- 1Institute for Immunological Research, University of Cartagena, Cartagena, Colombia
- 2Institute of Laboratory Medicine, Member of the German Center for Lung Research (DZL), Universities of Giessen and Marburg Lung Center (UGMLC), Philipps-University Marburg, Marburg, Germany
- 3John Paul II Hospital, Krakow, Poland
Background: Epigenetic changes in response to allergen exposure are still not well understood. The aim of this study was to evaluate histone acetylation levels in peripheral blood leukocytes from humans naturally infected by intestinal parasites and perennially exposed to house dust mites (HDM).
Methods: Peripheral blood mononuclear cells (PBMCs) were isolated by gradient centrifugation from 20 infected and 21 non-infected individuals living in a rural/village in Colombia. Histone 3 acetylation (H3Ac) and histone 4 acetylation (H4Ac) levels were measured in six immune genes previously associated with helminth immunity by chromatin immunoprecipitation (ChIP)-quantitative PCR. Then we analyzed the association between histone acetylation levels with total parasite egg burden and IgE levels.
Results: We found an inverse correlation between H4Ac levels in the IL13 gene and egg worm burden that remained significant after adjustment by age [−0.20 (−0.32 to −0.09), p < 0.0001]. Moreover, we found significant associations between H4Ac levels in IL4 [0.32 (0.05–0.60), p = 0.02] and CHI3L1 [0.29 (0.08–0.51), p = 0.008] with the IgE levels to Ascaris lumbricoides. In addition, the levels of specific IgE antibodies to HDM were associated with H4Ac levels in the gene TNFSF13B encoding the B cell activating factor (BAFF) [0.51 (0.26–0.76), p < 0.001]. All values are presented as beta (95% CI).
Conclusion: Histone acetylation levels at key type-2 immune genes in humans were modified by nematode infection and HDM allergens and are associated with the intensity of the IgE response.
Introduction
Epigenetic modifications and more specifically DNA methylation, have been associated with increased total IgE levels (1) and increased IgE sensitization to house dust mites (HDM) (2). In addition, allergen exposure induces epigenetic changes in immune cells affecting the inception and maintenance of type-2 skewed immune phenotypes (3). The IgE response to HDM allergens is very prominent in humans living in tropical environments, and even though perennial exposure may explain this observation, the co-exposure with intestinal helminth infection provides a unique opportunity to dissect key molecular events implicated in type 2 immunity (4). Indeed, a study in a mice model revealed that chronic helminth infection also reprograms T cell differentiation via histone acetylation changes (5), by the addition of acetyl groups to lysine residues (K) at the N-terminal tail of histones. Acetylation neutralizes the positive charge of lysine reducing histone affinity for DNA and (this way) opens chromatin. Also, by providing a tag in histone tails for transcription factors and regulatory proteins, histone acetylation affects the accessibility of promoters to the transcriptional machinery (6, 7). Several studies support that H3 acetylation at K9 and K14 (H3Ac) are a hallmark of gene activation and exhibit remarkable correlation with active promoters and active enhancers. Also, H4 acetylation has been associated with transcriptional activation and maintenance of euchromatin. Therefore, increased acetylation of lysine residues at H3 and H4 is informative on active transcription of the marked gene (6, 7).
Previous studies revealed that when naïve T cells differentiate into Th2 cells, the Th2 locus (chromosome 5q31) undergoes extensive epigenetic modifications that lead to a poised chromatin configuration (8), making chromatin accessible and promoting IL-4, IL-5, and IL-13 expression (9, 10). Regulatory regions, such as the IL4 and IL13 promoters, the Th2 locus control region (LCR), and enhancers are the primary targets of these modifications (11–13). The isotype class switching and specific IgE production resulting from these changes may be used as a proxy of Th2 locus activation. In the context of helminth infection, the magnitude of IgE production to parasite components depends on the individual predisposition toward type 2 immunity (14–17). Egg burden is also a marker of individual ability to resist parasite infection (18, 19). A quantitative trait locus (QTL) for Ascaris egg counts has been described in chromosome 13q33 in a region encoding for ligase IV (LIG4), abhydrolase domain containing 13 (ABDH13) and the B cell activating factor BAFF (TNFSF13B) (20). Genetic variants in this region are also associated with increased IgE against Ascaris and the HDM Dermatophagoides pteronyssinus (21), although the underlying mechanisms remain unclear. Since parasite immunity and allergic responses share several biological pathways, we hypothesized that the relative effects of these genes depend on environmental factors that could induce epigenetic modifications.
To date, no study has analyzed if exposure to Ascaris lumbricoides and HDM allergens can influence histone acetylation at these loci. In this study, we aimed to evaluate H3 and H4 acetylation levels in mononuclear leukocytes from humans living in a rural community exposed to A. lumbricoides and HDM, and to investigate the relationship of H3 and H4 acetylation with the specificity and intensity of the IgE response.
Materials and Methods
Study Population
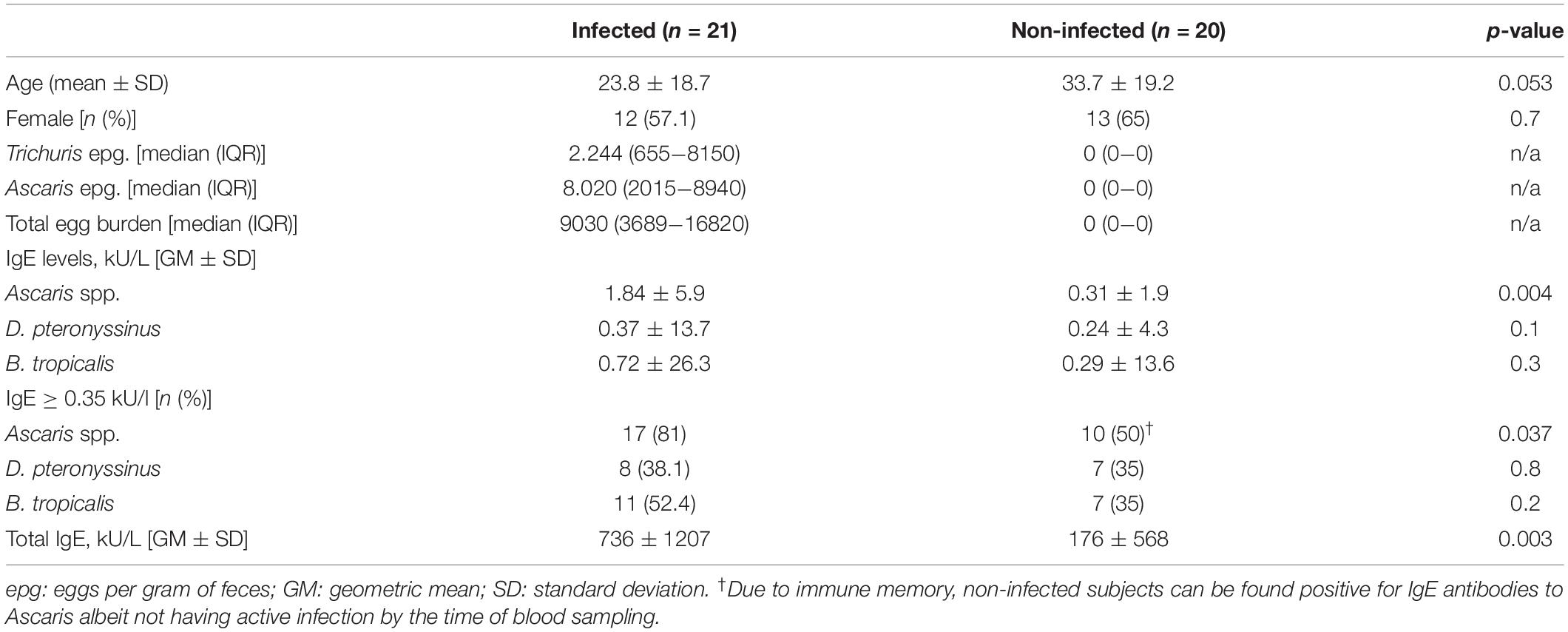
For this study we selected 41 subjects from a cohort of 739 well-characterized subjects living in Santa Catalina (Colombia) and previously described by Zakzuk et al. (20). This is a small tropical farming/fishing town in northern Colombia (10° 36′ 0″ N, 75° 18′ 0″ W) with a territorial extension of 153 km2 and a population of approximately 12,500 inhabitants. Half of the people have at least one unsatisfied basic need, only 4.5% of the population has a sewage system and 56% has tap water. This study included 20 non-infected subjects and 21 infected with A. lumbricoides (Table 1). Criteria for non-infected subjects included having two negative results in stool examinations conducted in 2014 (22), and when resampled in two consecutive stool tests collected for this study during May–June 2016. Criteria for infected subjects include active parasite infection as detected by fresh fecal smear in at least one stool test collected for this study in 2016. Parasite burden was quantified as eggs per gram (e.p.g) of feces by the Kato Katz method using a commercial kit (Copro Kit, C&M Medical, Campinas, Brazil). Blood samples were taken on the same day or within 2 days after the stool test. Albendazole treatment was prescribed after blood sampling in all infected subjects. This study was approved by the Ethics Committee of the University of Cartagena (nr. 1705-2012) and was conducted following the guidelines of the Declaration of Helsinki. All the participants gave their written informed consent prior to their inclusion in the study.
Isolation of Peripheral Blood Mononuclear Cells (PBMCs)
Blood samples were collected in EDTA tubes by standard phlebotomy and 3 mL of blood were mixed with 3 mL of RPMI-1640 based-medium (RPMI-1640 supplemented with 10% heat-inactivated Fetal Calf Serum (FCS), 1% antibiotic/antimycotic solution and 1% L-Glutamine) and then, layered over 3 mL of Histopaque (Sigma, city, United States). The sample was centrifuged at 800 × g for 20 min without a break. The mononuclear cell layer was aspirated, transferred to a new tube, and resuspended in 10 mL of RPMI-1640 based-medium. Cells were washed at 800 g for 10 min and the cell pellet resuspended in 2 mL of FCS-DMSO freezing medium and stored at −80°C until analysis.
Histone Modifications
Seven candidate genes were selected based on previous genetic association with helminth immunity: IL4 and IL13 at chr. 5q31 for their well-known involvement in helminth immunity (23, 24); LIG4/ABHD13 and TNFSF13B in the A. lumbricoides susceptibility locus at chr. 13q33 (20) and CHIA at chr. 1p13.2, and CHI3L1 at chr. 1q32.1 for their genetic association with anti-Ascaris IgE levels and protective immunity to helminths (21, 25). We also analyzed histone levels in the housekeeping gene RPL32 encoding the Ribosomal protein L32 as a non-immune related control. Chromatin immunoprecipitation (ChIP) followed by quantitative polymerase chain reaction (qPCR) using specific primers (Table 2) were used to assess histone H3 acetylation of K9 and K14 (H3Ac) and H4 acetylation of K5, K8, K12, and K16 (H4Ac) levels at the promoters of the selected loci, as described previously (26). H3Ac and H4Ac levels were expressed as percentage of the input control and corrected for the isotype control.
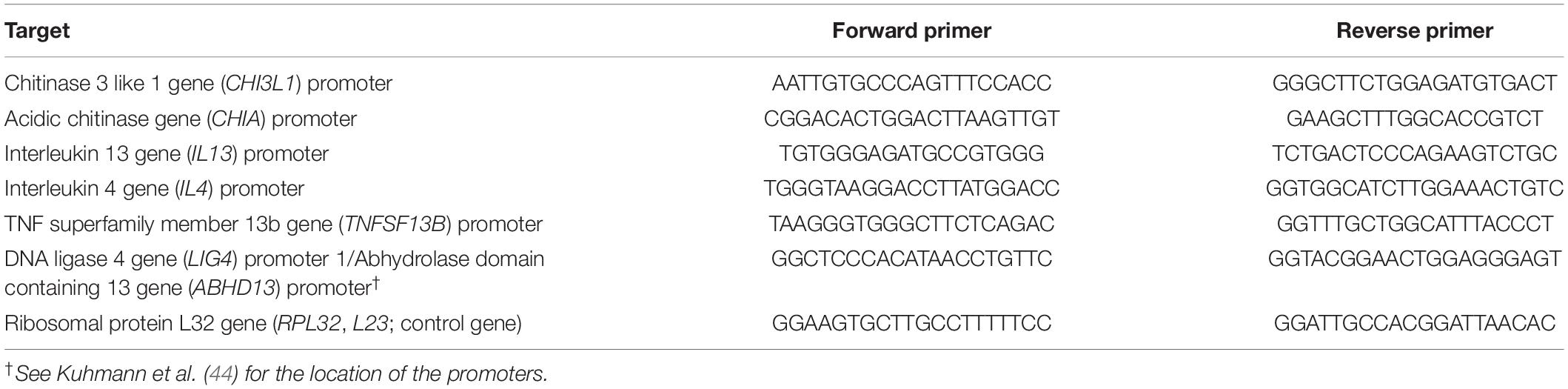
Table 2. Primers used for quantitative assessment of H3 and H4 histone acetylation by PCR following chromatin immunoprecipitation (ChIP).
IgE Levels
Total serum IgE levels, specific IgE levels to the nematode Ascaris, and specific IgE levels to Blomia tropicalis and D. pteronyssinus were measured by ImmunoCAP (Thermo Fisher, Uppsala, Sweden). Total IgE levels were reported in IU/mL. Specific IgE levels above 0.35 kUA/L were considered positive for IgE sensitization. Specific IgE levels to the purified nematode specific marker ABA-1 were determined by indirect ELISA as described previously (27). ABA-1 is an allergen of Ascaris sp., and a member of the nematode polyprotein allergen/antigens with fatty acid-binding properties (28). It has been found only in nematodes (29) and has been used as a serological marker of Ascaris infection (14, 16).
Statistical Analysis
Demographic data were compared between study subgroups by either Fisher’s exact test (binary variables) or Mann–Whitney U test (continuous variables). The correlation between acetylation levels with age, total egg burden, total IgE and specific IgE levels was calculated by Spearman correlation. Generalized linear models (GLM) were applied using the most appropriate function according to the distribution of the data to evaluate the relationship between histone acetylation and egg burden or IgE antibodies adjusting by the effect of age. Logistic regression was applied to model the relationship between histone acetylation and IgE sensitization to HDM as a categorical variable adjusting for age and gender. A p-value < 0.05 was considered significant. Model-based receiver operating characteristic (ROC) curves were drawn to test for the ability to predict sensitization to HDM allergens and the area under the curve (AUC) was calculated as a measure of performance using the logistic regression model described above.
Results
Study Population
The descriptive characteristics of infected and non-infected subjects are presented in Table 1. Of the twenty-one subjects infected by A. lumbricoides, nineteen were also infected by Trichuris trichiura. Median total IgE levels in infected subjects [902 IU/ml (IQR: 246-2097)] were higher than in non-infected [170 IU/ml (50.8–486), Mann–Whitney p = 0.003]. Median specific IgE to Ascaris spp. were significantly higher in the infected group [2.10 kU/l (0.57–8.72)] compared to non-infected [0.30 kU/l (0.08–1.14), Mann–Whitney p = 0.004], reflecting induction of the type 2 inflammation by the active helminth infection. Specific IgE levels to Ascaris significantly correlated with fecal egg counts of A. lumbricoides (rho 0.36, p = 0.02) and T. trichiura (rho 0.42, p = 0.007), thereby the sum of eggs of both helminths per individual was computed as total egg burden and this variable used in all subsequent analyses. This tropical population is also perennially exposed to HDM. A positive IgE response to B. tropicalis was detected in 43.9% of the individuals and to D. pteronyssinus in 36.6%. There was no difference in IgE levels to HDM between infected and non-infected subjects (Table 1).
Histone Acetylation and Nematode Infection
Age was inversely correlated with total egg burden (rho − 0.40, p = 0.01) and directly correlated with H4Ac (rho 0.38, p = 0.014) and H3Ac (rho 0.30, p = 0.05) levels in LIG4/ABDH13. Total egg burden was inversely correlated with H4Ac levels in IL13 (rho − 0.32, p = 0.03) and in LIG4/ABDH13 (rho − 0.31, p = 0.04). To further evaluate these relationships considering the effect of age as confounding factor we implemented multivariate GLM. These analyses confirmed that increased H4Ac levels in IL13 were associated with reduced total egg burden even after adjusting by age (Table 3). We also detected significant differences in the H3Ac acetylation levels and total egg burden in these genes, which remained significant after adjustment for IL13 [β−0.58 (−0.86 to −0.29), p < 0.0001] and LIG4/ABDH13 [β−0.28 (−0.40 to −0.16), p < 0.0001].
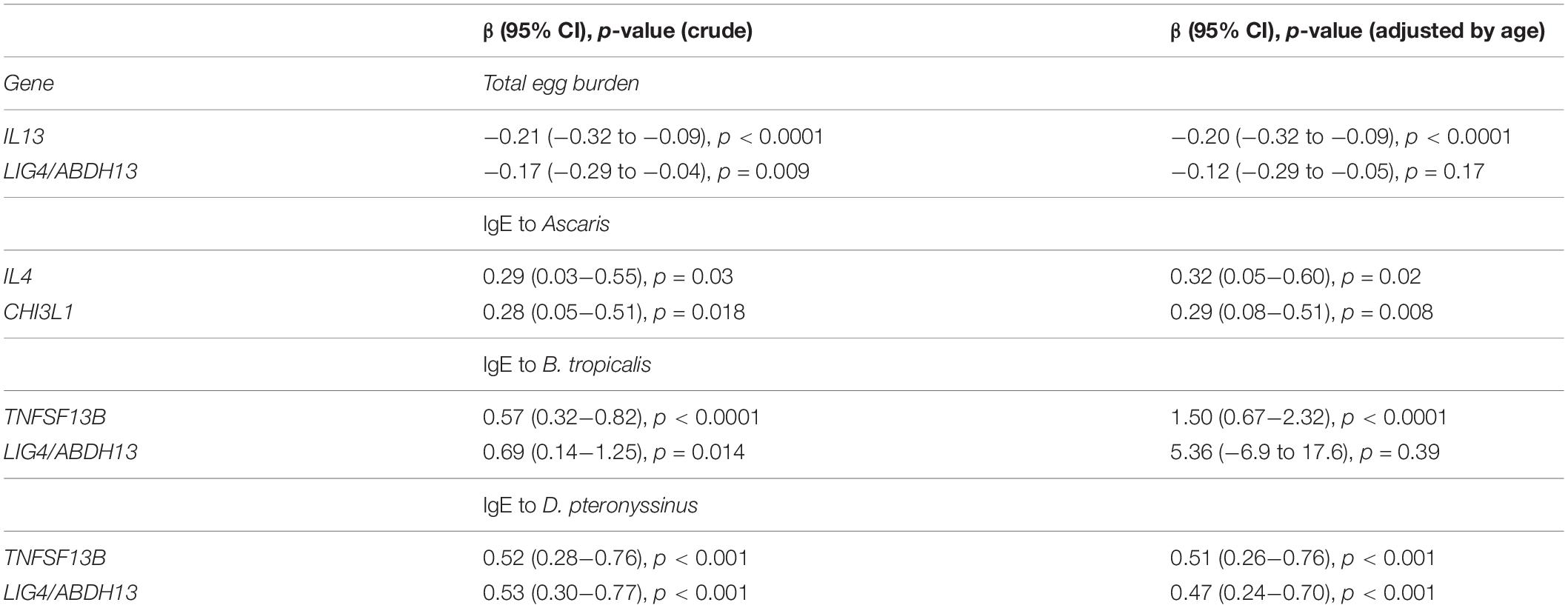
Table 3. Generalized linear regressions on the relationship between H4 acetylation with indicators of parasite and HDM exposure (n = 41).
Histone Acetylation and IgE Levels to Ascaris
Specific IgE levels to A. lumbricoides extract were directly correlated with H4Ac (rho 0.35, p = 0.025) and H3Ac (rho 0.33, p = 0.03) levels in IL4. In addition, specific IgE levels to A. lumbricoides correlated with H4Ac in CHI3L1 (rho 0.31, p = 0.049). The associations of the increased H4Ac levels in IL4 and CHI3L1 with increased IgE levels to A. lumbricoides also remained significant after adjusting by age (Table 3). Moreover, specific IgE levels to the nematode specific marker ABA-1 were directly correlated with histone acetylation levels in CHI3L1, affecting H4Ac (rho 0.38, p = 0.01) and H3Ac (rho 0.33, p = 0.03), supporting that epigenetic changes in these loci were induced by the infection with this nematode.
H4 Acetylation and IgE Sensitization to HDM
We found significant correlations between H4Ac levels in LIG4/ABHD13 and TNFSF13B with specific IgE levels to HDM (Figure 1). GLM adjusting by age, confirmed the association between H4Ac levels in TNFSF13B with specific IgE levels to B. tropicalis and D. pteronyssinus (Table 3). When sensitization was analyzed as a categorical variable, H4Ac levels in TNFSF13B were significantly higher in individuals sensitized to HDM (Mann–Whitney test p < 0.05, Figure 2). This association remained significant after adjustment by age and gender in a logistic regression model. Next, we computed model-based ROC curves to see how well the regression models can predict sensitization to HDM allergens and computed AUC as a measure of performance. These analyses suggested a good predictive value of H4 acetylation over HDM sensitization with an AUC = 0.76 (Figure 3). Interestingly, the differences in the H4Ac in TNFSF13B gene were only detected with the response to HDM extracts but not with the Ascaris extract (Figures 2, 3).
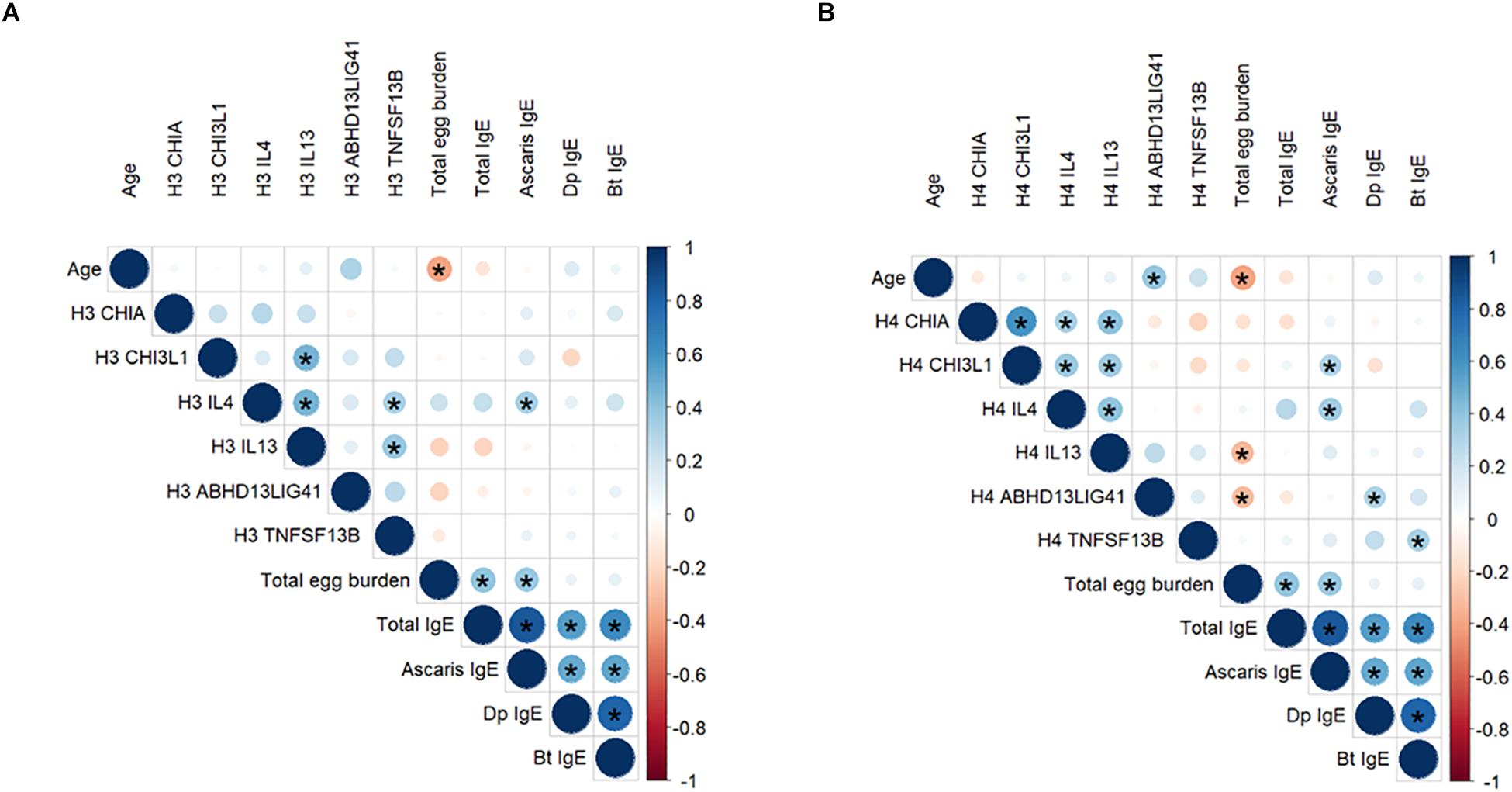
Figure 1. Correlation of H3Ac (A) and H4Ac (B) levels at six gene promoters with age, total egg burden, and total and specific IgE levels to Ascaris and HDM. The scale indicates the Spearman coefficient (Rho) from –1 to 1. Direct correlations are indicated in the blue scale and inverse correlations are indicated in the orange scale. Significant correlations (p < 0.05) are indicated with an asterisk. Dp: D. pteronyssinus; Bt: B. tropicalis.
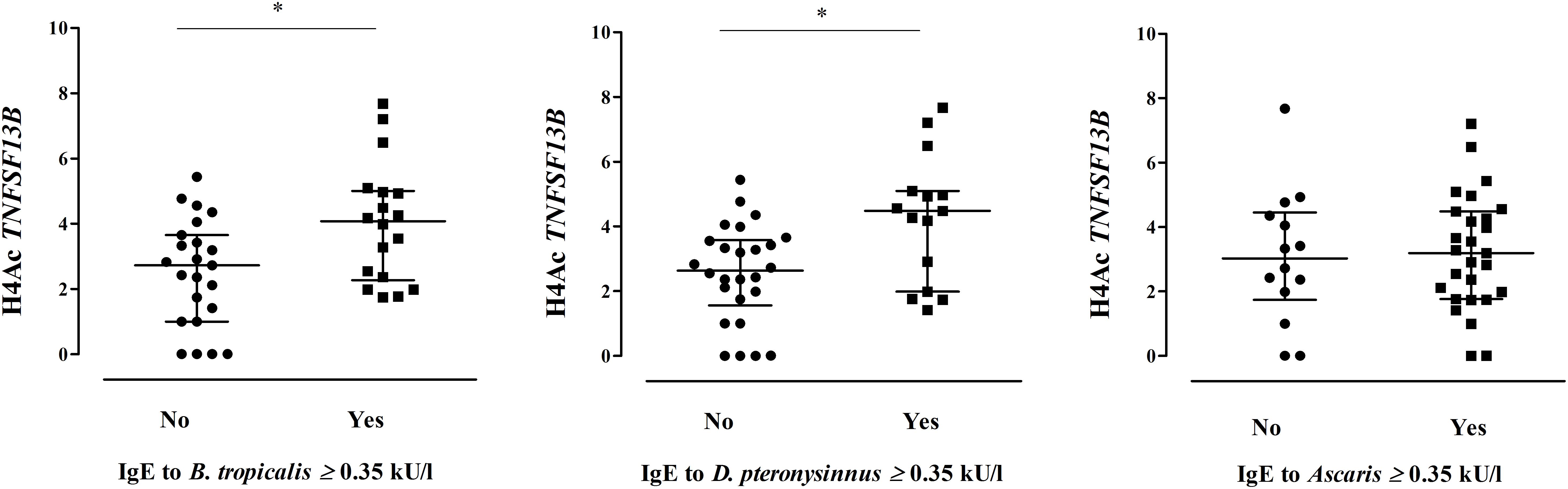
Figure 2. Comparison of the H4Ac levels in TNFSF13B according to HDM and Ascaris sensitization (*Mann–Whitney test p < 0.05). Each dot represents an individual, lines indicate median and IQR.
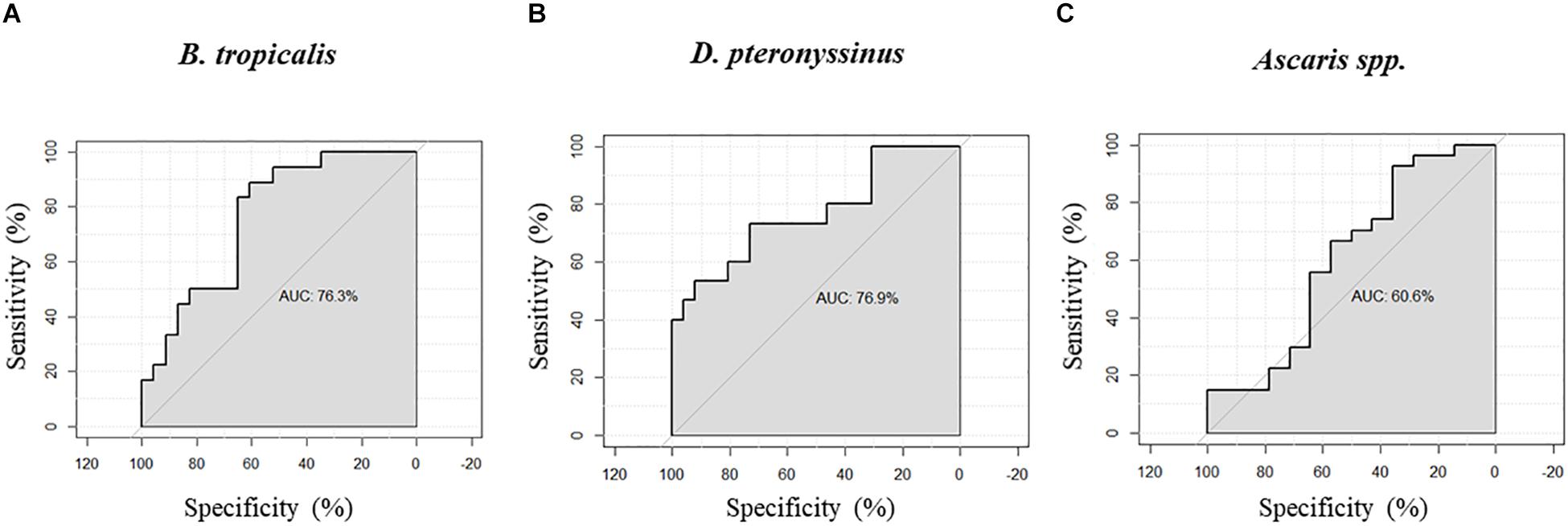
Figure 3. Receiver operating characteristic (ROC) curves and area under the curve (AUC) obtained from logistic regression models to predict the sensitization to HDM allergens. (A) H4 acetylation at the TNFSF13B gene versus IgE sensitization to B. tropicalis. (B) H4 acetylation at the TNFSF13B gene versus IgE sensitization to D. pteronyssinus. (C) H4 acetylation at the TNFSF13B gene versus IgE sensitization to Ascaris spp.
Discussion
The immune response to helminths and the allergic response share several biological pathways whose study have helped to understand the pathogenesis of both conditions. In the tropics, allergy and helminthiases are frequent, allowing the study of the mechanisms and clinical impact of their interactions (4). In addition to cellular and molecular mechanisms, genetic studies have provided evidence of genes involved in the same pathways activated in allergy and helminth immunity (19, 21). In contrast, epigenetic studies in this field are scarce, even considering the well-known importance of epigenetic mechanisms regulating gene expression. In this study, we present distinct epigenetic changes in Ascaris immunity and HDM IgE response. To our knowledge, this is the first report of association between differences in histone acetylation levels in the IL13 gene and parasite egg burden, which is expected because the great importance of IL-13 in helminth immunity (30). Reduced egg burden can be explained because increased H4Ac in IL13 may facilitate higher IL-13 production and suggests that this gene is sensitive to and modified by helminth infection. In our study asthmatic patients were not included; however, since IL-13 is also crucial for bronchial inflammation in asthma, this finding could help to explain why there is an increased severity of asthma in some Ascaris infected individuals (31, 32). Thus, our finding supports the traditional evolutionary hypothesis that Th2 allergic inflammation mechanisms are, at least partially explained, by helminth immunity legacy.
We also detected significant associations between H3 and H4 acetylation levels in the LIG4/ABHD13 at the 13q33 locus with total egg burden, supporting previous association of this chromosomic region with Ascaris egg counts (20) and suggesting that susceptibility to this infection is not only mediated by genetic but also by epigenetic effects. The mechanisms how these genes participate in parasite immunity and influence egg burden remain to be elucidated. The role of IgE on protective immunity to Ascaris has not been sufficiently explored; however, considering previous associations between LIG4/ABHD13 and the IgE responses to Ascaris (21), our results suggest that IgE may play a role in reducing egg burden during ascariasis (14, 16).
We also detected here a direct association between H4Ac in the IL4 gene and IgE levels against Ascaris suggesting that increased acetylation at the IL4 locus might influence IgE synthesis. H3 and H4 histone acetylation levels in IL13 and IL4 were directly correlated (Figure 1), however, the association between IL13 acetylation with egg burden was significant with both histone marks (H3 and H4) while the association between IL4 acetylation with specific IgE levels to Ascaris was only significant with H4Ac. The reason why each cytokine is associated to each of these outcomes is unknown, but a mice model revealed that a deletion in a DNase I-hypersensitive site 2 (HS2) element in the second intron of the interleukin 4 locus (Il4) impaired the acetylation of histone H3 at Lys9 and Lys14 in the Il4 locus and affected the production of IL-4 but not of other Th2 cytokines, suggesting that it may occur chromosomal modifications on Il4 that are independent of the Il5 and Il13 loci (33). Also in agreement with our previous genetic study (21), here we confirmed the association of CHI3L1 with the specific IgE levels to Ascaris and ABA-1, showing for the first time increased H4Ac levels in high IgE responders to Ascaris. Recent studies revealed that expression of CHI3L1 is modified upon contact with the Ascaris larvae (34). Further research is needed to elucidate the role of H4 acetylation in CHI3L1 expression and its contribution to boosting IgE synthesis upon A. lumbricoides infection.
Epigenetic changes leading to bronchial inflammation and hyperresponsiveness have been induced by HDM under experimental conditions in mice (35, 36) suggesting that those epigenetic mechanisms may also contribute to asthma pathogenesis. Performing this kind of research in humans is more difficult, however indirect analyses can be done using specific phenotypes in natural exposed individuals (37). In this study we found that increased H4Ac at the gene TNFSF13B encoding B-cell activating factor was associated with elevated IgE levels to HDM allergens, suggesting that perennial exposure to HDM might affect histone acetylation at this locus in those predisposed to IgE sensitization. The B cell activating factor plays a critical role in B cell development and immunoglobulins production (38, 39). We found no association between H4Ac at this gene and IgE to Ascaris, which is in contrast with a previous study suggesting that this gene is associated with the humoral responses to Ascaris extract (40). However, in a more detailed study we found that among 13q33.3 region-genes enriched in high responders to Ascaris, TNFSF13B was not associated with specificity but rather the strength of IgE levels (21). These findings indicate that more studies are needed to dissect the control of the IgE response by the Ascaris susceptibility locus chr. 13q33.3.
In this study we evaluated acetylation changes in amino acid residues of H3 and H4; both implicated in the regulation of cytokine gene expression (41, 42). Whether histone tails act independently or have synergistic effects is still disputed. Acetylation of histone H4 is often found to be anticorrelated with acetylation of H3 or the other histones in binding of transcription factors, expressing genes or remodeling the chromatin (42, 43). In our study we found significant direct correlations of H3Ac and H4Ac levels in IL-4, CHIA and CHI3L1 genes, while there were no significant correlations in the acetylation levels of these histones in IL-13 and the two regions in 13q33 (Supplementary Figure S1). Interestingly, the associations with total egg burden were detected with both H3 and H4 histones while the associations with IgE levels only remained significant with H4Ac levels, suggesting that H4 marks might be more informative for epigenetic effects associated to the allergen exposure at these genes. We also showed for the very first time the significant correlations between the H4 acetylation levels in gene regions that albeit being in different chromosomes seem to be related (Figure 1B). Still, how acetylation levels at chitinase related genes in chromosome 1 are mechanistically connected to acetylation levels in IL13 and IL4 at the chromosome 5q31 remains to be investigated (Figure 1B), but the results suggest that nematode infection may induce coordinated histone changes in type 2 immunity pathways. However, since our study is cross-sectional, the effects of other environmental factors on the acetylation patterns cannot be ruled out.
In conclusion, this study provides evidence that allergen exposure alters the patterns of epigenetic modifications in human mononuclear leukocytes. Increased H4 acetylation in key immune genes is reflected by increased IgE levels to nematode and HDM allergens, suggesting an additional explanation to the similarities between helminth immunity and the allergic response. Further studies are needed to elucidate the functional effects of these acetylation marks on gene expression and the mechanisms promoting the type-2 immune response.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Ethics Statement
This study was approved by the Ethics Committee of the University of Cartagena (nr. 1705-2012) and was conducted following the guidelines of the Declaration of Helsinki. All the participants gave their written informed consent prior to their inclusion in the study. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author Contributions
LC and DP conceived and designed the experiments. JZ, HH, and LE performed the experiments. JZ and NA analyzed the data. JZ, NA, HH, DP, HR, and LC contributed the reagents, materials, and analysis tools. NA, LC, and JZ wrote the manuscript. All authors involved in writing, reviewing, and editing. LC, JZ, HR, and DP involved in funding acquisition. LC overall responsibility of the project.
Funding
This work was funded by Colciencias (grants 699 2017 and 803-2018), the University of Cartagena, the Universities Giessen and Marburg Lung Center (UGMLC), the German Center for Lung Research (DZL; 82DZL00502/A2), and the Von Behring-Röntgen-Foundation (Von Behring-Röntgen-Stiftung; 62-LV04).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank all the individuals that voluntarily participate in this study.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2020.00756/full#supplementary-material
FIGURE S1 | Correlations between H3 and H4 acetylation levels in the six promoter regions analyzed in this study.
References
1. Liang L, Willis-Owen SA, Laprise C, Wong KC, Davies GA, Hudson TJ, et al. An epigenome-wide association study of total serum immunoglobulin E concentration. Nature. (2015) 520:670–4. doi: 10.1038/nature14125
2. Li JY, Zhang Y, Lin XP, Ruan Y, Wang Y, Wang CS, et al. Association between DNA hypomethylation at IL13 gene and allergic rhinitis in house dust mite-sensitized subjects. Clin Exp Allergy. (2016) 46:298–307. doi: 10.1111/cea.12647
3. North ML, Jones MJ, MacIsaac JL, Morin AM, Steacy LM, Gregor A, et al. Blood and nasal epigenetics correlate with allergic rhinitis symptom development in the environmental exposure unit. Allergy. (2018) 73:196–205. doi: 10.1111/all.13263
4. Caraballo L, Acevedo N, Zakzuk J. Ascariasis as a model to study the helminth/allergy relationships. Parasite Immunol. (2018) 2018:e12595. doi: 10.1111/pim.12595
5. Klar K, Perchermeier S, Bhattacharjee S, Harb H, Adler T, Istvanffy R, et al. Prazeres da Costa C: Chronic schistosomiasis during pregnancy epigenetically reprograms T-cell differentiation in offspring of infected mothers. Eur J Immunol. (2017) 47:841–7. doi: 10.1002/eji.201646836
6. Potaczek DP, Harb H, Michel S, Alhamwe BA, Renz H, Tost J. Epigenetics and allergy: from basic mechanisms to clinical applications. Epigenomics. (2017) 9:539–71. doi: 10.2217/epi-2016-0162
7. Alaskhar Alhamwe B, Khalaila R, Wolf J, von Bulow V, Harb H, Alhamdan F, et al. Histone modifications and their role in epigenetics of atopy and allergic diseases. Allergy Asthma Clin. (2018) 14:39.
8. Smale ST, Fisher AG. Chromatin structure and gene regulation in the immune system. Annu Rev Immunol. (2002) 20:427–62.
9. van Panhuys N, Le Gros G, McConnell MJ. Epigenetic regulation of Th2 cytokine expression in atopic diseases. Tissue Antigens. (2008) 72:91–7. doi: 10.1111/j.1399-0039.2008.01068.x
10. Kaneko T, Hosokawa H, Yamashita M, Wang CR, Hasegawa A, Kimura MY, et al. Chromatin remodeling at the Th2 cytokine gene loci in human type 2 helper T cells. Mol Immunol. (2007) 44:2249–56.
11. Kim K, Kim N, Lee GR. Transcription Factors Oct-1 and GATA-3 Cooperatively Regulate Th2 Cytokine Gene Expression via the RHS5 within the Th2 Locus Control Region. PLoS One. (2016) 11:e0148576. doi: 10.1371/journal.pone.0148576
12. Koh BH, Hwang SS, Kim JY, Lee W, Kang MJ, Lee CG, et al. Th2 LCR is essential for regulation of Th2 cytokine genes and for pathogenesis of allergic asthma. Proc Natl Acad Sci USA. (2010) 107:10614–9. doi: 10.1073/pnas.1005383107
13. Fields PE, Lee GR, Kim ST, Bartsevich VV, Flavell RA. Th2-specific chromatin remodeling and enhancer activity in the Th2 cytokine locus control region. Immunity. (2004) 21:865–76.
14. Turner JD, Faulkner H, Kamgno J, Kennedy MW, Behnke J, Boussinesq M, et al. Allergen-specific IgE and IgG4 are markers of resistance and susceptibility in a human intestinal nematode infection. Microbes Infect. (2005) 7:990–6.
15. Hagel I, Lynch NR, Di Prisco MC, Rojas E, Perez M, Alvarez N. Ascaris reinfection of slum children: relation with the IgE response. Clin Exp Immunol. (1993) 94:80–3.
16. McSharry C, Xia Y, Holland CV, Kennedy MW. Natural immunity to Ascaris lumbricoides associated with immunoglobulin E antibody to ABA-1 allergen and inflammation indicators in children. Infect Immun. (1999) 67:484–9.
17. Perzanowski MS, Ng’ang’a LW, Carter MC, Odhiambo J, Ngari P, Vaughan JW, et al. Atopy, asthma, and antibodies to Ascaris among rural and urban children in Kenya. J Pediatr. (2002) 140:582–8.
18. Wright JE, Werkman M, Dunn JC, Anderson RM. Current epidemiological evidence for predisposition to high or low intensity human helminth infection: a systematic review. Parasites & vectors. (2018) 11:65.
19. Moller M, Gravenor MB, Roberts SE, Sun D, Gao P, Hopkin JM. Genetic haplotypes of Th-2 immune signalling link allergy to enhanced protection to parasitic worms. Hum Mol Genet. (2007) 16:1828–36.
20. Williams-Blangero S, Vandeberg JL, Subedi J, Jha B, Correa-Oliveira R, Blangero J. Localization of multiple quantitative trait loci influencing susceptibility to infection with Ascaris lumbricoides. J Infect Dis. (2008) 197:66–71. doi: 10.1086/524060
21. Acevedo N, Bornacelly A, Mercado D, Unneberg P, Mittermann I, Valenta R, et al. Genetic Variants in CHIA and CHI3L1 Are Associated with the IgE Response to the Ascaris Resistance Marker ABA-1 and the Birch Pollen Allergen Bet v 1. PLoS One. (2016) 11:e0167453. doi: 10.1371/journal.pone.0167453
22. Zakzuk J, Casadiego S, Mercado A, Alvis-Guzman N, Caraballo L. Ascaris lumbricoides infection induces both, reduction and increase of asthma symptoms in a rural community. Acta Trop. (2018) 187:1–4. doi: 10.1016/j.actatropica.2018.07.016
23. Gerbe F, Sidot E, Smyth DJ, Ohmoto M, Matsumoto I, Dardalhon V, et al. Intestinal epithelial tuft cells initiate type 2 mucosal immunity to helminth parasites. Nature. (2016) 529:226–30. doi: 10.1038/nature16527
24. Finkelman FD, Shea-Donohue T, Morris SC, Gildea L, Strait R, Madden KB, et al. Interleukin-4- and interleukin-13-mediated host protection against intestinal nematode parasites. Immunol Rev. (2004) 201:139–55.
25. Vannella KM, Ramalingam TR, Hart KM, de Queiroz Prado R, Sciurba J, Barron L, et al. Acidic chitinase primes the protective immune response to gastrointestinal nematodes. Nat Immunol. (2016) 17:538–44. doi: 10.1038/ni.3417
26. Harb H, Amarasekera M, Ashley S, Tulic MK, Pfefferle PI, Potaczek DP, et al. Epigenetic Regulation in Early Childhood: A Miniaturized and Validated Method to Assess Histone Acetylation. Int Arch Allergy Immunol. (2015) 168:173–81. doi: 10.1159/000442158
27. Ahumada V, Garcia E, Dennis R, Rojas MX, Rondon MA, Perez A, et al. IgE responses to Ascaris and mite tropomyosins are risk factors for asthma. Clin Exp Allergy. (2015) 45:1189–200. doi: 10.1111/cea.12513
28. Xia Y, Spence HJ, Moore J, Heaney N, McDermott L, Cooper A, et al. The ABA-1 allergen of Ascaris lumbricoides: sequence polymorphism, stage and tissue-specific expression, lipid binding function, and protein biophysical properties. Parasitology. (2000) 120(Pt 2):211–24.
29. Christie JF, Dunbar B, Kennedy MW. The ABA-1 allergen of the nematode Ascaris suum: epitope stability, mass spectrometry, and N-terminal sequence comparison with its homologue in Toxocara canis. Clin Exp Immunol. (1993) 92:125–32.
30. Harris NL, Loke P. Recent Advances in Type-2-Cell-Mediated Immunity: Insights from Helminth Infection. Immunity. (2017) 47:1024–36. doi: 10.1016/j.immuni.2017.11.015
31. Hunninghake GM, Soto-Quiros ME, Avila L, Ly NP, Liang C, Sylvia JS, et al. Sensitization to Ascaris lumbricoides and severity of childhood asthma in Costa Rica. J Allergy Clin Immunol. (2007) 119:654–61.
32. Buendia E, Zakzuk J, Mercado D, Alvarez A, Caraballo L. The IgE response to Ascaris molecular components is associated with clinical indicators of asthma severity. World Allergy Organ J. (2015) 8:8. doi: 10.1186/s40413-015-0058-z
33. Tanaka S, Motomura Y, Suzuki Y, Yagi R, Inoue H, Miyatake S, et al. The enhancer HS2 critically regulates GATA-3-mediated Il4 transcription in T(H)2 cells. Nat Immunol. (2011) 12:77–85. doi: 10.1038/ni.1966
34. Ebner F, Kuhring M, Radonic A, Midha A, Renard BY, Hartmann S. Silent witness: dual-species transcriptomics reveals epithelial immunological quiescence to helminth larval encounter and fostered larval development. Front Immunol. (2018) 9:1868. doi: 10.3389/fimmu.2018.01868
35. Shang Y, Das S, Rabold R, Sham JS, Mitzner W, Tang WY. Epigenetic alterations by DNA methylation in house dust mite-induced airway hyperresponsiveness. Am J Respir Cell Mol Biol. (2013) 49:279–87. doi: 10.1165/rcmb.2012-0403OC
36. Cheng RY, Shang Y, Limjunyawong N, Dao T, Das S, Rabold R, et al. Alterations of the lung methylome in allergic airway hyper-responsiveness. Environ Mol Mutag. (2014) 55:244–55. doi: 10.1002/em.21851
37. Pascual M, Suzuki M, Isidoro-Garcia M, Padron J, Turner T, Lorente F, et al. Epigenetic changes in B lymphocytes associated with house dust mite allergic asthma. Epigenetics. (2011) 6:1131–7. doi: 10.4161/epi.6.9.16061
38. Dilidaer Zheng Y, Liu Z, Hu X, Zhang J, Hu L, Han M, et al. Increased BAFF expression in nasal polyps is associated with local IgE production, Th2 response and concomitant asthma. Eur Arch Oto Rhino Laryngol. (2017) 274:1883–90.
39. Smulski CR, Eibel H. BAFF and BAFF-Receptor in B Cell Selection and Survival. Front Immunol. (2018) 9:2285. doi: 10.3389/fimmu.2018.02285
40. Acevedo N, Mercado D, Jimenez S, Vergara C, Sanchez J, Fernandez A, et al. Insulin Receptor Substrate 2 (IRS2) and Ligase IV (LIG4) genes are associated with total IgE levels and specific IgE against Ascaris. Allergy. (2008) 63(Suppl. 88):15.
41. Falvo JV, Jasenosky LD, Kruidenier L, Goldfeld AE. Epigenetic control of cytokine gene expression: regulation of the TNF/LT locus and T helper cell differentiation. Adv Immunol. (2013) 118:37–128. doi: 10.1016/B978-0-12-407708-9.00002-9
42. Kurdistani SK, Tavazoie S, Grunstein M. Mapping global histone acetylation patterns to gene expression. Cell. (2004) 117:721–33.
43. Gansen A, Toth K, Schwarz N, Langowski J. Opposing roles of H3- and H4-acetylation in the regulation of nucleosome structure–a FRET study. Nucleic Acids Res. (2015) 43:1433–43.
Keywords: histone acetylation, IgE levels, nematode infection, H3Ac, H4Ac, house dust mites, epigenetics
Citation: Zakzuk J, Acevedo N, Harb H, Eick L, Renz H, Potaczek DP and Caraballo L (2020) IgE Levels to Ascaris and House Dust Mite Allergens Are Associated With Increased Histone Acetylation at Key Type-2 Immune Genes. Front. Immunol. 11:756. doi: 10.3389/fimmu.2020.00756
Received: 23 January 2020; Accepted: 03 April 2020;
Published: 28 April 2020.
Edited by:
Julia Szekeres-Bartho, University of Pécs, HungaryReviewed by:
Malcolm Kennedy, University of Glasgow, United KingdomMatthew Cook, Australian National University, Australia
Udo Jeschke, Ludwig-Maximilians-Universität München, Germany
Copyright © 2020 Zakzuk, Acevedo, Harb, Eick, Renz, Potaczek and Caraballo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Luis Caraballo, bGNhcmFiYWxsb2dAdW5pY2FydGFnZW5hLmVkdS5jbw==
 Josefina Zakzuk
Josefina Zakzuk Nathalie Acevedo
Nathalie Acevedo Hani Harb2
Hani Harb2 Luis Caraballo
Luis Caraballo