- 1Department of Parasitology of Zhongshan School of Medicine, Sun Yat-sen University, Guangzhou, China
- 2Key Laboratory of Tropical Disease Control (SYSU), Ministry of Education, Guangzhou, China
- 3Provincial Engineering Technology Research Center for Biological Vector Control, Guangzhou, China
Extracellular vesicles (EVs) are small membrane-surrounded structures released by different kinds of cells (normal, diseased, and transformed cells) in vivo and in vitro that contain large amounts of important substances (such as lipids, proteins, metabolites, DNA, RNA, and non-coding RNA (ncRNA), including miRNA, lncRNA, tRNA, rRNA, snoRNA, and scaRNA) in an evolutionarily conserved manner. EVs, including exosomes, play a role in the transmission of information, and substances between cells that is increasingly being recognized as important. In some infectious diseases such as parasitic diseases, EVs have emerged as a ubiquitous mechanism for mediating communication during host-parasite interactions. EVs can enable multiple modes to transfer virulence factors and effector molecules from parasites to hosts, thereby regulating host gene expression, and immune responses and, consequently, mediating the pathogenic process, which has made us rethink our understanding of the host-parasite interface. Thus, here, we review the present findings regarding EVs (especially exosomes) and recognize the role of EVs in host-parasite interactions. We hope that a better understanding of the mechanisms of parasite-derived EVs may provide new insights for further diagnostic biomarker, vaccine, and therapeutic development.
Introduction
To date, parasitic diseases remain a notable worldwide problem threatening human health; such diseases include schistosomiasis, malaria, toxoplasmosis, leishmaniasis, and trichomoniasis. Since these diseases mainly occur in developing countries and poor regions, they are somewhat neglected, although they burden many people, and cause multiple deaths every year. In addition, the problem of drug resistance has led to growing concerns due to the overuse of antiparasitic medicines (1). As a result, increasing awareness of parasites and finding better ways to cope with parasitic infections remain imperative. During long-term coevolution with different host species, parasites can adopt complex strategies of communication, manipulate, and even hijack the host immune system, and generally exhibit an ability to drive a physiological and immunological homeostasis that benefits their survival in the host (2). Thus, it is particularly important to recognize how parasites influence immune status through cell-cell communication and promote their survival in hosts to achieve long-term parasitism. Previous studies have mainly focused on the secretion of parasitic signaling molecules, such as proteins, glycans, lipids, and nucleic acids, involved in intercellular communication and promoting the host Th2 immune response to modulate the body's immune functions (3). However, long-term studies have not found a single parasite-derived substance that plays a decisive role in parasite-host interactions. A parasite is more likely to release these modulatory molecules in a variety of “packages” and express a dominant function (4). In recent years, with the discovery of extracellular vesicles (EVs) and their importance in cellular crosstalk, researchers have focused their attention on EVs in parasitic diseases.
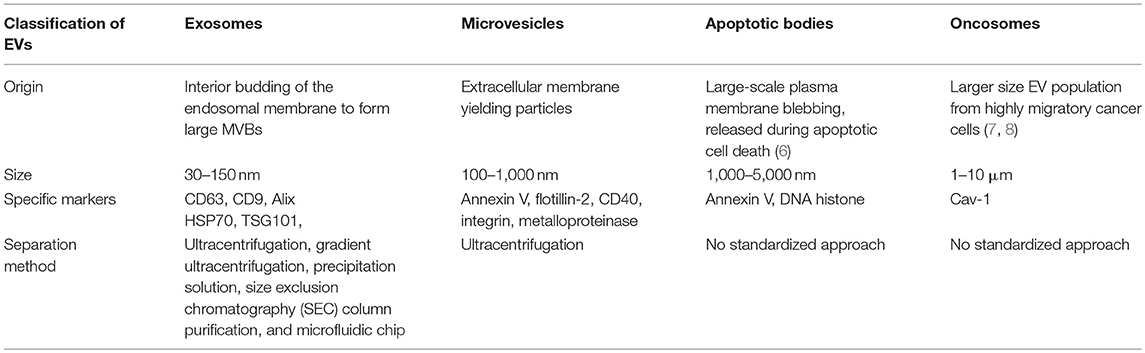
EVs are small membrane vesicles derived from the endocytic compartment of different kinds of cells such as reticulocytes, karyocytes, and platelets, which are present in most human bodily fluids, including blood, cerebrospinal fluid (CSF), sputum, saliva, ascites, amniotic fluid, bile, semen, breast milk, and urine (5). As pockets, EVs carry a series of bioactive cargos consisting of lipids, proteins, metabolites, DNA, and RNA (mRNA, miRNA, and ncRNA) and can be divided into four important types according to the mechanism of their generation and their size: (1) exosomes, (2) microvesicles, (3) apoptotic bodies, and (4) oncosomes (Table 1) (9).
Exosomes are the smallest EVs but have attracted attention from scientists (10). Exosomes measure between 40 and 100 nm, have a density between 1.13 and 1.19 g/mL, and consist of cytoplasm enclosed in a lipid bilayer with transmembrane proteins exposed to the extracellular environment (11). Exosomes are made up through the fusion of multivesicular bodies (MVBs) and intraluminal vesicles (ILVs) (12). Exosome release provides a mechanism of intercellular communication, and specific exosome functions depend on the derived-cell type and composition. More specifically, exosomes play roles in protein secretion, antigen presentation, shuttling of RNAs and infectious agents, and pathogen immune surveillance; furthermore, exosomes can even be used as vaccine candidates and biomarkers for the diagnosis and treatment of diseases. It is worth noting that not only parasite-derived exosomes but also exosomes released from host cells after parasite invasion are involved in the pathogenic mechanisms of parasitic diseases (13). On one hand, exosomes released from a parasite contain conserved parasite-specific information, including proteins, RNA, ncRNA, and nucleic acids, which are transferred to the host cells and can then modulate the host immune system, participate in immune escape by the parasite, and ultimately promote infection. On the other hand, when suffering from the stress of a parasitic infection, host cells also release exosomes that activate immune cells such as NK cells, macrophages, monocytes, T cells and B cells and play anti-infection roles. Thus, in this review, we give a comprehensive summary of EVs, especially exosomes, and focus mainly on their bidirectional regulatory effects on parasitic infections. Essential information about parasitic exosomes and exosomal molecules in this article is summarized in Table 2.
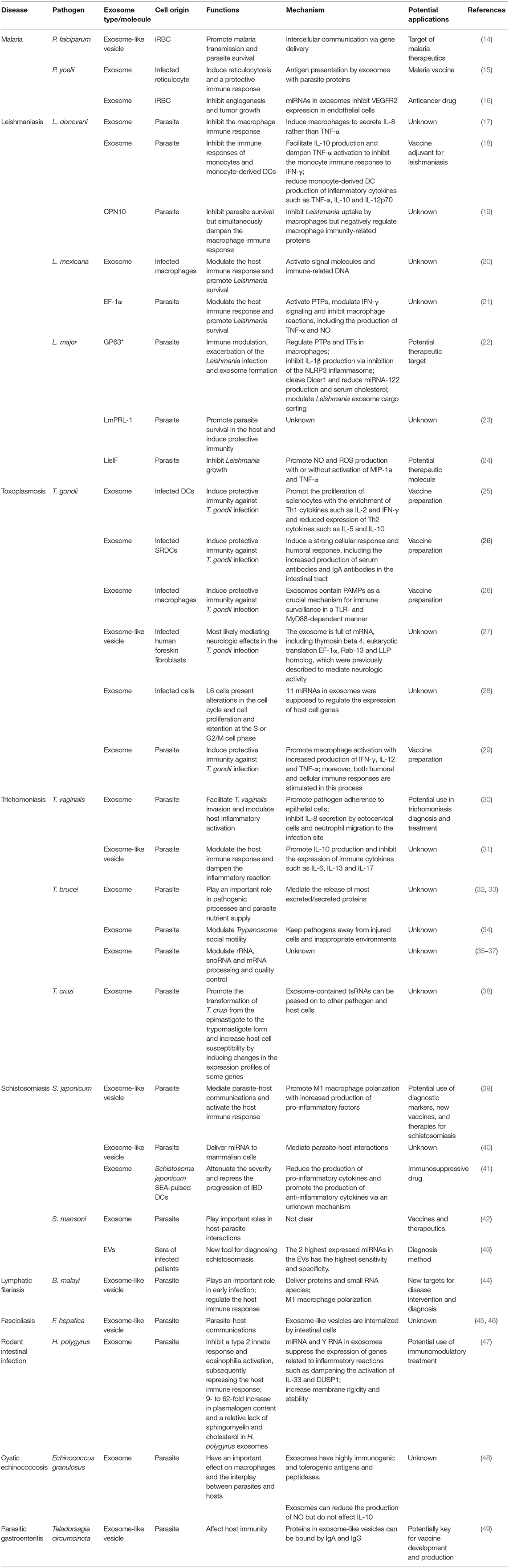
Table 2. Essential information on various exosomes and exosomal molecules in different parasitic diseases.
Background on Exosomes
Exosome Composition
The composition of exosomes varies depending on the originating cell type. However, all exosomes share many common protein components (50). Exosomes contain different proteins including Rabs, which cause exosome docking and fusion of the exosome membrane (51). Annexins and the lysobisphosphatidic acid (LBPA)-binding protein Alix may regulate membrane cytoskeleton dynamics and participate in vesicle formation, respectively (52). Heat shock proteins (HSPs) 70 and 90 can promote major histocompatibility complex (MHC)-I and MHC-II to cargo some peptides (53). Several adhesion molecules, such as CD146, CD9, CD18, and CD11, are also carried by exosomes (54, 55). In addition, the thioredoxin peroxidase II and galectin 3 proteins are involved in apoptosis. Tetraspanin markers, including CD9, CD63, CD81, and CD82, are important characteristic features of exosomes.
Lipids are essential components of exosomal membranes. Several studies have shown that exosomes contain 2–3 times the enrichment of cholesterol, SM, and glycosphingolipids relative to that in their parent cells (56). Moreover, lipid content also varies among different cell origins. For example, in mast cell-derived exosomes, the typical lipid composition includes lysophosphatidylcholine, sphingomyelin, phosphatidylcholine, phosphatidylethanolamine, phosphatidylserine, cholesterol, and diglyceride (56, 57). However, the ratios of these lipids in different cell types are different. Additionally, researchers have reported the presence of lipid rafts containing different acylated proteins, such as glycosyl-phosphatidylinositol-anchored proteins and tyrosine kinases of the Src family, on exosomes (58).
Furthermore, mRNAs, tRNAs, rRNAs, rRNA fragments such as natural antisense RNAs; microRNAs (miRNAs); small noncoding RNAs including piRNAs, snRNAs, snoRNAs, scaRNAs, and Y RNAs; and long noncoding RNAs have also been found in exosomes (59, 60). Single-stranded DNA, retrotransposon RNA transcripts, mitochondrial DNA, and oncogene amplifications have been detected in microvesicles, and previous studies have provided evidence that tumor-derived exosomes carry double-stranded DNA (61–63).
Exosome Biogenesis
Exosomes are membranous compartments that include various ILVs in MVBs for cargo degradation into lysosomes or for secretion as exosomes into the extracellular environment (64). The generation of exosomes is initiated in early endosomes upon endocytosis of extracellular material at the plasma membrane by intraluminal vesicle (ILV) formation, resulting in MVB formation (65). MVBs are intermediate but well-defined compartments that are formed from endosomes via invagination of the limiting endosomal membrane. The mechanisms for protein sorting to MVBs consist of target ubiquitination and preferential aggregation (12, 66). In addition, a key player in MVB biogenesis is the hetero-oligomeric protein complex, which is an endosomal-sorting complex required for transport (ESCRT). ESCRT-I, ESCRT-II, and ESCRT-III recognize monoubiquitinated cargos and promote their inclusion in MVBs (12, 56, 66, 67). Once MVBs are formed, their fusion with the plasma membrane is mediated by the cytoskeleton, fusion machinery such as SNARE proteins, and molecular switches such as low-molecular-weight GTPases. Upon fusion with the plasma membrane, the MVBs are released as exosomes (68).
Exosome Functions
Distinct functions of exosomes depend on their cellular origin. The roles of exosomes in immunoregulation can be summarized as antigen presentation, modulation of the host immune response, expression of some activated molecules, or complement factors causing immune surveillance and enhancing tumor cell invasion, and mediation of intercellular communication (69, 70).
In infectious diseases, several studies have demonstrated that pathogens such as viruses and prions can exploit exosomes to transfer some pathogen-derived molecules to host cells and cause immune evasion and virus spread (70, 71). In addition, pathogen-infected cells, or the pathogens themselves, release microvesicles or exosomes that can provide antigens to APCs and modulate both the innate and adaptive immune responses. For example, exosomes containing HIV Nef or Leishmania GP63 can block T cell activation and induce the apoptosis of certain immune effector cells. In some cases, this process benefits the pathogen, whereas in other cases, the host is benefited (15, 72).
Function of EVs in Parasite Diseases
EVs in Malaria
Malaria is a common parasitic disease caused by Plasmodium strains, whose clinical features include intermittent fever, vomiting, fatigue, and headache due to damage to erythrocytes. The extensive use of precautionary measures and various antimalarial drugs has decreased the morbidity and mortality of malaria dramatically, but to date, the disease still causes 2,000 human deaths every day (73). Previous research has shown that an increased level of circulating EVs is associated with malaria severity (74). Combes et al. found that knocking out the ABCA1 gene in a mouse model resulted in a decrease in EV production, protecting infected mice from cerebral malaria (75). These studies first demonstrated the importance of EVs in malaria. A pioneering study confirmed that microparticles derived from Plasmodium berghei-infected serum contained parasite antigens, which stimulated macrophage CD40 surface expression and TNF release in a dose-specific manner and induced remarkable immune responses via macrophage activation (76). Researchers isolated exosomes from the peripheral blood of BALB/c mice infected by reticulocyte-prone non-lethal Plasmodium yoelii, and proteomics revealed that these exosomes contained parasite proteins. Furthermore, immunization of mice with the abovementioned exosomes elicited IgG antibody production, inducing reticulocytosis, and a protective immune response (15). Due to their immunoregulatory functions, exosomes from Plasmodium types may be used as components in a malaria vaccine. In addition to regulating immune reactions, exosomes can mediate intercellular communication via nucleic acid delivery. It was reported that the exosome-like vesicles secreted by Plasmodium falciparum-infected red blood cells (iRBCs) can deliver DNA among themselves, resulting in an increased number of P. falciparum gametocytes and easier transmission to mosquito vectors (77, 78). Moreover, communication via exosome-like vesicles is beneficial to parasite survival in various situations such as stress or drug pressure. The P. falciparum-derived protein PfPTP2 is important in this process, and the disruption of PfPTP2 function reduces exosome-like vesicle production (78). Moreover, the exosome-like vesicles may be a potential therapeutic target to block parasite communication and reduce Plasmodium transmission. Immunization with P. yoelii-infected iRBC-derived EVs can be successfully used against lethal infection (15). Moreover, compared to stimulation by standard adjuvant vaccination, the administration of synthetic microparticles/microspheres loaded with antigens (e.g., merozoite surface protein-1, apical membrane antigen-1, or circumsporozoite protein) of Plasmodium vivax through the intranasal mucosal route can improve humoral and cell-mediated immune responses (79, 80). Furthermore, it was reported that exosomes from P. yoelii-infected plasma were capable of inhibiting the growth of Lewis lung cancer in a murine model. The researchers detected that a high level of miRNA in exosomes correlated with decreased VEGFR2 expression, thus restraining vessel formation in endothelial cells and ultimately leading to suppressed angiogenesis and tumor growth (16).
On the other hand, accumulating evidence suggests that host-origin EVs are related to the clinical symptoms and severity of malaria. The trans-bilayer distribution of phosphatidylserine at the outer leaflet of the plasma membrane can be modulated by the ATP-binding cassette transporter A1 (ABCA1). ABCA1 knockout mice, which have a lower ability to produce EVs, can significantly reduce the inflammatory response and relieve the severity of cerebral malaria (75). In human patients infected with P. falciparum, ABCA1 promoter haplotypes also influence the severity and complications of malaria (81). Moreover, many studies have reported that EV levels will increase during active Plasmodium infection. A significant increase in circulating EVs, including EVs originating from platelets, erythrocytes, and endothelial cells, has been shown in P. falciparum-infected patients with cerebral malaria in Cameroon and in India (82, 83). This relationship between active infection and increased EV release was also found in P. vivax-infected individuals in Brazil. A higher level of platelet-derived EVs correlated with high fever, suggesting that EV levels may play an important role in the inflammatory symptoms of P. vivax (84). These reports indicate that EVs may have applications as biomarkers of malaria severity.
EVs in Leishmaniasis
Leishmaniasis, a parasitic disease caused by the Leishmania genus (usually by Leishmania donovani, Leishmania infantum, or Leishmania chagasi) and spread through sandflies, can be divided into three types according to clinical features: cutaneous, mucocutaneous, and visceral leishmaniasis. Although it sickens millions of people in ~98 countries and causes 2 million new cases as well as 20–50 thousand deaths every year, leishmaniasis remains neglected because it mainly occurs in developing countries and poor regions (85, 86). Silverman et al. first identified 151 proteins that were apparently secreted by L. donovani through secretome analysis; interestingly, only 14% of these proteins were targeted for export and consisted of a classic eukaryotic amino-terminal secretion signal peptide. In contrast, a large number of eukaryotic exosome proteins were found, which suggested a vesicle-based pathway in Leishmania-derived intercellular communication (86). Sequentially, 329 Leishmania proteins have been detected and account for more than 52% of the general proteins secreted by Leishmania, which confirms vesicle-based secretion as the major protein secretion mechanism of Leishmania. Interestingly, changes in temperature and pH modulate the abundance and composition of Leishmania exosomes, and this phenomenon probably reflects different packages of virulence factors in different environments. In addition, the uptake of exosomes induces macrophages to secrete IL-8 rather than TNF-α through an unknown mechanism. This finding suggests that Leishmania exosomes function similarly to mammalian exosomes in long-range communication and immune modulation (17). Studies by Silverman et al. were the first to affirm exosome-based secretion in protozoans. Leishmania releases exosomes to modulate immune reactions, and macrophages also secrete exosomes to affect Leishmania organisms. Through a comparative proteomic and functional analysis, Leishmania mexicana was discovered to change the functions and targets of exosomes secreted by macrophages, resulting in the activation of signal molecules and immune-related DNA in a murine model (20). Recently, the role of exosomes in Leishmania-host interactions has been investigated in more detail. Similar to whole parasites, Leishmania exosomes can strongly influence macrophage cell signaling and function and were shown to be pro-inflammatory and to have the ability to recruit neutrophils at the inoculation site, exacerbating the resulting pathology (87). Leishmania exosomes may also impact the immune reactions of monocytes and dendritic cells (DCs) in addition to those of macrophages and neutrophils (88). Exosomes from Leishmania donovani facilitate IL-10 production and dampen TNF-α activation to inhibit the monocyte immune response to IFN-γ. In addition, monocyte-derived DCs are negatively regulated by exosomes, presenting decreased production of some inflammatory cytokines such as TNF-α and IL-12p70. Intriguingly, exosomes from L. donovani lacking HSP100 promote pro-inflammatory activation and protective immunity rather than suppressing the immune system, suggesting a significant role for HSP100 in exosomal protein sorting. Currently, one reason why vaccine preparations for Leishmania are problematic may be the deficiency of applicable adjuvants (18). Furthermore, Leishmania exosomes, as a kind of lipid adjuvant, may potentially be useful for vaccine preparations. It is worth mentioning that Leishmania exosomes are also related to antimony resistance (89).
Since many studies of Leishmania exosomes concern exosomal molecules, here, we focus on the descriptions of these molecules. Leishmania GP63, which is found on exosomes, is a zinc metalloprotease fixed on the surface of promastigotes by a GPI anchor. GP63 has been shown to play an important role in modulating the immune response of macrophages through the regulation of protein tyrosine phosphatases (PTPs) and transcription factors (TFs) (90). Studies have investigated differences in the macrophage immune reaction and exosome constitution between Leishmania major (WT) and L. major gp63-/- (KO). KO Leishmania presented a prominently decreased modulatory capacity and a large change in exosome protein sorting relative to WT Leishmania, which implied the critical role of GP63 in immune modulation and exosome formation (22). GP63 from L. donovani exosomes also cleaved the nuclease Dicer1 and consequently reduced miRNA-122 production, leading to a decreased level of serum cholesterol, and exacerbation of Leishmania infection (91). A recent study demonstrated that GP63 from L. mexicana exosomes decreases IL-1β production via inhibition of NLRP3 inflammasomes to suppress the host immune response (89). Another protein, elongation factor-1α (EF-1α), was also detected in Leishmania exosomes. EF-1α induces the activation of PTPs, leading to the negative modulation of IFN-γ signaling, and the inhibition of macrophage reactions, including the production of TNF-α and NO (21). A novel PRL-like phosphatase, LmPRL-1, was detected in L. major exosomes and was found to contribute to parasite survival in macrophages via an unknown mechanism (23). Another novel exosomal protein, chaperonin 10 (CPN10), which is released by L. donovani, was recently discovered to inhibit Leishmania uptake by macrophages and negatively regulate macrophage immunity-related proteins. Interestingly, it seems paradoxical that CPN10 inhibits parasite survival but simultaneously dampens the macrophage immune response, a phenomenon that is not well-explained by existing knowledge (19). Exosomal proteins such as GP63 and EF-1α function as immunosuppressive molecules and exacerbate Leishmania infection, and a recent study discovered a new type of exosomal molecule, L. infantum eukaryotic initiation factor (LieIF), which induces protective immunity (92). Both preinfection and postinfection applications of LieIF and IFN-γ promote NO and ROS production to inhibit Leishmania growth via a similar mechanism, although the former was correlated with the activation of macrophage inflammatory protein 1a (MIP-1a) and TNF-α, while the latter was not (24). The ability of LieIF to promote immune function and dampen Leishmania growth makes it a potential therapeutic molecule for leishmaniasis. In addition to proteins, non-coding RNAs (including rRNA, tRNA, and tRNA-derived small RNAs) were also found in exosomes secreted by Leishmania braziliensis and L. donovani, whereas siRNA was detected in only exosomes released by L. braziliensis (24). However, the functions of the non-coding RNAs in Leishmania exosomes remain unclear. In addition to producing exosomes in mammalian organisms, L. infantum and L. major were discovered to excrete exosomes in the midgut of sandflies via a mechanism resembling that in mammals (93). Exosomes and pathogens are coegested during biting by sandflies, probably accelerating the pathogenesis of leishmaniasis, especially cutaneous leishmaniasis. Coinjection of L. major and exosomes exacerbates inflammatory destruction, with increased levels of IL-17a and other inflammatory cytokines in the plasma. In Leishmania infection, IL-17a is involved in Th17 inflammation as well as neutrophil infiltration and leads to inflammatory lesions (94).
EVs in Toxoplasmosis
Toxoplasmosis is a common parasitic disease caused by Toxoplasma gondii. Most patients have no clinical symptoms, while people with impaired immune systems may develop severe symptoms, such as severe damage to the fetus in pregnant patients or life-threatening encephalitis in immunocompromised patients (95). An animal model experiment showed that the injection of exosomes released by T. gondii antigen-stimulated DCs promoted the proliferation of splenocytes with enrichment in Th1 cytokines such as IL-2 and IFN-rs and reduced the expression of Th2 cytokines such as IL-4, IL-5, and IL-10 (25). Potent Th1-biased immune responses protect the host from severe T. gondii infection, which indicates the potential use of antigen-stimulated DC-derived exosomes in vaccine preparations (96). Further studies revealed that exosomes secreted by SRDCs cause a murine protective immune response against T. gondii infection with a strong cellular response and humoral response in mice, including the increased production of serum antibodies and IgA antibodies in the intestinal tract (97). Macrophages may also excrete corresponding exosomes when infected by intracellular pathogens, including Salmonella typhimurium, M. tuberculosis, M. bovis BCG, and T. gondii. Exosomes contain pathogen-associated molecular patterns (PAMPs), which represent a crucial mechanism for immune surveillance that functions in a Toll-like receptor (TLR)-dependent and myeloid differentiation factor 88 (MyD88)-dependent manner (26). Another study showed that one kind of exosome-like vesicle was released by human foreskin fibroblasts infected by T. gondii. These vesicles were characterized by abundant miRNAs and a significant increase in mRNAs relative to those of uninfected fibroblasts. The most enriched mRNAs included thymosin beta 4, eukaryotic elongation factor-1α (EF-1α), Rab-13, and LLP homolog, which were previously described to mediate neurologic activity. These results indicated that T. gondii exosomes probably mediate neurologic effects in toxoplasmosis (27).
In addition, it was previously found that host cells infected by T. gondii present altered cell cycles and cell proliferation (98). Neighboring cells such as L6 cells, a rat myoblast cell line, manifested a similar phenomenon, and transient retention at the S or G2/M cell phase when incubated with exosomes secreted by T. gondii-infected cells, which suggested an exosome-mediated cell cycle and cell proliferation. Further experiments were conducted to detect 64 miRNAs with signal intensity changes, 11 of which were supposed to regulate the expression of host cell genes such as cyclin D2 and retinoblastoma 1, which are related to chromosome movement, centrosome movement, sister chromatid segregation, and other functions (28).
Wowk et al. first identified T. gondii-derived exosomes and performed proteomic profiling on them. A wide range of classical exosome proteins were discovered, but the exact functions of T. gondii exosomes and their proteins remained unknown (99). Further studies discovered that T. gondii-derived exosomes had a mean size of 50 nm and consisted of several notable protein markers, including HSP70, CD63, and P30 (29). HSP70 and CD63 have been previously described as exosome markers (100), and P30 is known as a major surface marker of T. gondii (101), which indicates that these isolated vesicles can be designated T. gondii-derived exosomes. In addition, the exosomes promoted macrophage activation with increased production of IFN-γ, IL-12, and TNF-α; moreover, both humoral and cellular immune responses were stimulated in this process, resulting in protective immunity against T. gondii infection (29).
EVs in Trichomoniasis
Trichomoniasis is a sexually transmitted disease caused by T. vaginalis with clinical genital symptoms such as itching, foul odor, and pain during sex or urination. Worse still, trichomoniasis can increase the incidence of HIV infection as well as prostate and cervical cancer. There are ~276 million new cases of trichomoniasis every year, and the disease mainly affects people who range from 15 to 49 years of age (102).
Previous studies have revealed three tetraspanins on the surface of T. vaginalis (103), and coincidently, tetraspanins are present in all mammalian exosomes and are used as a marker of exosomes (104). These results suggest that T. vaginalis may secrete exosomes as well. Olivia et al. first confirmed the existence of T. vaginalis exosomes and detected their composition, which not only included conserved exosomal proteins and RNA but also some specific parasite proteins (30). Adherence to epithelial cells is the first step for T. vaginalis to cause infection of the host (105). Exosomes released by highly adherent T. vaginalis were found to promote the adherence of poorly adherent species to epithelial cells. In addition to activating adherence, T. vaginalis exosomes may deliver regulatory molecules to the host and promote the modulation of host immune functions by inhibiting IL-8 secretion of ectocervical cells, consequently dampening neutrophil migration to infection sites (30). Another study demonstrated that exosome-like vesicles released by T. vaginalis were capable of inducing marked macrophage IL-10 production and a slight increase in IL-6 and TNF-α production. Pretreatment of these vesicles in a murine model also promoted production of IL-10 and inhibited the expression of immune cytokines such as IL-6, IL-13, and IL-17, suggesting that T. vaginalis-derived exosome-like vesicles modulate the host immune response and dampen inflammatory reactions (31).
EVs in Trypanosomiasis
Trypanosomiasis is a parasitic disease caused by organisms in the Trypanosoma genus, including Trypanosoma brucei, and Trypanosoma cruzi, of which the former causes African trypanosomiasis or sleeping sickness, while the latter causes American trypanosomiasis or Chagas disease (106). Trypanosomes were discovered to deliver various types of vesicles to the host, modulating the host immune response and promoting parasite survival (33). Most studies investigating Trypanosome exosomes have focused on T. brucei. A previous proteomic analysis revealed that a large portion of proteins secreted by T. brucei lack transit peptides, implying an unusual protein secretion pathway in this parasite (33). Later, investigators found that T. brucei mainly excreted proteins via an exosome-based pathway. T. brucei exosomes were found to mediate the release of most excreted/secreted proteins, including abundant proteases that functioned in the pathogenic process and affected parasite nutrient supply, indicating the important role of exosomes in T. brucei infection (32, 33). In addition, for the Trypanosoma genus, mRNA formation involves the process of trans-splicing, which needs the assistance of a kind of RNA termed a spliced leader RNA (SL RNA). When trans-splicing was compromised in T. brucei, SL RNA accumulated and was released via an exosome-based pathway (107). These exosomes mediated parasite-parasite communication, and only intact exosomes influenced Trypanosome social motility. It was hypothesized that these exosomes functioned as repellents to keep the pathogens away from injured cells and improper environments to influence parasite social motility (34). Moreover, a series of studies showed that exosomes secreted by T. brucei were capable of modulating rRNA, snoRNA, and mRNA processing and quality control (35–37). The experimental data also suggested that Trypanosome exosomes are present mainly in the nucleus, according to the expression of exosome subunits RRP6 and RRP44 (37).
In recent years, T. cruzi exosomes have also been discovered. Researchers identified small RNAs in T. cruzi exosomes and found that they were mainly derived from tRNAs and rRNAs. tRNA-derived small RNAs (tsRNAs) colocalize with an Argonaute protein that is distinctive of trypanosomatids and can be passed on to other T. cruzi or host cells, which may promote transformation of the parasite from the epimastigote form to the trypomastigote form and increase host cell susceptibility (108, 109). Further studies have found that tsRNA carried by exosomes may induce changes in the expression profiles of some genes in host cells (38).
EVs in Schistosomiasis
Schistosomiasis is caused by the Schistosoma genus (usually by Schistosoma japonicum, Schistosoma mansoni, or Schistosoma haematobium) and is one of the most important neglected tropical parasitic diseases worldwide. Schistosomiasis affects public health, second only to malaria, and sickens more than 200 million people and causes ~200 thousand deaths worldwide each year, especially in developing countries (110). The adult schistosome worm uses several immune-evasion strategies in the host and inhabits the mesenteric plexus in the portal system for a long time (111). Thus, detailed elucidation of the parasitism mechanism and biology of the schistosome are important for understanding the relationship between the host and schistosome, which may be useful for the identification of some novel biomarkers for schistosomiasis diagnosis and the development of new strategies to control schistosomiasis (112, 113). Recently, Wang et al. first isolated, identified and analyzed exosome-like vesicles derived from adult worms of S. japonicum and investigated their immune activities in macrophages. The results showed that the application of these vesicles prompted macrophage polarization into a classically activated form termed an M1 macrophage with increased production of pro-inflammatory factors such as TNF-α, CD16/32, and iNOS (39). Previous studies have demonstrated that the M1 macrophage plays an important role in killing parasites and preventing hepatic fibrosis (114). These results indicated that exosome-like vesicles secreted by adult worms of S. japonicum may be involved in activating the host immune response, which is different from the results of previous studies (39). Furthermore, 403 proteins in S. japonicum adult-worm-derived EVs were detected through proteomic analysis and found to possess the characteristics of catalytic activity, translation regulatory activity, binding activity and so on. In addition, these vesicles could also deliver miRNA to mammalian cells to mediate parasite-host interactions (40). Using the OptiPrep density gradient method, researchers have harvested a high yield of pure EVs in adult worms of S. mansoni and identified 130 schistosome proteins, as well as 143 miRNAs, some of which were detected in the sera of infected hosts (42, 115). In addition, to date, detecting eggs in stool or urine is still the “gold standard” for diagnosing schistosomiasis, although this detection is less sensitive in patients with a lower worm burden. On the other hand, using a serologic test increases the sensitivity but decreases the accuracy. Research by Meninger et al. provided a new diagnostic tool in patients with a low parasitic burden by detecting schistosome-specific miRNA, such as miR-2c-3p and bantam, isolated from EVs in sera from infected patients (43). Moreover, formerly DC-derived exosomes were found to modulate the overactivation of immune reactions in autoimmune diseases (69), and S. japonicum soluble egg antigen (SEA) was able to suppress colitis development in a murine model (116). Recent studies showed that the intraperitoneal injection of DC-derived exosomes pulsed by SEA was useful for attenuating the severity and repressing the progression of mouse inflammatory bowel disease (IBD), with decreased levels of pro-inflammatory cytokines such as IL-17a, IFN-γ, IL-22, IL-12, and TNF-α and increased levels of the anti-inflammatory cytokine TGF-β. Due to their anti-inflammatory effects, exosomes may have potential applications as immunosuppressive drugs (41). All of these results confirmed that schistosomes can directly secrete EVs, which may play important roles in host-schistosome interactions and be useful tools in the development of vaccines, diagnostics and therapeutics.
EVs in Lymphatic Filariasis
Lymphatic filariasis (LF) is caused by Filarioidea, including Brugia malayi, Wuchereria bancrofti, and Brugia timori, and is the most common vector-transmitted parasitic disease after malaria. LF is still a public health problem, affecting more than 120 million people in 74 countries to date (2016). Patients usually have no symptoms, but some of them may present with elephantiasis, a syndrome characterized by severe swelling of the arms, breasts, genitalia, or legs and affecting patients clinically and socially (117). However, although mass drug administration (MDA) has been widely used globally and has some effect on decreasing the infection rate, there is still no drug that is completely effective at killing adult filarial nematodes, which means there is still no useful cure for LF (118). Recently, an increasing number of studies have shown that EVs released from filaria may be involved in this process. Mostafa et al. first identified exosome-like vesicles secreted from the infective L3 stage of B. malayi by designating a set of proteins as exosome markers, such as actin, EF-1α, EF-2, Rab-1, and HSP70 (44). Furthermore, the vesicles were exosome-like because a proteomic analysis revealed that the proteins of B. malayi-derived vesicles and those of mammalian exosomes shared over 80% homology. Moreover, vesicles released by L3 B. malayi contained an abundance of small RNA species relative to those released by the adult stage of B. malayi, suggesting they played a more important role in early infection. When comparing miRNA expression, researchers discovered significantly increased expression of bma-let-7, bma-miR-9, bma-miR-1, bma-miR-92, and bma-miR-100b. Bma-let-7 was previously described to modulate the vertebrate immune response, suggesting that these vesicles regulate the host immune response through an miRNA-dependent pathway (119, 120). In addition, the internalization of exosome-like vesicles by macrophages can stimulate M1 macrophage polarization to regulate the host immune reaction (44). Recently, Hirunni H et al. found that the candidate site for EV release may be the excretory-secretory pore of microfilariae. Their results revealed quantitative and qualitative differences between male and female Brugia EVs and sex-specific differences in cargo consisting of immunomodulatory substances. Moreover, the results confirmed that inhibiting EV release may be a potential method to kill filaria (121).
EVs in Fascioliasis
Fascioliasis, a common helminthic zoonosis caused by Fasciola hepatica or Fasciola gigantica, mainly burdens ruminants such as sheep and cattle while sometimes affecting human beings. It is a noticeable public health problem with 2.4–17 million cases every year worldwide, especially in Europe, the Americas, Africa and Oceania (122). The parasite encysts in the small intestine and enters into the abdominal cavity after penetrating through the intestinal wall. Subsequently, the parasites in the abdominal cavity can migrate into the liver and ultimately penetrate into bile ducts (123).
Marcilla A. et al. first reported the presence of helminth-derived exosome-like vesicles that were found to contain species-specific proteins, including cytoskeletal proteins (i.e., actin, tubulin, myosin, paramyosin, tropomyosin), glycolytic enzymes (i.e., enolase, aldolase, GAPDH, PEPCK), calcium-binding proteins (i.e., calmodulin, calponin), and nuclear proteins (histones and elongation factors), as well as stress-related proteins (i.e., HSPs), detoxifying enzymes such as peroxiredoxins and host proteins including metabolic enzymes, immunoglobulins and typical exosomal molecules such as CD19 in F. hepatica. In addition, these exosome-like vesicles were found to be internalized by intestinal cells in culture and to mediate parasite-host communications (46). Researchers have confirmed that in addition to proteins, some potential immune-regulatory miRNAs were enriched in EVs of F. hepatica (124). Further studies confirmed the presence of exosome-like vesicles and demonstrated that these vesicles were shed from the apical plasma membrane via an ESCRT-dependent MVB pathway that occurred in the F. hepatica tegumental syncytium (45). Furthermore, the package of exosome-like vesicle cargo was regulated according to changes in the environment, probably implying an adaptive mechanism for F. hepatica survival and host immunosuppression (45).
EVs in Rodent Intestinal Nematode Infection
Heligmosomoides polygyrus is a kind of nematode parasite that mainly infects mice. It exclusively locates to the intestinal tract and promotes a Th2 immune reaction with immunosuppressive effects (125). It was discovered that H. polygyrus is capable of secreting miRNAs and Y RNAs through an exosome-based pathway. H. polygyrus exosomes contain various proteins such as HSPs and tetraspanins that are found naturally in mammalian exosomes. Local injection of these exosomes in a murine model inhibits the type 2 innate response and eosinophil activation, subsequently repressing the host immune response. In addition, an in vitro assay demonstrated that miRNAs and Y RNAs in exosomes internalized by epithelial cells dampen the activation of IL-33 (the alarmin receptor) and DUSP1 (the critical regulator of mitogen-activated protein kinase signaling) to inhibit immune reactions (47).
Further studies have compared the composition of exosomes secreted by H. polygyrus with those secreted by its murine host. Tandem mass spectrometry analysis showed a 9- to 62-fold increase in plasmalogen content and a relative lack of sphingomyelin and cholesterol in H. polygyrus exosomes. Biophysical analysis revealed that H. polygyrus exosomes consist of a stable and rigid membrane structure, although past studies have shown that decreased levels of sphingomyelin and cholesterol destabilize the membrane structure (126). These results suggested that plasmalogen might play an important role in increasing membrane rigidity and stability (127).
EVs in Cystic Echinococcosis
Cystic echinococcosis, a chronic zoonotic disease that is globally distributed in most pastoral and rangeland areas of the world, is induced by larval cestodes belonging to the genus Echinococcus, such as Echinococcus multilocularis and Echinococcus granulosus. Cystic echinococcosis features an asymptomatic incubation period that can last many years. Humans may contract this disease not only by ingesting soil or food contaminated with E. granulosus eggs but also by the hand-to-mouth transfer of eggs. The first cystic echinococcosis patient was observed in 1862 (128). From then on, cystic echinococcosis has become a noticeable parasitic disease that imposes an immense economic burden in a large number of countries (129). Mar Siles-Lucas et al. first reported that there were parasite exosome-enriched EV fractions in fertile hydatid cyst fluid (HF) and isolated, purified and characterized these exosomes. Both parasite-derived proteins (including antigen-5, severin/gelsolin/villin lipid transport protein, alpha-mannosidase, and malate-dehydrogenase) and host-origin proteins (including carbonic anhydrase, fructose-bisphosphate aldolase, peroxiredoxin, hemoglobin alpha and beta, pyruvate kinase, serum albumin, and triose phosphate isomerase) were identified in the EVs according to a proteomic analysis; the EVs carried virulence factors (including highly immunogenic and tolerogenic antigens and peptidases) that were associated with cyst survival. The results were the first to demonstrate that EVs may play an important role in infection by tape worms (130). In addition, Guilherme B. dos Santos et al. described a proteomic analysis and comparison of hydatid fertile samples that involved several proteins isolated from both the parasite and host during infection with E. granulosus fertile and infertile cysts in Bos Taurus lungs, aiming to highlight possible mechanisms involved in cyst fertility or infertility (131). In addition, the researchers found large differences between the proteomes. Therefore, the findings provided clues about the possible existence of an arms race involving parasites and host responses.
During early and chronic infections by Echinococcus species, nitric oxide (NO) is an important molecule that plays a role in downregulating host immunity (132). In particular, according to a report by Yadong Zheng (48), Ago1 and Ago4 expression by murine macrophage RAW264.7 cells was increased after transfection by E. multilocularis miR-71 (emu-miR-71) mimics. Interestingly, 12 h after treatment with LPS and IFN-γ, the production of NO was reduced, although the expression of IL-10 was not altered. Moreover, another study reported that the parasite can secrete exosomes containing miR-71 (133). EVs containing miR-71 may exert an important effect on macrophages, which introduces a new concept consisting of the interplay between parasites and hosts. However, the function of exosomes in host-parasite interactions in cystic echinococcosis is just beginning to be elucidated and still needs further investigation.
EVs in Teladorsagia circumcincta Infections
Teladorsagia circumcincta infection, a major cause of ovine parasitic gastroenteritis in temperate climatic regions, leads to considerable economic losses. Ovine parasitic gastroenteritis mainly occurs in temperate parts of the world. Patients contract the disease through contaminated fecal matter and show reduced weight and dehydration due to diarrhea. Recent research indicates a terrible phenomenon in which the anthelmintic resistance of T. circumcincta is significantly increasing due to the overuse or misuse of existing drugs. It is rather remarkable that the exact potential SNPs and mechanism of anthelmintic resistance need further investigation (134). Previous research has mainly involved sheep infected with T. circumcincta (133). Stear MJ et al. found that the important mechanism of pathogenesis in sheep infected with the nematode T. circumcincta was that the nematode can cause protein deficiency and a lower growth rate and thus lead to serious mucus production, hyperplasia and pepsinogenemia (135). T. Thomas Tzelos et al. isolated exosome-like EVs from excretory-secretory (ES) products secreted by fourth stage larvae (Tci-L4ES) of T. circumcincta and performed a preliminary proteomic characterization. In addition, the researchers found some specific proteins that were involved in structure, metabolism and other functions. Most importantly, in T. circumcincta-infected sheep, the research indicated that these proteins can be bound by IgA and IgG and are a potential key to vaccine development and production (49).
The Clinical Applications of Parasite EVs
Studies of EVs have been emerging constantly since their discovery in the 1980s. However, studies on parasite-derived EVs have been conducted for only a few years, and thus there is still much to be explored regarding the applications of parasite-derived EVs, giving rise to a new round of studies. All cell types can secrete EVs, and EVs are naturally found in different kinds of body fluids such as saliva, blood, urine, CSF, and even in milk. EVs were previously considered a waste disposal system but were subsequently found to be involved in cell-cell communication, thereby demonstrating multiple applications (136). Studies investigating the applications of parasite exosomes have mainly concentrated on the diagnosis and treatment of parasite diseases. Currently, we know that EVs are rich in lipids, proteins, small RNAs, and DNAs, many of which are available as molecular markers. Exosomes possess several major advantages as diagnostic markers, including (1) stable structure, (2) high content in plasma, and (3) alternative abundance and constitution under diverse conditions. Previously, EVs were found to be capable of helping diagnose malignant diseases because EVs contained a variety of tumor-specific molecules and shuttled them between cells to mediate intercellular communication. Specific EV molecules can be used as diagnostic markers for tumors, including NY-ESO-1 for lung cancer, HER2 for breast cancer, GGT1 for prostate cancer, glypican-1 for colorectal cancer, and CA-125 for ovarian cancer (137). For ovarian cancer, various exosomal cargos can be used as diagnostic markers, including (1) surface molecules such as EpCAM and L1CAM, (2) proteases such as ADAM10 and ADAM15, (3) tetraspanins, (4) HSPs, and (5) miRNAs such as miR-214, miR-140, and miR-147 (138). Unfortunately, in clinical practice, there is still no application for EVs in diagnosing parasite infections, and only a few studies have focused on the diagnostic use of EVs in parasitic diseases. Currently, we know that parasite EVs consist of a variety of molecules such as proteins, lipids, miRNAs, and DNAs (139). The detection of proteins, miRNAs or DNAs from isolated exosomes could represent a possible method to help diagnose parasitic infections. A recent study compared miRNA isolated from circulating exosomes in schistosomiasis patients and healthy controls and found a significant increase in 4 schistosomal miRNAs, including bantam, and miR-2c-3p (43). Traditional diagnostics for schistosomiasis are limited in patients with a low pathogen burden or cannot be used for patient follow-up (140, 141), indicating that the detection of miRNA from exosomes may have potential use for diagnosing patients with a low parasite burden or for follow-up of therapeutic effects (43).
Exosomes released by both parasites and host cells exert immunomodulatory properties, which has promoted investigations into their clinical applications for the treatment of diseases; currently, these applications mostly involve protection against tumors (142). For example, many researchers have demonstrated that DC-derived exosomes can stimulate patients' immune responses and promote the eradication of melanoma and non-small cell lung cancer (143, 144). With regard to parasite exosomes, as we mentioned, mice immunized with exosomes and infected by nonlethal P. yoelii elicit IgG antibody production, inducing reticulocytosis, and a protective immune response against malaria (15). T. gondii-derived exosomes may promote macrophage activation with increased production of IFN-γ, IL-12, and TNF-α; moreover, both humoral and cellular immune responses are stimulated in this process, resulting in protective immunity against T. gondii infection (29). Host cell-derived exosomes also execute immunoregulatory functions. For instance, DCs pulsed with T. gondii antigens induce the proliferation of splenocytes with Th1 cytokine enrichment and reduce the expression of Th2 cytokines, probably serving as a potent vaccine against toxoplasmosis (25). In addition to protecting against human parasitic infections, exosomes were discovered to induce a protective immune response against livestock parasites. Immunization of exosomes derived from Eimeria parasite antigen-loaded DCs protected chickens against avian coccidiosis, including Eimeria tenella, Eimeria maxima, and Eimeria acervulina infections, manifesting as symptom amelioration and mortality rate reduction (145).
Moreover, recent studies have revealed some new applications of exosomes in parasitic diseases. miRNAs in exosomes from the plasma of P. yoelii infectors inhibit VEGFR2 expression in endothelial cells and suppress regional angiogenesis, ultimately repressing the growth of Lewis lung cancer in a murine model (16). Further studies investigating P. yoelii-derived exosomal miRNAs may provide new therapies against tumors. Another investigation showed that the intraperitoneal injection of DC-derived exosomes pulsed by S. japonicum SEA is useful in attenuating the severity and repressing the progression of mouse IBD with decreased levels of pro-inflammatory cytokines and increased levels of anti-inflammatory cytokines (41). Due to their anti-inflammatory effects, exosomes may have potential applications as immunosuppressive drugs.
Conclusion
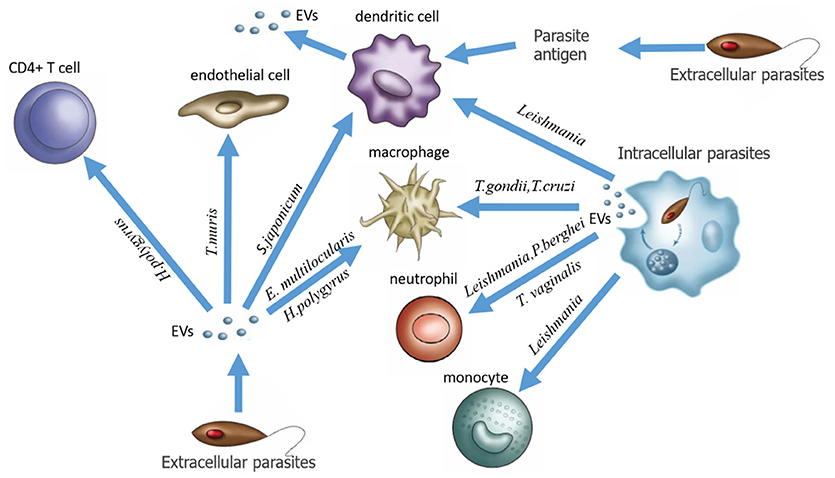
In this review, we give a general introduction to EVs and re-recognize the relationship between EVs and host-parasite interaction. Three groups of EVs are associated with parasitic infection: (1) EVs directly secreted from extracellular parasites, (2) EVs produced by host cells infected by intracellular parasites, and (3) EVs produced by host cells stimulated by parasite-derived antigens (Figure 1). Accumulating evidence indicates that parasite-derived EVs can act as signal molecules in parasite-host interactions to maintain normal parasitic physiology, leading to host pathogenesis. However, on the other hand, we should clearly recognize that EVs, as promising target drug delivery carriers, and useful biomarkers, have been extensively studied in other diseases such as cancer but still require more research in the context of parasitic infections. Although in recent years, a growing number of researchers have described the composition and function of EVs, the study of parasite EVs is still in its infancy, and the molecular mechanisms of EV formation, budding, and fusion as well as the function of EVs in host-parasite interactions remain to be further studied. Owing to the lack of consistent definitions and standards for the identification, isolation and analysis of EVs among laboratories, studies of EVs in different parasitic diseases seem somewhat confusing. We believe that after these problems are solved, more comprehensive studies of EVs will progressively reveal their exact roles in parasitic infections and provide new ideas and approaches for the application of exosomes in clinical practice.
Author Contributions
XS and ZheW constructed the manuscript. ZhoW wrote the manuscript. JL and LinW collected the information. LifW revised the manuscript.
Acknowledgments
These experiments were supported by grants from the National Research and Development Plan of China (No. 2016YFC1200500), the Pearl River Nova Program of Guangzhou (Grant No. 201710010030), the National Natural Science Foundation of China (Grant No. 81572014), and the National High Technology Research and Development Program of China (Grant No. 2015AA020934).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Clay GM, Sutterwala FS, Wilson ME. NLR proteins and parasitic disease. Immunol Res. (2014) 59:142–52. doi: 10.1007/s12026-014-8544-x
2. Coakley G, Buck AH, Maizels RM. Host parasite communications-Messages from helminths for the immune system: parasite communication and cell-cell interactions. Mol Biochem Parasitol. (2016) 208:33–40. doi: 10.1016/j.molbiopara.2016.06.003
3. Wu Z, Wang L, Tang Y, Sun X. Parasite-derived proteins for the treatment of allergies and autoimmune diseases. Front Microbiol. (2017) 8:2164. doi: 10.3389/fmicb.2017.02164
4. Hewitson JP, Grainger JR, Maizels RM. Helminth immunoregulation: the role of parasite secreted proteins in modulating host immunity. Mol Biochem Parasitol. (2009) 167:1–11. doi: 10.1016/j.molbiopara.2009.04.008
5. Robbins PD, Morelli AE. Regulation of immune responses by extracellular vesicles. Nat Rev Immunol. (2014) 14:195–208. doi: 10.1038/nri3622
6. van der Pol E, Boing AN, Harrison P, Sturk A, Nieuwland R. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol Rev. (2012) 64:676–705. doi: 10.1124/pr.112.005983
7. Al-Nedawi K, Meehan B, Micallef J, Lhotak V, May L, Guha A, et al. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat Cell Biol. (2008) 10:619–24. doi: 10.1038/ncb1725
8. Wendler F, Stamp GW, Giamas G. Tumor-stromal cell communication: small vesicles signal big changes. Trends Cancer (2016) 2:326–9. doi: 10.1016/j.trecan.2016.05.007
9. Devhare PB, Ray RB. Extracellular vesicles: Novel mediator for cell to cell communications in liver pathogenesis. Mol Aspects Med. (2018) 60:115–22. doi: 10.1016/j.mam.2017.11.001
10. Lasser C, Jang SC, Lotvall J. Subpopulations of extracellular vesicles and their therapeutic potential. Mol Aspects Med. (2018) 60:1–14. doi: 10.1016/j.mam.2018.02.002
11. Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles. J Biol Chem. (1987) 262:9412–20.
12. Schorey JS, Bhatnagar S. Exosome function: from tumor immunology to pathogen biology. Traffic (2008) 9:871–81. doi: 10.1111/j.1600-0854.2008.00734.x
13. Zhang W, Jiang X, Bao J, Wang Y, Liu H, Tang L. Exosomes in pathogen infections: a bridge to deliver molecules and link functions. Front Immunol. (2018) 9:90. doi: 10.3389/fimmu.2018.00090
14. Beghain J, Langlois AC, Legrand E, Grange L, Khim N, Witkowski B, et al. Plasmodium copy number variation scan: gene copy numbers evaluation in haploid genomes. Malaria J. (2016) 15:206. doi: 10.1186/s12936-016-1258-x
15. Martin-Jaular L, Nakayasu ES, Ferrer M, Almeida IC, Del PH. Exosomes from plasmodium yoelii-infected reticulocytes protect mice from lethal infections. PLoS ONE (2011) 6:e26588. doi: 10.1371/journal.pone.0026588
16. Yang Y, Liu Q, Lu J, Adah D, Yu S, Zhao S, et al. Exosomes from plasmodium-infected hosts inhibit tumor angiogenesis in a murine Lewis lung cancer model. Oncogenesis (2017) 6:e351. doi: 10.1038/oncsis.2017.52
17. Silverman JM, Clos JC, De'Oliveira C, Shirvani O, Fang Y, Wang C, et al. (2010). An exosome-based secretion pathway is responsible for protein export from Leishmania and communication with macrophages. J Cell Sci. 123:842–52. doi: 10.1242/jcs.056465
18. Khamesipour A. Therapeutic vaccines for leishmaniasis. Expert Opin Biol Ther. (2014) 14:1641–9. doi: 10.1517/14712598.2014.945415
19. Colineau L, Clos J, Moon KM, Foster LJ, Reiner NE. Leishmania donovani chaperonin 10 regulates parasite internalization and intracellular survival in human macrophages. Med Microbiol Immunol. (2017) 206:235–57. doi: 10.1007/s00430-017-0500-7
20. Hassani K, Olivier M. Immunomodulatory impact of leishmania-induced macrophage exosomes: a comparative proteomic and functional analysis. PLoS Negl Trop Dis. (2013) 7:e2185. doi: 10.1371/journal.pntd.0002185
21. Silverman JM, Reiner NE. Leishmania exosomes deliver preemptive strikes to create an environment permissive for early infection. Front Cell Infect Microbiol. (2011) 1:26. doi: 10.3389/fcimb.2011.00026
22. Hassani K, Shio MT, Martel C, Faubert D, Olivier M. Absence of metalloprotease GP63 alters the protein content of Leishmania exosomes. PLoS ONE (2014) 9:e95007. doi: 10.1371/journal.pone.0095007
23. Leitherer S, Clos J, Liebler-Tenorio EM, Schleicher U, Bogdan C, Soulat D. Characterization of the protein tyrosine phosphatase LmPRL-1 secreted by leishmania major via the exosome pathway. Infect Immun. (2017) 85:e00084–17. doi: 10.1128/IAI.00084-17
24. Bhattacharyya S, Ghosh S, Dasgupta B, Mazumder D, Roy S, Majumdar S. Chemokine-induced leishmanicidal activity in murine macrophages via the generation of nitric oxide. J Infect Dis. (2002) 185:1704–8. doi: 10.1086/340820
25. Aline F, Bout D, Amigorena S, Roingeard P, Dimier-Poisson I. Toxoplasma gondii antigen-pulsed-dendritic cell-derived exosomes induce a protective immune response against T. gondii infection. Infect Immun. (2004) 72:4127–37. doi: 10.1128/IAI.72.7.4127-4137.2004
26. Bhatnagar S, Shinagawa K, Castellino FJ, Schorey JS. Exosomes released from macrophages infected with intracellular pathogens stimulate a proinflammatory response in vitro and in vivo. Blood (2007) 110:3234–44. doi: 10.1182/blood-2007-03-079152
27. Pope SM, Lasser C. Toxoplasma gondii infection of fibroblasts causes the production of exosome-like vesicles containing a unique array of mRNA and miRNA transcripts compared to serum starvation. J Extracell Vesicles (2013):22484. doi: 10.3402/jev.v2i0.22484
28. Kim MJ, Jung BK, Cho J, Song H, Pyo KH, Chai JY. Exosomes secreted by Toxoplasma gondii-infected L6 cells: their effects on host cell proliferation and cell cycle changes. Korean J Parasitol. (2016) 54:147–54. doi: 10.3347/kjp.2016.54.2.147
29. Li Y, Liu Y, Xiu F, Wang J, Cong H, He S, et al. Characterization of exosomes derived from Toxoplasma gondii and their functions in modulating immune responses. Int J Nanomed. (2018) 13:467–77. doi: 10.2147/IJN.S151110
30. Twu O, de Miguel N, Lustig G, Stevens GC, Vashisht AA, Johnson PJ. Trichomonas vaginalis exosomes deliver cargo to host cells and mediate hostratioparasite interactions. PLoS Pathog. (2013) 9:e1003482. doi: 10.1371/journal.ppat.1003482
31. Olmos-Ortiz LM, Barajas-Mendiola MA, Barrios-Rodiles M, Castellano LE, Arias-Negrete S, Cuellar-Mata P, et al. Trichomonas vaginalis exosome-like vesicles modify the cytokine profile and reduce inflammation in parasite-infected mice. Parasite Immunol. (2017). 39:e12426. doi: 10.1111/pim.12426
32. Atyame NC, Sommerer N, Rofidal V, Hirtz C, Rossignol M, Cuny G, et al. Excreted/secreted proteins from trypanosome procyclic strains. J Biomed Biotechnol. 2010:212817. doi: 10.1155/2010/212817
33. Geiger A, Hirtz C, Becue T, Bellard E, Centeno D, Gargani D, et al. Exocytosis and protein secretion in Trypanosoma. BMC Microbiol. (2010) 10:20. doi: 10.1186/1471-2180-10-20
34. Eliaz D, Kannan S, Shaked H, Arvatz G, Tkacz ID, Michaeli S, et al. Exosome secretion affects social motility in Trypanosoma brucei. PLoS Pathog. (2017) 13:e1006245. doi: 10.1371/journal.ppat.1006245
35. Cristodero M, Clayton CE. Trypanosome MTR4 is involved in rRNA processing. Nucleic Acids Res. (2007) 35:7023–30. doi: 10.1093/nar/gkm736
36. Fadda A, Farber V, Droll D, Clayton C. The roles of 3'-exoribonucleases and the exosome in trypanosome mRNA degradation. RNA (2013) 19:937–47. doi: 10.1261/rna.038430.113
37. Kramer S, Piper S, Estevez A, Carrington M. Polycistronic trypanosome mRNAs are a target for the exosome. Mol Biochem Parasitol. (2016) 205:1–5. doi: 10.1016/j.molbiopara.2016.02.009
38. Garcia-Silva MR, Cabrera-Cabrera F, Das NR, Souto-Padron T, de Souza W, Cayota A. Gene expression changes induced by Trypanosoma cruzi shed microvesicles in mammalian host cells: relevance of tRNA-derived halves. Biomed Res Int. (2014) 2014:305239. doi: 10.1155/2014/305239
39. Wang L, Li Z, Shen J, Liu Z, Liang J, Wu X, et al. Exosome-like vesicles derived by Schistosoma japonicum adult worms mediates M1 type immune- activity of macrophage. Parasitol Res. (2015) 114:1865–73. doi: 10.1007/s00436-015-4373-7
40. Zhu L, Liu J, Dao J, Lu K, Li H, Gu H, et al. Molecular characterization of S. japonicum exosome-like vesicles reveals their regulatory roles in parasite-host interactions Sci Rep. (2016) 6:25885. doi: 10.1038/srep25885
41. Wang L, Yu Z, Wan S, Wu F, Chen W, Zhang B, et al. Exosomes derived from dendritic cells treated with schistosoma japonicum soluble egg antigen attenuate DSS-induced colitis. Front Pharmacol. (2017) 8:651. doi: 10.3389/fphar.2017.00651
42. Samoil V, Dagenais M, Ganapathy V, Aldridge J, Glebov A, Jardim A, et al. Vesicle-based secretion in schistosomes: Analysis of protein and microRNA (miRNA) content of exosome-like vesicles derived from Schistosoma mansoni. Sci Rep. (2018) 8:3286. doi: 10.1038/s41598-018-21587-4
43. Meningher T, Lerman G, Regev-Rudzki N, Gold D, Ben-Dov IZ, Sidi Y, et al. Schistosomal MicroRNAs isolated from extracellular vesicles in sera of infected patients: a new tool for diagnosis and follow-up of human schistosomiasis. J Infect Dis. (2017) 215:378–86. doi: 10.1093/infdis/jiw539
44. Zamanian M, Fraser LM, Agbedanu PN, Harischandra H, Moorhead AR, Kimber MJ, et al. Release of small RNA-containing exosome-like vesicles from the human filarial parasite Brugia malayi. PLoS Negl Trop Dis. (2015) 9:e0004069. doi: 10.1371/journal.pntd.0004069
45. Cwiklinski K, de la Torre-Escudero E, Trelis M, Bernal D, Dufresne P, Robinson, J, et al. The Extracellular vesicles of the helminth pathogen, Fasciola hepatica: biogenesis pathways and cargo molecules involved in parasite pathogenesis. Mol Cell Proteom. (2015) 14:3258–73. doi: 10.1074/mcp.M115.053934
46. Marcilla A, Trelis M, Cortes A, Sotillo J, Cantalapiedra F, Minguez M, et al. Extracellular vesicles from parasitic helminths contain specific excretory/secretory proteins and are internalized in intestinal host cells. PLoS ONE (2012) 7:e45974. doi: 10.1371/journal.pone.0045974
47. Buck AH, Coakley G, Simbari F, McSorley HJ, Quintana JF, Maizels RM, et al. Exosomes secreted by nematode parasites transfer small RNAs to mammalian cells and modulate innate immunity. Nat Commun. (2014) 5:5488. doi: 10.1038/ncomms6488
48. Zheng Y, Guo X, He W, Shao Z, Zhang X, Yang J, et al. Effects of Echinococcus multilocularis miR-71 mimics on murine macrophage RAW264.7 cells. Int Immunopharmacol. (2016) 34:259–62. doi: 10.1016/j.intimp.2016.03.015
49. Tzelos T, Matthews JB, Buck AH, Simbari F, Frew D, Inglis NF, et al. A preliminary proteomic characterisation of extracellular vesicles released by the ovine parasitic nematode, Teladorsagia circumcincta. Vet Parasitol. (2016) 221:84–92. doi: 10.1016/j.vetpar.2016.03.008
50. Thery C, Boussac M, Veron P, Ricciardi-Castagnoli P, Raposo G, Garin J, et al. Proteomic analysis of dendritic cell-derived exosomes: a secreted subcellular compartment distinct from apoptotic vesicles. J Immunol. (2001) 166:7309–18. doi: 10.4049/jimmunol.166.12.7309
51. Mears R, Craven RA, Hanrahan S, Totty N, Upton C, Young SL, et al. Proteomic analysis of melanoma-derived exosomes by two-dimensional polyacrylamide gel electrophoresis and mass spectrometry. Proteomics (2004) 4:4019–31. doi: 10.1002/pmic.200400876
52. Futter CE, White IJ. Annexins and endocytosis. Traffic (2007) 8:951–8. doi: 10.1111/j.1600-0854.2007.00590.x
53. Gastpar R, Gehrmann M, Bausero MA, Asea A, Gross C, Schroeder JA, et al. Heat shock protein 70 surface-positive tumor exosomes stimulate migratory and cytolytic activity of natural killer cells. Cancer Res. (2005) 65:5238–47. doi: 10.1158/0008-5472.CAN-04-3804
54. Patel AN, Vargas V, Revello P, Bull DA. Mesenchymal stem cell population isolated from the subepithelial layer of umbilical cord tissue. Cell Transplant. (2013) 22:513–9. doi: 10.3727/096368912X655064
55. Tang N, Sun B, Gupta A, Rempel H, Pulliam L. Monocyte exosomes induce adhesion molecules and cytokines via activation of NF-kappaB in endothelial cells. FASEB J. (2016) 30:3097–106. doi: 10.1096/fj.201600368RR
56. Skotland T, Sandvig K, Llorente A. Lipids in exosomes: Current knowledge and the way forward. Prog Lipid Res. (2017) 66:30–41. doi: 10.1016/j.plipres.2017.03.001
57. Laulagnier K, Motta C, Hamdi S, Roy S, Fauvelle F, Pageaux JF, et al. Mast cell- and dendritic cell-derived exosomes display a specific lipid composition and an unusual membrane organization. Biochem J. (2004) 380:161–71. doi: 10.1042/bj20031594
58. de Gassart A, Geminard C, Fevrier B, Raposo G, Vidal M. Lipid raft-associated protein sorting in exosomes. Blood (2003) 102:4336–44. doi: 10.1182/blood-2003-03-0871
59. Abdel-Maksoud MA, Abdel-Ghaffar FA, El-Amir A, Al-Quraishy S, Badr G. Infection with Plasmodium chabaudi diminishes plasma immune complexes and ameliorates the histopathological alterations in different organs of female BWF1 lupus mice. Eur Rev Med Pharmacol Sci. (2016) 20:733–44.
60. Li M, Zeringer E, Barta T, Schageman J, Cheng A, Vlassov AV. Analysis of the RNA content of the exosomes derived from blood serum and urine and its potential as biomarkers. Philos Trans R Soc Lond B Biol Sci. (2014) 369:20130502. doi: 10.1098/rstb.2013.0502
61. Balaj L, Lessard R, Dai L, Cho YJ, Pomeroy SL, Breakefield XO, et al. Tumour microvesicles contain retrotransposon elements and amplified oncogene sequences. Nat Commun. (2011) 2:180. doi: 10.1038/ncomms1180
62. Guescini M, Genedani S, Stocchi V, Agnati LF. Astrocytes and Glioblastoma cells release exosomes carrying mtDNA. J Neural Transm. (2010) 117:1–4. doi: 10.1007/s00702-009-0288-8
63. Thakur BK, Zhang H, Becker A, Matei I, Huang Y, Costa-Silva B, et al. Double-stranded DNA in exosomes: a novel biomarker in cancer detection. Cell Res. (2014) 24:766–9. doi: 10.1038/cr.2014.44
64. Mantel PY, Marti M. The role of extracellular vesicles in Plasmodium and other protozoan parasites. Cell Microbiol. (2014) 16:344–54. doi: 10.1111/cmi.12259
65. Roxrud I, Stenmark H, Malerod L. ESCRT and Co. Biol Cell (2010) 102:293–318. doi: 10.1042/BC20090161
66. Baietti MF, Zhang Z, Mortier E, Melchior A, Degeest G, Geeraerts A, et al. Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat Cell Biol. (2012) 14:677–85. doi: 10.1038/ncb2502
67. Piper RC, Katzmann DJ. Biogenesis and function of multivesicular bodies. Annu Rev Cell Dev Biol. (2007) 23:519–47. doi: 10.1146/annurev.cellbio.23.090506.123319
68. Dargan DJ, Patel AH, Subak-Sharpe JH. PREPs: herpes simplex virus type 1-specific particles produced by infected cells when viral DNA replication is blocked. J Virol. (1995) 69:4924–32.
69. Greening DW, Gopal SK, Xu R, Simpson RJ, Chen W. Exosomes and their roles in immune regulation and cancer. Semin Cell Dev Biol. (2015) 40:72–81. doi: 10.1016/j.semcdb.2015.02.009
70. Hood JL, San RS, Wickline SA. Exosomes released by melanoma cells prepare sentinel lymph nodes for tumor metastasis. Cancer Res. (2011) 71:3792–801. doi: 10.1158/0008-5472.CAN-10-4455
71. Pegtel DM, van de Garde MD, Middeldorp JM. Viral miRNAs exploiting the endosomal-exosomal pathway for intercellular cross-talk and immune evasion. Biochim Biophys Acta (2011) 1809:715–21. doi: 10.1016/j.bbagrm.2011.08.002
72. Schorey JS, Cheng Y, Singh PP, Smith VL. Exosomes and other extracellular vesicles in host-pathogen interactions. EMBO Rep. (2015) 16:24–43. doi: 10.15252/embr.201439363
73. White NJ, Pukrittayakamee S, Hien TT, Faiz MA, Mokuolu OA, Dondorp AM. Malaria. Lancet (2014) 383:723–35. doi: 10.1016/S0140-6736(13)60024-0
74. Combes V, Taylor TE, Juhan-Vague I, Mege JL, Mwenechanya J, Tembo M, et al. Circulating endothelial microparticles in malawian children with severe falciparum malaria complicated with coma. JAMA (2004) 291:2542–4. doi: 10.1001/jama.291.21.2542-b
75. Combes V, Coltel N, Alibert M, van Eck M, Raymond C, Juhan-Vague I, et al. ABCA1 gene deletion protects against cerebral malaria: potential pathogenic role of microparticles in neuropathology. Am J Pathol. (2005) 166:295–302. doi: 10.1016/S0002-9440(10)62253-5
76. Couper KN, Barnes T, Hafalla JC, Combes V, Ryffel B, Secher T, et al. Parasite-derived plasma microparticles contribute significantly to malaria infection-induced inflammation through potent macrophage stimulation. PLoS Pathog. (2010) 6:e1000744. doi: 10.1371/journal.ppat.1000744
77. Mantel PY, Hoang AN, Goldowitz I, Potashnikova D, Hamza B, Marti M, et al. Malaria-infected erythrocyte-derived microvesicles mediate cellular communication within the parasite population and with the host immune system. Cell Host Microbe (2013) 13:521–34. doi: 10.1016/j.chom.2013.04.009
78. Regev-Rudzki N, Wilson DW, Carvalho TG, Sisquella X, Coleman BM, Cowman AF, et al. Cell-cell communication between malaria-infected red blood cells via exosome-like vesicles. Cell (2013) 153:1120–33. doi: 10.1016/j.cell.2013.04.029
79. Bhat AA, Seth RK, Babu J, Biswas S, Rao DN. Induction of mucosal and systemic humoral immune responses in murine system by intranasal immunization with peptide antigens of P. vivax and CpG oligodeoxynucleotide (ODN) in microparticle delivery. Int Immunopharmacol. (2009) 9:1197–208. doi: 10.1016/j.intimp.2009.06.008
80. Bhat AA, Seth RK, Kumar S, Ali R, Mohan T, Biswas S, et al. Induction of cell-mediated immune responses to peptide antigens of P. vivax in microparticles using intranasal immunization. Immunol Invest. (2010) 39:483–99. doi: 10.3109/08820131003674826
81. Schofield L, Grau GE. Immunological processes in malaria pathogenesis. Nat Rev Immunol. (2005) 5:722–35. doi: 10.1038/nri1686
82. Pankoui Mfonkeu JB, Gouado I, Fotso Kuate H, Zambou O, Amvam Zollo PH, Grau GE, et al. Elevated cell-specific microparticles are a biological marker for cerebral dysfunctions in human severe malaria. PLoS ONE (2010) 5:e13415. doi: 10.1371/journal.pone.0013415
83. Sahu U, Sahoo PK, Kar SK, Mohapatra BN, Ranjit M. Association of TNF level with production of circulating cellular microparticles during clinical manifestation of human cerebral malaria. Hum Immunol. (2013) 74:713–21. doi: 10.1016/j.humimm.2013.02.006
84. Campos FM, Franklin BS, Teixeira-Carvalho A, Filho AL, de Paula SC, Fontes CJ, et al. Augmented plasma microparticles during acute Plasmodium vivax infection. Malaria J. (2010) 9:327. doi: 10.1186/1475-2875-9-327
86. Silverman JM, Chan SK, Robinson DP, Dwyer DM, Nandan D, Foster LJ, et al. Proteomic analysis of the secretome of Leishmania donovani. Genome Biol. (2008) 9:R35. doi: 10.1186/gb-2008-9-2-r35
87. Atayde VD, Hassani K, da Silva Lira Filho A, Borges AR, Adhikari A, Martel C, et al. Leishmania exosomes and other virulence factors: impact on innate immune response and macrophage functions. Cell Immunol. (2016) 309:7–18. doi: 10.1016/j.cellimm.2016.07.013
88. Silverman JM, Clos J, Horakova E, Wang AY, Wiesgigl M, Kelly I, et al. Leishmania exosomes modulate innate and adaptive immune responses through effects on monocytes and dendritic cells. J Immunol. (2010) 185:5011–22. doi: 10.4049/jimmunol.1000541
89. Tejera Nevado P, Bifeld E, Hohn K, Clos J. A telomeric cluster of antimony resistance genes on chromosome 34 of Leishmania infantum. Antimicrob Agents Chemother. (2016) 60:5262–75. doi: 10.1128/AAC.00544-16
90. Halle M, Gomez MA, Stuible M, Shimizu H, McMaster WR, Olivier M, et al. The Leishmania surface protease GP63 cleaves multiple intracellular proteins and actively participates in p38 mitogen-activated protein kinase inactivation. J Biol Chem. (2009) 284:6893–908. doi: 10.1074/jbc.M805861200
91. Ghosh J, Bose M, Roy S, Bhattacharyya SN. Leishmania donovani targets Dicer1 to downregulate miR-122, lower serum cholesterol, and facilitate murine liver infection. Cell Host Microbe (2013) 13:277–88. doi: 10.1016/j.chom.2013.02.005
92. Koutsoni O, Barhoumi M, Guizani I, Dotsika E. Leishmania eukaryotic initiation factor (LeIF) inhibits parasite growth in murine macrophages. PLoS ONE (2014) 9:e97319. doi: 10.1371/journal.pone.0097319
93. Atayde VD, Aslan H, Townsend S, Hassani K, Kamhawi S, Olivier M. Exosome secretion by the parasitic protozoan leishmania within the sand fly midgut. Cell Rep. (2015) 13:957–67. doi: 10.1016/j.celrep.2015.09.058
94. Boaventura VS, Santos CS, Cardoso CR, de Andrade J, Dos Santos WL, Clarencio J, et al. Human mucosal leishmaniasis: neutrophils infiltrate areas of tissue damage that express high levels of Th17-related cytokines. Eur J Immunol. (2010) 40:2830–6. doi: 10.1002/eji.200940115
95. Montoya JG, Liesenfeld O. Toxoplasmosis. Lancet (2004) 363:1965–76. doi: 10.1016/S0140-6736(04)16412-X
96. Dlugonska H, Gatkowska J. Exosomes in the context of Toxoplasma gondii - host communication. Ann Parasitol. (2016) 62:169–74. doi: 10.17420/ap6203.50
97. Beauvillain C, Ruiz S, Guiton R, Bout D, Dimier-Poisson I. A vaccine based on exosomes secreted by a dendritic cell line confers protection against T. gondii infection in syngeneic and allogeneic mice. Microbes Infect. (2007) 9:1614–22. doi: 10.1016/j.micinf.2007.07.002
98. Brunet J, Pfaff AW, Abidi A, Unoki M, Nakamura Y, Guinard M, et al. Toxoplasma gondii exploits UHRF1 and induces host cell cycle arrest at G2 to enable its proliferation. Cell Microbiol. (2008) 10:908–20. doi: 10.1111/j.1462-5822.2007.01093.x
99. Wowk PF, Zardo ML, Miot HT, Goldenberg S, Carvalho PC, Morking PA. Proteomic profiling of extracellular vesicles secreted from Toxoplasma gondii. Proteomics (2017) 17:1600477. doi: 10.1002/pmic.201600477
100. Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. (2013) 200:373–83. doi: 10.1083/jcb.201211138
101. Velge-Roussel F, Chardes T, Mevelec P, Brillard M, Hoebeke J, Bout D. Epitopic analysis of the Toxoplasma gondii major surface antigen SAG1. Mol Biochem Parasitol. (1994) 66:31–8. doi: 10.1016/0166-6851(94)90033-7
102. Menezes CB, Frasson AP, Tasca T. Trichomoniasis - are we giving the deserved attention to the most common non-viral sexually transmitted disease worldwide? Microb Cell (2016) 3:404–19. doi: 10.15698/mic2016.09.526
103. de Miguel N, Lustig G, Twu O, Chattopadhyay A, Wohlschlegel JA, Johnson PJ. Proteome analysis of the surface of Trichomonas vaginalis reveals novel proteins and strain-dependent differential expression. Mol Cell Proteom. (2010) 9:1554–66. doi: 10.1074/mcp.M000022-MCP201
104. Rana S, Zoller M. Exosome target cell selection and the importance of exosomal tetraspanins: a hypothesis. Biochem Soc Trans. (2011) 39:559–62. doi: 10.1042/BST0390559
105. Ryan CM, de Miguel N, Johnson PJ. Trichomonas vaginalis: current understanding of host-parasite interactions. Essays Biochem. (2011) 51:161–75. doi: 10.1042/bse0510161
106. Barrett MP, Burchmore RJ, Stich A, Lazzari JO, Frasch AC, Cazzulo JJ, et al. The trypanosomiases. Lancet (2003) 362:1469–80. doi: 10.1016/S0140-6736(03)14694-6
107. Gunzl A. The pre-mRNA splicing machinery of trypanosomes: complex or simplified? Eukaryot Cell (2010) 9:1159–70. doi: 10.1128/EC.00113-10
108. Bayer-Santos E, Lima FM, Ruiz JC, Almeida IC, da Silveira JF. Characterization of the small RNA content of Trypanosoma cruzi extracellular vesicles. Mol Biochem Parasitol. (2014) 193:71–4. doi: 10.1016/j.molbiopara.2014.02.004
109. Garcia-Silva MR, das Neves RF, Cabrera-Cabrera F, Sanguinetti J, Medeiros LC, Robello C, et al. Extracellular vesicles shed by Trypanosoma cruzi are linked to small RNA pathways, life cycle regulation, and susceptibility to infection of mammalian cells. Parasitol Res. (2014) 113:285–304. doi: 10.1007/s00436-013-3655-1
110. Lewis FA, Tucker MS. Schistosomiasis. Adv Exp Med Biol. (2014) 766:47–75. doi: 10.1007/978-1-4939-0915-5_3
111. Collins JJ III, Wang B, Lambrus BG, Tharp ME, Iyer H, Newmark PA. Adult somatic stem cells in the human parasite Schistosoma mansoni. Nature (2013) 494:476–9. doi: 10.1038/nature11924
112. Driguez P, McManus DP, Gobert GN. Clinical implications of recent findings in schistosome proteomics. Exp Rev Proteom. (2016) 13:19–33. doi: 10.1586/14789450.2016.1116390
113. Zhu L, Liu J, Cheng G. Role of microRNAs in schistosomes and schistosomiasis. Front Cell Infect Microbiol. (2014) 4:165. doi: 10.3389/fcimb.2014.00165
114. Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. (2004) 25:677–86. doi: 10.1016/j.it.2004.09.015
115. Sotillo J, Pearson M, Potriquet J, Becker L, Pickering D, Mulvenna J, et al. Extracellular vesicles secreted by Schistosoma mansoni contain protein vaccine candidates. Int J Parasitol. (2016) 46:1–5. doi: 10.1016/j.ijpara.2015.09.002
116. Hasby EA, Hasby Saad MA, Shohieb Z, El Noby K. FoxP3+ T regulatory cells and immunomodulation after Schistosoma mansoni egg antigen immunization in experimental model of inflammatory bowel disease. Cell Immunol. (2015) 295:67–76. doi: 10.1016/j.cellimm.2015.02.013
117. Cunningham NM. Lymphatic filariasis in immigrants from developing countries. Am Fam Phys. (1997) 55:1199–204.
118. Koudou BG, de Souza DK, Biritwum NK, Bougma R, Aboulaye M, Elhassan E, et al. Elimination of lymphatic filariasis in west African urban areas: is implementation of mass drug administration necessary? Lancet Infect Dis. (2018) 18:e214–20. doi: 10.1016/S1473-3099(18)30069-0
119. Banerjee S, Xie N, Cui H, Tan Z, Yang S, Icyuz M, et al. MicroRNA let-7c regulates macrophage polarization. J Immunol. (2013) 190:6542–9. doi: 10.4049/jimmunol.1202496
120. Chen XM, Splinter PL, O'Hara SP, LaRusso NF. A cellular micro-RNA, let-7i, regulates Toll-like receptor 4 expression and contributes to cholangiocyte immune responses against Cryptosporidium parvum infection. J Biol Chem. (2007) 282:28929–38. doi: 10.1074/jbc.M702633200
121. Harischandra H, Yuan W, Loghry HJ, Zamanian M, Kimber MJ. Profiling extracellular vesicle release by the filarial nematode Brugia malayi reveals sex-specific differences in cargo and a sensitivity to ivermectin. PLoS Negl Trop Dis. (2018) 12:e0006438. doi: 10.1371/journal.pntd.0006438
122. Mekky MA, Tolba M, Abdel-Malek MO, Abbas WA, Zidan M. Human fascioliasis: a re-emerging disease in upper Egypt. Am J Trop Med Hyg. (2015) 93:76–9. doi: 10.4269/ajtmh.15-0030
123. Robinson MW, Dalton JP. Zoonotic helminth infections with particular emphasis on fasciolosis and other trematodiases. Philos Trans R Soc Lond B Biol Sci. (2009) 364:2763–76. doi: 10.1098/rstb.2009.0089
124. Fromm B, Ovchinnikov V, Hoye E, Bernal D, Hackenberg M, Marcilla A. On the presence and immunoregulatory functions of extracellular microRNAs in the trematode Fasciola hepatica. Parasite Immunol. (2017) 39:e12399. doi: 10.1111/pim.12399
125. Mohrs K, Harris DP, Lund FE, Mohrs M. Systemic dissemination and persistence of Th2 and type 2 cells in response to infection with a strictly enteric nematode parasite. J Immunol. (2005) 175:5306–13. doi: 10.4049/jimmunol.175.8.5306
126. Ito J, Nagayasu Y, Yokoyama S. Cholesterol-sphingomyelin interaction in membrane and apolipoprotein-mediated cellular cholesterol efflux. J Lipid Res. (2000) 41:894–904.
127. Simbari F, McCaskill J, Coakley G, Millar M, Maizels RM, Fabrias G, et al. Plasmalogen enrichment in exosomes secreted by a nematode parasite versus those derived from its mouse host: implications for exosome stability and biology. J Extracell Vesicles (2016) 5:30741. doi: 10.3402/jev.v5.30741
128. Neghina R, Neghina AM, Marincu I, Iacobiciu I. Cystic echinococcosis in Romania: the pediatric approach. Vector Borne Zoonotic Dis. (2011) 11:993–9. doi: 10.1089/vbz.2010.0238
129. Venegas J, Espinoza S, Sanchez G. [Estimation of costs caused by cystic echinococcosis]. Rev Med Chil. (2014) 142:1023–33. doi: 10.4067/S0034-98872014000800010
130. Siles-Lucas M, Sanchez-Ovejero C, Gonzalez-Sanchez M, Gonzalez E, Falcon-Perez JM, Boufana B, et al. Isolation and characterization of exosomes derived from fertile sheep hydatid cysts. Vet Parasitol. (2017) 236:22–33. doi: 10.1016/j.vetpar.2017.01.022
131. Santos GBD, Monteiro KM, Da Silva ED, Battistella ME, Ferreira HB, Zaha A. Excretory/secretory products in the Echinococcus granulosus metacestode: is the intermediate host complacent with infection caused by the larval form of the parasite? Int J Parasitol. (2016) 46:843–56. doi: 10.1016/j.ijpara.2016.07.009
132. Zheng Y. Strategies of Echinococcus species responses to immune attacks: implications for therapeutic tool development. Int Immunopharmacol. (2013) 17:495–501. doi: 10.1016/j.intimp.2013.07.022
133. McNeilly TN, Devaney E, Matthews JB. Teladorsagia circumcincta in the sheep abomasum: defining the role of dendritic cells in T cell regulation and protective immunity. Parasite Immunol. (2009) 31:347–56. doi: 10.1111/j.1365-3024.2009.01110.x
134. Martinez-Valladares M, Geldhof P, Jonsson N, Rojo-Vazquez FA, Skuce P. Teladorsagia circumcincta: molecular characterisation of the avr-14B subunit and its relatively minor role in ivermectin resistance. Int J Parasitol Drugs Drug Resist. (2012) 2:154–61. doi: 10.1016/j.ijpddr.2012.03.005
135. Stear MJ, Bishop SC, Henderson NG, Scott I. A key mechanism of pathogenesis in sheep infected with the nematode Teladorsagia circumcincta. Anim Health Res Rev. (2003) 4:45–52. doi: 10.1079/AHRR200351
136. Colombo M, Raposo G, Thery C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. (2014) 30:255–89. doi: 10.1146/annurev-cellbio-101512-122326
137. Li A, Zhang T, Zheng M, Liu Y, Chen Z. Exosomal proteins as potential markers of tumor diagnosis. J Hematol Oncol. (2017) 10:175. doi: 10.1186/s13045-017-0542-8
138. Tang MK, Wong AS. Exosomes: emerging biomarkers and targets for ovarian cancer. Cancer Lett. (2015) 367:26–33. doi: 10.1016/j.canlet.2015.07.014
139. Coakley G, Maizels RM, Buck AH. Exosomes and other extracellular vesicles: the new communicators in parasite infections. Trends Parasitol. (2015) 31:477–89. doi: 10.1016/j.pt.2015.06.009
140. Gray DJ, Ross AG, Li YS, McManus DP. Diagnosis and management of schistosomiasis. BMJ (2011) 342:d2651. doi: 10.1136/bmj.d2651
141. Meltzer E, Schwartz E. Schistosomiasis: current epidemiology and management in travelers. Curr Infect Dis Rep. (2013) 15:211–5. doi: 10.1007/s11908-013-0329-1
142. Urbanelli L, Buratta S, Sagini K, Ferrara G, Lanni M, Emiliani C. Exosome-based strategies for diagnosis and therapy. Recent Pat CNS Drug Discov. (2015) 10:10–27. doi: 10.2174/1574889810666150702124059
143. Viaud S, Thery C, Ploix S, Tursz T, Lapierre V, Lantz O, et al. Dendritic cell-derived exosomes for cancer immunotherapy: what's next? Cancer Res. (2010) 70:1281–5. doi: 10.1158/0008-5472.CAN-09-3276
144. Zhang X, Pei Z, Chen J, Ji C, Xu J, Wang J. Exosomes for immunoregulation and therapeutic intervention in cancer. J Cancer (2016) 7:1081–7. doi: 10.7150/jca.14866
Keywords: parasite, extracellular vesicle, intercellular communication, parasite-host interaction, exosome
Citation: Wu Z, Wang L, Li J, Wang L, Wu Z and Sun X (2019) Extracellular Vesicle-Mediated Communication Within Host-Parasite Interactions. Front. Immunol. 9:3066. doi: 10.3389/fimmu.2018.03066
Received: 30 August 2018; Accepted: 11 December 2018;
Published: 15 January 2019.
Edited by:
Wanderley De Souza, Universidade Federal do Rio de Janeiro, BrazilReviewed by:
Eden Ramalho Ferreira, Federal University of São Paulo, BrazilEmile Santos Barrias, Instituto Nacional de Metrologia, Qualidade e Tecnologia, Brazil
Copyright © 2019 Wu, Wang, Li, Wang, Wu and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xi Sun, c3VueGkyQG1haWwuc3lzdS5lZHUuY24=
Zhongdao Wu, d3V6aGRAbWFpbC5zeXN1LmVkdS5jbg==
†These authors have contributed equally to this work and co-first authorship
 Zhenyu Wu
Zhenyu Wu Lingling Wang1,2,3†
Lingling Wang1,2,3† Zhongdao Wu
Zhongdao Wu Xi Sun
Xi Sun