
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
GENERAL COMMENTARY article
Front. Immunol., 23 July 2020
Sec. Viral Immunology
Volume 11 - 2020 | https://doi.org/10.3389/fimmu.2020.01400
A Commentary on
Antibodies to Human Herpesviruses in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Patients
by Blomberg, J., Rizwan, M., Böhlin-Wiener, A., Elfaitouri, A., Julin, P., Zachrisson, O., et al. (2019). Front. Immunol. 10:1945. doi: 10.3389/fimmu.2019.01946
Studies to ascertain a possible relationship between herpesviruses and myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) have relied heavily on classical approaches, specifically, serological examination for antibodies against virus proteins, primarily structural, and/or increases in viral load (1–21). These data have been conflicting due in part to several features: the heterogeneity of the disease, high prevalence of the herpesviruses in the population since they can establish lifelong infections, and differences between laboratories. Two additional problems lead to conflicting data in serological studies: which viral antigens are to be used for detection, and what is the possible relationship, if any, of these viral antigens to ME/CFS? These are important questions that must be addressed for any data to provide meaningful insight into the possible contribution of a virus to the pathophysiology of ME/CFS. Although the experimental techniques used in Blomberg's serological study were appropriate, the selection of specific herpesviruses and viral antigens studied gives a limited view of the humoral response in ME/CFS.
Blomberg et al. (22) used a suspension multiplex immunoassay to detect antibodies against various herpesviruses' antigens, derived from proteins expressed during latency or late lytic replication (Figure 1), with the goal of determining differences in antibody titers against these antigens between ME/CFS patients and controls. However, no rationale was given as to why these particular antigens were chosen and what association, if any, they may have with ME/CFS. This is important because the antigenic properties of the different virus proteins are not the same. As demonstrated in an eloquent study by Vaider-Shalt et al. (23), over the course of their evolution, herpes simplex virus 1 (HSV-1), Epstein-Barr virus (EBV), human herpesvirus 8 (HHV-8), and human cytomegalovirus have decreased the number of epitopes present in virus proteins in order to help them avoid immune detection. Thus, the ability of a virus protein to generate an antibody response is dependent upon the amount of protein present in the host and its antigenicity. It is also not clear why Blomberg et al. (22) included HSV-1/2, human cytomegalovirus, HHV-7, and varicella zoster virus (VZV) in their study since there are no up-to-date literature reports establishing a serological relationship between these viruses and ME/CFS.
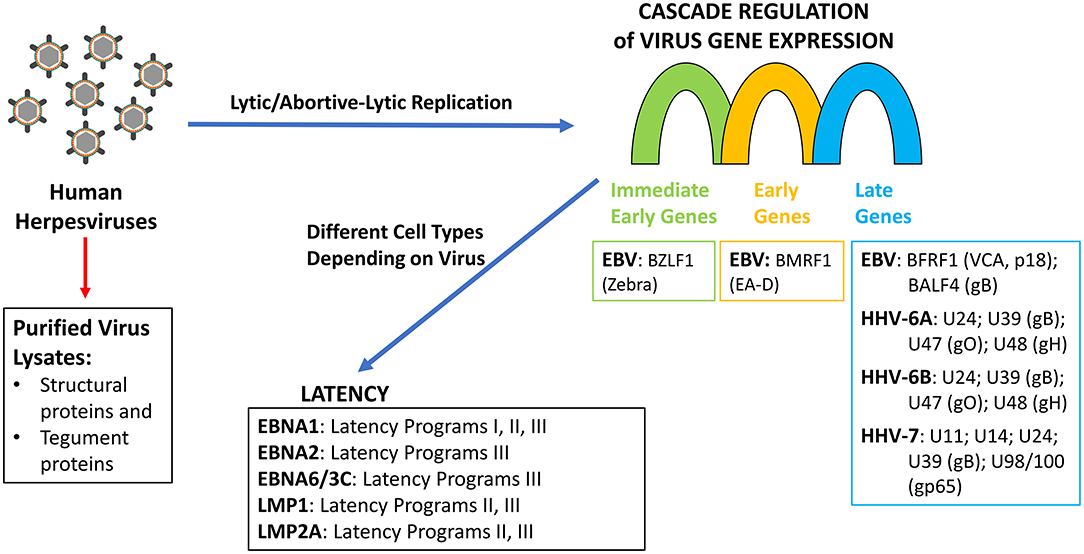
Figure 1. Herpesviruses replication and latency programs indicating the gene/gene products tested as antigens by Blomberg et al. (22).
Along these lines, measuring the response in patients' sera using purified virus lysate would only detect antibodies to proteins that are components of the virion (capsid proteins, glycoproteins, and tegument proteins) but not proteins expressed during virus lytic/abortive-lytic replication (Figure 1). Furthermore, since herpesviruses establish persistent infections in a significant proportion of the population (24), it is not surprising that antibody titers against purified virus lysates are not significantly different between ME/CFS cases and controls, especially since viremia has not been demonstrated in ME/CFS patients. Using “crude” antigen preparations make it impossible to ascertain whether there is a difference in the formation of specific antibodies against a single protein within the two populations under study. Although the epitope mapping studies provide qualitative data, the antigens have little relevance to ME/CFS, as they focused primarily on viral proteins that are expressed either during latency in the case of EBV or late in the lytic replicative cycles of EBV, HHV-6A/B, and HHV-7. Several studies have demonstrated that there is no increase in viral load of EBV or HHV-6A/B in ME/CFS patients (12, 14, 15, 17, 21), which suggests that there is no increase in the expression of late herpesvirus proteins in ME/CFS patients. Nor is there, in the case of EBV, an increase in viral capsid antigen (VCA) antibody titers, suggesting that abortive lytic replication is occurring in these patients (1, 17–19). Likewise, there is no evidence suggesting a role of any latency expressed gene products in ME/CFS. There is a single report showing a small non-significant difference in IgG response to Epstein-Barr nuclear antigen 3C (EBNA-3C; EBNA-6) by peptide microarray (25). EBNA-3C is the product of a gene only expressed in B cells undergoing the latency III gene-expression program, and there is no evidence to support latency type III in ME/CFS. Similarly, no rationale was provided for examining EBNA-1, although there are no studies linking it with ME/CFS. If a causal relationship is to be established between EBV, HHV-6, or any other herpesvirus and ME/CFS, it will require addressing the role of viral proteins produced during abortive-lytic replication of the herpesviruses, which does not lead to new viral progeny, and thus, no changes in viral load would be observed. Therefore, measuring humoral responses in patients' sera using virus lysate is not appropriate because it would only detect antibodies to proteins that are components of the virion but not to proteins expressed during abortive-lytic replication.
Lastly, Blomberg et al. (22) indicate in the Introduction and Discussion of their manuscript that a recent study (1) implicated VZV in ME/CFS. That is not the case. In that study, the investigators demonstrated that although ME/CFS patients' serum contained anti-VZV deoxyuridine triphosphate nucleotidohydrolase (dUTPase) antibodies, it was not significant (p < 0.857).
In conclusion, although the suspension multiplex immunoassay seems appropriate, it may lack the sensitivity of the luciferase immunoprecipitation system (26) and VirScan (27) serological platforms that have been utilized for the detection of viral infections including those caused by EBV, HSV-1, HSV-2, HHV-8, and VZV (28).
The author confirms being the sole contributor of this work and has approved it for publication.
This work was supported by the National Institutes of Health grant R01 A1084898 to MA.
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Halpin P, Williams M, Klimas NG, Fletcher MA, Barnes Z, Ariza ME. Differential antibody patterns to the herpesviruses-encoded dUTPases in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome and Gulf War Illness. J Med Virol. (2017) 89:1636–45. doi: 10.1002/jmv.24810
2. Tobi M, Morag A, Ravid Z, Chowers I, Feld-Weiss V, Michaeli Y, et al. Prolonged atypical illness associated with serological evidence of persistent Epstein-Barr virus infection. Lancet. (1982) 1:61–4. doi: 10.1016/S0140-6736(82)90210-0
3. DuBois RE, Seeley JR, Brus I, Sakamoto K, Ballow M, Harada S, et al. Chronic mononucleosis syndrome. Southern Med J. (1984) 77:1376–82. doi: 10.1097/00007611-198411000-00007
4. Buchwald D, Sullivan JL, Komaroff AL. Frequency of chronic active Epstein-Barr virus infection in a general medical practice. J Am Med Assoc. (1987) 257:2303–7. doi: 10.1001/jama.1987.03390170059028
5. Holmes GP, Kaplan JE, Stewart JA, Hunt B, Pinsky PF, Schonberger LB. A cluster of patients with a chronic mononucleosis-like syndrome. Is Epstein-Barr virus the cause? J Am Med Assoc. (1987) 257:2297–302. doi: 10.1001/jama.257.17.2297
6. Hellinger WC, Smith TF, Van Scoy RE, Spitzer PG, Forgacs P, Edson RS. Chronic fatigue syndrome and the diagnostic utility of antibody to Epstein-Barr early antigen. J. Am Med Assoc. (1988) 260:971–3. doi: 10.1001/jama.1988.03410070099036
7. Straus SE, Dale JK, Tobi M, Lawley T, Prebel O, Blaese M, et al. Acyclovir treatment of the Chronic Fatigue Syndrome. Lack of efficacy in a placebo-controlled trial. N Engl J Med. (1988) 319:1692–8. doi: 10.1056/NEJM198812293192602
8. Gold D, Bowden R, Sixbey J, Riggs R, Katon WJ, Ashley R, et al. Chronic Fatigue. A prospective clinical and virologic study. J Am Med Assoc. (1990) 264:48–53. doi: 10.1001/jama.264.1.48
9. Levine PH, Jacobson S, Pocinki AG, Cheney P, Peterson D, Connelly RR, et al. Clinical, epidemiological, and virological studies in four clusters of the Chronic Fatigue Syndrome. Arch Intern Med. (1992) 152:1611–16. doi: 10.1001/archinte.152.8.1611
10. Manian FA. Simultaneous measurement of antibodies to Epstein-Barr virus, human herpesvirus 6, herpes simplex virus types 1 and 2 and 14 enteroviruses in chronic fatigue syndrome: is there evidence of activation of a nonspecific polyclonal immune response? Clin Infect Dis. (1994) 19:448–53. doi: 10.1093/clinids/19.3.448
11. Sairenji T, Yamanishi K, Tachibaba Y, Bertoni G, Kurata T. Antibody responses to Epstein-Barr virus, human herpesvirus 6 and human herpesvirus 7 in patients with chronic fatigue syndrome. Intervirology. (1995) 38:269–73. doi: 10.1159/000150450
12. Di Duca D, Zorzenon M, Mirandola P, Colle R, Botta GA, Cassai E. Human herpesvirus 6 and human herpesvirus 7 in chronic fatigue syndrome. J Clin Microbiol. (1995) 33:1660–1. doi: 10.1128/JCM.33.6.1660-1661.1995
13. Buchwald D, Ashley RL, Pearlman T, Kith P, Komaroff AL. Viral serologies in patients with Chronic Fatigue and Chronic Fatigue Syndrome. J Med Virol. (1996) 50:25–30. doi: 10.1002/(SICI)1096-9071(199609)50:1<25::AID-JMV6>3.0.CO
14. Wallace HL, Natelson B, Gause W, Hay J. Human herpesviruses in chronic fatigue syndrome. Clin Diagn Lab Immunol. (1999) 6:216–23. doi: 10.1128/CDLI.6.2.216-223.1999
15. Reeves WC, Stamey FR, Black JB, Mawle AC, Stewart JA, Pellett PE. Human herpesviruses 6 and 7 in chronic fatigue syndrome: a case-control study. Clin Infect Dis. (2000) 31:48–52. doi: 10.1086/313908
16. Hickie I, Davenport T, Wakefield D, Vollmer-Conna U, Cameron B, Vernon SD, et al. Post-infective and chronic fatigue syndromes precipitated by viral and non-viral pathogens: prospective cohort study. BMJ. (2006) 333:575. doi: 10.1136/bmj.38933.585764.AE
17. Cameron B, Flamand L, Juwana H, Middeldrop J, Naing Z, Rawlinson W, et al. Serological and virological investigation of the role of the herpesviruses EBV, CMV and HHV-6 in post-infective fatigue syndrome. J Med Virol. (2010) 82:1684–8. doi: 10.1002/jmv.21873
18. Oakes B, Hoagland-Henefield M, Komaroff AL, Erkison JL, Huber BT. Human endogenous retrovirus-K18 superantigen expression and human herpesvirus-6 and human herpesvirus-7 viral loads in chronic fatigue syndrome patients. Clin Infect Dis. (2013) 56:1394–400. doi: 10.1093/cid/cit086
19. Swanink CM, van der Meer JW, Vancoulen JH, Bleijenberg G, Fennis JF, Galama JM. Epstein-Barr virus (EBV) and the chronic fatigue syndrome: normal virus load in blood and normal immunological reactivity in the EBV regression assay. Clin Infect Dis. (1995) 20:1390–2. doi: 10.1093/clinids/20.5.1390
20. Kristiansen MS, Stabursvik J, O'Leary C, Pedersen M, Asprusten TT, Leegaard T, et al. Clinical symptoms and markers of disease mechanisms in adolescent chronic fatigue following Epstein-Barr virus infections: an exploratory cross-sectional study. Brain Behav Immun. (2019) 80:551–63. doi: 10.1016/j.bbi.2019.04.040
21. Shikova E, Reshkova V, Kumanova A, Raleva S, Alexandrova D, Capo N, et al. Cytomegalovirus, Epstein-Barr virus and human herpesvirus-6 infections in patients with Myalgic encephalomyelitis/chronic fatigue syndrome. J Med Virol. (2020) 1–7. doi: 10.1002/jmv.25744. [Epub ahead of print].
22. Blomberg J., Rizwan M., Böhlin-Wiener A., Elfaitouri A., Julin P., Zachrisson O., et al. Antibodies to human herpesviruses in myalgic encephalomyelitis/chronic fatigue syndrome patients. Front Immunol. (2019) 10:1945. doi: 10.3389/fimmu.2019.01946
23. Vider-Shalit T, Fishbain V, Raffaeli S, Louzoun Y. Phase-dependent immune evasion of herpesviruses. J Virol. (2007) 81:9536–45. doi: 10.1128/JVI.02636-06
24. Pellett PE, Roizman B. Herpesviridae. In: Knipe DM, Howley PM, editors. Fields Virology, Vol. 2. 6th ed. Philadelphia, PA: Lippincott Williams & Wilkins (2013). p. 1802–22.
25. Loebel M, Eckey M, Sotzny F, Hahn E, Bauer S, Grabowski P, et al. Serological profiling of the EBV immune response using a peptide microarray. PLoS ONE. (2017) 12:e0179124. doi: 10.1371/journal.pone.0179124
26. Burbelo PD, Lebovitz EE, Notkins AL. Luciferase immunoprecipation systems for measuring antibodies in autoimmune and infectious diseases. Transl Res. (2015) 165:325–35. doi: 10.1016/j.trsl.2014.08.006
27. Xu GJ, Kula T, Xu Q, Li MZ, Vernon SD, Ndung'u T, et al. Comprehensive serological profiling of human populations using a synthetic human virome. Science. (2015) 348:aaa0698. doi: 10.1126/science.aaa0698
Keywords: Serology, EBV - Epstein-Barr Virus, Human herpesvirus (HHV)-6, Varicela zoster virus (VZV), Herpes Simplex virus type 1 and type 2 (HSV-1/2), Cytomegalovirus (CMV), Human herpesvirus 7 (HHV-7), Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS)
Citation: Ariza ME (2020) Commentary: Antibodies to Human Herpesviruses in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Patients. Front. Immunol. 11:1400. doi: 10.3389/fimmu.2020.01400
Received: 16 January 2020; Accepted: 01 June 2020;
Published: 23 July 2020.
Edited by:
Aurelio Cafaro, Istituto Superiore di Sanità (ISS), ItalyReviewed by:
Johan Van Weyenbergh, KU Leuven, BelgiumCopyright © 2020 Ariza. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maria Eugenia Ariza, bWFyaWEuYXJpemFAb3N1bWMuZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.