- Harvard Medical School, Brigham and Women's Hospital, Boston, MA, United States
Among the top priorities of the HIV field is the search for therapeutic interventions that can lead to sustained antiretroviral therapy (ART)-free HIV remission. Although the majority of HIV-infected persons will experience rapid viral rebound after ART interruption, there are rare individuals, termed post-treatment controllers (PTCs), who demonstrate sustained virologic suppression for months or years after treatment cessation. These individuals are considered an ideal example of durable HIV control, with direct implications for HIV cure research. However, understanding of the mechanisms behind the capacity of PTCs to control HIV remains incomplete. This is in part due to the scarcity of PTCs identified through any one research center or clinical trial, and in part because of the limited scope of studies that have been performed in these remarkable individuals. In this review, we summarize the results of both clinical and basic research studies of PTCs to date, explore key differences between PTCs and HIV spontaneous controllers, examine potential mechanisms of post-treatment control, and discuss unanswered questions and future research directions in this field.
Introduction
Within each medical field, there exist individuals who exhibit extreme responses to medical treatment. As an example, individuals who have an unexpectedly dramatic response to cancer therapy are termed “exceptional responders.” These exceptional responders represent an area of intense research interest within the oncology field (1) and have already made important contributions to the understanding of both basic tumor biology and drug development (2). In this review, we focus on a group of exceptional responders within the HIV field, specifically individuals who were treated with antiretroviral therapy (ART) and can subsequently maintain HIV remission even when the ART is discontinued.
HIV infection is characterized by sustained viral replication and progressive decline in CD4 cell counts (3). ART is effective in suppressing viral replication and decreasing HIV-associated morbidity and mortality, but it cannot completely eradicate all HIV-infected cells. Consequently, HIV viral load rebounds rapidly after treatment interruption in most HIV patients (4, 5). However, there are rare individuals, termed post-treatment controllers (PTCs), who are able to suppress the virus for a prolonged period of time after treatment interruption (Figure 1). These individuals are considered an ideal example of durable HIV control and have the potential to provide substantial insight into the “natural” mechanisms of functional cure and sustained HIV remission (7).
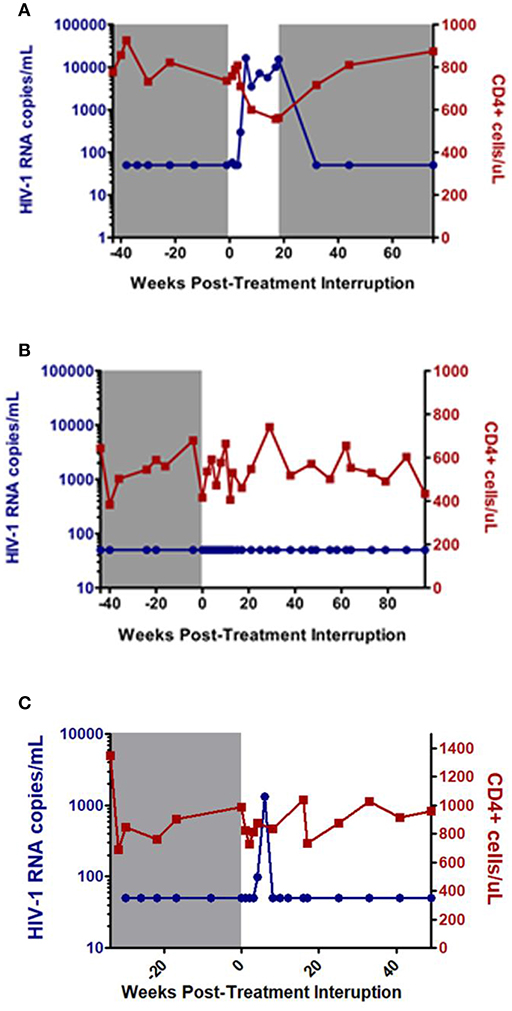
Figure 1. Examples of post-treatment non-controller (NC) (A), and post-treatment controllers (B,C). Gray shaded area represents time on antiretroviral therapy. Adapted from CHAMP study (6).
Interest in ways to induce post-treatment control were initially kindled by a report of an individual who was able to control HIV without ART after undergoing several sequential treatment interruptions (8) and in an in-depth report of 14 early-treated PTCs reported in the VISCONTI study (7). There have been a number of subsequent studies of PTCs with a wide range of reported frequency amongst those who discontinue ART (6, 7, 9–19). This variation in reported frequency of PTCs may be attributed to different baseline characteristics of the populations in which these studies were done, as well as the heterogeneous definitions applied for defining this rare group of HIV patients (18). In this review, we will summarize the most recent findings on the clinical and immunological characteristics of PTCs, differentiate them from HIV spontaneous controllers (SCs), and discuss the role of PTCs in the search for strategies toward HIV remission and cure.
Post-Treatment Controller Definitions
Since the initial description of the post-treatment controller phenotype, a number of observational studies and interventional clinical trials have been performed to investigate the characteristics of this rare group of patients and to determine the mediators of post-treatment control. However, the heterogeneities in study designs have made it challenging to compare studies and to gain a clear grasp of the PTC population. For example, the definition of post-treatment control has differed dramatically between studies. Some studies have considered virologic rebound to be a plasma viral load above 50 HIV-1 RNA copies/ml after treatment interruption, while others have used a threshold of 400 HIV-1 RNA copies/ml or 1,000 HIV-1 RNA copies/ml for this purpose (Table 1, Supplementary Table S1). The duration of viral control after treatment interruption has also differed dramatically between studies and ranged from a median of 6 month to more than 2 years (7, 9–32). Furthermore, the loss of viral control was also defined differently between previous studies. Some considered 2 consecutive viral loads above 50 HIV-1 RNA copies/ml to indicate the loss of post-treatment control (8–10), while others considered 1–4 consecutive viral loads higher than 400 HIV-1 RNA copies/ml as the definition for viral rebound post-treatment interruption (7, 12, 16–18). Of note, the largest PTC study to date has been the Control of HIV after Antiretroviral Medication Pause (CHAMP) study, which identified 67 PTCs through the pooled analysis of 14 clinical studies from the AIDS Clinical Trials Group (ACTG) and other North American cohorts (6, 14, 20–32). In this study, the PTCs were defined as individuals who maintained viral loads ≤ 400 copies/mL at two-thirds or more of time points for ≥24 weeks post treatment interruption (6).
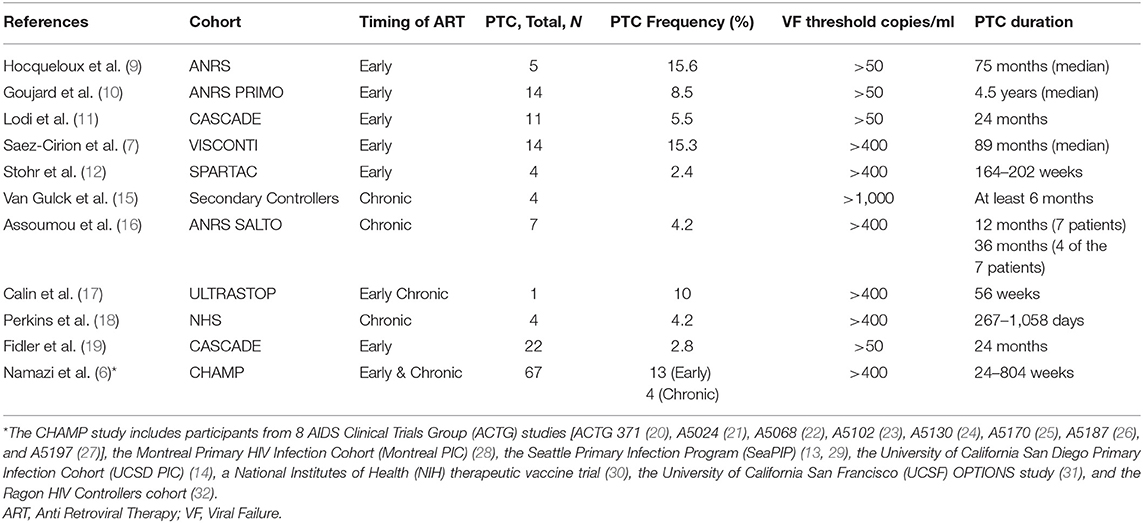
Table 1. Post-treatment controller (PTC) frequency after treatment interruption reported from previously published studies.
Demographic Characteristics of PTCs
The median age of PTCs in these studies ranged from 27 to 46 years old. The majority of PTCs identified were male, likely reflecting the sex distribution of the clinical trial participants (6, 7, 9, 11, 12, 15–18). Intriguingly, there have been reports that female gender may be associated with a higher chance of post-treatment HIV control (10) and spontaneous control (33, 34), highlighting the need for studies focusing on female participants of treatment interruption trials. In addition, the majority of PTCs have been reported by studies from North America and Europe (6, 7, 9–12, 15–18) and little is known about PTCs from outside of those regions. In an analysis of SPARTAC trial participants who initiated ART during early HIV infection, individuals with delayed viral rebound could be identified from participants enrolled in South Africa and Uganda (35). Furthermore, African participants tended to have lower pre-ART viral load and integrated HIV DNA levels, and after treatment interruption, Africans appeared to experience a longer duration of viral remission than non-Africans in the SPARTAC study (12, 36). These results provide a strong rationale for additional studies of PTCs from Africa and other regions to assess the impact of race and HIV subtype on barriers to HIV remission.
Clinical and Immunological Characteristics
Historically, the majority of PTCs have been identified in studies of patients who initiated ART during early HIV infection (7, 9–12, 20, 26, 29–31, 37). However, PTCs have also been identified in participants who were treated during chronic HIV infection (15, 16, 18, 21, 22, 25, 27, 38). The CHAMP study directly compared the frequency of post-treatment control between individuals who initiated ART during early and chronic HIV infection. This study found that individuals who were treated during early infection were far more likely to meet the PTC criteria after treatment interruption compared to those treated during chronic infection (13 vs. 4%, P < 0.01, Figure 2) (6).
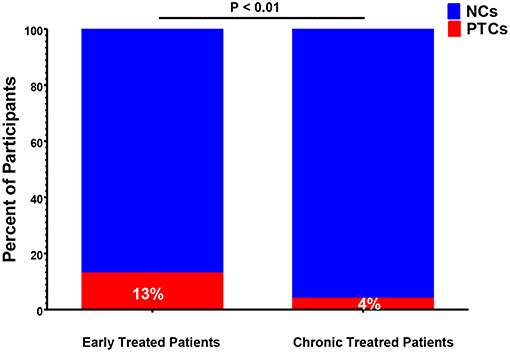
Figure 2. Frequency of post-treatment controllers (PTCs) identified in early-vs. chronic-treated participants of the CHAMP study (6). NCs, post-treatment non-controllers.
At the time of treatment interruption, CD4 cell counts for the PTCs were generally quite high with a median of 720 to 1,429 cells/mm3 amongst the studies (7, 9–12, 15–19). After ART discontinuation, PTCs can exhibit a range of viral load dynamics with a subset demonstrating persistent viral load suppression (Figure 1B) while others experience early viral rebound before subsequently regaining viral control (Figure 1C). In the CHAMP study, ~45% of PTCs had early viral load peaks ≥1,000 HIV-1 RNA copies/mL and 33% had early viral load peaks ≥10,000 HIV-1 RNA copies/mL amongst those with intensive weekly viral load monitoring (6).
The comparison of previously published PTC studies has also been difficult due to heterogeneity in the inclusion of PTCs with varying duration of viral control. To place the PTC studies in context, the median time of HIV rebound after ART interruption for post-treatment non-controllers (NCs) is ~3–4 weeks and only a small proportion of non-controllers are able to maintain viral suppression to 12 weeks or beyond (4). The VISCONTI study was one of the earliest and most comprehensive of the PTC studies (7). The inclusion criteria for the 14 VISCONTI participants were individuals who were treated during early HIV infection and maintained viral suppression <400 HIV-1 RNA copies/mL for at least 2 years after ART interruption. To assess the durability of HIV remission, the CHAMP study used a more inclusive definition of post-treatment control (viral suppression for 24 weeks). In this analysis, the median duration of post-treatment control was a little over 2 years and the proportion of PTCs who remained virologically suppressed in years 1–5 were 75, 55, 41, 30, and 22%, respectively (6). These results show that post-treatment control is not always durable and that PTCs will require continued clinical and virologic monitoring. These results highlight the heterogeneity in the post-treatment controller phenotype, with some individuals losing control within 1 year and others maintaining viral suppression for more than 10 years (6, 7). While the latter group may be the best model of sustained HIV remission, uncovering factors that lead to the loss of viral control in PTCs may also provide insight on the mechanisms behind their HIV remission. It should be noted though, that the rates of viral suppression reported in the PTCs are in the absence of any additional interventions and that strategies to augment key HIV-specific immune responses have the potential to improve the durability of post-treatment control.
Comparing Spontaneous and Post-Treatment Controllers
Without ART, most HIV-infected individuals will have high levels of HIV-1 RNA and experience progressive absolute CD4+ T-cell decline, clinical immunodeficiency, and death (39). However, a small proportion of those infected with HIV can spontaneously maintain very low levels of plasma viral load without the use of antiretroviral therapy (ART) (40, 41). The existence of these HIV spontaneous controllers (SCs), also known as elite controllers (ECs), represented the first indication that the goal of drug-free HIV remission is possible. Although these SCs have low or even undetectable viremia by conventional viral load assays, they generally harbor replication competent virus and have evidence of ongoing viral replication and evolution (42–45). Through robust genetic and functional studies, the most consistent mediator of spontaneous HIV control appears to be through the effects of cytotoxic CD8 T lymphocyte (CTL) responses (46, 47), and the protective effects of certain HLA alleles, such as HLA B*27 and B*57 (48–50). Similar to the PTCs, SCs appear to be a heterogeneous population of individuals with respect to the level and durability of HIV control (51, 52). While some SCs can maintain viral loads <50 copies/ml in absence of ART (i.e., elite controllers [ECs]) (41, 53). Viremic controllers (VCs) can maintain a less robust level of viral suppression, with detectable viral loads below 2,000 HIV-1 RNA copies/mL in the absence of ART (54).
However, even amongst the ECs, there is evidence of heterogeneity in immune responses (49, 55), and a subset will lose viral control and experience immunological and clinical progression over time (56–58). Low Gag-specific CD8 T cell response, high levels of inflammatory cytokines and high viral diversity have been reported as factors that predict loss of viral control in ECs (51).
Due to the rarity of individuals undergoing treatment interruption, PTCs have for a long time not been recognized as a separate entity from SCs. While it is possible that some PTCs treated during early HIV infection may have achieved spontaneous control in the absence of ART, there are now several lines of evidence that PTCs are indeed distinct from HIV SCs: (1) CTL responses have been found to be far weaker in PTCs compared to SCs (7); (2) Unlike SCs, PTCs do not appear to be enriched in protective HLA alleles (3, 10, 59), with the VISCONTI study reporting a high frequency of HLA alleles previously associated with less favorable clinical outcomes (7); (3) PTCs frequently present with symptomatic acute retroviral syndrome and have pre-ART viral loads that are similar to that of non-controllers, but significantly higher than that of HIV SCs (6, 7); and (4) Results from both the SPARTAC and CHAMP studies have demonstrated an ART-specific effect as early ART initiation significantly increases the chances of achieving post-treatment control (6, 35). Together, these findings support the concept that PTCs are largely distinct from SCs and represent individuals who would not have been able to achieve HIV remission without the period ART.
Mechanisms and Predictors of Post-Treatment Controllers
While the exact mechanism behind the ability of PTCs to maintain HIV remission remains unclear, there is evidence for an unusual degree of reservoir restriction and relatively weak HIV-specific CTL activity. In prior studies of ART-treated individuals, the HIV reservoir is primarily maintained within memory CD4 T cells, especially those of central memory (TCM) and transitional memory (TTM) cells (60). In prior treatment interruption studies, smaller total and active HIV reservoirs before treatment interruption have been associated with delayed HIV rebound after treatment interruption. Specifically, lower levels of pre-treatment interruption HIV proviral DNA have predicted delayed viral rebound (16, 61), as has lower levels of cell-associated HIV RNA (4, 30). In PTCs, levels of HIV DNA and cell-associated RNA have also been found to be low in some studies (10, 15) but not others (38). In the VISCONTI analysis, the predominant cellular subset contributing to the HIV reservoir has been reported to be the TTM cells (7), similar to that found in other early treated patients (62) and suggest that the low frequency of HIV infection within the longest-lived CD4 T cells (naïve and central memory) may contribute to post-treatment control. In studies of SCs, there have been reports that the HIV reservoir is also restricted within the TCM cell subset (63), although this has not been replicated in other studies (7). In ART-treated individuals, the vast majority of HIV proviral DNA are defective and until recently, the proviral landscape within PTCs had not been investigated. In an analysis of ACTG PTCs, Sharaf et al. reported near-full length proviral sequencing results showing that PTCs had an ~7-fold smaller HIV reservoir compared to NCs prior to the ATI, but that some PTCs had relatively large fractions of intact proviruses (64). In a separate case report, post-treatment control could be maintained despite the presence of a clonally-expanded population of HIV-infected cells harboring replication-competent virus (65). Overall, these results demonstrate that PTCs have a restricted HIV reservoir, especially within longer-lived cellular subsets, which may contribute to their ability to maintain HIV remission. Additional studies are needed to explore the role of viral fitness (15), clonal expansion and the integration sites of intact proviruses in HIV remission.
Primate studies have also provided insight on strategies for delaying viral rebound. In particular, early ART therapy restricts the seeding of SIV reservoirs and lead to delayed timing of viral rebound (66, 67). Similarly, early initiation of ART has been associated with a significantly increased chance of achieving post-treatment control both within CHAMP study and others (6, 19). Prior studies of early ART treatment have found that it is effective in dramatically reducing the size of the HIV reservoir (68–71). In addition, early ART may preserve HIV-specific T cell responses (72–74). However, the VISCONTI study and others have shown that HIV-specific CD8 T cell responses in PTCs are weak compared to either SCs or viremic individuals (7, 75, 76). These results are consistent with reports that pre-ART viral loads are generally quite high in PTCs (6, 7) and that they do not tend to harbor protective HLA alleles (7, 38, 59, 75). However, other studies have not found significant differences in T cell responses between PTCs and SCs (15). In addition, there are reports from the VISCONTI study that early HIV treatment in PTCs preserves robust poly-functional CD4+ responses to HIV (77). Finally, there have been several reports that early ART initiation in infants may also lead to long-term HIV remission (76, 78, 79). In the first reported case, known as the “Mississippi baby,” the infant initiated ART 30 h after delivery until 18 months of age. ART remission was achieved without detectable HIV-specific antibody or T cell responses (78), but viral rebound occurred ~2 years after ART discontinuation (80). In the second case, the infant became infected despite 6 weeks of Zidovudine prophylaxis after delivery and initiated ART at 3 months of age. ART was discontinued between 5 and 7 years of age and viral control has been documented for ~12 years despite several transient viral blips, a detectable replication-competent reservoir, and weak HIV-specific CD8+ T cell responses (76). The final report is that of a child who initiated 40 weeks of ART at day 61 after delivery as part of the Children with HIV Early antiretroviral therapy (CHER) trial (81, 82). The child has maintained viral suppression for almost 9 years after ART discontinuation, with detectable HIV DNA and residual viremia, low level of HIV-specific antibody and weak T cell response (79). Importantly, none of these children harbored the protective HLA class I alleles B*27 or B*57 associated with spontaneous viral control and levels of immune activation during HIV remission were low in all three children (76, 78, 79). These cases also highlight that post-treatment control in children can occur with a range of ART initiation times (between 30 h and 2–3 months after delivery), HIV subtypes (B, H, and C in the three cases, respectively), and duration of ART (10 months to 6 years) (76, 78, 79). Although these studies support the possibility of HIV remission in early-treated children, the frequency of post-treatment control appears to be rare as only 1 of 227 children in the CHER trial achieved this outcome (79) and smaller studies of treatment interruption in children have failed to detect any PTCs (83).
Early ART initiation has also been shown to preserve HIV-specific humoral immunity by preserving memory B cell numbers and function (84, 85). There are reports from a small case series that PTCs may harbor high levels of autologous neutralizing antibodies (15), although that has not been replicated in other studies (8, 75).
Knowledge Gaps and Unanswered Questions
Among the top priorities of the HIV field is the search for therapeutic interventions that can lead to sustained ART-free HIV remission (41). Understanding the mechanisms and predictors of post-treatment control would represent a key step toward that goal as PTCs represent a realistic model for the functional cure of HIV infection. Only in the past few years have interest heightened in the study of PTCs and a host of important questions remain unanswered. First, it has become clear that early initiation of ART is not only associated with personal health and public health benefits but may also lower the barrier to HIV remission and post-treatment control. However, the optimal timing of ART during early HIV infection is unknown. It is interesting to note that the vast majority of PTCs in the VISCONTI and CHAMP studies initiated ART during Fiebig stages III-V (6, 7) and that a small treatment interruption study of individuals who initiated ART during Fiebig I did not identify any PTCs as all individuals demonstrated rapid viral rebound (86). While extremely early initiation of ART will limit the extent of HIV reservoir seeding (87), additional research is needed to assess whether a slight delay in ART initiation allows for the further maturation of the HIV-specific immune response that may be important for post-treatment control.
As noted above, there is increasing evidence that PTCs do not appear to mediate HIV suppression through the same CTL and HLA-mediated mechanisms as SCs. While important, the favorable genetic profiles of SCs have not been easily translatable to therapeutics and the elucidation of the mechanisms of control in PTCs may have a greater impact on the design and evaluation of the next generation of HIV therapeutics. Studies of the HIV reservoir in PTCs have revealed the restricted size of the reservoir, including the intact proviral genomes (64). This, however, does not fully explain post-treatment control, especially given our experience in hematopoietic stem cell transplant participants who can dramatically lower their peripheral reservoir size, but are unable to maintain HIV remission (88). Additional studies are needed to assess potential differences in the distribution of infected cell types (7), cellular transcription environment, integration sites, and other factors that could contribute to the maintenance of a “deeper” state of viral latency (89).
Finally, little is known about the clinical implications of post-treatment control. While SCs can maintain low or undetectable viremia in the absence of ART, the ongoing viral replication and immune response in SCs may be associated with adverse consequences, including the progressive loss of CD4+ T cells in some individuals, increased T cell activation and inflammation (90–93). Chronic immune activation and systemic inflammation has been associated with poor clinical outcomes in non-controllers (94–97) but also in SCs, who are reported to have an increased risk of cardiovascular disease (98) and hospitalization (58), although the extent of this risk is still a matter of some uncertainty (99, 100). There is some evidence that PTCs may not exhibit the same heightened levels of immune activation as SCs (7, 10), but additional studies are needed to confirm these findings and to assess the long-term clinical implications of sustained HIV remission.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Funding
JL was funded by a grant from amfAR, The Foundation for AIDS Research.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2019.01749/full#supplementary-material
References
1. Exceptional Responders Initiative: Questions and Answers. National Cancer Institute. (2018) Available online at: http://www.cancer.gov/about-cancer/treatment/research/exceptional-responders-initiative-qa
2. Chau NG, Lorch JH. Exceptional responders inspire change: lessons for drug development from the bedside to the bench and back. Oncologist. (2015) 20:699–701. doi: 10.1634/theoncologist.2014-0476
3. Richey LE, Halperin J. Acute human immunodeficiency virus infection. Am J Med Sci. (2013) 345:136–42. doi: 10.1097/MAJ.0b013e31825d4b88
4. Li JZ, Etemad B, Ahmed H, Aga E, Bosch RJ, Mellors JW, et al. The size of the expressed HIV reservoir predicts timing of viral rebound after treatment interruption. AIDS. (2016) 30:343–53. doi: 10.1097/QAD.0000000000000953
5. Bongiovanni M, Casana M, Tincati C, d'Arminio Monforte A. Treatment interruptions in HIV-infected subjects. J Antimicrob Chemother. (2006) 58:502–5. doi: 10.1093/jac/dkl268
6. Namazi G, Fajnzylber JM, Aga E, Bosch RJ, Acosta EP, Sharaf R, et al. The Control of HIV After Antiretroviral Medication Pause (CHAMP) Study: Posttreatment Controllers Identified From 14 Clinical Studies. J Infect Dis. (2018) 218:1954–63. doi: 10.1093/infdis/jiy479
7. Saez-Cirion A, Bacchus C, Hocqueloux L, Avettand-Fenoel V, Girault I, Lecuroux C, et al. Post-treatment HIV-1 controllers with a long-term virological remission after the interruption of early initiated antiretroviral therapy ANRS VISCONTI Study. PLoS Pathog. (2013) 9:e1003211. doi: 10.1371/journal.ppat.1003211
8. Lisziewicz J, Rosenberg E, Lieberman J, Jessen H, Lopalco L, Siliciano R, et al. Control of HIV despite the discontinuation of antiretroviral therapy. N Engl J Med. (1999) 340:1683–4. doi: 10.1056/NEJM199905273402114
9. Hocqueloux L, Prazuck T, Avettand-Fenoel V, Lafeuillade A, Cardon B, Viard JP, et al. Long-term immunovirologic control following antiretroviral therapy interruption in patients treated at the time of primary HIV-1 infection. AIDS. (2010) 24:1598–601. doi: 10.1097/QAD.0b013e32833b61ba
10. Goujard C, Girault I, Rouzioux C, Lecuroux C, Deveau C, Chaix ML, et al. HIV-1 control after transient antiretroviral treatment initiated in primary infection: role of patient characteristics and effect of therapy. Antivir Ther. (2012) 17:1001–9. doi: 10.3851/IMP2273
11. Lodi S, Meyer L, Kelleher AD, Rosinska M, Ghosn J, Sannes M, et al. Immunovirologic control 24 months after interruption of antiretroviral therapy initiated close to HIV seroconversion. Arch Intern Med. (2012) 172:1252–5. doi: 10.1001/archinternmed.2012.2719
12. Stohr W, Fidler S, McClure M, Weber J, Cooper D, Ramjee G, et al. Duration of HIV-1 viral suppression on cessation of antiretroviral therapy in primary infection correlates with time on therapy. PLoS ONE. (2013) 8:e78287. doi: 10.1371/journal.pone.0078287
13. Maenza J, Tapia K, Holte S, Stekler JD, Stevens CE, Mullins JI, et al. How often does treatment of primary HIV lead to post-treatment control? Antivir Ther. (2015) 20:855–63. doi: 10.3851/IMP2963
14. Gianella S, Anderson CM, Richman DD, Smith DM, Little SJ. No evidence of posttreatment control after early initiation of antiretroviral therapy. AIDS. (2015) 29:2093–7. doi: 10.1097/QAD.0000000000000816
15. Van Gulck E, Bracke L, Heyndrickx L, Coppens S, Atkinson D, Merlin C, et al. Immune and viral correlates of “secondary viral control” after treatment interruption in chronically HIV-1 infected patients. PLoS ONE. (2012) 7:e37792. doi: 10.1371/journal.pone.0037792
16. Assoumou L, Weiss L, Piketty C, Burgard M, Melard A, Girard PM, et al. A low HIV-DNA level in peripheral blood mononuclear cells at antiretroviral treatment interruption predicts a higher probability of maintaining viral control. AIDS. (2015) 29:2003–7. doi: 10.1097/QAD.0000000000000734
17. Calin R, Hamimi C, Lambert-Niclot S, Carcelain G, Bellet J, Assoumou L, et al. Treatment interruption in chronically HIV-infected patients with an ultralow HIV reservoir. AIDS. (2016) 30:761–9. doi: 10.1097/QAD.0000000000000987
18. Perkins MJ, Bradley WP, Lalani T, Agan BK, Whitman TJ, Ferguson TM, et al. Brief report: prevalence of posttreatment controller phenotype is rare in HIV-infected persons after stopping antiretroviral therapy. J Acquir Immune Defic Syndr. (2017) 75:364–9. doi: 10.1097/QAI.0000000000001393
19. Fidler S, Olson AD, Bucher HC, Fox J, Thornhill J, Morrison C, et al. Virological blips and predictors of post treatment viral control after stopping ART started in primary HIV Infection. J Acquir Immune Defic Syndr. (2017) 74:126–33. doi: 10.1097/QAI.0000000000001220
20. Volberding P, Demeter L, Bosch RJ, Aga E, Pettinelli C, Hirsch M, et al. Antiretroviral therapy in acute and recent HIV infection: a prospective multicenter stratified trial of intentionally interrupted treatment. AIDS. (2009) 23:1987–95. doi: 10.1097/QAD.0b013e32832eb285
21. Kilby JM, Bucy RP, Mildvan D, Fischl M, Santana-Bagur J, Lennox J, et al. A randomized, partially blinded phase 2 trial of antiretroviral therapy, HIV-specific immunizations, and interleukin-2 cycles to promote efficient control of viral replication (ACTG A5024). J Infect Dis. (2006) 194:1672–6. doi: 10.1086/509508
22. Jacobson JM, Pat Bucy R, Spritzler J, Saag MS, Eron JJ Jr, Coombs RW, et al. Evidence that intermittent structured treatment interruption, but not immunization with ALVAC-HIV vCP1452, promotes host control of HIV replication: the results of AIDS Clinical Trials Group 5068. J Infect Dis. (2006) 194:623–32. doi: 10.1086/506364
23. Henry K, Katzenstein D, Cherng DW, Valdez H, Powderly W, Vargas MB, et al. A pilot study evaluating time to CD4 T-cell count <350 cells/mm(3) after treatment interruption following antiretroviral therapy +/- interleukin 2: results of ACTG A5102. J Acquir Immune Defic Syndr. (2006) 42:140–8. doi: 10.1097/01.qai.0000225319.59652.1e
24. Gandhi RT, O'Neill D, Bosch RJ, Chan ES, Bucy RP, Shopis J, et al. A randomized therapeutic vaccine trial of canarypox-HIV-pulsed dendritic cells vs. canarypox-HIV alone in HIV-1-infected patients on antiretroviral therapy. Vaccine. (2009) 27:6088–94. doi: 10.1016/j.vaccine.2009.05.016
25. Skiest DJ, Su Z, Havlir DV, Robertson KR, Coombs RW, Cain P, et al. Interruption of antiretroviral treatment in HIV-infected patients with preserved immune function is associated with a low rate of clinical progression: a prospective study by AIDS Clinical Trials Group 5170. J Infect Dis. (2007) 195:1426–36. doi: 10.1086/512681
26. Rosenberg ES, Graham BS, Chan ES, Bosch RJ, Stocker V, Maenza J, et al. Safety and immunogenicity of therapeutic DNA vaccination in individuals treated with antiretroviral therapy during acute/early HIV-1 infection. PLoS ONE. (2010) 5:e10555. doi: 10.1371/journal.pone.0010555
27. Schooley RT, Spritzler J, Wang H, Lederman MM, Havlir D, Kuritzkes DR, et al. AIDS clinical trials group 5197: a placebo-controlled trial of immunization of HIV-1-infected persons with a replication-deficient adenovirus type 5 vaccine expressing the HIV-1 core protein. J Infect Dis. (2010) 202:705–16. doi: 10.1086/655468
28. Routy JP, Vanhems P, Rouleau D, Tsoukas C, Lefebvre E, Cote P, et al. Comparison of clinical features of acute HIV-1 infection in patients infected sexually or through injection drug use. J Acquir Immune Defic Syndr. (2000) 24:425–32. doi: 10.1097/00042560-200008150-00004
29. Stekler JD, Wellman R, Holte S, Maenza J, Stevens CE, Corey L, et al. Are there benefits to starting antiretroviral therapy during primary HIV infection? Conclusions from the Seattle Primary Infection Cohort vary by control group. Int J STD AIDS. (2012) 23:201–6. doi: 10.1258/ijsa.2011.011178
30. Sneller MC, Justement JS, Gittens KR, Petrone ME, Clarridge KE, Proschan MA, et al. A randomized controlled safety/efficacy trial of therapeutic vaccination in HIV-infected individuals who initiated antiretroviral therapy early in infection. Sci Transl Med. (2017) 9:eaan8848. doi: 10.1126/scitranslmed.aan8848
31. Jain V, Liegler T, Vittinghoff E, Hartogensis W, Bacchetti P, Poole L, et al. Transmitted drug resistance in persons with acute/early HIV-1 in San Francisco, 2002–2009. PLoS ONE. (2010) 5:e15510. doi: 10.1371/journal.pone.0015510
32. Pereyra F, Jia X, McLaren PJ, Telenti A, de Bakker PI, Walker BD, et al. The major genetic determinants of HIV-1 control affect HLA class I peptide presentation. Science. (2010) 330:1551–7. doi: 10.1126/science.1195271
33. Yang OO, Cumberland WG, Escobar R, Liao D, Chew KW. Demographics and natural history of HIV-1-infected spontaneous controllers of viremia. AIDS. (2017) 31:1091–8. doi: 10.1097/QAD.0000000000001443
34. Vieira VA, Zuidewind P, Muenchhoff M, Roider J, Millar J, Clapson M, et al. Strong sex bias in elite control of paediatric HIV infection. AIDS. (2019) 33:67–75. doi: 10.1097/QAD.0000000000002043
35. Martin GE, Gossez M, Williams JP, Stohr W, Meyerowitz J, Leitman EM, et al. Post-treatment control or treated controllers? Viral remission in treated and untreated primary HIV infection. AIDS. (2017) 31:477–84. doi: 10.1097/QAD.0000000000001382
36. Gossez M, Martin GE, Pace M, Ramjee G, Premraj A, Kaleebu P, et al. Virological remission after antiretroviral therapy interruption in female African HIV seroconverters. AIDS. (2019) 33:185–97. doi: 10.1097/QAD.0000000000002044
37. Mehraj V, Cox J, Lebouche B, Costiniuk C, Cao W, Li T, et al. Socio-economic status and time trends associated with early ART initiation following primary HIV infection in Montreal, Canada: 1996 to 2015. J Int AIDS Soc. (2018) 21:e25034. doi: 10.1002/jia2.25034
38. Maggiolo F, Di Filippo E, Comi L, Callegaro A. Post-treatment controllers after treatment interruption in chronically HIV-infected patients. AIDS. (2018) 32:623–8. doi: 10.1097/QAD.0000000000001743
39. Mellors JW, Munoz A, Giorgi JV, Margolick JB, Tassoni CJ, Gupta P, et al. Plasma viral load and CD4+ lymphocytes as prognostic markers of HIV-1 infection. Ann Intern Med. (1997) 126:946–54. doi: 10.7326/0003-4819-126-12-199706150-00003
40. Lambotte O, Boufassa F, Madec Y, Nguyen A, Goujard C, Meyer L, et al. HIV controllers: a homogeneous group of HIV-1-infected patients with spontaneous control of viral replication. Clin Infect Dis. (2005) 41:1053–6. doi: 10.1086/433188
41. Deeks SG, Walker BD. Human immunodeficiency virus controllers: mechanisms of durable virus control in the absence of antiretroviral therapy. Immunity. (2007) 27:406–16. doi: 10.1016/j.immuni.2007.08.010
42. Blankson JN, Bailey JR, Thayil S, Yang HC, Lassen K, Lai J, et al. Isolation and characterization of replication-competent human immunodeficiency virus type 1 from a subset of elite suppressors. J Virol. (2007) 81:2508–18. doi: 10.1128/JVI.02165-06
43. O'Connell KA, Brennan TP, Bailey JR, Ray SC, Siliciano RF, Blankson JN. Control of HIV-1 in elite suppressors despite ongoing replication and evolution in plasma virus. J Virol. (2010) 84:7018–28. doi: 10.1128/JVI.00548-10
44. Bailey JR, Brennan TP, O'Connell KA, Siliciano RF, Blankson JN. Evidence of CD8+ T-cell-mediated selective pressure on human immunodeficiency virus type 1 nef in HLA-B*57+ elite suppressors. J Virol. (2009) 83:88–97. doi: 10.1128/JVI.01958-08
45. Bailey JR, Williams TM, Siliciano RF, Blankson JN. Maintenance of viral suppression in HIV-1-infected HLA-B*57+ elite suppressors despite CTL escape mutations. J Exp Med. (2006) 203:1357–69. doi: 10.1084/jem.20052319
46. Walker BD, Yu XG. Unravelling the mechanisms of durable control of HIV-1. Nat Rev Immunol. (2013) 13:487–98. doi: 10.1038/nri3478
47. Migueles SA, Osborne CM, Royce C, Compton AA, Joshi RP, Weeks KA, et al. Lytic granule loading of CD8+ T cells is required for HIV-infected cell elimination associated with immune control. Immunity. (2008) 29:1009–21. doi: 10.1016/j.immuni.2008.10.010
48. Migueles SA, Sabbaghian MS, Shupert WL, Bettinotti MP, Marincola FM, Martino L, et al. HLA B*5701 is highly associated with restriction of virus replication in a subgroup of HIV-infected long term nonprogressors. Proc Natl Acad Sci USA. (2000) 97:2709–14. doi: 10.1073/pnas.050567397
49. Pereyra F, Addo MM, Kaufmann DE, Liu Y, Miura T, Rathod A, et al. Genetic and immunologic heterogeneity among persons who control HIV infection in the absence of therapy. J Infect Dis. (2008) 197:563–71. doi: 10.1086/526786
50. Scheid JF, Mouquet H, Ueberheide B, Diskin R, Klein F, Oliveira TY, et al. Sequence and structural convergence of broad and potent HIV antibodies that mimic CD4 binding. Science. (2011) 333:1633–7. doi: 10.1126/science.1207227
51. Pernas M, Tarancon-Diez L, Rodriguez-Gallego E, Gomez J, Prado JG, Casado C, et al. Factors leading to the loss of natural elite control of HIV-1 infection. J Virol. (2018) 92:e01805–17. doi: 10.1128/JVI.01805-17
52. Jiang C, Lian X, Chevalier J, Chen SM, Gao C, Sun X, et al. Chracterizing the Proviral Landscape in HIV-1 Elite Controllers. Seattle, WA: CROI (2019).
53. Okulicz JF, Lambotte O. Epidemiology and clinical characteristics of elite controllers. Curr Opin HIV AIDS. (2011) 6:163–8. doi: 10.1097/COH.0b013e328344f35e
54. Okulicz JF, Marconi VC, Landrum ML, Wegner S, Weintrob A, Ganesan A, et al. Clinical outcomes of elite controllers, viremic controllers, and long-term nonprogressors in the US Department of Defense HIV natural history study. J Infect Dis. (2009) 200:1714–23. doi: 10.1086/646609
55. Lambotte O, Ferrari G, Moog C, Yates NL, Liao HX, Parks RJ, et al. Heterogeneous neutralizing antibody and antibody-dependent cell cytotoxicity responses in HIV-1 elite controllers. AIDS. (2009) 23:897–906. doi: 10.1097/QAD.0b013e328329f97d
56. Leon A, Perez I, Ruiz-Mateos E, Benito JM, Leal M, Lopez-Galindez C, et al. Rate and predictors of progression in elite and viremic HIV-1 controllers. AIDS. (2016) 30:1209–20. doi: 10.1097/QAD.0000000000001050
57. Dominguez-Molina B, Leon A, Rodriguez C, Benito JM, Lopez-Galindez C, Garcia F, et al. Analysis of non-AIDS-defining events in HIV controllers. Clin Infect Dis. (2016) 62:1304–9. doi: 10.1093/cid/ciw120
58. Crowell TA, Gebo KA, Blankson JN, Korthuis PT, Yehia BR, Rutstein RM, et al. Hospitalization Rates and Reasons Among HIV Elite Controllers and Persons With Medically Controlled HIV Infection. J Infect Dis. (2015) 211:1692–702. doi: 10.1093/infdis/jiu809
59. Etemad B, Sun X, Lederman MM, Gottlieb RZ, Aga E, Bosch R, et al. Viral and immune characteristics of HIV post-treatment controllers in ACTG Studies. In: Conference on Retroviruses and Opportunistic Infections. Boston, MA: CROI (2016).
60. Chomont N, El-Far M, Ancuta P, Trautmann L, Procopio FA, Yassine-Diab B, et al. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat Med. (2009) 15:893–900. doi: 10.1038/nm.1972
61. Williams JP, Hurst J, Stohr W, Robinson N, Brown H, Fisher M, et al. HIV-1 DNA predicts disease progression and post-treatment virological control. eLife. (2014) 3:e03821. doi: 10.7554/eLife.03821
62. Cheret A, Bacchus-Souffan C, Avettand-Fenoel V, Melard A, Nembot G, Blanc C, et al. Combined ART started during acute HIV infection protects central memory CD4+ T cells and can induce remission. J Antimicrob Chemother. (2015) 70:2108–20. doi: 10.1093/jac/dkv084
63. Descours B, Avettand-Fenoel V, Blanc C, Samri A, Melard A, Supervie V, et al. Immune responses driven by protective human leukocyte antigen alleles from long-term nonprogressors are associated with low HIV reservoir in central memory CD4 T cells. Clin Infect Dis. (2012) 54:1495–503. doi: 10.1093/cid/cis188
64. Sharaf R, Lee GQ, Sun X, Etemad B, Aboukhater LM, Hu Z, et al. HIV-1 proviral landscapes distinguish posttreatment controllers from noncontrollers. J Clin Invest. (2018) 128:4074–85. doi: 10.1172/JCI120549
65. Veenhuis RT, Kwaa AK, Garliss CC, Latanich R, Salgado M, Pohlmeyer CW, et al. Long-term remission despite clonal expansion of replication-competent HIV-1 isolates. JCI Insight. (2018) 3:122795. doi: 10.1172/jci.insight.122795
66. Okoye AA, Hansen SG, Vaidya M, Fukazawa Y, Park H, Duell DM, et al. Early antiretroviral therapy limits SIV reservoir establishment to delay or prevent post-treatment viral rebound. Nat Med. (2018) 24:1430–40. doi: 10.1038/s41591-018-0130-7
67. Whitney JB, Hill AL, Sanisetty S, Penaloza-MacMaster P, Liu J, Shetty M, et al. Rapid seeding of the viral reservoir prior to SIV viraemia in rhesus monkeys. Nature. (2014) 512:74–7. doi: 10.1038/nature13594
68. Murray JM, Zaunders JJ, McBride KL, Xu Y, Bailey M, Suzuki K, et al. HIV DNA subspecies persist in both activated and resting memory CD4+ T cells during antiretroviral therapy. J Virol. (2014) 88:3516–26. doi: 10.1128/JVI.03331-13
69. Rouzioux C, Hocqueloux L, Saez-Cirion A. Posttreatment controllers: what do they tell us? Curr Opin HIV AIDS. (2015) 10:29–34. doi: 10.1097/COH.0000000000000123
70. Hocqueloux L, Avettand-Fenoel V, Jacquot S, Prazuck T, Legac E, Melard A, et al. Long-term antiretroviral therapy initiated during primary HIV-1 infection is key to achieving both low HIV reservoirs and normal T cell counts. J Antimicrob Chemother. (2013) 68:1169–78. doi: 10.1093/jac/dks533
71. Ananworanich J, Chomont N, Eller LA, Kroon E, Tovanabutra S, Bose M, et al. HIV DNA Set Point is Rapidly Established in acute HIV infection and dramatically reduced by early ART. EBioMedicine. (2016) 11:68–72. doi: 10.1016/j.ebiom.2016.07.024
72. Oxenius A, Price DA, Easterbrook PJ, O'Callaghan CA, Kelleher AD, Whelan JA, et al. Early highly active antiretroviral therapy for acute HIV-1 infection preserves immune function of CD8+ and CD4+ T lymphocytes. Proc Natl Acad Sci USA. (2000) 97:3382–7. doi: 10.1073/pnas.97.7.3382
73. Altfeld M, Rosenberg ES, Shankarappa R, Mukherjee JS, Hecht FM, Eldridge RL, et al. Cellular immune responses and viral diversity in individuals treated during acute and early HIV-1 infection. J Exp Med. (2001) 193:169–80. doi: 10.1084/jem.193.2.169
74. Streeck H, Jessen H, Alter G, Teigen N, Waring MT, Jessen A, et al. Immunological and virological impact of highly active antiretroviral therapy initiated during acute HIV-1 infection. J Infect Dis. (2006) 194:734–9. doi: 10.1086/503811
75. Salgado M, Rabi SA, O'Connell KA, Buckheit RW III, Bailey JR, Chaudhry AA, et al. Prolonged control of replication-competent dual- tropic human immunodeficiency virus-1 following cessation of highly active antiretroviral therapy. Retrovirology. (2011) 8:97. doi: 10.1186/1742-4690-8-97
76. Frange P, Faye A, Avettand-Fenoel V, Bellaton E, Descamps D, Angin M, et al. HIV-1 virological remission lasting more than 12 years after interruption of early antiretroviral therapy in a perinatally infected teenager enrolled in the French ANRS EPF-CO10 paediatric cohort: a case report. Lancet HIV. (2016) 3:e49–54. doi: 10.1016/S2352-3018(15)00232-5
77. Samri A, Bacchus-Souffan C, Hocqueloux L, Avettand-Fenoel V, Descours B, Theodorou I, et al. Polyfunctional HIV-specific T cells in Post-Treatment Controllers. AIDS. (2016) 30:2299–302. doi: 10.1097/QAD.0000000000001195
78. Persaud D, Gay H, Ziemniak C, Chen YH, Piatak M Jr, Chun TW, et al. Absence of detectable HIV-1 viremia after treatment cessation in an infant. N Engl J Med. (2013) 369:1828–35. doi: 10.1056/NEJMoa1302976
79. Violari A, Cotton MF, Kuhn L, Schramm DB, Paximadis M, Loubser S, et al. A child with perinatal HIV infection and long-term sustained virological control following antiretroviral treatment cessation. Nat Commun. (2019) 10:412. doi: 10.1038/s41467-019-08311-0
80. Luzuriaga K, Gay H, Ziemniak C, Sanborn KB, Somasundaran M, Rainwater-Lovett K, et al. Viremic relapse after HIV-1 remission in a perinatally infected child. N Engl J Med. (2015) 372:786–8. doi: 10.1056/NEJMc1413931
81. Violari A, Cotton MF, Gibb DM, Babiker AG, Steyn J, Madhi SA, et al. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med. (2008) 359:2233–44. doi: 10.1056/NEJMoa0800971
82. Cotton MF, Violari A, Otwombe K, Panchia R, Dobbels E, Rabie H, et al. Early time-limited antiretroviral therapy versus deferred therapy in South African infants infected with HIV: results from the children with HIV early antiretroviral (CHER) randomised trial. Lancet. (2013) 382:1555–63. doi: 10.1016/S0140-6736(13)61409-9
83. Wamalwa D, Benki-Nugent S, Langat A, Tapia K, Ngugi E, Moraa H, et al. Treatment interruption after 2-year antiretroviral treatment initiated during acute/early HIV in infancy. AIDS. (2016) 30:2303–13. doi: 10.1097/QAD.0000000000001158
84. Moir S, Buckner CM, Ho J, Wang W, Chen J, Waldner AJ, et al. B cells in early and chronic HIV infection: evidence for preservation of immune function associated with early initiation of antiretroviral therapy. Blood. (2010) 116:5571–9. doi: 10.1182/blood-2010-05-285528
85. Pensieroso S, Cagigi A, Palma P, Nilsson A, Capponi C, Freda E, et al. Timing of HAART defines the integrity of memory B cells and the longevity of humoral responses in HIV-1 vertically-infected children. Proc Natl Acad Sci USA. (2009) 106:7939–44. doi: 10.1073/pnas.0901702106
86. Colby DJ, Trautmann L, Pinyakorn S, Leyre L, Pagliuzza A, Kroon E, et al. Rapid HIV RNA rebound after antiretroviral treatment interruption in persons durably suppressed in Fiebig I acute HIV infection. Nat Med. (2018) 24:923–6. doi: 10.1038/s41591-018-0026-6
87. Henrich TJ, Hatano H, Bacon O, Hogan LE, Rutishauser R, Hill A, et al. HIV-1 persistence following extremely early initiation of antiretroviral therapy (ART) during acute HIV-1 infection: An observational study. PLoS Med. (2017) 14:e1002417. doi: 10.1371/journal.pmed.1002417
88. Henrich TJ, Hanhauser E, Marty FM, Sirignano MN, Keating S, Lee TH, et al. Antiretroviral-free HIV-1 remission and viral rebound after allogeneic stem cell transplantation: report of 2 cases. Ann Intern Med. (2014) 161:319–27. doi: 10.7326/M14-1027
89. Yukl SA, Kaiser P, Kim P, Telwatte S, Joshi SK, Vu M, et al. HIV latency in isolated patient CD4(+) T cells may be due to blocks in HIV transcriptional elongation, completion, and splicing. Sci Transl Med. (2018) 10:eaap9927. doi: 10.1126/scitranslmed.aap9927
90. Mavigner M, Delobel P, Cazabat M, Dubois M, L'Faqihi-Olive FE, Raymond S, et al. HIV-1 residual viremia correlates with persistent T-cell activation in poor immunological responders to combination antiretroviral therapy. PLoS ONE. (2009) 4:e7658. doi: 10.1371/journal.pone.0007658
91. Ostrowski SR, Katzenstein TL, Pedersen BK, Gerstoft J, Ullum H. Residual viraemia in HIV-1-infected patients with plasma viral load ≤ 20 copies/ml is associated with increased blood levels of soluble immune activation markers. Scand J Immunol. (2008) 68:652–60. doi: 10.1111/j.1365-3083.2008.02184.x
92. Hunt PW, Brenchley J, Sinclair E, McCune JM, Roland M, Page-Shafer K, et al. Relationship between T cell activation and CD4+ T cell count in HIV-seropositive individuals with undetectable plasma HIV RNA levels in the absence of therapy. J Infect Dis. (2008) 197:126–33. doi: 10.1086/524143
93. Pereyra F, Palmer S, Miura T, Block BL, Wiegand A, Rothchild AC, et al. Persistent low-level viremia in HIV-1 elite controllers and relationship to immunologic parameters. J Infect Dis. (2009) 200:984–90. doi: 10.1086/605446
94. Liu Z, Cumberland WG, Hultin LE, Kaplan AH, Detels R, Giorgi JV. CD8+ T-lymphocyte activation in HIV-1 disease reflects an aspect of pathogenesis distinct from viral burden and immunodeficiency. J Acquir Immune Defic Syndr Hum Retrovirol. (1998) 18:332–40. doi: 10.1097/00042560-199808010-00004
95. Giorgi JV, Hultin LE, McKeating JA, Johnson TD, Owens B, Jacobson LP, et al. Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. J Infect Dis. (1999) 179:859–70. doi: 10.1086/314660
96. Kuller LH, Tracy R, Belloso W, De Wit S, Drummond F, Lane HC, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. (2008) 5:e203. doi: 10.1371/journal.pmed.0050203
97. Hazenberg MD, Otto SA, van Benthem BH, Roos MT, Coutinho RA, Lange JM, et al. Persistent immune activation in HIV-1 infection is associated with progression to AIDS. AIDS. (2003) 17:1881–8. doi: 10.1097/00002030-200309050-00006
98. Pereyra F, Lo J, Triant VA, Wei J, Buzon MJ, Fitch KV, et al. Increased coronary atherosclerosis and immune activation in HIV-1 elite controllers. AIDS. (2012) 26:2409–12. doi: 10.1097/QAD.0b013e32835a9950
99. Crowell TA, Ganesan A, Berry SA, Deiss RG, Agan BK, Okulicz JF. Hospitalizations among HIV controllers and persons with medically controlled HIV in the U.S. Military HIV Natural History Study. J Int AIDS Soc. (2016) 19:20524. doi: 10.7448/IAS.19.1.20524
Keywords: HIV, post-treatment controllers, remission, treatment interruption, elite controllers
Citation: Etemad B, Esmaeilzadeh E and Li JZ (2019) Learning From the Exceptions: HIV Remission in Post-treatment Controllers. Front. Immunol. 10:1749. doi: 10.3389/fimmu.2019.01749
Received: 04 June 2019; Accepted: 10 July 2019;
Published: 24 July 2019.
Edited by:
Mirko Paiardini, Emory University School of Medicine, United StatesReviewed by:
Ezequiel Ruiz-Mateos, Institute of Biomedicine of Seville (IBIS), SpainLisa A. Chakrabarti, Institut Pasteur, France
Copyright © 2019 Etemad, Esmaeilzadeh and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jonathan Z. Li, amxpQGJ3aC5oYXJ2YXJkLmVkdQ==
 Behzad Etemad
Behzad Etemad Jonathan Z. Li
Jonathan Z. Li