
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol., 05 February 2019
Sec. Immunological Tolerance and Regulation
Volume 10 - 2019 | https://doi.org/10.3389/fimmu.2019.00150
 Desiree J. Wendering1,2,3
Desiree J. Wendering1,2,3 Leila Amini1,2,3
Leila Amini1,2,3 Stephan Schlickeiser1,3
Stephan Schlickeiser1,3 Petra Reinke2,3
Petra Reinke2,3 Hans-Dieter Volk1,2,3
Hans-Dieter Volk1,2,3 Michael Schmueck-Henneresse1,2,3*
Michael Schmueck-Henneresse1,2,3*CD4+CD25+FoxP3+ human regulatory TCELLS (TREG) are promising candidates for reshaping undesired immunity/inflammation by adoptive cell transfer, yet their application is strongly dependent on robust assays testing their functionality. Several studies along with first clinical data indicate TREG to be auspicious to use for future cell therapies, e.g., to induce tolerance after solid organ transplantation. To this end, TREG suppressive capacity has to be thoroughly evaluated prior to any therapeutic application. A 7 h-protocol for the assessment of TREG function by suppression of the early activation markers CD154 and CD69 on CD4+CD25− responder TCELLS (TRESP) upon polyclonal stimulation via αCD3/28-coated activating microbeads has previously been published. Even though this assay has since been applied by various groups, the protocol comes with a critical pitfall, which is yet not corrected by the journal of its original publication. Our results demonstrate that the observed decrease in activation marker frequency on TRESP is due to competition for αCD3/28-coated microbeads as opposed to a TREG-attributable effect and therefore the protocol cannot further be used as a diagnostic test to assess suppressive TREG function.
Regulatory TCELLS (TREG) are key players in maintaining immune homeostasis, resolution of inflammation, and self (1). Exploiting those characteristics, TREG have gained plenty of attention as promising candidates in immunotherapeutic applications for the prevention or reshaping of undesired immune responses such as in autoimmune diseases, chronic inflammation, and allograft rejections. Data from clinical trials identify TREG as an encouraging cell type for use in cellular therapy (2). By the same token, a robust protocol to assess TREG function is of utmost importance to ensure their suppressive function prior to adoptive cell-therapeutic clinical trials, as well as for application in basic research. So far, for assessing TREG functionality, evaluating the suppressive capacity of TREG to inhibit the proliferation of responder TCELL (TRESP) after a 4-day co-cultivation period has been the gold-standard protocol since a decade (3, 4). Recently, Canavan et al. (5) and Ruitenberg et al. (6) described a rapid 7 h assay for the evaluation of TREG functionality by assessing their suppressive capacity using upregulation of the early TCELL activation makers CD154 (CD40L) and CD69 on conventional CD4+CD25− responder TCELLS (TRESP) upon CD3/28 engagement. CD3/28 stimulation is mediated by microbeads coupled with αCD3 and αCD28 antibodies. According to these studies, TREG alleviate CD154 and CD69 expression on TRESP in a dose-dependent manner. Even though this assay has since been frequently applied and cited more than 80 times (7, 8, 10), we observed that the protocol comes with a critical pitfall: TRESP and TREG both express the signaling molecule CD3 and TCELL co-stimulatory receptor CD28 on the plasma membrane, potentially competing for binding αCD3/28 TCELL activating microbeads applied in the rapid 7 h assay. We investigated whether the observed decreased frequencies of activated TRESP can be claimed to be a TREG-attributable effect or if it is rather a result of competition for αCD3/28-coated activating microbeads. We thus explored whether different ratios of αCD3/28 TCELL activation microbeads-to-TCELLS impact the outcome of this functional TREG assay.
The aim of this study was to investigate the influence of αCD3/CD28-coated activating microbeads on the expression of early activation markers CD69 and CD154, used for predicting TREG functionality in basic and translational research. We compared the expression of CD69 and CD154 of TRESP in TREG co-cultures, which were either activated via αCD3/CD28-coated microbeads adjusted to TRESP only or to the total cell number present in one well (TRESP + TREG). To verify the integrity of the TREG used in this study, as well as to demonstrate the TREG-mediated suppressive function in a bead-uncompetitive setting, TRESP proliferation suppression experiments were performed.
Peripheral blood mononuclear cells from healthy donors were purified using Ficoll-Paque separation (Biochrom). CD4+ cells were enriched by magnetic-activated cell sorting (Miltenyi) according to manufacturer's instructions (purity>90%). For fluorescence-activated cell sorting (FACS Aria II, BD) of CD4+CD25highCD127low TREG and CD4+CD25− TRESP, cells were stained with CD4 (SK3, Biolegend), CD25 (2A3, BD), and CD127 (R34.34, Beckman Coulter). Post-FACSort analysis by flow cytometry yielded CD25+FoxP3+ TCELL purity of >95%.
Assays were performed as described by Canavan et al. (5). Briefly, CFSE-labeled TRESP were co-cultured with autologous TREG at TRESP/TREG ratios ranging from 1:1 to 32:1. In two parallel setups, cells were either stimulated with αCD3/28-coated microbeads (Dynabeads® Human T-Activator CD3/CD28, Thermo Fisher Scientific) at a bead/cell ratio of 0.2 adjusted to the TRESP cell number per well (5, 6) or adapting the ratio of 0.2 to the total cell number per well including TREG. Stimulated and unstimulated TRESP without TREG were included as controls. For the microbead titration, TRESP were cultured alone at bead/TRESP ratios ranging from 0.1 to 0.4 (mimicking the presence of TREG). αCD154 (24–31) was added at start of incubation. Cells were incubated at 37°C for 7 h. All cell cultures were performed in X-Vivo-15 medium supplemented with 10% FCS (Lonza & Biochrom) and 100 IU/ml Penicillin/Streptomycin. After harvesting, cells were stained with CD3 (OKT3), CD4 (SK3), CD137 (4B4-4), and CD69 (FN50), all Biolegend. Dead cells were excluded (LIFE/DEADTM Fixable Blue Dead Cell Stain Kit, Thermo Fisher Scientific).
CFSE-labeled TRESP were cultured alone or with autologous TREGS at TRESP/TREG ratios ranging from 1:1 to 16:1. The cells were stimulated with αCD3/28-coated microbeads (TREG Suppression Inspector, Miltenyi) at a cell/bead ratio of 1:1 and 1:2 adjusted to the total cell number per well and incubated at 37°C for 96 h. Thereafter, cells were stained with CD3 (OKT3), CD4 (SK3), all Biolegend. Dead cells were excluded (Thermo Fisher Scientific). Proliferation was assessed by CFSE dilution and percentage suppression of proliferation was calculated by relating the percentage of proliferating TRESP in the presence and absence of TREG, respectively.
Data were acquired on a LSR-II Fortessa flow cytometer (BD) and analyzed using FlowJo V10 (TreeStar).
Analysis was performed with GraphPad Prism software (version 6, GraphPad, La Jolla, CA) and R (version 3.4.1) (9). We have tested for significant interaction, i.e., non-parallel response profiles of the two bead adjustment methods to the different TRESP:TREG ratios, using a non-parametric rank-based ANOVA-type statistic [as implemented in the nparLD package (11)] in a two-way factorial repeated measures design. For bead titration experiments, non-parametric two-tailed Wilcoxon matched-pairs signed rank tests were used to determine significance in pairwise comparison. Data indicate means ± SEMs in all bar graphs. P < 0.05 was considered significant.
We first examined TREG functionality according to the protocols published by Canavan et al. (5) and Ruitenberg et al. (6), whereby ex vivo FACSorted and CFSE-labeled TRESP were co-cultured in the presence and absence of autologous TREG and stimulated with αCD3/28-coated activating microbeads at a ratio of 0.2 microbeads per TRESP (Figure 1A). After 7 h, the mean frequency of CD154+ and CD69+ TCELLS of unstimulated TRESP was 0.14 and 0.45%, respectively and 57.25 and 78.26% on CD3/28-stimulated TRESP, respectively (Figure 1B). When TRESP were stimulated in the presence of TREG at ratio 1:1, the mean frequency of CD154+ and CD69+ TCELLS decreased to 47.77 and 69.86%, respectively. With increasing TRESP/TREG ratios both, CD154 and CD69 expression, increased in a linear fashion (Figure 1C, quantified in E, F, red columns). We next determined whether the total TCELL/bead ratio influences TREG-induced activation marker suppression. Accordingly, we adjusted the bead numbers to the total cell numbers, including TREG, thereby eluding the bead competition in contrast to Canavan et al. (5) and Ruitenberg et al. (6). In that case, TRESP activation in the presence of TREG equaled control TRESP cultures without TREG (Figure 1D, quantified in E, F, blue bars), indicating that indeed TRESP and TREG compete for CD3/28-binding microbeads. Serving as a negative control, we co-cultured TRESP with CD4+CD25− non-TREG/effector TCELLS in place of TREG. When the bead number was adjusted to TRESP only we observed similar reductions of CD154 and CD69 expression (Figures 1G,H, red bars) as when TRESP were co-cultured with TREG (Figures 1E,F, red bars). Correspondingly, when adjusting the bead number to the total cell number (Figures 1E,H, blue bars), the expression of CD154 and CD69 is similar to the conditions with TRESP only (Figures 1E–H, gray bars). To mimic the competition for the activating microbead stimuli, we stimulated TRESP with different amounts of αCD3/28-coated microbeads in the absence of TREG. We set the actual bead/TCELL ratio according to the published TRESP/TREG co-culture approach, in which the activation bead/TRESP ratio is adjusted to TRESP only, i.e., calculated the actual bead/TCELL ratio in each setting. CD154 and CD69 expression decreased in a dose-dependent manner with highest expression levels at a bead/TRESP ratio of 0.4 (69.83 and 89.47%, respectively) and lowest at a ratio of 0.1 (37.80 and 53.33%, respectively). The TRESP activation pattern with the different bead ratios ranging from 0.1 to 0.194 indicate a strong bead/TRESP ratio dependency (Figures 1I,J).
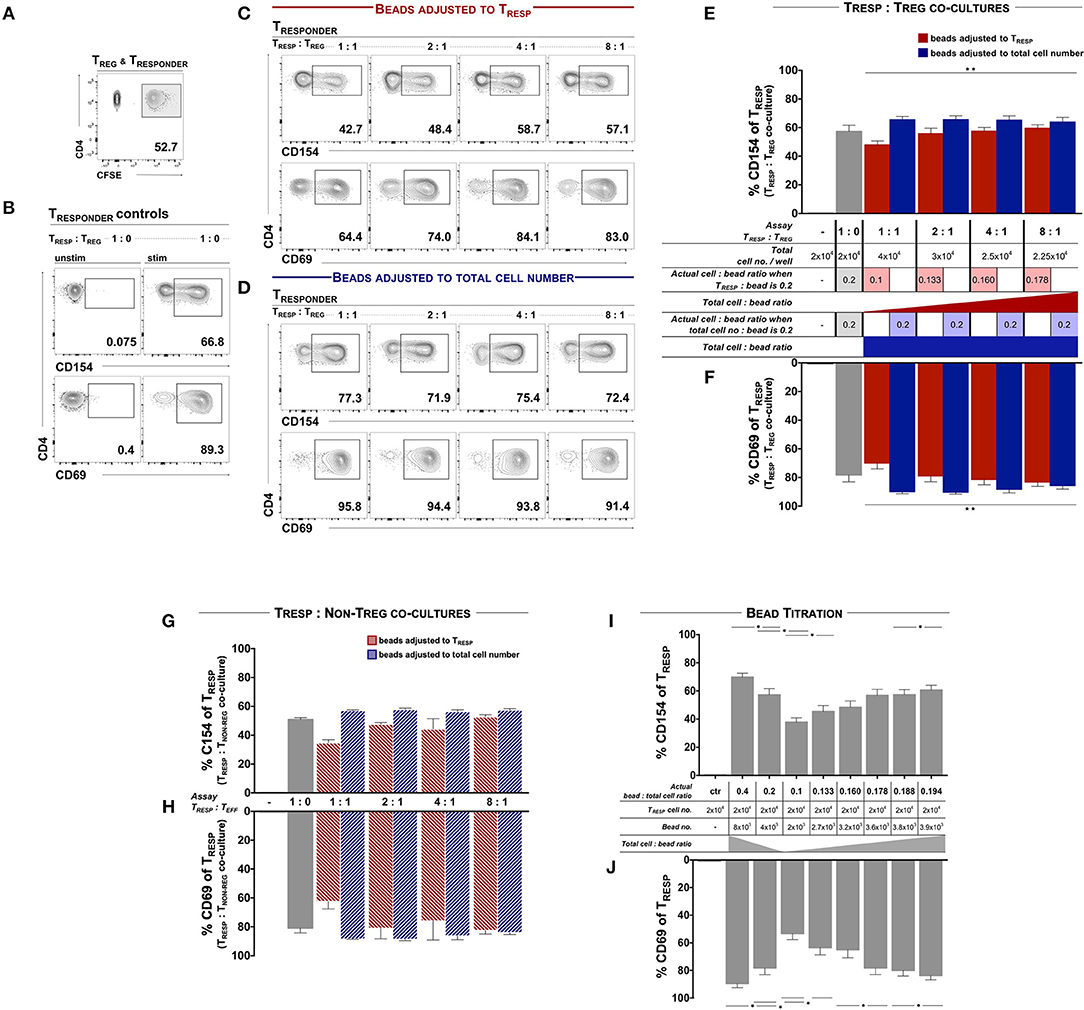
Figure 1. TCELL early activation marker expression is dependent of TCR engagement and cannot be used for TREG functional evaluation. FACSorted CD4+CD25− TRESP with and without autologous TREG co-culture were stimulated with anti-CD3/CD28-coated microbeads and analyzed for early activation marker expression. (A) For precise TRESP/TREG discrimination, TRESP were labeled with CFDA-SE (CFSE). (B) Representative plots of CD154 and CD69 expression on unstimulated and stimulated TRESP cultured without TREG. (C) Representative plots of CD154 and CD69 expression of TRESP co-cultured with TREG at different TRESP:TREG ratios and stimulated with anti-CD3/CD28-coated microbeads adjusted to TRESP. (D) Representative plots of CD154 and CD69 expression of TRESP co-cultured with TREG at different TRESP:TREG ratios and stimulated with anti-CD3/CD28-coated microbeads adjusted to total cell number. (E,F) Quantified data from (C,D), respectively. CD154 and CD69 of CFSE+TRESP co-cultured with FACSorted TREG at different TRESP:TREG ratios and stimulated with anti-CD3/CD28-coated microbeads adjusted to TRESP (red columns) and to total cell numbers (blue columns). For clarification, the table summarizes the experimental setups. n = 7. Non-parametric rank-based ANOVA-type statistic **p < 0.001 (CD154: p = 1.90E-06, CD69: p = 5.527256E-16). (G) Expression of CD154 and (H) expression of CD69 of CFSE+TRESP co-cultured with FACSorted TNON−TREG at different TRESP:TREG ratios and stimulated with anti-CD3/CD28-coated microbeads adjusted to TRESP (red columns) and to total cell numbers (blue columns). n = 3. (I) Expression of CD154 and (J) expression of CD69 of CFSE+TRESP after different anti-CD3/CD28-coated microbead:TRESP ratio stimulation. For clarification, the table summarizes the experimental setups. n = 7. *p < 0.05, Wilcoxon matched-pairs signed rank test. TRESP:TREG/TNON−TREG co-cultures (E,F) and corresponding bead titration (I,J) experiments were performed simultaneously using the same donor cells. Median data of independent experiments are shown and error bars represent SEM.
To confirm TREG functionality in an environment where the number of αCD3/28-activation microbeads is adjusted to the total cell number, the gold-standard TRESP proliferation suppression assay was performed. The proliferation assay was conducted with TCELLS of the same donors in parallel to the experiments shown in Figure 1. Following activation, TRESP proliferation alone yielded 52.03% and dose-dependently decreased in the presence of TREG to 15.51% at a TRESP/TREG ratio of 1:1 (Figures 2A–C, quantified in Figure 2D, green bars). Thus, we conclude that the TREG employed in this study are able to suppress TRESP proliferation in a standardized bead-competitive setting. To ascertain the reduction of proliferation to be TREG-mediated, we have added non-TREG/effector TCELLS instead of TREG to TRESP and observed no decrease in TRESP proliferation, indicating the suppression of TRESP proliferation to be a TREG-attributable effect (Figure 2D, blue bars). Even when TCELLS are stimulated with twice the number of activating αCD3/CD28 microbeads, the TREG-specific impact in suppressing TRESP proliferation can be seen (Figure 2E).
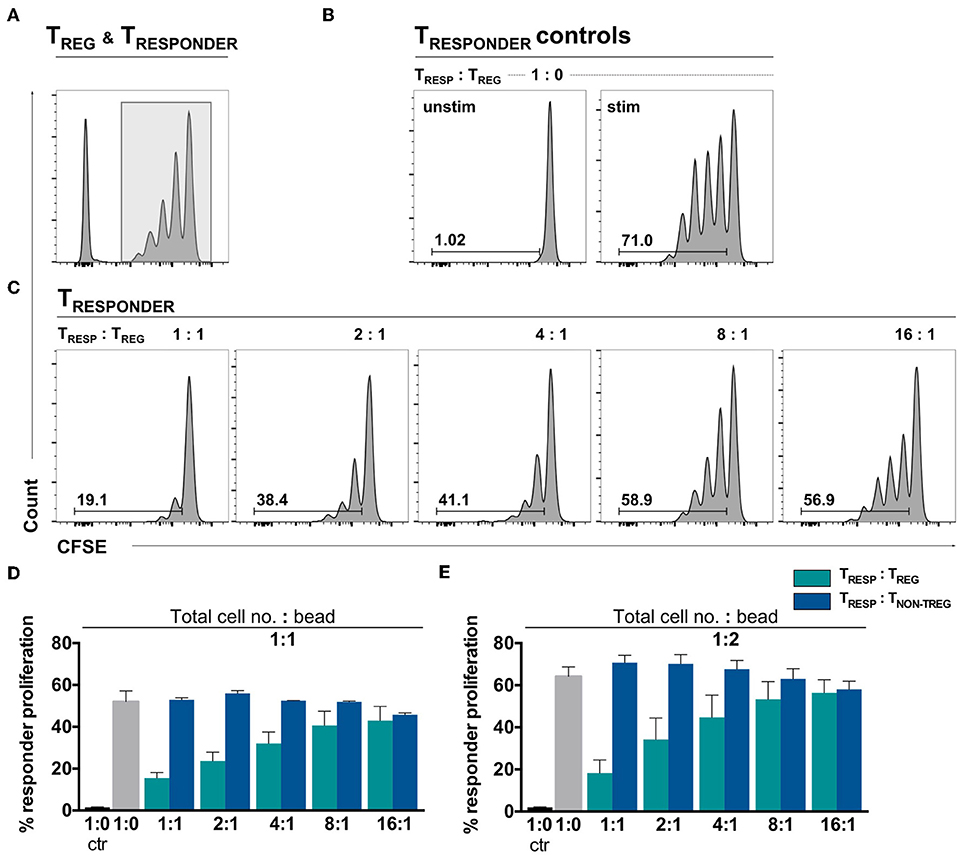
Figure 2. Ex vivo isolated TREG demonstrate a dose-dependent TRESP proliferation suppression in a bead-uncompetitive setting. FACSorted CFSE+CD4+CD25− TRESP were co-cultured with autologous FACSorted TREG and stimulated with anti-CD3/CD28-coated microbeads for 96 h. TRESP proliferation was analyzed by CFSE dilution. Representative plots depicting (A) CFSE-labeling strategy to accurately analyze TRESP proliferation; (B) proliferation of unstimulated and stimulated CFSE+TRESP cultured without TREG and (C) CFSE+TRESP proliferation after co-culture with different TREG ratios. (D) Percentage of TRESP proliferation after co-culture with decreasing TRESP:TREG ratios (green bars) and TRESP:TNON-TREG ratios (blue bars) stimulated with a total cell number:bead ratio of 1:1. n = 7 TRESP:TREG co-cultures, n = 3 TRESP:TNON−TREG co-cultures. (E) Percentage of TRESP proliferation after co-culture with decreasing TRESP:TREG ratios (green bars) and TRESP:TNON-TREG ratios (blue bars) stimulated with a total cell number:bead ratio of 1:2. n = 3. Median data of independent experiments are shown and error bars represent SEM.
In conclusion, when adjusting the αCD3/28-bead numbers to only TRESP in co-cultures of TRESP and TREG, activation marker expression was comparable to approaches where TRESP were cultured alone at same bead/total cell ratio present in the TRESP/TREG co-culture. When normalizing αCD3/28-bead competition by adjusting the bead number to total cell numbers, TREG-mediated suppression of activation marker upregulation is nullified. Even more strikingly, when titrating non-TREG/effector TCELLS to TRESP and adjusting the αCD3/28-bead numbers to TRESP only, we observe the same decrease in activation marker expression as in TRESP:TREG co-cultures. We thereby demonstrate that the suppression of activation marker expression on TRESP observed in co-cultures with TREG are due to competitive TCELL receptor and CD28 engagement limited by αCD3/28 microbead availability rather than by suppressive activity of TREG (Supplementary Figure 1). There is a pressing demand for a fast assay to evaluate TREG functionality, especially in the light of upcoming clinical trials needing a robust diagnostic test to assess the suppressive function as a release criterion for their TREG cell products. Nonetheless, the TRESP proliferation suppression analysis should still be considered as the gold-standard TREG functional assay as it is performed by adjusting the activation bead to TCELL ratios in experimental setups with decreasing TREG cell numbers (to assess TREG dose-dependent suppression). Since we firmly believe that activation bead to TCELL receptor competition should be kept constant throughout all conditions within a TREG functional assay, we claim that the rapid assessment for human TREG function proposed by Canavan et al. (5) and Ruitenberg et al. (6) does not result in reliable evidence of functional suppression since the putative TREG-mediated suppression of TRESP activation is to be ascribed to competitive TCELL receptor and CD28 engagement. Hence, we suggest that the previously published protocol is unsuitable as a diagnostic test to assess suppressive TREG function.
The Charité Ethics Committee (IRB) approved the study protocol and all blood donors provided written informed consent.
DW designed the research, performed experiments, analyzed and interpreted the data, and wrote the manuscript. LA performed experiments and revised the manuscript. SS performed statistical analyses. PR revised the manuscript. H-DV interpreted the data and revised the manuscript. MS-H led the project, designed the research, analyzed and interpreted the data, and wrote the manuscript.
The study was generously supported in parts by the Deutsche Forschungsgemeinschaft (DFG-SFB-TR36-project A3–H-DV, PR, and MS-H) and the German Federal Ministry of Education and Research (Berlin-Brandenburg Center for Regenerative Therapies grant—all authors). We acknowledge support from the German Research Foundation (DFG) and the Open Access Publication Fund of Charité—Universitätsmedizin Berlin.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We acknowledge the assistance of the BCRT Flow Cytometry Core Lab, Dr. D. Kunkel and J. Hartwig. Mathias Streitz for flow cytometry assistance. Karolina Grzeschik and Anke Jurisch for technical assistance. Dr. Toralf Roch for critical discussions. We likewise acknowledge support from the German Research Foundation (DFG) and the Open Access Publication Fund of Charité — Universitätsmedizin Berlin.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2019.00150/full#supplementary-material
1. Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell (2008) 133:775–87. doi: 10.1016/j.cell.2008.05.009
2. Desreumaux P, Foussat A, Allez M, Beaugerie L, Hébuterne X, Bouhnik Y, et al. Safety and efficacy of antigen-specific regulatory T-cell therapy for patients with refractory Crohn's disease. Gastroenterology (2012) 143:1207–17.e1–2. doi: 10.1053/j.gastro.2012.07.116
3. Brusko TM, Hulme MA, Myhr CB, Haller MJ, Atkinson MA. Assessing the in vitro suppressive capacity of regulatory T cells. Immunol Invest. (2007) 36:607–28. doi: 10.1080/08820130701790368
4. Venken K, Thewissen M, Hellings N, Somers V, Hensen K, Rummens J-L, et al. A CFSE based assay for measuring CD4+CD25+ regulatory T cell mediated suppression of auto-antigen specific and polyclonal T cell responses. J Immunol Methods (2007) 322:1–11. doi: 10.1016/j.jim.2007.01.025
5. Canavan JB, Afzali B, Scottà C, Fazekasova H, Edozie FC, Macdonald TT, et al. A rapid diagnostic test for human regulatory T-cell function to enable regulatory T-cell therapy. Blood (2012) 119:e57–66. doi: 10.1182/blood-2011-09-380048
6. Ruitenberg JJ, Boyce C, Hingorani R, Putnam A, Ghanekar SA. Rapid assessment of in vitro expanded human regulatory T cell function. J Immunol Methods (2011) 372:95–106. doi: 10.1016/j.jim.2011.07.001
7. Landwehr-Kenzel S, Issa F, Luu S-H, Schmück M, Lei H, Zobel A, et al. Novel GMP-compatible protocol employing an allogeneic B cell bank for clonal expansion of allospecific natural regulatory T cells. Am J Transplant. (2014) 14:594–606. doi: 10.1111/ajt.12629
8. Pedersen AE, Holmstrøm K, Jørgensen F, Jensen SS, Gad M. Development of assay platforms for in vitro screening of Treg modulating potential of pharmacological compounds. Immunopharmacol Immunotoxicol. (2014) 37:63–71. doi: 10.3109/08923973.2014.977449
9. R C Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing (2017).
10. Ruhnau J, Schulze J, von Sarnowski B, Heinrich M, Langner S, Pötschke C, et al. Reduced numbers and impaired function of regulatory T cells in peripheral blood of ischemic stroke patients. Mediat Inflamm. (2016) 2016:2974605. doi: 10.1155/2016/2974605
Keywords: regulatory T cell functional assay, αCD3/28-coated microbeads, competitive CD3/CD28 binding, nullified Treg-mediated suppression, correlation between T cell-to-αCD3/CD28-coated microbead ratio and activation marker frequency on responder T cells
Citation: Wendering DJ, Amini L, Schlickeiser S, Reinke P, Volk H-D and Schmueck-Henneresse M (2019) The Value of a Rapid Test of Human Regulatory T Cell Function Needs to be Revised. Front. Immunol. 10:150. doi: 10.3389/fimmu.2019.00150
Received: 01 November 2018; Accepted: 17 January 2019;
Published: 05 February 2019.
Edited by:
Lucienne Chatenoud, Université Paris Descartes, FranceReviewed by:
Karin Loser, University of Münster, GermanyCopyright © 2019 Wendering, Amini, Schlickeiser, Reinke, Volk and Schmueck-Henneresse. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michael Schmueck-Henneresse, bWljaGFlbC5zY2htdWVjay1oZW5uZXJlc3NlQGNoYXJpdGUuZGU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.