
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 10 October 2018
Sec. Inflammation
Volume 9 - 2018 | https://doi.org/10.3389/fimmu.2018.02230
 Isabella Jacomb1
Isabella Jacomb1 Clive Stanton1,2,3
Clive Stanton1,2,3 Rohini Vasudevan2
Rohini Vasudevan2 Hugh Powell2
Hugh Powell2 Maryanne O'Donnell1,2,3
Maryanne O'Donnell1,2,3 Rhoshel Lenroot1,2,3
Rhoshel Lenroot1,2,3 Jason Bruggemann1,3
Jason Bruggemann1,3 Ryan Balzan4,5
Ryan Balzan4,5 Cherrie Galletly4,6
Cherrie Galletly4,6 Dennis Liu4,6
Dennis Liu4,6 Cynthia S. Weickert1,3,7
Cynthia S. Weickert1,3,7 Thomas W. Weickert1,3*
Thomas W. Weickert1,3*There is increasing evidence for the role of inflammation in schizophrenia, yet the stability of increased peripheral inflammation in acute psychosis and the degree to which peripheral inflammation relates to cortical thickness, a measure of the degree of neuropathology, are unknown. In independent samples, we assessed the peripheral inflammation marker C-reactive protein (CRP) to determine the extent to which: (1) CRP was elevated and stable across admissions for acute psychosis, (2) cognition, daily function and symptom severity are characteristic of chronically ill patients with schizophrenia displaying elevated CRP, and (3) CRP levels predict cortical thickness. Study 1 assessed peripheral CRP (primary outcome) and other blood measures in 174/280 people with acute psychosis while Study 2 assessed peripheral CRP, cognition and cortical thickness (primary outcomes), symptoms, and daily function in 85/97 chronically ill patients with schizophrenia and 71/87 healthy controls. In acute psychosis, CRP and neutrophil-to-lymphocyte ratio were significantly elevated relative to a normal cutoff (with 59.8% of patients having elevated CRP) which remained elevated across admissions. CRP was significantly elevated in 43% of chronically ill patients with schizophrenia compared to 20% in controls. Elevated CRP patients displayed significantly worse working memory and CRP was inversely correlated with cortical thickness in frontal, insula, and temporal brain regions. This work supports the role of inflammation in psychotic illnesses and suggests that use of peripheral markers (e.g., CRP) in conjunction with diagnosis could be used to identify patients with more cortical neuropathology and cognitive deficits.
Schizophrenia is a severe psychiatric disorder characterized by positive (e.g., hallucinations and delusions) and negative (e.g., lack of motivation) symptoms, cognitive deficits, and functional decline. Presently there is no inexpensive, easily accessible diagnostic biomarker for the degree of cortical pathology in schizophrenia and substantial heterogeneity is associated with the disorder (1). While there are likely to be multiple causative pathways for developing schizophrenia (2), there is accumulating evidence to support the role of inflammation as increasing risk at least for a subset of patients (3). Therefore, improving interventions for schizophrenia may depend on identifying subgroups of patients characterized by inflammatory markers that can be targeted with specific treatments.
Recently, there has been increasing evidence that immunological and inflammatory mechanisms may play important roles in the pathophysiology of schizophrenia and related psychotic disorders (4–6). Epidemiological and preclinical evidence implicates prenatal infection and subsequent immune activation in the etiology of schizophrenia (7). Autoimmune disorders are significantly more prevalent in patients with schizophrenia compared to the general population (8, 9). It has been shown that antipsychotics, the most common treatment for psychosis, can decrease circulating levels of inflammatory markers which may, in turn, reduce psychotic symptoms (10, 11). However, some studies show an increase in peripheral inflammatory markers with second generation antipsychotics (12). Results from clinical trials of anti-inflammatory agents such as aspirin (13), non-steroidal anti-inflammatory drugs (14), and the microglial inhibitor minocycline (15, 16) suggest that adjunctive anti-inflammatory treatments may be a promising therapeutic approach for reducing symptoms and reversing cognitive deficits in people with schizophrenia.
Various immune and inflammatory alterations in the brain and blood have been found in subgroups of people with schizophrenia, including increased levels of pro-inflammatory cytokines (17–19), changes in white blood cell count (WBC) (20), a higher prevalence of positive antinuclear antibodies (ANA) (21, 22), increased neutrophil-to-lymphocyte ratio (NLR) (23, 24), and elevated C-reactive protein (CRP) (25, 26). Increases in inflammatory markers have been shown in subsets of individuals in both the prodromal stage (27, 28) and first-episode psychosis (29), suggesting that inflammation is not secondary to chronic illness or initiation of antipsychotics. The proportion of chronically ill patients with schizophrenia showing increased inflammation is generally quite substantial with ~40% who display elevated cytokine mRNA in the prefrontal cortex (17) and ~40% who display elevated cytokine mRNA in peripheral blood (30). These findings are consistent with meta-analytical results showing that cytokines are increased in the serum of people with schizophrenia (31). Furthermore, elevated proinflammatory cytokines, e.g., IL-6 and IL-1β, are inversely related to cognition and frontal brain volume in chronically ill people with schizophrenia (30). Thus, elevated inflammation markers appear to be a characteristic of psychosis in a substantial proportion of patients and they are related to brain structure and function.
CRP is an acute-phase reactant protein mainly produced by hepatocytes in response to an increase in circulating pro-inflammatory cytokines (32). CRP is attractive for use as a biomarker in psychiatry as it appears to be fairly stable, is easily and consistently measured by most diagnostic labs, and is considered a reliable biomarker of systemic inflammation (33–35). The healthy normal clinical range of CRP levels is generally < 3 mg/L (36), whilst CRP > 2 mg/L has been associated with increased risk of cardiovascular disease (37). Recent meta-analyses have reported a high prevalence of elevated CRP in schizophrenia (25, 36) and an association between adolescent CRP and adult schizophrenia diagnosis has been found in a recent longitudinal study (38). Elevated CRP has been associated with impaired cognitive functioning in chronically ill patients with schizophrenia (39, 40) and with acute psychotic episodes (41). An association between elevated CRP and more severe psychiatric symptoms in schizophrenia has also been shown (42); however, other studies have failed to replicate this finding (40, 43). Other studies suggest that CRP may be particularly elevated during exacerbations of psychosis (44) and may decrease following resolution of an acute psychotic episode (45). Whilst a small number of studies have shown an association between elevated CRP and decreased structural brain volume in healthy older adults (46, 47), there have been no studies to date assessing the potential link between CRP and cortical thickness in schizophrenia.
Here, we assess CRP as a marker of inflammation in two independent samples of patients with psychosis. The aims of Study 1 were to determine the extent to which: (1) CRP levels were elevated in a proportion of patients presenting with an acute psychotic episode, (2) relationships exist between CRP and other inflammation markers (including NLR, WBC, and ANA), and (3) peripheral inflammation markers remain stable over repeated admissions for acute psychotic episodes. We made the following three hypotheses: (1) CRP would be elevated above 3 mg/L in a subgroup of patients with acute psychosis, (2) there would be a significant difference in other peripheral inflammation markers between patients grouped on the basis of elevated and normal CRP, and (3) CRP would be consistently elevated during subsequent admissions for acute psychotic episodes in the same patients.
Given the limitations of assessing acutely psychotic patients for brain and cognitive measures, in Study 2 we tested the extent to which a peripheral inflammation marker (CRP) was related to cognition and a brain measure (cortical thickness) in a cohort of chronically ill patients with schizophrenia who were not acutely psychotic. The aims of Study 2 comparing chronically ill patients with schizophrenia to a healthy control group were to determine the extent to which: (1) CRP levels were elevated, (2) the proportions of patients versus controls having elevated CRP, (3) there were significant cognitive, symptom severity, and daily function differences between patients with elevated and patients with normal CRP levels, and (4) CRP levels predict cortical thickness. We made the following four hypotheses: (1) CRP would be significantly elevated in a subgroup of chronically ill patients with schizophrenia and schizoaffective disorder relative to healthy controls, (2) cognition, symptom severity, and functional abilities would be significantly affected in patients with schizophrenia who display elevated CRP relative to those patients who display normal CRP levels, (3) CRP levels would negatively correlate with cortical thickness, and (4) CRP would be elevated to a lesser extent in chronically ill patients compared to acutely ill patients (comparing results from Study 1 and Study 2).
Participants consisted of patients with an acute psychotic episode who were admitted to the Mental Health Intensive Care Unit (MHICU) at the Prince of Wales Hospital, Sydney, Australia, between March 2013 and February 2016. The MHICU is a 12-bed inpatient unit that provides treatment for patients who are acutely and severely mentally ill. A current comprehensive psychiatric assessment, current physical examination, and the last 7 days of progress notes were performed/documented prior to admission. The Prince of Wales Hospital MHICU admission criteria consisted of the following: (1) the patient is suffering with a mental disorder or illness which may have complex comorbidity, (2) the patient is presenting with behavioral difficulties, which seriously compromise the patient's physical wellbeing, psychological wellbeing, the physical wellbeing of others or the psychological wellbeing of others, (3) the patient's risk profile includes some or all of a significant risk of aggression, absconding with associated serious risk, suicide, or vulnerability, (4) it has been demonstrated that multidisciplinary management strategies have not succeeded in containing the presenting problems in the referring Mental Health Service, (5) the patient is detained under a section of the New South Wales Mental Health Act (2007), (6) the patient's risk profile is such that he/she does not require a higher level of security than that offered by the MHICU. The MHICU exclusion criteria (categories of patients who should not be treated on the MHICU) consisted of the following: (1) patients younger than 18 years, (2) patients older than 65 years, (3) patients who require a higher level of security by virtue of the risk profile, (4) patients with a primary diagnosis of substance misuse, where the current presenting behavior is a direct result of the substance misuse and not an exacerbation of a mental illness, (5) patients with a primary diagnosis of a personality disorder should not routinely be admitted to the MHICU, (6) patients with a primary diagnosis of dementia, (7) patients with a primary diagnosis of a learning disability, (8) patients with an exacerbated physical condition, which cannot be safely managed in the MHICU, (9) pregnant women, who would only be admitted to the MHICU in absolutely exceptional circumstances.
All patients were assessed and diagnosed using DSM-IV-TR criteria by trained psychiatrists. Individuals admitted to the MHICU without a psychotic disorder diagnosis were excluded. Patients who did not have CRP measured (n = 76; 27.1% of patients) were excluded. We also excluded patients suffering from any physical illness that may have influenced their immunological functioning (including non-Hodgkins lymphoma, Hashimoto's thyroiditis, HIV-AIDS and recent septicaemia; n = 5), and patients with inflammatory markers that were ≥ ± 2 standard deviations from the sample mean (n = 11).
Out of a total of 280 individuals screened for inclusion, 106 individuals were excluded based on the above criteria, leaving a total of 174 individuals (117 males, 57 females) for entry into analyses. Patients were between the ages of 15 and 76 years old. Table 1 provides diagnostic frequencies of the acutely psychotic sample.
Thirty patients with 2 admissions (23 males, 7 females), between 19 and 57 years of age (M = 34.8, SD = 10.3) at first admission, were included in the longitudinal analysis. Diagnoses included bipolar disorder (n = 10), schizoaffective disorder (n = 11), schizophrenia (n = 8), and psychosis NOS (n = 1). The mean length of time between admissions was 295.9 days (SD = 261.2; range = 13 to 898 days).
Venous peripheral blood was collected within 1 week of admission. Whole blood samples were shipped on ice to the South-Eastern Area Laboratory Service where they were processed using a chemiluminessence assay. Laboratory blood tests routinely performed upon admission included: full blood count, CRP and ANA. WBC and NLR were obtained from full blood count results. Results of these blood tests were collected from electronic medical records and entered into a separate database for analysis. CRP results recorded as < 1 mg/L (n = 25) were entered in the database at the minimum detection level (0.3 mg/L).
Clinical data collected from patients included body mass index (BMI), smoking status (smoker/non-smoker), illicit substance use (drug-user/non-drug user; polysubstance abuse/non-polysubstance abuse) and antipsychotic treatment upon admission. See Table 2 for the frequency of patients receiving different antipsychotics. The Health of the Nation Outcome Scales (HoNOS), an indicator of health and social functioning, was administered to the majority of patients (48).
All data analyses were performed using SPSS version 23. Antipsychotic dose was converted to mean daily chlorpromazine (CPZ) equivalent dose based on standard guidelines (49). Effect size calculations were measured as Cohen's d (50).
For single admission analyses, a one-sample t-test was used to compare the average levels of CRP with a clinical cut-off of 3 mg/L (36). Participants were also grouped into two categories; “normal CRP” with CRP < 3 mg/L and “elevated CRP” with CRP ≥ 3 mg/L. Demographics, clinical variables and other inflammatory markers were compared between elevated and normal CRP groups using ANOVA or χ2-tests where appropriate. ANCOVA was used to compare NLR and WBC between CRP groups, covarying for age and BMI. For the multiple admissions analyses, repeated measures ANCOVAs (with age at initial admission as a covariate) were used to determine significant differences in mean daily CPZ equivalent dose, HoNOS, CRP, NLR, and WBC between initial and repeat admissions.
This study was a retrospective analysis of patient's records blind to any personal identifying information. The study was conducted under protocol 15/028 approved by the South-Eastern Sydney Local Health District Human Research Ethics Committee.
Overall patient demographics, clinical, and inflammation measures are shown in Table 3. The numbers of patients classified by psychiatric diagnosis and the numbers of patients receiving different antipsychotic combinations are provided in Tables 1, 2. Mean CRP for the sample was 6.1 (SD = 6.7), which was significantly higher than the clinical cut-off of 3, t(173) = 6.15, p < 0.01, d = 0.47. Mean NLR of 2.7 (SD = 1.1) was also significantly elevated relative to the clinical cut-off for NLR of 2.2, t(170) = 5.88, p < 0.01, d = 0.45. Mean WBC was 7.8 (SD = 2.1), which was within the clinically normal range.
The majority of people with acute psychosis (n = 104, 59.8%) had CRP levels ≥ 3 mg/L. Elevated NLR (≥ 2.2) was present in 70.2% (n = 73) of individuals with elevated CRP, but only 6.7% (n = 7) had elevated WBC (≥ 11). The majority of the people with acute psychosis who were tested for signs of autoimmunity had positive ANA titers (66.7%, i.e., 42/63 patients).
As shown in Table 4, those with acute psychosis and elevated CRP were significantly older and had a significantly greater BMI than individuals who were acutely psychotic but had normal CRP at the time of blood draw. There were no significant differences between the CRP subgroups (≥3 mg/L vs. < 3 mg/L) in relation to antipsychotic dosage, smoking status, drug use status, polysubstance abuse (PSA), HoNOS scores, or percent ANA positive. After co-varying for age and BMI, NLR remained significantly higher in patients with elevated CRP compared to patients with normal CRP. There was a trend toward a significant increase in WBC in the elevated CRP group relative to the normal CRP group after covarying for age and BMI.
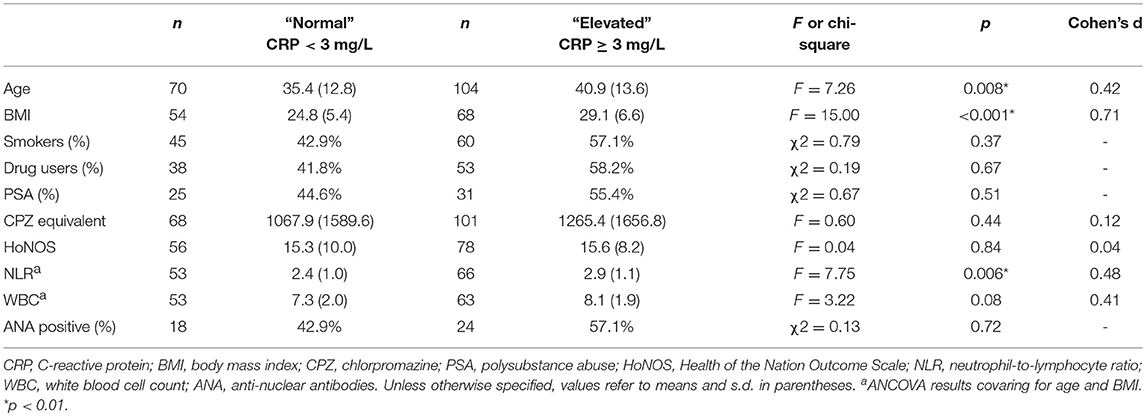
Table 4. Characteristics of the acute psychosis sample in the normal (CRP < 3 mg/L) and elevated (CRP ≥ 3 mg/L) CRP groups.
As shown in Table 5, CRP levels were significantly reduced at repeat admission compared to initial admission. However, mean CRP was significantly elevated relative to the clinical cut-off at both initial admission (M = 12.1, SD = 12.0), t(29) = 4.18, p < 0.001, and repeat admission (M = 7.8, SD = 8.0), t(29) = 3.29, p = 0.003. There were no significant differences in NLR and WBC between admissions. NLR was significantly elevated relative to the clinical cut-off at initial admission (M = 2.9, SD = 1.6), t(27) = 2.35, p = 0.03, and repeat admission (M = 2.9, SD = 1.2), t(27) = 3.02, p = 0.005. Mean WBC was within normal levels at both admissions. There were no significant differences in relation to mean daily CPZ equivalent dose and HONOS scores between admissions.
This study found CRP levels to be significantly elevated in patients with acute psychosis compared to clinical cut-offs, supporting previous work (45, 51). A large proportion (~60%) of the current sample had a CRP above the normal clinical range. NLR, which is the ratio of the number of most abundant white blood cells (neutrophils) to the total number of lymphocytes (which includes T cells, B cells. monocytes and Natural Killer Cells), was also significantly elevated in this acutely psychotic sample, confirming previous reports of increased NLR in schizophrenia (23, 24). WBC was within normal levels; however, there was a trend toward WBC being higher in the elevated CRP group compared to the normal CRP group, which is consistent with findings from a recent study that reported a positive relationship between leukocytes and CRP in 213 patients with schizophrenia (52). Furthermore, a large proportion of patients (~67%) in the current sample had positive ANA, which is a much higher frequency than previously reported (~20%) in chronically ill patients with schizophrenia (22) and higher than reported for healthy individuals (~26%) (53). Since known autoimmune disorders was an exclusion for being included in our analyses, elevated inflammatory markers would most likely not be attributed to other physical disorders in this instance.
We also found that there was a significant decrease in CRP levels at repeat admission compared to initial admission; however, CRP at both admissions was significantly elevated compared to the clinical cut-off. There was no significant change in NLR or WBC across both admissions. These findings demonstrate that CRP and NLR (but not WBC) are consistently elevated above normal levels during acute psychotic episodes. This may indicate that a subset of acutely psychotic individuals with elevated CRP or NLR may benefit most from adjunctive anti-inflammatory treatments initially at admission or later, possibly to prevent further admissions.
However, there are some potential confounding factors that make the connection between inflammation and psychotic illnesses uncertain. There was a significant difference between normal and elevated CRP groups in relation to age and BMI, which is consistent with previous findings that higher CRP level is associated with older age and greater BMI (25, 54). However, after co-varying for age and BMI, NLR remained significantly greater in patients with elevated relative to patients with normal CRP levels suggesting that CRP is a marker of brain illness/dysfunction in these cases and not simply a reflection of aging or excess weight. There was no significant difference between elevated and normal CRP groups in relation to antipsychotic dosage, supporting meta-analytical findings that CRP is not strongly modulated by antipsychotic dose (25). In contrast to previous studies that have shown smoking and drug use to be related to elevated CRP (55, 56), we found no significant difference between the patients with acute psychosis who displayed elevated and normal CRP in the proportion of smokers, drug users or poly substance abusers. Thus, the potential confounds did not appear to drive our findings in relation to the effects of CRP in this sample of acutely ill psychosis patients.
Other limitations include the lack of symptom severity measures, cognitive assessments and a restricted number of inflammation markers assessed. However, a common difficulty faced when working with acutely psychotic individuals admitted to inpatient hospitals is that these patients are frequently too unwell to complete standardized assessments. These patients met the clinical criteria for admission to the Mental Health Intensive Care Unit at intake, which is indicative of severe symptomatology and dysfunction.
In sum, after covarying for age and BMI, elevated CRP, and NLR appear to be characteristic of a substantial subset of people with acute psychosis. The findings from Study 1 support the hypothesis that a large subset (~60%) of individuals with acute psychosis display elevated markers of inflammation which is sustained across admissions for acute psychosis. Next, in Study 2 (below) we tested the relationship of CRP to symptoms, cognition and brain cortical thickness in a chronically ill sample of people with schizophrenia.
The second study is derived from baseline data collected in our previously published clinical trial of a hormonal based treatment for schizophrenia (57). The current work represents our first assessment of CRP as an assay of immune system function in schizophrenia and the degree to which cognitive, symptom, daily function, and brain volume variables differ in relation to CRP in this sample. Only participants who provided a sufficient quantity of blood for analysis of CRP were included.
Eighty-five adult outpatients (51 males, 34 females) with schizophrenia or schizoaffective disorder, between 20 and 51 years of age, were included. All participants had been receiving antipsychotics for at least 1 year before entering the study (see Table 6 for the numbers of people receiving each antipsychotic or combination of antipsychotics). A diagnosis of schizophrenia (n = 54) or schizoaffective disorder (n = 31) was determined using the Structured Clinical Interview for Diagnostic and Statistical Manual IV-TR Axis I Disorders (SCID) (58) by a clinician trained in administration of the SCID and was confirmed independently by another clinician. Patients with a concurrent Axis I psychiatric diagnosis, a history of substance abuse or dependence (within the past 5 years), head injuries with loss of consciousness, seizures, central nervous system infection, untreated diabetes, or hypertension, or mental retardation were excluded. Women were excluded if they were pregnant. Seventy-four patients had their height and weight collected for BMI calculation. Sixty-nine patients also provided information about whether they were a smoker or non-smoker.
Seventy-one healthy adults (34 males, 37 females) between 20 and 50 years of age were included. Exclusion criteria included a personal history of or a first-degree relative with a DSM-IV Axis I psychiatric diagnosis, history of substance abuse or dependence (within the past 5 years), head injuries with loss of consciousness, seizures, central nervous system infection, mental retardation, or untreated diabetes or hypertension.
All participants were administered a four subtest version of the Wechsler Adult Intelligence Scale – Third Edition (WAIS-III) (59) (consisting of Arithmetic, Similarities, Picture Completion and Digit Symbol subtests) as an estimate of current IQ, and the Wechsler Test of Adult Reading (WTAR) (60) as an estimate of premorbid IQ in schizophrenia. Working memory, verbal concept formation, visual perceptual organization, verbal fluency, attention/perceptual-motor processing speed and immediate and delayed verbal memory were assessed using WAIS-III Letter Number Sequencing (LNS) and Arithmetic, WAIS-III Similarities, WAIS-III Picture Completion, the Controlled Oral Word Association Test (COWAT) letter fluency (61), Form A of the Trail Making Test (TMT-A) (62) and Logical Memory I and II (LMI and LMII) of the Wechsler Memory Scale—Revised (63), respectively. TMT-A was incorporated into the battery after initiation of the study, which resulted in a lower total number of patients (n = 64) completing this measure.
Symptom severity was assessed in patients using the Positive and Negative Syndrome Scale (PANSS) (64). Inter-rater reliability for the PANSS was high with an average intra-class correlation coefficient of 0.90. Negative emotional states, daily function and quality of life were assessed with the 21-item version of the Depression, Anxiety and Stress Scale (DASS-21) (65), the Short Form 36 Version 2 Health Survey Questionnaire (SF-36v2) (66), and the Schizophrenia Quality of Life Scale (SQLS) (67), respectively.
Peripheral venous blood was collected from all participants in 8 ml Serum-separating tubes (SST Vacutainer, Becton Dickinson, Franklin Lakes, NJ) and 9 ml ethylenediaminetetraacetic acid (EDTA) tubes (Vacuette Vacutainer, Greiner Bio-One, Kremsmünster, Austria). Plasma was collected from EDTA tubes via centrifugation for 15 min at 2000 x g. SST tubes were incubated at room temperature for 30 min to allow for the blood to clot. Upon clotting, the serum was then collected via centrifugation for 5 min at 2000 x g. Plasma and serum were then aliquoted into protein low-binding tubes (Eppendorf, Hamburg, Germany) and stored at −80°C until the day of the assay.
CRP was measured in plasma using a high-sensitivity Enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's instructions (IBL-international, Hamburg, Germany). Ten microliters of plasma was diluted serially up to 1:1,000 and each assay was run back to back with samples in duplicate by the same investigator, who was blind to the diagnosis. A five-point standard curve was generated using 10, 5, 1, 0.4, and 0 μg/ml calibrators that were prepared by the manufacturer. The average coefficient variance across all plates was 3.23%. The sample reads ranged from 0.06 mg/L to 23.92 mg/L. A minimum detectable value replacement of 0.003 was used for 13 data points that were below the respective minimal detectable value on all protein analytes.
From the total number of participants, 32 patients and 19 controls did not receive an MRI scan for reasons including electing not to receive an MRI scan, not able to physically fit in the MRI scanner, and/or experiencing claustrophobia. Thus, a subsample of participants (44 patients, 51 healthy controls) received a structural MRI scan of the brain. The MRI scans were performed using a 3-Tesla Phillips Achieva scanner with an 8-channel head coil located at Neuroscience Research Australia (Randwick, NSW). T1 weighted high-resolution anatomical scans were obtained (1 mm slice thickness, no gap, 180 slices, TR = 5.4 ms, TE = 2.4 ms, field of view 256 mm). After visual inspection of scans for motion and other artifacts and neuroanatomical abnormalities, data from 41 patients and 51 controls were analyzed for cortical thickness. The scans were processed using Mac OSX 10.8 running FreeSurfer software (version 5.1.0, Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts, USA). A detailed methodological description of the FreeSurfer cortical reconstruction, volumetric segmentation and subsequent deformable procedures (including published references) is available at http://surfer.nmr.mgh.harvard.edu/. Processed scans were visually evaluated and manually edited as necessary by trained personnel. Cortical thickness data for each region were obtained based on the FreeSurfer Desikan-Killiany atlas.
Data analysis was performed using SPSS version 23. Where possible, age- and sex-adjusted scaled scores were used for the cognitive tests. Extreme outliers (≥ ± 2 standard deviations from the mean) for the CRP variable were excluded from the analysis (total excluded n = 10, schizophrenia group n = 9, healthy control group n = 1).
Demographic, clinical, cognitive, symptom severity, negative emotional states, quality of life, and daily function scores were compared between the chronically ill schizophrenia group and healthy control group using t-tests or χ2 as appropriate.
Based on clinical cut-off scores, all participants were grouped into two categories: “normal CRP” with CRP < 3 mg/L and “elevated CRP” with CRP ≥ 3 mg/L (36). χ2 tests were performed to determine if there were significant differences in the numbers of participants among the CRP groups in patients and controls. An ANOVA was performed between the acute psychosis patient group (from Study 1) and the chronically ill schizophrenia patient group (from Study 2) to determine the extent to which CRP levels differed between these patient groups. Demographic, clinical, cognitive, symptom severity, negative emotional states, quality of life, and daily function scores were compared among normal CRP groups in healthy controls and normal and elevated CRP groups in schizophrenia patients using univariate ANOVAs with False Discovery Rate (FDR) corrections for multiple comparisons (68). Effect size calculations were reported as Cohen's d (50).
Further analyses included paired t-tests in a subset of 46 patients with schizophrenia who had elevated CRP (n = 23) and normal CRP (n = 23) who were matched within 2 points on BMI to compare performance on any measures that were significantly different between the elevated and normal CRP groups (i.e., WAIS-III IQ and Arithmetic) to control for BMI.
FreeSurfer cortical thickness estimates for the left and right hemispheres were summed to create a composite measure for each brain region. Simple linear regression models (backward elimination) were used to model potential predictors of cortical thickness. Predictor variables consisted of CRP, diagnostic group, age at sampling and sex. The criterion for backward elimination was F ≥ 0.1.
All participants provided written informed consent consistent with the Declaration of Helsinki which was obtained after the nature and possible consequences of the study were explained. The study was conducted under protocols approved by the University of New South Wales (07/121 and 09/187), South Eastern Sydney Local Health District (07-259) Human Research Ethics Committees and the Queen Elizabeth Hospital Ethics and Human Research Committee, Adelaide (2010188).
The number of chronically ill patients with schizophrenia receiving different antipsychotics are provided in Table 6. Cohort demographics, cognitive, symptom, negative emotional states, and daily functioning scores, and CRP levels are provided in Table 7. Relative to healthy controls, patients with schizophrenia were significantly older (although the groups were only 9.4% different in age) and had significantly less education. Patients with schizophrenia also had significantly lower (worse) scores for cognitive, negative emotions, and daily functioning relative to controls. As predicted, the patients with schizophrenia had significantly elevated CRP levels relative to controls (an 88.9% increase, see Table 7). There were no significant differences between schizophrenia and control groups in relation to sex or ethnicity ratios. The patients displayed mild to moderate symptom severity based on Positive and Negative Syndrome Scale (PANSS) scores, were chronically ill with a typical mean age of onset and the majority of patients were receiving second generation antipsychotic medication (see Table 6).
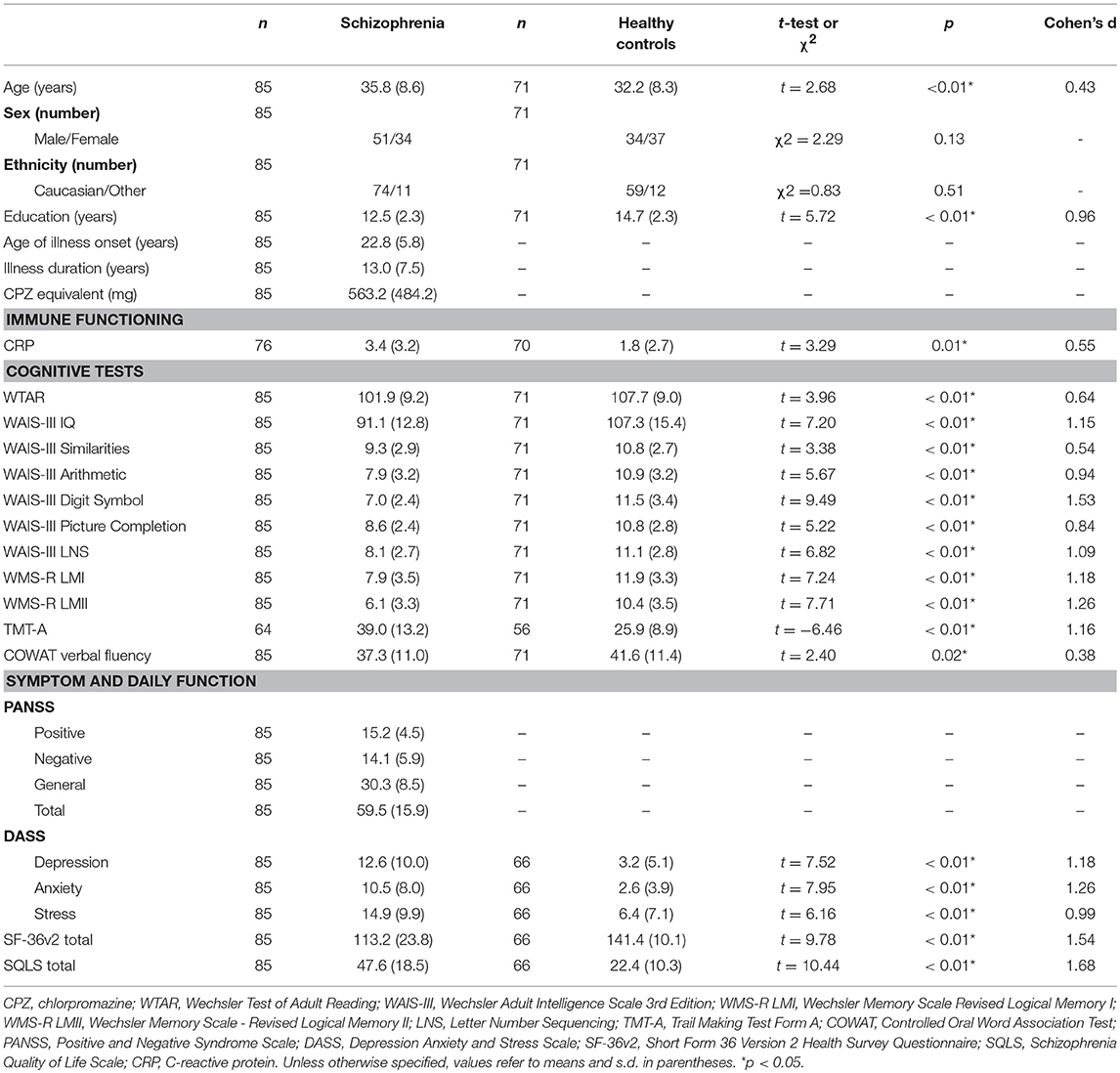
Table 7. Demographics, cognitive, symptom severity, negative emotional states, daily function measures, and C-reactive protein levels in chronically ill patients with schizophrenia and healthy controls.
There was a significant difference in the proportions of people with elevated CRP in schizophrenia and healthy control groups; = 9.16, p < 0.01. Forty three percent of people with schizophrenia had CRP ≥ 3 mg/L (n = 33; CRP range 3.0–12.3) compared to 20% of healthy controls with CRP ≥ 3 mg/L (n = 14; CRP range 3.0–13.5).
There was also a significantly higher CRP level in the acute psychosis patient group from Study 1 (M = 6.1, SD = 6.7) relative to the chronically ill patient group from Study 2 [M = 3.4, SD = 3.2, F(1, 248) = 11.36, p = 0.001, see Figure 1], as well as a significant difference in the proportions of people in the elevated versus normal CRP groups among acute psychosis patients, chronically ill patients, and healthy controls, = 11.2, p = 0.004.
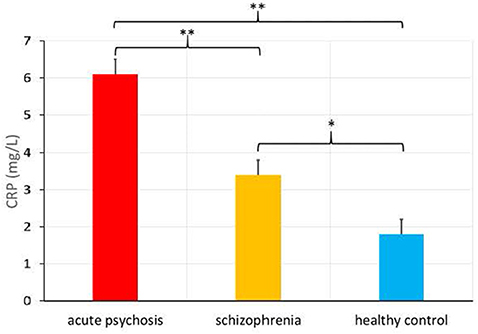
Figure 1. C-Reactive Protein (CRP) levels (in mg/L) in patients admitted for an acute psychotic episode, chronically ill patients with schizophrenia, and healthy controls (means provided with standard error), *p < 0.01, **p < 0.001.
As shown in Table 8, there were no significant differences between elevated and normal CRP groups in healthy controls in relation to any of the demographic, negative emotional states, daily function, quality of life, or cognitive measures after adjustment for multiple comparisons. Table 9 shows that after adjustment for multiple comparisons, there were significant differences between the elevated and normal CRP groups in patients with schizophrenia in relation to sex, BMI, current IQ (WAIS-III IQ) and working memory (i.e., WAIS-III Arithmetic subtest), with a large effect size for working memory such that performance was significantly poorer in the elevated CRP group of patients. Paired t-tests revealed a significant difference in working memory (WAIS-III Arithmetic) remained between the patients with schizophrenia displaying normal and elevated CRP who were matched on BMI (see Table 10). There was no significant difference between the matched groups on current IQ (WAIS-III IQ).
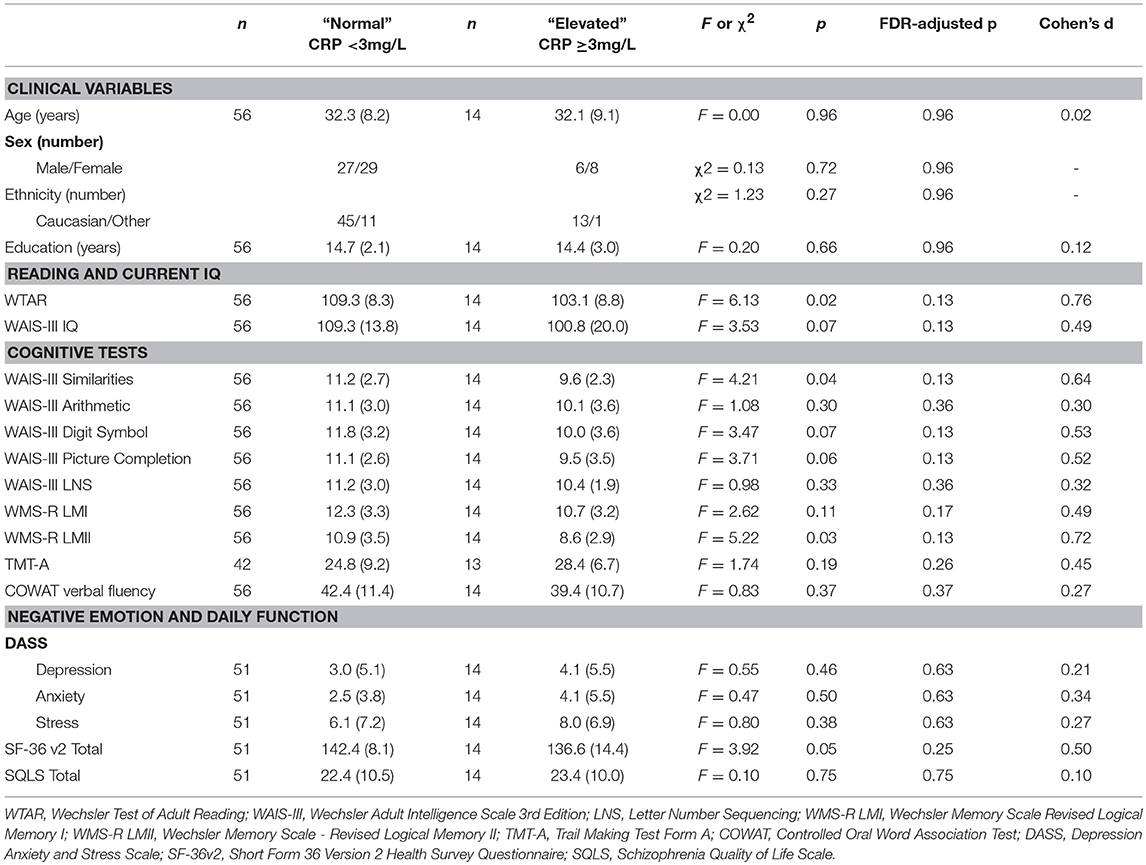
Table 8. Comparison of demographic, cognitive, negative emotional states, daily function, and quality of life measures between normal and elevated CRP groups in healthy controls.
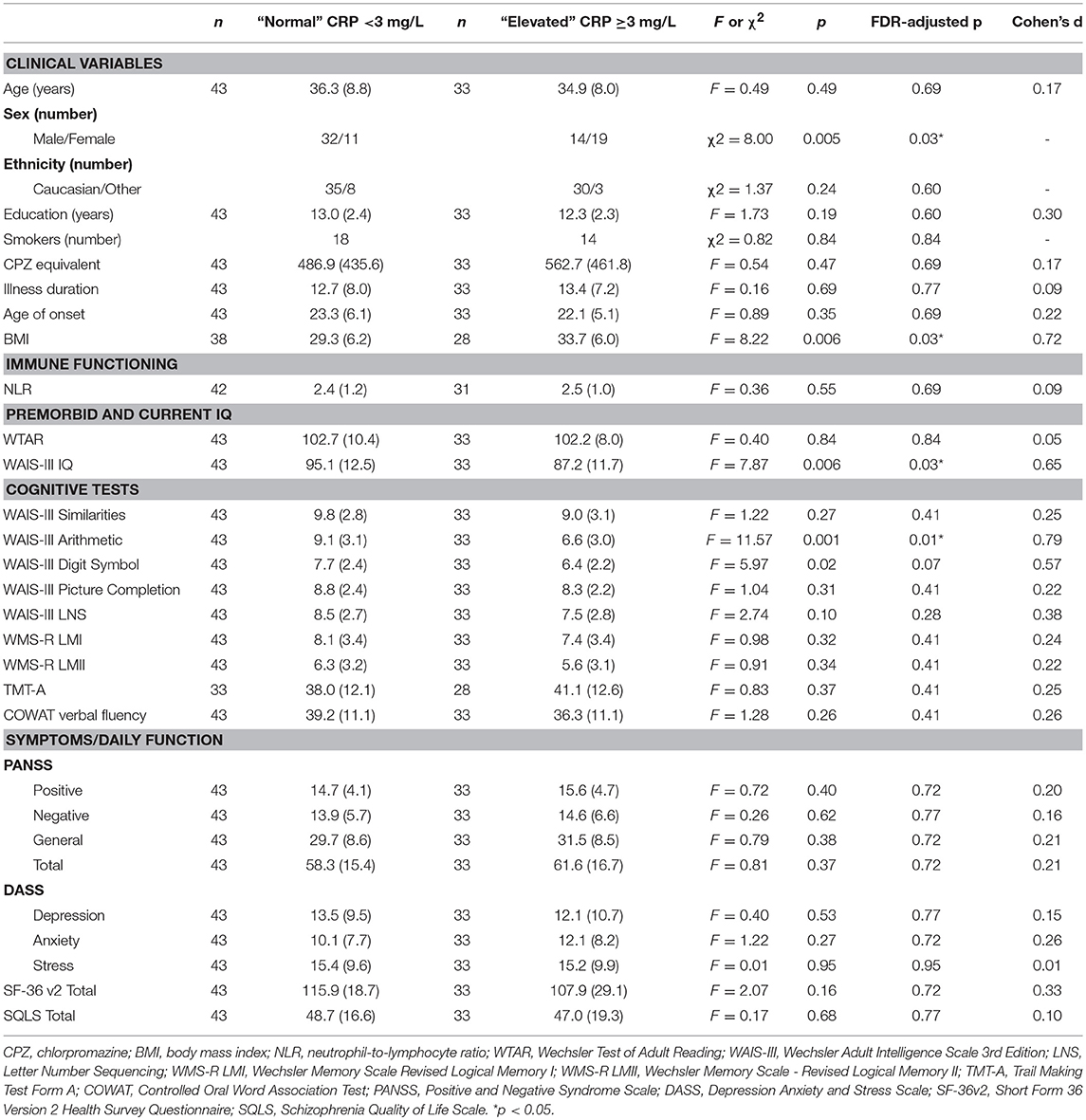
Table 9. Comparison of demographic, clinical, cognitive, symptom severity, negative emotional states, daily function and quality of life measures between normal and elevated CRP groups in patients with schizophrenia.
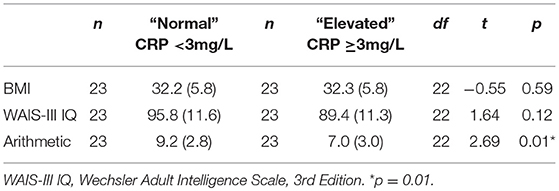
Table 10. Comparison of current IQ and working memory measures between normal and elevated CRP schizophrenia groups matched on BMI.
Results from the regression analysis using diagnostic group, age, sex, and CRP revealed significant contributions of the independent variables to the prediction of cortical thickness in several frontal and temporal regions in addition to the insula and paracentral gyrus (see Table 11 and Figure 2). In relation to our main variable of interest, CRP significantly predicted cortical thickness in most (8/9) regions, with CRP predicting from 3.9% of the variance in the frontal pole and middle temporal lobe to 7.7% of the variance in the temporal pole. The relationships between CRP levels and cortical thickness were inverse, demonstrating that as CRP levels increased, cortical thickness decreased.
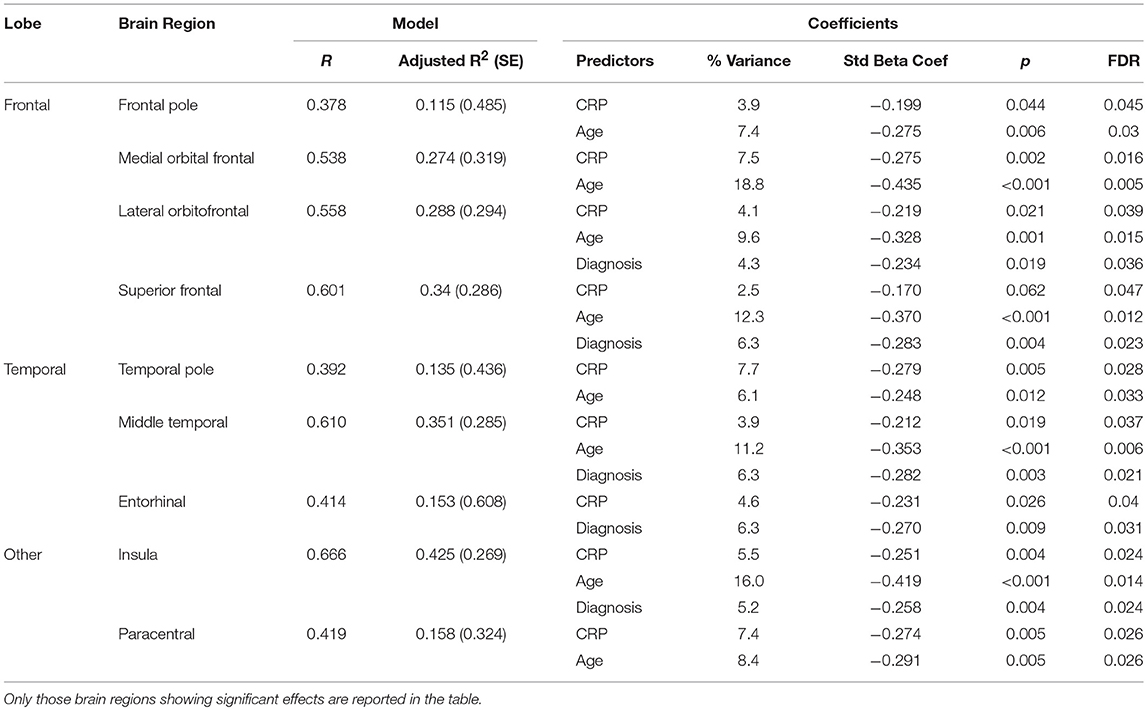
Table 11. Results of regression analysis showing significant contributions of diagnostic (Dx) group (schizophrenia or control), age, sex, and C-Reactive Protein (CRP) to cortical thickness.
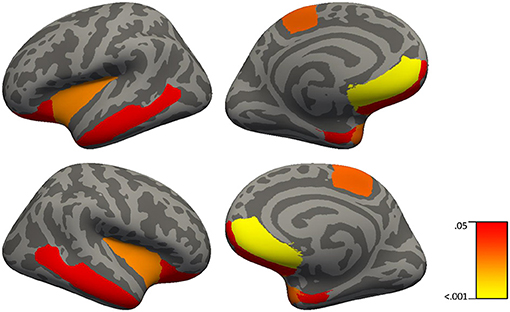
Figure 2. Brain regions in which C-reactive protein significantly predict cortical thickness in 51 healthy controls and 41 patients with schizophrenia using simple linear regressions with backward elimination (regions highlighted represent significant effects at FDR p < 0.04).
As would be expected, age was generally the strongest predictor of cortical thickness in most regions showing significant results, predicting from 6.1% of the variance in the temporal pole to 18.8% of the variance in the medial orbital frontal cortex. Also, as expected, diagnostic group predicted from 4.3% of cortical thickness in the lateral orbital frontal cortex to 6.3% of cortical thickness in superior frontal gyrus, middle temporal lobe, and entorhinal cortex.
In relation to elevated CRP, there was a significant difference between the proportions of chronically ill patients with schizophrenia (43%) and healthy controls (20%) who could be classified as having elevated levels of inflammation. These findings suggest the existence of a low-grade systemic inflammation in nearly half of the patients studied. This is consistent with our report of a similar proportion (~40%) of chronically ill patients displaying elevated cytokine mRNA expression in post-mortem prefrontal cortex tissue (17) and displaying elevated cytokine mRNA expression in blood (69). On average, the chronically ill patients with schizophrenia displayed significantly lower CRP levels than the acute psychosis group, supporting previous work showing that CRP may decrease following antipsychotic treatment and resolution of acute psychotic episodes (42). Thus, a substantial proportion of chronically ill patients with schizophrenia display elevated peripheral inflammation markers and CRP in particular.
Some studies have shown that elevated CRP is associated with poorer cognitive performance (verbal memory, global cognition) both in patients with schizophrenia (40) and healthy older adults (70). In the current study, the elevated CRP schizophrenia group displayed significantly worse performance on a measure of working memory compared to the normal CRP schizophrenia group after matching for BMI. These results suggest that immune-related mechanisms may be a contributing factor to working memory/reasoning deficits (which are known to be particularly dependent on a healthy prefrontal cortex) in schizophrenia. Given that working memory deficits are particularly predictive of long-term functional outcome (71), the influence of elevated CRP on working memory/reasoning suggests that adjunctive anti-inflammatory treatments may reduce cognitive deficits and improve daily function in a substantial subset of patients with schizophrenia. Our results suggest that measuring CRP levels in blood of chronically ill people with schizophrenia would also be an indicator of impaired prefrontal cortex function.
Contrary to our hypotheses, there were no significant differences between elevated and normal CRP patient groups in relation to symptom severity as measured by PANSS ratings. This finding is discordant with results from a previous study which reported greater severity of negative and general symptoms in patients with schizophrenia displaying elevated CRP levels (42). However, this difference may be due to inclusion/exclusion criteria and sampling differences. For instance, the sample used in the previous study consisted of patients with moderately high levels of symptom severity (42), whereas, the patients in the current sample displayed mild to moderate levels of symptom severity. In support of this view, the results of the current study are consistent with other studies that have failed to find an association between inflammation markers and symptom severity in patients with mild to moderate symptom severity (40). Thus, the presence of greater illness severity appears to be necessary to reveal the relationship between illness severity and inflammation.
Our study is the first to assess brain cortical thickness measures in relation to CRP levels in patients with schizophrenia and healthy controls. CRP levels made a significant contribution to cortical thickness in 8 out of 9 regions including the frontal pole, medial orbital frontal, lateral orbital frontal, middle temporal cortices, temporal pole, entorhinal cortex, insula, and paracentral gyrus in both patients and controls. An inverse relationship between CRP levels and cortical thickness in prefrontal regions is consistent with the elevated CRP patients with schizophrenia displaying a significantly worse working memory performance relative to patients with schizophrenia who display normal CRP levels; however, it is typically aberrant activity in the dorsolateral prefrontal cortex, not other prefrontal regions that is associated with changes in working memory in people with schizophrenia (72). However, others have shown activation of the inferior frontal gyrus in healthy adults and decreased blood flow to the middle temporal gyrus in coronary artery disease during mental stress induced by arithmetic (73) suggesting involvement of both ventral regions and temporal regions to math challenges. Since thinner cortex in temporal regions also showed a relationship to elevated CRP, CRP might have been expected to correlate with episodic memory performance. Although there was such a relationship (see Table 8 for delayed recall in controls) it did not survive correction for multiple comparisons suggesting that this relationship between CRP and delayed memory was less robust than that for working memory and prefrontal cortex. One limitation of this study would be in relation to the restricted number of inflammation markers assessed in our study; however, we have reported on the relationship of other peripheral inflammation markers (e.g., IL-1β) to cognition and brain volume in a smaller sample of chronically ill patients with schizophrenia (30). Overall, these results in relation to CRP levels and cortical thickness provide further support for the role of inflammatory processes especially influencing prefrontal brain structure and cognition in patients with schizophrenia.
We assessed the proportions of patients displaying a marker of inflammation (CRP) in two independent samples (in chronically ill patients with schizophrenia and over repeated admissions in patients experiencing an acute psychotic episode) and the ability of CRP to predict cortical thickness. The results of our study in patients admitted for an acute psychotic episode showed that acute psychosis is often associated with a relatively high level of systemic inflammation as measured by elevated CRP, NLR and positive ANA titers. In our study of chronically ill patients with schizophrenia, we showed that CRP was also significantly elevated relative to healthy controls. A larger proportion of individuals (~60%) with acute psychosis had elevated levels of CRP, compared to ~44% of the chronically ill patients with schizophrenia. This is consistent with findings that elevated CRP may decrease with resolution of psychotic symptoms (45). Some healthy controls also displayed elevated CRP, although the proportion (20%) was significantly lower than the proportions displaying elevated CRP in chronically ill patients with schizophrenia and those patients admitted for an acute psychotic episode. The finding of elevated CRP in some healthy controls is not unexpected as slightly increased and/or chronic immune activation can be found in otherwise healthy samples given that common inflammatory processes, including allergies, asthma, or arthritis may be present in otherwise healthy individuals, e.g., the prevalence of arthritis is 22.7% annually in the US (74).
The chronically ill patients with schizophrenia who displayed elevated CRP had significantly worse working memory performance. This is consistent with other studies indicating an association between CRP and cognitive deficits (such as global cognition, attention/psychomotor speed, language/executive function, memory, and visual spatial ability) in other populations including individuals with cardiovascular disease (75), obstructive sleep apnoea (76), and mild cognitive impairment (77). Given that BMI also differed between elevated and normal CRP patient groups in our study, we showed that after matching on BMI the significant difference in arithmetic performance remained between the elevated and normal CRP patients with schizophrenia; suggesting that factors intrinsic to schizophrenia and inflammation may underlie the cognitive deficits in this subgroup of patients. The presence of a significant difference in cognitive variables between elevated and normal CRP groups in schizophrenia coupled with the lack of significant cognitive differences in the healthy sample also supports the hypothesis suggesting a role of inflammatory processes in schizophrenia.
In conclusion, the current results show that CRP levels are substantially elevated in acute psychosis involving the majority of patients who presented to a mental health intensive care inpatient ward. While we did find some reduction of inflammation across admissions, we saw continued significantly increased CRP in these readmitted patients and a lower, but still significant CRP elevation in chronically ill patients with schizophrenia. NLR remained significantly elevated in the elevated CRP group of patients admitted for an acute psychotic episode after covarying for BMI, suggesting that the elevated inflammation would not be fully explained by BMI in these acutely ill patients. Similarly, significant working memory differences remained between elevated and normal CRP chronically ill patient groups after covarying for BMI, again suggesting that cognitive deficits associated with inflammation were not due to elevated BMI. Importantly, we provide further novel evidence that elevated CRP is not occurring in isolation of brain changes. We found significant relationships among peripheral CRP and measures of human brain structure and function especially involving the frontal lobe suggesting that this highly evolved human brain region may be especially sensitive to inflammation. This current work supports our earlier work (30) on the relationship between worse verbal fluency, lower Broca's area volume and higher IL-1β levels in patients with schizophrenia, suggesting that anti-inflammatory drugs may be useful to include as adjunctive treatments in those patients showing elevated inflammatory markers (15, 78).
IJ, CS, RV, HP, MO, RL, CG, CW, and TW designed and conducted the project. IJ and RB entered data, managed the database, analyzed and interpreted the data. RV and HP assisted with data entry and database management. RL, JB, CG, DL, CW, and TW assisted with interpretation of data. IJ wrote the initial draft of the manuscript, and all authors contributed comments and edited the manuscript.
This work was supported by the University of New South Wales School of Psychiatry, the National Health and Medical Research Council (NHMRC) of Australia Project Grant no. 568807, Neuroscience Research Australia, the Schizophrenia Research Institute utilizing infrastructure funding from NSW Ministry of Health and the Macquarie Group Foundation, and the Australian Schizophrenia Research Bank, which was supported by the NHMRC of Australia, the Pratt Foundation, Ramsay Health Care and the Viertel Charitable Foundation. CW is a recipient of a NHMRC (Australia) Principal Research Fellowship (#1117079).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank Michelle Hill, Julia Hill, and Bronwyn Overs for their work using Freesurfer to process the structural MRI data, Drs. Daniel Pellen and Peter Nichols for contributing to patient management, Julia Langton and Loretta Moore for administering cognitive and/or symptom assessments, Ans Vercammen for administering some cognitive, symptom, and psychiatric assessments, Nick Vella, Merribel Kyaw, Selena Hu, Alice Rothwell, and Beverly Hisee for screening participants, data entry, management, and monitoring, Roxanne Cadiz for blood collection and performing CRP assays, and Richard Morris and Ashley Skilleter for collection of the imaging data.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC. (2013).
2. Weickert CS, Weickert TW, Pillai A, Buckley P. Biomarkers in schizophrenia: A brief conceptual consideration. Dis Markers (2013) 35:3–9. doi: 10.1155/2013/510402
3. Weickert CS, Weickert TW. What's hot in schizophrenia research? Psychiatr Clin North Am. (2016) 39:343–51. doi: 10.1016/j.psc.2016.01.011
4. Gibney SM, Drexhage HA. Evidence for a dysregulated immune system in the etiology of psychiatric disorders. J Neuroimmune Pharmacol. (2013) 8:900–20. doi: 10.1007/s11481-013-9462-8
5. Horvath S, Mirnics K. Immune system disturbances in schizophrenia. Biol Psychiatry. (2014) 75:316–23. doi: 10.1016/j.biopsych.2013.06.010
6. Muller N. Immunology of schizophrenia. Neuroimmunomodulation (2014) 21:109–16. doi: 10.1159/000356538
7. Brown A, Derkits E. Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. Am J Psychiatry (2010) 167:261–80. doi: 10.1176/appi.ajp.2009.09030361
8. Benros ME, Pedersen MG, Rasmussen H, Eaton WW, Nordentoft M, Mortensen PB. A nationwide study on the risk of autoimmune diseases in individuals with a personal or a family history of schizophrenia and related psychosis. Am J Psychiatry (2014) 171:218–26. doi: 10.1176/appi.ajp.2013.13010086
9. Chen S-J, Chao Y-L, Chen C-Y, Chang C-M, Wu EC-H, Wu C-S, et al. Prevalence of autoimmune diseases in in-patients with schizophrenia: nationwide population-based study. Br J Psychiatry (2012) 200:374–80. doi: 10.1192/bjp.bp.111.092098
10. Cazzullo C, Sacchetti E, Galluzzo A, Panariello A, Adorni A, Pegoraro M, et al. Cytokine profiles in schizophrenic patients treated with risperidone: a 3-month follow-up study. Prog Neuropsychopharmacol Biol Psychiatry (2002) 26:33–9. doi: 10.1016/S0278-5846(01)00221-4
11. Zhang X, Zhou D, Cao L, Zhang P, Wu G, Shen Y. Changes in serum interleukin-2,−6, and−8 levels before and during treatment with risperidone and haloperidol: relationship to outcome in schizophrenia. J Clin Psychiatry (2004) 65:940–7. doi: 10.4088/JCP.v65n0710
12. Meyer J, McEvoy J, Davis V. Inflammatory markers in schizophrenia: comparing antipsychotic effects in Phase 1 of the CATIE Schizophrenia Trial. Biol Psychiatry (2009) 66:1013–22. doi: 10.1016/j.biopsych.2009.06.005
13. Laan W, Grobbee DE, Selten JP, Heijnen CJ, Kahn RS, Burger H. Adjuvant aspirin therapy reduces symptoms of schizophrenia spectrum disorders: results from a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry (2010) 71:520–7. doi: 10.4088/JCP.09m05117yel
14. Muller N, Riedel M, Scheppach C, Brandstatter B, Sokullu S, Krampe K, et al. Beneficial antipsychotic effects of celecoxib add-on therapy compared to risperidone alone in schizophrenia. Am J Psychiatry (2002) 159:1029–34. doi: 10.1176/appi.ajp.159.6.1029
15. Chaudhry I, Hallak J, Husain N, Minhas F, Stirling J, Richardson P, et al. Minocycline benefits negative symptoms in early schizophrenia: a randomised double-blind placebo-controlled clinical trial in patients on standard treatment. J Psychopharmacol. (2012) 26:1185–93. doi: 10.1177/0269881112444941
16. Levkovitz Y, Mendlovich S, Riwkes S, Yoram B, Levkovitch-Verbin H, Gal G. A double-blind, randomized study of minocycline for the treatment of negative and cognitive symptoms in early-phase schizophrenia. J Clin Psychiatry (2010) 71:138–49. doi: 10.4088/JCP.08m04666yel
17. Fillman SG, Cloonan N, Catts VS, Miller LC, Wong J, McCrossin T, et al. Increased inflammatory markers identified in the dorsolateral prefrontal cortex of individuals with schizophrenia. Mol Psychiatry (2013) 18:206–14. doi: 10.1038/mp.2012.110
18. Potvin S, Stip E, Sephry AA, Gendron A, Ramatoulaye B, Kouassi E. Inflammatory cytokine alterations in schizophrenia: a systematic quantitative review. Biol Psychiatry (2008) 63:801–8. doi: 10.1016/j.biopsych.2007.09.024
19. Volk D, Chitrapu A, Edelson J, Roman K, Moroco A, Lewis D. Molecular mechanisms and timing of cortical immune activation in schizophrenia. Am J Psychiatry (2015) 172:1112–21. doi: 10.1176/appi.ajp.2015.15010019
20. Miller BJ, Mellor A, Buckley P. Total and differential white blood cell counts, high-sensitivity C-reactive protien, and the metabolic syndrome in non-affective psychoses. Brain Behav Immun. (2013) 31:82–9. doi: 10.1016/j.bbi.2012.08.016
21. Schott K, Uhl A, Batra A, Bartels M, Eusterschulte B, Buchkremer G. Antinuclear antibodies in schizophrenia and major depressive disorder - A lasting puzzle. Eur Psychiatry (1996) 11:263–7. doi: 10.1016/0924-9338(96)82334-0
22. Spivak B, Radwan M, Bartur P, Mester R, Weizman A. Antinuclear autoantibodies in chronic schizophrenia. Acta Psychiatr Scand. (1995) 92:266–9. doi: 10.1111/j.1600-0447.1995.tb09581.x
23. Kulaksizoglu B, Kulaksizoglu S. Relationship between neutrophil/lymphocyte ratio with oxidative stress and psychopathology in patients with schizophrenia. Neuropsychiatr Dis Treat (2016) 12:1999–2005. doi: 10.2147/NDT.S110484
24. Semiz M, Yildirim O, Canan F, Demir S, Hasbek E, Tuman TC, et al. Elevated neutrophil/lymphocyte ratio in patients with schizophrenia. Psychiatr Danub. (2014) 26:220–5.
25. Fernandes BS, Steiner J, Bernstein H-G, Dodd S, Pasco JA, Dean OM, et al. C-reactive protein is increased in schizophrenia but is not altered by antipsychotics: meta-analysis and implications. Mol Psychiatry (2016) 21:554–64. doi: 10.1038/mp.2015.87
26. Singh B, Chaudhuri TK. Role of C-reactive protein in schizophrenia: an overview. Psychiatry Res. (2014) 216:277–85. doi: 10.1016/j.psychres.2014.02.004
27. Cannon T, Chung Y, He G, Sun D, Jacobson A, van Erp T, et al. Progressive reduction in cortical thickness as psychosis develops: a multisite longitudinal neuroimaging study of youth at elevated clinical risk. Biol Psychiatry (2015) 77:147–57. doi: 10.1016/j.biopsych.2014.05.023
28. Perkins D, Jeffries C, Addington J, Bearden C, Cadenhead K, Cannon T, et al. Towards a psychosis risk blood diagnostic for persons experiencing high-risk symptoms: preliminary results from the NAPLS project. Schizophr Bull. (2015) 41:419–28. doi: 10.1093/schbul/sbu099
29. Zajkowska Z, Mondelli V. First-episode psychosis: an inflammatory state? Neuroimmunomodulation. (2014) 21:102–8. doi: 10.1159/000356536
30. Fillman SG, Weickert TW, Lenroot RK, Catts SV, Bruggemann JM, Catts VS, et al. Elevated peripheral cytokines characterize a subgroup of people with schizophrenia displaying poor verbal fluency and reduced Broca's area volume. Mol Psychiatry (2016) 21:1090–8. doi: 10.1038/mp.2015.90
31. Miller BJ, Buckley P, Seabolt W, Mellor A, Kirkpatrick B. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry (2011) 70:663–71. doi: 10.1016/j.biopsych.2011.04.013
32. Pepys M, Hirschfield G. C-reactive protein: a critical update. J Clin Invest. (2003) 111:1805–12. doi: 10.1172/JCI200318921
33. Deodhar S. C-reactive protein: the best laboratory indicatory available for monitoring disease activity. Cleve Clin J Med. (1989) 56:126–30. doi: 10.3949/ccjm.56.2.126
34. Libby P, Ridker P. Inflammation and atherosclerosis: role of C-reactive protein in risk assessment. Am J Med. (2004) 116:9–16. doi: 10.1016/j.amjmed.2004.02.006
35. Tsimikas S, Willerson J, Ridker P. C-reactive protein and other emerging blood biomarkers to optimize risk stratification of vulnerable patients. J Am Coll Cardiol. (2006) 47:C19–C31. doi: 10.1016/j.jacc.2005.10.066
36. Miller BJ, Culpepper N, Rapaport MH. C-Reactive protein levels in schizophrenia: a review and meta-analysis. Clin Schizophr Relat Psychoses. (2014) 7:223–30. doi: 10.3371/CSRP.MICU.020813
37. Ridker P, Danielson E, Fonseca F, Genest J, Gotto A, Katstelein J, et al. Reduction in C-reactive protein and LDL cholesterol and cardiovascular event rates after initiation of rosuvastatin: a prospective study of the JUPITER trial. Lancet (2009) 373:1175–82. doi: 10.1016/S0140-6736(09)60447-5
38. Metcalf S, Jones PB, Nordstrom T, Timonen M, Maki P, Miettunen J, et al. Serum C-reactive protein in adolescence and risk of schizophrenia in adulthood: a prospective birth cohort study. Brain Behav Immun. (2017) 59:253–9. doi: 10.1016/j.bbi.2016.09.008
39. Bulzacka E, Boyer L, Schurhoff F, Godin O, Berna F, Brunel L, et al. Chronic peripheral inflammation is associated with cognitive impairment in schizophrenia: results from the multicentric FACE-SZ dataset. Schizophr Bull. (2016) 42:1290–302. doi: 10.1093/schbul/sbw029
40. Dickerson F, Stallings C, Origoni A, Boronow J, Yolken R. C-reactive protein is associated with the severity of cognitive impairment but not of psychiatric symptoms in individuals with schizophrenia. Schizophr Res. (2007) 93:261–5. doi: 10.1016/j.schres.2007.03.022
41. Johnsen E, Fathian F, Korken R, Steen V, Jorgensen H, Gjestad R, et al. The serum level of C-reactive protein (CRP) is associated with cognitive performance in acute phase psychosis. BMC Psychiatry (2016) 16:1–11. doi: 10.1186/s12888-016-0769-x
42. Fan X, Pristach C, Liu EY, Freudenreich O, Henderson DC, Goff DC. Elevated serum levels of C-reactive protein are associated with more severe psychopathology in a subgroup of patients with schizophrenia. Psychiatry Res. (2007) 149:267–71. doi: 10.1016/j.psychres.2006.07.011
43. Solanki R, Singh P, Singh M, Sinha M, Swami M, Saini S. C-reactive protein (CRP) in patients with schizophrenia: are they related with symptomatology? J Mental Health Hum Behav. (2009) 15:6–10.
44. Mazzarello V, Cecchini A, Fenu G, Rassu M, Dessy LA, Lorettu L, et al. Lymphocytes in schizophrenia patients under therapy: serological, morphological and cell subset findings. Ital J Anat Embryol. (2004) 109:177–88.
45. Ohaeri JU, Hedo CC, Lagundoye OO. The profile of C-reactive proteins in functional psychotic states in a cohort in Nigeria. Acta Psychiatr Scand. (1993) 88:252–5. doi: 10.1111/j.1600-0447.1993.tb03452.x
46. Bettcher B, Wilheim R, Rigby T, Green R, Miller J, Racine C, et al. C-reactive protein is related to memory and medial temporal brain volume in older adults. Brain Behav Immun. (2012) 26:103–8. doi: 10.1016/j.bbi.2011.07.240
47. Taki Y, Thyreau B, Kinomura S, Sato K, Goto R, Wu K, et al. Correlation between high-sensitivity C-reactive protein and brain gray matter volume in healthy elderly subjects. Hum Brain Mapp. (2013) 34:2418–24. doi: 10.1002/hbm.22073
48. Wing JK, Beevor AS, Curtis RH, Park SBG, Hadden S, Burns A. Health of the Nation Outcome Scales (HoNOS). Br J Psychiatry (1998) 172:11–8. doi: 10.1192/bjp.172.1.11
49. Woods S. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry (2003) 64:663–7. doi: 10.4088/JCP.v64n0607
50. Cohen, J. (1988). Statistical Power Analysis for the Behavioral Sciences. 2nd ed. New Jersey, USA: Lawrence Erlbaum.
51. Shcherbakova I, Neshkova E, Dotsenko V, Platonova T, Schcherbakova E, Yaravaya G. The possible role of plasma kallikrein-kinin system and leukocyte elastase in the pathogenesis of schizophrenia. Immunopharmacology (1999) 43:273–9. doi: 10.1016/S0162-3109(99)00099-5
52. Barzilay R, Lobel T, Krivoy A, Shloserg D, Weizman A, Katz N. Elevated C-reactive protein in schizophrenia inpatients is associated with aggressive behavior. Eur Psychiatry (2016) 31:8–12. doi: 10.1016/j.eurpsy.2015.09.461
53. Raj P, Li Q-Z, Karp D, Olsen N, Sivils K, James J, et al. Antinuclear antibodies in general population: what does that mean? J Immunol. (2013) 190:197–192.
54. Yamada S, Gotoh T, Nakashima Y, Kayaba K, Ishikawa S, Nago N, et al. Distribution of serum C-reactive protein and its association with atherosclerotic risk factors in a Japanese population: jichi medical school cohort study. Am J Epidemiol. (2001) 153:1183–90. doi: 10.1093/aje/153.12.1183
55. Costello E, Copeland W, Shanahan L, Worthman C, Angold A. C-reactive protein and substance use disorders in adolescence and early adulthood: a prospective analysis. Drug Alcohol Depend (2013) 133:712–7. doi: 10.1016/j.drugalcdep.2013.08.027
56. O'Loughlin J, Lambert M, Karp I, McGrath J, Gray-Donald K, Barnett T, et al. Association between cigarette smoking and C-reactive protein in a representative, population-based sample of adolescents. Nicotine Tob Res. (2008) 10:525–32. doi: 10.1080/14622200801901997
57. Weickert TW, Weinberg D, Lenroot R, Catts SV, Wells R, Vercammen A, et al. Adjunctive raloxifene treatment improves attention and memory in men and women with schizophrenia. Mol Psychiatry (2015) 20:685–94. doi: 10.1038/mp.2015.11
58. First MB, Spitzer RL, Miriam G, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. (SCID-I/P). New York, NY: Biometrics Research, New York State Psychiatric Institute (2002).
62. Reitan RWD. The Halstead-Reitan Neuropsychological Test Battery: Therapy and Clinical Interpretation (1985). Tucson, AZ: Neuropsychological Press.
64. Kay SR, Fiszbein A, Opfer LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. (1987) 13:261–76. doi: 10.1093/schbul/13.2.261
65. Henry JD, Crawford JR. The short-form version of the Depression Anxiety Stress Scales (DASS-21): construct validity and normative data in a large non-clinical sample. Br J Clin Psychol. (2005) 44:227–39. doi: 10.1348/014466505X29657
66. Ware JE, Kosinski M, Dewey JE, Gandek B. SF-36 Health Survey: Manual and Interpretation Guide. Lincoln, RI: Quality Metric Inc. (2000).
67. Wilkinson G, Hesdon B, Wild D, Cookson R, Farina C, Sharma V, et al. Self-report quality of life measure for people with schizophrenia: the SQLS. Br J Psychiatry (2000) 177:42–6. doi: 10.1192/bjp.177.1.42
68. Benjamin Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol. (1995) 57:289–300.
69. Boerrigter D, Weickert TW, Lenroot R, O'Donnell M, Galletly C, et al. Using blood cytokine measures to define high inflammatory biotype of schizophrenia and schizoaffective disorder. J Neuroinflammation. (2017) 14:188. doi: 10.1186/s12974-017-0962-y
70. Yaffe K, Lindquist K, Penninx BW, Simonsick EM, Pahor M, Kritchevsky S, et al. Inflammatory markers and cognition in well-functioning African-American and white elders. Neurology (2003) 61:76–80. doi: 10.1212/01.WNL.0000073620.42047.D7
71. Green M, Kern R, Braff D, Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: Are we measuring the “right stuff”? Schizophr Bull. (2000) 26:119–36. doi: 10.1093/oxfordjournals.schbul.a033430
72. Callicott JH, Mattay VS, Verchinski BA, Marenco S, Egan MF, Weinberger DR. Complexity of prefrontal cortical dysfunction in schizophrenia: more than up or down. Am J Psychiatry (2003) 160:2209–15. doi: 10.1176/appi.ajp.160.12.2209
73. Soufer R, Bremner JD, Arrighi JA, Cohen I, Zaret BL, Burg MM, et al. Cerebral cortical hyperactivation in response to mental stress in patients with cornary artery disease. Proc Natl Acad Sci USA. (1998) 95:6454–9. doi: 10.1073/pnas.95.11.6454
74. Barbour KE, Helmick CG, Boring MA, Brady TJ. Vital signs: prevalence of doctor-diagnosed arthritis and arthritis-attributable activity limitation - United States, 2013-2015. Morb Mortal Wkly Rep. (2017) 66:246–53. doi: 10.15585/mmwr.mm6609e1
75. Gunstad J, Bausserman L, Paul R, Tate D, Hoth K, Poppas A, et al. C-reactive protein, but not homocysteine, is related to cognitive dysfunction in older adults with cardiovascular disease. J Clin Neurosci. (2006) 13:540–6. doi: 10.1016/j.jocn.2005.08.010
76. Gozal D, Crabtree V, Capdevila O, Witcher L, Keirandish-Gozal L. C-reactive protein, obstructive sleep apnea, and cognitive dysfunction in school-aged children. Am J Respir Crit Care Med. (2007) 176:188–93. doi: 10.1164/rccm.200610-1519OC
77. Trollor J, Smith E, Baune B, Kochan N, Campbell L, Samaras K, et al. Systemic inflammation is associated with MCI and its subtypes: the Sydney memory and aging study. Dement Geriatr Cogn Disord. (2010) 30:569–78. doi: 10.1159/000322092
Keywords: schizophrenia, acute psychosis, c-reactive protein, cortical thickness, inflammation, working memory, cognition, CRP
Citation: Jacomb I, Stanton C, Vasudevan R, Powell H, O'Donnell M, Lenroot R, Bruggemann J, Balzan R, Galletly C, Liu D, Weickert CS and Weickert TW (2018) C-Reactive Protein: Higher During Acute Psychotic Episodes and Related to Cortical Thickness in Schizophrenia and Healthy Controls. Front. Immunol. 9:2230. doi: 10.3389/fimmu.2018.02230
Received: 24 June 2018; Accepted: 07 September 2018;
Published: 10 October 2018.
Edited by:
Valentin A. Pavlov, Northwell Health, United StatesReviewed by:
Sergio Iván Valdés-Ferrer, Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, MexicoCopyright © 2018 Jacomb, Stanton, Vasudevan, Powell, O'Donnell, Lenroot, Bruggemann, Balzan, Galletly, Liu, Weickert and Weickert. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Thomas W. Weickert, dC53ZWlja2VydEB1bnN3LmVkdS5hdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.