- 1Department of Abdominal Oncology, State Key Laboratory of Biotherapy, Cancer Center, West China Hospital, Sichuan University, Chengdu, China
- 2School of Pharmacy, Southwest Medical University, Luzhou, China
- 3Department of Breast Surgery, The First Affiliated Hospital of Chongqing Medical University, Chongqing, China
- 4Department of Pharmacy, The Affiliated Traditional Chinese Medicine Hospital of Southwest Medical University, Luzhou, China
- 5State Key Laboratory of Biotherapy, Lung Cancer Center, Cancer Center, West China Hospital of Sichuan University, Chengdu, China
Background: The performance of immune checkpoint inhibitor (ICI) monotherapy was proved to be disappointing in pancreatic ductal adenocarcinoma (PDAC). Increasing evidence has shown the promising efficacy of ICIs combined with systemic therapy in the first-line treatment in solid tumors.
Case presentation: We reported a case of a metastatic PDAC patient who had a long-term partial response and good tolerance to the combined approach of toripalimab (a novel PD-1 inhibitor) and gemcitabine plus nab-paclitaxel (GA). PD-L1 positive expression was detected in his liver metastases. Besides, we described a phenomenon of pseudo-progression of this patient during the course of therapy.
Conclusion: As the first-line treatment of metastatic PDAC patients, GA plus toripalimab may provide a novel combined approach with favorable response and manageable toxicity. Further clinical trials are needed to confirm the results. Pseudo-progression requires special attention and to be differentiated with true progression in patients undergoing immunotherapy.
Background
Metastatic pancreatic ductal adenocarcinoma (PDAC) is one of the most fatal diseases with increasing incidence and mortality. Between 2009 and 2016, the 5-year survival rate for PDAC fluctuated <9% (1). Insufficient selections are efficacious in this refractory disease due to its poor response. Since the MPACT trial indicated prolonged overall survival in first-line treatment of gemcitabine plus nab-paclitaxel (GA) compared to gemcitabine alone (2, 3), GA has substituted gemcitabine as the standard of care at the expense of the high possibility of side effects (4). Therefore, GA was recommended as the first choice to metastatic PDAC patients with Eastern Cooperative Oncology Group performance status (ECOG PS) 0 to 1, as well as on the condition of patients' preference and available support system (5). Despite some attempts of novel regimen, significant improvement in clinical outcomes of PDAC patients has remained absent.
Recently, immune checkpoint inhibitors (ICIs) have been approved in patients with mismatch repair-deficient (dMMR) (6) or microsatellite instability-high (MSI-H), irrespective of which types of tumor (7). Unfortunately, the success of ICIs has not been replicated in PDAC: no objective response was observed in either anti-PD-1/PD-L1 antibody or anti-CTLA-4 (cytotoxic T lymphocyte antigen-4) monotherapy in any research (8, 9). Plausible explanations contributing to poor efficacy of ICIs in PDAC mainly involve the tumor cell-intrinsic characteristics, including the low immunogenicity, such as low mutational burden and fewer neoantigens, as well as the prominent desmoplastic stroma surrounding PDAC tumors, which may impede the ability of CD8+ T effector cells to infiltrate into the tumor to exert their killing effect.
Herein, we report a case of a metastatic PDAC patient with high PD-L1 expression who had a partial response and good tolerance to combination of toripalimab, a novel PD-1 blockade, and GA chemotherapy. We also review relevant literature about combination therapy of ICIs and chemotherapy in PDAC.
Case Presentation
A 58-year-old man was found with some liver lumps by abdominal ultrasonography in his regular physical check-up in May 2019. Without any symptoms before, he went to the hospital for further examination. A test of tumor markers showed that serum CA125 was 1,898 U/ml and the CA199 level was out of the upper limit of detection (>1,000 U/ml). A computed tomography (CT) scan and magnetic resonance imaging (MRI) of the abdomen both indicated multiple liver lesions and a pancreatic tail mass at a size of 3.9 × 2.6 cm. He was referred to the Department of General Surgery and underwent a laparoscopic liver biopsy. Intraoperative findings showed multiple scattered nodules on the surface of the liver, whose diameters were <2 cm. Pathology showed metastatic ductal adenocarcinoma. Given these findings, his final diagnosis was pancreatic adenocarcinoma with multiple liver metastases (cT2N+M1, stage IV). The next-generation sequencing of his tumor showed an intermediate tumor mutation burden with 5.65 mutations/megabase and microsatellite stable (MSS) status. The immunohistochemistry (IHC) data of the tumor tissue of this patient indicated the positive expression of PD-L1 protein (30%), and the tumor proportion score (TPS) was 20% and combined positive score (CPS) was 30 (Figure 1). Additionally, deleterious alterations occurred in CDKN2A, KRAS, TP53, and VEGFA genes. There were not any applicable targeted drugs for these gene mutations.
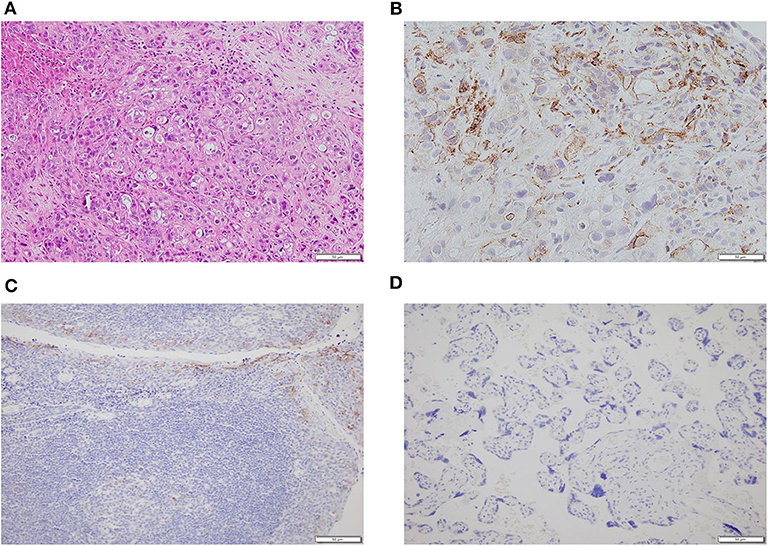
Figure 1. The histopathology and immunohistochemistry (IHC) of metastatic tumor tissues of this patient. (A) The H&E staining in the microscopic observation (100×). (B) Immunohistochemical staining for PD-L1 expression (400×) showed that the tumor cells were positive for PD-L1. (C) The positive control of the IHC of PD-L1 expression (200×). (D) The negative control of the IHC of PD-L1 expression (200×).
With his content, he was eligible for a clinical trial about the combination of doublet chemotherapy (gemcitabine 1,000 mg/m2 and nab-paclitaxel 125 mg/m2) and toripalimab (a novel PD-1 inhibitor, 240 mg) for the first-line treatment of metastatic PDAC conducted by our department. Therefore, he received gemcitabine 1,700 mg and nab-paclitaxel (Abraxane) 200 mg at day 1 and day 8, along with toripalimab 240 mg at day 1 every 3 weeks. After 2 cycles of the combination therapy, his metastatic liver lesions almost disappeared with an evaluation of partial response (PR) by Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 (Figure 2). Surprisingly, he did not suffer any serious side effects except mild nausea and loss of appetite (grade 1, CTCAE 3.0), which was self-cured at the interstitial period of therapy. The treatment continued and repeated CT scans after four cycles showed shrinkage of the primary lesion but an increase in the number of the liver metastases. However, the CA199 level plummeted from 8,015 to 553.7 U/ml after the first four cycles of treatment (Figure 2). He was still asymptomatic but had grade 1 myelosuppression, which was successfully treated with a recombinant human interleukin-11. Through multidisciplinary therapy (MDT) and communication with the patient, we thought that it was highly possible for him to have radiological pseudo-progression and suggested he continue the therapy regimen. As we expected, the subsequent two-cycle treatment brought new clinical benefits to this patient, which in turn confirmed the previous diagnosis of pseudo-progression. The patient's continuous PR is still ongoing at the time of this report (eight cycles after the initial of the combination therapy). Primary and metastatic lesions were significantly decreased or shrank to nearly invisible status as the last evaluation showed, and the level of CA 199 has maintained within the normal for a long period but a little increase at the last test (Figure 2). All treatment-related adverse events (TRAEs) of this patient throughout the clinical course were listed in Table 1. The most serious TRAEs he had was grade 2 leukocytopenia, which was recovered under drug intervention before the next cycle treatment. Overall, he did not suffer any grade 3 or higher toxicities and maintained good tolerance. With a history of hypertension and type II diabetes, the patient also kept his blood pressure and blood glucose under good control.
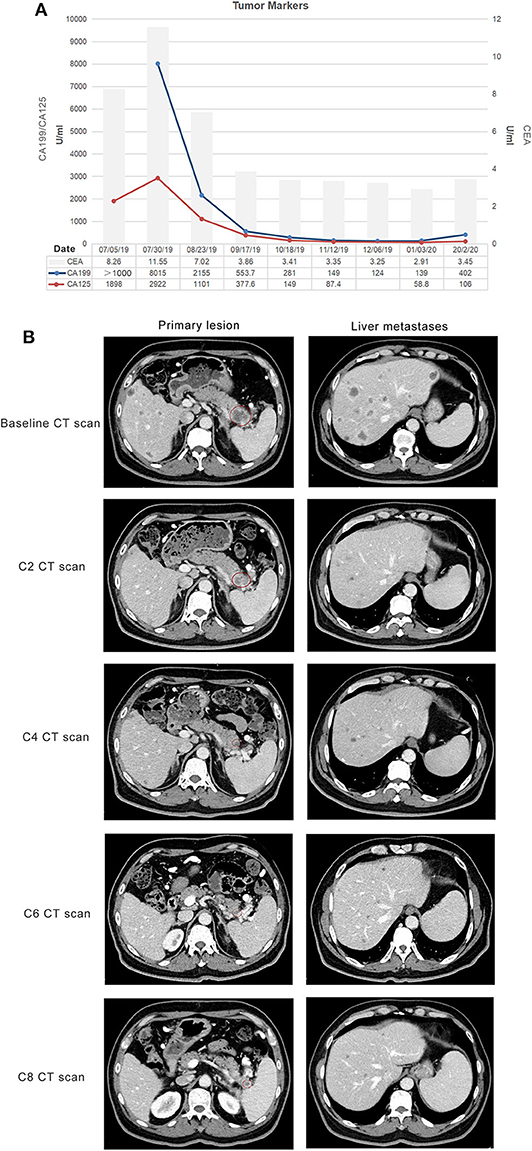
Figure 2. Response evaluation during the clinical course. (A) Trends in the level of tumor markers, including CA 199, CA 125 (left Y-axis), and CEA (right Y-axis) corresponding to the treatment timeline. X-axis showing the date of the disease course. The loss of the first value of CA 199 was due to the out of range of detection. (B) Representative images of the CT scan showed that both primary and metastatic lesions were shrunk and decreased after two cycles of gemcitabine plus nab-paclitaxel combined with a PD-1 antibody (toripalimab). Red circles indicate the primary pancreatic lesions.
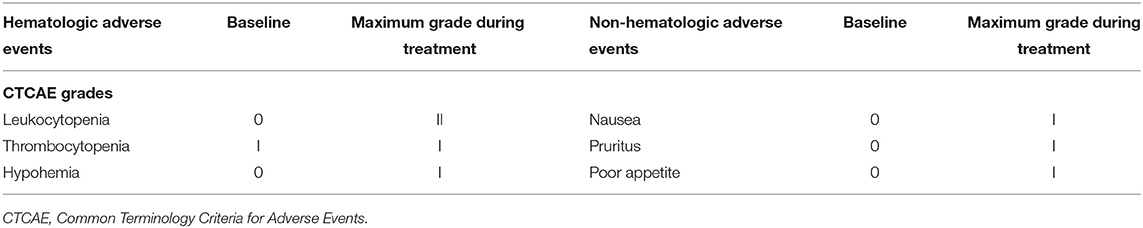
Table 1. Hematologic and non-hematologic adverse events in the therapeutic course presented in this patient, which were graded using CTCAE 3.0.
Discussion
Many cases of exceptional or durable responses to ICIs have been reported. To our knowledge, however, this is the first report showing the striking long-term response and safety of doublet chemotherapy combined with toripalimab in the first-line treatment of PDAC.
Toripalimab is the first recombinant humanized anti-PD-1 monoclonal antibody which was independently developed by Chinese companies. It was approved by the National Medical Products Administration (NMPA) of China in December 2018 for locally advanced or metastatic melanoma after systemic treatment failure. It has a high binding affinity, which enables it to bind its specific antigen PD-1 receptor more firmly and compete better with PD-L1 and PD-L2 binding on tumor cells. After binding, it can induce strong endocytosis of PD-1 receptor, thus reducing the expression of PD-1 on the cell membrane surface. A study revealed the different binding orientation of toripalimab compared to other PD-1 blockade, which binds PD-1 mainly on a loop that contributes multiple interactions with PD-L1 (10). The distinct biomolecular characteristics of toripalimab might result in different properties. Recently, increasing studies about various malignancies has proven the potential superiority of toripalimab, especially good tolerability, which may provide an opportunity to use concurrently with other anti-tumor drugs (11). In a phase II study, toripalimab combined with capecitabine and oxaliplatin (CapeOX) as the first-line treatment to treat patients with advanced gastric cancer, and the overall response rate (CR and PR) was 66.7% and the disease control rate (CR, PR, and SD) was 88.9%. Besides, nearly 38.9% of patients experienced grade 3 or 4 TRAEs (12). Compared to the ATTRACTION-4 trial, the encouraging efficacy of toripalimab was not inferior to Nivolumab with an ORR of 76.5%, but much more grade 3 or greater TRAEs occurred in 66.7% of patients with Nivolumab plus CapeOX (13).
This patient may not be sensitive to PD-1 blockade according to ASCO clinical practice guideline, which approved PD-1 blockade for patients with dMMR (6) or MSI-H (14). Given the predictive role of PD-L1 overexpression in PDAC was still controversial, our case suggested that PD-L1 overexpression may have the potential to select population. Moreover, emerging evidence supported combining systemic therapy on an ICIs backbone to overcome resistance due to the superior safety of ICIs (15). Theoretically, systemic chemotherapy was regarded as an immunogenic approach by stimulating anti-cancer immune effectors or inhibiting immunosuppressive factors (16). It may increase the expression or presentation of tumor-associated antigens on the surface of cancer cells, inducing signal emission to trigger immune response. As a method for priming the quiescent tumor microenvironment, chemotherapy has the potential to potentiate immunogenicity and antigenicity of tumors, thus enhancing the likelihood of recognition and killing of tumor cells by immune effector (17). For example, gemcitabine may upregulate the expression of class I human leukocyte antigen and promote the cross-presentation of tumor antigen, therefore selectively eliminating myeloid-derived suppressor cells (MDSCs) to overcome the immunosuppression. Paclitaxel was proved to stimulate antigen-presenting cells and improve the release of granzyme B by effector cells (18). Some phase I/II studies have confirmed the synergetic effects of cytotoxic chemotherapy with ICIs in other types of cancer (18–20). There are limited data about the safety and efficacy of combination of ICIs and chemotherapy in metastatic PDAC (Table 2). Results from a phase Ib/II study conducted in metastatic PDAC suggested that the efficacy of combined chemo-immunotherapy appears to be slightly improved over conventional standard chemotherapy (27). Others have highlighted the importance of combination therapy in the first-line treatment to obtain initial remission. The impressive results of this case need to be further confirmed by a large-scale randomized controlled study.
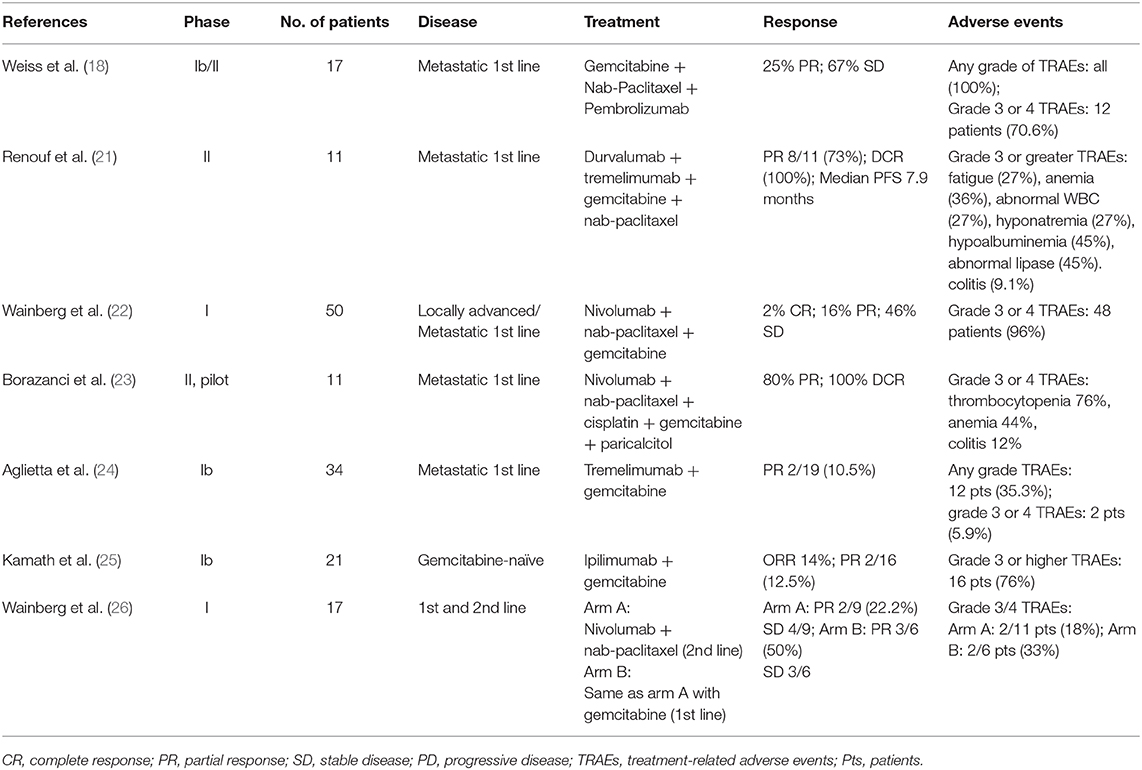
Table 2. Efficacy and safety of combined therapeutic approaches of immune checkpoint inhibitors and chemotherapy in pancreatic cancer.
Interestingly, the patient presented rare pseudo-progression on the CT scan in the treatment course. Pseudo-progression is defined as temporarily enlarging lesions or the appearance of new lesions detected by imaging tests undergoing cancer immunotherapy (28). As the term suggests, pseudo-progression is not a real progression of the disease, whereas it may be linked with a durable response to immunotherapy (29). Presentation of pseudo-progression may be explained as edema and necrosis of tumor tissues caused by the infiltration of immune cells (30), resulting in morphologically similar mass around the original lesions in the imaging. Besides, with the characteristics of a late response, immunotherapy may not induce tumor regression until CT evaluation after the next few cycles of treatment. Instead of treatment failure, this kind of transient tumor growth before the onset of immune response needs to be distinguished with the real progression. Exaggerating the occurrence of pseudo-progression is not advisable because over-treatment may damage life quality, especially for metastatic cancer patients whose main purpose is to alleviate their symptoms. In this case, the patient was found to have new liver lesions after four cycles of treatment (Figure 3). Given he was not accompanied by clinical deterioration and the continuously falling CA-199 level, we inferred that the emergence of new lesions may result from the pseudo enlargement of small lesions that were invisible on the baseline CT scan. As we expected, the newly presented lesions disappeared and original lesions shrank after the continuation of this therapy, which verified our diagnosis of pseudo-progression.
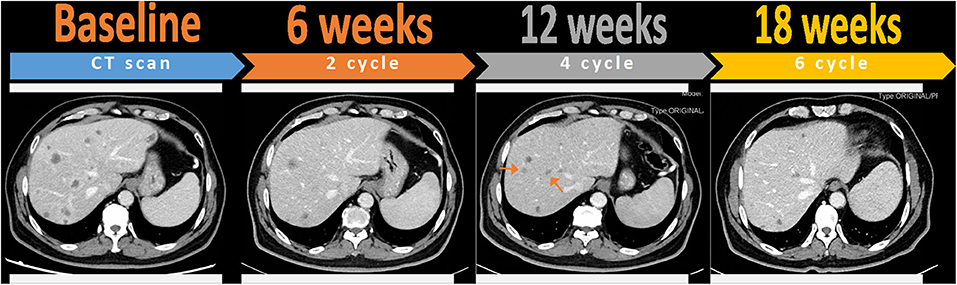
Figure 3. Pseudo-progression of liver metastases after four cycles of toripalimab combined with gemcitabine and nab-paclitaxel, which was confirmed by favorable outcomes at the next assessment of six cycles. Red arrows indicate the appearance of new lesions that were invisible at previous CT evaluation and then disappeared after the continuation of therapy.
Besides impressive efficacy, he also had a good tolerance to these triple anti-tumor drugs, especially PD-1 inhibitors whose immune-related adverse events need special attention. After the first 2 cycles of therapy, he encountered grade 1 myelosuppression, which was successfully treated with a recombinant human interleukin-11, along with a self-cured gastrointestinal tract reaction. Subsequently, he experienced no more overt toxicities and was well-tolerated to a total of 8 cycles of combination therapy. Taking concurrent anti-hypertensive drugs and metformin with this highly intensive anti-tumor regimen, his liver and renal function were still within the normal range.
In summary, combined therapy of toripalimab and standard chemotherapy is potentially effective and well-tolerated as the first-line treatment in metastatic PDAC. Although the data are limited to conclude, we presented a patient who had a striking response to this combination as well as a manageable safety profile. The favorable clinical outcome may be attributed to safer toripalimab or the synergistic function of chemotherapy and PD-1 blockade. Furthermore, this case also displayed the possibility of the phenomenon of pseudo-progression under this regimen, which needed to be taken into consideration in the design and process of clinical trials.
Ethics Statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Funding
This work was funded by the National Natural Science Foundation of China (No. 81773097).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
2. Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. New Engl J Med. (2013) 369:1691–703. doi: 10.1056/NEJMoa1304369
3. Goldstein D, El-Maraghi RH, Hammel P, Heinemann V, Kunzmann V, Sastre J, et al. nab-Paclitaxel plus gemcitabine for metastatic pancreatic cancer: long-term survival from a phase III trial. J Natl Cancer Inst. (2015) 107:dju413. doi: 10.1093/jnci/dju413
4. Chin V, Nagrial A, Sjoquist K, O'Connor CA, Chantrill L, Biankin AV, et al. Chemotherapy and radiotherapy for advanced pancreatic cancer. Cochrane Database Syst Rev. (2018) 3:Cd011044. doi: 10.1002/14651858.CD011044.pub2
5. Sohal DP, Mangu PB, Khorana AA, Shah MA, Philip PA, O'Reilly EM, et al. Metastatic pancreatic cancer: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. (2016) 34:2784–96. doi: 10.1200/JCO.2016.67.1412
6. Le DT, Durham JN. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. (2017) 357:409–13. doi: 10.1126/science.aan6733
7. Lemery S, Keegan P, Pazdur R. First FDA approval agnostic of cancer site - when a biomarker defines the indication. N Engl J Med. (2017) 377:1409–12. doi: 10.1056/NEJMp1709968
8. Patnaik A, Kang SP, Rasco D, Papadopoulos KP, Elassaiss-Schaap J, Beeram M, et al. Phase I study of pembrolizumab (MK-3475; Anti-PD-1 monoclonal antibody) in patients with advanced solid tumors. Clin Cancer Res. (2015) 21:4286–93. doi: 10.1158/1078-0432.CCR-14-2607
9. Royal RE, Levy C, Turner K, Mathur A, Hughes M, Kammula US, et al. Phase 2 trial of single agent Ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma. J Immunother. (2010) 33:828–33. doi: 10.1097/CJI.0b013e3181eec14c
10. Liu H, Guo L, Zhang J, Zhou Y, Zhou J, Yao J. Glycosylation-independent binding of monoclonal antibody toripalimab to FG loop of PD-1 for tumor immune checkpoint therapy. MAbs. (2019) 11:681–90. doi: 10.1080/19420862.2019.1596513
11. Sheng X, Yan X, Chi Z, Si L, Cui C, Tang B, et al. Axitinib in combination with toripalimab, a humanized immunoglobulin G4 monoclonal antibody against programmed cell death-1, in patients with metastatic mucosal melanoma: an open-label phase IB trial. J Clin Oncol. (2019) 37:2987–99. doi: 10.1200/JCO.19.00210
12. Wang F, Wei XL, Wang FH, Xu N, Shen L, Dai GH, et al. Safety, efficacy and tumor mutational burden as a biomarker of overall survival benefit in chemo-refractory gastric cancer treated with toripalimab, a PD-1 antibody in phase Ib/II clinical trial NCT02915432. Ann Oncol. (2019) 30:1479–86. doi: 10.1093/annonc/mdz197
13. Boku N, Ryu MH, Kato K, Chung HC, Minashi K, Lee KW, et al. Safety and efficacy of nivolumab in combination with S-1/capecitabine plus oxaliplatin in patients with previously untreated, unresectable, advanced, or recurrent gastric/gastroesophageal junction cancer: interim results of a randomized, phase II trial (ATTRACTION-4). Ann Oncol. (2019) 30:250–8. doi: 10.1093/annonc/mdy540
14. Sohal DPS, Kennedy EB, Khorana A, Copur MS, Crane CH, Garrido-Laguna I, et al. Metastatic pancreatic cancer: ASCO clinical practice guideline update. J Clin Oncol. (2018) 36:2545–56. doi: 10.1200/JCO.2018.78.9636
15. Gong J, Hendifar A, Tuli R, Chuang J, Cho M, Chung V, et al. Combination systemic therapies with immune checkpoint inhibitors in pancreatic cancer: overcoming resistance to single-agent checkpoint blockade. Clin Transl Med. (2018) 7:32. doi: 10.1186/s40169-018-0210-9
16. Zitvogel L, Kepp O, Kroemer G. Immune parameters affecting the efficacy of chemotherapeutic regimens. Nat Rev Clin Oncol. (2011) 8:151–60. doi: 10.1038/nrclinonc.2010.223
17. Zitvogel L, Galluzzi L, Smyth MJ, Kroemer G. Mechanism of action of conventional and targeted anticancer therapies: reinstating immunosurveillance. Immunity. (2013) 39:74–88. doi: 10.1016/j.immuni.2013.06.014
18. Weiss GJ, Waypa J, Blaydorn L, Coats J, McGahey K, Sangal A, et al. A phase Ib study of pembrolizumab plus chemotherapy in patients with advanced cancer (PembroPlus). Br J Cancer. (2017) 117:33–40. doi: 10.1038/bjc.2017.145
19. Langer CJ, Gadgeel SM, Borghaei H, Papadimitrakopoulou VA, Patnaik A, Powell SF, et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol. (2016) 17:1497–508. doi: 10.1016/S1470-2045(16)30498-3
20. Rizvi NA, Hellmann MD, Brahmer JR, Juergens RA, Borghaei H, Gettinger S, et al. Nivolumab in combination with platinum-based doublet chemotherapy for first-line treatment of advanced non-small-cell lung cancer. J Clin Oncol. (2016) 34:2969–79. doi: 10.1200/JCO.2016.66.9861
21. Renouf DJ, Dhani NC, Kavan P, Jonker DJ, Wei AC-C, Hsu T, et al. The canadian cancer trials group PA.7 trial: results from the safety run in of a randomized phase II study of gemcitabine (GEM) and nab-paclitaxel (Nab-P) versus GEM, nab-P, durvalumab (D), and tremelimumab (T) as frst-line therapy in metastatic pancreatic ductal adenocarcinoma (mPDAC). J Clin Oncol. (2018) 36:349. doi: 10.1200/JCO.2018.36.4_suppl.349
22. Wainberg ZA, Hochster HS, Kim E, George B, Kalyan A, Chiorean EG, et al. Phase I study of nivolumab (nivo) + nab-paclitaxel (nab-P) + gemcitabine (Gem) in advanced pancreatic cancer (APC). J Clin Oncol. (2019) 37(Suppl. 4):298. doi: 10.1200/JCO.2019.37.4_suppl.298
23. Borazanci EH, Jameson GS, Borad MJ, Ramanathan RK, Korn RL, Caldwell L, et al. A phase II pilot trial of nivolumab (N) + albumin bound paclitaxel (AP) + paricalcitol (P) + cisplatin (C) + gemcitabine (G) (NAPPCG) in patients with previously untreated metastatic pancreatic ductal adenocarcinoma (PDAC). J Clin Oncol. (2018) 36:358. doi: 10.1200/JCO.2018.36.4_suppl.358
24. Aglietta M, Barone C, Sawyer MB, Moore MJ, Miller WH Jr., et al. A phase I dose escalation trial of tremelimumab (CP-675,206) in combination with gemcitabine in chemotherapy-naive patients with metastatic pancreatic cancer. Ann Oncol. (2014) 25:1750–5. doi: 10.1093/annonc/mdu205
25. Kamath SD, Kalyan A, Kircher S, Nimeiri H, Fought AJ, Benson A, et al. Ipilimumab and gemcitabine for advanced pancreatic cancer: a phase Ib study. Oncologist. (2019) 25:e808–15. doi: 10.1634/theoncologist.2019-0473
26. Wainberg ZA, Hochster HS, George B, Gutierrez M, Johns ME, Chiorean EG, et al. Phase I study of nivolumab (nivo) + nab-paclitaxel (nab-P) ± gemcitabine (Gem) in solid tumors: interim results from the pancreatic cancer (PC) cohorts [abstract]. J Clin Oncol. (2017) 35:412. doi: 10.1200/JCO.2017.35.4_suppl.412
27. Weiss GJ, Blaydorn L, Beck J, Bornemann-Kolatzki K, Urnovitz H, Schutz E, et al. Phase Ib/II study of gemcitabine, nab-paclitaxel, and pembrolizumab in metastatic pancreatic adenocarcinoma. Invest New Drugs. (2018) 36:96–102. doi: 10.1007/s10637-017-0525-1
28. Wolchok JD, Hoos A, O'Day S, Weber JS, Hamid O, Lebbe C, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. (2009) 15:7412–20. doi: 10.1158/1078-0432.CCR-09-1624
29. Seymour L, Bogaerts J, Perrone A, Ford R, Schwartz LH, Mandrekar S, et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. (2017) 18:e143–52. doi: 10.1016/S1470-2045(17)30074-8
30. Kazandjian D, Keegan P, Suzman DL, Pazdur R, Blumenthal GM. Characterization of outcomes in patients with metastatic non-small cell lung cancer treated with programmed cell death protein 1 inhibitors past RECIST version 1.1-defined disease progression in clinical trials. Semin Oncol. (2017) 44:3–7. doi: 10.1053/j.seminoncol.2017.01.001
Keywords: PD-1 inhibitor, chemotherapy, combination therapy, metastatic pancreatic ductal adenocarcinoma, durable response, good tolerance, case report
Citation: Shui L, Cheng K, Li X, Shui P, Li S, Peng Y, Li J, Guo F, Yi C and Cao D (2020) Durable Response and Good Tolerance to the Triple Combination of Toripalimab, Gemcitabine, and Nab-Paclitaxel in a Patient With Metastatic Pancreatic Ductal Adenocarcinoma. Front. Immunol. 11:1127. doi: 10.3389/fimmu.2020.01127
Received: 22 February 2020; Accepted: 07 May 2020;
Published: 19 June 2020.
Edited by:
Sophie Lucas, Université Catholique de Louvain, BelgiumReviewed by:
Ying Ma, University of Texas MD Anderson Cancer Center, United StatesAlessandro Poggi, San Martino Hospital (IRCCS), Italy
Copyright © 2020 Shui, Cheng, Li, Shui, Li, Peng, Li, Guo, Yi and Cao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dan Cao, aHhjYW9kYW4yMDE5QDE2My5jb20=
†These authors have contributed equally to this work
 Lin Shui
Lin Shui Ke Cheng1†
Ke Cheng1† Jian Li
Jian Li Cheng Yi
Cheng Yi Dan Cao
Dan Cao