- 1National Institute of Infectious Diseases and Vaccinology, National Health Research Institutes, Zhunan, Taiwan
- 2Department of Biochemical Science and Technology, National Chiayi University, Chia-Yi, Taiwan
Pulmonary tuberculosis (TB) is a difficult-to-eliminate disease. Although the Bacille Calmette–Guérin (BCG) vaccine against Mycobacterium tuberculosis (MTB) has been available for decades, its efficacy is variable and has lessened over time. Furthermore, the BCG vaccine no longer protects against newly emerged Beijing strains which are responsible for many current infections in adults. Development of a novel vaccine is urgently needed. In this study, we first tested the efficacy of our recombinant BCG vaccines rBCG1 and rBCG2, compared to parental BCG, against MTB strain H37Ra in mice. Both the bacterial load and the level of lymphocyte infiltration decreased dramatically in the three groups treated with vaccine, especially rBCG1 and rBCG2. Furthermore, the Th1 and Th17 responses increased and macrophage numbers rose in the vaccination groups. Th1-mediated production of cytokines TNF-α, IFN-γ, and MCP-1 as well as M1-polarized cells all increased in lung tissue of the rBCG1 and rBCG2 groups. Clodronate-induced depletion of macrophages reduced the level of protection. Based on these results, we conclude that rBCG vaccines induce a significant increase in the number of M1 macrophages, which augments their potential as TB vaccine candidates.
Introduction
Tuberculosis is a difficult-to-treat disease that causes millions of deaths worldwide (1, 2). The disease is a major public health problem in many countries, consuming considerable societal and financial resources. The bacille Calmette–Guérin (BCG) vaccine, created in 1921, is the only licensed vaccine against Mycobacterium tuberculosis (MTB) (3). When administered during childhood, the vaccine provides ~10–15 years of protection, but this immunity wanes in adulthood (4). However, the protective efficacy of the BCG vaccine varies widely, from 0 to 88% (5–7). Based on this epidemiological evidence, BCG appears to lose its protective effect over time (4–7). Although most studies did not find any association, a study that subdivided Beijing MTB strains into typical and atypical lineages found typical Beijing strains to be more frequent among BCG-vaccinated persons compared to non-vaccinated persons (8), suggesting that BCG vaccination is less protective against typical Beijing strains, which has also been reported in animal models (9, 10). For these reasons, development of a novel, effective vaccine is urgently needed.
Antigen 85B (Ag85B) and culture filtrate protein 10 (CFP-10) are two candidates for effective MTB vaccine development. Ag85B, part of the Ag85 complex with antigens B and C, functions as a mycolyl transferase in MTB cell wall assembly (11). This major protective protein is recognized by T cells and is a strong MTB antigen (12, 13). Ag85B binds fibronectin, which then stimulates human monocytes to produce TNF-α (14). Overexpression of Ag85B reduces bacterial load (15) and protects mice against MTB infection (16). Recently, Prendergast et al. showed that Ag85B-deficient BCG had reduced capacity to infect macrophages, whereas an Ag85B-overexpressing BCG strain had improved uptake by macrophages (17). CFP-10, a secreted protein encoded by the RD-1 region of MTB, combines with the 6-kDa early secreted antigen target 6 (ESAT-6) protein to form a heterodimer (18). Loss of both Ag85B and CFP-10 has been associated with attenuated MTB infection (19, 20). Therefore, these targets have been used as trial vaccine candidates for years. However, some studies show that Ag85B and CFP-10 do not provide adequate protection (17) or do not have enough long-term data to support their efficacy (21, 22).
Recombinant BCG vaccines can be engineered to express immunodominant antigens such as Ag85 complex (Ag85A-C) and ESAT-6. Notably, CD8+ T cells recognizing CFP-10 are elicited following MTB infection in humans (23–26). Therefore, our strategy is to combine Ag85B and CFP-10 into a fusion protein that is expressed in BCG hosts. Human IL-12 provides a robust immune response to such fusion proteins and could enhance vaccine efficacy. Recently, our recombinant BCG vaccines rBCG1 and rBCG2 demonstrated higher stimulation of IFN-γ secretion compared to parental BCG vaccine (27). rBCG1 is BCG containing a fusion protein of Ag85B and CFP-10, whereas rBCG2 is BCG containing the same fusion protein plus human IL-12 (27). Prior to the present study, we had not tested the protective efficacy of these two recombinant vaccines in an animal infection model. In order to establish the efficacy of our rBCG vaccines in mice, we tested them against MTB strain H37Ra administered intravenously and compared their effects to that of the parental BCG vaccine; in this trial, the mice were given two vaccinations 2 weeks apart and then challenged with H37Ra 2 weeks later. This study was designed to evaluate rBCG1 and rBCG2 efficacy and long-term protection against MTB compared to the parental BCG vaccine. The study included characterization of immune cell responses, including cytokine-release profiles.
Materials and Methods
Bacterial Strains and Cultures
Parental BCG is Mycobacterium bovis BCG (Tokyo172) and was used to construct the recombinant BCG strains rBCG1 and rBCG2 in a previous study (27). Briefly, rBCG1 was constructed using MTB antigens Ag85B and CFP-10 in an M. bovis BCG Tokyo 172 strain, and rBCG2 was constructed using MTB antigens Ag85B and CFP-10 plus human IL-12 also in M. bovis BCG Tokyo 172. rBCG1 and rBCG2 were compared to M. tuberculosis strain H37Ra. H37Ra and BCG (Tokyo 172) were maintained on Middlebrook 7H9 medium (Difco Laboratories, Detroit, MI, USA) and supplemented with 0.5% glycerol, 0.05% Tween 80, and 10% albumin- dextrose-catalase or on solid Middlebrook 7H11 medium (Difco Laboratories) supplemented with oleic acid-albumin-dextrose-catalase. rBCG1 and rBCG2 were maintained on the media described above with 25 μg/ml kanamycin.
Mice and Immunization
Female C57BL/6 and SCID mice aged 6–8 weeks were purchased from the National Laboratory Animal Center (Taipei, Taiwan). All mice were kept in individually ventilated cage environments at the Animal Center of the National Health Research Institutes (Maoli, Taiwan). The temperature was maintained at 20–24°C with a relative humidity of 40–70% and a 12-h light/dark cycle. Animal experiments were reviewed and approved by the National Health Research Institutes Institutional Animal Care and Use Committee (NHRI-IACUC). We conducted experiments according to guidelines set out by the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC). Mice were given a two-dose vaccination of parental BCG, rBCG1, or rBCG2 (5 × 105 colony forming units [CFUs] per mouse) subcutaneously at weeks 0 and 2. Mice were then challenged with 106 CFU of H37Ra per mouse at week 4 by intravenous injection. For macrophage depletion, mice were given a two-dose vaccination of parental BCG, rBCG1, or rBCG2 (5 × 105 CFU per mouse) subcutaneously at weeks 0 and 2, and they received one drop of encapsome® (control) or clodrosome® (Encapsula Nano Sciences, TN, USA) intratracheally weekly for 8 weeks. For the biosafety assay, C57BL/6 and SCID mice aged 6–8 weeks were given a two-dose vaccination of parental BCG, rBCG1, or rBCG2 (5 × 105 CFU per mouse) subcutaneously at weeks 0 and 2, and then challenged with H37Ra at week 4. The mice were closely monitored and their body weights were measured every week until week 40 (or a humane endpoint).
Multiple Cytokine Testing
After sacrifice, blood was collected and plasma was stored at −20°C until use. Murine inflammatory cytokine testing was performed using a multiple mouse cytometric bead array (CBA) mouse inflammation kit (BD Biosciences, San Jose, CA, USA).
Immunohistochemical Staining (IHC) and Pathologic Score
Samples of lung tissue were fixed in formalin and embedded in paraffin using routine methods, and the sections were then stained with hematoxylin and eosin (H&E). Tissue processing was performed by the core pathology facility at the National Health Research Institutes (Maoli, Taiwan). Histopathologic parameters (i.e., peribronchiolitis, perivasculitis, alveolitis, and granuloma formation) were each semi-quantitatively scored as absent, minimal, slight, moderate, marked, or strong using numerical scores of 0, 1, 2, 3, 4, and 5, respectively (28). Lesion frequency and severity were incorporated into these scores. For each time point, lung tissue from six or seven animals was examined, and the mean score for each slide of five or six random views was calculated. For IHC, paraffin sections were rehydrated and retrieved with 100 mM citrate buffer (pH 6.0) at 100°C for 5 min. After blocking the peroxidase activity and background (IHC/ICC kit, BioVision, Milpitas, CA, USA), the serial sections were incubated with primary antibody anti-iNOS (Abcam, ab15323, Cambridge, UK, RRID:AB_301857) and anti-mannose receptor (Abcam, ab64693, Cambridge, UK, RRID:AB_1523910), and stained following the manufacturer's protocol (BioVision). Finally, the sections were colored with the chromogen DAB and counterstained with hematoxylin. For determination of positive cells, the number of DAB-positive plus hematoxylin- positive cells in six randomly selected fields per slide (each an area of 8.4 × 103 μm2) was calculated and analyzed.
Bacterial Culture
Half of each lung tissue sample was minced and passed through a MACS C tube to produce single-cell suspensions in 5 ml of saline (cell survival rate >99.5% by hemocytometer). CFU values were determined by using 100 μl of cell mixture dilution on 7H10 agar plates. Each plate contained 100 μl of tissue homogenate, and each sample was titrated for three dilutions (10×, 100× and 1000×) performed in triplicate. Plates were kept at 37°C for 3–4 weeks. The colony number was counted and presented as a value per one lung (one mouse).
Flow Cytometry
Lung and spleen tissues were minced and passed through a MACS C tube to produce single-cell suspensions (cell survival rate >99.5% by hemocytometer). The cells were incubated in 50 μl of RPMI medium that contained 2% fetal bovine serum (FBS) with Fc receptor for 15 min and then with several antibodies: CD4-FITC (cat. 553046, RRID:AB_394582), CD4-PerCP-Cy5.5(cat. 553052, RRID:AB_394587), CD8-PerCP-Cy5.5 (cat. 551162, RRID:AB_394081), CD25-BB515(cat.564424, RRID:AB_2738803), F4/80-PE(cat. 565410, RRID:AB_2687527), CD49b-APC, (cat.558295, RRID:AB_398658), IFN-γ-FITC(cat.554411), IL-17A-AF647(560184, RRID:AB_1645204), IL-4-PE(cat. 554435, RRID:AB_395391), Foxp3-PE(cat. 560408, RRID:AB_1645251) (all from BD Biosciences), and CD317-PerCP-Cy5.5 (pDCs; 120G8, Dendritics, San Diego, CA, USA, RRID:AB_2566646). For intracellular cytokine labeling, cells were stimulated with MTB-specific peptides (5 μg/ml of CD4 peptide Ag85B241−255aa QDAYNAAGGHAVFN (Mission Biotech, Taiwan, cat. 991172), CD8 peptide Ag85B1−19aa FSRPGLPVEYLQVPSMG, Mission Biotech, Taiwan, cat. 991171) for 68 h and then Golgi-stop (BD Biosciences) was added for an additional 4 h [28). Cells were then fixed, permeabilized and stained with cytokine antibodies (IFN-γ-FITC, IL-17A-AF647, IL-4-PE and Foxp3-PE) for 1 h following standard procedures. The gating strategy involved CD4+ T cells, and other relevant cell populations were gated from the single cell lymphocyte population in the forward scatter (FSC) and side scatter (SSC) sections (29). The samples were acquired and analyzed using a FACSCalibur system and CellQuest software (BD Biosciences).
RNA Extraction and Quantitative PCR
Total mRNA was extracted by Trizol reagent, and cDNA was prepared by using a reverse transcription kit (ThermoFisher Scientific, CA, USA). Primer sequences are shown in Table 1. The mRNA levels of TNF-α, IFN-γ, MCP-1, various transcription factors and GAPDH were detected by real-time quantitative PCR analysis using the ABI vii7 system (Applied Biosystems, Foster City, CA, USA). Cytokine and GAPDH levels were calculated relative to amounts found in a standard sample, and cytokine levels were corrected for GAPDH mRNA levels to normalize for RNA input. Relative expression (Relative index) is presented as 2−ΔCT.
Statistical Analysis
All results are presented as mean ± SEM for six or seven mice per group. The statistical significance between the experimental groups was assessed using a two-tailed unpaired Student's t-test, non-parametric analysis, one-way and two-way analysis of variance (ANOVA) with a Tukey or Bonferroni post hoc test. The differences were considered significant for P < 0.05. Statistical tests were performed using GraphPad Prism version 6.0 (GraphPad Software, La Jolla, CA, USA).
Results
rBCG1 and rBCG2 Vaccines Protect Mice Against Mycobacterium tuberculosis H37Ra
To determine whether our recombinant BCG vaccines rBCG1 and rBCG2 protect against MTB infection, we first conducted a vaccine efficacy test against the H37Ra strain in an ABSL2 facility. (We would have conducted this initial test in an ABSL3 facility with an aerosol virulent MTB Beijing strain, however our facility was not operational at the time.) We administered the vaccines to mice subcutaneously twice (at weeks 0 and 2) and then challenged the mice with MTB H37Ra via intravenous injection at week 4. The bacterial load and lung pathology were measured at weeks 8, 12, and 20 after the immunization. At week 2 (prior to H37Ra challenge), the lungs showed no sign of cellular infiltration (Figures 1B,C). At week 8 post-vaccination, the bacterial load of the PBS control group was high [CFU = (115.2 ± 47.91) × 1,000] compared to that of the BCG [CFU = (5.0 ± 2.822) × 1,000], rBCG1 [CFU = (0 ± 0) × 1,000] and rBCG2 [CFU = (1.112 ± 1.112) × 1,000] groups (**P < 0.001; Figure 1A). Inflammation in the PBS control group was more severe than in the vaccination groups (Figures 1B,C). At week 12, the bacterial load in the PBS control was still high [CFU = (74.72 ± 14.13) × 1,000], whereas the bacterial loads were lower in the BCG, rBCG1, and rBCG2 groups [CFU = (6.66 ± 2.974) × 1,000, (14.99 ± 5.867) × 1,000, and (1.667 ± 1.054) × 1,000, respectively, **P < 0.001; Figure 1A]. However, the cellular infiltration lesions had nearly disappeared by week 12 in the rBCG1 and rBCG2 groups (Figures 1B,C). Thus, the rBCG1 and rBCG2 vaccines protected mice against H37Ra infection at an early stage. At week 20, stable bacterial loads were detected for the PBS control group [CFU = (68.57 ± 6.904) × 1,000], but very low bacterial loads were measured in the rBCG1 and rBCG2 groups [CFU = (16.67 ± 5.236) × 1,000 and (11.11 ± 9.165) × 1,000, respectively; *P < 0.05]. At 20 weeks, the bacterial load had dramatically increased in the BCG group [CFU = (66.9 ± 29.23) × 1,000, P > 0.05; Figure 1A). These CFU results paralleled the H&E staining patterns and pathologic scores (Figures 1B,C). Taken together, these results suggest that, whereas the parental BCG vaccine does not provide adequate protection against H37Ra infection, the rBCG1 and rBCG2 vaccines do. Thus, the efficacies of the rBCG1 and rBCG2 vaccines are higher than that of the parental BCG.
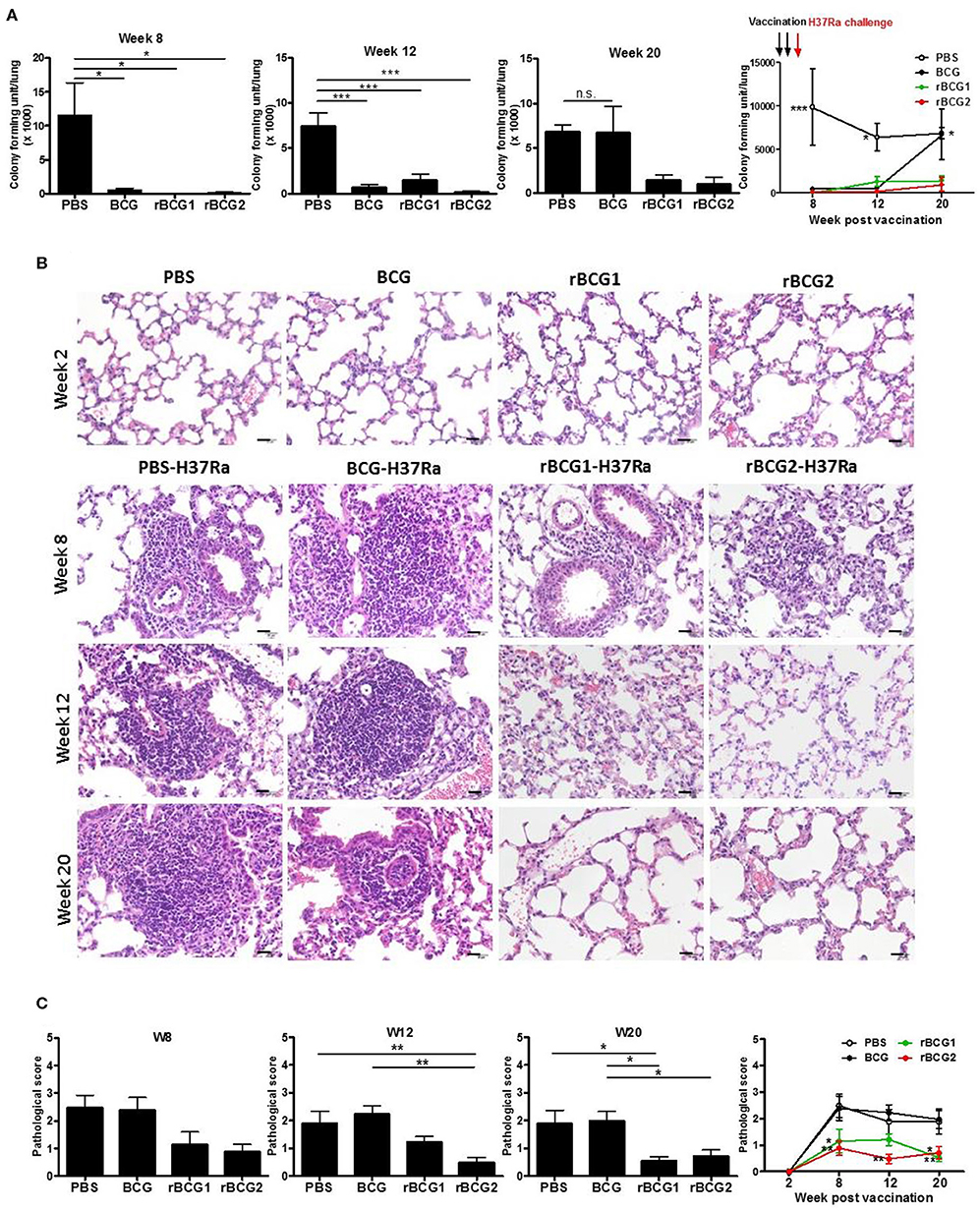
Figure 1. Protection provided by recombinant BCG vaccines rBCG1 and rBCG2 against Mycobacterium tuberculosis H37Ra in mice. C57BL/6 mice were immunized with BCG, rBCG1, or rBCG2 at weeks 0 and 2, and then challenged with H37Ra at week 4 (n = 6–7, in two independent experiments). At 2, 8, 12, and 20 weeks, the mice were sacrificed. One lung from each mouse was homogenized and the other was fixed in 3.7% formaldehyde. (A) Colony forming units (CFU) of bacterial growth in murine lung. Each plate contained 100 μl of tissue homogenate, and each sample was titrated for two dilutions (5× and 50×) and repeated in triplicate. (B) Lung samples were fixed in 3.7% formaldehyde, embedded in paraffin, sectioned, and stained with H&E; scale bar = 20 μm. Differences among groups were determined using a one-way, non-parametric test, or two-way ANOVA with a Tukey or Bonferroni post hoc test (*P < 0.05; **P < 0.01; ***P < 0.001; ns, non-significant). (C) Pathologic scores were determined and analyzed by H&E staining. For each time point, lung tissue from six or seven animals was examined and the mean score for each slide of five or six random views was calculated. Differences among groups were determined by one-way or two-way ANOVA with a Tukey or Bonferroni post hoc test (*P < 0.05, **P < 0.01).
rBCG1 and rBCG2 Vaccines Are Safe in SCID Mice as Well as in C57BL/6 Wild-Type Mice
To test the safety of the rBCG1 and rBCG2 vaccines, we gave the same dose of BCG and rBCG vaccines to severe combined immunodeficiency (SCID) and C57BL/6 wild-type mice subcutaneously at weeks 0 and 2. All of the mice were monitored very closely until week 40 or an earlier humane endpoint. Mouse weight was recorded every week. After the vaccination, neither the SCID nor wild-type mice showed any adverse physical signs. The weights of both groups increased slightly every week, but the differences between groups were not significant (Figures 2B,D, P > 0.05, ns). Starting at week 21, one SCID mouse in each of the BCG, rBCG1 and rBCG2 groups was euthanized for humane reasons but there were no deaths in the PBS group (Figure 2A) or in any of the wild-type groups (Figure 2C). These results suggest that the rBCG1 and rBCG2 vaccines are safe, even in immunodeficient individuals.
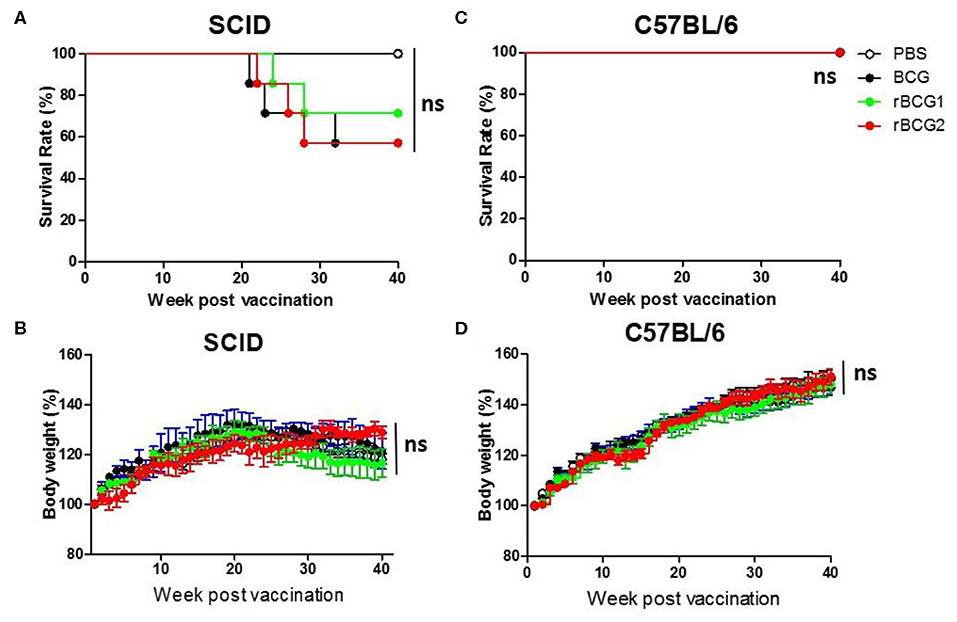
Figure 2. Test of rBCG vaccine safety in immunodeficient and immunocompetent mice. SCID and C57BL/6 wild-type mice were immunized with BCG, rBCG1, or rBCG2 at weeks 0 and 2 (n = 10). (A,C) Survival rate of SCID and C57BL/6 mice after vaccination. (B,D) The body weights of SCID and C57BL/6 mice were recorded every week. Body weight (%) was calculated as: weight (week n)/weight (week 0) per mouse. Survival curves were plotted by the Kaplan-Meier method. The Log-rank test was performed to compare each pair of survival curves (P > 0.05; ns, non-significant). Differences among groups were determined by two-way ANOVA with a Bonferroni post hoc test (P > 0.05; ns, non-significant).
mRNA Expression of Th1-Mediated Cytokines in Mouse Lung Tissue Following Immunization With BCG, rBCG1, and rBCG2
We next examined cytokine expression in the lungs using quantitative PCR (qPCR) to determine whether recombinant BCG vaccines stimulate Th1-mediated cytokine production. RNA was extracted from cells isolated from the lungs and spleens of the PBS control and vaccination groups before and after H37Ra challenge, at weeks 2, 8, 12, and 20, and qPCR was performed to detect cytokine gene expression in these tissues. TNF-α, IFN-γ, and MCP-1 were all increased in the BCG, rBCG1 and rBCG2 groups compared to the PBS control group, particularly during weeks 2 and 8 (Figures 3A–C). At weeks 12 and 20, TNF-α, IFN-γ, and MCP-1 were decreased in all groups (Figures 3A–C); presumably this reflects bacterial clearance, when cytokine production stops and normal resting levels are reached. These cytokines are the products of Th1-mediated immune responses. In contrast, IFN-α1, IFN-β1, and IDO-1, which are secreted by some of the surrounding infected immune cells, were detected at higher levels in the PBS and BCG groups compared to the rBCG1 and rBCG2 groups (Figures 3D–F). Cytokines IL-1β, IL-4, IL-6, IL-10, IL-12, IL-17, and IDO-2 were unchanged or undetectable for all groups (data not shown). These results suggest that rBCG1 and rBCG2 can induce host Th1 responses to clear MTB bacteria.
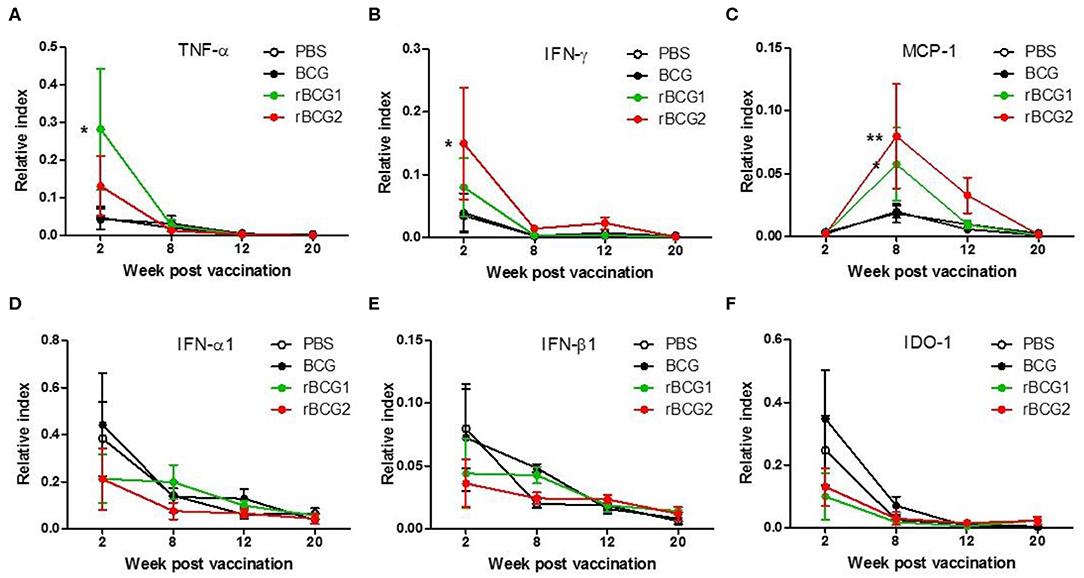
Figure 3. Th1-mediated cytokine profiles expressed in murine lung following immunization with rBCG1 or rBCG2. C57BL/6 mice were immunized with BCG, rBCG1 or rBCG2 at weeks 0 and 2. They were then challenged with H37Ra at week 4 (n = 6–7, in two independent experiments). At 2, 8, 12, and 20 weeks, the mice were sacrificed, lung RNA was extracted and cytokine gene expression profiles were determined using qPCR. (A) TNF-α, (B) IFN-γ, (C) MCP-1, (D) IFN-α1, (E) IFN-β1, (F) IDO-1. Differences among groups were determined using two-way ANOVA with a Bonferroni post hoc test (*P < 0.05; **P < 0.01).
Macrophages, Th1 Cells and Th17 Cells Dominate Following Immunization With BCG, rBCG1, and rBCG2
We analyzed immune cell profiles from murine lung and spleen tissue using flow cytometry. Single cells were isolated from the lung and spleen in the PBS control and vaccination groups before and after H37Ra challenge, at weeks 2, 8, 12, and 20. After conducting surface marker and intracellular staining, FACS was used to analyze immune cell profiles. At week 2, all immune cell levels were very low, with no differences among the four groups (Figures 4B,D,F,H). At week 8 (i.e., 4 weeks after H37Ra challenge), macrophage numbers (F4/80+ cells) had increased dramatically in the rBCG1 and rBCG2 vaccination groups compared to the PBS control and BCG groups (Figures 4A,B; *P < 0.05). There were few NK cells (CD49b+ cells) and resident macrophages in all groups at 2 weeks (data not shown). Increased IFN-γ+ T cells (Figures 4C,D) and IL-17A+ T cells (Figures 4G,H) (but not IL-4+ T cells, Figures 4E,F) were also detected in all BCG vaccination groups, but especially in the rBCG1 and rBCG2 groups. Thus, the induced cellular immunity was higher in mice immunized with rBCG1 and rBCG2 compared to parental BCG.
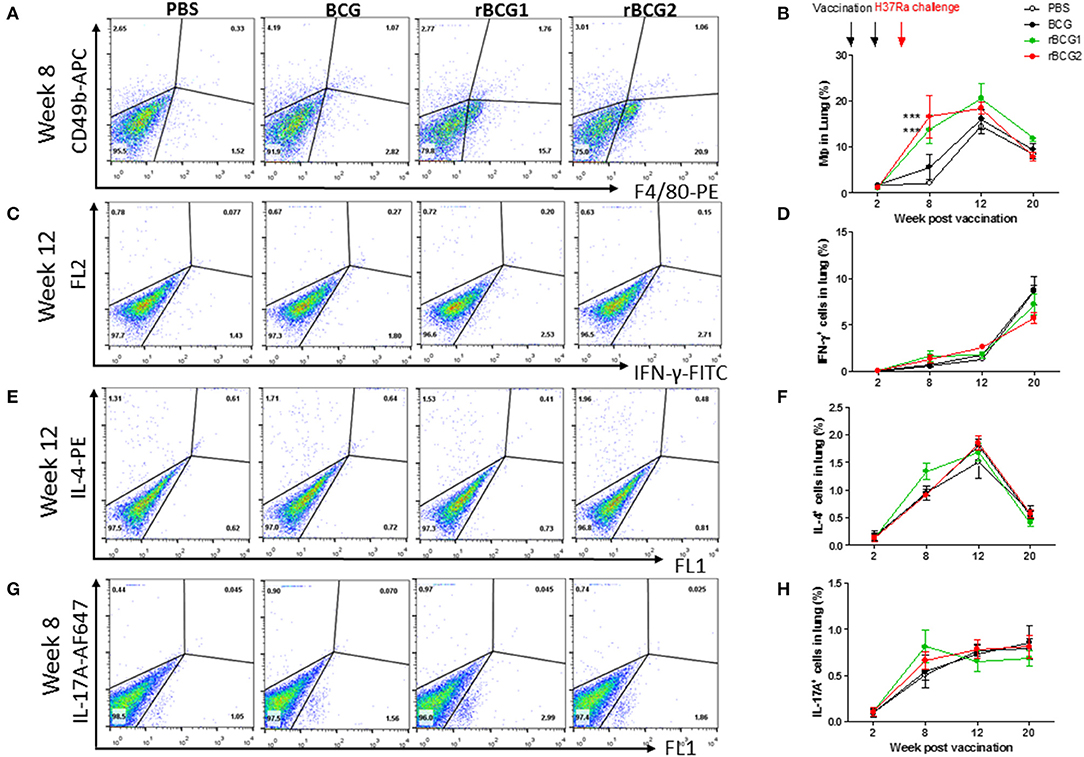
Figure 4. Innate and adaptive immune cell profiles pulsed with tuberculosis-specific peptides from mice immunized with BCG, rBCG1, or rBCG2. C57BL/6 mice were immunized with BCG, rBCG1 or rBCG2 at weeks 0 and 2, and then challenged with H37Ra at week 4 (n = 6–7, in two independent experiments). At 2, 8, 12, and 20 weeks, the mice were sacrificed, and lungs were homogenized to single-cell suspensions. The cells were stimulated with tuberculosis-specific TB peptides (described in the Materials and Methods) for 68 h and then Golgi-stop for 4 h, stained for surface and intracellular markers, and then subjected to flow cytometry to determine the percentage of cytokine-producing cells within CD4+ T cells. (A) Percentage of macrophages (F4/80+ cells) in lung post-vaccination at week 8. (B) Percentage of macrophages (F4/80+ cells) in lung post-vaccination at weeks 2–20. (C) Percentage of IFN-γ+ cells in lung post-vaccination at week 12. (D) Percentage of IFN-γ+ cells in lung post-vaccination at weeks 2–20. (E) Percentage of IL-4+ cells in lung post-vaccination at week 12. (F) Percentage of IL-4+ cells in lung post-vaccination at weeks 2–20. (G) Percentage of IL-17+ T cells in lung post-vaccination at week 8. (H) Percentage of IL-17+ T cells in lung post-vaccination at weeks 2–20. Differences among groups were determined by one-way or two-way ANOVA with a Tukey or Bonferroni post hoc test (***P < 0.001).
Monocyte Chemoattractant Protein-1 (MCP-1) Is the Dominant Cytokine Produced in Serum Following rBCG1 and rBCG2 Vaccination
Cytokine production was measured for serum and tissue (lung and spleen) using cultured supernatant samples and a multiple cytokine inflammation kit (BD Biosciences). The cytokine detection kit measured all inflammatory cytokines, including TNF-α, IFN-γ, IL-6, IL-10, IL-12, and MCP-1. No cytokines were found in tissue culture supernatant samples (data not shown) as they were below the limit of detection. Interestingly, MCP-1 was the only cytokine detected in serum. MCP-1 increased in the serum of mice immunized with rBCG1 and rBCG2 at 2 and 8 weeks (Figure 5; **P < 0.01). MCP-1 also increased in the PBS control and BCG groups beginning at week 8 and continuing until week 12. MCP-1 then decreased in all groups at week 20. The high MCP-1 production (weeks 2 and 8) coincided with reduced bacterial loads (Figure 1A) and increased lung macrophage levels in the rBCG1 and rBCG2 groups (Figure 4A).
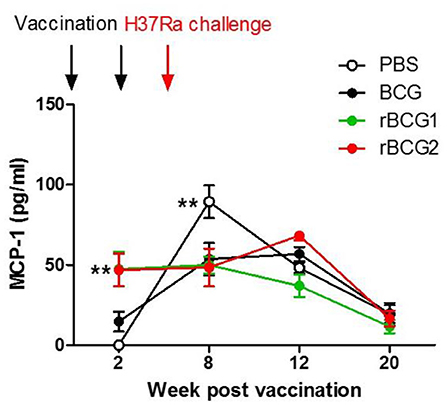
Figure 5. High MCP-1 expression in serum during early-stage recombinant BCG vaccination. C57BL/6 mice were immunized with BCG, rBCG1, and rBCG2 at weeks 0 and 2, and then challenged with H37Ra at week 4 (n = 6–7, in two independent experiments). At 2, 8, 12, and 20 weeks, sera were collected, diluted 20,000 times, and assayed using a multiple cytokine kit. Differences among groups were determined by two-way ANOVA with a Bonferroni post hoc test (**P < 0.01).
Macrophages Are Key Mediators of the Immune Response at the Early Stage of H37Ra Infection
Based on the qPCR results, the increased MCP-1 production, and the higher numbers of macrophages in the lungs, macrophage function is obviously of central importance in the response to MTB infection. To evaluate the role of macrophages early in infection, we attempted to deplete macrophages by administering clodronate-liposomes intratracheally every week following the vaccination and challenge (Figure 6A). After 3–4 weeks, we counted and calculated the bacterial load among the control and vaccination groups. The bacterial load of the PBS control group was still high [CFU = (7.611 ± 3.282) × 1,000] (Figure 6B); however, the bacterial loads of each of the vaccination groups (BCG [CFU = (0.9167 ± 0.4488) × 1,000], rBCG1 [CFU = (4.283 ± 3.722) × 1,000], rBCG2 [CFU = (0.6333± 0.178) × 1,000]) were dramatically increased (compared with the results shown in Figure 1A), but were not significantly different compared to the PBS group (Figure 6B, P > 0.05, ns). This result suggests that macrophages are the main immune cells involved in protection against Mycobacterium infection early on. After macrophage depletion, the mice were no longer protected against the infection.
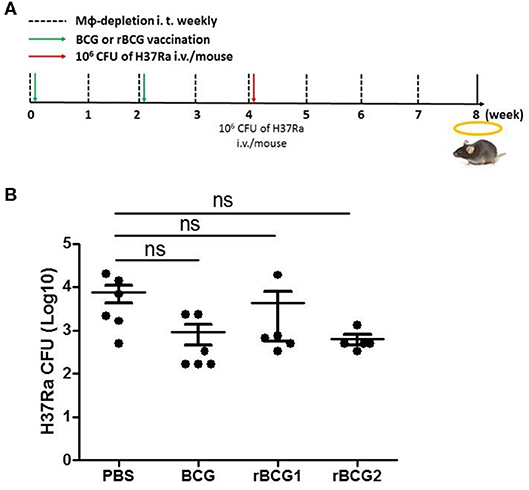
Figure 6. Macrophages are significant for protection against Mycobacterium infection. C57BL/6 mice were given a two-dose vaccination of parental BCG, rBCG1, and rBCG2 (5 × 105 CFU per mouse) subcutaneously at weeks 0 and 2 (n = 6), and a challenge of H37Ra at week 4; they also received 50 μl of encapsome® (control) or clodrosome® intratracheally weekly for 8 weeks to deplete macrophages. Mice were sacrificed at week 8. (A) Timeline for vaccination, challenge, and intratracheal inoculation in mice. (B) CFU of bacterial growth in murine lung at week 8. Each plate contained 100 μl of tissue homogenate, and each sample was titrated for two dilutions (5× and 50×) and repeated in triplicate. Differences among groups were determined using one-way ANOVA with a Tukey post hoc test (P > 0.05, ns, non-significant).
Increased Levels of Inflammatory M1-Polarized Macrophages Especially in rBCG1 and rBCG2 Vaccinated Mice
Macrophages adapt to the microenvironment and differentiate to express different functional phenotypes. M1 macrophages produce high levels of pro-inflammatory cytokines and have a strong ability to kill pathogens. In contrast, M2 macrophages are involved in clearance of parasites, tissue remodeling and immune regulation (30). We believe that rBCG1 and rBCG2 enhance the ability of M1 macrophages to clear pathogens. Using the remaining tissue samples fixed in paraffin blocks, we sought to compare the levels of M1 macrophages in the vaccination groups, especially in the rBCG1 and rBCG2 groups. We examined paraffin sections collected at week 8 because they showed the highest macrophage numbers and MCP-1 production. After deparaffinization, rehydration, antigen retrieval and blocking, the serial sections were stained to measure inducible nitric oxide synthase (iNOS, M1 macrophage marker) and mannose receptor (MR, M2 macrophage marker). In general, each treatment group expressed different levels of iNOS (Figure 7A); however, iNOS-expression was raised dramatically in the rBCG1 and rBCG2 groups (the mean M1 number for rBCG1 was ~35, for rBCG2 ~50, compared to PBS ~18 and BCG ~22; Figures 7A,B, *P < 0.05). The expression of MR was similar in all groups (the mean M2 value was ~15–20, Figures 7A,B). These results indicate that, among the tested vaccines, rBCG2 induces the greatest increase in the number of M1 macrophages to clear bacteria in the lungs.
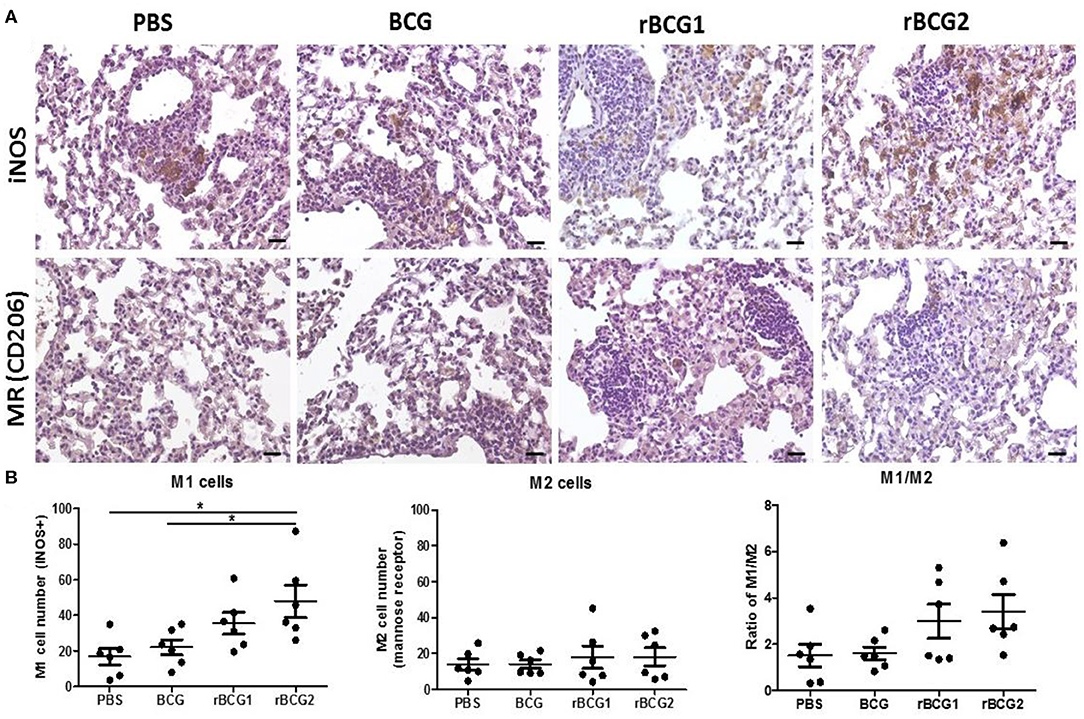
Figure 7. Increased M1-polarized macrophage cells in lung tissue sections from mice vaccinated with rBCG1 or rBCG2. C57BL/6 mice were immunized with BCG, rBCG1, or rBCG2 at weeks 0 and 2, and then challenged with H37Ra at week 4 (n = 6–7). At week 8, the mice were sacrificed. One lung from each mouse was homogenized and the other lung was fixed in 3.7% formaldehyde. (A) After fixation, lung samples were embedded in paraffin, sectioned, and stained for iNOS (M1 marker) and MR (M2 marker); scale bar = 20 μm. The images are representative of slides from the four groups. (B) Lung tissue from six or seven animals per group was examined, and the number of DAB+ plus hematoxylin-positive cells for each slide, based on five or six random views (each field of view has an area of 8.4 × 103 μm2), was calculated. Differences among groups were determined by one-way ANOVA with a Tukey post hoc test (*P < 0.05).
Discussion
Ag85B and CFP-10 are well-known MTB antigens that strongly induce IFN-γ production (31) and enhance T cell proliferation (31, 32). Both proteins are considered good vaccine candidates for tuberculosis. Notably, IL-12 is believed to play an important role in cell-mediated immunity against intracellular infection, primarily by acting on T and NK cells. Recent study findings reveal that IL-12 can activate macrophages potently during intracellular infection, and this activating effect is mediated primarily through its effect on macrophage IFN-γ release (33). Therefore, rBCG2 expressing Mycobacterium-specific Ag85B and CFP10 combined with human IL-12 was constructed and investigated in this study. In our previous study, protection efficacy was tested by using splenocytes from vaccine-immunized mice and co-culturing them with ex vivo murine bone-marrow-derived macrophages to measure the survival ability of the MTB strains (34). The results showed that rBCG2 induced a strong immune response against MTB infection compared with the parental BCG strain (34). In the present study, we first tested rBCG1 and rBCG2 against the MTB strain H37Ra. We found that rBCG1 and rBCG2 produced a stronger immune response to MTB infection compared to the parental BCG vaccine in mice. Previous ex vivo murine bone-marrow-derived macrophage infection experiments with H37Rv also showed that rBCG1 and rBCG2 provide stronger immunity compared to parental BCG (35, 36). In the present study, during early infection, all three tested vaccines (BCG, rBCG1 and rBCG2) inhibited H37Ra growth. However, at week 20, the bacterial load in the BCG group rebounded and increased. In contrast, the bacterial loads in the rBCG1 and rBCG2 groups remained low and nearly undetectable. If the murine age is converted to human age, the period of protection of BCG (20 weeks) found in this study would be equivalent to 15–20 years; this finding is consistent with that of a previous epidemiologic study of BCG vaccination in humans (37). Thus, rBCG1 and rBCG2 could provide more effective and longer protection against H37Ra compared to parental BCG.
We showed that macrophages, IFN-γ+ cells and IL-17+ cells increase soon after vaccination, particularly following rBCG1 and rBCG2 vaccination. Detection of high-level RNA expression of TNF-α, IFN-γ and MCP-1 in lung tissue of mice immunized with rBCG1 and rBCG2 further supports the immuno-protective results for these two candidate vaccines. MCP-1 produced in response to infection recruits monocytes, dendritic cells and T cells to the site of inflammation caused by infection. More importantly, in the present study, MCP-1 was the only cytokine detectable in mouse sera in the early stages following rBCG1 and rBCG2 vaccination. Although MCP-1 expression also increased in the PBS control group at 8 weeks, this finding is expected in the natural course of infection. These results also fit with our finding of increased macrophages in lung tissue following rBCG1 and rBCG2 vaccination. Thus, macrophages are vital to the early host immune response following rBCG1 and rBCG2 vaccination, but not parental BCG vaccination. Furthermore, the bacterial loads in the vaccination groups (BCG, rBCG1, and rBCG2) depleted of alveolar macrophages rose significantly, illustrating the importance of the protective role of macrophages early in MTB infection, which has not been shown previously. Moreover, we also found more inflammatory M1 cells in the two rBCG groups compared to the PBS control and parental BCG groups. This latter finding supports our argument that macrophages play a more important role in early and adaptive immunity to H37Ra than previously believed. Functional M1 macrophage assays will be needed to further clarify their roles. Over the past decades, most efforts to develop TB vaccine candidates have focused on enhancing adaptive immunity. However, in phase 2b clinical trials, while these vaccines have induced strong immune responses, they have been unable to confer significant protection against TB infection (38, 39). Recent studies suggest that trained immunity, particularly involving macrophages, may play a more important role than T cells in vaccine-mediated immunity (40–44), creating a new prospective view for vaccine development. Notably, our results suggest that both innate and adaptive immunity play important roles against Mycobacterium infection.
This study had some limitations. First, because our ABSL3 facility was not operational during the initial study period, we first conducted a vaccine efficacy test against the H37Ra strain in an ABSL2 facility. The present study not only focused on pathogenesis but also on the protective effect of vaccines against H37Ra, which is often used in vaccine or drug efficacy evaluation (45–47). To evaluate the efficacy of the new recombinant BCG, both in vivo (H37Ra infection, this study) and ex vivo models [H37Rv infection (34)] have been performed in our laboratory. Our results suggest that rBCG vaccines can provide good, safe and long-term protection against H37Ra infection. Second, functional M1 macrophage assays will be needed to further clarify the roles of macrophages. Our results showed that rBCG vaccination stimulated the greatest increase in the number of M1 macrophages, presumably to clear bacteria in the lungs, which may be involved in long-term protection.
In summary, this study showed that our rBCG vaccines are safe in SCID mice, and that they induced a significant increase in the number of M1 macrophages. Moreover, to further verify vaccine efficacy, an animal model incorporating a protective assay and challenged with virulent MTB strains should be investigated forthwith. Our results suggest that rBCG1 and rBCG2 may be good candidates as a novel TB vaccine.
Data Availability Statement
All datasets generated for this study are included in the article/supplementary material.
Ethics Statement
The animal study was reviewed and approved by National Health Research Institutes Institutional Animal Care and Use Committee (NHRI-IACUC).
Author Contributions
S-JY designed, performed the experiments, analyzed the data and wrote the manuscript. C-HH, Y-YC, C-WH, C-YC, and J-RC performed and assisted in the experiments. H-YD supervised this work and interpreted the data. All authors contributed to the article and approved the submitted version.
Funding
This project was supported by grants from Intramural grants provided by the National Health Research Institutes, Taiwan (IV-107PP-11 and IV-107-SP-09) and extramural grants supported by National Health Research Institute, and Central Government S & T grant, Taiwan (107-0324-01-19-13).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank the Core Instrument Center of Pathology, Flow Cytometry, and the Laboratory Animal Center of the National Health Research Institutes for technical assistance and consultation.
References
1. WHO. Tuberculosis. (2015). Available online at: http://www.whoint/mediacentre/factsheets/fs104/en/ (accessed March 24, 2016).
2. WHO. Tuberculosis. (2019). Available online at: https://www.whoint/tb/en/ (accessed March 24, 2020).
3. Calmette A. Preventive vaccination against tuberculosis with BCG. Proc R Soc Med. (1931) 24:1481–90. doi: 10.1177/003591573102401109
4. Sterne JA, Rodrigues LC, Guedes IN. Does the efficacy of BCG decline with time since vaccination? Int J Tuberc Lung Dis. (1998) 2:200–7.
5. Colditz GA, Brewer TF, Berkey CS, Wilson ME, Burdick E, Fineberg HV, et al. Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. JAMA. (1994) 271:698–702. doi: 10.1001/jama.271.9.698
6. Fine PEM. Variation in protection by BCG: implications of and for heterologous immunity. Lancet. (1995) 346:1339–45. doi: 10.1016/S0140-6736(95)92348-9
7. Brewer TF. Preventing tuberculosis with Bacillus Calmette-Guérin vaccine: a meta-analysis of the Literature. Clin Infect Dis. (2000) 31:S64–7. doi: 10.1086/314072
8. Kremer K, van-der-Werf MJ, Au BK, Anh DD, Kam KM, van-Doorn HR, et al. Vaccine-induced immunity circumvented by typical Mycobacterium tuberculosis Beijing strains. Emerg Infect Dis. (2009) 15:335–9. doi: 10.3201/eid1502.080795
9. Lopez B, Aguilar D, Orozco H, Burger M, Espitia C, Ritacco V, et al. A marked difference in pathogenesis and immune response induced by different Mycobacterium tuberculosis genotypes. Clin Exp Immunol. (2003) 133:30–7. doi: 10.1046/j.1365-2249.2003.02171.x
10. Tsenova L, Harbacheuski R, Sung N, Ellison E, Fallows D, Kaplan G. BCG vaccination confers poor protection against M. tuberculosis HN878-induced central nervous system disease. Vaccine. (2007) 25:5126–32. doi: 10.1016/j.vaccine.2006.11.024
11. Belisle JT, Vissa VD, Sievert T, Takayama K, Brennan PJ, Besra GS. Role of the major antigen of Mycobacterium tuberculosis in cell wall biogenesis. Science. (1997) 276:1420–2. doi: 10.1126/science.276.5317.1420
12. Roche PW, Triccas JA, Avery DT, Fifis T, Billman-Jacobe H, Britton WJ. Differential T cell responses to mycobacteria-secreted proteins distinguish vaccination with bacille Calmette-Guerin from infection with Mycobacterium tuberculosis. J Infect Dis. (1994) 170:1326–30. doi: 10.1093/infdis/170.5.1326
13. Mustafa AS, Amoudy HA, Wiker HG, Abal AT, Ravn P, Oftung F, et al. Comparison of antigen-specific T-cell responses of tuberculosis patients using complex or single antigens of Mycobacterium tuberculosis. Scand J Immunol. (1998) 48:535–43. doi: 10.1046/j.1365-3083.1998.00419.x
14. Aung H, Toossi Z, Wisnieski JJ, Wallis RS, Culp LA, Phillips NB, et al. Induction of monocyte expression of tumor necrosis factor alpha by the 30-kD alpha antigen of Mycobacterium tuberculosis and synergism with fibronectin. J Clin Invest. (1996) 98:1261–8. doi: 10.1172/JCI118910
15. Horwitz MA, Harth G, Dillon BJ, Masleša-Galić S. Recombinant bacillus Calmette–Guérin (BCG) vaccines expressing the Mycobacterium tuberculosis 30-kDa major secretory protein induce greater protective immunity against tuberculosis than conventional BCG vaccines in a highly susceptible animal model. Proc Natl Acad Sci USA. (2000) 97:13853–8. doi: 10.1073/pnas.250480397
16. Horwitz MA, Harth G. A new vaccine against tuberculosis affords greater survival after challenge than the current vaccine in the Guinea pig model of pulmonary tuberculosis. Infect Immun. (2003) 71:1672–9. doi: 10.1128/IAI.71.4.1672-1679.2003
17. Prendergast KA, Counoupas C, Leotta L, Eto C, Bitter W, Winter N, et al. The Ag85B protein of the BCG vaccine facilitates macrophage uptake but is dispensable for protection against aerosol Mycobacterium tuberculosis infection. Vaccine. (2016) 34:2608–15. doi: 10.1016/j.vaccine.2016.03.089
18. Berthet FX, Rasmussen PB, Rosenkrands I, Andersen P, Gicquel B. A Mycobacterium tuberculosis operon encoding ESAT-6 and a novel low-molecular-mass culture filtrate protein (CFP-10). Microbiology. (1998) 144 (Pt 11):3195–203. doi: 10.1099/00221287-144-11-3195
19. Pym AS, Brodin P, Brosch R, Huerre M, Cole ST. Loss of RD1 contributed to the attenuation of the live tuberculosis vaccines Mycobacterium bovis BCG and Mycobacterium microti. Mol Microbiol. (2002) 46:709–17. doi: 10.1046/j.1365-2958.2002.03237.x
20. Lewis KN, Liao R, Guinn KM, Hickey MJ, Smith S, Behr MA, et al. Deletion of RD1 from mycobacterium tuberculosis mimics bacille calmette-guérin attenuation. J Infect Dis. (2003) 187:117–23. doi: 10.1086/345862
21. Dietrich J, Aagaard C, Leah R, Olsen AW, Stryhn A, Doherty TM, et al. Exchanging ESAT6 with TB10.4 in an Ag85B fusion molecule-based tuberculosis subunit vaccine: efficient protection and ESAT6-based sensitive monitoring of vaccine efficacy. J Immunol. (2005) 174:6332–9. doi: 10.4049/jimmunol.174.10.6332
22. Larrouy-Maumus G, Layre E, Clark S, Prandi J, Rayner E, Lepore M, et al. Protective efficacy of a lipid antigen vaccine in a guinea pig model of tuberculosis. Vaccine. (2017) 35:1395–402. doi: 10.1016/j.vaccine.2017.01.079
23. Wiker HG, Harboe M, Bennedsen J, Closs O. The antigens of Mycobacterium tuberculosis, H37Rv, studied by crossed immunoelectrophoresis. Comparison with a reference system for Mycobacterium bovis, BCG. Scand J Immunol. (1988) 27:223–39. doi: 10.1111/j.1365-3083.1988.tb02342.x
24. Closs O, Harboe M, Axelsen NH, Bunch-Christensen K, Magnusson M. The antigens of Mycobacterium bovis, strain BCG, studied by crossed immunoelectrophoresis: a reference system. Scand J Immunol. (1980) 12:249–63. doi: 10.1111/j.1365-3083.1980.tb00065.x
25. O'Donnell MA, Aldovini A, Duda RB, Yang H, Szilvasi A, Young RA, et al. Recombinant Mycobacterium bovis BCG secreting functional interleukin-2 enhances gamma interferon production by splenocytes. Infect Immun. (1994) 62:2508–14. doi: 10.1128/IAI.62.6.2508-2514.1994
26. Murray PJ, Aldovini A, Young RA. Manipulation and potentiation of antimycobacterial immunity using recombinant bacille Calmette-Guérin strains that secrete cytokines. Proceedings of the National Academy of Sciences. (1996) 93:934. doi: 10.1073/pnas.93.2.934
27. Lin CW, Su IJ, Chang JR, Chen YY, Lu JJ, Dou HY. Recombinant BCG coexpressing Ag85B, CFP10, and interleukin-12 induces multifunctional Th1 and memory T cells in mice. Apmis. (2012) 120:72–82. doi: 10.1111/j.1600-0463.2011.02815.x
28. Dormans J, Burger M, Aguilar D, Hernandez-Pando R, Kremer K, Roholl P, et al. Correlation of virulence, lung pathology, bacterial load and delayed type hypersensitivity responses after infection with different Mycobacterium tuberculosis genotypes in a BALB/c mouse model. Clin Exp Immunol. (2004) 137:460–8. doi: 10.1111/j.1365-2249.2004.02551.x
29. Fett C, Zhao J, Perlman S. Measurement of CD8 and CD4 T cell responses in mouse lungs. Bio-protocol. (2014) 4:e1083. doi: 10.21769/BioProtoc.1083
30. Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Reports. (2014) 6:13. doi: 10.12703/P6-13
31. Arend SM, Andersen P, van Meijgaarden KE, Skjøt RLV, Subronto YW, van Dissel JT, et al. Detection of active tuberculosis infection by T cell responses to early-secreted antigenic target 6-kDa protein and culture filtrate protein 10. J Infect Dis. (2000) 181:1850–4. doi: 10.1086/315448
32. Arend SM, Geluk A, van Meijgaarden KE, van Dissel JT, Theisen M, Andersen P, et al. Antigenic equivalence of human T-Cell responses to Mycobacterium tuberculosis-specific RD1-encoded protein antigens ESAT-6 and culture filtrate protein 10 and to mixtures of synthetic peptides. Infect Immun. (2000) 68:3314–21. doi: 10.1128/IAI.68.6.3314-3321.2000
33. Xing Z, Zganiacz A, Santosuosso M. Role of IL-12 in macrophage activation during intracellular infection: IL-12 and mycobacteria synergistically release TNF-α and nitric oxide from macrophages via IFN-γ induction. J Leukoc Biol. (2000) 68:897–902. doi: 10.1189/jlb.68.6.897
34. Chen YY, Lin CW, Huang WF, Chang JR, Su IJ, Hsu CH, et al. Recombinant bacille Calmette–Guerin coexpressing Ag85b, CFP10, and interleukin-12 elicits effective protection against Mycobacterium tuberculosis. J Microbiol Immunol Infect. (2017) 50:90–6. doi: 10.1016/j.jmii.2014.11.019
35. Hu Z, Wong KW, Zhao HM, Wen HL, Ji P, Ma H, et al. Sendai virus mucosal vaccination establishes lung-resident memory CD8 T cell immunity and boosts BCG-primed protection against TB in mice. Mol Ther. (2017) 25:1222–33. doi: 10.1016/j.ymthe.2017.02.018
36. Moguche AO, Musvosvi M, Penn-Nicholson A, Plumlee CR, Mearns H, Geldenhuys H, et al. Antigen availability shapes T cell differentiation and function during tuberculosis. Cell Host Microbe. (2017) 21:695–706.e5. doi: 10.1016/j.chom.2017.05.012
37. Colditz GA, Brewer TF, Berkey CS, et al. Efficacy of bcg vaccine in the prevention of tuberculosis: Meta-analysis of the published literature. JAMA. (1994) 271:698–702. doi: 10.1001/jama.1994.03510330076038
38. Nemes E, Geldenhuys H, Rozot V, Rutkowski KT, Ratangee F, Bilek N, et al. Prevention of M. tuberculosis Infection with H4:IC31 Vaccine or BCG Revaccination. N Engl J Med. (2018) 379:138–49. doi: 10.1056/NEJMoa1714021
39. Tameris MD, Hatherill M, Landry BS, Scriba TJ, Snowden MA, Lockhart S, et al. Safety and efficacy of MVA85A, a new tuberculosis vaccine, in infants previously vaccinated with BCG: a randomised, placebo-controlled phase 2b trial. Lancet. (2013) 381:1021–8. doi: 10.1016/S0140-6736(13)60177-4
40. Kaufmann E, Sanz J, Dunn JL, Khan N, Mendonça LE, Pacis A, et al. BCG educates hematopoietic stem cells to generate protective innate immunity against tuberculosis. Cell. (2018) 172:176–90.e19. doi: 10.1016/j.cell.2017.12.031
41. Cheng SC, Quintin J, Cramer RA, Shepardson KM, Saeed S, Kumar V, et al. mTOR- and HIF-1α-mediated aerobic glycolysis as metabolic basis for trained immunity. Science. (2014) 345:1250684. doi: 10.1126/science.1250684
42. Yoshida K, Maekawa T, Zhu Y, Renard-Guillet C, Chatton B, Inoue K, et al. The transcription factor ATF7 mediates lipopolysaccharide-induced epigenetic changes in macrophages involved in innate immunological memory. Nat Immunol. (2015) 16:1034–43. doi: 10.1038/ni.3257
43. Arts RJW, Carvalho A, La Rocca C, Palma C, Rodrigues F, Silvestre R, et al. Immunometabolic pathways in BCG-induced trained immunity. Cell Rep. (2016) 17:2562–71. doi: 10.1016/j.celrep.2016.11.011
44. Kleinnijenhuis J, Quintin J, Preijers F, Benn CS, Joosten LAB, Jacobs C, et al. Long-lasting effects of BCG vaccination on both heterologous Th1/Th17 responses and innate trained immunity. J Innate Immun. (2014) 6:152–8. doi: 10.1159/000355628
45. Gupta N, Garg S, Vedi S, Kunimoto DY, Kumar R, Agrawal B. Future path toward TB vaccine development: boosting BCG or re-educating by a new subunit vaccine. Front Immunol. (2018) 9:2371. doi: 10.3389/fimmu.2018.02371
46. Kim BJ, Kim BR, Kook YH, Kim BJ. A temperature sensitive Mycobacterium paragordonae induces enhanced protective immune responses against mycobacterial infections in the mouse model. Sci Rep. (2017) 7:15230. doi: 10.1038/s41598-017-15458-7
47. Khalil ZG, Hill TA, De Leon Rodriguez LM, Lohman RJ, Hoang HN, Reiling N, et al. Structure-activity relationships of wollamide cyclic hexapeptides with activity against drug-resistant and intracellular Mycobacterium tuberculosis. Antimicrob Agents Chemother. (2019) 3:e01773. doi: 10.1128/AAC.01773-18
Keywords: recombinant Bacille Calmette–Guérin, Mycobacterium tuberculosis, innate immunity, macrophage, vaccine
Citation: Yang S-J, Chen Y-Y, Hsu C-H, Hsu C-W, Chang C-Y, Chang J-R and Dou H-Y (2020) Activation of M1 Macrophages in Response to Recombinant TB Vaccines With Enhanced Antimycobacterial Activity. Front. Immunol. 11:1298. doi: 10.3389/fimmu.2020.01298
Received: 06 March 2020; Accepted: 22 May 2020;
Published: 23 June 2020.
Edited by:
Juraj Ivanyi, King's College London, United KingdomReviewed by:
Hazel Marguerite Dockrell, University of London, United KingdomMarc Jacobsen, Heinrich Heine University of Düsseldorf, Germany
Copyright © 2020 Yang, Chen, Hsu, Hsu, Chang, Chang and Dou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Horng-Yunn Dou, aHlkb3VAbmhyaS5lZHUudHc=
 Shiu-Ju Yang
Shiu-Ju Yang Yih-Yuan Chen
Yih-Yuan Chen Chih-Hao Hsu1
Chih-Hao Hsu1 Horng-Yunn Dou
Horng-Yunn Dou