
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 31 January 2020
Sec. Microbial Immunology
Volume 11 - 2020 | https://doi.org/10.3389/fimmu.2020.00017
This article is part of the Research Topic The Role of Complement in Microbial Infections View all 15 articles
 Markus Korsholm Kristensen1
Markus Korsholm Kristensen1 Marco Bo Hansen1,2
Marco Bo Hansen1,2 Martin Bruun Madsen3
Martin Bruun Madsen3 Cecilie Bo Hansen1
Cecilie Bo Hansen1 Katrine Pilely1
Katrine Pilely1 Ole Hyldegaard2,3
Ole Hyldegaard2,3 Peter Garred1*
Peter Garred1*Aim: We assessed whether different complement factors and complement activation products were associated with poor outcome in patients with necrotizing soft-tissue infection (NSTI).
Methods: We conducted a prospective, observational study in an intensive care unit where treatment of NSTI is centralized at a national level. In 135 NSTI patients and 65 control patients, admission levels of MASP-1, MASP-2, MASP-3, C4, C3, complement activation products C4c, C3bc, and terminal complement complex (TCC) were assessed.
Results: The 90-day mortality was 23%. In a Cox regression model adjusted for sex, and SAPS II, a higher than median MASP-1 (HR 0.378, CI 95% [0.164–0.872], p = 0.0226) and C4 (HR 0.162, 95% CI [0.060–0.438], p = 0.0003), C4c/C4 ratio (HR 2.290 95% CI [1.078–4.867], p = 0.0312), C3bc (HR 2.664 95% CI [1.195–5.938], p = 0.0166), and C3bc/C3 ratio (HR 4.041 95% CI [1.673–9.758], p = 0.0019) were associated with 90-day mortality, while MASP-2, C4c, C3, and TCC were not. C4 had the highest ROC-AUC (0.748, [95% CI 0.649–0.847]), which was comparable to the AUC for SOFA score (0.753, [95% CI 0.649–0.857]), and SAPS II (0.862 [95% CI 0.795–0.929]).
Conclusion: In adjusted analyses, high admission levels of the C4c/C4 ratio, C3bc, and the C3bc/C3 ratio were significantly associated with a higher risk of death after 90 days while high admission levels of MASP-1 and C4 were associated with lower risk. In this cohort, these variables are better predictors of mortality in NSTI than C-reactive protein and Procalcitonin. C4's ability to predict mortality was comparable to the well-established scoring systems SAPS score II and SOFA on day 1.
Necrotizing soft-tissue infections (NSTIs) are infections with a necrotizing component involving any or all layers of the soft tissue compartment (1). They are rapidly progressive and can lead to sepsis, multisystem organ failure, and in 8–49% death (2). Surgical removal of all necrotic tissue is essential for survival and time to surgery an important determinant for the outcome (3–6). Yet it is common for patients to have additional areas of necrosis, and therefore require multiple operations with serial exploration and debridement until the infection is controlled (7). In 16% of patients, amputation is necessary (5).
If we had reliable tools to identify the patients requiring extensive surgery and discriminate them from the low-risk patients, we could improve survival rates, and avoid the extensive surgery in low-risk patients that currently leads to functional limitations in 30% of patients (8).
It is reasonable to look for such biomarkers in the complement system. Complement plays a crucial role in the microbial defense by mediating opsonization, sequestration, and lysis of pathogens (9), bridges the innate and adaptive system (10), and is activated by apoptotic and necrotic tissue (11). Uncontrolled complement activation is part of the hyperinflammatory response seen in sepsis and sepsis-related coagulopathy (12).
The complement system is divided into three converging proteolytic cascades; the classical, the lectin, and the alternative pathways. We have previously investigated the use of the lectin pathway initiator molecules and kinetic complement analyses as prognostic markers in NSTI patients (13), suggesting that pattern recognition molecules of the lectin pathway play an important role in these patients. Here, we further investigate the levels the lectin pathway associated enzymes MASP-1, MASP-2, MASP-3, the down-stream components C4, C3, the complement activation products C4c, C3bc, and the fluid phase analog of the terminal C5b-9 complement complex (TCC) in NSTI patients and a group of non-infected control patients.
This prospective, observational study was conducted from February 2013 to March 2015 at the Copenhagen University Hospital (Rigshospitalet) where the national treatment of NSTI has been centralized. This is a substudy of the European INFECT project (ClinicalTrials.gov Identifier: NCT01790698).
Patients were screened at arrival to Rigshospitalet. Inclusion criteria were: (1) NSTI based on surgical findings with necrosis engaging any layers of the soft tissue compartments and (2) age ≥18 years. Patients were excluded if the NSTI diagnosis could not be confirmed during surgery.
Control patients were eligible for inclusion if they were: (1) undergoing elective orthopedic surgery at Rigshospitalet; and (2) were aged ≥18 years. Patients with ongoing infection or inflammatory conditions were excluded.
We obtained clinical data from electronic records on age, sex, body mass index, chronic disease (diabetes, liver cirrhosis, chronic kidney disease, cardiovascular disease, chronic obstructive, pulmonary disease, peripheral vascular disease, immune deficiency, malignancy, rheumatoid disease), primary site of infection, microorganism, biochemistry, treatment, and the intensive care unit scoring systems; Simplified Acute Physiology Score II (SAPS II) and Sequential Organ Failure Assessment (SOFA) score day 1. SOFA score was altered as the Glasgow Coma Scale score was omitted. Vital status and time of death, if relevant, were extracted from the hospital database linked to the Danish Civil Registration System. The decision to amputate was based on the surgeon's evaluation. No protocol for amputation was used.
Standard blood analyses including platelet count, creatinine, leucocyte count, and C-reactive protein (CRP) levels were performed at the Department of Clinical Biochemistry, Rigshospitalet, as part of routine analyses, whereas sodium, potassium, hemoglobin, lactate, pH, base excess, pO2, and pCO2 were measured using an ABL 725 (Radiometer, Copenhagen, Denmark).
Upon admission, blood was drawn from an arterial line or central venous catheter into 9-mL vacuum tubes containing EDTA. For the control group, venous blood samples were drawn preoperatively. The samples were immediately put on ice until plasma was seperated from whole blood by centrifugation (within 40 min) at 2,400 G for 10 min and subsequently stored at −80°C.
MASPs, C4c, C3bc, and TCC were measured in the samples by Enzyme-linked Immunosorbent assays (ELISAs). MASP-1 and MASP-2 plasma levels were measured using commercial ELISA kits (MASP-1: USCN Life Sciences; catalog#: SEB895Hu, MASP-2: HycultBiotech Catalog #: HK326-02), as recommended by the manufacturer. Plasma levels of MASP-3, C4c, C3bc, and TCC were measured using previously described in house sandwich ELISAs (14–17). Total plasma concentrations of C4 and C3 were measured using an automated turbidimetric protein analyzer according to the manufacturer's instructions (SPAPLUS®, the Binding Site group LDT, Birmingham, UK).
Our primary analysis was the association between complement parameters measured upon admission, 90-day mortality, and disease severity. We compared patients with septic shock vs. patients without, amputated vs. non-amputated patients and survivors vs. non-survivors. Septic shock was defined according to the criteria of Bone et al. (18). Amputation was defined as any amputation of a limb or penis.
Secondarily, we dichotomized every complement parameter by its median and investigated for associations with 90-day mortality. Patients were followed from inclusion until death, loss to follow up or March 2015, whichever came first.
The significance level was set at 0.05. Kolmogorov-Smirnov and Shapiro-Wilk normality tests were performed for all variables. Due to non-parametric distribution, continuous data were reported as median (IQR) and compared by the Kruskal–Wallis test. For ad-hoc pairwise analyses, we used Dunn's test. For categorical data, we reported absolute numbers, with proportions in parentheses, and used Pearson's chi-square test or Fisher's exact test for comparisons.
The prognostic value of the complement parameters for 90-day-mortality was investigated using Cox regression. For multivariate analysis, the first model was adjusted for sex and SAPS II, the second for age, sex, chronic disease (yes/no), and SAPS II. Data from the Cox analyses were presented as hazard ratios (HR) with 95 % confidence intervals (95% CI). The proportional hazard assumption was met for all parameters in our regression models. We were unable to calculate SAPS II in five patients due to missing data. These patients were excluded from the multivariate analysis. Receiver operating characteristic (ROC) curves with area under the curve (AUC) were reported for the complement parameters for 90-day mortality. Nine patients were excluded from the ROC analyses due to missing SAPS II, pH, base excess, or lactate. Statistical analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, NC).
We included 135 NSTI patients (Figure 1) and 65 control patients, matched for age and sex. Ninety-six patients (71%) had septic shock, amputation was undertaken in 27 cases (20%), and 31 patients (23%) died within 90 days. Table 1 displays differences in the clinical data between surviving and non-surviving patients.
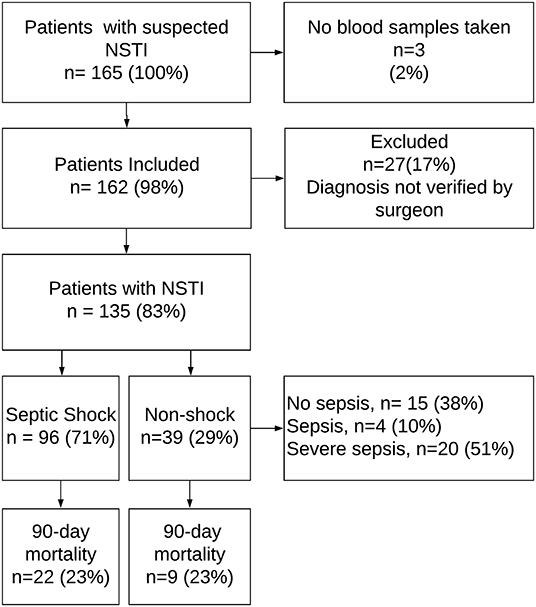
Figure 1. Flowchart of patient inclusion. NSTI, necrotizing soft-tissue infection. The figure is adapted from Figure 1 in Hansen et al. (19).
MASP-1, C4, C4c, C3, decreased gradually, and C3bc/C3 levels increased gradually from controls to survivors, and from survivors to non-survivors, with significant differences both overall (Kruskal Wallis, p < 0.0003, p < 0.0001, p < 0.0001, p < 0.0001, and p < 0.0001, respectively), and between all groups as revealed by the ad-hoc test (Table 2). MASP-2 levels differed significantly between the groups (Kruskal Wallis, p = 0.0025). The ad-hoc analysis revealed that MASP-2 was significantly lower in non-survivors compared to survivors and controls but did not differ between controls and survivors. MASP-3 and TCC differed significantly between the groups (Kruskal Wallis, p < 0.0001). The ad-hoc test showed significantly lower MASP-3 and higher TCC levels in surviving and non-surviving patients when compared to controls resulting in an overall difference between the groups (Kruskal Wallis, p < 0.0001).
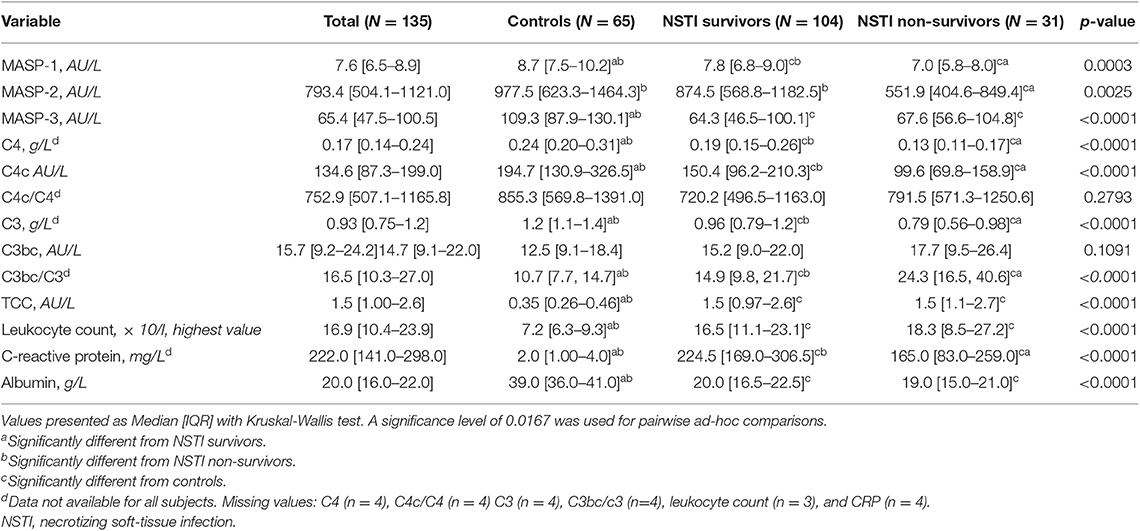
Table 2. Complement, leukocytes, and albumin in controls, surviving and non-surviving necrotizing soft-tissue infection patients.
Table 3 displays the differences in the complement parameters across control patients and NSTI patients with and without septic shock. The median MASP-1 and C4c levels differed significantly between the groups (Kruskal Wallis, p = 0.0017 and p < 0.0001), and the ad-hoc analyses revealed significantly lower levels in shock patients compared to controls. There was a significant difference in median MASP-2 level between controls, non-shock and shock patients (Kruskal Wallis, p = 0.0390), but with no differences in the pairwise ad-hoc analyses. The levels of MASP-3, C4, and C3bc/C3 differed significantly between the groups (Kruskal Wallis, p < 0.0001, p < 0.0001, and p = 0.0002, respectively), with ad-hoc analyses showing significantly lower levels in non-shock and shock patients compared with controls. The C3 levels decreased gradually, from controls to non-shock, and from non-shock to septic shock with significant differences both overall (Kruskal Wallis, p < 0.0001), and between all groups as revealed by the ad-hoc test. Similarly, the median TCC levels increased gradually from the control patients to non-shock and non-shock to shock patients with significant differences both overall (Kruskal Wallis, p < 0.0001), and between all groups as revealed by ad-hoc analyses.

Table 3. Complement, leukocytes, and albumin in controls and necrotizing soft-tissue infection patients with and without septic shock.
Admission levels of C4, C4c, and C3 were lower in patients who underwent amputation vs. non-amputated (p = 0.0081, 0.0066, 0.0464, respectively; Supplementary Table 1).
The univariate models revealed that an increase in age, SOFA score, and SAPS II were associated with a higher hazard of death before 90-days. Chronic disease and amputation were not associated with mortality (Supplementary Table 2). Regarding the complement parameters, significant associations with 90-day mortality were found for MASP-1 (HR 0.372, 95% CI [0.171–0.808], p = 0.0125), MASP-2 (HR 0.297, 95% CI [0.133–0.666], p = 0.0032), C4 (HR 0.215, 95% CI [0.088–0.525], p = 0.0007), C4c (HR 0.371, 95% CI [0.171–0.806], p = 0.0123), C3 (HR 0.434, 95% CI [0.204–0.921], p = 0.0297), and C3bc/C3 (HR 3.283, 95% CI [1.468–7.344], p = 0.0038; Table 4).
When adjusting the models for sex and SAPS II a level above the median MASP-1 (HR 0.378, CI 95% [0.164–0.872], p = 0.0226) and C4 (HR 0.162, 95% CI [0.060–0.438], p = 0.0003) remained significantly associated with lower 90-day mortality (Table 4). Additionally, a level above the median C4c/C4 ratio (HR 2.290 95% CI [1.078–4.867], p = 0.0312), C3bc (HR 2.664 95% CI [1.195–5.938], p = 0.0166), and C3bc/C3 ratio (HR 4.041 95% CI [1.673–9.758], p = 0.0019) were associated with an increased mortality. The remaining complement parameters were not associated with death in the adjusted models.
In a model where we adjusted for age, sex, chronic disease, and SAPS II, the same complement parameters yielded significant results (Table 4). Adding amputation as a covariate in our models did not change our results (data not shown).
We generated ROC curves for our explanatory variables predicting 90-day mortality and compared them to the ROC curves for SAPS II, SOFA score on day 1, and arterial blood gas values.
The ROC Area Under the Curve (AUC) for MASP-1 (AUC 0.657, [95% CI 0.544–0.770]), MASP-2 (AUC 0.683, [95% CI 0.573–0.793]), C4 (AUC 0.748, [95% CI 0.649–0.847]), C4c (AUC 0.639, [95% CI 0.526–0.751]), C3 (AUC 0.686, [95% CI 0.577–0.794]), and C3bc/C3 (AUC 0.683, [95% CI 0.570–0.796]) were significantly different from random chance (AUC 0.5) as illustrated in Figure 2.
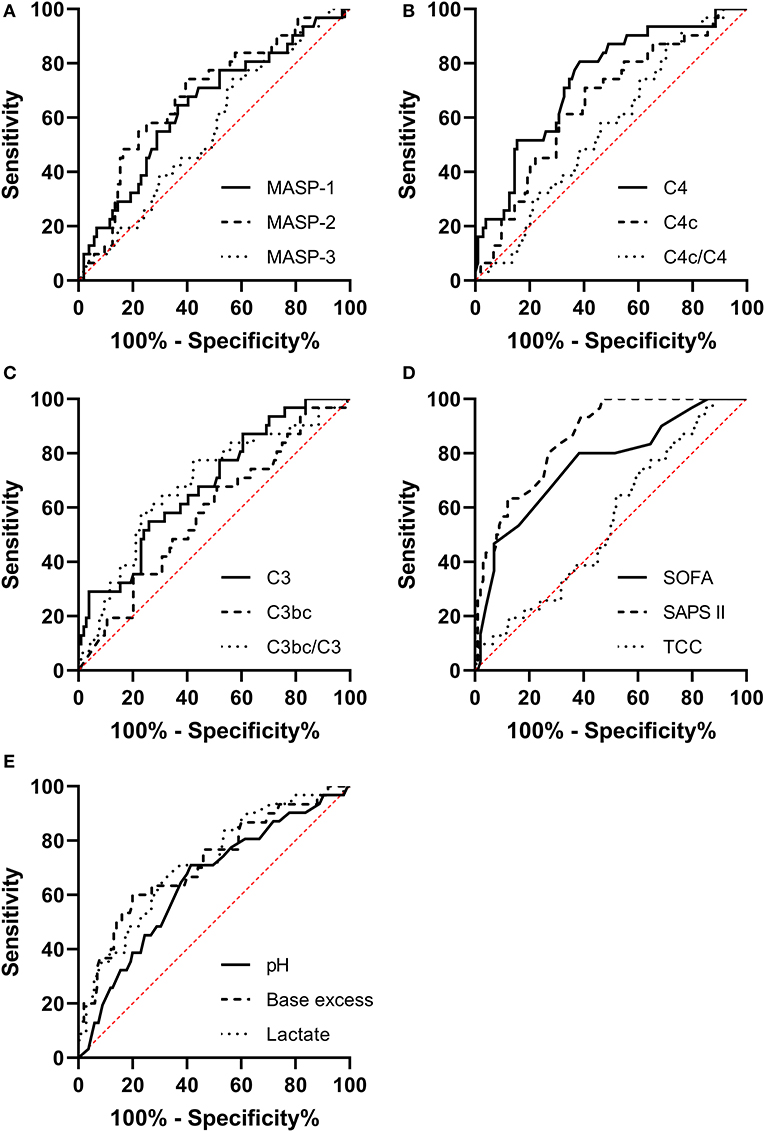
Figure 2. Receiver operating characteristic curves for predicting the 90-day mortality in necrotizing soft-tissue infection patients for (A) MASP-1, MASP-2, and MASP-3. (B) C4, C4c, and C4c/C4. (C) C3, C3bc, and C3bc/C3. (D) Sequential Organ Failure Assessment and score day 1 (SOFA), Simplified Acute Physiology Score II (SAPS II), and the terminal complement complex (TCC). (E) Arterial pH, base excess, and lactate.
The SOFA score had an AUC of 0.753, [95% CI 0.649–0.857], SAPS II had an AUC of 0.862, [95% CI 0.795–0.929]. The AUCs for the complement parameters combined with SOFA score or SAPS II were not significantly different from the AUC of SOFA or SAPS II alone (data not shown).
For pH the AUC was 0.704 [95% CI 0.589–0.818], for base excess the AUC was 0.716 [95% CI 0.603–0.829], and for lactate the AUC was 0.731 [95% CI 0.631–0.831]. The ROC curve for C4 combined with pH (AUC 0.777 [95% CI 0.674–0.880]) was significantly different than the ROC curve for pH alone (p = 0.0374). C4 combined with base excess or lactate was not better than base excess or lactate alone (data not shown).
Out of 135 patients, we could determine a responsible pathogen in 102 cases (76%). Two or more bacteria were responsible for 57 (56%) of these patients. The most common pathogen was Group A Streptococcus (GAS) with 36 infected patients (35%) (Table 1), but only 22 (22%) patients were only infected with GAS.
In this prospective, observational study we have shown that there's a relationship between the degree of complement activation and NSTI severity. Specifically, the levels of MASP-1, C4, C4c, C3, were significantly lower and C3bc/C3 significantly higher in non-survivors, than in survivors, who again differed significantly from controls. Similarly, the septic shock patients had significantly lower levels of C3, and higher levels of soluble TCC in their plasma, than the non-septic patients, who also differed significantly from the controls.
Interestingly, C4 and its breakdown product C4c both decreased with increasing severity. We expected an inverse proportionality between C4 and C4c, as we saw for C3 and its breakdown product C3bc. A probable explanation for this is a shorter half-life for C4c, leading to a faster clearance of C4c than of C3bc.
Overall, our findings are in line with studies on septic patients showing low C4 and C3 in non-survivors (20–22), and low MASP-1 in patients with disseminated intravascular coagulation (DIC) due to septic shock (23). This underlines that consumption of complement is directly linked to the pathophysiology in the hyperinflammatory reaction in NSTI as well as sepsis in general.
We have previously examined several complement system associated pattern recognition molecules (PRMs) such as CRP, Pentraxin-3, MBL, ficolin-1, ficolin-2, and ficolin-3 as biomarkers for risk stratification in the same cohort of NSTI patients (13, 19). In the survival analysis, ficolin-2 was the most promising of the above-mentioned PRMs, but the association with mortality disappeared after adjusting for SAPS II (13). Biomarkers outside of the complement system such as Procalcitonin and soluble urokinase plasminogen activator receptor (suPAR) have also been examined and they were not associated with mortality in the adjusted analyses (19, 24).
Here, we adjusted for the same covariates as previously and found above-median admission levels of MASP-1 and C4 to be independently associated with a lower risk of death before 90-days, when compared to below or equal to median admission levels. Above median admission levels of the C4c/C4 ratio, C3bc, and the C3bc/C3 ratio were independently associated with increased risk of death before 90-days from admission when compared to below or equal to median admission levels. These findings clearly suggest that complement consumption predicts a poor outcome in NSTI and that the ability to maintain circulating complement is linked to survival.
There are several, non-exclusive theories explaining the biological mechanism behind complement consumption in hyperinflammatory states. One theory is that consumption is a result of complement deposition in the necrotic and infected tissue. In addition, the complement protein production is probably affected in multiple organ failure, and there could also be a dilution effect with fluid resuscitation, although fluid resuscitation cannot explain why TCC concentration is higher in shock or non-survivors.
We lacked statistical power to examine the pathogens in the NSTI patients, but the MASP-1 findings indicate that the lectin pathway is involved in the pathophysiology. This is also supported by our previous findings showing that the NSTI patients had significantly lower baseline MBL and ficolin levels than the non-infected controls (13), indicating that the lectin pathway PRM's are also consumed in NSTI. To better understand the role of the classical pathway in NSTI we will have to proceed with C1q measurements.
If the depleted complement in plasma is reflecting an overactivation and dysregulation of complement in the infected and necrotic tissue; complement inhibition could possibly be beneficial. Complement inhibition has already been shown to improve survival in a septic baboon model (25). We think our findings warrant further studies on the use of C1-inhibitor and C5 inhibition in animal models.
The well-established clinical SOFA score and SAPS II also predicted the risk of death in adjusted Cox analyses. Scoring systems are necessary to monitor the patients over several days, however, using scoring systems as admission triage can be problematic as the necessary variables are often available after at least 24 h of admission. At that point, many interventions have already been made (26). The new SAPS 3, can be calculated quicker since it uses data available within 1 h of ICU admission (27), but, as Polzik et al. points out; it requires more variables to calculate, many of which may be missing upon admission (24). We wanted to compare the ROC curves for our complement parameters to the ROC curves for SAPS II, SOFA score on day 1, and the quickly available pH, base excess and lactate levels.
The prognostic value of MASP-1, MASP-2, C4, C4c, C3, C3bc/C3, pH, base excess, and lactate were similar to the prognostic value of SOFA score day 1, but only the AUC for C4, base excess and lactate had 95% CI's that overlapped with the AUC for SAPS II (0.862, [95% CI 0.795–0.929]). C4 had a higher AUC (0.748, [95% CI 0.649–0.847]) than the arterial gas values. Additionally, the AUC for C4 and pH combined (AUC 0.777 [95% CI 0.674–0.880]) was significantly higher than the AUC for pH alone (0.704, [95% CI 0.589–0.818]), further underlining C4's potential as a prognostic marker.
To our knowledge, this is the first study to examine complement parameters in a large, prospective cohort of NSTI patients. The study includes all NSTI patients transferred to our tertiary care hospital during the study period, which raises external validity. Besides, there was no loss to follow up, and the investigators performing analyses were blinded. Nevertheless, it is pertinent to mention some limitations of our study. NSTI patients are a heterogeneous group, and the complement parameters could be influenced by unknown confounders, as in other observational studies. Secondly, some patients might have died, before transfer to our tertiary hospital center, increasing the risk of selection bias. Fifteen patients (11%), did not fulfill the sepsis criteria according to Bone et al. which could be a result of this selection bias but could also be due to the variable time course to fulminant disease (18). Our controls were sampled preoperatively, while the NSTI patient's primary debridement often happened before transfer to our hospital. This means that the surgery, in and of itself, could bias the differences we measure between controls and patients. In addition, the controls were all elective orthopedic surgery patients. An extremity is the primary affected site in most NSTI cases but ideally, we should also have included controls from other surgical specialties. Lastly, we did not account for varying levels of complement proteins throughout the study, although the baseline level is most important for initial risk stratification and triage.
Since MASP-1, C4, the C4c/C4 ratio, C3bc, and the C3bc/C3 ratio showed great potential as prognostic markers next steps could be verification in a randomized control trial investigate differences in mortality, where one study arm uses complement admission levels to identify high-risk patients and then investigate for differences in mortality between groups. It would also be interesting to examine whether complement levels can discriminate non-necrotizing soft-tissue patients from NSTI to elucidate whether the complement levels could play a role diagnostically.
We have shown that there is a relationship between the degree of complement activation and NSTI severity suggesting that consumption of complement is directly linked to the pathophysiology in NSTI. Additionally, we have shown that low levels of MASP-1, C4 and high levels of the C4c/C4 ratio, C3bc and the C3bc/C3 ratio are associated with 90-day mortality in unadjusted and adjusted survival analyses, establishing these markers as better predictors of mortality than the currently used NSTI biomarkers C-reactive protein and Procalcitonin, at least in this cohort.
C4's ability to predict mortality was comparable to the well-established scoring systems SAPS score II and SOFA on day 1.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation, to any qualified researcher.
The studies involving human participants were reviewed and approved by the Regional Ethics Committee of the Capital Region of Denmark (H-2-2014-071) and the Danish Data Protection Agency (J. no. 30-1282) and registered at ClinicalTrials.gov (NCT02180906). The patients/participants or their legal guardians provided their written informed consent to participate in this study.
PG and OH conceived the study. PG, MH, and MM participated in study design and coordination. MH and MM participated in data acquisition and maintain the database for analysis. MH, KP, and MK analyzed the data. MK drafted the first manuscript. PG, OH, KP, CH, MH, MM, and MK contributed to critical revision of the work.
This INFECT project was supported by the European Seventh Framework Programme (Grant No. 305340). For more information about the European collaborative INFECT project, please visit https://cordis.europa.eu/project/rcn/106297/factsheet/en. This substudy was also supported by the Rigshospitalet Research Funds (grant number E-22514-02), the Aase and Ejnar Danielsens Foundation (Grant No. 10-001274), the Hans and Nora Buchards Foundation (Grant No. 7334), the Director Jacob Madsen and Olga Madsens Foundation (Grant No. 5323) and the Christian Larsen and judge Ellen Larsen's Foundation, the Tryg Foundation, the Danish Hearth Foundation [16-R107-A6650-22966], the Danish Research Foundation of Independent Research [DFF-6110-00489] and the Novo Nordisk Research Foundation.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank Jesper Andresen for help with the complement analyses and Nina Falcon Bærnthsen, Morten Hedetoft, Anna Mygind Wahl, Marie Warrer Petersen, Peter Polzik, and Isabel Guida Smidt-Nielsen for collecting blood samples. We also thank Peter Buhl Hjortrup, Matilde Jo Allingstrup, and Rasmus Müller for help with patient inclusion and the research nurses Diana Isaksen and Jette Fredlund Degn for help with data acquisition.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2020.00017/full#supplementary-material
AUC, area under the curve; CI, confidence interval; CRP, C-reactive protein; ELISA, enzyme-linked immunosorbent assays; HR, hazard ratio; IQR, interquartile range; NSTI, necrotizing soft-tissue infection; P, p-value; PRMs, pattern recognition molecules; ROC, Receiver Operating Characteristics; SAPS II, Simplified Acute Physiology Score II; SOFA, Sequential Organ Failure Assessment; suPAR, soluble urokinase plasminogen activator receptor; TCC, terminal C5b-9 complement complex.
1. Sartelli M, Guirao X, Hardcastle TC, Kluger Y, Boermeester MA, Raşa K, et al. 2018 WSES/SIS-E consensus conference: recommendations for the management of skin and soft-tissue infections. World J Emerg Surg. (2018) 13:58. doi: 10.1186/s13017-018-0219-9
2. Hua C, Sbidian E, Hemery F, Decousser JW, Bosc R, Amathieu R, et al. Prognostic factors in necrotizing soft-tissue infections (NSTI): a cohort study. J Am Acad Dermatol. (2015) 73:1006–12.e8. doi: 10.1016/j.jaad.2015.08.054
3. Bilton BD, Zibari GB, McMillan RW, Aultman DF, Dunn G, McDonald JC. Aggressive surgical management of necrotizing fasciitis serves to decrease mortality: a retrospective study. Am Surgeon. (1998) 64:397–401.
4. Wong C-H, Chang H-C, Pasupathy S, Khin L-W, Tan J-L. Necrotizing Fasciitis: Clinical Presentation, Microbiology, and Determinants of Mortality. J Bone Joint Surg Am. (2003) 85:1454–60. doi: 10.2106/00004623-200308000-00005
5. Goh T, Goh LG, Ang CH, Wong CH. Early diagnosis of necrotizing fasciitis. BJS. (2014) 101:e119–25. doi: 10.1002/bjs.9371
6. Latifi R, Patel AS, Samson DJ, Tilley EH, Gashi S, Bergamaschi R, et al. The roles of early surgery and comorbid conditions on outcomes of severe necrotizing soft-tissue infections. Eur J Trauma Emerg Surg. (2018) 45:919–26. doi: 10.1007/s00068-018-0958-z
7. Garcia NM, Cai J. Aggressive soft tissue infections. Surg Clin North Am. (2018) 98:1097–108. doi: 10.1016/j.suc.2018.05.001
8. Pham TN, Moore ML, Costa BA, Cuschieri J, Klein MB. Assessment of functional limitation after necrotizing soft tissue infection. J Burn Care Res. (2009) 30:301–6. doi: 10.1097/BCR.0b013e318198a241
9. Walport MJ. Complement - first of two parts. N Engl J Med. (2001) 344:1140–4. doi: 10.1056/NEJM200104123441506
10. Lubbers R, van Essen MF, van Kooten C, Trouw LA. Production of complement components by cells of the immune system. Clin Exp Immunol. (2017) 188:183–94. doi: 10.1111/cei.12952
11. Martin M, Blom AM. Complement in removal of the dead – balancing inflammation. Immunol Rev. (2016) 274:218–32. doi: 10.1111/imr.12462
12. Markiewski MM, DeAngelis RA, Lambris JD. Complexity of complement activation in sepsis. J Cell Mol Med. (2008) 12:2245–54. doi: 10.1111/j.1582-4934.2008.00504.x
13. Hansen MB, Rasmussen LS, Pilely K, Hellemann D, Hein E, Madsen MB, et al. The lectin complement pathway in patients with necrotizing soft tissue infection. JIN. (2016) 8:507–16. doi: 10.1159/000447327
14. Garred P, Mollnes TE, Lea T. Quantification in enzyme-linked immunosorbent assay of a C3 neoepitope expressed on activated human complement factor C3. Scand J Immunol. (1988) 27:329–35. doi: 10.1111/j.1365-3083.1988.tb02354.x
15. Pilely K, Skjoedt M-O, Nielsen C, Andersen TE, Louise Aabom A, Vitved L, et al. A specific assay for quantification of human C4c by use of an anti-C4c monoclonal antibody. J Immunol Methods. (2014) 405:87–96. doi: 10.1016/j.jim.2014.01.011
16. Mollnes TE, Lea T, Frøland SS, Harboe M. Quantification of the terminal complement complex in human plasma by an enzyme-linked immunosorbent assay based on monoclonal antibodies against a neoantigen of the complex. Scand J Immunol. (1985) 22:197–202. doi: 10.1111/j.1365-3083.1985.tb01871.x
17. Skjoedt M-O, Palarasah Y, Munthe-Fog L, Jie Ma Y, Weiss G, Skjodt K, et al. MBL-associated serine protease-3 circulates in high serum concentrations predominantly in complex with ficolin-3 and regulates ficolin-3 mediated complement activation. Immunobiology. (2010) 215:921–31. doi: 10.1016/j.imbio.2009.10.006
18. Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Chest. (1992) 101:1644–55. doi: 10.1378/chest.101.6.1644
19. Hansen MB, Rasmussen LS, Garred P, Bidstrup D, Madsen MB, Hyldegaard O. Pentraxin-3 as a marker of disease severity and risk of death in patients with necrotizing soft tissue infections: a nationwide, prospective, observational study. Crit Care. (2016) 20:40. doi: 10.1186/s13054-016-1210-z
20. Andaluz-Ojeda D, Iglesias V, Bobillo F, Almansa R, Rico L, Gandía F, et al. Early natural killer cell counts in blood predict mortality in severe sepsis. Crit Care. (2011) 15:R243. doi: 10.1186/cc10501
21. Nakae H, Endo S, Inada K, Yoshida M. Chronological changes in the complement system in sepsis. Surg Today. (1996) 26:225–9. doi: 10.1007/BF00311579
22. Ren J, Zhao Y, Yuan Y, Han G, Li W, Huang Q, et al. Complement depletion deteriorates clinical outcomes of severe abdominal sepsis: a conspirator of infection and coagulopathy in crime? PLoS ONE. (2012) 7:e47095. doi: 10.1371/journal.pone.0047095
23. Larsen JB, Laursen MA, Hvas CL, Larsen KM, Thiel S, Hvas A-M. Reduced mannose-binding lectin-associated serine protease (MASP)-1 is associated with disturbed coagulation in septic shock. Thromb Haemost. (2019) 119:952–61. doi: 10.1055/s-0039-1685140
24. Polzik P, Grøndal O, Tavenier J, Madsen MB, Andersen O, Hedetoft M, et al. SuPAR correlates with mortality and clinical severity in patients with necrotizing soft-tissue infections: results from a prospective, observational cohort study. Sci Rep. (2019) 9:5098. doi: 10.1038/s41598-019-41688-y
25. Keshari RS, Silasi R, Popescu NI, Patel MM, Chaaban H, Lupu C, et al. Inhibition of complement C5 protects against organ failure and reduces mortality in a baboon model of Escherichia coli sepsis. Proc Natl Acad Sci USA. (2017) 114:E6390–9. doi: 10.1073/pnas.1706818114
26. Awad A, Bader-El-Den M, McNicholas J, Briggs J, El-Sonbaty Y. Predicting hospital mortality for intensive care unit patients: time-series analysis. Health Informatics J. (2019). doi: 10.1177/1460458219850323. [Epub ahead of print].
27. Moreno RP, Metnitz PGH, Almeida E, Jordan B, Bauer P, Campos RA, et al. SAPS 3—from evaluation of the patient to evaluation of the intensive care unit. part 2: development of a prognostic model for hospital mortality at ICU admission. Intensive Care Med. (2005) 31:1345–55. doi: 10.1007/s00134-005-2763-5
Keywords: necrotizing fasciitis, soft tissue infection, sepsis, amputation, survival complement activation, immune system
Citation: Kristensen MK, Hansen MB, Madsen MB, Hansen CB, Pilely K, Hyldegaard O and Garred P (2020) Complement Activation Is Associated With Mortality in Patients With Necrotizing Soft-Tissue Infections—A Prospective Observational Study. Front. Immunol. 11:17. doi: 10.3389/fimmu.2020.00017
Received: 31 October 2019; Accepted: 07 January 2020;
Published: 31 January 2020.
Edited by:
Iara De Messias Reason, Federal University of Paraná, BrazilReviewed by:
Goran Zoran Stanojević, University of Niš, SerbiaCopyright © 2020 Kristensen, Hansen, Madsen, Hansen, Pilely, Hyldegaard and Garred. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peter Garred, cGV0ZXIuZ2FycmVkQHJlZ2lvbmguZGs=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.