- 1Division of Pharmacology, Utrecht Institute for Pharmaceutical Sciences, Faculty of Science, Utrecht University, Utrecht, Netherlands
- 2Danone Nutricia Research, Utrecht, Netherlands
Breastfeeding is indicated to support neonatal immune development and to protect against neonatal infections and allergies. Human milk composition is widely studied in relation to these unique abilities, which has led to the identification of various immunomodulating components in human milk, including various bioactive proteins. In addition to proteins, human milk contains free amino acids (FAAs), which have not been well-studied. Of those, the FAAs glutamate and glutamine are by far the most abundant. Levels of these FAAs in human milk sharply increase during the first months of lactation, in contrast to most other FAAs. These unique dynamics are globally consistent, suggesting that their levels in human milk are tightly regulated throughout lactation and, consequently, that they might have specific roles in the developing neonate. Interestingly, free glutamine and glutamate are reported to exhibit immunomodulating capacities, indicating that these FAAs could contribute to neonatal immune development and to the unique protective effects of breastfeeding. This review describes the current understanding of the FAA composition in human milk. Moreover, it provides an overview of the effects of free glutamine and glutamate on immune parameters relevant for allergic sensitization and infections in early life. The data reviewed provide rationale to study the role of free glutamine and glutamate in human milk in the protection against neonatal allergies and infections.
Introduction
Human milk is widely recognized as the best source of infant nutrition. It provides the infant with a highly diverse mix of nutrients that supports optimal development. The health benefits of human milk, however, go beyond that of providing nutrients. An increasing body of evidence suggests that human milk provides the neonate with a protection against a variety of immune-related conditions. For example, it is shown consistently that infants who were exclusively breastfed were less likely to develop respiratory and gastrointestinal infections than infants who fully or partially received an infant milk formula (1–5). This protective effect of breastfeeding against infections may extend well beyond infancy and is indicated to be enhanced upon prolonged breastfeeding (6, 7). Furthermore, studies have demonstrated that exclusive breastfeeding protects against various allergic diseases, including atopic dermatitis (8, 9), asthma (9–11) and food allergy (12–15), especially if there is a family history of allergic disease (16). For cow's milk allergy, which is one of the most common food allergies in infants, the incidence rate is reported to be up to seven times lower in exclusively breastfed infants, compared to infants fully or partially fed an infant milk formula (17–19). These unique protective capacities of human milk have driven scientific research into the underlying mechanisms in the past decades (15, 20, 21).
At birth, the immune system is immature (22). Compared to adults, the neonatal immune system is characterized by diminished innate effector cell functions, suppressed T-helper 1 (TH1) immune responses and skewed T cell responses to antigens toward T-helper 2 (TH2) immunity. These characteristics correlate with an increased susceptibility to infections and allergies in the neonatal period (23, 24). This susceptibility is further enhanced by an immature intestinal barrier function and an incomplete intestinal microbial colonization at birth (23). Various factors in human milk have been identified that could support the development of these immune functions, and thus may contribute to the protection against infections and allergies. For instance, human milk contains immunoglobulin A (IgA) antibodies, which confer protection against pathogens and are reported to induce tolerance to food allergens (25, 26). Moreover, various bioactive oligosaccharides, fatty acids and proteins have been identified in human milk that are capable of modulating immune responses directly, e.g., by regulating immune responses to pathogens (27–29), and indirectly, e.g., by shaping the gut microbiome (29–32). In addition to proteins, human milk also contains protein-unbound, free amino acids (FAAs). Accumulating evidence indicates that certain FAAs are bioactive, and more specifically have immunomodulating capacities (33, 34). Hence, FAAs in human milk may play an active part in an optimal immune development of the infant. However, whereas research on physiological functions of FAAs has made significant progress in recent years, FAAs are typically overlooked in human milk research.
Of the total content of amino acids (AAs) in human milk, 5–10% is present in free form. The FAAs glutamate and glutamine are by far the most abundant, both in absolute sense and relative to their protein-bound form, together comprising almost 70% of all FAAs present in human milk (35). Their levels display unique and consistent patterns over lactation, suggesting that secretion of these FAAs in human milk is a regulated process (35, 36). Interestingly, these structurally related FAAs have been widely associated with immunomodulation, including the modulation of immune mechanisms relevant for the development of allergies and infections. This review aims to describe the current understanding of the FAA composition in human milk, and to provide an overview of the effects of the FAAs glutamine and glutamate on immune parameters relevant for allergic diseases and infections in early life. Ultimately, a better understanding of the composition of FAAs in human milk and their immunomodulating capacities may contribute to the development of new avenues in the prevention of allergies and infectious diseases in infancy.
Amino Acids in Human Milk: Protein-Bound and Free Amino Acids
It is well known that protein quality and quantity are key aspects of the nutritional value of human milk. The total amino acid (TAA) composition of human milk, including protein-bound AAs and FAAs, is used to evaluate the quantity and the quality of the milk proteins and hence is well characterized (36, 37). However, many studies only report the TAA composition and do not distinguish between protein-bound and FAAs. As a result, data on FAAs in human milk are relatively limited.
FAAs in human milk have been reported to account for ~5–10% of the TAA content (35, 36). Despite their low abundance relative to protein-bound AA levels, the relevance of FAAs in human milk should not be underestimated. Their levels are approximately 100 times higher than the 0.05% FAA pool in tissues (38) and up to 30 times higher than the FAA levels in plasma of infants (39). Moreover, FAAs in human milk contribute significantly to the initial changes in plasma levels of FAAs following a feed (40, 41) and are indicated to be more readily absorbed (42–44), appear sooner in the circulation and thus might reach peripheral organs and tissues faster than protein-derived AAs. Indeed, differences in plasma FAA levels were observed between infants receiving an infant milk formula containing FAAs and infants receiving an equivalent portion of AAs in the form of intact protein, suggesting differences in absorption kinetics between FAAs and protein-derived AAs (45–47). In contrast to their protein-bound counterpart, FAAs can interact with specific receptors present on a wide variety of cells in various parts of the body, including the intestines, where they can activate specific intracellular pathways and confer physiological effects (34, 48).
While human milk directly supplies infants with FAAs, human milk proteins could also provide the infant with FAAs via proteolysis in the neonatal gastrointestinal tract. However, the contribution of proteolysis of human milk proteins to the FAA supply of infants might be relatively low, as (complete) proteolysis of these proteins in infants is shown to occur to a minimal extent (49–52). Factors contributing to the limited proteolysis of human milk proteins are the relatively low output of pepsin and gastric enzymes observed in infants, the relatively high gastric postprandial pH which leaves proteases largely inactive, as well as the high degree of glycosylation of these proteins (50). Accordingly, it has been argued that the availability of FAAs in the upper region of the gastrointestinal tract, including the upper parts of the small intestine, is almost entirely dependent on the dietary FAA content (48).
The unique abilities of FAAs compared to protein-bound AAs and the relatively inefficient proteolytic capacity of neonates underline the importance of understanding the FAA composition in human milk, separate from the TAA composition.
The FAA Composition in Human Milk is Dynamic and Seemingly Regulated
The composition of human milk is known to be dynamic over the course of lactation. The total protein content has been consistently shown to decrease in the first 3 months of lactation (35, 36). It is argued that this decrease correlates with the infant's protein requirements for growth and that it prevents overfeeding, as milk volume intake increases during this period (53, 54). Not surprisingly, similar dynamics are found for the protein-bound AA content in human milk. For each individual AA the protein-bound form decreases to a highly similar extent during lactation, indicating that the dynamics of protein-bound AAs in human milk during lactation are not AA-specific (35). In contrast, levels of FAAs in human milk display dynamics during lactation that are highly AA-specific: whereas levels of some FAAs decrease in the first 3 months of lactation, others remain stable or sharply increase (35, 36). Remarkably, these FAA dynamics during lactation are consistent in studies across various ethnic groups and geographical locations, indicating that these dynamics are globally consistent and thus seemingly regulated (35, 36, 55, 56).
The underlying mechanisms regulating the dynamics of FAA levels in human milk are poorly understood. Cells of the mammary gland secrete proteases and anti-proteases into human milk that together regulate the cleavage of specific AAs from human milk proteins, generating FAAs and peptides (57). Thus, it can be hypothesized that temporal changes in net proteolytic activity in human milk contribute to the FAA dynamics, although this is unlikely as levels of all major human milk proteases and anti-proteases decrease during lactation, along with levels of their substrates (50, 58). Mammary gland cells can also directly secrete FAAs into human milk via AA transporters present on their cell membranes. Interestingly, animal studies have shown that the expression of certain AA transporters in the mammary gland increases with progressing lactation, whereas that of others remains unchanged (59–62). These expression dynamics throughout lactation appear to be tightly regulated by multiple intracellular signaling pathways (63). Thus, it can be speculated that the dynamic expression of AA transporters on mammary gland cells along lactation contributes to the FAA dynamics in human milk.
To better understand the mechanisms underlying the secretion of FAAs in human milk, several studies examined the influence of maternal characteristics on the FAA composition in human milk. Whereas, FAA levels seem to be independent of the mothers' age (64), maternal body-mass index is reported to slightly influence levels of several FAAs (65, 66). Mechanisms underlying this effect are not known, but may involve the hormone prolactin, as prolactin is involved in regulating FAA transport in the mammary gland and levels of prolactin associate with maternal body-mass index (67–69). Studies investigating the effect of maternal diet on the AA composition in human milk indicate that the TAA composition is largely independent of the AA composition of the diet (70, 71). For FAAs, this relation remains to be examined in humans. However, studies across different geographical locations where different diets are consumed show largely similar levels and ratios of FAAs in human milk, suggesting that maternal diet is not of major influence (35, 36, 55, 56). This is supported by the finding that oral supplementation of a single load of glutamate (6g) in healthy lactating women did not alter levels of any of the FAAs in their breastmilk (72). Moreover, several studies reported that there was no association between maternal plasma levels of FAAs and the FAA levels in human milk (73, 74). In fact, some FAAs were 1- to 15-fold higher in plasma compared to milk, whereas levels of free glutamate were 40-fold higher in milk than in plasma.
All together, these findings indicate that selective FAA transport occurs in mammary tissues during lactation and that levels of FAAs in human milk might be highly regulated throughout lactation.
Correlations of FAAs in Human Milk With Lactation Stage, Gestational Age and Infant Anthropometrics: A Special Role for Free Glutamine and Glutamate?
The FAAs glutamine, glutamate, glycine, serine and alanine in human milk have consistently been shown to increase in the first 3 months of lactation, whereas the levels of most other FAAs remain relatively stable along lactation (35, 36, 55, 56). Of these, glutamate is by far the most abundant, accounting for more than 50% of the total FAA content at any stage of lactation. In addition, glutamate shows the highest absolute increase in concentration along lactation, increasing from ~1.25 to 1.75 mM from month 1 to 6 of lactation (35). Glutamine, the second-most abundant FAA, shows the highest relative increase in concentration, increasing almost 350% from month 1 to 6 of lactation and reaching a concentration of up to 0.6 mM (35, 64, 75). In addition to the stage of lactation, the gestational age of the infant has also been reported to be a determinant of the free glutamine levels in human milk. A meta-analysis has shown that free glutamine levels in milk for preterm infants are almost three times lower than those observed in milk for term infants in the first month of lactation (36). Levels of all other FAAs were similar in preterm and term human milk samples, indicating that this difference was AA-specific.
Studies investigating associations of FAAs with infant anthropometrics are scarce but do report consistent findings. It was recently reported that free glutamate levels in human milk were significantly higher for term infants that had faster weight gain (76). Moreover, glutamine levels also tended to be higher for fast growing children. Consistent with these findings, another study reported a positive association between free glutamine levels in human milk and infant length at 4 months of age (65). These findings are in line with studies indicating that milk for boys tends to have higher levels of free glutamine and glutamate than milk for girls in the first 3 to 4 months of lactation (35, 76), as boys are known to gain more weight and length than girls in this time period (77).
The finding that levels of free glutamine and glutamate in human milk are relatively high, display unique dynamics along lactation, and are associated with infant anthropometrics urges the need to understand the functions that these FAAs could have during infant development.
The Diversity in Physiological Functions of Free Glutamine and Glutamate
In the last decade, it has been recognized that glutamine and glutamate are essential AAs at key times in life, including the neonatal period when rapid growth occurs (78, 79). Although these two FAAs are structurally related, they appear to be different in terms of absorption by the infant. Whereas dietary glutamine supplementation in infants leads to higher plasma levels of this AA (80, 81), plasma levels of glutamate are largely unaffected by dietary glutamate (82, 83). This suggests that free glutamate in human milk is almost entirely used by splanchnic tissues, limiting its availability for other tissues, whereas glutamine might also exert direct effects elsewhere in the body. Despite these differences, most of the dietary glutamine and glutamate provided to neonates is consistently shown to be used by the intestines (84, 85). The intestines do not only form a physical barrier to protect against pathogens but are also home to the largest immune organ of the body: the gut-associated lymphoid tissue (GALT). This may explain why glutamine and glutamate are associated with a wide range of physiological functions, ranging from energy provision to cells to more specific immunomodulating functions, many of which could be relevant in the context of the prevention of neonatal allergies and infections. Figure 1 provides a summary of the demonstrated effects of free glutamine and glutamate in (developing) intestinal tissues, which are described in detail below.
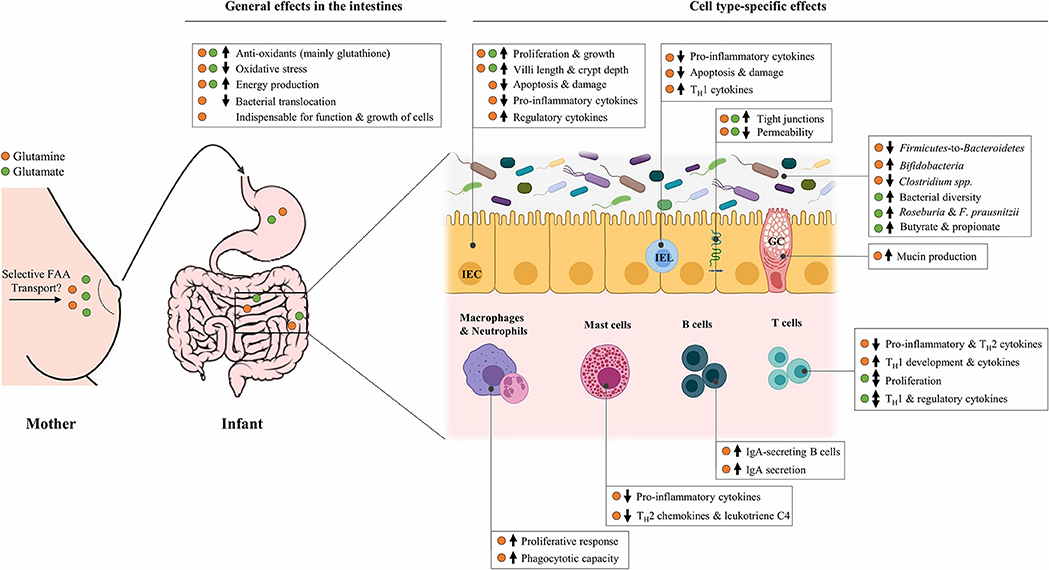
Figure 1. Overview of the potential effects of free glutamine and glutamate, selectively secreted in human milk by mammary gland cells, in the developing infant gut. The ↑ and ↓ indicate an upregulation and downregulation, respectively, of the corresponding target following in vitro and/or in vivo supplementation with glutamine (•) or glutamate (•). Effects are limited to those that are relevant in the context of allergic sensitization and infections. FAA, Free amino acid; IEC, Intestinal epithelial cell; IEL, Intraepithelial lymphocyte; GC, Goblet cell; TH1, T-helper 1 cell; TH2, T-helper 2 cell; IgA, Immunoglobulin A; F. prausnitzii, Faecalibacterium prausnitzii.
Metabolism of Glutamine and Glutamate in Intestinal Epithelial Cells and Immune Cells: Their Function as Energy Substrate and Protein Precursors
It is well-established that glutamine and glutamate are important energy substrates for intestinal epithelial cells (IECs) and immune cells, especially during periods of rapid growth (86). In fact, studies in young animals and infants have shown that approximately half of the dietary glutamate and glutamine is oxidized by intestinal and immune cells, ultimately leading to the generation of energy for the cells to adequately function and grow (87). Intestinal cells can convert glutamine into glutamate, which is crucial for the usage of glutamine for energy purposes (88). Whereas, human intestinal cells can also convert glutamate into glutamine, this process is limited due to the low glutamine synthetase activity in the small intestine (89, 90). In the neonatal period, this ability may be further limited as studies in young rats demonstrated that glutamine synthetase activity is particularly low in the pre-weaning period (91, 92). Remarkably, IECs as well as immune cells cannot function properly without the availability of exogenous glutamine (93). This, combined with their limited capacity to synthetize glutamine suggests that adequate functioning of these cells in the neonatal period might be partially dependent on dietary-derived glutamine.
Besides serving as energy substrates, free glutamine and glutamate are both specific precursors for glutathione, which is the main antioxidant in IECs and immune cells and critical for the prevention of cellular damage caused by pro-oxidants (94). An imbalance in pro- and antioxidants, known as oxidative stress, stimulates inflammatory responses that can lead to the development and maintenance of allergic disorders (95, 96). Hence, antioxidants like glutathione are considered as preventive or treatment strategy for food allergies (97). It has been reported that both dietary glutamine and dietary glutamate enhance glutathione production and, possibly as a result, reduce oxidative stress in the intestines of weaning piglets (98, 99). In addition, glutamine, but not glutamate, is an important specific precursor for the synthesis of mucins, which are critical for the defense against infections and are suggested to protect against allergic sensitization (100–103). Accordingly, oral glutamine supplementation has been shown to enhance mucin synthesis and to increase the number of mucin-secreting goblet cells in the small intestine of weaned piglets (104).
Effects of Free Glutamine and Glutamate on Intestinal Growth and Barrier Function
In the rapidly growing neonate where the intestines are not yet fully developed, it is crucial to achieve and maintain rapid growth of IECs. Moreover, it is well-established that intestinal barrier function is a crucial factor in the protection against allergies and infections, by preventing allergen and bacterial translocation from the gut lumen into the immune cell-populated lamina propria and mesenteric lymph nodes (105–107). In neonates where intestinal barrier function is immature, proper availability of nutrients that contribute to the growth of IECs and maturation of the intestinal barrier is critical to support this protective effect. Interestingly, free glutamine and glutamate have been shown consistently to influence these processes, by various mechanisms which are further explained in the following sections.
Impact of Glutamine on Intestinal Functions
Glutamine is by far the most widely examined AA in relation to growth and function of IECs. This FAA is known to stimulate IEC proliferation in a variety of ways, as demonstrated in various neonatal IEC lines in vitro. For instance, glutamine dose-dependently enhanced cell proliferation and differentiation of neonatal porcine and rat IECs, through activating multiple mitogen-activated protein kinases (MAPKs) (108–110). Moreover, studies in neonatal porcine and adult human IEC lines have indicated that glutamine also promotes growth through augmenting the effects of growth factors, including insulin-like growth factor 1 and epidermal growth factor (108, 111–113). In addition to promoting growth, glutamine has been reported to dose-dependently protect against inflammation-, endotoxin- and oxidant-induced cell death and damage in these IEC lines (114–116). Remarkably, glutamine completely blocked inflammation-induced apoptosis in the adult human epithelial cell line HT-29 when supplied at 0.5 mM, a concentration similar to that of free glutamine in human milk (115).
Multiple lines of evidence indicate that glutamine also specifically stimulates intestinal barrier function. For instance, in vitro studies with neonatal porcine and human adult IEC lines have revealed that glutamine restriction reduces the expression of the major tight junction proteins, including claudin and occludin proteins, which are vital for intestinal barrier function (110, 117, 118). This was accompanied by a reduced distribution of these proteins at the plasma membrane and an increase in IEC permeability. Remarkably, glutamine supplementation in these in vitro models completely reversed this process, suggesting that sufficient availability of free glutamine is crucial for optimal epithelial barrier functions. These effects were mediated through enhanced AMP-activated protein kinase signaling and diminished PI3K/Akt signaling, indicating that glutamine supports intestinal barrier function via modulation of specific intracellular pathways (110, 118).
Consistent with in vitro studies in neonatal cells, studies in young animals also suggest a potential role of glutamine in promoting a healthy intestinal development. In rat pups and young piglets, dietary deprivation of glutamine has been reported to diminish intestinal integrity, through breakdown of epithelial junctions and shortening of microvilli (119, 120). Conversely, dietary supplementation of glutamine in young piglets has been consistently reported to increase villus height, inhibit apoptosis and boost proliferation of IECs, increase tight junction protein expression and improve epithelial barrier function (98, 121–123). In addition, glutamine is shown to protect against pathogen-induced intestinal damage in vivo. For instance, weaning piglets fed a glutamine-enriched diet prior to challenge with E.coli completely maintained villus morphology and tight junction protein expression (124, 125). Moreover, oral supplementation of glutamine prevented endotoxin-induced intestinal damage in suckling piglets (114). Consistent with the ability of glutamine to promote intestinal barrier function, glutamine supplementation is reported to prevent bacterial translocation in various adult animal models of intestinal obstruction (126–131). Whether glutamine can also prevent bacterial translocation in neonatal animals remains to be examined.
Impact of Glutamate on Intestinal Functions
A growing body of evidence suggests that next to glutamine also glutamate has effects on IEC growth and intestinal barrier function. A recent in vitro study in neonatal porcine IECs has demonstrated that supplementation of glutamate dose-dependently enhances cell proliferation (132). Moreover, this study showed that glutamate supplementation prevented oxidative stress-induced changes in IEC viability, barrier function and membrane integrity by increasing the abundance of tight junction proteins (132). The ability of glutamate to improve intestinal barrier function is also demonstrated in a study using adult human IEC lines, where glutamate addition significantly reduced phorbol-induced hyperpermeability (133). Remarkably, these effects were observed at a glutamate concentration three times lower than that present in human milk, highlighting the potency of free glutamate in human milk to exert physiological effects.
In addition to in vitro studies, in vivo studies in young animals also indicate that free glutamate can promote intestinal development. Supplementation of dietary glutamate to healthy weaning piglets led to an increase in overall intestinal health, as evidenced by higher villus height and enhanced intestinal mucosal thickness and integrity (122, 134). Furthermore, dietary glutamate dose-dependently enhanced the weight of the small intestine, increased the depth of the crypts and the lamina propria, and improved intestinal antioxidative capacities in healthy weaning piglets (99). Finally, dietary glutamate prevented mycotoxin-induced impairments in intestinal barrier function and morphology in young piglets, suggesting that free glutamate may also play a role in the prevention of intestinal damage (135).
As glutamate can be converted into glutamine by IECs, although at limited rates, the effects observed for glutamate may be attributable to the effects of glutamine. However, studies examining effects of both glutamine and glutamate demonstrated differential effects of these FAAs on functions of IECs and intestinal morphology. For instance, weaning piglets supplemented with dietary glutamine alone had higher villi than those piglets supplemented with a combination of glutamate and glutamine, whereas the combination led to the deepest crypts (136). Moreover, glutamine was observed to have protective effects against oxidant- and endotoxin-induced death of porcine neonatal IECs in vitro, whereas glutamate had no effect (114). This indicates that the effects of glutamate on intestinal function are not solely exerted through conversion into glutamine.
Effects of Free Glutamine and Glutamate on Immune Cell Functions
In addition to epithelial cells, the immune cells of the GALT also play a crucial role in the prevention of neonatal allergies and infections. The immature neonatal GALT is characterized by the production of higher levels of pro-inflammatory cytokines (137, 138), whereas anti-inflammatory capacities are diminished (139). The pro-inflammatory milieu in the neonatal intestines is indicated to induce T-helper 2 (TH2) immune activity (140, 141). In contrast, T-helper 1 (TH1) immunity is highly limited and gradually develops during the postnatal period (142–144). The resulting TH2-dominant immune milieu is known to increase the susceptibility to allergic sensitization, whereas the minimal TH1 function correlates with the increased susceptibility of neonates to infections (144, 145). Thus, components in human milk with anti-inflammatory capacities, or components that enhance the development of TH1 immunity or suppress TH2 activity might contribute to the prevention of neonatal allergies and infections. Free glutamine and glutamate both have been associated with these immunomodulatory capacities, as described in detail below.
Impact of Glutamine on Immune Cell Functions
The importance of glutamine for the development and function of the immune system is well recognized. Although in vitro studies in neonatal cells are lacking, numerous in vitro studies in adult cells showed that various immune cells fail to develop and function without adequate glutamine availability (146). For instance, glutamine restriction impaired the growth and differentiation of B and T cells (147) and diminished antigen presentation and phagocytotic capacities of macrophages and neutrophils (148, 149). Conversely, glutamine supplementation dose-dependently enhanced phagocytotic capacities of human neutrophils in vitro (150, 151). Consistent with these findings, in vivo studies in young animals indicate that glutamine availability modifies intestinal immune cell populations. For example, dietary glutamine dose-dependently increased the number of neutrophils and macrophages in weaning piglets following an LPS-challenge (123, 152), suggestive of enhanced antimicrobial capacities. Moreover, in newly weaned piglets, dietary glutamine decreased the proportion of antigen-naïve T cells in the mesenteric lymph nodes (153), which are reported to be elevated in allergic patients and are proposed as an early life marker for future development of allergies (154, 155). Finally, dietary glutamine increased the number of IgA-secreting B cells in the small intestine of young mice (156) and enhanced intestinal levels of IgA in various weaning animals (157–161). Together, these results indicate that glutamine availability influences immune cell populations in developing intestinal tissues, which in turn may influence antimicrobial and anti-allergic immune processes.
A consistent body of evidence shows that glutamine also exhibits anti-inflammatory capacities. In vitro studies demonstrated that glutamine supplementation decreased the production of pro-inflammatory cytokines IL-6, IL-8, and/or TNFα, while increasing the production of anti-inflammatory/regulatory cytokine IL-10 in various activated adult human immune cells, including intra-epithelial lymphocytes (IELs), intestinal mast cells, peripheral mononuclear cells (PBMCs) and monocytes (162–165). Similar findings are reported in healthy young animals. For instance, dietary glutamine reduced levels of pro-inflammatory cytokines (including IL-1 and IL-8) while increasing levels of anti-inflammatory/regulatory cytokines (including IL-10) in the small intestine of healthy weaning piglets (123, 124, 166). Furthermore, in LPS-challenged piglets, dietary glutamine reduced intestinal expression of inflammatory markers, including Toll-like receptor-4 and the nuclear factor NF-κB, suggesting that glutamine might also have potent anti-inflammatory effects in immune-compromised conditions (114).
Glutamine has also been indicated to play a regulating role in the balance between TH1 and TH2 immunity, however, in vitro studies examining this aspect in neonatal immune cells are lacking. It is reported that adult murine naïve T cells are able to differentiate into TH2 cells under glutamine-restricted conditions, but not into functional TH1 cells, indicating that glutamine deprivation may favor TH2 differentiation (167). Conversely, supplementation of glutamine is reported to enhance TH1 and/or diminish TH2 responses of various activated adult immune cells in vitro. For instance, glutamine increased the production of TH1 cytokines IL-2 and IFNy by activated murine IELs and by human lymphocytes and PBMCs, while TH2 cytokines were unaltered (168–171). In activated human intestinal mast cells, glutamine did not alter the release of TH1 chemokines, but reduced the release of TH2 chemokine ligand 2 and leukotriene C4, which are both involved in the pathogenesis of various allergic diseases (164, 172). Although data are limited, in vivo studies in young animals also suggest a regulating role of glutamine in the TH1/TH2 immune balance. In young mice, dietary glutamine increased the expression of IL-2 and the IL-2 receptor by lymphocytes, indicative of increased activity of and responsiveness to TH1 stimuli (173). Moreover, dietary glutamine in healthy weaning piglets lowered the production of TH2 cytokine IL-4 and increased the IFNγ/IL-4 ratio in mesenteric lymph node cells (153). Finally, in weaning rabbits, dietary glutamine upregulated IL-2 and IL-10 expression by IELs, while inhibiting expression of IL-6, an inducer of TH2 differentiation of naïve T cells (174, 175). Although further confirmation in neonatal animals is critical, these data may indicate that glutamine plays a role in promoting a more balanced TH1/TH2 immune system in the neonatal period.
Impact of Glutamate on Immune Cell Functions
Despite dietary glutamate being almost completely used in intestinal tissues, studies investigating the effects of glutamate on intestinal immune cells are lacking. Yet, receptors for glutamate are found on a variety of immune cells, including lymphocytes and dendritic cells, suggesting that glutamate has a role in immune cell functioning (176). Studies using adult human peripheral T cells demonstrated that glutamate at low concentrations (<100 μM) dose-dependently increases the proliferative response of T cells to various stimuli (176, 177). At higher concentrations (>1 mM), however, this effect reversed, indicating that glutamate tends to have immunosuppressive properties at higher concentrations (176, 178). Accordingly, it is postulated that the high glutamate concentration in the intestinal microenvironment, which may reach the millimolar range, could prevent inappropriate responses to dietary antigens by exerting immunosuppressive effects on intestinal T cells (178).
Besides regulating T cell proliferation, glutamate availability is also indicated to influence the TH2 and TH1 cytokine production by T cells. Glutamate is released by dendritic cells during T cell interaction, where it has dual roles (179). In cases of non-specific antigen presentation, glutamate inhibits T cell activation. However, upon specific antigen presentation glutamate stimulates T cell proliferation and the production of IL-2, IFNγ and IL-10, thereby promoting a TH1 response (179). This latter process depends on glutamate released from dendritic cells, but also on extracellular glutamate concentrations, suggesting that this process could be influenced by dietary glutamate (179). Accordingly, it is reported that glutamate supplementation of up to 1-2 mM enhanced IFNy and IL-10 secretion by activated adult human peripheral T cells in vitro, whereas secretion of TH2 cytokines IL-4 and IL-5 was unaffected (180). When supplied at even higher concentrations (>5 mM), however, glutamate inhibited IFNy and IL-10 secretion by these cells. Unfortunately, in vitro studies in neonatal cells and in vivo studies investigating the effects of glutamate on immune cell functions are lacking. Nevertheless, the findings in adult immune cells suggest an immunoregulating role for glutamate, with effects that are highly dependent on the context and the concentration. At concentrations present in human milk, glutamate could be involved in promoting TH1 immunity and subsequently in reducing the susceptibility to allergic sensitization, although this remains speculative due the lack of evidence in neonatal cells or animals.
Effects of Free Glutamine and Glutamate on the Intestinal Microbiota
Accumulating evidence indicates that the gut microbiota plays a vital role in tolerance induction to dietary antigens (181–183). Accordingly, clinical studies have provided evidence for a link between the microbiota composition in the neonatal period and the development of allergic diseases. It is reported that a higher intestinal bacterial diversity in early life is associated with a lower risk of developing various allergic diseases, including food allergy (184–187). Moreover, infants with an increased colonization of Firmicutes and a decreased colonization of Bacteroidetes (corresponding to an increased Firmicutes-to-Bacteroidetes ratio), or a decreased colonization of Proteobacteria and Bifidobacteria are shown to be at increased risk of developing food allergies (188–191). Mechanisms by which gut microbes modify the susceptibility to allergies are poorly understood but may involve specific modulation of TH2 and TH1 immunity (192, 193). The colonization of intestinal microbiota is far from complete at birth and is influenced by various environmental factors, including breastfeeding duration (189). Thus, human milk components that shape the neonatal gut microbiota composition may play an active part in modifying the susceptibility to allergic sensitization. Although data are limited, several studies have shown that glutamine and glutamate can modulate the abundance of gut bacteria that have been associated with the protection against allergic diseases.
Impact of Glutamine on the Gut Microbiota Composition
The ability of dietary glutamine to modify the microbiota composition is shown in various young animals. A study in weaning mice demonstrated that dietary glutamine decreased the content of Firmicutes in the jejunum and ileum, and decreased the Firmicutes-to-Bacteroidetes ratio in the ileum (194). Similar findings are reported in studies in adult pigs and human (195, 196). In weaning rabbits, dietary glutamine specifically reduced the presence of Clostridium spp. in the ileum, of which colonization in early life has been associated with increased risk of allergic diseases (197, 198). Finally, a glutamine-enriched diet is also shown to increase the abundance of beneficial Bifidobacteria in the jejunum of healthy weaned mice (194), and to decrease potentially harmful microorganisms in adult pigs (196). The mechanisms underlying the effects of glutamine on the gut microbiota composition are poorly understood. It is postulated that glutamine supplementation regulates utilization and metabolism of a variety of AAs in a niche-specific manner, affecting the activity and number of specific microbes (157, 199).
Impact of Glutamate on the Gut Microbiota Composition
To our knowledge, only two animal studies examined the effects of dietary glutamate on the intestinal microbiota composition to date, both of which used animals in their post-weaning phase. It has been reported that dietary glutamate markedly enhanced the bacterial diversity in the intestinal flora of healthy post-weaning pigs (200). Moreover, the glutamate-enriched diet decreased the Firmicutes-to-Bacteroidetes ratio in the ileum, although this effect was only seen when given in combination with a high fat diet and was not observed in other intestinal sections. Perhaps more interestingly, dietary glutamate specifically promoted the colonization of prausnitzii and Faecalibacterium prausnitzii in post-weaning pigs (200, 201). The colonization of Roseburia in early life has been positively associated with the acquisition of tolerance to cow's milk (202), and Faecalibacterium prausnitzii is indicated to play a role in the prevention of food allergy (203–205). These intestinal microbes are some of the main producers of the short-chain fatty acids butyrate and propionate. Accordingly, a glutamate-enriched diet significantly increased colonic concentrations of these fatty acids in adult pigs (206). Butyrate and propionate both have been associated with the prevention of various allergic diseases and, consequently, high faecal levels of these fatty acids in early life have been associated with a decreased risk of developing atopy (207–209).
Concluding Remarks
Research indicates that breastfeeding during the first months of life provides protection against immune-related conditions in neonates and later in life. These conditions include gastrointestinal infections and several allergic diseases including food allergy. It is indicated that the transfer of specific immunomodulating components, such as bioactive proteins, from mother to infant through human milk contributes to this protective effect. In addition to proteins, human milk contains FAAs, which have unique characteristics. They are more readily absorbed than protein-derived AAs and can be recognized by specific receptors on various cells. Moreover, whereas protein-bound AAs decrease during the lactation period in a non-AA-specific manner, temporal changes of FAAs in human milk are highly AA-specific. These dynamics in FAA levels are globally consistent and thus seemingly independent of ethnicity, demographics and maternal diet. This suggests that selective FAA transport occurs in the mammary gland, that FAA levels in human milk are strictly regulated and, consequently, that FAAs are likely to be of physiological relevance in the developing infant.
With regards to individual FAAs in human milk, free glutamine and glutamate display particularly remarkable characteristics. They account for almost 70% of the FAA content in human milk, they both drastically increase in the first 3 months of lactation and their levels have been shown to positively correlate with infant growth, suggestive of important functions in the developing neonate. In neonates, dietary glutamine and glutamate are mainly used by the intestines. Remarkably, studies in neonatal immune cells and young animals demonstrate that these FAAs can have a wide range of effects on cells in developing intestines, also at concentrations similar to their levels in human milk. In short, they are reported to increase the growth of intestinal epithelial cells, enhance intestinal barrier function, influence immune cell development and populations in the gut-associated lymphoid tissue, exert anti-inflammatory and potentially TH1 promoting and/or TH2 inhibiting effects on various intestinal immune cells, and modify the abundance of gut microbiota that might play a role in allergic sensitization (Figure 1). Together, these effects could potentially support neonates in the protection against allergic sensitization and infections.
All together, the findings described in this review warrant further research into the contribution of free glutamine and glutamate in human milk to the protection against neonatal allergies and infections. Levels of free glutamine and glutamate, in addition to that of other bioactive factors that could influence early life immune development, are considerably higher in human milk than in standard infant milk formulas, leading to significant differences in the intake of these FAAs between breastfed and formula-fed children (210–212). As many of the effects of glutamine and glutamate described in this review were concentration-dependent, future studies should address whether this differential intake contributes to the differential occurrence in immune-related conditions between formula-fed and breastfed children.
Author Contributions
JS, AH, and SW: conceptualization and literature searches. AH, SW, and JG: supervision. JS: writing original draft. JS, AH, SW, and JG: review and editing. All authors read and approved the manuscript.
Conflict of Interest
SW is current employee of Danone Nutricia Research. JG is part-time employee of Danone Nutricia Research and Utrecht University.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Ajetunmobi OM, Whyte B, Chalmers J, Tappin DM, Wolfson L, Fleming M, et al. Breastfeeding is associated with reduced childhood hospitalization: Evidence from a scottish birth cohort (1997-2009). J Pediatr. (2015) 166:620–5.e624. doi: 10.1016/j.jpeds.2014.11.013
2. Duijts L, Jaddoe VW, Hofman A, Moll HA. Prolonged and exclusive breastfeeding reduces the risk of infectious diseases in infancy. Pediatrics. (2010) 126:e18–25. doi: 10.1542/peds.2008-3256
3. Shi T, Balsells E, Wastnedge E, Singleton R, Rasmussen ZA, Zar HJ, et al. Risk factors for respiratory syncytial virus associated with acute lower respiratory infection in children under five years: systematic review and meta-analysis. J Glob Health. (2015) 5:020416. doi: 10.7189/jogh.05.020416
4. Henrick BM, Yao X-D, Nasser L, Roozrogousheh A, Rosenthal KL. Breastfeeding behaviors and the innate immune system of human milk: working together to protect infants against inflammation, hiv-1, and other infections. Front Immunol. (2017) 8:1631. doi: 10.3389/fimmu.2017.01631
5. Kramer MS, Chalmers B, Hodnett ED, Sevkovskaya Z, Dzikovich I, Shapiro S, et al. Promotion of breastfeeding intervention trial (probit): a randomized trial in the republic of belarus. JAMA. (2001) 285:413–20. doi: 10.1001/jama.285.4.413
6. Li R, Dee D, Li C-M, Hoffman HJ, Grummer-Strawn LM. Breastfeeding and risk of infections at 6 years. Pediatrics. (2014) 134 (Suppl. 1):S13–20. doi: 10.1542/peds.2014-0646D
7. Wang J, Ramette A, Jurca M, Goutaki M, Beardsmore CS, Kuehni CE. Breastfeeding and respiratory tract infections during the first 2 years of life. ERJ Open Res. (2017) 3:00143–2016. doi: 10.1183/23120541.00143-2016
8. Bjorksten B, Ait-Khaled N, Innes Asher M, Clayton TO, Robertson C. Global analysis of breast feeding and risk of symptoms of asthma, rhinoconjunctivitis and eczema in 6-7 year old children: Isaac phase three. Allergol Immunopathol. (2011) 39:318–25. doi: 10.1016/j.aller.2011.02.005
9. Lodge CJ, Tan DJ, Lau MX, Dai X, Tham R, Lowe AJ, et al. Breastfeeding and asthma and allergies: a systematic review and meta-analysis. Acta Paediatr. (2015) 104:38–53. doi: 10.1111/apa.13132
10. Dogaru CM, Nyffenegger D, Pescatore AM, Spycher BD, Kuehni CE. Breastfeeding and childhood asthma: systematic review and meta-analysis. Am J Epidemiol. (2014) 179:1153–67. doi: 10.1093/aje/kwu072
11. Gdalevich M, Mimouni D, Mimouni M. Breast-feeding and the risk of bronchial asthma in childhood: a systematic review with meta-analysis of prospective studies. J Pediatr. (2001) 139:261–6. doi: 10.1067/mpd.2001.117006
12. Kull I, Melen E, Alm J, Hallberg J, Svartengren M, van Hage M, et al. Breast-feeding in relation to asthma, lung function, and sensitization in young schoolchildren. J Allergy Clin Immunol. (2010) 125:1013–9. doi: 10.1016/j.jaci.2010.01.051
13. Lucas A, Brooke OG, Morley R, Cole TJ, Bamford MF. Early diet of preterm infants and development of allergic or atopic disease: randomised prospective study. BMJ. (1990) 300:837–40. doi: 10.1136/bmj.300.6728.837
14. Saarinen UM, Kajosaari M. Breastfeeding as prophylaxis against atopic disease: prospective follow-up study until 17 years old. Lancet. (1995) 346:1065–9. doi: 10.1016/S0140-6736(95)91742-X
15. Järvinen KM, Martin H, Oyoshi MK. Immunomodulatory effects of breast milk on food allergy. Ann Aller Asthma Immunol. (2019) 123:133–43. doi: 10.1016/j.anai.2019.04.022
16. van Odijk J, Kull I, Borres MP, Brandtzaeg P, Edberg U, Hanson LA, et al. Breastfeeding and allergic disease: a multidisciplinary review of the literature (1966-2001) on the mode of early feeding in infancy and its impact on later atopic manifestations. Allergy. (2003) 58:833–43. doi: 10.1034/j.1398-9995.2003.00264.x
17. Greer FR, Sicherer SH, Burks AW. Effects of early nutritional interventions on the development of atopic disease in infants and children: the role of maternal dietary restriction, breastfeeding, timing of introduction of complementary foods, and hydrolyzed formulas. Pediatrics. (2008) 121:183–91. doi: 10.1542/peds.2007-3022
18. Fleischer DM, Spergel JM, Assa'ad AH, Pongracic JA. Primary prevention of allergic disease through nutritional interventions. J Aller Clin Immunol Pract. (2013) 1:29–36. doi: 10.1016/j.jaip.2012.09.003
19. Sambrook J. Incidence of cow's milk protein allergy. Br J Gen Pract. (2016) 66:512. doi: 10.3399/bjgp16X687277
20. Matheson MC, Allen KJ, Tang ML. Understanding the evidence for and against the role of breastfeeding in allergy prevention. Clin Exp Allergy. (2012) 42:827–51. doi: 10.1111/j.1365-2222.2011.03925.x
21. Munblit D, Verhasselt V. Allergy prevention by breastfeeding: possible mechanisms and evidence from human cohorts. Curr Opin Allergy Clin Immunol. (2016) 16:427–33. doi: 10.1097/ACI.0000000000000303
22. Newburg DS, Walker WA. Protection of the neonate by the innate immune system of developing gut and of human milk. Pediatr Res. (2007) 61:2–8. doi: 10.1203/01.pdr.0000250274.68571.18
23. Simon AK, Hollander GA, McMichael A. Evolution of the immune system in humans from infancy to old age. Proc Biol Sci. (2015) 282:20143085. doi: 10.1098/rspb.2014.3085
24. Marodi L. Down-regulation of th1 responses in human neonates. Clin Exp Immunol. (2002) 128:1–2. doi: 10.1046/j.1365-2249.2002.01873.x
25. Berin MC. Mucosal antibodies in the regulation of tolerance and allergy to foods. Semin Immunopathol. (2012) 34:633–42. doi: 10.1007/s00281-012-0325-9
26. Fagarasan S, Honjo T. Intestinal iga synthesis: regulation of front-line body defences. Nat Rev Immunol. (2003) 3:63–72. doi: 10.1038/nri982
27. Plaza-Diaz J, Fontana L, Gil A. Human milk oligosaccharides and immune system development. Nutrients. (2018) 10:1038. doi: 10.3390/nu10081038
28. Fritsche K. Fatty acids as modulators of the immune response. Ann Rev Nutr. (2006) 26:45–73. doi: 10.1146/annurev.nutr.25.050304.092610
29. Lönnerdal B. Bioactive proteins in human milk: health, nutrition, and implications for infant formulas. J Pediatr. (2016) 173:S4–9. doi: 10.1016/j.jpeds.2016.02.070
30. Chichlowski M, German JB, Lebrilla CB, Mills DA. The influence of milk oligosaccharides on microbiota of infants: opportunities for formulas. Annu Rev Food Sci Technol. (2011) 2:331–51. doi: 10.1146/annurev-food-022510-133743
31. Yu ZT, Chen C, Kling DE, Liu B, McCoy JM, Merighi M, et al. The principal fucosylated oligosaccharides of human milk exhibit prebiotic properties on cultured infant microbiota. Glycobiology. (2013) 23:169–77. doi: 10.1093/glycob/cws138
32. Parolini C. Effects of fish n-3 pufas on intestinal microbiota and immune system. Mar Drugs. (2019) 17:374. doi: 10.3390/md17060374
33. Roth E. Immune and cell modulation by amino acids. Clin Nutr. (2007) 26:535–44. doi: 10.1016/j.clnu.2007.05.007
34. Ruth MR, Field CJ. The immune modifying effects of amino acids on gut-associated lymphoid tissue. J Anim Sci Biotechnol. (2013) 4:27–. doi: 10.1186/2049-1891-4-27
35. van Sadelhoff JHJ, van BJM, Stahl B, Amodio S, Rings EHHM, Mearin ML, et al. Longitudinal variation of amino acid levels in human milk and their associations with infant gender. Nutrients. (2018) 10:1233. doi: 10.3390/nu10091233
36. Zhang Z, Adelman AS, Rai D, Boettcher J, Lonnerdal B. Amino acid profiles in term and preterm human milk through lactation: a systematic review. Nutrients. (2013) 5:4800–21. doi: 10.3390/nu5124800
37. Raiten DJ, Center for Food S, Applied N, Life Sciences Research O. Assessment of Nutrient Requirements For Infant Formulas: [lSRO Report]; Prepared for the Center for Food Safety and Applied Nutrition, Food and Drug Administration, Department of Health and Human Services, Washington, DC. Bethesda, MD: American Inst. of Nutrition (1998).
38. Christensen HN. Free amino acids and peptides in tissues. Mammal Protein Metab. (1964) 1:104–24.
39. Pohlandt F. Plasma amino acid concentrations in newborn infants breast-fed ad libitum. J Pediatr. (1978) 92:614–6. doi: 10.1016/S0022-3476(78)80305-9
40. Carratù B, Boniglia C, Scalise F, Ambruzzi A, Sanzini E. Nitrogenous components of human milk: non-protein nitrogen, true protein and free amino acids. Food Chem. (2003) 81:357–62. doi: 10.1016/S0308-8146(02)00430-2
41. Schanler RJ, Garza C. Plasma amino acid differences in very low birth weight infants fed either human milk or whey-dominant cow milk formula. Pediatr Res. (1987) 21:301–5. doi: 10.1203/00006450-198703000-00021
42. Boirie Y, Gachon P, Corny S, Fauquant J, Maubois JL, Beaufrere B. Acute postprandial changes in leucine metabolism as assessed with an intrinsically labeled milk protein. Am J Physiol. (1996) 271:E1083–91. doi: 10.1152/ajpendo.1996.271.6.E1083
43. Calbet JA, Holst JJ. Gastric emptying, gastric secretion and enterogastrone response after administration of milk proteins or their peptide hydrolysates in humans. Eur J Nutr. (2004) 43:127–39. doi: 10.1007/s00394-004-0448-4
44. Koopman R, Crombach N, Gijsen AP, Walrand S, Fauquant J, Kies AK, et al. Ingestion of a protein hydrolysate is accompanied by an accelerated in vivo digestion and absorption rate when compared with its intact protein. Am J Clin Nutr. (2009) 90:106–15. doi: 10.3945/ajcn.2009.27474
45. Rigo J, Salle BL, Picaud JC, Putet G, Senterre J. Nutritional evaluation of protein hydrolysate formulas. Eur J Clin Nutr. (1995) 49 (Suppl. 1):S26–38.
46. Hernell O, Lonnerdal B. Nutritional evaluation of protein hydrolysate formulas in healthy term infants: plasma amino acids, hematology, and trace elements. Am J Clin Nutr. (2003) 78:296–301. doi: 10.1093/ajcn/78.2.296
47. Isolauri E, Sutas Y, Makinen-Kiljunen S, Oja SS, Isosomppi R, Turjanmaa K. Efficacy and safety of hydrolyzed cow milk and amino acid-derived formulas in infants with cow milk allergy. J Pediatr. (1995) 127:550–7. doi: 10.1016/S0022-3476(95)70111-7
48. San Gabriel A, Uneyama H. Amino acid sensing in the gastrointestinal tract. Amino Acids. (2013) 45:451–61. doi: 10.1007/s00726-012-1371-2
49. Chatterton DEW, Rasmussen JT, Heegaard CW, Sørensen ES, Petersen TE. In vitro digestion of novel milk protein ingredients for use in infant formulas: research on biological functions. Trends Food Sci Technol. (2004) 15:373–83. doi: 10.1016/j.tifs.2003.12.004
50. Dallas DC, Underwood MA, Zivkovic AM, German JB. Digestion of protein in premature and term infants. J Nutr Disord Ther. (2012) 2:112. doi: 10.4172/2161-0509.1000112
51. Gan J, Bornhorst GM, Henrick BM, German JB. Protein digestion of baby foods: study approaches and implications for infant health. Mol Nutr Food Res. (2018) 62:10.1002/mnfr.201700231. doi: 10.1002/mnfr.201700231
52. Lindberg T, Borulf S, Jakobsson I. Digestion of milk proteins in infancy. Acta Paediatr Scand Suppl. (1989) 351:29–33. doi: 10.1111/j.1651-2227.1989.tb11205.x
53. da Costa TH, Haisma H, Wells JC, Mander AP, Whitehead RG, Bluck LJ. How much human milk do infants consume? Data from 12 countries using a standardized stable isotope methodology. J Nutr. (2010) 140:2227–32. doi: 10.3945/jn.110.123489
54. Dupont C. Protein requirements during the first year of life. Am J Clin Nutr. (2003) 77:1544s−9s. doi: 10.1093/ajcn/77.6.1544S
55. Garcia-Rodenas CL, Affolter M, Vinyes-Pares G, De Castro CA, Karagounis LG, Zhang Y, et al. Amino acid composition of breast milk from urban chinese mothers. Nutrients. (2016) 8:606. doi: 10.3390/nu8100606
56. Yamawaki N, Yamada M, Kan-no T, Kojima T, Kaneko T, Yonekubo A. Macronutrient, mineral and trace element composition of breast milk from japanese women. J Trace Elem Med Biol. (2005) 19:171–81. doi: 10.1016/j.jtemb.2005.05.001
57. Dallas DC, Murray NM, Gan J. Proteolytic systems in milk: perspectives on the evolutionary function within the mammary gland and the infant. J Mammary Gland Biol Neoplasia. (2015) 20:133–47. doi: 10.1007/s10911-015-9334-3
58. Chowanadisai W, Lönnerdal B. ?1-antitrypsin and antichymotrypsin in human milk: origin, concentrations, and stability. Am J Clin Nutr. (2002) 76:828–33. doi: 10.1093/ajcn/76.4.828
59. Lin Y, Duan X, Lv H, Yang Y, Liu Y, Gao X, et al. The effects of l-type amino acid transporter 1 on milk protein synthesis in mammary glands of dairy cows. J Dairy Sci. (2018) 101:1687–96. doi: 10.3168/jds.2017-13201
60. Lopez A, Torres N, Ortiz V, Aleman G, Hernandez-Pando R, Tovar AR. Characterization and regulation of the gene expression of amino acid transport system a (snat2) in rat mammary gland. Am J Physiol Endocrinol Metab. (2006) 291:E1059–66. doi: 10.1152/ajpendo.00062.2006
61. Laspiur JPr, Burton JL, Weber PSD, Moore J, Kirkwood RN, Trottier NL. Dietary protein intake and stage of lactation differentially modulate amino acid transporter mrna abundance in porcine mammary tissue. J Nutr. (2009) 139:1677–84. doi: 10.3945/jn.108.103549
62. Alemán G, López A, Ordaz G, Torres N, Tovar AR. Changes in messenger rna abundance of amino acid transporters in rat mammary gland during pregnancy, lactation, and weaning. Metabolism. (2009) 58:594–601. doi: 10.1016/j.metabol.2008.12.003
63. Shennan DB, Millar ID, Calvert DT. Mammary-tissue amino acid transport systems. Proc Nutr Soc. (1997) 56:177–91. doi: 10.1079/PNS19970020
64. Baldeón ME, Mennella JA, Flores N, Fornasini M, San Gabriel A. Free amino acid content in breast milk of adolescent and adult mothers in ecuador. Springerplus. (2014) 3:104. doi: 10.1186/2193-1801-3-104
65. Larnkjaer A, Bruun S, Pedersen D, Zachariassen G, Barkholt V, Agostoni C, et al. Free amino acids in human milk and associations with maternal anthropometry and infant growth. J Pediatr Gastroenterol Nutr. (2016) 63:374–8. doi: 10.1097/MPG.0000000000001195
66. Jochum F, Colling S, Meinardus P, Alteheld B, Stehle P, Fusch C. Total glutamine content in human milk is not influenced by gestational age. Acta Paediatr. (2006) 95:985–90. doi: 10.1080/08035250600729100
67. Rasmussen KM, Kjolhede CL. Prepregnant overweight and obesity diminish the prolactin response to suckling in the first week postpartum. Pediatrics. (2004) 113:e465–71. doi: 10.1542/peds.113.5.e465
68. Velázquez-Villegas LA, López-Barradas AM, Torres N, Hernández-Pando R, León-Contreras JC, Granados O, et al. Prolactin and the dietary protein/carbohydrate ratio regulate the expression of snat2 amino acid transporter in the mammary gland during lactation. Biochim Biophys Acta Biomembr. (2015) 1848:1157–64. doi: 10.1016/j.bbamem.2015.02.011
69. Roelfsema F, Pijl H, Keenan DM, Veldhuis JD. Prolactin secretion in healthy adults is determined by gender, age and body mass index. PLoS ONE. (2012) 7:e31305. doi: 10.1371/journal.pone.0031305
70. Ding M, Li W, Zhang Y, Wang X, Zhao A, Zhao X, et al. Amino acid composition of lactating mothers' milk and confinement diet in rural north china. Asia Pac J Clin Nutr. (2010) 19:344−9.
71. Lonnerdal B. Effects of maternal dietary intake on human milk composition. J Nutr. (1986) 116:499–513. doi: 10.1093/jn/116.4.499
72. Stegink LD, Filer LJ, Baker GL. Monosodium glutamate: effect on plasma and breast milk amino acid levels in lactating women. Proc Soc Exp Biol Med. (1972) 140:836–41. doi: 10.3181/00379727-140-36563
73. Ramirez I, DeSantiago S, Tovar AR, Torres N. Amino acid intake during lactation and amino acids of plasma and human milk. Adv Exp Med Biol. (2001) 501:415–21. doi: 10.1007/978-1-4615-1371-1_52
74. DeSantiago S, Ramirez I, Tovar AR, Alonso L, Ortiz-Olaya N, Torres N. [free amino acids in plasma and milk of mexican rural lactating women]. Rev Invest Clin. (1998) 50:405–12.
75. van Sadelhoff JHJ, Mastorakou D, Weenen H, Stahl B, Garssen J, Hartog A. Short communication: differences in levels of free amino acids and total protein in human foremilk and hindmilk. Nutrients. (2018) 10:1828. doi: 10.3390/nu10121828
76. Baldeon ME, Zertuche F, Flores N, Fornasini M. Free amino acid content in human milk is associated with infant gender and weight gain during the first four months of lactation. Nutrients. (2019) 11:2239. doi: 10.3390/nu11092239
77. Marques RF, Lopez FA, Braga JA. [growth of exclusively breastfed infants in the first 6 months of life]. J Pediatr. (2004) 80:99–105. doi: 10.2223/1147
78. Wu G. Functional amino acids in growth, reproduction, and health. Adv Nutr. (2010) 1:31–7. doi: 10.3945/an.110.1008
79. Watford M. Glutamine and glutamate: nonessential or essential amino acids? Anim Nutr. (2015) 1:119–22. doi: 10.1016/j.aninu.2015.08.008
80. Poindexter BB, Ehrenkranz RA, Stoll BJ, Koch MA, Wright LL, Oh W, et al. Effect of parenteral glutamine supplementation on plasma amino acid concentrations in extremely low-birth-weight infants. Am J Clin Nutr. (2003) 77:737–43. doi: 10.1093/ajcn/77.3.737
81. Parimi PS, Kalhan SC. Glutamine supplementation in the newborn infant. Semin Fetal Neonatal Med. (2007) 12:19–25. doi: 10.1016/j.siny.2006.10.003
82. Hays SP, Ordonez JM, Burrin DG, Sunehag AL. Dietary glutamate is almost entirely removed in its first pass through the splanchnic bed in premature infants. Pediatr Res. (2007) 62:353–6. doi: 10.1203/PDR.0b013e318123f719
83. Socha P, Grote V, Gruszfeld D, Janas R, Demmelmair H, Closa-Monasterolo R, et al. Milk protein intake, the metabolic-endocrine response, and growth in infancy: data from a randomized clinical trial. Am J Clin Nutr. (2011) 94:1776s−84s. doi: 10.3945/ajcn.110.000596
84. Riedijk MA, de Gast-Bakker DA, Wattimena JL, van Goudoever JB. Splanchnic oxidation is the major metabolic fate of dietary glutamate in enterally fed preterm infants. Pediatr Res. (2007) 62:468–73. doi: 10.1203/PDR.0b013e31813cbeba
85. Riedijk MA, van Goudoever JB. Splanchnic metabolism of ingested amino acids in neonates. Curr Opin Clin Nutr Metab Care. (2007) 10:58–62. doi: 10.1097/MCO.0b013e3280110183
86. Kong S, Zhang YH, Zhang W. Regulation of intestinal epithelial cells properties and functions by amino acids. Biomed Res Int. (2018) 2018:2819154. doi: 10.1155/2018/2819154
87. Burrin DG, Stoll B. Metabolic fate and function of dietary glutamate in the gut. Am J Clin Nutr. (2009) 90:850S-856S. doi: 10.3945/ajcn.2009.27462Y
88. Pinkus LM, Windmueller HG. Phosphate-dependent glutaminase of small intestine: localization and role in intestinal glutamine metabolism. Arch Biochem Biophys. (1977) 182:506–17. doi: 10.1016/0003-9861(77)90531-8
89. Cruzat V, Macedo Rogero M, Noel Keane K, Curi R, Newsholme P. Glutamine: metabolism and immune function, supplementation and clinical translation. Nutrients. (2018) 10:1564. doi: 10.3390/nu10111564
90. Blachier F, Boutry C, Bos C, Tomé D. Metabolism and functions of l-glutamate in the epithelial cells of the small and large intestines. Am J Clin Nutr. (2009) 90:814S−21S. doi: 10.3945/ajcn.2009.27462S
91. Meetze WH, Shenoy V, Martin G, Musy P, Neu J. Ontogeny of small intestinal glutaminase and glutamine synthetase in the rat: response to dexamethasone. Biol Neonate. (1993) 64:368–75. doi: 10.1159/000244013
92. Shenoy V, Roig JC, Chakrabarti R, Kubilis P, Neu J. Ontogeny of glutamine synthetase in rat small intestine. Pediatr Res. (1996) 39:643–8. doi: 10.1203/00006450-199604000-00014
93. Wu G. Amino acids: metabolism, functions, and nutrition. Amino Acids. (2009) 37:1–7. doi: 10.1007/s00726-009-0269-0
94. Wu G, Fang YZ, Yang S, Lupton JR, Turner ND. Glutathione metabolism and its implications for health. J Nutr. (2004) 134:489–92. doi: 10.1093/jn/134.3.489
95. Ober C, Yao TC. The genetics of asthma and allergic disease: a 21st century perspective. Immunol Rev. (2011) 242:10–30. doi: 10.1111/j.1600-065X.2011.01029.x
96. van Rijt LS, Utsch L, Lutter R, van Ree R. Oxidative stress: promoter of allergic sensitization to protease allergens? Int J Mol Sci. (2017) 18:1112. doi: 10.3390/ijms18061112
97. Antunes MM, Coelho BSL, Vichi TM, Santos EAd, Gondim FKB, Diniz AB, et al. Oral supplementation with capsaicin reduces oxidative stress and il-33 on a food allergy murine model. World Aller Org J. (2019) 12:100045. doi: 10.1016/j.waojou.2019.100045
98. Wang J, Chen L, Li P, Li X, Zhou H, Wang F, et al. Gene expression is altered in piglet small intestine by weaning and dietary glutamine supplementation. J Nutr. (2008) 138:1025–32. doi: 10.1093/jn/138.6.1025
99. Rezaei R, Knabe DA, Tekwe CD, Dahanayaka S, Ficken MD, Fielder SE, et al. Dietary supplementation with monosodium glutamate is safe and improves growth performance in postweaning pigs. Amino Acids. (2013) 44:911–23. doi: 10.1007/s00726-012-1420-x
100. Reeds PJ, Burrin DG. Glutamine and the bowel. J Nutr. (2001) 131:2505S−8S; discussion 2523S−2504S. doi: 10.1093/jn/131.9.2505S
101. Huang Y, Shao XM, Neu J. Immunonutrients and neonates. Eur J Pediatr. (2003) 162:122–8. doi: 10.1007/s00431-002-1128-0
102. Hansson GC. Role of mucus layers in gut infection and inflammation. Curr Opin Microbiol. (2012) 15:57–62. doi: 10.1016/j.mib.2011.11.002
103. van Ree R, Hummelshøj L, Plantinga M, Poulsen LK, Swindle E. Allergic sensitization: host-immune factors. Clin Transl Allergy. (2014) 4:12. doi: 10.1186/2045-7022-4-12
104. Xing S, Zhang B, Lin M, Zhou P, Li J, Zhang L, et al. Effects of alanyl-glutamine supplementation on the small intestinal mucosa barrier in weaned piglets. Asian Austr J Anim Sci. (2017) 30:236–45. doi: 10.5713/ajas.16.0077
105. Song D, Shi B, Xue H, Li Y, Yang X, Yu B, et al. Confirmation and prevention of intestinal barrier dysfunction and bacterial translocation caused by methotrexate. Dig Dis Sci. (2006) 51:1549–56. doi: 10.1007/s10620-005-9058-0
106. Assimakopoulos SF, Triantos C, Maroulis I, Gogos C. The role of the gut barrier function in health and disease. Gastroenterol Res. (2018) 11:261–3. doi: 10.14740/gr1053w
107. König J, Wells J, Cani PD, García-Ródenas CL, MacDonald T, Mercenier A, et al. Human intestinal barrier function in health and disease. Clin Transl Gastroenterol. (2016) 7:e196. doi: 10.1038/ctg.2016.54
108. Rhoads JM, Argenzio RA, Chen W, Rippe RA, Westwick JK, Cox AD, et al. L-glutamine stimulates intestinal cell proliferation and activates mitogen-activated protein kinases. Am J Physiol. (1997) 272:G943–53. doi: 10.1152/ajpgi.1997.272.5.G943
109. Jiang Q, Chen J, Liu S, Liu G, Yao K, Yin Y. L-glutamine attenuates apoptosis induced by endoplasmic reticulum stress by activating the ire1α-xbp1 axis in ipec-j2: a novel mechanism of l-glutamine in promoting intestinal health. Int J Mol Sci. (2017) 18:2617. doi: 10.3390/ijms18122617
110. Wang B, Wu Z, Ji Y, Sun K, Dai Z, Wu G. L-glutamine enhances tight junction integrity by activating camk kinase 2–amp-activated protein kinase signaling in intestinal porcine epithelial cells. J Nutr. (2016) 146:501–8. doi: 10.3945/jn.115.224857
111. Ko TC, Beauchamp RD, Townsend CM Jr, Thompson JC. Glutamine is essential for epidermal growth factor-stimulated intestinal cell proliferation. Surgery. (1993) 114:147–53; discussion 153–144.
112. Ziegler TR, Mantell MP, Chow JC, Rombeau JL, Smith RJ. Gut adaptation and the insulin-like growth factor system: regulation by glutamine and igf-i administration. Am J Physiol. (1996) 271:G866–75. doi: 10.1152/ajpgi.1996.271.5.G866
113. Blikslager AT, Rhoads JM, Bristol DG, Roberts MC, Argenzio RA. Glutamine and transforming growth factor-alpha stimulate extracellular regulated kinases and enhance recovery of villous surface area in porcine ischemic-injured intestine. Surgery. (1999) 125:186–94. doi: 10.1016/S0039-6060(99)70264-3
114. Haynes TE, Li P, Li X, Shimotori K, Sato H, Flynn NE, et al. L-glutamine or l-alanyl-l-glutamine prevents oxidant- or endotoxin-induced death of neonatal enterocytes. Amino Acids. (2009) 37:131–42. doi: 10.1007/s00726-009-0243-x
115. Evans ME, Jones DP, Ziegler TR. Glutamine prevents cytokine-induced apoptosis in human colonic epithelial cells. J Nutr. (2003) 133:3065–71. doi: 10.1093/jn/133.10.3065
116. Xue H, Sufit AJ, Wischmeyer PE. Glutamine therapy improves outcome of in vitro and in vivo experimental colitis models. JPEN J Parenter Enteral Nutr. (2011) 35:188–97. doi: 10.1177/0148607110381407
117. DeMarco VG, Li N, Thomas J, West CM, Neu J. Glutamine and barrier function in cultured caco-2 epithelial cell monolayers. J Nutr. (2003) 133:2176–9. doi: 10.1093/jn/133.7.2176
118. Li N, Neu J. Glutamine deprivation alters intestinal tight junctions via a pi3-k/akt mediated pathway in caco-2 cells. J Nutr. (2009) 139:710–4. doi: 10.3945/jn.108.101485
119. Potsic B, Holliday N, Lewis P, Samuelson D, DeMarco V, Neu J. Glutamine supplementation and deprivation: effect on artificially reared rat small intestinal morphology. Pediatr Res. (2002) 52:430–6. doi: 10.1203/00006450-200209000-00021
120. Rao R, Samak G. Role of glutamine in protection of intestinal epithelial tight junctions. J Epithel Biol Pharmacol. (2012) 5:47–54. doi: 10.2174/1875044301205010047
121. Domeneghini C, Di Giancamillo A, Bosi G, Arrighi S. Can nutraceuticals affect the structure of intestinal mucosa? Qualitative and quantitative microanatomy in l-glutamine diet-supplemented weaning piglets. Vet Res Commun. (2006) 30:331–42. doi: 10.1007/s11259-006-3236-1
122. Liu T, Peng J, Xiong Y, Zhou S, Cheng X. Effects of dietary glutamine and glutamate supplementation on small intestinal structure, active absorption and DNA, rna concentrations in skeletal muscle tissue of weaned piglets during d 28 to 42 of age. Asian Aust J Anim Sci. (2002) 15:238–42. doi: 10.5713/ajas.2002.238
123. Hanczakowska E, Niwinska B. Glutamine as a feed supplement for piglets: a review. Ann Anim Sci. (2013) 13:5–15. doi: 10.2478/v10220-012-0054-y
124. Ewaschuk JB, Murdoch GK, Johnson IR, Madsen KL, Field CJ. Glutamine supplementation improves intestinal barrier function in a weaned piglet model of escherichia coli infection. Br J Nutr. (2011) 106:870–7. doi: 10.1017/S0007114511001152
125. Yi GF, Carroll JA, Allee GL, Gaines AM, Kendall DC, Usry JL, et al. Effect of glutamine and spray-dried plasma on growth performance, small intestinal morphology, and immune responses of escherichia coli k88+-challenged weaned pigs. J Anim Sci. (2005) 83:634–43. doi: 10.2527/2005.833634x
126. Ding L-A, Li J-S. Effects of glutamine on intestinal permeability and bacterial translocation in tpn-rats with endotoxemia. World J Gastroenterol. (2003) 9:1327–32. doi: 10.3748/wjg.v9.i6.1327
127. de Oliveira MA, Lemos DS, Diniz SOF, Coelho JV, Cardoso VN. Prevention of bacterial translocation using glutamine: a new strategy of investigation. Nutrition. (2006) 22:419–24. doi: 10.1016/j.nut.2005.11.010
128. Santos RG, Quirino IE, Viana ML, Generoso SV, Nicoli JR, Martins FS, et al. Effects of nitric oxide synthase inhibition on glutamine action in a bacterial translocation model. Br J Nutr. (2014) 111:93–100. doi: 10.1017/S0007114513001888
129. Li Y, Chen Y, Zhang J, Zhu J-F, Liu Z-J, Liang S-Y, et al. Protective effect of glutamine-enriched early enteral nutrition on intestinal mucosal barrier injury after liver transplantation in rats. Am J Surg. (2010) 199:35–42. doi: 10.1016/j.amjsurg.2008.11.039
130. Karatzas T, Scopa S, Tsoni I, Panagopoulos K, Spiliopoulou I, Moschos S, et al. Effect of glutamine on intestinal mucosal integrity and bacterial translocation after abdominal radiation. Clin Nutr. (1991) 10:199–205. doi: 10.1016/0261-5614(91)90039-F
131. Karatepe O, Acet E, Battal M, Adas G, Kemik A, Altiok M, et al. Effects of glutamine and curcumin on bacterial translocation in jaundiced rats. World J Gastroenterol. (2010) 16:4313–20. doi: 10.3748/wjg.v16.i34.4313
132. Jiao N, Wu Z, Ji Y, Wang B, Dai Z, Wu G. L-glutamate enhances barrier and antioxidative functions in intestinal porcine epithelial cells. J Nutr. (2015) 145:2258–64. doi: 10.3945/jn.115.217661
133. Vermeulen MAR, de Jong J, Vaessen MJ, van Leeuwen PA, Houdijk APJ. Glutamate reduces experimental intestinal hyperpermeability and facilitates glutamine support of gut integrity. World J Gastroenterol. (2011) 17:1569–73. doi: 10.3748/wjg.v17.i12.1569
134. Wu X, Zhang Y, Liu Z, Li TJ, Yin YL. Effects of oral supplementation with glutamate or combination of glutamate and n-carbamylglutamate on intestinal mucosa morphology and epithelium cell proliferation in weanling piglets. J Anim Sci. (2012) 90 (Suppl. 4):337–9. doi: 10.2527/jas.53752
135. Duan J, Yin J, Wu M, Liao P, Deng D, Liu G, et al. Dietary glutamate supplementation ameliorates mycotoxin-induced abnormalities in the intestinal structure and expression of amino acid transporters in young pigs. PLoS ONE. (2014) 9:e112357. doi: 10.1371/journal.pone.0112357
136. Cabrera RA, Usry JL, Arrellano C, Nogueira ET, Kutschenko M, Moeser AJ, et al. Effects of creep feeding and supplemental glutamine or glutamine plus glutamate (aminogut) on pre- and post-weaning growth performance and intestinal health of piglets. J Anim Sci Biotechnol. (2013) 4:29. doi: 10.1186/2049-1891-4-29
137. MohanKumar K, Namachivayam K, Ho TTB, Torres BA, Ohls RK, Maheshwari A. Cytokines and growth factors in the developing intestine and during necrotizing enterocolitis. Semin Perinatol. (2017) 41:52–60. doi: 10.1053/j.semperi.2016.09.018
138. Erić Ž, Konjevic S. Proinflammatory cytokines in a newborn: a literature review. Signae Vitae. (2017) 13:10–13.
139. Nanthakumar N, Meng D, Goldstein AM, Zhu W, Lu L, Uauy R, et al. The mechanism of excessive intestinal inflammation in necrotizing enterocolitis: an immature innate immune response. PLoS ONE. (2011) 6:e17776. doi: 10.1371/journal.pone.0017776
141. Zhang Y, Collier F, Naselli G, Saffery R, Tang ML, Allen KJ, et al. Cord blood monocyte-derived inflammatory cytokines suppress il-2 and induce nonclassic “t(h)2-type” immunity associated with development of food allergy. Sci Transl Med. (2016) 8:321ra328. doi: 10.1126/scitranslmed.aad4322
142. Lee HH, Hoeman CM, Hardaway JC, Guloglu FB, Ellis JS, Jain R, et al. Delayed maturation of an il-12-producing dendritic cell subset explains the early th2 bias in neonatal immunity. J Exp Med. (2008) 205:2269–80. doi: 10.1084/jem.20071371
143. Zaghouani H, Hoeman CM, Adkins B. Neonatal immunity: faulty t-helpers and the shortcomings of dendritic cells. Trends Immunol. (2009) 30:585–91. doi: 10.1016/j.it.2009.09.002
144. Basha S, Surendran N, Pichichero M. Immune responses in neonates. Expert Rev Clin Immunol. (2014) 10:1171–84. doi: 10.1586/1744666X.2014.942288
145. Dowling DJ, Levy O. Ontogeny of early life immunity. Trends Immunol. (2014) 35:299–310. doi: 10.1016/j.it.2014.04.007
146. Newsholme P. Why is l-glutamine metabolism important to cells of the immune system in health, postinjury, surgery or infection? J Nutr. (2001) 131:2515S−22S; discussion 2523S-2514S. doi: 10.1093/jn/131.9.2515S
147. Heyse S, Connolly T, Doughty C, Chiles T. The regulation and role of l-glutamine in b-lymphocyte activation (lym7p.618). J Immunol. (2015) 194:200.210.
148. Spittler A, Winkler S, Gotzinger P, Oehler R, Willheim M, Tempfer C, et al. Influence of glutamine on the phenotype and function of human monocytes. Blood. (1995) 86:1564–9. doi: 10.1182/blood.V86.4.1564.bloodjournal8641564
149. Garcia C, Pithon-Curi TC, de Lourdes Firmano M, Pires de Melo M, Newsholme P, Curi R. Effects of adrenaline on glucose and glutamine metabolism and superoxide production by rat neutrophils. Clin Sci. (1999) 96:549–55. doi: 10.1042/cs0960549
150. Saito H, Furukawa S, Matsuda T. Glutamine as an immunoenhancing nutrient. J Parenter Enteral Nutr. (1999) 23:S59–61. doi: 10.1177/014860719902300515
151. Furukawa S, Saito H, Inoue T, Matsuda T, Fukatsu K, Han I, et al. Supplemental glutamine augments phagocytosis and reactive oxygen intermediate production by neutrophils and monocytes from postoperative patients in vitro. Nutrition. (2000) 16:323–9. doi: 10.1016/S0899-9007(00)00228-8
152. Pardo LA, Poveda PA, da Silva C, dos Santos A, Venâncio E, Arantes V, et al. Effect of l-glutamine levels in piglets diets challenged with escherichia coli lipopolysacharides. Revista MVZ Córdoba. (2014) 19:4328–37. doi: 10.21897/rmvz.94
153. Johnson IR, Ball RO, Baracos VE, Field CJ. Glutamine supplementation influences immune development in the newly weaned piglet. Dev Comp Immunol. (2006) 30:1191–202. doi: 10.1016/j.dci.2006.03.003
154. Powe DG, Huskisson RS, Carney AS, Jenkins D, McEuen AR, Walls AF, et al. Mucosal t-cell phenotypes in persistent atopic and nonatopic rhinitis show an association with mast cells. Allergy. (2004) 59:204–12. doi: 10.1046/j.1398-9995.2003.00315.x
155. Stencel-Gabriel K. Cd45ra/cd45r0 a probable marker for future development of allergy? J Aller Clin Immunol. (2004) 113:S290. doi: 10.1016/j.jaci.2004.01.522
156. Wu M, Xiao H, Liu G, Chen S, Tan B, Ren W, et al. Glutamine promotes intestinal siga secretion through intestinal microbiota and il-13. Mol Nutr Food Res. (2016) 60:1637–48. doi: 10.1002/mnfr.201600026
157. Ren W, Wang K, Yin J, Chen S, Liu G, Tan B, et al. Glutamine-induced secretion of intestinal secretory immunoglobulin a: a mechanistic perspective. Front Immunol. (2016) 7:503. doi: 10.3389/fimmu.2016.00503
158. Takechi H, Mawatari K, Harada N, Nakaya Y, Asakura M, Aihara M, et al. Glutamine protects the small intestinal mucosa in anticancer drug-induced rat enteritis model. J Med Investig. (2014) 61:59–64. doi: 10.2152/jmi.61.59
159. Zhou Y, Zhang P, Deng G, Liu X, Lu D. Improvements of immune status, intestinal integrity and gain performance in the early-weaned calves parenterally supplemented with l-alanyl-l-glutamine dipeptide. Vet Immunol Immunopathol. (2012) 145:134–42. doi: 10.1016/j.vetimm.2011.10.020
160. Bartell SM, Batal AB. The effect of supplemental glutamine on growth performance, development of the gastrointestinal tract, and humoral immune response of broilers. Poult Sci. (2007) 86:1940–7. doi: 10.1093/ps/86.9.1940
161. Zou X-P, Chen M, Wei W, Cao J, Chen L, Tian M. Effects of enteral immunonutrition on the maintenance of gut barrier function and immune function in pigs with severe acute pancreatitis. J Parent Enteral Nutr. (2010) 34:554–66. doi: 10.1177/0148607110362691
162. Wischmeyer PE, Riehm J, Singleton KD, Ren H, Musch MW, Kahana M, et al. Glutamine attenuates tumor necrosis factor-alpha release and enhances heat shock protein 72 in human peripheral blood mononuclear cells. Nutrition. (2003) 19:1–6. doi: 10.1016/S0899-9007(02)00839-0
163. Raspe C, Czeslick E, Weimann A, Schinke C, Leimert A, Kellner P, et al. Glutamine and alanine-induced differential expression of intracellular il-6, il-8, and tnf-alpha in lps-stimulated monocytes in human whole-blood. Cytokine. (2013) 62:52–7. doi: 10.1016/j.cyto.2013.02.020
164. Lechowski S, Feilhauer K, Staib L, Coeffier M, Bischoff SC, Lorentz A. Combined arginine and glutamine decrease release of de novo synthesized leukotrienes and expression of proinflammatory cytokines in activated human intestinal mast cells. Eur J Nutr. (2013) 52:505–12. doi: 10.1007/s00394-012-0353-1
165. Lee WY, Hu YM, Ko TL, Yeh SL, Yeh CL. Glutamine modulates sepsis-induced changes to intestinal intraepithelial gammadeltat lymphocyte expression in mice. Shock. (2012) 38:288–93. doi: 10.1097/SHK.0b013e3182655932
166. Zhong X, Li W, Huang X, Wang Y, Zhang L, Zhou Y, et al. Effects of glutamine supplementation on the immune status in weaning piglets with intrauterine growth retardation. Arch Anim Nutr. (2012) 66:347–56. doi: 10.1080/1745039X.2012.683325
167. Klysz D. Glutamine-dependent α-ketoglutarate production regulates the balance between t helper 1 cell and regulatory t cell generation. Sci Signal. (2015) 8:ra97. doi: 10.1126/scisignal.aab2610
168. Horio Y, Osawa S, Takagaki K, Hishida A, Furuta T, Ikuma M. Glutamine supplementation increases th1-cytokine responses in murine intestinal intraepithelial lymphocytes. Cytokine. (2008) 44:92–5. doi: 10.1016/j.cyto.2008.06.011
169. Rohde T, MacLean DA, Klarlund Pedersen B. Glutamine, lymphocyte proliferation and cytokine production. Scand J Immunol. (1996) 44:648–50. doi: 10.1046/j.1365-3083.1996.d01-352.x
170. Chang W-K, Yang KD, Shaio M-F. Effect of glutamine on th1 and th2 cytokine responses of human peripheral blood mononuclear cells. Clin Immunol. (1999) 93:294–301. doi: 10.1006/clim.1999.4788
171. Yaqoob P, Calder PC. Cytokine production by human peripheral blood mononuclear cells: differential sensitivity to glutamine availability. Cytokine. (1998) 10:790–4. doi: 10.1006/cyto.1998.0358
172. Jo-Watanabe A, Okuno T, Yokomizo T. The role of leukotrienes as potential therapeutic targets in allergic disorders. Int J Mol Sci. (2019) 20:3580. doi: 10.3390/ijms20143580
173. Kew S, Wells SM, Yaqoob P, Wallace FA, Miles EA, Calder PC. Dietary glutamine enhances murine t-lymphocyte responsiveness. J Nutr. (1999) 129:1524–31. doi: 10.1093/jn/129.8.1524
174. Delgado R, Abad-Guamán R, Nicodemus N, Diaz-Perales A, García J, Carabaño R, et al. Effect of pre- and post-weaning dietary supplementation with arginine and glutamine on rabbit performance and intestinal health. BMC Vet Res. (2019) 15:199. doi: 10.1186/s12917-019-1945-2
175. Dienz O, Rincon M. The effects of il-6 on cd4 t cell responses. Clin Immunol. (2009) 130:27–33. doi: 10.1016/j.clim.2008.08.018
176. Julio-Pieper M, Flor PJ, Dinan TG, Cryan JF. Exciting times beyond the brain: metabotropic glutamate receptors in peripheral and non-neural tissues. Pharmacol Rev. (2011) 63:35–58. doi: 10.1124/pr.110.004036
177. Lombardi G, Dianzani C, Miglio G, Canonico PL, Fantozzi R. Characterization of ionotropic glutamate receptors in human lymphocytes. Br J Pharmacol. (2001) 133:936–44. doi: 10.1038/sj.bjp.0704134
178. Xue H, Field CJ. New role of glutamate as an immunoregulator via glutamate receptors and transporters. Front Biosci. (2011) 3:1007–20. doi: 10.2741/s205
179. Pacheco R, Gallart T, Lluis C, Franco R. Role of glutamate on t-cell mediated immunity. J Neuroimmunol. (2007) 185:9–19. doi: 10.1016/j.jneuroim.2007.01.003
180. Lombardi G, Miglio G, Dianzani C, Mesturini R, Varsaldi F, Chiocchetti A, et al. Glutamate modulation of human lymphocyte growth: in vitro studies. Biochem Biophys Res Commun. (2004) 318:496–502. doi: 10.1016/j.bbrc.2004.04.053
181. Cahenzli J, Koller Y, Wyss M, Geuking MB, McCoy KD. Intestinal microbial diversity during early-life colonization shapes long-term ige levels. Cell Host Microbe. (2013) 14:559–70. doi: 10.1016/j.chom.2013.10.004
182. Hrncir T, Stepankova R, Kozakova H, Hudcovic T, Tlaskalova-Hogenova H. Gut microbiota and lipopolysaccharide content of the diet influence development of regulatory t cells: studies in germ-free mice. BMC Immunol. (2008) 9:65. doi: 10.1186/1471-2172-9-65
183. Ivanov II, Frutos RdL, Manel N, Yoshinaga K, Rifkin DB, Sartor RB, et al. Specific microbiota direct the differentiation of il-17-producing t-helper cells in the mucosa of the small intestine. Cell Host Microbe. (2008) 4:337–349. doi: 10.1016/j.chom.2008.09.009
184. Bisgaard H, Li N, Bonnelykke K, Chawes BL, Skov T, Paludan-Muller G, et al. Reduced diversity of the intestinal microbiota during infancy is associated with increased risk of allergic disease at school age. J Allergy Clin Immunol. (2011) 128:646–52.e641-645. doi: 10.1016/j.jaci.2011.04.060
185. Sjogren YM, Jenmalm MC, Bottcher MF, Bjorksten B, Sverremark-Ekstrom E. Altered early infant gut microbiota in children developing allergy up to 5 years of age. Clin Exp Allergy. (2009) 39:518–26. doi: 10.1111/j.1365-2222.2008.03156.x
186. Wang M, Karlsson C, Olsson C, Adlerberth I, Wold AE, Strachan DP, et al. Reduced diversity in the early fecal microbiota of infants with atopic eczema. J Allergy Clin Immunol. (2008) 121:129–34. doi: 10.1016/j.jaci.2007.09.011
187. Dong P, Feng J-j, Yan D-y, Lyu Y-j, Xu X. Early-life gut microbiome and cow's milk allergy- a prospective case - control 6-month follow-up study. Saudi J Biol Sci. (2018) 25:875–80. doi: 10.1016/j.sjbs.2017.11.051
188. Bjorksten B, Sepp E, Julge K, Voor T, Mikelsaar M. Allergy development and the intestinal microflora during the first year of life. J Allergy Clin Immunol. (2001) 108:516–20. doi: 10.1067/mai.2001.118130
189. Penders J, Gerhold K, Stobberingh EE, Thijs C, Zimmermann K, Lau S, et al. Establishment of the intestinal microbiota and its role for atopic dermatitis in early childhood. J Aller Clin Immunol. (2013) 132:601–7.e608. doi: 10.1016/j.jaci.2013.05.043
190. Penders J, Thijs C, van den Brandt PA, Kummeling I, Snijders B, Stelma F, et al. Gut microbiota composition and development of atopic manifestations in infancy: the koala birth cohort study. Gut. (2007) 56:661–7. doi: 10.1136/gut.2006.100164
191. Ling Z, Li Z, Liu X, Cheng Y, Luo Y, Tong X, et al. Altered fecal microbiota composition associated with food allergy in infants. Appl Environ Microbiol. (2014) 80:2546–54. doi: 10.1128/AEM.00003-14
192. Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell. (2005) 122:107–18. doi: 10.1016/j.cell.2005.05.007
193. Kim JY, Choi YO, Ji GE. Effect of oral probiotics (bifidobacterium lactis ad011 and lactobacillus acidophilus ad031) administration on ovalbumin-induced food allergy mouse model. J Microbiol Biotechnol. (2008) 18:1393–400.
194. Ren W, Duan J, Yin J, Liu G, Cao Z, Xiong X, et al. Dietary l-glutamine supplementation modulates microbial community and activates innate immunity in the mouse intestine. Amino Acids. (2014) 46:2403–13. doi: 10.1007/s00726-014-1793-0
195. de Souza AZ, Zambom AZ, Abboud KY, Reis SK, Tannihao F, Guadagnini D, et al. Oral supplementation with l-glutamine alters gut microbiota of obese and overweight adults: a pilot study. Nutrition. (2015) 31:884–9. doi: 10.1016/j.nut.2015.01.004
196. Zhang Y, Lu T, Han L, Zhao L, Niu Y, Chen H. L-glutamine supplementation alleviates constipation during late gestation of mini sows by modifying the microbiota composition in feces. Biomed Res Int. (2017) 2017:4862861. doi: 10.1155/2017/4862861
197. Chamorro S, de Blas C, Grant G, Badiola I, Menoyo D, Carabano R. Effect of dietary supplementation with glutamine and a combination of glutamine-arginine on intestinal health in twenty-five-day-old weaned rabbits. J Anim Sci. (2010) 88:170–80. doi: 10.2527/jas.2008-1698
198. van Nimwegen FA, Penders J, Stobberingh EE, Postma DS, Koppelman GH, Kerkhof M, et al. Mode and place of delivery, gastrointestinal microbiota, and their influence on asthma and atopy. J Allergy Clin Immunol. (2011) 128:948–55.e941-943. doi: 10.1016/j.jaci.2011.07.027
199. Dai ZL, Li XL, Xi PB, Zhang J, Wu G, Zhu WY. L-glutamine regulates amino acid utilization by intestinal bacteria. Amino Acids. (2013) 45:501–12. doi: 10.1007/s00726-012-1264-4
200. Feng Z-M, Li T-J, Wu L, Xiao D-F, Blachier F, Yin Y-L. Monosodium l-glutamate and dietary fat differently modify the composition of the intestinal microbiota in growing pigs. Obes Facts. (2015) 8:87–100. doi: 10.1159/000380889
201. Feng Z, Li T, Wu C, Tao L, Blachier F, Yin Y. Monosodium l-glutamate and dietary fat exert opposite effects on the proximal and distal intestinal health in growing pigs. Appl Physiol Nutr Metab. (2015) 40:353–63. doi: 10.1139/apnm-2014-0434
202. Berni Canani R, Sangwan N, Stefka AT, Nocerino R, Paparo L, Aitoro R, et al. Lactobacillus rhamnosus gg-supplemented formula expands butyrate-producing bacterial strains in food allergic infants. ISME J. (2016) 10:742–50. doi: 10.1038/ismej.2015.151
203. Fieten KB, Totté JEE, Levin E, Reyman M, Meijer Y, Knulst A, et al. Fecal microbiome and food allergy in pediatric atopic dermatitis: a cross-sectional pilot study. Int Arch Aller Immunol. (2018) 175:77–84. doi: 10.1159/000484897
204. Alameddine J, Godefroy E, Papargyris L, Sarrabayrouse G, Tabiasco J, Bridonneau C, et al. Faecalibacterium prausnitzii skews human dc to prime il10-producing t cells through tlr2/6/jnk signaling and il-10, il-27, cd39, and ido-1 induction. Front Immunol. (2019) 10:143. doi: 10.3389/fimmu.2019.00143
205. Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, et al. Induction of colonic regulatory t cells by indigenous clostridium species. Science. (2011) 331:337–41. doi: 10.1126/science.1198469
206. Hu C, Li F, Duan Y, Yin Y, Kong X. Glutamic acid supplementation reduces body fat weight in finishing pigs when provided solely or in combination with arginine and it is associated with colonic propionate and butyrate concentrations. Food Funct. (2019) 10:4693–704. doi: 10.1039/C9FO00520J
207. Vonk MM, Blokhuis BRJ, Diks MAP, Wagenaar L, Smit JJ, Pieters RHH, et al. Butyrate enhances desensitization induced by oral immunotherapy in cow's milk allergic mice. Mediators Inflamm. (2019) 2019:9062537. doi: 10.1155/2019/9062537
208. Aitoro R, Paparo L, Di Costanzo M, Nocerino R, Amoroso A, Amato F, et al. Breast milk butyrate as protective factor against food allergy. Dig Liver Dis. (2015) 47:e274. doi: 10.1016/j.dld.2015.07.150
209. Roduit C, Frei R, Ferstl R, Loeliger S, Westermann P, Rhyner C, et al. High levels of butyrate and propionate in early life are associated with protection against atopy. Allergy. (2019) 74:799–809. doi: 10.1111/all.13660
210. Agostoni C, Carratu B, Boniglia C, Riva E, Sanzini E. Free amino acid content in standard infant formulas: comparison with human milk. J Am Coll Nutr. (2000) 19:434–8. doi: 10.1080/07315724.2000.10718943
211. Ventura Alison K. Free amino acid content in infant formulas. Nutr Food Sci. (2012) 42:271–8. doi: 10.1108/00346651211248638
Keywords: human milk, free amino acids, glutamine, glutamate, neonates, immune development, allergies, infections
Citation: van Sadelhoff JHJ, Wiertsema SP, Garssen J and Hogenkamp A (2020) Free Amino Acids in Human Milk: A Potential Role for Glutamine and Glutamate in the Protection Against Neonatal Allergies and Infections. Front. Immunol. 11:1007. doi: 10.3389/fimmu.2020.01007
Received: 21 February 2020; Accepted: 28 April 2020;
Published: 28 May 2020.
Edited by:
Laxmi Yeruva, University of Arkansas for Medical Sciences, United StatesReviewed by:
Barbara Wróblewska, Institute of Animal Reproduction and Food Research (PAN), PolandJulio Villena, CONICET Centro de Referencia para Lactobacilos (CERELA), Argentina
Copyright © 2020 van Sadelhoff, Wiertsema, Garssen and Hogenkamp. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Joris. H. J. van Sadelhoff, ai5oLmoudmFuc2FkZWxob2ZmQHV1Lm5s
 Joris H. J. van Sadelhoff
Joris H. J. van Sadelhoff Selma P. Wiertsema2
Selma P. Wiertsema2 Johan Garssen
Johan Garssen Astrid Hogenkamp
Astrid Hogenkamp