- 1Department of Agricultural Biotechnology, Research Institute for Agriculture and Life Sciences, Seoul National University, Seoul, South Korea
- 2Department of Biological Science, College of Medical and Life Sciences, Silla University, Busan, South Korea
- 3Department of Animal Resources Technology, Gyeongnam National University of Science and Technology, Jinju, South Korea
- 4Department of Nano-Bioengineering, Incheon National University, Incheon, South Korea
- 5Department of Biochemistry, Institute of Cell Differentiation and Aging, College of Medicine, Hallym University, Chuncheon, South Korea
- 6Laboratory of Dendritic Cell Differentiation and Regulation, Department of Immunology, School of Medicine, Konkuk University, Chungju, South Korea
- 7Department of Oral Microbiology and Immunology, Dental Research Institute and Brain Korea 21 Plus Program, School of Dentistry, Seoul National University, Seoul, South Korea
- 8Institute of Green Bio Science Technology, Seoul National University, Seoul, South Korea
- 9Center for Food and Bioconvergence, Seoul National University, Seoul, South Korea
Probiotics can be an effective treatment for atopic dermatitis (AD), while their mechanism of action is still unclear. Here, we induced AD in mice with 2,4-dinitrochlorobenzene and administrated YK4, a probiotic mixture consisting of Lactobacillus acidophilus CBT LA1, L. plantarum CBT LP3, Bifidobacterium breve CBT BR3, and B. lactis CBT BL3. Then, we have validated the underlying mechanism for the alleviation of AD by YK4 from the intestinal and systematic immunological perspectives. Administration of YK4 in AD mice alleviated the symptoms of AD by suppressing the expression of skin thymic stromal lymphopoietin and serum immunoglobulin E eliciting excessive T-helper (Th) 2 cell-mediated responses. YK4 inhibited Th2 cell population through induce the proportion of Th1 cells in spleen and Treg cells in Peyer's patches and mesenteric lymph node (mLN). CD103+ dendritic cells (DCs) in mLN and the spleen were significantly increased in AD mice administered with YK4 when compared to AD mice. Furthermore, galectin-9 was significantly increased in the gut of AD mice administered with YK4. In vitro experiments were performed using bone marrow-derived DCs (BMDC) and CD4+ T cells to confirm the immune mechanisms of YK4 and galectin-9. The expression of CD44, a receptor of galectin-9, together with programmed death-ligand 1 was significantly upregulated in BMDCs following treatment with YK4. IL-10 and IL-12 were upregulated when BMDCs were treated with YK4. Cytokines together with co-receptors from DCs play a major role in the differentiation and activation of CD4+ T cells. Proliferation of Tregs and Th1 cell activation were enhanced when CD4+T cells were co-cultured with YK4-treated BMDCs. Galectin-9 appeared to contribute at least partially to the proliferation of Tregs. The results further suggested that DCs treated with YK4 induced the differentiation of naïve T cells toward Th1 and Tregs. At the same time, YK4 alleviated AD symptoms by inhibiting Th2 response. Thus, the present study suggested a potential role of YK4 as an effective immunomodulatory agent in AD patients.
Introduction
Atopic dermatitis (AD) is one of the most common chronic inflammatory skin diseases seen mostly in children, although it can occur at any age. The main symptoms of AD are destruction of the stratum corneum and increased eczema and itching. AD is known to be caused by complex interactions between genetic factors and extrinsic allergens (1, 2). When AD occurs, scratching of the skin due to itching causes severe damage to the epidermal barrier of the skin followed by dysregulation of immunoregulatory proteins, such as interleukin (IL)-1, IL-25, IL-33, and thymic stromal lymphopoietin (TSLP), in skin epithelial cells (3, 4). These immunomodulatory proteins are known to initiate and promote T-helper (Th) 2 cell-mediated immune responses (5). Activated Th2 cells release IL-4, IL-13, and IL-31, and stimulate B cells to undergo isotype switching of immunoglobulin (Ig) M to IgE (6). The increased IgE production causes eosinophil accumulation in the dermis (7).
The immune imbalance induces an increase of inflammation in the skin and exacerbates the symptoms of AD. Moreover, AD patients have increased risk of other atopic diseases, including asthma, allergic rhinitis, and food allergies (8). Several drugs are currently under development to improve itch-associated symptoms of AD, and to show anti-inflammatory or epithelial barrier repair action. Monoclonal antibodies and protein inhibitors are also under investigation to block the cytokine and/or its receptor signaling pathway and thereby alleviate symptoms of AD (9). Although steroids are widely accepted as the first choice of anti-inflammatory drugs and intermittent use can reduce disease recurrence, long-term drug therapy with steroids should be avoided due to side effects, such as nausea, vomiting, diarrhea, skin thinning, and purpura (10). Therefore, alternative therapies, including herbs, plants, vitamins, and probiotics, have been studied (11, 12).
Probiotics are live microorganisms that provide beneficial effects on host health and disease prevention and treatment (13). Among various probiotics, Lactobacillus, Bifidobacterium, Escherichia, and Saccharomyces have been widely studied and commonly used in humans and animals (14). Ingested probiotics competed with harmful microorganisms to prevent pathogens from adhering to the epithelium in the intestine (15). Probiotics also enhanced the survival of intestinal epithelial cells and improved the barrier function, and production of immunomodulatory substances (16). Some probiotics reach to the lamina propria through M cells and interact with immune cells to regulate gastrointestinal immune system (17). Dendritic cells (DCs) in the lamina propria layer was known to be the main cell type that recognizes probiotics (18). DCs are one of the antigen-presenting cells that play a key role in bridging innate and adaptive immune responses (19). Specifically, DCs that were specialized for inhibiting inflammation, called tolerogenic DCs (tDCs), and CD103+ DCs played a similar role in the gastrointestinal area (20). CD103+ DCs inhibited naive CD4+ T cell differentiation into Th2 cells and, at the same time, induced the differentiation of regulatory T cells (Tregs) through the production of IL-10 and TGF-β (20). Recently, the effects of DCs primed by probiotics to control T cell responses have been reported (21, 22). B. breve Yakult induced the production of IL-10 in DCs through TLR2/MyD88 signal transduction and promoted the differentiation of Tregs (23). In addition, L. plantarum WCFS1 induced CD103+ DCs infiltration and generation of Tregs in the spleen (24). Duolac ATP, a mixture of four probiotic strains; i.e., Lactobacillus casei CBT LC5, Lactobacillus plantarum CBT LP3, Lactobacillus rhamnosus CBT LR5, and Bifidobacterium lactis CBT BL3, was reported to modulate the expression of costimulatory molecules of DCs and downregulate Th2 responses in an AD mouse model (25). Mixed probiotic strains of Lactobacillus and Bifidobacterium reduced the atopic dermatitis index in young AD patients (26) and an AD mouse model (27, 28). However, the mechanism of action of probiotics is only partially understood.
Galectins are soluble forms of lectin that exhibit specific binding activity for beta-galactoside sugars (29). Galectin-1, -2, -3, -4, and -9 are generally expressed in the gastrointestinal tract (30) and appear to be involved in the regulation of intestinal homeostasis and immunity (31). Especially, galectin-9 binds directly to CD44 and promotes Foxp3 expression of Tregs, which have been reported to suppress excessive Th2 responses (32). Indeed, recombinant galectin-9 treatment resulted in improved clinical and immunological symptoms of allergic diseases in mouse models (33). Recently, Bifidobacterium breve M-16V, used as a dietary supplement, was shown to induce elevation of serum galectin-9 in mice and humans (34). However, the precise mechanism by which galectin-9 modulates immune cells in AD therapy is not yet clear.
In the present study, we designed YK4, a mixture of four probiotic strains; i.e., Lactobacillus acidophilus CBT LA1, L. plantarum CBT LP3, B. breve CBT BR3, and B. lactis CBT BL3, which were selected based on their anti-inflammatory activities in bone marrow-derived DCs (BMDCs). This study was performed to evaluate the effects of YK4 on regulation of the intestinal and systemic immune systems using an AD mouse model in relation to galectin-9 expression and CD4+ T cells.
Materials and Methods
Animal
Female, 6- to 9-week old, BALB/c mice were purchased from Orient Bio (Gapyeong, South Korea). The mice were randomized and housed at 2–3 mice per individually ventilated cages under the controlled environment with a 12 h light-dark cycle. Water and diet are the principle of unlimited supply, but stopped 3 h before the administration of probiotics or phosphate buffered saline (PBS; Thermo Fisher Scientific, Waltham, MA, USA). All the experimental procedures were carried out in accordance with the Animal Use and Care Protocol approved by the Institutional Animal Care and Use Committee at Seoul National University, Seoul, Korea (Approval No. SNU-170428-1-1).
Probiotics
Probiotic strains were obtained from Cell Biotech Co. Ltd (Gimpo, Korea) as a lyophilized powder form, containing 1 × 1011 CFU/g. YK4, used in the present study, is composed of four different strains of probiotics: L. acidophius CBT LA1 (KCTC11906BP), L. plantarum CBT LP3 (KCTC10782BP), B. breve CBT BR3 (KCTC12201BP), and B. lactis CBT BL3 (KCTC11904BP). The constituent probiotics of YK4 were mixed at the same weight ratio.
Mouse With Atopic Dermatitis Model
The protocol to induce AD-like skin lesion in mouse model was modified from Lim et al. (35). Before the atopic induction, the dorsal hair of the mice were shaved and divided into three groups (n = 5 per group): (1) PBS control (Control), (2) 2,4-dinitrochlorobenzene (DNCB; Sigma-Aldrich, St. Louis, MO, USA) + PBS (AD), and (3) DNCB + YK4 (1 × 109 CFU/day; AD + YK4). In order to induce the atopy, skin was topically sensitized with 1% DNCB that was dissolved in acetone-olive oil (3:1) twice for the first week, and 0.2% DNCB for three times in the second week. Once atopy was induced, 0.2% DNCB was applied for the maintenance purpose at once a week for 3–6 weeks. While maintaining the atopy, mice were administered a total of 12 times with PBS (200 μl/day) or YK4 (1 × 109 CFU/200 μl/day) using feeding needle for three times a week. At the end of the treatment, the mice were anesthetized by using CO2 (Figure 1A). Then, blood samples were taken from the mice and dorsal skin, intestines, spleen, mesenteric lymph node (mLN) and Peyer's patches (PP) were extracted.
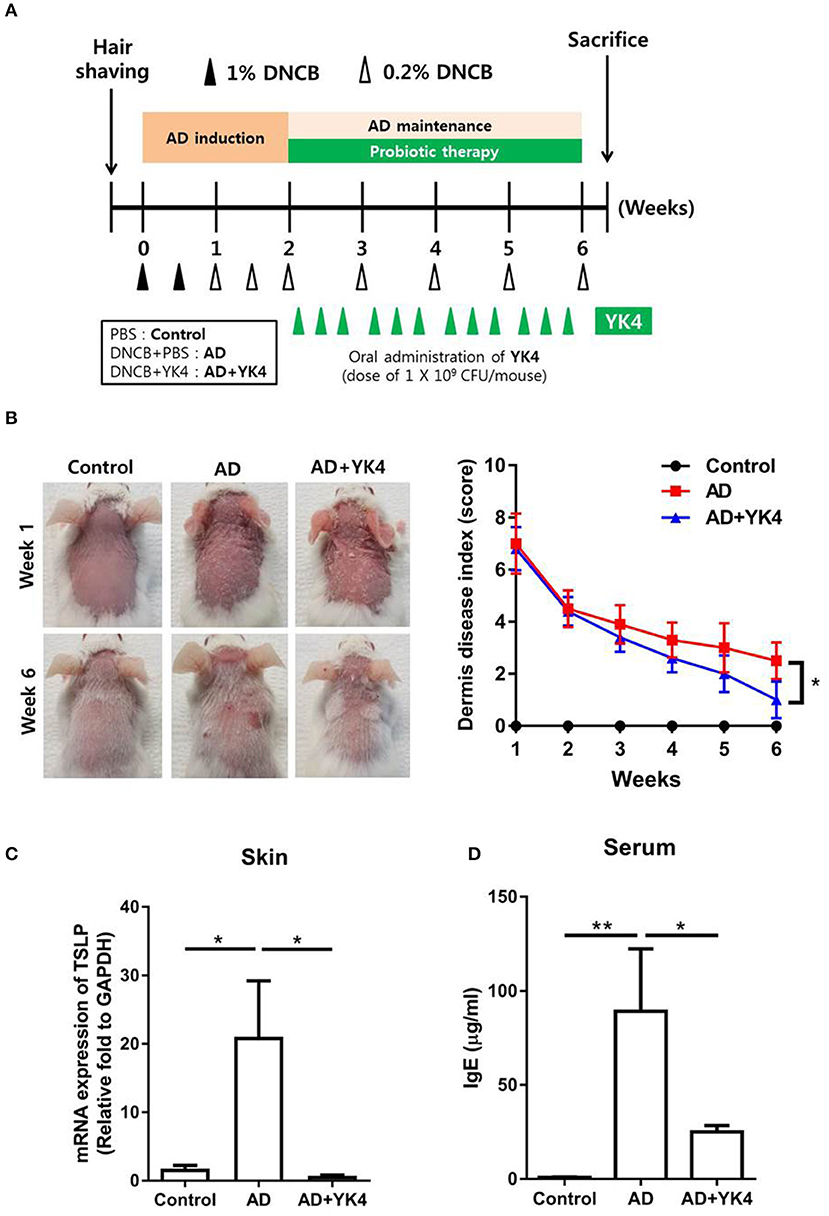
Figure 1. Amelioration of atopic dermatitis (AD)-like symptoms in mice treated with YK4. (A) Schematic diagram of 2,4-dinitrochlorobenzene (DNCB)-induced AD mouse model of AD-like skin lesions and oral administration of YK4 in BALB/c mice. DNCB was applied to the dorsal skin of BALB/c mice as described in the Materials and Methods. The mice were divided into three groups: (1) phosphate buffered saline (PBS) control (Control), (2) DNCB + PBS (AD), and (3) DNCB + YK4 (1 × 109 CFU/day; AD + YK4). (B) AD-like skin lesions were evaluated by visual observation. Scoring of the dermatitis index was performed as described in the Materials and Methods. (C) Dorsal skin was also collected on week 6, and RNA was extracted for cDNA synthesis. qPCR was performed to examine thymic stromal lymphopoietin (TSLP) mRNA expression. Relative fold changes of target genes were compared with the housekeeping gene, GAPDH. (D) Blood samples were acquired and serum IgE levels were measured by ELISA. *P < 0.05, **P < 0.01 by one-way ANOVA with Tukey's multiple comparison test. Bars indicate means ± standard error of the mean (SEM).
Dermatitis Index
The severity of dermatitis score was evaluated once a week after DNCB treatment. Scores of 0 (none), 1 (mild), 2 (moderate), and 3 (severe) were examined for each of the four symptoms: erythema/hemorrhage, scarring/dryness, edema and excoriation/erosion. The sum of the individual scores indicating clinical severity was taken as the dermatitis score (36).
Sample Preparation
Blood samples were collected by retro-orbital bleeding into heparinized tubes. The sera were then collected by centrifugation for 10 min at 1,500 × g and stored at −80°C, until further use. The mice were sacrificed at the end of the experiment. Spleen and large intestine were collected and the length was measured to determine the visual examination and inflammatory responses. Dorsal skin, and small and large intestines were collected for the mRNA expression of galectin-9. Spleen, mLN and PP were taken and rinsed with PBS. Then, they were placed on the 70 μm cell strainer (BD Biosciences) and ground using the back of the syringe to make single cells. Red blood cells were depleted using RBC-lysis buffer (Sigma-Aldrich) and the cells were stained with the proper combination of antibodies labeled with fluorescence and examined by using flow cytometry (FACScanto, BD Biosciences).
Generation and Culture of BMDCs
BM cells were isolated from femurs of mice. Red blood cells were depleted using RBC-lysis buffer (Sigma-Aldrich) and BM cells were cultured in a complete RPMI with 20 ng/ml GM-CSF (Creagene, Seongnam, Korea). The complete RPMI was composed of RPMI-1640 supplemented with 10% fatal bovine serum, 20 mM HEPES, 1 mM sodium pyruvate, 220 nM 2-mercaptoethanol, 100 μg/ml gentamicin (all from Sigma-Aldrich). BM cells were seeded at 3 × 106 cells/well in a 6-well plate in 3 ml media, and then 2 ml of fresh media was added at day 3 and 5. At day 6, a half of the culture supernatant was carefully discarded, and 3 ml of fresh media was added. At day 7, suspended BM cells were harvested and sorted by CD11c MicroBeads UltraPure kit (Miltenyi Biotec, Bergisch Gladbach, Germany). Suspended CD11c+ BM cells (i.e., BM-derived DCs, BMDCs) were seeded at 2 × 105 cells/well in 96-well plate and stimulated with LA1 (2 × 106 CFU/well), LP3 (2 × 106 CFU/well), BR3 (2 × 106 CFU/well), BL3 (2 × 106 CFU/well), YK4 (2 × 106 CFU/well), 100 ng/ml of Lipopolysaccharide (LPS, Sigma-Aldrich) and 1 μg/ml of recombinant mouse galectin-9 (R&D Systems, Minneapolis, MN, USA) in a complete RPMI. After the incubation for 24 h, the supernatant was collected for the examination of cytokine concentration.
In vitro CD4+ T Cell Stimulation
CD4+ T cells were isolated from mLN from wild type mice using mouse CD4+ T lymphocyte enrichment Set (BD Biosciences, San Jose, CA, USA). The CD4+ T cells were labeled with CellTrace™ Violet (CTV) Cell Proliferation Kit (Thermo Fisher Scientific). CD4+ T cells (2 × 105 cells/well) were co-cultured with BMDCs (2 × 104 cells/well) that had been treated with YK4 (2 × 105 CFU/well) and/or galectin-9 (1 μg/ml), on anti-CD3 mAbs (BD Biosciences)-coated (2 μg/ml) 96-well plate. After the incubation for 72 h, the cells were examined for proliferation of Foxp3+CD4+ T cells by using flow cytometry and the supernatant was collected and examined for IL-4, IL-10, IL-17, and IFN-γ concentration by using ELISA.
RNA Isolation and qPCR
Total RNA was isolated from dorsal skin and intestines by TRIzol® reagent (Thermo Fisher Scientific). One microgram of RNA was reverse-transcribed in a 20 μl reaction containing random primers (500 μg/ml), dNTP (10 mM), 5× first strand buffer, Dithiothreitol (0.1 M), Superscript III enzyme (200 U/μl) and RNase inhibitor (10 U/μl) (all from Thermo Fisher Scientific). The quantitative PCR (qPCR) was performed with the iQ SYBR Green Supermix (Bio-Rad Laboratories, Hercules, CA, USA) on the LightCycler 480 Real-Time PCR System (Roche, Mannheim, Germany). This was then used to calculate the relative amounts of target mRNA in test samples. Quantities of all targets in test samples were normalized to the corresponding GAPDH levels. Primers mouse galectin-9 (forward: 5′-CAG CAC CCC TGG ACA GAT GT-3′, reverse: 5′-ATG GAC TTG GAC GGG TAA AGC-3′), mouse TSLP (forward: 5′-TAC TAT ACT CTC AAT CCT ATC CCT G-3′, reverse: 5′-ACT TCT TGT GCC ATT TCC TG-3′), mouse GAPDH (forward: 5′-CTC CAC TCA CGG CAA ATT CA-3′, reverse: 5′-GCC TCA CCC CAT TTG ATG TT-3′) were synthesized from Bioneer Inc. (Daejeon, Korea).
Enzyme-Linked Immunosorbent Assay (ELISA)
The immunological response of the mice with DNCB-induced AD was monitored by measuring the levels of serum IL-4, IL-10, IL-12p40, IL-17, TGF-β, IFN-γ (all from R&D Systems) and IgE (BD Biosciences). Using the ELISA DuoSet kit (R&D Systems), mouse IL-6, IL-10 and IL-12p40 were measured in stimulated BMDCs supernatant, and IL-4, IL-10, IL-17 and IFN-γ were measured in stimulated T cells supernatant in vitro. Briefly, 96-well microplate (Thermo Fisher Scientific) was pre-coated with 100 μl/well of capture antibody. After blocking with 1% BSA for 1 h at room temperature, 100 μl/well of supernatant along with the standard solution diluted in diluent buffer was added and incubated for 2 h at room temperature. After the wash with PBS for three times, 100 μl/well of biotinylated detection antibody was added and incubated for 2 h at room temperature. Then, the plate was washed with PBS for three times and 100 μl of streptavidin-HRP in PBS was added. After the incubation for 20 min at room temperature, tetramethylbenzidine (TMB, Merck Millipore, Burlington, MA, USA) was added to develop the color and then the reaction was stopped by adding 50 μl of 2M H2SO4. The absorbance at wavelength 450 nm was measured by a microplate reader (Molecular Devices, San Jose, CA, USA).
Phenotypic and Functional Examination of Immune Cells by Using Flow Cytometry
In order to examine the activation status of the cells, BMDCs were treated with YK4 and/or galectin-9 for 24 h at 37°C. The cells were stained with anti-mouse CD44-FITC, CD86-FITC, PD-L1-PE, MHC II-PE-cy7, OX40L-APC, CD11c-APC (all from BD Biosciences) for 20 min at 4°C in the dark. To test the change of Tregs, CTV-labeled CD4+ T cells cultured with YK4 and/or galectin-9-treated BMDCs for 3 days were stained with anti-mouse CD25-FITC and CD4-PE (BD Biosciences). After surface staining, CD4+ T cells were fixed and stained with anti-mouse Foxp3-APC mAb (Biolegend, San Diego, MA, USA) using Foxp3 Fix/Perm Buffer Set (Biolegend). In vivo examination, spleen, mLN, and PP were isolated from the mice and single cells were prepared. Population changes of DCs were examined after the staining with combination of anti-mouse CD103-bv421, MHC II-PE-cy7, and CD11c-APC (all from BD Biosciences) for 20 min at 4°C in the dark. To examine the preferential subpopulation of CD4+ T cells, splenocytes, and mononuclear cells from mLN and PP were stimulated with 50 ng/ml of phorbol 12-myristate-13-acetate (PMA) and 750 ng/ml of ionomycin (Sigma-Aldrich) in the presence of brefeldin A (Sigma-Aldrich) for 4 h. Then, the cells were stained with appropriate combination of anti-mouse CD4-bv605, IFN-γ-PE, IL-4-bv605, and IL-17-APC-cy7 mAb (all from Biolegend). The cells were washed and the expression of fluorescence was examined using a FACS Canto II (BD Biosciences). All flow cytometric data acquired were analyzed with FlowJo software (Tree Star, Ashland, OR, USA).
Statistical Analysis
The levels of significance for the comparison between in vivo samples were determined by Tukey's multiple comparison test by using GradPad InStat software (Ver 5.01, GraphPad). The data were expressed as the mean ± SEM. In vitro data were evaluated using Student's t-test, and p < 0.05 was considered as statistically significant.
Results
Amelioration of AD in Mice Treated With YK4
To investigate the therapeutic properties of YK4 in vivo, we established a mouse model with AD-like skin lesions. As shown in Figure 1A, the mice were sensitized with DNCB for 2 weeks and then given phosphate buffered saline or 1 × 109 CFU of YK4 intragastrically. The AD group showed severe atopic symptoms including erythema/hemorrhage, edema, excoriation/erosion, and scaling/dryness. Oral administration of YK4 significantly ameliorated the severity of AD-like skin lesions compared to the AD group (Figure 1B). It has been suggested that inflammatory responses caused by AD may affect other organs. For example, strong Th2 inflammation causes intestinal contraction and splenomegaly (37, 38). No significant changes were found on visual or physical examination of the AD mice based on the length of the spleen and large intestine at the end of the feeding period (Supplementary Figures 1A,B). It has been suggested that the expression of TSLP in keratinocytes is one of the main features of AD (39). To investigate the induction of skin inflammation, we measured TSLP mRNA expression in the skin. The results showed that TSLP in the skin of the AD group was significantly increased, whereas it remained at a normal level in the AD group treated with YK4 (Figure 1C). Excessive production of serum IgE is a typical characteristic of AD symptoms in accordance with the induction of a Th2-type immune response (2, 40). The results of this study showed that serum IgE levels were significantly increased in the AD group compared to the control group, whereas the AD group administered YK4 showed significantly lower IgE (Figure 1D). These results suggested that YK4 effectively inhibited the expression of TSLP and IgE, thereby ameliorating the symptoms of AD.
YK4 Administration Induced a Decrease in the Th2 Response Coincident With an Increase in Tregs in vivo
AD is triggered by hypersensitivity of the Th2 response, which can be overcome by rebalancing CD4+ T cell subsets (25). To determine whether YK4 administration affects intestinal and systemic T cell responses, subpopulations of CD4+ T cells in mLN, PP, and the spleen obtained using the gating strategy shown in Figure 2A were examined in AD mice with/without YK4 administration. The results showed that the proportion of CD4+ T cells in PP was significantly increased in AD mice administered YK4, but no changes were observed in mLN and the spleen (Figure 2B). Next, we examined the other subtypes of helper T cells by testing the intracellular expression of interferon (IFN)-γ, IL-4, and IL-17 in CD4+ T cells. The ratio of Th1 to Th17 cells did not change, while the ratio of Th2 cells decreased significantly in PP and mLN from AD mice administered YK4. On the other hand, the Th1 response in the spleen was increased in AD mice administered YK4 (Figure 2C), suggesting that it potentially counteracted the decrease in the Th2 response. Th2-induced allergic reactions are known to be suppressed by Tregs (41). Therefore, we examined the changes in populations of Tregs in PP, mLN, and the spleen of AD mice administered YK4. The Tregs populations in PP and mLN were increased in AD mice administered YK4 compared to control and AD groups, while no changes were found in the spleen (Figure 2D).

Figure 2. Characterization of CD4+ T cells from Peyer's patches (PP), mesenteric lymph nodes (mLN), and the spleen of DNCB-sensitized BALB/c mice treated with YK4. DNCB-induced AD mice were fed YK4, and PP, mLN, and the spleen were collected on week 6. Single cells from each tissue were used for characterization of CD4+ T cells. (A) Gating strategy for CD4+ T cell subtypes. (B) The percentages of total CD4+ T cells from PP, mLN, and the spleen were examined by flow cytometry. (C) Single cells from PP, mLN, and the spleen were stimulated with PMA/ionomycin in the presence of brefeldin A for 4 h. IFN-γ-, IL-4-, and IL-17-producing CD4+ T cells from PP, mLN, and the spleen were examined following intracellular staining by flow cytometry. (D) The percentages of Foxp3+CD25+CD4+ T cells from PP, mLN, and the spleen were examined by flow cytometry. (E) Blood samples were taken and serum TGF-β levels were measured by ELISA. Data are representative of at least three experiments. *P < 0.05, **P < 0.01, ***P < 0.001 by one-way ANOVA with Tukey's multiple comparison test. Bars indicate means ± SEM.
Cytokine production in atopic disease is one of the indicators used to indirectly examine the systemic immune response. For example, IL-10 and transforming growth factor (TGF)-β affect the differentiation and function of Tregs (42). Therefore, we measured serum cytokines to assess their possible roles in the relationship between YK4 and T cell responses. The results showed that the levels of serum IFN-γ, IL-4, IL-10, and IL-17 were below the limits of detection in all groups (data not shown). However, the serum TGF-β level was significantly decreased at the onset of AD, and recovered following administration of YK4 (Figure 2E). These results indicated that YK4 induces a decrease in the Th2-type response coincident with an increase in Tregs in the intestine and induction of serum TGF-β expression.
YK4 Administration Induced an Increase in CD103+ Dendritic Cells in vivo
DCs are among the most important antigen presenting cells for the differentiation of naïve T cells into their specific subsets. In particular, CD103+ DCs are known to induce Tregs differentiation in the gastrointestinal tract (43). In the present study, the proportions of CD11c+MHCII+ and CD103+ DCs in AD mice administered YK4 were examined using the gating strategy shown in Figure 3A. The proportions of DCs in PP, mLN, and spleen were significantly increased in AD mice administered YK4 in comparison to AD mice (Figure 3B). Moreover, the increases in CD103+ DCs in mLN and the spleen were pronounced (Figure 3C). Taken together, these results suggested that CD103+ DCs were increased in AD mice administered YK4.
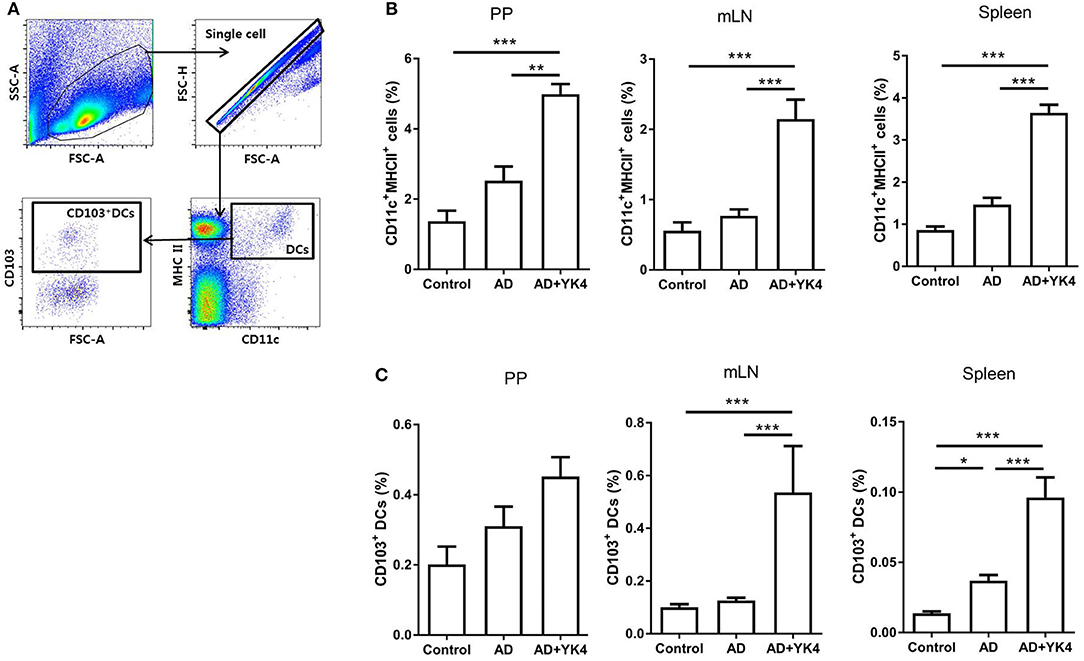
Figure 3. Composition of dendritic cells (DCs) from PP, mLN, and the spleen in DNCB-sensitized BALB/c mice treated with YK4. DNCB-induced AD mice were fed YK4, and PP, mLN, and the spleen were collected on week 6. Single cells from each tissue were used to examine the proportion of DCs. (A) Gating strategy for DC subtypes. The percentages of (B) CD11c+MHC II+ DCs and (C) CD103+ DCs from PP, mLN, and the spleen were examined by flow cytometry. Data are representative of at least three experiments. *P < 0.05, **P < 0.01, ***P < 0.001 by one-way ANOVA with Tukey's multiple comparison test. Bars indicate means ± SEM.
Galectin-9 in the Intestine Appears to Be Associated With Alleviation of AD Symptoms
Galectin-9 is known to regulate the immune response via modulation of DCs with sequential differentiation of Tregs (44, 45). We examined the expression of galectin-9 in the intestine to examine whether it plays a role in the suppression of AD-like symptoms. The results showed that there were no differences in the expression of galectin-9 in the small and large intestine between control and AD groups (Figure 4). However, galectin-9 expression was significantly increased in AD mice administered YK4 (Figure 4). These results suggested that YK4 induces an increase in galectin-9 in the intestine, suggesting the potential involvement of galectins in changes of CD103+ DCs and Tregs in AD mice.
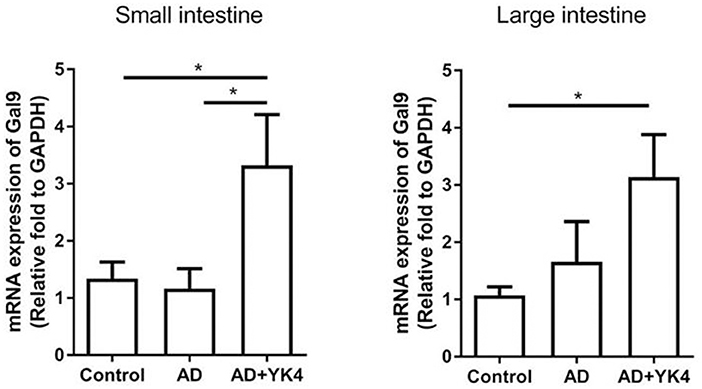
Figure 4. Expression of galectin-9 in the intestine of DNCB-sensitized mice treated with YK4. DNCB-induced AD mice were fed YK4. The small and large intestine were collected on week 6, and RNA was extracted for cDNA synthesis to examine the expression of galectin-9 mRNA (Gal9). Relative fold changes of target genes were compared with the housekeeping gene, GAPDH. *P < 0.05 by one-way ANOVA with Tukey's multiple comparison test. Bar indicates mean ± SEM.
YK4 and Galectin-9 Effectively Induced Regulatory Immune Responses by BMDCs
Next, we examined how YK4 and galectin-9 affect DCs. First, we investigated whether YK4 and/or galectin-9 affects the survival of BMDCs. The results showed that the survival of BMDCs was affected by neither YK4 at doses lower than 2 × 107 CFU (Supplementary Figure 2A) nor by galectin-9 at doses lower than 10 μg/ml (Supplementary Figure 2B). Based on these results, YK4 and galectin-9 were used at 2 × 106 CFU and 1 μg/ml, respectively. First, expression of the galectin-9 receptor, CD44, on BMDCs was examined. While treatment with galectin-9 alone did not alter the expression of CD44 on BMDCs, significant increases were observed when the cells were treated with YK4 alone or together with galectin-9 (Figure 5A). Galectin-9-treated BMDCs showed surface expression of CD86 similar to untreated cells, while its level slightly increased by YK4 treatment. MHC II expression was decreased when the cells were treated with both YK4 and galectin-9 (Figure 5A). Expression of OX40L, a ligand involved in induction of Th2 cell differentiation, was unaffected by treatment of BMDCs with galectin-9 and/or YK4 (Figure 5A). Expression of PD-L1, which is responsible for the immunosuppressive response, was increased following treatment of the cells with both YK4 and galectin-9 (Figure 5A). Immunomodulatory cytokines were examined in the culture supernatants of BMDCs treated with YK4 and/or galectin-9. The results showed that the expression level of the proinflammatory cytokine IL-6 was increased in BMDCs treated with YK4, and this was further increased by treatment with both YK4 and galectin-9 (Figure 5B). Expression of anti-inflammatory cytokine, IL-10 was also increased in BMDCs treated with YK4, while galectin-9 treatment with/without YK4 did not affect its expression (Figure 5B). IL-12p40, the expression of which was increased in BMDCs treated with YK4, was slightly decreased in cells treated with YK4 along with galectin-9 (Figure 5B). Taken together, these results suggest that YK4 makes BMDCs functionally tolerant. Moreover, galectin-9 further increased the tolerance of YK4-treated BMDCs.
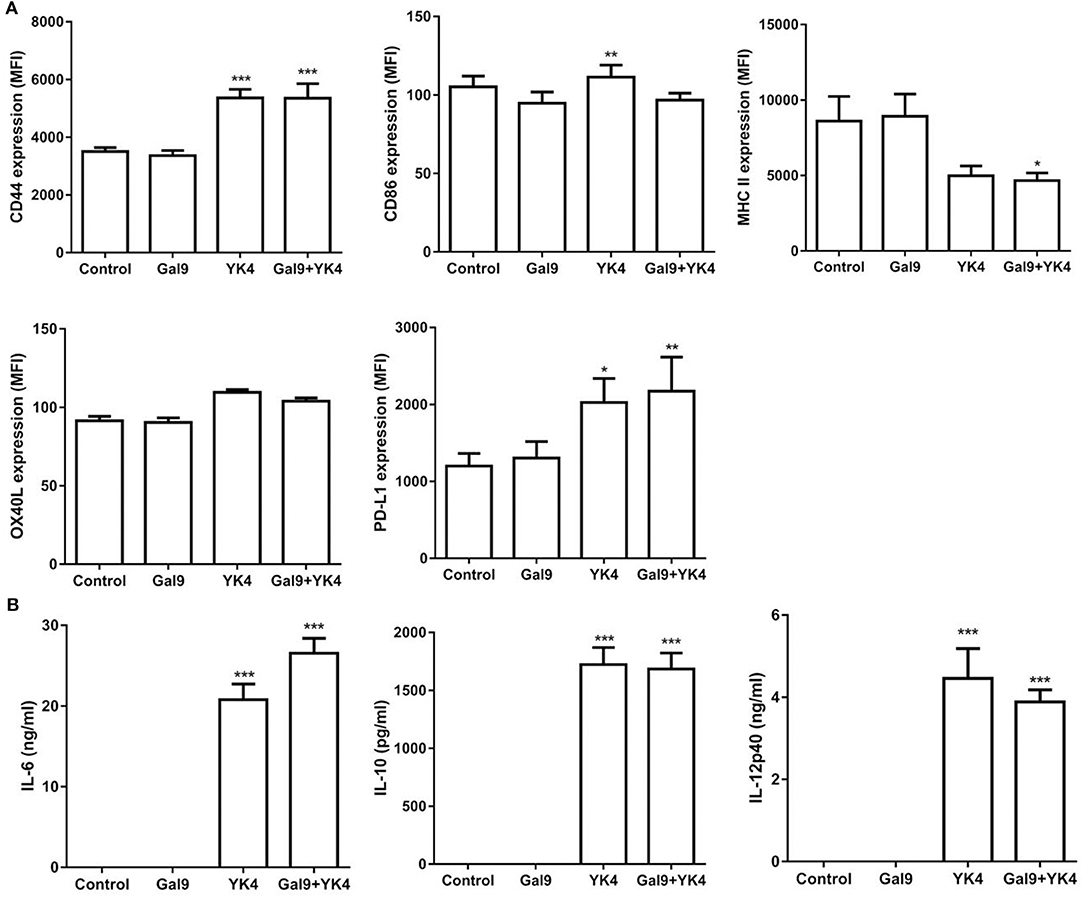
Figure 5. Changes of regulatory molecules in bone marrow-derived DCs (BMDCs) treated with YK4 and/or galectin-9. BMDCs were treated with galectin-9 and/or 2 × 106 CFU of YK4 for 24 h. (A) The expression levels of CD44, CD86, MHC II, OX40L, and PD-L1 on BMDCs were measured by flow cytometry. (B) The expression levels of cytokines in the supernatants were measured by ELISA. Data are representative of at least three experiments. *P < 0.05, **P < 0.01, ***P < 0.001 compared to the non-treated control group (Control) by Student's t-test.
YK4 and Galectin-9 Induced Proliferation of Tregs and Increased Immunomodulatory Cytokine Levels
Tregs are the main cells involved in induction of tolerance and suppression of excessive immune response. We investigated whether BMDCs treated with YK4 and/or galectin-9 could induce Treg proliferation. CD4+ T cells were isolated from mLN and co-cultured on anti-CD3 antibody-coated plates with BMDCs that had been treated with YK4 and/or galectin-9. The results showed that treatment of BMDCs with galectin-9 failed to inhibit proliferation of CD4+ T cells, while YK4-treated BMDCs showed inhibition of CD4+ T cell proliferation (Figure 6A). Next, we examined the proportion of Tregs among CD4+ T cells following co-culture with BMDCs. The proportion of Tregs increased when co-cultured with YK4-treated BMDCs, and a greater increase was observed in culture with YK4 and galectin-9-treated BMDCs (Figure 6B). To further confirm the activation of CD4+ T cells, the levels of immunomodulatory cytokines were investigated in the supernatants of CD4+ T cells co-cultured with YK4 and/or galectin-9-treated BMDCs. Galectin-9 alone did not affect the expression of immunomodulatory cytokines in CD4+ T cells (Figure 6C). However, the expression of IL-4 was reduced, while the levels of IL-10 and IL-17 were significantly increased in CD4+ T cells treated with YK4 and galectin-9 (Figure 6C). These results suggested that YK4 and galectin-9-treated BMDCs promoted IL-10 production and Tregs proliferation resulting in inhibition of other CD4+ T cells functions.
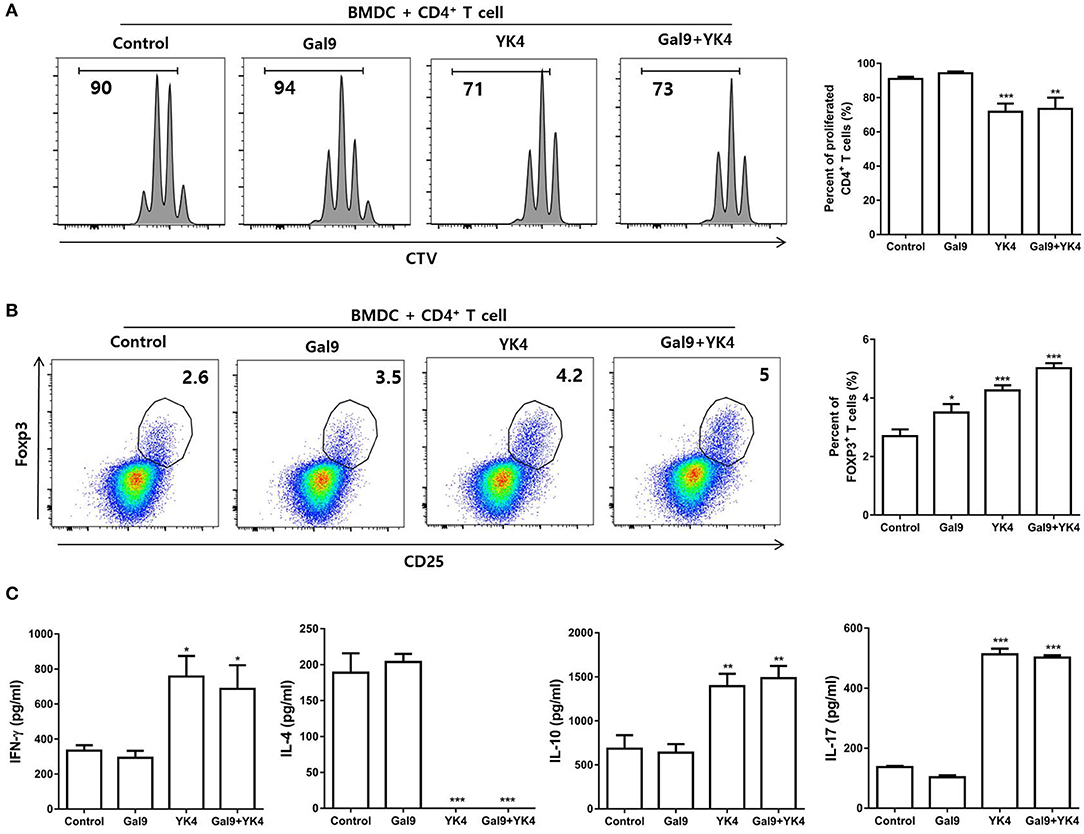
Figure 6. BMDCs treated with YK4 and galectin-9 promote Treg proliferation and immunomodulatory cytokine production. BMDCs treated with YK4 and/or galectin-9 were co-cultured with CD4+ T cells for 3 days on anti-CD3 mAb-coated plates. The percentage of (A) proliferated total CD4+ T cells and (B) proportion of CD4+CD25+Foxp3+ T cells among CD4+ T cells were analyzed by flow cytometry. At the same time, supernatants were harvested and examined for (C) the production of IFN-γ, IL-4, IL-10, and IL-17 in CD4+ T cells by ELISA. Data are representative of at least three experiments. *P < 0.05, **P < 0.01, ***P < 0.001 compared to the non-treated control group (Control) by Student's t-test.
Discussion
Probiotics are known to maintain health status upon ingestion in humans and animals by regulating intestinal immune homeostasis (46, 47). Despite numerous studies on allergies, the protective effects and precise mechanism of action of probiotics remain unclear. In the present study, we examined the regulatory effects against AD of YK4, a probiotic mixture with potent anti-inflammatory properties. Furthermore, we elucidated the mechanism underlying the anti-AD effect by showing that YK4 and galectin-9 regulate CD4+ T cells through DCs and Tregs.
In general, when the skin epidermal barrier is damaged by AD, TSLP is produced by keratinocytes. TSLP promotes activation of DCs and induces the expression of OX40L on the surface of DCs (48). The activated DCs together with IL-4 induce differentiation of naive T cells to form Th2 cells, which in turn contribute to the generation of IgE in B cells (49, 50). As expected, in the present study, DNCB-induced AD mice showed skin barrier disruption and inflammation due to excessive Th2 response followed by increases in serum IgE and skin TSLP levels. Previous studies have indicated that probiotics inhibit TSLP and IgE production to control AD symptoms (51). Intestinal microbiota plays a fundamental role in the induction and function of the host immune system (52). In particular, probiotics are known to affect the intestinal microbiota and to control disease propensity by the modulation of functional property of immune cells (53). It has been suggested that certain probiotics induce specific subsets of the intestinal CD4+ T cell population and alleviate AD symptoms. For example, the probiotic mixture, Duolac ATP, is known to downregulate Th2 cells in mLN and systemically inhibit Th2 responses (25). Treatment with L. plantarum WCFS1 was shown to downregulate Th2 cells in the intestinal lamina propria (54). Similarly, in the present study, the numbers of IL-4-producing Th2 cells decreased not only in mLN and PP but also in the spleen of DNCB-induced AD mice fed YK4. These findings suggested that YK4 might inhibit TSLP and IgE production by controlling intestinal and systemic Th2 cells.
Several strategies have been proposed for managing Th2 responses in AD, including the preferential differentiation of CD4+ T cells towards Th1 cells. Th1-type responses are known to counteract Th2 responses, thereby inhibiting the progression of inflammation in AD (55). Cytokines play an essential role in determining the direction and function of CD4+ T cells and their differentiation by activating STAT (56). IL-12, which is predominantly produced by antigen-presenting cells and phosphorylates STAT4 in CD4+ T cells, plays an important role in the differentiation of naïve T cells to Th1 cells (57). Phosphorylated STAT4 promotes T-bet expression and induces the production of IFN-γ, which again activates STAT1 in CD4+ T cells to further stabilize T-bet (57). This inhibits the expression of GATA3 and prevents differentiation of CD4+ T cells into IL-4-producing Th2 cells (58). Lactobacillus paracasei MoLac-1 has been shown to induce IL-12 production in DCs via Toll-like receptor (TLR) 9 signaling to inhibit the Th2 response (59, 60). In addition, the probiotic mixture, Duolac ATP, activated T-bet to induce a Th1 response and inhibited GATA3, thereby blocking the Th2 response in an AD mouse model (25). The levels of T-bet and GATA3 expression were reduced in the small intestine following treatment with L. plantarum WCFS1 in the mouse small intestinal lamina propria (54). The Weissella cibaria strain, WIKIM28, decreased IL-4 levels without affecting IFN-γ in peripheral lymph node cells (35). These results suggest that probiotics may contribute to a specific T cell response depending on the type and specific combination. In the present study, YK4 and its components induced IL-12 expression in DCs (Supplementary Figure 3A), which would directly affect the activity of STAT4 in CD4+ T cells. With this mechanism, YK4 increased IFN-γ and suppressed IL-4 production in CD4+ T cells through DCs. Furthermore, YK4 induced activation of Th1 cells, which produced IFN-γ and inhibited IL4-producing Th2 cells in the spleen of AD mice. These results suggest that YK4 induces differentiation of Th1 cells and downregulates the Th2 response in the spleen through IL-12 production by DCs.
Another mechanism underlying suppression of the Th2 response would involve Tregs-dependent regulation of unwanted inflammatory responses (61). Like other CD4+ T cells, Tregs are also stabilized and differentiated though specific cytokine signals, such as TGF-β and IL-10 (62). TGF-β activates Smad3 in Tregs and stabilizes Foxp3, which induces TGF-β and IL-10 production at the same time as differentiation, thereby suppressing unwanted immune responses (63). Certain probiotics can induce these immunosuppressive cytokines and promote the generation of Foxp3+ Tregs. For example, administration of WIKIM28 induced the differentiation of Tregs and the production of IL-10 in AD mice (35). Our study also showed that YK4 and its components probiotics induced IL-10 expression in BMDCs (Supplementary Figure 3B). Moreover, YK4 increased the expression of TGF-β, which was reduced in AD, to normal levels and increased the population of intestinal Foxp3+ Tregs. As a mechanism of action, tolerogenic DCs (tDCs) are essential to induce the differentiation of Tregs (64). Intake of probiotics can be recognized by TLRs on CD103+ DCs, which are abundant in the small intestine (65). Then, activated tDCs produce suppressive cytokines, including IL-10 and TGF-β, and cell-surface inhibitory molecules, PD-L1 and PD-L2 (66). Certain probiotics, such as Bifidobacterium bifidum PRI1, have been shown to increase the number of CD103+ DCs in the colonic lamina propria (67). Indeed, oral administration of YK4 in the present study induced upregulation of CD103+ DCs in mLN. Furthermore, DCs treated with YK4 induced increases in IL-10 and PD-L1 expression. Interestingly, when YK4-treated DCs were co-cultured with CD4+ T cells, the proportion of Tregs increased markedly. These results suggest that YK4 induces the activity of tDCs and affects the differentiation and activity of intestinal Tregs, thereby suppressing the intestinal Th2 response.
Probiotics may directly regulate immune cells, but they can also regulate the Th2 response indirectly by activating intestinal epithelial cells (68). When intestinal epithelial cells are activated by probiotics, effector molecules, including chemokines and galectins, are produced (69). In particular, galectin-9 is known to play a role in regulating AD through modulation of the immune response (70). The ingestion of dietary supplements with B. breve M-16V increased galectin-9 levels in intestinal epithelial cells thus preventing allergic symptoms (34). Similarly, we also observed an increase in the expression of intestinal galectin-9 in AD mice administered YK4. Galectin-9 binds directly to carbohydrate moieties of IgE to prevent IgE-antigen complex formation and mast cell degranulation. Moreover, galectin-9 binds to CD44 of DCs preventing their maturation and activation by inhibiting STAT1 activation (44). In the present study, galectin-9 did not directly affect the activity of DCs. However, when galectin-9 and YK4 were both administered, the expression of IL-12 was decreased in DCs. The activation of STAT1 by YK4 stimulation should precede the inhibitory effect of galectin-9 in DCs. The inhibition of IL-12 through galectin-9 also modulates the YK4-induced Th1 response. Galectin-9 is also known to bind CD44 on Tregs and phosphorylate Smad3 to stabilize Foxp3 followed by secretion of IL-10 and TGF-β (31). A number of reports have suggested a direct impact of galectin-9 on T cells; however, these studies did not consider the importance of the T cell–DC interaction for the best outcome of T cell activity. Thus, in the present study, we used BMDCs rather than anti-CD28 monoclonal antibodies to activate CD28 on CD4+ T cells. The results showed that galectin-9 did not cause BMDCs to become tolerogenic. Instead, treatment with galectin-9 together with YK4 induced proliferation of Tregs and promoted IL-10 production. TLR stimulation, such as by YK4 treatment, in DCs may be essential for galectin-9 to affect CD4+ T cells.
In this study, we investigated how YK4 affects immune cells in conjunction with cytokines and galectin-9 in the intestinal and systemic immune organs. YK4 effectively inhibited the Th2 response, but the intestinal and systemic immune responses were not the same. The intestines were immunologically tolerated compared with systemic immune organs (71). These fundamental environmental differences suggest that YK4 induced Th1 responses in the systemic organs and Tregs in the intestinal organs. In addition, metabolites of YK4 such as short-chain fatty acid may have a more direct effect on intestinal immune cells. Of course, further researches are needed to verify if the immune factors produced in the gut affect systemic organs. It has been reported that probiotics ingested would not settle effectively in the intestine, and its efficacy disappeared when ingestion is stopped (72). Therefore, further research is mandatory to determine if YK4 could influence the intestinal flora. It is important to mentioned that we are in need for comparative studies on females and males. For instance, under the certain condition, females have more CD4+ T cells than males (73). In addition, females are more sensitive to activation of TLRs and cytokines receptors under the influence of X chromosome (74). Male mice, on the other hand, have more numbers of Tregs than females and produce more IgE, especially during the fetal development via yet to be known mechanism (75).
In summary, our results showed that YK4 regulated intestinal galectin-9. Galectin-9, along with YK4, regulates the expression of IL-10 and IL-12 in DCs. These DCs, particularly CD103+ DCs, induced naïve T cell differentiation in Th1 and Tregs and inhibited Th2 responses (Figure 7). Taken together, these observations suggest that the probiotic mixture, YK4, has therapeutic potential to prevent AD symptoms and may act as an immunomodulator for AD patients.
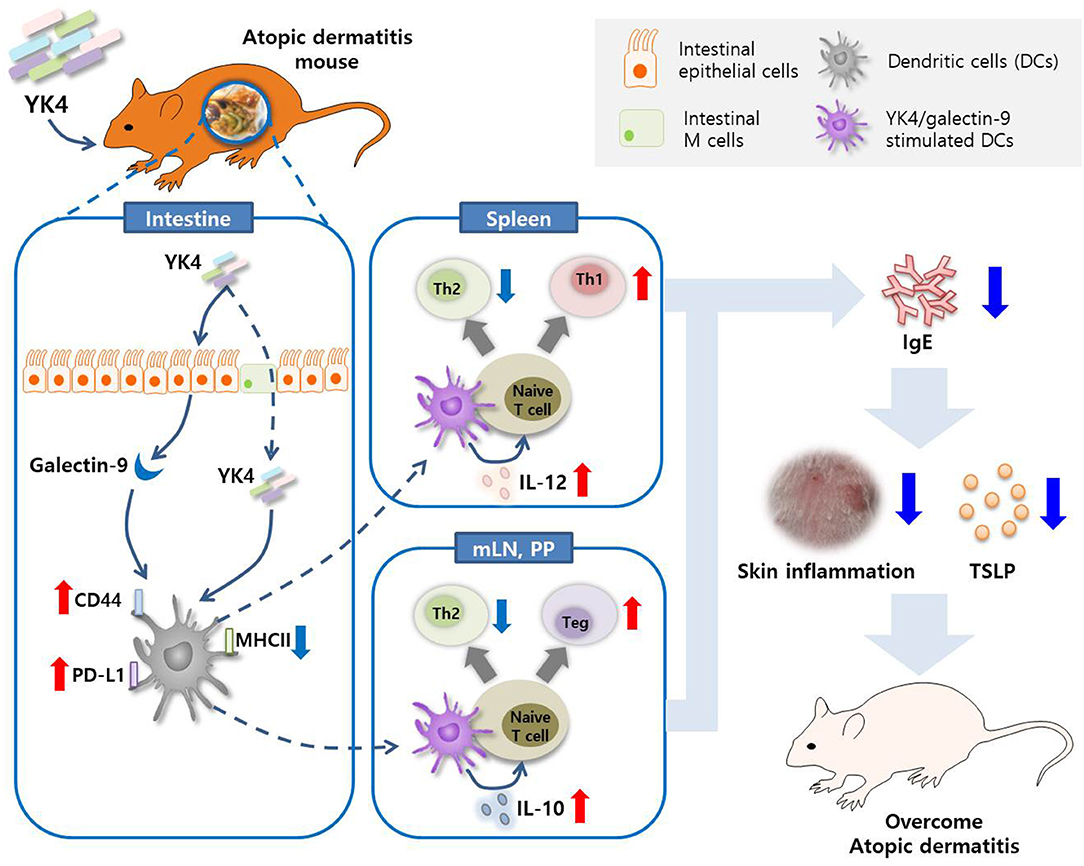
Figure 7. Graphical summary. The probiotic mixture, YK4, induced the production of galectin-9 in the intestine in a mouse model of AD. Galectin-9 together with YK4 induced the expression of PD-L1 and CD44 and the production of IL-10 and IL-12 in DCs. These DCs inhibited Th2 cell proliferation by induction of intestinal Tregs and splenic Th1 cell proliferation, thereby alleviating atopic symptoms.
Data Availability Statement
All datasets generated for this study are included in the article/Supplementary Material.
Ethics Statement
The animal study was reviewed and approved by the Institutional Animal Care and Use Committee at Seoul National University, Seoul, Korea (Approval No. SNU-170428-1-1).
Author Contributions
C-HY conceived and designed the experiments. HK, DJ, Y-CK, Y-JJ, CK, IL, and S-MP performed the experiments and analyzed the data. C-HY and HK wrote the draft of the manuscript. SH, IJ, SK, SL, KC, and IC contributed to analyses and directed the experimental work together with a critical revision of the manuscript. All authors discussed and finalized the manuscript.
Funding
This research was performed with funds from the National Research Foundation of Korea (NRF) (NRF-2018R1A2B2006793) and the Industry Core Technology Development Project (No. 10063302), Ministry of Trade, Industry and Energy, Republic of Korea. This research was also supported by the Cooperative Research Program for Agriculture Science and Technology Development (Project No. PJ01336401), Rural Development Administration, Republic of Korea. HK was supported by the BK21 Plus Program of the Department of Agricultural Biotechnology, Seoul National University, Seoul, Korea.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2019.03063/full#supplementary-material
Supplementary Figure 1. YK4 treatment does not induce inflammation in the spleen and large intestine. DNCB-induced AD mice were fed YK4. The (A) spleen and (B) large intestine were isolated on week 6. The length was measured by visual inspection to determine the inflammatory response.
Supplementary Figure 2. Apoptosis of BMDCs treated with YK4 and galectin-9. BMDCs (2 × 105 cells) were treated with various concentration of (A) YK4 and (B) galectin-9 for 24 h. Then, the percentage of apoptotic cells (the propidium iodide-positive fraction) was measured. H2O2 treatment was used as a positive control. A histogram of the results is shown. Data are representative of at least three experiments. *P < 0.05, **P < 0.01, ***P < 0.001 compared to the non-treated control group (Control) using Student's t-test.
Supplementary Figure 3. Changes of cytokine secretion in BMDCs treated with each component of YK4. BMDCs were treated with 100 ng/ml of LPS or 2 × 106 CFU of (YK4, BR3, BL3, LA1, LP3) for 24 h. The expression of (A) IL-12 and (B) IL-10 in the supernatants was measured by ELISA. Data are representative of at least three experiments. **P < 0.01, ***P < 0.001 compared to the YK4-treated group (Control) using Student's t-test.
References
1. Abramovits W. Atopic dermatitis. J Am Acad Dermatol. (2005) 53:S86–93. doi: 10.1016/j.jaad.2005.04.034
2. Weidinger S, Beck LA, Bieber T, Kabashima K, Irvine AD. Atopic dermatitis. Nat Rev Dis Primers. (2018) 4:1. doi: 10.1038/s41572-018-0001-z
3. Paternoster L, Standl M, Chen CM, Ramasamy A, Bonnelykke K, Duijts L, et al. Meta-analysis of genome-wide association studies identifies three new risk loci for atopic dermatitis. Nat Genet. (2011) 44:187–92. doi: 10.1038/ng.1017
4. Nygaard U, Hvid M, Johansen C, Buchner M, Folster-Holst R, Deleuran M, et al. TSLP, IL-31, IL-33 and sST2 are new biomarkers in endophenotypic profiling of adult and childhood atopic dermatitis. J Eur Acad Dermatol Venereol. (2016) 30:1930–8. doi: 10.1111/jdv.13679
5. Misery L. [TSLP, the key of pruritus in atopic dermatitis]. Med Sci. (2014) 30:142–4. doi: 10.1051/medsci/20143002009
6. Hellman LT, Akula S, Thorpe M, Fu Z. Tracing the origins of IgE, mast cells, and allergies by studies of wild. Animals. (2017) 8:1749. doi: 10.3389/fimmu.2017.01749
7. Long H, Zhang G, Wang L, Lu Q. Eosinophilic skin diseases: a comprehensive review. Clin Rev Allergy Immunol. (2016) 50:189–213. doi: 10.1007/s12016-015-8485-8
8. Moreno MA. Atopic diseases in childrenatopic diseases in children. JAMA Pediatr. (2016) 170:96. doi: 10.1001/jamapediatrics.2015.3886
9. Klonowska J, Glen J, Nowicki RJ, Trzeciak M. New cytokines in the pathogenesis of atopic dermatitis-new therapeutic targets. Int J Mol Sci. (2018) 19:E3086. doi: 10.3390/ijms19103086
10. Maghen P, Unrue EL, Oussedik E, Cline A, Cardwell LA, Feldman SR. Regardless of how risks are framed, patients seem hesitant to use topical steroids for atopic dermatitis. Br J Dermatol. (2019) 181:842–4. doi: 10.1111/bjd.17929
11. Zhou SL, Tan GH, Huang FY, Wang H, Lin YY, Chen SL. Sanpao herbs inhibit development of atopic dermatitis in Balb/c mice. Asian Pac J Allergy Immunol. (2014) 32:140–4. doi: 10.12932/AP0381.32.2.2013
12. Reynolds KA, Juhasz MLW, Mesinkovska NA. The role of oral vitamins and supplements in the management of atopic dermatitis: a systematic review. Int J Dermatol. (2019) 58:1371. doi: 10.1111/ijd.14404
13. Yan F, Polk DB. Probiotics and immune health. Curr Opin Gastroenterol. (2011) 27:496–501. doi: 10.1097/MOG.0b013e32834baa4d
14. Kechagia M, Basoulis D, Konstantopoulou S, Dimitriadi D, Gyftopoulou K, Skarmoutsou N, et al. Health benefits of probiotics: a review. ISRN Nutr. (2013) 2013:481651. doi: 10.5402/2013/481651
15. Vandenplas Y, Huys G, Daube G. Probiotics: an update. J Pediatr. (2015) 91:6–21. doi: 10.1016/j.jped.2014.08.005
16. Gill H, Prasad J. Probiotics, immunomodulation, and health benefits. Adv Exp Med Biol. (2008) 606:423–54. doi: 10.1007/978-0-387-74087-4_17
17. Mabbott NA, Donaldson DS, Ohno H, Williams IR, Mahajan A. Microfold (M) cells: important immunosurveillance posts in the intestinal epithelium. Mucosal Immunol. (2013) 6:666–77. doi: 10.1038/mi.2013.30
18. Macpherson G, Milling S, Yrlid U, Cousins L, Turnbull E, Huang FP. Uptake of antigens from the intestine by dendritic cells. Ann N Y Acad Sci. (2004) 1029:75–82. doi: 10.1196/annals.1309.010
19. Lee HK, Iwasaki A. Innate control of adaptive immunity: dendritic cells and beyond. Semin Immunol. (2007) 19:48–55. doi: 10.1016/j.smim.2006.12.001
20. Scott CL, Aumeunier AM, Mowat AM. Intestinal CD103+ dendritic cells: master regulators of tolerance? Trends Immunol. (2011) 32:412–9. doi: 10.1016/j.it.2011.06.003
21. Foligne B, Zoumpopoulou G, Dewulf J, Ben Younes A, Chareyre F, Sirard JC, et al. A key role of dendritic cells in probiotic functionality. PLoS ONE. (2007) 2:e313. doi: 10.1371/journal.pone.0000313
22. You J, Dong H, Mann ER, Knight SC, Yaqoob P. Probiotic modulation of dendritic cell function is influenced by ageing. Immunobiology. (2014) 219:138–48. doi: 10.1016/j.imbio.2013.08.012
23. Jeon SG, Kayama H, Ueda Y, Takahashi T, Asahara T, Tsuji H, et al. Probiotic Bifidobacterium breve induces IL-10-producing Tr1 cells in the colon. PLoS Pathog. (2012) 8:e1002714. doi: 10.1371/journal.ppat.1002714
24. Bermudez-Brito M, Borghuis T, Daniel C, Pot B, De Haan BJ, Faas MM, et al. L. plantarum WCFS1 enhances Treg frequencies by activating DCs even in absence of sampling of bacteria in the Peyer Patches. Sci Rep. (2018) 8:1785. doi: 10.1038/s41598-018-20243-1
25. Kim HW, Hong R, Choi EY, Yu K, Kim N, Hyeon JY, et al. A probiotic mixture regulates T cell balance and reduces atopic dermatitis symptoms in mice. Front Microbiol. (2018) 9:2414. doi: 10.3389/fmicb.2018.02414
26. Isolauri E, Arvola T, Sutas Y, Moilanen E, Salminen S. Probiotics in the management of atopic eczema. Clin Exp Allergy. (2000) 30:1604–10. doi: 10.1046/j.1365-2222.2000.00943.x
27. Kim MS, Kim JE, Yoon YS, Kim TH, Seo JG, Chung MJ, et al. Improvement of atopic dermatitis-like skin lesions by IL-4 inhibition of P14 protein isolated from Lactobacillus casei in NC/Nga mice. Appl Microbiol Biotechnol. (2015) 99:7089–99. doi: 10.1007/s00253-015-6455-y
28. Yeom M, Sur BJ, Park J, Cho SG, Lee B, Kim ST, et al. Oral administration of Lactobacillus casei variety rhamnosus partially alleviates TMA-induced atopic dermatitis in mice through improving intestinal microbiota. J Appl Microbiol. (2015) 119:560–70. doi: 10.1111/jam.12844
29. Thiemann S, Baum LG. Galectins and immune responses-just how do they do those things they do? Annu Rev Immunol. (2016) 34:243–64. doi: 10.1146/annurev-immunol-041015-055402
30. Sundblad V, Quintar AA, Morosi LG, Niveloni SI, Cabanne A, Smecuol E, et al. Galectins in intestinal inflammation: Galectin-1 expression delineates response to treatment in celiac disease patients. Front Immunol. (2018) 9:379. doi: 10.3389/fimmu.2018.00379
31. Blidner AG, Mendez-Huergo SP, Cagnoni AJ, Rabinovich GA. Re-wiring regulatory cell networks in immunity by galectin-glycan interactions. FEBS Lett. (2015) 589:3407–18. doi: 10.1016/j.febslet.2015.08.037
32. Wu C, Thalhamer T, Franca RF, Xiao S, Wang C, Hotta C, et al. Galectin-9-CD44 interaction enhances stability and function of adaptive regulatory T cells. Immunity. (2014) 41:270–82. doi: 10.1016/j.immuni.2014.06.011
33. Ikeda M, Katoh S, Shimizu H, Hasegawa A, Ohashi-Doi K, Oka M. Beneficial effects of Galectin-9 on allergen-specific sublingual immunotherapy in a Dermatophagoides farinae-induced mouse model of chronic asthma. Allergol Int. (2017) 66:432–9. doi: 10.1016/j.alit.2016.10.007
34. De Kivit S, Saeland E, Kraneveld AD, Van De Kant HJ, Schouten B, Van Esch BC, et al. Galectin-9 induced by dietary synbiotics is involved in suppression of allergic symptoms in mice and humans. Allergy. (2012) 67:343–52. doi: 10.1111/j.1398-9995.2011.02771.x
35. Lim SK, Kwon MS, Lee J, Oh YJ, Jang JY, Lee JH, et al. Weissella cibaria WIKIM28 ameliorates atopic dermatitis-like skin lesions by inducing tolerogenic dendritic cells and regulatory T cells in BALB/c mice. Sci Rep. (2017) 7:40040. doi: 10.1038/srep40040
36. Shin JH, Chung MJ, Seo JG. A multistrain probiotic formulation attenuates skin symptoms of atopic dermatitis in a mouse model through the generation of CD4(+)Foxp3(+) T cells. Food Nutr Res. (2016) 60:32550. doi: 10.3402/fnr.v60.32550
37. Lee HS, Choi EJ, Lee KS, Kim HR, Na BR, Kwon MS, et al. Oral Administration of p-hydroxycinnamic acid attenuates atopic dermatitis by downregulating Th1 and Th2 cytokine production and keratinocyte activation. PLoS ONE. (2016) 11:e0150952. doi: 10.1371/journal.pone.0150952
38. Kim M, Choi KH, Hwang SW, Lee YB, Park HJ, Bae JM. Inflammatory bowel disease is associated with an increased risk of inflammatory skin diseases: a population-based cross-sectional study. J Am Acad Dermatol. (2017) 76:40–8. doi: 10.1016/j.jaad.2016.08.022
39. Indra AK. Epidermal TSLP: a trigger factor for pathogenesis of atopic dermatitis. Expert Rev Proteomics. (2013) 10:309–11. doi: 10.1586/14789450.2013.814881
40. Brandt EB, Sivaprasad U. Th2 cytokines and atopic dermatitis. J Clin Cell Immunol. (2011) 2:110. doi: 10.4172/2155-9899.1000110
41. Kwon HK, Lee CG, So JS, Chae CS, Hwang JS, Sahoo A, et al. Generation of regulatory dendritic cells and CD4+Foxp3+ T cells by probiotics administration suppresses immune disorders. Proc Natl Acad Sci USA. (2010) 107:2159–64. doi: 10.1073/pnas.0904055107
42. Taylor A, Verhagen J, Blaser K, Akdis M, Akdis CA. Mechanisms of immune suppression by interleukin-10 and transforming growth factor-beta: the role of T regulatory cells. Immunology. (2006) 117:433–42. doi: 10.1111/j.1365-2567.2006.02321.x
43. Kushwah R, Hu J. Role of dendritic cells in the induction of regulatory T cells. Cell Biosci. (2011) 1:20. doi: 10.1186/2045-3701-1-20
44. Nagahara K, Arikawa T, Oomizu S, Kontani K, Nobumoto A, Tateno H, et al. Galectin-9 increases Tim-3+ dendritic cells and CD8+ T cells and enhances antitumor immunity via galectin-9-Tim-3 interactions. J Immunol. (2008) 181:7660–9. doi: 10.4049/jimmunol.181.11.7660
45. Kanzaki M, Wada J, Sugiyama K, Nakatsuka A, Teshigawara S, Murakami K, et al. Galectin-9 and T cell immunoglobulin mucin-3 pathway is a therapeutic target for type 1 diabetes. Endocrinology. (2012) 153:612–20. doi: 10.1210/en.2011-1579
46. Gareau MG, Sherman PM, Walker WA. Probiotics and the gut microbiota in intestinal health and disease. Nat Rev Gastroenterol Hepatol. (2010) 7:503–14. doi: 10.1038/nrgastro.2010.117
47. Sleator RD. Designer probiotics: development and applications in gastrointestinal health. World J Gastrointest Pathophysiol. (2015) 6:73–8. doi: 10.4291/wjgp.v6.i3.73
48. Leyva-Castillo JM, Hener P, Michea P, Karasuyama H, Chan S, Soumelis V, et al. Skin thymic stromal lymphopoietin initiates Th2 responses through an orchestrated immune cascade. Nat Commun. (2013) 4:2847. doi: 10.1038/ncomms3847
49. Ito T, Wang YH, Duramad O, Hori T, Delespesse GJ, Watanabe N, et al. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J Exp Med. (2005) 202:1213–23. doi: 10.1084/jem.20051135
50. Deo SS, Mistry KJ, Kakade AM, Niphadkar PV. Role played by Th2 type cytokines in IgE mediated allergy and asthma. Lung India. (2010) 27:66–71. doi: 10.4103/0970-2113.63609
51. Liu FT, Goodarzi H, Chen HY. IgE, mast cells, and eosinophils in atopic dermatitis. Clin Rev Allergy Immunol. (2011) 41:298–310. doi: 10.1007/s12016-011-8252-4
52. Belkaid Y, Harrison OJ. Homeostatic immunity and the microbiota. Immunity. (2017) 46:562–76. doi: 10.1016/j.immuni.2017.04.008
53. Hemarajata P, Versalovic J. Effects of probiotics on gut microbiota: mechanisms of intestinal immunomodulation and neuromodulation. Therap Adv Gastroenterol. (2013) 6:39–51. doi: 10.1177/1756283X12459294
54. Smelt MJ, De Haan BJ, Bron PA, Van Swam I, Meijerink M, Wells JM, et al. Probiotics can generate FoxP3 T-cell responses in the small intestine and simultaneously inducing CD4 and CD8 T cell activation in the large intestine. PLoS ONE. (2013) 8:e68952. doi: 10.1371/journal.pone.0068952
55. Brunner PM, Guttman-Yassky E, Leung DY. The immunology of atopic dermatitis and its reversibility with broad-spectrum and targeted therapies. J Allergy Clin Immunol. (2017) 139:S65–76. doi: 10.1016/j.jaci.2017.01.011
56. Haque SJ, Sharma P. Interleukins and STAT signaling. Vitam Horm. (2006) 74:165–206. doi: 10.1016/S0083-6729(06)74007-9
57. Morinobu A, Gadina M, Strober W, Visconti R, Fornace A, Montagna C, et al. STAT4 serine phosphorylation is critical for IL-12-induced IFN-gamma production but not for cell proliferation. Proc Natl Acad Sci USA. (2002) 99:12281–6. doi: 10.1073/pnas.182618999
58. Kanhere A, Hertweck A, Bhatia U, Gokmen MR, Perucha E, Jackson I, et al. T-bet and GATA3 orchestrate Th1 and Th2 differentiation through lineage-specific targeting of distal regulatory elements. Nat Commun. (2012) 3:1268. doi: 10.1038/ncomms2260
59. Iwabuchi N, Yonezawa S, Odamaki T, Yaeshima T, Iwatsuki K, Xiao JZ. Immunomodulating and anti-infective effects of a novel strain of Lactobacillus paracasei that strongly induces interleukin-12. FEMS Immunol Med Microbiol. (2012) 66:230–9. doi: 10.1111/j.1574-695X.2012.01003.x
60. Raso GM, Simeoli R, Iacono A, Santoro A, Amero P, Paciello O, et al. Effects of a Lactobacillus paracasei B21060 based synbiotic on steatosis, insulin signaling and toll-like receptor expression in rats fed a high-fat diet. J Nutr Biochem. (2014) 25:81–90. doi: 10.1016/j.jnutbio.2013.09.006
61. Venuprasad K, Kong YC, Farrar MA. Control of Th2-mediated inflammation by regulatory T cells. Am J Pathol. (2010) 177:525–31. doi: 10.2353/ajpath.2010.090936
62. Levings MK, Bacchetta R, Schulz U, Roncarolo MG. The role of IL-10 and TGF-beta in the differentiation and effector function of T regulatory cells. Int Arch Allergy Immunol. (2002) 129:263–76. doi: 10.1159/000067596
63. Palomares O, Martin-Fontecha M, Lauener R, Traidl-Hoffmann C, Cavkaytar O, Akdis M, et al. Regulatory T cells and immune regulation of allergic diseases: roles of IL-10 and TGF-beta. Genes Immun. (2014) 15:511–20. doi: 10.1038/gene.2014.45
64. Maldonado RA, Von Andrian UH. How tolerogenic dendritic cells induce regulatory T cells. Adv Immunol. (2010) 108:111–65. doi: 10.1016/B978-0-12-380995-7.00004-5
65. Ruane DT, Lavelle EC. The role of CD103(+) dendritic cells in the intestinal mucosal immune system. Front Immunol. (2011) 2:25. doi: 10.3389/fimmu.2011.00025
66. Yoo S, Ha SJ. Generation of tolerogenic dendritic cells and their therapeutic applications. Immune Netw. (2016) 16:52–60. doi: 10.4110/in.2016.16.1.52
67. Verma R, Lee C, Jeun EJ, Yi J, Kim KS, Ghosh A, et al. Cell surface polysaccharides of Bifidobacterium bifidum induce the generation of Foxp3(+) regulatory T cells. Sci Immunol. (2018) 3:eaat6975. doi: 10.1126/sciimmunol.aat6975
68. Allaire JM, Crowley SM, Law HT, Chang SY, Ko HJ, Vallance BA. The intestinal epithelium: central coordinator of mucosal immunity. Trends Immunol. (2018) 39:677–96. doi: 10.1016/j.it.2018.04.002
69. De Kivit S, Tobin MC, Forsyth CB, Keshavarzian A, Landay AL. Regulation of intestinal immune responses through TLR activation: implications for pro- and prebiotics. Front Immunol. (2014) 5:60. doi: 10.3389/fimmu.2014.00060
70. Nakajima R, Miyagaki T, Oka T, Nakao M, Kawaguchi M, Suga H, et al. Elevated serum galectin-9 levels in patients with atopic dermatitis. J Dermatol. (2015) 42:723–6. doi: 10.1111/1346-8138.12884
71. Chistiakov DA, Bobryshev YV, Kozarov E, Sobenin IA, Orekhov AN. Intestinal mucosal tolerance and impact of gut microbiota to mucosal tolerance. Front Microbiol. (2014) 5:781. doi: 10.3389/fmicb.2014.00781
72. Zmora N, Zilberman-Schapira G, Suez J, Mor U, Dori-Bachash M, Bashiardes S, et al. Personalized gut mucosal colonization resistance to empiric probiotics is associated with unique host and microbiome features. Cell. (2018) 174:1388–405.e1321. doi: 10.1016/j.cell.2018.08.041
73. Lee BW, Yap HK, Chew FT, Quah TC, Prabhakaran K, Chan GS, et al. Age- and sex-related changes in lymphocyte subpopulations of healthy Asian subjects: from birth to adulthood. Cytometry. (1996) 26:8–15. doi: 10.1002/(SICI)1097-0320(19960315)26:1<8::AID-CYTO2>3.0.CO;2-E
74. Libert C, Dejager L, Pinheiro I. The X chromosome in immune functions: when a chromosome makes the difference. Nat Rev Immunol. (2010) 10:594–604. doi: 10.1038/nri2815
Keywords: probiotics, atopic dermatitis, dendritic cell, T cell balance, galectin-9
Citation: Kim HW, Ju DB, Kye Y-C, Ju Y-J, Kim CG, Lee IK, Park S-M, Choi IS, Cho KK, Lee SH, Kim SC, Jung ID, Han SH and Yun C-H (2020) Galectin-9 Induced by Dietary Probiotic Mixture Regulates Immune Balance to Reduce Atopic Dermatitis Symptoms in Mice. Front. Immunol. 10:3063. doi: 10.3389/fimmu.2019.03063
Received: 20 August 2019; Accepted: 16 December 2019;
Published: 22 January 2020.
Edited by:
Caroline Elizabeth Childs, University of Southampton, United KingdomReviewed by:
Julio Villena, CONICET Centro de Referencia para Lactobacilos (CERELA), ArgentinaFrancisco José Pérez-Cano, University of Barcelona, Spain
Copyright © 2020 Kim, Ju, Kye, Ju, Kim, Lee, Park, Choi, Cho, Lee, Kim, Jung, Han and Yun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cheol-Heui Yun, Y3l1bkBzbnUuYWMua3I=
 Han Wool Kim
Han Wool Kim Do Bin Ju
Do Bin Ju Yoon-Chul Kye
Yoon-Chul Kye Young-Jun Ju1
Young-Jun Ju1 In Kyu Lee
In Kyu Lee Sung-Moo Park
Sung-Moo Park Seung Hyun Han
Seung Hyun Han Cheol-Heui Yun
Cheol-Heui Yun