- 1Department of Rheumatology and Immunology, Nanjing Drum Tower Hospital, The Affiliated Hospital of Nanjing University Medical School, Nanjing, China
- 2Department of Immunology, College of Basic Medical Science, Dalian Medical University, Dalian, Liaoning, China
Interleukin 27 (IL-27) plays diverse immune regulatory roles in autoimmune disorders and promotes the generation of IL-10–producing CD4+ T cells characterized by producing the immunosuppressive cytokine IL-10. However, whether IL-27 participates in pathological progress of Sjögren syndrome (SS) through regulating CD4+IL-10+ T cells remains unknown. Here we aimed to explore the potential role of IL-27 and CD4+IL-10+ T cells in the pathogenesis of SS. The IL-27 gene knockout non-obese diabetic (Il-27−/−NOD) mice were generated and injected with exogenous IL-27. Exogenous injection of IL-27 and neutralization of IL-27 with anti–IL-27 antibody in NOD mice were performed. The histopathologic changes in submandibular glands, lacrimal glands and lung, salivary flow rate, and percentages of CD4+IL-10+ T cells were determined. And, ovalbumin-immunized C57L/B6 mice were injected with IL-27 to detect the percentage of CD4+IL-10+ T cells. In vitro, splenic naive T cells from C57L/B6 mice were cultured with IL-27 for 4 days to induce the differentiation of CD4+IL-10+ T cells. In addition, IL-27, IL-10, and CD4+IL-10+ T cells were determined in health control and SS patients. The results showed that Il-27−/−NOD mice had more severe disease and lower level of CD4+IL-10+ T cells than control mice. And IL-27 promoted the generation and differentiation of CD4+IL-10+ T cells in vivo and in vitro significantly. In agreement with the findings in the SS-like mice, patients with SS showed lower levels of IL-27, IL-10, and CD4+IL-10+ T cells. Our findings indicated that IL-27 deficiency aggravated SS by regulating CD4+IL-10+ T cells. Targeting IL-27 and CD4+IL-10+ T cells may be a novel therapy for patients with SS.
Introduction
Sjögren syndrome (SS) is a chronic autoimmune disease that typically presents with dry eyes and mouth as a result of lymphocytic infiltration in salivary (SGs) and lacrimal glands (LGs). Sjögren syndrome is characterized by the presence of autoreactive T and B cells in exocrine glands and circulating antibodies against several autologous antigens, such as the autoantibodies against SS antigen A (SSA)/Ro and SS antigen B (SSB)/La and the Fc fragment of immunoglobulin G (1, 2). The current treatments of SS are limited to be symptomatic, as a result of the elusive pathogenesis and diverse syndrome. According to reports, the dysregulated cytokine network contributes to the occurrence and development of SS. Thus, targeting to cytokine as the potential therapies in SS should be explored (3).
Interleukin 27 (IL-27) is a heterodimeric immunological factor of the IL-12 cytokine family and consists of p28 and Epstein-Barr virus–induced gene 3 (EBi3) subunits. Interleukin 27 mainly produced by antigen-presenting cells (APCs) upon stimulation of innate immune receptors (4–7). The IL-27 receptor is composed of the unique chain IL-27Rα (also called TCCR or WSX-1) and gp130 (8). Interleukin 27Rα is widely expressed in the immune cells (9). The ligation of IL-27 and its receptor induce intracellular signaling via heterogeneous Jak/STAT pathways, with predominant activation of STAT1 and STAT3 (10–12). Interleukin 27 was preliminarily characterized as a pro-inflammatory cytokine with TH1 induction. However, studies with infectious and autoimmune inflammatory models had reported that IL-27Rα deficiency mice developed excessive, pathological inflammation responding to a variety of challenges (13). And the anti-inflammatory role of IL-27 signaling has been illustrated in many recent studies (6, 14–17).
Hunter and colleagues showed the molecular mechanisms underlying suppressive characters of IL-27, which indicated that IL-27 could reduce IL-2 production during TH1 differentiation and promote the development of regulatory T (Treg) cells specialized to control TH1-mediated immunity at local sites of inflammation (18, 19). Interleukin 27 signaling in DCs played a key role in antigen-induced peripheral tolerance, which relied on the ability of IL-27 to induce T cell–derived IL-10 and interferon γ (IFN-γ) (20). And the study found that IL-27 and IL-6 induced TH1 and TH2 cells, as well as TH17 cells to secrete IL-10 (21). Interleukin 27 promoted the expression of inhibitory receptors on T cells in vivo and in vitro (22). Furthermore, studies reported that IL-27 drove the generation (23) and differentiation of IL-10–producing murine type 1 regulatory T (Tr1) cells by inducing three key factors: the transcription factor c-Maf, the cytokine IL-21, and the costimulatory receptor ICOS (24, 25).
Tr1 cells are a subset of T cells that have strong immunosuppressive properties and predominantly produce IL-10 with variable amounts of IFN-γ, IL-2, and transforming growth factor β (TGF-β), but do not express transcription factor Fork head box 3 (Foxp3) (23, 26, 27). There is a lot of research focused on Tr1 cells to suppress innate and adaptive immunity to alleviate inflammatory pathologies, in particular autoimmune diseases (28–31). Interleukin 27 could limit autoimmune diseases by stimulating IL-10–secreting T cells (6, 16, 32). Nevertheless, the role of IL-27 and CD4+IL-10+ T cells in SS remained unknown.
Here, our results showed that CD4+IL-10+ T cells related to SS pathogenesis and reduced generation of CD4+IL-10+ T cells was ascribed to decreased IL-27 in SS. Our findings indicated that targeting to IL-27 and CD4+IL-10+ T cells is a new direction for the SS treatment.
Materials and Methods
Study Population
A total of 31 SS patients and 34 health controls (HCs) from the Department of Rheumatology and Immunology, The Affiliated Drum Tower Hospital of Nanjing University Medical School, Nanjing University, were enrolled. Written informed consent was obtained from all subjects. Whole-blood samples were obtained. Plasma was isolated and frozen at −80°C until use. The study was approved by the ethics committee of our institute.
Mice
Seven-week-old female NOD and IL-27 gene knockout female NOD (Il-27−/−NOD) and 5-week-old female C57L/B6 mice were purchased from the Model Animal Research Center of Nanjing University and maintained under specific-pathogen–free conditions in the animal center of the Affiliated Drum Tower Hospital of Nanjing University Medical School.
Salivary Flow Rate
After anesthetization, the mice were stimulated with pilocarpine 0.1 mg pilocarpine/kg body weight injected intraperitoneally (i.p.). The whole saliva was obtained from the oral cavity for 15 min. Saliva volume was determined gravimetrically.
Histological Analysis
For histological analysis, mice were euthanized. Submandibular glands, LGs, and lung tissues were collected and embedded in paraffin for hematoxylin and eosin staining.
Preparation of Single Cell Suspensions
Spleen was isolated, and the red blood cells were lysed with lysing buffer for single cell suspension preparation. Peripheral blood mononuclear cells in blood from SS patients and healthy volunteers were isolated with Ficoll-Hypaque by density gradient centrifugation.
Flow Cytometry
Antibodies (eBioscience) were diluted at optimal concentration for cell immunostaining. To avoid non-specific binding to Fc receptors, isotype-matched antibodies were used as controls. For analysis of intracellular IL-10, cells were stimulated with 20 ng/mL PMA plus 1 μg/mL ionomycin with 5 μg/mL of brefeldin A (Enzo LifeScience, East Farmingdale, NY, USA) at 37°C for 4 h before harvest. First, surface CD4 with anti–mouse/human CD4–fluorescein isothiocyanate was stained. After that, cells were fixed with Cytofix/Cytoperm solution (BD Pharmingen), incubated with anti–mouse IL-10–APC or anti–human IL-10–APC and analyzed on a FACS Calibur flow cytometer (BD Biosciences, Mountain View, CA, USA).
Immunization
Seven-week-old female C57L/B6 mice were sensitized with 10 μg ovalbumin (OVA) in complete Freund's adjuvant intradermally as the described methods (33).
In vivo Treatment With IL-27 and Anti–IL-27
In the IL-27 treatment experiments, 11-week-old female NOD mice or OVA-immunized 8-week-old female C57L/B6 mice were injected with 200 ng/mouse IL-27 (BioLegend) once a day for a total of seven times. The control mice were injected with same volume of phosphate-buffered saline (PBS). In the anti-IL-27 treatment experiments, female 11-week-old NOD mice were injected with 0.5 mg/mouse anti-IL-27 (BioLegend) or with commensurable IgG2a isotype once i.p. Mice were sacrificed on the seventh day.
In vitro Tr1 Cell Differentiation
Splenic CD4+CD62L+ naive T cells were purified with Micro Beads (Miltenyi Biotec) from 6- to 8-week-old female C57L/B6 mice, with purity of more than 90%. Then naive T cells are cultured in a 96-well plate bound with 4 μg/mL anti-CD3e antibodies (eBioscience) at a density of 1 × 106/mL in RPMI 1640 supplemented with 10% fetal bovine serum and 100 U/mL penicillin/streptomycin, in the presence of 2 μg/mL anti-CD28 antibodies with 20 ng/mL mouse recombinant IL-27 (rIL-27) (BioLegend) or not for 4 days.
Cytokine Quantification
The plasma IL-10 and IL-27 were measured by standard sandwich enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems) according to the manufacturer's instructions.
Statistical Analysis
Statistical analysis was performed with Prism software version 5.0 (GraphPad Software). Differences in means ± SEM were evaluated with Student t test or one-way analysis of variance followed by Dunnett test. P < 0.05 was considered statistically significant.
Results
Il-27−/−NOD Mice Displayed Severe SS
Interleukin 27 has been shown to attenuate multiple autoimmune disorders by regulating the response of T cells and decreasing the production of inflammatory cytokines (34–36). To investigate the potential role of IL-27 in SS, we compared the severity of SS-like symptoms in Il-27−/−NOD and wild-type NOD mice. We found that Il-27−/−NOD mice developed rash (Figure 1A) and had swollen SGs compared to NOD mice (Figures S1A,B). Histopathologic analysis results showed more severe inflammation in SG (Figure 1B and Figure S1C), LGs (Figure 1C and Figure S1D), and lung (Figure 1D and Figure S1E) of Il-27−/−NOD mice. The level of salivary flow rate decreased significantly in Il-27−/−NOD mice (Figure 2C). These data indicated that the IL-27 deficiency aggravated SS in NOD mice.
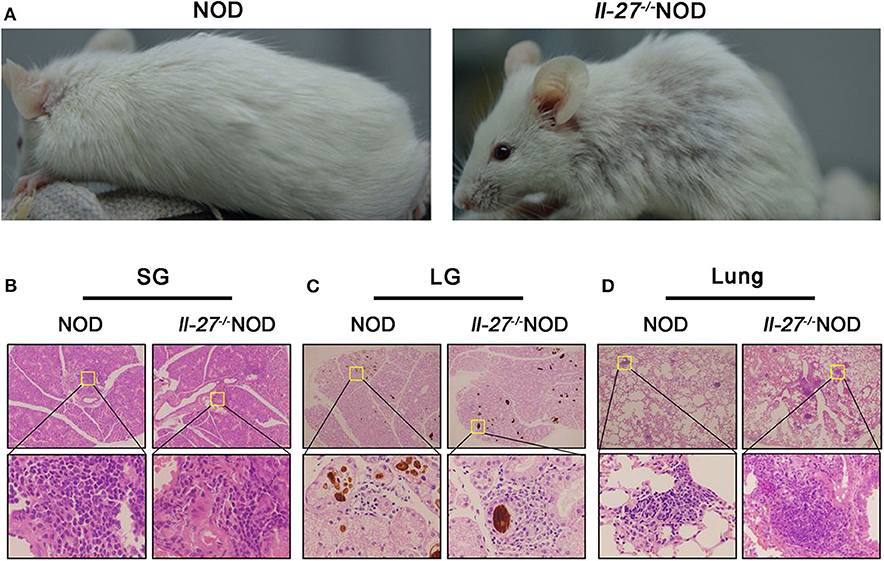
Figure 1. Interleukin 27 gene deficiency aggravated SS in NOD mice. (A) The physical appearance manifestation of rash in 12-week-old female NOD and Il-27−/−NOD mice. (B–D) Histological analysis, SGs, LGs, and lung from representative NOD and Il-27−/−NOD mice stained with hematoxylin and eosin to assess inflammation (top, magnification ×100; bottom, magnification ×400), n = 5.
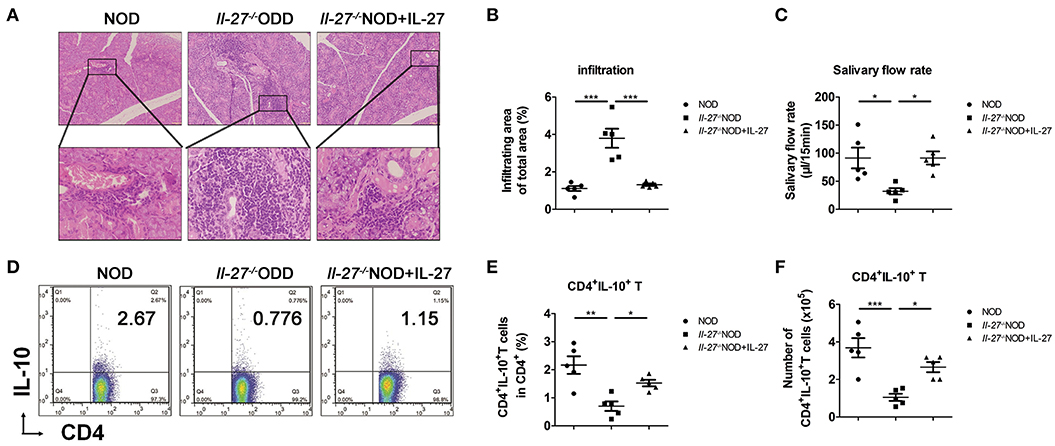
Figure 2. Lower levels of salivary flow rate and CD4+IL-10+ T cells in Il-27−/−NOD mice. (A) Histological analysis, SG from representative 12-week-old female NOD and Il-27−/−NOD and IL-27–treated Il-27−/−NOD mice stained with hematoxylin and eosin to assess inflammation (top, magnification ×100; bottom, magnification ×400). (B) The lymphocyte infiltration in SG of mice were evaluated for histological scores. (C) Salivary flow rate, (D) representative flow cytometry results, and (E) the percentage and (F) absolute number of splenic CD4+IL-10+ T cells in NOD and Il-27−/−NOD and IL-27–treated Il-27−/−NOD mice. Error bars indicate SEM. *p < 0.05, **p < 0.01, ***p < 0.001, n = 5.
CD4+IL-10+ T Cells Decreased in Il-27−/−NOD Mice
To explore the effects of IL-27 gene deficiency on inflammation in NOD mice, the mice were divided into three groups: NOD, Il-27−/−NOD, and Il-27−/−NOD mice with IL-27 treatment. More infiltrating lymphocytes and larger infiltrating area were seen in SG of Il-27−/− NOD mice compared to those of NOD and Il-27−/− NOD mice treated with IL-27 (Figures 2A,B). We collected the whole saliva from the oral cavity and found the saliva volume of Il-27−/− NOD mice reduced significantly, while exogenous IL-27 treatment could restore the salivary flow rate of Il-27−/−NOD mice (Figure 2C). The proportion and absolute number of splenic CD4+IL-10+ T cells of Il-27−/−NOD mice were lower than those of wild-type NOD mice. Exogenous IL-27 treatment restored the proportion (Figures 2D,E) and absolute number of splenic CD4+IL-10+ T cells (Figure 2F) significantly in Il-27−/−NOD mice. These findings suggested that IL-27 gene deficiency aggravated SS-like symptoms in NOD mice, and exogenous IL-27 treatment could reverse the decreasing salivary flow rate, splenic CD4+IL-10+ T cells, and serious inflammation.
Exogenous IL-27 Up-Regulated CD4+IL-10+ T Cells in NOD Mice
Given the aggravated SS-like symptoms and the decreased CD4+IL-10+ T cells in Il-27−/−NOD mice, we sought to investigate whether exogenous IL-27 treatment could up-regulate CD4+IL-10+ T cells and suppress inflammation in NOD mice. NOD mice were injected i.p. with recombinant IL-27, and the infiltration in SG, salivary flow rate, and splenic CD4+IL-10+ T cells were examined. We found NOD mice treated with IL-27 showed fewer lymphocytes in SG (Figure 3A) and LG (Figure S2A) and higher level of salivary flow rate (Figure 3B). Meanwhile, we used flow cytometry as the gating strategy (Figure S2C) to characterize the proportions and numbers of splenic CD4+IL-10+ T cells. We found significantly higher percentage, but not more numbers (Figure S2D), of splenic CD4+IL-10+ T cells in IL-27–treated NOD mice (Figures 3E,F). To further determine the significance of IL-27–induced of CD4+IL-10+ T cells in SS, anti-IL-27 and homologous isotype IgG2a were injected i.p. to NOD mice. As expected, we found the infiltration in SG (Figure 3C) and LG (Figure S2B) aggravated, the salivary flow rate reduced significantly (Figure 3D), and splenic CD4+IL-10+ T-cell proportion, but not the numbers (Figure S2E), decreased significantly (Figures 3G,H) in anti-IL-27–injected NOD mice. These results consist with our findings in Il-27−/−NOD mice and support the notion that IL-27 could inhibit SS-like symptoms in NOD by promoting CD4+IL-10+ T cells.
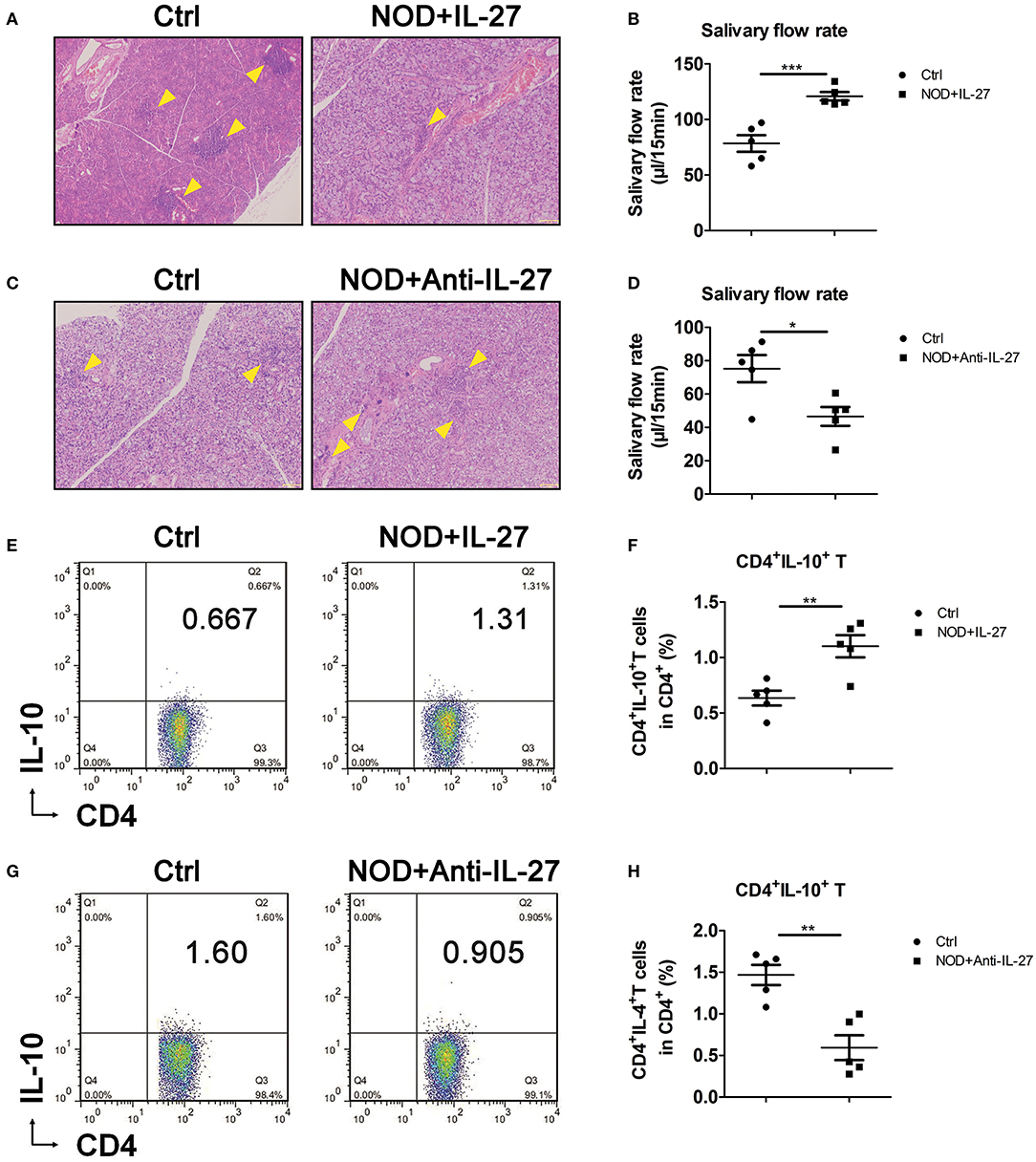
Figure 3. Interleukin 27 suppressed the inflammation in NOD mice. (A,C) Histological analysis, SG from representative mice stained with hematoxylin and eosin to assess inflammation (magnification ×100). Arrows indicate lymphocytes infiltrating focus. (B,D) Salivary flow rate of 12-week-old female NOD mice. (E,G) Representative flow cytometry results and (F,H) the percentage of splenic CD4+IL-10+ T cells in NOD mice. Error bars indicate SEM. *p < 0.05, **p < 0.01, ***p < 0.001, n = 5.
IL-27 Promoted the Development of CD4+IL-10+ T Cells
Next, the C57BL/6 mice were immunized with OVA and treated with PBS or IL-27 as schedule (Figure 4A). Results showed that IL-27 remarkably promoted the generation of CD4+IL-10+ T cells in vivo (Figures 4B,C). In addition, we cultured splenic naive T cells from C57BL/6 mice with rIL-27 in vitro to investigate the effects of IL-27 on the differentiation of CD4+IL-10+ T cells in vitro. And we found that IL-27 up-regulated the differentiation of CD4+IL-10+ T cells significantly (Figures 4D,E). Collectively, these results suggested that IL-27 significantly promoted generation and differentiation of CD4+IL-10+ T cells in vivo and in vitro.
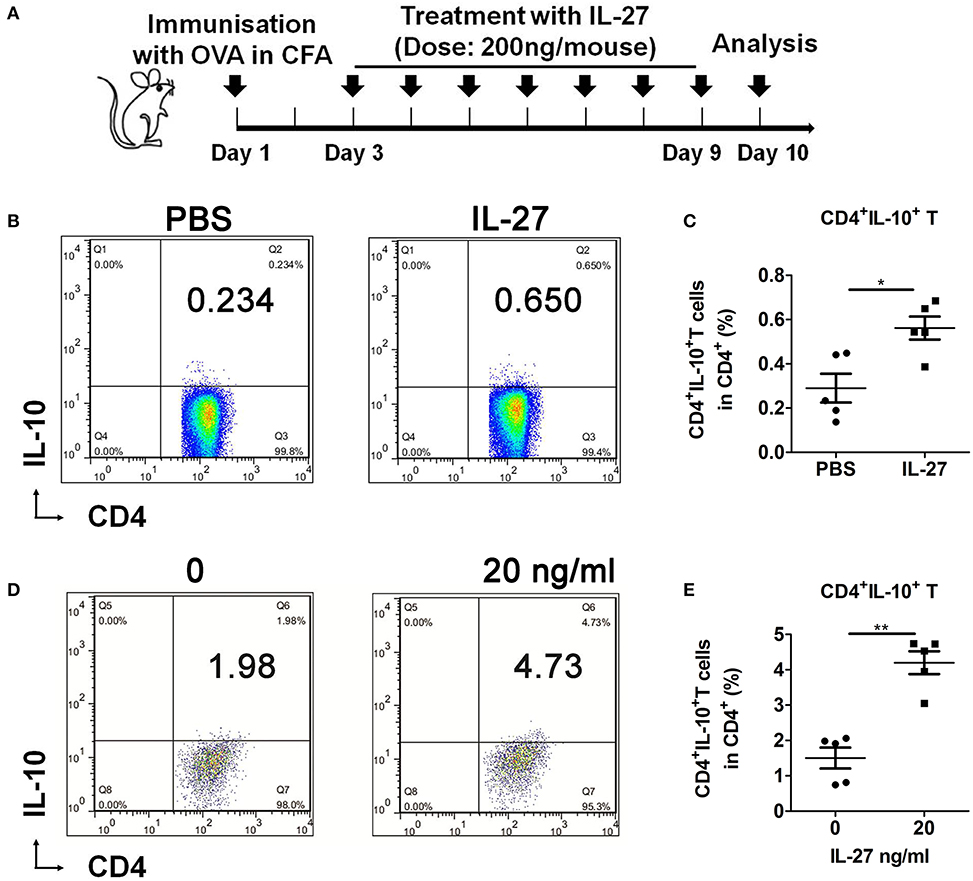
Figure 4. CD4+IL-10+ T cells generation and differentiation were promoted by IL-27. (A) IL-27 treatment schedule. (B) Representative flow cytometry results and (C) the percentage of splenic CD4+IL-10+ T cells in IL-27–treated or control OVA-immunized 9-week-old female C57BL/6 mice. (D) Representative flow cytometry results, and (E) the percentage of differentiated CD4+IL-10+ T cells. Error bars indicate SEM. *p < 0.05, **p < 0.01, n = 5.
IL-27 Correlated With CD4+IL-10+ T Cells in SS Patients
To examine the role and relationship of IL-27 and CD4+IL-10+ T cells in SS pathogenesis and whether they are correlated with known markers of SS disease, such as anti-SSA/Ro antibodies, anti-SSB/La antibodies, and antinuclear antibodies (ANAs). We detected the levels of plasma IL-27, IL-10, and peripheral CD4+IL-10+ T cells in HCs and SS patients. In addition, we analyzed the correlation between CD4+IL-10+ T cells and anti-SSA antibodies, anti-SSB antibodies, and ANAs in SS patients (Table S1). Results showed that the levels of plasma IL-27 (Figure 5A) and IL-10 (Figure 5B) and percentage of peripheral CD4+IL-10+ T cells (Figures 5C,D) in SS patients were lower than those in HCs. CD4+IL-10+ T cells were negatively correlated with anti-SSA antibodies (Figure 5E), but not anti-SSB antibodies (Figure 5F) and ANAs (Figure 5G). These data support the view that CD4+IL-10+ T cells regulated by IL-27 participated in SS pathogenesis.
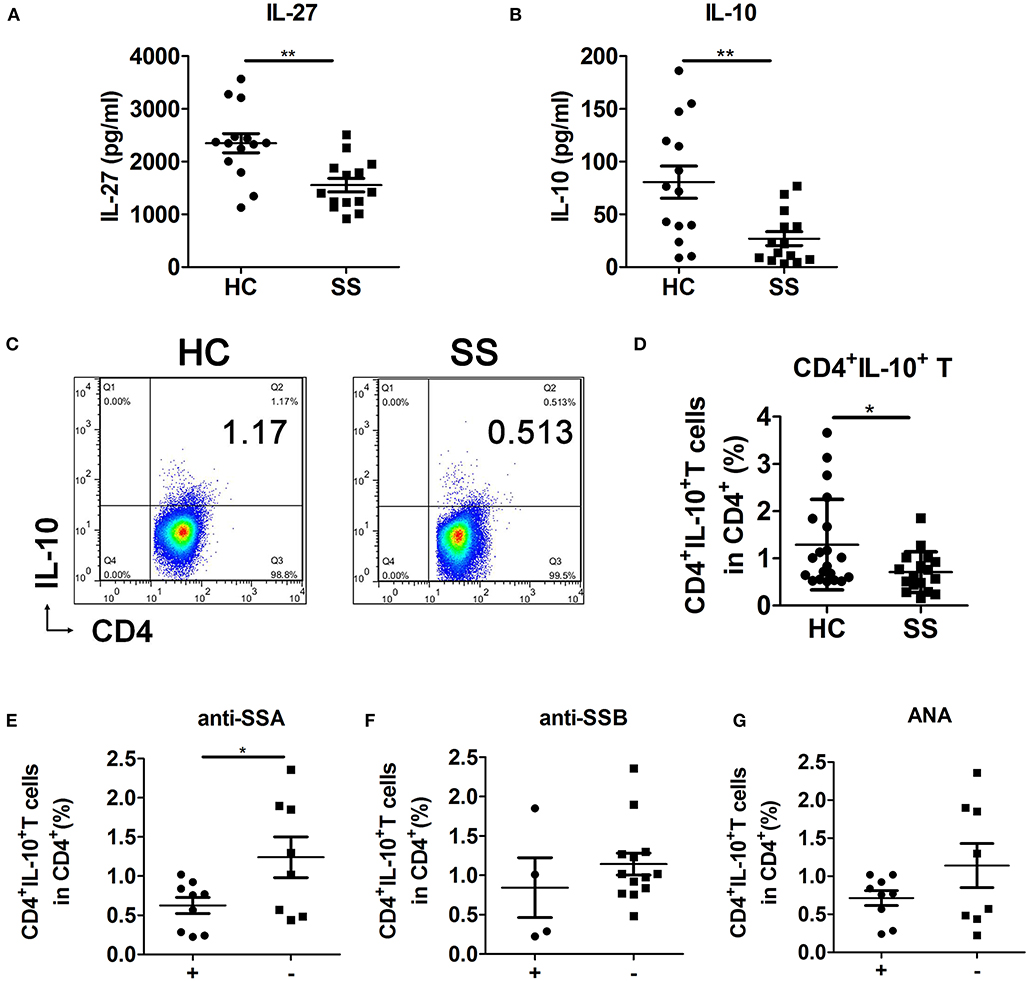
Figure 5. Interleukin 27 was related to decreased CD4+IL-10+ T cells in SS patients. (A,B) Plasma levels of IL-27 and IL-10 in HCs (n = 14) and SS patients (n = 14) were detected by ELISA. (C) Representative flow cytometry results and (D) the percentage of CD4+IL-10+ T cells in HCs (n = 20) and SS patients (n = 17). Sjögren syndrome patients were divided into antibodies-positive (+) and antibodies-negative (–) two groups. The correlations of CD4+IL-10+ T cells with (E) anti-SSA antibodies, (F) anti-SSB antibodies, and (G) ANAs were analyzed in SS patients. Error bars indicate SEM. *p < 0.05, **p < 0.01.
Discussion
Our study here has shown that IL-27–regulated CD4+IL-10+ T cells participated in SS pathogenesis. Il-27−/−NOD mice displayed severe SS disease. Exogenous IL-27 improved the symptoms of SS by promoting the generation of Tr1 cells in Il-27−/−NOD and NOD mice. And, antibody neutralization of IL-27 not only exacerbated inflammation but also reduced splenic CD4+IL-10+ T cells significantly in NOD mice. Moreover, SS patients have lower levels of IL-27, IL-10, and CD4+IL-10+ T cells. And, CD4+IL-10+ T cells were negatively correlated with anti-SSA antibodies. Together, these results have demonstrated a critical role of IL-27 in the pathogenesis of SS.
The anti-inflammatory properties of IL-27 have been reported in several autoimmune diseases, and IL-27 has been proposed as a therapy to modify inflammatory conditions by regulating T-cell responses (32). However, the contradictory effects of IL-27 have been reported in type 1 diabetes (T1D). Recent investigation reported that IL-27 not only showed immunomodulatory function, but also was a compensatory effort of dendritic cells against the ongoing inflammation in T1D patients (37). On the contrary, Ciecko et al. (38) reported that IL-27 contributes to the pathogenesis of T1D in NOD mice by altering the balance of Treg and TH1 cells and enhancing the effector function of CD8 T cells, although it was reported that serum IL-27 was strongly elevated in patients with SS (39), which is inconsistent with our results. This may be because SS patients were particularly associated with interstitial lung disease complications in their study. Another study reported that the levels of IL-27 and IL-23 increased significantly in the serum and urine of systemic lupus erythematosus patients with and without lupus nephritis compared with healthy control (40), which may account for the pleiotopic roles of IL-27 in several cases of autoimmunity. Up to date, many studies have reported that IL-27 showed a protective role in a variety of diseases, and exogenous IL-27 could suppress multiple autoimmune diseases by promoting Tr1 cells or other mechanisms (6, 16, 35, 36, 41, 42). Lee et al. (43) reported that exogenous IL-27 could induce a suppressive effect on SS development by regulating TH17 pro-inflammatory activity. Our previous study showed that IL-27 decreased significantly in SS patients and SS-like NOD mice, and mesenchymal stem cells (MSCs) alleviated SS by elevating the level of IL-27 (44). In this study, we found that IL-27 alleviated SS by inducing IL-10–producing CD4+ T cells. The different properties of IL-27 in the pathogenic conditions may account for the complex effects of IL-27 on different lymphocyte populations, which play a pleiotropic role in the development and progression of disease in NOD mice. Thus, the specific effects of IL-27 in SS need further investigation. In our studies, we compared the SS-like symptoms in NOD and Il-27−/−NOD mice and found the protective function of IL-27 by regulating Tr1 cells in SS.
Tr1 cells, a T-cell population with distinct suppressive function, are characterized by secreting high amounts of IL-10 and variable amounts of IFN-γ, IL-2, and TGF-β, depending on the microenvironment and the disease context (23), while none of the biomarkers are yet the ideal candidates for Tr1 cells. Studies suggested that IL-10–producing CD4+ T cells may represent a heterogeneous cell population reflecting different cell origins, maturation stage, or different functions of Tr1 cells (45). Tr1 cells suppressed immune responses mainly by producing IL-10 (23). A number of investigators have also documented that Tr1 cells could prevent T cell–mediated diseases via a TGF-β-dependent mechanism in addition to IL-10 (46, 47). Interleukin 27 was the dominant factor for IL-10–producing T cells and worked together with TGF-β to further enhance Tr1 differentiation (23). And IL-27 limited autoimmune disorders by promoting Tr1 cells. In this study, exogenous IL-27 ameliorated SS-like syndromes in NOD mice by promoting the generation of CD4+IL-10+ T cells in NOD and Il-27−/−NOD mice, whereas IFN-γ and TGF-β as the important factors involved in the induction and function of Tr1 cells should be considered and need to be identified.
Although the deficiency of IL-27 signal resulted in more serious SS-like symptoms and fewer splenic CD4+IL-10+ T cells in NOD mice, the percentage of CD4+IL-10+ T cells was very low and showed no significant difference in SG of NOD and Il-27−/−NOD mice (Figure S3). The improved SS-like syndromes in IL-27–treated NOD mice might be due to that splenic CD4+IL-10+ T cells could affect already established inflammation in SG and saliva flow indirectly via IL-10 to regulate other immune responses in the immune system.
Many different cytokines can induce JAKs and STATs activation to regulate fundamental biological processes (48). It has been reported that IL-27 induced IL-10–producing Tr1 cells generation by activating STAT1 and STAT3. While other cytokines that signal, such as IFN-α or IL-6, alone or in combination, could promote the generation of Tr1 cells via STAT3 (49, 50). Brockmann et al. reported that IL-10 signaling controlled the differentiation and regulatory activity of Tr1 cells via P38MAPK and not by STAT3 (51). These studies suggested that STAT1 and/or STAT3 were activated in the Tr1 cells depending on different cytokine signals.
In summary, we have demonstrated that IL-27 could promote the development and differentiation of CD4+IL-10+ T cells in vitro and in vivo. Interleukin 27 gene deficiency resulted in decreased CD4+IL-10+ T cells and aggravated the severity of SS-like symptoms of NOD mice. Exogenous IL-27 ameliorated Il-27−/−NOD and NOD mice by regulating CD4+IL-10+ T cells. Notably, CD4+IL-10+ T cells decreased in SS patients, which may be correlated to IL-27. These findings suggested that IL-27 played a crucial role in the pathogenesis of SS by regulating CD4+IL-10+ T cells. We propose that targeting IL-27 and CD4+IL-10+ T cells may be a new direction for the SS treatment.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies were reviewed and approved by Nanjing Drum Tower Hospital, The Affiliated Hospital of Nanjing University Medical School. Written informed consent was obtained from all subjects.
Author Contributions
JQ and ZZ participated in study design, data collection, data analysis, data interpretation, and drafting the paper. XT, WL, and WC participated in patient recruitment, animal experiments, and data collection. GY supervised the whole research, designed the study, interpreted the data, and wrote the paper. All authors read and approved the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (NSFC) (grant nos. 81770061, 81970062, and 81571583 to GY) and Key Project supported by Medical Science and technology development Foundation, Nanjing Department of Health (grant no. ZKX18024 to GY).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2020.01699/full#supplementary-material
References
1. Mavragani CP. Mechanisms and new strategies for primary Sjogren's syndrome. Annu Rev Med. (2017) 68:331–43. doi: 10.1146/annurev-med-043015-123313
2. Mavragani CP, Moutsopoulos HM. Sjogren's syndrome. Annu Rev Pathol. (2014) 9:273–85. doi: 10.1146/annurev-pathol-012513-104728
3. Roescher N, Tak PP, Illei GG. Cytokines in Sjogren's syndrome. Oral Dis. (2009) 15:519–26. doi: 10.1111/j.1601-0825.2009.01582.x
4. Kimura D, Miyakoda M, Kimura K, Honma K, Hara H, Yoshida H, et al. Interleukin-27-producing CD4+ T cells regulate protective immunity during malaria parasite infection. Immunity. (2016) 44:672–82. doi: 10.1016/j.immuni.2016.02.011
5. Yoshida H, Hunter CA. The immunobiology of interleukin-27. Annu Rev Immunol. (2015) 33:417–43. doi: 10.1146/annurev-immunol-032414-112134
6. Batten M, Li J, Yi S, Kljavin NM, Danilenko DM, Lucas S, et al. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat Immunol. (2006) 7:929–36. doi: 10.1038/ni1375
7. Pflanz S, Timans JC, Cheung J, Rosales R, Kanzler H, Gilbert J, et al. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4+ T cells. Immunity. (2002) 16:779–90. doi: 10.1016/S1074-7613(02)00324-2
8. Pflanz S, Hibbert L, Mattson J, Rosales R, Vaisberg E, Bazan JF, et al. WSX-1 and glycoprotein 130 constitute a signal-transducing receptor for IL-27. J Immunol. (2004) 172:2225–31. doi: 10.4049/jimmunol.172.4.2225
9. Villarino AV, Larkin J III, Saris CJ, Caton AJ, Lucas S, Wong T, et al. Positive and negative regulation of the IL-27 receptor during lymphoid cell activation. J Immunol. (2005) 174:7684–91. doi: 10.4049/jimmunol.174.12.7684
10. Artis D, Villarino A, Silverman M, He W, Thornton EM, Mu S, et al. The IL-27 receptor (WSX-1) is an inhibitor of innate and adaptive elements of type 2 immunity. J Immunol. (2004) 173:5626–34. doi: 10.4049/jimmunol.173.9.5626
11. Takeda A, Hamano S, Yamanaka A, Hanada T, Ishibashi T, Mak TW, et al. Cutting edge: role of IL-27/WSX-1 signaling for induction of T-bet through activation of STAT1 during initial Th1 commitment. J Immunol. (2003) 170:4886–90. doi: 10.4049/jimmunol.170.10.4886
12. Hibbert L, Pflanz S, De Waal Malefyt R, Kastelein RA. IL-27 and IFN-alpha signal via Stat1 and Stat3 and induce T-Bet and IL-12Rbeta2 in naive T cells. J Interferon Cytokine Res. (2003) 23:513–22. doi: 10.1089/10799900360708632
13. Yoshida H, Hamano S, Senaldi G, Covey T, Faggioni R, Mu S, et al. WSX-1 is required for the initiation of Th1 responses and resistance to L. major infection. Immunity. (2001) 15:569–78. doi: 10.1016/S1074-7613(01)00206-0
14. Chen X, Deng R, Chi W, Hua X, Lu F, Bian F, et al. IL-27 signaling deficiency develops Th17-enhanced Th2-dominant inflammation in murine allergic conjunctivitis model. Allergy. (2019) 74:910–21. doi: 10.1111/all.13691
15. Wang L, Cao J, Li C, Zhang L. IL-27/IL-27 receptor signaling provides protection in Clostridium difficile-induced colitis. J Infect Dis. (2018) 217:198–207. doi: 10.1093/infdis/jix581
16. Fitzgerald DC, Zhang GX, El-Behi M, Fonseca-Kelly Z, Li H, Yu S, et al. Suppression of autoimmune inflammation of the central nervous system by interleukin 10 secreted by interleukin 27-stimulated T cells. Nat Immunol. (2007) 8:1372–9. doi: 10.1038/ni1540
17. Do J, Kim D, Kim S, Valentin-Torres A, Dvorina N, Jang E, et al. Treg-specific IL-27Ralpha deletion uncovers a key role for IL-27 in Treg function to control autoimmunity. Proc Natl Acad Sci USA. (2017) 114:10190–5. doi: 10.1073/pnas.1703100114
18. Hall AO, Beiting DP, Tato C, John B, Oldenhove G, Lombana CG, et al. The cytokines interleukin 27 and interferon-gamma promote distinct Treg cell populations required to limit infection-induced pathology. Immunity. (2012) 37:511–23. doi: 10.1016/j.immuni.2012.06.014
19. Villarino AV, Stumhofer JS, Saris CJ, Kastelein RA, de Sauvage FJ, Hunter CA. IL-27 limits IL-2 production during Th1 differentiation. J Immunol. (2006) 176:237–47. doi: 10.4049/jimmunol.176.1.237
20. Thome R, Moore JN, Mari ER, Rasouli J, Hwang D, Yoshimura S, et al. Induction of peripheral tolerance in ongoing autoimmune inflammation requires interleukin 27 signaling in dendritic cells. Front Immunol. (2017) 8:1392. doi: 10.3389/fimmu.2017.01392
21. Stumhofer JS, Silver JS, Laurence A, Porrett PM, Harris TH, Turka LA, et al. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat Immunol. (2007) 8:1363–71. doi: 10.1038/ni1537
22. DeLong JH, O'Hara Hall A, Rausch M, Moodley D, Perry J, Park J, et al. IL-27 and TCR stimulation promote T cell expression of multiple inhibitory receptors. Immunohorizons. (2019) 3:13–25. doi: 10.4049/immunohorizons.1800083
23. Awasthi A, Carrier Y, Peron JP, Bettelli E, Kamanaka M, Flavell RA, et al. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat Immunol. (2007) 8:1380–9. doi: 10.1038/ni1541
24. Pot C, Jin H, Awasthi A, Liu SM, Lai CY, Madan R, et al. Cutting edge: IL-27 induces the transcription factor c-Maf, cytokine IL-21, and the costimulatory receptor ICOS that coordinately act together to promote differentiation of IL-10-producing Tr1 cells. J Immunol. (2009) 183:797–801. doi: 10.4049/jimmunol.0901233
25. Murugaiyan G, Mittal A, Lopez-Diego R, Maier LM, Anderson DE, Weiner HL. IL-27 is a key regulator of IL-10 and IL-17 production by human CD4+ T cells. J Immunol. (2009) 183:2435–43. doi: 10.4049/jimmunol.0900568
26. Vieira PL, Christensen JR, Minaee S, O'Neill EJ, Barrat FJ, Boonstra A, et al. IL-10-secreting regulatory T cells do not express Foxp3 but have comparable regulatory function to naturally occurring CD4+CD25+ regulatory T cells. J Immunol. (2004) 172:5986–93. doi: 10.4049/jimmunol.172.10.5986
27. Mbongue JC, Rawson J, Garcia PA, Gonzalez N, Cobb J, Kandeel F, et al. Reversal of new onset type 1 diabetes by oral salmonella-based combination therapy and mediated by regulatory T-cells in NOD mice. Front Immunol. (2019) 10:320. doi: 10.3389/fimmu.2019.00320
28. Cook L, Stahl M, Han X, Nazli A, MacDonald KN, Wong MQ, et al. Suppressive and gut reparative functions of human type 1 T-regulatory cells. Gastroenterology. (2019) 157:1584–98. doi: 10.1053/j.gastro.2019.09.002
29. Yu H, Gagliani N, Ishigame H, Huber S, Zhu S, Esplugues E, et al. Intestinal type 1 regulatory T cells migrate to periphery to suppress diabetogenic T cells and prevent diabetes development. Proc Natl Acad Sci USA. (2017) 114:10443–8. doi: 10.1073/pnas.1705599114
30. Mfarrej B, Tresoldi E, Stabilini A, Paganelli A, Caldara R, Secchi A, et al. Generation of donor-specific Tr1 cells to be used after kidney transplantation and definition of the timing of their in vivo infusion in the presence of immunosuppression. J Transl Med. (2017) 15:40. doi: 10.1186/s12967-017-1133-8
31. Gagliani N, Jofra T, Stabilini A, Valle A, Atkinson M, Roncarolo MG, et al. Antigen-specific dependence of Tr1-cell therapy in preclinical models of islet transplant. Diabetes. (2010) 59:433–9. doi: 10.2337/db09-1168
32. Tait Wojno ED, Hunter CA, Stumhofer JS. The immunobiology of the interleukin-12 family: room for discovery. Immunity. (2019) 50:851–70. doi: 10.1016/j.immuni.2019.03.011
33. Suzuki M, Yokota M, Ozaki S, Matsumoto T. Intranasal administration of IL-27 ameliorates nasal allergic responses and symptoms. Int Arch Allergy Immunol. (2019) 178:101–5. doi: 10.1159/000493398
34. Zhu X, Liu Z, Liu JQ, Zhu J, Zhang J, Davis JP, et al. Systemic delivery of IL-27 by an adeno-associated viral vector inhibits T cell-mediated colitis and induces multiple inhibitory pathways in T cells. J Leukocyte Biol. (2016) 100:403–11. doi: 10.1189/jlb.3A1215-540R
35. Moon SJ, Park JS, Heo YJ, Kang CM, Kim EK, Lim MA, et al. In vivo action of IL-27: reciprocal regulation of Th17 and Treg cells in collagen-induced arthritis. Exp Mol Med. (2013) 45:e46. doi: 10.1038/emm.2013.89
36. Niedbala W, Cai B, Wei X, Patakas A, Leung BP, McInnes IB, et al. Interleukin 27 attenuates collagen-induced arthritis. Ann Rheum Dis. (2008) 67:1474–9. doi: 10.1136/ard.2007.083360
37. Parackova Z, Vrabcova P, Zentsova I, Kayserova J, Richtrova I, Sojka L, et al. Enhanced STAT3 phosphorylation and PD-L1 expression in myeloid dendritic cells indicate impaired IL-27Ralpha signaling in type 1 diabetes. Sci Rep. (2020) 10:493. doi: 10.1038/s41598-020-57507-8
38. Ciecko AE, Foda B, Barr JY, Ramanathan S, Atkinson MA, Serreze DV, et al. Interleukin-27 is essential for type 1 diabetes development and Sjogren syndrome-like inflammation. Cell Rep. (2019) 29:3073–86.e5. doi: 10.1016/j.celrep.2019.11.010
39. Xia L, Shen H, Zhao L, Lu J. Elevated levels of interleukin-27 in patients with Sjogren's syndrome. Scand J Rheumatol. (2012) 41:73–4. doi: 10.3109/03009742.2011.620574
40. Xia LP, Li BF, Shen H, Lu J. Interleukin-27 and interleukin-23 in patients with systemic lupus erythematosus: possible role in lupus nephritis. Scand J Rheumatol. (2015) 44:200–5. doi: 10.3109/03009742.2014.962080
41. Sugiyama N, Nakashima H, Yoshimura T, Sadanaga A, Shimizu S, Masutani K, et al. Amelioration of human lupus-like phenotypes in MRL/lpr mice by overexpression of interleukin 27 receptor alpha (WSX-1). Ann Rheum Dis. (2008) 67:1461–7. doi: 10.1136/ard.2007.077537
42. Fitzgerald DC, Ciric B, Touil T, Harle H, Grammatikopolou J, Das Sarma J, et al. Suppressive effect of IL-27 on encephalitogenic Th17 cells and the effector phase of experimental autoimmune encephalomyelitis. J Immunol. (2007) 179:3268–75. doi: 10.4049/jimmunol.179.5.3268
43. Lee BH, Carcamo WC, Chiorini JA, Peck AB, Nguyen CQ. Gene therapy using IL-27 ameliorates Sjogren's syndrome-like autoimmune exocrinopathy. Arthritis Res Ther. (2012) 14:R172. doi: 10.1186/ar3925
44. Yao G, Qi J, Liang J, Shi B, Chen W, Li W, et al. Mesenchymal stem cell transplantation alleviates experimental Sjogren's syndrome through IFN-beta/IL-27 signaling axis. Theranostics. (2019) 9:8253–65. doi: 10.7150/thno.37351
45. Sakaguchi S, Mikami N, Wing JB, Tanaka A, Ichiyama K, Ohkura N. Regulatory T cells and human disease. Annu Rev Immunol. (2020) 38:541–66. doi: 10.1146/annurev-immunol-042718-041717
46. Levings MK, Sangregorio R, Galbiati F, Squadrone S, de Waal Malefyt R, Roncarolo MG. IFN-alpha and IL-10 induce the differentiation of human type 1 T regulatory cells. J Immunol. (2001) 166:5530–9. doi: 10.4049/jimmunol.166.9.5530
47. Singh B, Krawetz MD, De Lima RM, Mukherjee R, Chaturvedi P, Lee-Chan E, et al. Role of TGF-beta in self-peptide regulation of autoimmunity. Arch Immunol Ther Exp. (2018) 66:11–19. doi: 10.1007/s00005-017-0482-6
48. Salas A, Hernandez-Rocha C, Duijvestein M, Faubion W, McGovern D, Vermeire S, et al. JAK-STAT pathway targeting for the treatment of inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. (2020) 17:323–37. doi: 10.1038/s41575-020-0273-0
49. Jin JO, Han X, Yu Q. Interleukin-6 induces the generation of IL-10-producing Tr1 cells and suppresses autoimmune tissue inflammation. J Autoimmun. (2013) 40:28–44. doi: 10.1016/j.jaut.2012.07.009
50. Corre B, Perrier J, El Khouri M, Cerboni S, Pellegrini S, Michel F. Type I interferon potentiates T-cell receptor mediated induction of IL-10-producing CD4+ T cells. Eur J Immunol. (2013) 43:2730–40. doi: 10.1002/eji.201242977
Keywords: Sjögren syndrome, CD4+IL-10+ T cells, interleukin-27, interleukin-10, immunosuppression
Citation: Qi J, Zhang Z, Tang X, Li W, Chen W and Yao G (2020) IL-27 Regulated CD4+IL-10+ T Cells in Experimental Sjögren Syndrome. Front. Immunol. 11:1699. doi: 10.3389/fimmu.2020.01699
Received: 07 April 2020; Accepted: 25 June 2020;
Published: 11 August 2020.
Edited by:
Huji Xu, Tsinghua University, ChinaReviewed by:
Howard A. Young, National Cancer Institute at Frederick, United StatesUmesh S. Deshmukh, Oklahoma Medical Research Foundation, United States
Copyright © 2020 Qi, Zhang, Tang, Li, Chen and Yao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Genhong Yao, eWFvZ2VuaG9uZ0BuanUuZWR1LmNu
†These authors have contributed equally to this work
 Jingjing Qi1,2†
Jingjing Qi1,2† Genhong Yao
Genhong Yao