
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Immunol., 05 June 2020
Sec. Microbial Immunology
Volume 11 - 2020 | https://doi.org/10.3389/fimmu.2020.00972
This article is part of the Research TopicImmunity in Compromised NewbornsView all 30 articles
 Eduardo Villamor-Martinez
Eduardo Villamor-Martinez George A. Lubach
George A. Lubach Owais Mohammed Rahim
Owais Mohammed Rahim Pieter Degraeuwe
Pieter Degraeuwe Luc J. Zimmermann
Luc J. Zimmermann Boris W. Kramer
Boris W. Kramer Eduardo Villamor*
Eduardo Villamor*Chorioamnionitis (CA) is considered a key risk factor for very preterm birth and for developing early onset sepsis (EOS) in preterm infants, but recent data suggest that CA might be protective against late onset sepsis (LOS). We performed a systematic review and meta-analysis of studies exploring the association between CA and sepsis. A comprehensive literature search was performed in PubMed/MEDLINE and EMBASE, from their inception to December 1, 2018. A random-effects model was used to calculate odds ratios (OR) and 95% confidence intervals (CI). Sources of heterogeneity were analyzed by subgroup and meta-regression analyses. The following categories of sepsis were analyzed: EOS, LOS, unspecified onset sepsis (UOS), culture-proven, and clinical sepsis. CA was subdivided into clinical and histological chorioamnionitis. Funisitis was also analyzed. We found 3,768 potentially relevant studies, of which 107 met the inclusion criteria (387,321 infants; 44,414 cases of CA). Meta-analysis showed an association between any CA and any EOS (OR 4.29, CI 3.63–5.06), any LOS (OR 1.29, CI 1.11–1.54), and any UOS (OR 1.59, CI 1.11–1.54). Subgroup analysis showed that CA was associated with culture-proven EOS (OR 4.69, CI 3.91–5.56), clinical EOS (OR 3.58, CI 1.90–6.76), and culture-proven LOS (OR 1.31, CI 1.12–1.53), but not with clinical LOS (OR 1.52, CI 0.78–2.96). The presence of funisitis did not increase the risk of either EOS or LOS when compared with CA without funisitis. CA-exposed infants had lower gestational age (−1.11 weeks, CI −1.37 to −0.84) than the infants not exposed to CA. Meta-regression analysis showed that the lower gestational age of the CA group correlated with the association between CA and LOS but not with the association between CA and EOS. In conclusion, our data suggest that the positive association between chorioamnionitis and LOS may be modulated by the effect of chorioamnionitis on gestational age.
Very preterm birth is defined by a gestational age (GA) below 32 weeks, and extremely preterm birth is defined by a GA below 28 weeks (1). The etiological background of very/extremely preterm birth can be divided into two main categories: intrauterine infection/inflammation and placental vascular dysfunction (2–4). The first category is associated with chorioamnionitis (CA), preterm labor, premature rupture of membranes (PROM), placental abruption, and cervical insufficiency, whereas the second category is associated with gestational hypertensive disorders and condition known as fetal indication/fetal growth restriction (2–4). Belonging to the first group, CA is the maternal response to an intrauterine infection/inflammation and implies the presence of inflammatory cells in the extraplacental membranes (chorion and amnion) (5, 6). Acute CA generally represents the presence of intraamniotic infection or “amniotic fluid infection syndrome” but can also occur in the in the absence of proven infection (6). This may partly be due to lack of detection of some bacterial species by the culturing methods routinely employed (7, 8).
CA is not only considered to be a leading cause of very/extremely preterm birth but also a main factor in the development of subsequent neonatal complications (9–11). Numerous individual studies and meta-analyses have addressed the association between CA and complications of very/extremely preterm birth such as bronchopulmonary dysplasia (12, 13), necrotizing enterocolitis (14), retinopathy of prematurity (15, 16), patent ductus arteriosus (17, 18), intraventricular hemorrhage (19), cerebellar hemorrhage (20), neonatal brain injury (21), or cerebral palsy (22).
Very/extremely preterm infants are at high risk for neonatal sepsis (23–27). Early onset sepsis (EOS) is defined as a blood or cerebrospinal fluid culture obtained within 72 h after birth. EOS is typically caused by microorganisms transmitted vertically from the mother to the infant before birth or during delivery (23, 24). Frequently, preterm EOS begins in the uterus and the microbial-induced maternal inflammation initiates labor and elicits an inflammatory response in the fetus (23–25). Therefore, CA and/or intraamniotic infection are strongly associated with EOS in preterm infants (23–25).
Late-onset sepsis (LOS) occurs after 72 h of life and may be caused by microorganisms acquired at delivery or during the course of hospital care (23, 25). Very/extremely preterm infants are at an increased risk of LOS because of the relative immaturity of their immune system as well as the frequently required prolonged hospitalization, with ongoing risk of infection, and exposure to invasive procedures and devices (23, 25, 26, 28). Coagulase-negative staphylococci (CoNS) are the most frequent pathogens causing nosocomial sepsis among preterm infants. Interestingly, Strunk et al. reported in a cohort of infants with a GA below 30 weeks that histological CA was associated with reduced risk of developing LOS, both with CoNS and other bacteria (25). They speculate that “chorioamnionitis may result in maturation of the fetal and neonatal immune system and therefore indirectly modulates the risk of LOS with nosocomial organisms” (25).
Surprisingly, to the best of our knowledge, the association between CA and neonatal sepsis has not yet been the subject of a systematic review. We therefore aimed to carry out a systematic review and meta-analysis of observational studies reporting on the association between CA and EOS and/or LOS in preterm infants. We paid particular attention to how the criteria used to define CA and sepsis affected the potential association between the two conditions. We also analyzed the role of potential confounders or intermediate factors, such as GA, birth weight (BW), presence of fetal inflammatory response (i.e., funisitis), or exposure to antenatal corticosteroids, on the association between CA and neonatal sepsis.
We used a similar methodology to earlier meta-analyses on the association of CA and short-term outcomes of prematurity (13, 16, 17, 19). A protocol (available at https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=117190) was developed and published a priori in which the objectives, inclusion criteria, method for evaluating study quality, included outcomes and covariates, and statistical methodology were specified (29) We report the study according to the guidelines for the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) (30).
A comprehensive literature search was performed in the PubMed/MEDLINE and EMBASE databases from their inception to December 1, 2018. The search strategy involved the following keywords in various combinations: “chorioamnionitis,” “intrauterine infection,” “intrauterine inflammation,” “antenatal infection,” antenatal inflammation,” “funisitis,” “preterm infant,” “prematurity,” “cohort,” “sepsis,” “(neonatal) early-onset sepsis,” and “(neonatal) late-onset sepsis.” The full search strategy can be found in the Supplementary Data 1.
Studies were included if they examined preterm (GA <37 weeks) infants and reported primary data that could be used to measure the association between exposure to CA and the development of neonatal sepsis. Studies using BW instead of GA as the inclusion criteria were included only when inclusion BW was below 1,500 g. Therefore, we selected studies assessing the outcomes of infants exposed to CA when sepsis was one of the reported outcomes, and studies assessing the risk factors for sepsis when CA was one of the reported risk factors. We also included studies reporting on intra-amniotic infection resulting from microbial invasion of the amniotic cavity (MIAC) because MIAC is also within the spectrum of CA (31). MIAC was defined as a positive amniotic fluid culture for microorganisms. The results of the total search were screened independently by two reviewers (G. A. L. and O. M. R.) in several rounds: first by title only, second by title and abstract and thirdly by consulting the full text. The reviewers resolved discrepancies in inclusion through discussion and by consulting a third reviewer (P. D.).
Utilizing a predetermined worksheet, data was extracted from the included studies by three researchers (G. A. L., O. M. R., and E. V.-M). Two additional researchers (P. D. and E. V.) checked the extracted data for accuracy and completeness. Discrepancies were resolved by checking the primary data report and by discussion. We extracted the following data from each study: citation information, the language of the publication, the location where research was conducted, the time period of the study, study objectives, study design, inclusion/exclusion criteria, patient characteristics, definitions of CA (clinical, histological, or microbiological), definitions of sepsis (culture-proven or clinical), and results (including raw numbers, summary statistics, and adjusted analyses on CA and sepsis where available). Onset of sepsis was classified in three groups: EOS, LOS, and unspecified onset sepsis (UOS).
The Newcastle-Ottawa Scale (NOS) for cohort or case-control studies was used to assess the methodological quality of the included studies (32). The NOS evaluates three aspects of a given study: selection, comparability, and exposure/outcome. These are scored individually and tallied up to a possible total of 9 points. The NOS was independently used by two researchers (G. A. L. and E. V.-M) to evaluate the quality of each study. Discrepancies were resolved by reaching consensus through discussion.
Studies were combined and analyzed using COMPREHENSIVE META-ANALYSIS V 3.0 software (CMA, RRID:SCR_012779, Biostat Inc., Englewood, NJ, USA). The odds ratio (OR) and the 95% confidence interval (CI) for dichotomous variables were calculated from the extracted data of the studies. For continuous variables, the mean difference (MD) was calculated together with the 95% CI. When studies reported continuous variables as the median and the range/interquartile range, we estimated the mean and standard deviation using the method of Wan et al. (33). Due to anticipated heterogeneity, summary statistics were calculated with a random-effects model. This accounts for the variability between studies as well as within studies. For subgroup analyses the mixed-effects model was used (34). With this approach, a random-effects model is used within each subgroup, while a fixed-effect model is used to combine subgroups to generate the overall effect. The study-to-study variance (tau-squared) was calculated across all studies. Statistical heterogeneity was tested using Cochran's Q statistic and the I2 statistic (34). Publication bias was assessed with Egger's regression test (35) and visual inspection of funnel plots. K represents the number of studies used in each analysis.
Univariate random-effects meta-regression was used to explore whether the differences in covariates between studies might influence the outcome effect size (34). We carried out meta-regression analysis only if there were more than 10 studies that reported on a covariate. The following possible sources of variability were defined beforehand: CA type (clinical or histological), sepsis type (culture-proven or clinical), differences in GA and BW between infants with and without CA, use of antenatal corticosteroids, mode of delivery, rate of GA, rate of PROM, and rate of preeclampsia.
We screened 3,768 studies after removing duplicates, of which 107 studies met the inclusion criteria (4, 25, 36–141). The PRISMA flow diagram of the search is shown in Figure 1. The included studies evaluated 387,321 infants, including 44,414 infants with CA. An overview of the characteristics of the included studies can be found in Supplementary Table 1. There were 75 studies that evaluated the outcomes of CA, and sepsis was one of these outcomes, and 26 studies that looked at potential risk factors for sepsis, including CA. Seven studies were designed to primarily examine the association between CA and sepsis. From the included studies, 58 used a histological definition of CA and 28 studies used a clinical definition of CA. Nine studies distinguished between both definitions of CA in their reporting (36, 45, 65, 80, 93, 97, 104, 106, 108). Five studies reported on MIAC (77, 83, 84, 105, 113). In six studies, the CA definition was not further specified and categorized as “unspecified” for further analysis (37, 42, 75, 103, 129). Nine studies (37, 46, 77, 98, 115, 118, 129, 139, 141) used the 7-day limit to differentiate between EOS and LOS.
A summary of the NOS quality assessment can be found in Supplementary Table 2. Four studies received a quality score of 5 points, 24 studies a score of 6 points, 57 studies a score of 7 points, 9 studies a score of 8 points and 13 studies received a score 9 points. Studies lost points for quality for not adjusting the risk of sepsis for confounders (k = 90), for not defining sepsis clearly (k = 6), for not defining CA clearly (k = 21), and for adjusting the risk of sepsis only for one confounder (k = 6).
As shown in Figure 2A, meta-analysis found a positive association between CA (any type) and EOS (any type) (k = 70, OR 4.29, 95% CI 3.63–5.06). When subdividing by CA definition, meta-analysis showed that histological CA (k = 43, OR 3.46, 95% CI 2.74–4.4.38, Figure 3), clinical CA (k = 20, OR 4.76, 95% CI 3.68–6.15, Figure 4), histological/clinical CA (k = 1, OR 6.17, 95% CI 1.18–32.23, Figure 4), MIAC (k = 3, OR 5.38, 95% CI 1.76–16.44, Figure 4), and unspecified CA (k = 3, OR 14.14, 95% CI 6.46–30.95, Figure 4) were associated with EOS (any type). The exclusion of the studies reporting on MIAC, combined clinical/histological CA, and unspecified CA did not substantially affect the OR of the association between CA and EOS (Table 1).
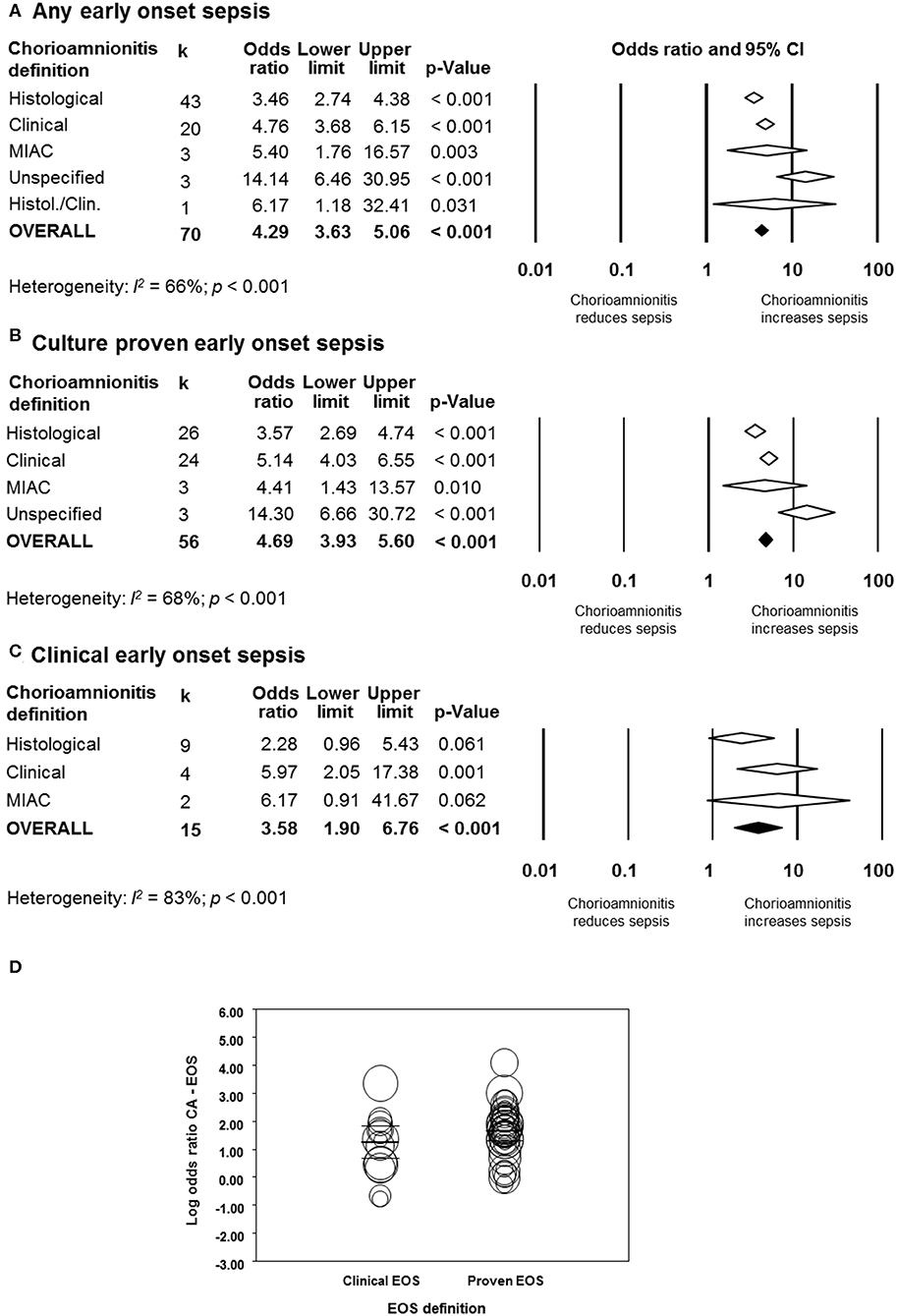
Figure 2. Random effects meta-analyses of chorioamnionitis and early onset sepsis (EOS), subdivided by definition of chorioamnionitis. (A) Any EOS; (B) culture-proven EOS; (C) clinical EOS; (D) meta-regression comparing culture-proven and clinical EOS. MIAC, microbial invasion of the amniotic cavity.
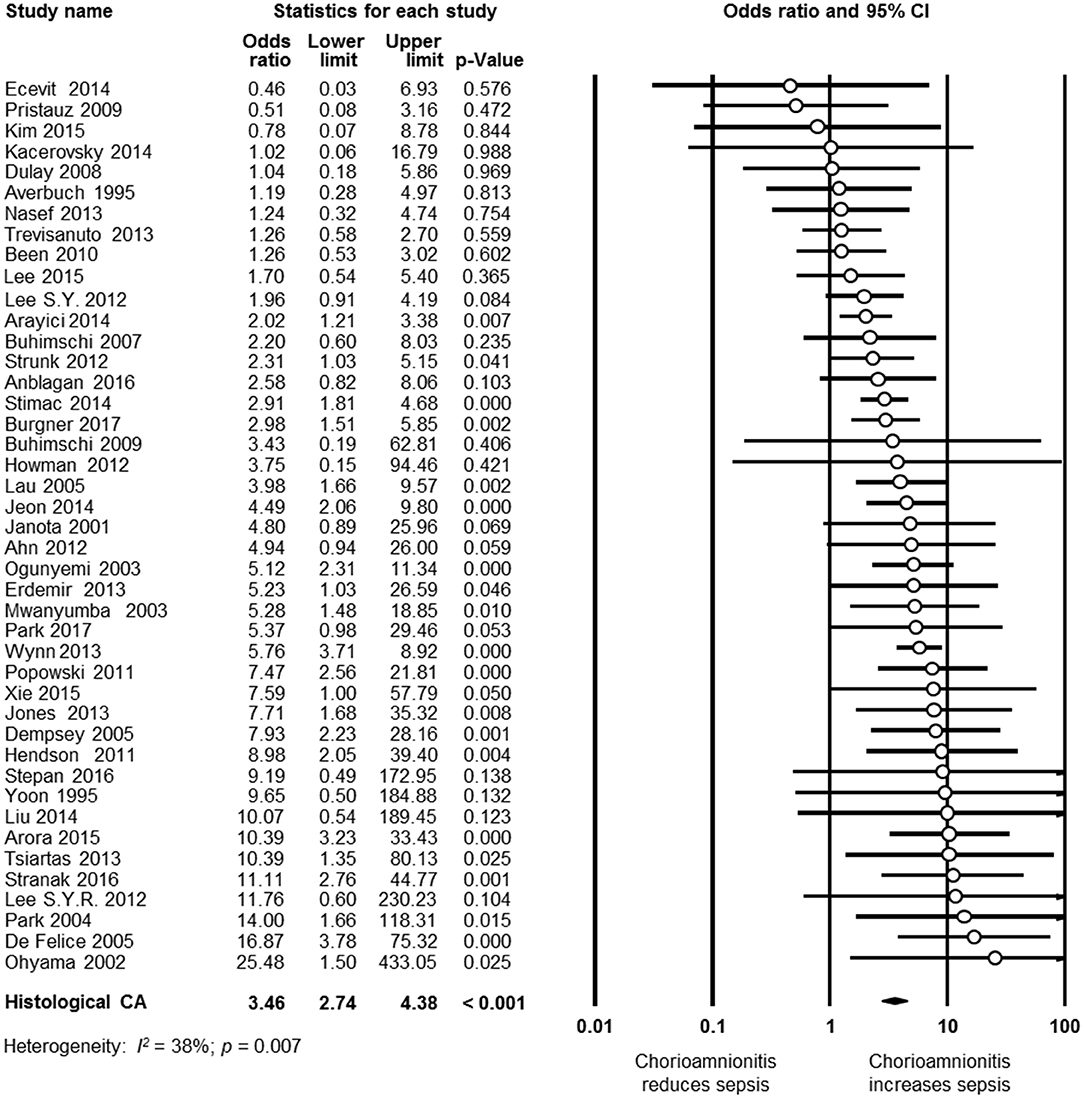
Figure 3. Random effects meta-analysis of histological chorioamnionitis (CA) and all early onset sepsis (culture proven or clinical).
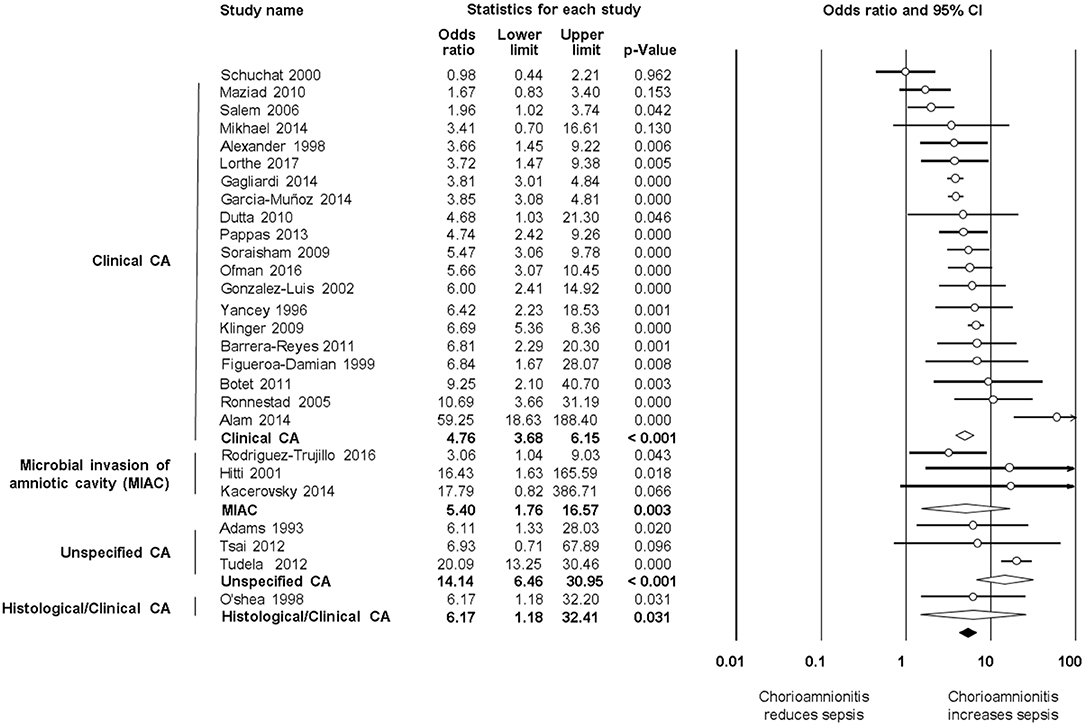
Figure 4. Random effects meta-analyses of clinical, microbiological, unspecified, and histological/clinical chorioamnionitis (CA) and all early onset sepsis (culture proven or clinical).
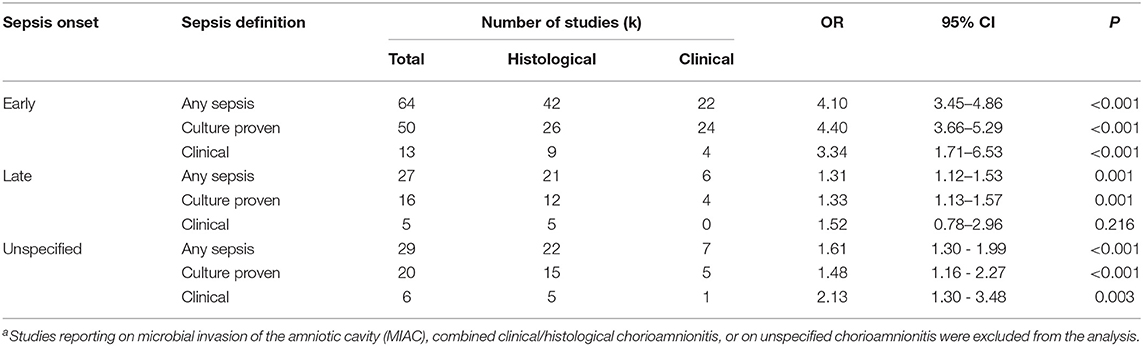
Table 1. Meta-analysis on the association between any chorioamnionitisa and sepsis, divided by onset, and definition of sepsis.
When subdividing by EOS definition, meta-analysis showed that CA (any type) was associated with culture-proven EOS (k = 56, OR 4.69, 95% CI 3.93–5.60, Figure 2B) and clinical EOS (k = 15, OR 3.58, 95% CI 1.90–6.76, Figure 2C). As shown in Figure 2B, subgroup analysis based on CA type showed that histological CA (k = 26, Supplementary Figure 1), clinical CA (k = 24 Supplementary Figure 2), MIAC (k = 3, Supplementary Figure 2), and unspecified CA (k = 3, Supplementary Figure 2) were associated with culture-proven EOS. In contrast, as shown in Figure 2C and Supplementary Figure 3, clinical EOS was associated with clinical CA (k = 4), but not with histological CA (k = 9), or MIAC (k = 2). Meta-regression could not find differences between the effect size of the association CA-culture-proven EOS and the effect size of the association CA-clinical EOS (p = 0.150, Figure 2D). There was no evidence of publication bias for studies reporting on EOS (Supplementary Figure 4), assessed with Egger's regression test and visual inspection of the funnel plots.
As shown in Figures 5A, 6, meta-analysis found a positive association between CA (any type) and LOS (any type) (k = 29, OR 1.29, 95% CI 1.11–1.54). When subdividing by CA definition, meta-analysis showed that histological CA (k = 21, OR 1.38, 95% CI 1.13–1.68, Figure 6), and MIAC (k = 1, OR 9.90, 95% CI 1.90–51.54, Figure 6) were associated with LOS (any type). In contrast, subgroup analysis could not find an association between LOS (any type) and clinical CA (k = 6, OR 1.21, 95% CI 0.95–1.55, Figure 6), or unspecified CA (k = 1 OR 0.61, 95% CI 0.28–1.33, Figure 6). The exclusion of the studies reporting on MIAC, combined clinical/histological CA, and unspecified CA did not substantially affect the OR of the association between CA and LOS (Table 1).
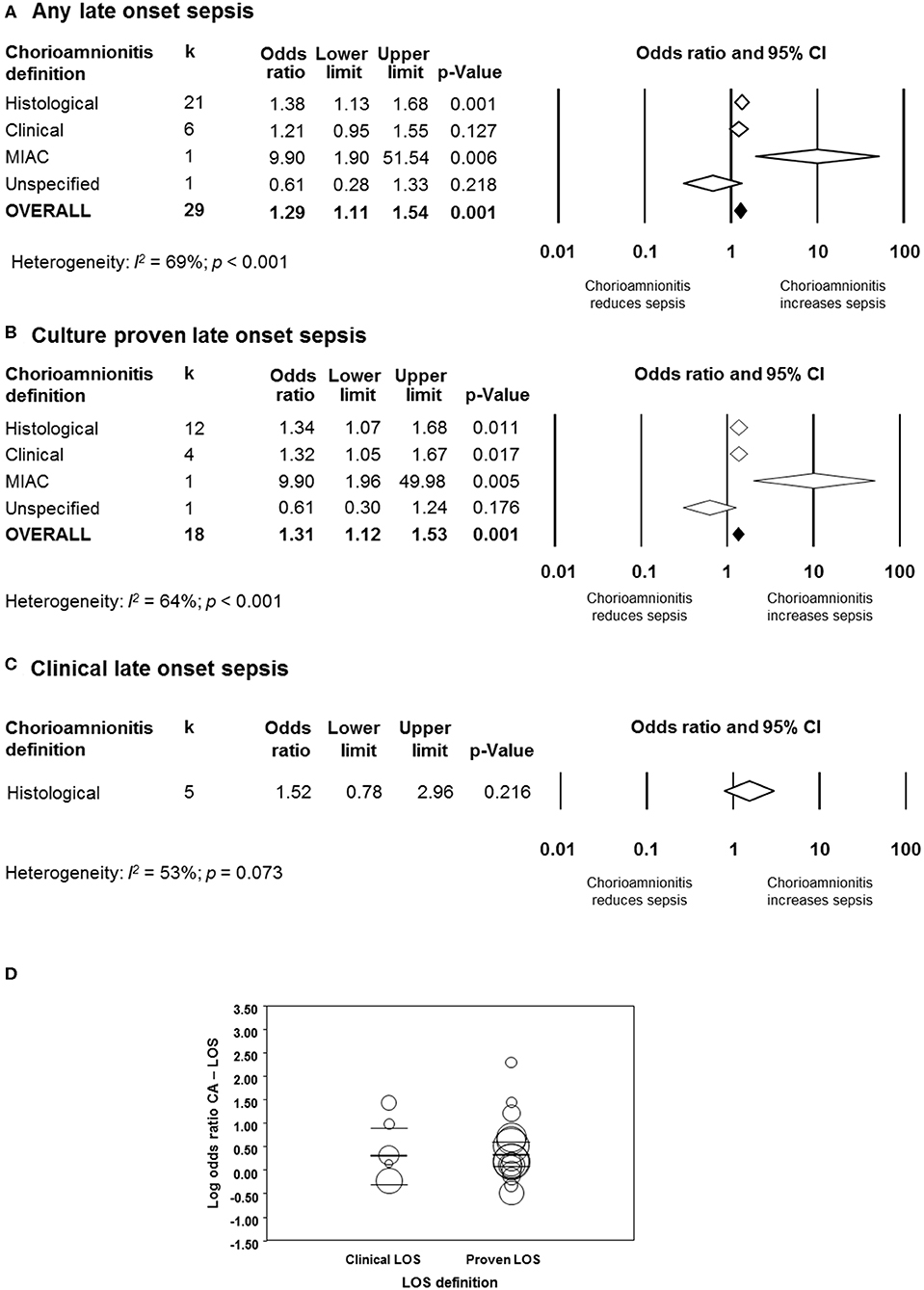
Figure 5. Random effects meta-analyses of chorioamnionitis and late onset sepsis (LOS), subdivided by definition of chorioamnionitis. (A) Any LOS; (B) culture-proven LOS; (C) clinical LOS; (D) meta-regression comparing culture-proven and clinical LOS. MIAC, microbial invasion of the amniotic cavity.
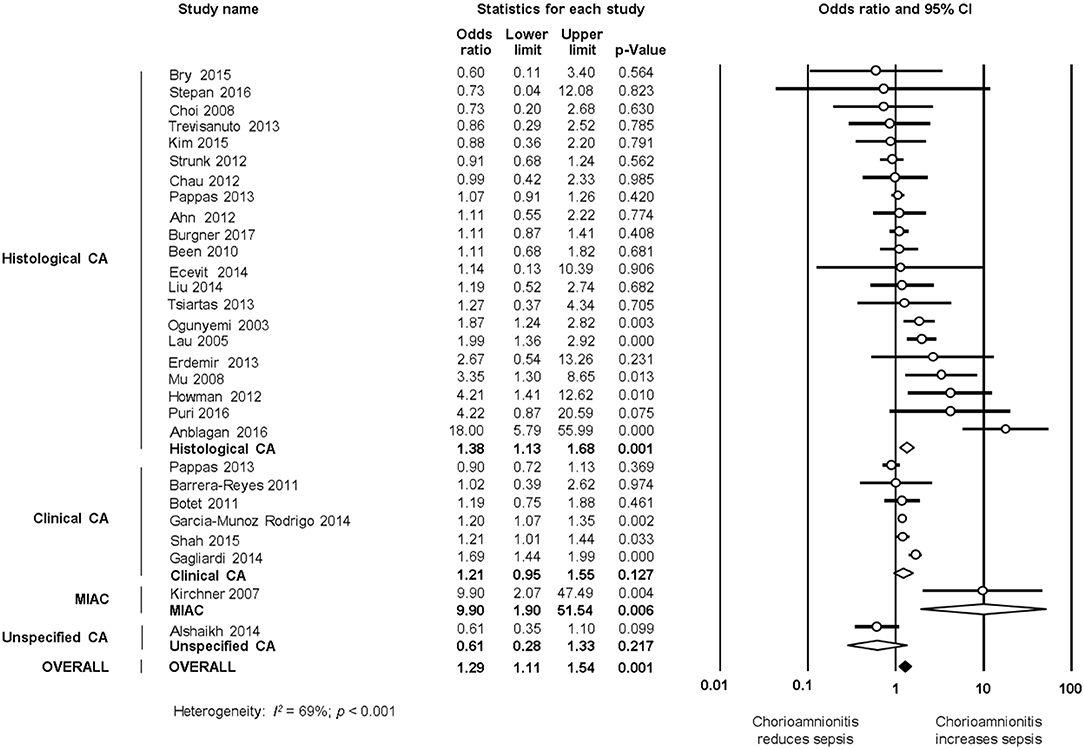
Figure 6. Random effects meta-analyses of histological, clinical, microbiological, and unspecified chorioamnionitis (CA) and all late onset sepsis.
When subdividing by LOS definition, meta-analysis showed that CA (any type) was associated with culture-proven LOS (k = 18, OR 1.31, 95% CI 1.12–1.53, Figure 5B and Supplementary Figure 5), but not with clinical LOS (k = 5, OR 1.52, 95% CI 0.78–2.96, Figure 5C and Supplementary Figure 6). The analysis on the association between clinical LOS and CA was exclusively based on data on histological CA. As shown in Figure 5B and Supplementary Figure 5, subgroup analysis based on CA type showed that histological CA (k = 12), clinical CA (k = 4), and MIAC (k = 1) were associated with culture-proven LOS. Meta-regression could not find differences between the effect size of the association CA-culture-proven LOS and the effect size of the association CA-clinical LOS (p = 0.920, Figure 5D). There was no evidence of publication bias for studies reporting on LOS (Supplementary Figure 4), assessed with Egger's regression test and visual inspection of funnel plots.
As shown in Figure 7A and Supplementary Figure 7, meta-analysis found a significant positive association between CA (any type) and UOS (any type) (k = 31, OR 1.59, 95% CI 1.11–1.54). When subdividing by CA definition, meta-analysis showed that histological CA (k = 22, OR 1.61, 95% CI 1.30–1.99), and clinical CA (k = 7, OR 1.63, 95% CI 1.16–2.29) were significantly associated with UOS (any type, Supplementary Figure 7).
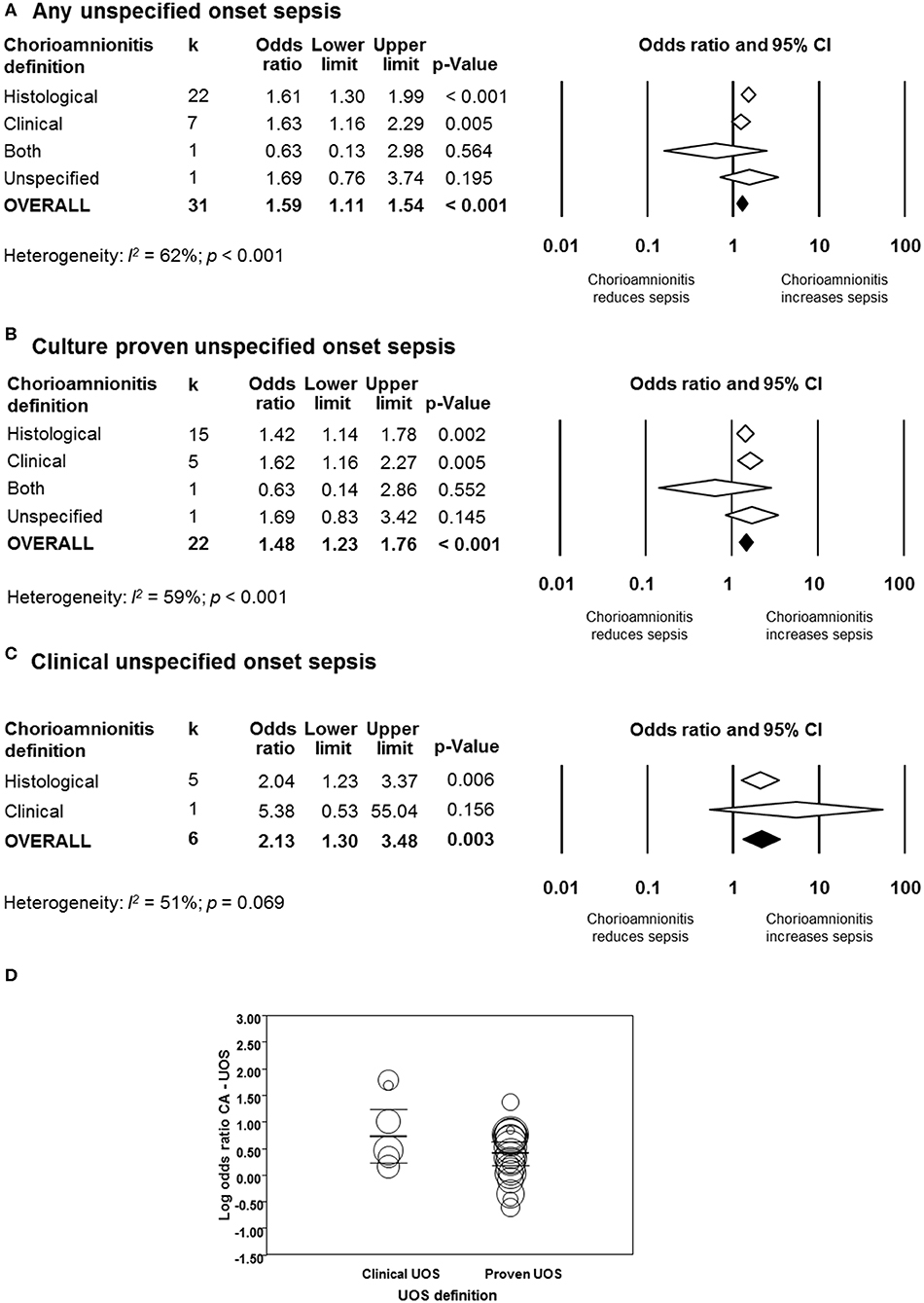
Figure 7. Random effects meta-analyses of unspecified onset sepsis (UOS), subdivided by definition of chorioamnionitis. (A) Any UOS; (B) culture-proven UOS; (C) clinical UOS; (D) meta-regression comparing culture-proven and clinical UOS.
When subdividing by UOS definition, meta-analysis showed that CA (any type) was associated with culture-proven UOS (k = 22, OR 1.48, 95% CI 1.23–1.76, Figure 7B and Supplementary Figure 8) and clinical UOS (k = 6, OR 2.13, 95% CI 1.30–3.48, Figure 7C and Supplementary Figure 9). As shown in Figure 7B and Supplementary Figure 8, subgroup analysis based on CA type showed that histological CA (k = 15), and clinical CA (k = 5) were associated with culture-proven UOS. In contrast, only histological CA was associated with clinical UOS (k = 5, Figure 7C and Supplementary Figure 9). Meta-regression could not find differences between the effect size of the association CA-culture proven UOS and the effect size of the association CA-clinical UOS (Figure 7D). There was no evidence of publication bias for studies reporting on UOS (Supplementary Figure 4), assessed with Egger's regression test and visual inspection of funnel plots.
Additional meta-analyses were performed to determine if funisitis, as a fetal-inflammatory response, was associated with the development of neonatal sepsis. As shown in Figure 8, 10 studies reported on EOS and infants with histological CA with or without funisitis; eight studies on LOS and infants with histological CA with or without funisitis; and five studies on UOS and infants with histological CA with or without funisitis. Meta-analysis showed that funisitis did not increase the risk of sepsis (EOS, LOS, or UOS), when compared with CA without funisitis (Figure 8).
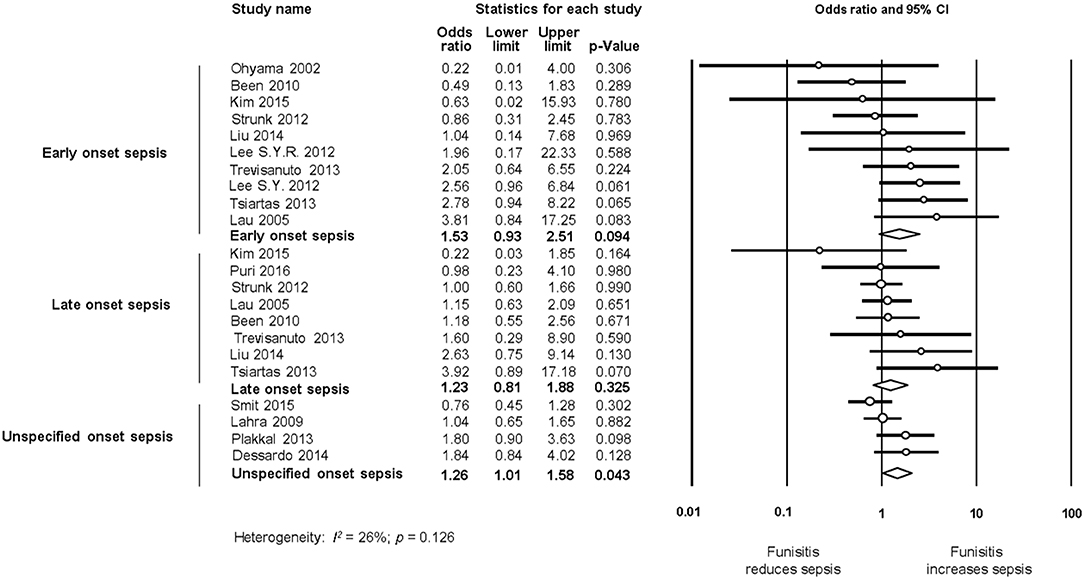
Figure 8. Random effects meta-analyses of funisitis (compared with chorioamnionitis without funisitis) and sepsis (culture proven or clinical), subdivided by onset of sepsis.
We performed additional meta-analyses to explore the possible differences in baseline characteristics between the groups exposed and non-exposed to CA. Exposure to CA was significantly associated with lower GA and BW, as shown in Table 2. Additionally, when compared with CA-unexposed, CA-exposed infants had significantly higher rates of exposure to antenatal corticosteroids and PROM, and significantly increased rates of mortality, but significantly lower rates of preeclampsia, cesarean section, or small for GA (Table 3).
Meta-regression was performed to determine the potential modulatory effect of GA and BW on the association between sepsis and CA. As shown in Figure 9A, the effect size of the association between CA and EOS was not affected by the differences in GA between the CA-exposed and CA-unexposed group (R2 = 0.0; p = 0.490). In contrast, meta-regression showed that mean differences in GA modified the effect size of the association between CA and LOS (R2 = 0.54, p = 0.001, Figure 9B). Meta-regression for mean differences in BW could not show a correlation with the effect size of the association CA-EOS (Supplementary Figure 10A) or with the effect size of the association CA-LOS (Supplementary Figure 10B). Meta-regressions of the associations CA-EOS and CA-LOS with other covariates (antenatal corticosteroids, cesarean section, small for GA, PROM) did not show correlations with these covariates (Supplementary Table 2).
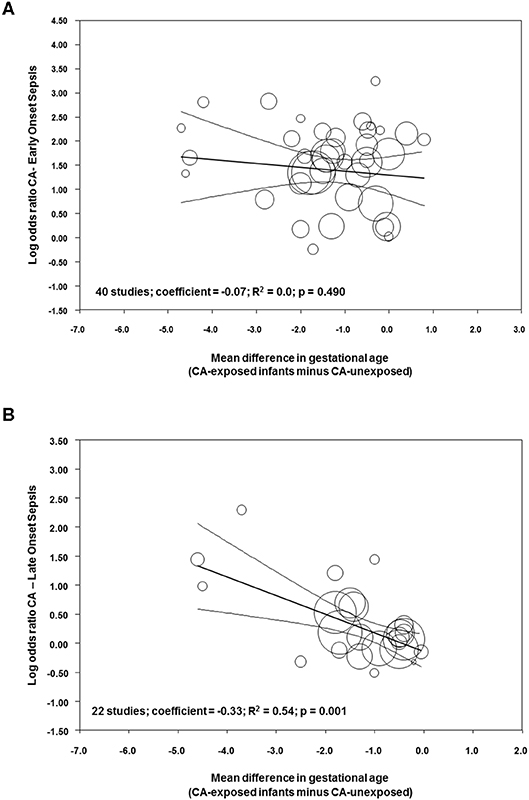
Figure 9. Meta-regression plot of association between chorioamnionitis and (A) early onset sepsis, and (B) late onset sepsis controlling for difference in gestational age between exposed and non-exposed groups.
To further assess the effect of GA on the association CA-sepsis, we pooled the studies where the mean difference in GA was, according to the classical threshold of p-value (p ≥ 0.05) (142), “non-significant” and compared it with meta-analysis of studies where the mean difference in GA was “statistically significant” (p < 0.05). Meta-analysis found that CA was a risk factor for EOS both in studies where infants had a similar GA across groups (k = 15, OR 3.76, 95% CI 2.66–5.32, Supplementary Figure 11), and in studies where infants had significantly lower GA in the CA group (k = 22, OR 3.95, 95% CI 3.11–5.02, Supplementary Figure 11). In contrast, the association between CA and LOS was only observed in the subgroup meta-analysis of studies where CA-exposed infants had lower GA (k = 16, OR 1.33, 95% CI 1.11–1.60, Supplementary Figure 12), but not in the subgroup of studies where infants had similar GA across groups (k = 7, OR 1.06, 95% CI 0.72–1.57, Supplementary Figure 12).
We pooled studies that provided adjusted data on the association between CA and sepsis. Meta-analysis of adjusted data found an association between CA and EOS (k = 15, OR 2.51, 95% CI 1.51–4.14, Supplementary Figure 13), but not between CA and LOS (k = 7, OR 1.04, 95% CI 0.79–1.38, Supplementary Figure 14). Meta-analysis of unadjusted data of the same group of studies found that CA was associated with both EOS (OR 5.10, 95% CI 3.07–8.46, Supplementary Figure 13) and LOS (OR 1.42, 95% CI 1.08–1.87, Supplementary Figure 14).
This is the first meta-analysis investigating the association between CA and sepsis in preterm infants. The meta-analysis based on unadjusted data showed a strong association between CA and EOS and a weaker, but still positive, association between CA and LOS. Exposure to funisitis was not associated with a higher risk of sepsis when compared with exposure to CA in the absence of funisitis. As in previous meta-analysis on the association between CA and short term outcomes of prematurity (12, 13, 16, 17, 19), we observed differences in basal characteristics between the CA-exposed and the CA-unexposed group. CA was associated with a lower GA and BW, higher rates of exposure to antenatal corticosteroids, and the preterm rupture of membranes, as well as lower rates of cesarean section and small for gestational age. Meta-regression analysis showed that the lower GA of the CA group correlated with the association between CA and LOS but not with the association between CA and EOS. Therefore, the pathogenic effect of CA on LOS appears to be modulated by the effect of chorioamnionitis on GA.
We chose to combine studies by using ORs rather than risk ratios (RRs) because it allowed us to compare the unadjusted ORs with the adjusted ORs reported in some studies. However, while the RR has a relatively simple interpretation, OR interpretation is less intuitive, as the concept of “odds” is less easy to grasp (143, 144). Interpretation of ORs in the same way as RRs can lead to overestimation of effect sizes when the risk in either group is high (above 20%) and the OR is large (144). In order to check if there were important discrepancies between ORs and RRs in our study, we calculated the RRs for some analyses. In the case of the association between CA and EOS, the RR was 3.45, whereas the OR was 4.29 (~24% higher). In the case of the association between CA and LOS, the RR was 1.21 whereas the OR was 1.29 (~6.6% higher). Therefore, in CA-exposed infants, the risk of EOS was 3.5-fold higher and the risk of LOS was 1.2-fold higher than the respective risk in infants non-exposed to CA. These increases in risk were slightly overestimated by the OR.
Since very/extremely preterm birth is always a pathological condition, any study aimed to analyze the association between its etiology and its outcome will face the limitation of the absence of a healthy control group (145). As mentioned in the introduction, very/extremely preterm birth etiology can be divided into two main categories: intrauterine infection/inflammation and placental vascular dysfunction (2–4). The distribution of these two etiological entities across the different gestational ages is not uniform: the lower the GA, the greater the possibility that an infectious/inflammatory process is the trigger for preterm birth (2, 146). Our meta-analysis showed that infants exposed to CA were born ~1.1 weeks earlier than “control” infants. Meta-regression showed that this difference in GA did not affect the association between CA and EOS but modulated the association between CA and LOS. The difference in GA was associated with 54% (R2) of the variance in the association between CA and LOS across studies and each week that infants with CA were born earlier than control infants resulted in an increase in LOS log OR of 0.33 (the equivalent of going from an OR of 1.00 to an OR of 2.14). This modulatory role of GA may be related to the maturation of the immunological system (26, 147), but also to the fact that CA-exposed preterm infants would require longer hospitalization and more days of invasive therapies, rendering them more susceptible to LOS.
Besides the meta-regression analysis, other data of the present study underline the modulatory role of GA on the association between CA and LOS. When we performed a subgroup analysis of studies without a significant difference (p > 0.05) in GA between the CA-exposed group and the CA-unexposed group, the association between CA and EOS was still strong (odds ratio 3.76), but the association between CA and LOS could not be demonstrated. When we pooled the few studies that corrected for GA, as well as for other potential confounders, the association between CA and EOS was tempered but still positive, and the association between CA and LOS could not be further demonstrated. This effect of the use of adjusted data has been previously described in meta-analyses on the association between CA and bronchopulmonary dysplasia (12, 13), cerebral palsy (22), retinopathy of prematurity (16), patent ductus arteriosus (17), and intraventricular hemorrhage (19). Moreover, the decreased risk of LOS in CA-exposed infants previously reported by Strunk et al. was only observed after correction for GA (25). Adjustment for GA is commonly used in observational studies examining predictors of outcomes in preterm infants (3, 148, 149). However, GA may represent both a risk factor per se and a mediator in the causal pathway linking cause of preterm birth (i.e., CA) to outcome (i.e., LOS). As pointed out by Ananth and Schisterman, GA is often mislabeled as a confounder when it may be an intermediate (149). Adjustment for GA in the presence of unmeasured factors that may affect both GA and neonatal outcome, may result in bias (3, 148–151). This bias may even change the direction of estimates, unless all mediator–outcome confounders are taken into account in the analysis, a condition that is unlikely to be achieved (3, 148–152). Therefore, observational studies and meta-analyses of observational studies that analyze the association between conditions like CA or pre-eclampsia and outcome of preterm birth provide valuable information for descriptive or prognostic purposes. However, inferring causal effects is not possible (145, 149).
There is a reasonable biological plausibility for the immunomodulatory role of intrauterine infection/inflammation on the etiopathogenesis of neonatal sepsis. Nevertheless, our comprehension of the mechanisms that link intrauterine infection/inflammation and preterm birth is still incomplete. The maternal lower genital tract is generally considered to be the primary source of bacteria, but other potential sources, such as the oral cavity, need to be considered (7, 8). The microorganisms more frequently cultivated from amniotic fluid in pregnancies complicated by preterm birth are Ureaplasma urealyticum, Mycoplasma hominis, Fusobacterium nucleatum, Gardnerella vaginalis, and Bacteroides spp. (7, 8). These microorganisms are able to stimulate the intrauterine inflammatory process but have relatively low virulence and rarely induce EOS, which is mainly related to such bacteria as group B streptococcus or Escherichia coli (153). In addition, an important proportion of the organisms associated with intra-amniotic infection are uncultivated or difficult-to-cultivate bacteria (7, 8). Nevertheless, as suggested by Strunk et al., even culture-negative, asymptomatic CA that does not result in EOS might lead to a persistent alteration of the neonatal immune system (11). Accordingly, evidence from animal and human studies supports that CA could diminish innate immune responses and thereby increase the susceptibility of preterm infants to LOS (154–157).
Not all the situations of intrauterine infection/inflammation will lead to an inflammatory process reaching the fetus (158). Funisitis is considered the histologic counterpart of the fetal inflammatory response syndrome (9, 158). Our meta-analysis could not demonstrate a stronger association between funisitis and the risk of developing neonatal sepsis (EOS, LOS, or UOS), when compared with CA in the absence of funisitis (Figure 8). This is an argument against the role of the immunomodulation induced by the fetal inflammatory response in the etiopathogenesis of neonatal sepsis. Similarly, we observed in previous meta-analyses that funisitis was not an additional risk factor for developing intraventricular hemorrhage (19), patent ductus arteriosus (17), respiratory distress syndrome (13), or bronchopulmonary dysplasia (13) in preterm infants. In contrast, funisitis significantly increased the risk of retinopathy of prematurity (16). However, all these meta-analyses, as well as the present one, are limited by the small number of studies providing data on funisitis.
Besides the immunomodulatory role of prenatal infection/inflammation, some differences between CA-exposed infants and CA-unexposed infants may also play a role in the association between CA and neonatal sepsis. Our meta-analysis showed a higher rate of exposure to antenatal corticosteroids among CA-exposed infants, but meta-regression could not demonstrate that this affected the association between CA and sepsis. In addition, alterations in infant's microbiome due to mode of delivery, or antibiotic exposure in early life may be related to the increased risk of LOS in CA-exposed infants (159). Concerns that an intrauterine infection is the trigger for prematurity lead to the initiation of empirical antibiotics in the majority of very and extremely preterm infants (160). If the newborn has a true infection, these antibiotics will save his life, but overuse may lead to the development of antibiotic resistance. Moreover, growing evidence shows that prolonged initial empirical antibiotic treatment may be associated with adverse outcomes, such as LOS, necrotizing enterocolitis, bronchopulmonary dysplasia, or death (160, 161).
Our study has several limitations. The studies showed great heterogeneity in their definition of CA, particularly pertaining to criteria used in defining clinical CA. Recent recommendations propose to restrict the term CA to pathologic diagnosis (162). With regard to histological CA, the definition and staging criteria of Redline et al. (163) were the most frequently used but only 11 studies (25, 41, 43, 50, 55, 61, 81, 90, 111, 120, 141) used this classification and only two studies (81, 141) stratified the outcomes according to the grade of histological CA. In addition, few studies had the association between CA and sepsis as their main study objective, while at the same time this may have acted in favor of avoiding publication bias. Furthermore, the generalized definition of EOS, LOS, clinical, and culture-proven sepsis did not allow for analysis of individual pathogen associations. Such an approach was simply not feasible given the overall lack of such specific data across all analyzed studies. Currently, no consensus exists on the definition of neonatal bacterial sepsis unlike pediatric sepsis (164, 165). Pediatric sepsis is defined as a “systemic inflammatory response syndrome in the presence of a suspected or proven infection,” conditions that do not necessarily apply to neonatal sepsis (166). Neonatal sepsis in most cases may present itself with a negative blood culture, and with non-specific clinical symptoms (167). Providing a clear disease definition of neonatal sepsis remains therefore in part challenging due to current limitations of ancillary diagnostics (165, 168).
Infant's immaturity plays a key role in the morbidity associated with very/extremely preterm birth but the pathological processes causing preterm birth may also influence the outcome. Our data suggest that when infection/inflammation is the trigger of preterm birth, infants are more susceptible to develop sepsis not only immediately after birth, but also during the first weeks of life. The association between CA and EOS seems to be GA-independent, whereas the lower GA of CA-exposed infants modulated the effect size of the association between CA and LOS. CA may initiate the immunomodulatory sequence leading to LOS but also may alter the rate of exposure to other stimuli such as antenatal and post-natal corticosteroids, antenatal and post-natal antibiotics, invasive therapies, lung damage, patent ductus arteriosus, or necrotizing enterocolitis, which render very/extremely preterm infants more vulnerable to sepsis. A meta-analysis of individual patient data would help determine the role of some of these factors in the different outcomes of preterm children exposed to chorioamnionitis.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation, to any qualified researcher.
This study was presented at the 3rd Congress of Joint European Neonatal Societies (jENS 2019). Abstract available at https://www.nature.com/articles/s41390-019-0521-6.
EV-M contributed to the search and inclusion of studies, carried out data collection, carried out statistical analyses, assessed methodological quality, contributed to interpretation of results, helped draft the initial manuscript, and reviewed and revised the manuscript. GL contributed to the search and inclusion of studies, contributed to data collection, assessed methodological quality, contributed to interpretation of results, and drafted the initial manuscript. OR selected studies for inclusion, carried out data collection, and carried out statistical analyses. PD carried out and supervised data collection, contributed to interpretation of results, and reviewed and revised the manuscript. LZ contributed to interpretation of results and reviewed and revised the manuscript. BK contributed to interpretation of results, contributed to drafting the manuscript, and reviewed and revised the manuscript. EV conceptualized and designed the study, carried out the search and selected studies for inclusion, supervised data collection, contributed to statistical analyses and interpretation of results, helped draft the initial manuscript, and reviewed and revised the manuscript. All authors approved the final manuscript as submitted.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling Editor declared a past co-authorship with one of the authors BK.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2020.00972/full#supplementary-material
1. Moutquin JM. Classification and heterogeneity of preterm birth. BJOG. (2003) 110(Suppl. 20):30–3. doi: 10.1046/j.1471-0528.2003.00021.x
2. McElrath TF, Hecht JL, Dammann O, Boggess K, Onderdonk A, Markenson G, et al. Pregnancy disorders that lead to delivery before the 28th week of gestation: an epidemiologic approach to classification. Am J Epidemiol. (2008) 168:980–9. doi: 10.1093/aje/kwn202
3. Gagliardi L, Rusconi F, Da Fre M, Mello G, Carnielli V, Di Lallo D, et al. Pregnancy disorders leading to very preterm birth influence neonatal outcomes: results of the population-based ACTION cohort study. Pediatr Res. (2013) 73:794–801. doi: 10.1038/pr.2013.52
4. Gagliardi L, Rusconi F, Bellu R, Zanini R, Italian Neonatal N. Association of maternal hypertension and chorioamnionitis with preterm outcomes. Pediatrics. (2014) 134:e154–61. doi: 10.1542/peds.2013-3898
5. Park CW, Moon KC, Park JS, Jun JK, Romero R, Yoon BH. The involvement of human amnion in histologic chorioamnionitis is an indicator that a fetal and an intra-amniotic inflammatory response is more likely and severe: clinical implications. Placenta. (2009) 30:56–61. doi: 10.1016/j.placenta.2008.09.017
6. Kim CJ, Romero R, Chaemsaithong P, Chaiyasit N, Yoon BH, Kim YM. Acute chorioamnionitis and funisitis: definition, pathologic features, and clinical significance. Am J Obstet Gynecol. (2015) 213(4 Suppl.):S29–52. doi: 10.1016/j.ajog.2015.08.040
7. Han YW, Shen T, Chung P, Buhimschi IA, Buhimschi CS. Uncultivated bacteria as etiologic agents of intra-amniotic inflammation leading to preterm birth. J Clin Microbiol. (2009) 47:38–47. doi: 10.1128/JCM.01206-08
8. DiGiulio DB. Diversity of microbes in amniotic fluid. Semin Fetal Neonatal Med. (2012) 17:2–11. doi: 10.1016/j.siny.2011.10.001
9. Gantert M, Been JV, Gavilanes AW, Garnier Y, Zimmermann LJ, Kramer BW. Chorioamnionitis: a multiorgan disease of the fetus? J Perinatol. (2010) 30(Suppl):S21–30. doi: 10.1038/jp.2010.96
10. Thomas W, Speer CP. Chorioamnionitis: important risk factor or innocent bystander for neonatal outcome? Neonatology. (2011) 99:177–87. doi: 10.1159/000320170
11. Strunk T, Inder T, Wang X, Burgner D, Mallard C, Levy O. Infection-induced inflammation and cerebral injury in preterm infants. Lancet Infect Dis. (2014) 14:751–62. doi: 10.1016/S1473-3099(14)70710-8
12. Hartling L, Liang Y, Lacaze-Masmonteil T. Chorioamnionitis as a risk factor for bronchopulmonary dysplasia: a systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed. (2012) 97:F8–17. doi: 10.1136/adc.2010.210187
13. Villamor-Martinez E, Alvarez-Fuente M, Ghazi AMT, Degraeuwe P, Zimmermann LJI, Kramer BW, et al. Association of chorioamnionitis with bronchopulmonary dysplasia among preterm infants: a systematic review, meta-analysis, and metaregression. JAMA Netw Open. (2019) 2:e1914611. doi: 10.1001/jamanetworkopen.2019.14611
14. Been JV, Lievense S, Zimmermann LJ, Kramer BW, Wolfs TG. Chorioamnionitis as a risk factor for necrotizing enterocolitis: a systematic review and meta-analysis. J Pediatr. (2013) 162:236–42.e2. doi: 10.1016/j.jpeds.2012.07.012
15. Mitra S, Aune D, Speer CP, Saugstad OD. Chorioamnionitis as a risk factor for retinopathy of prematurity: a systematic review and meta-analysis. Neonatology. (2014) 105:189–99. doi: 10.1159/000357556
16. Villamor-Martinez E, Cavallaro G, Raffaeli G, Mohammed Rahim OMM, Gulden S, Ghazi AMT, et al. Chorioamnionitis as a risk factor for retinopathy of prematurity: an updated systematic review and meta-analysis. PLoS ONE. (2018) 13:e0205838. doi: 10.1371/journal.pone.0205838
17. Behbodi E, Villamor-Martinez E, Degraeuwe PL, Villamor E. Chorioamnionitis appears not to be a risk factor for patent ductus arteriosus in preterm infants: a systematic review and meta-analysis. Sci Rep. (2016) 6:37967. doi: 10.1038/srep37967
18. Park HW, Choi YS, Kim KS, Kim SN. Chorioamnionitis and patent ductus arteriosus: a systematic review and meta-analysis. PLoS ONE. (2015) 10:e0138114. doi: 10.1371/journal.pone.0138114
19. Villamor-Martinez E, Fumagalli M, Mohammed Rahim O, Passera S, Cavallaro G, Degraeuwe P, et al. Chorioamnionitis is a risk factor for intraventricular hemorrhage in preterm infants: a systematic review and meta-analysis. Front Physiol. (2018) 9:1253. doi: 10.3389/fphys.2018.01253
20. Villamor-Martinez E, Fumagalli M, Alomar YI, Passera S, Cavallaro G, Mosca F, et al. Cerebellar hemorrhage in preterm infants: a meta-analysis on risk factors and neurodevelopmental outcome. Front Physiol. (2019) 10:800. doi: 10.3389/fphys.2019.00800
21. De Felice C, Toti P, Laurini RN, Stumpo M, Picciolini E, Todros T, et al. Early neonatal brain injury in histologic chorioamnionitis. J Pediatr. (2001) 138:101–4. doi: 10.1067/mpd.2001.109605
22. Wu YW, Colford JM Jr. Chorioamnionitis as a risk factor for cerebral palsy: a meta-analysis. JAMA. (2000) 284:1417–24. doi: 10.1001/jama.284.11.1417
23. Hornik CP, Fort P, Clark RH, Watt K, Benjamin DK Jr, Smith PB, et al. Early and late onset sepsis in very-low-birth-weight infants from a large group of neonatal intensive care units. Early Hum Dev. (2012) 88(Suppl. 2):S69–74. doi: 10.1016/S0378-3782(12)70019-1
24. Puopolo KM, Benitz WE, Zaoutis TE, Committee On F Newborn Committee On Infectious D. Management of neonates born at </=34 6/7 weeks' gestation with suspected or proven early-onset bacterial sepsis. Pediatrics. (2018) 142:e20182894. doi: 10.1542/peds.2018-2896
25. Strunk T, Doherty D, Jacques A, Simmer K, Richmond P, Kohan R, et al. Histologic chorioamnionitis is associated with reduced risk of late-onset sepsis in preterm infants. Pediatrics. (2012) 129:e134–41. doi: 10.1542/peds.2010-3493
26. Schuller SS, Kramer BW, Villamor E, Spittler A, Berger A, Levy O. Immunomodulation to prevent or treat neonatal sepsis: past, present, and future. Front Pediatr. (2018) 6:199. doi: 10.3389/fped.2018.00199
27. Ng S, Strunk T, Jiang P, Muk T, Sangild PT, Currie A. Precision medicine for neonatal sepsis. Front Mol Biosci. (2018) 5:70. doi: 10.3389/fmolb.2018.00070
28. Hocevar SN, Edwards JR, Horan TC, Morrell GC, Iwamoto M, Lessa FC. Device-associated infections among neonatal intensive care unit patients: incidence and associated pathogens reported to the national healthcare safety network, 2006–2008. Infect Control Hosp Epidemiol. (2012) 33:1200–6. doi: 10.1086/668425
29. Villamor-Martinez E, Lubach G, Mohammed Rahim OM, Degraeuwe P, Kramer BW, Villamor E. Chorioamnionitis as a Risk Factor for Neonatal Sepsis: A Systematic Review and Meta-Analysis CRD42018117190 2018. (2019). Available online at: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=117190 (accessed November 15, 2019).
30. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
31. Kachikis A, Eckert LO, Walker C, Bardají A, Varricchio F, Lipkind HS, et al. Chorioamnionitis: case definition & guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine. (2019) 37:7610–22. doi: 10.1016/j.vaccine.2019.05.030
32. Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomized Studies in Meta-Analyses. Available online at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm (accessed February 02, 2019).
33. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. (2014) 14:135. doi: 10.1186/1471-2288-14-135
34. Borenstein M (editor). Introduction to Meta-Analysis. Chichester, WS: John Wiley & Sons Ltd (2009). doi: 10.1002/9780470743386
35. Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
36. Abu-Maziad A, Schaa K, Bell EF, Dagle JM, Cooper M, Marazita ML, et al. Role of polymorphic variants as genetic modulators of infection in neonatal sepsis. Pediatr Res. (2010) 68:323–9. doi: 10.1203/PDR.0b013e3181e6a068
37. Adams WG, Kinney JS, Schuchat A, Collier CL, Papasian CJ, Kilbride HW, et al. Outbreak of early onset group B streptococcal sepsis. Pediatr Infect Dis J. (1993) 12:565–70. doi: 10.1097/00006454-199307000-00003
38. Ahn HM, Park EA, Cho SJ, Kim YJ, Park HS. The association of histological chorioamnionitis and antenatal steroids on neonatal outcome in preterm infants born at less than thirty-four weeks' gestation. Neonatology. (2012) 102:259–64. doi: 10.1159/000339577
39. Alam MM, Saleem AF, Shaikh AS, Munir O, Qadir M. Neonatal sepsis following prolonged rupture of membranes in a tertiary care hospital in Karachi, Pakistan. J Infect Dev Ctries. (2014) 8:67–73. doi: 10.3855/jidc.3136
40. Alexander JM, Gilstrap LC, Cox SM, McIntire DM, Leveno KJ. Clinical chorioamnionitis and the prognosis for very low birth weight infants. Obstet Gynecol. (1998) 91(5 Pt 1):725–9. doi: 10.1097/00006250-199805000-00016
41. Alfiero Bordigato M, Piva D, Di Gangi IM, Giordano G, Chiandetti L, Filippone M. Asymmetric dimethylarginine in ELBW newborns exposed to chorioamnionitis. Early Hum Dev. (2011) 87:143–5. doi: 10.1016/j.earlhumdev.2010.11.004
42. Alshaikh B, Yee W, Lodha A, Henderson E, Yusuf K, Sauve R. Coagulase-negative staphylococcus sepsis in preterm infants and long-term neurodevelopmental outcome. J Perinatol. (2014) 34:125–9. doi: 10.1038/jp.2013.155
43. Anblagan D, Pataky R, Evans MJ, Telford EJ, Serag A, Sparrow S, et al. Association between preterm brain injury and exposure to chorioamnionitis during fetal life. Sci Rep. (2016) 6:37932. doi: 10.1038/srep37932
44. Arayici S, Kadioglu Simsek G, Oncel MY, Eras Z, Canpolat FE, Oguz SS, et al. The effect of histological chorioamnionitis on the short-term outcome of preterm infants </=32 weeks: a single-center study. J Matern Fetal Neonatal Med. (2014) 27:1129–33. doi: 10.3109/14767058.2013.850668
45. Arora P, Bagga R, Kalra J, Kumar P, Radhika S, Gautam V. Mean gestation at delivery and histological chorioamnionitis correlates with early-onset neonatal sepsis following expectant management in pPROM. J Obstet Gynaecol. (2015) 35:235–40. doi: 10.3109/01443615.2014.958143
46. Averbuch B, Mazor M, Shoham-Vardi I, Chaim W, Vardi H, Horowitz S, et al. Intra-uterine infection in women with preterm premature rupture of membranes: maternal and neonatal characteristics. Eur J Obstet Gynecol Reprod Biol. (1995) 62:25–9. doi: 10.1016/0301-2115(95)02176-8
47. Aziz N, Cheng YW, Caughey AB. Neonatal outcomes in the setting of preterm premature rupture of membranes complicated by chorioamnionitis. J Matern Fetal Neonatal Med. (2009) 22:780–4. doi: 10.3109/14767050902922581
48. Ballard AR, Mallett LH, Pruszynski JE, Cantey JB. Chorioamnionitis and subsequent bronchopulmonary dysplasia in very-low-birth weight infants: a 25-year cohort. J Perinatol. (2016) 36:1045–8. doi: 10.1038/jp.2016.138
49. Barrera-Reyes RH, Ruiz-Macias H, Segura-Cervantes E. Neurodevelopment at one year of age [corrected] in preterm newborns with history of maternal chorioamnionitis. Ginecol Obstet Mex. (2011) 79:31–7.
50. Been JV, Rours IG, Kornelisse RF, Lima Passos V, Kramer BW, Schneider TA, et al. Histologic chorioamnionitis, fetal involvement, and antenatal steroids: effects on neonatal outcome in preterm infants. Am J Obstet Gynecol. (2009) 201:587.e1–8. doi: 10.1016/j.ajog.2009.06.025
51. Botet F, Figueras J, Carbonell-Estrany X, Arca G, The Castrillo Study G. Effect of maternal clinical chorioamnionitis on neonatal morbidity in very-low birthweight infants: a case-control study. J Perinat Med. (2010) 38:269–73. doi: 10.1515/jpm.2010.029
52. Bry KJ, Jacobsson B, Nilsson S, Bry K. Gastric fluid cytokines are associated with chorioamnionitis and white blood cell counts in preterm infants. Acta Paediatr. (2015) 104:575–80. doi: 10.1111/apa.12947
53. Buhimschi CS, Buhimschi IA, Abdel-Razeq S, Rosenberg VA, Thung SF, Zhao G, et al. Proteomic biomarkers of intra-amniotic inflammation: relationship with funisitis and early-onset sepsis in the premature neonate. Pediatr Res. (2007) 61:318–24. doi: 10.1203/01.pdr.0000252439.48564.37
54. Buhimschi CS, Dulay AT, Abdel-Razeq S, Zhao G, Lee S, Hodgson EJ, et al. Fetal inflammatory response in women with proteomic biomarkers characteristic of intra-amniotic inflammation and preterm birth. BJOG. (2009) 116:257–67. doi: 10.1111/j.1471-0528.2008.01925.x
55. Burgner DP, Doherty D, Humphreys J, Currie A, Simmer K, Charles A, et al. Maternal chorioamnionitis and postneonatal respiratory tract infection in ex-preterm infants. J Pediatr. (2017) 184:62–7.e2. doi: 10.1016/j.jpeds.2017.01.037
56. Chau V, Brant R, Poskitt KJ, Tam EW, Synnes A, Miller SP. Post-natal infection is associated with widespread abnormalities of brain development in premature newborns. Pediatr Res. (2012) 71:274–9. doi: 10.1038/pr.2011.40
57. Choi CW, Kim BI, Joung KE, Lee JA, Lee YK, Kim EK, et al. Decreased expression of transforming growth factor-beta1 in bronchoalveolar lavage cells of preterm infants with maternal chorioamnionitis. J Korean Med Sci. (2008) 23:609–15. doi: 10.3346/jkms.2008.23.4.609
58. Churgay CA, Smith MA, Blok B. Maternal fever during labor–what does it mean? J Am Board Fam Pract. (1994) 7:14–24.
59. De Felice C, Toti P, Parrini S, Del Vecchio A, Bagnoli F, Latini G, et al. Histologic chorioamnionitis and severity of illness in very low birth weight newborns. Pediatr Crit Care Med. (2005) 6:298–302. doi: 10.1097/01.PCC.0000160658.35437.65
60. Dempsey E, Chen MF, Kokottis T, Vallerand D, Usher R. Outcome of neonates less than 30 weeks gestation with histologic chorioamnionitis. Am J Perinatol. (2005) 22:155–9. doi: 10.1055/s-2005-865020
61. Dessardo NS, Dessardo S, Mustac E, Banac S, Petrovic O, Peter B. Chronic lung disease of prematurity and early childhood wheezing: is foetal inflammatory response syndrome to blame? Early Hum Dev. (2014) 90:493–9. doi: 10.1016/j.earlhumdev.2014.07.002
62. Dexter SC, Malee MP, Pinar H, Hogan JW, Carpenter MW, Vohr BR. Influence of chorioamnionitis on developmental outcome in very low birth weight infants. Obstet Gynecol. (1999) 94:267–73. doi: 10.1097/00006250-199908000-00022
63. Dexter SC, Pinar H, Malee MP, Hogan J, Carpenter MW, Vohr BR. Outcome of very low birth weight infants with histopathologic chorioamnionitis. Obstet Gynecol. (2000) 96:172–7. doi: 10.1097/00006250-200008000-00004
64. Dollner H, Vatten L, Halgunset J, Rahimipoor S, Austgulen R. Histologic chorioamnionitis and umbilical serum levels of pro-inflammatory cytokines and cytokine inhibitors. BJOG. (2002) 109:534–9. doi: 10.1111/j.1471-0528.2002.01028.x
65. Dulay AT, Buhimschi IA, Zhao G, Luo G, Abdel-Razeq S, Cackovic M, et al. Nucleated red blood cells are a direct response to mediators of inflammation in newborns with early-onset neonatal sepsis. Am J Obstet Gynecol. (2008) 198:426.e1–9. doi: 10.1016/j.ajog.2008.01.040
66. Dutta S, Reddy R, Sheikh S, Kalra J, Ray P, Narang A. Intrapartum antibiotics and risk factors for early onset sepsis. Arch Dis Child Fetal Neonatal Ed. (2010) 95:F99–103. doi: 10.1136/adc.2009.163220
67. Ecevit A, Anuk-Ince D, Yapakci E, Kupana-Ayva S, Kurt A, Yanik FF, et al. Association of respiratory distress syndrome and perinatal hypoxia with histologic chorioamnionitis in preterm infants. Turk J Pediatr. (2014) 56:56–61.
68. Elimian A, Verma U, Beneck D, Cipriano R, Visintainer P, Tejani N. Histologic chorioamnionitis, antenatal steroids, and perinatal outcomes. Obstet Gynecol. (2000) 96:333–6. doi: 10.1097/00006250-200009000-00003
69. Erdemir G, Kultursay N, Calkavur S, Zekioglu O, Koroglu OA, Cakmak B, et al. Histological chorioamnionitis: effects on premature delivery and neonatal prognosis. Pediatr Neonatol. (2013) 54:267–74. doi: 10.1016/j.pedneo.2013.03.012
70. Figueroa-Damian R, Arredondo-Garcia JL, Mancilla-Ramirez J. Amniotic fluid interleukin-6 and the risk of early-onset sepsis among preterm infants. Arch Med Res. (1999) 30:198–202. doi: 10.1016/S0188-0128(99)00015-9
71. Garcia-Munoz Rodrigo F, Galan Henriquez G, Figueras Aloy J, Garcia-Alix Perez A. Outcomes of very-low-birth-weight infants exposed to maternal clinical chorioamnionitis: a multicentre study. Neonatology. (2014) 106:229–34. doi: 10.1159/000363127
72. Gonzalez-Luis G, Jordan Garcia I, Rodriguez-Miguelez J, Botet Mussons F, Figueras Aloy J. [Neonatal morbidity and mortality in very low birth weight infants according to exposure to chorioamnionitis]. An Esp Pediatr. (2002) 56:551–5. doi: 10.1016/S1695-4033(02)77863-6
73. Group E. Incidence of and risk factors for neonatal morbidity after active perinatal care: extremely preterm infants study in Sweden (EXPRESS). Acta Paediatr. (2010) 99:978–92. doi: 10.1111/j.1651-2227.2010.01846.x
74. Guzick DS, Winn K. The association of chorioamnionitis with preterm delivery. Obstet Gynecol. (1985) 65:11–6.
75. Gyamfi-Bannerman C, Son M. Preterm premature rupture of membranes and the rate of neonatal sepsis after two courses of antenatal corticosteroids. Obstet Gynecol. (2014) 124:999–1003. doi: 10.1097/AOG.0000000000000460
76. Hendson L, Russell L, Robertson CM, Liang Y, Chen Y, Abdalla A, et al. Neonatal and neurodevelopmental outcomes of very low birth weight infants with histologic chorioamnionitis. J Pediatr. (2011) 158:397–402. doi: 10.1016/j.jpeds.2010.09.010
77. Hitti J, Tarczy-Hornoch P, Murphy J, Hillier SL, Aura J, Eschenbach DA. Amniotic fluid infection, cytokines, and adverse outcome among infants at 34 weeks' gestation or less. Obstet Gynecol. (2001) 98:1080–8. doi: 10.1016/S0029-7844(01)01567-8
78. Howman RA, Charles AK, Jacques A, Doherty DA, Simmer K, Strunk T, et al. Inflammatory and haematological markers in the maternal, umbilical cord and infant circulation in histological chorioamnionitis. PLoS ONE. (2012) 7:e51836. doi: 10.1371/journal.pone.0051836
79. Janota J, Stranak Z, Belohlavkova S, Mudra K, Simak J. Post-natal increase of procalcitonin in premature newborns is enhanced by chorioamnionitis and neonatal sepsis. Eur J Clin Invest. (2001) 31:978–83. doi: 10.1046/j.1365-2362.2001.00912.x
80. Jeon JH, Namgung R, Park MS, Park KI, Lee C. Positive maternal C-reactive protein predicts neonatal sepsis. Yonsei Med J. (2014) 55:113–7. doi: 10.3349/ymj.2014.55.1.113
81. Jones MH, Corso AL, Tepper RS, Edelweiss MI, Friedrich L, Pitrez PM, et al. Chorioamnionitis and subsequent lung function in preterm infants. PLoS ONE. (2013) 8:e81193. doi: 10.1371/journal.pone.0081193
82. Kacerovsky M, Musilova I, Andrys C, Hornychova H, Pliskova L, Kostal M, et al. Prelabor rupture of membranes between 34 and 37 weeks: the intraamniotic inflammatory response and neonatal outcomes. Am J Obstet Gynecol. (2014) 210:325.e1–e10. doi: 10.1016/j.ajog.2013.10.882
83. Kim SY, Choi CW, Jung E, Lee J, Lee JA, Kim H, et al. Neonatal morbidities associated with histologic chorioamnionitis defined based on the site and extent of inflammation in very low birth weight infants. J Korean Med Sci. (2015) 30:1476–82. doi: 10.3346/jkms.2015.30.10.1476
84. Kirchner L, Helmer H, Heinze G, Wald M, Brunbauer M, Weninger M, et al. Amnionitis with ureaplasma urealyticum or other microbes leads to increased morbidity and prolonged hospitalization in very low birth weight infants. Eur J Obstet Gynecol Reprod Biol. (2007) 134:44–50. doi: 10.1016/j.ejogrb.2006.09.013
85. Klinger G, Levy I, Sirota L, Boyko V, Reichman B, Lerner-Geva L, et al. Epidemiology and risk factors for early onset sepsis among very-low-birthweight infants. Am J Obstet Gynecol. (2009) 201:38.e1–6. doi: 10.1016/j.ajog.2009.03.006
86. Lahra MM, Beeby PJ, Jeffery HE. Intrauterine inflammation, neonatal sepsis, and chronic lung disease: a 13-year hospital cohort study. Pediatrics. (2009) 123:1314–9. doi: 10.1542/peds.2008-0656
87. Lau J, Magee F, Qiu Z, Hoube J, Von Dadelszen P, Lee SK. Chorioamnionitis with a fetal inflammatory response is associated with higher neonatal mortality, morbidity, and resource use than chorioamnionitis displaying a maternal inflammatory response only. Am J Obstet Gynecol. (2005) 193(3 Pt 1):708–13. doi: 10.1016/j.ajog.2005.01.017
88. Lee SY, Leung CW. Histological chorioamnionitis - implication for bacterial colonization, laboratory markers of infection, and early onset sepsis in very-low-birth-weight neonates. J Matern Fetal Neonatal Med. (2012) 25:364–8. doi: 10.3109/14767058.2011.579208
89. Lee SY, Park KH, Jeong EH, Oh KJ, Ryu A, Park KU. Relationship between maternal serum C-reactive protein, funisitis and early-onset neonatal sepsis. J Korean Med Sci. (2012) 27:674–80. doi: 10.3346/jkms.2012.27.6.674
90. Liu Z, Tang Z, Li J, Yang Y. Effects of placental inflammation on neonatal outcome in preterm infants. Pediatr Neonatol. (2014) 55:35–40. doi: 10.1016/j.pedneo.2013.05.007
91. Lorthe E, Ancel PY, Torchin H, Kaminski M, Langer B, Subtil D, et al. Impact of latency duration on the prognosis of preterm infants after preterm premature rupture of membranes at 24 to 32 weeks' gestation: a national population-based cohort study. J Pediatr. (2017) 182:47–52.e2. doi: 10.1016/j.jpeds.2016.11.074
92. Mehta R, Nanjundaswamy S, Shen-Schwarz S, Petrova A. Neonatal morbidity and placental pathology. Indian J Pediatr. (2006) 73:25–8. doi: 10.1007/BF02758255
93. Mikhael M, Brown LS, Rosenfeld CR. Serial neutrophil values facilitate predicting the absence of neonatal early-onset sepsis. J Pediatr. (2014) 164:522–8.e1–3. doi: 10.1016/j.jpeds.2013.10.080
94. Miyazaki K, Furuhashi M, Ishikawa K, Tamakoshi K, Hayashi K, Kai A, et al. Impact of chorioamnionitis on short- and long-term outcomes in very low birth weight preterm infants: the neonatal research network Japan. J Matern Fetal Neonatal Med. (2016) 29:331–7. doi: 10.3109/14767058.2014.1000852
95. Mu SC, Lin CH, Chen YL, Ma HJ, Lee JS, Lin MI, et al. Impact on neonatal outcome and anthropometric growth in very low birth weight infants with histological chorioamnionitis. J Formos Med Assoc. (2008) 107:304–10. doi: 10.1016/S0929-6646(08)60091-1
96. Mwanyumba F, Inion I, Gaillard P, Mandaliya K, Praet M, Temmerman M. Placental inflammation and perinatal outcome. Eur J Obstet Gynecol Reprod Biol. (2003) 108:164–70. doi: 10.1016/S0301-2115(02)00438-4
97. Nasef N, Shabaan AE, Schurr P, Iaboni D, Choudhury J, Church P, et al. Effect of clinical and histological chorioamnionitis on the outcome of preterm infants. Am J Perinatol. (2013) 30:59–68. doi: 10.1055/s-0032-1321501
98. O'Shea TM, Klinepeter KL, Meis PJ, Dillard RG. Intrauterine infection and the risk of cerebral palsy in very low-birthweight infants. Paediatr Perinat Epidemiol. (1998) 12:72–83. doi: 10.1111/j.1365-3016.1998.00081.x
99. Ofman G, Vasco N, Cantey JB. Risk of early-onset sepsis following preterm, prolonged rupture of membranes with or without chorioamnionitis. Am J Perinatol. (2016) 33:339–42. doi: 10.1055/s-0035-1556758
100. Ogunyemi D, Murillo M, Jackson U, Hunter N, Alperson B. The relationship between placental histopathology findings and perinatal outcome in preterm infants. J Matern Fetal Neonatal Med. (2003) 13:102–9. doi: 10.1080/jmf.13.2.102.109
101. Oh S-H, Kim J-J, Do H-J, Lee BS, Kim K-S, Kim EA-R. Preliminary study on neurodevelopmental outcome and placental pathology among extremely low birth weight infants. Korean J Perinatol. (2015) 26:67–77. doi: 10.14734/kjp.2015.26.1.67
102. Ohyama M, Itani Y, Yamanaka M, Goto A, Kato K, Ijiri R, et al. Re-evaluation of chorioamnionitis and funisitis with a special reference to subacute chorioamnionitis. Hum Pathol. (2002) 33:183–90. doi: 10.1053/hupa.2002.31291
103. Ozkan H, Cetinkaya M, Koksal N, Celebi S, Hacimustafaoglu M. Culture-proven neonatal sepsis in preterm infants in a neonatal intensive care unit over a 7 year period: coagulase-negative Staphylococcus as the predominant pathogen. Pediatr Int. (2014) 56:60–6. doi: 10.1111/ped.12218
104. Pappas A, Kendrick DE, Shankaran S, Stoll BJ, Bell EF, Laptook AR, et al. Chorioamnionitis and early childhood outcomes among extremely low-gestational-age neonates. JAMA Pediatr. (2014) 168:137–47. doi: 10.1001/jamapediatrics.2013.4248
105. Park JW, Park KH, Jung EY. Clinical significance of histologic chorioamnionitis with a negative amniotic fluid culture in patients with preterm labor and premature membrane rupture. PLoS ONE. (2017) 12:e0173312. doi: 10.1371/journal.pone.0173312
106. Park KH, Yoon BH, Shim SS, Jun JK, Syn HC. Amniotic fluid tumor necrosis factor-alpha is a marker for the prediction of early-onset neonatal sepsis in preterm labor. Gynecol Obstet Invest. (2004) 58:84–90. doi: 10.1159/000078492
107. Plakkal N, Soraisham AS, Trevenen C, Freiheit EA, Sauve R. Histological chorioamnionitis and bronchopulmonary dysplasia: a retrospective cohort study. J Perinatol. (2013) 33:441–5. doi: 10.1038/jp.2012.154
108. Popowski T, Goffinet F, Maillard F, Schmitz T, Leroy S, Kayem G. Maternal markers for detecting early-onset neonatal infection and chorioamnionitis in cases of premature rupture of membranes at or after 34 weeks of gestation: a two-center prospective study. BMC Pregnancy ChildB. (2011) 11:26. doi: 10.1186/1471-2393-11-26
109. Prendergast M, May C, Broughton S, Pollina E, Milner AD, Rafferty GF, et al. Chorioamnionitis, lung function and bronchopulmonary dysplasia in prematurely born infants. Arch Dis Child Fetal Neonatal Ed. (2011) 96:F270–4. doi: 10.1136/adc.2010.189480
110. Pristauz G, Bader AA, Schwantzer G, Kutschera J, Lang U. Assessment of risk factors for survival of neonates born after second-trimester PPROM. Early Hum Dev. (2009) 85:177–80. doi: 10.1016/j.earlhumdev.2008.09.012
111. Puri K, Taft DH, Ambalavanan N, Schibler KR, Morrow AL, Kallapur SG. Association of chorioamnionitis with aberrant neonatal gut colonization and adverse clinical outcomes. PLoS ONE. (2016) 11:e0162734. doi: 10.1371/journal.pone.0162734
112. Rocha G, Proenca E, Quintas C, Rodrigues T, Guimaraes H. Chorioamnionitis and neonatal morbidity. Acta Med Port. (2006) 19:207–12. doi: 10.1016/j.ajog.2004.11.035
113. Rodriguez-Trujillo A, Cobo T, Vives I, Bosch J, Kacerovsky M, Posadas DE, et al. Gestational age is more important for short-term neonatal outcome than microbial invasion of the amniotic cavity or intra-amniotic inflammation in preterm prelabor rupture of membranes. Acta Obstet Gynecol Scand. (2016) 95:926–33. doi: 10.1111/aogs.12905
114. Ronnestad A, Abrahamsen TG, Medbo S, Reigstad H, Lossius K, Kaaresen PI, et al. Septicemia in the first week of life in a norwegian national cohort of extremely premature infants. Pediatrics. (2005) 115:e262–8. doi: 10.1542/peds.2004-1834
115. Salem SY, Sheiner E, Zmora E, Vardi H, Shoham-Vardi I, Mazor M. Risk factors for early neonatal sepsis. Arch Gynecol Obstet. (2006) 274:198–202. doi: 10.1007/s00404-006-0135-1
116. Sato M, Nishimaki S, Yokota S, Seki K, Horiguchi H, An H, et al. Severity of chorioamnionitis and neonatal outcome. J Obstet Gynaecol Res. (2011) 37:1313–9. doi: 10.1111/j.1447-0756.2010.01519.x
117. Schlapbach LJ, Ersch J, Adams M, Bernet V, Bucher HU, Latal B. Impact of chorioamnionitis and preeclampsia on neurodevelopmental outcome in preterm infants below 32 weeks gestational age. Acta Paediatr. (2010) 99:1504–9. doi: 10.1111/j.1651-2227.2010.01861.x
118. Schuchat A, Zywicki SS, Dinsmoor MJ, Mercer B, Romaguera J, O'Sullivan MJ, et al. Risk factors and opportunities for prevention of early-onset neonatal sepsis: a multicenter case-control study. Pediatrics. (2000) 105(1 Pt 1):21–6. doi: 10.1542/peds.105.1.21
119. Shah J, Jefferies AL, Yoon EW, Lee SK, Shah PS, Canadian Neonatal N. Risk factors and outcomes of late-onset bacterial sepsis in preterm neonates born at <32 weeks' gestation. Am J Perinatol. (2015) 32:675–82. doi: 10.1055/s-0034-1393936
120. Smit AL, Been JV, Zimmermann LJ, Kornelisse RF, Andriessen P, Vanterpool SF, et al. Automated auditory brainstem response in preterm newborns with histological chorioamnionitis. J Matern Fetal Neonatal Med. (2015) 28:1864–9. doi: 10.3109/14767058.2014.971747
121. Smulian JC, Shen-Schwarz S, Vintzileos AM, Lake MF, Ananth CV. Clinical chorioamnionitis and histologic placental inflammation. Obstet Gynecol. (1999) 94:1000–5. doi: 10.1097/00006250-199912000-00018
122. Soraisham AS, Singhal N, McMillan DD, Sauve RS, Lee SK, Canadian Neonatal N. A multicenter study on the clinical outcome of chorioamnionitis in preterm infants. Am J Obstet Gynecol. (2009) 200:372.e1–6. doi: 10.1016/j.ajog.2008.11.034
123. Soraisham AS, Trevenen C, Wood S, Singhal N, Sauve R. Histological chorioamnionitis and neurodevelopmental outcome in preterm infants. J Perinatol. (2013) 33:70–5. doi: 10.1038/jp.2012.49
124. Stepan M, Cobo T, Maly J, Navratilova M, Musilova I, Hornychova H, et al. Neonatal outcomes in subgroups of women with preterm prelabor rupture of membranes before 34 weeks. J Matern Fetal Neonatal Med. (2016) 29:2373–7. doi: 10.3109/14767058.2015.1086329
125. Stimac M, Juretic E, Vukelic V, Matasic NP, Kos M, Babic D. Effect of chorioamnionitis on mortality, early onset neonatal sepsis and bronchopulmonary dysplasia in preterm neonates with birth weight of <1,500 grams. Coll Antropol. (2014) 38:167–71.
126. Stranak Z, Feyereisl J, Korcek P, Feyereislova S, Krofta L. Procalcitonin is more likely to be released by the fetus rather than placental tissue during chorioamnionitis. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. (2016) 160:499–502. doi: 10.5507/bp.2016.041
127. Sung JH, Choi SJ, Oh SY, Roh CR, Kim JH. Revisiting the diagnostic criteria of clinical chorioamnionitis in preterm birth. BJOG. (2017) 124:775–83. doi: 10.1111/1471-0528.14176
128. Trevisanuto D, Peruzzetto C, Cavallin F, Vedovato S, Cosmi E, Visentin S, et al. Fetal placental inflammation is associated with poor neonatal growth of preterm infants: a case-control study. J Matern Fetal Neonatal Med. (2013) 26:1484–90. doi: 10.3109/14767058.2013.789849
129. Tsai CH, Chen YY, Wang KG, Chen CY, Chen CP. Characteristics of early-onset neonatal sepsis caused by Escherichia coli. Taiwan J Obstet Gynecol. (2012) 51:26–30. doi: 10.1016/j.tjog.2012.01.006
130. Tsiartas P, Kacerovsky M, Musilova I, Hornychova H, Cobo T, Savman K, et al. The association between histological chorioamnionitis, funisitis and neonatal outcome in women with preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med. (2013) 26:1332–6. doi: 10.3109/14767058.2013.784741
131. Tudela CM, Stewart RD, Roberts SW, Wendel GD Jr, Stafford IA, McIntire DD, et al. Intrapartum evidence of early-onset group B streptococcus. Obstet Gynecol. (2012) 119:626–9. doi: 10.1097/AOG.0b013e31824532f6
132. Van Marter LJ, Dammann O, Allred EN, Leviton A, Pagano M, Moore M, et al. Chorioamnionitis, mechanical ventilation, and post-natal sepsis as modulators of chronic lung disease in preterm infants. J Pediatr. (2002) 140:171–6. doi: 10.1067/mpd.2002.121381
133. van Vliet EO, de Kieviet JF, van der Voorn JP, Been JV, Oosterlaan J, van Elburg RM. Placental pathology and long-term neurodevelopment of very preterm infants. Am J Obstet Gynecol. (2012) 206:489.e1–7. doi: 10.1016/j.ajog.2012.03.024
134. Vander Haar E, Gyamfi-Bannerman C. Chorioamnionitis and neurocognitive development at age 2 years. Obstet Gynecol. (2016) 127:437–41. doi: 10.1097/AOG.0000000000001295
135. Vinnars MT, Papadogiannakis N, Nasiell J, Holmstrom G, Westgren M. Placental pathology in relation to stillbirth and neonatal outcome in an extremely preterm population: a prospective cohort study. Acta Obstet Gynecol Scand. (2015) 94:584–90. doi: 10.1111/aogs.12610
136. Watterberg KL, Gerdes JS, Gifford KL, Lin HM. Prophylaxis against early adrenal insufficiency to prevent chronic lung disease in premature infants. Pediatrics. (1999) 104:1258–63. doi: 10.1542/peds.104.6.1258
137. Wynn JL, Hansen NI, Das A, Cotten CM, Goldberg RN, Sanchez PJ, et al. Early sepsis does not increase the risk of late sepsis in very low birth weight neonates. J Pediatr. (2013) 162:942–8.e1–3. doi: 10.1016/j.jpeds.2012.11.027
138. Xie A, Zhang W, Chen M, Wang Y, Wang Y, Zhou Q, et al. Related factors and adverse neonatal outcomes in women with preterm premature rupture of membranes complicated by histologic chorioamnionitis. Med Sci Monit. (2015) 21:390–5. doi: 10.12659/MSM.891203
139. Yancey MK, Duff P, Kubilis P, Clark P, Frentzen BH. Risk factors for neonatal sepsis. Obstet Gynecol. (1996) 87:188–94. doi: 10.1016/0029-7844(95)00402-5
140. Yoon BH, Romero R, Kim CJ, Jun JK, Gomez R, Choi JH, et al. Amniotic fluid interleukin-6: a sensitive test for antenatal diagnosis of acute inflammatory lesions of preterm placenta and prediction of perinatal morbidity. Am J Obstet Gynecol. (1995) 172:960–70. doi: 10.1016/0002-9378(95)90028-4
141. Lee Y, Kim H-J, Choi S-J, Oh S-Y, Kim J-S, Roh C-R, et al. Is there a stepwise increase in neonatal morbidities according to histological stage (or grade) of acute chorioamnionitis and funisitis?: effect of gestational age at delivery. J Perinatal Med. (2015) 43:259–67. doi: 10.1515/jpm-2014-0035
142. McShane BB, Gal D, Gelman A, Robert C, Tackett JL. Abandon statistical significance. Am Stat. (2019) 73(Supp1):235–45. doi: 10.1080/00031305.2018.1527253
143. Ciolino JD, Martin RH, Zhao W, Jauch EC, Hill MD, Palesch YY. Covariate imbalance and adjustment for logistic regression analysis of clinical trial data. J Biopharm Stat. (2013) 23:1383–402. doi: 10.1080/10543406.2013.834912
144. Davies HTO, Crombie IK, Tavakoli M. When can odds ratios mislead? BMJ. (1998) 316:989–91. doi: 10.1136/bmj.316.7136.989
145. Snowden JM, Basso O. Causal inference in studies of preterm babies: a simulation study. BJOG. (2018) 125:686–92. doi: 10.1111/1471-0528.14942
146. Durrmeyer X, Kayem G, Sinico M, Dassieu G, Danan C, Decobert F. Perinatal risk factors for bronchopulmonary dysplasia in extremely low gestational age infants: a pregnancy disorder-based approach. J Pediatr. (2012) 160:578–83.e2. doi: 10.1016/j.jpeds.2011.09.025
147. van Well GTJ, Daalderop LA, Wolfs T, Kramer BW. Human perinatal immunity in physiological conditions and during infection. Mol Cell Pediatr. (2017) 4:4. doi: 10.1186/s40348-017-0070-1
148. Wilcox AJ, Weinberg CR, Basso O. On the pitfalls of adjusting for gestational age at birth. Am J Epidemiol. (2011) 174:1062–8. doi: 10.1093/aje/kwr230
149. Ananth CV, Schisterman EF. Confounding, causality, and confusion: the role of intermediate variables in interpreting observational studies in obstetrics. Am J Obstet Gynecol. (2017) 217:167–75. doi: 10.1016/j.ajog.2017.04.016
150. Basso O, Wilcox A. Mortality risk among preterm babies: immaturity vs. underlying pathology. Epidemiology (Cambridge, Mass). (2010) 21:521–7. doi: 10.1097/EDE.0b013e3181debe5e
151. Hernández-Díaz S, Schisterman EF, Hernán MA. The birth weight “paradox” uncovered? Am J Epidemiol. (2006) 164:1115–20. doi: 10.1093/aje/kwj275
152. Braun D, Bromberger P, Ho NJ, Getahun D. Low rate of perinatal sepsis in term infants of mothers with chorioamnionitis. Am J Perinatol. (2016) 33:143–50. doi: 10.1055/s-0035-1560045
153. Puopolo KM, Mukhopadhyay S, Hansen NI, Cotten CM, Stoll BJ, Sanchez PJ, et al. Identification of extremely premature infants at low risk for early-onset sepsis. Pediatrics. (2017) 140:e20170925. doi: 10.1542/peds.2017-0925
154. Azizia M, Lloyd J, Allen M, Klein N, Peebles D. Immune status in very preterm neonates. Pediatrics. (2012) 129:e967–74. doi: 10.1542/peds.2011-1579
155. Wolfs TG, Jellema RK, Turrisi G, Becucci E, Buonocore G, Kramer BW. Inflammation-induced immune suppression of the fetus: a potential link between chorioamnionitis and post-natal early onset sepsis. J Matern Fetal Neonatal Med. (2012) 25(Suppl. 1):8–11. doi: 10.3109/14767058.2012.664447
156. Kallapur SG, Kramer BW, Knox CL, Berry CA, Collins JJ, Kemp MW, et al. Chronic fetal exposure to ureaplasma parvum suppresses innate immune responses in sheep. J Immunol. (2011) 187:2688–95. doi: 10.4049/jimmunol.1100779
157. Kramer BW, Kallapur SG, Moss TJ, Nitsos I, Newnham JP, Jobe AH. Intra-amniotic LPS modulation of TLR signaling in lung and blood monocytes of fetal sheep. Innate Immun. (2009) 15:101–7. doi: 10.1177/1753425908100455
158. Revello R, Alcaide MJ, Dudzik D, Abehsera D, Bartha JL. Differential amniotic fluid cytokine profile in women with chorioamnionitis with and without funisitis. J. Matern. Fetal Neonatal Med. (2015) 29:2161–5. doi: 10.3109/14767058.2015.1077512
159. Groer MW, Luciano AA, Dishaw LJ, Ashmeade TL, Miller E, Gilbert JA. Development of the preterm infant gut microbiome: a research priority. Microbiome. (2014) 2:38. doi: 10.1186/2049-2618-2-38
160. Ting JY, Roberts A, Sherlock R, Ojah C, Cieslak Z, Dunn M, et al. Duration of initial empirical antibiotic therapy and outcomes in very low birth weight infants. Pediatrics. (2019) 143:e20182286. doi: 10.1542/peds.2018-2286
161. Flannery DD, Dysart K, Cook A, Greenspan J, Aghai ZH, Jensen EA. Association between early antibiotic exposure and bronchopulmonary dysplasia or death. J Perinatol. (2018) 38:1227–34. doi: 10.1038/s41372-018-0146-3
162. Higgins RD, Saade G, Polin RA, Grobman WA, Buhimschi IA, Watterberg K, et al. Evaluation and management of women and newborns with a maternal diagnosis of chorioamnionitis: summary of a workshop. Obst Gynecol. (2016) 127:426–36. doi: 10.1097/AOG.0000000000001246
163. Redline RW, Heller D, Keating S, Kingdom J. Placental diagnostic criteria and clinical correlation-a workshop report. Placenta. (2005) 26:S114–S7. doi: 10.1016/j.placenta.2005.02.009
164. Wynn JL. Defining neonatal sepsis. Curr Opin Pediatr. (2016) 28:135–40. doi: 10.1097/MOP.0000000000000315
165. Hofer N, Zacharias E, Muller W, Resch B. Performance of the definitions of the systemic inflammatory response syndrome and sepsis in neonates. J Perinat Med. (2012) 40:587–90. doi: 10.1515/jpm-2011-0308
166. Wynn JL, Wong HR, Shanley TP, Bizzarro MJ, Saiman L, Polin RA. Time for a neonatal-specific consensus definition for sepsis. Pediatr Crit Care Med. (2014) 15:523–8. doi: 10.1097/PCC.0000000000000157
167. Wynn JL, Guthrie SO, Wong HR, Lahni P, Ungaro R, Lopez MC, et al. Post-natal age is a critical determinant of the neonatal host response to sepsis. Mol Med. (2015) 21:496–504. doi: 10.2119/molmed.2015.00064
Keywords: chorioamnionitis, neonatal sepsis, immunomodulation, very preterm birth, extremely preterm birth, meta-analysis, meta-regression, systematic review
Citation: Villamor-Martinez E, Lubach GA, Rahim OM, Degraeuwe P, Zimmermann LJ, Kramer BW and Villamor E (2020) Association of Histological and Clinical Chorioamnionitis With Neonatal Sepsis Among Preterm Infants: A Systematic Review, Meta-Analysis, and Meta-Regression. Front. Immunol. 11:972. doi: 10.3389/fimmu.2020.00972
Received: 15 November 2019; Accepted: 24 April 2020;
Published: 05 June 2020.
Edited by:
Duc Ninh Nguyen, University of Copenhagen, DenmarkReviewed by:
David Burgner, Royal Children's Hospital, AustraliaCopyright © 2020 Villamor-Martinez, Lubach, Rahim, Degraeuwe, Zimmermann, Kramer and Villamor. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Eduardo Villamor, ZS52aWxsYW1vckBtdW1jLm5s
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.