- Organ Transplantation Unit, First Affiliated Hospital of Sun Yat-sen University, Guangzhou, China
The liver is one of the most important immunological organs that remains tolerogenic in homeostasis yet promotes rapid responses to pathogens in the presence of a systemic infection. The composition of leucocytes in the liver is highly distinct from that of the blood and other lymphoid organs, particularly with respect to enrichment of innate T cells, i.e., invariant NKT cells (iNKT cells) and Mucosal-Associated Invariant T cells (MAIT cells). In recent years, studies have revealed insights into their biology and potential roles in maintaining the immune-environment in the liver. As the primary liver-resident immune cells, they are emerging as significant players in the human immune system and are associated with an increasing number of clinical diseases. As such, innate T cells are promising targets for modifying host defense and inflammation of various liver diseases, including viral, autoimmune, and those of tumor origin. In this review, we emphasize and discuss some of the recent discoveries and advances in the biology of innate T cells, their recruitment and diversity in the liver, and their role in various liver diseases, postulating on their potential application in immunotherapy.
Introduction
The liver is a primary internal organ that plays a unique role in pathogen defense. Approximately 1/3 of total blood passes through the liver every minute (1, 2). Once the blood enters, it circulates at a reduced flow rate through the sinusoids, which comprise a complex vascular network of capillary-like vessels. The reduced flow rate maximizes the opportunity for pathogens to recognize the hepatic immune environment.
The ability to restrict and eliminate invading pathogens is one of the main features of the immune system. Much hepatology literature has focused on the adaptive, antigen-specific classical T-cell populations and their role in the protection and pathogenesis of liver disease. However, human liver is selectively enriched in innate T cells, including natural killer T (NKT) cells (3) and Mucosal-Associated Invariant T (MAIT) cells (4, 5). These innate T cells are unconventional T cells with diverse functions that play an essential role in liver immune surveillance. Similar to other innate cells, recognition of antigens from microbial, endogenous glycolipids or metabolites activates innate T cells, allowing them to produce cytokines and cytolytic proteins (4, 5). One of the most important features of innate T cells is bridging the innate and adaptive immune response, and various types of cytokines produced after innate T cell activation can modulate CD4+ and CD8+ T cell immune response (6). During liver injury, innate T cells infiltrating into the inflammatory site after neutrophils and monocytes are proposed to be sensors that control the local immune response (7). Although innate T cells have previously been defined primarily by phenotypic markers, recent emerging evidence has revealed considerable functional complexity in this population. This review summarizes the current literature regarding iNKT, type II NKT and MAIT cells, which have important roles in a variety of liver diseases, particularly focusing on their role in human liver diseases.
The Biology of Innate T Cells
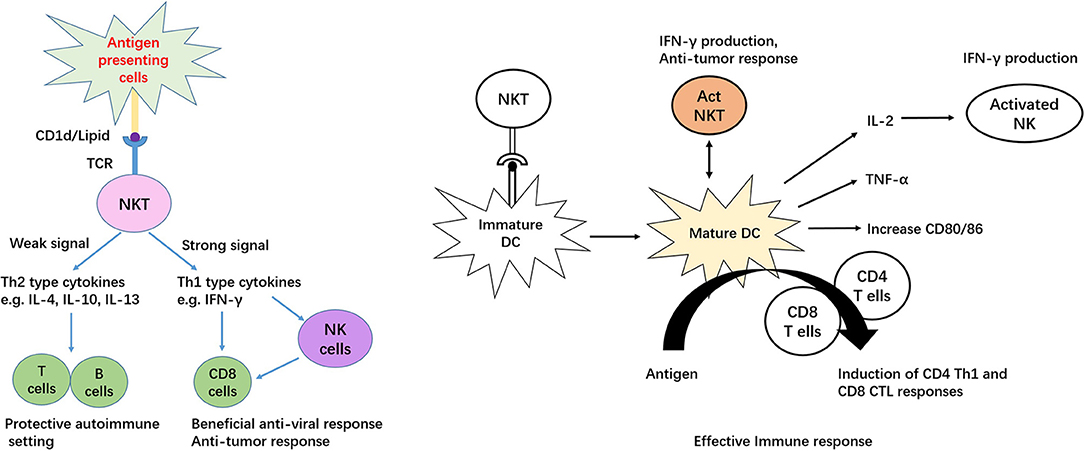
iNKT cells, also known as type I NKT cells or classic NKT cells, are a subset of natural killer T (NKT) cells, while another subset of NKT cells is type II NKT cells. iNKT cells are characterized by signatures of both T and NK cells, including broad range expression of molecular markers that are typically associated with NK cells, for example, NK1.1 in mouse and CD161/CD56 in human (8, 9). Notably, these markers alone may be insufficient to distinguish iNKT cells, because in mouse, NK1.1 is not expressed in certain strains, including AKR, BALB/c, CBA/J, C3H, DBA/1, DBA/2, NOD, SJL, and 129 (10). There are reasonable amounts of MAIT cells in human peripheral blood and liver that also express CD56 (11); therefore, co-staining with CD1d tetramer should precisely identify iNKT populations. The so-called invariant is based on limited TCR arrangement, Vα14Jα18/Vβ2, Vβ7, and Vβ8 in mouse and Vα24Jα18/Vβ11 in human (12, 13). Mouse and human iNKT cells can recognize lipid and glycolipid antigens of self or microbial origin presented on MHC class-I-like CD1d molecules (14, 15). Activation by the agonist α-galactosylceramide (α-Galcer) allows mice and human iNKT cells to readily proliferate, undergoing significant remodeling of their surface expression patterns with regard to several markers, such as NK1.1 and the semi-invariant TCR, resulting in production of abundant Th1, Th2 and Th17 type cytokines, including IFN-gamma, IL-4, IL-13, and IL-17 (16–18). Apart from the TCR dependent pathway, human iNKT cells can recognize and eliminate target cells expressing NKG2D ligands in a TCR-independent manner (19). Cytokines released by stimulated iNKT cells are able to transactivate other innate and innate-like immune cell subsets, thereby amplifying their initial responses (20–24). In addition, iNKT cells provide both antigen-specific cognate and non-cognate aid to B cells (25) and in turn, are activated by B cells (26, 27). Interestingly, unlike the non-cognate iNKT cell–B cell interactions Figure 1, antigen-specific cognate iNKT cells induce a more innate-biased B cell response characterized by a discontinuous germinal center B cell expansion and rapid initial proliferation of IL-10-producing B cells that fails to induce humoral memory (28).
There are three functional subsets of iNKT cells in mouse and human, which produce a distinct combination of cytokines and lineage-specific transcription factors, namely, NKT1, NKT2, and NKT17. Murine studies have demonstrated that T-box 21 (T-bet), GATA binding protein-3 (GATA3), and retinoic acid receptor-related orphan nuclear receptor gamma (RORγt) are expressed on iNKT1, iNKT2, and iNKT17 cells, respectively, and these transcription factors are correlated with the function of iNKT cells (29). Mirroring T helper cell subtypes, iNKT1, iNKT2, and iNKT17 cells produce IFN-γ, IL-4, and IL-17 (29). These cytokines allow iNKT cells to interact with other immune cells. For example, IL-4 produced by NKT2 cells at steady state through phosphorylation of signal transducer and activator of transcription 6 (STAT6) regulates CD8 T cells developing to a memory-like phenotype in the thymus (30). Additionally, IL-4 promotes antibody production by B cells and induces dendritic cells to secrete T helper (Th) 2-type chemokines, such as chemokine (C-C motif) ligand (CCL) 17 and CCL22 (30). Moreover, in lymphoid organs, each iNKT subset displays different anatomic localization, which determines their responsiveness to intravenous or oral antigenic challenges (31). Recently, RNAseq analysis has suggested that each iNKT subset has a unique genetic signature, and these footprints are more similar to γδ T cells and innate lymphoid cells (ILCs) than to conventional T cells (32).
Compared to iNKT cells, type II NKT cells express relatively diverse TCRs that can recognize antigens derived from microbial, endogenous glycolipids, phospholipids and endogenous hydrophobic peptides presented by CD1d molecules (33). Type II NKT cells are unable to recognize α-linked glycolipids, for example, α-Galcer, but are responded to β-linked glycolipids (34). Igor Maricic et al. found that mice type II NKT cells are activated by self-phospholipids, for example, lysophosphatidylcholine (LPC), lysosphingomyelin (LSM) and lyso-platelet-activating factor (LPAF) (34). According to their function, type II NKT cells can be divided into pro-inflammatory and anti-inflammatory subsets (34). Stimulating type II NKT cells with self-glycolipid sulfatide inhibits inflammatory responses induced by CD4+ T (35) and iNKT cells (36) in mice. In a mouse bone marrow transplantation model, donor type II NKT cells inhibit graft-vs.-host disease via releasing IL-4 (37). In a rat model, activating type II NKT cells with SCP2 peptide promotes the inflammatory response through production of inflammatory cytokines IL-5 and IL-6 (38). In an iNKT cell-deficient (Jα18−/−) mouse model, Sagami et al. found adoptive transfer of type II NKT cells exacerbated DSS-induced colitis (39).
MAIT cells are a subset of αβ T cells that possess both innate and effector-like qualities (40, 41). They are preferentially located in the gut lamina propria and express an invariant α chain (42). Similar to other αβ T cells, MAIT cells undergoing conventional TCR arrangement express canonical Vα7.2-Jα33 TCRs paired with variable β-chains in human (43, 44) and Vα19-Jα33 TCR in mice (42). In general, MAIT cells are equipped with effector properties before exiting from the thymus (45, 46). These cells were initially discovered as invariant α chain-expressing cells in the double-negative T cell fraction of human peripheral blood by Steven Porcelli et al. in 1993 (47). Later studies found that MAIT cells express high levels of CD161 and are also present in CD4-positive, as well as CD8-positive, lymphocytes (48).
Distribution of MAIT cells differs between humans and mice. The frequency of MAIT cells in C57BL/6 mice accounts for ~0.1% of the peripheral T cell population (49), whereas 1–10% of them are identified in human peripheral blood. Moreover, the occurrence of MAIT cells varies widely among tissues in healthy adults, ranging from 2% (ileum) to 60% (jejunum), and they can make up ~20–50% of intrahepatic T cells.
Interestingly, MAIT cells are absent in germ-free mice, indicating that their expansion in the periphery depends on the presence of microbial ligands (40, 42). MAIT cells are MR1, an MHC class I-like protein, restricted-T cells. Recognizing bacterial-produced vitamin B metabolites presented by MR1 allows MAIT cells to secrete a vast amount of pro-inflammatory cytokines, including IFN-γ, TNF-α, IL-2, and IL-17 (11, 41, 50), which lyse bacterially infected cells (51, 52). Additionally, comparative genomic analysis has demonstrated that MR1 is only expressed in marsupial and placental mammals and is exceptionally highly conserved, particularly at the α1 and α2 domains of ligand-binding grooves (53). Both MR1 and MAIT TCR genes are extremely highly conserved, implying that evolutionary pressure is involved in maintaining conservation of these genes (42).
MAIT cells can defend against microbial activity and infections caused by bacteria or yeast through activating the vitamin B2/riboflavin pathway in an innate manner (42, 45, 54). A study by Michael S. Bennett et al. provided evidence that supernatants from stimulated human MAIT cells promote B cell plasmablast differentiation and IgA, IgG, and IgM production (55). Additionally, human MAIT cells respond to mycobacterium tuberculosis infection and provide an early source of IFN-γ required for activation of the Th1 response (56). Together, these results indicate the potentially important role of MAIT cells in the defense against microbial invasion.
CD1D Restricted T Cells in Liver Health and Disease
iNKT cells contribute to a significant subset of lymphocytes in the liver. In mice, iNKT cells are most abundant in the liver (10–30%), with lower frequencies found in the thymus, blood, bone marrow and lymph nodes (0.1–0.2%). In humans, high iNKT cell numbers are detected in the liver (1%) (57, 58), compared to 0.01–0.5% in their peripheral counterparts (59, 60). The distribution of type II NKT cells is difficult to investigate due to their lack of specific surface markers (61). The literature suggests that type II NKT cells may outnumber iNKT cells in humans (61).
iNKT cells mediate various functions in the liver, including hepatic injury, fibrogenesis, and carcinogenesis. As one of the important immune subsets in the liver, iNKT cells demonstrate a pathogenic role in IRI, primary biliary cirrhosis, non-alcoholic fatty liver, and hepatitis. Interestingly, a protective role was identified for these cells in an acute liver injury model (Figure 2). To date, literature appears to suggest that iNKT cells exert a protective role during the acute phase of liver injury and a pathogenic role in chronic conditions (62). Some attention has also been focused on the implication of NKT cells in liver transplantation, including their role in ischaemia/reperfusion injury and transplantation rejection (63, 64). The study of type II NKT cells in liver disease progression is limited, focusing on hepatitis viral infection, where type II NKT cells appear to play a controversial role in controlling liver injury. A new subset of type II NKT cells, II NKT-Tfh cells, have been found to regulate metabolic lipid disorders (65).
Hepatocellular Carcinoma
HCC is often linked to chronic inflammatory liver diseases, such as NASH and viral hepatitis (66). A murine model suggested that TLR4 and canonical nuclear factor-κB signaling in the liver facilitate NASH-to-HCC conversion. Fundamentally, iNKT cells have a dual role in cancer that either promotes an anti-tumor response or elevates tumor growth via activation of effector T cells promoting Th1 responses or recruitment of regulatory T cells to induce Th2 responses (67, 68). Although hepatic iNKT cells are rich in number, relatively few studies have attempted to clarify their role in HCC, and results from these studies appear contradictory.
HCC patients exhibit increased iNKT cell numbers in the tumor site compared to the peripheral blood. More importantly, hepatic iNKT cells are found to secrete Th2 cytokines, thus inhibiting tumor-specific CD8+ T-cell responses (66, 69). In contrast, murine studies identified that CD4+ iNKT cells could mediate anti-tumor responses through inhibition of the inflammatory response triggered by activation of the oncogenic β-catenin pathway (70). Additionally, iNKT cells are able to suppress tumor growth after adoptive transfer of HCC tumor lines in mice (71, 72).
Very recently, a study by Ma et al. found that commensal bacteria are important regulators of anti-tumor immunity that alter hepatic natural killer T cells. This regulation strengthens IFN-gamma production by hepatic natural killer T cells and promotes anti-tumor effects (73).
Current studies have suggested that type II NKT cells may play an immune regulatory role in cancer settings. In CD1d knockout and Jα18 knockout mice, Terabe et al. found that activation of CD1d-restricted type II NKT cells is sufficient for downregulation of tumor immunosurveillance in mouse fibrosarcoma, mammary carcinoma, colon carcinoma, and lung metastases of the CT26 colon carcinoma models (74). Using the same knockout method, Renukaradhya et al. demonstrated that type II NKT cells supress anti-tumor immunity against B-cell lymphoma (75). In addition, there are higher frequencies of IL-13 releasing type II NKT cells in myeloma patients than in healthy donors (76).
Hepatitis Viral Infection (HCV and HBV)
Overall, 57% of liver cirrhosis cases and 78% of liver cancers are caused by chronic HBV and HCV infections, accounting for almost a million deaths every year. A few studies have attempted to identify the role of iNKT cells in controlling HCV infections, in particular, during the initial phase of infection. In human hepatic CD3+CD56+ cells, including iNKT cells, HCV replication is inhibited in hepatocytes by IFN-γ secretion, and this activity is positively correlated with disease progression (77). Furthermore, it modulates the effectiveness of IFN-alpha in late HCV infection. However, studies have shown that iNKT cells are considerably depleted in chronic HCV infection (78–80). This finding may suggest that iNKT cells contribute to the early phase of HCV infection but not as much to disease progression.
Similarly, high numbers of activated type I NKT cells have been identified in the early stages of HBV infection in humans (78–80). In agreement with those results, CD1d expression is elevated in HBV+ve liver tissue compared to HBV–ve counterparts (81). Similar to their action in HCV infections, the inhibitory effect of iNKT cells on HBV occurs through secretion of IFN-γ as well, activating the adaptive immune response and inhibiting viral replication (82). Recently, Xu and colleagues have shown that exhaustion marker Tim-3 is upregulated on hepatic iNKT cells from HBV-transgenic mice (83). Blockade of Tim-3 by anti-Tim-3 antibody strongly enhances expression of IL-4, IFN-γ, TNFα, and CD107a in iNKT cells and augments α-Galcer-induced inhibition of HBV replication (83). Interestingly, researchers have found that on the one hand, iNKT cells control the replication of hepatic viruses, while on the other hand, they contribute to virally induced liver injury through production of pro-inflammatory cytokines that induce hepatocyte apoptosis and inhibit proliferation (84–86).
The function of type II NKT cells in hepatitis viral infection is debatable. In a ConA-induced mouse hepatitis model, activation of type II NKT cells with sulfatide or LPC evoke anergy in iNKT cells that suppresses inflammation-triggered liver damage (34). In contrast, hepatic type II NKT cells promote development of liver injury in a transgenic mouse model of acute hepatitis B virus infection (87). Stimulation of type II NKT cells triggers conventional T-cell activation and pro-inflammatory cytokine production, resulting in augmentation of hepatic injury in murine autoimmune hepatitis models (88).
Non-Alcoholic Fatty Liver Disease
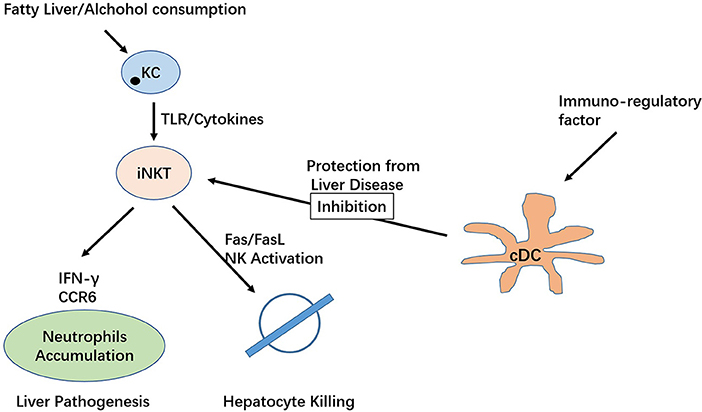
In the modern era, NAFLD is considered the most frequent chronic liver disease in developed countries, affecting ~10–20% of the population. NAFLD is characterized by abnormal accumulation of fat in the liver, leading to infiltration of inflammatory cells accompanied by fibrosis or necrosis progressing to liver cirrhosis or hepatocellular carcinoma (HCC) (89, 90). Current studies on systemic analysis of iNKT cell subsets in non-alcoholic fatty liver disease are very limited, with a few studies confirming their importance. In human hepatic CD1d cells, the number of CD3+CD56+ cells are elevated in NASH patients (91). Reduced iNKT cell counts were found in mice fed high-fat diets and in obese mice (92, 93). In addition, mice with NAFLD lacking iNKT cells showed increased pro-inflammatory mediator factor and increased levels of TLR4 and PDGF2 mRNA (94). Activation of Kupffer cells (KCs) could cause apoptosis in these cells and further contribute to steatosis and insulin resistance (92, 95). Depletion of KCs could reduce hepatic IL-12 expression and rescue iNKT cells from apoptosis, preventing further pathological changes in the disease. Tim-3/galectin-9 is known to regulate the homeostasis of liver iNKT cells in the murine system (96).
Indeed, depletion of KCs via treatment with gadolinium chloride reduces hepatic IL-12 expression and does not lead to iNKT apoptosis, thereby preventing diet-induced hepatic steatosis and insulin resistance. Consistently, activation of the Hedgehog pathway and HSCs have been revealed to be associated with iNKT cells in mice fed an MCD diet or a combination of a CD-HFD (97–99). Using a diet-induced mouse obesity model, Satoh and colleague show that type II NKT cells trigger inflammation in the liver and exacerbate obesity (100).
Alcoholic Liver Disease
ALD is caused by chronic alcohol abuse resulting in alcoholic fibrosis or cirrhosis. The disease currently one of the most frequent causes of death. Activation of KCs via LPS/TLR signaling-dependent mechanisms following alcohol consumption result in increased secretion of a variety of pro-inflammatory cytokines and chemokines, in addition to eicosanoids and reactive oxygen species (101, 102). Mechanisms underlying ALD include a complex network of hepatocytes, KCs, DCs and innate T cells (103). Studies found activation of KCs via the LPS/TLR pathway following alcohol intake, which increases secretion of a variety of pro-inflammatory substances (101, 102, 104). In a murine model, increasing TNFα and IL-1β production were observed in alcohol-fed mice that neutralize IL-1β in KCs to allowed iNKT cell accumulation and steatosis. The study also demonstrated that NLRP3 inflammasome and IL-1β secretion are essential factors for hepatic iNKT cells to accumulate and activate in ALD (105). Consistently, gut microbes can also trigger KC NLRP3 activation, resulting in iNKT cell activation (105). A study by Mariric et al. examined the role of both type I and type II NKT cells in alcoholic liver disease, demonstrating that only iNKT cells became activated following heavy alcohol consumption, resulting in inflammation and liver tissue damage. This study suggests that type I and II NKT cells are functionally distinct in liver inflammation and tissue injury (106). A more recent study identified the interplay between IL-10-producing iNKT cells silencing the productive roles of NK cells in alcoholic liver disease (107).
Despite these findings, the role of iNKT cells in human ALD has not been well-examined. In agreement with the murine data, pro-inflammatory cytokine levels were increased in alcoholic hepatitis human subjects, suggesting a correlation with disease severity (57). In addition, in patients with alcoholic hepatitis, NKG2D expression in NK and iNKT cells has been found to correlate with disease severity, suggesting these cells are involved in promoting liver damage (108).
MAIT Cells in Liver Health and Disease
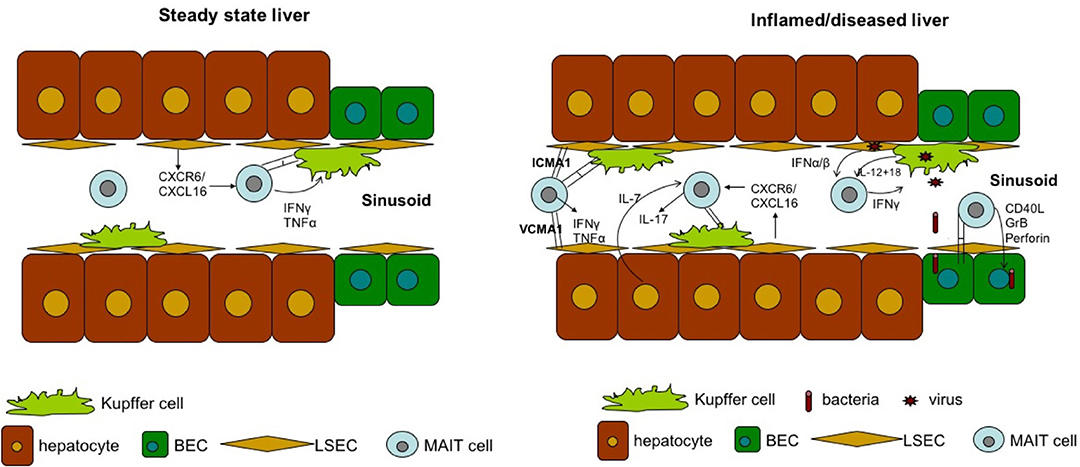
MAIT cells are significantly enriched in the liver, where they comprise up to 50% of liver-resident lymphocytes. These cells are located primarily in the biliary tract, and in the context of liver infection, MAIT cells can be activated by MR1-presenting bacterial ligands or indirectly via IL-12 and IL-18 produced by antigen-presenting cells in response to Toll-like receptor 8 signaling triggered by viral RNA (109, 110). The importance of MAIT cells in liver immunosurveillance is highlighted by three findings. First, liver MAIT cells are highly activated and express the activation marker CD69, as well as HLA-DR and CD38 (11, 110). This activation status suggests that liver MAIT cells are in a highly activated state, poised to respond to incoming antigens from the gut. Second, intra-hepatic MAIT cells, along with CD56bright NK cells, are the main source of IFN-γ post-TLR8 stimulation by liver-derived mononuclear cells through IL-12 and IL-18 activation (80). Finally, MAIT cells are the predominant IL-17 producers among intrahepatic T cells (~65% of IL-17+ T cells) in response to phorbol 12-myristate 13-acetate/ionomycin stimulation (11). As IL-17 targets multiple cell types in the liver, including Kupffer cells and BECs, to produce pro-inflammatory cytokines and chemokines (111), MAIT cells may be important regulators of hepatic inflammation and fibrosis Figure 3.
Hepatocellular Carcinoma
Recent studies have found that MAIT cells are recruited from peripheral blood to solid tumors in several cancers (112–114). Infiltration and accumulation of MAIT cells into tumor sites suggest that MAIT cells play an essential role in tumor development. MAIT cells are highly enriched in human liver (109) but are resisted to skew to an IL-17-producing phenotype, as they fail to release to IL-17 upon TCR stimulation (11). On the other hand, the function of tumor-infiltrating MAIT cells is proposed to be impaired in response to a panel of TCR ligands and cytokines (115). In a colorectal liver metastasis setting, IFN-γ produced by hepatic tumor-infiltrating MAIT cells is significantly suppressed (115). Taken together, the role of MAIT cells in hepatocellular carcinoma is still obscure, and further studies investigating the phenotype of tumor-infiltrating MAIT cells and their interactions with liver-resident cells will help to understand the role of MAIT cells in HCC.
Hepatitis B Virus (HBV)
To date, the role of hepatic MAIT cells in HBV is still poorly understood. Two studies have compared peripheral MAIT cells in healthy controls and chronic HBV patients, showing opposing results. The first study found that MAIT cells were not deleted nor functionally impaired in HBV patients (116). In contrast, there was a higher frequency of MAIT cells expressing CD38 and releasing granzyme B in HBV patients, suggesting that MAIT cells were more activated in the HBV setting (116). The second study demonstrates that in HBV patients, MAIT cells are in an exhausted phenotype, where the frequency of cells in circulation is reduced, the expression of the early activation marker CD69 is inhibited, and the production of IFN-γ and granzyme B are significantly suppressed (43). Why there are discrepancies between these two studies is not clear. The opposite observations on granzyme B production may be explained by different activation methods, as Boeijen et al. activated MAIT cells with IL-12/IL-18/CD28+ Escherichia coli (116), while Yong et al. stimulated MAIT cells with PMA/ionomycin (43). It should be taken into consideration that the size of both studies is relatively small. Therefore, the patients could have been in various clinical phases and undergoing different treatments. Indeed, MAIT cells are abundant in the peripheral blood but account for only a small percent of T cells (1–10%) (117). MAIT cells are further enriched in the liver (20% to 50% of T cells), which is also the primary site of infection (117). Therefore, further research with larger cohorts that focus on intrahepatic MAIT cells is required to solve the mystery of MAIT cells in HBV.
Hepatitis C Virus (HCV)
Several studies have shown that CD8+, rather than CD4+, MAIT cells in the peripheral blood were significantly reduced in the setting of chronic HCV (118, 119). These results may be due to CD8+ MAIT cells belonging to a newly defined pro-apoptotic phenotype expressing high levels of caspase 3 and 7 (120). Further phenotypic and functional studies reveal that the remaining CD8+ MAIT cells represent a chronic activation phenotype with signs of immune exhaustion, which is characterized by elevated levels of CD38, HLA-DR, CD69, PD-1, TIM-3, CTLA-4, and Granzyme B (118, 119). Notably, the function of these MAIT cells is also impaired, as reflected by the production of IFN-γ and TNFα being actively suppressed upon stimulation with TCR-dependent E. coli but not TCR-independent IL-12+IL-18 (118, 121). This result suggests that the loss and functional impairment of MAIT cells is a non-reversible process in chronic HCV patients, as antiviral treatment cannot reinvigorate these MAIT cells (118, 121, 122). Arguably, Ben Youssef et al found that adult MAIT cells in peripheral blood expand from cord blood Vα7.2+ CD161high T cells, and this process lasts ~5 years before filling up the adult MAIT pool (123). Therefore, the dysfunction and loss of MAIT cells after antiviral therapy may be due to the slow kinetics of differentiation and proliferation in MAIT cells.
There is an inverse correlation between the frequency of hepatic MAIT cells with liver inflammation and liver fibrosis in the setting of chronic HCV, demonstrating that MAIT cells are crucial mediators against HCV infection in the liver (121). Similarly, the percentage of hepatic MAIT cells is also reduced in chronic HCV patients (121). Importantly, the expression of HLA-DR and CD69 on MAIT cells is higher in the liver, suggesting that intrahepatic MAIT cells are more activated than are peripheral MAIT cells (121). This difference may because there is a higher frequency of activated monocytes in the liver, as they are an important source of IL-18 (121). MAIT cells are deleted in both blood and liver in the setting of HCV, and it is hypothesized that blood MAIT cells migrate to the organ, where they are further stimulated by inflammatory cytokines, resulting in activation-induced death, a mechanism that has been observed and well-characterized in HIV-induced MAIT cell depletion (121, 124).
Non-Alcoholic Fatty Liver Disease
The major cause of NASH/ NAFLD is chronic liver inflammation induced by tissue damage or pathogen infection (125). Hegde et al. finds that the number of hepatic MAIT cells is decreased in patients with non-alcoholic fatty liver disease-related cirrhosis (126). Compared with controls, cirrhotic liver MAIT cells exhibit an activated phenotype characterized by increasing IL-17 production with no differences in the percentage of MAIT cells producing granzyme B, IFN-γ, or TNF (126). Another study demonstrated that MAIT cells in NASH patients also display an activated phenotype defined by enhanced cytotoxicity but reduced cytokine production (127). These experiments suggest that MAIT cells are activated and contribute to pathogenesis in NAFLD/NASH.
Alcoholic Liver Disease
One of the most frequent complications of ALD is bacterial infection. One study has shown that over 50% of severe alcoholic hepatitis patients suffer from bacterial infection (128). As potent antibacterial lymphocytes in the liver, the number, cytokine production (IL-17) and cytotoxic response (Granzyme B, CD107a) of MAIT cell are impaired in peripheral blood of severe alcoholic hepatitis and alcoholic cirrhosis patients (129). Dysfunction of MAIT cells in ALD patients occurs from exposure to bacterial antigens and metabolites, but not ethanol (129). Importantly, in the liver, microarray data show that expression of transcription factors RORC/RORγt, ZBTB16/PLZF, and Eomes that mediate the function of MAIT cells is lower in ALD patients than in controls (129). Together, these results suggest that MAIT cells in ALD display a defective phenotype, which may explain why there is a high rate of bacterial infection complications in ALD patients.
Conclusions
Herein, we have discussed several key aspects of innate T cells and their potential role in liver diseases. Their enrichment in the liver suggests their unique role in liver disease progression and protection. The distinctive features and functions of innate T cells impart both pathogenic and protective abilities to the host. Thus, modulation of these cells represents a very attractive therapeutic strategy in liver diseases.
Our current knowledge of these cell subsets in the liver and their potential role in liver disease mainly comes from studies in animal models. Data from human and clinical studies are insufficient and are primarily complicated by the opposing effect these cell types have, both pathogenic and protective. In addition, most liver diseases are chronic disorders, and this further complicates analysis of these cells. The dynamic effect of these cells at different time points during the progression of disease could be significantly different regarding both number and function. Another critical factor is that a vast number of immune-regulatory cells resides in the liver, where they all modulate the activity level of iNKT and MAIT cells. In turn, these two innate T-cell subtypes also act as key modulators of other immune cell activity, including KCs, classical innate cells (macrophages and DCs) and conventional T cells. These factors form an involved local liver environment; thus, a molecular understanding of these cross-regulatory effects is key to understanding the liver immune system. Understanding liver immunity and function is also key to maintaining a proper balance between immune tolerance and immunity in the liver. To further understand the mechanism of these cells, it will be essential to develop more specific and reliable reagents to characterize and analyse these cells.
Author Contributions
WHu, WHe, XS, LD, XH and YG contributed to the writing of the manuscript.
Funding
YG is supported by Natural Science Foundation of Guangdong Province (Grant number: 2018A030313019).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Sheth K, Bankey P. The liver as an immune organ. Curr Opin Crit Care (2001) 7:99–104. doi: 10.1097/00075198-200104000-00008
2. McNamara HA, Cockburn IA. The three Rs: recruitment, retention and residence of leukocytes in the liver. Clin Transl Immunol. (2016) 5:e123. doi: 10.1038/cti.2016.84
3. Bandyopadhyay K, Marrero I, Kumar V. NKT cell subsets as key participants in liver physiology and pathology. Cell Mol Immunol. (2016) 13:337–46. doi: 10.1038/cmi.2015.115
4. Klugewitz K, Blumenthal-Barby F, Eulenburg K, Emoto M, Hamann A. The spectrum of lymphoid subsets preferentially recruited into the liver reflects that of resident populations. Immunol Lett. (2004) 93:159–62. doi: 10.1016/j.imlet.2004.03.007
5. Norris S, Collins C, Doherty DG, Smith F, McEntee G, Traynor O, et al. Resident human hepatic lymphocytes are phenotypically different from circulating lymphocytes. J Hepatol. (1998) 28:84–90. doi: 10.1016/S0168-8278(98)80206-7
6. Taniguchi M, Harada M, Kojo S, Nakayama T, Wakao H. The regulatory role of Vα14 NKT cells in innate and acquired immune response. Ann Rev Immunol. (2003) 21:483–513. doi: 10.1146/annurev.immunol.21.120601.141057
7. Liew PX, Lee WY, Kubes P. iNKT cells orchestrate a switch from inflammation to resolution of sterile liver injury. Immunity (2017) 47:752–765.e5. doi: 10.1016/j.immuni.2017.09.016
8. Godfrey DI, MacDonald HR, Kronenberg M, Smyth MJ, Van Kaer L. NKT cells: what's in a name? Nat Rev Immunol. (2004) 4:231–7. doi: 10.1038/nri1309
9. Kumar V. NKT-cell subsets: promoters and protectors in inflammatory liver disease. J Hepatol. (2013) 59:618–20. doi: 10.1016/j.jhep.2013.02.032
10. Moodycliffe AM, Maiti S, Ullrich SE. Splenic NK1.1-negative, TCRαβ intermediate CD4+ T cells exist in naive NK1.1 allelic positive and negative mice, with the capacity to rapidly secrete large amounts of IL-4 and IFN-γ upon primary TCR stimulation. J Immunol. (1999) 162:5156.
11. Tang X-Z, Jo J, Tan AT, Sandalova E, Chia A, Tan KC, et al. IL-7 licenses activation of human liver intrasinusoidal mucosal-associated invariant T cells. J Immunol. (2013) 190:3142. doi: 10.4049/jimmunol.1203218
12. Adams EJ, Lopez-Sagaseta J. The immutable recognition of CD1d. Immunity (2011) 34:281–3. doi: 10.1016/j.immuni.2011.03.006
13. Brigl M, Brenner MB. CD1: antigen presentation and T cell function. Annu Rev Immunol. (2004) 22:817–90. doi: 10.1146/annurev.immunol.22.012703.104608
14. Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. (2007) 25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711
15. Kawano T, Tanaka Y, Shimizu E, Kaneko Y, Kamata N, Sato H, et al. A novel recognition motif of human NKT antigen receptor for a glycolipid ligand. Int Immunol. (1999) 11:881–7. doi: 10.1093/intimm/11.6.881
16. Wilson MT, Johansson C, Olivares-Villagomez D, Singh AK, Stanic AK, Wang CR, et al. The response of natural killer T cells to glycolipid antigens is characterized by surface receptor down-modulation and expansion. Proc Natl Acad Sci USA. (2003) 100:10913–8. doi: 10.1073/pnas.1833166100
17. Brigl M, Tatituri RV, Watts GF, Bhowruth V, Leadbetter EA, Barton N, et al. Innate and cytokine-driven signals, rather than microbial antigens, dominate in natural killer T cell activation during microbial infection. J Exp Med. (2011) 208:1163–77. doi: 10.1084/jem.20102555
18. Kikuchi A, Nieda M, Schmidt C, Koezuka Y, Ishihara S, Ishikawa Y, et al. In vitro anti-tumour activity of α-galactosylceramide-stimulated human invariant Vα24+NKT cells against melanoma. Br J Cancer (2001) 85:741–746. doi: 10.1054/bjoc.2001.1973
19. Kuylenstierna C, Bjorkstrom NK, Andersson SK, Sahlstrom P, Bosnjak L, Paquin-Proulx D, et al. NKG2D performs two functions in invariant NKT cells: direct TCR-independent activation of NK-like cytolysis and co-stimulation of activation by CD1d. Eur J immunol. (2011) 41:1913–23. doi: 10.1002/eji.200940278
20. Carnaud C, Lee D, Donnars O, Park SH, Beavis A, Koezuka Y, et al. Cutting edge: cross-talk between cells of the innate immune system: NKT cells rapidly activate NK cells. J Immunol. (1999) 163:4647–50.
21. Paget C, Bialecki E, Fontaine J, Vendeville C, Mallevaey T, Faveeuw C, et al. Role of invariant NK T lymphocytes in immune responses to CpG oligodeoxynucleotides. J Immunol. (2009) 182:1846–53. doi: 10.4049/jimmunol.0802492
22. Schneiders FL, de Bruin RC, Santegoets SJ, Bonneville M, Scotet E, Scheper RJ, et al. Activated iNKT cells promote Vgamma9Vdelta2-T cell anti-tumor effector functions through the production of TNF-alpha. Clin Immunol. (2012) 142:194–200. doi: 10.1016/j.clim.2011.10.006
23. Smyth MJ, Crowe NY, Pellicci DG, Kyparissoudis K, Kelly JM, Takeda K, et al. Sequential production of interferon-gamma by NK1.1(+) T cells and natural killer cells is essential for the antimetastatic effect of alpha-galactosylceramide. Blood (2002) 99:1259–66. doi: 10.1182/blood.V99.4.1259
24. Smyth MJ, Wallace ME, Nutt SL, Yagita H, Godfrey DI, Hayakawa Y. Sequential activation of NKT cells and NK cells provides effective innate immunotherapy of cancer. J Exp Med. (2005) 201:1973–85. doi: 10.1084/jem.20042280
25. Chang PP, Barral P, Fitch J, Pratama A, Ma CS, Kallies A, et al. Identification of Bcl-6-dependent follicular helper NKT cells that provide cognate help for B cell responses. Nat Immunol. (2011) 13:35–43. doi: 10.1038/ni.2166
26. Zietara N, Lyszkiewicz M, Krueger A, Weiss S. ICOS-dependent stimulation of NKT cells by marginal zone B cells. Eur J Immunol. (2011) 41:3125–34. doi: 10.1002/eji.201041092
27. Bialecki E, Paget C, Fontaine J, Capron M, Trottein F, Faveeuw C. Role of marginal zone B lymphocytes in invariant NKT cell activation. J Immunol. (2009) 182:6105–13. doi: 10.4049/jimmunol.0802273
28. Vomhof-DeKrey EE, Yates J, Hagglof T, Lanthier P, Amiel E, Veerapen N, et al. Cognate interaction with iNKT cells expands IL-10-producing B regulatory cells. Proc Natl Acad Sci USA. (2015) 112:12474–9. doi: 10.1073/pnas.1504790112
29. Gapin L. Development of invariant natural killer T cells. Curr opin immunol. (2016) 39:68–74. doi: 10.1016/j.coi.2016.01.001
30. Lee YJ, Holzapfel KL, Zhu J, Jameson SC, Hogquist KA. Steady-state production of IL-4 modulates immunity in mouse strains and is determined by lineage diversity of iNKT cells. Nat Immunol. (2013) 14:1146–54. doi: 10.1038/ni.2731
31. Lee YJ, Wang H, Starrett GJ, Phuong V, Jameson SC, Hogquist KA. Tissue-specific distribution of iNKT cells impacts their cytokine response. Immunity (2015) 43:566–78. doi: 10.1016/j.immuni.2015.06.025
32. Lee YJ, Starrett GJ, Lee ST, Yang R, Henzler CM, Jameson SC, et al. Lineage-specific effector signatures of invariant NKT cells are shared amongst gammadelta t, innate lymphoid, and Th cells. J Immunol. (2016) 197:1460–70. doi: 10.4049/jimmunol.1600643
33. Nishioka Y, Masuda S, Tomaru U, Ishizu A. CD1d-restricted type II NKT cells reactive with endogenous hydrophobic peptides. Front Immunol. (2018) 9:548. doi: 10.3389/fimmu.2018.00548
34. Maricic I, Girardi E, Zajonc DM, Kumar V: Recognition of lysophosphatidylcholine by type II NKT cells and protection from an inflammatory liver disease. J Immunol. (2014) 193:4580–9. doi: 10.4049/jimmunol.1400699
35. Arrenberg P, Maricic I, Kumar V. Sulfatide-mediated activation of type II natural killer T cells prevents hepatic ischemic reperfusion injury in mice. Gastroenterology (2011) 140:646–55. doi: 10.1053/j.gastro.2010.10.003
36. Halder RC, Aguilera C, Maricic I, Kumar V. Type II NKT cell-mediated anergy induction in type I NKT cells prevents inflammatory liver disease. J Clin Invest. (2007) 117:2302–12. doi: 10.1172/JCI31602
37. Kim JH, Choi EY, Chung DH. Donor bone marrow type II (non-Valpha14Jalpha18 CD1d-restricted) NKT cells suppress graft-versus-host disease by producing IFN-gamma and IL-4. J Immunol. (2007) 179:6579–87. doi: 10.4049/jimmunol.179.10.6579
38. Iinuma C, Waki M, Kawakami A, Yamaguchi M, Tomaru U, Sasaki N, et al. Establishment of a vascular endothelial cell-reactive type II NKT cell clone from a rat model of autoimmune vasculitis. Int Immunol. (2015) 27:105–14. doi: 10.1093/intimm/dxu088
39. Sagami S, Ueno Y, Tanaka S, Fujita A, Niitsu H, Hayashi R, et al. Choline deficiency causes colonic type II natural killer T (NKT) cell loss and alleviates murine colitis under Type I NKT cell deficiency. PLoS ONE (2017) 12:e0169681. doi: 10.1371/journal.pone.0169681
40. Napier RJ, Adams EJ, Gold MC, Lewinsohn DM. The role of mucosal associated invariant T cells in antimicrobial immunity. Front Immunol. (2015) 6:344. doi: 10.3389/fimmu.2015.00344
41. Dusseaux M, Martin E, Serriari N, Peguillet I, Premel V, Louis D, et al. Human MAIT cells are xenobiotic-resistant, tissue-targeted, CD161hi IL-17-secreting T cells. Blood (2011) 117:1250–9. doi: 10.1182/blood-2010-08-303339
42. Treiner E, Duban L, Bahram S, Radosavljevic M, Wanner V, Tilloy F, et al. Selection of evolutionarily conserved mucosal-associated invariant T cells by MR1. Nature (2003) 422:164–9. doi: 10.1038/nature01433
43. Yong YK, Tan HY, Saeidi A, Rosmawati M, Atiya N, Ansari AW, et al. Decrease of CD69 levels on TCR Valpha7.2(+)CD4(+) innate-like lymphocytes is associated with impaired cytotoxic functions in chronic hepatitis B virus-infected patients. Innate Immun. (2017) 23:459–67. doi: 10.1177/1753425917714854
44. Reantragoon R, Corbett AJ, Sakala IG, Gherardin NA, Furness JB, Chen Z, et al. Antigen-loaded MR1 tetramers define T cell receptor heterogeneity in mucosal-associated invariant T cells. J Exp Med. (2013) 210:2305–20. doi: 10.1084/jem.20130958
45. Gold MC, Ehlinger HD, Cook MS, Smyk-Pearson SK, Wille PT, Ungerleider RM, et al. Human innate Mycobacterium tuberculosis-reactive alphabetaTCR+ thymocytes. PLoS Pathog. (2008) 4:e39. doi: 10.1371/journal.ppat.0040039
46. Gold MC, Eid T, Smyk-Pearson S, Eberling Y, Swarbrick GM, Langley SM, et al. Human thymic MR1-restricted MAIT cells are innate pathogen-reactive effectors that adapt following thymic egress. Mucosal Immunol. (2013) 6:35–44. doi: 10.1038/mi.2012.45
47. Porcelli S, Yockey CE, Brenner MB, Balk SP. Analysis of T cell antigen receptor (TCR) expression by human peripheral blood CD4−8− alpha/beta T cells demonstrates preferential use of several V beta genes and an invariant TCR alpha chain. J Exp Med. (1993) 178:1. doi: 10.1084/jem.178.1.1
48. Mondot S, Boudinot P, Lantz O. MAIT, MR1, microbes and riboflavin: a paradigm for the co-evolution of invariant TCRs and restricting MHCI-like molecules? Immunogenetics (2016) 68:537–48. doi: 10.1007/s00251-016-0927-9
49. Kumar V, Ahmad A. Role of MAIT cells in the immunopathogenesis of inflammatory diseases: new players in old game. Int Rev Immunol. (2018) 37:90–110. doi: 10.1080/08830185.2017.1380199
50. Lepore M, Kalinichenko A, Colone A, Paleja B, Singhal A, Tschumi A, et al. Parallel T-cell cloning and deep sequencing of human MAIT cells reveal stable oligoclonal TCRbeta repertoire. Nat Commun. (2014) 5:3866. doi: 10.1038/ncomms4866
51. Eckle SB, Corbett AJ, Keller AN, Chen Z, Godfrey DI, Liu L, et al. Recognition of Vitamin B precursors and byproducts by mucosal associated invariant T Cells. J Biol Chem. (2015) 290:30204–11. doi: 10.1074/jbc.R115.685990
52. Ussher JE, Klenerman P, Willberg CB. Mucosal-associated invariant T-cells: new players in anti-bacterial immunity. Front Immunol. (2014) 5:450. doi: 10.3389/fimmu.2014.00450
53. Tsukamoto K, Deakin JE, Graves JA, Hashimoto K. Exceptionally high conservation of the MHC class I-related gene, MR1, among mammals. Immunogenetics (2013) 65:115–24. doi: 10.1007/s00251-012-0666-5
54. Dias J, Leeansyah E, Sandberg JK. Multiple layers of heterogeneity and subset diversity in human MAIT cell responses to distinct microorganisms and to innate cytokines. Proc Natl Acad Sci USA. (2017) 114:E5434–43. doi: 10.1073/pnas.1705759114
55. Bennett MS, Trivedi S, Iyer AS, Hale JS, Leung DT. Human mucosal-associated invariant T (MAIT) cells possess capacity for B cell help. J Leukoc Biol. (2017) 102:1261–9. doi: 10.1189/jlb.4A0317-116R
56. Gold MC, Napier RJ, Lewinsohn DM. MR1-restricted mucosal associated invariant T (MAIT) cells in the immune response to Mycobacterium tuberculosis. Immunol Rev. (2015) 264:154–66. doi: 10.1111/imr.12271
57. Gao B, Radaeva S, Park O. Liver natural killer and natural killer T cells: immunobiology and emerging roles in liver diseases. J Leukoc Biol. (2009) 86:513–28. doi: 10.1189/JLB.0309135
58. Schipper HS, Rakhshandehroo M, van de Graaf SF, Venken K, Koppen A, Stienstra R, et al. Natural killer T cells in adipose tissue prevent insulin resistance. J Clin Invest. (2012) 122:3343–54. doi: 10.1172/JCI62739
59. Watarai H, Nakagawa R, Omori-Miyake M, Dashtsoodol N, Taniguchi M. Methods for detection, isolation and culture of mouse and human invariant NKT cells. Nat Protoc. (2008) 3:70–8. doi: 10.1038/nprot.2007.515
60. Berzins SP, Smyth MJ, Baxter AG. Presumed guilty: natural killer T cell defects and human disease. Nat Rev Immunol. (2011) 11:131–42. doi: 10.1038/nri2904
61. Rhost S, Sedimbi S, Kadri N, Cardell SL. Immunomodulatory type II natural killer T lymphocytes in health and disease. Scand J Immunol. (2012) 76:246–55. doi: 10.1111/j.1365-3083.2012.02750.x
62. Zeissig S, Peuker K, Iyer S, Gensollen T, Dougan SK, Olszak T, et al. CD1d-Restricted pathways in hepatocytes control local natural killer T cell homeostasis and hepatic inflammation. Proc Natl Acad Sci USA. (2017) 114:10449–54. doi: 10.1073/pnas.1701428114
63. Ware R, Kumar V. Complexity and function of natural killer T cells with potential application to hepatic transplant survival. Liver Transpl. (2017) 23:1589–92. doi: 10.1002/lt.24950
64. Lan P, Fan Y, Zhao Y, Lou X, Monsour HP, Zhang X, et al. TNF superfamily receptor OX40 triggers invariant NKT cell pyroptosis and liver injury. J Clin Invest. (2017) 127:2222–34. doi: 10.1172/JCI91075
65. Nair S, Boddupalli CS, Verma R, Liu J, Yang R, Pastores GM, et al. Type II NKT-TFH cells against Gaucher lipids regulate B-cell immunity and inflammation. Blood (2015) 125:1256–71. doi: 10.1182/blood-2014-09-600270
66. Sachdeva M, Chawla YK, Arora SK. Immunology of hepatocellular carcinoma. World J Hepatol. (2015) 7:2080–90. doi: 10.4254/wjh.v7.i17.2080
67. Bricard G, Cesson V, Devevre E, Bouzourene H, Barbey C, Rufer N, et al. Enrichment of human CD4+ V(alpha)24/Vbeta11 invariant NKT cells in intrahepatic malignant tumors. J Immunol. (2009) 182:5140–51. doi: 10.4049/jimmunol.0711086
68. Zhu S, Zhang H, Bai L. NKT cells in liver diseases. Front Med. (2018) 12:249–61. doi: 10.1007/s11684-018-0622-3
69. Wolf MJ, Adili A, Piotrowitz K, Abdullah Z, Boege Y, Stemmer K, et al. Metabolic activation of intrahepatic CD8+ T cells and NKT cells causes nonalcoholic steatohepatitis and liver cancer via cross-talk with hepatocytes. Cancer Cell (2014) 26:549–64. doi: 10.1016/j.ccell.2014.09.003
70. Anson M, Crain-Denoyelle AM, Baud V, Chereau F, Gougelet A, Terris B, et al. Oncogenic beta-catenin triggers an inflammatory response that determines the aggressiveness of hepatocellular carcinoma in mice. J Clin Invest. (2012) 122:586–99. doi: 10.1172/JCI43937
71. Shibolet O, Alper R, Zlotogarov L, Thalenfeld B, Engelhardt D, Rabbani E, et al. NKT and CD8 lymphocytes mediate suppression of hepatocellular carcinoma growth via tumor antigen-pulsed dendritic cells. Int J Cancer (2003) 106:236–43. doi: 10.1002/ijc.11201
72. Miyagi T, Takehara T, Tatsumi T, Kanto T, Suzuki T, Jinushi M, et al. CD1d-mediated stimulation of natural killer T cells selectively activates hepatic natural killer cells to eliminate experimentally disseminated hepatoma cells in murine liver. Int J Cancer (2003) 106:81–9. doi: 10.1002/ijc.11163
73. Ma C, Han M, Heinrich B, Fu Q, Zhang Q, Sandhu M, et al. Gut microbiome-mediated bile acid metabolism regulates liver cancer via NKT cells. Science (2018) 360:eaan5931. doi: 10.1126/science.aan5931
74. Terabe M, Swann J, Ambrosino E, Sinha P, Takaku S, Hayakawa Y, et al. A nonclassical non-Valpha14Jalpha18 CD1d-restricted (type II) NKT cell is sufficient for down-regulation of tumor immunosurveillance. J Exp Med. (2005) 202:1627–33. doi: 10.1084/jem.20051381
75. Renukaradhya GJ, Khan MA, Vieira M, Du W, Gervay-Hague J, Brutkiewicz RR. Type I NKT cells protect (and type II NKT cells suppress) the host's innate antitumor immune response to a B-cell lymphoma. Blood (2008) 111:5637–5645. doi: 10.1182/blood-2007-05-092866
76. Chang DH, Deng H, Matthews P, Krasovsky J, Ragupathi G, Spisek R, et al. Inflammation-associated lysophospholipids as ligands for CD1d-restricted T cells in human cancer. Blood (2008) 112:1308–1316. doi: 10.1182/blood-2008-04-149831
77. Ye L, Wang X, Wang S, Wang Y, Song L, Hou W, et al. CD56+ T cells inhibit hepatitis C virus replication in human hepatocytes. Hepatology (2009) 49:753–62. doi: 10.1002/hep.22715
78. Fisicaro P, Valdatta C, Boni C, Massari M, Mori C, Zerbini A, et al. Early kinetics of innate and adaptive immune responses during hepatitis B virus infection. Gut (2009) 58:974–82. doi: 10.1136/gut.2008.163600
79. Webster GJ, Reignat S, Maini MK, Whalley SA, Ogg GS, King A, et al. Incubation phase of acute hepatitis B in man: dynamic of cellular immune mechanisms. Hepatology (2000) 32:1117–24. doi: 10.1053/jhep.2000.19324
80. Kimura K, Kakimi K, Wieland S, Guidotti LG, Chisari FV. Interleukin-18 inhibits hepatitis B virus replication in the livers of transgenic mice. J Virol. (2002) 76:10702–7. doi: 10.1128/JVI.76.21.10702-10707.2002
81. Tan X, Ding Y, Zhu P, Dou R, Liang Z, Yang D, et al. Elevated hepatic CD1d levels coincide with invariant NKT cell defects in chronic hepatitis B virus infection. J Immunol. (2018) 200:3530–8. doi: 10.4049/jimmunol.1701801
82. Sprengers D, Sille FC, Derkow K, Besra GS, Janssen HL, Schott E, et al. Critical role for CD1d-restricted invariant NKT cells in stimulating intrahepatic CD8 T-cell responses to liver antigen. Gastroenterology (2008) 134:2132–43. doi: 10.1053/j.gastro.2008.02.037
83. Xu Y, Wang Z, Du X, Liu Y, Song X, Wang T, et al. Tim-3 blockade promotes iNKT cell function to inhibit HBV replication. J Cell Mol Med. (2018) 22:3192–201. doi: 10.1111/jcmm.13600
84. Dunn SE, Ousman SS, Sobel RA, Zuniga L, Baranzini SE, Youssef S, et al. Peroxisome proliferator-activated receptor (PPAR)alpha expression in T cells mediates gender differences in development of T cell-mediated autoimmunity. J Exp Med (2007) 204:321–30. doi: 10.1084/jem.20061839
85. Malhi H, Gores GJ. Cellular and molecular mechanisms of liver injury. Gastroenterology (2008) 134:1641–54. doi: 10.1053/j.gastro.2008.03.002
86. Vilarinho S, Ogasawara K, Nishimura S, Lanier LL, Baron JL. Blockade of NKG2D on NKT cells prevents hepatitis and the acute immune response to hepatitis B virus. Proc Natl Acad Sci USA. (2007) 104:18187–92. doi: 10.1073/pnas.0708968104
87. Baron JL, Gardiner L, Nishimura S, Shinkai K, Locksley R, Ganem D. Activation of a nonclassical NKT cell subset in a transgenic mouse model of hepatitis B virus infection. Immunity (2002) 16:583–94. doi: 10.1016/S1074-7613(02)00305-9
88. Weng X, He Y, Visvabharathy L, Liao C-M, Tan X, Balakumar A, et al. Crosstalk between type II NKT cells and T cells leads to spontaneous chronic inflammatory liver disease. J Hepatol. (2017) 67:791–800. doi: 10.1016/j.jhep.2017.05.024
89. Michelotti GA, Machado MV, Diehl AM. NAFLD, NASH and liver cancer. Nat Rev Gastroenterol Hepatol. (2013) 10:656–65. doi: 10.1038/nrgastro.2013.183
90. Rinella ME, Sanyal AJ. NAFLD in 2014: genetics, diagnostics and therapeutic advances in NAFLD. Nat Rev Gastroenterol Hepatol. (2015) 12:65–66. doi: 10.1038/nrgastro.2014.232
91. Tajiri K, Shimizu Y, Tsuneyama K, Sugiyama T. Role of liver-infiltrating CD3+CD56+ natural killer T cells in the pathogenesis of nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol. (2009) 21:673–80. doi: 10.1097/MEG.0b013e32831bc3d6
92. Li Z, Soloski MJ, Diehl AM. Dietary factors alter hepatic innate immune system in mice with nonalcoholic fatty liver disease. Hepatology (2005) 42:880–5. doi: 10.1002/hep.20826
93. Valenti L, Fracanzani AL, Fargion S. The immunopathogenesis of alcoholic and nonalcoholic steatohepatitis: two triggers for one disease? Semin Immunopathol (2009) 31:359–69. doi: 10.1007/s00281-009-0152-9
94. Alhasson F, Dattaroy D, Das S, Chandrashekaran V, Seth RK, Schnellmann RG, et al. NKT cell modulates NAFLD potentiation of metabolic oxidative stress-induced mesangial cell activation and proximal tubular toxicity. Am J Physiol Renal Physiol. (2016) 310:F85–101. doi: 10.1152/ajprenal.00243.2015
95. Kremer M, Thomas E, Milton RJ, Perry AW, van Rooijen N, Wheeler MD, et al. Kupffer cell and interleukin-12-dependent loss of natural killer T cells in hepatosteatosis. Hepatology (2010) 51:130–41. doi: 10.1002/hep.23292
96. Tang ZH, Liang S, Potter J, Jiang X, Mao HQ, Li Z. Tim-3/galectin-9 regulate the homeostasis of hepatic NKT cells in a murine model of nonalcoholic fatty liver disease. J Immunol. (2013) 190:1788–96. doi: 10.4049/jimmunol.1202814
97. Syn WK, Oo YH. Supporting evidence for natural killer T cell accumulation in progressive nonalcoholic fatty liver disease? Hepatology (2010) 51:345–6; author reply 346. doi: 10.1002/hep.23404
98. Syn WK, Oo YH, Pereira TA, Karaca GF, Jung Y, Omenetti A, et al. Accumulation of natural killer T cells in progressive nonalcoholic fatty liver disease. Hepatology (2010) 51:1998–2007. doi: 10.1002/hep.23599
99. Syn WK, Agboola KM, Swiderska M, Michelotti GA, Liaskou E, Pang H, et al. NKT-associated hedgehog and osteopontin drive fibrogenesis in non-alcoholic fatty liver disease. Gut (2012) 61:1323–9. doi: 10.1136/gutjnl-2011-301857
100. Satoh M, Andoh Y, Clingan CS, Ogura H, Fujii S, Eshima K, et al. Type II NKT cells stimulate diet-induced obesity by mediating adipose tissue inflammation, steatohepatitis and insulin resistance. PLoS ONE (2012) 7:e30568. doi: 10.1371/journal.pone.0030568
101. Szabo G, Bala S. Alcoholic liver disease and the gut-liver axis. World J Gastroenterol. (2010) 16:1321–9. doi: 10.3748/wjg.v16.i11.1321
102. Szabo G, Mandrekar P, Petrasek J, Catalano D. The unfolding web of innate immune dysregulation in alcoholic liver injury. Alcohol Clin Exp Res. (2011) 35:782–6. doi: 10.1111/j.1530-0277.2010.01398.x
103. Jeong WI, Gao B. Innate immunity and alcoholic liver fibrosis. J Gastroenterol Hepatol. (2008) 23(Suppl. 1):S112–8. doi: 10.1111/j.1440-1746.2007.05274.x
104. Mathews S, Feng D, Maricic I, Ju C, Kumar V, Gao B. Invariant natural killer T cells contribute to chronic-plus-binge ethanol-mediated liver injury by promoting hepatic neutrophil infiltration. Cell Mol Immunol. (2016) 13:206–16. doi: 10.1038/cmi.2015.06
105. Cui K, Yan G, Xu C, Chen Y, Wang J, Zhou R, et al. Invariant NKT cells promote alcohol-induced steatohepatitis through interleukin-1beta in mice. J Hepatol. (2015) 62:1311–8. doi: 10.1016/j.jhep.2014.12.027
106. Maricic I, Sheng H, Marrero I, Seki E, Kisseleva T, Chaturvedi S, et al. Inhibition of type I natural killer T cells by retinoids or following sulfatide-mediated activation of type II natural killer T cells attenuates alcoholic liver disease in mice. Hepatology (2015) 61:1357–69. doi: 10.1002/hep.27632
107. Cui K, Yan G, Zheng X, Bai L, Wei H, Sun R, et al. Suppression of natural killer cell activity by regulatory NKT10 cells aggravates alcoholic hepatosteatosis. Front Immunol. (2017) 8:1414. doi: 10.3389/fimmu.2017.01414
108. Stoy S, Dige A, Sandahl TD, Laursen TL, Buus C, Hokland M, et al. Cytotoxic T lymphocytes and natural killer cells display impaired cytotoxic functions and reduced activation in patients with alcoholic hepatitis. Am J Physiol Gastrointest Liver Physiol. (2015) 308:G269–76. doi: 10.1152/ajpgi.00200.2014
109. Jo J, Tan AT, Ussher JE, Sandalova E, Tang XZ, Tan-Garcia A, et al. Toll-like receptor 8 agonist and bacteria trigger potent activation of innate immune cells in human liver. PLoS Pathog. (2014) 10:e1004210. doi: 10.1371/journal.ppat.1004210
110. Jeffery HC, van Wilgenburg B, Kurioka A, Parekh K, Stirling K, Roberts S, et al. Biliary epithelium and liver B cells exposed to bacteria activate intrahepatic MAIT cells through MR1. J Hepatol. (2016) 64:1118–27. doi: 10.1016/j.jhep.2015.12.017
111. Harada K, Shimoda S, Sato Y, Isse K, Ikeda H, Nakanuma Y. Periductal interleukin-17 production in association with biliary innate immunity contributes to the pathogenesis of cholangiopathy in primary biliary cirrhosis. Clin Exp Immunol. (2009) 157:261–70. doi: 10.1111/j.1365-2249.2009.03947.x
112. Ling L, Lin Y, Zheng W, Hong S, Tang X, Zhao P, et al. Circulating and tumor-infiltrating mucosal associated invariant T (MAIT) cells in colorectal cancer patients. Sci Rep. (2016) 6:20358. doi: 10.1038/srep20358
113. Sundstrom P, Ahlmanner F, Akeus P, Sundquist M, Alsen S, Yrlid U, et al. Human mucosa-associated invariant T cells accumulate in colon adenocarcinomas but produce reduced amounts of IFN-gamma. J Immunol. (2015) 195:3472–81. doi: 10.4049/jimmunol.1500258
114. Peterfalvi A, Gomori E, Magyarlaki T, Pal J, Banati M, Javorhazy A, et al. Invariant Vα7.2-Jα33 TCR is expressed in human kidney and brain tumors indicating infiltration by mucosal-associated invariant T (MAIT) cells. Int Immunol. (2008) 20:1517–25. doi: 10.1093/intimm/dxn111
115. Shaler CR, Tun-Abraham ME, Skaro AI, Khazaie K, Corbett AJ, Mele T, et al. Mucosa-associated invariant T cells infiltrate hepatic metastases in patients with colorectal carcinoma but are rendered dysfunctional within and adjacent to tumor microenvironment. Cancer Immunol Immunother. (2017) 66:1563–75. doi: 10.1007/s00262-017-2050-7
116. Boeijen LL, Montanari NR, de Groen RA, van Oord GW, van der Heide-Mulder M, de Knegt RJ, et al. Mucosal-associated invariant T cells are more activated in chronic hepatitis B, but not depleted in blood: reversal by antiviral therapy. J Infect Dis. (2017) 216:969–76. doi: 10.1093/infdis/jix425
117. Kurioka A, Walker LJ, Klenerman P, Willberg CB. MAIT cells: new guardians of the liver. Clin Trans Immunol. (2016) 5:e98. doi: 10.1038/cti.2016.51
118. Hengst J, Strunz B, Deterding K, Ljunggren HG, Leeansyah E, Manns MP, et al. Nonreversible MAIT cell-dysfunction in chronic hepatitis C virus infection despite successful interferon-free therapy. Eur J Immunol. (2016) 46:2204–10. doi: 10.1002/eji.201646447
119. Barathan M, Mohamed R, Vadivelu J, Chang LY, Saeidi A, Yong YK, et al. Peripheral loss of CD8(+) CD161(++) TCRValpha7.2(+) mucosal-associated invariant T cells in chronic hepatitis C virus-infected patients. Eur J Clin Invest. (2016) 46:170–80. doi: 10.1111/eci.12581
120. Gerart S, Siberil S, Martin E, Lenoir C, Aguilar C, Picard C, et al. Human iNKT and MAIT cells exhibit a PLZF-dependent proapoptotic propensity that is counterbalanced by XIAP. Blood (2013) 121:614–23. doi: 10.1182/blood-2012-09-456095
121. Bolte FJ, O'Keefe AC, Webb LM, Serti E, Rivera E, Liang TJ, et al. Intra-hepatic depletion of mucosal-associated invariant T cells in hepatitis C virus-induced liver inflammation. Gastroenterology (2017) 153:1392–403.e2. doi: 10.1053/j.gastro.2017.07.043
122. Eberhard JM, Kummer S, Hartjen P, Hufner A, Diedrich T, Degen O, et al. Reduced CD161(+) MAIT cell frequencies in HCV and HIV/HCV co-infection: is the liver the heart of the matter? J Hepatol. (2016) 65:1261–3. doi: 10.1016/j.jhep.2016.07.031
123. Ben Youssef G, Tourret M, Salou M, Ghazarian L, Houdouin V, Mondot S, et al. Ontogeny of human mucosal-associated invariant T cells and related T cell subsets. J Exp Med. (2018) 215:459. doi: 10.1084/jem.20171739
124. Cosgrove C, Ussher JE, Rauch A, Gartner K, Kurioka A, Huhn MH, et al. Early and nonreversible decrease of CD161++/MAIT cells in HIV infection. Blood (2013) 121:951–61. doi: 10.1182/blood-2012-06-436436
125. Arrese M, Cabrera D, Kalergis AM, Feldstein AE. Innate immunity and inflammation in NAFLD/NASH. Digest Diseas Sci. (2016) 61:1294–303. doi: 10.1007/s10620-016-4049-x
126. Hegde P, Weiss E, Paradis V, Wan J, Mabire M, Sukriti S, et al. Mucosal-associated invariant T cells are a profibrogenic immune cell population in the liver. Nat Commun. (2018) 9:2146. doi: 10.1038/s41467-018-04450-y
127. Bolte FJ, Rehermann B. Mucosal-associated invariant T cells in chronic inflammatory liver disease. Semin Liver Dis. (2018) 38:60–5. doi: 10.1055/s-0037-1621709
128. Louvet A, Wartel F, Castel H, Dharancy S, Hollebecque A, Canva-Delcambre V, et al. Infection in patients with severe alcoholic hepatitis treated with steroids: early response to therapy is the key factor. Gastroenterology (2009) 137:541–8. doi: 10.1053/j.gastro.2009.04.062
Keywords: iNKT cells, MAIT cells, liver diseases, innate T cells, CD1d restriction
Citation: Huang W, He W, Shi X, He X, Dou L and Gao Y (2018) The Role of CD1d and MR1 Restricted T Cells in the Liver. Front. Immunol. 9:2424. doi: 10.3389/fimmu.2018.02424
Received: 25 April 2018; Accepted: 01 October 2018;
Published: 30 October 2018.
Edited by:
Luc Van Kaer, Vanderbilt University, United StatesReviewed by:
Paul Klenerman, University of Oxford, United KingdomPhilipp Hackstein, University of Oxford, United Kingdom, in collaboration with reviewer PK
Rafael Solana, Universidad de Córdoba, Spain
Moriya Tsuji, Aaron Diamond AIDS Research Center, United States
Vipin Kumar, University of California, San Diego, United States
Copyright © 2018 Huang, He, Shi, He, Dou and Gao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yifang Gao, Z2FveWYyNkBzeXN1LmVkdS5jbg==
 Wenyong Huang
Wenyong Huang Wenjing He
Wenjing He Yifang Gao
Yifang Gao