- 1Department of Immunology, School of Basic Medical Sciences, Fudan University, Shanghai, China
- 2RIKEN Center for Integrative Medical Sciences, Kanagawa, Japan
Immunoglobulin (Ig) M is the first antibody isotype to appear during evolution, ontogeny and immune responses. IgM not only serves as the first line of host defense against infections but also plays an important role in immune regulation and immunological tolerance. For many years, IgM is thought to function by binding to antigen and activating complement system. With the discovery of the IgM Fc receptor (FcμR), it is now clear that IgM can also elicit its function through FcμR. In this review, we will describe the molecular characteristics of FcμR, its role in B cell development, maturation and activation, humoral immune responses, host defense, and immunological tolerance. We will also discuss the functional relationship between IgM-complement and IgM-FcμR pathways in regulating immunity and tolerance. Finally, we will discuss the potential involvement of FcμR in human diseases.
Introduction
B cells produce different classes of antibodies (Ab), including IgM, IgD, IgG, IgA, and IgE. Ab constitutes a variable F(ab) region that binds to antigen (Ag) and a constant Fc region that mediates effector function. Cellular receptors for the Fc region mediate a variety of functions including phagocytosis of Ab-opsonized pathogens and induction of cellular cytotoxicity. Recent studies have unveiled three Fc receptors for IgM, including Fcα/μ receptor (Fcα/μR), polymeric immunoglobulin receptor (pIgR), and Fcμ receptor (FcμR). Fcα/μR, pIgR, and FcμR are all type I transmembrane proteins belonging to the immunoglobulin (Ig) gene superfamily. Fcα/μR is expressed by both hematopoietic and non-hematopoietic cells (1, 2), and has been shown to play an important role in humoral immune responses, especially in pro-inflammatory functions of marginal zone B cells in sepsis (3). pIgR is expressed on the basolateral surface of ciliated epithelial cell in the mucosal epithelium (4, 5), but not in hematopoietic cells (6). The main function of pIgR is to transport dimeric IgA and polymeric IgM from the lamina propria across the epithelial barrier to mucosal surfaces (7). FcμR was discovered relatively recently and its function has not been fully elucidated. Here we summarize the results of FcμR published over the past several years, and discuss how it contributes to immunity and tolerance.
Molecular Characteristics of FcμR
The existence of a receptor for IgM was noted more than 40 years ago (8–16). Biochemical analysis revealed that human FcμR had a molecular weight of ~60-kDa (17). Molecular cloning of FCMR, the gene encoding human FcμR, revealed that it is a single copy gene located on chromosome 1q32.2, adjacent to two other IgM associated Fc receptor genes, polymeric Ig receptor gene (PIGR) and the gene of FcR for IgA and IgM (FCAMR) (18). Human FcμR is a type I transmembrane protein of 390 amino acids (aa), composed of a 234-aa extracellular domain, a 21-aa transmembrane segment, and a 118-aa cytoplasmic tail (19, 20). BW5147 T cells ectopically expressing human FcμR exhibited specific binding to IgM but not any other Ab isotypes, demonstrating that FcμR is the bona-fide receptor for IgM (18). Unlike many other FcRs, the cytoplasmic tail of human FcμR does not contain any immunoreceptor tyrosine-based activation (ITAM) or inhibitory (ITIM) motifs. Instead, it contains conserved serine and tyrosine residues, which match the recently described Ig-tail tyrosine (ITT) motif (21, 22). Crosslinking human FcμR with either anti-FcμR monoclonal antibodies or preformed IgM immune complexes triggered the phosphorylation of these serine and tyrosine residues in FcμR-overexpressing BW5147 T cells, suggesting that FcμR could serve as an ITT phosphorylation molecule to interact with and influence the B cell receptor (BCR) signaling (23). Human FcμR is predominantly expressed by B, T, and NK cells, but not by monocytes, granulocytes, erythrocytes, and platelets (18). Human FcμR binds more efficiently to the Fc portion of IgM reactive with surface proteins than to the Fc portion of free IgM (24), suggesting that FcμR might modulate the signal of B, T, and NK cell surface receptors or proteins recognized by natural or immune IgM.
The mouse FcμR gene (Fcmr) is also a single copy gene located on chromosome 1 (56.89 cM), adjacent to Pigr and Fcamr (25). Although mouse and human FcμR have similar molecular structure, they share only 54% aa identity. Mouse FcμR also specifically binds to IgM (25, 26). Unlike human FcμR, we found that mouse FcμR is predominantly expressed in B lymphocytes by both microarray of a panel of immune cell types and FACS analyses (25, 27, 28). However, others have reported that monocytes, macrophages, granulocytes, and dendritic cells also express FcμR (29, 30). The expression levels of Mouse FcμR are different among different B cell subsets. The hierarchy of FcμR levels on various B cell subsets is as follows: marginal zone precursor (MZP, IgMhiCD21hiCD23hi) > follicular B (FOB, IgMloCD21loCD23hi) > marginal zone B (MZB, IgMhiCD21hiCD23lo) > newly formed B (CD93+CD21−CD23−) cells (28, 31). FcμR expression level is indistinguishable between B1 (CD5+) and B2 (CD5−) cells in the spleen. In the peritoneal cavity, FcμR expression level in each B cell subsets follows the order: B2 (CD11b−CD5−) ≅ B1a (CD5+) > B1b (CD11b+CD5−) cells (31, 32). In addition, FcμR expression is very low in pro-B (B220+CD43+) and pre-B (B220+CD43−IgM−) cells, and slightly upregulated in immature B cells (B220dullIgM+) in the bone marrow (BM) (27, 31, 33). FcμR expression in the germinal center (GC) B cells (CD95+GL7+) is much lower than that in naïve B cells (27), suggesting that FcμR is down-modulated during GC reaction. FcμR is expressed at higher levels in plasmablasts compared to plasma cells. Intriguingly, FcμR is also expressed by IgG- or IgA-positive B cells, suggesting that it may play a role in switched B cells (32).
It is intriguing that genes encoding FcμR, Fcα/μR, and pIgR are located in the same chromosomal region (18, 25), suggesting that these genes are evolutionarily related and might have derived from a common ancestor gene. However, in contrast to FcμR which only binds to IgM, Fcα/μR binds both IgM and IgA (3, 34, 35). Moreover, pIgR binds both IgM and IgA via their associated J chains and is essential for the transcytosis of polymeric IgA and IgM to the gut (36). The expression pattern is also quite different among these receptors. FcμR is predominantly expressed by B cells in mice and by B, T, and NK cells in humans (18, 25). In contrast, Fcα/μR is expressed by macrophages, B cells, intestinal lamina propria and several other cell types (35), and pIgR is mainly expressed on the intestinal epithelial cells (4, 5). Although FcμR was originally designated as Fas apoptotic inhibitory molecule 3 or TOSO (37), it is now clear that both human and mouse FcμR have no inhibitory activity against Fas-mediated apoptosis (38, 39).
FcμR in B Cell Development and Maturation
Several Fcmr-deficient (KO) and B-cell-specific deletion of Fcmr (BKO) mouse strains have been generated. (1) We and Kubagawa et al. share the constitutive FcμR knockout strain (Fcmrtm1Ohno) in which exons 2–4 were deleted in 129/Sv ES cells and the mutant mice had been backcrossed to C57BL/6 mice for > 12 generations. The neo gene used in drug selection was removed by crossing with Cre-Tg mice (27, 28, 32, 40–42); (2) Mak et al. and Coligan et al. share the constitutive FcμR knockout strain (Fcmrtm1Mak) where exons 2–8 were deleted in 129/Sv ES cells and the mutant mice had been backcrossed to C57BL/6 mice. The neo gene remained in the targeted allele (29–31, 43, 44); (3) Lee et al. have the constitutive FcμR knockout strain (Fcmrtm1.2Khl) and a strain with floxed Fcmr allele, with exons 4–7 were deleted or flanked by loxP sites, respectively. No neo gene remained in the targeted allele and both mice are on a pure B6 background (45–47); Baumgarth et al. generated the Fcmrflx/flxCd19-Cre+ strain in which exon 4 was deleted by CD19-driven Cre. The mutant mice are on a pure B6 background (33, 48). A comparison of the phenotypes of Fcmr−/− mice generated and/or analyzed by different groups is shown in Table 1.
B cell development proceeds from pro-B, pre-B to immature B cells in BM (49). Immature B cells then migrate to the periphery where they further differentiate into various mature B cell subsets that play distinct roles. The survival and maturation of B cells are dependent on the strength of tonic BCR signal (50, 51). Studies from our group, Honjo et al. and Nguyen et al. revealed that FcμR deficiency did not significantly affect B cell development, but altered the numbers of different B cell subsets (32, 33). We and Honjo et al. found that MZB were severely reduced in KO mice (27, 32) whereas Nguyen et al. found decreased proportion of MZB but the absolute numbers of MZB were not affected (Table 1) (33). Honjo et al., Choi et al., and Nguyen et al. reported that the splenic B1 cells were increased in KO mice (31–33). More recently, we found reduced tonic BCR signaling in FcμR-deficient MZB, which we think led to their decreased numbers in KO mice (28). In contrast, Honjo et al. suggested that the reduction of MZB in KO mice was due to their rapid differentiation into plasma cells (41). Lee et al. found decreased numbers of B cells in the spleen and lymph nodes (47). Choi et al. found that B-1a were increased but B-2 were decreased in the peritoneal cavity and that FOB were decreased in the spleen (Table 1) (31), which were similar to the phenotypes found in Sμ−/− mice that lack secreted IgM (52, 53). Taken together, these results indicate that FcμR affects the maturation or differentiation of various B cell subsets.
FcμR in B Cell Survival and Activation
We found that FcμR cell surface expression was upregulated after BCR cross-linking with anti-IgM Abs but only moderately increased by CD40L or LPS stimulation under in vitro culture conditions (40). Choi et al. reported that FcμR transcript levels were markedly reduced by stimulation of spleen B cells with anti-IgM, LPS or anti-CD40 (31), suggesting that FcμR expression is regulated at both transcriptional and posttranscriptional levels. Moreover, we and others demonstrated that FcμR specifically enhanced B cell survival induced by anti-IgM stimulation (Table 1) (27, 31, 40). Immunofluorescence and co-immunoprecipitation revealed physical interaction between FcμR and BCR on the plasma membrane of primary B cells (40). Although FcμR deficient B cells exhibited normal Ca2+ influx after BCR crosslinking, their survival was reduced compared with WT B cells (27), indicating that FcμR did not affect the early BCR signaling event such as Ca2+ influx but affected the late response such as B cell survival. Analysis of signaling molecules downstream of BCR revealed that FcμR promoted the activation of the non-canonical NF-κB pathway and the induction of BCL-xL (40). These results suggest that FcμR and BCR cooperate in signal transduction to promote B cell survival. FcμR does not contain any ITAM motifs but instead contains several conserved tyrosine and serine residues in its cytoplasmic tail (19, 20, 23, 26). A detailed mutational analysis has revealed that the tyrosines 315, 366, and 385 are not required for ligand (IgM) binding. However, tyrosine 315, as well as the entire intracellular domain, was shown to be required for inhibiting an IgM anti-FAS Ab-induced apoptosis (24). It remains to be investigated how FcμR specifically affects the late phase of BCR signaling and whether these tyrosine and serine residues are involved.
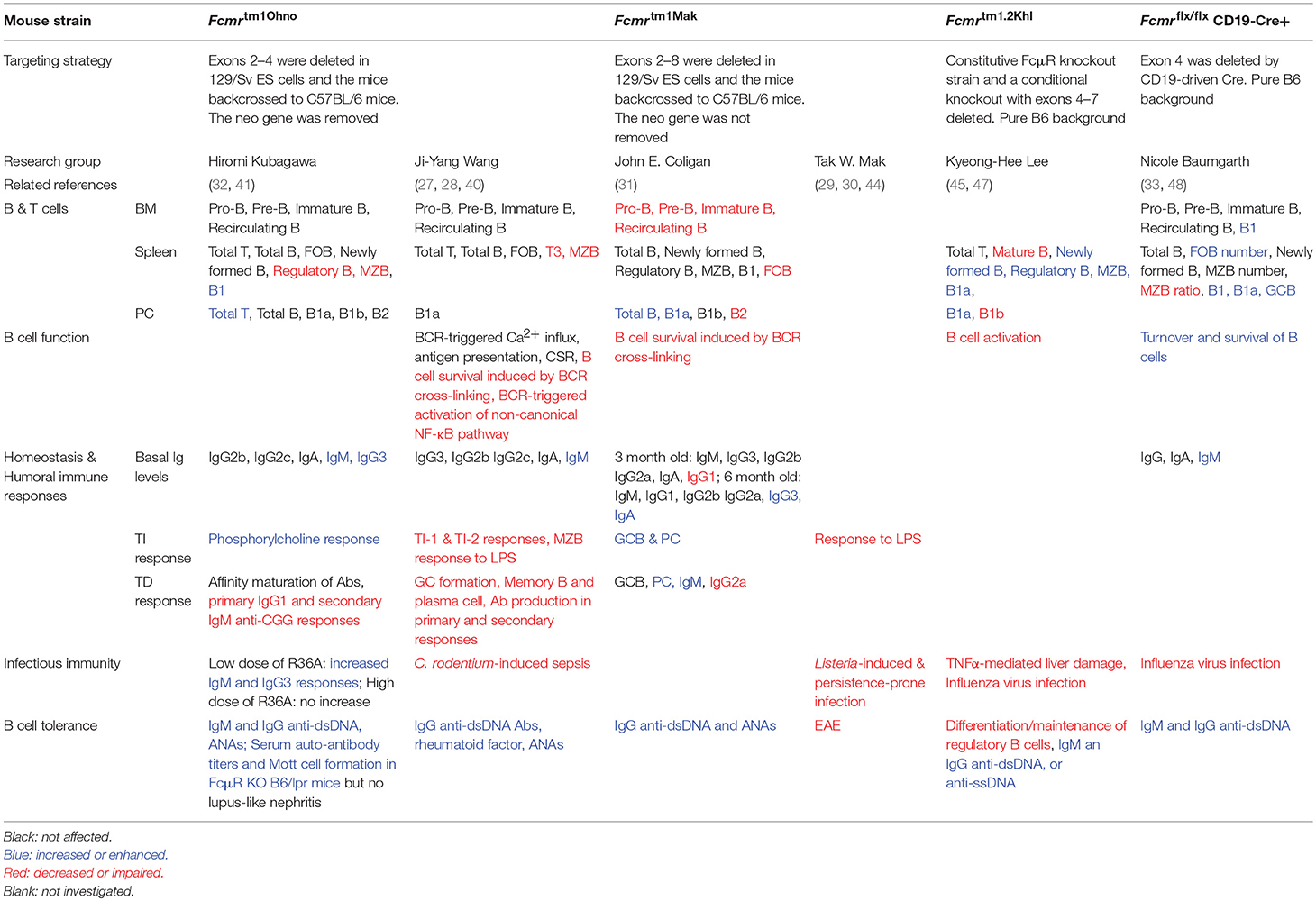
It is well-known that B cells express FcγRIIB, which inhibits BCR signaling and B cell activation upon binding IgG-Ag immune complexes, which then results in colligation of FcγRIIB and the BCR. Therefore, B cells express two types of Fc receptors, FcμR and FcγRIIB, which promotes and inhibits BCR signaling and B cell activation, respectively (Figure 1). More recently, Nguyen et al. reported that FcμR limited tonic BCR signaling in immature B cells by regulating the expression of IgM BCR (33). Therefore, FcμR regulates both the cell surface expression and the function of BCR.
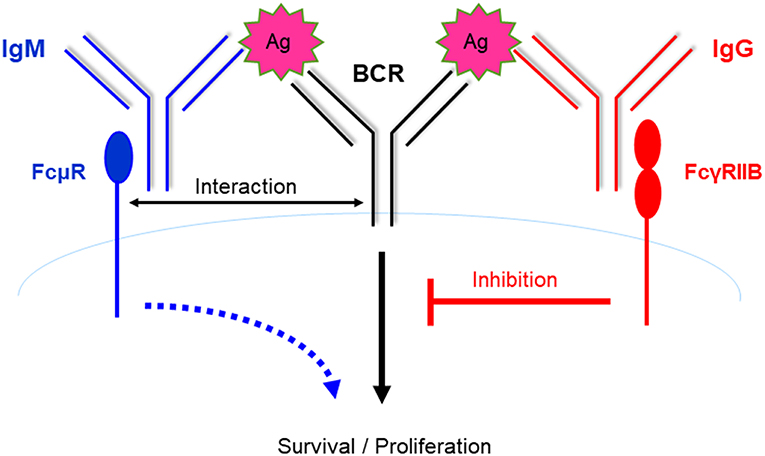
Figure 1. B cells express two types of Fc receptors of opposing functions. FcμR promotes B cell activation via interacting with the BCR and potentiating BCR signaling. In contrast, B cells express FcγRIIB, which inhibits B cell activation upon binding to immune complexes containing IgG and the cognate Ag, which then results in colligation of FcγRIIB and the BCR. Therefore, B cells express two types of Fc receptors of opposing functions. It is suggested that these two receptors function in a spatial-temporal manner to positively and negatively regulate B cell activation during humoral immune responses (see Figure 2).
Role of FcμR in Humoral Immune Responses
The basal Ig levels reflect the immune homeostasis at the steady state. We found that basal serum IgM levels were elevated in the absence of FcμR in a gene dosage-dependent manner, suggesting that a portion of the serum IgM actually binds to the FcμR in WT mice (27). Nguyen et al. found the same results and attributed the high IgM level to the elevated numbers and hyper-activation of B1 cells in the spleen (33). In addition, Honjo et al. found that IgM levels were elevated and that the IgG3 levels were slightly elevated in KO mice (32). In contrast, Choi et al. reported that only IgG1 levels were reduced in 3-month old mice and IgG3 and IgA levels were slightly elevated in 6-month old mice (31). Therefore, FcμR-deficient mice generated by different groups all exhibited increased levels of serum IgM and/or IgG3 (Table 1). These results implicate a role for FcμR in B cell homeostasis.
We found that KO mice had significantly decreased production of NP-specific IgG1 during both primary and secondary responses against a T-dependent (T-D) Ag, NP-CGG (27, 28), likely due to impaired GC formation and reduced memory and plasma cell differentiation. Similarly, Honjo et al. found impaired primary IgG1 and secondary IgM anti-CGG responses, but normal Ab affinity maturation (32). During humoral immune responses to T-D Ag, Ag-specific IgM is first produced, which is followed by the production of Ag-specific IgG. Based on our results that FcμR is required for efficient Ab production and the earlier findings that FcγRIIB inhibits B cell activation and Ab production, we propose an autoregulatory mechanism for T-D humoral immune responses [(27) and Figure 2]. During the early phase of the response, when the amount of Ag-specific IgM is greater than that of Ag-specific IgG, B cell activation is enhanced by FcμR-mediated positive signals. However, during the later phase of the response, when the amount of Ag-specific IgG is greater than that of Ag-specific IgM, further B cell activation is suppressed by FcγRIIB-mediated inhibitory signal (Figure 2). B cell activation and Ab production can thus be positively and negatively regulated by Ag-specific IgM and IgG present in the local environment, respectively.
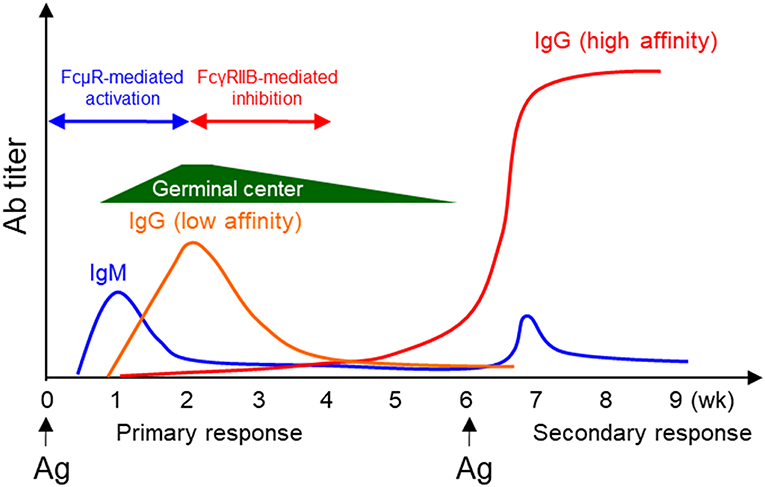
Figure 2. Positive and negative regulation of humoral immune responses by FcμR and FcγRIIB. During a typical T-D humoral immune response, Ag-specific IgM is produced first, followed by IgG production. Based on the results that FcμR promotes B cell activation and Ab production and the earlier findings that FcγRIIB inhibits B cell activation and Ab production, we propose an autoregulatory mechanism for T-D humoral immune responses. During the early phase of the response, B cell activation is enhanced by FcμR-mediated positive signals. However, at a later phase of the response, further B cell activation is suppressed by FcγRIIB-mediated inhibitory signal.
Consistent with the reduced survival in FcμR-deficient B cells after BCR crosslinking, FcμR KO mice had decreased Ab production against a type 2 T-independent (T-I) Ag, NP-FICOLL (27), since response to this type of Ag is largely dependent on BCR signal. Additionally, we found that FcμR KO mice had impaired Ab production against a type 1 T-I Ag, NP-LPS (28), which activates B cells through both BCR and toll-like receptor 4. Moreover, we found that MZB in KO were not activated upon LPS injection (28). Since MZB cells are thought to participate in the response to LPS, the reduced Ab production to NP-LPS immunization could be due to both a reduction in the number of MZB cells and their impaired response to LPS. Our results are consistent with the earlier finding by Lang et al. that FcμR-deficient mice had reduced LPS response in vivo (29). Choi et al. found elevated numbers of GC B cells and accelerated plasma cell formation during type 1 and 2 T-I immune responses and secondary T-D immune responses (31). In addition, the plasma cell formation in primary T-D immune response was also increased (summarized in Table 1). The reason for the discrepancies among results from different groups is unclear but could in part be attributable to the differences in the targeting strategy, the immunization protocol, and the genetic background as well as rearing environment of these mutant mice. Collectively, these results suggest that FcμR regulates humoral immune responses.
FcμR in Infectious Immunity
As summarized in Table 1, FcμR-deficient mice generated a higher titer of anti-phosphorylcholine Ab and a lower titer of anti-protein Ab than did WT mice when infected with a low dose of live non-encapsulated strain of Streptococcus pneumoniae (R36A) (32). However, a high dose of pathogen infection induced no significant difference in Ab production between WT and KO mice. We found that FcμR protected mice against sepsis induced by Citrobacter rodentium, a gram-negative bacterium that has LPS on the outer membrane (28). Similarly, Lang et al. found that the absence of FcμR resulted in limited cytokine production after Listeria monocytogenes (a gram-positive bacterium) infection and increased death of the infected KO mice (29). They also found that FcμR was required for the control of persistence-prone virus infection in a lymphocytic choriomeningitis virus model system (44). In addition, Yu et al. reported that FcμR deficiency resulted in increased numbers of IL-10–producing B cells, which mediated regulation of T cell immunity during influenza infection (45). On the contrary, Nguyen et al. found that FcμR expression on B cells, but not Fcα/uR expression or complement activation, was important for the antiviral IgG responses (48). B cell-specific KO mice lacked robust clonal expansion of influenza hemagglutinin-specific B cells early after infection and developed fewer IgG plasma cells and memory B cells in the spleen and BM, compared with WT mice (48). These results suggest that FcμR has important roles in B cell responses to protein and non-protein determinants of live pathogens and in cooperating with other immune cells to protect the mice against infection.
FcμR in B Cell Tolerance
B cell central tolerance ensures autoreactive immature B cells to undergo clonal deletion, anergy or receptor editing while peripheral tolerance functions to delete autoreactive B cells generated during GC reaction. We and others found that KO generated autoreactive antibodies including anti-dsDNA, rheumatoid factor, and anti-nuclear antibodies (27, 32, 33, 41, 45). Honjo et al. crossed FcμR-deficient mice with the Fas-deficient autoimmune-prone B6.MRL Faslpr/lpr mice (B6/lpr), and found that the double mutant mice had accelerated development of autoreactive Ab including anti-dsDNA and anti-Sm Ab (41). They also found enhanced formation of Mott cells, aberrant plasma cells which accumulate large amount of Ig in the rough endoplasmic reticulum, in KO mice. Nevertheless, KO mice with autoimmune-prone background have normal kidney function and equal mortality compared to control group (41). Brenner et al. reported that KO mice were protected from the development of severe experimental autoimmune encephalomyelitis (EAE), a mouse model for human multiple sclerosis. Their results suggested that FcμR regulated the function of dendritic and regulatory T cells (30). Collectively, a common feature of KO and BKO generated by different groups is the production of various autoantibodies (Table 1). It remains to be investigated how FcμR regulates B cell tolerance. We have shown that FcμR promotes B cell survival and activation by interacting with BCR and potentiating Ag-triggered BCR signaling (Figure 3, left panel). By analogy, we think that FcμR might also promote self Ag-triggered BCR signaling in immature B cells and contribute to the deletion/anergy of autoreactive immature B cells in the BM (Figure 3, right panel). Further studies are required to clarify whether and how FcμR contributes to B cell central or peripheral tolerance.
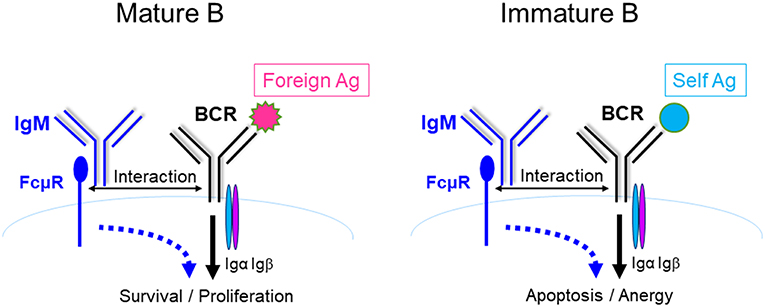
Figure 3. A model for FcμR-mediated immunity and tolerance. FcμR promotes the survival and activation of mature B cells by interacting with the BCR and potentiating foreign Ag-triggered BCR signaling (left). By analogy, FcμR might also promote self Ag-triggered BCR signaling in immature B cells and contribute to the deletion/anergy of autoreactive immature B cells in the BM (right). Ag-specific IgM/IgG are illustrated in the scheme shown in Figures 1, 2 and 4 to suggest that those reactions occur during an immune response. In contrast, IgM shown in this scheme is not Ag-specific to implicate that these reactions can occur in the absence of Ag-specific IgM.
Functional Relationship Between IgM-Complement and IgM-FcμR Pathways
IgM is the first Ab to appear during evolution and the only isotype produced by all species of jawed vertebrates (54–56). It is also the first isotype produced during a T-D immune response and is the first line of host defense (57). IgM is not only an effector molecule, but also regulates humoral immune response. Earlier studies suggested that IgM promotes the production of antigen-specific IgG via activating complement. However, a recent study by Heyman's group demonstrated that mice expressing a mutant IgM unable to activate complement (Cμ13) had completely normal humoral immune responses (58), thus raising the possibly that in addition to complement activation, there are alternative pathways by which IgM elicits its function. As discussed above, IgM can elicit its function through FcμR. Therefore, both IgM-FcμR and IgM-complement pathways function to regulate B cell survival and activation (Figure 4). It remains to be investigated whether these two pathways function cooperatively, independently, or competitively.
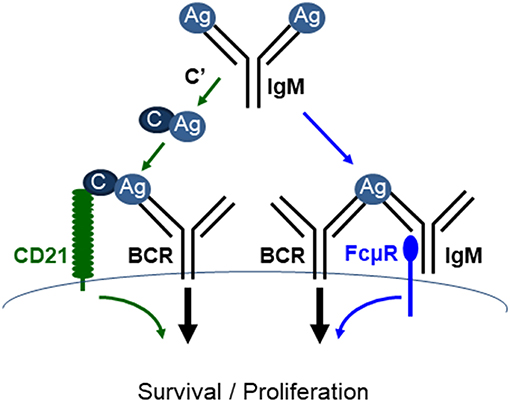
Figure 4. FcμR and complement receptor promote mature B cell survival and activation. IgM (pentamer; for simplicity a monomeric IgM is depicted) binds to antigens (Ag) and the resulting IgM-Ag complexes can enhance B cell survival and activation through at least two pathways. (1) IgM-Ag complexes can activate complement (C') cascade, resulting in C' fixation on the Ag, which can crosslink BCR and C' receptor (CD21) on B cells (green arrows). (2) IgM-Ag complexes can crosslink BCR and FcμR on B cells (blue arrows). It remains to be elucidated whether these two pathways function cooperatively, independently or competitively [adapted from Ouchida et al. (27)].
FcμR in Human Diseases
Human FcμR was shown to be overexpressed and associated with the anti-apoptotic characteristic in chronic lymphocytic leukemia (CLL) (59, 60). CLL is a malignancy of mature IgM+ B cells that exhibit features of polyreactive, partially anergized B cells related to memory B cells (60). Several studies showed that FCMR expression in CLL was significantly higher than that in healthy controls and other B cell lymphoproliferative diseases (59, 61–63). In addition, CLL patients also had higher serum titers of FcμR compared with healthy donors. The serum FcμR, a 40-kDa soluble form of the receptor generated by alternative splicing, was produced by both CLL B and non-CLL B cells (64). Cox regression analysis indicated that high expression of FCMR was an independent indicator for shorter treatment-free survival in CLL (64). Thus, FcμR is associated with the disease progression and patient survival and may serve as a prognostic factor. Interestingly, FcμR can even be used as a target for a more selective treatment of CLL by T cells expressing a chimeric antigen receptor (CAR-T), and initial studies have implicated a superior therapeutic index with anti-FcμR CAR-T cells for the treatment of CLL compared with the currently used therapies (65).
The reason that causes FcμR upregulation in CLL remains unclear. A negative correlation was observed between age and FcμR expression (59). In addition, overexpression of FCMR seemed to promote the chromosomal abnormalities (61). These shreds of evidence suggest that FcμR expression is related to the degree of genomic activity. Intriguingly, surface FcμR levels were also significantly elevated in the non-CLL B cells and T cells, suggesting that abnormal expression of FcμR is associated with systemic gene regulation (64). FcμR expression is significantly upregulated by BCR stimulation but decreased by CD40 ligation, which suggested that autoreactive BCR signaling as a key mediator of apoptosis resistance in CLL (63). Besides, FcμR expression on CLL cells is downmodulated at both the mRNA and protein levels by TLR7 and TLR9 agonists (60). This study also revealed that FcμR not only localized to the cell membrane but also accumulated in the trans-Golgi network (60). FcμR may internalize IgM-Ag complexes and thus serve as a receptor for the delivery of therapeutic Ab–drug conjugates into CLL cells (60). In addition, based on the findings in mice, human FcμR may have some roles in TNFα-mediated liver damage (47), malaria vaccine promotion (46), and the function of pancreatic islets (66).
Conclusion
IgM is an old immunoglobulin isotype, which can bind to Ag with high avidity and activate the complement cascade. Its authentic and specific Fc receptor (FcμR) is the last one to be explored after Fcα/μR and pIgR. Although there are some discrepancies regarding the function of FcμR published by different groups, the following common abnormal phenotypes have been observed: (1) alterations in B cell maturation and differentiation; (2) impaired humoral immune responses; (3) autoantibody production. In addition, FcμR appears to contribute to the initiation/progression of human CLL and has recently been tested as a therapeutic target for treating CLL. Yet still many questions remain to be answered, including the function of FcμR in the generation, maintenance and activation of memory B cells, and in host defense mediated by natural IgM produced by B-1 and Ag-specific IgM produced by B-2 cells. Further studies are required to fully uncover the function of FcμR in immunity and tolerance.
Author Contributions
JL provided a draft of the manuscript. YW completed the references. EX and RH provided all the figures. QL revised the manuscript. HO corrected the manuscript. J-YW designed the outline and made the final corrections of the manuscript.
Funding
This work was supported by the National Basic Research Program of China (2015CB943300 to J-YW), the National Natural Science Foundation of China (81571529, 81811540035, and 31870898 to J-YW), a postdoctoral grant (2018M641929 to JL), an International Joint Research Project FY2018 to JL, JSPS Bilateral Collaborations (Joint Research Projects to HO) and a grant-in-aid for scientific research (C) from Japan Society for the Promotion of Science (17K08878 to J-YW).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Luming Zhang for secretarial assistance, Fudan University Animal Facility for maintaining the mice, and the members in Wang lab for helpful suggestions.
References
1. Shibuya A, Sakamoto N, Shimizu Y, Shibuya K, Osawa M, Hiroyama T, et al. Fc alpha/mu receptor mediates endocytosis of IgM-coated microbes. Nat Immunol. (2000) 1:441–6. doi: 10.1038/80886
2. Shibuya A, Honda S. Molecular and functional characteristics of the Fcalpha/muR, a novel Fc receptor for IgM and IgA. Springer Semin Immunopathol. (2006) 28:377–82. doi: 10.1007/s00281-006-0050-3
3. Honda S, Sato K, Totsuka N, Fujiyama S, Fujimoto M, Miyake K, et al. Marginal zone B cells exacerbate endotoxic shock via interleukin-6 secretion induced by Fcalpha/muR-coupled TLR4 signalling. Nat Commun. (2016) 7:11498. doi: 10.1038/ncomms11498
4. Stadtmueller BM, Huey-Tubman KE, Lopez CJ, Yang Z, Hubbell WL, Bjorkman PJ. The structure and dynamics of secretory component and its interactions with polymeric immunoglobulins. eLife. (2016) 5:e10640. doi: 10.7554/eLife.10640
5. Schneeman TA, Bruno ME, Schjerven H, Johansen FE, Chady L, Kaetzel CS. Regulation of the polymeric Ig receptor by signaling through TLRs 3 and 4: linking innate and adaptive immune responses. J Immunol. (2005) 175:376–84. doi: 10.4049/jimmunol.175.1.376
6. Mostov KE. Transepithelial transport of immunoglobulins. Ann Rev Immunol. (1994) 12:63–84. doi: 10.1146/annurev.iy.12.040194.000431
7. Kaetzel CS, Robinson JK, Chintalacharuvu KR, Vaerman JP, Lamm ME. The polymeric immunoglobulin receptor (secretory component) mediates transport of immune complexes across epithelial cells: a local defense function for IgA. Proc Natl Acad Sci USA. (1991) 88:8796–800.
9. Kikutani H, Inui S, Sato R, Barsumian EL, Owaki H, Yamasaki K, et al. Molecular structure of human lymphocyte receptor for immunoglobulin E. Cell. (1986) 47:657–65.
10. Ikuta K, Takami M, Kim CW, Honjo T, Miyoshi T, Tagaya Y, et al. Human lymphocyte Fc receptor for IgE: sequence homology of its cloned cDNA with animal lectins. Proc Natl Acad Sci USA. (1987) 84:819–23.
11. Ludin C, Hofstetter H, Sarfati M, Levy CA, Suter U, Alaimo D, et al. Cloning and expression of the cDNA coding for a human lymphocyte IgE receptor. EMBO J. (1987) 6:109–14.
12. Moretta L, Ferrarini M, Durante ML, Mingari MC. Expression of a receptor for IgM by human T cells in vitro. Eur J Immunol. (1975) 5:565–9. doi: 10.1002/eji.1830050812
13. Lamon EW, Andersson B, Whitten HD, Hurst MM, Ghanta V. IgM complex receptors on subpopulations of murine lymphocytes. J Immunol. (1976) 116:1199–203.
14. Pichler WJ, Knapp W. Receptors for IgM-coated erythrocytes on chronic lymphatic leukemia cells. J Immunol. (1977) 118:1010–5.
15. Ferrarini M, Hoffman T, Fu SM, Winchester R, Kunkel HG. Receptors for IgM on certain human B lymphocytes. J Immunol. (1977) 119:1525–9.
16. Burns GF, Cawley JC, Barker CR. Characterization of the receptor for IgM present on human B lymphocytes. Immunology. (1979) 36:569–77.
17. Ohno T, Kubagawa H, Sanders SK, Cooper MD. Biochemical nature of an Fc mu receptor on human B-lineage cells. J Exp Med. (1990) 172:1165–75.
18. Kubagawa H, Oka S, Kubagawa Y, Torii I, Takayama E, Kang DW, et al. Identity of the elusive IgM Fc receptor (FcmuR) in humans. J Exp Med. (2009) 206:2779–93. doi: 10.1084/jem.20091107
19. Kubagawa H, Kubagawa Y, Jones D, Nasti TH, Walter MR, Honjo K. The old but new IgM Fc receptor (FcmuR). Curr Top Microbiol Immunol. (2014) 382:3–28. doi: 10.1007/978-3-319-07911-0_1
20. Kubagawa H, Oka S, Kubagawa Y, Torii I, Takayama E, Kang DW, et al. The long elusive IgM Fc receptor, FcmuR. J Clin Immunol. (2014) 34(Suppl. 1):S35–45. doi: 10.1007/s10875-014-0022-7
21. Engels N, Konig LM, Heemann C, Lutz J, Tsubata T, Griep S, et al. Recruitment of the cytoplasmic adaptor Grb2 to surface IgG and IgE provides antigen receptor-intrinsic costimulation to class-switched B cells. Nat Immunol. (2009) 10:1018–25. doi: 10.1038/ni.1764
22. Engels N, Wienands J. The signaling tool box for tyrosine-based costimulation of lymphocytes. Curr Opin Immunol. (2011) 23:324–9. doi: 10.1016/j.coi.2011.01.005
23. Wang H, Coligan JE, Morse HC III. Emerging functions of natural IgM and its Fc receptor FCMR in immune homeostasis. Front Immunol. (2016) 7:99. doi: 10.3389/fimmu.2016.00099
24. Honjo K, Kubagawa Y, Kearney JF, Kubagawa H. Unique ligand-binding property of the human IgM Fc receptor. J Immunol. (2015) 194:1975–82. doi: 10.4049/jimmunol.1401866
25. Shima H, Takatsu H, Fukuda S, Ohmae M, Hase K, Kubagawa H, et al. Identification of TOSO/FAIM3 as an Fc receptor for IgM. Int Immunol. (2010) 22:149–56. doi: 10.1093/intimm/dxp121
26. Kubagawa H, Skopnik CM, Zimmermann J, Durek P, Chang HD, Yoo E, et al. Authentic IgM Fc Receptor (FcmuR). Curr Top Microbiol Immunol. (2017) 408:25–45. doi: 10.1007/82_2017_23
27. Ouchida R, Mori H, Hase K, Takatsu H, Kurosaki T, Tokuhisa T, et al. Critical role of the IgM Fc receptor in IgM homeostasis, B-cell survival, and humoral immune responses. Proc Natl Acad Sci USA. (2012) 109:E2699–706. doi: 10.1073/pnas.1210706109
28. Liu J, Zhu H, Qian J, Xiong E, Zhang L, Wang YQ, et al. Fcmicro receptor promotes the survival and activation of marginal zone B cells and protects mice against bacterial sepsis. Front Immunol. (2018) 9:160. doi: 10.3389/fimmu.2018.00160
29. Lang KS, Lang PA, Meryk A, Pandyra AA, Boucher LM, Pozdeev VI, et al. Involvement of Toso in activation of monocytes, macrophages, and granulocytes. Proc Natl Acad Sci USA. (2013) 110:2593–8. doi: 10.1073/pnas.1222264110
30. Brenner D, Brustle A, Lin GH, Lang PA, Duncan GS, Knobbe-Thomsen CB, et al. Toso controls encephalitogenic immune responses by dendritic cells and regulatory T cells. Proc Natl Acad Sci USA. (2014) 111:1060–5. doi: 10.1073/pnas.1323166111
31. Choi SC, Wang H, Tian L, Murakami Y, Shin DM, Borrego F, et al. Mouse IgM Fc receptor, FCMR, promotes B cell development and modulates antigen-driven immune responses. J Immunol. (2013) 190:987–96. doi: 10.4049/jimmunol.1202227
32. Honjo K, Kubagawa Y, Jones DM, Dizon B, Zhu Z, Ohno H, et al. Altered Ig levels and antibody responses in mice deficient for the Fc receptor for IgM (FcmuR). Proc Natl Acad Sci USA. (2012) 109:15882–7. doi: 10.1073/pnas.1206567109
33. Nguyen TT, Klasener K, Zurn C, Castillo PA, Brust-Mascher I, Imai DM, et al. The IgM receptor FcmuR limits tonic BCR signaling by regulating expression of the IgM BCR. Nat Immunol. (2017) 18:321–33. doi: 10.1038/ni.3677
34. Shibuya A, Honda S. Immune regulation by Fcalpha/mu receptor (CD351) on marginal zone B cells and follicular dendritic cells. Immunol Rev. (2015) 268:288–95. doi: 10.1111/imr.12345
35. Shibuya A, Honda SI, Shibuya K. A pro-inflammatory role of Fcalpha/muR on marginal zone B cells in sepsis. Int Immunol. (2017) 29:519–24. doi: 10.1093/intimm/dxx059
36. Turula H, Wobus CE. The role of the polymeric immunoglobulin receptor and secretory immunoglobulins during mucosal infection and immunity. Viruses. (2018) 10:E237. doi: 10.3390/v10050237
37. Hitoshi Y, Lorens J, Kitada SI, Fisher J, LaBarge M, Ring HZ, et al. Toso, a cell surface, specific regulator of Fas-induced apoptosis in T cells. Immunity. (1998) 8:461–71.
38. Honjo K, Kubagawa Y, Kubagawa H. Is toso an antiapoptotic protein or an Fc receptor for IgM? Blood. (2012) 119:1789–90. doi: 10.1182/blood-2011-09-380782
39. Ouchida R, Mori H, Ohno H, Wang JY. FcmuR (Toso/Faim3) is not an inhibitor of Fas-mediated cell death in mouse T and B cells. Blood. (2013) 121:2368–70. doi: 10.1182/blood-2012-12-470906
40. Ouchida R, Lu Q, Liu J, Li Y, Chu Y, Tsubata T, et al. FcmuR interacts and cooperates with the B cell receptor To promote B cell survival. J Immunol. (2015) 194:3096–101. doi: 10.4049/jimmunol.1402352
41. Honjo K, Kubagawa Y, Suzuki Y, Takagi M, Ohno H, Bucy RP, et al. Enhanced auto-antibody production and Mott cell formation in FcmuR-deficient autoimmune mice. Int Immunol. (2014) 26:659–72. doi: 10.1093/intimm/dxu070
42. Ding Z, Bergman A, Rutemark C, Ouchida R, Ohno H, Wang JY, et al. Complement-activating IgM enhances the humoral but not the T cell immune response in mice. PLoS ONE. (2013) 8:e81299. doi: 10.1371/journal.pone.0081299
43. Murakami Y, Narayanan S, Su S, Childs R, Krzewski K, Borrego F, et al. Toso, a functional IgM receptor, is regulated by IL-2 in T and NK cells. J Immunol. (2012) 189:587–97. doi: 10.4049/jimmunol.1200840
44. Lang PA, Meryk A, Pandyra AA, Brenner D, Brustle A, Xu HC, et al. Toso regulates differentiation and activation of inflammatory dendritic cells during persistence-prone virus infection. Cell Death Differ. (2015) 22:164–73. doi: 10.1038/cdd.2014.138
45. Yu J, Duong VHH, Westphal K, Westphal A, Suwandi A, Grassl GA, et al. Surface receptor Toso controls B cell-mediated regulation of T cell immunity. J Clin Invest. (2018) 128:1820–36. doi: 10.1172/jci97280
46. Lapke N, Tartz S, Lee KH, Jacobs T. The application of anti-Toso antibody enhances CD8(+) T cell responses in experimental malaria vaccination and disease. Vaccine. (2015) 33:6763–70. doi: 10.1016/j.vaccine.2015.10.065
47. Nguyen XH, Lang PA, Lang KS, Adam D, Fattakhova G, Foger N, et al. Toso regulates the balance between apoptotic and non-apoptotic death receptor signaling by facilitating RIP1 ubiquitination. Blood. (2011) 118:598–608. doi: 10.1182/blood-2010-10-313643
48. Nguyen TTT, Graf BA, Randall TD, Baumgarth N. sIgM-FcmuR interactions regulate early B cell activation and plasma cell development after influenza virus infection. J Immunol. (2017) 199:1635–46. doi: 10.4049/jimmunol.1700560
49. Schwickert TA, Tagoh H, Gultekin S, Dakic A, Axelsson E, Minnich M, et al. Stage-specific control of early B cell development by the transcription factor Ikaros. Nat Immunol. (2014) 15:283–93. doi: 10.1038/ni.2828
50. Yu M, Chen Y, Zeng H, Zheng Y, Fu G, Zhu W, et al. PLCgamma-dependent mTOR signalling controls IL-7-mediated early B cell development. Nat Commun. (2017) 8:1457. doi: 10.1038/s41467-017-01388-5
51. Schweighoffer E, Vanes L, Nys J, Cantrell D, McCleary S, Smithers N, et al. The BAFF receptor transduces survival signals by co-opting the B cell receptor signaling pathway. Immunity. (2013) 38:475–88. doi: 10.1016/j.immuni.2012.11.015
52. Notley CA, Baker N, Ehrenstein MR. Secreted IgM enhances B cell receptor signaling and promotes splenic but impairs peritoneal B cell survival. J Immunol. (2010) 184:3386–93. doi: 10.4049/jimmunol.0902640
53. Baker N, Ehrenstein MR. Cutting edge: selection of B lymphocyte subsets is regulated by natural IgM. J Immunol. (2002) 169:6686–90. doi: 10.4049/jimmunol.169.12.6686
54. Fellah JS, Wiles MV, Charlemagne J, Schwager J. Evolution of vertebrate IgM: complete amino acid sequence of the constant region of Ambystoma mexicanum mu chain deduced from cDNA sequence. Eur J Immunol. (1992) 22:2595–601. doi: 10.1002/eji.1830221019
56. Ehrenstein MR, Notley CA. The importance of natural IgM: scavenger, protector and regulator. Nat Rev Immunol. (2010) 10:778–86. doi: 10.1038/nri2849
57. Dorner T, Radbruch A. Antibodies and B cell memory in viral immunity. Immunity. (2007) 27:384–92. doi: 10.1016/j.immuni.2007.09.002
58. Rutemark C, Alicot E, Bergman A, Ma M, Getahun A, Ellmerich S, et al. Requirement for complement in antibody responses is not explained by the classic pathway activator IgM. Proc Natl Acad Sci USA. (2011) 108:E934–42. doi: 10.1073/pnas.1109831108
59. Yi S, Yu Z, Zhou K, Wang Y, An G, Li Z, et al. TOSO is overexpressed and correlated with disease progression in Chinese patients with chronic lymphocytic leukemia. Leuk Lymphom. (2011) 52:72–8. doi: 10.3109/10428194.2010.531411
60. Vire B, David A, Wiestner A. TOSO, the Fcmicro receptor, is highly expressed on chronic lymphocytic leukemia B cells, internalizes upon IgM binding, shuttles to the lysosome, and is downregulated in response to TLR activation. J Immunol. (2011) 187:4040–50. doi: 10.4049/jimmunol.1100532
61. Hancer VS, Diz-Kucukkaya R, Aktan M. Overexpression of Fc mu receptor (FCMR, TOSO) gene in chronic lymphocytic leukemia patients. Med Oncol. (2012) 29:1068–72. doi: 10.1007/s12032-011-9821-3
62. Proto-Siqueira R, Panepucci RA, Careta FP, Lee A, Clear A, Morris K, et al. SAGE analysis demonstrates increased expression of TOSO contributing to Fas-mediated resistance in CLL. Blood. (2008) 112:394–7. doi: 10.1182/blood-2007-11-124065
63. Pallasch CP, Schulz A, Kutsch N, Schwamb J, Hagist S, Kashkar H, et al. Overexpression of TOSO in CLL is triggered by B-cell receptor signaling and associated with progressive disease. Blood. (2008) 112:4213–9. doi: 10.1182/blood-2008-05-157255
64. Li FJ, Kubagawa Y, McCollum MK, Wilson L, Motohashi T, Bertoli LF, et al. Enhanced levels of both the membrane-bound and soluble forms of IgM Fc receptor (FcmuR) in patients with chronic lymphocytic leukemia. Blood. (2011) 118:4902–9. doi: 10.1182/blood-2011-04-350793
65. Faitschuk E, Hombach AA, Frenzel LP, Wendtner CM, Abken H. Chimeric antigen receptor T cells targeting Fc mu receptor selectively eliminate CLL cells while sparing healthy B cells. Blood. (2016) 128:1711–22. doi: 10.1182/blood-2016-01-692046
Keywords: IgM, FcμR, BCR signal, humoral immune response, complement
Citation: Liu J, Wang Y, Xiong E, Hong R, Lu Q, Ohno H and Wang J-Y (2019) Role of the IgM Fc Receptor in Immunity and Tolerance. Front. Immunol. 10:529. doi: 10.3389/fimmu.2019.00529
Received: 30 September 2018; Accepted: 27 February 2019;
Published: 22 March 2019.
Edited by:
Gestur Vidarsson, Sanquin Research, NetherlandsReviewed by:
Jorg Hermann Fritz, McGill University, CanadaLars Torkel Hellman, Uppsala University, Sweden
Renato C. Monteiro, Paris Diderot University, France
Copyright © 2019 Liu, Wang, Xiong, Hong, Lu, Ohno and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ji-Yang Wang, wang@fudan.edu.cn
 Jun Liu
Jun Liu Ying Wang
Ying Wang Ermeng Xiong
Ermeng Xiong Rongjian Hong
Rongjian Hong Qing Lu1
Qing Lu1 Hiroshi Ohno
Hiroshi Ohno Ji-Yang Wang
Ji-Yang Wang