- 1Hunan Key Laboratory of Medical Epigenomics, Department of Dermatology, Second Xiangya Hospital, Central South University, Changsha, China
- 2Immunology Section, Lund University, Lund, Sweden
- 3Department of Dermatology, Peking University People's Hospital, Beijing, China
- 4Department of Dermatology, The Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, China
- 5Beijing Wenfeng Tianji Pharmaceuticals Ltd., Beijing, China
- 6Department of Dermatology, Beijing Friendship Hospital, Capital Medical University, Beijing, China
- 7Department of Emergency, Second Xiangya Hospital of Central South University, Changsha, China
Follicular helper T cells (Tfh) are specialized helper T cells that are predominantly located in germinal centers and provide help to B cells. The development and differentiation of Tfh cells has been shown to be regulated by transcription factors, such as B-cell lymphoma 6 protein (Bcl-6), signal transducer and activator of transcription 3 (STAT3) and B lymphocyte-induced maturation protein-1 (Blimp-1). In addition, cytokines, including IL-21, have been found to be important for Tfh cell development. Moreover, several epigenetic modifications have also been reported to be involved in the determination of Tfh cell fate. The regulatory network is complicated, and the number of novel molecules demonstrated to control the fate of Tfh cells is increasing. Therefore, this review aims to summarize the current knowledge regarding the molecular regulation of Tfh cell development and differentiation at the protein level and at the epigenetic level to elucidate Tfh cell biology and provide potential targets for clinical interventions in the future.
Introduction
A subset of CD4+ T cells, which help B cells and are a resident in B follicles, has been described in the early 1990s (1–4). The existence of follicular helper T (Tfh) cells was proposed in 2000 (5, 6). However, the existence of these cells was not widely accepted until the identification of the Tfh cell linage-specific transcription factor, B-cell lymphoma 6 protein (Bcl-6), in follicular T cells in 2009 (7, 8). High expression of CXCR5 and low expression of CCR7 enable T cells to enter and stay in germinal centers (GCs) (6, 9–11). Bcl-6 deficient T cells have been shown to fail to differentiate into follicular helper T cells (8), indicating the importance of Bcl-6 in the determination of Tfh cell fate. Under the effects of CCL19 and CCL21, expression of the receptor CCR7 on naïve CD4+ T cells enables these cells to migrate into T cell zones in the secondary lymph nodes (9, 12). With stimulation from antigens and CD80, CD86 and ICOSL expressed on dendritic cells (DCs), these cells differentiate into pre-Tfh cells with high expression of PD-1, CXCR5 and signaling lymphocytic activation molecule adapter protein (SAP) (13) and low expression of CCR7 and P selectin glycoprotein ligand 1 (PSGL1) (14, 15) (Figure 1). Generally, Tfh cells provide signals for B cell maturation, differentiation and survival via ICOS, CD40L, IL-4, and IL-21 (16, 17). ICOS and ICOSL ligation is involved in T-B cell interactions, further promoting calcium spikes in T-cells and CD40-CD40L signaling in B cells (18). ICOS-deficient T cells fail to express CXCR5 and are unable to migrate into follicles, a finding also observed during antibody-blockade of ICOS-ligand (19, 20). PD-1 has been found to limit the number of Tfh cells (21). More evidence is needed to address the role of PD-1 in the migration and function of Tfh cells. SAP has been found to stabilize the interaction between B cells and Tfh cells (22). Therefore, Tfh cells can be distinguished from Th1, Th2 and Th17 cells using surface markers with a profile of CCR7loPSGL1loCXCR5hiPD-1hiICOShi. Activated by antigens and ICOSL expressed by DCs, the expression of Bcl-6 is upregulated in CD4+ T cells, and it represses other Th cell transcription factors, such as T-bet, GATA-3, and RORγT. Next, Bcl-6 promotes the transcription of Tfh cell migration and function-related genes, such as CXCR5, PD-1, and CXCR4 (8).
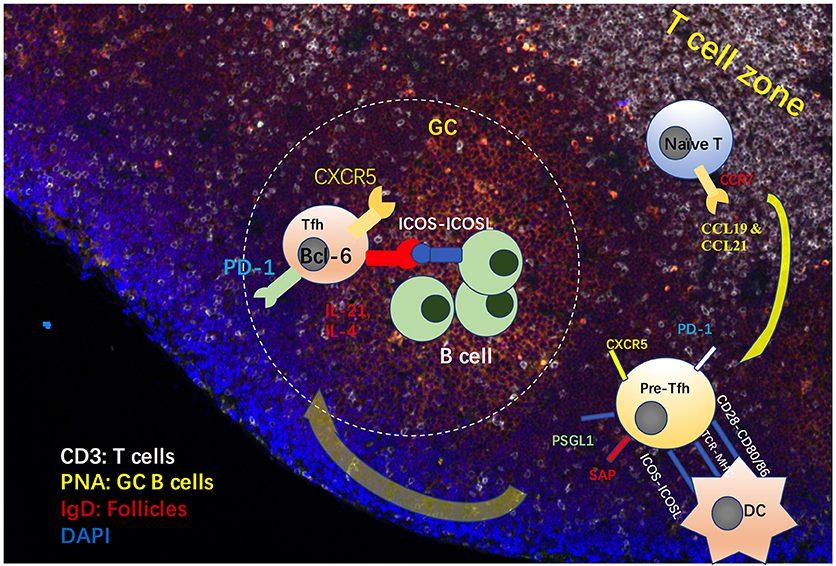
Figure 1. Process of Tfh cell differentiation and migration in GCs. Under the effects of CCL19 and CCL21, expression of the receptor CCR7 on naïve CD4+ T cells allows these cells to migrate into T cell zones in the secondary lymph nodes. With stimulation from antigens and CD80, CD86 and ICOSL expressed on dendritic cells (DCs), these cells differentiate into pre-Tfh cells, with high expression of CXCR5, PD-1 and signaling lymphocytic activation molecule adapter protein (SAP) and low expression of CCR7 and P selectin glycoprotein ligand 1 (PSGL1). Generally, Tfh cells provide signals for B cell maturation, differentiation and survival via ICOS, CD40L, IL-4, and IL-21 (16, 17). ICOS and ICOSL ligation is involved in Tfh-B cell interactions, which promotes calcium spikes in T-cells and CD40-CD40L signaling in B cells.
Tfh cells have been found to be regulated by a complex network of transcription factors, including the Bcl-6-Blimp1 axis, STAT1, STAT3, STAT4, STAT5b, B-cell activating transcription factor (Batf), v-maf avian musculoaponeurotic fibrosarcoma oncogene homolog (c-Maf), interferon regulatory factor 4 (IRF4), Achaete-scute homolog 2 (Acl2), and T-cell-specific transcription factor 1 (TCF-1)-lymphoid enhancer binding factor 1 (LEF-1). Since the study of Tfh cells began, certain proteins have been identified to participate in the development of Tfh cells. In addition, such cytokines as IL-2, IL-6, IL-7, IL-9, IL-12, IL-21, IL-23, IL-27, and TGF-β have been reported to enhance or impair the differentiation and survival of Tfh cells. Therefore, this review will comprehensively describe the current knowledge of Tfh cells, hoping to provide potential targets for Tfh cell-mediated autoimmune disease.
Transcription Factor Network
Bcl-6-Blimp-1 Axis
The discovery of Bcl-6 in Tfh cells is a hallmark for the identification of Tfh cells. The essential role of Bcl-6 has been confirmed in a mice study, indicating that CD4+ T cells deficient in Bcl-6 fail to differentiate into Tfh cells (8). Forced expression of Bcl-6 in CD4+ T cells promotes the expression of CXCR5, CXCR4, and PD-1(8). Bcl-6 can bind to the promoters of Th1 and Th17 cell transcriptional regulators T-bet and RORγT, thereby repressing the production of IFN-γ and IL-17 (8). The key role of Bcl-6 in Tfh cell fate determination has been further confirmed in subsequent studies (23, 24), and one of them reveals that Bcl-6 regulates Tfh cell early differentiation in an IL-21- and IL-6-independent manner (24). Conversely, Bcl-6 can bind to the promoters and enhancers of several migration-related genes, such as CCR7, CCR6, PSGL-1, CXCR5, CXCR4, PD-1, and SAP (24, 25). In addition, Bcl-6-targeted genes are enriched in the MAPK and JAK-STAT signaling pathways and cytokine-cytokine receptor ligations, which are involved in cell activation, metabolism and maintenance (26).
Blimp-1 has been found to be a critical antagonist for Tfh cell differentiation but an important transcription factor for other effector cells, such as Th1, Th2, Th17, and regulatory T cells (7). In a mouse study, Blimp-1-deficient CD4+ T cells preferentially develop into Tfh cells in vivo, while Blimp-1-expressing CD4+ T cells failed to aid in germinal center formation (7). Therefore, Tfh cell differentiation is believed to be a distinct pathway independently regulated by Bcl-6, in contrast to other effector T cells regulated by Blimp-1 (27). More importantly, constitutive expression of Blimp-1 has an inhibitory effect on Bcl-6 expression and thus represses Tfh cell differentiation (7), indicating that Bcl-6 and Blimp-1 are antagonistic regulators in Tfh cells. The results in B cells might shed light on this mechanism. In plasma cell differentiation, the release of Bcl-6-bound histone deacetylases (HDACs) may increase the histone acetylation levels on the promoter region of Blimp-1, promoting the expression of this gene (28, 29). Thus, HDACs might be the competitive substrate for these two genes. In autoimmune status, Bcl-6 deficiency in lupus-prone mice has been found to impair lupus-like symptoms (30), and increased Bcl-6 has been observed in lupus circulating Tfh-like cells, which is positively correlated with disease activity (31).
Bcl-6 and STAT5
Similar to the Bcl-6-Blimp-1 axis, Bcl-6 and STAT5 also inhibit each other due to their overlapping binding sites in many Tfh cell-related genes, including Socs2, IL7r, and Tcf7. In a mouse study, Bcl-6 has been found to repress both IL-7R and STAT5 expression, as well as inhibiting IL-2-induced STAT5 activation (32). This inhibitory effect on STAT5 by Bcl-6 is due to the abrogation of STAT5 phosphorylation (32). In contrast, signals through IL-2-CD25 activate STAT5 and inhibit Bcl-6 and CXCR5 via inducing Blimp-1 (20, 33, 34), and lack of IL-2R signaling leads to Bcl-6 expression (35). A high concentration of IL-2 has been found to inhibit Bcl-6 expression in polarized Th1 cells, in which the Bcl-6 DNA-binding domain is masked by the T-bet-Bcl-6 complex and normally shows low levels of Bcl-6 expression in response to limited IL-2 (36). However, in response to low IL-2, besides increased Bcl-6 and IL-6R, Th1 cells can also increase the expression of IL-7R, which can repress Tfh-related genes, including cxcr5 and bcl6 via IL-7-dependent STAT5 activation (37). In addition, Bcl-6 in Tfh cells has been observed to have a decreased level of 5-hydroxymethylcytosine (5hmC), which might explain the markedly high level of Bcl-6 in Tfh cells (32). Conversely, Bcl-6 deficiency results in increased STAT5 signaling and promotes the differentiation of non-Tfh effector T cells. The inhibitory effects of STAT5 have been found to be Blimp-1-independent. In addition, inhibition of IL-2 results in the reduction of Blimp-1 expression (38), indicating that IL-2, STAT5 and Blimp-1 collaboratively inhibit Tfh cell differentiation (39).
STAT3
IL-21 and IL-6/STAT3 are first described to be essential for Th17 cell differentiation (40). Next, STAT3 has found to be critical for Tfh cell differentiation. The evidence come from the fact that reduced IL-21 production is reported in mouse STAT3-deficient T cells, and only a STAT3 mutation, rather than Il12RB1, reduce the frequency of Tfh cells in vivo (41). Similarly, in CD4+ T cell-conditional STAT3 knockout mice, fewer CXCR5+ Tfh cells, as well as defective GCs and reduced IgG and IgM antibody production, have been observed after KLH immunization (42, 43). In another study, the gene expression of Cxcr5 and Icos is shown to be downregulated in STAT3-deficient mice, while the expression of Blimp-1 is increased (44). More importantly, cluster analysis showed that STAT3-deficient Ly6Clo PSGL-1hi T cells in the T cell zone more closely resemble Th1 cells, with a high expression of IFN-induced genes (44). More direct evidence is that STAT3 can form a complex with Ikaros zinc finger transcription factor Aiolos to regulate Bcl-6 expression (45). In a human study, rather than in a mouse system, TGF-beta has been found to provide critical additional signals for STAT3 and STAT4 to initiate Tfh cell differentiation (46), emphasizing the important role of STAT3 in Tfh cell development. Unlike the critical role of IL-6 in early Tfh cell differentiation, STAT3 deficiency fails to recapitulate the impaired Tfh frequency. However, in this study, STAT1 activity has been found to be required for Bcl-6 induction and initiating Tfh cell differentiation (47). In addition, STAT3 can suppress type 1 IFN induced CD25 expression and can compete with STAT5 to bind to the Bcl6 locus (48). However, it might be difficult to distinguish whether the effects of STAT3 is intrinsic to the Tfh cell or a reflection of diminished capacity for other cell subset differentiation. The forced overexpression of STAT3 in T cell may provide an explanation to this issue, which is still lacking at this moment.
TCF-1 and LEF-1
TCF-1 and LEF-1 belong to the TCF-LEF subfamily and have been well-documented to be necessary for the maturation of double negative T cells to the double positive stage in thymus. In addition, TCF-1 has been reported to restrain mature T cell-mediated Th17 responses via suppressing IL-17 expression (49). TCF-1 and LEF-1 have been reported as critical transcription factors in Tfh cell differentiation by two independent studies published in the same year (50, 51). The loss of either TCF-1 or LEF-1 in mice leads to defects in Tfh cells, and the depletion of both TCF-1 and LEF-1 results in the impairment of Tfh cell differentiation and GC formation. In addition, the important role of LEF-1 has been emphasized by the observation that forced LEF-1 expression promotes the differentiation of Tfh cells (51). In another study, TCF-1 and LEF-1 are revealed to regulate the Bcl-6/Blimp-1 axis. TCF-1 has been identified as a positive regulator for Bcl-6 and it displays negative effects on Blimp-1 via directly binding to the Bcl-6 promoter to form a complex and regulatory region known as intron 3 of Prdm1 (51). In addition, TCF-1 has been found to upregulate IL-6R expression and inhibit IL-2R expression (51), indicating that TCF-1 might be upstream of STAT3 and STAT5. The exact function of LEF-1 in Tfh cells remains unclear. However, evidence shows that LEF-1 synergistically works with TCF-1 to regulate Tfh cells, and TCF-1 can inhibit LEF-1 expression (51). Furthermore, TCF-1 and LEF-1 have been found to promote early Tfh cell differentiation by maintaining the expression of IL-6Rα and gp130 and enhancing ICOS and Bcl-6 expression (52).
Ascl2
Ascl2 is a basic helix-loop helix (bHLH) transcription factor that has been reported to initiate Tfh cell differentiation via upregulating CXCR5 but not Bcl-6 in T cells in vitro (53). In addition, in vivo, Ascl2 can promote T cell migration to the border of B cell follicles and can promote Tfh cell differentiation by inhibiting Th1 and Th17 signature genes and upregulating Tfh cell-related genes (53). In other studies, Ascl2 has been shown to be responsible for low CD25 expression on regulatory follicular T cells (Tfr) (54). Ascl2 displays the active chromatin marker trimethylated histone H3 lysine 4 (H3K4me3), which has not been observed in other T cell subsets. In contrast, other Tfh cell-related genes, such as Bcl-6, Maf, Batf, and Irf4, are associated with H3K4me3 in all T-cell subsets (55).
C-Maf
c-Maf, a member of the activator protein 1 (AP-1) transcription factor family, has been found to be highly expressed by Th17 and mature Tfh cells compared with CD4+ICOShiCXCR5− or CD4+ICOSloCXCR5− non-Tfh cells. During Th17 cell differentiation, IL-6 plus TGF-β or IL-21 plus TGF-β can increase the expression of c-Maf, which is ICOS-dependent (56). As mentioned before, Bcl-6 controls the expression of migration genes that are important for the migration of T cells to the follicles. However, the introduction of Bcl-6 cannot alter the production of IL-21 and IL-4, which are the key cytokines produced by Tfh cells. c-Maf has been found to affect the production of IL-21 and CXCR5 (57). In addition, c-Maf and Bcl-6 have been reported to cooperate in the expression of Tfh cell-related genes, such as CXCR4, PD-1, and ICOS (57). The selective loss of c-Maf expression in T cells leads to the inhibition of Tfh cell differentiation in response to vaccinations and bacteria, and it is also critical for high-affinity antibody secretion in vaccinated animals (58). In addition, in Tfh cells, c-Maf has been shown to positively regulate IL-4 production via binding to the conserved noncoding sequence 2 (CNS2) region of the IL-4 locus and via the induction of IRF4 (59–61); however, this effect is c-Maf-independent (61).
Batf
Batf is also a member of the AP-1 family, which lacks transcriptional activation domains (TADs). Batf has been found to be highly expressed by Tfh cells and directly regulates the expression Bcl-6 and c-Maf (62). The expression of Batf has been observed to be regulated by IL-4-STAT6 in Th9 cells and IL-6-STAT3 signaling in M1 mouse myeloid leukemia cells (63–65). In Batf-deficient mouse T cells, the expression of Bcl-6 and c-Maf decreased dramatically, and Bcl-6 alone is not sufficient for Tfh cell differentiation in the absence of Batf (62). In addition, Batf can cooperate with IRF4 along with STAT3 and STAT4 to promote IL-4 production in Tfh cells via binding to the CNS2 region in the IL-4 locus. BATF does not impair IL-4 in Th2 cells but only Tfh cells (61). However, other studies show that the loss of Batf impairs IL-4 production in both Tfh and Th2 cells (66, 67).
IRF4
IRF4 has been well-documented as an important transcription factor in the differentiation of helper T cells and B cells via promoting cell development (68). IRF4 expression in mouse T cells has also been found to promote GC formation by promoting Tfh cell differentiation (69). In IRF4 knockout mice, CD4+ T cells in lymph nodes and Peyer's patches failed to express Bcl-6 and Tfh cell-related genes. In addition, the adoptive transfer of wild-type Tfh cells cannot rescue the failed IRF4−/− Tfh cell differentiation (69), indicating a critical role for IRF4 in Tfh cell development. In wild-type mice, IRF4 can interact with JUN and Batf to form a heterotrimer that can bind to AP1-IRF4 complexes and regulate Tfh cell differentiation (69). In another study, IRF4−/− CD4+ T cells have impaired STAT3 binding and fail to differentiate into Tfh cells (70). In a recent study, the Irf4 locus is reported to “sense” the intensity of TCR signaling to determine the Irf4 expression level. The binding of IRF4 to divergent DNA sequences is regulated by the expression levels of IRF4 and controls Th cell fate determination (71). In Th2 cells, enhancers show a spectrum of occupancy by the Batf-IRF4 complex, which correlates with the sensitivity of gene expression to TCR signal strength (72). The adaptor molecule LAT has been revealed to export the repressor HDAC7 from the nucleus of CD4(+) T cells. The loss of LAT results in impaired TCR signal and the repression of HDAC7 targeted gene Nur77 and Irf4 (73). Furthermore, IRF4 has been reported to be induced in a TCR-affinity dependent manner, and it is critical for clonal expansion (74).
In addition, other transcription factors have also been reported to be involved in Tfh cell differentiation. Foxo1, which has been found to negatively regulate Tfh cell differentiation in the early stages of differentiation (75), has also been identified to positively promote Tfh cell differentiation in the late stage of this process (76). However, the molecular mechanism remains unclear. FOXP1 negatively regulates Tfh cell differentiation by directly inhibiting ICOS expression and IL-21 production (77). Kruppel-like factor 2 (KLF2), a transcription factor, has been found to be involved in T cell trafficking, survival and homeostasis. KLF2 deficiency has been linked with increased number of Tfh cells, and forced expression of KLF2 results in reduced Tfh cell differentiation and GC formation (78). KLF2 can negatively control Tfh cell differentiation by inhibiting the homing receptors, such as CXCR5, CCR7, S1PR1 and PSGL1 (79), via induction of negative regulators for Tfh cells, including Blimp-1, T-bet and GATA3 (78).
Other Proteins Regulating Tfh Cell Differentiation E3 Ubiquitin Ligase
Roquin is an RNA binding protein, which has been revealed to play a critical role in innate and adoptive immune systems. The lack of Roquin activity results in numerous autoimmune diseases, such as lupus and inflammatory bowel disease. It is well-known that sanroque mice, which have the mutant ROQUINM199R that promotes Tfh cells, show a spontaneous germinal center (GC) and accumulation of plasma cells (30, 80, 81). The ubiquitin E3 ligase Roquin-1 negatively regulates Tfh cell differentiation by recognizing and directly binding a cis-element in the 3' untranslated region of ICOS mRNA, thereby repressing ICOS expression (82). The combined loss of Roquin-1 and 2 results in spontaneous Tfh cell and germinal center development (83). Other Tfh-related genes, such as Il6, Irf4, Ox40, (84, 85) and Ifng (86), are repressed by Roquin. The loss of the RUNG domain of Roquin has been found to reduce the number of Tfh cells, which might be a result of impaired mTOR signaling (87) and reduced Bcl-6 expression (88). In addition, the E3 ubiquitin ligase Itch has also been reported to regulate Tfh cells by regulating the ubiquitination and degradation of Foxo1 (89), and the effect of Itch has been revealed to be upstream of Bcl-6, which is validated by the fact that forced Bcl-6 in Itch deficient mice can restore Tfh cell differentiation (89). Moreover, the E3 ubiquitin ligase Cullin3 acts as a negative regulator by directly binding to Bcl-6 and regulating the ubiquitination of histone proteins (90). Furthermore, in transplantation, herpesvirus entry mediator/B-and T-lymphocyte attenuator (HVEM/BTLA) signaling pathway has been found to be dispensable for the expansion of Tfh cells and formation of de novo host anti-donor isotype-specific antibodies (91).
Notch−1 and −2
The T cell-specific deletion of Notch-1 and Notch-2 results in the reduced number of fully mature Tfh cells and the absence of high-affinity Abs (92). These mature Tfh cells produce low levels of IL-21 and displayed low expression of Bcl-6 and C-Maf. However, the effect of the loss of Notch on Tfh cell differentiation is in an IL-4-independent manner (92). In a recent study, Notch signaling has been identified as an early lineage-determining factor between Tfh and Th2 cell fate (93). In addition, Delta-L 1/4-mediated signals to Tfh cells occur from stroma cells, and follicular dendritic cells are not required (93, 94). Fasnacht et al. (94) shows DLL4 in stromal cells is important for Tfh development. In a previous study, fibroblasts, rather than hematopoietic or endothelial cells, as niche cells, support Notch-2 driven differentiation of marginal-zone B cells, ESAMDCs, and Tfh cells (94).
Surface Molecule Regulation
CXCR5
CXCR5 is a hallmark of Tfh cells that guides T cells to migrate to the B cell zone by binding to CXCL13 that is expressed by follicular dendritic cells (95). CXCL13 is expressed in the follicular mantle zone and not in the endothelial venules and paracortical T cell zone, where ligands for CCR7 exist. Unlike CCR7 ligands, CCL19 and CCL21, CXCL13 controls the segregation between T and B cells, rather than recruiting T cells and B cells to lymph nodes (96). Therefore, these CXCR5 hi T cells express a low level of CCR7, which helps these T cells to migrate to GCs (6, 9, 10, 97). Moreover, CXCR5 has been found to help the maintenance of PD-1 hi Tfh population in GCs (9). CXCR5-deficient mice have a low GC number and antibody production (95), which shows the important role of CXCR5 in Tfh cell differentiation. In addition to being controlled by Bcl-6, CXCR5 expression is also regulated by nuclear factor of activated T cells (NFAT2) (98).
ICOS/ICOSL
With signals from MHC-antigen-TCR and CD28 stimulation, ICOS expression is induced on activated T cells. Therefore, ICOS is not a reliable marker for Tfh cells not only due to its expression on precursor Tfh cells but also due to its high expression on activated T cells. Signals through ICOS-ICOSL are critical for Tfh cell differentiation, B cell survival and activation, antibody class switching and GC formation (99). In human Tfh cells, ICOS is used as a marker of GC Tfh cells (100). However, ICOS is probably not a reliable marker for GC Tfh cells in mice due to its similar expression in Tfh cells and precursor Tfh cells (101). It has been found that initial DC priming is sufficient to differentiate CXCR5+Bcl-6+ Tfh cells, but this process depends on consistent ICOS/ICOSL signaling from DCs (102). Further ICOSL signals from B cells are necessary for the complete differentiation and maintenance of GC-Tfh cells (14). ICOS has been found to be capable of regulating the migration of T cells to GCs via the induction of filopodia (17). Signals through ICOS/ICOSL activate phosphoinositide-3 kinase (PI3K) (103), which is also a critical kinase for Tfh cell differentiation via the AKT-mediated inactivation of FOXO (104). In addition, ICOS is able to maintain the Tfh cell phenotype via FOXO1-mediated KLF2 expression (79), and FOXO1 is also inhibited by ICOS-induced mTORc2 (75). ICOS signaling can also affect IL-21 production via c-Maf, thereby regulating Tfh cell differentiation (56). The importance of ICOS in Tfh cells has been demonstrated by a study showing that ICOS-deficient mice have impaired GCs, a reduced level of CXCR5+ memory T cells (19, 105), impaired immunoglobulin class switching and low levels of IL-4 when primed in vivo and restimulated in vitro with a specific antigen (106, 107).
OX40/OX40L
OX40 belongs to the TNFR family and is transiently expressed by T cells during chronic virus infection (108). OX40 has been found to play a critical role in Tfh cell differentiation. Reinforcing OX40 stimulation promotes the expression of Blimp-1 in LCMV-specific T cells and inhibits the differentiation of Tfh cells (108). However, OX40-deficient mice display impaired generation of Tfh cells and GCs (109), indicating that OX40 is important for the Tfh cell differentiation. Indeed, OX40L has been reported to contribute to lupus by promoting Tfh cell responses (110). In addition, TSLP-activated dendritic cells have been found to be able to induce Tfh cell generation via OX40L (111). In addition, OX40 can cooperate with ICOS to amplify Tfh cell development during vaccinia virus infection (112).
Other Important Surface Markers
PD-1, which is usually expressed by exhausted T cells, is highly expressed on Tfh cells. PD-1/PD-Ls signals are generally considered as negative regulatory signals that dephosphorylate TCR signaling, thereby inhibiting activation and cytokine production by T cells (113). PD-1 expressed by Tfh cells is believed to balance the negative regulation from IL-2-mediated STAT5 signaling (114). In addition, Tfr cells also express PD-1, which regulates Tfr cells (115). In a recent report, PD-1 has been found to inhibit follicular T cell recruitment via limiting CXCR3 expression to confine Tfh cell localization in GCs and increase the stringency of GC affinity selection through PD-1-PD-L1 ligation (116). Cytotoxic T lymphocyte antigen 4 (CTLA-4) is another negative regulator of T cells that has been reported to be expressed by Tfh cells and Tfr cells (117). Tfr and Treg cells regulate Tfh cells via B7-1 and B7-2 binding to CTLA-4. Loss of CTLA-4 in Tfh cells results in the promotion of B cell responses (118). Moreover, mice deficient in the SLAM-associated protein (SAP) show impaired GC formation and defects in T-B cell interaction (22, 119–121). Although SAP deficient T cells can express Tfh markers initially, reduced Tfh cells have been found in GCs from SAP deficient mice (30, 122), suggesting that SAP is required for the generation of functional Tfh cells and the differentiation of Tfh cell contains multiple steps.
Cytokine Regulation
Signals from follicular DCs and the cytokine milieu produced by DCs provide instructions for Tfh cell differentiation. Various cytokines, including IL-6, IL-21, IL-12, IL-23, IL-2, TGF-β, IL-1β, can regulate Bcl-6, STAT5, and Blimp-1 expression via the JAK- STAT signaling pathway (55). In the early stage of human Tfh cell differentiation, IL-12, IL-23, and TGF-β initiate this process. In addition, other STAT3-activating cytokines, such as IL-1β and IL-6, support this process in the presence of IL-12, IL-23, and TGF-β. The precursors of Tfh cells share similarities with other Th subsets and can further differentiate into Th1 and Th17 cells dependent on the balance of cytokines (123). Following interactions with B cells, precursor cells can differentiate into Th1-like Tfh cells and Th17-like Tfh cells (123). In addition, some reports have shown that Tfh cells can express IFN-gamma and IL-4, which provides help for cytokine-driven patterns of immunoglobulin class switching (124). In some autoimmune conditions, such as an experimental autoimmune encephalomyelitis (EAE) model of multiple sclerosis (MS), cells that display a Tfh cell phenotype produce IL-17 (56), and during helminth infection, Tfh cells in lymph nodes produce IL-4 (124–126). These IL-4 producing Tfh cells located in B cell follicles are found to be functionally different form Th2 cells found in peripheral region (124). These IL-4 producing Tfh cells express a low level of GATA3 and no IL-13 (127).
It has been well-established that IL-6-mediated STAT3 activation is critical for IL-21 expression in TCR stimulated mice and human T cells (40, 128). STAT3 can also respond to IL-21 and IL-23. Following cytokine stimulation, STAT3 is phosphorylated by JAK and binds to the Bcl-6 promoter to further promote Bcl-6 transcription (129). In addition, IL-12 has been reported to induce Bcl-6 expression in human T cells via STAT4 activation and has a greater effect on IL-21 production compared to IL-6 and IL-21 (130). In addition, the IL-12-STAT4 pathway can also regulate CXCR5, ICOS, c-Maf, and Batf expression in human T cells (131, 132). TGF-β has been found to enhance the function of STAT3-STAT4 to help T cells to express CXCR5, ICOS, Bcl-6, c-Maf, IL-21, and Batf, as well as to repress the expression of Blimp-1 (42). However, in mice, TGF-β has been reported to have negative regulatory effects on Bcl-6 expression via mir-10a (133). The positive regulation of TGF-β might be restricted to human in vitro studies. However, in mice, cytokines and TCR stimulation are insufficient, and the T-B interaction is necessary to generate Tfh cells (134).
Epigenetic Regulations
Epigenetic regulation refers to a modification that will not change the DNA sequence but alters the gene expression through several modifications, such as DNA methylation, histone modification and non-coding RNA-mediated regulations. Increasing evidence has shown the cooperation between epigenetic modifications with transcription factors to determine T cell fate (135).
Unsurprisingly, Tfh cell differentiation is also regulated by epigenetic modifications. DNA methylation refers to silencing gene expression, and demethylation/hydroxymethylation is related to gene reactivation. In Tfh cells, Bcl-6 binding to gene loci has been found to be associated with reduced recruitment of translocation methylcytosine dioxygenase 1 (TET1), which is a hydroxymethyltransferase. Bcl-6 binding is also observed to result in reduced 5-hydroxymethylcytosine (5-hmC) (32), which is a mechanism for DNA demethylation. In addition, in our previous study, we found that IL-21 can increase TET2 enrichment on the promoter region of Bcl-6, which might explain the increased levels of Bcl-6 in lupus T cells (31). In addition, methylated H3K27 has been reported to prevent Tfh-related gene expression, while the H3K27me3 demethylase UTX sustains Tfh cells and antibody production (136). Positive histone modifications have been detected at the Bcl-6 locus in Tfh cells, but negative marks are present at Bcl-6 in other Th subsets (137). In addition, positive and negative histone modifications can be detected on Prdm1 in all Tfh cell populations. These positive and negative histone modifications might provide clues for Tfh cell plasticity. miRNAs, which are non-coding RNAs, regulate gene expression at the posttranscriptional and posttranslational levels. miRNAs silence gene expression by targeting the 3'-untranslated regions of mRNA, causing mRNA cleavage and translational repression. In Tfh cells, the miR-17-92 cluster has been observed to be downregulated, which might contribute to the overexpression of Bcl-6 (138). miR-155 can regulate Tfh cell accumulation in miR-146a-deficient mice, resulting in abnormal Tfh cell accumulation (138). miR-146a can directly targets ICOS and the overexpression of ICOS mediated by the loss of miR-146a results in spontaneous and cell-autonomous Tfh cell accumulation (139). The molecules regulating Tfh cell differentiation are summarized in Figure 2.
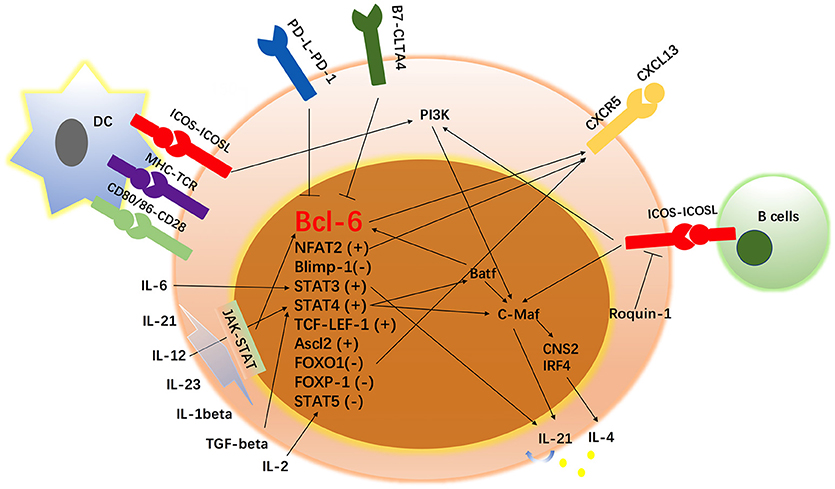
Figure 2. Network of transcription factors, cytokines and surface markers in Tfh cell regulation. In addition to the signals from surface markers, Tfh cells have been found to be regulated by a complex network of transcription factors, including the Bcl-6-Blimp1 axis, STAT1, STAT3, STAT4, STAT5, B-cell activating transcription factor (Batf), v-maf avian musculoaponeurotic fibrosarcoma oncogene homolog (c-Maf), interferon regulatory factor 4 (IRF4), Achaete-scute homolog 2 (Acl2), and T-cell-specific transcription factor 1 (TCF-1)-LEF-1, FOXO-1, FOXP-1, and NFAT-2. Since the study of Tfh cells began, some proteins have been identified to participate in the development of Tfh cells. In addition, cytokines such as IL-1 beta, IL-2, IL-6, IL-12, IL-21, IL-23, and TGF-β have been reported to be involved in the differentiation and survival of Tfh cells. “+” means positively regulates Tfh cell differentiation and “–” means negatively regulates Tfh cell development.
Increasing evidence has shown the plasticity of Tfh cells, which can be explained by epigenetic regulations. Tfh cells display repressive histone markings (H2K27me3) on Il4, Ifng, and Il17a, while permissive active chromatin H3K4me3 on Il21 locus (137, 140). Interestingly, the evidence that Tfh cells can produce effector T cell cytokines in response to the polarization cytokines and maintain the ability to produce IL-21 (137), can be explained by the fact that Tfh cells also display detectable H3K4me3 on Tbx21, Gata3, and Rorc locus (137). The positive H3K4me3 has been observed on the Bcl6 gene in Tfh cells from an in vivo and ex vivo system. Other in vitro differentiated Th cells also show permissive markers on Bcl6, which enables these cells to acquire Tfh cell phenotypes and the capacity to produce IL-21 (137).
Conclusions
Tfh cell differentiation is regulated by multiple transcription factors, receptors, cytokines, and epigenetic modifications. Unlike other Th cells, mouse Tfh cells are difficult to generate in vitro by cytokines and TCR stimulation, possibly reflecting a requirement for T-B cell interactions. ICOS/ICOSL signals might be an underlying explanation for the difficulty mentioned above. In addition, the cytokines driving differentiation in mouse and human systems are different; for example, TGF-β is a negative regulator in mice but a positive regulator in human Tfh cells. Tfh cells are heterogenic populations. Certain Th1, Th2, and Th17-like Tfh cells have been identified in GCs. In addition, Tfr cells have also been reported and regulate Tfh cell homeostasis. In addition, in certain inflammatory sites, such as synovium from rheumatoid arthritis patients, non-classic Tfh-like cells have been identified, which are CXCR5low but have high expression levels of Bcl-6, PD-1, and IL-21. Single-cell mRNA sequencing should facilitate studies aiming at dissecting Tfh cell subset heterogeneity and distribution in tissues and blood. Our understanding of epigenetic regulation of Tfh cells is limited. Due to the development of new technologies, new molecules might be identified in the near future.
Author Contributions
HW wrote the manuscript. YD, MZ, JZ, MZ, LL, and GC edited the manuscript. ZH and QL revised the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 81602767, No. 81430074, No. 91442116, No. 81373195, and No. 81771761), the National Basic Research Program of China (No. 2014CB541904), the Natural Science Foundation of Hunan Province (2017JJ3453, 2017SK2042, 2018JJ3756) the National Key Research and Development Program of China (2016YFC0903900), and the Natural Key Clinical Specialty Construction Project of National Health and Family Planning Commission of the People's Republic of China. Many thanks to Prof. Bengt Johansson Lindbom (Lund University, Sweden) for providing valuable suggestions for this review.
References
1. MacLennan IC, Gulbranson-Judge A, Toellner KM, Casamayor-Palleja M, Chan E, Sze DM, et al. The changing preference of T and B cells for partners as T-dependent antibody responses develop. Immunol Rev. (1997)156:53–66. doi: 10.1111/j.1600-065X.1997.tb00958.x
2. Zheng B, Han S, Kelsoe G. T helper cells in murine germinal centers are antigen-specific emigrants that downregulate Thy-1. J Exp Med. (1996)184:1083–91. doi: 10.1084/jem.184.3.1083
3. Bowen MB, Butch AW, Parvin CA, Levine A, Nahm MH. Germinal center T cells are distinct helper-inducer T cells. Hum Immunol. (1991)31:67–75. doi: 10.1016/0198-8859(91)90050-J
4. Kelly KA, Bucy RP, Nahm MH. Germinal center T cells exhibit properties of memory helper T cells. Cell Immunol. (1995)163:206–14. doi: 10.1006/cimm.1995.1118
5. Schaerli P, Willimann K, Lang AB, Lipp M, Loetscher P, Moser B. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J Exp Med. (2000)192:1553–62. doi: 10.1084/jem.192.11.1553
6. Breitfeld D, Ohl L, Kremmer E, Ellwart J, Sallusto F, Lipp M, et al. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J Exp Med. (2000)192:1545–52. doi: 10.1084/jem.192.11.1545
7. Johnston RJ, Poholek AC, DiToro D, Yusuf I, Eto D, Barnett B, et al. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science (2009)325:1006–10. doi: 10.1126/science.1175870
8. Yu D, Rao S, Tsai LM, Lee SK, He Y, Sutcliffe EL, et al. The transcriptional repressor Bcl-6 directs T follicular helper cell lineage commitment. Immunity (2009)31:457–68. doi: 10.1016/j.immuni.2009.07.002
9. Haynes NM, Allen CD, Lesley R, Ansel KM, Killeen N, Cyster JG. Role of CXCR5 and CCR7 in follicular Th cell positioning and appearance of a programmed cell death gene-1high germinal center-associated subpopulation. J Immunol. (2007)179:5099–108. doi: 10.4049/jimmunol.179.8.5099
10. Hardtke S, Ohl L, Forster R. Balanced expression of CXCR5 and CCR7 on follicular T helper cells determines their transient positioning to lymph node follicles and is essential for efficient B-cell help. Blood (2005)106:1924–31. doi: 10.1182/blood-2004-11-4494
11. Kim CH, Rott LS, Clark-Lewis I, Campbell DJ, Wu L, Butcher EC. Subspecialization of CXCR5+ T cells: B helper activity is focused in a germinal center-localized subset of CXCR5+ T cells. J Exp Med. (2001)193:1373–81. doi: 10.1084/jem.193.12.1373
12. Luther SA, Bidgol A, Hargreaves DC, Schmidt A, Xu Y, Paniyadi J, et al. Differing activities of homeostatic chemokines CCL19, CCL21, and CXCL12 in lymphocyte and dendritic cell recruitment and lymphoid neogenesis. J Immunol. (2002)169:424–33. doi: 10.4049/jimmunol.169.1.424
13. Hu J, Havenar-Daughton C, Crotty S. Modulation of SAP dependent T:B cell interactions as a strategy to improve vaccination. Curr Opin Virol. (2013)3:363–70. doi: 10.1016/j.coviro.2013.05.015
14. Crotty S. T follicular helper cell differentiation, function, and roles in disease. Immunity (2014)41:529–42. doi: 10.1016/j.immuni.2014.10.004
15. Yu D, Vinuesa CG. The elusive identity of T follicular helper cells. Trends Immunol. (2010)31:377–83. doi: 10.1016/j.it.2010.07.001
16. Walker LS, Gulbranson-Judge A, Flynn S, Brocker T, Raykundalia C, Goodall M, et al. Compromised OX40 function in CD28-deficient mice is linked with failure to develop CXC chemokine receptor 5-positive CD4 cells and germinal centers. J Exp Med. (1999)190:1115–22. doi: 10.1084/jem.190.8.1115
17. Xu H, Li X, Liu D, Li J, Zhang X, Chen X, et al. Follicular T-helper cell recruitment governed by bystander B cells and ICOS-driven motility. Nature (2013)496:523–7. doi: 10.1038/nature12058
18. Liu D, Xu H, Shih C, Wan Z, Ma X, Ma W, et al. T-B-cell entanglement and ICOSL-driven feed-forward regulation of germinal centre reaction. Nature (2015)517:214–8. doi: 10.1038/nature13803
19. Akiba H, Takeda K, Kojima Y, Usui Y, Harada N, Yamazaki T, et al. The role of ICOS in the CXCR5+ follicular B helper T cell maintenance in vivo. J Immunol. (2005)175:2340–8. doi: 10.4049/jimmunol.175.4.2340
20. Choi YS, Kageyama R, Eto D, Escobar TC, Johnston RJ, Monticelli L, et al. ICOS receptor instructs T follicular helper cell versus effector cell differentiation via induction of the transcriptional repressor Bcl6. Immunity (2011)34:932–46. doi: 10.1016/j.immuni.2011.03.023
21. Kawamoto S, Tran TH, Maruya M, Suzuki K, Doi Y, Tsutsui Y, et al. The inhibitory receptor PD-1 regulates IgA selection and bacterial composition in the gut. Science (2012)336:485–9. doi: 10.1126/science.1217718
22. Qi H, Cannons JL, Klauschen F, Schwartzberg PL, Germain RN. SAP-controlled T-B cell interactions underlie germinal centre formation. Nature (2008)455:764–9. doi: 10.1038/nature07345
23. Nurieva RI, Chung Y, Martinez GJ, Yang XO, Tanaka S, Matskevitch TD, et al. Bcl6 mediates the development of T follicular helper cells. Science (2009)325:1001–5. doi: 10.1126/science.1176676
24. Poholek AC, Hansen K, Hernandez SG, Eto D, Chandele A, Weinstein JS, et al. In vivo regulation of Bcl6 and T follicular helper cell development. J Immunol. (2010)185:313–26. doi: 10.4049/jimmunol.0904023
25. Hatzi K, Nance JP, Kroenke MA, Bothwell M, Haddad EK, Melnick A, et al. BCL6 orchestrates Tfh cell differentiation via multiple distinct mechanisms. J Exp Med. (2015)212:539–53. doi: 10.1084/jem.20141380
26. Barish GD, Yu RT, Karunasiri M, Ocampo CB, Dixon J, Benner C, et al. Bcl-6 and NF-kappaB cistromes mediate opposing regulation of the innate immune response. Genes Dev. (2010)24:2760–5. doi: 10.1101/gad.1998010
27. Crotty S, Johnston RJ, Schoenberger SP. Effectors and memories: Bcl-6 and Blimp-1 in T and B lymphocyte differentiation. Nat Immunol. (2010)11:114–20. doi: 10.1038/ni.1837
28. Ochiai K, Muto A, Tanaka H, Takahashi S, Igarashi K. Regulation of the plasma cell transcription factor Blimp-1 gene by Bach2 and Bcl6. Int Immunol. (2008)20:453–60. doi: 10.1093/intimm/dxn005
29. Wu H, Deng Y, Feng Y, Long D, Ma K, Wang X, et al. Epigenetic regulation in B-cell maturation and its dysregulation in autoimmunity. Cell Mol Immunol. (2018)15:676–84. doi: 10.1038/cmi.2017.133
30. Linterman MA, Rigby RJ, Wong RK, Yu D, Brink R, Cannons JL, et al. Follicular helper T cells are required for systemic autoimmunity. J Exp Med. (2009)206:561–76. doi: 10.1084/jem.20081886
31. Huang X, Wu H, Qiu H, Yang H, Deng Y, Zhao M, et al. The expression of Bcl-6 in circulating follicular helper-like T cells positively correlates with the disease activity in systemic lupus erythematosus. Clin Immunol. (2016)173:161–70. doi: 10.1016/j.clim.2016.10.017
32. Liu X, Lu H, Chen T, Nallaparaju KC, Yan X, Tanaka S, et al. Genome-wide analysis identifies Bcl6-controlled regulatory networks during T follicular helper cell differentiation. Cell Rep. (2016)14:1735–47. doi: 10.1016/j.celrep.2016.01.038
33. Gong D, Malek TR. Cytokine-dependent Blimp-1 expression in activated T cells inhibits IL-2 production. J Immunol. (2007)178:242–52. doi: 10.4049/jimmunol.178.1.242
34. Shaffer AL, Lin KI, Kuo TC, Yu X, Hurt EM, Rosenwald A, et al. Blimp-1 orchestrates plasma cell differentiation by extinguishing the mature B cell gene expression program. Immunity (2002)17:51–62. doi: 10.1016/S1074-7613(02)00335-7
35. Pepper M, Pagan AJ, Igyarto BZ, Taylor JJ, Jenkins MK. Opposing signals from the Bcl6 transcription factor and the interleukin-2 receptor generate T helper 1 central and effector memory cells. Immunity (2011)35:583–95. doi: 10.1016/j.immuni.2011.09.009
36. Oestreich KJ, Mohn SE, Weinmann AS. Molecular mechanisms that control the expression and activity of Bcl-6 in TH1 cells to regulate flexibility with a TFH-like gene profile. Nat Immunol. (2012)13:405–11. doi: 10.1038/ni.2242
37. McDonald PW, Read KA, Baker CE, Anderson AE, Powell MD, Ballesteros-Tato A, et al. IL-7 signalling represses Bcl-6 and the TFH gene program. Nat Commun. (2016) 7:10285. doi: 10.1038/ncomms10285
38. Nurieva RI, Podd A, Chen Y, Alekseev AM, Yu M, Qi X, et al. STAT5 protein negatively regulates T follicular helper (Tfh) cell generation and function. J Biol Chem. (2012)287:11234–9. doi: 10.1074/jbc.M111.324046
39. Johnston RJ, Choi YS, Diamond JA, Yang JA, Crotty S. STAT5 is a potent negative regulator of TFH cell differentiation. J Exp Med. (2012)209:243–50. doi: 10.1084/jem.20111174
40. Nurieva R, Yang XO, Martinez G, Zhang Y, Panopoulos AD, Ma L, et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature (2007)448:480–3. doi: 10.1038/nature05969
41. Ma CS, Avery DT, Chan A, Batten M, Bustamante J, Boisson-Dupuis S, et al. Functional STAT3 deficiency compromises the generation of human T follicular helper cells. Blood (2012)119:3997–4008. doi: 10.1182/blood-2011-11-392985
42. Nurieva RI, Chung Y, Hwang D, Yang XO, Kang HS, Ma L, et al. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity (2008)29:138–49. doi: 10.1016/j.immuni.2008.05.009
43. Liu X, Nurieva RI, Dong C. Transcriptional regulation of follicular T-helper (Tfh) cells. Immunol Rev. (2013)252:139–45. doi: 10.1111/imr.12040
44. Edelmann SL, Heissmeyer V. Tfh cell differentiation: missing Stat3 uncovers interferons' interference. Immunity (2014)40:307–9. doi: 10.1016/j.immuni.2014.02.008
45. Read KA, Powell MD, Baker CE, Sreekumar BK, Ringel-Scaia VM, Bachus H, et al. Integrated STAT3 and ikaros zinc finger transcription factor activities regulate Bcl-6 expression in CD4(+) Th cells. J Immunol. (2017)199:2377–87. doi: 10.4049/jimmunol.1700106
46. Schmitt N, Liu Y, Bentebibel SE, Munagala I, Bourdery L, Venuprasad K, et al. The cytokine TGF-beta co-opts signaling via STAT3-STAT4 to promote the differentiation of human TFH cells. Nat Immunol. (2014)15:856–65. doi: 10.1038/ni.2947
47. Choi YS, Eto D, Yang JA, Lao C, Crotty S. Cutting edge: STAT1 is required for IL-6-mediated Bcl6 induction for early follicular helper cell differentiation. J Immunol. (2013)190:3049–53. doi: 10.4049/jimmunol.1203032
48. Ray JP, Marshall HD, Laidlaw BJ, Staron MM, Kaech SM, Craft J. Transcription factor STAT3 and type I interferons are corepressive insulators for differentiation of follicular helper and T helper 1 cells. Immunity (2014)40:367–77. doi: 10.1016/j.immuni.2014.02.005
49. Zhang J, He Z, Sen S, Wang F, Zhang Q, Sun Z. TCF-1 Inhibits IL-17 gene Expression to restrain Th17 immunity in a stage-specific manner. J Immunol. (2018)200:3397–406. doi: 10.4049/jimmunol.1800193
50. Choi YS, Gullicksrud JA, Xing S, Zeng Z, Shan Q, Li F, et al. LEF-1 and TCF-1 orchestrate T(FH) differentiation by regulating differentiation circuits upstream of the transcriptional repressor Bcl6. Nat Immunol. (2015)16:980–90. doi: 10.1038/ni.3226
51. Xu L, Cao Y, Xie Z, Huang Q, Bai Q, Yang X, et al. The transcription factor TCF-1 initiates the differentiation of T(FH) cells during acute viral infection. Nat Immunol. (2015)16:991–9. doi: 10.1038/ni.3229
52. Wu T, Shin HM, Moseman EA, Ji Y, Huang B, Harly C, et al. TCF1 is required for the T follicular helper cell response to viral infection. Cell Rep. (2015)12:2099–110. doi: 10.1016/j.celrep.2015.08.049
53. Liu X, Chen X, Zhong B, Wang A, Wang X, Chu F, et al. Transcription factor achaete-scute homologue 2 initiates follicular T-helper-cell development. Nature (2014)507:513–8. doi: 10.1038/nature12910
54. Wing JB, Kitagawa Y, Locci M, Hume H, Tay C, Morita T, et al. A distinct subpopulation of CD25(-) T-follicular regulatory cells localizes in the germinal centers. Proc Natl Acad Sci USA. (2017) 114:E6400–E9. doi: 10.1073/pnas.1705551114
55. Qiu H, Wu H, Chan V, Lau CS, Lu Q. Transcriptional and epigenetic regulation of follicular T-helper cells and their role in autoimmunity. Autoimmunity (2017)50:71–81. doi: 10.1080/08916934.2017.1284821
56. Bauquet AT, Jin H, Paterson AM, Mitsdoerffer M, Ho IC, Sharpe AH, et al. The costimulatory molecule ICOS regulates the expression of c-Maf and IL-21 in the development of follicular T helper cells and TH-17 cells. Nat Immunol. (2009)10:167–75. doi: 10.1038/ni.1690
57. Kroenke MA, Eto D, Locci M, Cho M, Davidson T, Haddad EK, et al. Bcl6 and Maf cooperate to instruct human follicular helper CD4 T cell differentiation. J Immunol. (2012)188:3734–44. doi: 10.4049/jimmunol.1103246
58. Andris F, Denanglaire S, Anciaux M, Hercor M, Hussein H, Leo O. The transcription factor c-Maf promotes the differentiation of follicular helper T cells. Front Immunol. (2017) 8:480. doi: 10.3389/fimmu.2017.00480
59. Vijayanand P, Seumois G, Simpson LJ, Abdul-Wajid S, Baumjohann D, Panduro M, et al. Interleukin-4 production by follicular helper T cells requires the conserved Il4 enhancer hypersensitivity site V. Immunity (2012)36:175–87. doi: 10.1016/j.immuni.2011.12.014
60. Harada Y, Tanaka S, Motomura Y, Harada Y, Ohno S, Ohno S, et al. The 3' enhancer CNS2 is a critical regulator of interleukin-4-mediated humoral immunity in follicular helper T cells. Immunity (2012)36:188–200. doi: 10.1016/j.immuni.2012.02.002
61. Sahoo A, Alekseev A, Tanaka K, Obertas L, Lerman B, Haymaker C, et al. Batf is important for IL-4 expression in T follicular helper cells. Nat Commun. (2015) 6:7997. doi: 10.1038/ncomms8997
62. Ise W, Kohyama M, Schraml BU, Zhang T, Schwer B, Basu U, et al. The transcription factor BATF controls the global regulators of class-switch recombination in both B cells and T cells. Nat Immunol. (2011)12:536–43. doi: 10.1038/ni.2037
63. Ellyard JI, Vinuesa CG. A BATF-ling connection between B cells and follicular helper T cells. Nat Immunol. (2011)12:519–20. doi: 10.1038/ni.2042
64. Senga T, Iwamoto T, Humphrey SE, Yokota T, Taparowsky EJ, Hamaguchi M. Stat3-dependent induction of BATF in M1 mouse myeloid leukemia cells. Oncogene. (2002)21:8186–91. doi: 10.1038/sj.onc.1205918
65. Jabeen R, Goswami R, Awe O, Kulkarni A, Nguyen ET, Attenasio A, et al. Th9 cell development requires a BATF-regulated transcriptional network. J Clin Invest. (2013)123:4641–53. doi: 10.1172/JCI69489
66. Bao K, Carr T, Wu J, Barclay W, Jin J, Ciofani M, et al. BATF Modulates the Th2 locus control region and regulates CD4+ T cell fate during antihelminth immunity. J Immunol. (2016)197:4371–81. doi: 10.4049/jimmunol.1601371
67. Betz BC, Jordan-Williams KL, Wang C, Kang SG, Liao J, Logan MR, et al. Batf coordinates multiple aspects of B and T cell function required for normal antibody responses. J Exp Med. (2010)207:933–42. doi: 10.1084/jem.20091548
68. Huber M, Lohoff M. IRF4 at the crossroads of effector T-cell fate decision. Eur J Immunol. (2014)44:1886–95. doi: 10.1002/eji.201344279
69. Bollig N, Brustle A, Kellner K, Ackermann W, Abass E, Raifer H, et al. Transcription factor IRF4 determines germinal center formation through follicular T-helper cell differentiation. Proc Natl Acad Sci USA. (2012)109:8664–9. doi: 10.1073/pnas.1205834109
70. Kwon H, Thierry-Mieg D, Thierry-Mieg J, Kim HP, Oh J, Tunyaplin C, et al. Analysis of interleukin-21-induced Prdm1 gene regulation reveals functional cooperation of STAT3 and IRF4 transcription factors. Immunity (2009)31:941–52. doi: 10.1016/j.immuni.2009.10.008
71. Krishnamoorthy V, Kannanganat S, Maienschein-Cline M, Cook SL, Chen J, Bahroos N, et al. The IRF4 gene regulatory module functions as a read-write integrator to dynamically coordinate T helper cell fate. Immunity (2017)47:481–97 e7. doi: 10.1016/j.immuni.2017.09.001
72. Iwata A, Durai V, Tussiwand R, Briseno CG, Wu X, Grajales-Reyes GE, et al. Quality of TCR signaling determined by differential affinities of enhancers for the composite BATF-IRF4 transcription factor complex. Nat Immunol. (2017)18:563–72. doi: 10.1038/ni.3714
73. Myers DR, Lau T, Markegard E, Lim HW, Kasler H, Zhu M, et al. Tonic LAT-HDAC7 signals sustain Nur77 and Irf4 expression to tune naive CD4 T cells. Cell Rep. (2017)19:1558–71. doi: 10.1016/j.celrep.2017.04.076
74. Shi W, Man K, Smyth GK, Nutt SL, Kallies A. Whole transcriptome analysis for T cell receptor-affinity and IRF4-regulated clonal expansion of T cells. Genom Data (2014)2:396–8. doi: 10.1016/j.gdata.2014.10.019
75. Zeng H, Cohen S, Guy C, Shrestha S, Neale G, Brown SA, et al. mTORC1 and mTORC2 kinase signaling and glucose metabolism drive follicular helper T cell differentiation. Immunity (2016)45:540–54. doi: 10.1016/j.immuni.2016.08.017
76. Stone EL, Pepper M, Katayama CD, Kerdiles YM, Lai CY, Emslie E, et al. ICOS coreceptor signaling inactivates the transcription factor FOXO1 to promote Tfh cell differentiation. Immunity (2015)42:239–51. doi: 10.1016/j.immuni.2015.01.017
77. Wang H, Geng J, Wen X, Bi E, Kossenkov AV, Wolf AI, et al. The transcription factor Foxp1 is a critical negative regulator of the differentiation of follicular helper T cells. Nat Immunol. (2014)15:667–75. doi: 10.1038/ni.2890
78. Lee JY, Skon CN, Lee YJ, Oh S, Taylor JJ, Malhotra D, et al. The transcription factor KLF2 restrains CD4(+) T follicular helper cell differentiation. Immunity (2015)42:252–64. doi: 10.1016/j.immuni.2015.01.013
79. Weber JP, Fuhrmann F, Feist RK, Lahmann A, Al Baz MS, Gentz LJ, et al. ICOS maintains the T follicular helper cell phenotype by down-regulating Kruppel-like factor 2. J Exp Med. (2015)212:217–33. doi: 10.1084/jem.20141432
80. Vinuesa CG, Cook MC, Angelucci C, Athanasopoulos V, Rui L, Hill KM, et al. A RING-type ubiquitin ligase family member required to repress follicular helper T cells and autoimmunity. Nature (2005)435:452–8. doi: 10.1038/nature03555
81. Bertossi A, Aichinger M, Sansonetti P, Lech M, Neff F, Pal M, et al. Loss of Roquin induces early death and immune deregulation but not autoimmunity. J Exp Med. (2011)208:1749–56. doi: 10.1084/jem.20110578
82. Heissmeyer V, Vogel KU. Molecular control of Tfh-cell differentiation by Roquin family proteins. Immunol Rev. (2013)253:273–89. doi: 10.1111/imr.12056
83. Pratama A, Ramiscal RR, Silva DG, Das SK, Athanasopoulos V, Fitch J, et al. Roquin-2 shares functions with its paralog Roquin-1 in the repression of mRNAs controlling T follicular helper cells and systemic inflammation. Immunity (2013)38:669–80. doi: 10.1016/j.immuni.2013.01.011
84. Tan D, Zhou M, Kiledjian M, Tong L. The ROQ domain of Roquin recognizes mRNA constitutive-decay element and double-stranded RNA. Nat Struct Mol Biol. (2014)21:679–85. doi: 10.1038/nsmb.2857
85. Vogel KU, Edelmann SL, Jeltsch KM, Bertossi A, Heger K, Heinz GA, et al. Roquin paralogs 1 and 2 redundantly repress the Icos and Ox40 costimulator mRNAs and control follicular helper T cell differentiation. Immunity (2013)38:655–68. doi: 10.1016/j.immuni.2012.12.004
86. Lee SK, Silva DG, Martin JL, Pratama A, Hu X, Chang PP, et al. Interferon-gamma excess leads to pathogenic accumulation of follicular helper T cells and germinal centers. Immunity (2012)37:880–92. doi: 10.1016/j.immuni.2012.10.010
87. Ramiscal RR, Parish IA, Lee-Young RS, Babon JJ, Blagih J, Pratama A, et al. Attenuation of AMPK signaling by ROQUIN promotes T follicular helper cell formation. Elife (2015) 4:e08698. doi: 10.7554/eLife.08698
88. Ding Y, Li J, Yang P, Luo B, Wu Q, Zajac AJ, et al. Interleukin-21 promotes germinal center reaction by skewing the follicular regulatory T cell to follicular helper T cell balance in autoimmune BXD2 mice. Arthritis Rheumatol. (2014)66:2601–12. doi: 10.1002/art.38735
89. Xiao N, Eto D, Elly C, Peng G, Crotty S, Liu YC. The E3 ubiquitin ligase Itch is required for the differentiation of follicular helper T cells. Nat Immunol. (2014)15:657–66. doi: 10.1038/ni.2912
90. Mathew R, Mao AP, Chiang AH, Bertozzi-Villa C, Bunker JJ, Scanlon ST, et al. A negative feedback loop mediated by the Bcl6-cullin 3 complex limits Tfh cell differentiation. J Exp Med. (2014)211:1137–51. doi: 10.1084/jem.20132267
91. Rodriguez-Barbosa JI, Fernandez-Renedo C, Moral AMB, Buhler L, Del Rio ML. T follicular helper expansion and humoral-mediated rejection are independent of the HVEM/BTLA pathway. Cell Mol Immunol. (2017)14:497–510. doi: 10.1038/cmi.2015.101
92. Auderset F, Schuster S, Fasnacht N, Coutaz M, Charmoy M, Koch U, et al. Notch signaling regulates follicular helper T cell differentiation. J Immunol. (2013)191:2344–50. doi: 10.4049/jimmunol.1300643
93. Dell'Aringa M, Reinhardt RL. Notch signaling represents an important checkpoint between follicular T-helper and canonical T-helper 2 cell fate. Mucosal Immunol. (2018)11:1079–91. doi: 10.1038/s41385-018-0012-9
94. Fasnacht N, Huang HY, Koch U, Favre S, Auderset F, Chai Q, et al. Specific fibroblastic niches in secondary lymphoid organs orchestrate distinct Notch-regulated immune responses. J Exp Med. (2014)211:2265–79. doi: 10.1084/jem.20132528
95. Ansel KM, McHeyzer-Williams LJ, Ngo VN, McHeyzer-Williams MG, Cyster JG. In vivo-activated CD4 T cells upregulate CXC chemokine receptor 5 and reprogram their response to lymphoid chemokines. J Exp Med. (1999)190:1123–34. doi: 10.1084/jem.190.8.1123
96. Ngo VN, Korner H, Gunn MD, Schmidt KN, Riminton DS, Cooper MD, et al. Lymphotoxin alpha/beta and tumor necrosis factor are required for stromal cell expression of homing chemokines in B and T cell areas of the spleen. J Exp Med. (1999)189:403–12. doi: 10.1084/jem.189.2.403
97. Arnold CN, Campbell DJ, Lipp M, Butcher EC. The germinal center response is impaired in the absence of T cell-expressed CXCR5. Eur J Immunol. (2007)37:100–9. doi: 10.1002/eji.200636486
98. Vaeth M, Muller G, Stauss D, Dietz L, Klein-Hessling S, Serfling E, et al. Follicular regulatory T cells control humoral autoimmunity via NFAT2-regulated CXCR5 expression. J Exp Med. (2014)211:545–61. doi: 10.1084/jem.20130604
99. Vinuesa CG, Tangye SG, Moser B, Mackay CR. Follicular B helper T cells in antibody responses and autoimmunity. Nat Rev Immunol. (2005)5:853–65. doi: 10.1038/nri1714
100. Bentebibel SE, Schmitt N, Banchereau J, Ueno H. Human tonsil B-cell lymphoma 6 (BCL6)-expressing CD4+ T-cell subset specialized for B-cell help outside germinal centers. Proc Natl Acad Sci USA. (2011) 108:E488–97. doi: 10.1073/pnas.1100898108
101. Shulman Z, Gitlin AD, Targ S, Jankovic M, Pasqual G, Nussenzweig MC, et al. T follicular helper cell dynamics in germinal centers. Science (2013)341:673–7. doi: 10.1126/science.1241680
102. Qi H, Liu D, Ma W, Wang Y, Yan H. Bcl-6 controlled TFH polarization and memory: the known unknowns. Curr Opin Immunol. (2014)28:34–41. doi: 10.1016/j.coi.2014.01.016
103. Rolf J, Fairfax K, Turner M. Signaling pathways in T follicular helper cells. J Immunol. (2010)184:6563–8. doi: 10.4049/jimmunol.1000202
105. Bossaller L, Burger J, Draeger R, Grimbacher B, Knoth R, Plebani A, et al. ICOS deficiency is associated with a severe reduction of CXCR5+CD4 germinal center Th cells. J Immunol. (2006)177:4927–32. doi: 10.4049/jimmunol.177.7.4927
106. Tafuri A, Shahinian A, Bladt F, Yoshinaga SK, Jordana M, Wakeham A, et al. ICOS is essential for effective T-helper-cell responses. Nature (2001)409:105–9. doi: 10.1038/35051113
107. Dong C, Temann UA, Flavell RA. Cutting edge: critical role of inducible costimulator in germinal center reactions. J Immunol. (2001)166:3659–62. doi: 10.4049/jimmunol.166.6.3659
108. Boettler T, Choi YS, Salek-Ardakani S, Cheng Y, Moeckel F, Croft M, et al. Exogenous OX40 stimulation during lymphocytic choriomeningitis virus infection impairs follicular Th cell differentiation and diverts CD4 T cells into the effector lineage by upregulating Blimp-1. J Immunol. (2013)191:5026–35. doi: 10.4049/jimmunol.1300013
109. Boettler T, Moeckel F, Cheng Y, Heeg M, Salek-Ardakani S, Crotty S, et al. OX40 facilitates control of a persistent virus infection. PLoS Pathog. (2012) 8:e1002913. doi: 10.1371/journal.ppat.1002913
110. Jacquemin C, Schmitt N, Contin-Bordes C, Liu Y, Narayanan P, Seneschal J, et al. OX40 ligand contributes to human lupus pathogenesis by promoting T follicular helper response. Immunity (2015)42:1159–70. doi: 10.1016/j.immuni.2015.05.012
111. Pattarini L, Trichot C, Bogiatzi S, Grandclaudon M, Meller S, Keuylian Z, et al. TSLP-activated dendritic cells induce human T follicular helper cell differentiation through OX40-ligand. J Exp Med. (2017)214:1529–46. doi: 10.1084/jem.20150402
112. Tahiliani V, Hutchinson TE, Abboud G, Croft M, Salek-Ardakani S. OX40 cooperates with ICOS to amplify follicular Th cell development and germinal center reactions during infection. J Immunol. (2017)198:218–28. doi: 10.4049/jimmunol.1601356
113. Bardhan K, Anagnostou T, Boussiotis VA. The PD1:PD-L1/2 pathway from discovery to clinical implementation. Front Immunol. (2016) 7:550. doi: 10.3389/fimmu.2016.00550
114. Jogdand GM, Mohanty S, Devadas S. Regulators of Tfh cell differentiation. Front Immunol. (2016) 7:520. doi: 10.3389/fimmu.2016.00520
115. Sage PT, Francisco LM, Carman CV, Sharpe AH. The receptor PD-1 controls follicular regulatory T cells in the lymph nodes and blood. Nat Immunol. (2013)14:152–61. doi: 10.1038/ni.2496
116. Shi J, Hou S, Fang Q, Liu X, Liu X, Qi H. PD-1 controls follicular T helper cell positioning and function. Immunity (2018)49:264–74.e4. doi: 10.1016/j.immuni.2018.06.012.
117. Linterman MA, Pierson W, Lee SK, Kallies A, Kawamoto S, Rayner TF, et al. Foxp3+ follicular regulatory T cells control the germinal center response. Nat Med. (2011)17:975–82. doi: 10.1038/nm.2425
118. Wing JB, Ise W, Kurosaki T, Sakaguchi S. Regulatory T cells control antigen-specific expansion of Tfh cell number and humoral immune responses via the coreceptor CTLA-4. Immunity (2014)41:1013–25. doi: 10.1016/j.immuni.2014.12.006
119. Cannons JL, Qi H, Lu KT, Dutta M, Gomez-Rodriguez J, Cheng J, et al. Optimal germinal center responses require a multistage T cell:B cell adhesion process involving integrins, SLAM-associated protein, and CD84. Immunity (2010)32:253–65. doi: 10.1016/j.immuni.2010.01.010
120. Crotty S, Kersh EN, Cannons J, Schwartzberg PL, Ahmed R. SAP is required for generating long-term humoral immunity. Nature (2003)421:282–7. doi: 10.1038/nature01318
121. Czar MJ, Kersh EN, Mijares LA, Lanier G, Lewis J, Yap G, et al. Altered lymphocyte responses and cytokine production in mice deficient in the X-linked lymphoproliferative disease gene SH2D1A/DSHP/SAP. Proc Natl Acad Sci USA. (2001)98:7449–54. doi: 10.1073/pnas.131193098
122. Yusuf I, Kageyama R, Monticelli L, Johnston RJ, Ditoro D, Hansen K, et al. Germinal center T follicular helper cell IL-4 production is dependent on signaling lymphocytic activation molecule receptor (CD150). J Immunol. (2010)185:190–202. doi: 10.4049/jimmunol.0903505
123. Ueno H, Banchereau J, Vinuesa CG. Pathophysiology of T follicular helper cells in humans and mice. Nat Immunol. (2015)16:142–52. doi: 10.1038/ni.3054
124. Reinhardt RL, Liang HE, Locksley RM. Cytokine-secreting follicular T cells shape the antibody repertoire. Nat Immunol. (2009)10:385–93. doi: 10.1038/ni.1715
125. King IL, Mohrs M. IL-4-producing CD4+ T cells in reactive lymph nodes during helminth infection are T follicular helper cells. J Exp Med. (2009)206:1001–7. doi: 10.1084/jem.20090313
126. Glatman Zaretsky A, Taylor JJ, King IL, Marshall FA, Mohrs M, Pearce EJ. T follicular helper cells differentiate from Th2 cells in response to helminth antigens. J Exp Med. (2009)206:991–9. doi: 10.1084/jem.20090303
127. Liang HE, Reinhardt RL, Bando JK, Sullivan BM, Ho IC, Locksley RM. Divergent expression patterns of IL-4 and IL-13 define unique functions in allergic immunity. Nat Immunol. (2011) 13:58–66. doi: 10.1038/ni.2182
128. Yang Y, Ochando J, Yopp A, Bromberg JS, Ding Y. IL-6 plays a unique role in initiating c-Maf expression during early stage of CD4 T cell activation. J Immunol. (2005)174:2720–9. doi: 10.4049/jimmunol.174.5.2720
129. Eto D, Lao C, DiToro D, Barnett B, Escobar TC, Kageyama R, et al. IL-21 and IL-6 are critical for different aspects of B cell immunity and redundantly induce optimal follicular helper CD4 T cell (Tfh) differentiation. PLoS ONE (2011) 6:e17739. doi: 10.1371/journal.pone.0017739
130. Ma CS, Suryani S, Avery DT, Chan A, Nanan R, Santner-Nanan B, et al. Early commitment of naive human CD4+ T cells to the T follicular helper (T(FH)) cell lineage is induced by IL-12. Immunol Cell Biol. (2009)87:590–600. doi: 10.1038/icb.2009.64
131. Nakayamada S, Kanno Y, Takahashi H, Jankovic D, Lu KT, Johnson TA, et al. Early Th1 cell differentiation is marked by a Tfh cell-like transition. Immunity (2011)35:919–31. doi: 10.1016/j.immuni.2011.11.012
132. Wei L, Vahedi G, Sun HW, Watford WT, Takatori H, Ramos HL, et al. Discrete roles of STAT4 and STAT6 transcription factors in tuning epigenetic modifications and transcription during T helper cell differentiation. Immunity (2010)32:840–51. doi: 10.1016/j.immuni.2010.06.003
133. McCarron MJ, Marie JC. TGF-beta prevents T follicular helper cell accumulation and B cell autoreactivity. J Clin Invest. (2014)124:4375–86. doi: 10.1172/JCI76179
134. Kolenbrander A, Grewe B, Nemazee D, Uberla K, Temchura V. Generation of T follicular helper cells in vitro: requirement for B-cell receptor cross-linking and cognate B- and T-cell interaction. Immunology (2018)153:214–24. doi: 10.1111/imm.12834
135. Kitagawa Y, Wing JB, Sakaguchi S. Transcriptional and epigenetic control of regulatory T cell development. Prog Mol Biol Transl Sci. (2015)136:1–33. doi: 10.1016/bs.pmbts.2015.07.011
136. Cook KD, Shpargel KB, Starmer J, Whitfield-Larry F, Conley B, Allard DE, et al. T Follicular Helper cell-dependent clearance of a persistent virus infection requires T cell expression of the histone demethylase UTX. Immunity (2015)43:703–14. doi: 10.1016/j.immuni.2015.09.002
137. Lu KT, Kanno Y, Cannons JL, Handon R, Bible P, Elkahloun AG, et al. Functional and epigenetic studies reveal multistep differentiation and plasticity of in vitro-generated and in vivo-derived follicular T helper cells. Immunity (2011)35:622–32. doi: 10.1016/j.immuni.2011.07.015
138. Kang SG, Liu WH, Lu P, Jin HY, Lim HW, Shepherd J, et al. MicroRNAs of the miR-17 approximately 92 family are critical regulators of T(FH) differentiation. Nat Immunol. (2013)14:849–57. doi: 10.1038/ni.2648
139. Pratama A, Srivastava M, Williams NJ, Papa I, Lee SK, Dinh XT, et al. MicroRNA-146a regulates ICOS-ICOSL signalling to limit accumulation of T follicular helper cells and germinal centres. Nat Commun. (2015) 6:6436. doi: 10.1038/ncomms7436
Keywords: Tfh, Bcl-6, Blimp-1, transcription factors, epigenetics
Citation: Wu H, Deng Y, Zhao M, Zhang J, Zheng M, Chen G, Li L, He Z and Lu Q (2018) Molecular Control of Follicular Helper T cell Development and Differentiation. Front. Immunol. 9:2470. doi: 10.3389/fimmu.2018.02470
Received: 15 June 2018; Accepted: 05 October 2018;
Published: 25 October 2018.
Edited by:
Shahram Salek-Ardakani, Pfizer, United StatesReviewed by:
Betty Diamond, Feinstein Institute for Medical Research, United States (Sun Jung Kim, Northwell Health, United States, in collaboration with reviewer BD)Richard Lee Reinhardt, National Jewish Health, United States
Copyright © 2018 Wu, Deng, Zhao, Zhang, Zheng, Chen, Li, He and Lu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qianjin Lu, cWlhbmx1NTg2MEBnbWFpbC5jb20=; cWlhbmx1NTg2MEBjc3UuZWR1LmNu
 Haijing Wu1
Haijing Wu1 Qianjin Lu
Qianjin Lu