- 1West African Centre for Cell Biology of Infectious Pathogens, University of Ghana, Accra, Ghana
- 2Department of Immunology and Infection, Faculty of Infectious and Tropical Diseases, London School of Hygiene and Tropical Medicine, London, United Kingdom
- 3Department of Epidemiology and Biostatistics, School of Public Health, University of Health and Allied Sciences, Ho, Ghana
- 4Faculty of Biological Sciences, Liverpool School of Tropical Medicine, Liverpool, United Kingdom
- 5Department of Biomedical Sciences, School of Basic and Biomedical Sciences, University of Health and Allied Sciences, Ho, Ghana
Malaria infections remain a serious global health problem in the world, particularly among children and pregnant women in Sub-Saharan Africa. Moreover, malaria control and elimination is hampered by rapid development of resistance by the parasite and the vector to commonly used antimalarial drugs and insecticides, respectively. Therefore, vaccine-based strategies are sorely needed, including those designed to interrupt disease transmission. However, a prerequisite for such a vaccine strategy is the understanding of both the human and vector immune responses to parasite developmental stages involved in parasite transmission in both man and mosquito. Here, we review the naturally acquired humoral and cellular responses to sexual stages of the parasite while in the human host and the Anopheles vector. In addition, updates on current anti-gametocyte, anti-gamete, and anti-mosquito transmission blocking vaccines are given. We conclude with our views on some important future directions of research into P. falciparum sexual stage immunity relevant to the search for the most appropriate transmission-blocking vaccine.
Introduction
Malaria is one of the most important parasitic infections with the highest burden of mortality and morbidity in sub-Saharan Africa. Despite progress and advances in the strategies to control the disease, malaria claimed the lives of approximately 445,000 people from among 216 million clinical cases globally in 2016; mostly in children under 5 years and pregnant women as reported by WHO (1). The increasing challenges posed by the emergence of resistance to antimalarials by malaria parasites and to insecticides by mosquitoes (2, 3) suggest the need for additional interventions aiming at transmission reduction such as vaccines. Moreover, targeting of multiple stages of the parasites might be the best strategy for any successful malaria vaccine (4), further highlighting the need for continuous identification and validation of alternative and effective targets.
Transmission blocking interventions either targeting gametocytes while in the human host or gametes in the mosquito are considered an essential part of malaria control strategies especially in the quest to eradicate malaria (5, 6). Malaria parasites (sporozoites) are transmitted through the bite of Anopheline mosquitoes. Once in the human system, the sporozoites migrate to the liver where they undergo pre-erythrocytic multiplication (schizogony) leading to the production of merozoites that move into the bloodstream (erythrocytic stage; Figure 1). The pathology results from red blood cell (RBCs) invasion and further asexual replication of parasites within RBCs (erythrocytic schizogony) leading to massive RBC lysis, disrupted blood flow due to cytoadherence of parasite-infected RBCs to endothelial surfaces, anemia, and inflammation that may be lethal if untreated. Gametocytes are specialized stages of Plasmodium parasites that are essential for transmission from humans to mosquitoes. Initially, a certain proportion of the erythrocytic stage parasites undergoes a permanent differentiation also referred to as sexual commitment into both male (microgametocyte) and female (macrogametocyte) gametocytes (Figure 1). This process is known as gametocytogenesis (7, 8).
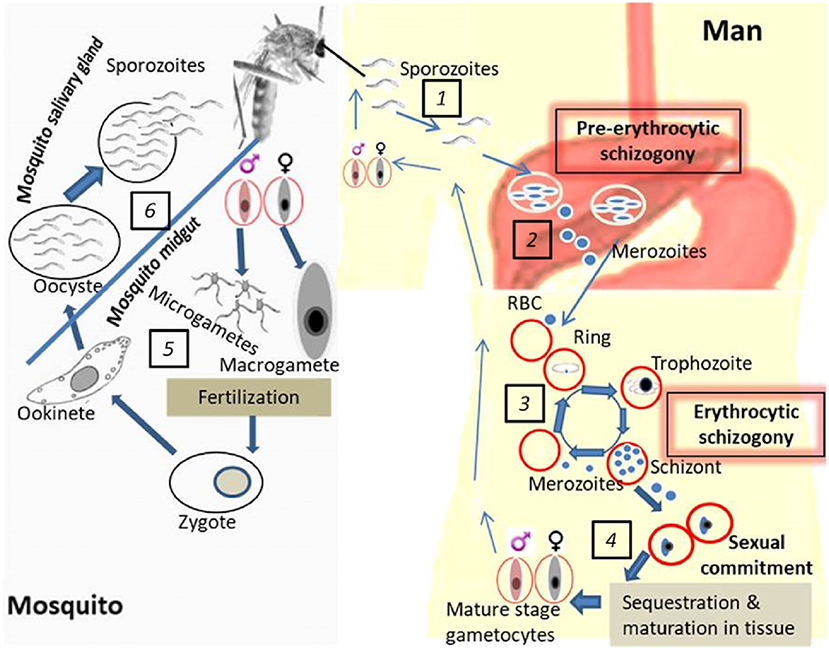
Figure 1. Life cycle of P. falciparum development in the human host and mosquito vector. (1). Mosquito's bite and release sporozoites into the human host followed by migration into the liver. (2). Pre-erythrocytic schizogony: infection of hepatocytes and asexual multiplication of the parasites in the liver. (3). Erythrocytic schizogony: translocation of parasites from the liver into the bloodstream accompanied by asexual multiplication and release of merozoites upon RBC rupture. (4). Gametocyte generation: sexual commitment, sequestration of early gametocytes, maturation in tissues and release of mature gametocytes in blood (ready to be picked up by the vector). (5). Parasite development in the mosquito midgut: exflagellation of male gametocytes prior to fertilization which yields the zygote which undergoes further development into a motile ookinete. (6). Parasite development in the mosquito salivary gland: oocyst formation, sporozoite development, and release in the mosquito salivary gland (ready to be transmitted to the human host during subsequent mosquito bites).
Sexually committed ring stage trophozoites from erythrocytic stages in peripheral circulation (9, 10) progress into gametocyte developmental stages 1 to IV while sequestered in bone marrow compartments (11–14). This constitutes the main reason why only late gametocyte stages are found in peripheral circulation. Early gametocytes are thought to sequester in tissues such as spleen and bone marrow through parasite-host interactions via parasite molecules less elucidated but probably PfEMP1, STEVORS, or RIFINS (14–16). The human host endothelial receptors mediating sequestration of developing gametocytes in the bone marrow and other organs however remain unidentified (17). Differentiation of male and female gametocytes occur during sexual commitment where the asexual precursor, schizont, give rise to either male or female gametocytes (7, 8).
After about 10–12 days of sequestered development, mature, male, and female gametocytes emerge and circulate in peripheral blood for a variable amount of time until taken up by mosquitoes (18, 19). Gametocytes do not replicate; however, hemoglobin digestion continues until they reach stage IV (20). In addition, gametocyte-specific mRNAs are produced and a subset of these, important for their stage development in the mosquito, are translationally repressed until gametocytes are taken up by the vector when they go back to peripheral circulation (21). The phenomenon governing the return of mature gametocytes in the peripheral blood is not clearly understood. Once ingested, gametocytes rapidly transform into male (microgamete) and female gametes (macrogamete) in response to environmental cues such as a rise in pH, reduction in temperature and exposure to xanthurenic acid (22). Exflagellation (male gamete induction) is followed by the expression of gamete-specific proteins (23). Fertilization of macrogametocytes by microgametes is preceded by 3 rounds of DNA replication by male gametocytes giving rise to 8 motile microgametes resulting in a zygote (Figure 1). The zygote elongates to form an ookinete which crosses the midgut wall to develop into an oocyst. Further cell divisions and development of the oocyst give rise to sporozoites. Following oocyst capsule rupture, thousands of sporozoites emerge and invade the mosquito salivary glands which then render the vector infectious to humans during a bloodmeal, thus completing the transmission cycle (24–26) (Figure 1).
The infectiousness and transmission potential of gametocytes is influenced by their prevalence and density (27), degree of maturity (28), and both mosquito and human immune responses (29, 30). Furthermore, the efficiency of transmission depends on the generation of sporozoites and therefore level of infectivity or sporozoite dose transmitted (31). Moreover, the sporogonic stages are exposed to the vector's natural immune responses (32–34). It should be pointed out that gametocyte infectiousness refers to the amount of mature gametocytes that can potently infect the mosquito (demonstrated by their ability to undergo further development) after ingestion whereas sporozoite infectivity refers to the dose of potent sporozoites capable of being transmitted to humans during subsequent blood meals.
Here, we review the available evidence for naturally acquired human immune responses against the sexual stages of Plasmodium parasites targeting gametocytes and gametes in human and mosquito hosts, respectively. The mosquito immune responses against the development of these sexual stages in the midgut are also discussed, and propositions are made for future research directions toward the design of appropriate transmission blocking vaccines.
Naturally Acquired Antibody Responses to Gametocyte and Gamete Antigens
For over three decades now there have been some efforts to illuminate antibody responses to gametocyte and gamete development in mosquitoes and their potential for transmission reducing immunity (TRI). TRI is based on observations of naturally acquired antibodies against gametocytes that are produced in the human host in response to proteins of gametocytes that were not taken up by mosquitoes (35). When these gametocytes die, they release intracellular proteins/antigens into the host circulation. Among these are proteins produced in gametocytes which are crucial for the extracellular parasite development in the mosquito midgut (36, 37). These antigens are then processed and presented by antigen presenting cells eventually eliciting humoral immune responses, which can cause substantial or complete blockade of parasite development (gametogenesis, fertilization) in the mosquito. This is the essence of TRI and forms the basis for the development of transmission-blocking vaccines (TBV). TRI occurs when human antibodies, taken up by a mosquito in a potentially infectious blood-meal containing male and female gametocytes, are able to prevent fertilization and/or development of ookinetes/oocyts/sporozoites in the mosquito and thus infection of the mosquito (38, 39).
Extensively studied antigens to date include gametocyte/gamete proteins such as Pfs230 and Pfs45/48 and the zygote/ookinete proteins Pfs25 and Pfs28 (37) also known as the TBV candidate (30, 37, 40–43). Anti-Pfs230 and Pfs48/45 antibodies target the so-called pre-fertilization phase while anti-Pfs25 and anti-Pfs28 antibodies represent the post-fertilization phases marked by the differences in the parasite stage and target antigens. As such parasite proteins are referred to as Pre- and post-fertilization antigens, respectively. Binding of these antibodies to their antigen either blocks their function essential for parasite development or facilitates complement-mediated gamete killing as shown for antibodies against Pfs230 (44).
Naturally occurring antibodies targeting Pfs230 and Pfs45/48 have been observed in field studies in The Gambia, Kenya, and Cameroon and were associated with reduced malaria transmission (30, 45). However, other studies reported that transmission reduction correlated with antibody responses to Pfs230 only (46) or with anti-Pfs48/45 antibodies only (6, 42, 43). These conflicting results may be due to differences in the history of exposure of study participants or existence of other co-infections.
A recent study by Stone et al. using field-based mosquito-feeding assays found mosquito infection rate to be significantly reduced for people harboring naturally acquired anti-Pfs48/45 and anti-Pfs230 antibodies. In addition, these antibodies were shown to be host gametocyte density-dependent and mechanistically associated with transmission reducing activity (TRA) (47, 48). In the same study, using protein microarray, 43 novel gametocyte proteins whose specific antibodies were associated with TRA were also identified (48). Among these 43 proteins, 16 predicted to be surface-expressed showed responses more similar to those of Pfs48/45 and Pfs230 in terms of TRA and as such warrant further investigations and characterization as TBV candidates (48, 49). However, the increase of natural seroprevalence to Pfs48/45 and Pfs230 with age found by Stone and colleagues did not corroborate a previous study by Ouedraogo et al. (50).
It is worth noting that antibodies against the post-fertilization antigens Pfs25 and Pfs28 have not been observed because these antigens are not exposed to the human immune system. If utilized in a vaccine, post-fertilization antibodies would not be boosted by natural malaria infections in vaccinated individuals. Nevertheless, antibodies against Pfs28 and Pfs25 have shown promise in blocking mosquito stage development and therefore transmission in in vitro experiments and are currently being evaluated in clinical trials (51).
The development and evaluation of antibody responses to all gametocyte/gamete-specific antigens and their effect on sexual stages in the mosquito face several challenges (51). First, evaluation of transmission reducing immunity relies heavily on mosquito feeding experiments, otherwise known as standard membrane feeding assays, where gametocyte-infected blood is fed to mosquitoes with or without antibodies to the respective antigens. Dissection of mosquitoes 7 days after blood feeding for oocyt counts is used as an indirect measure of transmission blocking activity. These assays are time-consuming and labor-intensive. Second, it is not known what ingested antibody levels in the mosquito are required that would lead to a subsequent transmission blockade. The identification and validation of gametocyte surface antigens as vaccine candidates with transmission reducing activity (TRA) directly measurable in the human host will overcome these challenges, complement and strengthen current transmission blocking vaccine efforts (6, 52).
Naturally Acquired Antibody Responses to The Surface of Gametocyte-Infected Erythrocytes
It has been difficult to elucidate naturally acquired antibody responses to gametocyte-infected erythrocyte surface antigens (GSAs), distinct from those recognizing internally expressed gametocyte and gamete antigens, in natural human infections. This is largely due to the indirect effect of asexual stage immunity on the prevalence and density of gametocytes.
Naturally acquired sexual stage antibodies are known to be produced against P. falciparum gametocyte-infected erythrocyte surface antigens in human peripheral circulation (anti-gametocyte immunity) (4, 53). There are very few studies on human immune responses recognizing gametocyte-infected erythrocyte surface antigens referred to as anti- gametocyte immunity. This is in contrast to anti-gamete immunity which is raised against intracellular proteins of dead gametocytes which have some function at the gamete stages, or more broadly immune responses to gamete surface antigens (4, 6, 31, 52–54).
In the first investigation of its kind, plasma antibodies from gametocytemic Gambian children donated after antimalarial treatment were used to detect antigens on the surface of 3D7 cultured mature stage V gametocytes. Surprisingly, no antibody recognition of the surface of erythrocytes infected with developing gametocytes, stages I-IV, representing the stages known to be sequestered in deep tissues, were found (44, 54). In addition, children harboring these anti-GSA antibody responses were significantly less likely to carry gametocytes after subsequent infections suggesting an ability to control gametocytemia in these patients. It was also shown that malaria patient plasma samples with strong anti-GSA plasma antibody recognition of the mature gametocyte-infected erythrocyte surface were not more likely to recognize the surface of erythrocytes infected with asexual parasites and vice versa (54).
This was a proof of concept for the rationale to develop an anti-GSA transmission blocking vaccine. It derived its basis from epidemiological observations of specific immune suppression of gametocytes in Indonesia (53). P. falciparum gametocyte rates were reduced among semi-immune native Papuans, independent of immune control of asexual parasitemia, when compared to a transmigrant Javanese population with a history of lower malaria exposure. These findings suggest specific immune control of gametocytemia as the observations could not be explained by differences in the frequency or grade of parasitemia, illness or by known patterns of antimalarial treatment. Further, immunofluorescence tests with acetone-fixed whole gametocytes showed a correlation between antibody levels and reduced gametocytemia among the native Irianese (53).
The important observations by Saeed et al. (54), the ability of patient plasma to recognize GSA and the significant association with reduced gametocyte carriage, required further investigation. In order to rule out the fact that patient plasma antibody recognition of GSA on mature stage V gametocytes was not a deficiency or artifact of the 3D7 clone, we carried out recognition studies in plasma antibody samples from Ghanaian school children from a high endemicity region, against both a recent isolate and 3D7. In this study, plasma from asymptomatic school children collected over 5 sampling times at weekly intervals were tested against the surface of 3D7 mature gametocyte-infected erythrocytes as well as mature gametocytes derived from a 2012 clinical isolate of Kenyan origin, HL1204. Interestingly, we found plasma antibodies from all children bind to GSA of gametocytes derived from both clones to at least some extent. It was striking to note that plasma from Ghanaian children recognized the GSA on mature gametocytes of Kenyan origin, suggesting that perhaps the antigens detected might be conserved across geographical locations. Immature gametocytes from the clinical isolate were also tested against a selected number of plasma samples from Ghanaian children with strong anti-GSA antibody responses. Similar to the observations of Saeed et al. (54), no detectable recognition of GSA to asynchronous immature gametocytes was observed. These findings were corroborated by some gametocyte adhesion studies (15, 55), which posit that maturing gametocytes do not, as previously thought, sequester from peripheral circulation through adhesion to human bone marrow-derived endothelial surfaces and receptors (56–58). Nevertheless, further studies with tightly synchronized immature gametocyte preparations are required before we can rule out the possibility that developing gametocytes express adhesins involved in parasite ligand-host receptor interactions which mediates sequestration and elicit gametocyte-specific immunity (4).
To further test the prevalence of anti-GSA antibodies in the general endemic population, plasma donated by microscopically-confirmed parasite negative individuals were tested for antibody recognition to GSA. Forty-eight percent (24/50) of parasite-negative children and adults recognized the surface of mature gametocyte-infected erythrocytes (4, 59). Since submicroscopic gametocytemia could not be excluded, anti-GSA antibody carriage in cohort studies utilizing sensitive gametocyte detection methods such as RT-qPCR or QT-NASBA are needed to fully illuminate this relationship. Moreover, testing plasma donated from both gametocyte positive and negative children showed that our findings could possibly represent the general malaria-infected population. In addition, evidence was found that children who harbored anti-GSA antibodies were significantly but weakly associated with lower risk of gametocyte carriage (4). In addition, preliminary indirect evidence suggest that anti-GSA antibodies may be maintained over a period of time (4, 59).
Cellular Immune Responses to Gametocytes While in The Human Host
Studies aiming at evaluating the cellular immune responses to the sexual stages compared to asexual ones of Plasmodium species are limited. However, there is evidence that such immunity exists. The transfer of T-cells from gamete-immunized mice was shown in the 1980s to markedly reduce gametocytemia in the recipient mice using the rodent species P. yoelii nigeriensis (60). Recipient mice also failed to effectively infect the mosquito vector A. stephensi as demonstrated by direct blood feeding (up to 95% transmission reduction) suggesting the direct impact of T-cells on transmission. However, the T-cell transfer had no effects on asexual stages (60). Good et al. (61) further showed that peripheral blood from non-exposed individuals contains T cells (clone) which proliferate and up-regulate interferon-gamma production upon stimulation with mature gametocyte-infected RBCs lysate. Similar results were also obtained in a hyper-endemic region in The Gambia by stimulation of peripheral blood mononuclear cells (PBMCs) of volunteers by gametocyte lysate (62). The detection of gametocyte-specific antibodies in the study participants by ELISA implied previous exposure to sexual stage parasites. Findings from this study suggest a T cell-dependent suppression of gametocytes or T cells helping B cells to fight against the malaria infection. Both asexual and sexual stage-specific antigens have equally been shown to elicit polyclonal T-cell responses in malaria non-exposed individuals (63). However, the reaction is not peculiar to gametocytes since it has been demonstrated that CD4 T Cells from non-exposed individuals react with PfEMP-1 via a Major Histocompatibility Complex (MHC) Class II-T cell receptor-independent Pathway (64). This could be associated to cross-reactivity from other infections. Whether this phenomenon is protective in children is not known.
In other studies, an increase in cytokine production such as TNF-α and IFN-γ was demonstrated in monkeys and humans infected with P. cynomolgi and P. vivax, respectively (65, 66). The increase in cytokine secretion correlated with the decrease in parasitemia and the inability of gametocytes to infect the mosquito vector and as such, cytokines and other PMBC-derived components (nitric oxide, antibodies) were believed to play a role in the loss of infectivity (66, 67). In their study in 1993, Naotunne et al. showed that the effect of PBMC-derived components on gametocyte infectivity was closely linked to the presence of white blood cells as no effect was seen in their absence (67). This negative effect on the infectivity of gametocytes appeared to be reversed in the presence of high concentration of an L-arginine analog (NGL-monomethyl arginine acetate) (68). This suggests that gametocyte inactivation in the presence of WBCs is achieved through an L-arginine-associated pathway mechanism. In the same line, an in vitro study conducted by Smith et al. (69) revealed that P. falciparum stage I and IIA gametocytes are to a large extent eliminated from the circulation by non-opsonic phagocytosis mediated by monocytes and macrophages. They showed through antibody inhibition assays and enzyme treatment that the interaction of PfEMP-1 and CD36 plays a major role in this innate defense against early gametocytes stages. This is in line with a previous study reporting the interaction of P. falciparum early gametocytes with CD36 receptor (70). A recent study conducted in India demonstrated a significant negative association between gametocytemia and IFN-γ in children (71).
Gametocyte-specific exoantigens (from gametocyte culture supernatants) have been shown to be able to stimulate the proliferation and activation of lymphocytes from P. falciparum exposed individual (72). In this study, T cell receptors gamma/delta (TCR γδ+), and CD3+ CD8+ and CD3+ CD4− CD8− T cells were found to be up-regulated upon sensitization with these exoantigens. Particularly, the expression of the activation marker CD25+ increased on stimulated CD3+ and γδ T cells. The frequency of γδ T cells had previously been found to increase in the course of acute malaria (73). However, it is difficult to ascertain the specificity of the exoantigens because they could as well be coming from ruptured or dead asexual parasite iRBCs. As already indicated, there is a paucity of information on cellular immunity to gametocytes. Therefore, further investigations into the role of cellular immune responses to gametocytes and malaria transmission; and the identification/validation of the antigens involved, in a bid to contribute toward the development of an effective transmission blocking vaccine are required.
Cellular Immunity to Sexual Stages While in the Mosquito Vector
In the mosquito vector, killing of malaria parasites is not only mediated by vertebrate host-derived molecules but also by mosquito components as has previously been demonstrated (32–34). Studies have shown that only a small proportion of gametocytes ingested in the blood meal by the mosquito vector is transformed into oocysts and sporozoites; and only about 38% of mosquitoes that take gametocyte-containing blood become infected (32, 33, 74). This is largely due to the peritrophic membrane or matrix (PM) which constitutes a physical barrier to Plasmodium species and other pathogens (33, 75, 76). This membrane is formed after the ingestion of a potentially infectious blood meal by the mosquito and surrounds the ingested blood. It prevents direct contact between the pathogens in the blood and the midgut epithelium and by so doing interferes with midgut invasion (33, 76). However, ookinetes secrete the enzyme chitinase which destroys the chitineous PM and allows it to invade the midgut (33, 77). The midgut epithelial cells are also thought to secrete high amounts of nitric oxide synthase and peroxidases, which in turn leads to nitration of the gut epithelium with subsequent tagging of ookinetes for destruction by the complement system (33, 78, 79).
The innate immune response in the malaria mosquito vector is mediated mainly by hemocytes which eliminate pathogens such as bacteria, fungi, and protozoa by phagocytosis (80–82). The Anopheles species and other insects are known to have a complement C3-like protein called thioester-containing proteins (TEP) (80). TEP of A. gambiae (AgTEP1) has been shown to be valuable for the initiation of immune defense against P. berghei. TEP1 plays the role of opsonins and facilitates the interaction between the parasite and the hemocytes with subsequent encapsulation, and killing of the parasite (80). Double knock-out of the TEP1 gene renders genetically selected refractory Anopheles strain susceptible to infection and increases the infectivity rates in susceptible A. gambiae (80). This vector defense mechanism has been shown recently to be by-passed by P. falciparum through its 6-cysteine protein P47-like (83). This protein is invaluable for P. berghei female gamete fertility (84) but in P. falciparum, it promotes the gametocyte-to-ookinete development and protects the ookinete from complement-dependent lysis (83).
In addition, infection of A. gambiae mosquito by ookinetes of P. berghei has been demonstrated to modulate the mosquito's immune system by up-regulating the expression of the antibacterial peptide defensin and a putative gram-negative bacteria-binding protein (85), and a TNF-α factor-like transcription factor (LL3) (86). Silencing of the LL3 gene was found to be associated with an increase in parasite survival, confirming its role in conferring mosquito resistance to the Plasmodium parasite. LL3 also affects the expression of another protein, a serine protease inhibitor (SRPN6), which equally confers resistance to invasion by Plasmodium (86).
Genomic and transcriptomic analyses of bacterial lipopolysaccharide-stimulated A. gambiae mosquitos revealed 23 immune-regulated genes which include putative protease inhibitors, serine proteases, and regulatory molecules (87). Interestingly, the protease inhibitor α-2-macroglobulin was found to be more specific in response to malaria parasite than bacterial infection as observed with mosquitoes fed on a P. berghei-infected hamster. This suggests that the immune response mounted by the mosquito vector may be pathogen specific, and other authors have reported similar findings (88). RNA gene interference (RNAi) experiments on P. falciparum- and P. berghei-infected An. gambiae revealed some common genes that confer resistance to both parasite species. However, other genes were found to exhibit species-specificity, conferring resistance only to one parasite species, namely a pattern recognition receptor (MD2-like receptor, AgMDL1) and an immunolectin, FBN39 for P. falciparum and the antimicrobial peptide gambicin and a novel putative short secreted peptide, IRSP5 for P. berghei (88). Together, these findings show that mosquitoes express molecules with anti-plasmodial properties which act as self-defense mechanism in the vector.
Anti-gametocyte and Anti-gamete Transmission Blocking Vaccines
Up to date, it has been a difficult task developing an effective vaccine against malaria. This is due both to the complexity of the Plasmodium parasite life cycle and the polymorphic nature of its antigens (89, 90). However, the hope that an effective malaria vaccine is feasible is based on the observation that in endemic regions, clinically immune adults are protected from severe malaria and death compared to children (89). This could be attributed to the fact that natural immunity in adults is probably complex and dependent on immune responses to many stages. Interestingly, sera from immune individuals have been shown to inhibit gamete fertilization and development in the mosquito vector thereby interfering with disease transmission (91–93). This constitutes the basis of the development of malaria transmission blocking vaccines (TBVs). An emerging concept is to develop vaccines against antigens expressed solely in the mosquito's midgut to which the host immune system is not naturally exposed. Antibodies against those antigens from vaccinated individuals and animals have been shown to interfere with parasite viability and development in the mosquito midgut interaction (94–96).
As a limitation, TBVs are different from the other vaccine types (liver and blood stage vaccines) in the sense that they do not protect against disease in the vaccinees. However, they reduce the risk of transmission to other people by the mosquito vector and by so doing favor herd immunity; as such they have sometimes been referred to as altruistic vaccines (95). Two groups of target antigens (gene superfamilies) exist, namely pre-fertilization and post-fertilization antigens (Table 1 and Figure 2) (48, 97, 107–111, 121). The list (Table 1) is not exhaustive both for pre- and post-fertilization antigens as some proteins remain unidentified to date. Some of these target antigens were characterized back in 1983 by Kaushal et al. (98), among them, Pfs48/45, Pfs47, Pfs230, and Pfs25 are immunogenic and less polymorphic, making them good vaccine candidates (122). Their use in combination with strong adjuvants or carrier proteins has been shown to boost their immunogenicity. Some of the adjuvants/carrier proteins used included Maltose Binding Protein (MBP)—Exoprotein A (EPA) from P. aeruginosa—Outer Membrane Protein Complex (OMPC)—modified Lickenase carrier (LiKM)—Virus-like particle (VLP)—Alhydrogel. Only two of these vaccine candidates, namely Pfs 230 and Pfs25, have entered clinical trial stage (123) and are reviewed in this paper. The potential of the other antigens (e.g., Pfs48/45) as TBV candidates has been recently reviewed by Chaturvedi et al. (122). Although Pfs 45/48 has not yet attained the clinical trial phase, previous studies demonstrated that antibodies against this antigen elicit up to 99% inhibition of oocyst intensity and 85% inhibition of oocyst prevalence (99). Hence the necessity to pursue studies with the Pfs 48/45 TBV candidate. Moreover, recent studies have identified new sexual stage antigens that require more attention (48, 121)
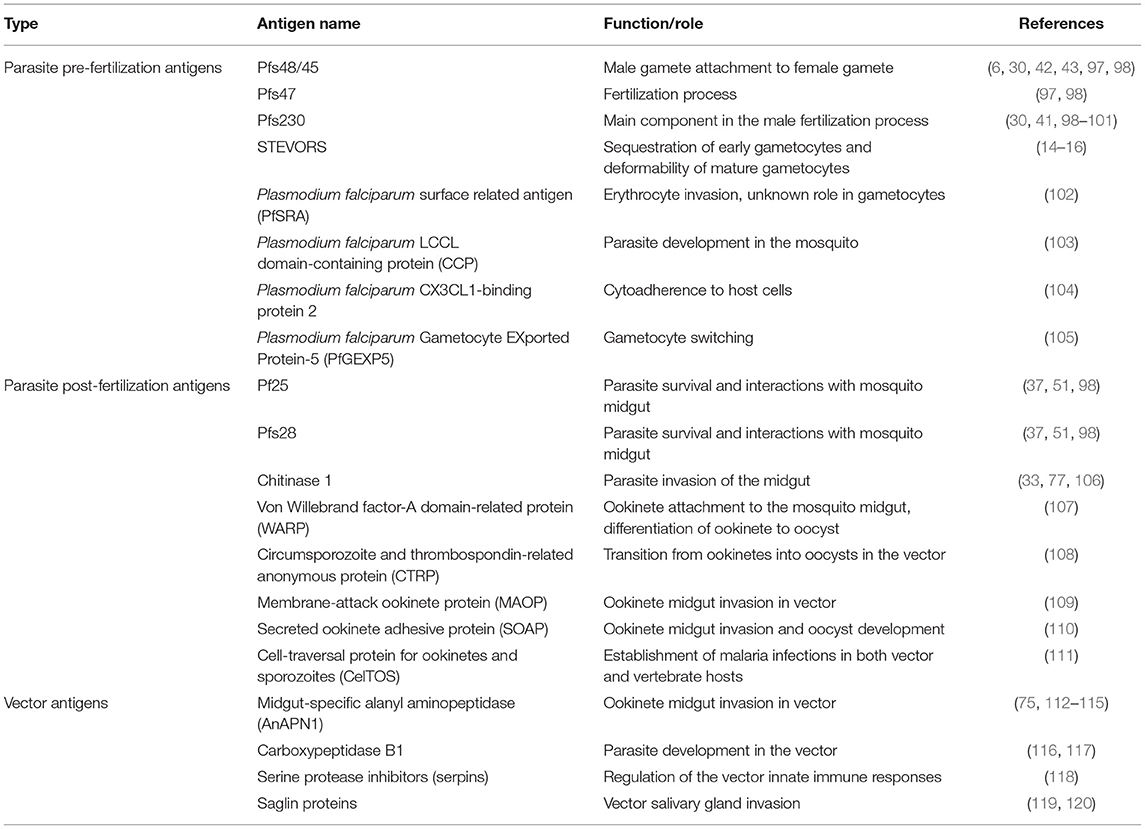
Table 1. Sexual stage antigens in the human host and mosquito vector with transmission reducing activity/potentials.
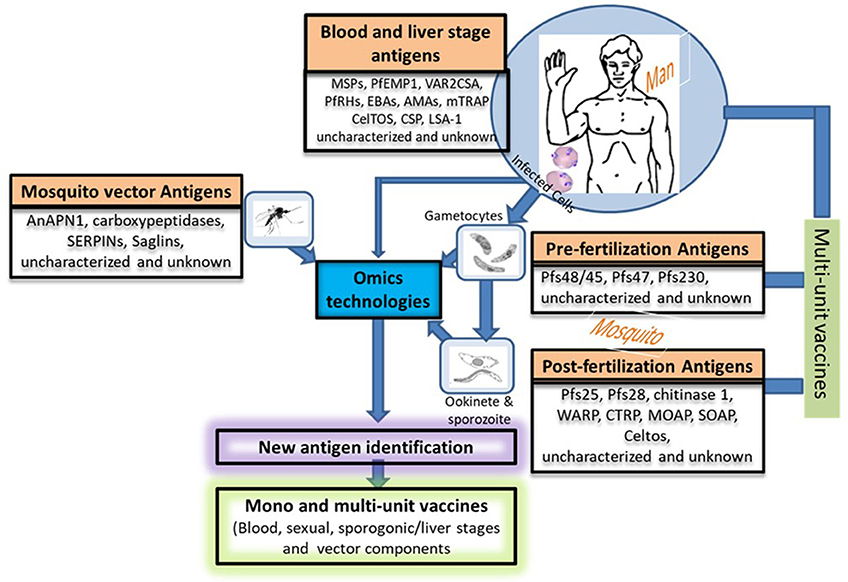
Figure 2. Potential approaches for new vaccine candidate identification and vaccine development. This figure describes in a nutshell the life cycle of the Plasmodium species providing different stage-specific antigens and the strategies that could be used to develop more efficacious vaccine by combining potential vaccine candidates from various stages (host's and vector's). Uncharacterized and unknown antigens refer to proteins of unknown functions or proteins that have only been partially characterized such as the multigene families (var, rif, and stevor). The function of the proteins encoded by these multigene families have been tremendously studied in the asexual stages of Plasmodium but poorly exploited in their sexual counterparts. We propose that more attention should be paid to the sexual stages in terms of vaccine candidate identification and characterization. Nowadays, this could easily be done with the advent of Omics technologies. PfEMP-1, Plasmodium falciparum erythrocyte membrane protein-1; RIFINs, repetitive interspersed families of polypeptides; STEVORs, Subtelomeric variable open reading frame polypeptides; MSPs, Merozoite surface proteins; VAR2CSA, A variant of PfEMP-1 with high affinity to placental tissues; PfRHs, Plasmodium falciparum reticulocyte binding protein homologs; EBAs, Erythrocyte binding antigens; AMAs, Apical membrane antigens; CSP, Circumsporozoite protein; LSA-1, Liver-Stage Antigen; mTRAP, Merozoite thrombospondin-related anonymous protein; Pfs (230, 48/45, 25,28), Plasmodium falciparum gamete surface proteins (molecular weight in dalton); WARP, von Willebrand factor-A domain-related protein; CTRP, Circumsporozoite and TRAP-related protein; MAOP, Membrane-attack ookinete protein; SOAP, Secreted ookinete adhesive protein; CelTos, Cell-traversal protein for ookinetes and sporozoites; AnAPN1, An. Gambiae alanyl aminopeptidase; serpins, Serine protease inhibitors; saglin proteins.
Pfs230 is a 363 kDa protein and a potent antigen of malaria TBV. It is a main component in the fertilization process as male gametes with impaired Pfs230 gene are incapable of interacting with red blood cells (RBCs) and forming exflagellation centers (100). This results in marked reduction in oocyst production and mosquito infectivity. Similar observations were made with genetically modified P. falciparum with a truncated chitinase 1 (PfCHT1) gene which could be due to the inability of affected ookinetes to invade the mosquito midgut (106). Administration of recombinant Pfs230 + Alhydrogel induced high titers of antibodies in rabbits which were found to have significant transmission reducing activity (101). It should be pointed out that only certain fragments of the recombinant Pfs230 antigen induce responses that lead to TRA (99). Significant associations between suppression of mosquito infectivity and anti-Pfs230 antibody levels were also found using membrane feeding assays with sera collected from African populations (43, 45, 46, 124) and in mice (99). This vaccine candidate (125) has entered a phase 1 clinical trial in which the Safety and Immunogenicity of Pfs230D1M-EPA/Alhydrogel is being evaluated in adults in the US and Mali (data unpublished, https://clinicaltrials.gov/ct2/show/NCT02334462).
Pf25 is relevant for parasite survival and interactions with mosquito midgut molecules prior to invasion. Anti-Pf25 antibodies have been shown to halt parasite growth within the mosquito in membrane feeding assays as reviewed by Chaturvedi et al. (122). This vaccine candidate has undergone clinical trial phase 1 in combination with different carriers and adjuvants. Administration of Pf25-Viral-like-particles plus Alhydrogel® to mice resulted in high antibody titers with 100% transmission reducing activity (TRA) throughout the study (126). A clinical trial phase 1a with this combination was recently carried out in the United States (https://clinicaltrials.gov/ct2/show/NCT02013687). It appeared that the combination is safe with no serious adverse reactions observed in healthy volunteers even when higher doses are administered. However, the antibodies generated showed low TRA hence the necessity to prioritize vaccine adjuvant formulations for further investigations (127).
Another combination of Pfs25 and EPA (Pseudomonas aeruginosa ExoProtein A) plus Alhydrogel® was also demonstrated to be well tolerated by naïve individuals after several doses in a phase 1a dose-response clinical trial in the US which correlated with antibody titers (128). A Phase 1b trial of Pfs25-EPA/Alhydrogel® is currently ongoing in Malian adults (122). New promising multimeric Pf25-based Vaccine Candidates ChAd63 Pfs25-IMX313 and MVA Pfs25-IMX313 have recently been developed with promising results (129) and are now undergoing clinical trial phase 1a in the UK. These vaccine candidates consist of attenuated viruses (ChAd63-chimpanzee adenovirus 63 and MVA- modified vaccinia Ankara) encoding the parasite protein Pf25, which are fused to a carrier protein (IMX313-multimerization technology) as adjuvant (NCT02532049). In mouse models, Chad63Pfs25-IMX313 was safe and significantly more immunogenic with higher TRA than monomeric Pfs25 (130). Similar results were obtained with the P. vivax antigen (Pvs25H/Alhydrogel), and the P. falciparum ortholog Pfs25 in a mouse model. Anti-Pvs25H antibody levels peaked after the third vaccination and vaccine-induced antibodies were functional, giving significant TRA (96). This combination has proven to be safe in humans in a clinical trial phase 1 with similar immunogenicity and TRA as previously shown in mice (131).
The main limitations of these sexual stage vaccine candidates have been the systemic reactogenicity observed in some clinical trials, short-lasting antibody responses owing to the fact that the host has no or limited exposure to the antigens requiring multiple boosting doses and strong adjuvants. In addition the recombinant antigens are difficult to express in their native form (122). To circumvent the issue of limited exposure of pre-fertilization and post-fertilization antigens to the human immune system, it might be good to develop DNA or viral vector-based vaccines containing different antigens (132, 133). This ensures continuous production of the antigens of interest in the host hence permanent stimulation of the immune system, thus bypassing the necessity for multiple immunizations (132, 133). However, the main problem is that the attenuated virus used can revert and cause infections, it can also get integrated in the vaccinee's genome and lead to unforeseen consequences with the associated ethical issues. It would be valuable to make use of high class adjuvants such as the Polymeric nanoparticle- and microparticle-based adjuvant systems that ensure long term delivery of the antigen to the host system when administered (134). It would be good also to include some blood and liver stage antigens to such combinations so that the vaccinee benefits from the process (Figure 2).
Anti-mosquito Transmission Blocking Vaccines
Some mosquito components are invaluable for the sporogonic development of malaria parasites as they are involved in parasite invasion through interaction with parasite receptors. Antibodies raised against these components could be very useful in blocking parasite development in the mosquito vector (95). Thus, these components constitute another class of TBV candidates as their inhibition would likely minimize the risk of new infection in the community (135). An alternative transmission blocking vaccine strategy could be to interfere with the interactions between the parasite and the midgut molecules of the mosquito vector which will lead to the inhibition of ookinete invasion and the development of mosquito stages. Many of such molecules have been identified and characterized (Table 1 and Figure 2). These molecules have been shown to be more conserved than the parasite antigens and are immunogenic in non-human primates (75, 112–115).
Midgut-specific alanyl aminopeptidase (AnAPN1) is a Glycosylphosphatidyl inositol (GPI)-anchored antigen which plays a valuable role in ookinete invasion in A. gambiae as previously shown by Dinglasan et al. (113). Administration of a recombinant fragment of rAnAPN160–195 with Alhydrogel has been demonstrated to stimulate a sustained production of antibodies with transmission blocking activities as revealed by membrane feeding assays (112). The TRA was dose dependent with higher antibody levels attaining 100% efficacy, and functional in both the chromosomal M and S forms of A. gambiae vectors. Moreover, the P. falciparum-infected blood samples used for membrane feeding assays were collected directly from gametocyte positive individuals and the results obtained exceeded those that had been reported previously with laboratory strains (113). More importantly, anti rAnAPN160–195 antibodies had effects on both P. falciparum and P. vivax and based on that evidence the AnAPN1 TBV has been recommended for phase I clinical trials (112). This antigen is immunogenic in mice and rabbits even in the absence of adjuvants (113, 115). However, it is worth mentioning that another study by Kapulu et al. failed to replicate the finding reported here. In their study, anti-AgAPN1 IgG had no significant impact on oocyst prevalence (99). Antibodies to another GPI-anchored vector midgut protein, α-AgSGU, were also confirmed to have an effect on P. falciparum and P. vivax development in An. gambiae and An. Dirus (114). However, high doses of α-AgSGU antibodies were required to achieve 80% TRA rendering α-AgSGU less promising as a TBV target.
In the same line, the midgut carboxypeptidase gene of A. gambiae (cpbAg) has been shown to be up-regulated following P. falciparum gametocyte ingestion by the vector (116). In addition, anti-CPBAG antibodies were shown to inhibit the development of both P. falciparum and P. berghei in the vector's midgut. Antibodies directed against CPBA have also been demonstrated to be vector-unspecific in the sense that they also inhibit the development of the P. falciparum gametocytes in A. stephensi mosquitoes, which is the main malaria vector in Iran and neighboring countries (117). This confirmed the conserved nature of molecules across different vector as predicted using genomic and proteomic approaches (117) and implies that a vaccine designed with CPBA could provide cross-species protection. Thus, CPBA constitutes another promising TBV candidate.
The interaction between the Plasmodium sporozoite Thrombospondin Related Anonymous Protein (TRAP) and the mosquito Saglin proteins is a prerequisite for vector salivary gland invasion (119). This has been confirmed by in vivo down regulation experiments of saglin gene expression which revealed a negative association with salivary gland invasion (119). Moreover, in silico analysis of saglin revealed the presence of a signal peptide suggesting that it may be a secreted protein. If verified in vitro and in vivo, Saglin proteins could constitute a new promising candidate for TBV design (120). Similarly, RNA interference silencing and knock-down experiments have demonstrated the essentiality of the serine protease inhibitors (serpins) in the survival of An. gambiae and An. stephensi as well as in the development of parasites (P. berghei) within these vectors (136, 137). Serpins are regulators of the vector innate immune responses and they are involved in the clearance of protozoan parasites (137). Antibodies raised against the An. gambiae serpin-2 (AgSRPN2) have been shown to be P. berghei-specific in An. gambiae and An. stephensi as they failed to interfere with P. falciparum oocyst formation (118). This study demonstrated that mosquito innate immune response-related molecules could be used as targets for TBV design; however, further investigations are needed to identify and/or validate the right antigens. A limitation here will be that all of these proteins are likely to suffer from the same problems as gamete/ookinete antigens in the sense that several booster doses are required and antibodies may be short-lived.
Future Directions in Sexual Stage Immunity and Vaccine Development
Apart from studies reported in these reviews (60–62, 69, 72, 73), studies on host cellular and humoral immunity to gametocytes are scarce if not inexistent. Given promising results in clinical trials of TBV experimental vaccines for malaria eradication, the antigens involved should be characterized further to explore their suitability as vaccine candidates. The generation of long-lived antibodies depends on the generation of long-lived plasma cells and memory B cells (MBCs) within germinal centers (GCs) of secondary lymphoid organs (138). The prerequisite for plasma cells and MBCs is the interaction between follicular T helper cells and B cells. Further investigations of these cell types vis-à-vis the identified antigens in the context of malaria infections are needed. Similarly, further studies aiming at identifying new antigens (mainly vector-, gametocyte-, ookinete-, and/or oocyste-related) using genomics, transcriptomics and proteomics approaches, the Sanger center parasite gene knock-out library and other bioinformatics strategies are warranted (139–141) (Figure 2). These approaches take advantage of next generation sequencing (NGS) and the availability of growing numbers of P. falciparum whole genome sequences to identify new antigens (142). These methods are relatively fast and high throughput, leading to the identification of a plethora of essential genes or antigens through comparative analyses (139, 141–143). The implications of omics in the fight against infectious disease was recently reviewed by Bah et al. (144). But this would be strengthened by the concomitant ability to cultivate the sequenced lines and generate sexual stages from them for phenotypic studies. Bioinformatic strategies can also overcome some of the difficulties in studying parasites such as Plasmodium spp. or Trypanosoma spp. which are genetically diverse (140, 145–147). In addition, computer-based algorithms have been developed to delineate T-cell epitopes on essential parasite proteins directly from genome sequence data (148–150). Vaccine developers should consider designing multi-unit or multi-stage TBVs with components from both the parasite (precisely gametocyte antigens) and the vector as this will broaden their spectrum of action.
As far as the search for a vaccine is concerned, much has been done to understand the multigene family PfEMP-1. However, the other multigene family proteins such as RIFIN and STEVOR also constitute an important class of parasite molecules that deserve attention. These form part of the uncharacterized or partially characterized parasite antigen repertoire with respect to the sexual stages (Figure 2). The stevor multicopy family is made up of a set of 39 genes with 2–3 copies expressed at a time (151) while about 150–200 genes code for RIFINs with many copies expressed at a time as well (152). STEVOR and RIFIN proteins were recently shown to be implicated in rosetting which is a phenomenon associated with sequestration and clinical complications of the malaria (153–157). STEVORs are suspected to be implicated in the sequestration of early gametocytes in tissues such as the bone marrow and spleen as well as the deformability of the mature gametocytes (14–16). There is also evidence that STEVORs alter RBC membrane rigidity since RBC deformability has been shown to be linked to STEVOR dissociation from the mature gametocyte-infected RBC membrane (15). This implies that inhibiting the functions of STEVORS might negatively affect the development of gametocytes and by so-doing will also reduce disease transmission due to a reduced number of sexual stages being ingested by the mosquito vector during its blood meal. Despite their variable nature, the putative role of STEVORs in gametocyte development and sequestration certainly make this family a possible new class of TBV vaccine targets. Humoral responses to these antigens have been demonstrated (158). We therefore recommend further characterization (both humoral and cellular) of anti-STEVOR immune response, in the hope of finding additional clues in the search for efficient TBVs. However, the most pressing task is to develop further STEVOR-specific reagents demonstrating their relevance in anti-gametocyte immune responses and therefore transmission reducing immunity/activities.
In addition to STEVORS, many other Plasmodium antigens such as LCCL domain-containing protein family (103), Plasmodium falciparum Surface Related Antigen (PfSRA) (102), CX3CL1-binding protein 2 (104), Gametocyte EXported Protein-5 (PfGEXP5) (105) have been described as potential TBV candidates and as such deserve further characterizations.
Author Contributions
JK-O and BD designed and drafted the manuscript. CS, FB, GA, and BU reviewed and edited the manuscript. All authors approved the final version of manuscript for publication.
Funding
JK-O is supported by a DELTAS Africa grant (DEL-15-007: Awandare). The DELTAS Africa Initiative is an independent funding scheme of the African Academy of Sciences (AAS)'s Alliance for Accelerating Excellence in Science in Africa (AESA) and supported by the New Partnership for Africa's Development Planning and Coordinating Agency (NEPAD Agency) with funding from the Wellcome Trust (107755/Z/15/Z: Awandare) and the UK government. BD is funded by a Wellcome Trust Training Fellowship in Public Health and Tropical Medicine (110090/Z/15/Z). The views expressed in this publication are those of the authors and not necessarily those of AAS, NEPAD Agency, Wellcome Trust or the UK government.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are grateful to Prof. Kirk W. Deitsch of Weill Cornell Medical College, New York, for providing critical reviews of the manuscripts which helped improve it.
References
1. WHO. World Malaria Day 2018 “Ready to Beat Malaria”: key messages. No. WHO/CDS/GMP/2018.06, World Health Organization (2018).
2. Ashley EA, Dhorda M, Fairhurst RM, Amaratunga C, Lim P, Suon S, et al. Spread of artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. (2014) 371:411–23. doi: 10.1056/NEJMoa1314981
3. Dondorp AM, Nosten F, Yi P, Das D, Phyo AP, Tarning J, et al. Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. (2009) 361:455–67. doi: 10.1056/NEJMoa0808859
4. Dinko B, King E, Targett GA, Sutherland CJ. Antibody responses to surface antigens of Plasmodium falciparum gametocyte-infected erythrocytes and their relation to gametocytaemia. Parasite Immunol. (2016) 38:352–64. doi: 10.1111/pim.12323
5. Alonso PL, Brown G, Arevalo-Herrera M, Binka F, Chitnis C, Collins F, et al. A research agenda to underpin malaria eradication. PLoS Med. (2011) 8:e1000406. doi: 10.1371/journal.pmed.1000406
6. Gebru T, Ajua A, Theisen M, Esen M, Ngoa UA, Issifou S, et al. Recognition of Plasmodium falciparum mature gametocyte-infected erythrocytes by antibodies of semi-immune adults and malaria-exposed children from Gabon. Malar J. (2017) 16:176. doi: 10.1186/s12936-017-1827-7
7. Sinha A, Hughes KR, Modrzynska KK, Otto TD, Pfander C, Dickens NJ, et al. A cascade of DNA-binding proteins for sexual commitment and development in Plasmodium. Nature (2014) 507:253. doi: 10.1038/nature12970
8. Smith T, Lourenco P, Carter R, Walliker D, Ranford-Cartwright L. Commitment to sexual differentiation in the human malaria parasite, Plasmodium falciparum. Parasitology (2000) 121:127–33. doi: 10.1017/S0031182099006265
9. Dinko B, Ansah F, Agyare-Kwabi C, Tagboto S, Amoah LE, Urban BC, et al. Gametocyte development and carriage in Ghanaian individuals with uncomplicated Plasmodium falciparum malaria. Am J Trop Med Hyg. (2018) 99:57–64. doi: 10.4269/ajtmh.18-0077
10. Pelle KG, Oh K, Buchholz K, Narasimhan V, Joice R, Milner DA, et al. Transcriptional profiling defines dynamics of parasite tissue sequestration during malaria infection. Genome Med. (2015) 7:19. doi: 10.1186/s13073-015-0133-7
11. Aguilar R, Magallon-Tejada A, Achtman AH, Moraleda C, Joice R, Cisteró P, et al. Molecular evidence for the localization of Plasmodium falciparum immature gametocytes in bone marrow. Blood (2014) 123:959–66. doi: 10.1182/blood-2013-08-520767
12. Farfour E, Charlotte F, Settegrana C, Miyara M, Buffet P. The extravascular compartment of the bone marrow: a niche for Plasmodium falciparum gametocyte maturation? Malar J. (2012) 11:285. doi: 10.1186/1475-2875-11-285
13. Joice R, Nilsson SK, Montgomery J, Dankwa S, Egan E, Morahan B, et al. Plasmodium falciparum transmission stages accumulate in the human bone marrow. Sci Transl Med. (2014) 6:244re245. doi: 10.1126/scitranslmed.3008882
14. Smalley M, Abdalla S, Brown J. The distribution of Plasmodium falciparum in the peripheral blood and bone marrow of Gambian children. Trans R Soc Trop Med Hyg. (1981) 75:103–5. doi: 10.1016/0035-9203(81)90019-5
15. Tibúrcio M, Niang M, Deplaine G, Perrot S, Bischoff E, Ndour PA, et al. A switch in infected erythrocyte deformability at the maturation and blood circulation of Plasmodium falciparum transmission stages. Blood (2012) 119:e172–e180. doi: 10.1182/blood-2012-03-414557
16. Tibúrcio M, Silvestrini F, Bertuccini L, Sander AF, Turner L, Lavstsen T, et al. Early gametocytes of the malaria parasite Plasmodium falciparum specifically remodel the adhesive properties of infected erythrocyte surface. Cell Microbiol. (2013) 15:647–59. doi: 10.1111/cmi.12062
17. Messina V, Valtieri M, Rubio M, Falchi M, Mancini F, Mayor A, et al. Gametocytes of the malaria parasite Plasmodium falciparum interact with and stimulate bone marrow mesenchymal cells to secrete angiogenetic factors. Front Cell Infect Microbiol. (2018) 8:50. doi: 10.3389/fcimb.2018.00050
18. Bruce M, Alano P, Duthie S, Carter R. Commitment of the malaria parasite Plasmodium falciparum to sexual and asexual development. Parasitology (1990) 100:191–200. doi: 10.1017/S0031182000061199
19. Silvestrini F, Alano P, Williams J. Commitment to the production of male and female gametocytes in the human malaria parasite Plasmodium falciparum. Parasitology (2000) 121:465–71. doi: 10.1017/S0031182099006691
20. Hanssen E, Knoechel C, Dearnley M, Dixon MWA, Le Gros M, Larabell C, et al. Soft X-ray microscopy analysis of cell volume and hemoglobin content in erythrocytes infected with asexual and sexual stages of Plasmodium falciparum. J Struct Biol. (2012) 177:224–32. doi: 10.1016/j.jsb.2011.09.003
21. Mair GR, Lasonder E, Garver LS, Franke-Fayard BM, Carret CK, Wiegant JC, et al. Universal features of post-transcriptional gene regulation are critical for Plasmodium zygote development. PLoS Pathog. (2010) 6:e1000767. doi: 10.1371/journal.ppat.1000767
22. Billker O, Shaw M, Margos G, Sinden R. The roles of temperature, pH and mosquito factors as triggers of male and female gametogenesis of Plasmodium berghei in vitro. Parasitology (1997) 115:1–7. doi: 10.1017/S0031182097008895
23. Billker O, Lindo V, Panico M, Etienne A, Paxton T, Dell A, et al. Identification of xanthurenic acid as the putative inducer of malaria development in the mosquito. Nature (1998) 392:289. doi: 10.1038/32667
24. Sinden R. The cell biology of sexual development in Plasmodium. Parasitology (1983) 86:7–28. doi: 10.1017/S0031182000050824
25. Sinden R. Sexual development of malarial parasites. Adv Parasitol. (1983) 22:153–216. doi: 10.1016/S0065-308X(08)60462-5
26. Vaughan JA. Population dynamics of Plasmodium sporogony. Trends Parasitol. (2007) 23:63–70. doi: 10.1016/j.pt.2006.12.009
27. Schneider P, Bousema JT, Gouagna LC, Otieno S, Van De Vegte-Bolmer M, Omar SA, et al. Submicroscopic Plasmodium falciparum gametocyte densities frequently result in mosquito infection. Am J Trop Med Hyg. (2007) 76:470–4. doi: 10.4269/ajtmh.2007.76.470
28. Targett G, Drakeley C, Jawara M, von Seidlein L, Coleman R, Deen J, et al. Artesunate reduces but does not prevent posttreatment transmission of Plasmodium falciparum to Anopheles gambiae. J Infect Dis. (2001) 183:1254–9. doi: 10.1086/319689
29. Bousema J, Drakeley C, Sauerwein R. Sexual-stage antibody responses to P. falciparum in endemic populations. Curr Mol Med. (2006) 6:223–9. doi: 10.2174/156652406776055140
30. Bousema T, Okell L, Shekalaghe S, Griffin JT, Omar S, Sawa P, et al. Revisiting the circulation time of Plasmodium falciparum gametocytes: molecular detection methods to estimate the duration of gametocyte carriage and the effect of gametocytocidal drugs. Malar J. (2010) 9:136. doi: 10.1186/1475-2875-9-136
31. Stone WJ, Dantzler KW, Nilsson SK, Drakeley CJ, Marti M, Bousema T, et al. Naturally acquired immunity to sexual stage P. falciparum parasites. Parasitology (2016) 143:187–98. doi: 10.1017/S0031182015001341
32. Gouagna LC, Mulder B, Noubissi E, Tchuinkam T, Verhave JP, Boudin C. The early sporogonic cycle of Plasmodium falciparum in laboratory-infected Anopheles gambiae: an estimation of parasite efficacy. Trop Med Int Health (1998) 3:21–8. doi: 10.1046/j.1365-3156.1998.00156.x
33. Smith RC, Vega-Rodríguez J, Jacobs-Lorena M. The Plasmodium bottleneck: malaria parasite losses in the mosquito vector. Mem Inst Oswaldo Cruz. (2014) 109:644–61. doi: 10.1590/0074-0276130597
34. Whitten MM, Shiao SH, Levashina EA. Mosquito midguts and malaria: cell biology, compartmentalization and immunology. Parasite Immunol. (2006) 28:121–30. doi: 10.1111/j.1365-3024.2006.00804.x
35. Mendis K, Munesinghe Y, De Silva Y, Keragalla I, Carter R. Malaria transmission-blocking immunity induced by natural infections of Plasmodium vivax in humans. Infect Immun. (1987) 55:369–72.
36. Alano P. Plasmodium sexual stage antigens. Parasitol Today (1991) 7:199–203. doi: 10.1016/0169-4758(91)90138-E
37. Sinden R. A biologist's perspective on malaria vaccine development. Hum Vaccin. (2010) 6:3–11. doi: 10.4161/hv.6.1.9604
38. Mendis KN, David PH, Carter R. Human immune responses against sexual stages of malaria parasites: considerations for malaria vaccines. Int J Parasitol. (1990) 20:497–502. doi: 10.1016/0020-7519(90)90197-U
39. Mendis KN, Munesinghe YD, de Silva YN, Keragalla I, Carter R. Malaria transmission-blocking immunity induced by natural infections of Plasmodium vivax in humans. Infect Immun. (1987) 55:369–72.
40. Drakeley C, Bousema J, Akim N, Teelen K, Roeffen W, Lensen A, et al. Transmission-reducing immunity is inversely related to age in Plasmodium falciparum gametocyte carriers. Parasite Immunol. (2006) 28:185–90. doi: 10.1111/j.1365-3024.2005.00818.x
41. Graves P, Wirtz R, Carter R, Burkot T, Looker M, Targett G. Naturally occurring antibodies to an epitope on Plasmodium falciparum gametes detected by monoclonal antibody-based competitive enzyme-linked immunosorbent assay. Infect Immun. (1988) 56:2818–21.
42. Mulder B, Lensen T, Tchuinkam T, Roeffen W, Verhave JP, Boudin C, et al. Plasmodium falciparum: membrane feeding assays and competition ELISAs for the measurement of transmission reduction in sera from Cameroon. Exp Parasitol. (1999) 92:81–6. doi: 10.1006/expr.1999.4398
43. van der Kolk M, de Vlas SJ, Sauerwein RW. Reduction and enhancement of Plasmodium falciparum transmission by endemic human sera. Int J Parasitol. (2006) 36:1091–5. doi: 10.1016/j.ijpara.2006.05.004
44. Sutherland CJ. Surface antigens of Plasmodium falciparum gametocytes—a new class of transmission-blocking vaccine targets? Mol Biochem Parasitol. (2009) 166:93–8. doi: 10.1016/j.molbiopara.2009.03.007
45. Bousema T, Sutherland CJ, Churcher TS, Mulder B, Gouagna LC, Riley EM, et al. Human immune responses that reduce the transmission of Plasmodium falciparum in African populations. Int J Parasitol. (2011) 41:293–300. doi: 10.1016/j.ijpara.2010.09.008
46. Graves PM, Carter R, Burkot TR, Quakyi IA, Kumar N. Antibodies to Plasmodium falciparum gamete surface antigens in Papua New Guinea sera. Parasite Immunol. (1988) 10:209–18. doi: 10.1111/j.1365-3024.1988.tb00215.x
47. Ouedraogo AL, Eckhoff PA, Luty AJF. Modeling the impact of Plasmodium falciparum sexual stage immunity on the composition and dynamics of the human infectious reservoir for malaria in natural settings. PLoS Pathog. (2018) 14:e1007034. doi: 10.1371/journal.ppat.1007034
48. Stone WJR, Campo JJ, Ouedraogo AL, Meerstein-Kessel L, Morlais I, Da D, et al. Unravelling the immune signature of Plasmodium falciparum transmission-reducing immunity. Nat Commun. (2018) 9:558. doi: 10.1038/s41467-017-02646-2
49. Jones S, Grignard L, Nebie I, Chilongola J, Dodoo D, Sauerwein R, et al. Naturally acquired antibody responses to recombinant Pfs230 and Pfs48/45 transmission blocking vaccine candidates. J Infect. (2015) 71:117–27. doi: 10.1016/j.jinf.2015.03.007
50. Ouédraogo AL, Roeffen W, Luty AJ, de Vlas SJ, Nebie I, Ilboudo-Sanogo E, et al. (2011). Naturally acquired immune responses to Plasmodium falciparum sexual stage antigens Pfs48/45 and Pfs230 in an area of seasonal transmission. Infect. Immun. 79, 4957–64. doi: 10.1128/iai.05288-11
51. Nunes JK, Woods C, Carter T, Raphael T, Morin MJ, Diallo D, et al. Development of a transmission-blocking malaria vaccine: progress, challenges, and the path forward. Vaccine (2014) 32:5531–9. doi: 10.1016/j.vaccine.2014.07.030
52. Tonwong N, Sattabongkot J, Tsuboi T, Iriko H, Takeo S, Sirichaisinthop J, et al. Natural infection of Plasmodium falciparum induces inhibitory antibodies against gametocyte development in human hosts. Jpn J Infect Dis. (2012) 65:152–6. doi: 10.1186/1475-2875-9-S2-P53
53. Baird JK, Jones TR, Masbar PS, Ratiwayanto S, Leksana B. Evidence for specific suppression of gametocytemia by Plasmodium falciparum in residents of hyperendemic Irian Jaya. Am J Trop Med Hyg. (1991) 44:183–90. doi: 10.4269/ajtmh.1991.44.183
54. Saeed M, Roeffen W, Alexander N, Drakeley CJ, Targett GA, Sutherland CJ. Plasmodium falciparum antigens on the surface of the gametocyte-infected erythrocyte. PLoS ONE (2008) 3:e2280. doi: 10.1371/journal.pone.0002280
55. Silvestrini F, Tibúrcio M, Bertuccini L, Alano P. Differential adhesive properties of sequestered asexual and sexual stages of Plasmodium falciparum on human endothelial cells are tissue independent. PLoS ONE (2012) 7:e31567. doi: 10.1371/journal.pone.0031567
56. Alano P. Plasmodium falciparum gametocytes: still many secrets of a hidden life. Mol Microbiol. (2007) 66:291–302. doi: 10.1111/j.1365-2958.2007.05904.x
57. Day KP, Hayward RE, Smith D, Culvenor JG. CD36-dependent adhesion and knob expression of the transmission stages of Plasmodium falciparum is stage-specific. Mol Biochem Parasitol. (1998) 93:167–77. doi: 10.1016/S0166-6851(98)00040-1
58. Rogers NJ, Hall BS, Obiero J, Targett GA, Sutherland CJ. A model for sequestration of the transmission stages of Plasmodium falciparum: adhesion of gametocyte-infected erythrocytes to human bone marrow cells. Infect Immun. (2000) 68:3455–62. doi: 10.1128/IAI.68.6.3455-3462.2000
59. Bousema J, Drakeley C, Kihonda J, Hendriks J, Akim N, Roeffen W, et al. A longitudinal study of immune responses to Plasmodium falciparum sexual stage antigens in Tanzanian adults. Parasite Immunol. (2007) 29:309–17. doi: 10.1111/j.1365-3024.2007.00948.x
60. Harte PG, Rogers NC, Targett GA. Role of T cells in preventing transmission of rodent malaria. Immunology (1985) 56:1–7.
61. Good M, Quakyi I, Saul A, Berzofsky J, Carter R, Miller L. Human T clones reactive to the sexual stages of Plasmodium falciparum malaria. High frequency of gamete-reactive T cells in peripheral blood from nonexposed donors. J Immunol. (1987) 138:306–11.
62. Riley EM, Ong C, Olerup O, Eida S, Allen S, Bennett S, et al. Cellular and humoral immune responses to Plasmodium falciparum gametocyte antigens in malaria-immune individuals. Limited response to the 48/45-kilodalton surface antigen does not appear to be due to MHC restriction. J Immunol. (1990) 144:4810–6.
63. Goodier MR, Targett GA. Polyclonal T-cell responses to Plasmodium falciparum gametocytes in malaria nonexposed donors. Parasite Immunol. (1997) 19:419–25. doi: 10.1046/j.1365-3024.1997.d01-238.x
64. Ndungu FM, Sanni L, Urban B, Stephens R, Newbold CI, Marsh K, et al. CD4 T cells from malaria-nonexposed individuals respond to the CD36-binding domain of Plasmodium falciparum erythrocyte membrane protein-1 via an MHC class II-TCR-independent pathway. J Immunol. (2006) 176:5504–12. doi: 10.4049/jimmunol.176.9.5504
65. Karunaweera ND, Carter R, Grau G, Kwiatkowski D, Giudice G, Mendis K. Tumour necrosis factor-dependent parasite-killing effects during paroxysms in non-immune Plasmodium vivax malaria patients. Clin Exp Immunol. (1992) 88:499–505. doi: 10.1111/j.1365-2249.1992.tb06478.x
66. Naotunne TDS, Karunaweera ND, Del Giudice G, Kularatne M, Grau G, Carter R, et al. Cytokines kill malaria parasites during infection crisis: extracellular complementary factors are essential. J Exp Med. (1991) 173:523–9. doi: 10.1084/jem.173.3.523
67. Naotunne TS, Karunaweera ND, Mendis KN, Carter R. Cytokine-mediated inactivation of malarial gametocytes is dependent on the presence of white blood cells and involves reactive nitrogen intermediates. Immunology (1993) 78:555–62.
68. Motard A, Landau I, Nussler A, Grau G, Baccam D, Mazier D, et al. The role of reactive nitrogen intermediates in modulation of gametocyte infectivity of rodent malaria parasites. Parasite Immunol. (1993) 15:21–6. doi: 10.1111/j.1365-3024.1993.tb00568.x
69. Smith TG, Serghides L, Patel SN, Febbraio M, Silverstein RL, Kain KC. CD36-mediated nonopsonic phagocytosis of erythrocytes infected with stage I and IIA gametocytes of Plasmodium falciparum. Infect Immun. (2003) 71:393–400. doi: 10.1128/IAI.71.1.393-400.2003
70. Hayward RE, Tiwari B, Piper KP, Baruch DI, Day KP. Virulence and transmission success of the malarial parasite Plasmodium falciparum. Proc Natl Acad Sci USA. (1999) 96:4563–8. doi: 10.1073/pnas.96.8.4563
71. Jain V, Singh PP, Silawat N, Patel R, Saxena A, Bharti PK, et al. A preliminary study on pro- and anti-inflammatory cytokine profiles in Plasmodium vivax malaria patients from central zone of India. Acta Trop. (2010) 113:263–8. doi: 10.1016/j.actatropica.2009.11.009
72. Ramsey JM, Tello A, Contreras CO, Ordonez R, Chirino N, Rojo J, et al. Plasmodium falciparum and P. vivax gametocyte-specific exoantigens stimulate proliferation of TCR gammadelta+ lymphocytes. J Parasitol. (2002) 88:59–68. doi: 10.1645/0022-3395(2002)088[0059:PFAPVG]2.0.CO;2
73. Ho M, Webster HK, Tongtawe P, Pattanapanyasat K, Weidanz WP. Increased gamma delta T cells in acute Plasmodium falciparum malaria. Immunol Lett. (1990) 25:139–41. doi: 10.1016/0165-2478(90)90105-Y
74. Sinden RE, Billingsley PF. Plasmodium invasion of mosquito cells: hawk or dove? Trends Parasitol. (2001) 17:209–12. doi: 10.1016/S1471-4922(01)01928-6
75. Dinglasan RR, Devenport M, Florens L, Johnson JR, McHugh CA, Donnelly-Doman M, et al. The Anopheles gambiae adult midgut peritrophic matrix proteome. Insect Biochem Mol Biol. (2009) 39:125–34. doi: 10.1016/j.ibmb.2008.10.010
76. Shao L, Devenport M, Jacobs-Lorena M. The peritrophic matrix of hematophagous insects. Arch Insect Biochem Physiol. (2001) 47:119–25. doi: 10.1002/arch.1042
77. Vinetz JM, Dave SK, Specht CA, Brameld KA, Xu B, Hayward R, et al. The chitinase PfCHT1 from the human malaria parasite Plasmodium falciparum lacks proenzyme and chitin-binding domains and displays unique substrate preferences. Proc Natl Acad Sci USA. (1999) 96:14061–6. doi: 10.1073/pnas.96.24.14061
78. Garver LS, de Almeida Oliveira G, Barillas-Mury C. The JNK pathway is a key mediator of Anopheles gambiae antiplasmodial immunity. PLoS Pathog. (2013) 9:e1003622. doi: 10.1371/journal.ppat.1003622
79. Kumar S, Gupta L, Han YS, Barillas-Mury C. Inducible peroxidases mediate nitration of anopheles midgut cells undergoing apoptosis in response to Plasmodium invasion. J Biol Chem. (2004) 279:53475–82. doi: 10.1074/jbc.M409905200
80. Blandin S, Shiao SH, Moita LF, Janse CJ, Waters AP, Kafatos FC, et al. Complement-like protein TEP1 is a determinant of vectorial capacity in the malaria vector Anopheles gambiae. Cell (2004) 116:661–70. doi: 10.1016/S0092-8674(04)00173-4
81. Moita LF, Wang-Sattler R, Michel K, Zimmermann T, Blandin S, Levashina EA, et al. In vivo identification of novel regulators and conserved pathways of phagocytosis in A. gambiae. Immunity (2005) 23:65–73. doi: 10.1016/j.immuni.2005.05.006
82. Shokal U, Eleftherianos I. Evolution and function of thioester-containing proteins and the complement system in the innate immune response. Front Immunol. (2017) 8:759. doi: 10.3389/fimmu.2017.00759
83. Ukegbu CV, Giorgalli M, Yassine H, Ramirez JL, Taxiarchi C, Barillas-Mury C, et al. Plasmodium berghei P47 is essential for ookinete protection from the Anopheles gambiae complement-like response. Sci Rep. (2017) 7:6026. doi: 10.1038/s41598-017-05917-6
84. van Dijk MR, van Schaijk BC, Khan SM, van Dooren MW, Ramesar J, Kaczanowski S, et al. Three members of the 6-cys protein family of Plasmodium play a role in gamete fertility. PLoS Pathog. (2010) 6:e1000853. doi: 10.1371/journal.ppat.1000853
85. Richman AM, Dimopoulos G, Seeley D, Kafatos FC. Plasmodium activates the innate immune response of Anopheles gambiae mosquitoes. EMBO J. (1997) 16:6114–9. doi: 10.1093/emboj/16.20.6114
86. Smith RC, Eappen AG, Radtke AJ, Jacobs-Lorena M. Regulation of anti-Plasmodium immunity by a LITAF-like transcription factor in the malaria vector Anopheles gambiae. PLoS Pathog. (2012) 8:e1002965. doi: 10.1371/journal.ppat.1002965
87. Oduol F, Xu J, Niare O, Natarajan R, Vernick KD. Genes identified by an expression screen of the vector mosquito Anopheles gambiae display differential molecular immune response to malaria parasites and bacteria. Proc Natl Acad Sci USA. (2000) 97:11397–402. doi: 10.1073/pnas.180060997
88. Dong Y, Aguilar R, Xi Z, Warr E, Mongin E, Dimopoulos G. Anopheles gambiae immune responses to human and rodent Plasmodium parasite species. PLoS Pathog. (2006) 2:e52. doi: 10.1371/journal.ppat.0020052
89. Greenwood B. Malaria vaccines. Evaluation and implementation. Acta Trop. (2005) 95:298–304. doi: 10.1016/j.actatropica.2005.04.017
90. Webster D, Hill AV. Progress with new malaria vaccines. Bull World Health Organ. (2003) 81:902–9. Available online at: https://www.scielosp.org/article/bwho/2003.v81n12/902-909/#ModalArticles
91. Healer J, McGuinness D, Hopcroft P, Haley S, Carter R, Riley E. Complement-mediated lysis of Plasmodium falciparum gametes by malaria-immune human sera is associated with antibodies to the gamete surface antigen Pfs230. Infect Immun. (1997) 65:3017–23.
92. Kaslow DC. Transmission-blocking immunity against malaria and other vector-borne diseases. Curr Opin Immunol. (1993) 5:557–65. doi: 10.1016/0952-7915(93)90037-S
93. Ponnudurai T, Van Gemert G, Bensink T, Lensen A, Meuwissen JT. Transmission blockade of Plasmodium falciparum: its variability with gametocyte numbers and concentration of antibody. Trans R Soc Trop Med Hyg. (1987) 81:491–3. doi: 10.1016/0035-9203(87)90172-6
94. Kaslow DC. Immunogenicity of Plasmodium falciparum sexual stage antigens: implications for the design of a transmission blocking vaccine. Immunol Lett. (1990) 25:83–6. doi: 10.1016/0165-2478(90)90096-9
95. Stowers A, Carter R. Current developments in malaria transmission-blocking vaccines. Expert Opin Biol Ther. (2001) 1:619–28. doi: 10.1517/14712598.1.4.619
96. Tsuboi T, Tachibana M, Kaneko O, Torii M. Transmission-blocking vaccine of vivax malaria. Parasitol Int. (2003) 52:1–11. doi: 10.1016/S1383-5769(02)00037-5
97. Carter R, Graves PM, Keister DB, Quakyi IA. Properties of epitopes of Pfs 48/45, a target of transmission blocking monoclonal antibodies, on gametes of different isolates of Plasmodium falciparum. Parasite Immunol. (1990) 12:587–603. doi: 10.1111/j.1365-3024.1990.tb00990.x
98. Kaushal DC, Carter R, Howard RJ, McAuliffe FM. Characterization of antigens on mosquito midgut stages of Plasmodium gallinaceum. I Zygote surface antigens. Mol Biochem Parasitol. (1983) 8:53–69. doi: 10.1016/0166-6851(83)90034-8
99. Kapulu M, Da D, Miura K, Li Y, Blagborough A, Churcher T, et al. Comparative assessment of transmission-blocking vaccine candidates against Plasmodium falciparum. Sci Rep. (2015) 5:11193. doi: 10.1038/srep11193
100. Eksi S, Czesny B, van Gemert GJ, Sauerwein RW, Eling W, Williamson KC. Malaria transmission-blocking antigen, Pfs230, mediates human red blood cell binding to exflagellating male parasites and oocyst production. Mol Microbiol. (2006) 61:991–8. doi: 10.1111/j.1365-2958.2006.05284.x
101. Farrance CE, Rhee A, Jones RM, Musiychuk K, Shamloul M, Sharma S, et al. A plant-produced Pfs230 vaccine candidate blocks transmission of Plasmodium falciparum. Clin Vaccine Immunol. (2011) 18:1351–7. doi: 10.1128/CVI.05105-11
102. Amlabu E, Mensah-Brown H, Nyarko PB, Akuh OA, Opoku G, Ilani P, et al. Functional characterization of Plasmodium falciparum surface related antigen (PfSRA) as a potential blood-stage vaccine target. J Infect Dis. (2018) 218:778–90. doi: 10.1093/infdis/jiy222
103. Scholz SM, Simon N, Lavazec C, Dude M-A, Templeton TJ, Pradel G. PfCCp proteins of Plasmodium falciparum: gametocyte-specific expression and role in complement-mediated inhibition of exflagellation. Int J Parasitol. (2008) 38:327–40. doi: 10.1016/j.ijpara.2007.08.009
104. Hermand P, Cicéron L, Pionneau C, Vaquero C, Combadière C, Deterre P. Plasmodium falciparum proteins involved in cytoadherence of infected erythrocytes to chemokine CX3CL1. Sci Rep. (2016) 6:33786. doi: 10.1038/srep33786
105. Tibúrcio M, Dixon MW, Looker O, Younis SY, Tilley L, Alano P. Specific expression and export of the Plasmodium falciparum gametocyte exported protein-5 marks the gametocyte ring stage. Malar J. (2015) 14:334. doi: 10.1186/s12936-015-0853-6
106. Tsai YL, Hayward RE, Langer RC, Fidock DA, Vinetz JM. Disruption of Plasmodium falciparum chitinase markedly impairs parasite invasion of mosquito midgut. Infect Immun. (2001) 69:4048–54. doi: 10.1128/IAI.69.6.4048-4054.2001
107. Yuda M, Yano K, Tsuboi T, Torii M, Chinzei Y. von Willebrand Factor A domain-related protein, a novel microneme protein of the malaria ookinete highly conserved throughout Plasmodium parasites. Mol Biochem Parasitol. (2001) 116:65–72. doi: 10.1016/S0166-6851(01)00304-8
108. Ramakrishnan C, Dessens JT, Armson R, Pinto SB, Talman AM, Blagborough AM, et al. Vital functions of the malarial ookinete protein CTRP reside in the A domains. Int J Parasitol. (2011) 41:1029–39. doi: 10.1016/j.ijpara.2011.05.007
109. Kadota K, Ishino T, Matsuyama T, Chinzei Y, Yuda M. Essential role of membrane-attack protein in malarial transmission to mosquito host. Proc Natl Acad Sci USA. (2004) 101:16310–5. doi: 10.1073/pnas.0406187101
110. Dessens JT, Siden-Kiamos I, Mendoza J, Mahairaki V, Khater E, Vlachou D, et al. SOAP, a novel malaria ookinete protein involved in mosquito midgut invasion and oocyst development. Mol Microbiol. (2003) 49:319–29. doi: 10.1046/j.1365-2958.2003.03566.x
111. Espinosa DA, Vega-Rodriguez J, Flores-Garcia Y, Noe AR, Muñoz C, Coleman R, et al. The Plasmodium falciparum cell-traversal protein for ookinetes and sporozoites as a candidate for preerythrocytic and transmission-blocking vaccines. Infect Immun. (2017) 85:e00498–16. doi: 10.1128/IAI.00498-16
112. Armistead JS, Morlais I, Mathias DK, Jardim JG, Joy J, Fridman A, et al. Antibodies to a single, conserved epitope in Anopheles APN1 inhibit universal transmission of Plasmodium falciparum and Plasmodium vivax malaria. Infect Immun. (2014) 82:818–29. doi: 10.1128/IAI.01222-13
113. Dinglasan RR, Kalume DE, Kanzok SM, Ghosh AK, Muratova O, Pandey A, et al. Disruption of Plasmodium falciparum development by antibodies against a conserved mosquito midgut antigen. Proc Natl Acad Sci USA. (2007) 104:13461–6. doi: 10.1073/pnas.0702239104
114. Mathias DK, Jardim JG, Parish LA, Armistead JS, Trinh HV, Khumpitak C, et al. Differential roles of an Anopheline midgut GPI-anchored protein in mediating Plasmodium falciparum and Plasmodium vivax ookinete invasion. Infect Genet Evol. (2014) 28:635–47. doi: 10.1016/j.meegid.2014.05.025
115. Mathias DK, Plieskatt JL, Armistead JS, Bethony JM, Abdul-Majid KB, McMillan A, et al. Expression, immunogenicity, histopathology, and potency of a mosquito-based malaria transmission-blocking recombinant vaccine. Infect Immun. (2012) 80:1606–14. doi: 10.1128/IAI.06212-11
116. Lavazec C, Boudin C, Lacroix R, Bonnet S, Diop A, Thiberge S, et al. Carboxypeptidases B of Anopheles gambiae as targets for a Plasmodium falciparum transmission-blocking vaccine. Infect Immun. (2007) 75:1635–42. doi: 10.1128/IAI.00864-06
117. Raz A, Dinparast Djadid N, Zakeri S. Molecular characterization of the Carboxypeptidase B1 of Anopheles stephensi and its evaluation as a target for transmission-blocking vaccines. Infect Immun. (2013) 81:2206–16. doi: 10.1128/IAI.01331-12
118. Williams AR, Zakutansky SE, Miura K, Dicks MJD, Churcher TS, Jewell KE, et al. Immunization against a serine protease inhibitor reduces intensity of Plasmodium berghei infection in mosquitoes. Int J Parasitol. (2013) 43:869–74. doi: 10.1016/j.ijpara.2013.06.004
119. Ghosh AK, Devenport M, Jethwaney D, Kalume DE, Pandey A, Anderson VE, et al. Malaria parasite invasion of the mosquito salivary gland requires interaction between the Plasmodium TRAP and the Anopheles saglin proteins. PLoS Pathog. (2009) 5:e1000265. doi: 10.1371/journal.ppat.1000265
120. Okulate MA, Kalume DE, Reddy R, Kristiansen T, Bhattacharyya M, Chaerkady R, et al. Identification and molecular characterization of a novel protein Saglin as a target of monoclonal antibodies affecting salivary gland infectivity of Plasmodium sporozoites. Insect Mol Biol. (2007) 16:711–22. doi: 10.1111/j.1365-2583.2007.00765.x
121. Nikolaeva D, Illingworth JJ, Miura K, Alanine DG, Brian IJ, Li Y, et al. Functional characterization and comparison of Plasmodium falciparum proteins as targets of transmission-blocking antibodies. Mol Cell Proteomics (2017) doi: 10.1074/mcp.RA117.000036. [Epub ahead of print].
122. Chaturvedi N, Bharti PK, Tiwari A, Singh N. Strategies and recent development of transmission-blocking vaccines against Plasmodium falciparum. Indian J Med Res. (2016) 143:696–711. doi: 10.4103/0971-5916.191927
123. Mahmoudi S, Keshavarz H. Efficacy of phase 3 trial of RTS, S/AS01 malaria vaccine: the need for an alternative development plan. Hum Vaccin Immunother. (2017) 13:2098–101. doi: 10.1080/21645515.2017.1295906
124. Drakeley CJ, Eling W, Teelen K, Bousema JT, Sauerwein R, Greenwood BM, et al. Parasite infectivity and immunity to Plasmodium falciparum gametocytes in Gambian children. Parasite Immunol. (2004) 26:159–65. doi: 10.1111/j.0141-9838.2004.00696.x
125. Shimp RLJr, Rowe C, Reiter K, Chen B, Nguyen V, Aebig J, et al. Development of a Pfs25-EPA malaria transmission blocking vaccine as a chemically conjugated nanoparticle. Vaccine (2013) 31:2954–62. doi: 10.1016/j.vaccine.2013.04.034
126. Jones RM, Chichester JA, Mett V, Jaje J, Tottey S, Manceva S, et al. A plant-produced Pfs25 VLP malaria vaccine candidate induces persistent transmission blocking antibodies against Plasmodium falciparum in immunized mice. PLoS ONE (2013) 8:e79538. doi: 10.1371/journal.pone.0079538
127. Chichester JA, Green BJ, Jones RM, Shoji Y, Miura K, Long CA, et al. Safety and immunogenicity of a plant-produced Pfs25 virus-like particle as a transmission blocking vaccine against malaria: A Phase 1 dose-escalation study in healthy adults. Vaccine (2018) 36:5865–71. doi: 10.1016/j.vaccine.2018.08.033
128. Talaat KR, Ellis RD, Hurd J, Hentrich A, Gabriel E, Hynes NA, et al. Safety and immunogenicity of Pfs25-EPA/Alhydrogel(R), a transmission blocking vaccine against Plasmodium falciparum: an open label study in malaria naive adults. PLoS ONE (2016) 11:e0163144. doi: 10.1371/journal.pone.0163144
129. Menon V, Kapulu MC, Taylor I, Jewell K, Li Y, Hill F, et al. Assessment of antibodies induced by multivalent transmission-blocking malaria vaccines. Front Immunol. (2018) 8:1998. doi: 10.3389/fimmu.2017.01998
130. Li Y, Leneghan DB, Miura K, Nikolaeva D, Brian IJ, Dicks MD, et al. Enhancing immunogenicity and transmission-blocking activity of malaria vaccines by fusing Pfs25 to IMX313 multimerization technology. Sci Rep. (2016) 6:18848. doi: 10.1038/srep18848
131. Malkin EM, Durbin AP, Diemert DJ, Sattabongkot J, Wu Y, Miura K, et al. Phase 1 vaccine trial of Pvs25H: a transmission blocking vaccine for Plasmodium vivax malaria. Vaccine (2005) 23:3131–8. doi: 10.1016/j.vaccine.2004.12.019
132. Pereira VB, Zurita-Turk M, Saraiva TDL, De Castro CP, Souza BM, Agresti PM, et al. DNA vaccines approach: from concepts to applications. World J Vaccines (2014) 4:50. doi: 10.4236/wjv.2014.42008
133. Ura T, Okuda K, Shimada M. Developments in viral vector-based vaccines. Vaccines (2014) 2:624–41. doi: 10.3390/vaccines2030624
134. Azmi F, Ahmad Fuaad AAH, Skwarczynski M, Toth I. Recent progress in adjuvant discovery for peptide-based subunit vaccines. Hum Vaccin Immunother. (2014) 10:778–96. doi: 10.4161/hv.27332
135. Wykes M, Good MF. A case for whole-parasite malaria vaccines. Int J Parasitol. (2007) 37:705–12. doi: 10.1016/j.ijpara.2007.02.007
136. An C, Budd A, Kanost MR, Michel K. Characterization of a regulatory unit that controls melanization and affects longevity of mosquitoes. Cell Mol Life Sci. (2011) 68:1929–39. doi: 10.1007/s00018-010-0543-z
137. Michel K, Budd A, Pinto S, Gibson TJ, Kafatos FC. Anopheles gambiae SRPN2 facilitates midgut invasion by the malaria parasite Plasmodium berghei. EMBO Rep. (2005) 6:891–7. doi: 10.1038/sj.embor.7400478
138. Tarlinton D, Good-Jacobson K. Diversity among memory B cells: origin, consequences, and utility. Science (2013) 341:1205–11. doi: 10.1126/science.1241146
139. Lasonder E, Rijpma SR, van Schaijk BC, Hoeijmakers WA, Kensche PR, Gresnigt MS, et al. Integrated transcriptomic and proteomic analyses of P. falciparum gametocytes: molecular insight into sex-specific processes and translational repression. Nucleic Acids Res. (2016) 44:6087–101. doi: 10.1093/nar/gkw536
140. Reid AJ, Talman AM, Bennett HM, Gomes AR, Sanders MJ, Illingworth CJR. Single-cell RNA-seq reveals hidden transcriptional variation in malaria parasites. Elife (2018) 7:e33105. doi: 10.7554/eLife.33105
141. Suarez-Cortes P, Sharma V, Bertuccini L, Costa G, Bannerman NL, Sannella AR, et al. Comparative proteomics and functional analysis reveal a role of Plasmodium falciparum osmiophilic bodies in malaria parasite transmission. Mol Cell Proteomics (2016) 15:3243–55. doi: 10.1074/mcp.M116.060681
142. Conway DJ. Paths to a malaria vaccine illuminated by parasite genomics. Trends Genet. (2015) 31:97–107. doi: 10.1016/j.tig.2014.12.005
143. He Y. Omics-based systems vaccinology for vaccine target identification. Drug Dev Res. (2012) 73:559–68. doi: 10.1002/ddr.21049
144. Bah SY, Morang'a CM, Kengne-Ouafo JA, Amenga–Etego L, Awandare GA. Highlights on the application of genomics and bioinformatics in the fight against infectious diseases: challenges and opportunities in Africa. Front Genet. (2018) 9:575. doi: 10.3389/fgene.2018.00575
145. Masignani V, Rappuoli R, Pizza M. Reverse vaccinology: a genome-based approach for vaccine development. Expert Opin Biol Ther. (2002) 2:895–905. doi: 10.1517/14712598.2.8.895
146. Rappuoli R. Reverse vaccinology, a genome-based approach to vaccine development. Vaccine (2001) 19:2688–91. doi: 10.1016/S0264-410X(00)00554-5
147. Rappuoli R. Bridging the knowledge gaps in vaccine design. Nat Biotechnol. (2007) 25:1361. doi: 10.1038/nbt1207-1361
148. Davies DH, Duffy P, Bodmer J-L, Felgner PL, Doolan DL. Large screen approaches to identify novel malaria vaccine candidates. Vaccine (2015) 33:7496–505. doi: 10.1016/j.vaccine.2015.09.059
149. Grubaugh D, Flechtner JB, Higgins DE. Proteins as T cell antigens: methods for high-throughput identification. Vaccine (2013) 31:3805–10. doi: 10.1016/j.vaccine.2013.06.046
150. Sidney J, Assarsson E, Moore C, Ngo S, Pinilla C, Sette A, et al. Quantitative peptide binding motifs for 19 human and mouse MHC class I molecules derived using positional scanning combinatorial peptide libraries. Immunome Res. (2008) 4:2. doi: 10.1186/1745-7580-4-2
151. Lavazec C, Sanyal S, Templeton TJ. Expression switching in the stevor and Pfmc-2TM superfamilies in Plasmodium falciparum. Mol Microbiol. (2007) 64:1621–34. doi: 10.1111/j.1365-2958.2007.05767.x
152. Chan J-A, Fowkes FJI, Beeson JG. Surface antigens of Plasmodium falciparum-infected erythrocytes as immune targets and malaria vaccine candidates. Cell Mol Life Sci. (2014) 71:3633–57. doi: 10.1007/s00018-014-1614-3
153. Goel S, Palmkvist M, Moll K, Joannin N, Lara P, Akhouri RR, et al. RIFINs are adhesins implicated in severe Plasmodium falciparum malaria. Nat Med. (2015) 21:314. doi: 10.1038/nm.3812
154. Khattab A, Bonow I, Schreiber N, Petter M, Schmetz C, Klinkert MQ. Plasmodium falciparum variant STEVOR antigens are expressed in merozoites and possibly associated with erythrocyte invasion. Malar J. (2008) 7:137. doi: 10.1186/1475-2875-7-137
155. Niang M, Bei AK, Madnani KG, Pelly S, Dankwa S, Kanjee U, et al. STEVOR is a Plasmodium falciparum erythrocyte binding protein that mediates merozoite invasion and rosetting. Cell Host Microbe (2014) 16:81–93. doi: 10.1016/j.chom.2014.06.004
156. Nunes-Alves C. RIFINs promote rosette formation during malaria. Nat Rev Microbiol. (2015) 13:250. doi: 10.1038/nrmicro3472
157. Yam XY, Niang M, Madnani KG, Preiser PR. Three is a crowd–new insights into rosetting in Plasmodium falciparum. Trends Parasitol. (2017) 33:309–20. doi: 10.1016/j.pt.2016.12.012
Keywords: Plasmodium falciparum, gametocytes, humoral immunity, cellular immunity, mosquito immunity
Citation: Kengne-Ouafo JA, Sutherland CJ, Binka FN, Awandare GA, Urban BC and Dinko B (2019) Immune Responses to the Sexual Stages of Plasmodium falciparum Parasites. Front. Immunol. 10:136. doi: 10.3389/fimmu.2019.00136
Received: 20 August 2018; Accepted: 16 January 2019;
Published: 11 February 2019.
Edited by:
Ashraful Haque, QIMR Berghofer Medical Research Institute, AustraliaReviewed by:
Adrian John Frederick Luty, Institut de recherche pour le développement (IRD), FranceMelissa Chola Kapulu, University of Oxford, United Kingdom
Copyright © 2019 Kengne-Ouafo, Sutherland, Binka, Awandare, Urban and Dinko. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bismarck Dinko, YmRpbmtvQHVoYXMuZWR1Lmdo
 Jonas A. Kengne-Ouafo
Jonas A. Kengne-Ouafo Colin J. Sutherland
Colin J. Sutherland Fred N. Binka3
Fred N. Binka3 Bismarck Dinko
Bismarck Dinko