (2016) 8:361ra138. doi: 10.1126/scitranslmed.aag1711
138. Wu CF, Andzinski L, Kasnitz N, Kroger A, Klawonn F, Lienenklaus S, et al. The lack of type I interferon induces neutrophil-mediated pre-metastatic niche formation in the mouse lung. Int J Cancer. (2015) 137:837–47. doi: 10.1002/ijc.29444
139. Albrengues J, Shields MA, Ng D, Park CG, Ambrico A, Poindexter ME, et al. Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. Science. (2018) 361:eaao4227. doi: 10.1126/science.aao4227
140. Tosolini M, Pont F, Poupot M, Vergez F, Nicolau-Travers ML, Vermijlen D, et al. Assessment of tumor-infiltrating TCRV. Oncoimmunology. (2017) 6:e1284723. doi: 10.1080/2162402X.2017.1284723
141. Wagner J, Rapsomaniki MA, Chevrier S, Anzeneder T, Langwieder C, Dykgers A, et al. A single-cell atlas of the tumor and immune ecosystem of human breast cancer. Cell. (2019) 177:1330–45.e18. doi: 10.1016/j.cell.2019.03.005
142. Zilionis R, Engblom C, Pfirschke C, Savova V, Zemmour D, Saatcioglu HD, et al. Single-cell transcriptomics of human and mouse lung cancers reveals conserved myeloid populations across individuals and species. Immunity. (2019) 50:1317–34.e10. doi: 10.1016/j.immuni.2019.03.009
143. Azizi E, Carr AJ, Plitas G, Cornish AE, Konopacki C, Prabhakaran S, et al. Single-cell map of diverse immune phenotypes in the breast tumor microenvironment. Cell. (2018) 174:1293–308.e36. doi: 10.1016/j.cell.2018.05.060
144. Drooger JC, van Pelt-Sprangers JM, Leunis C, Jager A, de Jongh FE. Neutrophil-guided dosing of anthracycline-cyclophosphamide-containing chemotherapy in patients with breast cancer: a feasibility study. Med Oncol. (2015) 32:113. doi: 10.1007/s12032-015-0550-x
145. Janni WJ, Rack B, Terstappen LWMM, Pierga JY, Taran FA, Fehm T, et al. Pooled analysis of the prognostic relevance of circulating tumor cells in primary breast cancer. Clin Cancer Res. (2016) 22:2583–93. doi: 10.1158/1078-0432.CCR-15-1603
146. Mendonca MAO, Souto FO, Micheli DC, Alves-Filho JC, Cunha FQ, Murta EFC, et al. Mechanisms affecting neutrophil migration capacity in breast cancer patients before and after chemotherapy. Cancer Chemother Pharmacol. (2014) 73:317–24. doi: 10.1007/s00280-013-2348-x
147. Lautenschlager F, Paschke S, Schinkinger S, Bruel A, Beil M, Guck J. The regulatory role of cell mechanics for migration of differentiating myeloid cells. Proc Natl Acad Sci USA. (2009) 106:15696–701. doi: 10.1073/pnas.0811261106
148. Murakami R, Matsumura N, Brown JB, Wang Z, Yamaguchi K, Abiko K, et al. Prediction of taxane and platinum sensitivity in ovarian cancer based on gene expression profiles. Gynecol Oncol. (2016) 141:49–56. doi: 10.1016/j.ygyno.2016.02.027
149. Bruno PM, Liu Y, Park GY, Murai J, Koch CE, Eisen TJ, et al. A subset of platinum-containing chemotherapeutic agents kills cells by inducing ribosome biogenesis stress. Nat Med. (2017) 23:461–71. doi: 10.1038/nm.4291
150. Poggio F, Bruzzone M, Ceppi M, Ponde NF, La Valle G, Del Mastro L, et al. Platinum-based neoadjuvant chemotherapy in triple-negative breast cancer: a systematic review and meta-analysis. Ann Oncol. (2018) 29:1497–508. doi: 10.1093/annonc/mdy127
151. Onier N, Hilpert S, Arnould L, Saint-Giorgio V, Davies JG, Jeannin JF, et al. Cure of colon cancer metastasis in rats with the new lipid A OM 174. apoptosis of tumor cells and immunization of rats. Clin Exp Metastasis. (1999) 17:299–306. doi: 10.1023/A:1006663017149
152. Gautier T, Paul C, Deckert V, Desrumaux C, Klein A, Labbe J, et al. Innate immune response triggered by triacyl lipid A is dependent on phospholipid transfer protein (PLTP) gene expression. FASEB J. (2010) 24:3544–54. doi: 10.1096/fj.09-152876
153. Seignez C, Martin A, Rollet CE, Racoeur C, Scagliarini A, Jeannin JF, et al. Senescence of tumor cells induced by oxaliplatin increases the efficiency of a lipid A immunotherapy via the recruitment of neutrophils. Oncotarget. (2014) 5:11442–51. doi: 10.18632/oncotarget.2556
154. Yang Y, Swierczak A, Ibahim M, Paiva P, Cann L, Stevenson AW, et al. Synchrotron microbeam radiotherapy evokes a different early tumor immunomodulatory response to conventional radiotherapy in EMT6.5 mammary tumors. Radiother Oncol. (2019) 133:93–9. doi: 10.1016/j.radonc.2019.01.006
155. Krombach J, Hennel R, Brix N, Orth M, Schoetz U, Ernst A, et al. Priming anti-tumor immunity by radiotherapy: dying tumor cell-derived DAMPs trigger endothelial cell activation and recruitment of myeloid cells. Oncoimmunology. (2019) 8:e1523097. doi: 10.1080/2162402X.2018.1523097
156. Gandhi KA, Goda JS, Gandhi VV, Sadanpurwala A, Jain VK, Joshi K, et al. Oral administration of 3,3'-diselenodipropionic acid prevents thoracic radiation induced pneumonitis in mice by suppressing NF-kB/IL-17/G-CSF/neutrophil axis. Free Radic Biol Med. (2019) 145:8–19. doi: 10.1016/j.freeradbiomed.2019.09.009
157. Lavigne J, Suissa A, Verger N, Dos Santos M, Benadjaoud M, Mille-Hamard L, et al. Lung stereotactic arc therapy in mice: development of radiation pneumopathy and influence of hif-1alpha endothelial deletion. Int J Radiat Oncol Biol Phys. (2019) 104:279–90. doi: 10.1016/j.ijrobp.2019.01.081
158. Wang F, Luo Y, Tian X, Ma S, Sun Y, You C, et al. Impact of radiotherapy concurrent with anti-PD-1 therapy on the lung tissue of tumor-bearing Mice. Radiat Res. (2019) 191:271–7. doi: 10.1667/RR15182.1
159. Sherry AD, von Eyben R, Newman NB, Gutkin P, Mayer I, Horst K, et al. Systemic inflammation after radiation predicts locoregional recurrence, progression, and mortality in stage ii-iii triple-negative breast cancer. Int J Radiat Oncol Biol Phys. (2019) doi: 10.1016/j.ijrobp.2019.11.398
160. Garcia-Mendoza MG, Inman DR, Ponik SM, Jeffery JJ, Sheerar DS, Van Doorn RR, et al. Neutrophils drive accelerated tumor progression in the collagen-dense mammary tumor microenvironment. Breast Cancer Res. (2016) 18:49. doi: 10.1186/s13058-016-0703-7
161. Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. (2009) 16:183–94. doi: 10.1016/j.ccr.2009.06.017
162. Sagiv JY, Michaeli J, Assi S, Mishalian I, Kisos H, Levy L, et al. Phenotypic diversity and plasticity in circulating neutrophil subpopulations in cancer. Cell Rep. (2015) 10:562–73. doi: 10.1016/j.celrep.2014.12.039
163. Jablonska J, Leschner S, Westphal K, Lienenklaus S, Weiss S. Neutrophils responsive to endogenous IFN-beta regulate tumor angiogenesis and growth in a mouse tumor model. J Clin Invest. (2010) 120:1151–64. doi: 10.1172/JCI37223
164. Hesari Z, Nourbakhsh M, Hosseinkhani S, Abdolvahabi Z, Alipour M, Tavakoli-Yaraki M, et al. Down-regulation of NAMPT expression by mir-206 reduces cell survival of breast cancer cells. Gene. (2018) 673:149–58. doi: 10.1016/j.gene.2018.06.021
165. Pylaeva E, Harati MD, Spyra I, Bordbari S, Strachan S, Thakur BK, et al. NAMPT signaling is critical for the proangiogenic activity of tumor-associated neutrophils. Int J Cancer. (2019) 144:136–49. doi: 10.1002/ijc.31808
166. Gong L, Cumpian AM, Caetano MS, Ochoa CE, De la Garza MM, Lapid DJ, et al. Promoting effect of neutrophils on lung tumorigenesis is mediated by CXCR2 and neutrophil elastase. Mol Cancer. (2013) 12:154. doi: 10.1186/1476-4598-12-154
Keywords: breast cancer, immuno-therapy, neutrophils, neutrophil-to-lymphocyte ratio, tumor microenvironment
Citation: Zhang W, Shen Y, Huang H, Pan S, Jiang J, Chen W, Zhang T, Zhang C and Ni C (2020) A Rosetta Stone for Breast Cancer: Prognostic Value and Dynamic Regulation of Neutrophil in Tumor Microenvironment. Front. Immunol. 11:1779. doi: 10.3389/fimmu.2020.01779
Received: 06 May 2020; Accepted: 03 July 2020;
Published: 07 August 2020.
Edited by:
Brahm Segal, University at Buffalo, United StatesReviewed by:
Olga Sizova, University of Texas MD Anderson Cancer Center, United StatesXuyao Zhang, University of Pennsylvania, United States
Copyright © 2020 Zhang, Shen, Huang, Pan, Jiang, Chen, Zhang, Zhang and Ni. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chao Ni, bmljYWhvNDI4QHpqdS5lZHUuY24=; Chao Zhang, emhhbmdjaGFvbWVkaWMyMDExQDE2My5jb20=
†These authors have contributed equally to this work
 Wei Zhang1†
Wei Zhang1† Chao Ni
Chao Ni