
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 30 October 2018
Sec. Vaccines and Molecular Therapeutics
Volume 9 - 2018 | https://doi.org/10.3389/fimmu.2018.02439
 Yaqi Wu1†
Yaqi Wu1† Ming Cai2†
Ming Cai2† Jilei Ma1
Jilei Ma1 Xindong Teng3
Xindong Teng3 Maopeng Tian1
Maopeng Tian1 Eman Borham Mohamed Borham Bassuoney1
Eman Borham Mohamed Borham Bassuoney1 Xionglin Fan1*
Xionglin Fan1*Adults are the leading population affected by tuberculosis (TB) epidemic and death. Developing an effective vaccine against adult TB is urgently needed. Mycobacterium bovis Bacillus Calmette-Guerin (BCG) prime-heterologous boost strategy has been explored extensively to protect adults against primary TB infection, but the majority of experimental regimens have not improved the protection primed by the BCG vaccine. The reason attributed to the failure remains unknown. In this study, CTT3H-based vaccines, namely DMT adjuvanted CTT3H subunit or DNA vaccine (pCTT3H-DMT), and recombinant adenovirus rAdCTT3H were constructed. Protective efficacy and immunogenicity of BCG prime-CTT3H based boosters were compared in C57BL/c mice models of primary or late persistent TB infection. Similar protective efficacy against early intranasal infection was provided by different CTT3H-based vaccines alone in vaccinated mice, and their protection was inferior to that of the BCG vaccine. In addition, CTT3H-based heterologous boosters did not enhance the protection conferred by the BCG vaccine against primary infection. However, all of these three boosters provided stronger protection against late persistent TB infection than BCG alone, regardless of vaccine types. Although BCG prime-boosters elicited Th1-biased responses to the antigen CTT3H, the number of CTT3H-sepcific IFN-γ-expressing TEM (CD62LloCD44hi) and IL-2-expressing TCM (CD62LhiCD44hi) cells in the spleen was not improved before exposure to Mycobacterium tuberculosis infection. In contrast, IFN-γ+ TEM and IL-2+ TCM cells in spleens, especially in lungs were significantly increased in BCG prime-boosters after exposure vaccination. Our results indicate that BCG prime-boost strategy might be a promising measure for the prevention against late persistent TB infection by induction of IFN-γ+ TEM and IL-2+ TCM cells in the lung, which can be used as alternative biomarkers for guiding the clinical practice and future development of TB vaccine for adults.
Despite significant progress in the pipeline for new diagnostics, drugs, and vaccine candidates, tuberculosis (TB) remains one of the deadliest killers among infectious diseases (1). As the only licensed vaccine for TB, Mycobacterium bovis Bacillus Calmette-Guerin (BCG) is recommended to vaccinate infants worldwide and can provide effective protection against several serious forms of TB in children even though its protection wanes with time and only lasts 10–15 years (2). Accordingly, BCG vaccine cannot control the prevalence and transmission of adult, mostly pulmonary TB. As estimated by WHO in 2017, there were ~ 10 million new TB cases, and 90% occurred in adults with only 10% in children (1). Furthermore, one-third of the world population is estimated to have latent TB infection (LTBI), of which 5–10% would progress to active TB during their lifetime (3). Therefore, there is an urgent need to develop an effective vaccine against adult TB.
Adult TB is primarily caused by endogenous reactivation of LTBI secondarily by exogenous contagion or reinfection. Vaccine candidates such as DNA and subunit vaccines, recombinant viral vectors, genetically modified BCG, or attenuated Mycobacterium tuberculosis strains have been explored extensively for adult TB (4). However, it remains elusive to replace the BCG vaccine because few vaccine candidates can provide vastly superior protection than the BCG vaccine in vaccinated animals (5). Furthermore, extensive research has been carried out on the heterologous boost strategy, aimed to extend the protection period primed by the BCG vaccine (5, 6). Unfortunately, almost half of heterologous boost regimens could not demonstrate better efficacy than the BCG vaccine alone against primary M. tuberculosis infection in various animal models, such as mice, guinea pigs, cattle, and non-human primates (5, 6). BCG prime and a modified vaccinia virus Ankara expressing antigen 85A (MVA85A) boost regimen might elicit a stronger Th1-typed immune response than the BCG vaccine alone, but MVA85A boosted BCG-primed infants did not show any improved efficacy against TB in a clinical trial (7). The reasons attributed to these failures remain unknown, thus significantly hindering the development of BCG prime-boost strategy.
Recently, we reported that a regimen of BCG prime and DMT (DDA-MPLA-TDB) adjuvanted multistage subunit protein WH121 showed a stronger ability to protect mice against M. tuberculosis post-exposure infection than BCG alone or BCG repeat vaccination in mice (8). Furthermore, subunit vaccine CMFO in adjuvant of DMT enhanced the BCG vaccine to thwart the reactivation of LTBI (9). Therefore, whether heterologous boost regimens are more efficacious to prevent against LTBI than primary infection remains to be investigated. CTT3H consists of five antigens of M. tuberculosis containing CD8+ epitopes: CFP10, TB10.4, TB8.4, Rv3165c, and HBHA (10). In addition, CFP10 and TB10.4-specific cytolytic CD8+T cells were found in the lung of mice as early as 3 weeks after infection (11). Besides CD8+ T cells, Rv3165c induced specific-CD4+T cells both in active TB patients and LTBI individuals (12). HBHA, expressed by both M. tuberculosis and BCG, is an adhesion molecule mediating adherence to epithelial cells and plays an important role in extrapulmonary dissemination (13). In this study, CTT3H was chosen to develop recombinant adenovirus vector, the adjuvant DMT emulsified DNA or protein subunit vaccines in this study. Differential protective efficacy of three vaccines against primary M. tuberculosis infection was compared with BCG in vaccinated C57BL/6 mice. More importantly, the immunogenicity and protection of BCG prime-boost regimens against primary infection and late persistent infection were compared and assessed in mouse models.
Recombinant plasmid pDC316-CTT3H, composed of the tandem-fusion of genes encoding the signal peptide of human tissue plasminogen activator (SPtPA) and the fusion protein CTT3H, was commercially synthesized (Life Technologies) (10). A recombinant replication-deficient adenovirus rAdCTT3H was packaged and constructed by co-transfecting plasmids pDC316-CTT3H and pBHGloxΔE1, 3 Cre into human embryonic kidney 293 (HEK293, ATCC® CRL-1573 ™) cells with Lipofactamine 2000 reagent (Invitrogen), as previously described (14). To construct DNA vaccine, the fusion gene was obtained by polymerase chain reaction (PCR) with the specific primers targeting to the plasmid pDC316-CTT3H and subcloned into the eukaryotic expression vector pVAX-1 (Thermo Fisher Scientific), as previously described (15). The resultant construction was named pCTT3H and the inserted gene was verified by enzyme digestion and DNA sequencing.
1 × 106 HEK293T cells (ATCC® CRL-3216 ™) were plated in each well of 6-well plates and cultured at 5% CO2, 37°C in Dulbecco's modified Eagle medium (DMEM) (Hyclone), supplemented with 10% fetal bovine serum (FBS) (Hyclone), 100 U/mL penicillin and streptomycin. 24 h later, cells were transfected with the plasmid pCTT3H using Lipofectamine 2000 reagent (Invitrogen). The pVAX1-transfected cells were used as a negative control. Alternatively, cells were infected with either rAdCTT3H or wild-type adenovirus serotype 5 Adnull (Neuron Biotech) at a multiplicity of infection (MOI) of 10 for 48 h. Then, supernatant and cell lysates were collected and prepared, respectively. Fifty micrograms of proteins from either supernatant or cell lysates were analyzed by 12% SDS-PAGE. Western blotting was explored to confirm the expression of the fusion protein CTT3H using mouse anti-CTT3H sera (10) as the primary antibody and peroxidase conjugated goat anti-mouse IgG (Proteintech Biotech) as the secondary antibody. The immunoblots were visualized using a BeyoECL Plus kit (Beyotime Biotech).
Both recombinant replication-deficient adenovirus serotype 5-based rAdCTT3H and recombinant eukaryotic expressing plasmid pCTT3H were successfully constructed (Figures 1A,B). Secretion of the recombinant fusion protein CTT3H with a size of about 61kDa was detected only in supernatants rather than cellular lysates generated from the rAdCTT3H infected- or the plasmid pCTT3H transfected- HEK293T cells, as confirmed by western blotting with anti-CTT3H mouse polyclonal antibody (Figures 1C,D). In contrast, no specific expression in culture supernatants or cellular lysates from Adnull or pVAX-1 treated HEK293T cells was detected, respectively (Figures 1C,D).
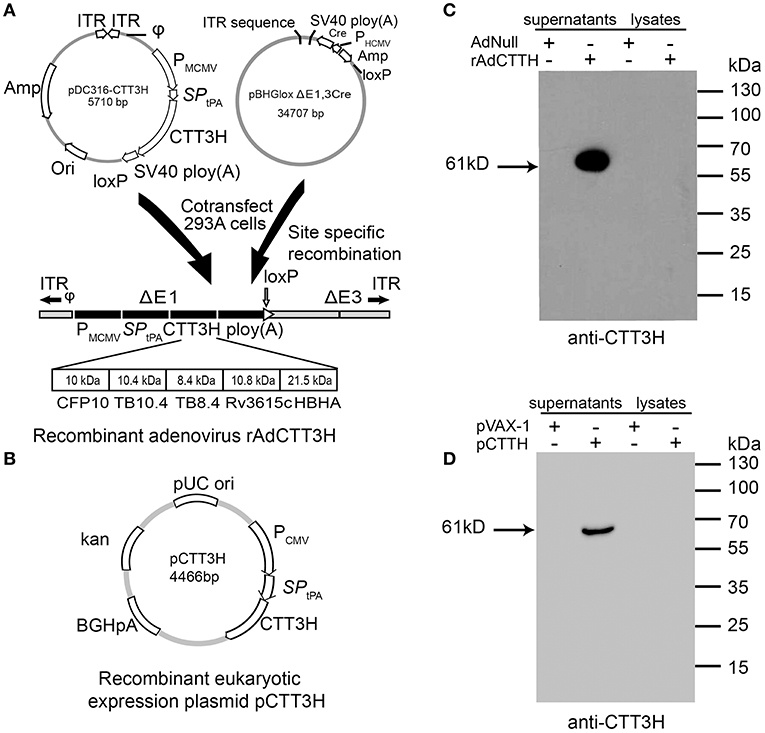
Figure 1. Construction and identification of rAdCTT3H and pCTT3H. (A) The structural diagram of recombinant replication-deficient adenovirus serotype 5-based rAdCTT3H. (B) The structural diagram of the recombinant eukaryotic expression plasmid pCTT3H. Identification of the recombinant protein CTT3H expressed in the supernatant or lysates of rAdCTT3H infected HEK293T cells (C) or pCTT3H transfected HEK293T cells (D) by western blotting with anti-CTT3H antibody.
The purification and quantitation of recombinant adenovirus rAdCTT3H, recombinant protein CTT3H, and recombinant eukaryotic plasmid pCTT3H was performed as previously described, respectively (10, 14, 15). The concentration of the endotoxin in each purified products was detected by ToxinSensorTM Chromogenic LAL Endotoxin Assay Kit (Genscript). The adjuvant DMT liposome was prepared by lipid film hydration method and rehydration in 10 mM sterile Tris-buffer (pH 7.4) as previously described (10). DMT adjuvanted subunit vaccine CTT3H-DMT and DNA vaccine pCTT3H-DMT were prepared by emulsifying 100 μL of the DMT liposome and 100 μL of either protein CTT3H (50 μg/100 μL) or plasmid pCTT3H solution (50 μg/100 μL), respectively.
Specific pathogen-free (SPF) C57BL/6 female mice, aged 6–8 weeks, were purchased from the Center for Animal Experiments of Wuhan University (Wuhan, China). Mice were randomly divided into different groups and were housed on the animal feeding cabinet (VentiRack) in an ABSL-3 laboratory. The immunization regimens were listed in Figure 2. For different regimens, a dose of 200 μL either pCTT3H-DMT or CTT3H-DMT was immunized intramuscularly (i.m.) twice at 3-weeks intervals. rAdCTT3H was immunized intranasally (i.n.) once with a dose of 5 × 108 PFU. 1 × 106 CFU of BCG China in a volume of 200 μL PBS was vaccinated subcutaneously (s.c.) once at the time of the first vaccination and used as a positive control. PBS, DMT, Adnull, or pVAX-1 alone were used as negative controls.
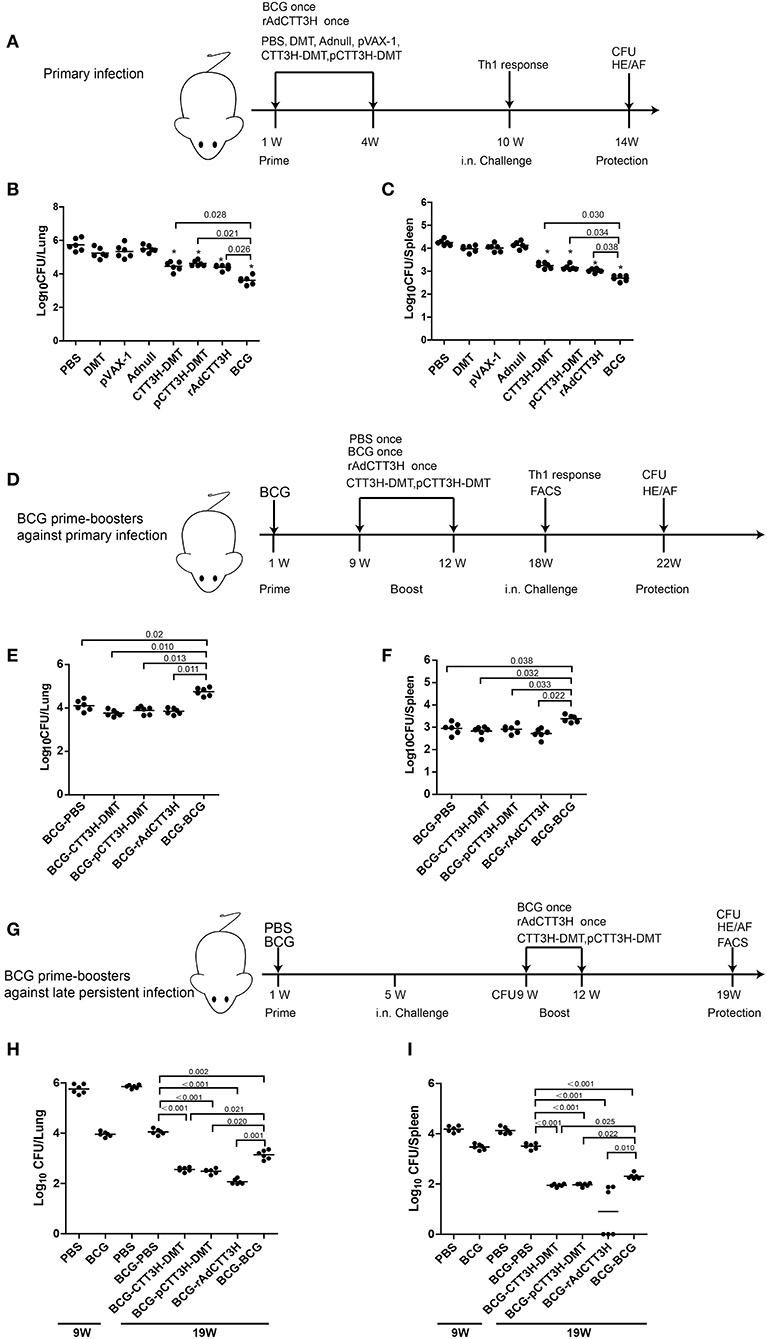
Figure 2. Comparison of protective efficacy among different regimens. (A) The immunization and challenge schedules of three CTT3H-based vaccines alone immunized C57BL/6 mice against primary M. tuberculosis infection (n = 6). C57BL/6 mice were immunized with CTT3H-DMT, pCTT3H-DMT, and rAdCTT3H, respectively. BCG was used as positive control. PBS, DMT, pVAX-1, or Adnull were used as negative controls. At the tenth week after immunization, mice were challenged i.n. with approximately 100 CFU virulent M. tuberculosis H37Rv strain. Four weeks post-challenge, spleen, and lung were aseptically removed from each mouse and the number of M. tuberculosis in both organs was cultured and enumerated. The results are shown as (mean ± SEM) log10 CFU/lung (B) or spleen (C) of different groups. (D) The protocol of BCG prime-CTT3H-based boosters in mice against primary infection (n = 6). Bacterial load in the lung (E) and spleen (F) of the different groups were shown as (mean ± SEM) log10 CFU/organ. (G) The protocol of BCG prime-CTT3H-based boosters against mice late persistent TB infection (n = 6). Bacterial load in the lung (H) and the spleen (I) of different groups was shown as (mean ± SEM) log10 CFU/organ. All experiments were repeated twice with similar results.
Mice models of primary infection and late persistent TB infection were established as previously described (8, 9). Vaccinated mice were infected i.n. with about 100 CFU live M. tuberculosis H37Rv as mentioned in different regimens (Figure 2). At different time-points post-challenge, mice were sacrificed. Lung and spleen from six mice in each group were aseptically removed, respectively. The bacterial load per organ was enumerated by plating serial dilutions of whole organ homogenates on Middlebrook 7H11 agar. When required, 2 μg/ml of 2-thiophenecarboxylic acid hydrazide (TCH, Beijing Luqiao Corp.) was added to selectively inhibit the growth of the residual BCG. The lung from three mice in each group was fixed in 4% paraformaldehyde solution, embedded in paraffin, sectioned and stained with hematoxylin and eosin (HE) and acid-fast (AF) staining. Lung histopathological scores of three mice in different groups were evaluated as previously described (9).
Serum from each mouse in different vaccinated groups was collected and the CTT3H-specific endpoint titers for IgG, IgG1, and IgG2a (Abcam) were tested by ELISA, as previously described (10).
2.5 × 106 of splenocytes were seeded into each well of 24-well microtiter plates and incubated with 10 μg/mL of CTT3H protein at 37°C in a humidified CO2 incubator for 72 h (10). Supernatants from each well were collected and a commercial mouse IFN-γ ELISA kit (Multi Sciences LTD) was used to measure the concentration of IFN-γ in the supernatants with a detection limit of 5 pg/ml. CTT3H-specifc IFN-γ concentration was calculated by subtracting the background of medium control. The results are showed as the mean ± SD (pg/mL) for each group (n = 6).
The CFSE method was used to evaluate the CTL effects against TB10.4 (Qiangyao Biotech) of different vaccinated mice as previously described (10) and analyzed by a flow cytometer (Figure S1). The specific rate of killing of in vivo CTL assay was calculated by the formula: 1 – (%CFSE high cells/%CFSE low cells) × 100 (10). The results are expressed as the mean ± SEM of six mice per group.
Total splenocytes or pneumonocytes from each mouse were prepared. These cells were stained for CD4 or CD8, intracellular cytokines IFN-γ and IL-2, and surface markers CD62L and CD44. The absolute number of IFN-γ+ and IL-2+ T cells, central memory T cells (TCM, CD62LhiCD44hi) and effector memory T cells (TEM, CD62LloCD44hi) was detected as previously described (9). The results are shown as mean ± SD per group (n = 6).
A two-tailed Student's T-test was performed for pair-wise comparisons with SPSS 19.0 software (SPSS 19.0, Chicago, IL, USA). One-way ANOVA test was used for analysis, when three or more experimental groups were compared, with Tukey's honestly significant difference (HSD) post-hoc test in SPSS 19.0. A value of p < 0.05 was considered significant.
To evaluate the effect of vaccine types on the protection, the protective efficacy against primary M. tuberculosis infection between DMT adjuvanted CTT3H subunit protein vaccine, DNA vaccine pCTT3H in adjuvant of DMT, and recombinant adenovirus rAdCTT3H vaccine were firstly compared in different vaccinated mice. At the tenth week after immunization, different vaccinated C57BL/6 mice were challenged i.n. with about 100 CFU of virulent M. tuberculosis H37Rv strain. Four weeks after infection, bacterial load in the lung, spleen, and lung histopathology were used to assess vaccine-induced protection (Figure 2A). Among all groups, PBS control mice, as well as Adnull and pVAX-1 groups, had the highest bacterial load in the both lung (Figure 2B) and spleen (Figure 2C) (p < 0.05), while the most significant inhibition of the growth of M. tuberculosis in both organs was obtained in BCG vaccinated mice (p < 0.05). Interestingly, both DMT adjuvanted CTT3H and pCTT3H vaccinated mice conferred much more significant protection than the DMT alone treated mice (p < 0.05). rAdCTT3H vaccinated mice also more significantly reduced the bacterial load in the lung and spleen than PBS or Adnull controls (p < 0.05). In addition, there was no statistical difference of organ bacterial load among rAdCTT3H, CTT3H-DMT, and pCTT3H-DMT groups.
To further investigate the boost effect and the effect of booster vaccine types on the BCG-primed protection against primary infection, mice were first vaccinated with the BCG vaccine and then boosted with three CTT3H-based vaccines at the ninth week. Nine weeks later, mice were challenged i.n. with about 100 CFU of virulent M. tuberculosis. Four weeks post-challenge, protection was evaluated in different groups (Figure 2D). BCG repeat vaccinated mice had the highest bacterial load in both lung (Figure 2E) and spleen (Figure 2F) of all groups. Interestingly, there was no statistical difference of the bacterial load in both lung and spleen between heterogeneous CTT3H boosters and the BCG-PBS control group, respectively. Therefore, our results suggest that different CTT3H-based vaccines could not enhance BCG primed mice to protect over the BCG vaccine alone against primary infection, regardless of vaccine types.
To explore the protective efficacy of heterologous boost strategy against LTBI, BCG primed mice were challenged with almost 100 CFU M. tuberculosis H37Rv at the fifth week after immunization (Figure 2G). Four weeks later, about 104 CFU and 103.5 CFU of M. tuberculosis, respectively, persisted in the lung and the spleen of BCG-primed mice and remained stable during the whole experimental period (Figures 2H,I). Consistent with the previous report (9), the mouse models of late persistent TB infection to mimic LTBI were established successfully, and then boosted with CTT3H-based vaccines or BCG. At the nineteenth week, bacterial load in both lung and spleen from heterologous boosted mice was decreased more significantly than the BCG-PBS control group (Figures 2H,I). In particular, almost half of rAdCTT3H-boosted mice significantly inhibited the growth of M. tuberculosis in their spleens (Figure 2I). Unlike protection against primary infection, repeated BCG vaccination also resulted in a more significant decrease in bacterial load of both lung and spleen than in the BCG-PBS group, even if that protection was inferior to those of heterologous boosters. Taken together, our results demonstrated that different CTT3H-based vaccines, regardless of vaccine types, could enhance BCG primed mice to reduce the bacteria load in the organs from late persistent but early stage of intranasal M. tuberculosis infection.
To assess the immunogenicity of different CTT3H-based vaccines, vaccinated mice were sacrificed for immunological analysis at the tenth week after immunization. PBS, DMT, Adnull, and pVAX-1 treated control mice did not produce any antibodies, including IgG, IgG1, or IgG2a against the recombinant protein CTT3H, as expected (Figure 3). In contrast, higher levels of CTT3H-specific IgG, IgG1, and IgG2a antibodies as well as the higher ratio of IgG2a/IgG1 were induced in mice vaccinated with all three CTT3H-based vaccines vs. BCG (p < 0.05) (Figures 3A–D). Among all groups, CTT3H-DMT elicited the highest titers of IgG, IgG1, and IgG2a, while rAdCTT3H vaccinated mice elicited the highest ratio of IgG2a/IgG1. In addition, splenocytes from control mice also did not secrete any INF-γ. Compared to control groups, BCG-vaccinated mice produced much higher levels of both CTT3H specific IFN-γ and CTL response to TB10.4. Both levels in three groups of CTT3H-based vaccinated mice were also much higher than those in the BCG group. Of all groups, the highest concentration of CTT3H specific IFN-γ was secreted by the rAdCTT3H group (Figure 3E), while the strongest CTL response to TB10.4 was detected in pCTT3H-DMT vaccinated mice (Figure 3F).
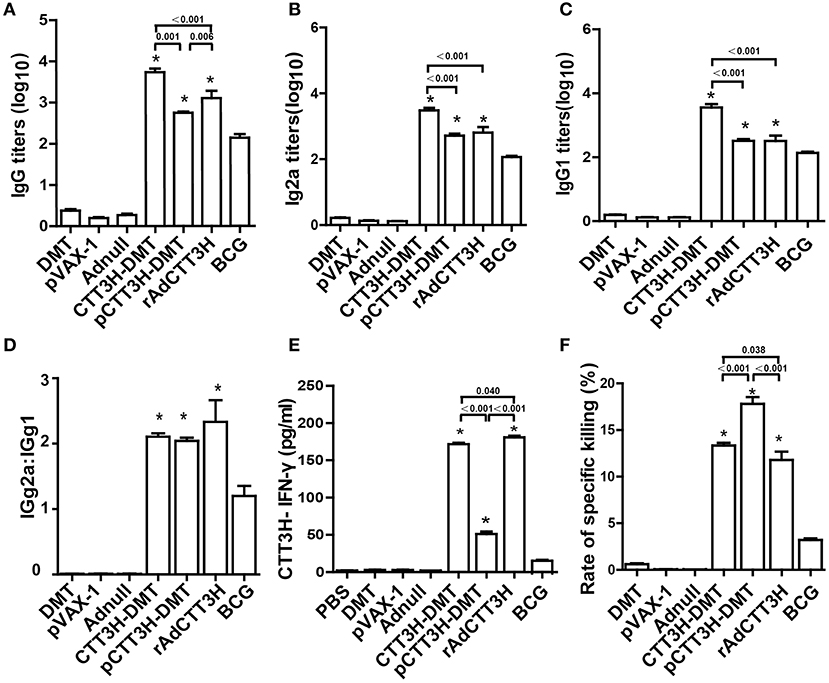
Figure 3. Comparison of CTT3H-specific Th1 responses among three CTT3H-based vaccines immunized C57BL/6 mice (n = 6). The immunization schedule was described in Figure 2A. At the tenth week after immunization, sera from each C57BL/6 mice of different groups were collected and titers of anti-CTT3H antibodies IgG (A), IgG2a (B), and IgG1 (C) were detected by ELISA. The results are shown as (mean ± SEM) log10 endpoint titers. (D) The ratio of IgG2a/IgG1 of different vaccinated groups. (E) The levels of CTT3H-specific IFN-γ secreted by splenocytes. The concentration of IFN-γ in supernatants was detected by ELISA and the results are expressed as mean ± SD (pg/mL). (F) Comparison of CTL response to TB10.4 among different vaccinated groups (mean ± SEM). *p < 0.05 vs. the BCG group. All experiments were repeated twice with similar results.
To investigate the immunological mechanism related with failures of boost strategy against primary infection, Th1-typed response was also analyzed in different vaccinated mice before challenge. Higher levels of anti-CTT3H IgG, IgG1, IgG2a, IgG2a/IgG1, IFN-γ, and CTL responses were induced in mice vaccinated with BCG and CTT3H-based boosters than the BCG-PBS control group (p < 0.05) (Figures 4A–D). Among three types of boosters, the highest level of IgG2a/IgG1 and the strongest IFN-γ response were obtained in rAdCTT3H boosted mice (Figures 4D,E), while the most significant CTL response to TB10.4 was detected in DMT adjuvanted DNA or protein boosted mice (Figure 4F). However, BCG repeat vaccination did not enhance any of these immunological biomarkers, compared to the BCG-PBS group.
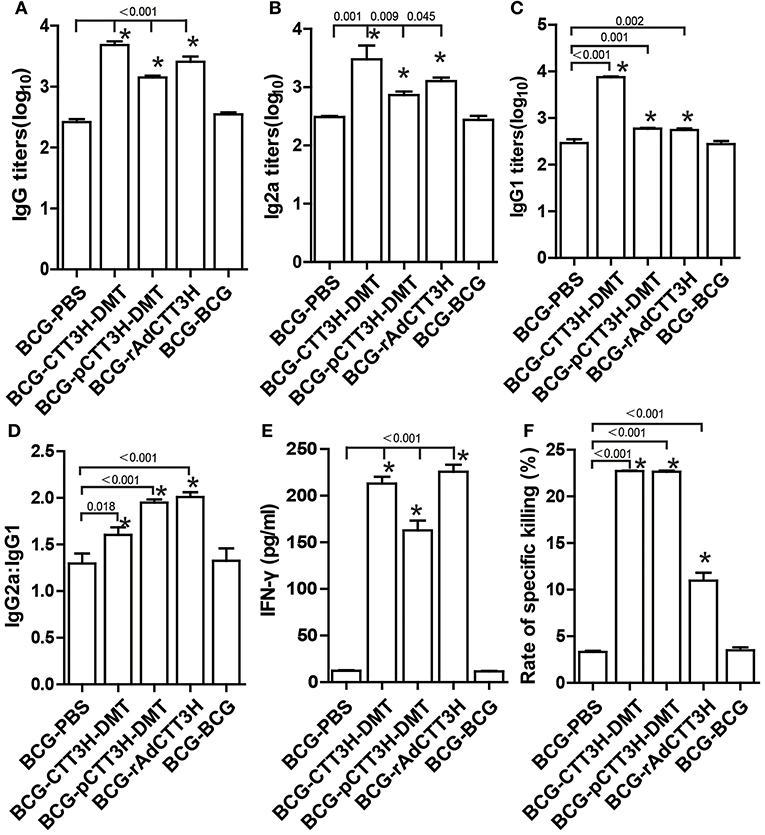
Figure 4. Th1 biased response induced by BCG prime-CTT3H-based boosters (n = 6). The immunization schedule was described in Figure 2D. ELISA was used to determine titers of anti-CTT3H IgG (A), IgG2a (B), and IgG1(C) in the serum from each C57BL/6 mouse in BCG prime-booster vaccinated groups at the eighteenth week. (D) The ratio of IgG2a/IgG1 of different vaccinated groups. (E) The levels of CTT3H-specific IFN-γ secreted by splenocytes (mean ± SD pg/mL). (F) CTL response to TB10.4 (mean ± SEM). *p < 0.05 vs. the BCG repeated vaccination group. All experiments were repeated twice with similar results.
IFN-γ+ TEM and IL-2+ TCM cells in the spleen are thought as effective biomarkers to predict the vaccine-induced early and persistent protection (8). To further establish the immunological mechanism related with the failure of boost strategy against primary infection, the number of CTT3H-specific IFN-γ or IL-2 secreting T cells (Figure 5A), IL-2-expressing TCM (CD62LhiCD44hi) cells, and IFN-γ-positive TEM (CD62LloCD44hi) cells (Figure 5B) in splenocytes between BCG prime-boosted mice was determined. BCG repeat vaccination more significantly decreased the number of IFN-γ+ or IL-2+ CD4+ T cells, and IFN-γ+ CD8+TEM cells in the spleen vs. BCG-PBS control mice (p < 0.05, Figure 5C). Interestingly, BCG prime-heterologous boosters also did not improve the levels of all biomarkers, but resulted in a more significant decrease of the levels of IFN-γ+ TEM cells, IL-2+ T cells, and IL-2+ TCM cells in the spleen, when compared with the respective booster control mice. Compared to the BCG-PBS control group, CTT3H-based boosters only more significantly increased the levels of IFN-γ+ T cells and IFN-γ+ TEM cells in the spleen (Figures 5D–F), regardless of vaccine types (Figure S2).
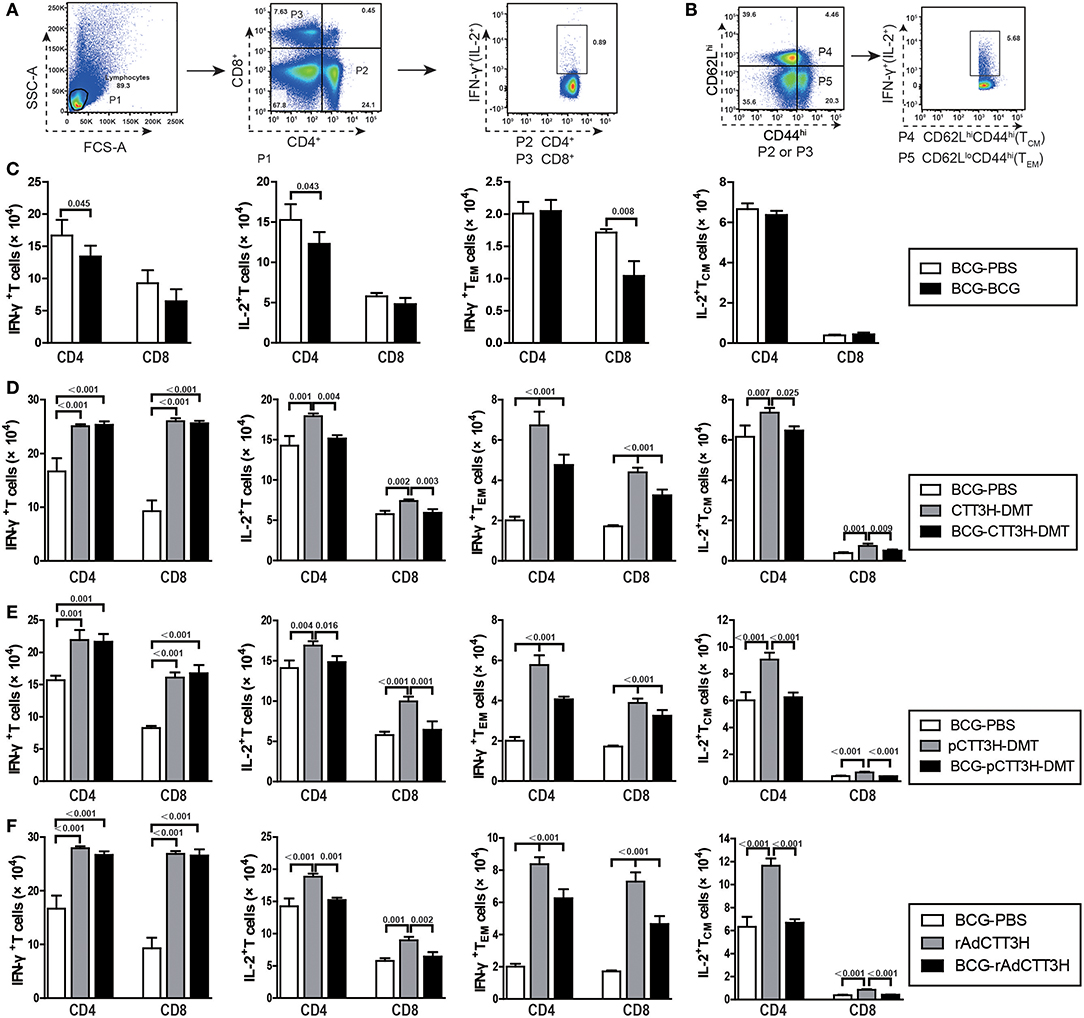
Figure 5. CTT3H antigen-specific T cells in spleens induced by BCG prime-CTT3H-based boosters before exposure (n = 6). The immunization protocol was described in Figure 2D. At the eighteenth week, splenocytes from heterologous boosters with PBS, CTT3H-DMT, pCTT3H-DMT, rAdCTT3H, and BCG vaccinated mice were collected and counted. 2.5 × 106 cells were seeded in each well of a 24-well plate and stimulated with CTT3H (10 μg/mL) as described in detail in the section of Materials and Methods. The absolute number of CTT3H-specific IFN-γ+ (or IL-2 +) CD4 + (or CD8 +) T cells, IFN-γ+ CD4 + (or CD8 +) TEM cells, and IL-2+ CD4 + (or CD8 +) TCM cells from spleens were detected by flow cytometry. Results are shown as mean ± SD (n = 6). (A,B) Flow cytometry analysis strategy, (C) comparing BCG prime and BCG repeated boost strategy, (D,F) comparing BCG prime, subunit vaccine prime, and BCG prime and boost with subunit vaccine including protein vaccine (D), DNA vaccine (E), and recombinant adenovirus vaccine (F).
To investigate immunological effects related with the protection against late persistent TB infection, IFN-γ+ or IL-2+ T cells, and memory T cells in the spleen were further analyzed 14 weeks post-challenge (Figure 6). Among all the treatment, repeat BCG vaccination induced the most significant increase in the number of IFN-γ+ or IL-2+ T cells, IFN-γ+ TEM and IL-2+ TCM cells in the spleen, except the levels of IL-2+ CD8+ T cells in both DMT adjuvanted boosters and IFN-γ+ TEM in CTT3H- DMT boost group (p < 0.05, Figure 6). Regardless of vaccine types (Figure S3A), heterologous boosters with CTT3H-based vaccines induced a more significant increase of all these biomarkers than BCG-PBS control group (Figure 8). In particular, IFN-γ+ T cells and IFN-γ+ TEM cells were dominated in the spleen of all BCG prime-CTT3H boost groups.
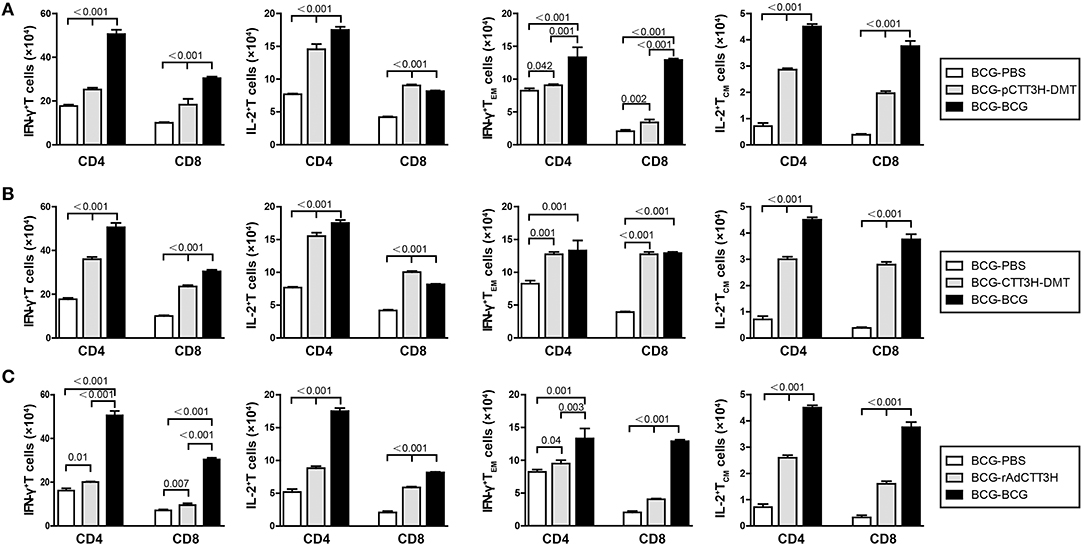
Figure 6. CTT3H antigen-specific T cells in spleens induced by BCG prime-CTT3H-based boosters after exposure (n = 6). The immunization and infection protocols were described in Figure 2G. At the nineteenth week, the absolute number of CTT3H-specific IFN-γ+ (or IL-2 +) CD4 + (or CD8 +) T cells, IFN-γ+ CD4 + (or CD8 +) TEM cells, and IL-2+ CD4 + (or CD8 +) TCM cells from spleens were detected by flow cytometry. Results are shown as mean ± SD (n = 6). (A–C) Comparing BCG prime, BCG repeated boosting, and BCG prime and boosted with subunit vaccine strategies including DNA vaccine (A), protein vaccine (B), and recombinant adenovirus vaccine (C).
Furthermore, sterilizing immunity is attributed to drive IFN-γ+ TEM and IL-2+ TCM cells from the spleen to the infected lung (9). Comparatively, the levels of IFN-γ+ or IL-2+ T cells, IFN-γ+ TEM, and IL-2+ TCM cells in the lung of each group were much lower than that in the respective spleen. Compared with BCG-PBS control mice, repeat BCG vaccination induced a more significant increase in the number of IFN-γ+ or IL-2+ T cells, IFN-γ+ TEM, and IL-2+ TCM cells in the lung (p < 0.05, Figure 7). Regardless of vaccine types (Figure S3B), heterologous boosters with CTT3H-based vaccines induced the most numbers of all these biomarkers, especially both IL-2+ TCM and IFN-γ+ TEM cells than BCG-PBS control group as well as repeat BCG vaccination (Figure 7). In addition, IFN-γ+ T cells and IFN-γ+ TEM cells of all biomarkers were dominated in the lung of all BCG prime-CTT3H boost groups.
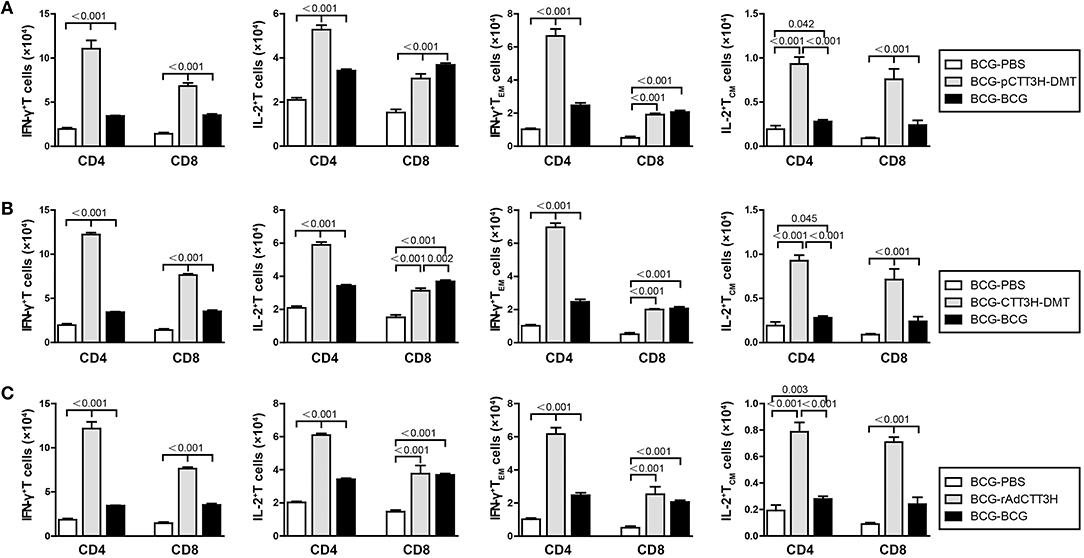
Figure 7. CTT3H antigen-specific T cells in lungs induced by BCG prime-CTT3H-based boosters after exposure (n = 6). The immunization and infection protocols were described in Figure 2G. At the nineteenth week, the absolute number of CTT3H-specific IFN-γ+ (or IL-2 +) CD4 + (or CD8 +) T cells, IFN-γ+ CD4 + (or CD8 +) TEM cells, and IL-2+ CD4 + (or CD8 +) TCM cells in pneumonocytes were detected by flow cytometry. Results are shown as mean ± SD (n = 6). (A–C) Comparing BCG prime, BCG repeated boosting, and BCG prime and boosted with subunit vaccine strategies including DNA vaccine (A), protein vaccine (B), and recombinant adenovirus vaccine (C).
As shown in Figure 8A, in the primary M. tuberculosis infection model, the most serious lung pathology with extensive fibrosis, perivasculitis, and alveolitis was obtained in all control groups, which also had the highest pathological score with acid-fast bacilli (AFB) throughout the whole lung tissue section. Compared to controls, three CTT3H-based vaccines displayed far fewer pathological lung changes with only a few AFB dispersed through the tissue section (Figure 8A). Consistent with the results of enumeration in organs, the lowest lung pathological score and the fewest AFB were found in the lung from BCG vaccinated mice among all groups (Figures 8A,B).
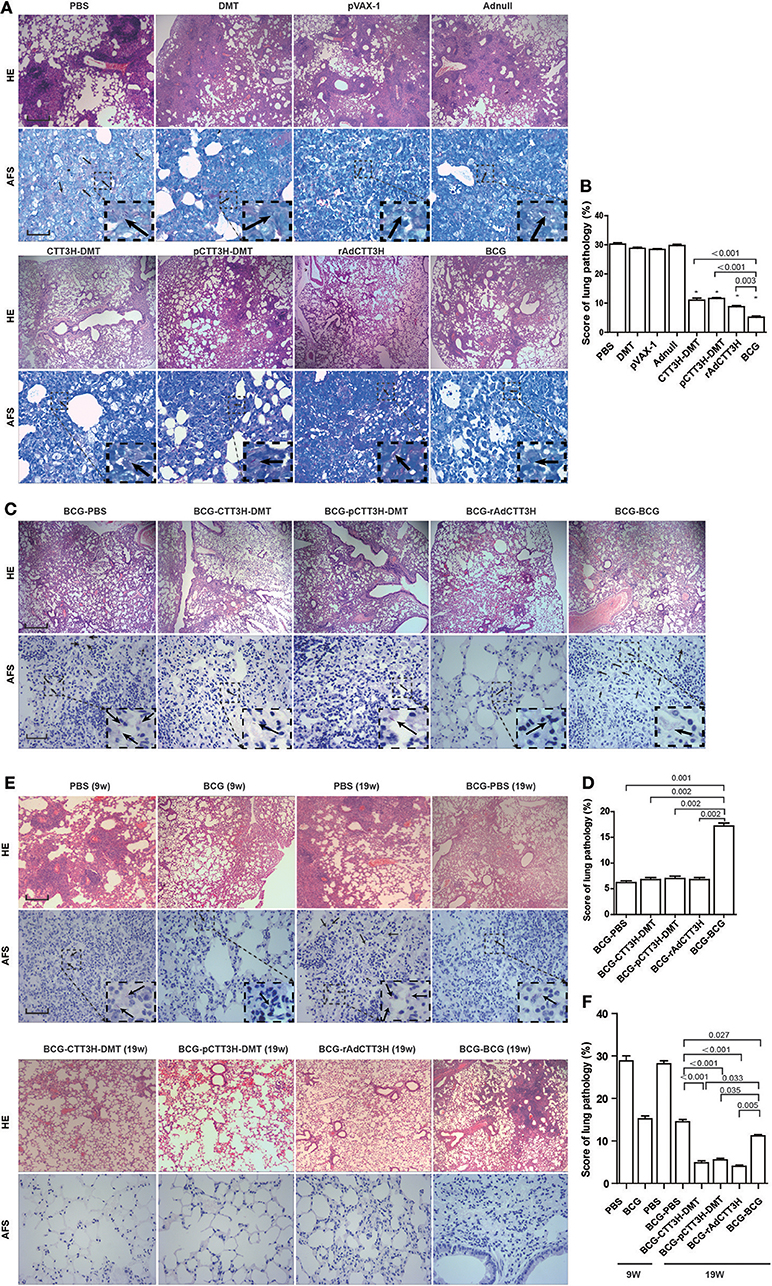
Figure 8. Comparison of histopathological changes in lungs among different regimens. The immunization and challenge schedules were shown in Figures 2A,D,G. Arrowheads indicate AF positive bacteria. HE, scale bar = 400 μm; AF staining, scale bar = 50 μm. (A) The representative lung pathological changes of three CTT3H-based vaccines alone immunized C57BL/6 mice against primary M. tuberculosis infection and (B) lung pathological scores (n = 3). (C) The representative lung pathological changes of BCG prime-CTT3H-based boosters in mice against primary infection and (D) lung pathological scores (n = 3). (E) The representative lung pathological changes of BCG prime-CTT3H-based boosters against mice late persistent TB infection and (F) lung pathological scores (n = 3).
When pre-exposure boost BCG with CTT3H-based vaccines to against primary M. tuberculosis infection, consistent with the results of bacterial enumeration, more AFB stained bacteria were observed in the lung section from BCG repeat vaccinated mice, which also showed the most serious pathological changes (Figures 8C,D) with extensive fibrosis, perivasculitis, and alveolitis (Figures 8C,D). However, heterogeneous CTT3H boosters did not enhance the protection of the BCG vaccine against late persistent TB when compared to BCG-PBS control group. Interestingly, compared with BCG repeat vaccination, mice boosted with CTT3H-based vaccines or PBS resulted in fewer pathological changes and fewer AFB present in the lung (Figures 8C,D).
While post-exposure boost BCG with CTT3H-based vaccines to against persistent M. tuberculosis infection, among all groups, the most serious lung pathology with extensive fibrosis was found in the BCG-PBS group, and a few AFB were found in their lung tissue sections. Compared with the BCG-PBS group, lower lung pathological scores and fewer AFB in the lung tissue were obtained in CTT3H-based vaccines boosted mice (Figures 8E,F), consistent with the results of HE staining. Together with these data, heterologous post-exposure boost BCG with CTT3H-based vaccines significant improve lung pathological changes of late persistent M. tuberculosis infected mice.
In this study, we compared the efficacy of different vaccine types, namely CTT3H-DMT, pCTT3H-DMT, and rAdCTT3H, as boosters to BCG vaccine-primed protection. In particular, immunogenicity and protection efficacy against primary TB infection and late persistent TB infection were evaluated. Our results demonstrated that similar protective efficacy was provided by CTT3H-DMT, pCTT3H-DMT, and rAdCTT3H alone in vaccinated mice against primary infection but that protection was inferior to that of the BCG vaccine. CTT3H-based vaccines as heterologous boosters did not enhance the protection primed by the BCG vaccine against primary infection, however, boost regimens conferred stronger protection against late persistent TB infection than BCG alone, regardless of vaccine types. Our findings demonstrate that a BCG prime-boost strategy might be a promising measure for the prevention against late persistent TB infection, rather than primary infection.
During past decades, BCG prime and heterologous boost strategy has been explored to protect adults against primary TB infection (6). Th1-biased response, especially increasing IFN-γ secreting CD4+T cells in the spleen of vaccinated mice were generally used as the biomarker to assess the immunogenicity of the strategy (5, 6). The majority of boosters (5, 6), as well as all heterologous boosters in this study, could elicit Th1-typed response, which cannot enhance the protection of the BCG vaccine against primary TB infection in mice. Therefore, it may not be a reliable biomarker to guide the development of booster vaccine for adult TB (16–18). In fact, cell-mediated immunity against M. tuberculosis always is slow to form when compared with other pulmonary pathogens such as Influenza virus (19) or Streptococcus pneumoniae (20), which benefits the replication of M. tuberculosis in the lung during the first 3 weeks after primary infection and subsequently promotes the establishment of a latent infection (21). IL-2+ TCM cells are located in secondary lymphoid tissue. They are characterized by highly expressing IL-2 and can turn into effector T cells when they encounter the antigen again (22). TEM cells are located in infection sites and can express IFN-γ very rapidly (22). Previously, we found that IFN-γ+ TEM cells and IL-2+ T cells, especially IL-2+ TCM cells in the spleen are related with the vaccine-induced protection (8). Further, sterilizing immunity plays a critical in the clearance of LTBI, which might be achieved by driving IFN-γ+ CD4+TEM and IL-2+ TCM cells to the infected lung (9). In this study, CTT3H-based boosters did not enhance the protection of the BCG vaccine against primary infection, which might contribute to the decreased levels of IFN-γ+ TEM and IL-2+ TCM cells in the spleen. A recent study also supported this conclusion that an improved protection conferred by an BCG prime-recombinant Sendai virus expressing antigen Ag85A and Ag85B boost regimen was related with the increasing levels of CD4+ TCM cells (23). Furthermore, both IFN-γ+ TEM cells and IL-2+ TCM cells were increased significantly in the spleen and lung by post-exposure vaccination, regardless of BCG or CTT3H-based vaccines as boosters. Correspondingly, BCG primed mice boosted with BCG or CTT3H-based vaccines strongly inhibited the growth of persistent M. tuberculosis and provided more significant protection against late persistent TB infection than the BCG vaccine alone. The improved protection of all boosters was related with increasing number of IFN-γ+ TEM cells and IL-2+ TCM cells in the infected lung, consistent with our previous reports (8, 9). However, BCG re-vaccination or CTT3H based-boosters cannot thwart the reactivation as CMFO/DMT does (9). Our results were also supported by other previous reports. For example, TCM cells are associated with LTBI, while TEM cells are related with active TB (24). Moreover, an experiment of adoptive transferring of T cells from “immune” or vaccinated mice into immunodeficient recipient mice also demonstrated that central memory-like CD4+ T cells (CD62Lhi) rather than effector memory T cells (CD62Llo) play an important role in the protection against TB (25). VPM1002, a modified BCG ΔureC::hly conferred superior protection against TB vs. BCG, which is also related with higher numbers of TCM cells (26). In addition, mice chronically infected with M. tuberculosis could elicit higher number of TCM cells than mice vaccinated with the BCG vaccine, which may contribute to the failure of the BCG vaccine to provide long-lasting protection (27). Taken together, IFN-γ+ TEM cells, and especially IL-2+ TCM cells and their distribution are more effective biomarkers to assess vaccine-induced protection, regardless of models of primary infection and late persistent infection.
In conclusion, the levels of IFN-γ+ TEM cells and IL-2+ TCM cells are related with vaccine-elicited protective efficacy and might be used as alternative biomarkers for management of LTBI or guiding preclinical and clinical trials of vaccine candidates. Antigens that can stimulate IL-2+ TCM cell responses should be suitable targets for controlling of LTBI. For example, ESAT-6 might not be a suitable antigen candidate, because it induces ESAT-6-specific T cells driven toward terminal differentiation in persistent TB infections (28). Several subunit vaccine candidates, such as CMFO (9), ID93 (29), and H56 (30) might provide longer-lasting protection against primary infection than the BCG vaccine alone in different animal models. In the future, they could be developed to replace the BCG vaccine for the immunization of infants. Therefore, our results have important implications in guiding the clinical practice and future development of TB vaccine for adults.
The study protocols involving animal experiments were performed in accordance with the guidelines of the Chinese Council on Animal Care. The Committee on the Ethics of Animal Experiments and the School Committee on Biosafety of Tongji Medical College (Wuhan, China) approved the research protocols.
YW and MC performed immunological detection, collection, and analysis of data. JM, XT, and MT performed the experiments with TB animal models. EB prepared the purification of antigen. XF designed the experiments, interpreted the data, and wrote the manuscript.
This work was supported by grants of the National Megaproject of Science Research for both the 13th 5-year Plan of China (No. 2018ZX10302302002-001) and the 12th 5-year Plan of China (No. 2012ZX10003008-005), and the National Natural Science Foundation of China (No.81772147). The funders had no role in study design, data collection, data analysis, interpretation, and writing of the report.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank Zhihui Liang (Department of Immunology, Huazhong University of Science and Technology) for FACS analysis, Qiaoyang Xian, Yan Rao, Qijie Zhang, and Ming Guo (Wuhan University) for their technical assistance with mouse model with primary TB infection.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2018.02439/full#supplementary-material
Figure S1. TB10.4 CD8+ peptide-pulsed, CFSE-labeled target cells were analyzed for in vivo CTL activities from different immunized C57BL/6 mouse (n = 6). Representative histograms of the peptide-pulsed splenocyte targets with the right and left peaks being CFSE-labeled TB10.4 peptide-pulsed splenocytes and unpulsed splenocytes analyzed by a flow cytometer, respectively.
Figure S2. Comparison of CTT3H antigen-specific T cells in spleens between BCG prime-different CTT3H-based boosters before exposure (n = 6).
Figure S3. Comparison of CTT3H antigen-specific T cells in spleens and lungs between BCG prime-different CTT3H-based boosters after exposure (n = 6). Comparison of CTT3H antigen-specific T cells in spleens (A) and lungs (B) between BCG prime-different CTT3H-based boosters after exposure.
1. WHO. Global Tuberculosis Report 2017. Geneva: United Nations, World Health Organization (WHO) (2017). Available online at: http://www.who.int/tb/publications/global_report/en/
2. Mangtani P, Abubakar I, Ariti C, Beynon R, Pimpin L, Fine PE, et al. Protection by BCG vaccine against tuberculosis: a systematic review of randomized controlled trials. Clin Infect Dis. (2014) 58:470–80. doi: 10.1093/cid/cit790
3. WHO. Guidelines on the Management of Latent Tuberculosis Infection. Geneva: World Health Organization (2015).
4. Kaufmann SH, Lange C, Rao M, Balaji KN, Lotze M, Schito M, et al. Progress in tuberculosis vaccine development and host-directed therapies–a state of the art review. Lancet Respir Med. (2014) 2:301–20. doi: 10.1016/S2213-2600(14)70033-5
5. Nieuwenhuizen NE, Kaufmann SHE. Next-generation vaccines based on Bacille Calmette-Guerin. Front Immunol. (2018) 9:121. doi: 10.3389/fimmu.2018.00121
6. Brennan MJ, Clagett B, Fitzgerald H, Chen V, Williams A, Izzo AA, et al. Preclinical evidence for implementing a prime-boost vaccine strategy for tuberculosis. Vaccine (2012) 30:2811–23. doi: 10.1016/j.vaccine.2012.02.036
7. Tameris MD, Hatherill M, Landry BS, Scriba TJ, Snowden MA, Lockhart S, et al. MVA85A 020 trial study team, safety and efficacy of MVA85A, a new tuberculosis vaccine, in infants previously vaccinated with BCG: a randomised, placebo-controlled phase 2b trial. Lancet (2013) 381:1021–8. doi: 10.1016/S0140-6736(13)60177-4
8. Ma J, Tian M, Fan X, Yu Q, Jing Y, Wang W, et al. Mycobacterium tuberculosis multistage antigens confer comprehensive protection against pre- and post-exposure infections by driving Th1-type T cell immunity. Oncotarget (2016) 7:63804–15. doi: 10.18632/oncotarget.11542
9. Ma J, Teng X, Wang X, Fan X, Wu Y, Tian M, et al. A multistage subunit vaccine effectively protects mice against primary progressive tuberculosis, latency and reactivation. EBioMed. (2017) 22:143–154. doi: 10.1016/j.ebiom.2017.07.005
10. Teng X, Tian M, Li J, Tan S, Yuan X, Yu Q, et al. Immunogenicity and protective efficacy of DMT liposome-adjuvanted tuberculosis subunit CTT3H vaccine. Hum Vaccin Immunother. (2015) 11:1456–64. doi: 10.1080/21645515.2015.1037057
11. Kamath AB, Woodworth J, Xiong X, Taylor C, Weng Y, Behar SM. Cytolytic CD8+ T cells recognizing CFP10 are recruited to the lung after Mycobacterium tuberculosis infection. J Exp Med. (2004) 200:1479–89. doi: 10.1084/jem.20041690
12. Millington KA, Fortune SM, Low J, Garces A, Hingley-Wilson SM, Wickremasinghe M, et al. Rv3615c is a highly immunodominant RD1 (Region of Difference 1)-dependent secreted antigen specific for Mycobacterium tuberculosis infection. Proc Natl Acad Sci USA. (2011) 108:5730–5. doi: 10.1073/pnas.1015153108
13. Temmerman S, Pethe K, Parra M, Alonso S, Rouanet C, Pickett T, et al. Methylation-dependent T cell immunity to Mycobacterium tuberculosis heparin-binding hemagglutinin. Nat Med. (2004) 10:935–41. doi: 10.1038/nm1090
14. Ma J, Lu J, Huang H, Teng X, Tian M, Yu Q, et al. Inhalation of recombinant adenovirus expressing granulysin protects mice infected with Mycobacterium tuberculosis. Gene Ther. (2015) 22:968–76. doi: 10.1038/gt.2015.73
15. Tian M, Zhou Z, Tan S, Fan X, Li L, Ullah N. Formulation in DDA-MPLA-TDB liposome enhances the immunogenicity and protective efficacy of a DNA vaccine against Mycobacterium tuberculosis infection. Front Immunol. (2018) 9:310. doi: 10.3389/fimmu.2018.00310
16. Mittrücker HW, Steinhoff U, Köhler A, Krause M, Lazar D, Mex P, et al. Poor correlation between BCG vaccination-induced T cell responses and protection against tuberculosis. Proc Natl Acad Sci USA. (2007) 104:12434–9. doi: 10.1073/pnas.0703510104
17. Kagina BM, Abel B, Scriba TJ, Hughes EJ, Keyser A, Soares A, et al. Specific T cell frequency and cytokine expression profile do not correlate with protection against tuberculosis after bacillus Calmette-Guérin vaccination of newborns. Am J Respir Crit Care Med. (2010) 182:1073–79. doi: 10.1164/rccm.201003-0334OC
18. Orr MT, Windish HP, Beebe EA, Argilla D, Huang PW, Reese VA, et al. Interferon γ and tumor necrosis factor are not essential parameters of CD4+ T-cell responses for vaccine control of tuberculosis. J Infect Dis. (2015) 212:495–504. doi: 10.1093/infdis/jiv055
19. Ho AW, Prabhu N, Betts RJ, Ge MQ, Dai X, Hutchinson PE, et al. Lung CD103+ dendritic cells efficiently transport influenza virus to the lymph node and load viral antigen onto MHC class I for presentation to CD8 T cells. J Immunol. (2011) 187:6011–21. doi: 10.4049/jimmunol.1100987
20. Kirby AC, Newton DJ, Carding SR, Kaye PM. Evidence for the involvement of lung-specific gammadelta T cell subsets in local responses to Streptococcus pneumoniae infection. Eur J Immunol. (2007) 37:3404–13. doi: 10.1002/eji.200737216
21. Ernst DJ. The immunological life cycle of tuberculosis. Nat Rev Immunol. (2012) 12:581–91. doi: 10.1038/nri3259
22. Seder RA, Darrah PA, Roederer M. T-cell quality in memory and protection: implications for vaccine design. Nat Rev Immunol. (2008) 8:247–58. doi: 10.1038/nri2274
23. Hu Z, Gu L, Li CL, Shu T, Lowrie DB, Fan XY. The profile of T cell responses in Bacille Calmette-Guérin-primed mice boosted by a novel sendai virus vectored anti-tuberculosis vaccine. Front Immunol. (2018) 9:1796. doi: 10.3389/fimmu.2018.01796
24. Pathakumari B, Devasundaram S, Raja A. Altered expression of antigen-specific memory and regulatory T-cell subsets differentiate latent and active tuberculosis. Immunology (2018) 153:325–36. doi: 10.1111/imm.12833
25. Kipnis A, Irwin S, Izzo AA, Basaraba RJ, Orme IM. Memory T lymphocytes generated by Mycobacterium bovis BCG vaccination reside within a CD4 CD44lo CD62 ligand(hi) population. Infect Immun. (2005) 73:7759–64. doi: 10.1128/IAI.73.11.7759-7764.2005
26. Vogelzang A, Perdomo C, Zedler U, Kuhlmann S, Hurwitz R, Gengenbacher M, et al. Central memory CD4+ T cells are responsible for the recombinant Bacillus Calmette-Guerin DeltaureC::hly vaccine's superior protection against tuberculosis. J Infect Dis. (2014) 210:1928–37. doi: 10.1093/infdis/jiu347
27. Henao-Tamayo MI, Ordway DJ, Irwin SM, Shang S, Shanley C, Orme IM. Phenotypic definition of effector and memory T-lymphocyte subsets in mice chronically infected with Mycobacterium tuberculosis. Clin Vaccine Immunol. (2010) 17:618–25. doi: 10.1128/CVI.00368-09
28. Moguche AO, Musvosvi M, Penn-Nicholson A, Plumlee CR, Mearns H, Geldenhuys H, et al. Antigen availability shapes T cell differentiation and function during tuberculosis. Cell Host Microbe (2017) 21:695–706. doi: 10.1016/j.chom.2017.05.012
29. Bertholet S, Ireton GC, Ordway DJ, Windish HP, Pine SO, Kahn M, et al. A defined tuberculosis vaccine candidate boosts BCG and protects against multidrug-resistant Mycobacterium tuberculosis. Sci Transl Med. (2010) 2:53ra74. doi: 10.1126/scitranslmed.3001094
Keywords: BCG, heterologous boost, memory T cells, early stage of tuberculosis, late persistent tuberculosis
Citation: Wu Y, Cai M, Ma J, Teng X, Tian M, Bassuoney EBMB and Fan X (2018) Heterologous Boost Following Mycobacterium bovis BCG Reduces the Late Persistent, Rather Than the Early Stage of Intranasal Tuberculosis Challenge Infection. Front. Immunol. 9:2439. doi: 10.3389/fimmu.2018.02439
Received: 17 July 2018; Accepted: 02 October 2018;
Published: 30 October 2018.
Edited by:
Jeffrey K. Actor, University of Texas Health Science Center at Houston, United StatesReviewed by:
Juraj Ivanyi, King's College London, United KingdomCopyright © 2018 Wu, Cai, Ma, Teng, Tian, Bassuoney and Fan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xionglin Fan, eGxmYW5AaHVzdC5lZHUuY24=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.