- 1Division of Pathology, ICAR-Indian Veterinary Research Institute, Bareilly, India
- 2Central University Laboratory, Tamil Nadu Veterinary and Animal Sciences University, Chennai, India
- 3Department of Biochemistry and Genetics, Barkatullah University, Bhopal, India
- 4Department of Veterinary Microbiology, College of Veterinary Sciences and Animal Husbandry, Agartala, India
- 5Immunology Section, ICAR-Indian Veterinary Research Institute, Bareilly, India
- 6Division of Veterinary Biotechnology, ICAR-Indian Veterinary Research Institute, Bareilly, India
- 7Division of Virology, ICAR-Indian Veterinary Research Institute, Nainital, Uttarakhand, India
- 8Division of Biological Standardization, ICAR-Indian Veterinary Research Institute, Bareilly, India
- 9Division of Veterinary Public Health, ICAR-Indian Veterinary Research Institute, Bareilly, India
- 10ICAR-Indian Veterinary Research Institute, Bareilly, Uttar Pradesh, India
- 11Center of Research Excellence on Therapeutic Proteins and Antibody Engineering, Department of Parasitology, Faculty of Medicine SIriraj Hospital, Mahidol University, Bangkok, Thailand
Ebola virus (EBOV), a member of the family Filoviridae, is responsible for causing Ebola virus disease (EVD) (formerly named Ebola hemorrhagic fever). This is a severe, often fatal illness with mortality rates varying from 50 to 90% in humans. Although the virus and associated disease has been recognized since 1976, it was only when the recent outbreak of EBOV in 2014–2016 highlighted the danger and global impact of this virus, necessitating the need for coming up with the effective vaccines and drugs to counter its pandemic threat. Albeit no commercial vaccine is available so far against EBOV, a few vaccine candidates are under evaluation and clinical trials to assess their prophylactic efficacy. These include recombinant viral vector (recombinant vesicular stomatitis virus vector, chimpanzee adenovirus type 3-vector, and modified vaccinia Ankara virus), Ebola virus-like particles, virus-like replicon particles, DNA, and plant-based vaccines. Due to improvement in the field of genomics and proteomics, epitope-targeted vaccines have gained top priority. Correspondingly, several therapies have also been developed, including immunoglobulins against specific viral structures small cell-penetrating antibody fragments that target intracellular EBOV proteins. Small interfering RNAs and oligomer-mediated inhibition have also been verified for EVD treatment. Other treatment options include viral entry inhibitors, transfusion of convalescent blood/serum, neutralizing antibodies, and gene expression inhibitors. Repurposed drugs, which have proven safety profiles, can be adapted after high-throughput screening for efficacy and potency for EVD treatment. Herbal and other natural products are also being explored for EVD treatment. Further studies to better understand the pathogenesis and antigenic structures of the virus can help in developing an effective vaccine and identifying appropriate antiviral targets. This review presents the recent advances in designing and developing vaccines, drugs, and therapies to counter the EBOV threat.
Introduction
Ebola virus (EBOV; Zaire ebolavirus) is the causative agent of a severe hemorrhagic fever disease, Ebola virus disease (EVD; formerly called Ebola hemorrhagic fever). It was first recognized in 1976 in northern Democratic Republic of Congo, at that time Zaire (1–3). Since then, EVD is endemic in Africa. Fruit bats are the best-known reservoirs of EBOV (4). EVD is a well-established zoonotic disease; the initial cases of the EVD outbreaks occur after contact with reservoir or materials contaminated with the virus and followed by human-to-human transmission (5). EBOV is not only a serious public health issue but now also designated as category A pathogen and considered as a potential bioterrorism agent (6, 7). EBOV causes high mortality rates of up to 88% in the infected humans (8); therefore, it is classified as a risk group 4 agent and handled under biosafety level-4 containment. The risk of mortality is relatively greater in the elderly and/or patients with high viral load and poor immune response at the initial stage of the infection (9).
The EBOV belongs to the Filoviridae family and has a unique thin filamentous structure that is 80-nm wide and up to 14-µm long. Its envelope is decorated with spikes of trimeric glycoprotein (GP1,2) which are responsible for mediating viral entry into target cells (function of GP1) (10) and release of viral ribonucleoprotein from endosome to cytoplasm for replication (function of GP2) (11, 12). EBOV infects primarily humans, simians, and bats; but other species such as mice, shrew, and duikers may also contact infection (3, 13). Of the five identified EBOV species, four species, viz., EBOV, Sudan virus (SUDV; Sudan ebolavirus), Tai Forest virus (TAFV; Tai Forest ebolavirus, formerly Côte d’Ivoire ebolavirus), and Bundibugyo virus (BDBV; Bundibugyo ebolavirus), are known to infect humans and cause disease, whereas Reston virus (RESTV; Reston ebolavirus) is non-human primate (NHP) pathogen.
After an initial incubation period of 3–21 days, the disease progresses quickly to fever, intense fatigue, diarrhea, anorexia, abdominal pain, hiccups, myalgia, vomiting, confusion, and conjunctivitis (14) which may lead to the loss of vision (15). EBOV can spread from males to females through semen (16) and from mother to fetus and infant during gestation and lactation, respectively (17). Of the note, in an EBOV-infected patient, higher concentration of Ebola viral RNA in semen was noticed during the recovery period than the viral concentration in the blood during peak time of infection, suggesting male genital organ as virus predilection site for replication (18). Usually the human immune system mounts a response against infectious pathogens by sensing the pathogen-associated molecular patterns via a variety of pathogen-recognition receptors. Nevertheless, in the case of EBOV, innate immunity is impaired by the immunosuppressive viral proteins including VP35 and VP24, and lymphocytes are depleted as a result of apoptosis caused by inappropriate dendritic cell (DC)–T-cell interaction (7, 19). A thorough understanding on the pathogenesis of this deadly virus is essential because of its severe health impacts (20).
The increased incidences and fast spread of EBOV paving into a pandemic flight has compelled more focus of research to develop strategies and remedial measures for mitigating the impact and consequential severity of the viral infection. Even before delineating the less studied Ebola viral genome fully, researchers throughout the globe and health industry were pressured to focus on the development of effective and safe Ebola vaccines and therapeutics (21, 22). As of now, no licensed vaccines and direct-acting anti-EBOV agents are available to protect against the lethal viral infection or to treat the disease. To minimize the suffering, EBOV-infected patients are only provided with symptomatic treatment and supportive care. Because of its high pathogenicity and mortality rate, preventive measures, prophylactics, and therapeutics are essential, and researchers worldwide are working to develop effective vaccines, drug, and therapeutics, including passive immunization and antibody-based treatments for EVD (23–26). Prior to the 2014–2016 EBOV outbreak in West Africa, which has been the deadliest EBOV outbreak to date, convalescent blood products from survivors of EVD represented the only recommended treatment option for newly infected persons. Administration of monoclonal antibody (mAb) cocktails (ZMapp, ZMAb, and MB-003) as post-exposure prophylactics have been found to reverse the advanced EVD in NHPs and/or effectively prevented morbidity and mortality in NHPs (27–30).
There is the need for an effective vaccine against EBOV, especially in high-risk areas, to prevent infections in physicians, nurses, and other health-care workers who come into contact with diseased patients (31). Regular monitoring and surveillance of EBOV is essential to control this disease. In the EBOV outbreak, novel surveillance approaches include contact tracing with coordination at the national level and “lockdown” periods, during which household door-to-door reviews are conducted to limit the spread of the virus. Swift identification and confirmation of the Ebola cases and immediate follow-up of appropriate prevention and control measures, including safe burial of dead persons, are crucial practices to counter EBOV (32).
After the onset of EVD, treatment is required, whereas, when EBOV is circulating in population dense areas before infection, prophylactic measures like vaccination are necessary. One of the main challenges in containing EBOV is its presence in remote areas that lack technology and equipment to limit the virus spread. Because of its lethality, EBOV can only be handled in laboratories with biosecurity level-4 containment; thus, only few laboratories in the world can conduct EBOV research and testing of the counter measures against the authentic virus. Recent efforts by several organizations have focused on identifying effective therapies and developing appropriate vaccination strategies (33). Several drugs and vaccines have been developed against EBOV, and the production of low-cost drugs and vaccines against EBOV is essential for everyone, including those in the high-risk areas of the world, to be protected (26, 34). As of the acquisition of better knowledge against the pathogen due to improvement in the field of genomics and proteomics, there has been expansion in the field of vaccine synthesis where epitope-based vaccines are gaining top priority (35–37).
The present review aims to discuss advances in designing and development of EBOV vaccines, drugs, antibody-based treatments, and therapeutics, and their clinical efficacy in limiting EVD, thereby providing protection against the disease and alleviating high public health concerns associated with EBOV.
Advances in Developing Vaccines Against EBOV
There is a clear need for an effective vaccine to prevent the rapid spread of EVD. An inactivated EBOV vaccine was first produced in 1980. This vaccine was tested for efficacy in guinea pigs (7). Since that time, several vaccines against EBOV have been developed, but no vaccine is licensed and available in the market (7). After the massive 2014–2016 outbreak of EBOV, several researchers have begun working to develop an effective vaccine (38). For an EBOV vaccine candidate, a long-lasting immune response is essential; as EBOV remains in the seminal fluid of EVD survivors as long as 401 days post-infection (39, 40). Keeping this window of virus persistence, a vaccine conferring immunity at least for 2 years is recommended by the Wellcome Trust-CIDRAP Ebola Vaccine Team B initiative (41). Vaccines like the chimpanzee adenovirus type 3 (ChAd3)-based non-replicating ChAd3-EBO vaccine, prime-boost recombinant adenovirus type 26 vector (Ad26.ZEBOV) followed by the modified vaccinia Ankara vector (MVA-BN-Filoa) vaccine, adenovirus 5-vectored EBOV vaccine, EBOV DNA vaccine, and recombinant vesicular stomatitis virus (rVSV) vector-based vaccine are undergoing clinical trials to evaluate their efficacy against EVD (38). The RNA-dependent RNA polymerase (L) epitope-based vaccine was designed using immunoinformatics. Various software have been used to analyze immunological parameters, and this epitope vaccine was found to be a good candidate for use against EVD (42). Two conserved peptides of EBOV, 79VPSATKRWGFRSGVPP94 from GP1 and 515LHYWTTQDEGAAIGLA530 from GP2, were identified as targets for the development of an epitope-based vaccine (43). Collection of the sequences of EBOV glycoproteins and examination for determining the proteins with greatest immunogenicity have been performed using in silico methods. The best corresponding B and T cell epitopes included peptide regions encompassing residues 186–220 and 154HKEGAFFLY162, respectively. Such predicted epitopes can confer the long-lasting immunity against EBOV with better ability of protection (36).
Ebola virus-GP fused with the Fc fragment of a human IgG1 subunit vaccine administrated with alum, QS-21, or polyinosinic-polycytidylic acid-poly-l-lysine carboxymethylcellulose adjuvant induced strong humoral immunity in guinea pigs (44). Effectiveness of a ring vaccine using rVSVΔG/EBOVGP in cases of simulated EBOV disease was studied and even this approach can be employed during an outbreak situation (45). Notably, the neutralizing antibodies play a major role in conferring protection against EBOV infections. Thus, an EBOV vaccine capable of effectively inducing a long-lasting neutralizing antibody response is desirable for developing appropriate prevention strategies in combating the infection. In this line, the mucin-like domain of EBOV envelope glycoprotein GP1 has been identified to be critical in induction of protective humoral immune response (46, 47). Filorab 1 vaccine revealed desirable immunogenicity without the side effects. The main advantage of this vaccine is its higher immune response induction in chimpanzees (captive) when given orally and also with a single dose [instead of multiple doses as is required by virus-like particle (VLP) vaccine] (48). Modified mRNA-based vaccine constructs, formulated with lipid nanoparticles (LNPs) to facilitate delivery, are being tested against EBOV challenge in guinea pigs. These mRNAs induced robust immune responses and conferred up to 100% protection from the infection (49).
It is important to note that compilation of data in relation to immune responses (both induced by vaccines and natural infection) and the records of community members showing IgG seropositivity should be kept systematically. Assimilation of such information will help to handle next outbreaks with more rigidity, thereby helping to check EVD-associated disasters at an early stage (50). Vis-à-vis public health workers should also be vaccinated and mass vaccination programs should be undertaken through standardized and coordinated efforts (51).
The following section describes the various types of vaccines and vaccine platforms which are being explored for the development of a successful EBOV vaccine.
Inactivated Vaccines
Even though inactivated vaccines suffer with the problem of reversion to virulence due to inadequate viral inactivation, various strategies have been constantly explored in developing safe and potent non-replicating vaccine candidates for combating the EBOV infection (52). Both heat- and formalin-inactivated EBOV have been found protective against EBOV infection in a guinea pig model. Inclusion of inactivated vaccine with EBOV E-178 along with interferon (IFN) and immune plasma saved the life of a scientist working on EBOV (53). The protective efficacy of liposome-encapsulated irradiated EBOV, tested in a mouse model, was 100%. However, these viral particles failed to protect NHPs (54). This suggests that murine model is excellent for evaluating vaccine efficacy, but the level of protection might be different in different species and, hence, it is essential to test vaccines in NHPs before proceeding to clinical trials in humans. Heat-, formalin-, or gamma irradiation-killed EBOV vaccines have been found ineffective against EBOV disease; thus, the novel effective vaccine is essentially required (55).
DNA Vaccines
In DNA vaccines, plasmids are used to express immunogenic antigens. This is an attractive vaccine approach because of the ease of production and simplicity. In addition, DNA vaccine induces both humoral and cellular immune responses. A three-plasmid DNA vaccine comprising the transmembrane-deleted GP sequences from EBOV species Zaire and SUDV-Gulu as well as nucleoprotein (NP) sequence from EBOV was tested in healthy adults. The vaccine was well tolerated, and both CD4+ and CD8+ T cell responses were elicited (56). An EBOV GP DNA vaccine designed on a consensus alignment of GPs (from strains obtained during 1976–2014), delivered intramuscularly and then electroporated, elicited a strong T cell response, and protected 100% of experimental mice from lethal challenge with EBOV (57). The DNA from three strains of EBOV was used to prime human volunteers and boosted with attenuated adenovirus, which acted as delivery vehicle for EBOV DNA into antigen-presenting cells, induced significant humoral- and cell-mediated immune (CMI) responses (58). Intramuscular inoculation of the DNA vaccine through electroporation with DNA plasmid containing codon-optimized GP genes of EBOV elicited high levels of IgG and a strong CMI response (measured by IFN-γ ELISpot assay) in cynomolgus macaques (59).
Though the preliminary trials using DNA constructs have provided the acceptable safety profiles, the development of low immune titer for a shorter window necessitates repeated vaccinations to overcome this problem. Thus, the use of a potent vaccination regimen based on DNA vaccine platforms does not appear logical for a large population (60, 61).
Virus-Like Particles
Ebola VLPs (EBOV-VLPs or eVLPs) are generated from the expression of viral transmembrane glycoprotein (GP) and structural matrix protein (VP40) in mammalian cells, which undergo self-assembling and budding from host cells and display morphological similarity to infectious EBOV particles (47). Baculovirus-derived eVLPs comprising GP, VP40, and NP of EBOV have been found to induce human myeloid DC maturation, suggesting their immunogenicity. Baculovirus-generated VLPs were able to elicit similar levels of protection as 293T cell-derived VLPs and showed protection against virus challenge in a dose-dependent manner (62). Nano-VLPs, produced by sonication of VLPs and filtering to have a mean diameter of approximately 230 nm, increased their thermostability. Unlike native VLPs where GP protein is denatured in a solution by heating, the nano-VLP maintained the conformational integrity of the GP protein at temperature up to 70°C and could confer protection in a mouse model (63). VLP containing only VP40 was sufficient to protect mice from EBOV infection. VLP injection leads to an enhanced number of natural killer (NK) cells, which play a crucial role in innate immune protection against lethal EBOV. NK cell protection is dependent on perforin, but not recombinant viral vector vaccines on IFN-gamma secretion (64).
Ebola virus VP40 and GP have been demonstrated to interact with the host protein, BST2, and are associated with viral infections by trapping the newly assembled enveloped virions at the plasma membrane in the infected cells, ultimately induce NF-κB activity. The effects of EBOV GP1,2, VP40, and BST2 converge on an intracellular signaling pathway leads to neddylation, resulting in the additive response with respect to the induction of NF-κB activity. Exploring the dynamics of this interaction could provide targets for vaccine developments and therapies that can modulate the inflammatory response during EVD (65).
Quantitation of EBOV antigenic particles using proteomic assays like liquid chromatography high resolution mass spectrometry method can be employed for determining the batch quality of vaccine constructs as well as in optimizing the dosages by assessing the amount of GP1 needed to confer effective protection (47).
It is to be noted that though anti-EBOV antibody can mediate effective protection, VLP-vaccinated murine models were shown to survive the EBOV challenge in the absence of detectable serum anti-EBOV antibodies (66). It could also be revealed that adjuvant signaling may circumvent the necessity for B-cell immunity in conferring protection against EBOV. These studies can be valuable for the future characterization, development, and optimization of effective EBOV vaccine candidates (66).
Virus-Like Replicon Particles (VRPs)
The VRPs are the alternative to live-attenuated vaccines. The use of VRPs eliminates the risk of reversion to the original pathogenic form of live vaccine strains. To generate VRPs, generally filoviruses or alphaviruses are required. Here, while keeping the genes essential for replication, viral structural genes are deleted from full-length genomic cDNA clones. Viral structural genes are replaced with alternative gene(s) coding for an immunogen. Such replicons are able to replicate and transcribe upon transfection in competent cells. The resulting VRPs are able to infect cells only for one cycle. Because of the lack of structural genes, viral progeny are not formed. Viruses such as Venezuelan equine encephalitis virus (VEEV) can be used for production of EBOV antigen instead of structural proteins for the replicon vector. Thus, such vaccines are also quite safe (67). The gene inserted is typically GP, the main target of neutralizing antibodies. VRPs expressing EBOV VP24, VP30, VP35, and VP40 have been evaluated for their protective efficacy in a mouse model, but these were found not to be as protective as EBOV GP and NP antigens. VEEV replicons containing GPs from both EBOV and SUDV showed promising results in cynomolgus macaques after administration of a single dose. Here, two VRPs were constructed that contained the GP of EBOV or SUDV. The animals intramuscularly injected with both of the VRPs, survived viral challenge without exhibiting any clinical signs. The final results indicated that VRP-EBOV GP was able to confer cross-protection against SUDV, whereas VRP-SUDV GP was unable to provide complete protection against EBOV-Zaire challenge (68).
Recently, Ren et al. (69) constructed an alphavirus Semliki forest virus based recombinant replicon vector DREP for efficient and unchecked ex vivo co-expression of EBOV GP and VP40. Active immunization with recombinant DREP vectors possessing GP and VP40 induced cellular and humoral immune responses in murine model against EBOV antigens. This path breaking approach may provide key insights and strategies for designing further effective vaccines to contain EBOV permanently.
Reverse Genetics System for EBOV Vaccine
A full-length recombinant EBOV infectious clone was constructed using cDNA. By employing reverse genetics method, viable but replication incompetent virus lacking entire VP30 ORF was constructed. The resultant EbolaΔVP30 is biologically contained and replication deficient, until VP30 is provided extraneously. Virus replication in cell culture was allowed by growing the virus in Vero cell line that stably expresses VP30, designated VeroVP30 (70). The safety of EbolaΔVP30 has been evaluated in mice and guinea pig model and was able to protect from lethal infection (71). The EbolaΔVP30 virus inactivated by using hydrogen peroxide protected NHPs after a single immunization. To avoid any incidence of potential recombination events that might result in regaining the replicative efficiency, the vaccine candidate was inactivated by hydrogen peroxide, that creates nicks and breakages in single- or double-stranded DNA or RNA and the virus is completely inactivated while retaining antigenic determinants unaffected (72).
Recombinant Viral Vector Vaccines
Engineered viruses are gaining popularity because of their ability to efficiently induce CMI responses (a major part of adaptive immunity along with humoral response), as the antigen is expressed and processed in the cytoplasm. Replication-competent rVSV and chimpanzee adenovirus 3 (ChAd-3/cAd3) are the most efficient platforms for designing new vaccines (73). A recombinant vesiculovirus vector containing EBOV GP region (rVSVΔG/EBOVGP) was found to be highly effective after a single injection in NHPs (74, 75). The vaccine evaluated in pigs showed no disease development and no viral shedding. This indicated that the vaccine could be utilized for herd immunization and it also suggested the safety of the live-attenuated rVSVΔG/EBOVGP vaccine (76). Recently, this rVSVΔG/EBOVGP vaccine was evaluated in a randomized double blinded placebo phase III trial in 1,197 humans. There were no adverse effects or death following vaccination, supporting its use as a vaccine (77). The vaccine protected immunocompromised rhesus macaques that had a high number of CD4+ T cells (78). The rVSVΔG/EBOVGP vaccine was also studied for its efficacy as a therapy in rhesus monkeys after exposure to EBOV-Makona. This vaccine showed minimal prophylactic efficacy after exposure (79). Efficacy trials initiated to test the rVSV-vectored EBOV vaccine showed greater efficiency at the time of EVD outbreak, if deployed following the strategy of ring vaccination (80).
Another recombinant vaccine (VSV based), i.e., rVSV-Zaire EBOV has been shown to provide substantial protection. From 10th day of vaccination with this vaccine, no report of any disease was documented, which proved efficacy and effectiveness of rVSV-vectored vaccine in preventing EVD (81). It is interesting to note that seroconversion has been noticed in recipients of recombinant VSV-EBOV (rVSV-EBOV) vaccine by the end of fourth week (i.e., by 28 days) against the Kikwit strain glycoprotein (82). Another recombinant vaccine viz., rVSV-EBOV vaccine was tested as a candidate vaccine. This particular vaccine is under trial in human (phase II/III). It provides protection against only EBOV and is clinically efficient in the clinical set up of ring vaccination format (38, 83, 84). EBOV and SUDV glycoproteins have been assimilated into a cAdVax vector (adenovirus-based vaccine). In mice, this vaccine has provided full protection (85, 86). During recent outbreak in Democratic Republic of the Congo (DRC), rVSVΔG-EBOV-GP is being used for ring vaccination in the affected area. Though the vaccine is yet not approved and still under investigation.
In Russia, clinical trial of a vaccine, GamEvac-Combi, has been performed and has been approved to enter in phase III clinical trial (87). The vaccine GamEvac-Combi contained two heterologous expression systems. One is live-attenuated rVSV and the second is a recombinant replication-defective adenovirus type-5 (Ad5). Both the vectors are expressing the same glycoprotein. The rationale to use a combination of two vectors expressing glycoprotein of EBOV is that widely present preexisting immunity to Ad5 limits the use of Ad5 and also a negative correlation between EBOV glycoprotein-specific immune response and preexisting antibodies to Ad5 has been reported (88). Hence, prime immunization with VSV vectored vaccine and then boosting with AD5 vectored vaccine might contribute in compensating negative impacts of preexisting immune response to Ad5. This heterologous vaccine evoked glycoprotein-specific immune response in 100% volunteers on day 28th. Also, the vaccine is well tolerated and did not significantly altered the body physiological parameters and vital organs. In Liberia, Sierra Leone, and Guinea; the VSV and ChAd3 vectored vaccine are in focus (89).
Another study in mice models has reported that the adoption of a heterologous prime-boost vaccine strategy can result in a durable EBOV-neutralizing antibody response. The chimpanzee serotype 7 adenovirus vectors expressing EBOV GP (AdC7-GP) was used for priming and a truncated version of EBOV GP1 protein (GP1t) was used for boosting. Vaccination response studies showed that AdC7-GP prime/GP1t boost strategy was more potent in generating a sustained and strong immune response as compared to using an individual vaccine construct (90).
Replication-defective recombinant chimpanzee adenovirus type 3-vectored EBOV vaccine (cAd3-EBO) elicited both cell-mediated and humoral immunity in NHPs. A vaccine dose of 2 × 1011 particle units was found sufficient to induce protective immunity in the NHPs and to eliminate the effect of prior immunity to cAd3 (91). Recombinant VSV vaccine expressing EBOV GP and A/Hanoi/30408/2005 H5N1 hemagglutinin (VSVΔG-HA-ZGP) protected mice against challenge with both viruses and also cross-protected against H5N1 viruses (92).
The utility of adenovirus-vectored EBOV vaccines is limited with preexisting anti-adenoviral antibodies, which significantly lower the GP-specific humoral and T cell responses (88). Six mutations in the genome of MVA virus restrict its host specificity and make it unable to replicate in mammalian cells. A randomized study of a multivalent MVA vaccine encoding GPs from EBOV, SUDV, Marburg virus (MARV), and TAFV NP (MVA-BN-Filo) conducted in 87 participants resulted in no fever. The quadrivalent vaccine formulation has demonstrated the boosting up of both cellular and humoral immune responses against EBOV to several folds (93). Twenty-eight days after immunization, GP-specific IgG was detected with EBOV-specific T cell responses (94). EBOV GP and TAFV NP expressed in an MVA platform assembles into VLPs. Heterologous NPs enhanced VLP formation and offered GP-specific IgG1/IgG2a ratios comparable to those of MVA-BN-Filo (95).
Recombinant cytomegalovirus expressing EBOV GP was found to evoke protective immunity in rhesus monkeys challenged with EBOV (79). Baculovirus-expressed EBOV-Makona strain GP administered with Matrix-M (saponin adjuvant) showed better immunogenicity. Administration of Matrix M-adjuvanted vaccine resulted in increased IgG production and CD4+ and CD8+ T cell production (96). A human parainfluenza virus type 3-vectored vaccine expressing the GP of EBOV (HPIV3/EboGP) was developed as an aerosolized vaccine, and studies in Rhesus macaques showed 100% protection against challenge with EBOV (97).
Adenovirus 26 vectored glycoprotein/MVA-BN vaccine has recently passed the phase I trial (94). In the European countries including United Kingdom and United States, for the purpose of clinical trial, administration of ChAd-3 vectored vaccine has been adopted. This vaccine expresses the EBOV GP and is available in monovalent and divalent forms (91, 98).
Ebola vaccine potency trials employing replication defective adenoviral vectors (rAd) encoding EBOV GP have come up with promising results in NHP models. Based on such studies, multiple mutant glycoproteins were developed (such as glycoprotein with deleted transmembrane domain) which offers reduced in vitro cytopathogenicity but possessed reduced vaccine-mediated protection. In contrast to this, a point mutated glycoprotein has been reported to offer minimal cytopathogenicity and appropriate immune protection even with a two logs lower vaccine dose (99).
Plant-Based Vaccines and Antibodies
Viral antigens, including GP, VP40, and NP, elicit protective immune responses. ZMapp, the cocktail of antibodies being used to treat EBOV, is a biopharmaceutical drug. To note, the component antibodies in ZMapp are manufactured in Nicotiana benthamiana using a rapid antibody manufacturing platform. Gene transfer is mediated by a viral vector, and the expression is transient. N. benthamiana-derived antibodies produced stronger antibody-dependent cellular cytotoxicity than the analogous anti-EBOV mAbs produced in a mammalian Chinese hamster kidney cell line (100). Phoolcharoen (101) expressed a GP1 chimera with the heavy chain of 6D8 mAb, forming an immune complex that was co-expressed with the light chain of the same mAb in leaves of tobacco plant. The ammonium sulfate-precipitated purified antibodies, along with poly(I:C) adjuvant, a synthetic analog of double-stranded RNA capable of interacting with toll-like receptor (TLR)-3, was found to elicit strong neutralizing anti-EBOV IgG. In addition, the immune complex along with poly(I:C) adjuvant was capable of stimulating a Th1/Th2 response. The experiment suggested the potential application of plant-produced Ebola immune complexes as vaccine candidates. EBOV VP40 was expressed in tobacco plants, and a mouse immunization study showed results that suggested this approach can be used to produce an EBOV vaccine (102).
The utility of plants as bioreactors for the bulk production of ZMapp could be considered to meet the required demand. The glycosylation pattern of mAbs may alter their efficiency and bioactivity, including their binding with the antigenic epitope. Several glycoforms of EBOV mAb13F6 have been prepared using a magnICON expression system. These glycoforms have human-like biantennary N-glycans with terminal N-acetylglucosamine, resulting in a structure similar to that of human mAbs. Hence, these are beneficial for humans (103).
Both RNA and DNA viruses have been modified to serve as plant-based vectors for the expression of heterologous proteins. Bean yellow dwarf virus, a single stranded-DNA virus, can replicate inside the nucleus of plant cells using their cellular machinery. A vector containing deletions in the coat-encoding genes and gene for the desired antigen may be inserted to form an expression cassette. The delivery of vectors to plants is Agrobacterium-mediated (23). mAbs against EBOV are produced by the process of agroinfiltration. In this context, it is noteworthy that lettuce acts as a very good host for the process of agroinfiltration. In lettuce cells, Agrobacterium tumefaciens has been used for delivering viral vectors (104). Neutralizing and protective mAb6D8 against EBOV has been expressed at a concentration of 0.5 mg/g of leaf mass. This quantity is similar to that generated in magnICON expression system (105). The plant-derived approach to vaccine development is attractive because of the large amount of transient proteins that can be expressed, with the potential for use during high demand for therapeutics and prophylactics (106). Advances in the field of vector expression like plant transient expression system and associated host cell engineering and manufacturing processes paved way for developing biopharmaceutical proteins and therapeutics in commercial basis. The great potentials of such novel approaches have been exploited for evolving therapeutics to counter emerging pandemics of EBOV and influenza that is evidenced from the production of experimental ZMapp antibodies (107).
An overview of various types of vaccines for countering EVD is presented in Table 1 and depicted in Figure 1.
Figure 1. Various vaccine platforms under progress for the development of a successful Ebola virus (EBOV) vaccine. Platforms like inactivated vaccine, DNA vaccine, virus-like particles, virus-like replicon particles (VRPs), plant-based vaccine, and recombinant viral-vectored vaccines are available.
Advances in Developing Drugs and Therapies Against EBOV
Momentous leap has been witnessed toward the designing of efficacious EBOV drugs and therapeutics during the short span of only few years, even though the efficacy of several biologicals and vaccines were evaluated during the recent West African outbreak, it remained elusive to ratify a licensed EBOV disease treatment regimen (123).
Management of suspected or confirmed EVD patients includes quarantine, symptomatic, and supportive treatments, including fluid replacement, electrolyte imbalance correction, treating complicated infections, and preventing shock (124). For mitigation of the huge fluid loss and resultant hypovolemia, oral rehydration solutions should be provided adequately and if required anti-diarrheal and anti-emetic drugs need to be administered (125). Brincidofovir, a drug used to treat dsDNA viruses such as adenovirus, herpesviruses, orthopoxviruses, papillomavirus, and polyomaviruses, was approved for emergency treatment of two patients with EBOV infection; however, the clinical efficacy of the drug is unknown (126). Many drugs are being tested to identify specific antiviral drugs to treat EBOV, and new drug candidates are being developed by researchers worldwide (26, 127, 128).
Favipiravir (T-705), an antiviral drug found useful in treating influenza, has been studied and found effective against EBOV (129–131). Insertional mutagenesis, a high-throughput method to identify genes responsible for virus replication, can be used to develop drug candidates (132). Molecular docking experiments with EBOV GPs can be used for drug designing and the development of therapeutics (133, 134). Novel flexible nucleosides called fleximers were found to be effective against recombinant EBOV in Huh7 cells (135). Ribavirin antiviral can be recommended for the treatment of EBOV, since in mouse and monkey models, treatment with ribavirin delayed the death and increased survival rate (136). However, adverse effects associated with its use may limit ribavirin use (137). Lamivudine, an anti-retroviral drug, has been tested by Liberian doctor on 15 EBOV patients with survival of 13 patients (138). However, study by Cong et al. (139) found no survival benefits in Guinea pig model. Similar results were obtained by Hensley et al. (140), with no significant antiviral activity of lamivudine against EBOV in Vero E6 cells. Hence, the use of lamivudine may not be advocated.
The docking of VP40, VP35, VP30, and VP24 has been achieved using small molecules belonging to the class of flavonoids and derivatives. Gossypetin and Taxifolin (the two flavonoids of top ranks) showed higher docking scores for every EBOV receptor (141). A virtual analysis of more phytochemicals could help to identify plant-derived products with comparatively higher efficacy and lower toxicity. Adenosine nucleoside analogs such as BCX4430 have been found effective against EBOV in a mouse model. GS-5734, also a nucleoside analog, was found effective against EBOV in a NHP model (142). Using molecular dynamics simulations, graphene sheets are found to associate strongly with VP40 (matrix) protein of EBOV and disrupt VP40 hexamer–hexamer association, crucial to form virus matrix, thereby graphene and similar nanopolymers may be used as therapy or at least disinfectant to reduce the risk of transmission at time of epidemic (143).
The potential of retro-type drugs (molecules that block the retrograde trafficking of bacterial and plant toxins within mammalian cells) must be explored for designing novel therapy against filovirus (144). Retro-2 along with its other two derivatives, Retro-2.1 and compound 25 could effectively block EBOV and MARV progression in vitro. The derivatives were shown to be more potent inhibitors of filoviral penetration, replication, and progression when compared with their parent compound, as evidenced by pseudo-typed virus assays (144).
EBOV Entry and Inhibitors
The cell entry of EBOV involves virus binding to the cell surface receptors followed by internalization through macropinocytosis, processing by endosomal proteases, and transport to Niemann–Pick C1 (NPC1; an internal receptor for EBOV) containing endolysosomes. Phosphatidylinositol-3-phosphate 5-kinase is essential for maturation of endosome, a critical step to the EBOV infection (145). In vitro studies using apilimod, an antagonist of phosphatidylinositol-3-phosphate 5-kinase, showed inhibition of EBOV by blocking the viral particle trafficking to NPC1 containing endolysosomes (146). IFNs are natural antivirals, and type I (IFN-α/β), particularly, is being widely used for the treatment of viral diseases. Type I IFN-α2b has been evaluated for the treatment of EBOV; however, IFN-α2b was not successful, as only delayed death but could not prevent mortality in EBOV-exposed monkeys. IFN-γ reduced the mortality rate in mice when administered either before or after EBOV infection (147), suggesting its promise as a prophylactic and/or therapeutic drug for use in EBOV infections. As IFN-γ has already been used to treat certain chronic medical conditions and has been approved by the FDA, it can be readily adapted for use against EBOV infections.
Silvestrol extracted from Aglaia foveolata was found to inhibit replication of EBOV and has been suggested as a therapeutic drug to treat acute EBOV infection (148). Supportive treatments like oral rehydration therapy are recommended for children under 5 years of age (149). Web-based identification of therapeutic agents indicated that a single siRNA can inhibit mRNA transcription of three species of EBOV, whereas 75 siRNAs can inhibit at least two species of EBOV. The web server Ebola VCR has been developed, with details available for the development of suitable therapeutic agents (150). Numerous treatment options for EVD are discussed below.
Ebola virus possesses only one surface protein and is responsible for both the receptor binding and fusion of virus-to-host cell endosomal membrane. EBOV glycoprotein binds with lectin receptor DC-SIGN (151). The infection initiated with the binding of EBOV glycoprotein to lectin receptors and internalization of virus majorly through macropinocytosis and as alternative mechanism through clathrin-dependent endocytosis (152). In the low pH of endosome, cysteine proteases including cathepsin B and L proteolytically cleave GP. Possibly this proteolytic cleavage exposes the putative receptor-binding region that interacts with NPC1, a receptor facilitating the filovirus entry (153). TIM-1 receptors directly interact with phosphatidylserine on the viral envelope, suggestive of GP independent virus attachment onto the cells. In poorly permissive cells, EBOV infection enhanced by exogenously expressed TIM-1 by 10- to 30-folds (154). Other phosphatidylserine interacting proteins like TIM-4 and Axl (a receptor tyrosine kinase) also have been demonstrated to enhance the infection of several enveloped virus. A benzylpiperazine adamantane diamide-derived compound obtained after screening of a library of small molecules, targets endosomal NPC1, and inhibit infection by VSV particles (VSV) pseudo-typed with EBOV GP (155).
Tetrandrin is a potent drug that inhibits the EBOV entry into the cells (156). Two estrogen receptor drugs, clomiphene and toremifene, have been reported to hinder EBOV infection in mice by blocking cell entry and fusion with host cells (157). Amiodarone, an ion channel blocker, has been found to inhibit EBOV entry into cells (158, 159). Dendrimers and fullerene C60 have unique symmetrical properties and were recently found effective in inhibiting EBOV entry in vitro (160). Clarithromycin, an antibiotic, inhibits the release of calcium (stimulated by nicotinic acid adenine dinucleotide phosphate) from lysosome and exhibit anti-EBOV activity. Alike clarithromycin, posaconazole, an anti-fungal agent also shows similar anti-EBOV activity. In addition, it also inhibits the functions of NPC1 protein and acid sphingomyelinase activity. Both drugs, i.e., clarithromycin and posaconazole, ultimately inhibit the entry of EBOV into the host cell (161). The drug 5-(N-ethyl-N-isopropyl) amiloride inhibits the process of macropinocytosis (a process required for uptake of large filamentous virions, like EBOV) and thus interferes with the viral entry into the cell. Compounds like MLS000394177 and MLS000733230 also inhibit the viral entry into cells (162).
Prunella vulgaris, a Chinese herb, was found to inhibit EBOV entry into cells, using an EBOV-GP-pseudotyped-human immunodeficiency virus (HIV)-1-based vector system (163). Pseudovirions containing EBOV-GP were used for screening of the Prestwick chemical library, which contains 1,200 FDA approved drugs. The assay was based on cell entry of HIV-1-based surrogate in 384-well format. Twenty chemicals were found to inhibit more than 80% entry and 16 out of them were identified as G protein-coupled receptor (GPCR) antagonists, which target a range of GPCRs including adrenergic receptors, 5-HT (serotonin) receptors, histamine receptors, muscarinic and acetylcholine receptors. The time-of-addition studies suggested that EBOV entry is stopped at level of initial attachment prior to fusion of virus and cell membrane (164). Quercetin 3-β-O-d-glucoside (Q3G), a flavonoid derivate, was found to protect mice against EBOV challenge by targeting viral entry (165).
During replication of EBOV, surface GPs undergo proteolytic cleavage in the endosome by several proteases, including cathepsin B (CatB) (166). Thus, proteases may be a good target for the inhibition of EBOV replication. One study showed that, using a synthetic serine protease inhibitor, nafamostat mesilate (NM), caused a reduction in CatB release in rat pancreases. NM was also found to have anti-coagulant properties, which would also be useful in EBOV infections, as EBOV causes disseminated intravascular coagulation. Thus, this drug should be examined in clinical trials to be approved for the treatment of EVD (167). Chemically modified human serum albumin with 3-hydroxyphthalic anhydride (HP-HSA) has been demonstrated with the potential of a therapeutic candidate in resisting the EBOV infection (168).
Transfusion of Convalescent Blood/Serum
Convalescent serum by definition contains immunoglobulins IgM and IgG but is devoid of red blood cells and clotting factors. Transfusing convalescent whole blood and convalescent plasma from disease survivors has been found to neutralize EBOV and reduce its load; thereafter, the immune response of the patient can provide protection against EBOV (169, 170). The use of whole blood and convalescent serum was approved by the World Health Organization (WHO) during critical EBOV conditions (171, 172). Screening of plasma is needed to rule out the presence of residual EBOV RNA and other blood-transmitted pathogens such as HIV, hepatitis B virus, and hepatitis C virus. Protection is conferred in NHPs through antibody therapy (post-exposure). In humans, this has ultimately paved the way for filovirus therapy by the use of polyclonal/mAb (approved by Food and Drug Administration) (173).
Valuable emergency therapeutics for the treatment of EBOV-infected persons include passive immunization with neutralizing antibodies by the transfer of sera from individuals recovering from EVD (174, 175), although it is not considered to render 100% protection especially after exposure (3 days post-exposure) to EBOV (e.g., Zaire Ebolavirus Makona) (176). Precise immunoglobulins retrieved from equine serum against EBOV were found safe and effective as prophylactic therapy in non-allergic patients (177). Recently developed mAb-based treatments for EVD include mAb114 and MB-003, ZMAb, ZMapp, and MIL-77E cocktails (25). ZMAb, consisting of three murine mAbs (1H3, 2G4, and 4G7), administered at a dose of 25 mg/kg three times, completely protected cynomolgus macaques against EVD. Administration of ZMAb with adenovirus-vectored IFN-α resulted in 75 and 100% survival of cynomolgus and rhesus macaques, respectively (29). mAbs that bind to the base of GP (4G7 and 2G4) are neutralizing antibodies, whereas mAbs that bind to the glycan cap (mAb114, 1H3, and 13C6) are non-neutralizing antibodies. The chimeric human mAbs 13C6, 6D8, and 13F6 possess the variable region from mice and Fc region of human; mAbs 13C6 and 6D8 neutralize EBOV in the presence of complement proteins (24). By repeated immunization of mice with glycoproteins of filovirus, generation of pan-EBOV-specific (as well as pan-filovirus) mAbs have been obtained. These pan-EBOV mAbs have shown reaction with RESTV, SUDV, and other viruses (178).
The components of ZMapp are mAbs (chimeric), viz., c13C6 from MB-003 (already known cocktail of antibody) and c2G4 as well as c4G7 from ZMab (different cocktail of antibody). This drug reversed clinical signs in 100% of rhesus macaques, even when administered as late as 5 days after EBOV exposure (30). Use of ZMapp, humanized-mouse antibodies, as a therapeutic agent has shown promise in NHPs (30, 84, 179) and it is a WHO-approved treatment regimen for EVD. Recently, a baby born to an EBOV-infected mother was found positive for EBOV on the first day of life. The baby was treated with ZMapp and the broad spectrum antiviral GS-5734, and, on day 20, the baby was found negative for EBOV (180). MB-003 is another mAb cocktail which is found to be effective in NHPs against variants of EBOV that are resistant to ZMapp (181).
The mechanism of action of mAbs is that they identify the inter-protomer epitope of the GP fusion loop, which is essential for viral membrane fusion, and also neutralize the entry of virus (182). Although several mAbs are available that can neutralize EBOV, there are few mAbs that can neutralize GPs from different EBOV species. In a study by Duehr et al. (183), a panel of eight murine mAbs derived from animals immunized with Zaire ebolavirus was evaluated. The mAbs were tested for binding breadth using a set of recombinant surface GPs from RESTV, TAFV, BDBV, EBOV, SUDV, and MARV. Of the eight, two mAbs (KL-2E5 and KL-2H7) showed binding ability. These two mAbs did not neutralize EBOV; however, they protected mice from infection with a VSV expressing the Zaire ebolavirus GP. Duehr et al. (183) also suggested that Fc-FcR interactions are responsible for the protection of mice in the absence of neutralization. Although ZMapp was found to be effective against Zaire EBOV, it has not shown cross-protection against other species of EBOV. FVM04 (a mAb) has shown cross neutralizing activity against SUDV. So, it can be used to replace one of the components of ZMapp, thereby increasing the range of protection against SUDV, ultimately leading to generation of cross-protective mAbs cocktail (184).
One Fab, KZ52, obtained by panning of phage display library, was derived from the bone marrow of an EVD survivor. Fab KZ52 exhibited 50% neutralization at a concentration of 8 nM (185). The mAb KZ52 protected guinea pigs from lethal Zaire ebolavirus challenge; however, when an experiment was carried out in rhesus macaques, the antibody failed to protect animals prophylactically and did not inhibit viremia (186). EBOV GP is processed by cathepsins, and the cleaved GP fuses with host cells to form a fusion pore, a passage for the EBOV genome to enter the cytosol for replication. Human mAb KZ52 and monkey mAb JP3K11 bind to conformation-dependent epitopes of GP. KZ52 is directed to bind a conformational non-glycosylated epitope at base of GP and a total 23 residues of GP residues remain in contact with antibody. Out of 23, 15 are contacted through van der Waals interactions and remaining 8 through direct hydrogen bonds (187). At 0.4 µg/ml dose, KZ52 lead to 50% neutralization. KZ52 protective efficacy is due to inhibition of cathepsin mediated cleavage of GP (23).
Exploring the synergistic effect of different pairs of neutralizing and non-neutralizing anti-EBOV mAbs could provide 100% protection in mice, revealing the scope of this approach in designing and developing immunotherapeutics and vaccines (188).
Bispecific Trojan-horse antibodies neutralizing other filoviruses have been found to provide protection in mice from multiple EBOV infection (189). Cell-penetrable human scFvs (HuscFvs) (transbodies) that bind to EBOV VP40, a matrix protein pivotal for viral assembly and budding, produced by phage display technology, revealed inhibition of the EBOV-like particles (VLPs) egress from hepatic cells (190). These transbodies were effective in blocking viral assembly and budding within the cells as they bind to several cationic patches in the VP40 C-terminal domain. The transbodies inhibit the function of VP40 by additional mechanisms also; such as binding to N-terminal domain and L-domain peptide WW binding motifs, suggesting the potential of these transbodies as direct acting anti-EBOV agents in future (190). Cell-penetrable HuscFvs specific to a highly conserved interferon-inhibitory domain (IID) of VP35 of EBOV inhibited the VP35 biofunctions in the EBOV replication cycle including polymerase cofactor activity and host IFN–antagonism by forming interface contact with residues of the first basic patch, the central basic patch, end-cap, and residues important for IID multimeric formation for dsRNA binding (191). The cell-penetrable small antibody fragments (HuscFvs) or superantibodies [the term coined by Kohler and Paul (192)] can cross the membrane of all cells but get accumulated intracellularly only where the target antigen is present. Thus, disappearance of the superantibodies from the blood circulation does not imply that they are eliminated from the body. The transbodies to the highly conserved EBOV VP40 and VP35 should be evaluated further using authentic EBOV in animal models of EVD and clinical trials before they can be considered a broadly effective and promising alternative to existing treatment approaches for EVD.
Recently, three mAbs produced in tobacco plants that target the EBOV GP were tested and showed good results in humans (193). Human mAbs against BDBV GP were isolated from patients who survived during the 2007 Uganda outbreak. These mAbs were found to have a neutralizing effect against multiple EBOV species, suggesting the possibility of the use of single mAbs as cross-protecting antibodies (194). Another investigation showed that EBOV GPs were conserved across different EBOV species. ELISA revealed that four mAbs namely S3, S12, S17, and S33 were found to show cross-reaction with GPs of five different species of EBOV (195). The discovery of cross-protective antibodies can aid in the development of therapeutic strategies for treatment of EBOV disease (196). In another study, 349 EBOV GP mAbs were isolated from survivors of EVD in an outbreak in Zaire, and 77% of the mAbs were found to neutralize EBOV (197). Three mAbs of EBOV-GP (Q206, Q314, and Q411) were isolated during the West African EVD outbreak in 2014. Recognition of the novel epitopes has been performed for Q206 and Q411, wherein these mAbs were found protecting mice against EBOV (198). A therapeutic vaccine based on mAbs has been proposed to sufficiently resolve replication of invasive EBOV, even if administered as a single dose 4 days post-infection (199). Non-neutralizing mAb 5D2 or 7C9 expressing adeno-associated virus (AAV), consistently released mAb in body and was found 100% protective against mice adapted EBOV strain. Neutralizing mAb 2G4 conferred 83% protection and a cocktail of these two mAbs provided 100% protection when given 7 days prior to infection and sustained protection when immunized animals were challenged 5 months post AAV-mAb immunization (200).
Potential limitations of mAb-based therapies include the requirement for high doses and mAb mixtures that are outbreak-specific owing to constant viral evolution. Furthermore, epitope mutations could reduce efficacy of the therapeutic mAbs used. Hence, these limiting factors need to be taken care of accordingly with mAbs usages.
EBOV Gene Expression Inhibitors
Viral gene expression is dependent on host cell machinery and is critical for virus replication. A conserved guanine-rich sequence in the EBOV L gene has been reported to assemble into quadruplex RNA, targeted by cationic porphyrin TmPyP4 that directs inhibition of the expression of L gene at the RNA level (86, 201). BCX4430 (a nucleoside analog) is a viral RNA polymerase inhibitor, and it has been found effective in protecting mice against lethal challenge of EBOV (202–204). In addition, double-stranded RNA binding protein 76 has been reported to inhibit EBOV polymerase activity (205).
Small molecular inhibitors needed for the synthesis of polyamine have been found to block the expression of EBOV gene. The eukaryotic initiation factor 5A (eIF5A) hypusination and spermidine (a polyamine) are essentially required for the replication of EBOV. However, if eIF5A hypusination is blocked, the gene expression of EBOV is inhibited which subsequently blocks the replication of the virus. Therefore, in-depth understanding of this mechanism at molecular level is essential for developing anti-EBOV drugs (205).
Repurposed Drugs
It is time taking task to develop a new therapeutic against an infectious agent and till that time new therapy divulged; drug repurposing, i.e., already existing drugs may be screened for their efficacy against pathogen. Owing to the lack of approved EBOV therapies, the screening of potentially efficacious drugs revealed that few of the drugs could be repurposed for EBOV treatment (206) (Table 2). Amiodarone, dronedarone, and verapamil, which are used for tachycardia, arrhythmias, and high blood pressure or angina, respectively, have been screened for their ability to inhibit the entry of filoviruses into cells and found efficacious in in vitro models (158). The use of statins, angiotensin-converting enzyme inhibitors, and angiotensin receptor blockers has also been suggested to attenuate EBOV infection (126). Phosphoinositide 3-kinases inhibitor LY294002 and calcium/calmodulin kinase (CAMK2) inhibitor KN-93 have been reported to reduce EBOV infection in Vero E6 cells. The p38 mitogen-activated protein kinase inhibitor SB202190 was shown to check virus-mediated cytokine storm, as studied in monocyte-derived DC of humans (207). Estrogen re-uptake modulators, viz., toremiphene and clomiphene, although cause in vitro inhibition of the virus entry, but are not free from unwanted side effects like ocular adverse reaction (in case of clomiphene) and serious derangements of electrolytes (in case of toremiphene) at the higher doses. To overcome this, combination therapy is suggested while using such drugs (206). Brincidofovir, a cidofovir analog conjugated with a lipid, can prevent EBOV replication; however, its exact efficacy in an in vivo model needs to be determined (208). Because cyclophilin A (CypA) is not essential for EBOV replication, alisporivir, which inhibits the host protein CypA, has shown limited antiviral effects against EBOV strains (Makona, Mayinga) (209). Emetine, an anti-protozoal agent, and its desmethyl analog cephaeline have potently inhibited EBOV replication and cephaeline is well tolerated in patients than emetine (210).
Rosuvastatin, atorvastatin, and pravastatin have been reported to alleviate inflammation, reduce C-reactive protein and TNFα levels, and impede cholesterol-supported EBOV membrane biosynthesis (232). In EBOV infection, overexpression of the procoagulant tissue factor in monocytes and macrophages and participation of endothelial cells leads to an imbalance in coagulation. The use of recombinant nematode anticoagulant protein c2, an inhibitor of tissue factor-mediated blood coagulation, was found to improve survival of macaques from Ebola hemorrhagic fever, and hence suggested to act as a good treatment module targeting the disease development (228). Anti-malarial drugs such as chloroquine and its structural analogs (hydroxychloroquine, pamaquine, primaquine, and plasmaquine) also act as lysosomotropic agents, preventing endosomal/lysosomal acidification, and thus limiting certain viral infections (233). There are conflicting reports on the therapeutic effects of chloroquine in mouse, hamster, and guinea pig models of EVD. Chloroquine was found to inhibit virus replication in in vitro studies but failed to protect against EBOV infection and disease development in mice, hamsters, and guinea pigs (234, 235). Esomeprazole and omeprazole were also found to inhibit viral entry during in vitro studies but higher concentrations of these drugs may be required when to be used in vivo (236). During the EBOV outbreak in Liberia in 2014, a reduction in fever cases was observed following mass administration of malaria chemoprevention drugs (237).
Because of its competitive anti-heparin potential and interference with viral replication and entry into the cell, the anti-trypanosomal agent, Suramin (Germanin or Bayer-205) has been proposed to treat EVD (229). A pyrazine carboxamide derivative namely Favipiravir (an anti-flu medicine), which was used earlier as an inhibitor of influenza virus replication, has been found useful in both therapy and prophylaxis during EBOV epidemic in West Africa (238–240). Favipiravir and the pyrazine carboxamide derivative T-705 showed positive results in treating patients with medium to high viremias, although these drugs were not found to be effective with very high viremias, but revealed acceptable results during EBOV infection in mouse (219, 221, 241).
The microtubule inhibitor drugs (vinblastine, vinorelbine/navelbine, and vincristine), commonly used as anticancer agents, have been found effective in inhibiting EBOV VLP entry into HeLa cells even at low concentrations (48–140 nM). Colchicine, a microtubule modulator primarily used for gout, has also been found to show anti-EBOV activity (225, 242).
Screening of 1766 FDA approved drugs and 259 experimental drugs revealed that Indinavir, an HIV protease inhibitor, may be effective in reducing the severity of EVD (230). The antiviral drugs including Maraviroc, Abacavir, Telbivudine, and Cidofovir could target the MTase domain of EBOV and inhibit the viral RNA-directed RNA polymerase (230). The Computational Analysis of Novel Drug Opportunities platform was recently developed to screen drugs approved by the FDA. Drugs like enfuvirtide, vancomycin, bleomycin, octreotide, lanreotide, somatostatin, and ubidecarenone (CoQ10) have shown higher activity against EBOV (231). Recently, virtual screening of several thousands of repurposing drugs from Drug Bank has been performed and ibuprofen was selected by realizing its possible inhibitory effect on EBOV infection. The drug has been found to show detectable antiviral effect in cell culture and can thus be used as a very useful molecular template for anti-Ebola viral drug development (243).
Nucleotide Analog Prodrug
GS-5734 developed by Gilead Sciences falls under this category. Interestingly, clinical trials have been conducted and it has been found that the drug is effective in clearing virus from semen (181, 244). Administration of GS-5734 in rhesus monkey through intravenous route resulted in suppression of replication of EBOV. It is also important to note that in NHPs, this compound can provide protection post-exposure (245).
Interferons
Interferons act as potent inhibitors of EBOV as has been proved through in vitro studies conducted involving various types of cells. IFN-β-1a treatment protected mice against a lethal challenge of EBOV (206). The virus clearance from the blood stream is enhanced by IFN β-1a leading to resolution of the symptoms of the disease at an early stage (246). Even though there is increased therapeutic usage of IFNs, but certain side effects are also associated with such treatment, viz., fever and myalgia, which must be kept in mind while opting for their use. Moreover, the occurrence of malaria additionally should be ruled out before initiating IFN therapy (206). Tilorone hydrochloride induces IFN response in mice and has been found effective against EBOV due to its action mainly mediated through pathway of innate immunity (IFN related) (247).
Oligomer-Mediated Inhibition
RNAi and advanced antisense therapies have been reported to provide post-exposure protection against lethal filovirus infections (248). Small interfering (si)RNA targeting RNA polymerase L protein has shown inhibition of EBOV replication and promising results for its use as a post-exposure therapeutic option (249). siRNAs and phosphorodiamidate morpholino oligomers (PMOs) targeting the EBOV RNA polymerase (L) protein protected NHPs against EVD (248, 249). PMOs target co-polymerase protein VP35 and membrane-associated protein VP24 for this protection (250). Antisense PMO-based drugs like AVI 6002, AVI-6003, and LNPs/siRNA (TKM-Ebola) are in clinical trials. TKM-Ebola is a mixture of three siRNAs that target the L, VP24, and VP35 proteins of Zaire EBOV. In NHPs, it efficiently provided post-exposure protection (249, 251). Although the results were promising, clinicians did not use much TKM-Ebola as it could lead to lethal overproduction of cytokines (a dangerous EBOV-induced inflammatory response) (232). A siRNA LNP product named TKM-130803 has been developed for EVD therapy. Although, the infusion of TKM-130803 at a dosage of 0.3 mg/kg/day through intravenous route to adult patients with severe clinical signs of EVD was comparable, it did not show an improved protection to the existing and classical controls (252). LNP-encapsulated short siRNAs protected 100% of rhesus monkeys exhibiting viremia and clinical illness (253). LNP encapsulation is also an effective drug delivery system (127, 253).
The inhibitors of hsa-miR-1246, hsa-miR-320a, and hsa-miR-196b-5p have been found to decrease EBOV GP cytotoxicity during in vitro studies; hence, miRNA technology can be used to develop useful therapeutics (254). Antiviral drug AVI-7537, targeting the VP24 gene of EBOV, has been shown to be efficacious in mice and monkeys (255). The EBOV-GP (cleaved) molecule acts as a ligand for NPC1, a transmembrane transfer protein. For entry of the virus into the target cell, an interaction between EBOV-GP and NPC1 domain C is necessary. Two small molecules, a sulfonamide (MBX2254) and a triazole thioether (MBX2270), have been identified as novel EBOV inhibitors suppressing EBOV infection in an in vitro model by blocking the entry of virus into target cell via inhibiting GP–NPC1 protein interaction (256). This strategy of targeting viral entry could pave way for the development of an anti-EBOV therapeutic agent.
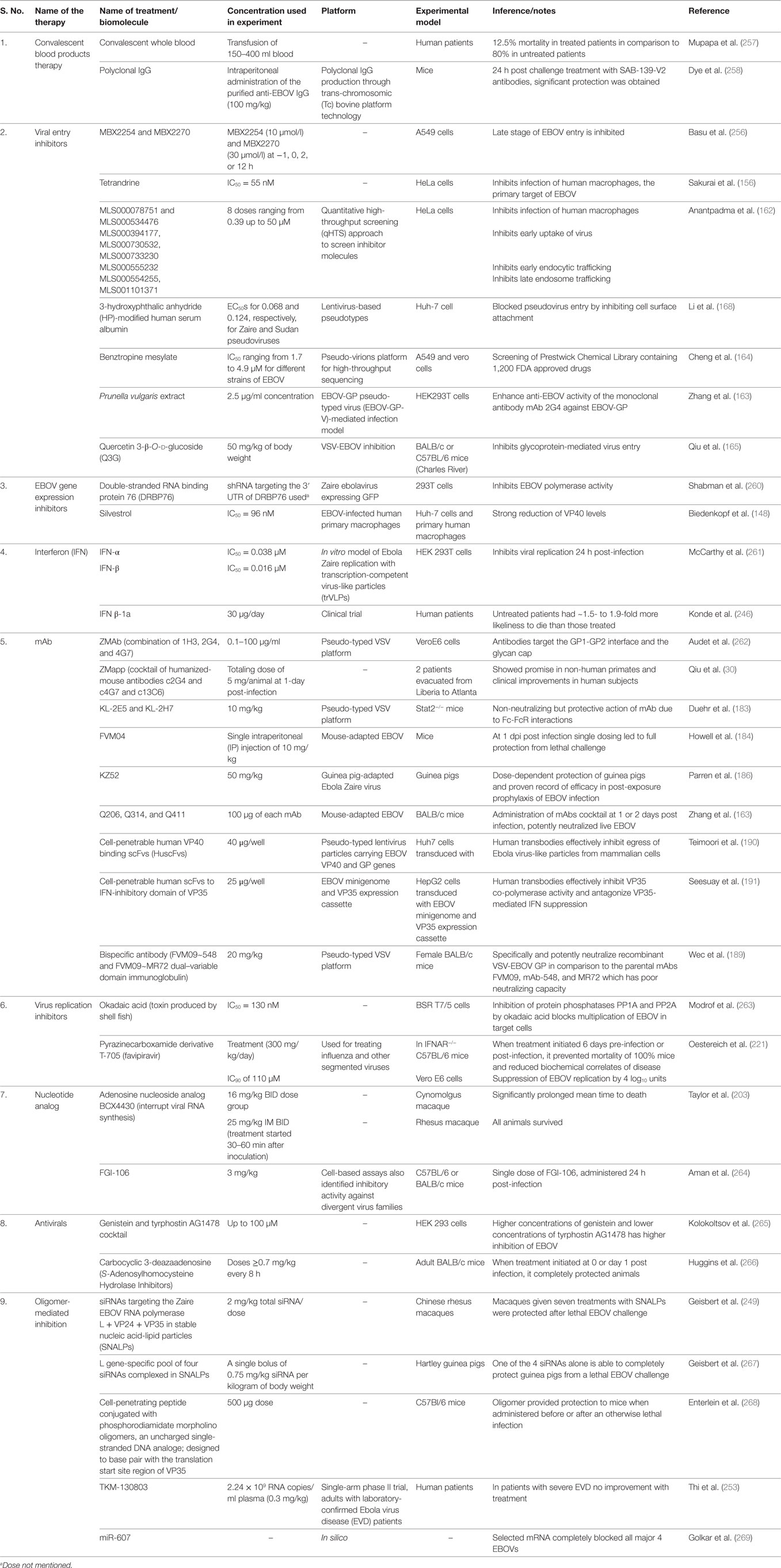
A brief summary of investigated drugs/biomolecules and therapeutics to treat EBOV infection is presented in Table 3 and depicted in Figure 2.
Figure 2. Different therapeutic agents and drugs available for the treatment of Ebola virus disease (EVD). Some agents block the viral entry, some block the RNA polymerase, while some inhibit gene expression. Neutralizing antibodies and mAbs have shown the potential to effectively inhibit Ebola virus (EBOV).
Of the note, a luciferase reporter system (EBOV-like particle/EBOVLP) has been developed. It helps in evaluating the in vivo anti-EBOV agents, viz., vaccines and drugs without the necessity of biosafety level-4 facilities. The system appears suitable in studying the process of viral entry also (259). The molecular tweezer CLR01 has been recently reported to inhibit EBOV and Zika virus infection. CLR01 interacts with the lipids in the viral envelop but not with the cellular membrane, thereby it is having very less effect on viability of cells (270). This small molecule has earlier been shown to possess antiviral activity against HIV-1 and herpes viruses. Such broad-spectrum antiviral agents need to be further explored to develop an effective drug against EBOV.
Currently, priority is being given toward investigating various proteins in the host system and viral targets (druggable) (271). Further research works need to be strengthened to identify potent viral or host targets that can be exploited to treat EVD or inhibit EBOV. With advances in bioinformatics tools, it is now possible to identify the active sites of the viral targets which can be utilized as a critical step toward designing and discovering anti-EBOV drugs (272). The involvement of computational tools has widened our approach toward designing drugs (target based) widely. Computational approaches can also countervail the endemic burdens in development of drugs traditionally (271, 273). Large libraries can now be effectively screened, ultimately stimulating research activities toward identifying potent anti-EBOV drugs. Therapeutic applications of cytokines, recombinant proteins, RNAi technology/RNA interference, TLRs, avian egg yolk antibodies, plant-based pharmaceuticals, nanomedicines, immunomodulatory agents, probiotics, herbs/plant extracts, and others may be explored appropriately to combat EBOV, as these have been found promising against other viral pathogens (2, 249, 274–282).
Conclusion and Future Perspectives
The 2014 EBOV outbreak has been marked as the most widespread lethal viral hemorrhagic attack and prompted a hasty leap in the researches for developing effective vaccines and therapies to counter it. In the case of Ebola, deviations in the touchstone drug/vaccine research approaches may be permitted by authorities to an appropriate extent, considering the devastating and alarming pandemic threat from the disease. In recent years, several therapies have emerged to tackle lethal EBOV infections. A plant-derived formulation of humanized mAbs: “ZMapp” has been used to treat some patients. However, the shortage of ZMapp supply warrants the evaluation and development of new mAbs. Various drugs have been repurposed to treat potentially lethal disease like EVD. There is a long list of repurposed compounds that have been evaluated as inhibitors of EBOV, including microtubule inhibitors, estrogen receptor and reuptake modulators, kinase inhibitors, histamine antagonists, and ion channel blockers. In-depth studies are still required to understand the pathogenesis and the role of different EBOV peptides, proteins, and antigens and host–virus interactions in EVD. There is also a need to develop economic and effective antivirals and vaccines against EBOV having approach/utility to any part of the world including resource poor countries.
Although the development of vaccines against EBOV began in 1980, there is still no effective vaccine available to prevent this deadly disease. Hence, the hunt for an effective vaccine is still on. Ebola VLPs play an imperative role in high-throughput screening of anti-EBOV compounds. Because five EBOV species have been reported, a polyvalent vaccine having immunogenic determinants such as GP from each of species would provide broader immunity; indeed, in nonhuman primate experimental studies with a DNA vaccine, this is commonly true. The best first-generation vaccine candidates for EBOV are rVSV and ChAd3, as reflected by their application in providing long duration protection during sporadic outbreaks. Various combinations of antigens from different species of EBOV may be explored to achieve higher protective immune response. The rVSV-based vaccine is being used in Democratic Republic of the Congo. Due to absence of preexisting immunity to VSV, it eliminates several drawbacks and safety concerns associated Ad5-based vaccine. Also, it has show long-term protection in several NHP models, it is an ideal vaccine platform to be used at time of outbreak. Together, the GamEvac-Combi vaccine also seems to be equally promising as it generated immune response in 100% volunteers.
In addition, mAbs with broad cross-reactivity that will neutralize all five species of EBOV are required to be developed and evaluated for prophylactic and therapeutic uses. Furthermore, effective antibodies may be engineered for homogeneity with human antibodies. Many nucleic acid-based modalities like siRNA, miRNA, and PMOs have been tested against EBOV and found functional. In the era of genomics, a computational approach may also be employed to screen large numbers of inhibitory molecules to safeguard human health. Available treatments within the disaster settings; mostly combination of appropriate supportive care and boosting of patient’s immune responses, need to be optimized to ensure minimum research/medical ethics being followed in such settings.
There is always scope for future investigations on the basis of clinical studies that are designed well and statistically supported. Maximum use of supportive therapy (MUST) should be introduced for studying the effects of new therapeutics. The side effects of newer drugs can also be revealed very efficiently by MUST and for this more resources are needed for the Ebola clinics. Though several drugs have been evaluated and vaccines are in development; however, more research is required to develop potent therapeutic and prophylactic agents against EBOV. Apart from these advances, adaptation of appropriate preventive measures and strict biosecurity principles are essential to stop the EBOV outbreaks, limit the spread of virus, and address its public health significance.
Author Contributions
All the authors substantially contributed to the conception, design, analysis and interpretation of data, checking and approving final version of the manuscript, and agreed to be accountable for its contents. KD, RK, AM, and KK initiated this review compilation. SC, SL, and RK updated various sections. RKS, YM, DK, and MR reviewed virology and biotechnology aspects. RKS, SM, RS, and WC reviewed recent vaccines and therapies. RK designed tables. KK designed the figures. WC, AM, and KD overviewed and edited final.
Conflict of Interest Statement
All authors declare that there exist no commercial or financial relationships that could in any way lead to a potential conflict of interest.
Acknowledgments
All the authors acknowledge and thank their respective Institutes and Universities.
Funding
This compilation is a review article written, analyzed and designed by its authors, and required no substantial funding to be stated.
References
1. Vogel WH, Viale PH. What you need to know about the Ebola virus. J Adv Pract Oncol (2014) 5(6):471–3.
2. Dhama K, Malik YS, Malik SV, Singh RK. Ebola from emergence to epidemic: the virus and the disease, global preparedness and perspectives. J Infect Dev Ctries (2015) 9:441–55. doi:10.3855/jidc.6197
3. Singh RK, Dhama K, Malik YS, Ramakrishnan MA, Karthik K, Khandia R, et al. Ebola virus – epidemiology, diagnosis, and control: threat to humans, lessons learnt, and preparedness plans – an update on its 40 year’s journey. Vet Q (2017) 37(1):98–135. doi:10.1080/01652176.2017.1309474
4. Leroy EM, Epelboin A, Mondonge V, Pourrut X, Gonzalez J-P, Muyembe-Tamfum J-J, et al. Human Ebola outbreak resulting from direct exposure to fruit bats in Luebo, Democratic Republic of Congo. Vector Borne Zoonotic Dis (2007) 9:6. doi:10.1089/vbz.2008.0167
5. Judson SD, Fischer R, Judson A, Munster VJ. Ecological contexts of index cases and spill over events of different ebolaviruses. PLoS Pathog (2016) 12(8):e1005780. doi:10.1371/journal.ppat.1005780
6. Kosal ME. A new role for public health in bioterrorism deterrence. Front Public Health (2014) 2:278. doi:10.3389/fpubh.2014.00278
7. Wu XX, Yao HP, Wu NP, Gao HN, Wu HB, Jin CZ, et al. Ebolavirus vaccines: progress in the fight against Ebola virus disease. Cell Physiol Biochem (2015) 37(5):1641–58. doi:10.1159/000438531
8. Johnson KM, Lange JV, Webb PA, Murphy FA. Isolation and partial characterisation of a new virus causing acute haemorrhagic fever in Zaire. Lancet (1977) 1(8011):569–71. doi:10.1016/S0140-6736(77)92000-1
9. Skrable K, Roshania R, Mallow M, Wolfman V, Siakor M, Levine AC. The natural history of acute Ebola virus disease among patients managed in five Ebola treatment units in West Africa: a retrospective cohort study. PLoS Negl Trop Dis (2017) 11(7):e0005700. doi:10.1371/journal.pntd.0005700
10. Elliott LH, Kiley MP, McCormick JB. Descriptive analysis of Ebola virus proteins. Virology (1985) 147:169–76. doi:10.1016/0042-6822(85)90236-3
11. Adam B, Lins L, Stroobant V, Thomas A, Brasseur R. Distribution of hydrophobic, residues is crucial for the fusogenic properties of the Ebola virus GP2 fusion peptide. J Virol (2004) 78:2131–6. doi:10.1128/JVI.78.4.2131-2136.2004
12. Kuhn JH, Andersen KG, Bào Y, Bavari S, Becker S, Bennett RS, et al. Filovirus RefSeq entries: evaluation and selection of filovirus type variants, type sequences, and names. Viruses (2014) 6(9):3663–82. doi:10.3390/v6093663
13. Morvan JM, Deubel V, Gounon P, Nakouné E, Barrière P, Murri S, et al. Identification of Ebola virus sequences present as RNA or DNA in organs of terrestrial small mammals of the Central African Republic. Microbes Infect (1999) 1:1193–201. doi:10.1016/S1286-4579(99)00242-7
14. Rosales-Mendoza S, Nieto-Gómez R, Angulo C. A perspective on the development of plant-made vaccines in the fight against Ebola virus. Front Immunol (2017) 8:252. doi:10.3389/fimmu.2017.00252
15. Varkey JB, Shantha JG, Crozier I, Kraft CS, Lyon GM, Mehta AK, et al. Persistence of Ebola virus in ocular fluid during convalescence. Persistence of Ebola virus in ocular fluid during. N Engl J Med (2015) 372(25):2423–7. doi:10.1056/NEJMoa1500306
16. Uyeki TM, Erickson BR, Brown S, McElroy AK, Cannon D, Gibbons A, et al. Ebola virus persistence in semen of male survivors. Clin Infect Dis (2016) 62(12):1552–5. doi:10.1093/cid/ciw202
17. Black BO, Caluwaerts S, Achar J. Ebola viral disease and pregnancy. Obstet Med (2015) 8(3):108–13. doi:10.1177/1753495X15597354
18. Barnes KG, Kindrachuk J, Lin AE, Wohl S, Qu J, Tostenson SD, et al. Evidence of Ebola virus replication and high concentration in semen of a patient during recovery. Clin Infect Dis (2017) 65(8):1400–3. doi:10.1093/cid/cix518
19. Falasca L, Agrati C, Petrosillo N, Di Caro A, Capobianchi MR, Ippolito G, et al. Molecular mechanisms of Ebola virus pathogenesis: focus on cell death. Cell Death Differ (2015) 22:1250–9. doi:10.1038/cdd.2015.67
20. He F, Melén K, Maljanen S, Lundberg R, Jiang M, Österlund P, et al. Ebolavirus protein VP24 interferes with innate immune responses by inhibiting interferon-λ1 gene expression. Virology (2017) 509:23–34. doi:10.1016/j.virol.2017.06.002
21. Babalola MO. The strengths, weaknesses, opportunities, and threats (SWOTS) analyses of the Ebola virus – paper retracted. Afr J Infect Dis (2016) 10(2):69–88. doi:10.21010/ajid.v10i2.2
22. Banadyga L, Wong G, Qiu X. Small animal models for evaluating filovirus countermeasures. ACS Infect Dis (2018). doi:10.1021/acsinfecdis.7b00266
23. Hiatt A, Pauly M, Whaley K, Qiu X, Kobinger G, Zeitlin L. The emergence of antibody therapies for Ebola. Hum Antibodies (2015) 23(3–4):49–56. doi:10.3233/HAB-150284
24. Moekotte AL, Huson MA, van der Ende AJ, Agnandji ST, Huizenga E, Goorhuis A, et al. Monoclonal antibodies for the treatment of Ebola virus disease. Expert Opin Investig Drugs (2016) 25(11):1325–35. doi:10.1080/13543784.2016.1240785
25. Mendoza EJ, Racine T, Kobinger GP. The ongoing evolution of antibody-based treatments for Ebola virus infection. Immunotherapy (2017) 9(5):435–50. doi:10.2217/imt-2017-0010
26. Wu W, Liu S. The drug targets and antiviral molecules for treatment of Ebola virus infection. Curr Top Med Chem (2017) 17(3):361–70. doi:10.2174/1568026616666160829161318
27. Marzi A, Yoshida R, Miyamoto H, Ishijima M, Suzuki Y, Higuchi M, et al. Protective efficacy of neutralizing monoclonal antibodies in a nonhuman primate model of Ebola hemorrhagic fever. PLoS One (2012) 7:e36192. doi:10.1371/journal.pone.0036192
28. Olinger GG Jr, Pettitt J, Kim D, Working C, Bohorov O, Bratcher B, et al. Delayed treatment of Ebola virus infection with plant-derived monoclonal antibodies provides protection in rhesus macaques. Proc Natl Acad Sci U S A (2012) 109:18030–5. doi:10.1073/pnas.1213709109
29. Qiu X, Audet J, Wong G, Pillet S, Bello A, Cabral T, et al. Successful treatment of Ebola virus-infected cynomolgus macaques with monoclonal antibodies. Sci Transl Med (2012) 4(138):138ra81. doi:10.1126/scitranslmed.3003876
30. Qiu X, Wong G, Audet J, Bello A, Fernando L, Alimonti JB, et al. Reversion of advanced Ebola virus disease in nonhuman primates with ZMapp. Nature (2014) 514(7520):47–53. doi:10.1038/nature13777
31. Kanapathipillai R, Henao Restrepo AM, Fast P, Wood D, Dye C, Kieny M-P, et al. Ebola vaccine — an urgent international priority. New Engl J Med (2014) 371:2249–51. doi:10.1056/NEJMp1412166
32. Houlihan CF, Youkee D, Brown CS. Novel surveillance methods for the control of Ebola virus disease. Int Health (2017) 9(3):139–41. doi:10.1093/inthealth/ihx010
33. Joffe S. Evaluating novel therapies during the Ebola epidemic. JAMA (2014) 312:1299–300. doi:10.1001/jama.2014.12867
34. Rios-Huerta R, Monreal-Escalante E, Govea-Alonso DO, Angulo C, Rosales-Mendoza S. Expression of an immunogenic LTB-based chimeric protein targeting Zaire ebolavirus epitopes from GP1 in plant cells. Plant Cell Rep (2017) 36(2):355–65. doi:10.1007/s00299-016-2088-6
35. Sharmin R, Islam AB. A highly conserved WDYPKCDRA epitope in the RNA directed RNA polymerase of human coronaviruses can be used as epitope-based universal vaccine design. BMC Bioinformatics (2014) 15(161):1471–2105. doi:10.1186/1471-2105-15-161
36. Dash R, Das R, Junaid M, Akash MFC, Islam A, Hosen SMZ. In-silico based vaccine design against Ebola virus glycoprotein. Adv Appl Bioinform Chem (2017) 10:11–28. doi:10.2147/AABC.S115859
37. Gera P, Gupta A, Verma P, Singh J, Gupta J. Recent advances in vaccine development against Ebola threat as bioweapon. Virus disease (2017) 28(3):242–6. doi:10.1007/s13337-017-0398-0
38. Medaglini D, Siegrist CA. Immunomonitoring of human responses to the rVSV-ZEBOV Ebola vaccine. Curr Opin Virol (2017) 23:88–94. doi:10.1016/j.coviro.2017.03.008
39. Mate SE, Kugelman JR, Nyenswah TG, Ladner JT, Wiley MR, Cordier-Lassalle T, et al. Molecular evidence of sexual transmission of Ebola virus. N Engl J Med (2015) 373(25):2448–54. doi:10.1056/NEJMoa1509773
40. Sissoko D, Duraffour S, Kerber R, Kolie JS, Beavogui AH, Camara AM, et al. Persistence and clearance of Ebola virus RNA from seminal fluid of Ebola virus disease survivors: a longitudinal analysis and modelling study. Lancet Glob Health (2017) 5(1):e80–8. doi:10.1016/S2214-109X(16)30243-1
41. Osterholm M, Moore K, Ostrowsky J, Kimball-Baker K, Farrar J, Wellcome Trust CEVTB. The Ebola Vaccine Team B: a model for promoting the rapid development of medical countermeasures for emerging infectious disease threats. Lancet Infect Dis (2016) 16:e1–9. doi:10.1016/S1473-3099(15)00416-8
42. Oany AR, Sharmin T, Chowdhury AS, Jyoti TP, Hasan MA. Highly conserved regions in Ebola virus RNA dependent RNA polymerase may be act as a universal novel peptide vaccine target: a computational approach. In silico Pharmacol (2015) 3(1):7. doi:10.1186/s40203-015-0011-4
43. Yasmin T, Nabi AH. B and T cell epitope-based peptides predicted from evolutionarily conserved and whole protein sequences of Ebola virus as vaccine targets. Scand J Immunol (2016) 83(5):321–37. doi:10.1111/sji.12425
44. Konduru K, Shurtleff AC, Bradfute SB, Nakamura S, Bavari S, Kaplan G. Ebolavirus glycoprotein Fc fusion protein protects guinea pigs against lethal challenge. PLoS One (2016) 11(9):e0162446. doi:10.1371/journal.pone.0162446
45. Merler S, Ajelli M, Fumanelli L, Parlamento S, Pastore Y, Piontti A, et al. Containing Ebola at the source with ring vaccination. PLoS Negl Trop Dis (2016) 10(11):e0005093. doi:10.1371/journal.pntd.0005093
46. Cook JD, Lee JE. The secret life of viral entry glycoproteins: moonlighting in immune evasion. PLoS Pathog (2013) 9(5):e1003258. doi:10.1371/journal.ppat.1003258
47. Cazares LH, Ward MD, Brueggemann EE, Kenny T, Demond P, Mahone CR, et al. Development of a liquid chromatography high resolution mass spectrometry method for the quantitation of viral envelope glycoprotein in Ebola virus-like particle vaccine preparations. Clin Proteomics (2016) 13(1):18. doi:10.1186/s12014-016-9119-8
48. Walsh PD, Kurup D, Hasselschwert DL, Wirblich C, Goetzmann JE, Schnell MJ. The final (oral Ebola) vaccine trial on captive Chimpanzees. Sci Rep (2017) 7:43339. doi:10.1038/srep43339
49. Meyer M, Huang E, Yuzhakov O, Ramanathan P, Ciaramella G, Bukreyev A. Modified mRNA-based vaccines elicit robust immune responses and protect guinea pigs from Ebola virus disease. J Infect Dis (2018) 217(3):451–5. doi:10.1093/infdis/jix592
50. Lambe T, Bowyer G, Ewer KJ. A review of phase I trials of Ebola virus vaccines: what can we learn from the race to develop novel vaccines? Phil Trans R Soc B (2016) 372:20160295. doi:10.1098/rstb.2016.0295
51. Walldorf JA, Cloessner EA, Hyde TB, MacNeil A; CDC Emergency Ebola Vaccine Taskforce. Considerations for use of Ebola vaccine during an emergency response. Vaccine (2017). doi:10.1016/j.vaccine.2017.08.058
52. Blaney JE, Wirblich C, Papaneri AB, Johnson RF, Myers CJ, Juelich TL, et al. Inactivated or live-attenuated bivalent vaccines that confer protection against rabies and Ebola viruses. J Virol (2011) 85(20):10605–16. doi:10.1128/JVI.00558-11
53. Lupton HW, Lambert RD, Bumgardner DL, Moe JB, Eddy GA. Inactivated vaccine for Ebola virus efficacious in guineapig model. Lancet (1980) 2(8207):1294–5. doi:10.1016/S0140-6736(80)92352-1
54. Rao M, Bray M, Alving CR, Jahrling P, Matyas GR. Induction of immune responses in mice and monkeys to Ebola virus after immunization with liposome-encapsulated irradiated Ebola virus: protection in mice requires CD4(+) T cells. J Virol (2002) 76(18):9176–85. doi:10.1128/JVI.76.18.9176-9185.2002
55. Ohimain EI. Recent advances in the development of vaccines for Ebola virus disease. Virus Res (2016) 211:174–85. doi:10.1016/j.virusres.2015.10.021
56. Martin JE, Sullivan NJ, Enama ME, Gordon IJ, Roederer M, Koup RA, et al. A DNA vaccine for Ebola virus is safe and immunogenic in a phase I clinical trial. Clin Vaccine Immunol (2006) 13(11):1267–77. doi:10.1128/CVI.00162-06
57. Patel A, Scott V, Wong G, Reuschel E, Villarreal D, Muthumani K, et al. A single immunization with optimized DNA vaccines protects against lethal Ebola virus challenge in mice (VAC8P.1057). J Immunol (2015) 194(1 Suppl):144.13.
58. Vastag B. Ebola vaccines tested in humans, monkeys. JAMA (2004) 291:549–50. doi:10.1001/jama.291.5.549
59. Grant-Klein RJ, Altamura LA, Badger CV, Bounds CE, Van Deusen NM, Kwilas SA, et al. Codon-optimized filovirus DNA vaccines delivered by intramuscular electroporation protect cynomolgus macaques from lethal Ebola and Marburg virus challenges. Hum Vaccin Immunother (2015) 11(8):1991–2004. doi:10.1080/21645515.2015.1039757
60. Kibuuka H, Berkowitz NM, Millard M, Enama ME, Tindikahwa A, Sekiziyivu AB, et al. Safety and immunogenicity of Ebola virus and Marburg virus glycoprotein DNA vaccines assessed separately and concomitantly in healthy Ugandan adults: a phase 1b, randomised, double-blind, placebo-controlled clinical trial. Lancet (2015) 385(9977):1545–54. doi:10.1016/S0140-6736(14)62385-0
61. Sarwar UN, Costner P, Enama ME, Berkowitz N, Hu Z, Hendel CS, et al. Safety and immunogenicity of DNA vaccines encoding Ebola virus and Marburg virus wild-type glycoproteins in a phase I clinical trial. J Infect Dis (2015) 211(4):549–57. doi:10.1093/infdis/jiu511
62. Warfield KL, Posten NA, Swenson DL, Olinger GG, Esposito D, Gillette WK, et al. Filovirus-like particles produced in insect cells: immunogenicity and protection in rodents. J Infect Dis (2007) 196(Suppl 2):S421–9. doi:10.1086/520612
63. Carra JH, Martins KAO, Schokman RD, Robinson CG, Steffens JT, Bavari S. A thermostable, chromatographically purified Ebola nano-VLP vaccine. J Transl Med (2015) 13:228. doi:10.1186/s12967-015-0593-y
64. Warfield KL, Perkins JG, Swenson DL, Deal EM, Bosio CM, Aman MJ, et al. Role of natural killer cells in innate protection against lethal Ebola virus infection. J Exp Med (2004) 200(2):169–79. doi:10.1084/jem.20032141
65. Rizk MG, Basler CF, Guatelli J. Cooperation of the Ebola virus proteins VP40 and GP1,2 with BST2 to activate NF-κB independently of virus-like particle trapping. J Virol (2017) 91(22):e1308–17. doi:10.1128/JVI.01308-17
66. Cooper CL, Martins KA, Stronsky SM, Langan DP, Steffens J, Van Tongeren S, et al. T-cell-dependent mechanisms promote Ebola VLP-induced antibody responses, but are dispensable for vaccine-mediated protection. Emerg Microbes Infect (2017) 6(6):e46. doi:10.1038/emi.2017.31
67. Reynolds P, Marzi A. Ebola and Marburg virus vaccines. Virus Genes (2017) 53(4):501–15. doi:10.1007/s11262-017-1455-x
68. Herbert AS, Kuehne AI, Barth JF, Ortiz RA, Nichols DK, Zak SE, et al. Venezuelan equine encephalitis virus replicon particle vaccine protects nonhuman primates from intramuscular and aerosol challenge with ebolavirus. J Virol (2013) 87(9):4952–64. doi:10.1128/JVI.03361-12
69. Ren S, Wei Q, Cai L, Yang X, Xing C, Tan F, et al. Alphavirus replicon DNA vectors expressing Ebola GP and VP40 antigens induce humoral and cellular immune responses in mice. Front Microbiol (2018) 8:2662. doi:10.3389/fmicb.2017.02662
70. Halfmann P, Kim JH, Ebihara H, Noda T, Neumann G, Feldmann H, et al. Generation of biologically contained Ebola viruses. Proc Natl Acad Sci U S A (2008) 105(4):1129–33. doi:10.1073/pnas.0708057105
71. Halfmann P, Ebihara H, Marzi A, Hatta Y, Watanabe S, Suresh M, et al. Replication-deficient ebolavirus as a vaccine candidate. J Virol (2009) 83(8):3810–5. doi:10.1128/JVI.00074-09
72. Marzi A, Halfmann P, Hill-Batorski L, Feldmann F, Shupert WL, Neumann G, et al. An Ebola whole-virus vaccine is protective in nonhuman primates. Science (2015) 348(6233):439–42. doi:10.1126/science.aaa4919
73. Sameem R, Dias S. Ebola virus: promising vaccine candidates. Vaccination Res (2017) 1(1):33–8. doi:10.1080/21645515.2016.1225637
74. Geisbert TW, Feldmann H. Recombinant vesicular stomatitis virus-based vaccines against Ebola and Marburg virus infections. J Infect Dis (2011) 204(Suppl 3):S1075–81. doi:10.1093/infdis/jir349
75. Wong G, Qiu X. Designing efficacious vesicular stomatitis virus-vectored vaccines against Ebola virus. Methods Mol Biol (2016) 1403:245–57. doi:10.1007/978-1-4939-3387-7_12
76. Wit ED, Marzi A, Bushmaker T, Brining D, Scott D, Richt JA, et al. Safety of recombinant VSV–Ebola virus vaccine vector in pigs. Emerg Infect Dis (2015) 21(4):702–4. doi:10.3201/eid2104.142012
77. Halperin SA, Arribas JR, Rupp R, Andrews CP, Chu L, Das R, et al. Six-month safety data of recombinant vesicular stomatitis virus-Zaire Ebola virus envelope glycoprotein vaccine in a phase 3 double-blind, placebo-controlled randomized study in healthy adults. J Infect Dis (2017) 215(12):1789–98. doi:10.1093/infdis/jix189
78. Geisbert TW, Daddario-Dicaprio KM, Lewis MG, Geisbert JB, Grolla A, Leung A, et al. Vesicular stomatitis virus-based Ebola vaccine is well-tolerated and protects immunocompromised nonhuman primates. PLoS Pathog (2008) 4(11):e1000225. doi:10.1371/journal.ppat.1000225
79. Marzi A, Murphy AA, Feldmann F, Parkins CJ, Haddock E, Hanley PW, et al. Cytomegalovirus-based vaccine expressing Ebola virus glycoprotein protects nonhuman primates from Ebola virus infection. Sci Rep (2016) 6:21674. doi:10.1038/srep21674
80. Venkatraman N, Silman D, Folegatti PM, Hill AVS. Vaccines against Ebola virus. Vaccine (2017). doi:10.1016/j.vaccine.2017.07.054
81. Henao-Restrepo A, Camacho A, Longini IM, Watson CH, Edmunds WJ, Egger M, et al. Efficacy and effectiveness of an rVSV-vectored vaccine in preventing Ebola virus disease: final results from the Guinea ring vaccination, open-label, cluster-randomized trial (Ebola Ca Suffit!). Lancet (2017) 389(10068):505–18. doi:10.1016/S0140-6736(16)32621-6
82. Regules JA, Beigel JH, Paolino KM, Voell J, Castellano AR, Hu Z, et al. A recombinant vesicular stomatitis virus Ebola vaccine. N Engl J Med (2017) 376:330–41. doi:10.1056/NEJMoa1414216
83. Folayan MO, Yakubu A, Haire B, Peterson K. Ebola vaccine development plan: ethics, concerns and proposed measures. BMC Med Ethics (2016) 17:10. doi:10.1186/s12910-016-0094-4
84. Trad MA, Naughton W, Yeung A, Mazlin L, O’sullivan M, Gilroy N, et al. Ebola virus disease: an update on current prevention and management strategies. J Clin Virol (2017) 86:5–13. doi:10.1016/j.jcv.2016.11.005
85. Wang D, Raja NU, Trubey CM, Juompan LY, Luo M, Woraratanadharm J, et al. Development of a cAdVax-based bivalent Ebola virus vaccine that induces immune responses against both the Sudan and Zaire species of Ebola virus. J Virol (2006) 80(6):2738–46. doi:10.1128/JVI.80.6.2738-2746.2006
86. Wang SR, Zhang QY, Wang JQ, Ge XY, Song YY, Wang YF, et al. Chemical targeting of a G-quadruplex RNA in the Ebola virus L gene. Cell Chem Biol (2016) 23(9):1113–22. doi:10.1016/j.chembiol.2016.07.019
87. Dolzhikova IV, Zubkova OV, Tukhvatulin AI, Dzharullaeva AS, Tukhvatulina NM, Shcheblyakov DV, et al. Safety and immunogenicity of GamEvac-Combi, a heterologous VSV- and Ad5-vectored Ebola vaccine: an open phase I/II trial in healthy adults in Russia. Hum Vaccin Immunother (2017) 13(3):613–20. doi:10.1080/21645515.2016.1238535
88. Zhu FC, Hou LH, Li JX, Wu SP, Liu P, Zhang GR, et al. Safety and immunogenicity of a novel recombinant adenovirus type-5 vector-based Ebola vaccine in healthy adults in China: preliminary report of a randomised, double-blind, placebo-controlled, phase 1 trial. Lancet (2015) 385(9984):2272–9. doi:10.1016/S0140-6736(15)60553-0
89. Wong G, Mendoza EJ, Plummer FA, Gao GF, Kobinger GP, Qiu X. From bench to almost bedside: the long road to a licensed Ebola virus vaccine. Expert Opin Biol Ther (2018) 18(2):159–73. doi:10.1080/14712598.2018.1404572
90. Chen T, Li D, Song Y, Yang X, Liu Q, Jin X, et al. A heterologous prime-boost Ebola virus vaccine regimen induces durable neutralizing antibody response and prevents Ebola virus-like particle entry in mice. Antiviral Res (2017) 145:54–9. doi:10.1016/j.antiviral.2017.07.009
91. Ledgerwood JE, DeZure AD, Stanley DA, Coates EE, Novik L, Enama ME, et al. Chimpanzee adenovirus vector Ebola vaccine. N Engl J Med (2017) 376(10):928–38. doi:10.1056/NEJMoa1410863
92. Wong G, Qiu X, Ebihara H, Feldmann H, Kobinger GP. Characterization of a bivalent vaccine capable of inducing protection against both Ebola and cross-clade H5N1 influenza in mice. J Infect Dis (2015) 212(Suppl 2):S435–42. doi:10.1093/infdis/jiv257
93. Ewer K, Rampling T, Venkatraman N, Bowyer G, Wright D, Lambe T, et al. A monovalent chimpanzee adenovirus Ebola vaccine boosted with MVA. New Engl J Med (2016) 374(17):1635–46. doi:10.1056/NEJMoa1411627
94. Milligan ID, Gibani MM, Sewell R, Clutterbuck EA, Campbell D, Plested E, et al. Safety and immunogenicity of novel adenovirus type 26- and modified vaccinia Ankara-vectored Ebola vaccines: a randomized clinical trial. JAMA (2016) 315(15):1610–23. doi:10.1001/jama.2016.4218
95. Schweneker M, Laimbacher AS, Zimmer G, Wagner S, Schraner EM, Wolferstätter M, et al. Recombinant modified vaccinia virus Ankara generating Ebola virus-like particles. J Virol (2017) 91(12). doi:10.1128/JVI.00343-17
96. Bengtsson KL, Song H, Stertman L, Liu Y, Flyer DC, Massare MJ, et al. Matrix-M adjuvant enhances antibody, cellular and protective immune responses of a Zaire Ebola/Makona virus glycoprotein (GP) nanoparticle vaccine in mice. Vaccine (2016) 34(16):1927–35. doi:10.1016/j.vaccine.2016.02.033
97. Meyer M, Garron T, Lubaki NM, Mire CE, Fenton KA, Klages C, et al. Aerosolized Ebola vaccine protects primates and elicits lung-resident T cell responses. J Clin Invest (2015) 125(8):3241–55. doi:10.1172/JCI81532
98. De Santis O, Audran R, Pothin E, Warpelin-Decrausaz L, Vallotton L, Wuerzner G, et al. Safety and immunogenicity of a chimpanzee adenovirus-vectored Ebola vaccine in healthy adults: a randomised, double-blind, placebo controlled, dose-finding, phase 1/2a study. Lancet Infect Dis (2016) 16(3):311–20. doi:10.1016/S1473-3099(15)00486-7
99. Sullivan NJ, Geisbert TW, Geisbert JB, Shedlock DJ, Xu L, Lamoreaux L, et al. Immune protection of nonhuman primates against Ebola virus with single low-dose adenovirus vectors encoding modified GPs. PLoS Med (2006) 3(6):e177. doi:10.1371/journal.pmed.0030177
101. Phoolcharoen W. Plant-Produced Ebola Immune Complex as an Ebola Vaccine Candidate. Ph.D. thesis. Arizona State University (2010).
102. Monreal-Escalante E, Ramos-Vega AA, Salazar-González JA, Bañuelos-Hernández B, Angulo C, Rosales-Mendoza S. Expression of the VP40 antigen from the Zaire ebolavirus in tobacco plants. Planta (2017) 246(1):123–32. doi:10.1007/s00425-017-2689-5
103. Zeitlin L, Pettitt J, Scully C, Bohorova N, Kim D, Pauly M, et al. Enhanced potency of a fucose-free monoclonal antibody being developed as an Ebola virus immunoprotectant. Proc Natl Acad Sci U S A (2011) 108(51):20690–4. doi:10.1073/pnas.1108360108
104. Laere E, Ling APK, Wong YP, Koh RY, Lila MAM, Hussein S. Plant-based vaccines: production and challenges. J Botany (2016) 2016:1–11. doi:10.1155/2016/4928637
105. Huang Z, Phoolcharoen W, Lai H, Piensook K, Cardineau G, Zeitlin L, et al. High-level rapid production of full-size monoclonal antibodies in plants by a single-vector DNA replicon system. Biotechnol Bioeng (2010) 106:9–17. doi:10.1002/bit.22652
106. Dhama K, Wani MY, Deb R, Karthik K, Tiwari R, Barathidasan R, et al. Plant based oral vaccines for human and animal pathogens – a new era of prophylaxis: current and future perspectives. J Exp Biol Agric Sci (2013) 1(1):1–12. doi:10.1177/2051013615613272
107. Kopertekh L, Schiemann J. Transient production of recombinant pharmaceutical proteins in plants: evolution and perspectives. Curr Med Chem (2017). doi:10.2174/0929867324666170718114724
108. Johnson RF, Kurup D, Hagen KR, Fisher C, Keshwara R, Papaneri A, et al. An inactivated rabies virus-based Ebola vaccine, filorab1, adjuvanted with glucopyranosyl lipid A in stable emulsion confers complete protection in nonhuman primate challenge models. J Infect Dis (2016) 214(Suppl 3):S342–54. doi:10.1093/infdis/jiw231
109. Blaney JE, Marzi A, Willet M, Papaneri AB, Wirblich C, Feldmann F, et al. Antibody quality and protection from lethal Ebola virus challenge in nonhuman primates immunized with rabies virus based bivalent vaccine. PLoS Pathog (2013) 9(5):e1003389. doi:10.1371/journal.ppat.1003389
110. Grant-Klein RJ, Van Deusen NM, Badger CV, Hannaman D, Dupuy LC, Schmaljohn CS. A multiagent filovirus DNA vaccine delivered by intramuscular electroporation completely protects mice from Ebola and Marburg virus challenge. Hum Vaccin Immunother (2012) 8(11):1703–6. doi:10.4161/hv.21873
111. Shedlock DJ, Aviles J, Talbott KT, Wong G, Wu SJ, Villarreal DO, et al. Induction of broad cytotoxic T cells by protective DNA vaccination against Marburg and Ebola. Mol Ther (2013) 21(7):1432–44. doi:10.1038/mt.2013.61
112. Warfield KL, Bosio CM, Welcher BC, Deal EM, Mohamadzadeh M, Schmaljohn A, et al. Ebola virus-like particles protect from lethal Ebola virus infection. Proc Natl Acad Sci U S A (2003) 100(26):15889–94. doi:10.1073/pnas.2237038100
113. Swenson DL, Warfield KL, Negley DL, Schmaljohn A, Aman MJ, Bavari S. Virus-like particles exhibit potential as a pan-filovirus vaccine for both Ebola and Marburg viral infections. Vaccine (2005) 23(23):3033–42. doi:10.1016/j.vaccine.2004.11.070
114. Warfield KL, Swenson DL, Olinger GG, Kalina WV, Aman MJ, Bavari S. Ebola virus-like particle-based vaccine protects nonhuman primates against lethal Ebola virus challenge. J Infect Dis (2007) 196(Suppl 2):S430–7. doi:10.1086/520583
115. Domi A, Feldmann F, Basu R, McCurley N, Shifflett K, Emanuel J, et al. A single dose of modified vaccinia Ankara expressing Ebola virus like particles protects nonhuman primates from lethal Ebola virus challenge. Sci Rep (2018) 8:864. doi:10.1038/s41598-017-19041-y
116. Pushko P, Bray M, Ludwig GV, Parker M, Schmaljohn A, Sanchez A, et al. Recombinant RNA replicons derived from attenuated Venezuelan equine encephalitis virus protect guinea pigs and mice from Ebola hemorrhagic fever virus. Vaccine (2000) 19(1):142–53. doi:10.1016/S0264-410X(00)00113-4
117. Tsuda Y, Caposio P, Parkins CJ, Botto S, Messaoudi I, Cicin-Sain L, et al. A replicating cytomegalovirus-based vaccine encoding a single Ebola virus nucleoprotein CTL epitope confers protection against Ebola virus. PLoS Negl Trop Dis (2011) 5(8):e1275. doi:10.1371/journal.pntd.0001275
118. Reynard O, Mokhonov V, Mokhonova E, Leung J, Page A, Mateo M, et al. Kunjin virus replicon-based vaccines expressing Ebola virus glycoprotein GP protect the guinea pig against lethal Ebola virus infection. J Infect Dis (2011) 204(Suppl 3):S1060–5. doi:10.1093/infdis/jir347
119. Bukreyev AA, Dinapoli JM, Yang L, Murphy BR, Collins PL. Mucosal parainfluenza virus-vectored vaccine against Ebola virus replicates in the respiratory tract of vector-immune monkeys and is immunogenic. Virology (2010) 399(2):290–8. doi:10.1016/j.virol.2010.01.015
120. DiNapoli JM, Yang L, Samal SK, Murphy BR, Collins PL, Bukreyev A. Respiratory tract immunization of non-human primates with a Newcastle disease virus-vectored vaccine candidate against Ebola virus elicits a neutralizing antibody response. Vaccine (2010) 29(1):17–25. doi:10.1016/j.vaccine.2010.10.024
121. Ledgerwood JE, Costner P, Desai N, Holman L, Enama ME, Yamshchikov G, et al. A replication defective recombinant Ad5 vaccine expressing Ebola virus GP is safe and immunogenic in healthy adults. Vaccine (2010) 29(2):304–13. doi:10.1016/j.vaccine.2010.10.037
122. Agnandji ST, Huttner A, Zinser ME, Njuguna P, Dahlke C, Fernandes JF, et al. Phase 1 trials of rVSV Ebola vaccine in Africa and Europe. N Engl J Med (2016) 374:1647–60. doi:10.1056/NEJMoa1502924
123. Cardile AP, Downey LG, Wiseman PD, Warren TK, Bavari S. Antiviral therapeutics for the treatment of Ebola virus infection. Curr Opin Pharmacol (2016) 30:138–43. doi:10.1016/j.coph.2016.08.016
124. Bishop BM. Potential and emerging treatment options for Ebola virus disease. Ann Pharmacother (2015) 49(2):196–206. doi:10.1177/1060028014561227
125. Shao X, Ren W, Zhou F. Clinical presentation and care for patients with Ebola virus disease in China Ebola treatment unit, Liberia. Jpn J Infect Dis (2016) 70(1):32–7. doi:10.7883/yoken.JJID.2015.597
126. Madariaga MG. Ebola virus disease: a perspective for the United States. Am J Med (2015) 128(7):682–91. doi:10.1016/j.amjmed.2015.01.035
127. Choi JH, Croyle MA. Emerging targets and novel approaches to Ebola virus prophylaxis and treatment. Bio Drugs (2013) 27(6):565–83. doi:10.1007/s40259-013-0046-1
128. Kilgore PE, Grabenstein JD, Salim AM, Rybak M. Treatment of Ebola virus disease. Pharmacotherapy (2015) 35:43–53. doi:10.1002/phar.1545
129. Smither SJ, Eastaugh LS, Steward JA, Nelson M, Lenk RP, Lever MS. Post-exposure efficacy of oral T-705 (Favipiravir) against inhalational Ebola virus infection in a mouse model. Antiviral Res (2014) 104:153–5. doi:10.1016/j.antiviral.2014.01.012
130. Duraffour S, Malvy D, Sissoko D. How to treat Ebola virus infections? A lesson from the field. Curr Opin Virol (2017) 24:9–15. doi:10.1016/j.coviro.2017.03.003
131. Zhang T, Zhai M, Ji J, Zhang J, Tian Y, Liu X. Recent progress on the treatment of Ebola virus disease with Favipiravir and other related strategies. Bioorg Med Chem Lett (2017) 27(11):2364–8. doi:10.1016/j.bmcl.2017.04.028
132. Cheng F, Murray JL, Zhao J, Sheng J, Zhao Z, Rubin DH. Systems biology-based investigation of cellular antiviral drug targets identified by gene-trap insertional mutagenesis. PLoS Comput Biol (2016) 12:e1005074. doi:10.1371/journal.pcbi.1005074
133. Ahmad N, Farman A, Badshah SL, Ur Rahman A, Ur Rashid H, Khan K. Molecular modeling, simulation and docking study of Ebola virus glycoprotein. J Mol Graph Model (2016) 72:266–71. doi:10.1016/j.jmgm.2016.12.010
134. Alam El-Din HM, Loutfy SA, Fathy N, Elberry MH, Mayla AM, Kassem S, et al. Molecular docking based screening of compounds against VP40 from Ebola virus. Bioinformation (2016) 12:192–6. doi:10.6026/97320630012192
135. Yates MK, Raje MR, Chatterjee P, Spiropoulou CF, Bavari S, Flint M, et al. Flex-nucleoside analogues – novel therapeutics against filoviruses. Bioorg Med Chem Lett (2017) 27(12):2800–2. doi:10.1016/j.bmcl.2017.04.069
136. Alfson KJ, Worwa G, Carrion R Jr, Griffiths A. Determination and therapeutic exploitation of Ebola virus spontaneous mutation frequency. J Virol (2015) 90(5):2345–55. doi:10.1128/JVI.02701-15
137. Tseng CP, Chan YJ. Overview of Ebola virus disease in 2014. J Chin Med Assoc (2015) 78:51–5. doi:10.1016/j.jcma.2014.11.007
138. AllAfrica. (2014). Available from: http://allafrica.com/stories/201409292050.html (Accessed: March, 2018).
139. Cong Y, Dyall J, Hart BJ, DeWald LE, Johnson JC, Postnikova E, et al. Evaluation of the activity of lamivudine and zidovudine against Ebola virus. PLoS One (2016) 11(11):e0166318. doi:10.1371/journal.pone.0166318
140. Hensley LE, Dyall J, Olinger GG, Jahrling PB. Lack of effect of lamivudine on Ebola virus replication. Emerg Infect Dis (2015) 21(3):550–2. doi:10.3201/eid2103.141862
141. Raj U, Varadwaj PK. Flavonoids as multi-target inhibitors for proteins associated with Ebola virus: in silico discovery using virtual screening and molecular docking studies. Interdiscip Sci (2016) 8(2):142. doi:10.1007/s12539-015-0125-8
142. Bradfute SB. The early clinical development of Ebola virus treatments. Expert Opin Investig Drugs (2017) 26(1):1–4. doi:10.1080/13543784.2017.1260545
143. Pokhrel R, Jeevan GC, Bhattarai N, Chapagain P, Gerstman B. Potential disruption of Ebola virus matrix by graphene nano-sheets. Biophysical J (2018) 114(3):218a. doi:10.1016/j.bpj.2017.11.1217
144. Shtanko O, Sakurai Y, Reyes AN, Noel R, Cintrat JC, Gillet D, et al. Retro-2 and its dihydroquinazolinone derivatives inhibit filovirus infection. Antiviral Res (2018) 149:154–63. doi:10.1016/j.antiviral.2017.11.016
145. Qiu S, Leung A, Bo Y, Kozak RA, Anand SP, Warkentin C, et al. Ebola virus requires phosphatidylinositol (3,5) bisphosphate production for efficient viral entry. Virology (2018) 513:17–28. doi:10.1016/j.virol.2017.09.028
146. Nelson EA, Dyall J, Hoenen T, Barnes AB, Zhou H, Liang JY, et al. The phosphatidylinositol-3-phosphate 5-kinase inhibitor apilimod blocks filoviral entry and infection. PLoS Negl Trop Dis (2017) 11(4):e0005540. doi:10.1371/journal.pntd.0005540
147. Jerebtsova M, Nekhai S. Therapeutics for postexposure treatment of Ebola virus infection. Future Virol (2015) 10(3):221–32. doi:10.2217/fvl.14.109
148. Biedenkopf N, Lange-Grunweller K, Schulte FW, Weißer A, Müller C, Becker D, et al. The natural compound silvestrol is a potent inhibitor of Ebola virus replication. Antiviral Res (2017) 137:76–81. doi:10.1016/j.antiviral.2016.11.011
149. Yuan S, Zhang ZW, Li ZL. Improvements in treatment of children younger than age 5 years infected with Ebola virus. J Pediatr (2017) 185:251–2. doi:10.1016/j.jpeds.2017.02.025
150. Dhanda SK, Chaudhary K, Gupta S, Brahmachari SK, Raghava GP. A web-based resource for designing therapeutics against Ebola virus. Sci Rep (2016) 6:24782. doi:10.1038/srep24782
151. Matsuno K, Kishida N, Usami K, Igarashi M, Yoshida R, Nakayama E, et al. Different potential of C-type lectin-mediated entry between Marburg virus strains. J Virol (2010) 84:5140–7. doi:10.1128/JVI.02021-09
152. Aleksandrowicz P, Marzi A, Biedenkopf N, Beimforde N, Becker S, Hoenen T, et al. Ebola virus enters host cells by macropinocytosis and clathrin-mediated endocytosis. J Infect Dis (2011) 204(Suppl 3):S957–67. doi:10.1093/infdis/jir326
153. Miller EH, Obernosterer G, Raaben M, Herbert AS, Deffieu MS, Krishnan A, et al. Ebola virus entry requires the host-programmed recognition of an intracellular receptor. EMBO J (2012) 31:1947–60. doi:10.1038/emboj.2012.53
154. Kondratowicz AS, Lennemann NJ, Sinn PL, Davey RA, Hunt CL, Moller-Tank S, et al. T-cell immunoglobulin and mucin domain 1 (TIM-1) is a receptor for Zaire Ebolavirus and Lake Victoria Marburgvirus. Proc Natl Acad Sci U S A (2011) 108(20):8426–31. doi:10.1073/pnas.1019030108
155. Côté M, Misasi J, Ren T, Bruchez A, Lee K, Filone CM. Small molecule inhibitors reveal Niemann-Pick C1 is essential for Ebola virus infection. Nature (2011) 477(7364):344–8. doi:10.1038/nature10380
156. Sakurai Y, Kolokoltsov AA, Chen CC, Tidwell MW, Bauta WE, Klugbauer N, et al. Two-pore channels control Ebola virus host cell entry and are drug targets for disease treatment. Science (2015) 347:995–8. doi:10.1126/science.1258758
157. Johansen LM, Brannan JM, Delos SE, Shoemaker CJ, Stossel A, Lear C, et al. FDA-approved selective estrogen receptor modulators inhibit Ebola virus infection. Sci Transl Med (2013) 5:190ra79. doi:10.1126/scitranslmed.3005471
158. Gehring G, Rohrmann K, Atenchong N, Mittler E, Becker S, Dahlmann F, et al. The clinically approved drugs amiodarone, dronedarone and verapamil inhibit filovirus cell entry. J Antimicrob Chemother (2014) 69:2123–31. doi:10.1093/jac/dku091
159. Salata C, Munegato D, Martelli F, Parolin C, Calistri A, Baritussio A, et al. Amiodarone affects Ebola virus binding and entry into target cells. New Microbiol (2018) 41(2):162–4.
160. Illescas BM, Rojo J, Delgado R, Martín N. Multivalent glycosylated nanostructures to inhibit Ebola virus infection. J Am Chem Soc (2017) 139(17):6018–25. doi:10.1021/jacs.7b01683
161. Sun W, He S, Martínez-Romero C, Kouznetsova J, Tawa G, Xu M, et al. Synergistic drug combination effectively blocks Ebola virus infection. Antiviral Res (2016) 137:165–72. doi:10.1016/j.antiviral.2016.11.017
162. Anantpadma M, Kouznetsova J, Wang H, Huang R, Kolokoltsov A, Guha R, et al. Large-scale screening and identification of novel Ebola virus and Mardburg virus entry inhibitors. Antimicrob Agents Chemother (2016) 60(8):4471–81. doi:10.1128/AAC.00543-16
163. Zhang X, Ao Z, Bello A, Ran X, Liu S, Wigle J, et al. Characterization of the inhibitory effect of an extract of Prunella vulgaris on Ebola virus glycoprotein (GP)-mediated virus entry and infection. Antiviral Res (2016) 127:20–31. doi:10.1016/j.antiviral.2016.01.001
164. Cheng H, Lear-Rooney CM, Johansen L, Varhegyi E, Chen ZW, Olinger GG, et al. Inhibition of Ebola and Marburg virus entry by G protein-coupled receptor antagonists. J Virol (2015) 89(19):9932–8. doi:10.1128/JVI.01337-15
165. Qiu X, Kroeker A, He S, Kozak R, Audet J, Mbikay M, et al. Prophylactic efficacy of quercetin 3-β-O-d-glucoside against Ebola virus infection. Antimicrob Agents Chemother (2016) 60(9):5182–8. doi:10.1128/AAC.00307-16
166. Bornholdt ZA, Ndungo E, Fusco ML, Bale S, Flyak AI, Crowe JE Jr, et al. Host-primed Ebola virus GP exposes a hydrophobic NPC1 receptor-binding pocket, revealing a target for broadly neutralizing antibodies. mBio (2016) 7(1):e2154–2115. doi:10.1128/mBio.02154-15
167. Nishimura H, Yamaya M. A synthetic serine protease inhibitor, nafamostat mesilate, is a drug potentially applicable to the treatment of Ebola virus disease. Tohoku J Exp Med (2015) 237(1):45–50. doi:10.1620/tjem.237.45
168. Li H, Yu F, Xia S, Yu Y, Wang Q, Lv M, et al. Chemically modified human serum albumin potently blocks entry of Ebola pseudoviruses and virus like particles. Antimicrob Agents Chemother (2017) 61(4):e2168–2116. doi:10.1128/AAC.02168-16
169. van Griensven J, De Weiggheleire A, Delamou A, Smith PG, Edwards T, Vandekerckhove P, et al. The use of Ebola convalescent plasma to treat Ebola virus disease in resource constrained set-tings: a perspective from the field. Clin Infect Dis (2015) 62:69–74. doi:10.1093/cid/civ680
170. Garraud O. Use of convalescent plasma in Ebola virus infection. Transfus Apher Sci (2017) 56(1):31–4. doi:10.1016/j.transci.2016.12.014
171. Gulland A. First Ebola treatment is approved by WHO. British Med J (2014) 349:g5539. doi:10.1136/bmj.g5539
172. Geisen C, Kann G, Strecker T, Wolf T, Schüttfort G, van Kraaij M, et al. Pathogen-reduced Ebola virus convalescent plasma: first steps towards standardization of manufacturing and quality control including assessment of Ebola-specific neutralizing antibodies. Vox Sang (2016) 110(4):329–35. doi:10.1111/vox.12376
173. Dye JM, Herbert AS, Kuehne AI, Barth JF, Muhammad MA, Zak SE, et al. Postexposure antibody prophylaxis protects nonhuman primates from filovirus disease. Proc Natl Acad Sci U S A (2012) 109:5034–9. doi:10.1073/pnas.1200409109
174. Saphire EO. An update on the use of antibodies against the filoviruses. Immunotherapy (2013) 5:1221–33. doi:10.2217/imt.13.124
175. Corti D, Misasi J, Mulangu S, Stanley DA, Kanekiyo M, Wollen S, et al. Protective monotherapy against lethal Ebola virus infection by a potently neutralizing antibody. Science (2016) 351:1339–42. doi:10.1126/science.aad5224
176. Mire CE, Geisbert JB, Agans KN, Thi EP, Lee AC, Fenton KA, et al. Passive immunotherapy: assessment of convalescent serum against Ebola virus Makona infection in nonhuman primates. J Infect Dis (2016) 214(S3):S367–74. doi:10.1093/infdis/jiw333
177. Borisevich IV, Chemikova NK, Markov VI, Krasnianskiy VP, Borisevich SV, Rozhdestvenskiy EV. An experience in the clinical use of specific immunoglobulin from horse blood serum for prophylaxis of Ebola haemorrhagic fever. Vopr Virusol (2017) 62(1):25–9.
178. Holtsberg FW, Shulenin S, Vu H, Howell KA, Patel SJ, Gunn B, et al. Pan-ebolavirus and pan-filovirus mouse monoclonal antibodies: protection against Ebola and Sudan viruses. J Virol (2016) 90(1):266–78. doi:10.1128/JVI.02171-15
179. Zhang Y, Li D, Jin X, Huang Z. Fighting Ebola with ZMapp: spotlight on plant-made antibody. Sci China Life Sci (2014) 57:987–8. doi:10.1007/s11427-014-4746-7
180. Dornemann J, Burzio C, Ronsse A, Sprecher A, De Clerck H, Van Herp M, et al. First newborn baby to receive experimental therapies survives Ebola virus disease. J Infect Dis (2017) 215(2):171–4. doi:10.1093/infdis/jiw493
181. Hayden FG, Friede M, Bausch DG. Experimental therapies for Ebola virus disease: what have we learned? J Infect Dis (2017) 215:167–70. doi:10.1093/infdis/jiw496
182. Wec AZ, Herbert AS, Murin CD, Nyakatura EK, Abelson DM, Fels JM, et al. Antibodies from a human survivor define sites of vulnerability for broad protection against Ebola viruses. Cell (2017) 169(5):878.e–90.e. doi:10.1016/j.cell.2017.04.037
183. Duehr J, Wohlbold TJ, Oestereich L, Chromikova V, Amanat F, Rajendran M, et al. Novel cross-reactive monoclonal antibodies against Ebolavirus glycoproteins show protection in a murine challenge model. J Virol (2017) 91(16). doi:10.1128/JVI.00652-17
184. Howell KA, Qiu X, Brannan JM, Bryan C, Davidson E, Holtsberg FW, et al. Antibody treatment of Ebola and Sudan virus infection via a uniquely exposed epitope within the glycoprotein receptor-binding site. Cell Rep (2016) 15(7):1514–26. doi:10.1016/j.celrep.2016.04.026
185. Maruyama T, Rodriguez LL, Jahrling PB, Sanchez A, Khan AS, Nichol ST, et al. Ebola virus can be effectively neutralized by antibody produced in natural human infection. J Virol (1999) 73(7):6024–30.
186. Parren PWHI, Geisbert TW, Maruyama T, Jahrling PB, Burton DR. Pre- and postexposure prophylaxis of Ebola virus infection in an animal model by passive transfer of a neutralizing human antibody. J Virol (2002) 76(12):6408–12. doi:10.1128/JVI.76.12.6408-6412.2002
187. Lee JE, Fusco ML, Hessell AJ, Oswald WB, Burton DR, Saphire EO. Structure of the Ebola virus glycoprotein bound to an antibody from a human survivor. Nature (2008) 454(7201):177–82. doi:10.1038/nature07082
188. Howell KA, Brannan JM, Bryan C, McNeal A, Davidson E, Turner HL, et al. Cooperativity enables non-neutralizing antibodies to neutralize Ebolavirus. Cell Rep (2017) 19(2):413–24. doi:10.1016/j.celrep.2017.03.049
189. Wec AZ, Nyakatura EK, Herbert AS, Howell KA, Holtsberg FW, Bakken RR, et al. A “Trojan horse” bispecific antibody strategy for broad protection against ebolaviruses. Science (2016) 354:350–4. doi:10.1126/science.aag3267
190. Teimoori S, Seesuay W, Jittavisutthikul S, Chaisri U, Sookrung N, Densumite J, et al. Human transbodies to VP40 inhibit cellular egress of Ebola virus-like particles. Biochem Bioph Res Co (2016) 479:245–52. doi:10.1016/j.bbrc.2016.09.052
191. Seesuay S, Jittavisutthikul S, Sae-lim N, Sookrung N, Sakolvaree Y, Chaicumpa W. Human transbodies that interfere with the functions of Ebolavirus VP35 protein in genome replication and transcription and innate immune antagonism. Emerg Microbe Infect (2018) 7(1):41. doi:10.1038/s41426-018-0031-3
192. Kohler H, Paul S. Superantibody activities: new players in innate and adaptive immune response. Immunol Today (1998) 9(5):221–7. doi:10.1016/S0167-5699(97)01234-6
193. González-González E, Alvarez MM, Márquez-Ipiña AR, Trujillo-de Santiago G, Rodríguez-Martínez LM, Annabi N, et al. Anti-Ebola therapies based on monoclonal antibodies: current state and challenges ahead. Crit Rev Biotechnol (2017) 37(1):53–68. doi:10.3109/07388551.2015.1114465
194. Flyak AI, Shen X, Murin CD, Turner HL, David JA, Fusco ML, et al. Cross-reactive and potent neutralizing antibody responses in human survivors of natural Ebola virus infection. Cell (2016) 164(3):392–405. doi:10.1016/j.cell.2015.12.022
195. Hernandez H, Marceau C, Halliday H, Callison J, Borisevich V, Escaffre O, et al. Development and characterization of broadly cross-reactive monoclonal antibodies against all known Ebolavirus species. J Infect Dis (2015) 212(Suppl 2):S410–3. doi:10.1093/infdis/jiv209
196. Furuyama W, Marzi A, Nanbo A, Haddock E, Maruyama J, Miyamoto H, et al. Discovery of an antibody for pan-ebolavirus therapy. Sci Rep (2016) 6:20514. doi:10.1038/srep20514
197. Bornholdt ZA, Turner HL, Murin CD, Li W, Sok D, Souders CA, et al. Isolation of potent neutralizing antibodies from a survivor of the 2014 Ebola virus outbreak. Science (2016) 351(6277):1078–83. doi:10.1126/science.aad5788
198. Zhang Q, Gui M, Niu X, He S, Wang R, Feng Y, et al. Potent neutralizing monoclonal antibodies against Ebola virus infection. Sci Rep (2016) 6:25856. doi:10.1038/srep25856
199. Nguyen VK, Hernandez-Vargas EA. Windows of opportunity for Ebola virus infection treatment and vaccination. Sci Rep (2017) 7:8975. doi:10.1038/s41598-017-08884-0
200. van Lieshout LP, Soule G, Sorensen D, Frost KL, He S, Tierney K, et al. Intramuscular adeno-associated virus-mediated expression of monoclonal antibodies provides 100% protection against Ebola virus infection in mice. J Infect Dis (2018) 217(6):916–25. doi:10.1093/infdis/jix644
201. Ruggiero E, Richter SN. G-quadruplexes and G-quadruplex ligands: targets and tools in antiviral therapy. Nucleic Acids Res (2018) 46(7):3270–83. doi:10.1093/nar/gky187
202. Warren TK, Wells J, Panchal RG, Stuthman KS, Garza NL, Van Tongeren SA, et al. Protection against filovirus diseases by a novel broad-spectrum nucleoside analogue BCX4430. Nature (2014) 508:402–5. doi:10.1038/nature13027
203. Taylor R, Kotian P, Warren T, Panchal R, Bavari S, Julander J, et al. BCX4430—a broad-spectrum antiviral adenosine nucleoside analog under development for the treatment of Ebola virus disease. J Infect Public Health (2016) 9:220–6. doi:10.1016/j.jiph.2016.04.002
204. Cardile AP, Warren TK, Martins KA, Reisler RB, Bavari S. Will there be a cure for Ebola? Annu Rev Pharmacol Toxicol (2017) 57:329–48. doi:10.1146/annurev-pharmtox-010716-105055
205. Olsen ME, Filone CM, Rozelle D, Mire CE, Agans KN, Hensley L, et al. Polyamines and hypusination are required for Ebolavirus gene expression and replication. mBio (2016) 7(4):e882–816. doi:10.1128/mBio.00882-16
206. Sweiti H, Ekwunife O, Jaschinski T, Lhachimi SK. Repurposed therapeutic agents targeting the Ebola virus: a systematic review. Curr Therap Res (2017) 84:10–21. doi:10.1016/j.curtheres.2017.01.007
207. Johnson JC, Martinez O, Honko AN, Hensley LE, Olinger GG, Basler CF. Pyridinyl imidazole inhibitors of p38 MAP kinase impair viral dendritic cells. Antiviral Res (2014) 107:102–9. doi:10.1016/j.antiviral.2014.04.014
208. Dunning J, Kennedy SB, Antierens A, Whitehead J, Ciglenecki I, Carson G, et al. Experimental treatment of Ebola virus disease with brincidofovir. PLoS One (2016) 11:e0162199. doi:10.1371/journal.pone.0162199
209. Chiramel AI, Banadyga L, Dougherty JD, Falzarano D, Martellaro C, Brees D, et al. Alisporivir has limited antiviral effects against Ebola virus strains Makona and Mayinga. J Infect Dis (2016) 214(S3):S355–9. doi:10.1093/infdis/jiw241
210. Yang S, Xu M, Lee EM, Gorshkov K, Shiryaev SA, He S, et al. Emetine inhibits Zika and Ebola virus infections through two molecular mechanisms: inhibiting viral replication and decreasing viral entry. Cell Discov (2018) 4:31. doi:10.1038/s41421-018-0034-1
211. Wang Y, Cui R, Li G, Gao Q, Yuan S, Altmeyer R, et al. Teicoplanin inhibits Ebola pseudovirus infection in cell culture. Antiviral Res (2016) 125:1–7. doi:10.1016/j.antiviral.2015.11.003
212. Yonezawa A, Cavrois M, Greene WC. Studies of Ebola virus glycoprotein-mediated entry and fusion by using pseudotyped human immunodeficiency virus type 1 virions: involvement of cytoskeletal proteins and enhancement by tumor necrosis factor alpha. J Virol (2005) 79(2):918–26. doi:10.1128/JVI.79.2.918-926.2005
213. Kinch MS, Yunus AS, Lear C, Mao H, Chen H, Fesseha Z, et al. FGI-104: a broad-spectrum small molecule inhibitor of viral infection. Am J Transl Res (2009) 1(1):87–98.
214. Ekins S, Freundlich JS, Coffee M. A common feature pharmacophore for FDA-approved drugs inhibiting the Ebola virus. Version 2. F1000Res (2014) 3:277. doi:10.12688/f1000research.5741.2
215. Madrid PB, Panchal RG, Warren TK, Shurtleff AC, Endsley AN, Green CE, et al. Evaluation of Ebola virus inhibitors for drug repurposing. ACS Infect Dis (2015) 1(7):317–26. doi:10.1021/acsinfecdis.5b00030
216. Salata C, Baritussio A, Munegato D, Calistri A, Ha HR, Bigler L, et al. Amiodarone and metabolite MDEA inhibit Ebola virus infection by interfering with the viral entry process. Pathog Dis (2015) 73(5):ftv032. doi:10.1093/femspd/ftv032
217. Yuan S. Possible FDA-approved drugs to treat Ebola virus infection. Infect Dis Poverty (2015) 4:23. doi:10.1186/s40249-015-0055-z
218. Johansen LM, DeWald LE, Shoemaker CJ, Hoffstrom BG, Lear-Rooney CM, Stossel A, et al. A screen of approved drugs and molecular probes identifies therapeutics with anti-Ebola virus activity. Sci Transl Med (2015) 7(290):290ra89. doi:10.1126/scitranslmed.aaa5597
219. Takada A, Robison C, Goto H, Sanchez A, Murti KG, Whitt MA, et al. A system for functional analysis of Ebola virus glycoprotein. Proc Natl Acad Sci U S A (1997) 94(26):14764–9. doi:10.1073/pnas.94.26.14764
220. Sissoko D, Laouenan C, Folkesson E, M’Lebing AB, Beavogui AH, Baize S, et al. Experimental treatment with favipiravir for Ebola virus disease (the JIKI Trial): a historically controlled, single-arm proof-of-concept trial in Guinea. PLoS Med (2016) 13:e1001967. doi:10.1371/journal.pmed.1001967
221. Oestereich L, Ludtke A, Wurr S, Rieger T, Muñoz-Fontela C, Gunther S. Successful treatment of advanced Ebola virus infection with T-705 (favipiravir) in a small animal model. Antiviral Res (2014) 105:17–21. doi:10.1016/j.antiviral.2014.02.014
222. Smith DR, McCarthy S, Chrovian A, Olinger G, Stossel A, Geisbert TW, et al. Inhibition of heat-shock protein 90 reduces Ebola virus replication. Antiviral Res (2010) 87(2):187–94. doi:10.1016/j.antiviral.2010.04.015
223. García M, Cooper A, Shi W, Bornmann W, Carrion R, Kalman D, et al. Productive replication of Ebola virus is regulated by the c-Abl1 tyrosine kinase. Sci Transl Med (2012) 4(123):123ra24. doi:10.1126/scitranslmed.3003500
224. Nelson EA, Barnes AB, Wiehle RD, Fontenot GK, Hoenen T, White JM. Clomiphene and its isomers block Ebola virus particle entry and infection with similar potency: potential therapeutic implications. Viruses (2016) 8(8):E206. doi:10.3390/v8080206
225. Kouznetsova J, Sun W, Martínez-Romero C, Tawa G, Shinn P, Chen CZ, et al. Identification of 53 compounds that block Ebola virus-like particle entry via a repurposing screen of approved drugs. Emerg Microbes Infect (2014) 3:e84. doi:10.1038/emi.2014.88
226. Madrid PB, Chopra S, Manger ID, Gilfillan L, Keepers TR, Shurtleff AC, et al. A systematic screen of FDA-approved drugs for inhibitors of biological threat agents. PLoS One (2013) 8(4):e60579. doi:10.1371/journal.pone.0060579
227. Panchal RG, Reid SP, Tran JP, Bergeron AA, Wells J, Kota KP, et al. Identification of an antioxidant small-molecule with broad-spectrum antiviral activity. Antiviral Res (2012) 93(1):23–9. doi:10.1016/j.antiviral.2011.10.011
228. Geisbert TW, Hensley LE, Jahrling PB, Larsen T, Geisbert JB, Paragas J, et al. Treatment of Ebola virus infection with a recombinant inhibitor of factor VIIa/tissue factor: a study in rhesus monkeys. Lancet (2003) 362:1953–8. doi:10.1016/S0140-6736(03)15012-X
229. Henß L, Beck S, Weidner T, Biedenkopf N, Sliva K, Weber C, et al. Suramin is a potent inhibitor of Chikungunya and Ebola virus cell entry. Virol J (2016) 13:149. doi:10.1186/s12985-016-0607-2
230. Zhao Z, Martin C, Fan R, Bourne PE, Xie L. Drug repurposing to target Ebola virus replication and virulence using structural systems pharmacology. BMC Bioinformatics (2016) 17:90. doi:10.1186/s12859-016-0941-9
231. Chopra G, Kaushik S, Elkin PL, Samudrala R. Combating Ebola with repurposed therapeutics using the CANDO platform. Molecules (2016) 21(12):E1537. doi:10.3390/molecules21121537
232. Haque A, Hober D, Blondiaux J. Addressing therapeutic options for Ebola virus infection in current and future outbreaks. Antimicrob Agents Chemother (2015) 59:5892–902. doi:10.1128/AAC.01105-15
233. Olsen NJ, Schleich MA, Karp DR. Multifaceted effects of hydroxychloroquine in human disease. Semin Arthritis Rheum (2013) 43:264–72. doi:10.1016/j.semarthrit.2013.01.001
234. Dowall SD, Bosworth A, Watson R, Bewley K, Taylor I, Rayner E, et al. Chloroquine inhibited Ebola virus replication in vitro but failed to protect against infection anddisease in the in vivo guinea pig model. J Gen Virol (2015) 96(12):3484–92. doi:10.1099/jgv.0.000309
235. Falzarano D, Safronetz D, Prescott J, Marzi A, Feldmann F, Feldmann H. Lack of protection against Ebola virus from chloroquine in mice and hamsters. Emerg Infect Dis (2015) 21(6):1065–7. doi:10.3201/eid2106.150176
236. Long J, Wright E, Molesti E, Temperton N, Barclay W. Antiviral therapies against Ebola and other emerging viral diseases using existing medicines that block virus entry. Version 2. F1000Res (2015) 4:30. doi:10.12688/f1000research.6085.2
237. Kuehne A, Tiffany A, Lasry E, Janssens M, Besse C, Okonta C, et al. Impact and lessons learned from mass drug administrations of malaria chemoprevention during the Ebola outbreak in Monrovia, Liberia, 2014. PLoS One (2016) 11:e0161311. doi:10.1371/journal.pone.0161311
238. Furuta Y, Takahashi K, Fukuda Y, Kuno M, Kamiyama T, Kozaki K, et al. In vitro and in vivo activities of anti-influenza virus compound T-705. Antimicrob Agents Chemother (2002) 46:977–81. doi:10.1128/AAC.46.4.977-981.2002
239. Van Herp M, Declerck H, Decroo T. Favipiravir–a prophylactic treatment for Ebola contacts? Lancet (2015) 385:2350. doi:10.1016/S0140-6736(15)61095-9
240. Chinello P, Petrosillo N, Pittalis S, Biava G, Ippolito G, Nicastri E, et al. QTc interval prolongation during favipiravir therapy in an Ebolavirus-infected patient. PLoS Negl Trop Dis (2017) 11(12):e0006034. doi:10.1371/journal.pntd.0006034
241. Nagata T, Lefor AK, Hasegawa M, Ishii M. Favipiravir: a new medication for the Ebola virus disease pandemic. Disaster Med Public Health Prep (2015) 9:79–81. doi:10.1017/dmp.2014.151
242. Veljkovic V, Loiseau PM, Figadere B, Glisic S, Veljkovic N, Perovic VR, et al. Virtual screen for repurposing approved and experimental drugs for candidate inhibitors of EBOLA virus infection. F1000Res (2015) 4:34. doi:10.12688/f1000research.6110.1
243. Paessler S, Huang C, Sencanski M, Veljkovic N, Perovic V, Glisic S, et al. Ibuprofen as a template molecule for drug design against Ebola virus. Front Biosci (Landmark Ed) (2018) 23:947–53. doi:10.2741/4627
244. Siegel D, Hui HC, Doerffler E, Clarke MO, Chun K, Zhang L, et al. Discovery and synthesis of a phosphoramidate prodrug of a Pyrollo[2,1f] [triazin-4-amino] Adenine C-nucleoside (GS-5734) for the treatment of Ebola and emerging viruses. J Med Chem (2017) 60(5):1648–61. doi:10.1021/acs.jmedchem.6b01594
245. Warren TK, Jordan R, Lo MK, Ray AS, Mackman RL, Soloveva V, et al. Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature (2016) 531(7594):381–5. doi:10.1038/nature17180
246. Konde MK, Baker DP, Traore FA, Sow MS, Camara A, Barry AA, et al. Interferon β-1a for the treatment of Ebola virus disease: a historically controlled, single-arm proof-of-concept trial. PLoS One (2017) 12(2):e0169255. doi:10.1371/journal.pone.0169255
247. Ekins S, Lingerfelt MA, Comer JE, Freiberg AN, Mirsalis JC, O’Loughlin K, et al. Efficacy of tilorone dihydrochloride against Ebola virus infection. Antimicrob Agents Chemother (2017) 62:e1711–7. doi:10.1128/AAC.01711-17
248. Warren TK, Warfield KL, Wells J, Swenson DL, Donner KS, Van Tongeren SA, et al. Advanced antisense therapies for postexposure protection against lethal filovirus infections. Nat Med (2010) 16:991–4. doi:10.1038/nm.2202
249. Geisbert TW, Lee AC, Robbins M, Geisbert JB, Honko AN, Sood V, et al. Post exposure protection of non-human primates against a lethal Ebola virus challenge with RNA interference: a proof-of-concept study. Lancet (2010) 375:1896–905. doi:10.1016/S0140-6736(10)60357-1
250. Warren TK, Whitehouse CA, Wells J, Welch L, Heald AE, Charleston JS, et al. A single phosphorodiamidate morpholino oligomer targeting VP24 protects rhesus monkeys against lethal Ebola virus infection. mBio (2015) 6:e2344–2314. doi:10.1128/mBio.02344-14
251. Bixler SL, Duplantier AJ, Bavari S. Discovering drugs for the treatment of Ebola virus. Curr Treat Options Infect Dis (2017) 9(3):299–317. doi:10.1007/s40506-017-0130-z
252. Dunning J, Sahr F, Rojek A, Gannon F, Carson G, Idriss B, et al. Experimental treatment of Ebola virus disease with TKM-130803: a single-arm phase 2 clinical trial. PLoS Med (2016) 13(4):e1001997. doi:10.1371/journal.pmed.1001997
253. Thi EP, Mire CE, Lee AC, Geisbert JB, Zhou JZ, Agans KN, et al. Lipid nanoparticle siRNA treatment of Ebola-virus-Makona-infected nonhuman primates. Nature (2015) 521:362–5. doi:10.1038/nature14442
254. Sheng M, Zhong Y, Chen Y, Du J, Ju X, Zhao C, et al. Hsa-miR-1246, hsamiR-320a and hsa-miR-196b-5p inhibitors can reduce the cytotoxicity of Ebola virus glycoprotein in vitro. Sci China Life Sci (2014) 57:959–72. doi:10.1007/s11427-014-4742-y
255. Iversen PL, Warren TK, Wells JB, Garza NL, Mourich DV, Welch LS, et al. Discovery and early development of AVI-7537 and AVI-7288 for the treatment of Ebola virus and Marburg virus infections. Viruses (2012) 4:2806–30. doi:10.3390/v4112806
256. Basu A, Mills DM, Mitchell D, Ndungo E, Williams JD, Herbert AS, et al. Novel small molecule entry inhibitors of Ebola virus. J Infect Dis (2015) 212(Suppl 2):S425–34. doi:10.1093/infdis/jiv223
257. Mupapa K, Massamba M, Kibadi K, Kuvula K, Bwaka A, Kipasa M, et al. Treatment of Ebola hemorrhagic fever with blood transfusions from convalescent patients. International Scientific and Technical Committee. J Infect Dis (1999) 179(Suppl 1):S18–23. doi:10.1086/514298
258. Dye JM, Wu H, Hooper JW, Khurana S, Kuehne AI, Coyle EM, et al. Production of potent fully human polyclonal antibodies against Ebola Zaire virus in transchromosomal cattle. Sci Rep (2016) 6:24897. doi:10.1038/srep24897
259. Li D, Chen T, Hu Y, Zhou Y, Liu Q, Zhou D, et al. An Ebola virus-like particle-based reporter system enables evaluation of antiviral drugs in vitro under non-biosafety level 4 conditions. J Virol (2016) 90(19):8720–8. doi:10.1128/JVI.01239-16
260. Shabman RS, Leung DW, Johnson J, Glennon N, Gulcicek EE, Stone KL, et al. DRBP76 associates with Ebola virus VP35 and suppresses viral polymerase function. J Infect Dis (2011) 204:S911–8. doi:10.1093/infdis/jir343
261. McCarthy SD, Majchrzak-Kita B, Racine T, Kozlowski HN, Baker DP, Hoenen T, et al. A rapid screening assay identifies monotherapy with interferon-ß and combination therapies with nucleoside analogs as effective inhibitors of Ebola virus. PLoS Negl Trop Dis (2016) 10(1):e0004364. doi:10.1371/journal.pntd.0004364
262. Audet J, Wong G, Wang H, Lu G, Gao GF, Kobinger G, et al. Molecular characterization of the monoclonal antibodies composing ZMAb: a protective cocktail against Ebola virus. Sci Rep (2014) 4:6881. doi:10.1038/srep06881
263. Modrof J, Mühlberger E, Klenk HD, Becker S. Phosphorylation of VP30 impairs Ebola virus transcription. J Biol Chem (2002) 277(36):33099–104. doi:10.1074/jbc.M203775200
264. Aman MJ, Kinch MS, Warfield K, Warren T, Yunus A, Enterlein S, et al. Development of a broad-spectrum antiviral with activity against Ebola virus. Antiviral Res (2009) 83(3):245–51. doi:10.1016/j.antiviral.2009.06.001
265. Kolokoltsov AA, Adhikary S, Garver J, Johnson L, Davey RA, Vela EM. Inhibition of Lassa virus and Ebola virus infection in host cells treated with the kinase inhibitors genistein and tyrphostin. Arch Virol (2012) 157(1):121–7. doi:10.1007/s00705-011-1115-8
266. Huggins J, Zhang ZX, Bray M. Antiviral drug therapy of filovirus infections: S-adenosylhomocysteine hydrolase inhibitors inhibit Ebola virus in vitro and in a lethal mouse model. J Infect Dis (1999) 179(Suppl 1):S240–7. doi:10.1086/514316
267. Geisbert TW, Hensley LE, Kagan E, Yu EZ, Geisbert JB, Daddario-DiCaprio K, et al. Postexposure protection of guinea pigs against a lethal Ebola virus challenge is conferred by RNA interference. J Infect Dis (2006) 193(12):1650–7. doi:10.1086/504267
268. Enterlein S, Warfield KL, Swenson DL, Stein DA, Smith JL, Gamble CS, et al. VP35 knockdown inhibits Ebola virus amplification and protects against lethal infection in mice. Antimicrob Agents Chemother (2006) 50(3):984–93. doi:10.1128/AAC.50.3.984-993.2006
269. Golkar Z, Battaria R, Pace DG, Bagasra O. Inhibition of Ebola virus by anti-Ebola miRNAs in silico. J Infect Dev Ctries (2016) 10(6):626–34. doi:10.3855/jidc.7127
270. Rocker AE, Müller JA, Dietzel E, Harms M, Krüger F, Heid C, et al. The molecular tweezer CLR01 inhibits Ebola and Zika virus infection. Antiviral Res (2018) 152:26–35. doi:10.1016/j.antiviral.2018.02.003
271. Balmith M, Faya M, Soliman ME. Ebola virus: a gap in drug design and discovery – experimental and computational perspective. Chem Biol Drug Des (2017) 89(3):297–308. doi:10.1111/cbdd.12870
272. Balmith M, Soliman MES. Potential Ebola drug targets – filling the gap: a critical step forward towards the design and discovery of potential drugs. Biologia (2017) 72(1):1–13. doi:10.1515/biolog-2017-0012
273. Schuler J, Hudson ML, Schwartz D, Samudrala R. A systematic review of computational drug discovery, development, and repurposing for Ebola virus disease treatment. Molecules (2017) 22(1777):1–27. doi:10.3390/molecules22101777
274. Dhama K, Chakraborty S, Mahima, Wani MY, Verma AK, Deb R, et al. Novel and emerging therapies safeguarding health of humans and their companion animals: a review. Pak J Biol Sci (2013) 16:101–11. doi:10.3923/pjbs.2013.101.111
275. Dhama K, Karthik K, Khandia R, Munjal A, Tiwari R, Rana R, et al. Medicinal and therapeutic potential of herbs and plant metabolites/extracts countering viral pathogens – current knowledge and future prospects. Curr Drug Metab (2018). doi:10.2174/1389200219666180129145252
276. Malik YS, Sharma K, Jeena LM, Kumar N, Sircar S, Rajak KK, et al. Toll-like receptors: the innate immune receptors with ingenious anti-viral paradigm. South Asian J Exp Biol (2013) 3:207–13.
277. Gao J, Yin L. Drug development for controlling Ebola epidemic – a race against time. Drug Discov Ther (2014) 8:229–31. doi:10.5582/ddt.2014.01040
278. Arntzen C. Plant-made pharmaceuticals: from “Edible Vaccines” to Ebola therapeutics. Plant Biotechnol (2015) 13:1013–6. doi:10.1111/pbi.12460
279. Streatfield SJ, Kushnir N, Yusibov V. Plant-produced candidate countermeasures against emerging and re-emerging infections and bioterror agents. Plant Biotechnol J (2015) 13:1136–59. doi:10.1111/pbi.12475
280. Yao J, Weng Y, Dickey A, Wang KY. Plants as factories for human pharmaceuticals: applications and challenges. Int J Mol Sci (2015) 16:28549–65. doi:10.3390/ijms161226122
281. Iqbal HM, Villalba A, Khandia R, Munjal A, Dhama K. Recent trends in nanotechnology-based drugs and formulations for targeted therapeutic delivery. Recent Pat Inflamm Allergy Drug Discov (2016) 10(2):86–93. doi:10.2174/1872213X10666161213162823
Keywords: Ebola virus, Ebola virus disease, vaccines, prophylactics, drugs, therapeutics, treatment
Citation: Dhama K, Karthik K, Khandia R, Chakraborty S, Munjal A, Latheef SK, Kumar D, Ramakrishnan MA, Malik YS, Singh R, Malik SVS, Singh RK and Chaicumpa W (2018) Advances in Designing and Developing Vaccines, Drugs, and Therapies to Counter Ebola Virus. Front. Immunol. 9:1803. doi: 10.3389/fimmu.2018.01803
Received: 01 April 2018; Accepted: 23 July 2018;
Published: 10 August 2018
Edited by:
Hiroyuki Oshiumi, Kumamoto University, JapanReviewed by:
Oscar Negrete, Sandia National Laboratories (SNL), United StatesPei Xu, Sun Yat-sen University, China
Yohei Kurosaki, Nagasaki University, Japan
Copyright: © 2018 Dhama, Karthik, Khandia, Chakraborty, Munjal, Latheef, Kumar, Ramakrishnan, Malik, Singh, Malik, Singh and Chaicumpa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kuldeep Dhama, a2RoYW1hJiN4MDAwNDA7cmVkaWZmbWFpbC5jb20=;
Yashpal Singh Malik, bWFsaWt5cHMmI3gwMDA0MDtnbWFpbC5jb20=
 Kuldeep Dhama
Kuldeep Dhama Kumaragurubaran Karthik
Kumaragurubaran Karthik Rekha Khandia
Rekha Khandia Sandip Chakraborty
Sandip Chakraborty Ashok Munjal
Ashok Munjal Shyma K. Latheef
Shyma K. Latheef Deepak Kumar
Deepak Kumar Muthannan Andavar Ramakrishnan
Muthannan Andavar Ramakrishnan Yashpal Singh Malik
Yashpal Singh Malik Rajendra Singh
Rajendra Singh Satya Veer Singh Malik
Satya Veer Singh Malik Raj Kumar Singh
Raj Kumar Singh Wanpen Chaicumpa
Wanpen Chaicumpa