- 1Department for Immunobiochemistry, Universitätsmedizin Mannheim, Medizinische Fakultät Mannheim, Universität Heidelberg, Mannheim, Germany
- 2Innate Immunity, German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany
Natural Killer (NK) cells are cytotoxic innate lymphoid cells serving at the front line against infection and cancer. In inflammatory microenvironments, multiple soluble and contact-dependent signals modulate NK cell responsiveness. Besides their innate cytotoxic and immunostimulatory activity, it has been uncovered in recent years that NK cells constitute a heterogeneous and versatile cell subset. Persistent memory-like NK populations that mount a robust recall response were reported during viral infection, contact hypersensitivity reactions, and after stimulation by pro-inflammatory cytokines or activating receptor pathways. In this review, we highlight recent findings on the generation, functionality, and clinical applicability of memory-like NK cells and describe common features in comparison to other recent concepts of memory NK cells. Understanding of these features will facilitate the conception and design of novel NK cell-based immunotherapies.
Introduction
NK cells were discovered in the 1970s, when it was concluded that NK cells are able to naturally lyse certain tumor target cells without prior sensitization and mediate lysis of antibody-opsonized tumor cells (1). NK cells were characterized to respond to cells that have a loss (“missing-self”) or reduced levels in cognate self-MHC class I molecules and, thus, are unable to engage inhibitory NK cell receptors, contrary to the MHC class I–restricted recognition of foreign antigens by cytotoxic T cells (2, 3). Nowadays, it is well-established that the threshold for NK cell cytotoxicity is dictated by the surplus of activating over inhibiting signals from target cells and the microenvironment (4). A multitude of NK cell activating and inhibitory surface receptors regulates target cell elimination and the production of immunostimulatory cytokines like IFN-γ. Aside from their classical innate immune functionality, NK cells can promote adaptive immune responses or elicit regulatory functions under certain conditions (5). For instance, tissue-resident decidual NK cells can give rise to a specifically enriched NK cell subset upon repeated pregnancies, exerting enhanced IFN-γ and VEGF production, which might improve placentation (6, 7). The uncovering of immunological memory has added to the complexity of NK cell biology (8). Following MCMV infection, murine NK cells acquire traits of adaptive immunity such as expansion of virus/m157-specific NK cell subsets and long-lasting enhanced secondary responses including improved protection against MCMV compared to that of naïve NK cells (9). NK cells can exert antigen-specific memory against previously sensitized haptens or viruses in a T/B cell-independent manner (10). In essence, virus and hapten-specific memory NK cells in mice resemble antigen-specific immunological memory of T and B cells to some extent with phases of expansion and contraction but a more limited selection of antigen-specific recall responses (11). Similarly, human NKG2C+ NK cells enriched in patients with a history of HCMV infection, referred to as “adaptive” NK cells, may rely at least in part on the specific recognition of HLA-E–loaded HCMV peptides (12, 13). Besides NK cells, allergen- or IL-33-experienced group 2 innate lymphoid cells acquire antigen unspecific memory-like properties (14).
The cytokines IL-2 and IL-15 drive NK cell differentiation, proliferation, and activation, while IL-15 trans-presented by activated dendritic cells is critical for NK cell survival (15–17). IL-2 has long been known to prime the cytotoxic function of NK cells toward cancer cells (18). IL-2 produced by antigen-specific memory CD4 T cells has been implicated in NK cell activation during anti-viral recall responses (19–21). IL-12 and IL-18 secreted during viral infections by e.g., dendritic cells induce potent NK cell IFN-γ production and cytotoxicity, in particular in combination, and synergistically augment IL-2 and IL-15–induced NK cell activation (20, 22–26). The direct effects of cytokine-mediated NK cell activation may involve a reduced threshold of activating receptor signaling (27–31), increased expression of activating receptors (32, 33), a lower (but not absent) responsiveness to cognate inhibitory MHC class I ligands (34, 35), a ready-to-execute cytotoxic machinery by granule convergence (36), and changes in NK cell metabolism (31, 37).
In addition to the direct effects of cytokines on NK cell activation, there is emerging evidence that pre-activation by IL-12 and IL-18 plus IL-15 can endow murine and human NK cells with long-lasting enhanced NK cell functionality even after discontinuation and in the absence of the initial stimulus (Figure 1). This so-called memory-like functionality is antigen-unspecific and characterized by an enhanced proliferative capacity, prolonged persistence in vivo for up to 3 months, and superior IFN-γ production and potent cytotoxic activity upon ex vivo restimulation (8, 38). The generation, mechanistic insight, physiological relevance and therapeutic potential of antigen-unspecific memory-like NK cells are the prime focus of this review.
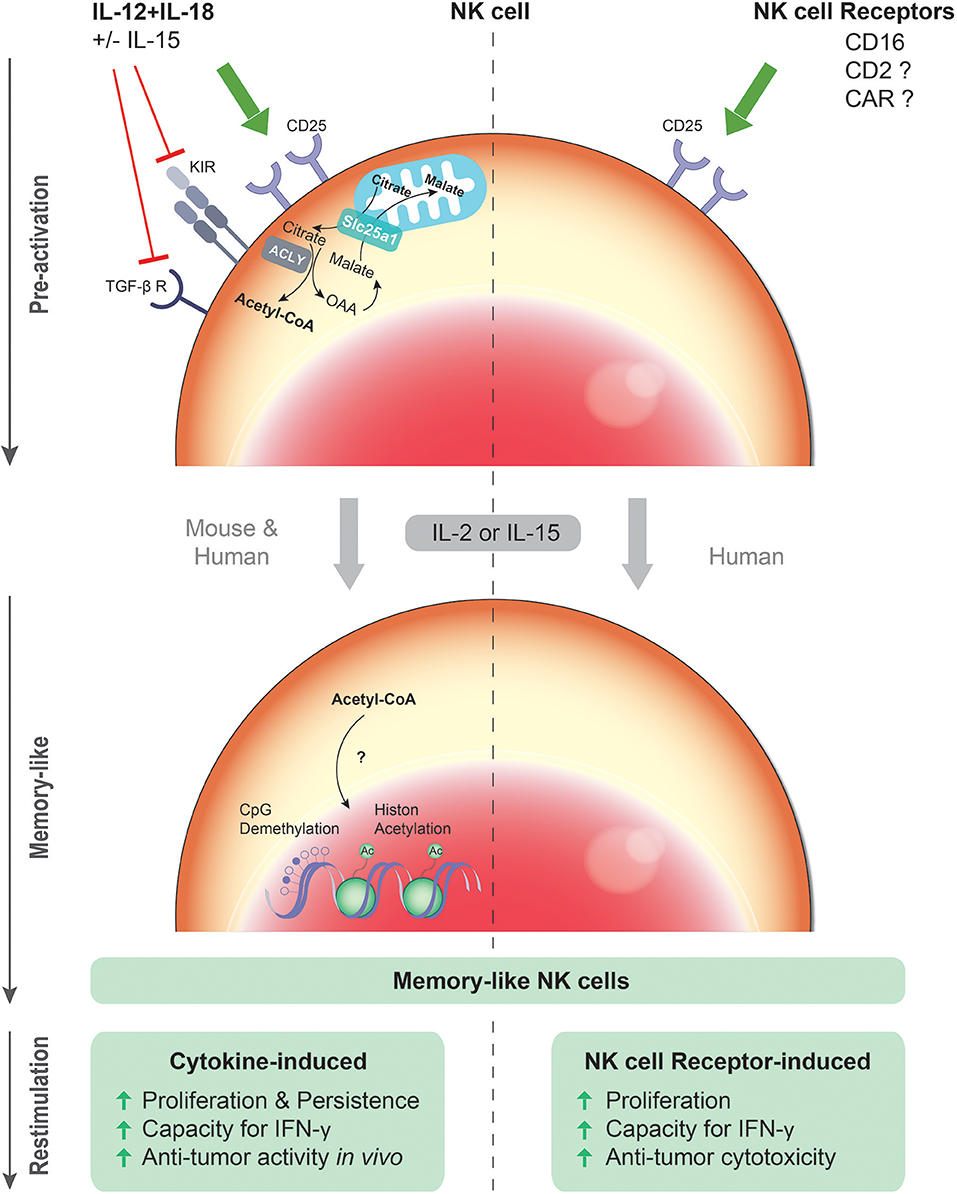
Figure 1. Cytokine- and NK cell receptor-induced memory-like NK cells. Upon primary exposure to the cytokine combination IL-12/18 plus IL-15, murine and human NK cells up-regulate the IL-2 receptor α chain (CD25), and undergo rapid proliferation and expansion in response to IL-2 or IL-15. Moreover, down-regulation of the TGF-β receptor and certain inhibitory KIRs by IL-12/15/18 might contribute to the superior effector function of the cytokine pre-activated NK cells. After restimulation with cytokines or tumor cells, these cytokine pre-activated NK cells have an enhanced capacity to produce IFN-γ and a more robust and sustained anti-tumor activity in vivo. Epigenetic modification such as CpG demethylation and histone acetylation (Ac) induced by the cytokines might be crucial for the persistent competence of enhanced gene transcription upon restimulation. Similarly, upon primary exposure to ITAM-associated NK cell activating receptors such as CD16, human NK cells undergo in response to IL-2 or IL-15 more rapid proliferation and expansion in vitro. After restimulation with cytokines or tumor cells, these CD16 pre-activated NK cells have an enhanced capacity to produce IFN-γ and a more robust cytotoxic activity. Hence, both cytokine-induced and CD16-induced memory-like functionalities are antigen-unspecific and share the property of “remembering” a previous activated state induced by cytokine or antibody exposure.
Properties of Memory-Like NK Cells
Cytokine-Induced Memory-Like Functionality
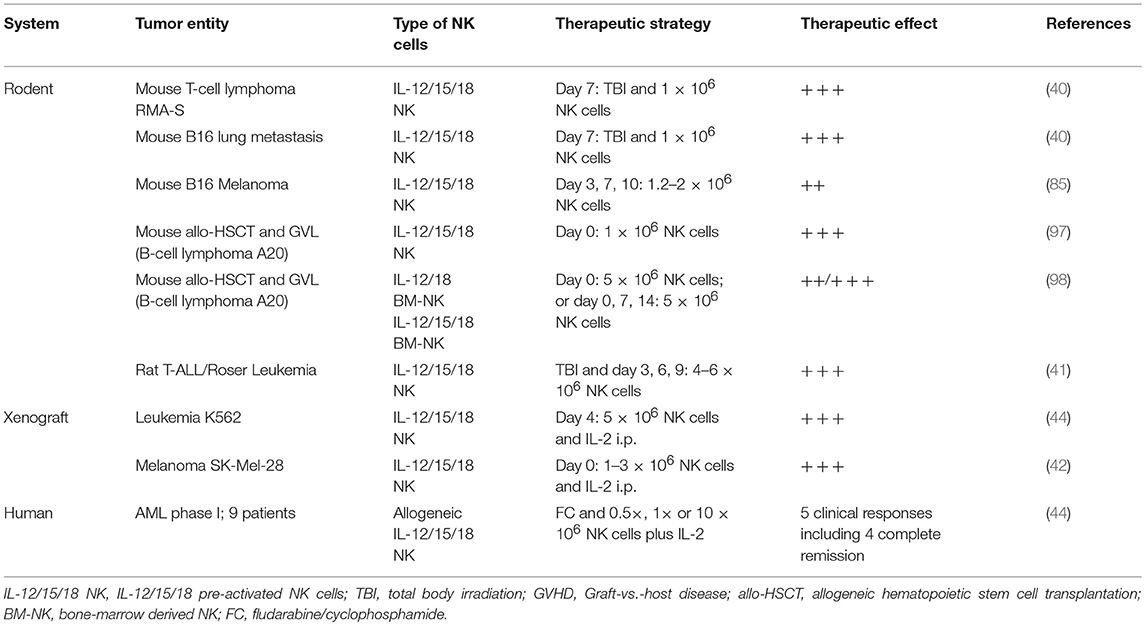
In 2009, Cooper et al. demonstrated that mouse NK cells pre-activated with the cytokine cocktail IL-12, IL-15, and IL-18 persisted at high numbers several weeks after transfer into RAG-1−/− mice and produced higher levels of IFN-γ upon restimulation ex vivo compared to control NK cells (39). Later, our group and others showed that mouse and rat IL-12/15/18 pre-activated NK cells could mount a more robust and long-lived anti-tumor response after adoptive transfer (40, 41). This memory-like NK cell activity required extrinsic help from IL-2 producing CD4 T cells and was associated with intrinsic demethylation of the IFNG locus, facilitating IFN-γ transcription and production upon restimulation (42).
Analogous to murine NK cells, activation of human NK cells with IL-12/18 plus IL-15 for 16 h conferred memory-like functionality after in vitro re-culture in IL-15 or IL-2 for several days. IL-12/15/18 pre-activated NK cells produced more IFN-γ upon restimulation with cytokines, K562 cells or primary acute myeloid leukemia (AML) blasts in comparison to control NK cells, which had been pre-activated with an equivalent dose of IL-15 (40, 43) or with low-dose IL-15 (44). Importantly, 6 days after transfer into tumor-free T/B/NK cell-deficient NSG mice (supplemented daily with IL-2), IL-12/15/18 pre-activated NK cells were superior in IFN-γ production when restimulated ex vivo with K562 cells or cytokines (24, 42, 44). In xenograft mouse models, adoptively-transferred IL-12/15/18 pre-activated NK cells significantly ablated melanoma growth in the lung (42) and reduced systemic K562 tumor burden associated with improved survival (44).
NK cells pre-activated with IL-12/18 +/− IL-15 were more sensitive to low concentrations of IL-2 due to increased surface density of the high-affinity IL-2 receptor α chain (CD25) (Figure 1), resulting in more rapid proliferation and a higher NK cell recovery upon IL-2 culture (24, 40). Accordingly, in an immunocompetent tumor microenvironment, IL-12/15/18 pre-activated NK cells might be superior in competing for low amounts of IL-2 with CD25+ regulatory T cells, which restrain IL-2–dependent expansion of NK cells and T cells after adoptive cell transfer (45, 46). Of note, IL-2 was critical for the profound proliferation of IL-12/15/18 pre-activated NK cells, their anti-tumor activity and persistence in several organs such as blood, spleen, liver, and lung after adoptive transfer (42). IL-2 may be provided by host CD4 T cells activated by homeostatic proliferation in tumor-bearing non-lethally irradiated mice (40). Furthermore, the concerted activation of CD4 T cells and myeloid cells co-transferred within autologous PBMC could substitute IL-2 injections after adoptive transfer (42).
Directly after cytokine stimulation, IL-12/15/18 pre-activated NK cells mediated more potent cytotoxicity as compared to IL-15 activated NK cells (42, 47). Of note, this difference may be more pronounced against target cells displaying cognate self-MHC class I ligands, since IL-12/15/18 pre-activation for at least 48 h has been shown to reduce inhibitory KIR expression (35) (Figure 1). The difference compared to IL-15 pre-activated NK cells might merely reflect a prolonged state of potent activation. After in vitro re-culture, low-dose IL-15 pre-activated NK cells exhibited lower DNAM-1-dependent cytotoxicity against primary AML blasts than IL-12/15/18 pre-activated NK cells (44). In contrast, degranulation of NK cells pre-activated with IL-12/15/18 or an equivalent dose of IL-15 was comparable against NK cell-sensitive K562 cells (43), which are mainly recognized through the NK cell receptors NKG2D and NKp30 (48, 49). Thus, it remains to be resolved whether IL-12/15/18 pre-activated memory-like NK cells, i.e., when restimulated after adoptive transfer or after re-culture with IL-2 or IL-15 in vitro, indeed possess higher cytolytic activity, in particular against target cells that are poorly killed by IL-2 or IL-15 pre-activated or resting NK cells. Interestingly, IL-12, IL-15, and IL-18 are known to down-regulated the TGF-β receptor and its signaling pathway (Figure 1). Hence, resistance to TGF-β might add to the potency of IL-12/15/18-induced memory-like NK cells (50). Moreover, it has been reported that IL-12 and IL-18 injections reversed the anergic phenotype of NK cells in tumor-bearing mice (51). Whether this treatment would induce memory-like properties in vivo in endogenous NK cells is unknown. In spite of the up-regulation of several surface markers such as CD25, CD69, KLRG1 on a more mature CD11bhighCD27low NK cell subpopulation (40, 52) and down-regulation of the KIRs as well as the TGF-β receptor, an unequivocal biomarker profile is lacking to discriminate IL-12/15/18 pre-activated NK cells in vivo.
NK Cell Receptor-Mediated Memory-Like Functionality
Cancer cells might confer contact-dependent priming signals through NK cell activating receptors that enhance NK cell activation. It has been suggested that NK cells (cultured without/with IL-2/15) displayed enhanced cytotoxicity after a previous co-culture with certain tumor cells, although the exact priming stimulus was not identified (53, 54). In this context, it has been shown that pre-activation of NK cells with tumor cells through CD2 and its ligand CD15 on tumor cells could enable subsequent lysis of otherwise poorly susceptible target cells (55). CD2 is a co-stimulatory receptor for ITAM-coupled NK cell receptors like NKp46 and CD16 (29, 56). Upon cross-linking CD2 can associate with CD3ζ signaling by forming a complex with CD16 at the immunological synapse (57), and CD2 can lead to STAT5 phosphorylation similar to IL-2 and IL-15 (58, 59). While the adoptive transfer of these tumor cell-primed NK cells has been tested in a phase 1 study in AML patients (60), it is unknown whether priming of NK cells by tumor cells can occur in vivo in an NK cell immunosuppressive microenvironment.
Recently, our group has revealed that FcγRIIIa/CD16 engagement by therapeutic (bispecific) antibodies can prime enhanced memory-like NK cell functionality in addition to direct activation known as classical antibody-dependent cellular cytotoxicity (ADCC). Following 5-day IL-2 re-culture, CD16 pre-activated NK cells exerted enhanced antibody-independent cytotoxicity and IFN-γ production upon restimulation with cytokines or tumor cells compared to IL-2 cultured NK cells (47) (Figure 1). Similar to cytokine-induced memory-like NK cells, CD16 pre-activated NK cells up-regulate CD25 expression (Figure 1) in particular in the presence of IL-12, resulting in increased sensitivity to low-dose IL-2 and more vigorous proliferation and expansion in response to IL-2 (47, 61, 62). Potential common mechanisms of cytokines and CD16-engaging antibodies in inducing memory-like NK cells require further investigation. Altogether, the concept of CD16-induced memory-like functionality of NK cells needs to be investigated and confirmed in experimental in vivo systems.
Since IL-12/15/18 pre-activated NK cells are sufficient in antibody-mediated cytotoxicity despite partial CD16 shedding (35, 47, 63), a scenario of NK cell activation through both CD16 and IL-12 may synergistically improve NK cell activity like IFN-γ production (64). It has been inferred from mouse and human studies that tumor-reactive therapeutic antibodies may promote uptake and presentation of tumor antigens by dendritic cells, resulting in the formation of antigen-specific T cell memory (65–67). In contrast, the physiological existence and role of CD16-induced memory-like NK cells in vivo is currently unknown. However, it would require the presence of ADCC-sufficient therapeutic antibodies or killer engagers applied in cancer patients (68). In this context, CD16-induced memory-like NK cell functionality might be preferentially induced in patients carrying the high affinity CD16-158V/V genotype, which confers better clinical efficacy in cancer patients treated with IgG-type therapeutic antibodies (69, 70). Moreover, CD16-induced memory-like NK cells might support T/B cell responses during primary infection or reinfection, when endogenous IgG antibodies are produced e.g., against HCMV (71–73). While (HCMV-specific) antibodies have been shown to promote the expansion of pre-existing HCMV-associated NKG2C+ “adaptive” NK cells (74–76), antibodies probably do not mediate in the initial generation of NKG2C+ “adaptive” NK cells in HCMV-seronegative individuals (76). In contrast to the NKG2C+ “adaptive” NK cells (75), CD16-induced memory-like NK cells maintained expression of FcεR1γ and NK cell activating receptors and exerted enhanced IFN-γ in response to IL-12 and enhanced antibody-independent cytotoxicity (47). It is unknown whether HCMV-specific antibodies can induce CD16-induced memory-like NK cell functionality. Finally, it is unknown whether other ITAM-coupled activating receptors or chimeric antigen receptors in human NK cells have the potential to induce memory-like functionality similar to FcγRIII/CD16. In this regard, engagement of the murine ITAM-coupled activating receptor Ly49H has been shown to mediate the expansion of virus/m157-specific memory NK cells in mice with long-lasting enhanced responsiveness to secondary stimulation (9).
Epigenetic Regulation
NK cells intrinsically “remember” a previous exposure to cytokines, since IL-12/15/18 pre-activated NK cells pass their enhanced IFN-γ producing capacity to daughter cells (39, 42). Epigenetic imprinting, e.g., demethylated CNS1 region of the IFNG gene, was detected in HCMV-associated NKG2C+ “adaptive” NK cells (75, 77) and IL-12/15/18 pre-activated NK cells (42) (Figure 1), which was shown to lead to a remarkable stability of the IFN-γ-producing phenotype even after adoptive transfer. Both IL-12 and IL-18 are essential for the pronounced demethylation of the CNS1 region (42, 77), while IL-15 might serve as a survival factor. Besides the IFNG gene, CpG demethylation of the PRDM1/BLIMP1 and ZBTB32/TZFP genes or hypermethlyation of FCER1G (Fc fragment of IgE receptor Ig) were also detected in NKG2C+ “adaptive” NK cells (75, 77). Recently, stable epigenetic changes were also found in MCMV-specific memory NK cells, some of which are shared by memory CD8 T cells (78). Particularly, IL-12 during MCMV infection induces epigenetic remodeling of the IRF8 gene, an important regulator for the proliferation of MCMV-specific NK cells (79). This finding sheds light on NK cell deficiencies in individuals with IRF8 mutations associated with severe viral infections (80). However, it is not clear how IRF8 intrinsically/extrinsically affects NK cell function (that is impaired in IRF8−/− patients), or the formation and maintenance of the long-lived MCMV-specific NK cell memory compartment including protection against re-infection.
Metabolic Regulation
Metabolic regulation is pivotal for the development, maintenance and recall responses of memory T cells (81). Similar to T cells, activation of NK cells by cytokines such as IL-2, IL-15, IL-12, and IL-18 or activating receptors lead to elevated oxidative phosphorylation (OXPHOS) and elevated glycolysis (82, 83). The in vitro IFN-γ production from IL-12/18 stimulated NK cells was not affected by inhibition of glycolysis and mitochondrial OXPHOS under certain in vitro activating condition (83). In fact, increased rates of glycolysis are required for NK cell mediated control of MCMV infection (84). Hence, it is of interest whether increases in glycolysis regulate the generation and function of IL-12/15/18-induced memory-like NK cells including their recall responsiveness.
The Srebp-controlled increased citrate-malate shuttle is required for elevated glycolysis and oxidative phosphorylation in cytokine-stimulated NK cells (85). Anti-tumor activity of adoptively transferred IL-12/15/18 pre-activated NK cells was lost when Srebp inhibitors were present during NK cell activation, suggesting the importance of the citrate-malate shuttle in cytokine-induced memory-like function of NK cells. The citrate-malate shuttle exports acetyl-CoA into the cytosol via citrate. Of note, it was shown in a recent study that histone acetylation was controlled by changing levels of nuclear acetyl-CoA (86). This finding links the metabolic regulation to the epigenetic modification, which might be crucial for the persistent competence of enhanced gene transcription upon restimulation. Further investigation of the impact of acetyl-CoA on histone modification in NK cells would help to reveal the possible association of the NK cell memory-like phenotype with cytokine-induced metabolic changes (Figure 1).
Recently, it has been shown that the fundamental metabolic regulator cMyc controls metabolic and functional activation of NK cells upon cytokine stimulation (87). Whether cMyc is involved in regulating NK cell memory similar as in memory CD8 T cells awaits further investigation (88).
Physiological Relevance
The physiological relevance and existence of human memory-like NK cells in vivo remains to be resolved. In mice, long-lived NK cells were generated during respiratory syncytial virus infection that undergo homeostatic proliferation or virus-induced proliferation in the bone marrow but not at the primary sites of infection like respiratory tissues (89). In mice, IL-12, IL-18 are essential co-stimulatory factors for the generation of murine CMV-specific Ly49H+ memory NK cells (11). Furthermore, IL-12 and IL-18 are important for the expansion of HCMV-associated NKG2C+ “adaptive” NK cells in vitro (12, 73). Hence, a coordinated availability of IL-12, IL-15, and IL-18, derived from dendritic cells and myeloid cells during viral infections such as CMV or influenza (90), might support generation of cytokine-induced memory-like NK cells in vivo. Of note, a study of tracking the fate of NK cells upon MCMV infection showed the induction of both antigen-specific memory Ly49H+ NK cells and cytokine-activated antigen-unspecific long-lived Ly49H− NK cells, and the differentiation of both subsets critically relied on IL-12. This study highlighted the in vivo relevance of cytokine-induced memory-like features in NK cells. These cytokine-activated persistent Ly49H− NK cells were less responsive to restimulation by activating receptors in vitro or tumor cells in vivo but survived longer in an MCMV-free environment (91).
Interestingly, durable enhanced IFN-γ responses by NK cells (and NKT cells) have been reported in humans up to 1 year after Bacillus Calmette-Guérin (BCG) revaccination in response to BCG restimulation, at a time point when BCG-specific IL-2 producing CD4 T cells were reduced (92). The enhanced NK cell IFN-γ response involved IL-12 and IL-18, probably derived from myeloid peripheral blood cells. The contribution of IL-2 was low, suggesting that IL-2 producing memory T cells were dispensable in BCG infection (92), unlike in certain viral infections (19, 20). IL-21 can potentiate the expansion and anti-tumor activity of IL-2 stimulated NK cells (93). In mice, BCG vaccination was suggested to generate long-lived NK cells, which possessed high proliferative capacity and anti-bacterial activity when restimulated with the mycobacteria tuberculosis antigen complex (94). Notably, this observation required the presence of T/B cells or IL-21 during BCG vaccination. Overall, it remains to be determined whether BCG-reactive NK cells have indeed intrinsic memory-like functionality and/or require the contribution of myeloid cells, which are known to undergo epigenetic modifications and immune training (akin to innate immune memory) in response to BCG vaccination (95, 96). Mechanistically, it will be of relevance to determine whether BCG-induced memory NK cells share regulatory mechanisms such as epigenetic imprinting with cytokine-induced memory-like NK cells or MCMV-specific memory NK cells (8, 78).
Clinical Potential
It has been demonstrated in different mouse and rat tumor models that IL-12/15/18 pre-activated NK cells can confer favorable therapeutic effects after adoptive cell transfer, such as enhanced IFN-γ production, cytotoxicity, and long-lived capacity for antigen-unspecific immunological memory (40–42, 44) (Table 1). Importantly, IL-12/15/18 pre-activated NK cells have been suggested to alleviate severe acute graft-vs.-host disease (GvHD). In a mouse model of a fully mismatched hematopoietic cell transplant, co-transfer of autologous IL-12/15/18 pre-activated NK cells limited the severity of acute GvHD by presumably restricting the proliferation of adoptively-transferred allogeneic T cells while the graft-vs.-leukemia (GvL) effect of the T cells was retained (Table 1) (97). Still, the role of IL-12/15/18 pre-activated NK cells on delayed or failed stem cell engraftment and development of chronic GvHD remains incompletely understood (97). Consistent with the previous study it has been recently reported that adoptive transfer of murine NK cells pre-activated with IL-12/18+/− IL-15, which had been expanded with IL-2 before, suppressed severe acute GvHD (Table 1) (98). However, IL-12/15/18 pre-activated NK cells, in contrast to IL-12/18 pre-activated NK cells, did not have a protective effect against mild acute GvHD. The absence of a potent direct anti-tumor activity of IL-12/15/18 pre-activated NK cells in these mouse studies might be due to the lack of host CD4 T cells and insufficient IL-2 that may be instrumental in maintaining durable anti-tumor responses (40). Hence, with respect to the variegated NK cell pre-activation protocols, it needs to be refined how the different cytokine pre-activation protocols, dosing regiments and in vivo cytokine supplementation maximize GvL effects and minimize acute and chronic GvHD.
In patients with AML it has been shown that alloreactive NK cells generated from haploidentical hematopoietic stem cell grafts reduced leukemia recurrence and lowered the risk for GvHD while contributing to GvL effects (99). Recently, Romee et al. has pioneered the adoptive transfer of haploidentical IL-12/15/18 pre-activated memory-like NK cells in a phase I clinical study in nine heavily pre-treated relapsed/refractory AML patients (Table 1). In this trial, IL-12/15/18 pre-activated NK cells displayed an enhanced proliferative state, leading to increased frequencies in the recipients after IL-2 supplementation in vivo (44). Importantly, 7 days after adoptive transfer, IL-12/15/18 pre-activated NK cells exerted potent anti-tumor activity ex vivo after restimulation, correlating with improved survival in the absence of GvHD in a subset of AML patients. Thus, adoptive transfer of human IL-12/15/18 pre-activated memory-like NK cells into AML patients with active disease is considered safe and feasible, resulting in donor NK cell expansion, GvL activity and favorable clinical responses. Thus, this study initiated a promising translational immunotherapy approach for durable NK cell anti-cancer responses not only for AML but also for other NK cell-sensitive tumors. Overall, the use of donor NK cells for adoptive transfer may be more favorable than autologous NK cells from cancer patients, which are often functionally impaired or less responsive to cytokine activation (25, 100, 101).
Conclusion
Pre-activation of NK cells by the cytokines IL-12/18 plus IL-15 or by engagement of FcγRIII/CD16 via therapeutic antibodies can induce similar memory-like functionalities: an enhanced proliferative capacity toward IL-2 due to CD25 up-regulation as well as a strengthened responsiveness to restimulation by tumor cells. Importantly, both memory-like functionalities are antigen-unspecific and imply “remembering” a previous state of heightened activation induced by cytokine exposure or stimulation via activating NK cell receptors. These memory-like functionalities have unveiled a previously unappreciated potential for NK cell-based cancer immunotherapy. Several aspects may improve the translation of the recent findings into clinical application. First, clear criteria for NK cell-sensitive tumors are pivotal, which requires proper genetic and protein markers. Second, optimal NK cell activation protocols as well as pre-conditioning regimens of patients need to be established to improve engraftment, expansion, persistence and durable anti-cancer activity after adoptive transfer. Third, epigenetic and metabolic parameters should be monitored and manipulated during cancer therapy to sustain NK cell reactivity in the tumor microenvironment. In future clinical studies, it remains to be determined whether clinical responses by memory-like NK cells may be augmented by the combination with therapeutic tumor-reactive antibodies or checkpoint immunotherapy.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Funding
This work is supported by the Deutsche Krebshilfe (#109174 to AC). We acknowledge financial support by Deutsche Forschungsgemeinschaft within the funding program Open Access Publishing, by the Baden-Württemberg Ministry of Science, Research and the Arts and by Ruprecht-Karls-Universität Heidelberg.
Conflict of Interest Statement
JP and AC received a commercial research grant from Affirmed. AC is a consultant/advisory board member for SAB Dragonfly Therapeutics.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor declared a past co-authorship with AC.
References
1. Herberman RB, Ortaldo JR. Natural killer cells: their roles in defenses against disease. Science (1981) 214:24–30. doi: 10.1126/science.7025208
2. Moretta L, Ciccone E, Moretta A, Hoglund P, Ohlen C, Karre K. Allorecognition by NK cells: nonself or no self? Immunol Today (1992) 13:300–6. doi: 10.1016/0167-5699(92)90042-6
3. Raulet DH. Recognition events that inhibit and activate natural killer cells. Curr Opin Immunol. (1996) 8:372–7. doi: 10.1016/S0952-7915(96)80127-0
4. Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL, et al. Innate or adaptive immunity? The example of natural killer cells. Science (2011) 331:44–9. doi: 10.1126/science.1198687
5. Schuster IS, Coudert JD, Andoniou CE, Degli-Esposti MA. “Natural Regulators”: NK cells as modulators of T cell immunity. Front Immunol. (2016) 7:235. doi: 10.3389/fimmu.2016.00235
6. Moffett A, Colucci F. Uterine NK cells: active regulators at the maternal-fetal interface. J Clin Invest. (2014) 124:1872–9. doi: 10.1172/JCI68107
7. Gamliel M, Goldman-Wohl D, Isaacson B, Gur C, Stein N, Yamin R, et al. Trained memory of human uterine NK cells enhances their function in subsequent pregnancies. Immunity (2018) 48:951–62.e5. doi: 10.1016/j.immuni.2018.03.030
8. Cerwenka A, Lanier LL. Natural killer cell memory in infection, inflammation and cancer. Nat Rev Immunol. (2016) 16:112–23. doi: 10.1038/nri.2015.9
9. Sun JC, Beilke JN, Lanier LL. Adaptive immune features of natural killer cells. Nature (2009) 457:557–61. doi: 10.1038/nature07665
10. Paust S, Gill HS, Wang BZ, Flynn MP, Moseman EA, Senman B, et al. Critical role for the chemokine receptor CXCR6 in NK cell-mediated antigen-specific memory of haptens and viruses. Nat Immunol. (2010) 11:1127–35. doi: 10.1038/ni.1953
11. Geary CD, Sun JC. Memory responses of natural killer cells. Semin Immunol. (2017) 31:11–9. doi: 10.1016/j.smim.2017.08.012
12. Hammer Q, Ruckert T, Borst EM, Dunst J, Haubner A, Durek P, et al. Peptide-specific recognition of human cytomegalovirus strains controls adaptive natural killer cells. Nat Immunol. (2018) 19:453–63. doi: 10.1038/s41590-018-0082-6
13. Rolle A, Meyer M, Calderazzo S, Jager D, Momburg F. Distinct HLA-E peptide complexes modify antibody-driven effector functions of adaptive NK cells. Cell Rep. (2018) 24:1967–76.e4. doi: 10.1016/j.celrep.2018.07.069
14. Martinez-Gonzalez I, Matha L, Steer CA, Ghaedi M, Poon GF, Takei F. Allergen-experienced group 2 innate lymphoid cells acquire memory-like properties and enhance allergic lung inflammation. Immunity (2016) 45:198–208. doi: 10.1016/j.immuni.2016.06.017
15. Becknell B, Caligiuri MA. Interleukin-2, interleukin-15, and their roles in human natural killer cells. Adv Immunol. (2005) 86:209–39. doi: 10.1016/S0065-2776(04)86006-1
16. Waldmann TA. The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat Rev Immunol. (2006) 6:595–601. doi: 10.1038/nri1901
17. Rautela J, Huntington ND. IL-15 signaling in NK cell cancer immunotherapy. Curr Opin Immunol. (2017) 44:1–6. doi: 10.1016/j.coi.2016.10.004
18. Trinchieri G, Matsumoto-Kobayashi M, Clark SC, Seehra J, London L, Perussia B. Response of resting human peripheral blood natural killer cells to interleukin 2. J Exp Med. (1984) 160:1147–69. doi: 10.1084/jem.160.4.1147
19. He XS, Draghi M, Mahmood K, Holmes TH, Kemble GW, Dekker CL, et al. T cell-dependent production of IFN-gamma by NK cells in response to influenza A virus. J Clin Invest. (2004) 114:1812–9. doi: 10.1172/JCI22797
20. Horowitz A, Behrens RH, Okell L, Fooks AR, Riley EM. NK cells as effectors of acquired immune responses: effector CD4+ T cell-dependent activation of NK cells following vaccination. J Immunol. (2010) 185:2808–18. doi: 10.4049/jimmunol.1000844
21. Pahl JH, Verhoeven DH, Kwappenberg KM, Vellinga J, Lankester AC, Van Tol MJ, et al. Adenovirus type 35, but not type 5, stimulates NK cell activation via plasmacytoid dendritic cells and TLR9 signaling. Mol Immunol. (2012) 51:91–100. doi: 10.1016/j.molimm.2012.02.119
22. Fehniger TA, Shah MH, Turner MJ, Vandeusen JB, Whitman SP, Cooper MA, et al. Differential cytokine and chemokine gene expression by human NK cells following activation with IL-18 or IL-15 in combination with IL-12: implications for the innate immune response. J Immunol. (1999) 162:4511–20.
23. Chaix J, Tessmer MS, Hoebe K, Fuseri N, Ryffel B, Dalod M, et al. Cutting edge: priming of NK cells by IL-18. J Immunol. (2008) 181:1627–31. doi: 10.4049/jimmunol.181.3.1627
24. Leong JW, Chase JM, Romee R, Schneider SE, Sullivan RP, Cooper MA, et al. Preactivation with IL-12, IL-15, and IL-18 induces CD25 and a functional high-affinity IL-2 receptor on human cytokine-induced memory-like natural killer cells. Biol Blood Marrow Transplant. (2014) 20:463–73. doi: 10.1016/j.bbmt.2014.01.006
25. Mirjacic Martinovic K. Babovic N, Dzodic R, Jurisic V, Matkovic S, Konjevic G. Favorable in vitro effects of combined IL-12 and IL-18 treatment on NK cell cytotoxicity and CD25 receptor expression in metastatic melanoma patients. J Transl Med. (2015) 13:120. doi: 10.1186/s12967-015-0479-z
26. Terren I, Mikelez I, Odriozola I, Gredilla A, Gonzalez J, Orrantia A, et al. Implication of interleukin-12/15/18 and ruxolitinib in the phenotype, proliferation, and polyfunctionality of human cytokine-preactivated natural killer cells. Front Immunol. (2018) 9:737. doi: 10.3389/fimmu.2018.00737
27. Barber DF, Faure M, Long EO. LFA-1 contributes an early signal for NK cell cytotoxicity. J Immunol. (2004) 173:3653–9. doi: 10.4049/jimmunol.173.6.3653
28. Bryceson YT, March ME, Barber DF, Ljunggren HG, Long EO. Cytolytic granule polarization and degranulation controlled by different receptors in resting NK cells. J Exp Med. (2005) 202:1001–12. doi: 10.1084/jem.20051143
29. Bryceson YT, March ME, Ljunggren HG, Long EO. Synergy among receptors on resting NK cells for the activation of natural cytotoxicity and cytokine secretion. Blood (2006) 107:159–66. doi: 10.1182/blood-2005-04-1351
30. Horng T, Bezbradica JS, Medzhitov R. NKG2D signaling is coupled to the interleukin 15 receptor signaling pathway. Nat Immunol. (2007) 8:1345–52. doi: 10.1038/ni1524
31. Jensen H, Potempa M, Gotthardt D, Lanier LL. Cutting edge: IL-2-induced expression of the amino acid transporters SLC1A5 and CD98 Is a prerequisite for NKG2D-mediated activation of human NK cells. J Immunol. (2017) 199:1967–72. doi: 10.4049/jimmunol.1700497
32. Fujisaki H, Kakuda H, Shimasaki N, Imai C, Ma J, Lockey T, et al. Expansion of highly cytotoxic human natural killer cells for cancer cell therapy. Cancer Res. (2009) 69:4010–7. doi: 10.1158/0008-5472.CAN-08-3712
33. Pahl JH, Ruslan SE, Kwappenberg KM, Van Ostaijen-Ten Dam MM, Van Tol MJ, Lankester AC, et al. Antibody-dependent cell lysis by NK cells is preserved after sarcoma-induced inhibition of NK cell cytotoxicity. Cancer Immunol Immunother. (2013) 62:1235–47. doi: 10.1007/s00262-013-1406-x
34. Draghi M, Yawata N, Gleimer M, Yawata M, Valiante NM, Parham P. Single-cell analysis of the human NK cell response to missing self and its inhibition by HLA class I. Blood (2005) 105:2028–35. doi: 10.1182/blood-2004-08-3174
35. Ewen EM, Pahl JHW, Miller M, Watzl C, Cerwenka A. KIR downregulation by IL-12/15/18 unleashes human NK cells from KIR/HLA-I inhibition and enhances killing of tumor cells. Eur J Immunol. (2018) 48:355–65. doi: 10.1002/eji.201747128
36. James AM, Hsu HT, Dongre P, Uzel G, Mace EM, Banerjee PP, et al. Rapid activation receptor- or IL-2-induced lytic granule convergence in human natural killer cells requires Src, but not downstream signaling. Blood (2013) 121:2627–37. doi: 10.1182/blood-2012-06-437012
37. Mao Y, Van Hoef V, Zhang X, Wennerberg E, Lorent J, Witt K, et al. IL-15 activates mTOR and primes stress-activated gene expression leading to prolonged antitumor capacity of NK cells. Blood (2016) 128:1475–89. doi: 10.1182/blood-2016-02-698027
38. Fehniger TA, Cooper MA. Harnessing NK cell memory for cancer immunotherapy. Trends Immunol. (2016) 37:877–88. doi: 10.1016/j.it.2016.09.005
39. Cooper MA, Elliott JM, Keyel PA, Yang L, Carrero JA, Yokoyama WM. Cytokine-induced memory-like natural killer cells. Proc Natl Acad Sci USA. (2009) 106:1915–9. doi: 10.1073/pnas.0813192106
40. Ni J, Miller M, Stojanovic A, Garbi N, Cerwenka A. Sustained effector function of IL-12/15/18-preactivated NK cells against established tumors. J Exp Med. (2012) 209:2351–65. doi: 10.1084/jem.20120944
41. Boieri M, Ulvmoen A, Sudworth A, Lendrem C, Collin M, Dickinson AM, et al. IL-12, IL-15, and IL-18 pre-activated NK cells target resistant T cell acute lymphoblastic leukemia and delay leukemia development in vivo. Oncoimmunology (2017) 6:e1274478. doi: 10.1080/2162402X.2016.1274478
42. Ni J, Holsken O, Miller M, Hammer Q, Luetke-Eversloh M, Romagnani C, et al. Adoptively transferred natural killer cells maintain long-term antitumor activity by epigenetic imprinting and CD4(+) T cell help. Oncoimmunology (2016) 5:e1219009. doi: 10.1080/2162402X.2016.1219009
43. Romee R, Schneider SE, Leong JW, Chase JM, Keppel CR, Sullivan RP, et al. Cytokine activation induces human memory-like NK cells. Blood (2012) 120:4751–60. doi: 10.1182/blood-2012-04-419283
44. Romee R, Rosario M, Berrien-Elliott MM, Wagner JA, Jewell BA, Schappe T, et al. Cytokine-induced memory-like natural killer cells exhibit enhanced responses against myeloid leukemia. Sci. Transl. Med. (2016) 8:357ra123. doi: 10.1126/scitranslmed.aaf2341
45. Pandiyan P, Zheng L, Ishihara S, Reed J, Lenardo MJ. CD4+CD25+Foxp3+ regulatory T cells induce cytokine deprivation-mediated apoptosis of effector CD4+ T cells. Nat Immunol. (2007) 8:1353–62. doi: 10.1038/ni1536
46. Gasteiger G, Hemmers S, Firth MA, Le Floc'h A, Huse M, Sun JC, et al. IL-2-dependent tuning of NK cell sensitivity for target cells is controlled by regulatory T cells. J. Exp. Med. (2013) 210:1167–78. doi: 10.1084/jem.20122462
47. Pahl JHW, Koch J, Gotz JJ, Arnold A, Reusch U, Gantke T, et al. CD16A activation of NK cells promotes NK cell proliferation and memory-like cytotoxicity against cancer cells. Cancer Immunol Res. (2018) 6:517–27. doi: 10.1158/2326-6066.CIR-17-0550
48. Brandt CS, Baratin M, Yi EC, Kennedy J, Gao Z, Fox B, et al. The B7 family member B7-H6 is a tumor cell ligand for the activating natural killer cell receptor NKp30 in humans. J Exp Med. (2009) 206:1495–503. doi: 10.1084/jem.20090681
49. Kuylenstierna C, Bjorkstrom NK, Andersson SK, Sahlstrom P, Bosnjak L, Paquin-Proulx D, et al. NKG2D performs two functions in invariant NKT cells: direct TCR-independent activation of NK-like cytolysis and co-stimulation of activation by CD1d. Eur J Immunol. (2011) 41:1913–23. doi: 10.1002/eji.200940278
50. Yu J, Wei M, Becknell B, Trotta R, Liu S, Boyd Z, et al. Pro- and antiinflammatory cytokine signaling: reciprocal antagonism regulates interferon-gamma production by human natural killer cells. Immunity (2006) 24:575–90. doi: 10.1016/j.immuni.2006.03.016
51. Ardolino M, Azimi CS, Iannello A, Trevino TN, Horan L, Zhang L, et al. Cytokine therapy reverses NK cell anergy in MHC-deficient tumors. J Clin Invest. (2014) 124:4781–94. doi: 10.1172/JCI74337
52. Chiossone L, Chaix J, Fuseri N, Roth C, Vivier E, Walzer T. Maturation of mouse NK cells is a 4-stage developmental program. Blood (2009) 113:5488–96. doi: 10.1182/blood-2008-10-187179
53. North J, Bakhsh I, Marden C, Pittman H, Addison E, Navarrete C, et al. Tumor-primed human natural killer cells lyse NK-resistant tumor targets: evidence of a two-stage process in resting NK cell activation. J Immunol. (2007) 178:85–94. doi: 10.4049/jimmunol.178.1.85
54. Pal M, Schwab L, Yermakova A, Mace EM, Claus R, Krahl AC, et al. Tumor-priming converts NK cells to memory-like NK cells. Oncoimmunology (2017) 6:e1317411. doi: 10.1080/2162402X.2017.1317411
55. Sabry M, Tsirogianni M, Bakhsh IA, North J, Sivakumaran J, Giannopoulos K, et al. Leukemic priming of resting NK cells is killer Ig-like receptor independent but requires CD15-mediated CD2 ligation and natural cytotoxicity receptors. J Immunol. (2011) 187:6227–34. doi: 10.4049/jimmunol.1101640
56. Liu LL, Landskron J, Ask EH, Enqvist M, Sohlberg E, Traherne JA, et al. Critical role of CD2 co-stimulation in adaptive natural killer cell responses revealed in NKG2C-deficient humans. Cell Rep. (2016) 15:1088–99. doi: 10.1016/j.celrep.2016.04.005
57. Grier JT, Forbes LR, Monaco-Shawver L, Oshinsky J, Atkinson TP, Moody C, et al. Human immunodeficiency-causing mutation defines CD16 in spontaneous NK cell cytotoxicity. J Clin Invest. (2012) 122:3769–80. doi: 10.1172/JCI64837
58. Gonsky R, Deem RL, Bream J, Young HA, Targan SR. Enhancer role of STAT5 in CD2 activation of IFN-gamma gene expression. J Immunol. (2004) 173:6241–7. doi: 10.4049/jimmunol.173.10.6241
59. Waldmann TA. The shared and contrasting roles of IL2 and IL15 in the life and death of normal and neoplastic lymphocytes: implications for cancer therapy. Cancer Immunol Res. (2015) 3:219–27. doi: 10.1158/2326-6066.CIR-15-0009
60. Kottaridis PD, North J, Tsirogianni M, Marden C, Samuel ER, Jide-Banwo S, et al. Two-stage priming of allogeneic natural killer cells for the treatment of patients with acute myeloid leukemia: a phase I trial. PLoS ONE (2015) 10:e0123416. doi: 10.1371/journal.pone.0123416
61. Warren HS, Kinnear BF. Quantitative analysis of the effect of CD16 ligation on human NK cell proliferation. J Immunol. (1999) 162:735–42.
62. Duggan MC, Campbell AR, Mcmichael EL, Opheim KS, Levine KM, Bhave N, et al. Co-stimulation of the fc receptor and interleukin-12 receptor on human natural killer cells leads to increased expression of cd25. Oncoimmunology (2018) 7:e1381813. doi: 10.1080/2162402X.2017.1381813
63. Romee R, Foley B, Lenvik T, Wang Y, Zhang B, Ankarlo D, et al. NK cell CD16 surface expression and function is regulated by a disintegrin and metalloprotease-17 (ADAM17). Blood (2013) 121:3599–608. doi: 10.1182/blood-2012-04-425397
64. Kondadasula SV, Roda JM, Parihar R, Yu J, Lehman A, Caligiuri MA, et al. Colocalization of the IL-12 receptor and FcgammaRIIIa to natural killer cell lipid rafts leads to activation of ERK and enhanced production of interferon-gamma. Blood (2008) 111:4173–83. doi: 10.1182/blood-2007-01-068908
65. Kim PS, Armstrong TD, Song H, Wolpoe ME, Weiss V, Manning EA, et al. Antibody association with HER-2/neu-targeted vaccine enhances CD8 T cell responses in mice through Fc-mediated activation of DCs. J Clin Invest. (2008) 118:1700–11. doi: 10.1172/JCI34333
66. Abes R, Gelize E, Fridman WH, Teillaud JL. Long-lasting antitumor protection by anti-CD20 antibody through cellular immune response. Blood (2010) 116:926–34. doi: 10.1182/blood-2009-10-248609
67. Srivastava RM, Lee SC, Andrade Filho PA, Lord CA, Jie HB, Davidson HC, et al. Cetuximab-activated natural killer and dendritic cells collaborate to trigger tumor antigen-specific T-cell immunity in head and neck cancer patients. Clin Cancer Res. (2013) 19:1858–72. doi: 10.1158/1078-0432.CCR-12-2426
68. Guillerey C, Huntington ND, Smyth MJ. Targeting natural killer cells in cancer immunotherapy. Nat Immunol. (2016) 17:1025–36. doi: 10.1038/ni.3518
69. Cartron G, Dacheux L, Salles G, Solal-Celigny P, Bardos P, Colombat P, et al. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood (2002) 99:754–8. doi: 10.1182/blood.V99.3.754
70. Bibeau F, Lopez-Crapez E, Di Fiore F, Thezenas S, Ychou M, Blanchard F, et al. Impact of Fc{gamma}RIIa-Fc{gamma}RIIIa polymorphisms and KRAS mutations on the clinical outcome of patients with metastatic colorectal cancer treated with cetuximab plus irinotecan. J Clin Oncol. (2009) 27:1122–9. doi: 10.1200/JCO.2008.18.0463
71. Wu Z, Sinzger C, Frascaroli G, Reichel J, Bayer C, Wang L, et al. Human cytomegalovirus-induced NKG2C(hi) CD57(hi) natural killer cells are effectors dependent on humoral antiviral immunity. J Virol. (2013) 87:7717–25. doi: 10.1128/JVI.01096-13
72. Zhang T, Scott JM, Hwang I, Kim S. Cutting edge: antibody-dependent memory-like NK cells distinguished by FcRgamma deficiency. J Immunol. (2013) 190:1402–6. doi: 10.4049/jimmunol.1203034
73. Rolle A, Pollmann J, Ewen EM, Le VT, Halenius A, Hengel H, et al. IL-12-producing monocytes and HLA-E control HCMV-driven NKG2C+ NK cell expansion. J Clin Invest. (2014) 124:5305–16. doi: 10.1172/JCI77440
74. Lee J, Zhang T, Hwang I, Kim A, Nitschke L, Kim M, et al. Epigenetic modification and antibody-dependent expansion of memory-like NK cells in human cytomegalovirus-infected individuals. Immunity (2015) 42:431–42. doi: 10.1016/j.immuni.2015.02.013
75. Schlums H, Cichocki F, Tesi B, Theorell J, Beziat V, Holmes TD, et al. Cytomegalovirus infection drives adaptive epigenetic diversification of NK cells with altered signaling and effector function. Immunity (2015) 42:443–56. doi: 10.1016/j.immuni.2015.02.008
76. Capuano C, Battella S, Pighi C, Franchitti L, Turriziani O, Morrone S, et al. Tumor-targeting anti-CD20 antibodies mediate in vitro expansion of memory natural killer cells: impact of CD16 affinity ligation conditions and in vivo priming. Front Immunol. (2018) 9:1031. doi: 10.3389/fimmu.2018.01031
77. Luetke-Eversloh M, Hammer Q, Durek P, Nordstrom K, Gasparoni G, Pink M, et al. Human cytomegalovirus drives epigenetic imprinting of the IFNG locus in NKG2Chi natural killer cells. PLoS Pathog. (2014) 10:e1004441. doi: 10.1371/journal.ppat.1004441
78. Lau CM, Sun JC. The widening spectrum of immunological memory. Curr Opin Immunol. (2018) 54:42–9. doi: 10.1016/j.coi.2018.05.013
79. Adams NM, Lau CM, Fan X, Rapp M, Geary CD, Weizman OE, et al. Transcription factor IRF8 orchestrates the adaptive natural killer cell response. Immunity (2018) 48:1172–82.e6. doi: 10.1016/j.immuni.2018.04.018
80. Mace EM, Bigley V, Gunesch JT, Chinn IK, Angelo LS, Care MA et al. Biallelic mutations in IRF8 impair human NK cell maturation and function. J Clin Invest. (2017) 127:306–20. doi: 10.1172/JCI86276
81. Almeida L, Lochner M, Berod L, Sparwasser T. Metabolic pathways in T cell activation and lineage differentiation. Semin Immunol. (2016) 28:514–24. doi: 10.1016/j.smim.2016.10.009
82. Marcais A, Cherfils-Vicini J, Viant C, Degouve S, Viel S, Fenis A, et al. The metabolic checkpoint kinase mTOR is essential for IL-15 signaling during the development and activation of NK cells. Nat Immunol. (2014) 15:749–57. doi: 10.1038/ni.2936
83. Keppel MP, Saucier N, Mah AY, Vogel TP, Cooper MA. Activation-specific metabolic requirements for NK Cell IFN-gamma production. J Immunol. (2015) 194:1954–62. doi: 10.4049/jimmunol.1402099
84. Mah AY, Rashidi A, Keppel MP, Saucier N, Moore EK, Alinger JB, et al. Glycolytic requirement for NK cell cytotoxicity and cytomegalovirus control. JCI Insight (2017) 2. doi: 10.1172/jci.insight.95128
85. Assmann N, O'brien KL, Donnelly RP, Dyck L, Zaiatz-Bittencourt V, Loftus RM, et al. Srebp-controlled glucose metabolism is essential for NK cell functional responses. Nat. Immunol. (2017) 18:1197–206. doi: 10.1038/ni.3838
86. Mews P, Donahue G, Drake AM, Luczak V, Abel T, Berger SL. Acetyl-CoA synthetase regulates histone acetylation and hippocampal memory. Nature (2017) 546:381–6. doi: 10.1038/nature22405
87. Loftus RM, Assmann N, Kedia-Mehta N, O'brien KL, Garcia A, Gillespie C, et al. Amino acid-dependent cMyc expression is essential for NK cell metabolic and functional responses in mice. Nat. Commun. (2018) 9:2341. doi: 10.1038/s41467-018-04719-2
88. Bianchi T, Gasser S, Trumpp A, Macdonald HR. c-Myc acts downstream of IL-15 in the regulation of memory CD8 T-cell homeostasis. Blood (2006) 107:3992–9. doi: 10.1182/blood-2005-09-3851
89. Van Helden MJ, De Graaf N, Boog CJ, Topham DJ, Zaiss DM, Sijts AJ. The bone marrow functions as the central site of proliferation for long-lived NK cells. J Immunol. (2012) 189:2333–7. doi: 10.4049/jimmunol.1200008
90. Mogensen TH, Paludan SR. Molecular pathways in virus-induced cytokine production. Microbiol Mol Biol Rev. (2001) 65:131–50. doi: 10.1128/MMBR.65.1.131-150.2001
91. Nabekura T, Lanier LL. Tracking the fate of antigen-specific versus cytokine-activated natural killer cells after cytomegalovirus infection. J Exp Med. (2016) 213:2745–58. doi: 10.1084/jem.20160726
92. Suliman S, Geldenhuys H, Johnson JL, Hughes JE, Smit E, Murphy M, et al. Bacillus Calmette-Guerin (BCG) revaccination of adults with latent Mycobacterium tuberculosis infection induces long-lived BCG-reactive NK cell responses. J Immunol. (2016) 197:1100–10. doi: 10.4049/jimmunol.1501996
93. Granzin M, Stojanovic A, Miller M, Childs R, Huppert V, Cerwenka A. Highly efficient IL-21 and feeder cell-driven ex vivo expansion of human NK cells with therapeutic activity in a xenograft mouse model of melanoma. Oncoimmunology (2016) 5:e1219007. doi: 10.1080/2162402X.2016.1219007
94. Venkatasubramanian S, Cheekatla S, Paidipally P, Tripathi D, Welch E, Tvinnereim AR, et al. IL-21-dependent expansion of memory-like NK cells enhances protective immune responses against Mycobacterium tuberculosis. Mucosal Immunol. (2017) 10:1031–42. doi: 10.1038/mi.2016.105
95. Kleinnijenhuis J, Quintin J, Preijers F, Joosten LA, Jacobs C, Xavier RJ, et al. BCG-induced trained immunity in NK cells: role for non-specific protection to infection. Clin Immunol. (2014) 155:213–9. doi: 10.1016/j.clim.2014.10.005
96. Netea MG, Joosten LA, Latz E, Mills KH, Natoli G, Stunnenberg HG, et al. Trained immunity: a program of innate immune memory in health and disease. Science (2016) 352:aaf1098. doi: 10.1126/science.aaf1098
97. Huber CM, Doisne JM, Colucci F. IL-12/15/18-preactivated NK cells suppress GvHD in a mouse model of mismatched hematopoietic cell transplantation. Eur J Immunol. (2015) 45:1727–35. doi: 10.1002/eji.201445200
98. Song Y, Hu B, Liu Y, Jin Z, Zhang Y, Lin D, et al. IL-12/IL-18-preactivated donor NK cells enhance GVL effects and mitigate GvHD after allogeneic hematopoietic stem cell transplantation. Eur J Immunol. (2018) 48:670–82. doi: 10.1002/eji.201747177
99. Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science (2002) 295:2097–100. doi: 10.1126/science.1068440
100. Parkhurst MR, Riley JP, Dudley ME, Rosenberg SA. Adoptive transfer of autologous natural killer cells leads to high levels of circulating natural killer cells but does not mediate tumor regression. Clin Cancer Res. (2011) 17:6287–97. doi: 10.1158/1078-0432.CCR-11-1347
Keywords: memory, NK cell, IL-12 cytokines, IL-12/15/18, CD16, recall (memory), adoptive transfer, immunotherapy
Citation: Pahl JHW, Cerwenka A and Ni J (2018) Memory-Like NK Cells: Remembering a Previous Activation by Cytokines and NK Cell Receptors. Front. Immunol. 9:2796. doi: 10.3389/fimmu.2018.02796
Received: 01 August 2018; Accepted: 13 November 2018;
Published: 28 November 2018.
Edited by:
Alexander Rölle, Nationales Centrum für Tumorerkrankungen (NCT), GermanyReviewed by:
Aimee Beaulieu, Rutgers, The State University of New Jersey, United StatesErik Dissen, University of Oslo, Norway
Alessandra Zingoni, La Sapienza University of Rome, Italy
Copyright © 2018 Pahl, Cerwenka and Ni. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jens H. W. Pahl, amVucy5wYWhsQG1lZG1hLnVuaS1oZWlkZWxiZXJnLmRl
 Jens H. W. Pahl
Jens H. W. Pahl Adelheid Cerwenka1
Adelheid Cerwenka1