
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Immunol., 30 April 2020
Sec. T Cell Biology
Volume 11 - 2020 | https://doi.org/10.3389/fimmu.2020.00773
This article is part of the Research TopicNew Insights into Thymic Functions during Stress, Aging, and in Disease SettingsView all 17 articles
The thymus is the central lymphoid organ for T cell development, a cradle of T cells, and for central tolerance establishment, an educator of T cells, maintaining homeostatic cellular immunity. T cell immunity is critical to control cancer occurrence, relapse, and antitumor immunity. Evidence on how aberrant thymic function influences cancer remains largely insufficient, however, there has been recent progress. For example, the involuted thymus results in reduced output of naïve T cells and a restricted T cell receptor (TCR) repertoire, inducing immunosenescence and potentially dampening immune surveillance of neoplasia. In addition, the involuted thymus relatively enhances regulatory T (Treg) cell generation. This coupled with age-related accumulation of Treg cells in the periphery, potentially provides a supportive microenvironment for tumors to escape T cell-mediated antitumor responses. Furthermore, acute thymic involution from chemotherapy can create a tumor reservoir, resulting from an inflammatory microenvironment in the thymus, which is suitable for disseminated tumor cells to hide, survive chemotherapy, and become dormant. This may eventually result in cancer metastatic relapse. On the other hand, if thymic involution is wisely taken advantage of, it may be potentially beneficial to antitumor immunity, since the involuted thymus increases output of self-reactive T cells, which may recognize certain tumor-associated self-antigens and enhance antitumor immunity, as demonstrated through depletion of autoimmune regulator (AIRE) gene in the thymus. Herein, we briefly review recent research progression regarding how altered thymic function modifies T cell immunity against tumors.
T cells are key players in cell-mediated antitumor immunity (1–4) as they have a diverse TCR repertoire specifically recognizing tremendous numbers of tumor neo-antigens, termed TSAs (5, 6), resulting from genomic mutations or viral infection. They can directly kill malignant cells in cytotoxic manners (1, 7, 8) and interact with other tumor-infiltrating immune cells (9) influencing immune surveillance. T cells are thymus-derived, heterogeneous lymphocytes, mainly including αβ-TCR CD4+/CD8+ and γδ-TCR T cells (10). As αβ-TCR T cells are the most abundant and comprehensively studied sub-population involved in antitumor immunity, we focus on this population.
The thymus mediates T cell development and the signals received by thymocytes from thymic stromal cells, primarily TECs, determine thymocyte fate. For example, Notch ligands expressed by TECs provide continuous Notch signals to thymocytes to decide each stage of T-lineage development (11, 12). Interleukin (IL)-7 is a second indispensable factor produced by TECs for the survival, proliferation and differentiation in early stages of T cell development (13, 14). After the completion of TCR rearrangement, the development and differentiation of T cells depend on the interaction between TCR and major histocompatibility complex (MHC)/self-antigens. This interaction leads to establishment of central tolerance via negative selection and regulatory T (Treg) cell selection (15–17). Thymic involution induced by primary TEC defects affects this signaling by impacting lymphostromal interactions. The process of T cell development in the thymus is complex, but there are several important checkpoints that decide successful establishment of immune surveillance and antitumor immunity: (a) αβ-TCR rearrangement to acquire various specificities of antigen recognition; (b) positive selection to achieve MHC restriction; and (c) negative selection/Treg cell generation to establish central tolerance to self (13, 17).
Thymic involution resulting from primary TEC defects occurs in the age-related phenotype, and not only reduces output of naïve T cells (18, 19), but also perturbs the interactions between MHC-II/self-peptide complexes on mTECs and TCRs on thymocytes, thereby altering TCR signaling strength, which impairs thymic negative selection and relatively enhances CD4+ thymic Treg (tTreg) cell generation (20, 21). These changes could lead to declined tumor immune surveillance, potentially attributed to a reduced capacity to recognize neo-antigens and deplete neoplasia. On the other hand, deliberately increasing release of self-reactive conventional T (Tcon) cells that are able to recognize tumor-borne self-antigens could enhance antitumor immunity (22–24). In addition, during aging, the involuted thymus generates relatively increased polyclonal tTreg cells (20), which, coupled with accumulated peripheral Treg (pTreg) cells (25, 26), may infiltrate to tumor mass and establish a microenvironment that suppresses both CD8+ and CD4+ T cell-mediated antitumor immunity, facilitating tumor cell survival (16, 27, 28). This could be related to the higher cancer incidence observed in the elderly (29).
Further, tumor-bearing individuals could be afflicted with cancer-related contributors of acute thymic involution, including (a) increased apoptosis of TECs and thymocytes (30–34) and obstruction of thymocyte maturation (32, 35, 36); and/or (b) chemotherapy-induced non-malignant thymic cellular apoptosis and senescence response (37–39). These will further disrupt antitumor immunity by disrupting T cell development and creating a tumor reservoir in the involuted thymus, allowing for tumor cell dormancy and eventually metastatic relapse (37, 38).
Therefore, thymic conditions impacting T cell immunity are critical issues underlying the high risk for late-life tumor development and the effectiveness (or lack thereof) of antitumor immunotherapy. Revealing the relationship between thymic conditions and T cell-mediated antitumor immunity may facilitate further studies in tumor immunology.
Tumor immune surveillance is an interaction between tumor development and antitumor immunity. The process of tumor immunoediting has three phases: elimination, equilibrium, and escape (40, 41). Elimination is an effective process of immune recognition via antigen-specific identification, and responsiveness to remove neoplasia. However, if T cells are senescent and/or tumor cells evolve into less targetable variants by genetic mutation or epigenetic modifications, the adaptive immune system might only restrain tumor growth, reaching a state of equilibrium. As this process continues it results in the selection of tumor cell variants that are resistant to antitumor response, ushering in the escape phase (40, 41).
T cell immunosenescence is largely attributed to reduced output of naïve T cells from the aged, involuted thymus (18, 42–44), resulting in increased oligoclonal expansion of peripheral memory T cells (45, 46), thereby, restricting TCR repertoire diversity (47, 48). This hampers T cell ability to recognize tumor neo-antigens, resulting from high frequency of somatic mutations in proto-oncogenes and tumor suppressors in tumor cells, and/or from viral antigens produced by virus-induced cancers. These abnormal proteins are called TSAs (5), which are regarded by T cells as foreign antigens. Normally, the T effector (Teff) cell population can recognize tremendous numbers of tumor antigens (5, 6), while the senescent T cell population, with a reduced TCR repertoire diversity, might overlook these antigens. Therefore, one of the potential mechanisms of the reduced cancer immune surveillance is a compromised TCR repertoire generated first by the involuted thymus and exacerbated by age-related peripheral memory cell expansion, which neglects to recognize certain TSAs and fails to eliminate tumors (47, 49, 50) (Figure 1A).
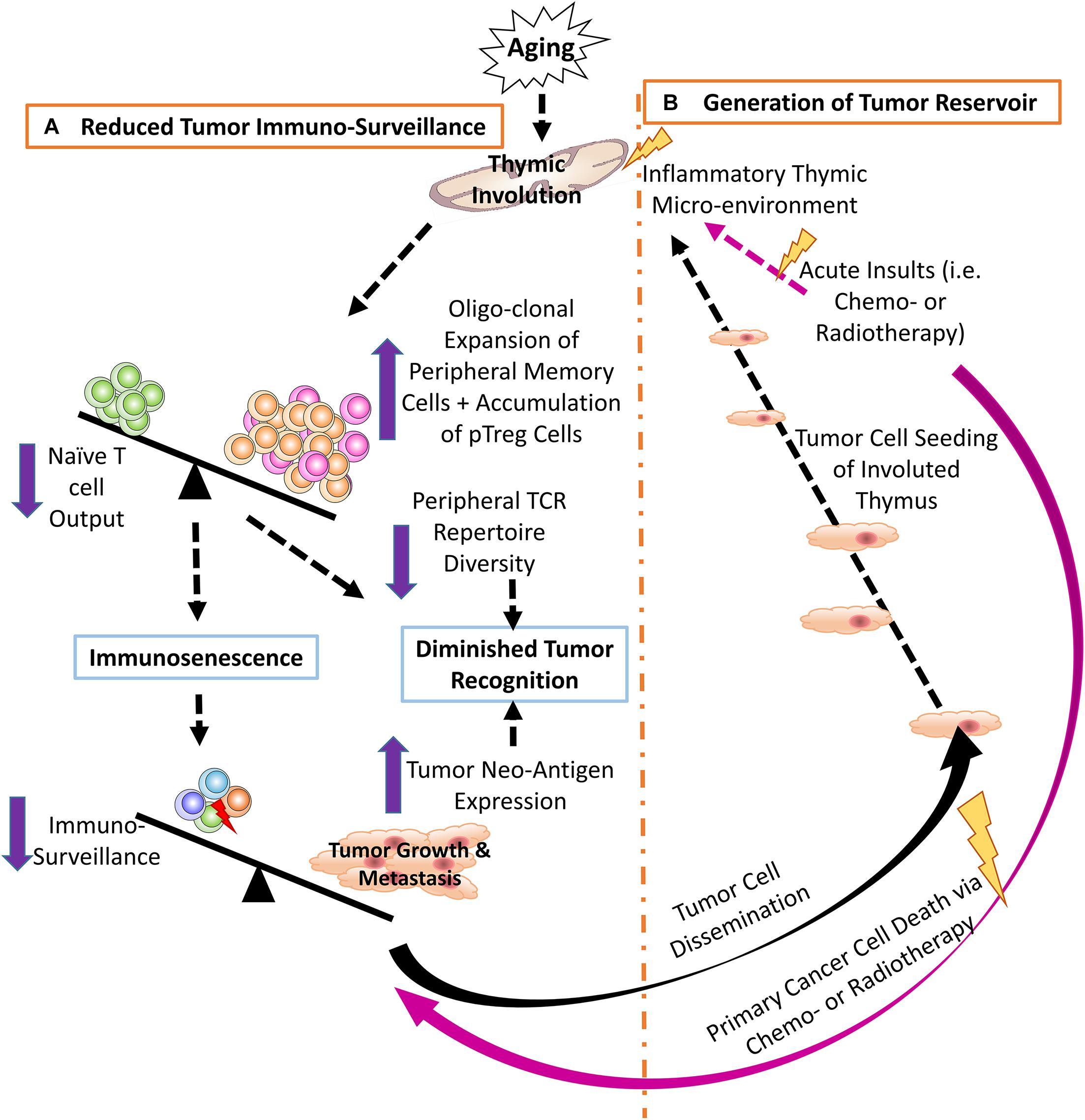
Figure 1. (A) Thymic involution contributes to reduced tumor immune surveillance by participating in immunosenescence and constricting the peripheral TCR repertoire diversity. Additionally, neo-antigens produced by either genomic mutation and/or viral infection create novel tumor antigens that may not be recognized by the reduced pool of naïve T cells in the aged periphery. (B) The involuted thymus acts as a pre-metastatic reservoir for disseminating tumor cells and the inflammatory thymic microenvironment promotes heterogeneous tumor cell dormancy, at both the cellular and population levels.
Recent studies identified several senescent T cell markers: PD-1 and CD153 in murine senescence-associated T (SA-T) cells (51–53). Previously, our knowledge was limited to T cell secondary signaling molecule CD28, which is reduced or absent in senescent T cells. CD28–neg “exhausted” peripheral T cells are accumulated in aged humans (54, 55). These T cells not only lose responsiveness to co-stimulation (56), but also are involved in chronic inflammation (57). The PD-1+CD153+ senescent T cells in mice also exhibit impaired TCR-mediated proliferation and defective IL-2 production, and are biased toward the secretion of pro-inflammatory cytokines, such as IFN-γ (45). It is not clear, however, whether increased PD-1 is directly involved in senescent T cell dysfunction. The generation of SA-T cells is generally attributed to thymic involution and the aged environment (53).
There are two major immunosuppressive mechanisms blocking antitumor immunity: the intrinsic PD-1—PD-L1 axis and the extrinsic Treg—Teff axis (58). A recent finding showed that senescent T cells express increased PD-1 (51–53, 59). This, coupled with increased PD-L1 on tumor cells (60), could lead to an enhanced PD-1/PD-L1 signaling (61), in which the interaction between PD-1/PD-L1 provides a possibility for the anergy, exhaustion, and apoptosis of tumor-reactive T cells (62), thereby, reducing cancer immune surveillance associated with senescent T cells (63). We will discuss the Treg—Teff axis in the following section.
Taken together, thymic involution, immunosenescence, and the declined TCR repertoire diversity, coupled with increased age-related genomic mutations in somatic cells and increased PD-1 expression on senescent T cells in the elderly, contribute to compromised immune surveillance of tumors and the higher late-life tumor incidence.
Thymic involution not only reduces naïve T cell output, but also relatively enhances tTreg generation as displayed by an increased ratio of tTreg versus tTcon in the aged, involuted thymus (20). The basic mechanism is potentially due to altered TCR signaling strength, which may skew CD4+ single positive thymocytes from negative selection to Treg cell generation in the involuted thymus (43). Strong TCR signaling strength, generated by interactions between MHC-II/self-peptide complexes and self-reactive TCRs, induce clonal depletion by negative selection, while intermediate TCR signaling strength induces thymocyte differentiation into CD4+FoxP3+ tTreg cells (17, 64, 65). MHC-II/self-peptide complexes expressed by mTECs are reduced due to mTEC decline in the involuted thymus, resulting in weakened interactions (20, 43, 66). Thus, some self-reactive T clones, which should be negatively selected with strong signaling, survive and differentiate into tTreg cells due to intermediate signaling (20). In addition, such skewing of thymocytes from clonal depletion to Treg generation could modify the TCR repertoire of Treg cells to include certain self-antigens that are also expressed by tumors, enabling these Treg cells to suppress antitumor immunity.
In light of the age-related accumulation of pTreg cells in the periphery (25) and the aging-related enhancement of FoxP3 expression (67), the underlying mechanisms may not be simply due to relatively enhanced tTreg cell generation in the involuted thymus, but also potentially due to declined activation of pro-apoptotic BIM gene (Bcl2 homology-3, BH3-only) (68) via increased methylation (68, 69) during aging. BIM should be activated after each immune reaction (after infection or inflammation, etc.) in order to deplete excess immune cells and return the expanded immune cell numbers to normal levels (70). However, with age, BIM activation in T cells is declined and homeostatic immune rebalance is hindered, resulting in an accumulation of “exhausted” senescent T cells and pTreg cells (25, 26, 71, 72). In addition, conversion of effector memory cells into memory Treg cells might occur in aged people (73). These all increase the pTreg pool (25, 74, 75).
Although Treg cells maintain immunological tolerance by suppressing excessive or aberrant immune responses mediated by Teff cells (76–78), they are opponents of antitumor immunity (79, 80) via their highly immunosuppressive functions against CD8+ cytotoxic T lymphocytes (CTLs) (27, 81, 82). Our current understanding is that Treg cells primarily infiltrate the tumor mass and execute suppressive function (77, 83, 84). Generally, T cell infiltration into the tumor mass correlates to tumor antigen expression. If the cancer mass expresses few neo-antigens, then greater numbers of Treg cells infiltrate to form a Treg-dominant tumor microenvironment; whereas, if the cancer mass expresses abundant neo-antigens, fewer Treg cells infiltrate, and more effector cells including CD8+ T cells can be primed and expand in the tumor tissues (16, 85, 86). Tumor-infiltrating Treg cells are thought to be recruited from the preexisting thymus-derived Treg population, including autoimmune regulator gene (Aire)-dependent TAA-specific Treg cells (87–89), rather than from peripherally induced tumor-specific Teff cells. Therefore, central tolerance is implicated in impaired antitumor responses.
Removal of Treg cells (with monoclonal antibodies, such as anti-CD25 (90), or other means) enhances T cell antitumor responses (15, 16, 91). However, anti-CD25 antibodies potentially eliminate activated Teff cells, expressing CD25 (92). Targeted functional inactivation of Treg cells based on constitutively expressed molecules including CTLA-4, GITR, TLR8 and OX40 (93–97) is a better means to nullify Treg cell function without decreasing Treg cell numbers from surrounding Teff cells (15), nor effecting Teff cell numbers. That is why anti-CTLA-4 (98) can serve as another immune checkpoint inhibitor to reduce Treg cell activation and be used for tumor immunotherapy (99).
Although direct evidence is still lacking about whether increased tTreg cells play a role in suppressing antitumor immunity, 80 – 95% of pTreg cells are derived from thymic generated tTreg cells bearing a thymic imprint (17, 64). Therefore, relative enhancement of tTreg cell generation resulting from thymic involution is a risk factor for suppressing antitumor immunity that ought not be overlooked.
Metastatic relapse occurs when the same type of cancer recurs at a distant location (100) several years after removal of the primary tumor and adjuvant chemotherapy (101, 102), and this results mainly from chemo-resistance obtained by cancer cells in an inflammatory microenvironment (37, 38). Relapse, an immense clinical challenge, is responsible for 90% of cancer-associated mortality (103, 104). It means that cancer cells may still exist for a silent period after the primary treatment. Tumor pre-metastatic niches or reservoirs permit these silent cancer cells to hide and acquire chemo-resistance. Recently, several organs, such as the perivascular space of blood vessels in the lung and liver (105, 106) and bone marrow (107, 108), have been determined to be such reservoirs. We (37) and others (38) found that the involuted thymus is another tumor reservoir that allows for silent primary tumor cells to find safe-harbor.
Cancer cells circulating in the blood stream (109, 110) enter the thymus creating a heterogeneous environment, including malignant cancer cells and non-malignant thymic cells (TECs and thymocytes). Since the thymus contains mostly immature T cells and possesses semi-immune privilege, the cancer cells cannot be thoroughly eradicated by immune surveillance (37). In addition, the thymus is sensitive to many insults that cause involution. One of strongest insults is chemotherapy. In addition to killing cancer cells, systemic chemotherapy also results in non-malignant cell death and/or senescence due to DNA damage (111, 112), which produces an inflammatory microenvironment. This induces chemo-resistant dormancy in the sojourning cancer cells (38, 105, 113, 114) (Figure 1B). Dormancy occurs at two levels (101, 108): (a) at the single-cell level, in which the dormant cancer cells exist in a quiescent state of G0 – G1 arrest (101), with increased MAPK p38 and decreased ERK, (conventional dormancy); and (b) at the population level, in which cancer cell proliferation is balanced by apoptosis (dynamic dormancy) resulting in an overall unchanged total cancer cell number (115), i.e., immune equilibrium (116, 117).
Our research found that thymic-sojourning disseminated solid tumor cells show a heterogeneous dormancy phenotype, some being quiescent with features of conventional dormancy, such as increased ratio of p38/ERK (activation of p38 and inhibition of ERK), inducing tumor growth arrest (113, 118, 119), while some either propagate or undergo apoptosis with features of dynamic dormancy (37). Together, chemotherapy-induced acute thymic involution provides a chemo-resistant microenvironment for tumor dormancy, creating a pre-metastatic reservoir. Although the distinct dormancy mechanism underlying the heterogeneity of dormant tumor cells (being quiescent and dynamic) needs further investigation, these observations provide a new therapeutic target for preventing cancer relapse and metastasis.
Since cancer is derived from self-tissues, pathogenic tumor cells are oftentimes carrying “self”-antigens, i.e., TAAs, and can be recognized by most self-reactive Teff cells that are deleted by negative selection in the thymus. Thus, this has led several groups to posit that disruption of central tolerance might further the ability of the T cell compartment to combat cancers (87, 120–122). In this regard, most of the recent studies focus on targeting Aire-expressing mTECs in the thymus.
Medullary TECs highly express Aire, allowing them to promiscuously present self-antigens to self-reactive T clones during negative selection for central tolerance establishment (13, 21, 123). Though the full scope of this process remains to be elucidated, it is readily accepted that Aire deficiency facilitates increased self-reactive T cell release enhancing immunity to certain cancers. One recent technique targets mTECs specifically via anti-RANK-Ligand treatments, which transiently deplete Aire-expressing mTECs (22, 121, 124). Because the anti-RANK-Ligand reagent is already FDA-approved, albeit for osteoporosis (125), it has potential to be easily translated to cancer patients. This strategy is also promising because the depletion is brief, with mTECs normally replenished within 2 weeks (126, 127) and full recovery observed 10 weeks after cessation of anti-RANK-Ligand treatment (22). This tactic was tested in animal models of melanoma, since several of the melanoma antigens, including gp100 and TRP-1, are controlled by Aire (23, 122) and up-regulated in melanoma cells (122). Importantly, many of these studies used anti-RANK-Ligand in combination with peripheral therapies, such as checkpoint inhibitors, demonstrating greatly improved outcome in comparison to peripheral treatment alone. However, it is obvious that central therapy alone is not sufficient for tumor immunotherapy (121).
One caveat to this type of strategy is the recent finding that other transcriptional regulators are implicated in promiscuous self-antigen expression in the thymus, for example, forebrain embryonic zinc fingerlike protein 2 (Fezf2) (128). There are not many reports on what Fezf2 disruption would accomplish in regards to heightened TAA targeting as observed with the above Aire-targeting studies. There is evidence that Fezf2 is independent of the RANK/CD40/Aire axis which implies that an anti-RANK-Ligand therapy may not be as effective for disrupting Fezf2-dependent self-antigen expression (129).
The obvious risk for disruption of central tolerance is increased incidence of autoimmunity (130, 131), which is one of the underlying players in inflammaging in the elderly (66). This is clearly seen in patients who have mutations in AIRE (132) and has been recently demonstrated in mice who lack Fezf2 (128). Another challenge to strategies that manipulate central tolerance is that some TAAs are not under the control of Aire, such as TRP-2 (122), and some may be under the regulation of factors that have yet to be identified.
Additionally, we know that tumor antigens not only include TAAs (“self”-antigens), but also TSAs (“foreign”/neo-antigens), which are recognized by T cells as foreign antigens (133, 134). Therefore, deletion of Aire expression cannot induce antitumor immunity to non-Aire-controlled TAA-bearing tumors nor for TSA-bearing tumors. This limits the scope of cancers that would benefit from such a strategy, and also supports studies that use combinative central and peripheral immunotherapies. Finally, it is important to also take age-related peripheral changes into account, as many other age-related changes may offset the potential benefits of such central tolerance manipulation therapies. Therefore, several potential avenues of research remain for this type of cancer immunotherapy.
We have briefly reviewed some of the potential impacts of thymic involution (chronic age-related or acute chemotherapy-induced) on cancer and attempt to pave the way for further studies in tumor immunology. Since cancer and thymic atrophy are both associated with age, there is potential for a deeper connection. For instance, it is interesting to consider that most cancers develop in older adults, long after thymic involution has progressed. Since thymic involution is associated with declined mTEC cellularity and Aire expression in mTECs (66, 135), it raises the question of why there is not a natural increase in antitumor immunity in the elderly due to the defects in negative selection in the aged thymus. In addition, chemotherapy also induces TEC-impaired thymic involution (37) and declined Aire expression in tumor-bearing mice treated with doxorubicin (our unpublished observation). Why, then, do we not see enhanced antitumor T cell generation? Further, estrogen has recently been identified as a repressor of Aire (136, 137), possibly explaining the sex-related tendencies for higher autoimmune disease incidence in women. Does this correlate with a lower incidence for development of certain TAA-expressing cancers in post-menopausal women? In addition, whether we can manipulate thymic function to better target tumor-infiltrating Treg cells by weakening tTreg generation or harness newly generated Teff cells to home to the tumor is in need of further study. Finally, since the tumor microenvironment exerts such strong immunosuppressive signals, how can immunotherapies be tailored to overcome those signals in a tumor-specific manner without breaking peripheral tolerance completely. Moreover, many important questions remain in our understanding of the crosstalk of aging, cancer, and the impacts of thymic involution on late-life cancers.
D-MS: conceptualization and supervision. WW, RT, and D-MS: writing the original draft. OS: provide assistances. RT: visualization and proofreading.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
AIRE, autoimmune regulator; ERK, extracellular signal-regulated kinases; MAPK, mitogen-activated protein kinases; MHC-II, major histocompatibility complex class-II; mTECs, medullary TECs; PD-1, programmed cell death protein 1; PD-L1, programmed death-ligand 1; pTreg, peripheral Treg cells; SA-T, senescence-associated T cells; TAA, tumor-associated antigen; Tcon, conventional T cells; TCR, T cell receptor; TECs, thymic epithelial cells; Teff, T effector cells; Treg, regulatory T cells; TSA, tumor-specific antigen; tTreg, thymic regulatory T cells.
1. Durgeau A, Virk Y, Corgnac S, Mami-Chouaib F. Recent advances in targeting cd8 t-cell immunity for more effective cancer immunotherapy. Front Immunol. (2018) 9:14. doi: 10.3389/fimmu.2018.00014
2. Kunzmann V, Bauer E, Feurle J, Weissinger F, Tony HP, Wilhelm M. Stimulation of gammadelta T cells by aminobisphosphonates and induction of antiplasma cell activity in multiple myeloma. Blood. (2000) 96:384–92. doi: 10.1182/blood.V96.2.384
3. Minetto P, Guolo F, Pesce S, Greppi M, Obino V, Ferretti E, et al. Harnessing NK cells for cancer treatment. Front Immunol. (2019) 10:2836. doi: 10.3389/fimmu.2019.02836
4. Godfrey DI, Koay HF, McCluskey J, Gherardin NA. The biology and functional importance of MAIT cells. Nat Immunol. (2019) 20:1110–28. doi: 10.1038/s41590-019-0444-8
5. Coulie PG, Van den Eynde BJ, van der Bruggen P, Boon T. Tumour antigens recognized by T lymphocytes: at the core of cancer immunotherapy. Nat Rev Cancer. (2014) 14:135–46. doi: 10.1038/nrc3670
6. Zhang H, Liu L, Zhang J, Chen J, Ye J, Shukla S, et al. Investigation of antigen-specific T-cell receptor clusters in human cancers. Clin Cancer Res. (2019) 26:3249. doi: 10.1158/1078-0432.CCR-19-3249
7. Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. (2004) 21:137–48. doi: 10.1016/j.immuni.2004.07.017
8. Farhood B, Najafi M, Mortezaee K. CD8(+) cytotoxic T lymphocytes in cancer immunotherapy: a review. J Cell Physiol. (2019) 234:8509–21. doi: 10.1002/jcp.27782
9. Eisel D, Das K, Dickes E, Konig R, Osen W, Eichmuller SB. Cognate interaction with CD4(+) T cells instructs tumor-associated macrophages to acquire M1-like phenotype. Front Immunol. (2019) 10:219. doi: 10.3389/fimmu.2019.00219
10. Kreslavsky T, Gleimer M, von Boehmer H. Alphabeta versus gammadelta lineage choice at the first TCR-controlled checkpoint. Curr Opin Immunol. (2010) 22:185–92. doi: 10.1016/j.coi.2009.12.006
11. Sambandam A, Maillard I, Zediak VP, Xu L, Gerstein RM, Aster JC, et al. Notch signaling controls the generation and differentiation of early T lineage progenitors. Nat Immunol. (2005) 6:663–70. doi: 10.1038/ni1216
12. Rothenberg EV. Programming for T-lymphocyte fates: modularity and mechanisms. Genes Dev. (2019) 33:1117–35. doi: 10.1101/gad.327163.119
13. Koch U, Radtke F. Mechanisms of T cell development and transformation. Annu Rev Cell Dev Biol. (2011) 27:539–62. doi: 10.1146/annurev-cellbio-092910-154008
14. Boudil A, Matei IR, Shih HY, Bogdanoski G, Yuan JS, Chang SG, et al. IL-7 coordinates proliferation, differentiation and Tcra recombination during thymocyte beta-selection. Nat Immunol. (2015) 16:397–405. doi: 10.1038/ni.3122
15. Colombo MP, Piconese S. Regulatory-T-cell inhibition versus depletion: the right choice in cancer immunotherapy. Nat Rev Cancer. (2007) 7:880–7. doi: 10.1038/nrc2250
16. Takeuchi Y, Nishikawa H. Roles of regulatory T cells in cancer immunity. Int Immunol. (2016) 28:401–9. doi: 10.1093/intimm/dxw025
17. Klein L, Robey EA, Hsieh CS. Central CD4(+) T cell tolerance: deletion versus regulatory T cell differentiation. Nat Rev Immunol. (2019) 19:7–18. doi: 10.1038/s41577-018-0083-6
18. Hale JS, Boursalian TE, Turk GL, Fink PJ. Thymic output in aged mice. Proc Natl Acad Sci USA. (2006) 103:8447–52. doi: 10.1073/pnas.0601040103
19. Petrie HT. Role of thymic organ structure and stromal composition in steady-state postnatal T-cell production. Immunol Rev. (2002) 189:8–19. doi: 10.1034/j.1600-065x.2002.18902.x
20. Oh J, Wang W, Thomas R, Su DM. Capacity of tTreg generation is not impaired in the atrophied thymus. PLoS Biol. (2017) 15:e2003352. doi: 10.1371/journal.pbio.2003352
21. Hinterberger M, Aichinger M, Prazeres O, da Costa D, Hoffmann R, Klein L. Autonomous role of medullary thymic epithelial cells in central CD4(+) T cell tolerance. Nat Immunol. (2010) 11:512–9. doi: 10.1038/ni.1874
22. Khan IS, Mouchess ML, Zhu ML, Conley B, Fasano KJ, Hou Y, et al. Enhancement of an anti-tumor immune response by transient blockade of central T cell tolerance. J Exp Med. (2014) 211:761–8. doi: 10.1084/jem.20131889
23. Zhu ML, Nagavalli A, Su MA. Aire deficiency promotes TRP-1-specific immune rejection of melanoma. Cancer Res. (2013) 73:2104–16. doi: 10.1158/0008-5472.CAN-12-3781
24. Su MA, Anderson MS. Breaking through the central tolerance ceiling to unleash anticancer immune responses. Oncoimmunology. (2014) 3:e950169. doi: 10.4161/21624011.2014.950169
25. Raynor J, Lages CS, Shehata H, Hildeman DA, Chougnet CA. Homeostasis and function of regulatory T cells in aging. Curr Opin Immunol. (2012) 24:482–7. doi: 10.1016/j.coi.2012.04.005
26. Chougnet CA, Tripathi P, Lages CS, Raynor J, Sholl A, Fink P, et al. A major role for Bim in regulatory T cell homeostasis. J Immunol. (2011) 186:156–63. doi: 10.4049/jimmunol.1001505
27. Liu F, Lang R, Zhao J, Zhang X, Pringle GA, Fan Y, et al. CD8(+) cytotoxic T cell and FOXP3(+) regulatory T cell infiltration in relation to breast cancer survival and molecular subtypes. Breast Cancer Res Treat. (2011) 130:645–55. doi: 10.1007/s10549-011-1647-3
28. Curiel TJ. Regulatory T cells and treatment of cancer. Curr Opin Immunol. (2008) 20:241–6. doi: 10.1016/j.coi.2008.04.008
29. White MC, Holman DM, Boehm JE, Peipins LA, Grossman M, Henley SJ. Age and cancer risk: a potentially modifiable relationship. Am J Prev Med. (2014) 46(3 Suppl. 1):S7–15. doi: 10.1016/j.amepre.2013.10.029
30. Song Y, Yu R, Wang C, Chi F, Guo Z, Zhu X. Disruption of the thymic microenvironment is associated with thymic involution of transitional cell cancer. Urol Int. (2014) 92:104–15. doi: 10.1159/000353350
31. Carrio R, Torroella-Kouri M, Iragavarapu-Charyulu V, Lopez DM. Tumor-induced thymic atrophy: alteration in interferons and Jak/Stats signaling pathways. Int J Oncol. (2011) 38:547–53. doi: 10.3892/ijo.2010.870
32. Mandal D, Bhattacharyya A, Lahiry L, Choudhuri T, Sa G, Das T. Failure in peripheral immuno-surveillance due to thymic atrophy: importance of thymocyte maturation and apoptosis in adult tumor-bearer. Life Sci. (2005) 77:2703–16. doi: 10.1016/j.lfs.2005.05.038
33. Prins RM, Graf MR, Merchant RE, Black KL, Wheeler CJ. Thymic function and output of recent thymic emigrant T cells during intracranial glioma progression. J Neurooncol. (2003) 64:45–54. doi: 10.1007/bf02700019
34. Lopez DM, Charyulu V, Adkins B. Influence of breast cancer on thymic function in mice. J Mammary Gland Biol Neoplasia. (2002) 7:191–9. doi: 10.1023/a:1020356020542
35. Ohm JE, Gabrilovich DI, Sempowski GD, Kisseleva E, Parman KS, Nadaf S, et al. VEGF inhibits T-cell development and may contribute to tumor-induced immune suppression. Blood. (2003) 101:4878–86. doi: 10.1182/blood-2002-07-1956
36. Sun QL, Charyulu V, Lobo D, Lopez DM. Role of thymic stromal cell dysfunction in the thymic involution of mammary tumor-bearing mice. Anticancer Res (2002) 22:91–6.
37. Sizova O, Kuriatnikov D, Liu Y, Su DM. Atrophied thymus, a tumor reservoir for harboring melanoma cells. Mol Cancer Res. (2018) 16:1652–64. doi: 10.1158/1541-7786.MCR-18-0308
38. Gilbert LA, Hemann MT. DNA damage-mediated induction of a chemoresistant niche. Cell. (2010) 143:355–66. doi: 10.1016/j.cell.2010.09.043
39. Bent EH, Gilbert LA, Hemann MT. A senescence secretory switch mediated by PI3K/AKT/mTOR activation controls chemoprotective endothelial secretory responses. Genes Dev. (2016) 30:1811–21. doi: 10.1101/gad.284851.116
40. Swann JB, Smyth MJ. Immune surveillance of tumors. J Clin Invest. (2007) 117:1137–46. doi: 10.1172/JCI31405
41. Miller JF, Sadelain M. The journey from discoveries in fundamental immunology to cancer immunotherapy. Cancer Cell. (2015) 27:439–49. doi: 10.1016/j.ccell.2015.03.007
42. Linton PJ, Dorshkind K. Age-related changes in lymphocyte development and function. Nat Immunol. (2004) 5:133–9. doi: 10.1038/ni1033
43. Thomas R, Wang W, Su DM. Contributions of age-related thymic involution to immunosenescence and inflammaging. Immun Ageing. (2020) 17:2. doi: 10.1186/s12979-020-0173-8
44. Pawelec G. Age and immunity: What is “immunosenescence”? Exp Gerontol. (2018) 105:4–9. doi: 10.1016/j.exger.2017.10.024
45. Fukushima Y, Minato N, Hattori M. The impact of senescence-associated T cells on immunosenescence and age-related disorders. Inflamm Regen. (2018) 38:24. doi: 10.1186/s41232-018-0082-9
46. Fulop T, Larbi A, Dupuis G, Le Page A, Frost EH, Cohen AA, et al. Immunosenescence and inflamm-aging as two sides of the same coin: friends or foes? Front Immunol. (2017) 8:1960. doi: 10.3389/fimmu.2017.01960
47. Pawelec G. Does patient age influence anti-cancer immunity? Semin Immunopathol. (2019) 41:125–31. doi: 10.1007/s00281-018-0697-6
48. Hurez V, Padron AS, Svatek RS, Curiel TJ. Considerations for successful cancer immunotherapy in aged hosts. Clin Exp Immunol. (2017) 187:53–63. doi: 10.1111/cei.12875
49. Schreiber K, Karrison TG, Wolf SP, Kiyotani K, Steiner M, Littmann ER, et al. Impact of TCR diversity on the development of transplanted or chemically induced tumors. Cancer Immunol Res. (2020) 8:192–202. doi: 10.1158/2326-6066.CIR-19-0567
50. Palmer S, Albergante L, Blackburn CC, Newman TJ. Thymic involution and rising disease incidence with age. Proc Natl Acad Sci USA. (2018) 115:1883–8. doi: 10.1073/pnas.1714478115
51. Shimatani K, Nakashima Y, Hattori M, Hamazaki Y, Minato N. PD-1+ memory phenotype CD4+ T cells expressing C/EBPalpha underlie T cell immunodepression in senescence and leukemia. Proc Natl Acad Sci USA. (2009) 106:15807–12. doi: 10.1073/pnas.0908805106
52. Tahir S, Fukushima Y, Sakamoto K, Sato K, Fujita H, Inoue J, et al. A CD153+CD4+ T follicular cell population with cell-senescence features plays a crucial role in lupus pathogenesis via osteopontin production. J Immunol. (2015) 194:5725–35. doi: 10.4049/jimmunol.1500319
53. Sato K, Kato A, Sekai M, Hamazaki Y, Minato N. Physiologic thymic involution underlies age-dependent accumulation of senescence-associated CD4(+) T Cells. J Immunol. (2017) 199:138–48. doi: 10.4049/jimmunol.1602005
54. Weng NP, Akbar AN, Goronzy J. CD28(-) T cells: their role in the age-associated decline of immune function. Trends Immunol. (2009) 30:306–12. doi: 10.1016/j.it.2009.03.013
55. Vallejo AN. CD28 extinction in human T cells: altered functions and the program of T-cell senescence. Immunol Rev. (2005) 205:158–69. doi: 10.1111/j.0105-2896.2005.00256.x
56. Engwerda CR, Handwerger BS, Fox BS. Aged T cells are hyporesponsive to costimulation mediated by CD28. J Immunol. (1994) 152:3740–7.
57. Callender LA, Carroll EC, Beal RWJ, Chambers ES, Nourshargh S, Akbar AN, et al. Human CD8(+) EMRA T cells display a senescence-associated secretory phenotype regulated by p38 MAPK. Aging Cell. (2018) 17:e12675. doi: 10.1111/acel.12675
58. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. (2012) 12:252–64. doi: 10.1038/nrc3239
59. Kasakovski D, Xu L, Li Y. T cell senescence and CAR-T cell exhaustion in hematological malignancies. J Hematol Oncol. (2018) 11:91. doi: 10.1186/s13045-018-0629-x
60. Dong P, Xiong Y, Yue J, Hanley SJB, Watari H. Tumor-Intrinsic PD-L1 signaling in cancer initiation, development and treatment: beyond immune evasion. Front Oncol. (2018) 8:386. doi: 10.3389/fonc.2018.00386
61. Sharpe AH, Pauken KE. The diverse functions of the PD1 inhibitory pathway. Nat Rev Immunol. (2018) 18:153–67. doi: 10.1038/nri.2017.108
62. Ostrand-Rosenberg S, Horn LA, Haile ST. The programmed death-1 immune-suppressive pathway: barrier to antitumor immunity. J Immunol. (2014) 193:3835–41. doi: 10.4049/jimmunol.1401572
63. Minato N, Hattori M, Hamazaki Y. Physiology and pathology of T-cell aging. Int Immunol (2020) dxaa006. doi: 10.1093/intimm/dxaa006
64. Pohar J, Simon Q, Fillatreau S. Antigen-specificity in the thymic development and peripheral activity of cd4(+)foxp3(+) t regulatory cells. Front Immunol. (2018) 9:1701. doi: 10.3389/fimmu.2018.01701
65. Wirnsberger G, Hinterberger M, Klein L. Regulatory T-cell differentiation versus clonal deletion of autoreactive thymocytes. Immunol Cell Biol. (2011) 89:45–53. doi: 10.1038/icb.2010.123
66. Coder BD, Wang H, Ruan L, Su DM. Thymic involution perturbs negative selection leading to autoreactive T cells that induce chronic inflammation. J Immunol. (2015) 194:5825–37. doi: 10.4049/jimmunol.1500082
67. Garg SK, Delaney C, Toubai T, Ghosh A, Reddy P, Banerjee R, et al. Aging is associated with increased regulatory T-cell function. Aging Cell. (2014) 13:441–8. doi: 10.1111/acel.12191
68. Sionov RV, Vlahopoulos SA, Granot Z. Regulation of bim in health and disease. Oncotarget. (2015) 6:23058–134. doi: 10.18632/oncotarget.5492
69. Paschos K, Smith P, Anderton E, Middeldorp JM, White RE, Allday MJ. Epstein-barr virus latency in B cells leads to epigenetic repression and CpG methylation of the tumour suppressor gene Bim. PLoS Pathog. (2009) 5:e1000492. doi: 10.1371/journal.ppat.1000492
70. Bouillet P, O’Reilly LA. CD95, BIM and T cell homeostasis. Nat Rev Immunol. (2009) 9:514–9. doi: 10.1038/nri2570
71. Tsukamoto H, Clise-Dwyer K, Huston GE, Duso DK, Buck AL, Johnson LL, et al. Age-associated increase in lifespan of naive CD4 T cells contributes to T-cell homeostasis but facilitates development of functional defects. Proc Natl Acad Sci USA. (2009) 106:18333–8. doi: 10.1073/pnas.0910139106
72. Tsukamoto H, Huston GE, Dibble J, Duso DK, Swain SL. Bim dictates naive CD4 T cell lifespan and the development of age-associated functional defects. J Immunol. (2010) 185:4535–44. doi: 10.4049/jimmunol.1001668
73. Vukmanovic-Stejic M, Zhang Y, Cook JE, Fletcher JM, McQuaid A, Masters JE, et al. Human CD4+ CD25hi Foxp3+ regulatory T cells are derived by rapid turnover of memory populations in vivo. J Clin Invest. (2006) 116:2423–33. doi: 10.1172/JCI28941
74. Gregg R, Smith CM, Clark FJ, Dunnion D, Khan N, Chakraverty R, et al. The number of human peripheral blood CD4+ CD25high regulatory T cells increases with age. Clin Exp Immunol. (2005) 140:540–6. doi: 10.1111/j.1365-2249.2005.02798.x
75. Fessler J, Ficjan A, Duftner C, Dejaco C. The impact of aging on regulatory T-cells. Front Immunol. (2013) 4:231. doi: 10.3389/fimmu.2013.00231
76. Kumar P, Bhattacharya P, Prabhakar BS. A comprehensive review on the role of co-signaling receptors and Treg homeostasis in autoimmunity and tumor immunity. J Autoimmun. (2018) 95:77–99. doi: 10.1016/j.jaut.2018.08.007
77. Yamaguchi T, Sakaguchi S. Regulatory T cells in immune surveillance and treatment of cancer. Semin Cancer Biol. (2006) 16:115–23. doi: 10.1016/j.semcancer.2005.11.005
78. Okeke EB, Uzonna JE. The pivotal role of regulatory T cells in the regulation of innate immune cells. Front Immunol. (2019) 10:680. doi: 10.3389/fimmu.2019.00680
79. Wolf D, Sopper S, Pircher A, Gastl G, Wolf AM. Treg(s) in cancer: friends or foe? J Cell Physiol. (2015) 230:2598–605. doi: 10.1002/jcp.25016
80. Wang HY, Wang RF. Regulatory T cells and cancer. Curr Opin Immunol. (2007) 19:217–23. doi: 10.1016/j.coi.2007.02.004
81. Turk MJ, Guevara-Patino JA, Rizzuto GA, Engelhorn ME, Sakaguchi S, Houghton AN. Concomitant tumor immunity to a poorly immunogenic melanoma is prevented by regulatory T cells. J Exp Med. (2004) 200:771–82. doi: 10.1084/jem.20041130
82. Nishikawa H, Sakaguchi S. Regulatory T cells in tumor immunity. Int J Cancer. (2010) 127:759–67. doi: 10.1002/ijc.25429
83. Liu Q, Du F, Huang W, Ding X, Wang Z, Yan F, et al. Epigenetic control of Foxp3 in intratumoral T-cells regulates growth of hepatocellular carcinoma. Aging (Albany NY). (2019) 11:2343–51. doi: 10.18632/aging.101918
84. Chaudhary B, Abd Al Samid M, Al-Ramadi BK, Elkord E. Phenotypic alterations, clinical impact and therapeutic potential of regulatory T cells in cancer. Expert Opin Biol Ther. (2014) 14:931–45. doi: 10.1517/14712598.2014.900539
85. Nishikawa H, Kato T, Tawara I, Saito K, Ikeda H, Kuribayashi K, et al. Definition of target antigens for naturally occurring CD4(+) CD25(+) regulatory T cells. J Exp Med. (2005) 201:681–6. doi: 10.1084/jem.20041959
86. Nishikawa H, Tanida K, Ikeda H, Sakakura M, Miyahara Y, Aota T, et al. Role of SEREX-defined immunogenic wild-type cellular molecules in the development of tumor-specific immunity. Proc Natl Acad Sci USA. (2001) 98:14571–6. doi: 10.1073/pnas.251547298
87. Malchow S, Leventhal DS, Nishi S, Fischer BI, Shen L, Paner GP, et al. Aire-dependent thymic development of tumor-associated regulatory T cells. Science. (2013) 339:1219–24. doi: 10.1126/science.1233913
88. Hindley JP, Ferreira C, Jones E, Lauder SN, Ladell K, Wynn KK, et al. Analysis of the T-cell receptor repertoires of tumor-infiltrating conventional and regulatory T cells reveals no evidence for conversion in carcinogen-induced tumors. Cancer Res. (2011) 71:736–46. doi: 10.1158/0008-5472.CAN-10-1797
89. Wainwright DA, Sengupta S, Han Y, Lesniak MS. Thymus-derived rather than tumor-induced regulatory T cells predominate in brain tumors. Neuro Oncol. (2011) 13:1308–23. doi: 10.1093/neuonc/nor134
90. Onizuka S, Tawara I, Shimizu J, Sakaguchi S, Fujita T, Nakayama E. Tumor rejection by in vivo administration of anti-CD25 (interleukin-2 receptor alpha) monoclonal antibody. Cancer Res. (1999) 59:3128–33.
91. Freeman ZT, Nirschl TR, Hovelson DH, Johnston RJ, Engelhardt JJ, Selby MJ, et al. A conserved intratumoral regulatory T cell signature identifies 4-1BB as a pan-cancer target. J Clin Invest. (2020) 130:1405–16. doi: 10.1172/JCI128672
92. Betts G, Twohig J, Van den Broek M, Sierro S, Godkin A, Gallimore A. The impact of regulatory T cells on carcinogen-induced sarcogenesis. Br J Cancer. (2007) 96:1849–54. doi: 10.1038/sj.bjc.6603824
93. Takahashi T, Tagami T, Yamazaki S, Uede T, Shimizu J, Sakaguchi N, et al. Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. (2000) 192:303–10. doi: 10.1084/jem.192.2.303
94. Shimizu J, Yamazaki S, Takahashi T, Ishida Y, Sakaguchi S. Stimulation of CD25(+)CD4(+) regulatory T cells through GITR breaks immunological self-tolerance. Nat Immunol. (2002) 3:135–42. doi: 10.1038/ni759
95. Ko K, Yamazaki S, Nakamura K, Nishioka T, Hirota K, Yamaguchi T, et al. Treatment of advanced tumors with agonistic anti-GITR mAb and its effects on tumor-infiltrating Foxp3+CD25+CD4+ regulatory T cells. J Exp Med. (2005) 202:885–91. doi: 10.1084/jem.20050940
96. Peng G, Guo Z, Kiniwa Y, Voo KS, Peng W, Fu T, et al. Toll-like receptor 8-mediated reversal of CD4+ regulatory T cell function. Science. (2005) 309:1380–4. doi: 10.1126/science.1113401
97. Valzasina B, Guiducci C, Dislich H, Killeen N, Weinberg AD, Colombo MP. Triggering of OX40 (CD134) on CD4(+)CD25+ T cells blocks their inhibitory activity: a novel regulatory role for OX40 and its comparison with GITR. Blood. (2005) 105:2845–51. doi: 10.1182/blood-2004-07-2959
98. Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. (1996) 271:1734–6. doi: 10.1126/science.271.5256.1734
99. Seidel JA, Otsuka A, Kabashima K. Anti-PD-1 and Anti-CTLA-4 therapies in cancer: mechanisms of action. Efficacy, and limitations. Front Oncol. (2018) 8:86. doi: 10.3389/fonc.2018.00086
100. Giancotti FG. Mechanisms governing metastatic dormancy and reactivation. Cell. (2013) 155:750–64. doi: 10.1016/j.cell.2013.10.029
101. Aguirre-Ghiso JA. Models, mechanisms and clinical evidence for cancer dormancy. Nat Rev Cancer. (2007) 7:834–46. doi: 10.1038/nrc2256
102. Pantel K, Brakenhoff RH. Dissecting the metastatic cascade. Nat Rev Cancer. (2004) 4:448–56. doi: 10.1038/nrc1370
103. Steeg PS. Tumor metastasis: mechanistic insights and clinical challenges. Nat Med. (2006) 12:895–904. doi: 10.1038/nm1469
104. Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, et al. Cancer statistics, 2006. CA Cancer J Clin. (2006) 56:106–30. doi: 10.3322/canjclin.56.2.106
105. Ghajar CM, Peinado H, Mori H, Matei IR, Evason KJ, Brazier H, et al. The perivascular niche regulates breast tumour dormancy. Nat Cell Biol. (2013) 15:807–17. doi: 10.1038/ncb2767
106. Piccioli A, Maccauro G, Spinelli MS, Biagini R, Rossi B. Bone metastases of unknown origin: epidemiology and principles of management. J Orthop Traumatol. (2015) 16:81–6. doi: 10.1007/s10195-015-0344-0
107. Shiozawa Y, Eber MR, Berry JE, Taichman RS. Bone marrow as a metastatic niche for disseminated tumor cells from solid tumors. Bonekey Rep. (2015) 4:689. doi: 10.1038/bonekey.2015.57
108. Marlow R, Honeth G, Lombardi S, Cariati M, Hessey S, Pipili A, et al. A novel model of dormancy for bone metastatic breast cancer cells. Cancer Res. (2013) 73:6886–99. doi: 10.1158/0008-5472.CAN-13-0991
109. Pantel K, Alix-Panabieres C. Circulating tumour cells in cancer patients: challenges and perspectives. Trends Mol Med. (2010) 16:398–406. doi: 10.1016/j.molmed.2010.07.001
110. Alix-Panabieres C, Schwarzenbach H, Pantel K. Circulating tumor cells and circulating tumor DNA. Annu Rev Med. (2012) 63:199–215. doi: 10.1146/annurev-med-062310-094219
111. Perez-Mancera PA, Young AR, Narita M. Inside and out: the activities of senescence in cancer. Nat Rev Cancer. (2014) 14:547–58. doi: 10.1038/nrc3773
112. Klemm F, Joyce JA. Microenvironmental regulation of therapeutic response in cancer. Trends Cell Biol. (2015) 25:198–213. doi: 10.1016/j.tcb.2014.11.006
113. Yumoto K, Eber MR, Berry JE, Taichman RS, Shiozawa Y. Molecular pathways: niches in metastatic dormancy. Clin Cancer Res. (2014) 20:3384–9. doi: 10.1158/1078-0432.CCR-13-0897
114. Sun Y, Campisi J, Higano C, Beer TM, Porter P, Coleman I, et al. Treatment-induced damage to the tumor microenvironment promotes prostate cancer therapy resistance through WNT16B. Nat Med. (2012) 18:1359–68. doi: 10.1038/nm.2890
115. Klein CA. Framework models of tumor dormancy from patient-derived observations. Curr Opin Genet Dev. (2011) 21:42–9. doi: 10.1016/j.gde.2010.10.011
116. Koebel CM, Vermi W, Swann JB, Zerafa N, Rodig SJ, Old LJ, et al. Adaptive immunity maintains occult cancer in an equilibrium state. Nature. (2007) 450:903–7. doi: 10.1038/nature06309
117. Romero I, Garrido C, Algarra I, Collado A, Garrido F, Garcia-Lora AM. T lymphocytes restrain spontaneous metastases in permanent dormancy. Cancer Res. (2014) 74:1958–68. doi: 10.1158/0008-5472.CAN-13-2084
118. Aguirre-Ghiso JA, Estrada Y, Liu D, Ossowski L. ERK(MAPK) activity as a determinant of tumor growth and dormancy; regulation by p38(SAPK). Cancer Res. (2003) 63:1684–95. doi: 10.1016/j.urolonc.2003.12.012
119. Gelao L, Criscitiello C, Fumagalli L, Locatelli M, Manunta S, Esposito A, et al. Tumour dormancy and clinical implications in breast cancer. Ecancermedicalscience. (2013) 7:320. doi: 10.3332/ecancer.2013.320
120. Malchow S, Leventhal DS, Savage PA. Organ-specific regulatory T cells of thymic origin are expanded in murine prostate tumors. Oncoimmunology. (2013) 2:e24898. doi: 10.4161/onci.24898
121. Bakhru P, Zhu ML, Wang HH, Hong LK, Khan I, Mouchess M, et al. Combination central tolerance and peripheral checkpoint blockade unleashes antimelanoma immunity. JCI Insight. (2017) 2:e93265. doi: 10.1172/jci.insight.93265
122. Trager U, Sierro S, Djordjevic G, Bouzo B, Khandwala S, Meloni A, et al. The immune response to melanoma is limited by thymic selection of self-antigens. PLoS One. (2012) 7:e35005. doi: 10.1371/journal.pone.0035005
123. Derbinski J, Schulte A, Kyewski B, Klein L. Promiscuous gene expression in medullary thymic epithelial cells mirrors the peripheral self. Nat Immunol. (2001) 2:1032–9. doi: 10.1038/ni723
124. Su MA, Anderson MS. Pulling RANK on cancer: blocking aire-mediated central tolerance to enhance immunotherapy. Cancer Immunol Res. (2019) 7:854–9. doi: 10.1158/2326-6066.cir-18-0912
125. Cummings SR, San Martin J, McClung MR, Siris ES, Eastell R, Reid IR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. (2009) 361:756–65. doi: 10.1056/NEJMoa0809493
126. Gray D, Abramson J, Benoist C, Mathis D. Proliferative arrest and rapid turnover of thymic epithelial cells expressing Aire. J Exp Med. (2007) 204:2521–8. doi: 10.1084/jem.20070795
127. Gray DH, Seach N, Ueno T, Milton MK, Liston A, Lew AM, et al. Developmental kinetics, turnover, and stimulatory capacity of thymic epithelial cells. Blood. (2006) 108:3777–85. doi: 10.1182/blood-2006-02-004531
128. Takaba H, Morishita Y, Tomofuji Y, Danks L, Nitta T, Komatsu N, et al. Fezf2 orchestrates a thymic program of self-antigen expression for immune tolerance. Cell. (2015) 163:975–87. doi: 10.1016/j.cell.2015.10.013
129. Alexandropoulos K, Danzl NM. Thymic epithelial cells: antigen presenting cells that regulate T cell repertoire and tolerance development. Immunol Res. (2012) 54:177–90. doi: 10.1007/s12026-012-8301-y
130. Karimi S, Chattopadhyay S, Chakraborty NG. Manipulation of regulatory T cells and antigen-specific cytotoxic T lymphocyte-based tumour immunotherapy. Immunology. (2015) 144:186–96. doi: 10.1111/imm.12387
131. Turk MJ, Wolchok JD, Guevara-Patino JA, Goldberg SM, Houghton AN. Multiple pathways to tumor immunity and concomitant autoimmunity. Immunol Rev. (2002) 188:122–35. doi: 10.1034/j.1600-065x.2002.18811.x
132. Akirav EM, Ruddle NH, Herold KC. The role of AIRE in human autoimmune disease. Nat Rev Endocrinol. (2011) 7:25–33. doi: 10.1038/nrendo.2010.200
133. McGranahan N, Furness AJ, Rosenthal R, Ramskov S, Lyngaa R, Saini SK, et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science. (2016) 351:1463–9. doi: 10.1126/science.aaf1490
134. Franzese O, Torino F, Fuggetta MP, Aquino A, Roselli M, Bonmassar E, et al. Tumor immunotherapy: drug-induced neoantigens (xenogenization) and immune checkpoint inhibitors. Oncotarget. (2017) 8:41641–69. doi: 10.18632/oncotarget.16335
135. Palmer DB. The effect of age on thymic function. Front Immunol. (2013) 4:316. doi: 10.3389/fimmu.2013.00316
136. Bakhru P, Su MA. Estrogen turns down “the AIRE”. J Clin Invest. (2016) 126:1239–41. doi: 10.1172/JCI86800
Keywords: thymic involution, negative selection and regulatory T (Treg) cell generation, cancer immunity, tumor microenvironment, tumor reservoir
Citation: Wang W, Thomas R, Sizova O and Su D-M (2020) Thymic Function Associated With Cancer Development, Relapse, and Antitumor Immunity – A Mini-Review. Front. Immunol. 11:773. doi: 10.3389/fimmu.2020.00773
Received: 17 February 2020; Accepted: 06 April 2020;
Published: 30 April 2020.
Edited by:
Wanjun Chen, National Institutes of Health (NIH), United StatesReviewed by:
Qing Ge, Peking University, ChinaCopyright © 2020 Wang, Thomas, Sizova and Su. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dong-Ming Su, ZG9uZy1taW5nLnN1QHVudGhzYy5lZHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.