
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Immunol., 20 December 2018
Sec. Immunological Memory
Volume 9 - 2018 | https://doi.org/10.3389/fimmu.2018.03059
This article is part of the Research TopicDifferentiation, Tissue Adaptation and Function of Memory T CellsView all 12 articles
 Chunmei Fu
Chunmei Fu Aimin Jiang*
Aimin Jiang*Dendritic cells (DCs) play a central role in the regulation of the balance between CD8 T cell immunity vs. tolerance to tumor antigens. Cross-priming, a process which DCs activate CD8 T cells by cross-presenting exogenous antigens, plays a critical role in generating anti-tumor CD8 T cell immunity. However, there are compelling evidences now that the tumor microenvironment (TME)-mediated suppression and modulation of tumor-infiltrated DCs (TIDCs) impair their function in initiating potent anti-tumor immunity and even promote tumor progression. Thus, DC-mediated cross-presentation of tumor antigens in tumor-bearing hosts often induces T cell tolerance instead of immunity. As tumor-induced immunosuppression remains one of the major hurdles for cancer immunotherapy, understanding how DCs regulate anti-tumor CD8 T cell immunity in particular within TME has been under intensive investigation. Recent reports on the Batf3-dependent type 1 conventional DCs (cDC1s) in anti-tumor immunity have greatly advanced our understanding on the interplay of DCs and CD8 T cells in the TME, highlighted by the critical role of CD103+ cDC1s in the cross-priming of tumor antigen-specific CD8 T cells. In this review, we will discuss recent advances in anti-tumor CD8 T cell cross-priming by CD103+ cDC1s in TME, and share perspective on future directions including therapeutic applications and memory CD8 T cell responses.
Cancer is characterized by the accumulation of genetic mutations and the loss of normal cellular regulatory functions (1). The identification of tumor-associated antigens (TAAs) that separated cancerous cells from non-transformed healthy cells, and the observation of tumor antigen-specific CD8 T cells in cancer patients have greatly advanced our understanding on tumor immunology and formed the basis for antigen-specific immunotherapy (2). The first human tumor antigen recognized by CD8 cytotoxic T lymphocytes (CTL) was identified in melanoma and was designated melanoma-associated antigen (MAGE)-1 (3). The isolation of tumor-specific CTL from peripheral blood or tumor tissue of patients from various cancer patients provided evidence for existence of CD8 T cell-mediated anti-tumor immunity (4–7). The detection of TAA-specific CD8 T cells in spontaneously regressing tumors further supported the importance of tumor-specific CD8 T cell responses (5). It is well accepted now that CD8 T cells play a central role in mediating anti-tumor immunity, and their effector CTLs eliminate tumor cells by recognizing tumor-associated antigens presented on major histocompatibility complex class I (MHCI) by their expressed T cell receptor (TCR). Indeed, studies have shown that infiltration of T cells, especially CD8 T cells into tumor microenvironment, correlates with better prognosis in multiple malignancies such as breast, lung, melanoma, colorectal, and brain cancer (8, 9). However, even when tumor-specific CD8 T cell responses were observed, they rarely provided protective immunity as tumors often evade immune surveillance by dampening T cell effector and memory functions (10, 11). Promising cancer immunotherapies that aim to boost CD8 T cell-mediated anti-tumor immunity include DC cancer vaccines, adaptive cell transfer (ACT) of tumor-reactive T cells, either native (CTL clones or Tumor infiltrated lymphocytes–TIL) or engineered to express tumor antigen-specific TCR or chimeric antigen receptors (CAR), and immune checkpoint blockade (ICB) such as anti-PD-1, anti-PD-L1, and anti-CTLA-4 (2). Among them, immunotherapies with ICB and CAR T cells have achieved unprecedented clinical efficacy leading to a number of drugs being approved by the FDA. However, a majority of patients still fail to respond to these checkpoint or CAR T cell therapies, and many patients that do respond often experience relapse (12). While direct presentation of tumor antigens onto their MHCI by tumor cells play an important role in effector function of CD8 T cells, cross-presentation by professional antigen presenting cells in particular DCs are required for prime naive CD8 T cells and sustaining the cytotoxic immune responses (13). Thus, increasing efforts has been made to repair and enhance insufficient T cell priming by DCs to further improve the efficacy of immunotherapies with ICB and CAR T cells due to DCs' critical role in priming and directing CD8 T cells to target tumor cells (12, 14). Indeed, the ability of DCs to cross-present exogenous tumor-associated antigens onto MHCI molecule to prime CD8 T cells is the foundation of the “Cancer-Immunity cycle” proposed by Chen and Mellman (11). Thus, better understanding the interaction of CD8 T cells and DCs would be critical to improve the efficacy of current cancer immunotherapies.
Ralph Steinman was awarded the 2011 Nobel Prize for Medicine or Physiology for his pioneering work on DCs (15). As the sentinel of the immune system, DCs play a central role in linking innate and adaptive immune responses (16). Known as the most potent professional antigen presenting cells (APCs), DCs initiate all adaptive immune responses by uptaking, processing and presenting antigens including tumor antigens to activate naive antigen-specific CD4 and CD8 T cells (17). Since their identification in 1973 (18), DC development and the regulation of their function have been under intensive study. DCs originate in bone marrow from macrophage/DC progenitors (MDP) that give rise to common DC progenitors (CDP), which then differentiate into two major DC subsets: classical/conventional DCs (cDCs) and plasmacytoid DCs (pDCs) (19–26). Murine cDCs consist of two subtypes currently described as cDC1s (XCR1hiCD24hiCD26hiCD11chiMHCIIhi CD11bloCD172aloF4/80loCD64loLinlo,type 1 cDCs)andcDC2s(CD11bhiCD172ahiCD26hiCD11chiMHCIIhiXCR1loF4/80loCD64loLinlo, type 2 cDCs), and their human counterparts are CD141+ DCs (also known as BDCA3+) and CD1c+ DCs (also known as BDCA1+), respectively (27, 28). These two subtypes of cDCs differ in their transcriptional factor dependency, function and phenotypes (23, 24). cDC1 cells include lymphoid tissue CD8α+ cDC1s and migratory CD103+ cDC1s (29). cDC1 cells rely on interferon regulatory factor 8 (IRF8) and basic leucine zipper transcriptional factor ATF-like 3 (Batf3) for their development, and are specialized in presenting internalized exogenous antigens onto MHCI to prime CD8 T cells by cross-presentation (30). cDC2s depend on interferon regulatory factor 4 (IRF4) for their development and comprise a heterogeneous population that are very efficient in presenting internalized antigens on MHCII to activate CD4 T cells (31–34).
pDCs are a multifunctional population best known for their specialized ability in producing and secreting large amount of type I interferons (IFNs) (35–37). pDCs also express high level of IRF8 similar to cDC1s, but require the E2-2 transcription factor for their development (38). E2-2, encoded by TCF4, is a member of the E family of basic helix–loop–helix transcription factors (39). In both mice and humans, E2-2 is required for the differentiation of pDCs from CDPs (38). Induced deletion of E2-2 in mature pDCs results in the acquisition of cDC-like properties, such as dendritic morphology, MHCII and CD8α expression, and the ability to induce proliferation of allogeneic CD4 T cells (40). Deletion of E2-2 in pDCs also induces the expression of ID2, which is required for cDC1 development. Murine pDCs express Siglec-H, B220, Ly6c, PDCA1 (CD317) and intermediate level of CD11c, and human pDCs express HLA-DR, CD123, BDCA2 (CD303), and BDCA4 (CD304) but not CD11c (36, 41). Initially reported as IFN-producing cells (IPCs), pDCs have been extensively studied for their function in sensing viral RNA and DNA by toll-like receptor (TLR)-7 and−9 (42, 43). In addition to their function in producing IFNs, pDCs have also been shown to play an important role in immune tolerance. In autoimmune diseases, aberrant activation of pDCs has been implicated in the pathogenesis of psoriasis, systemic lupus erythematosus (SLE), and IFN-related autoimmune diseases (36, 44, 45).
Monocytes that arise from MDPs could also differentiate into another DC subset named Monocyte-derived inflammatory DCs (inf-DC) under conditions such as inflammation, cancer and infection (46). The inf-DCs have been shown to both activate antigen-specific CD4 T cells and cross-present tumor antigens to activate CD8 T cells, and their presence has been found to be important for the efficacy of cancer immunotherapy (47–49). Recently, TNF/iNOS-producing DCs (TIP-DCs), a novel type of inf-DCs that produce TNF-α and nitric oxide (NO) was shown to be critical for tumor growth control upon treatment with adaptive CD8 T cell transfer (50).
The TME is a specialized niche composed of tumor cells, fibroblasts, endothelial cells, infiltrating leukocytes, and extracellular matrix components. TIDCs have been found in many cancer types including breast, lung, renal, head and neck, gastric, colorectal, bladder and ovarian cancers (51). However, in general within the TME tumor cells are able to adapt their environment to favor tumor growth, evade immune surveillance and confer resistance to immunotherapies (52, 53). A key mechanism in achieving tumor immune evasion is through modulation of DC function by tumors and tumor-associated cells/factors in the TME. Thus, despite the presence of DCs in TME and their potential in generating anti-tumor immunity, TIDCs often exhibit impaired or defective function, thus might mediate immunosuppression instead (41, 54). Indeed, the TME employs a variety of mechanisms to modulate DCs to suppress their ability to induce anti-tumor responses.
A number of factors such as IL-6, Macrophage colony-stimulating factor (M-CSF), IL-10, Vascular endothelial growth factor (VEGF), and Transforming growth factor beta (TGF-β) that are present in TME have been shown to negatively regulate DC functions (55, 56). IL-6 and M-CSF, cytokines secreted by tumor cells, have been shown to switch the differentiation of CD34+ progenitors from DCs to CD14+ monocytes that failed to mediate allogeneic T cell proliferation (57, 58). Tumor-derived IL-6 has been shown to negatively regulate DC function by inhibiting their maturation and migration, affect the differentiation of hematopoietic progenitor cells from DCs to macrophage, and induce tolerogenic phenotypes of DCs (59–61). In the TME, a variety of cells such as tumor cells, myeloid-derived suppressor cells (MDSCs), tumor-associated macrophages (TAMs), DCs, and Tregs, have been shown to produce IL-10 (62). IL-10 has been shown to suppress DC function by inhibiting different aspects of DC biology, such as DC maturation, their ability to secret IL-12, their capacity in antigen presentation and priming of T cells (63, 64). IL-10 has also been shown to convert immunogenic DCs into tolerogenic DCs leading to the induction of anergic cytotoxic CD8 T cells (65). In addition, IL-10 derived from tumors has also been shown to switch differentiation from monocytic precursors to immunosuppressive TAMs rather than DCs (66). VEGF has been shown to inhibit differentiation and maturation of DCs (67, 68). Tumor-derived TGF-β significantly suppresses DC function and their ability to initiate anti-tumor immune responses by inhibiting DC maturation (69, 70).
Several factors such as VEGF, TGFβ, IL-1β, IL-13, Granulocyte-macrophage colony-stimulating factor (GM-CSF) and prostaglandins, that are produced by tumor cells and other cells in the TME, have been shown to inhibit DC differentiation from progenitors and promote their differentiation into immunosuppressive cells such as MDSCs and TAMs (71).
Another mechanism used by TME to evade immune detection is by modulating DC function to skew T cell differentiation. Factors in TME, such as Matrix metalloproteinase 2 (MMP-2) and Thymic Stromal Lymphopoietin (TSLP) have been shown to modulate DC function to induce detrimental Th2 responses (72, 73). Tumor-produced TSLP has been shown to up-regulate OX40L expression on DCs, thus inducing the generation of Th2 cells that produce IL-4 and IL-13 that have been shown to promote tumor growth in breast and pancreatic cancer (74, 75).
Several signaling pathways such as β-catenin, MAPK and STATs that are active in cancers also play critical roles in crosstalk between tumor cells and DCs in the TME (76, 77). β-catenin signaling in melanoma cells has been shown to inhibit the recruitment of T cells and DCs into tumors (78). Melanoma-derived Wnt ligand Wnt5α has been shown to increase the production of Indoleamine 2,3-dioxygenase (IDO) by TIDCs via β-catenin signaling, leading to increased generation of Treg cells (79). Conditional knockout of Wnt co-receptors LRP5 and LRP6 on DCs, on the other hand, enhanced DC-mediated anti-tumor immunity leading to delayed tumor growth (80). In addition, activation of β-catenin in DCs from tumor-bearing mice exhibited a more tolerogenic phenotype and mediated the suppression of DC vaccine-induced cross-priming of anti-tumor CD8 T cells through IL-10 (81, 82).
Regulatory T cells (Tregs), working in concert with tolerogenic DCs, play critical roles in the establishment and maintenance of an immunosuppressive TME to inhibit anti-tumor immunity (83). Tregs are comprised of a heterogeneous population of T lymphocytes that have shared the ability to suppress immune responses, with the CD4+CD25+Foxp3+ Tregs being most studied. These Tregs express the inhibitory receptors CTLA-4, Tim-3, PD-1, GITR, LAG3, and BTLA that exert their suppressive function on DCs through different mechanisms. For example, Tregs have been shown to inhibit DC maturation by down-regulating the expression of co-stimulatory molecules such as CD80 and CD86 through CTLA-4 (84). Engagement of CTLA-4 on Treg by CD80/CD86 on DCs has been shown to up-regulate both human and murine DCs' production of IDO (85), which then activate antigen-specific regulatory T cells to induce potent suppressor activity (86, 87). In turn, IDO-activated Tregs have been shown to induce the up-regulation of the inhibitory PD-L1 on DCs (88). In addition, Tregs secrete IL-10 and TGF-β, two of the main immunosuppressive cytokines that are known to induce DC dysfunction (89, 90).
The expression of inhibitory molecules, such as PD-L1, PD-L2, Tim3, LAG3 contributes to the suppressed function of DCs in tumors and tumor-draining LNs. It has been reported that tumor-derived factors up-regulate Tim3 expression in tumor DCs (91). TIM-3 on DCs then inhibits anti-tumor responses and reduces the efficacy of cancer treatments by binding to high-mobility group box 1 protein (HMGB1), a damage-associated molecular pattern molecule involved in cytosolic nucleic acid recognition in the TME. In addition, signaling via TIM-3 on both BMDCs and splenic DCs has been shown to inhibit DC activation and maturation (92). For PD-L1, CD103+ DCs from tumor-draining LNs have recently been shown to have increased expression of PD-L1 compared to non-draining LN DCs (93). PD-L1 and PD-1 blockade has been shown to reverse DC dysfunction leading to enhanced T cell immunity (94, 95), suggesting that PD-L1/PD-1 signaling negatively regulates DC function.
Tumor cells escape immune surveillance by disabling the process of tumor antigen presentation. Recent studies have shown that DCs in TME often exhibited impaired capacity in cross-presentation (25, 96). The TME can specifically modulate DCs' antigen presentation function by targeting the molecules and machinery directly involved in antigen presentation, for example, decreasing the expression of their MHCI and MHCII molecules and their regulators such as CIITA, down-regulation of genes such as ER-resident aminopeptidases (ERAP) and transporter associated with antigen processing (TAP) (97). Abnormal accumulation of lipids in DCs has emerged as an important mechanism for DC dysfunction, as TIDCs from multiple tumor models and cancer patients exhibited reduced capacity in cross-presentation because of lipid accumulation (98, 99). Supporting this notion, a recent study has shown that the accumulation of lipids in TIDCs was involved inplay arole in blunting inhibiting anti-tumor T cell responses in ovarian cancer (100).
Recruitment of pDCs to the tumor microenvironment has been reported in a variety of cancers, however, these tumor-infiltrated pDCs are often tolerogenic, favoring tumor progression. High tumor infiltration by pDCs has been associated with poor prognosis in melanoma, head and neck, breast, and ovarian cancers (45, 101–103). pDCs have been shown to induce the generation of Tregs in the TME and tumor-draining LNs (88, 104). pDCs can also stimulate the generation of Tregs by their expression of ICOS-L, and ICOS-L expression on pDCs has also been shown to be associated with breast cancer progression (105, 106). On the other hand, pDCs have also been shown to promote immunogenic anti-tumor responses if properly stimulated, as therapeutic activation of pDCs have shown efficacy in melanoma, basal cell carcinoma, and T cell lymphoma (25, 41, 103, 107).
Cross-priming, a process which DCs activate CD8 T cells by cross-presenting exogenous antigens (108, 109), plays a critical role in generating anti-tumor CD8 T cell immunity (110–115). Anti-tumor CD8 T cell responses are induced in three sequential steps: (1) tumor antigen uptake and cross-presentation; (2) tumor antigen-specific CD8 T cell priming by DCs, and (3) elimination of tumor cells by effector CTLs (116). However, TME-mediated suppression and modulation of TIDCs often leads to their dysfunction, resulting in failure in cross-priming (step 1 and 2) and suppressed anti-tumor CD8 T cell immunity. Indeed, DC-mediated cross-presentation of tumor antigens in tumor-bearing hosts often induces T cell tolerance instead of immunity (110). However, not all TIDCs within TME exhibit suppressive and/or regulatory functions. For example, the infiltration of BDCA3+ cDC1s in the TME has been shown to correlate with increased T cell infiltration and improved prognosis in cancer patients and better efficacy of cancer immunotherapies, highlighting the critical positive role of cDC1 in generating anti-tumor immunity in the TME (78, 117). Thus, recent discoveries on the critical role of cDC1s in particular CD103+ cDC1s in CD8 T cell cross-priming in tumors have generated much interest, and have offered opportunities for improved cancer immunotherapies (96).
The generation of Batf3−/− mice that selectively lack cDC1s has greatly advanced our understanding of their function in CD8 T cell cross-priming in tumors (30). Batf3−/− mice exhibited defective cross-presentation and impaired anti-tumor immunity, suggesting that cDC1s play a critical role in initiating CD8 T cell-mediated anti-tumor immunity through cross-presentation (30). The mechanisms that make cDC1s superior in cross-presentation are only being uncovered recently. While cDC1s exhibit high efficiency at endocytosis of cell-associated antigens, their superior capacity in cross-presentation is thought to due to their specialized capability in processing antigens (96). In addition, the cross-presentation capacity of cDC1s is further enhanced by their expression of Clec9A, which facilitate the cross-presentation of antigens from dead cells by binding filamentous actin (118–120).
Examining the TME, Broz et al. have identified CD103+ cDC1s as the only population with the capability to induce proliferation of both naive CD8 T cells and established CTLs, suggesting that CD103+ cDC1s are the APCs that cross-prime CTLs in the TME (117) (Figure 1). More importantly, analysis of The Cancer Genome Atlas (TCGA) database indicated that the CD103+/CD103− gene ratio correlates strongly with increased patient survival across 12 different cancer types (117). Consistently with cDC1s' critical role in anti-tumor immunity, a recent study has shown that activation of β-catenin signaling in melanoma cells reduces the numbers of intratumoral CD103+ cDC1 cells, thus preventing tumor-specific T cell priming, suggesting that CD103+ cDC1s might not only promote anti-tumor immunity but also be suppressed by cancer cells for immune evasion (78). In both B16 and Braf-mutant mouse melanoma models, CD103+ cDC1s have been shown to play a critical role in the efficacy of immunotherapy with PD-L1 and Braf inhibition (93). A combined treatment of systemic FMS-like tyrosine kinase 3 ligand (FLT3L) and poly I:C at the tumor sites, which induced the expansion and maturation of CD103+ cDC1s, improved the efficacy of BRAF and PD-L1 blockade, suggesting that combined FLT3L and poly I:C therapy might be a promising approach that could improve the efficacy of current ICB immunotherapy in cancer patients (93). Similarly, efficacy of immunotherapy using PD-1 and CD137 blockade has been shown to depend on CD103+ cDC1s, likely due to their function in cross-priming (121).
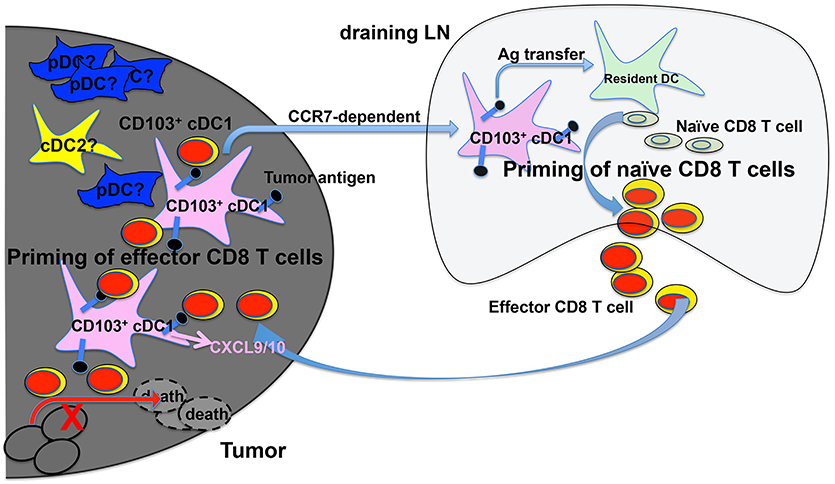
Figure 1. cDC1s and priming of tumor-antigen-specific CD8 T cells in the tumor microenvironment (TME) and tumor-draining lymph nodes (tdLNs). Migratory CD103+ cDC1s in the TME take up tumor antigens (black dots), and transport tumor antigens to tdLN by migrating to the tdLN in a CCR7-dependent mechanism. Once in the tdLN, cross-presenting CD103+ cDC1s prime naive tumor antigen-specific CD8 T cells to become effector CD8 T cells. Cross-presenting CD103+ cDC1s also transfer tumor antigens to other resident myeloid cells including CD8α+ cDC1s that are also likely involved in priming naive CD8 T cells in tdLN. cDC1s in the TME produce CXCL9/10 to recruit primed effector CD8 T cells into TME, where they are re-stimulated by CD103+ cDC1s leading to the efficient killing of tumor cells. The function of other DCs such as pDCs and cDC2s in CD8 T cell priming is less understood.
Recent studies have also shown that CD103+ cDC1s are the only population that mediates the transport of solid tumor antigens from TME to tumor draining lymph nodes for cross-priming of CD8 T cells (93, 122) (Figure 1). DC migration to LNs is mediated by the CCR7 chemokine receptor expressed on CD103+ cDC1s, as CCR7−/− CD103+ cDC1s exhibited reduced function in migration and T cell priming (122). In addition, intratumoral CD103+ cDC1s also play a critical role in the trafficking of tumor-specific effector T cells into tumors, as effector T cell recruitment into tumors depends on the presence of CXCL9/10-producing CD103+ cDC1s (123, 124) (Figure 1). Importantly, the expression of Batf3-dependent DC transcripts in human melanoma tumors correlates with CXCL9/10 expression and CD8 T cell infiltration, suggesting that Batf3-dependent cDC1s might regulate T cell recruitment to tumors in both mice and human (124).
The role of cDC2s and pDCs in CD8 T cell cross-priming in tumors are less well understood. cDC2s isolated from the TME have been shown to engulf tumor antigens and induce T cell proliferation in vitro, suggesting cDC2s may play a role in cross-priming CD8 T cells in TME (117). Given the dominant role of cDC1s as described above, however, cDC2s likely play a minor role in promoting anti-tumor immunity. pDCs can present antigens to activate CD4 T cells as well as activate CD8 T cells through cross-presentation (125, 126). Recruitment of pDCs to the TME has been reported in a number of cancers, although high tumor infiltration of pDCs has been shown to correlate with poor prognosis in melanoma, head and neck, breast, and ovarian cancers (45, 101–103). Activation of pDCs has been shown to promote anti-tumor immunity, likely through the production of type 1 IFNs (127, 128). The role of tumor infiltrated pDCs in the cross-priming of tumor-specific CD8 T cells, however, remains underinvestigated and poorly understood. Interesting, several recent reports have shown the cooperation of pDCs and cDCs in achieving optimal cross-priming (129, 130), suggesting that pDCs could play a positive role in generating anti-tumor CD8 T cell immunity.
It's worth noting that the best efficacy for anti-CTLA-4 blockade was achieved in combination with GM-CSF+ tumor cell vaccination two decades ago by the lab of the newly Nobel laureate Dr. James Allison (131, 132). In the 1998 PNAS paper, the authors suggested that “the most effective and synergistic vaccine strategy targets treatments that enhance T cell priming at the level of host-derived antigen-presenting cells” (131), which quite accurately predicted the direction of cancer immunotherapy as combining ICB with DC-based cancer immunotherapy. In light of the recent discovery of the critical role of cDC1s in priming tumor-specific CD8 T cells, repairing and/or enhancing DC-mediated CD8 T cell priming represents an exciting approach to improve the efficacy of current T cell-based cancer immunotherapies including ICB and ACT (12, 14, 96). Indeed, Spranger et al. have shown that vaccination with in vitro-generated DCs improved the efficacy of anti-PD-L1 and anti-CTLA-4 immunotherapy (78). Similarly, treatment of FLT3L/poly I:C, which expands and induces the maturation and activation of CD103+ cDC1s at the tumor sites, has been shown to enhance anti-tumor responses and improve efficacy when combined with BRAF and PD-L1 blockade (93). Recently, we have genetically engineered tumor-specific CD8 T cells with a second T-cell receptor (TCR) that recognizes a Listeria antigen. And we have shown that Listeria infection led to the eradication of primary tumors and development of immunological memory against tumor re-challenge in combination with adoptive cell transfer (ACT) of these dual-specific T cells, likely due to the substantially enhanced T cell priming involving DCs (133). In vivo DC-targeted vaccines that deliver tumor antigens to cross-presenting DCs with monoclonal antibodies carrying tumor antigens is another attractive approach to enhance cross-priming of tumor-specific CD8 T cells. As multiple clinical trials with human anti-DEC-205 monoclonal antibody fused with antigens such as tumor antigen NY-ESO-1 have shown promising results (134–137), it will be interesting to combine in vivo DC-targeted vaccines with T cell-based cancer immunotherapies such as ICB and ACT to further improve their efficacy. Another intriguing approach is the manipulation of pDCs. While tumors are known to prevent the infiltration of cDCs exemplified by recent reports involving β-catenin signaling pathway (78), accumulation of pDCs has been reported in multiple tumors including melanoma, head and neck, breast, and ovarian cancers (45, 101–103), thus offering an opportunity to manipulate these pDCs to generate anti-tumor immunity in the tumor microenvironment (TME). Indeed, therapeutic activation of pDCs have been reported to induce immunogenic anti-tumor responses and shown efficacy in multiple human cancers (25, 41, 103, 107). While the roles of cross-priming by pDCs in vivo are still under debate (29, 138–140), recent studies have shown that the co-operation of pDCs and cDCs was required to achieve optimal cross-priming of CD8 T cells (129, 130, 141). Thus, studies are warrantied to further understand the contribution of other DC subsets including pDCs and cDC2s in CD8 T cell priming in TME and tumor-draining LN, which will help develop better strategies to improve efficacy of cancer immunotherapies by enhancing DC function in CD8 T cell priming.
Generation of durable memory CD8 T cells responses that are capable of protection from recurrence and relapse is the ultimate goal of cancer immunotherapy. Memory CD8 T cells are heterogeneous populations that include both circulating memory CD8 T cells and non-circulating tissue resident memory CD8 T cells (Trm) (142). Circulating memory CD8 T cells can be further divided into stem cell memory (Tscm), central memory (Tcm) and effector memory (Tem). Tumor infiltrated Tcm and Tem cells have been reported in multiple cancers such as colorectal and breast cancer (143–145). However, memory CD8 T cells in tumors often exhibit dysfunctional phenotypes and their dysfunction correlates with cancer progression (142). Highlighting their role in anti-tumor immunity, intratumoral expansion of Tem cells in patient samples have been associated with improved responses to anti-PD-L1 therapy (146). For the recently identified Trm cells, tumor infiltrated CD8+CD103+ Trm cells have been reported in tumor samples of ovarian, endometrial, breast and lung cancer patients, and their number correlates with prolonged survival and better prognosis (147–152). While the presence of the memory CD8 T cells in tumors is clear, whether and how TIDCs in particular CD103+ cDC1s regulate the generation and function of memory CD8 T cells remains largely unexplored. Under certain conditions, cross-priming of CD8 T cells by CD103+ cDC1s in TME does lead to memory CD8 T cell responses. For instance, Salmon et al. have shown that FLT3L/poly I:C treatment synergized with PD-L1 blockade to prevent the secondary melanoma lesions after Braf inhibition, as well as provide protection against tumor re-challenge, indicated the generation of memory CD8 T cell responses after CD8 T cell priming (93). Thus, further studies on memory CD8 T cells in TME are warrantied to understand how to better achieve memory CD8 T cell responses in TME.
DC-mediated cross-priming of tumor-specific CD8 T cells plays a critical role in initiating and sustaining anti-tumor immunity (110–115). TME employs an array of mechanisms to modify the phenotype and function of TIDC to transform them into immunosuppressive DCs. Insufficient T cell priming likely contributes to cold tumors (no T cell infiltration in TME) and unresponsiveness to immune checkpoint blockade (ICB) therapy, and is under intensive investigation (12). Recently, a number of studies have shown that CD103+ cDC1s in TME are critical in cross-priming CD8 T cells to generate anti-tumor immunity. These CD103+ cDC1s mediate cross-presentation and transport tumor antigens from tumors to draining LN to prime naive CD8 T cells, have the capacity to prime tumor-reactive CTLs in TME, play a critical role in trafficking of effector CD8 T cells to tumors, thus impact all three steps of anti-tumor CD8 T cell responses required for tumor eradication (78, 93, 117, 121–124, 153). In addition, the presence of CD103+ cDC1s has been shown to be critical for efficacy of multiple ICB therapies (93, 121). Thus, manipulating CD8 T cell cross-priming by cDC1s, by employing strategies to increase the number of cDC1s and enhancing their capacity of cross-priming in tumors and tumor draining LNs, represents an exciting approach to enhance anti-tumor CD8 T cell immunity and improve the efficacy of current cancer immunotherapies including ICB and ACT (see reference 13 for an excellent recent review on DC-based cancer immunotherapy). Of note, combination treatment of FLT3L/poly I:C, which expands and induces the maturation and activation of CD103+ cDC1s at the tumor sites, has already been shown to enhance anti-tumor responses to BRAF and PD-L1 blockade (93).
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This work was supported by the NCI Cancer Center Support Grant 5P30 CA016056, an award from the Roswell Park Alliance Foundation and R01CA198105 (To AJ).
1. Tian T, Olson S, Whitacre JM, Harding A. The origins of cancer robustness and evolvability. Integr Biol. (2011) 3:17–30. doi: 10.1039/C0IB00046A
2. Durgeau A, Virk Y, Corgnac S, Mami-Chouaib F. Recent advances in targeting CD8 T-cell immunity for more effective cancer immunotherapy. Front Immunol. (2018) 9:14. doi: 10.3389/fimmu.2018.00014
3. van der Bruggen P, Traversari C, Chomez P, Lurquin C, De Plaen E, Van den Eynde B, et al. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science (1991) 254:1643–7. doi: 10.1126/science.1840703
4. Slingluff CL Jr, Cox AL, Stover JM Jr, Moore MM, Hunt DF, Engelhard VH. Cytotoxic T-lymphocyte response to autologous human squamous cell cancer of the lung: epitope reconstitution with peptides extracted from HLA-Aw68. Cancer Res. (1994) 54:2731–7.
5. Boon T, Coulie PG, Van den Eynde B. Tumor antigens recognized by T cells. Immunol Today (1997) 18:267–8. doi: 10.1016/S0167-5699(97)80020-5
6. Echchakir H, Vergnon I, Dorothee G, Grunenwald D, Chouaib S, Mami-Chouaib F. Evidence for in situ expansion of diverse antitumor-specific cytotoxic T lymphocyte clones in a human large cell carcinoma of the lung. Int Immunol. (2000) 12:537–46. doi: 10.1093/intimm/12.4.537
7. Karanikas V, Colau D, Baurain JF, Chiari R, Thonnard J, Gutierrez-Roelens I, et al. High frequency of cytolytic T lymphocytes directed against a tumor-specific mutated antigen detectable with HLA tetramers in the blood of a lung carcinoma patient with long survival. Cancer Res. (2001) 61:3718–24.
8. Fridman WH, Pages F, Sautes-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer (2012) 12:298–306. doi: 10.1038/nrc3245
9. Reiser J, Banerjee A. Effector, memory, and dysfunctional CD8(+) T cell fates in the antitumor immune response. J Immunol Res. (2016) 2016:8941260. doi: 10.1155/2016/8941260
10. Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ. Natural innate and adaptive immunity to cancer. Annu Rev Immunol. (2011) 29:235–71. doi: 10.1146/annurev-immunol-031210-101324
11. Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity (2013) 39:1–10. doi: 10.1016/j.immuni.2013.07.012
12. Vonderheide RH. The immune revolution: a case for priming, not checkpoint. Cancer Cell (2018) 33:563–9. doi: 10.1016/j.ccell.2018.03.008
13. Arina A, Tirapu I, Alfaro C, Rodriguez-Calvillo M, Mazzolini G, Inoges S, et al. Clinical implications of antigen transfer mechanisms from malignant to dendritic cells. exploiting cross-priming. Exp Hematol. (2002) 30:1355–64. doi: 10.1016/S0301-472X(02)00956-6
14. Saxena M, Bhardwaj N. Re-emergence of dendritic cell vaccines for cancer treatment. Trends Cancer (2018) 4:119–37. doi: 10.1016/j.trecan.2017.12.007
15. Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer (2012) 12:265–77. doi: 10.1038/nrc3258
16. Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature (1998) 392:245–52. doi: 10.1038/32588
17. Ma Y, Shurin GV, Peiyuan Z, Shurin MR. Dendritic cells in the cancer microenvironment. J Cancer (2013) 4:36–44. doi: 10.7150/jca.5046
18. Steinman RM, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J Exp Med. (1973) 137:1142–62. doi: 10.1084/jem.137.5.1142
19. Diao J, Winter E, Chen W, Cantin C, Cattral MS. Characterization of distinct conventional and plasmacytoid dendritic cell-committed precursors in murine bone marrow. J Immunol. (2004) 173:1826–33. doi: 10.4049/jimmunol.173.3.1826
20. Fogg DK, Sibon C, Miled C, Jung S, Aucouturier P, Littman DR, et al. A clonogenic bone marrow progenitor specific for macrophages and dendritic cells. Science (2006) 311:83–7. doi: 10.1126/science.1117729
21. Naik SH, Sathe P, Park HY, Metcalf D, Proietto AI, Dakic A, et al. Development of plasmacytoid and conventional dendritic cell subtypes from single precursor cells derived in vitro and in vivo. Nat Immunol. (2007) 8:1217–26. doi: 10.1038/ni1522
22. Onai N, Kurabayashi K, Hosoi-Amaike M, Toyama-Sorimachi N, Matsushima K, Inaba K, et al. A clonogenic progenitor with prominent plasmacytoid dendritic cell developmental potential. Immunity (2013) 38:943–57. doi: 10.1016/j.immuni.2013.04.006
23. Murphy TL, Grajales-Reyes GE, Wu X, Tussiwand R, Briseno CG, Iwata A, et al. Transcriptional control of dendritic cell development. Annu Rev Immunol. (2016) 34:93–119. doi: 10.1146/annurev-immunol-032713-120204
24. Anderson DA 3rd Murphy KM, Briseno CG. Development, diversity, and function of dendritic cells in mouse and human. Cold Spring Harb Perspect Biol. (2017) 10:a028613. doi: 10.1101/cshperspect.a028613
25. Bandola-Simon J, Roche PA. Dysfunction of antigen processing and presentation by dendritic cells in cancer. Mol Immunol. (2018). doi: 10.1016/j.molimm.2018.03.025. [Epub ahead of print].
26. Chrisikos TT, Zhou Y, Slone N, Babcock R, Watowich SS, Li HS. Molecular regulation of dendritic cell development and function in homeostasis, inflammation, and cancer. Mol Immunol (2018). doi: 10.1016/j.molimm.2018.01.014. [Epub ahead of print].
27. Guilliams M, Ginhoux F, Jakubzick C, Naik SH, Onai N, Schraml BU, et al. Dendritic cells, monocytes and macrophages: a unified nomenclature based on ontogeny. Nat Rev Immunol. (2014) 14:571–8. doi: 10.1038/nri3712
28. Guilliams M, Dutertre CA, Scott CL, McGovern N, Sichien D, Chakarov S, et al. Unsupervised high-dimensional analysis aligns dendritic cells across tissues and species. Immunity (2016) 45:669–84. doi: 10.1016/j.immuni.2016.08.015
29. Gutierrez-Martinez E, Planes R, Anselmi G, Reynolds M, Menezes S, Adiko AC, et al. Cross-presentation of cell-associated antigens by MHC class I in dendritic cell subsets. Front Immunol. (2015) 6:363. doi: 10.3389/fimmu.2015.00363
30. Hildner K, Edelson BT, Purtha WE, Diamond M, Matsushita H, Kohyama M, et al. Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity. Science (2008) 322:1097–100. doi: 10.1126/science.1164206
31. Suzuki S, Honma K, Matsuyama T, Suzuki K, Toriyama K, Akitoyo I, et al. Critical roles of interferon regulatory factor 4 in CD11bhighCD8alpha- dendritic cell development. Proc Natl Acad Sci USA (2004) 101:8981–6. doi: 10.1073/pnas.0402139101
32. Mildner A, Jung S. Development and function of dendritic cell subsets. Immunity (2014) 40:642–56. doi: 10.1016/j.immuni.2014.04.016
33. Merad M, Sathe P, Helft J, Miller J, Mortha A. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu Rev Immunol. (2013) 31:563–604. doi: 10.1146/annurev-immunol-020711-074950
34. Gardner A, Ruffell B. Dendritic cells and cancer immunity. Trends Immunol. (2016) 37:855–65. doi: 10.1016/j.it.2016.09.006
35. Reizis B, Colonna M, Trinchieri G, Barrat F, Gilliet M. Plasmacytoid dendritic cells: one-trick ponies or workhorses of the immune system? Nat Rev Immunol. (2011) 11:558–65. doi: 10.1038/nri3027
36. Swiecki M, Colonna M. The multifaceted biology of plasmacytoid dendritic cells. Nat Rev Immunol. (2015) 15:471–85. doi: 10.1038/nri3865
37. Mitchell D, Chintala S, Dey M. Plasmacytoid dendritic cell in immunity and cancer. J Neuroimmunol. (2018) 322:63–73. doi: 10.1016/j.jneuroim.2018.06.012
38. Cisse B, Caton ML, Lehner M, Maeda T, Scheu S, Locksley R, et al. Transcription factor E2-2 is an essential and specific regulator of plasmacytoid dendritic cell development. Cell (2008) 135:37–48. doi: 10.1016/j.cell.2008.09.016
40. Ghosh HS, Cisse B, Bunin A, Lewis KL, Reizis B. Continuous expression of the transcription factor e2-2 maintains the cell fate of mature plasmacytoid dendritic cells. Immunity (2010) 33:905–16. doi: 10.1016/j.immuni.2010.11.023
41. Veglia F, Gabrilovich DI. Dendritic cells in cancer: the role revisited. Curr Opin Immunol. (2017) 45:43–51. doi: 10.1016/j.coi.2017.01.002
42. Colonna M, Trinchieri G, Liu YJ. Plasmacytoid dendritic cells in immunity. Nat Immunol. (2004) 5:1219–26. doi: 10.1038/ni1141
43. Gilliet M, Cao W, Liu YJ. Plasmacytoid dendritic cells: sensing nucleic acids in viral infection and autoimmune diseases. Nat Rev Immunol. (2008) 8:594–606. doi: 10.1038/nri2358
44. Lande R, Gregorio J, Facchinetti V, Chatterjee B, Wang YH, Homey B, et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature (2007) 449:564–9. doi: 10.1038/nature06116
45. Li S, Wu J, Zhu S, Liu YJ, Chen J. Disease-associated plasmacytoid dendritic cells. Front Immunol (2017) 8:1268. doi: 10.3389/fimmu.2017.01268
46. Segura E, Amigorena S. Inflammatory dendritic cells in mice and humans. Trends Immunol (2013) 34:440–5. doi: 10.1016/j.it.2013.06.001
47. Kuhn S, Hyde EJ, Yang J, Rich FJ, Harper JL, Kirman JR, et al. Increased numbers of monocyte-derived dendritic cells during successful tumor immunotherapy with immune-activating agents. J Immunol. (2013) 191:1984–92. doi: 10.4049/jimmunol.1301135
48. Kuhn S, Yang J, Ronchese F. Monocyte-derived dendritic cells are essential for CD8(+) T cell activation and antitumor responses after local immunotherapy. Front Immunol. (2015) 6:584. doi: 10.3389/fimmu.2015.00584
49. Ma Y, Adjemian S, Mattarollo SR, Yamazaki T, Aymeric L, Yang H, et al. Anticancer chemotherapy-induced intratumoral recruitment and differentiation of antigen-presenting cells. Immunity (2013) 38:729–41. doi: 10.1016/j.immuni.2013.03.003
50. Marigo I, Zilio S, Desantis G, Mlecnik B, Agnellini AH, Ugel S, et al. T cell cancer therapy requires CD40-CD40L activation of tumor necrosis factor and inducible nitric-oxide-synthase-producing dendritic cells. Cancer Cell (2016) 30:651. doi: 10.1016/j.ccell.2016.09.009
51. Karthaus N, Torensma R, Tel J. Deciphering the message broadcast by tumor-infiltrating dendritic cells. Am J Pathol. (2012) 181:733–42. doi: 10.1016/j.ajpath.2012.05.012
52. Ocana MC, Martinez-Poveda B, Quesada AR, Medina MA. Metabolism within the tumor microenvironment and its implication on cancer progression: an ongoing therapeutic target. Med Res Rev. (2018) 39:70–113. doi: 10.1002/med.21511
53. Almeida FV, Douglass SM, Fane ME, Weeraratna AT. Bad company: Microenvironmentally mediated resistance to targeted therapy in melanoma. Pigment Cell Melanoma Res. (2018). doi: 10.1111/pcmr.12736. [Epub ahead of print].
54. Tran Janco JM, Lamichhane P, Karyampudi L, Knutson KL. Tumor-infiltrating dendritic cells in cancer pathogenesis. J Immunol. (2015) 194:2985–91. doi: 10.4049/jimmunol.1403134
55. Zong J, Keskinov AA, Shurin GV, Shurin MR. Tumor-derived factors modulating dendritic cell function. Cancer Immunol Immunother. (2016) 65:821–33. doi: 10.1007/s00262-016-1820-y
56. Tang M, Diao J, Cattral MS. Molecular mechanisms involved in dendritic cell dysfunction in cancer. Cell Mol Life Sci. (2017) 74:761–76. doi: 10.1007/s00018-016-2317-8
57. Menetrier-Caux C, Montmain G, Dieu MC, Bain C, Favrot MC, Caux C, et al. Inhibition of the differentiation of dendritic cells from CD34(+) progenitors by tumor cells: role of interleukin-6 and macrophage colony-stimulating factor. Blood (1998) 92:4778–91.
58. Bharadwaj U, Li M, Zhang R, Chen C, Yao Q. Elevated interleukin-6 and G-CSF in human pancreatic cancer cell conditioned medium suppress dendritic cell differentiation and activation. Cancer Res. (2007) 67:5479–88. doi: 10.1158/0008-5472.CAN-06-3963
59. Chomarat P, Banchereau J, Davoust J, Palucka AK. IL-6 switches the differentiation of monocytes from dendritic cells to macrophages. Nat Immunol. (2000) 1:510–4. doi: 10.1038/82763
60. Alshamsan A. Induction of tolerogenic dendritic cells by IL-6-secreting CT26 colon carcinoma. Immunopharmacol Immunotoxicol. (2012) 34:465–9. doi: 10.3109/08923973.2011.625034
61. Pahne-Zeppenfeld J, Schroer N, Walch-Ruckheim B, Oldak M, Gorter A, Hegde S, et al. Cervical cancer cell-derived interleukin-6 impairs CCR7-dependent migration of MMP-9-expressing dendritic cells. Int J Cancer (2014) 134:2061–73. doi: 10.1002/ijc.28549
62. O'Garra A, Barrat FJ, Castro AG, Vicari A, Hawrylowicz C. Strategies for use of IL-10 or its antagonists in human disease. Immunol Rev. (2008) 223:114–31. doi: 10.1111/j.1600-065X.2008.00635.x
63. Huang LY, Reis e Sousa C, Itoh Y, Inman J, Scott DE. IL-12 induction by a TH1-inducing adjuvant in vivo: dendritic cell subsets and regulation by IL-10. J Immunol. (2001) 167:1423–30. doi: 10.4049/jimmunol.167.3.1423
64. Yang AS, Lattime EC. Tumor-induced interleukin 10 suppresses the ability of splenic dendritic cells to stimulate CD4 and CD8 T-cell responses. Cancer Res (2003) 63:2150–7.
65. Steinbrink K, Jonuleit H, Muller G, Schuler G, Knop J, Enk AH. Interleukin-10-treated human dendritic cells induce a melanoma-antigen-specific anergy in CD8(+) T cells resulting in a failure to lyse tumor cells. Blood (1999) 93:1634–42.
66. Allavena P, Piemonti L, Longoni D, Bernasconi S, Stoppacciaro A, Ruco L, et al. IL-10 prevents the differentiation of monocytes to dendritic cells but promotes their maturation to macrophages. Eur J Immunol. (1998) 28:359–69. doi: 10.1002/(SICI)1521-4141(199801)28:01<359::AID-IMMU359>3.0.CO;2-4
67. Gabrilovich D, Ishida T, Oyama T, Ran S, Kravtsov V, Nadaf S, et al. Vascular endothelial growth factor inhibits the development of dendritic cells and dramatically affects the differentiation of multiple hematopoietic lineages in vivo. Blood (1998) 92:4150–66.
68. Shi Y, Yu P, Zeng D, Qian F, Lei X, Zhao Y, et al. Suppression of vascular endothelial growth factor abrogates the immunosuppressive capability of murine gastric cancer cells and elicits antitumor immunity. FEBS J. (2014) 281:3882–93. doi: 10.1111/febs.12923
69. Brown RD, Pope B, Murray A, Esdale W, Sze DM, Gibson J, et al. Dendritic cells from patients with myeloma are numerically normal but functionally defective as they fail to up-regulate CD80 (B7-1) expression after huCD40LT stimulation because of inhibition by transforming growth factor-beta1 and interleukin-10. Blood (2001) 98:2992–8. doi: 10.1182/blood.V98.10.2992
70. Kel JM, Girard-Madoux MJ, Reizis B, Clausen BE. TGF-beta is required to maintain the pool of immature Langerhans cells in the epidermis. J Immunol. (2010) 185:3248–55. doi: 10.4049/jimmunol.1000981
71. Hargadon KM. Tumor-altered dendritic cell function: implications for anti-tumor immunity. Front Immunol. (2013) 4:192. doi: 10.3389/fimmu.2013.00192
72. Ito T, Wang YH, Duramad O, Hori T, Delespesse GJ, Watanabe N, et al. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J Exp Med. (2005) 202:1213–23. doi: 10.1084/jem.20051135
73. Godefroy E, Manches O, Dreno B, Hochman T, Rolnitzky L, Labarriere N, et al. Matrix metalloproteinase-2 conditions human dendritic cells to prime inflammatory T(H)2 cells via an IL-12- and OX40L-dependent pathway. Cancer Cell (2011) 19:333–46. doi: 10.1016/j.ccr.2011.01.037
74. Aspord C, Pedroza-Gonzalez A, Gallegos M, Tindle S, Burton EC, Su D, et al. Breast cancer instructs dendritic cells to prime interleukin 13-secreting CD4+ T cells that facilitate tumor development. J Exp Med. (2007) 204:1037–47. doi: 10.1084/jem.20061120
75. Lo Kuan E, Ziegler SF. Thymic stromal lymphopoietin and cancer. J Immunol. (2014) 193:4283–8. doi: 10.4049/jimmunol.1400864
76. Kawakami Y, Yaguchi T, Sumimoto H, Kudo-Saito C, Iwata-Kajihara T, Nakamura S, et al. Improvement of cancer immunotherapy by combining molecular targeted therapy. Front Oncol. (2013) 3:136. doi: 10.3389/fonc.2013.00136
77. Hansen M, Andersen MH. The role of dendritic cells in cancer. Semin Immunopathol. (2017) 39:307–16. doi: 10.1007/s00281-016-0592-y
78. Spranger S, Bao R, Gajewski TF. Melanoma-intrinsic beta-catenin signalling prevents anti-tumour immunity. Nature (2015) 523:231–5. doi: 10.1038/nature14404
79. Holtzhausen A, Zhao F, Evans K, Tsutsui M, Orabona C, Tyler DS, et al. Melanoma-derived Wnt5a promotes local dendritic-cell expression of IDO and immunotolerance: opportunities for pharmacologic enhancement of immunotherapy. Cancer Immunol Res. (2015) 3:1082–95. doi: 10.1158/2326-6066.CIR-14-0167
80. Hong Y, Manoharan I, Suryawanshi A, Majumdar T, Angus-Hill ML, Koni PA, et al. β-catenin promotes regulatory T-cell responses in tumors by inducing vitamin A metabolism in dendritic cells. Cancer Res. (2015) 75:656–65. doi: 10.1158/0008-5472.CAN-14-2377
81. Liang X, Fu C, Cui W, Ober-Blobaum JL, Zahner SP, Shrikant PA, et al. beta-Catenin mediates tumor-induced immunosuppression by inhibiting cross-priming of CD8+ T cells. J Leukoc Biol. (2014) 95:179–90. doi: 10.1189/jlb.0613330
82. Fu C, Liang X, Cui W, Ober-Blobaum JL, Vazzana J, Shrikant PA, et al. β-Catenin in dendritic cells exerts opposite functions in cross-priming and maintenance of CD8+ T cells through regulation of IL-10. Proc Natl Acad Sci USA (2015) 112:2823–8. doi: 10.1073/pnas.1414167112
83. Janikashvili N, Bonnotte B, Katsanis E, Larmonier N. The dendritic cell-regulatory T lymphocyte crosstalk contributes to tumor-induced tolerance. Clin Dev Immunol. (2011) 2011:430394. doi: 10.1155/2011/430394
84. Oderup C, Cederbom L, Makowska A, Cilio CM, Ivars F. Cytotoxic T lymphocyte antigen-4-dependent down-modulation of costimulatory molecules on dendritic cells in CD4+ CD25+ regulatory T-cell-mediated suppression. Immunology (2006) 118:240–9. doi: 10.1111/j.1365-2567.2006.02362.x
85. Fallarino F, Grohmann U, Hwang KW, Orabona C, Vacca C, Bianchi R, et al. Modulation of tryptophan catabolism by regulatory T cells. Nat Immunol. (2003) 4:1206–12. doi: 10.1038/ni1003
86. Manches O, Munn D, Fallahi A, Lifson J, Chaperot L, Plumas J, et al. HIV-activated human plasmacytoid DCs induce Tregs through an indoleamine 2,3-dioxygenase-dependent mechanism. J Clin Invest. (2008) 118:3431–9. doi: 10.1172/JCI34823
87. Munn DH, Sharma MD, Lee JR, Jhaver KG, Johnson TS, Keskin DB, et al. Potential regulatory function of human dendritic cells expressing indoleamine 2,3-dioxygenase. Science (2002) 297:1867–70. doi: 10.1126/science.1073514
88. Sharma MD, Baban B, Chandler P, Hou DY, Singh N, Yagita H, et al. Plasmacytoid dendritic cells from mouse tumor-draining lymph nodes directly activate mature Tregs via indoleamine 2,3-dioxygenase. J Clin Invest. (2007) 117:2570–82. doi: 10.1172/JCI31911
89. Larmonier N, Marron M, Zeng Y, Cantrell J, Romanoski A, Sepassi M, et al. Tumor-derived CD4+CD25+ regulatory T cell suppression of dendritic cell function involves TGF-beta and IL-10. Cancer Immunol Immunother. (2007) 56:48–59. doi: 10.1007/s00262-006-0160-8
90. Gallois A, Bhardwaj N. Dendritic cell-targeted approaches to modulate immune dysfunction in the tumor microenvironment. Front Immunol. (2013) 4:436. doi: 10.3389/fimmu.2013.00436
91. Chiba S, Baghdadi M, Akiba H, Yoshiyama H, Kinoshita I, Dosaka-Akita H, et al. Tumor-infiltrating DCs suppress nucleic acid-mediated innate immune responses through interactions between the receptor TIM-3 and the alarmin HMGB1. Nat Immunol. (2012) 13:832–42. doi: 10.1038/ni.2376
92. Maurya N, Gujar R, Gupta M, Yadav V, Verma S, Sen P. Immunoregulation of dendritic cells by the receptor T cell Ig and mucin protein-3 via Bruton's tyrosine kinase and c-Src. J Immunol. (2014) 193:3417–25. doi: 10.4049/jimmunol.1400395
93. Salmon H, Idoyaga J, Rahman A, Leboeuf M, Remark R, Jordan S, et al. Expansion and activation of CD103+ dendritic cell progenitors at the tumor site enhances tumor responses to therapeutic PD-L1 and BRAF inhibition. Immunity (2016) 44:924–38. doi: 10.1016/j.immuni.2016.03.012
94. Karyampudi L, Lamichhane P, Scheid AD, Kalli KR, Shreeder B, Krempski JW, et al. Accumulation of memory precursor CD8 T cells in regressing tumors following combination therapy with vaccine and anti-PD-1 antibody. Cancer Res. (2014) 74:2974–85. doi: 10.1158/0008-5472.CAN-13-2564
95. Karyampudi L, Lamichhane P, Krempski J, Kalli KR, Behrens MD, Vargas DM, et al. PD-1 blunts the function of ovarian tumor-infiltrating dendritic cells by inactivating NF-kappaB. Cancer Res. (2016) 76:239–50. doi: 10.1158/0008-5472.CAN-15-0748
96. Sanchez-Paulete AR, Teijeira A, Cueto FJ, Garasa S, Perez-Gracia JL, Sanchez-Arraez A, et al. Antigen cross-presentation and T-cell cross-priming in cancer immunology and immunotherapy. Ann Oncol. (2017) 28(Suppl_12):xii44-xii55. doi: 10.1093/annonc/mdx237
97. Gabrilovich D. Mechanisms and functional significance of tumour-induced dendritic-cell defects. Nat Rev Immunol. (2004) 4:941–52. doi: 10.1038/nri1498
98. Herber DL, Cao W, Nefedova Y, Novitskiy SV, Nagaraj S, Tyurin VA, et al. Lipid accumulation and dendritic cell dysfunction in cancer. Nat Med. (2010) 16:880–6. doi: 10.1038/nm.2172
99. Ramakrishnan R, Tyurin VA, Veglia F, Condamine T, Amoscato A, Mohammadyani D, et al. Oxidized lipids block antigen cross-presentation by dendritic cells in cancer. J Immunol. (2014) 192:2920–31. doi: 10.4049/jimmunol.1302801
100. Cubillos-Ruiz JR, Silberman PC, Rutkowski MR, Chopra S, Perales-Puchalt A, Song M, et al. ER stress sensor XBP1 controls anti-tumor immunity by disrupting dendritic cell homeostasis. Cell (2015) 161:1527–38. doi: 10.1016/j.cell.2015.05.025
101. Treilleux I, Blay JY, Bendriss-Vermare N, Ray-Coquard I, Bachelot T, Guastalla JP, et al. Dendritic cell infiltration and prognosis of early stage breast cancer. Clin Cancer Res. (2004) 10:7466–74. doi: 10.1158/1078-0432.CCR-04-0684
102. Swiecki M, Colonna M. Accumulation of plasmacytoid DC: Roles in disease pathogenesis and targets for immunotherapy. Eur J Immunol. (2010) 40:2094–8. doi: 10.1002/eji.201040602
103. Demoulin S, Herfs M, Delvenne P, Hubert P. Tumor microenvironment converts plasmacytoid dendritic cells into immunosuppressive/tolerogenic cells: insight into the molecular mechanisms. J Leukoc Biol. (2013) 93:343–52. doi: 10.1189/jlb.0812397
104. Munn DH, Sharma MD, Hou D, Baban B, Lee JR, Antonia SJ, et al. Expression of indoleamine 2,3-dioxygenase by plasmacytoid dendritic cells in tumor-draining lymph nodes. J Clin Invest. (2004) 114:280–90. doi: 10.1172/JCI21583
105. Ito T, Yang M, Wang YH, Lande R, Gregorio J, Perng OA, et al. Plasmacytoid dendritic cells prime IL-10-producing T regulatory cells by inducible costimulator ligand. J Exp Med. (2007) 204:105–15. doi: 10.1084/jem.20061660
106. Faget J, Bendriss-Vermare N, Gobert M, Durand I, Olive D, Biota C, et al. ICOS-ligand expression on plasmacytoid dendritic cells supports breast cancer progression by promoting the accumulation of immunosuppressive CD4+ T cells. Cancer Res. (2012) 72:6130–41. doi: 10.1158/0008-5472.CAN-12-2409
107. Lombardi VC, Khaiboullina SF, Rizvanov AA. Plasmacytoid dendritic cells, a role in neoplastic prevention and progression. Eur J Clin Invest. (2015) 45 (Suppl. 1):1–8. doi: 10.1111/eci.12363
108. Bevan MJ. Cross-priming for a secondary cytotoxic response to minor H antigens with H-2 congenic cells which do not cross-react in the cytotoxic assay. J Exp Med. (1976) 143:1283–8. doi: 10.1084/jem.143.5.1283
109. Bevan MJ. Minor H antigens introduced on H-2 different stimulating cells cross-react at the cytotoxic T cell level during in vivo priming. J Immunol. (1976) 117:2233–8.
110. Melief CJ. Cancer immunotherapy by dendritic cells. Immunity (2008) 29:372–83. doi: 10.1016/j.immuni.2008.08.004
111. Kurts C, Robinson BW, Knolle PA. Cross-priming in health and disease. Nat Rev Immunol. (2010) 10:403–14. doi: 10.1038/nri2780
112. Andersen BM, Ohlfest JR. Increasing the efficacy of tumor cell vaccines by enhancing cross priming. Cancer Lett. (2012). doi: 10.1016/j.canlet.2012.07.012
113. Chen L, Fabian KL, Taylor JL, Storkus WJ. Therapeutic use of dendritic cells to promote the extranodal priming of anti-tumor immunity. Front Immunol. (2013) 4:388. doi: 10.3389/fimmu.2013.00388
114. Fuertes MB, Woo SR, Burnett B, Fu YX, Gajewski TF. Type I interferon response and innate immune sensing of cancer. Trends Immunol. (2013) 34:67–73. doi: 10.1016/j.it.2012.10.004
115. Schiavoni G, Mattei F, Gabriele L. Type I interferons as stimulators of DC-mediated cross-priming: impact on anti-tumor response. Front Immunol. (2013) 4:483. doi: 10.3389/fimmu.2013.00483
116. Nouri-Shirazi M, Banchereau J, Bell D, Burkeholder S, Kraus ET, Davoust J, et al. Dendritic cells capture killed tumor cells and present their antigens to elicit tumor-specific immune responses. J Immunol. (2000) 165:3797–803. doi: 10.4049/jimmunol.165.7.3797
117. Broz ML, Binnewies M, Boldajipour B, Nelson AE, Pollack JL, Erle DJ, et al. Dissecting the tumor myeloid compartment reveals rare activating antigen-presenting cells critical for T cell immunity. Cancer Cell (2014) 26:638–52. doi: 10.1016/j.ccell.2014.09.007
118. Sancho D, Mourao-Sa D, Joffre OP, Schulz O, Rogers NC, Pennington DJ, et al. Tumor therapy in mice via antigen targeting to a novel, DC-restricted C-type lectin. J Clin Invest. (2008) 118:2098–110. doi: 10.1172/JCI34584
119. Ahrens S, Zelenay S, Sancho D, Hanc P, Kjaer S, Feest C, et al. F-actin is an evolutionarily conserved damage-associated molecular pattern recognized by DNGR-1, a receptor for dead cells. Immunity (2012) 36:635–45. doi: 10.1016/j.immuni.2012.03.008
120. Zhang JG, Czabotar PE, Policheni AN, Caminschi I, Wan SS, Kitsoulis S, et al. The dendritic cell receptor Clec9A binds damaged cells via exposed actin filaments. Immunity (2012) 36:646–57. doi: 10.1016/j.immuni.2012.03.009
121. Sanchez-Paulete AR, Cueto FJ, Martinez-Lopez M, Labiano S, Morales-Kastresana A, Rodriguez-Ruiz ME, et al. Cancer immunotherapy with immunomodulatory Anti-CD137 and Anti-PD-1 monoclonal antibodies requires BATF3-dependent dendritic cells. Cancer Discov. (2016) 6:71–9. doi: 10.1158/2159-8290.CD-15-0510
122. Roberts EW, Broz ML, Binnewies M, Headley MB, Nelson AE, Wolf DM, et al. Critical role for CD103+/CD141+ dendritic cells bearing CCR7 for tumor antigen trafficking and priming of T cell immunity in melanoma. Cancer Cell (2016) 30:324–36. doi: 10.1016/j.ccell.2016.06.003
123. Mikucki ME, Fisher DT, Matsuzaki J, Skitzki JJ, Gaulin NB, Muhitch JB, et al. Non-redundant requirement for CXCR3 signalling during tumoricidal T-cell trafficking across tumour vascular checkpoints. Nat Commun. (2015) 6:7458. doi: 10.1038/ncomms8458
124. Spranger S, Dai D, Horton B, Gajewski TF. Tumor-residing Batf3 dendritic cells are required for effector T cell trafficking and adoptive T cell therapy. Cancer Cell (2017) 31:711–23 e4. doi: 10.1016/j.ccell.2017.04.003
125. Colonna M, Cella M. Crosspresentation: plasmacytoid dendritic cells are in the business. Immunity (2007) 27:419–21. doi: 10.1016/j.immuni.2007.08.006
126. Villadangos JA, Young L. Antigen-presentation properties of plasmacytoid dendritic cells. Immunity (2008) 29:352–61. doi: 10.1016/j.immuni.2008.09.002
127. Aspord C, Leccia MT, Charles J, Plumas J. Melanoma hijacks plasmacytoid dendritic cells to promote its own progression. Oncoimmunology (2014) 3:e27402. doi: 10.4161/onci.27402
128. Le Mercier I, Poujol D, Sanlaville A, Sisirak V, Gobert M, Durand I, et al. Tumor promotion by intratumoral plasmacytoid dendritic cells is reversed by TLR7 ligand treatment. Cancer Res. (2013) 73:4629–40. doi: 10.1158/0008-5472.CAN-12-3058
129. Rogers GL, Shirley JL, Zolotukhin I, Kumar SRP, Sherman A, Perrin GQ, et al. Plasmacytoid and conventional dendritic cells cooperate in crosspriming AAV capsid-specific CD8(+) T cells. Blood (2017) 129:3184–95. doi: 10.1182/blood-2016-11-751040
130. Brewitz A, Eickhoff S, Dahling S, Quast T, Bedoui S, Kroczek RA, et al. CD8(+) T cells orchestrate pDC-XCR1(+) dendritic cell spatial and functional cooperativity to optimize priming. Immunity (2017) 46:205–19. doi: 10.1016/j.immuni.2017.01.003
131. Hurwitz AA, Yu TF, Leach DR, Allison JP. CTLA-4 blockade synergizes with tumor-derived granulocyte-macrophage colony-stimulating factor for treatment of an experimental mammary carcinoma. Proc Natl Acad Sci USA (1998) 95:10067–71. doi: 10.1073/pnas.95.17.10067
132. Hurwitz AA, Foster BA, Kwon ED, Truong T, Choi EM, Greenberg NM, et al. Combination immunotherapy of primary prostate cancer in a transgenic mouse model using CTLA-4 blockade. Cancer Res. (2000) 60:2444–8.
133. Xin G, Schauder DM, Jing W, Jiang A, Joshi NS, Johnson B, et al. Pathogen boosted adoptive cell transfer immunotherapy to treat solid tumors. Proc Natl Acad Sci USA (2017) 114:740–5. doi: 10.1073/pnas.1614315114
134. Caminschi I, Maraskovsky E, Heath WR. Targeting dendritic cells in vivo for cancer therapy. Front Immunol. (2012) 3:13. doi: 10.3389/fimmu.2012.00013
135. Trumpfheller C, Longhi MP, Caskey M, Idoyaga J, Bozzacco L, Keler T, et al. Dendritic cell-targeted protein vaccines: a novel approach to induce T-cell immunity. J Intern Med. (2012) 271:183–92. doi: 10.1111/j.1365-2796.2011.02496.x
136. Sehgal K, Dhodapkar KM, Dhodapkar MV. Targeting human dendritic cells in situ to improve vaccines. Immunol Lett. (2014) 162(1 Pt A):59–67. doi: 10.1016/j.imlet.2014.07.004
137. Dhodapkar MV, Sznol M, Zhao B, Wang D, Carvajal RD, Keohan ML, et al. Induction of antigen-specific immunity with a vaccine targeting NY-ESO-1 to the dendritic cell receptor DEC-205. Sci Transl Med. (2014) 6:232ra51. doi: 10.1126/scitranslmed.3008068
138. Joffre OP, Segura E, Savina A, Amigorena S. Cross-presentation by dendritic cells. Nat Rev Immunol. (2012) 12:557–69. doi: 10.1038/nri3254
139. Nierkens S, Tel J, Janssen E, Adema GJ. Antigen cross-presentation by dendritic cell subsets: one general or all sergeants? Trends Immunol. (2013) 34:361–70. doi: 10.1016/j.it.2013.02.007
140. Embgenbroich M, Burgdorf S. Current concepts of antigen cross-presentation. Front Immunol. (2018) 9:1643. doi: 10.3389/fimmu.2018.01643
141. Liu C, Lou Y, Lizee G, Qin H, Liu S, Rabinovich B, et al. Plasmacytoid dendritic cells induce NK cell-dependent, tumor antigen-specific T cell cross-priming and tumor regression in mice. J Clin Invest. (2008) 118:1165–75. doi: 10.1172/JCI33583
142. Reading JL, Galvez-Cancino F, Swanton C, Lladser A, Peggs KS, Quezada SA. The function and dysfunction of memory CD8(+) T cells in tumor immunity. Immunol Rev. (2018) 283:194–212. doi: 10.1111/imr.12657
143. Feuerer M, Rocha M, Bai L, Umansky V, Solomayer EF, Bastert G, et al. Enrichment of memory T cells and other profound immunological changes in the bone marrow from untreated breast cancer patients. Int J Cancer (2001) 92:96–105. doi: 10.1002/1097-0215(200102)9999:9999<::AID-IJC1152>3.0.CO;2-Q
144. Pages F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. (2005) 353:2654–66. doi: 10.1056/NEJMoa051424
145. Beckhove P, Feuerer M, Dolenc M, Schuetz F, Choi C, Sommerfeldt N, et al. Specifically activated memory T cell subsets from cancer patients recognize and reject xenotransplanted autologous tumors. J Clin Invest. (2004) 114:67–76. doi: 10.1172/JCI200420278
146. Ribas A, Shin DS, Zaretsky J, Frederiksen J, Cornish A, Avramis E, et al. PD-1 blockade expands intratumoral memory T cells. Cancer Immunol Res. (2016) 4:194–203. doi: 10.1158/2326-6066.CIR-15-0210
147. Webb JR, Wick DA, Nielsen JS, Tran E, Milne K, McMurtrie E, et al. Profound elevation of CD8+ T cells expressing the intraepithelial lymphocyte marker CD103 (alphaE/beta7 Integrin) in high-grade serous ovarian cancer. Gynecol Oncol. (2010) 118:228–36. doi: 10.1016/j.ygyno.2010.05.016
148. Webb JR, Milne K, Watson P, Deleeuw RJ, Nelson BH. Tumor-infiltrating lymphocytes expressing the tissue resident memory marker CD103 are associated with increased survival in high-grade serous ovarian cancer. Clin Cancer Res. (2014) 20:434–44. doi: 10.1158/1078-0432.CCR-13-1877
149. Webb JR, Milne K, Nelson BH. PD-1 and CD103 are widely coexpressed on prognostically favorable intraepithelial CD8 T cells in human ovarian cancer. Cancer Immunol Res. (2015) 3:926–35. doi: 10.1158/2326-6066.CIR-14-0239
150. Wang ZQ, Milne K, Derocher H, Webb JR, Nelson BH, Watson PH. CD103 and intratumoral immune response in breast cancer. Clin Cancer Res. (2016) 22:6290–7. doi: 10.1158/1078-0432.CCR-16-0732
151. Ganesan AP, Clarke J, Wood O, Garrido-Martin EM, Chee SJ, Mellows T, et al. Tissue-resident memory features are linked to the magnitude of cytotoxic T cell responses in human lung cancer. Nat Immunol. (2017) 18:940–50. doi: 10.1038/ni.3775
152. Smazynski J, Webb JR. Resident memory-like tumor-infiltrating lymphocytes (TILRM): latest players in the immuno-oncology repertoire. Front Immunol. (2018) 9:1741. doi: 10.3389/fimmu.2018.01741
Keywords: CD103+ cDC1s, CD8 T cell immunity, anti-tumor immunity, cross-priming, tumor microenvironment, cancer immunotherapy
Citation: Fu C and Jiang A (2018) Dendritic Cells and CD8 T Cell Immunity in Tumor Microenvironment. Front. Immunol. 9:3059. doi: 10.3389/fimmu.2018.03059
Received: 10 September 2018; Accepted: 10 December 2018;
Published: 20 December 2018.
Edited by:
Carmen Gerlach, Karolinska Institute (KI), SwedenReviewed by:
Thomas Brocker, Ludwig Maximilian University of Munich, GermanyCopyright © 2018 Fu and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Aimin Jiang, YWltaW5qaWFAYnVmZmFsby5lZHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.