
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 30 June 2020
Sec. Inflammation
Volume 11 - 2020 | https://doi.org/10.3389/fimmu.2020.01321
This article is part of the Research Topic Immune Aging and HIV View all 4 articles
 Thomas A. Premeaux1
Thomas A. Premeaux1 Shireen Javandel2
Shireen Javandel2 Kalei R. J. Hosaka1
Kalei R. J. Hosaka1 Meredith Greene3
Meredith Greene3 Nicholas Therrien1
Nicholas Therrien1 Isabel E. Allen4
Isabel E. Allen4 Michael J. Corley5
Michael J. Corley5 Victor G. Valcour2,3
Victor G. Valcour2,3 Lishomwa C. Ndhlovu1,5*
Lishomwa C. Ndhlovu1,5*The prevalence of age-related comorbidities is increased in people living with HIV, even in those well-controlled on combination antiretroviral therapy (ART). Persistent immune activation and inflammation may play pivotal roles in the pathogenesis; however, the burden of morbidities in the older HIV infected population may be exacerbated and driven by distinct mechanisms. In a cross sectional study of 45 HIV-infected participants 60 years or older, we examined the relationships between 14 immunomodulatory and inflammatory factors and the Veterans Aging Cohort Study (VACS) Index, a metric of multimorbidity and mortality comprised of age, CD4 count, hemoglobin, Fibrosis-4 [FIB-4], and estimated glomerular filtration rate [eGFR], by linear regression analysis. All participants were virally suppressed (<50 HIV RNA copies/mL), on ART, and primarily Caucasian (86.7%), and male (91.1%). Plasma levels of monocyte/macrophage-associated (neopterin, IP-10, sCD163, sCD14, and MCP-1) and glycan-binding immunomodulatory factors (galectin (Gal)-1, Gal-3, and Gal-9) were assessed, as well as inflammatory biomarkers previously linked to the VACS Index (i.e., CRP, cystatin C, TNF-α, TNFRI, IL-6, and D-dimer) for comparison. In regression analysis, higher VACS index scores were associated with higher levels of neopterin, cystatin C, TNFRI, and Gal-9 (all p < 0.05), potentially driven by correlations found with individual VACS components, including age, CD4 count, FIB-4, and eGFR. Gal-9, cystatin C, and TNFRI directly correlated with the extent of multimorbidity. Multiple correlations among markers were observed, suggesting an interplay of overlapping, but distinct, pathways. Collectively, in addition to cystatin C and TNFRI, both galectin-9 and neopterin, independently emerged as novel fluid markers of the VACS Index and burden of comorbidity and may further guide in understanding pathogenic mechanisms of age-related disorders in older HIV-infected individuals on suppressive ART.
In the current era of effective suppressive combination anti-retroviral therapy (ART) regimens people aging with HIV (PAWH) have an increased risk for and earlier onset of age-related comorbidities including cardiovascular, kidney, liver, bone, and neurologic disease (1, 2). Nearly half of the population living with HIV in the United States is older than 50 years of age and non-AIDS-defined events and age-related comorbidities are now the leading cause of mortality in the ART era (3–5). This demographic shift in the HIV-infected population further complicates the clinical care and management of PAWH, particularly as the increased burden of multimorbidity, frailty, geriatric syndromes, and polypharmacy become the norm (6, 7). Although multifactorial, evidence suggests chronic inflammation, particularly monocyte activation, is a key driver of early development of these comorbidities (8–11). This chronic inflammation is thought to stem from occult viral replication and the senescence, exhaustion, and premature aging of the immune system (12–14).
Soluble biomarkers of immune activation, inflammation, and coagulation, such as TNF-α and D-dimer, have been previously associated with clinical indices of morbidity and mortality in people with HIV on suppressive ART (15, 16). As organ system injury is strongly related to immune perturbations, linking clinical predictors and outcomes of morbidity and mortality with mediators of chronic inflammation and immune dysfunction can inform potential molecular pathways involved in the early onset of these comorbidities and, thus, guide therapeutic interventions. The Veterans Aging Cohort Study (VACS) Index is a validated predictor of morbidity and mortality for people with HIV by incorporating markers of organ system injury with traditional HIV disease estimates (17–19). The VACS Index is shown to correlate with age-related complications (i.e., frailty and cognitive impairment) and can inform mechanistic studies on the pathophysiologic effects of aging with HIV (15, 20–25). Evaluating soluble mediators with these clinical index scores and discovering novel correlations can provide a deeper understanding of the interplay between biological function and outcomes of morbidity and mortality in PAWH (26).
In order to inform our understanding of the biology of morbidity risk in older PAWH, we assessed correlations between the VACS Index and soluble monocyte/macrophage activation (neopterin, IP-10) and glycan-binding (galectins) immunomodulatory factors. Several of these factors are elevated in the plasma of untreated people living with HIV and, to a lesser degree, those on ART (27–29). Furthermore, many of these factors are shown to be involved in modulating metabolism and inflammation, directly involved in HIV replication, and linked to comorbidities in the general population (30–52). We investigated these immunomodulatory factors in addition to biomarkers traditionally associated with the VACS Index in a cohort of older adults living with HIV (over the age of 60) with long-term viral suppression to better understand the relationship between aging, HIV, and inflammation.
We conducted a cross sectional study of HIV-infected participants enrolled in the UCSF HIV Elders Study or HIV Over 60 Cohort. These studies were approved by the UCSF Institutional Review Board at the University of California, San Francisco. All participants were aged 60 or older, virally suppressed (defined as plasma HIV RNA <50 copies/mL), and reported adherence to ART for at least 12 months. All participants underwent neuropsychological testing and had endorsed cognitive symptoms on the Patient Assessment of Own Functioning questionnaire as previously described (53). Individuals with confounding conditions such as major neurological or psychiatric conditions were excluded. VACS index was calculated using age, current CD4 t-lymphocyte count, plasma HIV RNA level, hemoglobin, Fibrosis-4 (FIB-4) index, estimated glomerular filtration rate (eGFR), and active hepatitis C infection in a weighted manner, per the scoring system developed by the VACS Project Team (19). Self-reported current CD4 T-lymphocyte count was employed for two individuals as laboratory measures were not available. FIB-4 was calculated using routine liver function tests (AST and ALT), platelet count, and age. eGFR was calculated using serum creatinine levels, age, gender, and race.
Plasma aliquots were thawed and prepared following kit manufacturer guidelines. All samples were analyzed in duplicate. MCP-1, soluble (s)CD163, IP-10, galectin-1, galectin-3, galectin-9, IL-6, TNF-α, TNFR1, D-dimer, and cystatin C were measured using custom Luminex kits from (R&D Systems) and CRP was measured using the Procartaplex kit (Thermofisher). Data was acquired on a Luminex 200TM analyzer (Luminex) and analyzed using MILLIPLEX® Analyst software (Millipore). Neopterin and sCD14 were measured via ELISA (Neopterin competitive enzyme immunoassay, ALPCO, NH, USA; Human CD14 Quantikine ELISA kit, R&D Systems). Optical density was read with a microplate spectrophotometer (Bio-Rad) and data analysis, including four parameter logistic standard interpolation, was carried out using the online MyAssays Ltd. data analysis tool.
Demographic and HIV-related characteristics were described using the median, first quartile (Q1), and third quartile (Q3) for continuous variables, and frequency for categorical variables. Relationships among soluble markers and clinical parameters were examined by Pearson correlation for continuous variables or Spearman correlation for count variables. A multiple linear regression model was fit to examine predictors of the VACS index. Soluble marker values were log transformed to conform to normality prior to analysis. All statistical tests were performed with GraphPad Prism version 8.0 (Graphpad Software Inc., CA, USA) or SPSS version 26 (IBM SPSS Statistics, NY, USA). Correlation matrix was constructed using GraphPad Prism. Statistical significance is indicated as *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. P-values ≤ 0.100, but not significant, are noted as statistical trends.
Participants were 60 years of age or older, predominantly male (91.1%), and Caucasian (86.7%) (Table 1). All individuals were on ART and had undetectable plasma HIV RNA (viral load; VL). Most participants self-reported being infected with HIV for 20+ years (89%). More than half of participants (65%) had CD4 T-lymphocyte counts over 500 cells/μL; only one individual had CD4 T-lymphocyte count <200 cells/μL. Only one individual had a hemoglobin level <12 g/dL, three individuals had a FIB-4 score consistent with liver fibrosis (>3.25), 23% had some compromise in renal function (eGFR <60), and six had past co-infection with hepatitis C virus (HCV) and at least one active infection. We observed a mean VACS index score of 30 for the cohort. Based on prior reports, this suggests a 11.9% all-cause 5-years mortality risk (19, 54). Recent comorbid conditions of cohort participants were also assessed (Table 2), showing 14% were obese defined by a BMI >30, three had coronary disease, almost half (49%) had hyperlipidemia/hypercholesterolemia, more than half (58%) had hypertension, 22% had diabetes mellitus, one individual had liver disease, 22% had chronic obstructive pulmonary disease (COPD), three previously experienced kidney failure (two had stage III chronic kidney disease, and one had chronic renal insufficiency), and one individual had cancer.
We found several significant correlations between VACS Index scores and the plasma levels of inflammatory mediators, including the monocyte/macrophage-associated markers, glycan-binding immunomodulatory proteins, as well as inflammatory biomarkers previously linked to the VACS Index (Table 3). VACS index scores were significantly associated with neopterin (r = 0.40, p = 0.007), Gal-9 (r = 0.38, p = 0.012), cystatin C (r = 0.54, p = 0.0002), and TNFRI (r = 0.50, p = 0.0007). Furthermore, these correlations found with neopterin, Gal-9, cystatin C, and TNFRI remained statistically significant when adjusting for estimated duration of infection (EDI). Additionally, we observed trends between VACS index scores with IP-10 (r = 0.30, p = 0.051), Gal-1 (r = 0.30, p = 0.053), and TNF-α (r = 0.27, p = 0.082). However, markers previously being associated with the VACS index, including sCD163, sCD14, CRP, D-dimer, and IL-6, were not observed here.
Upon correlation analysis of individual VACS components (Figure 1), we observed that levels of neopterin, cystatin C, TNF-α, and TNFRI were positively linearly associated with age (all p < 0.05). Neopterin, IP-10, and TNF-α inversely related with CD4 count (all p < 0.01). Hemoglobin levels were inversely associated with Gal-1 and cystatin C levels, but directly correlated with levels of IP-10 (all p < 0.05). Direct correlations between FIB-4 and IP-10, sCD163, Gal-9, cystatin C, TNF-α, and TNFRI were present (all p < 0.05). And finally, levels of neopterin, MCP-1, Gal-1, Gal-9, cystatin C, and TNFRI inversely related with eGFR (all p < 0.05). No soluble markers differed significantly among HCV status, except for higher Gal-3 levels observed in individuals with past HCV co-infection (p = 0.048; data not shown). Soluble markers levels were compared among individuals differing in CD4 nadir (>100, 100–200, >200) and no differences were observed (Supplementary Figure 1). Associations among sCD14, CRP, and IL-6 with individual VACS components were not found. We next evaluated these markers with the burden of multimorbidity (Figure 2). We observed that levels of Gal-9 (rho = 0.34, p = 0.021), TNFRI (rho = 0.36, p = 0.015), and cystatin C (rho = 0.44, p = 0.003) were directly correlated with the total number of comorbid conditions. Differences in soluble marker levels for specific comorbidities were also evaluated (Supplementary Figure 2). Only higher levels of CRP in individuals with obesity (p = 0.022) and higher Gal-3 with COPD (p = 0.023) were observed.
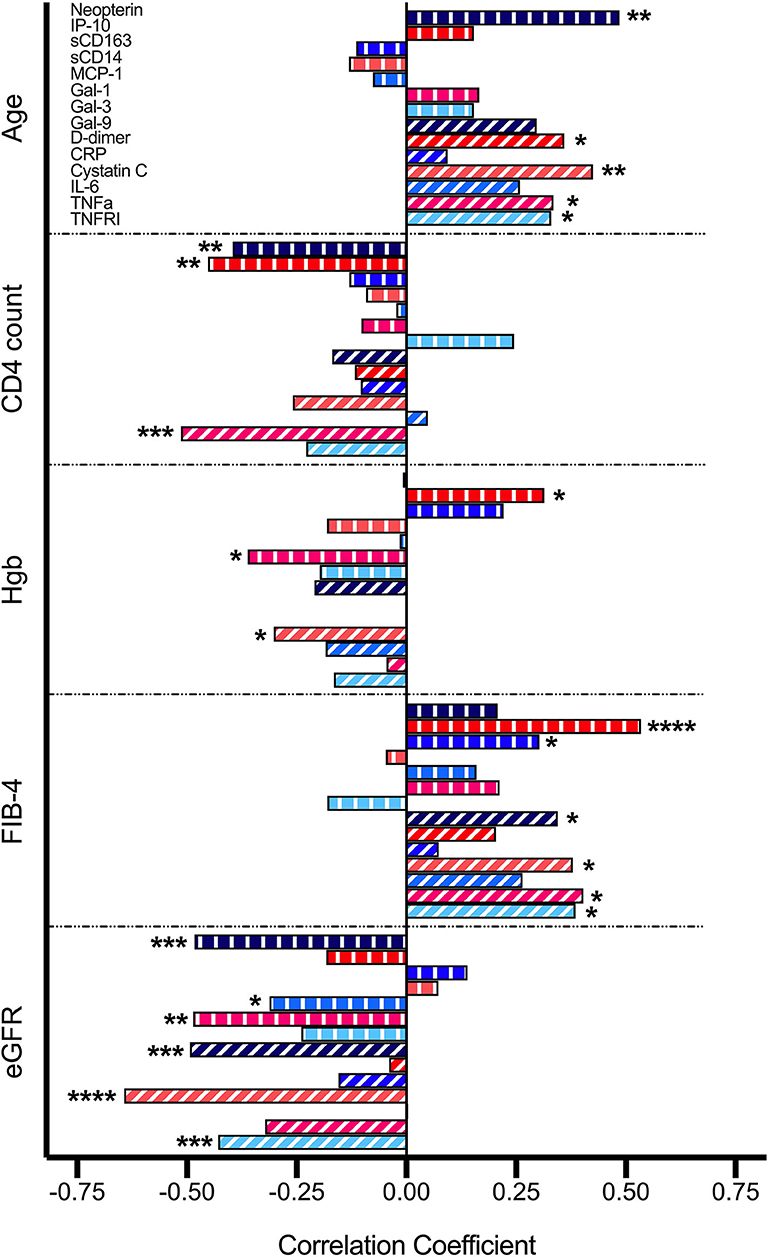
Figure 1. Correlations between soluble mediators and VACS Index components. Relationship between variables were analyzed by Pearson correlation. Statistical significance is indicated as *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
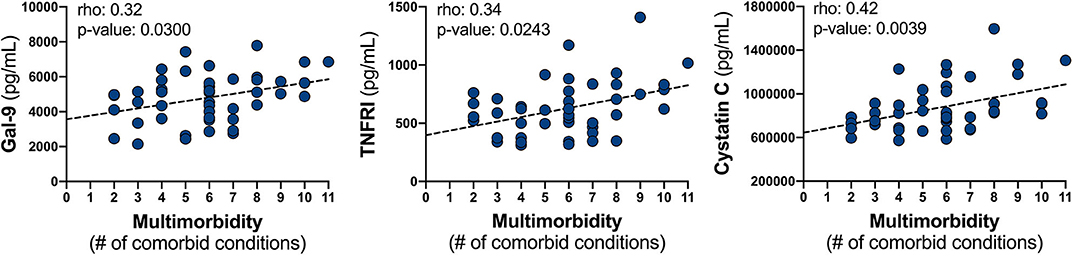
Figure 2. Relationship between soluble mediator levels and the burden of multimorbidity. Plasma levels of Gal-9, TNFRI, and cystatin C were correlated with the number of comorbid conditions by Spearman correlation; represented line is indicative only.
We also conducted an exploratory analysis to determine if there exists an interplay of multiple immune pathways by evaluating intercorrelations among the 14 soluble mediators quantified (Figure 3). A positive interaction between neopterin and Gal-9 was noted (p < 0.001) and multiple correlations among several plasma markers were observed, including neopterin levels positively associating with IP-10, cystatin C, TNF-α, and TNFRI (p < 0.05). Gal-9 levels positively associated with IP-10, CRP, cystatin C, IL-6, TNF-α, TNFRI, and Gal-1 (p < 0.05). Among the other monocyte/macrophage activation markers, IP-10 also correlated with sCD163, cystatin C, and TNF-α; sCD14 only correlated with cystatin C (p < 0.05). And Gal-1 correlated with Gal-3, CRP, cystatin C, IL-6, TNF-α, and TNFRI (p < 0.05).
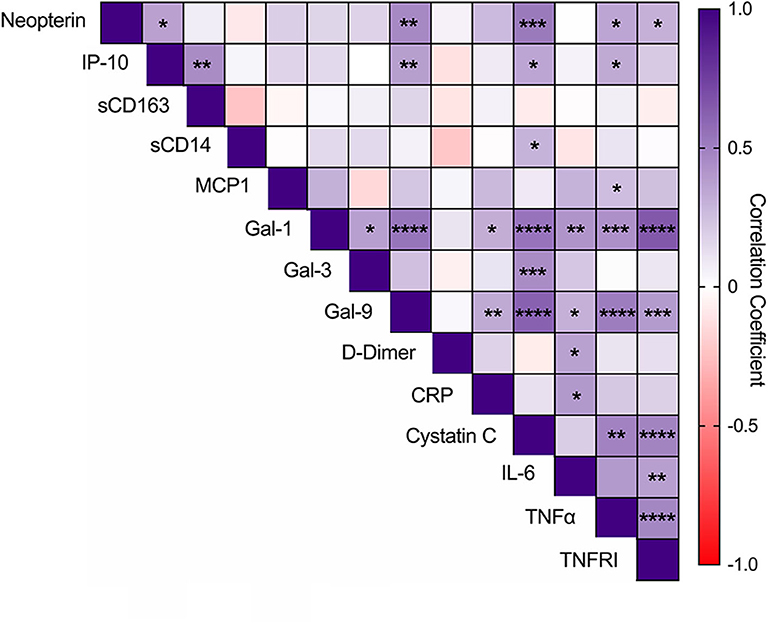
Figure 3. Intercorrelations among glycan-binding immunomodulators, myeloid-associated markers, and markers of coagulation and inflammation. Intercorrelations among soluble markers were analyzed by Pearson correlation. Statistical significance is indicated as *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
In an assessment of immunomodulatory factors in older PAWH on suppressive ART, we illuminated potential plasma mediators while highlighting the importance of previously evaluated markers that could lead to a better understanding of the biological complexity of the aging process within the context of HIV and assess those most vulnerable of experiencing morbidity and mortality. In addition to cystatin C and TNFRI, we identified a relationship between myeloid-associated and glycan-binding immunomodulators neopterin and Gal-9, and to a lesser extent IP-10 and Gal-1, with the VACS Index and several key VACS components. Furthermore, several of these factors correlated with the extent of comorbidities and with one another.
Chronic inflammation, particularly through monocyte/macrophage activation, is hypothesized as a key predictor of increased morbidity and all-cause mortality in HIV infection even in the context of viral suppression (55–57). Neopterin and IP-10 are markers of monocyte/macrophage activation that are associated with IFN-γ-induced pathways and demonstrate immune regulatory functions (58–61). Prior literature has demonstrated that both neopterin and IP-10 are implicated in the progression of HIV disease, principally associating with viremia, decreased CD4 counts, and all-cause mortality (62–64). We extend these observations by demonstrating that neopterin and, to a lesser extent, IP-10 associated with increased risk of mortality in older PAWH on suppressive ART. Furthermore, our correlative findings between eGFR, an indicator of kidney function, with these markers are consistent with previous findings demonstrating that neopterin associates with chronic kidney disease severity and IP-10 blockade promotes renal dysfunction (65, 66). Interventions that target monocyte activation, specifically IFN-γ-induced pathways, may potentially be useful in lowering chronic immune activation, and reduce morbidity incidence in PAWH during suppressive ART.
Glycan-glycan-binding protein mediated immune responses, particularly through the galectin family of proteins, is an emerging field in HIV research and implicated to play a role in HIV pathogenesis (67). Gal-9 and Gal-1, are demonstrated as drivers of HIV transcription and cell entry, respectively, as well as pro-inflammatory mediators (33–35, 37, 38). Gal-9 specifically has been shown to rapidly increase in the plasma as part of the cytokine storm in early HIV infection and remains elevated during chronic infection despite viral suppression (28). Furthermore, in the cerebrospinal fluid of people living with HIV higher Gal-9 levels were closely linked to increased viremia and immune activation as well as lower cognitive performance (68). Mechanistically, Gal-9 is hypothesized to potentially contribute to NK cell dysfunction and the non-specific activation of T cells in HIV infection (69, 70). Whether elevated endogenous Gal-9 levels contribute or sustain the state of chronic inflammation and immune activation and contribute to the progression of comorbid conditions during suppressive ART warrants further investigation. Here, we observed Gal-9 directly associated with morbidity risk and the extent of multimorbidity and our findings with VACS components are consistent with correlations previously demonstrated with FIB-4, a predictor of liver fibrosis, and eGFR in people without HIV (42, 44). As Gal-9 plays significant roles in both immune function and HIV transcription, elevated levels in PAWH may provide additional insights beyond biomarkers previously assessed with the VACS Index.
In this study we reveal additional inflammatory-induced pathways that could be contributing to the persistent inflammatory milieu and morbidity risk in treated HIV in the older population. We also conducted an evaluation of markers previously shown to associate with the VACS Index based on a diverse population in terms of age and ART treatment status. In our virally suppressed cohort, we found correlations among the VACS Index with cystatin C and TNFRI levels, as well as a trends with D-dimer and TNF-α, complementing the idea that perturbations in inflammation and coagulation mechanisms are linked to morbidity and mortality progression in PAWH (5). However, we did not observe VACS index correlations with CRP, IL-6, sCD14, and sCD163 as previously described (71). The absence of these correlations could stem from a low incidence of comorbid complications in our cohort that these biomarkers are found to be associated with, such as cardiovascular disease, bone disease, and cancer (72–78). Additionally, previous links between these biomarkers and the VACS Index were observed in relatively younger participants either with incomplete viral suppression (VL detectable but <500 c/mL) or an immunologic non-responder profile (20, 21).
As people living with HIV advance in age measures to determine increased risk for morbidity and mortality are warranted and soluble mediators may be particularly useful as screening tools to identify high-risk groups. However, the synergistic effects of HIV, aging, and multimorbidity complicates the ability to identify “universal” biomarkers. Previous studies demonstrate that PAWH display accelerated or early aging and accentuated inflammatory responses in advancing age, seroconversion at an earlier age associates with higher rates of multiple comorbidities, and specific mediators are associated with distinct comorbidities observed in HIV infection (79–82). Many comorbidities were common in our cohort, similar to other studies of older PAWH, which makes many of these soluble markers informative, as some are disease specific and others are common among many conditions. Furthermore, we discovered intercorrelations among many of the soluble mediators analyzed, suggesting an interplay of overlapping, but distinct, pathways, leading to the conclusion that a composite panel of clinical and immunomodulatory markers might be a more comprehensive approach for monitoring comorbid progression and the discovery of an effective means of intervention.
Chronic inflammation and immune activation are proposed as significant drivers of comorbidity development in PAWH, but this could be propelled by several factors, including biological aging, ARV regimens, HIV infection, or rather a synergistic effect. Chronic, low-grade inflammation occurs as people age, termed inflammaging, and soluble markers shown to be elevated with age, such as Gal-9 and neopterin, could be a reflection of this normal biological aging process (83). Adverse effects of several ARVs are linked to inflammation and the development of specific comorbidities; however, initiation of ART at diagnosis irrespective of CD4 count is now mandated given the overriding benefit of viral suppression in reducing transmission and lowering immune damage and higher states of inflammation (84–89). Current efforts to use ARV drugs that are safer and in combinations with anti-inflammatory agents are being pursued. To date, no therapy has been approved to decrease inflammation in PAWH despite numerous clinical trials targeting multiple proposed mechanisms of chronic inflammation in HIV (90–97). Any successes in interventions to lower immune activation and inflammation would be of significant value for PAWH and our panel of identified markers could potentially assist in monitoring interventions and identifying novel pathways to target.
Our study has several limitations that should be acknowledged. Our sample was relatively small and, thus, limited the power for multivariate regression analysis. Participants were primarily white males and, thus, we are limited in our ability to generalize our data to all affected populations. Our cohort consisted of participants who were virally suppressed and ≥ 60 years old, which may limit the predictive capabilities of the VACS Index. The availability of an age-matched comparison group comprised of people without HIV could have added more clarity to the relevance of these soluble mediators specifically in the context of aging with HIV. Additionally, many of these soluble mediators are shown to associate with age (98, 99). As calculations for VACS, FIB-4, and eGFR use age as a component adjustments for this variable in regression models were not feasible. Finally, we cannot determine the predictive nor diagnostic ability of these identified soluble mediators for specific non-AIDS clinical events.
In conclusion, we identified several key mediators in our population of PAWH on ART who were over 60, some of which were consistent with previous studies and in particular, novel factors such as neopterin and Gal-9. Further evaluation of these identified immunomodulatory factors could determine if suitable to implement in clinical practice to monitor those at an increased risk of developing comorbidities or mortality in aging HIV-infected individuals on suppressive ART. In light of our studies either independently or collectively, these immune markers could also emerge as novel mediators in understanding the pathogenic molecular mechanisms of age-related disorders in treated HIV that may ultimately lead to identifying potential targets to prevent, slow, or reverse these complications in this population.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by UCSF Institutional Review Board at the University of California, San Francisco. The patients/participants provided their written informed consent to participate in this study.
TP contributed to biomarker data acquisition, statistical analysis, and wrote the manuscript. SJ, KH, IA, and MG contributed to clinical data acquisition and interpretation. NT contributed to biomarker data acquisition and interpretation. MC, VV, and LN contributed to study design and concept. All authors reviewed the manuscript.
This work was supported by R01NR015223 (VV).
VV has served as a consultant to ViiV Healthcare and Merck on issues related to HIV and aging. LN has served as an advisory board member to ViiV Healthcare related to ART and HIV.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank the study participants and the support of the Memory and Aging Center at UCSF.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2020.01321/full#supplementary-material
Supplementary Figure 1. Differences in soluble mediator levels in individuals according to CD4 nadir count. Associations among groups were analyzed via Kruskal-Wallis with Dunn's multiple comparison test. Neopterin concentration is in nMol/L.
Supplementary Figure 2. Differences in soluble mediator levels according to comorbidity. Neopterin concentration is in nMol/L. Associations among groups were analyzed via Mann-Whitney tests. Statistical significance is indicated as *p < 0.05.
1. Deeks SG, Tracy R, Douek DC. Systemic effects of inflammation on health during chronic HIV infection. Immunity. (2013) 39:633–45. doi: 10.1016/j.immuni.2013.10.001
2. Heaton RK, Clifford DB, Franklin DR Jr, Woods SP, Ake C, Vaida F, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER study. Neurology. (2010) 75:2087–96. doi: 10.1212/WNL.0b013e318200d727
3. Justice AC. HIV and aging: time for a new paradigm. Curr HIV/AIDS Rep. (2010) 7:69–76. doi: 10.1007/s11904-010-0041-9
4. Mills EJ, Barnighausen T, Negin J. HIV and aging–preparing for the challenges ahead. N Engl J Med. (2012) 366:1270–3. doi: 10.1056/NEJMp1113643
5. Montano M, Bhasin S, D'Aquila RT, Erlandson KM, Evans WJ, Funderburg NT, et al. Harvard HIV and aging workshop: perspectives and priorities from Claude D. Pepper Centers and Centers for AIDS Research. AIDS Res Hum Retroviruses. (2019) 35:999–1012. doi: 10.1089/aid.2019.0130
6. Greene M, Covinsky KE, Valcour V, Miao Y, Madamba J, Lampiris H, et al. Geriatric syndromes in older HIV-infected adults. J Acquir Immune Defic Syndr. (2015) 69:161–7. doi: 10.1097/QAI.0000000000000556
7. Guaraldi G, Malagoli A, Calcagno A, Mussi C, Celesia BM, Carli F, et al. The increasing burden and complexity of multi-morbidity and polypharmacy in geriatric HIV patients: a cross sectional study of people aged 65—74 years and more than 75 years. BMC Geriatr. (2018) 18:99. doi: 10.1186/s12877-018-0789-0
8. Campbell JH, Hearps AC, Martin GE, Williams KC, Crowe SM. The importance of monocytes and macrophages in HIV pathogenesis, treatment, and cure. AIDS. (2014) 28:2175–87. doi: 10.1097/QAD.0000000000000408
9. Hart BB, Nordell AD, Okulicz JF, Palfreeman A, Horban A, Kedem E, et al. Inflammation-related morbidity and mortality among HIV-positive adults: how extensive is it? J Acquir Immune Defic Syndr. (2018) 77:1–7. doi: 10.1097/QAI.0000000000001554
10. Hunt PW, Lee SA, Siedner MJ. Immunologic biomarkers, morbidity, and mortality in treated HIV infection. J Infect Dis. (2016) 214(Suppl.2):S44–50. doi: 10.1093/infdis/jiw275
11. Wilson EM, Singh A, Hullsiek KH, Gibson D, Henry WK, Lichtenstein K, et al. Monocyte-activation phenotypes are associated with biomarkers of inflammation and coagulation in chronic HIV infection. J Infect Dis. (2014) 210:1396–406. doi: 10.1093/infdis/jiu275
12. Duffau P, Wittkop L, Lazaro E, le Marec F, Cognet C, Blanco P, et al. Association of immune-activation and senescence markers with non-AIDS-defining comorbidities in HIV-suppressed patients. AIDS. (2015) 29:2099–108. doi: 10.1097/QAD.0000000000000807
13. Desai S, Landay A. Early immune senescence in HIV disease. Curr HIV/AIDS Rep. (2010) 7:4–10. doi: 10.1007/s11904-009-0038-4
14. Appay V, Kelleher AD. Immune activation and immune aging in HIV infection. Curr Opin HIV AIDS. (2016) 11:242–9. doi: 10.1097/COH.0000000000000240
15. Mooney S, Tracy R, Osler T, Grace C. Elevated biomarkers of inflammation and coagulation in patients with HIV are associated with higher framingham and VACS risk index scores. PLoS ONE. (2015) 10:e0144312. doi: 10.1371/journal.pone.0144312
16. Duffau P, Ozanne A, Bonnet F, Lazaro E, Cazanave C, Blanco P, et al. Multimorbidity, age-related comorbidities and mortality: association of activation, senescence and inflammation markers in HIV adults. AIDS. (2018) 32:1651–60. doi: 10.1097/QAD.0000000000001875
17. Tate JP, Justice AC, Hughes MD, Bonnet F, Reiss P, Mocroft A, et al. An internationally generalizable risk index for mortality after 1 year of antiretroviral therapy. AIDS. (2013) 27:563–72. doi: 10.1097/QAD.0b013e32835b8c7f
18. Bebu I, Tate J, Rimland D, Mesner O, Macalino GE, Ganesan A, et al. The VACS index predicts mortality in a young, healthy HIV population starting highly active antiretroviral therapy. J Acquir Immune Defic Syndr. (2014) 65:226–30. doi: 10.1097/QAI.0000000000000045
19. Justice AC, Modur SP, Tate JP, Althoff KN, Jacobson LP, Gebo KA, et al. Predictive accuracy of the Veterans Aging Cohort Study index for mortality with HIV infection: a North American cross cohort analysis. J Acquir Immune Defic Syndr. (2013) 62:149–63. doi: 10.1097/QAI.0b013e31827df36c
20. Williams B, Livak B, Bahk M, Keating SM, Adeyemi OM. Short communication: SCD14 and SCD163 levels are correlated with VACS index scores: initial data from the Blunted Immune Recovery in CORE Patients with HIV (BIRCH) Cohort. AIDS Res Hum Retroviruses. (2016) 32:144–7. doi: 10.1089/aid.2015.0012
21. Justice AC, Freiberg MS, Tracy R, Kuller L, Tate JP, Goetz MB, et al. Does an index composed of clinical data reflect effects of inflammation, coagulation, and monocyte activation on mortality among those aging with HIV? Clin Infect Dis. (2012) 54:984–94. doi: 10.1093/cid/cir989
22. Marquine MJ, Montoya JL, Umlauf A, Fazeli PL, Gouaux B, Heaton RK, et al. The Veterans Aging Cohort Study (VACS) index and neurocognitive change: a longitudinal study. Clin Infect Dis. (2016) 63:694–702. doi: 10.1093/cid/ciw328
23. Akgun KM, Tate JP, Crothers K, Crystal S, Leaf DA, Womack J, et al. An adapted frailty-related phenotype and the VACS index as predictors of hospitalization and mortality in HIV-infected and uninfected individuals. J Acquir Immune Defic Syndr. (2014) 67:397–404. doi: 10.1097/QAI.0000000000000341
24. Escota GV, Patel P, Brooks JT, Bush T, Conley L, Baker J, et al. Short communication: the Veterans Aging Cohort Study Index is an effective tool to assess baseline frailty status in a contemporary cohort of HIV-infected persons. AIDS Res Hum Retroviruses. (2015) 31:313–7. doi: 10.1089/aid.2014.0225
25. Marquine MJ, Sakamoto M, Dufour C, Rooney A, Fazeli P, Umlauf A, et al. The impact of ethnicity/race on the association between the Veterans Aging Cohort Study (VACS) Index and neurocognitive function among HIV-infected persons. J Neurovirol. (2016) 22:442–54. doi: 10.1007/s13365-015-0411-6
26. Tenorio AR, Zheng Y, Bosch RJ, Krishnan S, Rodriguez B, Hunt PW, et al. Soluble markers of inflammation and coagulation but not T-cell activation predict non-AIDS-defining morbid events during suppressive antiretroviral treatment. J Infect Dis. (2014) 210:1248–59. doi: 10.1093/infdis/jiu254
27. Wirleitner B, Schroecksnadel K, Winkler C, Fuchs D. Neopterin in HIV-1 infection. Mol Immunol. (2005) 42:183–94. doi: 10.1016/j.molimm.2004.06.017
28. Tandon R, Chew GM, Byron MM, Borrow P, Niki T, Hirashima M, et al. Galectin-9 is rapidly released during acute HIV-1 infection and remains sustained at high levels despite viral suppression even in elite controllers. AIDS Res Hum Retroviruses. (2014) 30:654–64. doi: 10.1089/aid.2014.0004
29. Pastor L, Casellas A, Ruperez M, Carrillo J, Maculuve S, Jairoce C, et al. Interferon-gamma-Inducible Protein 10 (IP-10) as a screening tool to optimize human immunodeficiency virus RNA monitoring in resource-limited settings. Clin Infect Dis. (2017) 65:1670–5. doi: 10.1093/cid/cix600
30. Brinchmann MF, Patel DM, Iversen MH. The role of galectins as modulators of metabolism and inflammation. Mediators Inflamm. (2018) 2018:9186940. doi: 10.1155/2018/9186940
31. Liu M, Guo S, Hibbert JM, Jain V, Singh N, Wilson NO, et al. CXCL10/IP-10 in infectious diseases pathogenesis and potential therapeutic implications. Cytokine Growth Factor Rev. (2011) 22:121–30. doi: 10.1016/j.cytogfr.2011.06.001
32. Gieseg SP, Baxter-Parker G, Lindsay A. Neopterin, inflammation, and oxidative stress: what could we be missing? Antioxidants. (2018) 7:80. doi: 10.3390/antiox7070080
33. Abdel-Mohsen M, Chavez L, Tandon R, Chew GM, Deng X, Danesh A, et al. Human Galectin-9 Is a potent mediator of HIV transcription and reactivation. PLoS Pathog. (2016) 12:e1005677. doi: 10.1371/journal.ppat.1005677
34. Bi S, Hong PW, Lee B, Baum LG. Galectin-9 binding to cell surface protein disulfide isomerase regulates the redox environment to enhance T-cell migration and HIV entry. Proc Natl Acad Sci USA. (2011) 108:10650–5. doi: 10.1073/pnas.1017954108
35. Mercier S, St-Pierre C, Pelletier I, Ouellet M, Tremblay MJ, Sato S. Galectin-1 promotes HIV-1 infectivity in macrophages through stabilization of viral adsorption. Virology. (2008) 371:121–9. doi: 10.1016/j.virol.2007.09.034
36. Okamoto M, Hidaka A, Toyama M, Baba M. Galectin-3 is involved in HIV-1 expression through NF-kappaB activation and associated with Tat in latently infected cells. Virus Res. (2019) 260:86–93. doi: 10.1016/j.virusres.2018.11.012
37. Ouellet M, Mercier S, Pelletier I, Bounou S, Roy J, Hirabayashi J, et al. Galectin-1 acts as a soluble host factor that promotes HIV-1 infectivity through stabilization of virus attachment to host cells. J Immunol. (2005) 174:4120–6. doi: 10.4049/jimmunol.174.7.4120
38. St-Pierre C, Manya H, Ouellet M, Clark GF, Endo T, Tremblay MJ, et al. Host-soluble galectin-1 promotes HIV-1 replication through a direct interaction with glycans of viral gp120 and host CD4. J Virol. (2011) 85:11742–51. doi: 10.1128/JVI.05351-11
39. Wang SF, Tsao CH, Lin YT, Hsu DK, Chiang ML, Lo CH, et al. Galectin-3 promotes HIV-1 budding via association with Alix and Gag p6. Glycobiology. (2014) 24:1022–35. doi: 10.1093/glycob/cwu064
40. Avanzas P, Arroyo-Espliguero R, Kaski JC. Neopterin and cardiovascular disease: growing evidence for a role in patient risk stratification. Clin Chem. (2009) 55:1056–7. doi: 10.1373/clinchem.2009.127084
41. Drechsler C, Delgado G, Wanner C, Blouin K, Pilz S, Tomaschitz A, et al. Galectin-3, renal function, and clinical outcomes: results from the LURIC and 4D studies. J Am Soc Nephrol. (2015) 26:2213–21. doi: 10.1681/ASN.2014010093
42. Fujita K, Niki T, Nomura T, Oura K, Tadokoro T, Sakamoto T, et al. Correlation between serum galectin-9 levels and liver fibrosis. J Gastroenterol Hepatol. (2018) 33:492–9. doi: 10.1111/jgh.13851
43. Gudowska M, Gruszewska E, Cylwik B, Panasiuk A, Rogalska M, Flisiak R, et al. Galectin-3 concentration in liver diseases. Ann Clin Lab Sci. (2015) 45:669–73.
44. Kurose Y, Wada J, Kanzaki M, Teshigawara S, Nakatsuka A, Murakami K, et al. Serum galectin-9 levels are elevated in the patients with type 2 diabetes and chronic kidney disease. BMC Nephrol. (2013) 14:23. doi: 10.1186/1471-2369-14-23
45. Rebholz CM, Selvin E, Liang M, Ballantyne CM, Hoogeveen RC, Aguilar D, et al. Plasma galectin-3 levels are associated with the risk of incident chronic kidney disease. Kidney Int. (2018) 93:252–9. doi: 10.1016/j.kint.2017.06.028
46. Seropian IM, Gonzalez GE, Maller SM, Berrocal DH, Abbate A, Rabinovich GA. Galectin-1 as an emerging mediator of cardiovascular inflammation: mechanisms and therapeutic opportunities. Mediators Inflamm. (2018) 2018:8696543. doi: 10.1155/2018/8696543
47. Unuvar S, Aslanhan H. Clinical significance of increased serum neopterin in chronic kidney failure as a biomarker of cell-mediated immunity. J Med Biochem. (2019) 38:1–5. doi: 10.2478/jomb-2018-0019
48. Vaidya VS, Ferguson MA, Bonventre JV. Biomarkers of acute kidney injury. Annu Rev Pharmacol Toxicol. (2008) 48:463–93. doi: 10.1146/annurev.pharmtox.48.113006.094615
49. van den Borne P, Quax PH, Hoefer IE, Pasterkamp G. The multifaceted functions of CXCL10 in cardiovascular disease. Biomed Res Int. (2014) 2014:893106. doi: 10.1155/2014/893106
50. Wilmer A, Nolchen B, Tilg H, Herold M, Pechlaner C, Judmaier G, et al. Serum neopterin concentrations in chronic liver disease. Gut. (1995) 37:108–12. doi: 10.1136/gut.37.1.108
51. You CR, Park SH, Jeong SW, Woo HY, Bae SH, Choi JY, et al. Serum IP-10 levels correlate with the severity of liver histopathology in patients infected with genotype-1 HCV. Gut Liver. (2011) 5:506–12. doi: 10.5009/gnl.2011.5.4.506
52. Zhong X, Qian X, Chen G, Song X. The role of galectin-3 in heart failure and cardiovascular disease. Clin Exp Pharmacol Physiol. (2019) 46:197–203. doi: 10.1111/1440-1681.13048
53. Chang K, Premeaux TA, Cobigo Y, Milanini B, Hellmuth J, Rubin LH, et al. Plasma inflammatory biomarkers link to diffusion tensor imaging metrics in virally suppressed HIV-infected individuals. AIDS. (2020) 34:203–13. doi: 10.1097/QAD.0000000000002404
54. Brown ST, Tate JP, Kyriakides TC, Kirkwood KA, Holodniy M, Goulet JL, et al. The VACS index accurately predicts mortality and treatment response among multi-drug resistant HIV infected patients participating in the options in management with antiretrovirals (OPTIMA) study. PLoS ONE. (2014) 9:e92606. doi: 10.1371/journal.pone.0092606
55. Knudsen TB, Ertner G, Petersen J, Moller HJ, Moestrup SK, Eugen-Olsen J, et al. Plasma soluble CD163 level independently predicts all-cause mortality in HIV-1-infected individuals. J Infect Dis. (2016) 214:1198–204. doi: 10.1093/infdis/jiw263
56. High KP, Brennan-Ing M, Clifford DB, Cohen MH, Currier J, Deeks SG, et al. HIV and aging: state of knowledge and areas of critical need for research. A report to the NIH Office of AIDS Research by the HIV and Aging Working Group. J Acquir Immune Defic Syndr. (2012) 60(Suppl.1):S1–18. doi: 10.1097/QAI.0b013e31825a3668
57. Sandler NG, Wand H, Roque A, Law M, Nason MC, Nixon DE, et al. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis. (2011) 203:780–90. doi: 10.1093/infdis/jiq118
58. Ramirez LA, Arango TA, Thompson E, Naji M, Tebas P, Boyer JD. High IP-10 levels decrease T cell function in HIV-1-infected individuals on ART. J Leukoc Biol. (2014) 96:1055–63. doi: 10.1189/jlb.3A0414-232RR
59. Huber C, Batchelor JR, Fuchs D, Hausen A, Lang A, Niederwieser D, et al. Immune response-associated production of neopterin. Release from macrophages primarily under control of interferon-gamma. J Exp Med. (1984) 160:310–6. doi: 10.1084/jem.160.1.310
60. Hoffmann G, Frede S, Kenn S, Smolny M, Wachter H, Fuchs D, et al. Neopterin-induced tumor necrosis factor-alpha synthesis in vascular smooth muscle cells in vitro. Int Arch Allergy Immunol. (1998) 116:240–5. doi: 10.1159/000023950
61. Hoffmann G, Kenn S, Wirleitner B, Deetjen C, Frede S, Smolny M, et al. Neopterin induces nitric oxide-dependent apoptosis in rat vascular smooth muscle cells. Immunobiology. (1998) 199:63–73. doi: 10.1016/S0171-2985(98)80064-8
62. Lei J, Yin X, Shang H, Jiang Y. IP-10 is highly involved in HIV infection. Cytokine. (2019) 115:97–103. doi: 10.1016/j.cyto.2018.11.018
63. Mhandire K, Mlambo T, Zijenah LS, Duri K, Mateveke K, Tshabalala M, et al. Plasma IP-10 concentrations correlate positively with viraemia and inversely with CD4 counts in untreated HIV infection. Open AIDS J. (2017) 11:24–31. doi: 10.2174/1874613601711010024
64. Valverde-Villegas JM, de Medeiros RM, Ellwanger JH, Santos BR, Melo MG, Almeida SEM, et al. High CXCL10/IP-10 levels are a hallmark in the clinical evolution of the HIV infection. Infect Genet Evol. (2018) 57:51–8. doi: 10.1016/j.meegid.2017.11.002
65. Yadav AK, Sharma V, Jha V. Association between serum neopterin and inflammatory activation in chronic kidney disease. Mediators Inflamm. (2012) 2012:476979. doi: 10.1155/2012/476979
66. Nakaya I, Wada T, Furuichi K, Sakai N, Kitagawa K, Yokoyama H, et al. Blockade of IP-10/CXCR3 promotes progressive renal fibrosis. Nephron Exp Nephrol. (2007) 107:e12–21. doi: 10.1159/000106505
67. Colomb F, Giron LB, Trbojevic-Akmacic I, Lauc G, Abdel-Mohsen M. Breaking the Glyco-Code of HIV persistence and immunopathogenesis. Curr HIV/AIDS Rep. (2019) 16:151–68. doi: 10.1007/s11904-019-00433-w
68. Premeaux TA, D'Antoni ML, Abdel-Mohsen M, Pillai SK, Kallianpur KJ, Nakamoto BK, et al. Elevated cerebrospinal fluid Galectin-9 is associated with central nervous system immune activation and poor cognitive performance in older HIV-infected individuals. J Neurovirol. (2019) 25:150–61. doi: 10.1007/s13365-018-0696-3
69. Colomb F, Giron LB, Premeaux TA, Mitchell BI, Niki T, Papasavvas E, et al. Galectin-9 Mediates HIV Transcription by Inducing TCR-Dependent ERK Signaling. Front Immunol. (2019) 10:267. doi: 10.3389/fimmu.2019.00267
70. Jost S, Moreno-Nieves UY, Garcia-Beltran WF, Rands K, Reardon J, Toth I, et al. Dysregulated Tim-3 expression on natural killer cells is associated with increased Galectin-9 levels in HIV-1 infection. Retrovirology. (2013) 10:74. doi: 10.1186/1742-4690-10-74
71. Miller CJ, Baker JV, Bormann AM, Erlandson KM, Huppler Hullsiek K, Justice AC, et al. Adjudicated morbidity and mortality outcomes by age among individuals with HIV infection on suppressive antiretroviral therapy. PLoS ONE. (2014) 9:e95061. doi: 10.1371/journal.pone.0095061
72. Borges AH, O'Connor JL, Phillips AN, Ronsholt FF, Pett S, Vjecha MJ, et al. Determinants of IL-6 levels during HIV infection. J Int AIDS Soc. (2014) 17(4 Suppl.3):19482. doi: 10.7448/IAS.17.4.19482
73. Borges AH, Silverberg MJ, Wentworth D, Grulich AE, Fatkenheuer G, Mitsuyasu R, et al. Predicting risk of cancer during HIV infection: the role of inflammatory and coagulation biomarkers. AIDS. (2013) 27:1433–41. doi: 10.1097/QAD.0b013e32835f6b0c
74. Duprez DA, Neuhaus J, Kuller LH, Tracy R, Belloso W, De Wit S, et al. Inflammation, coagulation and cardiovascular disease in HIV-infected individuals. PLoS ONE. (2012) 7:e44454. doi: 10.1371/journal.pone.0044454
75. Ford ES, Greenwald JH, Richterman AG, Rupert A, Dutcher L, Badralmaa Y, et al. Traditional risk factors and D-dimer predict incident cardiovascular disease events in chronic HIV infection. AIDS. (2010) 24:1509–17. doi: 10.1097/QAD.0b013e32833ad914
76. Hileman CO, Labbato DE, Storer NJ, Tangpricha V, McComsey GA. Is bone loss linked to chronic inflammation in antiretroviral-naive HIV-infected adults? A 48-week matched cohort study. AIDS. (2014) 28:1759–67. doi: 10.1097/QAD.0000000000000320
77. Musselwhite LW, Sheikh V, Norton TD, Rupert A, Porter BO, Penzak SR, et al. Markers of endothelial dysfunction, coagulation and tissue fibrosis independently predict venous thromboembolism in HIV. AIDS. (2011) 25:787–95. doi: 10.1097/QAD.0b013e3283453fcb
78. Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. (2007) 92:2506–12. doi: 10.1210/jc.2006-2190
79. Gallant J, Hsue PY, Shreay S, Meyer N. Comorbidities among US patients with prevalent HIV infection-A trend analysis. J Infect Dis. (2017) 216:1525–33. doi: 10.1093/infdis/jix518
80. Guaraldi G, Zona S, Brothers TD, Carli F, Stentarelli C, Dolci G, et al. Aging with HIV vs. HIV seroconversion at older age: a diverse population with distinct comorbidity profiles. PLoS ONE. (2015) 10:e0118531. doi: 10.1371/journal.pone.0118531
81. Schouten J, Wit FW, Stolte IG, Kootstra NA, van der Valk M, Geerlings SE, et al. Cross-sectional comparison of the prevalence of age-associated comorbidities and their risk factors between HIV-infected and uninfected individuals: the AGEhIV cohort study. Clin Infect Dis. (2014) 59:1787–97. doi: 10.1093/cid/ciu701
82. Hasse B, Ledergerber B, Furrer H, Battegay M, Hirschel B, Cavassini M, et al. Morbidity and aging in HIV-infected persons: the Swiss HIV cohort study. Clin Infect Dis. (2011) 53:1130–9. doi: 10.1093/cid/cir626
83. Franceschi C, Garagnani P, Parini P, Giuliani C, Santoro A. Inflammaging: a new immune-metabolic viewpoint for age-related diseases. Nat Rev Endocrinol. (2018) 14:576–90. doi: 10.1038/s41574-018-0059-4
84. Brown TT, Qaqish RB. Antiretroviral therapy and the prevalence of osteopenia and osteoporosis: a meta-analytic review. AIDS. (2006) 20:2165–74. doi: 10.1097/QAD.0b013e32801022eb
85. Daugas E, Rougier JP, Hill G. HAART-related nephropathies in HIV-infected patients. Kidney Int. (2005) 67:393–403. doi: 10.1111/j.1523-1755.2005.67096.x
86. Friis-Moller N, Sabin CA, Weber R, d'Arminio Monforte A, El-Sadr WM, Reiss P, et al. Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med. (2003) 349:1993–2003. doi: 10.1056/NEJMoa030218
87. Villarroya F, Domingo P, Giralt M. Drug-induced lipotoxicity: lipodystrophy associated with HIV-1 infection and antiretroviral treatment. Biochim Biophys Acta. (2010) 1801:392–9. doi: 10.1016/j.bbalip.2009.09.018
88. Group ISS, Lundgren JD, Babiker AG, Gordin F, Emery S, Grund B, et al. Initiation of Antiretroviral Therapy in Early Asymptomatic HIV Infection. N Engl J Med. (2015) 373:795–807. doi: 10.1056/NEJMoa1506816
89. Lifson AR, Grund B, Gardner EM, Kaplan R, Denning E, Engen N, et al. Improved quality of life with immediate versus deferred initiation of antiretroviral therapy in early asymptomatic HIV infection. AIDS. (2017) 31:953–63. doi: 10.1097/QAD.0000000000001417
90. group As. Vorapaxar for HIV-associated inflammation and coagulopathy (ADVICE): a randomised, double-blind, placebo-controlled trial. Lancet HIV. (2018) 5:e553–9. doi: 10.1016/S2352-3018(18)30214-5
91. Aberg JA, Sponseller CA, Ward DJ, Kryzhanovski VA, Campbell SE, Thompson MA. Pitavastatin versus pravastatin in adults with HIV-1 infection and dyslipidaemia (INTREPID): 12 week and 52 week results of a phase 4, multicentre, randomised, double-blind, superiority trial. Lancet HIV. (2017) 4:e284–94. doi: 10.1016/S2352-3018(17)30075-9
92. Toribio M, Fitch KV, Sanchez L, Burdo TH, Williams KC, Sponseller CA, et al. Effects of pitavastatin and pravastatin on markers of immune activation and arterial inflammation in HIV. AIDS. (2017) 31:797–806. doi: 10.1097/QAD.0000000000001427
93. Hsue PY, Ribaudo HJ, Deeks SG, Bell T, Ridker PM, Fichtenbaum C, et al. Safety and impact of low-dose methotrexate on endothelial function and inflammation in individuals with treated human immunodeficiency virus: AIDS clinical trials group study A5314. Clin Infect Dis. (2019) 68:1877–86. doi: 10.1093/cid/ciy781
94. Jacobson JM, Bosinger SE, Kang M, Belaunzaran-Zamudio P, Matining RM, Wilson CC, et al. The effect of chloroquine on immune activation and interferon signatures associated with HIV-1. AIDS Res Hum Retroviruses. (2016) 32:636–47. doi: 10.1089/aid.2015.0336
95. Murray SM, Down CM, Boulware DR, Stauffer WM, Cavert WP, Schacker TW, et al. Reduction of immune activation with chloroquine therapy during chronic HIV infection. J Virol. (2010) 84:12082–6. doi: 10.1128/JVI.01466-10
96. O'Brien MP, Hunt PW, Kitch DW, Klingman K, Stein JH, Funderburg NT, et al. A randomized placebo controlled trial of aspirin effects on immune activation in chronically human immunodeficiency virus-infected adults on virologically suppressive antiretroviral therapy. Open Forum Infect Dis. (2017) 4:ofw278. doi: 10.1093/ofid/ofw278
97. Utay NS, Kitch DW, Yeh E, Fichtenbaum CJ, Lederman MM, Estes JD, et al. Telmisartan therapy does not improve lymph node or adipose tissue fibrosis more than continued antiretroviral therapy alone. J Infect Dis. (2018) 217:1770–81. doi: 10.1093/infdis/jiy064
98. Capuron L, Geisler S, Kurz K, Leblhuber F, Sperner-Unterweger B, Fuchs D. Activated immune system and inflammation in healthy ageing: relevance for tryptophan and neopterin metabolism. Curr Pharm Des. (2014) 20:6048–57. doi: 10.2174/1381612820666140317110217
Keywords: HIV, aging, inflammation, morbidity, anti-retroviral therapy
Citation: Premeaux TA, Javandel S, Hosaka KRJ, Greene M, Therrien N, Allen IE, Corley MJ, Valcour VG and Ndhlovu LC (2020) Associations Between Plasma Immunomodulatory and Inflammatory Mediators With VACS Index Scores Among Older HIV-Infected Adults on Antiretroviral Therapy. Front. Immunol. 11:1321. doi: 10.3389/fimmu.2020.01321
Received: 06 April 2020; Accepted: 26 May 2020;
Published: 30 June 2020.
Edited by:
Ujjwal Neogi, Karolinska Institute, SwedenReviewed by:
Jose Artur Chies, Federal University of Rio Grande do Sul, BrazilCopyright © 2020 Premeaux, Javandel, Hosaka, Greene, Therrien, Allen, Corley, Valcour and Ndhlovu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lishomwa C. Ndhlovu, bG5kaGxvdnVAbWVkLmNvcm5lbGwuZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.