- Section of Dermatooncology, Department of Dermatology and National Center for Tumor Diseases (NCT), University Hospital Heidelberg, Heidelberg, Germany
Background: Immune checkpoint inhibition (ICI) with anti-CTLA-4 and/or anti-PD-1 antibodies is standard treatment for metastatic melanoma. Anti-PD-1 (pembrolizumab, nivolumab) and anti-PD-L1 antibodies (atezolizumab, durvalumab, and avelumab) have been approved for treatment of several other advanced malignancies, including non-small-cell lung cancer (NSCLC); renal cell, and urothelial carcinoma; head and neck cancer; gastric, hepatocellular, and Merkel-cell carcinoma; and classical Hodgkin lymphoma. In some of these malignancies approval was based on the detection of biomarkers such as PD-L1 expression or high microsatellite instability.
Methods: We review the current status of prognostic and predictive biomarkers used in ICI for melanoma and other malignancies. We include clinical, tissue, blood, and stool biomarkers, as well as imaging biomarkers.
Results: Several biomarkers have been studied in ICI for metastatic melanoma. In clinical practice, pre-treatment tumor burden measured by means of imaging and serum lactate dehydrogenase level is already being used to estimate the likelihood of effective ICI treatment. In peripheral blood, the number of different immune cell types, such as lymphocytes, neutrophils, and eosinophils, as well as different soluble factors, have been correlated with clinical outcome. For intra-tumoral biomarkers, expression of the PD-1 ligand PD-L1 has been found to be of some predictive value for anti-PD-1-directed therapy for NSCLC and melanoma. A high mutational load, particularly when accompanied by neoantigens, seems to facilitate immune response and correlates with patient survival for all entities treated by use of ICI. Tumor microenvironment also seems to be of major importance. Interestingly, even the gut microbiome has been found to correlate with response to ICI, most likely through immuno-stimulatory effects of distinct bacteria. New imaging biomarkers, e.g., for PET, and magnetic resonance imaging are also being investigated, and results suggest they will make early prediction of patient response possible.
Conclusion: Several promising results are available regarding possible biomarkers for response to ICI, which need to be validated in large clinical trials. A better understanding of how ICI works will enable the development of biomarkers that can predict the response of individual patients.
Introduction
In the last decade, treatment of metastatic melanoma and other malignancies has improved significantly. In addition to targeted treatment options, immunotherapy with immune checkpoint inhibitors (ICI) has contributed greatly to this development.
The anti-CTLA-4 antibody (CTLA4ab) ipilimumab was first approved by the U.S. Food and Drug Administration (FDA) for the treatment of metastatic melanoma in 2011 (1), followed by the anti-PD-1 antibodies (PD1ab) pembrolizumab and nivolumab in 2014 (2–4). Combined ICI with CTLA4ab and PD1ab for melanoma was introduced with enormous success, but was also accompanied by significant immune-related adverse events (irAEs) (5, 6). PD1ab treatment is currently approved for treatment of several other advanced malignancies including non-small-cell lung cancer (NSCLC), urothelial cancer, renal cell carcinoma (RCC), squamous cell carcinoma of head and neck (SCCHN), gastric carcinoma, hepatocellular carcinoma, and classical Hodgkin lymphoma (7–12). The anti-PD-L1 antibodies (PD-L1ab) atezolizumab (urothelial carcinoma and NSCLC), durvalumab (urothelial carcinoma), and avelumab [Merkel-cell carcinoma (MCC) and urothelial carcinoma] have also recently been approved by the FDA (13–18). In 2017 the FDA also announced a biomarker-based approval for pembrolizumab for patients with unresectable or metastatic solid tumors, and for colorectal carcinoma (CRC) with high microsatellite instability or mismatch repair deficiency (dMMR) (19).
Despite this enormous success, ICI does not achieve long-lasting responses for all patients. Response varies between different entities, and between different patients. For melanoma, PD1ab monotherapy can achieve a response of 26–32% (2, 4) and the combination of PD1ab and CTLA4ab achieves a response as high as 60% (5). Some subsets of patients achieve durable responses with PD1ab monotherapy and do not require combined ICI, and could therefore be protected from the higher risk of irAEs.
There remains the medical need to find reliable biomarkers that could help to identify both, the patients who would benefit from ICI and the primary resistant patients. Biomarkers are also needed to help decide the type of first-line treatment, e.g., whether BRAF-mutant melanoma should be treated by use of targeted or immunotherapy, therapy sequencing, and/or by a combination of treatments. Here, we review biomarkers in the field of ICI therapy for metastatic melanoma and other malignancies. We have not performed a review of pre-analytic, analytic, and clinical validation techniques for biomarkers because these have been reviewed elsewhere (20, 21).
Clinical Biomarkers
Tumor Burden
Tumor burden and metastatic site, e.g., liver or brain metastases, significantly affect patient prognosis, particularly in terms of overall survival (OS), as described in the TNM classification (22). Because the prognostic effect of tumor burden and metastatic site is well known, they are used to stratify clinical trials and are the object of sub-group analyses. Several authors have found an association between metastatic site and incidence of response, progression-free survival (PFS), and OS for PD1ab treatment of melanoma (23–26) (Table 1). Response to PD1ab therapy is better for lung and skin metastases than for metastases in other organs, particularly those in the liver. Response for melanoma brain metastases is lower compared with response for extracranial sites, particularly for PD-1ab monotherapy (27). This could be because T cell infiltrate in cerebral metastases is less dense compared with other anatomic sites (28). For combined ICI with ipilimumab and nivolumab, response for brain metastases that were asymptomatic was similar to response for extracranial sites (27, 29). Peripheral blood biomarkers which correlate with tumor burden, such as serum lactate dehydrogenase (LDH), circulating tumor cells (CTCs), and circulating tumor DNA (ctDNA), are of significance for biomarker investigations, as described below.
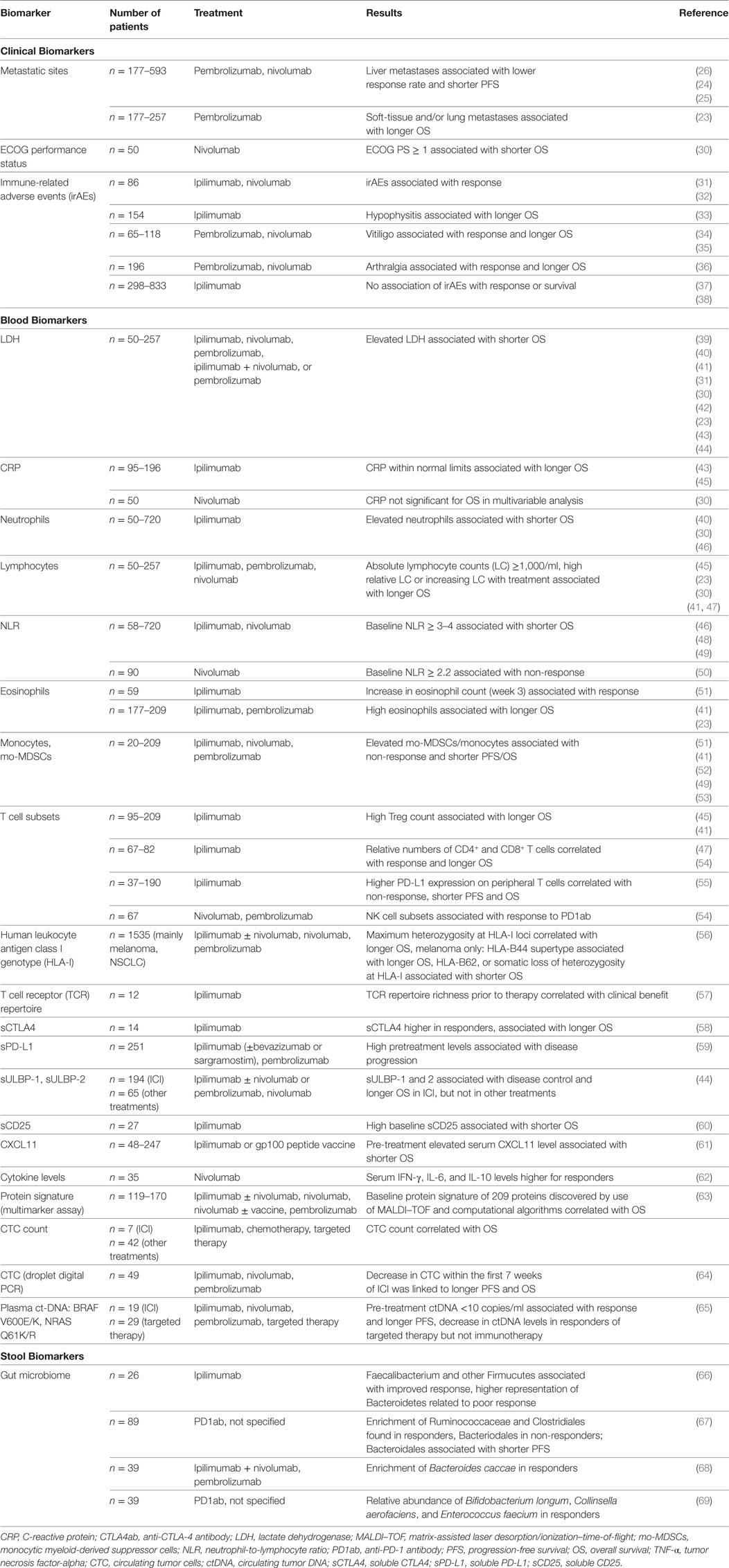
Table 1. Clinical, blood, and stool biomarkers for clinical outcome under checkpoint blockage for metastatic melanoma patients.
Clinical Condition (Performance Status)
A good clinical condition expressed by the ECOG (Eastern Cooperative Oncology Group) performance status (ECOG PS 0) is associated with prolonged OS for patients receiving PD1ab treatment, as well as for patients receiving other melanoma treatments such as BRAF inhibitors (30, 70). For other entities such as NSCLC (71), association between performance status and OS is also well known. Because of its prognostic character, performance status is frequently used as a biomarker in enrichment designs of clinical trials, i.e., only biomarker-positive patients are included, in this case only patients with good performance status. Because patients with poorer performance are not included and are therefore unavailable for further analysis, enrichment design prevents information from being gathered on the prognostic versus predictive value of performance status (20). Significance of other patient characteristics for ICI response, such as sex and age, has only been found in single studies with PD1ab for melanoma (24).
Immune-Related Adverse Events
Immune-Related Adverse Events are side effects caused by the activated immune system and are, therefore, a possible sign of successful immune checkpoint blockage. Several retrospective analyses have reported an association between CTLA4ab-induced irAE and a more favorable clinical outcome (Table 1). From a cohort of 86 patients, occurrence of irAE ≥ grade 2 CTC-AE was associated with improved response, PFS, and OS (31). In contrast, other studies focusing on irAE of any grade could not find this association for large ipilimumab-treated cohorts (37, 38). The development of autoimmune hypophysitis was found to be associated with prolonged OS (33). It has been observed in retrospective analyses of several groups receiving PD1ab therapy that incidence of irAEs is associated with a more favorable outcome. In a large cohort of 576 PD1ab-treated patients, response but not PFS was associated with irAE manifestation of any grade (32). For certain adverse events, vitiligo was found to be associated with response (34) and, similar to exanthema, with longer OS (35). Arthralgia of any grade was associated with response and significantly longer PFS (36). Here, median onset of arthralgia was 100 days after start of treatment and was caused by either arthritis or reactivated osteoarthritis in pre-damaged joints. It is worth noting that all these analyses were performed retrospectively and that there is a risk of guarantee-time bias, i.e., patients with early progression are less likely to develop irAEs because of a shorter treatment period. This bias can be controlled to some degree by use of landmark analysis, which was used in the above-mentioned reports.
Blood Biomarkers
Blood-based biomarkers have several preferential characteristics and are, therefore, the focus of biomarker research. First, they are easily accessible, which enables analysis at several time-points. Second, they might be independent from intra and inter-tumor heterogeneity. Third, they might reflect multiple sites of interest, e.g., tumor cells, tumor microenvironment, and the patient’s immune system.
Serum Biomarkers Correlating With Tumor Load
Lactate dehydrogenase (LDH) is a house-keeping enzyme which is released by rapidly growing tumors. Serum LDH therefore correlates with tumor burden. For melanoma, the prognostic significance of this biomarker is expressed by its inclusion in the American Joint Committee on Cancer classification (72). Serum LDH levels correlate with patients’ OS in various treatment regimens (73), including ICI (Table 1) (23, 30, 31, 39–45). Nearly all studies have found no correlation between baseline LDH and response. Only a dynamic change in LDH from baseline to week 12 was found to be associated with response (31, 45). Hence, despite the prognostic value of LDH, patients with elevated serum LDH can respond to ICI; LDH elevation does not, therefore, lead to an exclusion of patients from ICI treatment. Patients with very high levels of more than twice the upper limit of normal, however, did not benefit from either CTLA-4ab or PD1ab monotherapy (23, 31, 39). Importantly, even though targeted treatments by use of BRAF/MEK inhibitors are known to lead to fast tumor responses in highly advanced patients with BRAF-V600-mutated melanoma, patients with normal LDH still achieve the better clinical outcome from treatment (74). Hence, the best treatment sequencing of targeted and immunotherapy for patients with BRAF-mutated melanoma and normal LDH is not clear.
Another serum biomarker which correlates with tumor burden is the acute-phase protein C-reactive protein (CRP). It is a prognostic marker for melanoma, and elevated concentrations are linked to worse PFS and OS (75). CRP also significantly affects prognosis for other malignancies such as renal, gastrointestinal, lung, pancreas, hepatocellular, and bladder cancer (76). For ICI, only retrospective analyses are available. A normal CRP level at the start of treatment was associated with longer OS for ipilimumab-treated melanoma patients (43). Decreasing CRP levels from baseline to week 12 of CTLA4ab therapy were, as for LDH, associated with longer PFS and OS (45). In multivariate analysis of a small study of PD1ab-treated melanoma patients, baseline CRP levels were no independent biomarkers for OS (30). For PD1ab treatment of NSCLC, in contrast, elevated baseline CRP levels were shown to be associated with shorter PFS (77).
Differential Blood Count Biomarkers
Immune checkpoint inhibition works via activation of T lymphocytes. Hence, the number of lymphocytes and other immune cells circulating might affect its efficacy. Several retrospective analyses have focused on this question. The role of neutrophils, which can display heterogeneous phenotypes and diverse functionality, is also important (78). Increased levels of neutrophils have been found in the peripheral blood of cancer patients; they might possibly be induced by cytokines such as granulocyte-colony stimulating factor (G-CSF), although no definite cause for neutrophilia in malignancies has been clearly shown (78). Pretreatment elevation of neutrophil count has been found to correlate with worse OS in ICI treatment of melanoma (Table 1). Increasing lymphocyte counts, in contrast, correlated with prolonged OS in ICI-treated patients (Table 1). The neutrophil-to-lymphocyte ratio (NLR) has been more frequently reported to be of prognostic, and potentially predictive, value by several authors using various cutoffs (NLR > 2–5). For ipilimumab-treated melanoma patients, high baseline NLR was associated with shorter PFS and OS (46, 48). For PD1ab treatment, high baseline NLR was linked to non-response (50) and to worse OS for melanoma (49, 79) and for several other types of cancer being investigated in phase I studies with PD1ab/PD-L1ab treatment (79). For example, NLR was associated with lower incidence of response, poor PFS, and OS for NSCLC (71, 80) and for RCC (81). It is worth noting that an association was also found between NLR and prognosis for melanoma patients treated by use of BRAF inhibitors (82). Overall, NLR certainly has prognostic value but is probably not treatment specific and no predictive ability has been observed so far. It has, however, also been shown that eosinophils correlate with clinical outcome in ICI treatment of melanoma. A high pre-ICI absolute or relative eosinophil count was associated with prolonged OS (23, 41). Dynamically, for melanoma patients treated by use of ipilimumab, eosinophil counts that increased with treatment correlated with response to ipilimumab (51).
Myeloid-derived suppressor cells (MDSCs) are important in melanoma and other malignancies. MDSCs have immunosuppressive potential, particularly by inhibiting activated T cells, and can be divided into two subgroups: granulocytic and monocytic myeloid-derived suppressor cell (mo-MDSC) (78). The number of mo-MDSC in the peripheral blood in particular has been correlated with prognosis for melanoma patients (51). In CTLA4ab treatment, the number of mo-MDSC has been found to negatively affect incidence of response and survival (41, 47, 51). In addition, mo-MDSC was negatively correlated with OS for CTLA4ab-pretreated melanoma patients receiving PD1ab (52) (Table 1). The development of cytometry by time-of-flight (CyTOF) has enabled in-depth analysis of peripheral blood immune cells. CyTOF can measure up to 50 proteins per cell. Use of CyTOF for ICI patients has shown that high incidence of classical monocytes (CD14+CD16−HLA-DRhi) are associated with response and improved PFS in PD1ab therapy for melanoma (53).
It is worth mentioning that all these potential markers have been found by retrospective exploratory analyses. They potentially have prognostic features but their predictive potential remains unclear. Furthermore, the above-mentioned publications used several different cutoffs. Prospective studies are needed to investigate a possible predictive value of these biomarkers.
Biomarkers on Peripheral T Cells
T cells are the effector cells of ICI treatment. Therefore, in addition to the pure cell number of several subsets of T cells in the peripheral blood, a more detailed analysis might be beneficial. Retrospective examination of peripheral blood T cell subsets in ipilimumab-treated melanoma patients revealed that higher pre-treatment CD4+/CD25+/FoxP3+ Tregs was associated with favorable survival (41, 45) (Table 1). Tregs express high levels of CTLA-4 and might, therefore, be one of the main targets of ipilimumab. It was shown that more melanoma-reactive CD8+ cytotoxic T cells in the peripheral blood were detected in patients after treatment than before treatment (83). Preexisting immune responses were only infrequently boosted.
Most studies have focused on PD-L1 expression on tumor cells and macrophages in the tumor microenvironment, whereas PD-L1-expression on peripheral T cells has been studied to a lesser extent. High PD-L1 expression on peripheral T cells (CD4+ and CD8+) has been shown to be associated with worse PFS and OS for CTLA4ab treatment of melanoma (55). For an NSCLC cohort treated mainly by chemotherapy, high PD-1/PD-L1/PD-L2 expression on peripheral blood T cells was associated with shorter OS (84). PD-L1 expression on peripheral T cells might, therefore, be a mechanism for tumor immune escape. Expression of co-stimulatory molecules on peripheral T cells was also studied. Detectable levels of CD137+CD8+ cytotoxic T cells in the peripheral blood were found in patients with relapse-free status after adjuvant combined ICI, but this was not investigated in the therapeutic setting (55). CyTOF analysis revealed that high pre-treatment incidence of memory T cells was a potential marker for response to CTLA4ab, whereas higher incidence of distinct NK cell subsets was found to be associated with response to PD1ab treatment for melanoma (54).
Tumor antigen presentation by human leukocyte antigen class I (HLA-I) molecules is a prerequisite for cancer cell attack by cytotoxic T cells. Maximum heterozygosity at HLA-I loci (A, B, C) opposed to homozygosity for at least one HLA locus was shown to be associated with longer OS after ICI for mainly NSCLC and melanoma (56). Furthermore, HLA-B44 supertype was linked to prolonged OS, whereas the HLA-B62 supertype or somatic loss of heterozygosity at HLA-I were associated with worse OS for melanoma (56). Although assessed in test and validation cohorts, these biomarkers have also not been prospectively tested for their potential predictive versus prognostic value. Nevertheless, this investigation indicates that diversity in antigen presentation might improve tumor defense. Tumor cell antigens presented on MHC molecules are recognized by T cells via the T cell receptor (TCR). The TCR is, therefore, of great interest in ICI. TCR diversity and clonality can be investigated by use of sequencing methods. Most investigations focus on TCR sequencing in tumor tissue specimens (see “Tissue Biomarkers”). TCR sequencing data in the peripheral blood are limited. In ipilimumab-treated melanoma patients, patients with a positive clinical outcome had a higher degree of TCR repertoire richness prior to therapy (57). In patients with urothelial carcinoma, TCR sequencing in peripheral blood was done before and after atezolizumab administration. Here, a pretreatment TCR clonality below the median was associated with improved PFS and OS (85). Furthermore, a long-lasting clinical benefit was found in patients with a more substantial expansion of tumor-associated TCR clones after three weeks (85). T cells carrying the γδ-TCR-subtype—as opposed to the more common αβ-subtype—play a distinct role in anti-tumor immunology. A study found that higher incidence of Vδ2+ cells (versus Vδ1+ cells) was linked to longer OS in melanoma patients, and suggested that Vδ2+ cells potentially have tumor-killing capability (86). However, this has not yet been investigated for ICI.
In summary, T cells as the effector cells of ICI are the focus of biomarker research for melanoma and other malignancies. Some approaches are promising, but no biomarker has yet been evaluated in a prospective clinical setting. Their predictive ability therefore remains to be determined.
Soluble Serum Biomarkers
Soluble serum biomarkers that might correlate with clinical benefit of ICI treatment include immune regulatory molecules such as cytokines or soluble checkpoint receptors and binding partners. Biomarker potential in ICI treatment of melanoma (Table 1) and other malignancies has been found for several soluble serum factors.
Soluble CTLA-4 (sCTLA4), which is mainly secreted by regulatory T cells (Tregs), has inhibitory effects on T cell immune responses (87). An association has been found between higher sCTLA4 levels and both response and prolonged OS for a small cohort of ipilimumab-treated melanoma patients; this was not found for patients who did not receive ipilimumab (58). In view of its inhibitory function on T cells, neutralization by CTLA4ab therapy might be responsible for this finding. Higher levels of soluble PD-L1 (sPD-L1) can be found in tumor patients compared with healthy individuals (88, 89). Its physiological role has not yet been identified (89). It holds some prognostic value because high pre-treatment concentrations are associated with shorter OS for NSCLC (88), and for hepatocellular carcinoma (90), and it is linked to disease progression in ICI for melanoma (59). sPD-L1 is, however, likely to be only prognostic and not predictive for melanoma, because assessments pre- and during early ICI did not reveal significant associations with response or OS (59). In addition to sPD-L1, a soluble form of PD-1 (sPD-1) also exists (89) which is currently being investigated in a clinical trial (NCT03197636).
Soluble ligands of the transmembrane receptor NKG2D (sULBP-1, sULBP-2), which affect induction or reactivation of T cell responses, were associated with OS for ICI-treated but not for BRAF inhibitor-treated melanoma patients (44). They are interesting biomarkers with treatment-specific potential. Further data are, however, needed to confirm the significance of these markers. In addition, soluble CD25 (sCD25), the alpha unit of the IL-2 receptor, was found to be a biomarker in ipilimumab therapy. The interleukin (IL)-2/IL-2 receptor pathway is essential for the antitumor activity of CTLA4ab (91). High pre-treatment serum levels of sCD25 were shown to be associated with shorter OS for CTLA4ab-treated patients (60). A possible explanation for this finding could be direct binding of sCD25 to IL-2, which would amplify Tregs and inhibit tumor immune response.
It has been shown that other serum factors including vascular epithelial growth factor and chemokines such as C-X-C chemokine motif ligand (CXCL)8 are of prognostic significance for PFS and OS of melanoma of different stages, regardless of treatment (92). For CTLA4ab therapy, elevated pre-treatment levels of CXCL11 were associated with poor OS (61). The gene expression of CXCL11 is induced by interferon-(IFN)-γ, and CXCL11 binds to its chemokine receptor CXCR3, which is mainly expressed on activated T cells. CXCR3 is highly important for the migration of cytotoxic T cells, and its tissue expression correlates with poorer prognosis for several malignancies (93). Elevated CXCL11 in blood has been linked to poorer outcome for ipilimumab-treated melanoma (61). For PD1ab treatment, serum IFN-γ, IL-6 and IL-10 levels were significantly higher for responders than for non-responders (62).
In contrast to single-biomarker searches, serum-based multi-marker assays are of current and future interest. One group developed a test based on 119 patients with pre-PD1ab therapy for metastatic melanoma using matrix-assisted laser desorption/ionization-time of flight (MALDI–TOF) mass spectrometry, which was validated in four independent cohorts (63). Computational algorithms were used for data analysis, resulting in a protein signature of 209 proteins that appears to differentiate patients with three-year OS of over 50% from patients with three-year OS of less than 20%. Further analysis revealed that acute phase proteins, complement, and wound healing pathways were associated with poor outcome (63). Because this test has also not been prospectively evaluated yet, distinction of prognostic versus predictive ability is warranted.
Liquid Biopsy
Liquid biopsy, including CTCs, ctDNA, and circulating tumor RNA (ctRNA) has only been studied in small patient cohorts treated by use of ICI (mainly CTLA4ab) for melanoma. An association between treatment response and a decrease in CTCs and ctDNA has been found for targeted therapy, but not for ICI (94). Methodological improvements offer new opportunities for CTC detection, as has been very recently described for microfluidic enrichment of melanoma CTCs combined with RNA-based droplet digital PCR quantitation (64). That study found that a decrease in CTCs within the first seven weeks of ICI was linked to prolonged PFS and OS in CTLA4ab or PD1ab-treated melanoma patients (64). Another approach to CTCs is characterization of subsets of CTCs that express specific markers. The heterogeneity of melanoma CTCs and the significance of CTC subsets (e.g., receptor activator of NF-κβ (RANK) expressing CTCs) as biomarkers has been found to affect targeted treatment; this was not, however, observed for ICI (65). For NSCLC, high expression of carcinoembryonic antigen and telomerase reverse transcriptase was linked to non-response to nivolumab (95). For urothelial carcinoma, CTCs with high PD-L1 expression were associated with worse OS (96), and for chemotherapeutically treated SCCHN, high levels of CTCs with PD-L1 expression were linked to poor PFS and OS (97). CTCs are being studied as part of a recruiting clinical trial on prospective biomarkers for melanoma, and this will hopefully shed light on a potential predictive function of CTCs for ICI.
Not only CTCs but also ctDNA has been investigated in PD1ab therapy. A proof-of-concept study found that detectable levels of ctDNA in week 8 of PD1ab therapy were linked to worse PFS and OS for NSCLC, uveal melanoma, and microsatellite-instable colorectal cancer (98). Furthermore, an association was found between high hypermutated ctDNA levels and response, PFS, and OS for diverse malignancies treated by use of ICI (99).
Other Blood Biomarkers
Several other blood biomarkers have been investigated in ICI patients; for example, blood-based testing of gene-expression profiles of cathepsin D, phopholipase A2 group VII, thioredoxin reductase 1, and interleukin 1 receptor-associated kinase 3 were found to be associated with OS for CTLA4ab-treated patients (100). Ongoing studies on blood biomarkers for ICI treatment of melanoma include assessment of different T cell subsets, cytokines, and CTCs (Table 1). The challenge is to select the most promising biomarkers, ideally identified by several different investigators, and to study them in prospective clinical trials.
Stool Biomarkers
The effect of gut microbiota on anti-tumor response has recently been observed in both murine and human studies for several cancers, including melanoma, NSCLC, and RCC. Unsurprisingly, microorganisms are prevalent in primary CRC, but distant metastases are also colonized with Fusobacterium and its associated microbiome, including Bacteroides, Selenomonas, and Prevotella species (101). Gut flora composition can stimulate or inhibit immune response. Immunostimulatory effects of Bacteroidales, particularly Bacteroides fragilis, have been observed for CTLA4ab therapy in mice (102). Similarly, Bifidobacterium improved anti-tumor responses for PD-1/PD-L1 blockade in a murine melanoma model (103). In contrast, ICI treatment itself can affect the population of gut microbiota (102). Baseline gut microbiota have been investigated in small CTLA4ab-treated melanoma cohorts (Table 1). Enrichment with Faecalibacterium and other Firmucutes was associated with improved response and with development of colitis, whereas a higher representation of Bacteroidetes was related to poorer response to CTLA4ab therapy (66). For melanoma patients receiving PD1ab therapy, enrichment of Ruminococcaceae and Clostridiales was found in responders whereas Bacteriodales were enriched in non-responders (67). Shortened PFS was observed for patients with high abundance of Bacteroidales, which is in agreement with another publication on melanoma patients treated with CTLA4ab (66). Another analysis found enrichment of Bacteroides caccae in all ICI responders, and specifically Faecalibacterium prausnitzii, Bacteroides thetaiotaomicron, and Holdemania filiformis if treated with ipilimumab plus nivolumab combination therapy. Dorea formicogenerans was enriched in pembrolizumab responders (68). Other authors found relative abundance of Bifidobacterium longum, Collinsella aerofaciens, and Enterococcus faecium in PD1ab responders with melanoma (69). An imbalance in gut microbiota correlating with impaired immune cell activity was observed for non-responders. Treatment by use of antibiotics before or shortly after ICI was associated with poorer response and worse OS for patients with RCC and NSCLC; in this analysis, a higher percentage of non-responders (69%) had a particularly low level of Akkermansia muciniphila compared with responders (34%) (104). It is worth nothing that in a mouse model, fecal transplants of responders into germ-free mice restored the anti-tumor effect of PD-1/PD-L1 blockade (67, 69, 104).
In summary, gut microbiota affect anti-tumor immune response. However, there is only partial overlap between the potentially relevant microorganisms (Table 1). It is unclear if this is because of methodological reasons, or if it depends on the individual tumor entity, or the geographical region and associated dietary habits of the investigated patients. Further prospective studies are needed to evaluate the prognostic or predictive effect of gut microbiota on ICI outcome (currently ongoing: NCT02960282, NCT03370861). A study on fecal microbiota transplantation for metastatic melanoma patients who failed ICI is also being conducted (NCT03353402).
Tissue Biomarkers
PD-L1 Expression
In the initial phase I study of nivolumab for patients with solid tumors, an association between PD-L1 expression and probability of response was observed for NSCLC, melanoma, and RCC (105, 106) (Table 2). Because clinical significance was greatest for NSCLC, this led to further PD1ab studies using enrichment designs with different antibodies and expression cutoffs (107–109). Because patients with PD-L1 negative (or PD-L1 expression below cutoff) value cannot be followed in clinical trials of enriched design, it is not possible to distinguish between prognostic and predictive value (20). It is worth mentioning that in a retrospective analysis of metastatic melanoma patients, PD-L1 expression was linked to improved OS irrespective of treatment type; this raises the possibility of a prognostic rather than a predictive value for ICI (28). Throughout several clinical trials for NSCLC and urothelial cancer, no association was observed between PD-L1 expression and ICI therapy outcome (8, 110, 111). There was one exception: for urothelial carcinoma, a composite biomarker of either ≥25% positive tumor cells or ≥25% positive immune cells indicated tumor response to durvalumab, and is a pre-requisite for treatment according to FDA-approval (15). PD-L1 positivity was also associated with higher probability of response in a subgroup analysis of PD-L1ab therapy for MCC (14). In addition, PD-L1 expression on tumor and immune cells was linked to higher incidence of response for SCCHN (112). There is growing evidence that response is associated more with PD-L1 expression on tumor-infiltrating immune cells than it is with tumor cell PD-L1 expression (113).
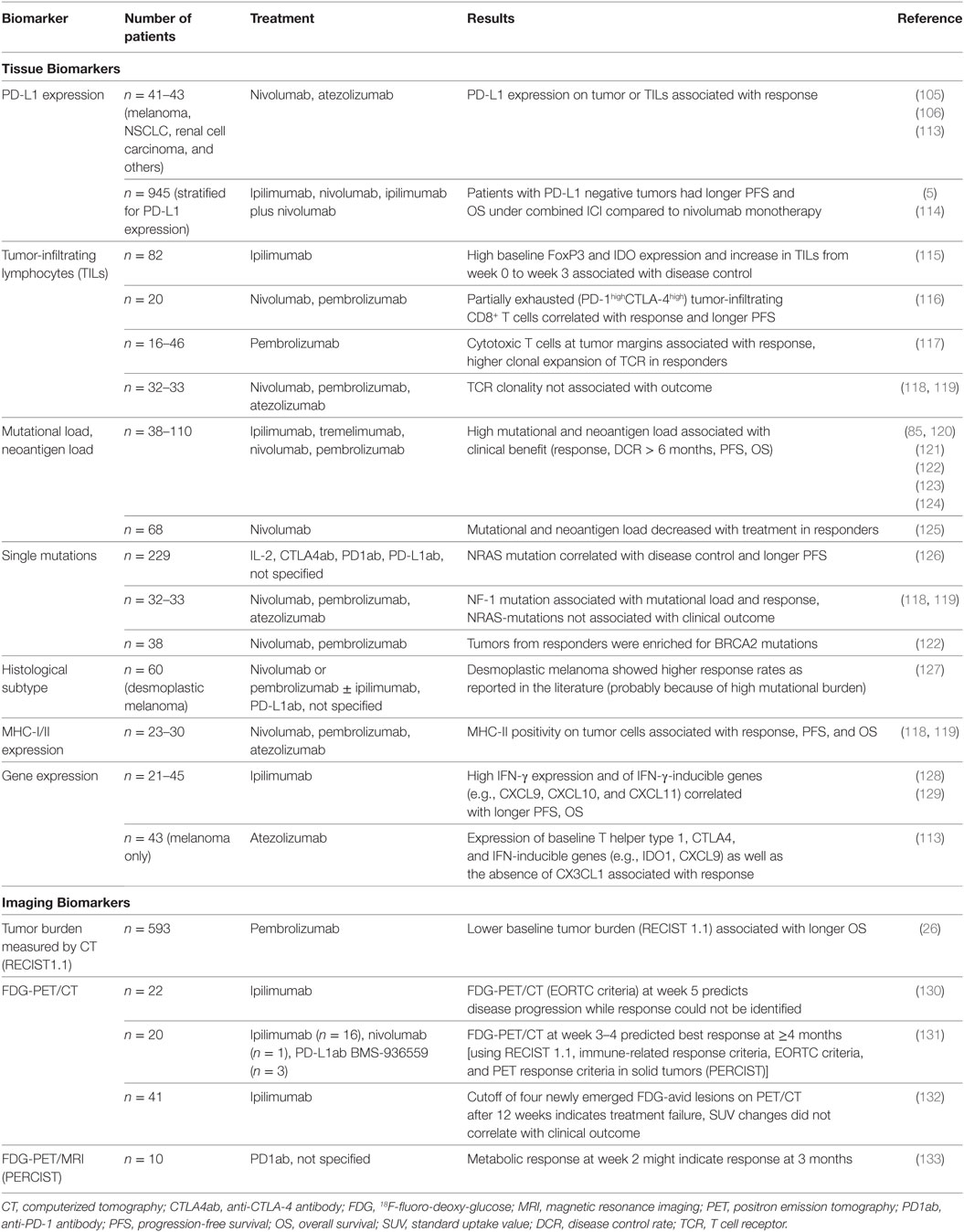
Table 2. Tissue and imaging biomarkers for clinical outcome under checkpoint blockage for metastatic melanoma patients.
PD-L1 expression on tumor cells was used to stratify the design of the Checkmate-067 trial investigating the combination of ipilimumab plus nivolumab compared with nivolumab and ipilimumab monotherapies (5). Although not designed for this purpose, this study revealed that patients with tumors expressing PD-L1 had similar PFS and OS compared with PD1ab monotherapy and the combination of CTLA4ab plus PD1ab (5, 114). Response to combined ICI was still higher, however, compared with response to PD1ab monotherapy. The study was not designed to compare the two nivolumab-containing treatment arms, but it shows possible limitations of PD-L1 as a biomarker for treatment decisions for melanoma.
General problems associated with PD-L1 as a biomarker are: use of different immunohistochemical (IHC) assays, different cutoffs, intra-tumor heterogeneity, and dynamic changes of PD-L1 expression. In summary, there are conflicting data in diverse tumor entities. It is worth noting that treatment responses can be found in PD-L1-negative tumors.
Tumor-Infiltrating Lymphocytes (TIL)
The presence of TILs has prognostic potential for different tumor entities regardless of tumor stage (134, 135). For patients with metastatic melanoma, TILs were associated with a better outcome for primary melanoma and metastatic disease, irrespective of treatment type (28, 136). A prospective biomarker study of ipilimumab-treated patients with melanoma found an association between early increase in TILs and disease control (115) (Table 2). A more detailed assessment of the T cell infiltrate at baseline revealed an association between high baseline FoxP3+ Tregs and indoleamine-2,3-dioxygenase (IDO) expression and favorable outcome (115). For PD1ab-treated melanoma patients, no association was found between baseline TILs and response to PD1ab (106). Abundance of partially exhausted (PD-1highCTLA-4high) tumor-infiltrating CD8+ T cells correlated with response and PFS in PD1ab therapy (116). Cytotoxic T cells at tumor margins were also linked to response to PD1abs (117). Preliminary investigations on TCR clonality of TILs in melanoma metastases revealed higher clonal expansion of TCR for PD1ab-responders compared with non-responders (117). This finding was not, however, confirmed by other authors (119).
Interferon-γ is one of the cytokines secreted by activated T cells and is known to upregulate PD-L1 expression. This might be one reason why PD-L1 expression could be co-localized with TIL infiltrates in melanoma metastases (117, 137). In a retrospective analysis, pretreatment tumor samples from NSCLC and melanoma patients treated with PD1ab were evaluated for IFN-γ expression (129). A significantly longer PFS and OS were observed for patients with high IFN-γ expression. High pre-treatment expression of IFN-inducible genes (e.g., IDO1, CXCL9, CXCL10, and CXCL11 among others) was associated with response and prolonged OS for PD-L1ab treatment of melanoma, but this was less pronounced for NSCLC or RCC (113, 128). Primary mutations in IFN-γ signaling pathways (e.g., JAK1 and JAK2 mutations) have been described for several tumor entities. For cutaneous melanomas, JAK1/2 mutations were detected before treatment in 21% of tissue specimens (138). Interestingly, patients with resistance to ICI were found to harbor JAK mutations with consecutive loss of IFN-γ pathways (139, 140).
The following challenges apply for all potential tissue biomarkers described above: possible dependency on biopsy site, the specific time of the biopsy, and intratumor-heterogeneity. It should be noted that, when considering multiple potential biomarkers, large multivariable analyses are required to exclude a significant overlap of markers (138). Moreover, future models might include transcriptome-derived stromal and immune cell scores exceeding a pure TIL assessment (141).
Mutational Analysis
The first notion that mutational changes might affect tumor response came with the observation that melanoma patients with a high mutation rate benefitted more from ipilimumab treatment than patients with a low one, resulting in longer OS (120). In agreement with this, tumors with a naturally high mutation rate because of exogenous cancerogens, such as UV light, smoking, and alcohol (melanoma, lung cancer, SCCHN, and bladder cancer), belong to the entities that respond best to ICI treatment (123, 142–145). A small biomarker-stratified trial was performed for non-colorectal CRC and mismatch-repair deficient cancers. Stratification according to mismatch repair deficiency and mismatch-repair proficiency revealed a 40% response for patients with mismatch-repair deficiency (MSI high, dMMR), whereas mismatch-repair proficient patients did not respond at all (146). Whereas mismatch-repair deficiency can be found in gastrointestinal and genitourinary tumors (147, 148), it is of minor significance for melanoma. Colli et al. suggested a cutoff of 192 nonsynonymous mutations for a potential clinical benefit of ICI (149). Here, a high mutational load seems to result in prolonged OS in particular, whereas no correlation with response to PD1ab was observed (122). This is in agreement with the observation that melanoma patients treated with PD1ab survive longer even when not responding to the treatment (150). This was found to change, however, for ipilimumab treatment prior to PD1ab therapy; in contrast to ipilimumab-naïve patients, no association between tumor mutational burden and response/OS was observed (125). Most likely, the difference is not because of the number of mutations, but because of the increasing chance of tumor neoepitopes which might be easier recognized by the immune system. Clonal neoantigens in particular might significantly affect ICI outcome (120, 123).
A considerable difficulty of using mutational load as a biomarker is its practical implementation in clinical routine. In addition to the high costs incurred by whole exome sequencing, most neoantigens are probably patient-specific and not recurrent (121). Specific types of mutations might be more frequent, e.g., the frameshift insertion and deletion count was found to be associated with ICI response for melanoma (124). Furthermore, several groups have proposed fitness models to describe neoantigen qualities that could be possibly employed as biomarkers in the future (151, 152). It is not clear, however, that neoantigen burden will add significant value to mutational burden testing, as shown in the examples of urothelial carcinoma and melanoma (85, 153).
Tumor antigen presentation is essential for the immune defense of cancer, and mutations affecting pathways important for antigen presentation, e.g., beta-2-microglobuline (B2M) loss, might result in ICI resistance (139). B2M mutations are more frequent for melanoma, bladder, gastric, and lung cancer in particular, with 27–50% found for these cancers in The Cancer Genome Atlas dataset compared with 1.8% across all tumor types (138). The MHC-II-expression itself (HLA-DR+) was found to be associated with PD1ab/PD-L1ab response in melanoma (118).
It would be easy to use biomarkers for mutations that are routinely assessed in clinical care. BRAF-V600 mutations, which are found in 40–50% of melanomas, are not associated with ICI outcome (154–157). A subgroup survival analysis of combined ipilimumab plus nivolumab versus PD1ab monotherapy showed a trend toward longer OS for combined ICI treatment of BRAF-mutant patients, but this needs to be addressed further (114). In a retrospective analysis from the pre-ICI era, NRAS mutations, which are seen in up to 20% of melanomas, were found to be associated with worse OS (158). After introduction of ICI, a retrospective study investigating patients treated by immunotherapy, including IL-2, CTLA4ab, PD1ab, and PD-L1ab revealed greater disease control and longer PFS for NRAS-mutant melanoma (126). This result was not, however, found for a smaller cohort of patients with PD1ab/PD-L1ab therapy (119). The NF-1 mutation, which is associated with UV damage and high mutational load, was linked to higher incidence of response and prolonged survival for PD1ab-treated patients (119). A more favorable response to PD1ab therapy was observed for desmoplastic melanomas which are characterized by a high mutational load and frequent NF-1 mutations (127); it should be mentioned that this observation was also made from retrospective assessment. For NSCLC, single-gene mutation analysis showed that the presence of an EGFR-mutation seems to be a negative predictor for PD1ab response (159). NGS data, however, revealed a lower mutational burden for EGFR-mutant NSCLC, which could be one reason for this finding (160).
Objectives for the future include exploration and validation of a panel of genetic biomarkers detected by next-generation sequencing. Definition of cutoffs is a current challenge because absolute values depend on the depth of sequencing. Furthermore, gene translocations/fusions and other variants will not be detected by use of targeted sequencing techniques. Development of multi-marker assays is more complex and specific computational algorithms must be used for validation (20).
Imaging Biomarkers
Anatomic Imaging
The use of imaging enables non-invasive assessment of tumor dimensions and can also provide biologic tumor data. The current standard assessment procedure for metastatic melanoma and other advanced malignancies is computerized tomography (CT) with iodine contrast dye, evaluated according to response evaluation criteria in solid tumors (RECIST) 1.1 (161). It has been shown that the size of baseline tumor lesions is associated with OS (26, 162). This is probably of prognostic value because it correlates with tumor load. However, RECIST 1.1 might be insufficient to evaluate response to ICI therapy, in particular cases of initial tumor progression or occurrence of new lesions during ICI. To overcome this problem, immune-related response criteria (irRC and irRECIST) have been introduced as alternative response criteria (163). Pure anatomic imaging is, however, unlikely to be sufficient to predict tumor response to ICI. New imaging biomarkers for metabolic and immune imaging are discussed below.
Metabolic Imaging
In addition to anatomic imaging, metabolic imaging by use of 18F-fluoro-deoxy-glucose-positron emission tomography (FDG-PET) can add clinically meaningful data when imaging malignancies. Two different response criteria for FDG-PET imaging are currently used in clinical routine: European Organisation for Research and Treatment of Cancer (EORTC) criteria and positron emission tomography response criteria in solid tumors (PERCIST) (164, 165). It has been shown that FDG-PET combined with CT is of clinical value for assessment of ICI responses in melanoma (130–132) (Table 2). The assumption for metabolic imaging is that metabolic changes in tumors occur prior to anatomic changes, which might enable prediction of response to ICI earlier during the course of treatment. This can be prevented by the failure to discriminate between inflammation and tumor metabolism (166). For ipilimumab, one clinical trial showed that FDG-PET/CT 5 weeks after treatment initiation could predict disease progression to CTLA4ab; patients responding to treatment could not be identified at this time (130). In another trial it was possible to predict best response by use of PERCIST and EORTC criteria (131). To evaluate ICI response, a new PET-CT classification, the PET response evaluation criteria for immunotherapy criteria, was developed to reflect the fact that single new lesions do not define disease progression. The absolute number of new lesions was, however, more important than changes in standardized uptake values (SUV) (132). During PD1ab therapy for melanoma, use of FDG-PET/magnetic resonance imaging (MRI) as early as 2 weeks after the start of treatment might identify patients with complete response at the 3-month time-point (133). For FDG-PET/CT for NSCLC, maximum SUV at 4 weeks after commencement of PD1ab therapy was associated with PFS and OS (167). However, these are case series and small prospective studies. Larger prospective trials are needed to investigate these findings further.
18F-fluorothymidine-PET (FLT-PET) uses a thymidine analog that accumulates in proliferating tissues, including malignant and immune cells (168). Positive findings were published for e.g., differentiation of cerebral radionecrosis from glioma progression (169), and correlation of mean SUV with OS for resectable pancreatic carcinoma (170). In contrast, no association was found between early changes in FLT uptake after the first cycle of chemotherapy for CRC and the response evaluated from subsequent CT scans (171). Its value as a biomarker for ICI in melanoma has only been reported in one case of FLT-PET/MRI; this precludes general conclusions (172). Further clinical evaluation of FLT-PET in ICI is, therefore, warranted. O-(2-[18F]fluoroethyl)-l-tyrosine (FET-)PET can be used to specifically image primary brain tumors and metastases to the brain which can be differentiated from healthy, inflamed, and radionecrotic tissue (173, 174). FET-uptake correlates with ki-67 expression and could be a potential biomarker for early response assessment (175). For melanoma, a case report showed that pseudo-progression of melanoma brain metastases could be detected by use of FET-PET (176) but more data are needed to assess the value of FET-PET in ICI.
Modern MRI techniques, including dynamic contrast-enhanced MRI, are also available for high resolution imaging of tumor perfusion or cell-membrane permeability (177). This technique could be particularly useful for assessment of specific metastatic sites, e.g., hepatic metastasis (178). Another potential application could be MRI-based immune-cell tracking and assessment of drug delivery (179).
With the exception of FDG-PET/CT, all the methods described above have been studied in only a few patients, and rarely in the setting of ICI. Results of ongoing studies will reveal a potential prognostic or predictive value of PET-biomarkers.
Immuno-Imaging
Several immuno-PET tracers, namely monoclonal antibodies, scaffold proteins, or peptides have been evaluated in preclinical tumor models. Potential targets are, for example, CD8+ cytotoxic T cells, PD-1, and PD-L1. Immuno-PET tracers have been studied in preclinical models. A 89Zr-labeled PEGylated single-domain anti-CD8 antibody was used for longitudinal evaluation of CTLA4ab treatment in the B16-melanoma mouse model. A homogeneous distribution of the anti-CD8 PET signal was observed for responding animals, whereas a heterogeneous signal was associated with lower response and faster tumor growth (180). Another group studied a 89Zr-desferrioxamine-labeled anti-CD8 cys-diabody in PD1ab treatment of Balb/c mice with CRC. They found a higher SUV in responding animals compared with non-responding ones. They also found that uptake for responders tended to be intra-tumoral, whereas uptake for non-responders was in the margins of the tumor (181); this is in agreement with the role of intra-tumoral CD8+ cytotoxic T cells in PD1ab response.
Another T cell imaging approach is via visualization of PD-1. The feasibility of this method has been proven in murine studies. Natarajan et al. developed the anti-PD-1 tracers 89Zr-keytruda and 64Cu-keytruda; these were evaluated in a humanized NOD-scid mouse model, and uptake in tumors and lymphoid tissue was observed for human melanoma tumors (182). Other groups developed radiotracers which target PD-L1 expressed on tumor cells and on immune cells of the tumor microenvironment (183–185). The feasibility of PD-L1 imaging was shown by use of 64Cu-atezolizumab in mice with tumors constitutively expressing PD-L1, and in two breast cancer mouse models (183). Investigation of irradiated versus non-irradiated tumors in a HPV + SCHNN and a B16F10 melanoma mouse models by use of an 89Zr-labeled anti-PD-L1 monoclonal antibody revealed PD-L1 upregulation in irradiated tumors specifically (184). In a patient-derived xenograft model of NSCLC, the 89Zr-C4-PD-L1 antibody revealed PD-L1 changes after chemotherapy (185).
These immuno-PET tracers have been investigated in animal models, which can certainly improve understanding of response or non-response mechanisms to ICI treatment. Studies in humans are under way. A possible predictive ability of immuno-PET in the setting of ICI, however, needs to be explored in the future (NCT03313323, NCT02760225).
Conclusion
Several factors might affect response to ICI treatment, including mutational load, tumor microenvironment, and stool microbiome. Upfront exclusion of metastatic melanoma patients from ICI therapy on the basis of biomarkers is not currently possible. It also remains unclear which patients will need combined ICI and which patients will benefit from use of PD1ab only. Although there are several potential biomarkers, their predictive versus prognostic abilities have not yet been validated by prospective clinical trials. In particular, the best sequence of treatment to follow, e.g., targeted versus immunotherapy for melanoma, cannot be answered on the basis of the biomarker data currently available.
Peripheral blood immune-cell analysis, e.g., by use of CyTOF, enables investigation of multiple markers, and will hopefully reveal predictive biomarkers in future. A multi-marker assay is more likely than a single biomarker. Future challenges include the development and validation of multi-marker assays, which will require detailed pre-analytics, computation algorithms, and, most importantly, well-designed clinical trials with large numbers of patients.
Author Contributions
KB-B and JH contributed to the conception and design of the article, acquisition and interpretation of data, drafting of the article, critical revision, and final approval. Both are accountable for the content of this manuscript.
Conflict of Interest Statement
KB-B has received honoraria and travel reimbursements from MSD Sharp & Dome GmbH Oncology (MSD) and Roche Pharma AG (Roche). JH has received consultancy fees from Amgen GmbH and MSD, payment for lectures from BMS, MSD, Roche, GSK, Novartis, and Pfizer, and travel reimbursements from BMS, MSD, Amgen, and GSK.
Funding
We acknowledge financial support from the Deutsche Forschungsgemeinschaft within the funding program Open Access Publishing; from the Baden-Württemberg Ministry of Science, Research and the Arts; and from the Ruprecht-Karls-Universität Heidelberg.
References
1. Robert C, Thomas L, Bondarenko I, O’Day S, Weber J, Garbe C, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med (2011) 364:2517–26. doi:10.1056/NEJMoa1104621
2. Robert C, Ribas A, Wolchok JD, Hodi FS, Hamid O, Kefford R, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet (2014) 384:1109–17. doi:10.1016/S0140-6736(14)60958-2
3. Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med (2015) 372:320–30. doi:10.1056/NEJMoa1412082
4. Weber JS, D’Angelo SP, Minor D, Hodi FS, Gutzmer R, Neyns B, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol (2015) 16:375–84. doi:10.1016/S1470-2045(15)70076-8
5. Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med (2015) 373:23–34. doi:10.1056/NEJMoa1504030
6. Hassel JC, Heinzerling L, Aberle J, Bahr O, Eigentler TK, Grimm MO, et al. Combined immune checkpoint blockade (anti-PD-1/anti-CTLA-4): evaluation and management of adverse drug reactions. Cancer Treat Rev (2017) 57:36–49. doi:10.1016/j.ctrv.2017.05.003
7. Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med (2015) 373:1627–39. doi:10.1056/NEJMoa1507643
8. Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WE, Poddubskaya E, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med (2015) 373:123–35. doi:10.1056/NEJMoa1504627
9. Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med (2015) 372:2018–28. doi:10.1056/NEJMoa1501824
10. Armand P, Shipp MA, Ribrag V, Michot JM, Zinzani PL, Kuruvilla J, et al. Programmed death-1 blockade with pembrolizumab in patients with classical Hodgkin lymphoma after brentuximab vedotin failure. J Clin Oncol (2016) 34:3733–9. doi:10.1200/JCO.2016.67.3467
11. El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet (2017) 389:2492–502. doi:10.1016/S0140-6736(17)31046-2
12. Kasamon YL, De Claro RA, Wang Y, Shen YL, Farrell AT, Pazdur R. FDA approval summary: nivolumab for the treatment of relapsed or progressive classical Hodgkin lymphoma. Oncologist (2017) 22:585–91. doi:10.1634/theoncologist.2017-0004
13. Fehrenbacher L, Spira A, Ballinger M, Kowanetz M, Vansteenkiste J, Mazieres J, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet (2016) 387:1837–46. doi:10.1016/S0140-6736(16)00587-0
14. Kaufman HL, Russell J, Hamid O, Bhatia S, Terheyden P, D’Angelo SP, et al. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. Lancet Oncol (2016) 17:1374–85. doi:10.1016/S1470-2045(16)30364-3
15. Massard C, Gordon MS, Sharma S, Rafii S, Wainberg ZA, Luke J, et al. Safety and efficacy of durvalumab (MEDI4736), an anti-programmed cell death ligand-1 immune checkpoint inhibitor, in patients with advanced urothelial bladder cancer. J Clin Oncol (2016) 34:3119–25. doi:10.1200/JCO.2016.67.9761
16. Apolo AB, Infante JR, Balmanoukian A, Patel MR, Wang D, Kelly K, et al. Avelumab, an anti-programmed death-ligand 1 antibody, in patients with refractory metastatic urothelial carcinoma: results from a multicenter, phase Ib study. J Clin Oncol (2017) 35:2117–24. doi:10.1200/JCO.2016.71.6795
17. Gulley JL, Rajan A, Spigel DR, Iannotti N, Chandler J, Wong DJL, et al. Avelumab for patients with previously treated metastatic or recurrent non-small-cell lung cancer (JAVELIN solid tumor): dose-expansion cohort of a multicentre, open-label, phase 1b trial. Lancet Oncol (2017) 18:599–610. doi:10.1016/S1470-2045(17)30240-1
18. Ning YM, Suzman D, Maher VE, Zhang L, Tang S, Ricks T, et al. FDA approval summary: atezolizumab for the treatment of patients with progressive advanced urothelial carcinoma after platinum-containing chemotherapy. Oncologist (2017) 22:743–9. doi:10.1634/theoncologist.2017-0087
19. Overman MJ, Mcdermott R, Leach JL, Lonardi S, Lenz HJ, Morse MA, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol (2017) 18:1182–91. doi:10.1016/S1470-2045(17)30422-9
20. Dobbin KK, Cesano A, Alvarez J, Hawtin R, Janetzki S, Kirsch I, et al. Validation of biomarkers to predict response to immunotherapy in cancer: volume II – clinical validation and regulatory considerations. J Immunother Cancer (2016) 4:77. doi:10.1186/s40425-016-0179-0
21. Masucci GV, Cesano A, Hawtin R, Janetzki S, Zhang J, Kirsch I, et al. Validation of biomarkers to predict response to immunotherapy in cancer: volume I – pre-analytical and analytical validation. J Immunother Cancer (2016) 4:76. doi:10.1186/s40425-016-0178-1
22. Brierley JD, Gospodarowicz MK, Wittekind C. TNM Classification of Malignant Tumours. 8th ed. Oxford, UK: Wiley Blackwell (2017).
23. Weide B, Martens A, Hassel JC, Berking C, Postow MA, Bisschop K, et al. Baseline biomarkers for outcome of melanoma patients treated with pembrolizumab. Clin Cancer Res (2016) 22:5487–96. doi:10.1158/1078-0432.CCR-16-0127
24. Nosrati A, Tsai KK, Goldinger SM, Tumeh P, Grimes B, Loo K, et al. Evaluation of clinicopathological factors in PD-1 response: derivation and validation of a prediction scale for response to PD-1 monotherapy. Br J Cancer (2017) 116:1141–7. doi:10.1038/bjc.2017.70
25. Tumeh PC, Hellmann MD, Hamid O, Tsai KK, Loo KL, Gubens MA, et al. Liver metastasis and treatment outcome with anti-PD-1 monoclonal antibody in patients with melanoma and NSCLC. Cancer Immunol Res (2017) 5:417–24. doi:10.1158/2326-6066.CIR-16-0325
26. Joseph RW, Elassaiss-Schaap J, Kefford RF, Hwu WJ, Wolchok JD, Joshua AM, et al. Baseline tumor size is an independent prognostic factor for overall survival in patients with melanoma treated with pembrolizumab. Clin Cancer Res (2018). doi:10.1158/1078-0432.CCR-17-2386
27. Long GV, Atkinson V, Lo S, Sandhu S, Guminski AD, Brown MP, et al. Combination nivolumab and ipilimumab or nivolumab alone in melanoma brain metastases: a multicentre randomised phase 2 study. Lancet Oncol (2018) 19:672–81. doi:10.1016/S1470-2045(18)30139-6
28. Kluger HM, Zito CR, Barr ML, Baine MK, Chiang VL, Sznol M, et al. Characterization of PD-L1 expression and associated T-cell infiltrates in metastatic melanoma samples from variable anatomic sites. Clin Cancer Res (2015) 21:3052–60. doi:10.1158/1078-0432.CCR-14-3073
29. Tawbi HA-H, Forsyth PAJ, Algazi AP, Hamid O, Hodi FS, Moschos SJ, et al. Efficacy and safety of nivolumab (NIVO) plus ipilimumab (IPI) in patients with melanoma (MEL) metastatic to the brain: results of the phase II study CheckMate 204. J Clin Oncol (2017) 35:9507. doi:10.1200/JCO.2017.35.15_suppl.9507
30. Nakamura Y, Kitano S, Takahashi A, Tsutsumida A, Namikawa K, Tanese K, et al. Nivolumab for advanced melanoma: pretreatment prognostic factors and early outcome markers during therapy. Oncotarget (2016) 7:77404–15. doi:10.18632/oncotarget.12677
31. Dick J, Lang N, Slynko A, Kopp-Schneider A, Schulz C, Dimitrakopoulou-Strauss A, et al. Use of LDH and autoimmune side effects to predict response to ipilimumab treatment. Immunotherapy (2016) 8:1033–44. doi:10.2217/imt-2016-0083
32. Weber JS, Hodi FS, Wolchok JD, Topalian SL, Schadendorf D, Larkin J, et al. Safety profile of nivolumab monotherapy: a pooled analysis of patients with advanced melanoma. J Clin Oncol (2017) 35:785–92. doi:10.1200/JCO.2015.66.1389
33. Faje AT, Sullivan R, Lawrence D, Tritos NA, Fadden R, Klibanski A, et al. Ipilimumab-induced hypophysitis: a detailed longitudinal analysis in a large cohort of patients with metastatic melanoma. J Clin Endocrinol Metab (2014) 99:4078–85. doi:10.1210/jc.2014-2306
34. Hua C, Boussemart L, Mateus C, Routier E, Boutros C, Cazenave H, et al. Association of vitiligo with tumor response in patients with metastatic melanoma treated with pembrolizumab. JAMA Dermatol (2016) 152:45–51. doi:10.1001/jamadermatol.2015.2707
35. Freeman-Keller M, Kim Y, Cronin H, Richards A, Gibney G, Weber JS. Nivolumab in resected and unresectable metastatic melanoma: characteristics of immune-related adverse events and association with outcomes. Clin Cancer Res (2016) 22:886–94. doi:10.1158/1078-0432.CCR-15-1136
36. Buder-Bakhaya K, Benesova K, Schulz C, Anwar H, Dimitrakopoulou-Strauss A, Weber TF, et al. Characterization of arthralgia induced by PD-1 antibody treatment in patients with metastasized cutaneous malignancies. Cancer Immunol Immunother (2018) 67:175–82. doi:10.1007/s00262-017-2069-9
37. Ascierto PA, Simeone E, Sileni VC, Pigozzo J, Maio M, Altomonte M, et al. Clinical experience with ipilimumab 3 mg/kg: real-world efficacy and safety data from an expanded access programme cohort. J Transl Med (2014) 12:116. doi:10.1186/1479-5876-12-116
38. Horvat TZ, Adel NG, Dang TO, Momtaz P, Postow MA, Callahan MK, et al. Immune-related adverse events, need for systemic immunosuppression, and effects on survival and time to treatment failure in patients with melanoma treated with ipilimumab at memorial Sloan Kettering Cancer Center. J Clin Oncol (2015) 33:3193–8. doi:10.1200/JCO.2015.60.8448
39. Kelderman S, Heemskerk B, Van Tinteren H, Van Den Brom RR, Hospers GA, Van Den Eertwegh AJ, et al. Lactate dehydrogenase as a selection criterion for ipilimumab treatment in metastatic melanoma. Cancer Immunol Immunother (2014) 63:449–58. doi:10.1007/s00262-014-1528-9
40. Valpione S, Martinoli C, Fava P, Mocellin S, Campana LG, Quaglino P, et al. Personalised medicine: development and external validation of a prognostic model for metastatic melanoma patients treated with ipilimumab. Eur J Cancer (2015) 51:2086–94. doi:10.1016/j.ejca.2015.06.130
41. Martens A, Wistuba-Hamprecht K, Geukes Foppen M, Yuan J, Postow MA, Wong P, et al. Baseline peripheral blood biomarkers associated with clinical outcome of advanced melanoma patients treated with ipilimumab. Clin Cancer Res (2016) 22:2908–18. doi:10.1158/1078-0432.CCR-15-2412
42. Diem S, Kasenda B, Spain L, Martin-Liberal J, Marconcini R, Gore M, et al. Serum lactate dehydrogenase as an early marker for outcome in patients treated with anti-PD-1 therapy in metastatic melanoma. Br J Cancer (2016) 114:256–61. doi:10.1038/bjc.2015.467
43. Krajsova I, Arenberger P, Lakomy R, Kubala E, Brezinova I, Poprach A, et al. Long-term survival with ipilimumab: experience from a national expanded access program for patients with melanoma. Anticancer Res (2015) 35:6303–10.
44. Maccalli C, Giannarelli D, Chiarucci C, Cutaia O, Giacobini G, Hendrickx W, et al. Soluble NKG2D ligands are biomarkers associated with the clinical outcome to immune checkpoint blockade therapy of metastatic melanoma patients. Oncoimmunology (2017) 6:e1323618. doi:10.1080/2162402X.2017.1323618
45. Simeone E, Gentilcore G, Giannarelli D, Grimaldi AM, Caraco C, Curvietto M, et al. Immunological and biological changes during ipilimumab treatment and their potential correlation with clinical response and survival in patients with advanced melanoma. Cancer Immunol Immunother (2014) 63:675–83. doi:10.1007/s00262-014-1545-8
46. Ferrucci PF, Ascierto PA, Pigozzo J, Del Vecchio M, Maio M, Antonini Cappellini GC, et al. Baseline neutrophils and derived neutrophil-to-lymphocyte ratio: prognostic relevance in metastatic melanoma patients receiving ipilimumab. Ann Oncol (2016) 27:732–8. doi:10.1093/annonc/mdw016
47. Martens A, Wistuba-Hamprecht K, Yuan J, Postow MA, Wong P, Capone M, et al. Increases in absolute lymphocytes and circulating CD4+ and CD8+ T cells are associated with positive clinical outcome of melanoma patients treated with ipilimumab. Clin Cancer Res (2016) 22:4848–58. doi:10.1158/1078-0432.CCR-16-0249
48. Zaragoza J, Caille A, Beneton N, Bens G, Christiann F, Maillard H, et al. High neutrophil to lymphocyte ratio measured before starting ipilimumab treatment is associated with reduced overall survival in patients with melanoma. Br J Dermatol (2016) 174:146–51. doi:10.1111/bjd.14155
49. Chasseuil E, Saint-Jean M, Chasseuil H, Peuvrel L, Quereux G, Nguyen JM, et al. Blood predictive biomarkers for nivolumab in advanced melanoma. Acta Derm Venereol (2017) 98(4):406–10. doi:10.2340/00015555-2872
50. Fujisawa Y, Yoshino K, Otsuka A, Funakoshi T, Fujimura T, Yamamoto Y, et al. Baseline neutrophil to lymphocyte ratio combined with serum LDH level associated with outcome of nivolumab immunotherapy in a Japanese advanced melanoma population. Br J Dermatol (2018). doi:10.1111/bjd.16427
51. Gebhardt C, Sevko A, Jiang H, Lichtenberger R, Reith M, Tarnanidis K, et al. Myeloid cells and related chronic inflammatory factors as novel predictive markers in melanoma treatment with ipilimumab. Clin Cancer Res (2015) 21:5453–9. doi:10.1158/1078-0432.CCR-15-0676
52. Weber J, Gibney G, Kudchadkar R, Yu B, Cheng P, Martinez AJ, et al. Phase I/II study of metastatic melanoma patients treated with nivolumab who had progressed after ipilimumab. Cancer Immunol Res (2016) 4:345–53. doi:10.1158/2326-6066.CIR-15-0193
53. Krieg C, Nowicka M, Guglietta S, Schindler S, Hartmann FJ, Weber LM, et al. High-dimensional single-cell analysis predicts response to anti-PD-1 immunotherapy. Nat Med (2018) 24:144–53. doi:10.1038/nm.4466
54. Subrahmanyam PB, Dong Z, Gusenleitner D, Giobbie-Hurder A, Severgnini M, Zhou J, et al. Distinct predictive biomarker candidates for response to anti-CTLA-4 and anti-PD-1 immunotherapy in melanoma patients. J Immunother Cancer (2018) 6:18. doi:10.1186/s40425-018-0328-8
55. Jacquelot N, Roberti MP, Enot DP, Rusakiewicz S, Ternes N, Jegou S, et al. Predictors of responses to immune checkpoint blockade in advanced melanoma. Nat Commun (2017) 8:592. doi:10.1038/s41467-017-00608-2
56. Chowell D, Morris LGT, Grigg CM, Weber JK, Samstein RM, Makarov V, et al. Patient HLA class I genotype effects cancer response to checkpoint blockade immunotherapy. Science (2018) 359:582–7. doi:10.1126/science.aao4572
57. Postow MA, Manuel M, Wong P, Yuan J, Dong Z, Liu C, et al. Peripheral T cell receptor diversity is associated with clinical outcomes following ipilimumab treatment in metastatic melanoma. J Immunother Cancer (2015) 3:23. doi:10.1186/s40425-015-0070-4
58. Leung AM, Lee AF, Ozao-Choy J, Ramos RI, Hamid O, O’Day SJ, et al. Clinical benefit from ipilimumab therapy in melanoma patients may be associated with serum CTLA4 levels. Front Oncol (2014) 4:110. doi:10.3389/fonc.2014.00110
59. Zhou J, Mahoney KM, Giobbie-Hurder A, Zhao F, Lee S, Liao X, et al. Soluble PD-L1 as a biomarker in malignant melanoma treated with checkpoint blockade. Cancer Immunol Res (2017) 5:480–92. doi:10.1158/2326-6066.CIR-16-0329
60. Hannani D, Vetizou M, Enot D, Rusakiewicz S, Chaput N, Klatzmann D, et al. Anticancer immunotherapy by CTLA-4 blockade: obligatory contribution of IL-2 receptors and negative prognostic impact of soluble CD25. Cell Res (2015) 25:208–24. doi:10.1038/cr.2015.3
61. Koguchi Y, Hoen HM, Bambina SA, Rynning MD, Fuerstenberg RK, Curti BD, et al. Serum immunoregulatory proteins as predictors of overall survival of metastatic melanoma patients treated with ipilimumab. Cancer Res (2015) 75:5084–92. doi:10.1158/0008-5472.CAN-15-2303
62. Yamazaki N, Kiyohara Y, Uhara H, Iizuka H, Uehara J, Otsuka F, et al. Cytokine biomarkers to predict antitumor responses to nivolumab suggested in a phase 2 study for advanced melanoma. Cancer Sci (2017) 108:1022–31. doi:10.1111/cas.13226
63. Weber JS, Sznol M, Sullivan RJ, Blackmon S, Boland G, Kluger HM, et al. A serum protein signature associated with outcome after anti-PD-1 therapy in metastatic melanoma. Cancer Immunol Res (2018) 6:79–86. doi:10.1158/2326-6066.CIR-17-0412
64. Hong X, Sullivan RJ, Kalinich M, Kwan TT, Giobbie-Hurder A, Pan S, et al. Molecular signatures of circulating melanoma cells for monitoring early response to immune checkpoint therapy. Proc Natl Acad Sci U S A (2018) 115:2467–72. doi:10.1073/pnas.1719264115
65. Gray ES, Reid AL, Bowyer S, Calapre L, Siew K, Pearce R, et al. Circulating melanoma cell subpopulations: their heterogeneity and differential responses to treatment. J Invest Dermatol (2015) 135:2040–8. doi:10.1038/jid.2015.127
66. Chaput N, Lepage P, Coutzac C, Soularue E, Le Roux K, Monot C, et al. Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann Oncol (2017) 28:1368–79. doi:10.1093/annonc/mdx108
67. Gopalakrishnan V, Spencer CN, Nezi L, Reuben A, Andrews MC, Karpinets TV, et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science (2018) 359:97–103. doi:10.1126/science.aan4236
68. Frankel AE, Coughlin LA, Kim J, Froehlich TW, Xie Y, Frenkel EP, et al. Metagenomic shotgun sequencing and unbiased metabolomic profiling identify specific human gut microbiota and metabolites associated with immune checkpoint therapy efficacy in melanoma patients. Neoplasia (2017) 19:848–55. doi:10.1016/j.neo.2017.08.004
69. Matson V, Fessler J, Bao R, Chongsuwat T, Zha Y, Alegre ML, et al. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science (2018) 359:104–8. doi:10.1126/science.aao3290
70. Hassel JC, Buder-Bakhaya K, Bender C, Zimmer L, Weide B, Loquai C, et al. Progression patterns under BRAF inhibitor treatment and treatment beyond progression in patients with metastatic melanoma. Cancer Med (2018) 7:95–104. doi:10.1002/cam4.1267
71. Bagley SJ, Kothari S, Aggarwal C, Bauml JM, Alley EW, Evans TL, et al. Pretreatment neutrophil-to-lymphocyte ratio as a marker of outcomes in nivolumab-treated patients with advanced non-small-cell lung cancer. Lung Cancer (2017) 106:1–7. doi:10.1016/j.lungcan.2017.01.013
72. Gershenwald JE, Scolyer RA, Hess KR, Sondak VK, Long GV, Ross MI, et al. Melanoma staging: evidence-based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin (2017) 67:472–92. doi:10.3322/caac.21409
73. Manola J, Atkins M, Ibrahim J, Kirkwood J. Prognostic factors in metastatic melanoma: a pooled analysis of Eastern Cooperative Oncology Group trials. J Clin Oncol (2000) 18:3782–93. doi:10.1200/JCO.2000.18.22.3782
74. Long GV, Grob JJ, Nathan P, Ribas A, Robert C, Schadendorf D, et al. Factors predictive of response, disease progression, and overall survival after dabrafenib and trametinib combination treatment: a pooled analysis of individual patient data from randomised trials. Lancet Oncol (2016) 17:1743–54. doi:10.1016/S1470-2045(16)30578-2
75. Fang S, Wang Y, Sui D, Liu H, Ross MI, Gershenwald JE, et al. C-reactive protein as a marker of melanoma progression. J Clin Oncol (2015) 33:1389–96. doi:10.1200/JCO.2014.58.0209
76. Shrotriya S, Walsh D, Bennani-Baiti N, Thomas S, Lorton C. C-reactive protein is an important biomarker for prognosis tumor recurrence and treatment response in adult solid tumors: a systematic review. PLoS One (2015) 10:e0143080. doi:10.1371/journal.pone.0143080
77. Oya Y, Yoshida T, Kuroda H, Mikubo M, Kondo C, Shimizu J, et al. Predictive clinical parameters for the response of nivolumab in pretreated advanced non-small-cell lung cancer. Oncotarget (2017) 8:103117–28. doi:10.18632/oncotarget.21602
78. Rosales C. Neutrophil: a cell with many roles in inflammation or several cell types? Front Physiol (2018) 9:113. doi:10.3389/fphys.2018.00113
79. Bigot F, Castanon E, Baldini C, Hollebecque A, Carmona A, Postel-Vinay S, et al. Prospective validation of a prognostic score for patients in immunotherapy phase I trials: the gustave roussy immune score (GRIm-score). Eur J Cancer (2017) 84:212–8. doi:10.1016/j.ejca.2017.07.027
80. Diem S, Schmid S, Krapf M, Flatz L, Born D, Jochum W, et al. Neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) as prognostic markers in patients with non-small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer (2017) 111:176–81. doi:10.1016/j.lungcan.2017.07.024
81. Jeyakumar G, Kim S, Bumma N, Landry C, Silski C, Suisham S, et al. Neutrophil lymphocyte ratio and duration of prior anti-angiogenic therapy as biomarkers in metastatic RCC receiving immune checkpoint inhibitor therapy. J Immunother Cancer (2017) 5:82. doi:10.1186/s40425-017-0287-5
82. Finon A, Zaragoza J, Maillard H, Beneton N, Bens G, Samimi M, et al. A high neutrophil to lymphocyte ratio prior to BRAF inhibitor treatment is a predictor of poor progression-free survival in patients with metastatic melanoma. Eur J Dermatol (2018) 28:38–43. doi:10.1684/ejd.2017.3167
83. Kvistborg P, Philips D, Kelderman S, Hageman L, Ottensmeier C, Joseph-Pietras D, et al. Anti-CTLA-4 therapy broadens the melanoma-reactive CD8+ T cell response. Sci Transl Med (2014) 6:254ra128. doi:10.1126/scitranslmed.3008918
84. Arrieta O, Montes-Servin E, Hernandez-Martinez JM, Cardona AF, Casas-Ruiz E, Crispin JC, et al. Expression of PD-1/PD-L1 and PD-L2 in peripheral T-cells from non-small cell lung cancer patients. Oncotarget (2017) 8:101994–2005. doi:10.18632/oncotarget.22025
85. Snyder A, Nathanson T, Funt SA, Ahuja A, Buros Novik J, Hellmann MD, et al. Contribution of systemic and somatic factors to clinical response and resistance to PD-L1 blockade in urothelial cancer: an exploratory multi-omic analysis. PLoS Med (2017) 14:e1002309. doi:10.1371/journal.pmed.1002309
86. Wistuba-Hamprecht K, Di Benedetto S, Schilling B, Sucker A, Schadendorf D, Garbe C, et al. Phenotypic characterization and prognostic impact of circulating gammadelta and alphabeta T-cells in metastatic malignant melanoma. Int J Cancer (2016) 138:698–704. doi:10.1002/ijc.29818
87. Ward FJ, Dahal LN, Wijesekera SK, Abdul-Jawad SK, Kaewarpai T, Xu H, et al. The soluble isoform of CTLA-4 as a regulator of T-cell responses. Eur J Immunol (2013) 43:1274–85. doi:10.1002/eji.201242529
88. Okuma Y, Hosomi Y, Nakahara Y, Watanabe K, Sagawa Y, Homma S. High plasma levels of soluble programmed cell death ligand 1 are prognostic for reduced survival in advanced lung cancer. Lung Cancer (2017) 104:1–6. doi:10.1016/j.lungcan.2016.11.023
89. Zhu X, Lang J. Soluble PD-1 and PD-L1: predictive and prognostic significance in cancer. Oncotarget (2017) 8:97671–82. doi:10.18632/oncotarget.18311
90. Finkelmeier F, Canli O, Tal A, Pleli T, Trojan J, Schmidt M, et al. High levels of the soluble programmed death-ligand (sPD-L1) identify hepatocellular carcinoma patients with a poor prognosis. Eur J Cancer (2016) 59:152–9. doi:10.1016/j.ejca.2016.03.002
91. Shrikant P, Khoruts A, Mescher MF. CTLA-4 blockade reverses CD8+ T cell tolerance to tumor by a CD4+ T cell- and IL-2-dependent mechanism. Immunity (1999) 11:483–93. doi:10.1016/S1074-7613(00)80123-5
92. Ugurel S, Rappl G, Tilgen W, Reinhold U. Increased serum concentration of angiogenic factors in malignant melanoma patients correlates with tumor progression and survival. J Clin Oncol (2001) 19:577–83. doi:10.1200/JCO.2001.19.2.577
93. Van Raemdonck K, Van Den Steen PE, Liekens S, Van Damme J, Struyf S. CXCR3 ligands in disease and therapy. Cytokine Growth Factor Rev (2015) 26:311–27. doi:10.1016/j.cytogfr.2014.11.009
94. Buder-Bakhaya K, Machiraju D, Hassel JC. Liquid biopsy: value for melanoma therapy. Oncol Res Treat (2017) 40:430–4. doi:10.1159/000478893
95. Bao H, Bai T, Takata K, Yokobori T, Ohnaga T, Hisada T, et al. High expression of carcinoembryonic antigen and telomerase reverse transcriptase in circulating tumor cells is associated with poor clinical response to the immune checkpoint inhibitor nivolumab. Oncol Lett (2018) 15:3061–7. doi:10.3892/ol.2017.7671
96. Anantharaman A, Friedlander T, Lu D, Krupa R, Premasekharan G, Hough J, et al. Programmed death-ligand 1 (PD-L1) characterization of circulating tumor cells (CTCs) in muscle invasive and metastatic bladder cancer patients. BMC Cancer (2016) 16:744. doi:10.1186/s12885-016-2758-3
97. Strati A, Koutsodontis G, Papaxoinis G, Angelidis I, Zavridou M, Economopoulou P, et al. Prognostic significance of PD-L1 expression on circulating tumor cells in patients with head and neck squamous cell carcinoma. Ann Oncol (2017) 28:1923–33. doi:10.1093/annonc/mdx206
98. Cabel L, Riva F, Servois V, Livartowski A, Daniel C, Rampanou A, et al. Circulating tumor DNA changes for early monitoring of anti-PD1 immunotherapy: a proof-of-concept study. Ann Oncol (2017) 28:1996–2001. doi:10.1093/annonc/mdx212
99. Khagi Y, Goodman AM, Daniels GA, Patel SP, Sacco AG, Randall JM, et al. Hypermutated circulating tumor DNA: correlation with response to checkpoint inhibitor-based immunotherapy. Clin Cancer Res (2017) 23:5729–36. doi:10.1158/1078-0432.CCR-17-1439
100. Saenger Y, Magidson J, Liaw B, De Moll E, Harcharik S, Fu Y, et al. Blood mRNA expression profiling predicts survival in patients treated with tremelimumab. Clin Cancer Res (2014) 20:3310–8. doi:10.1158/1078-0432.CCR-13-2906
101. Bullman S, Pedamallu CS, Sicinska E, Clancy TE, Zhang X, Cai D, et al. Analysis of Fusobacterium persistence and antibiotic response in colorectal cancer. Science (2017) 358:1443–8. doi:10.1126/science.aal5240
102. Vetizou M, Pitt JM, Daillere R, Lepage P, Waldschmitt N, Flament C, et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science (2015) 350:1079–84. doi:10.1126/science.aad1329
103. Sivan A, Corrales L, Hubert N, Williams JB, Aquino-Michaels K, Earley ZM, et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science (2015) 350:1084–9. doi:10.1126/science.aac4255
104. Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillere R, et al. Gut microbiome effects efficacy of PD-1-based immunotherapy against epithelial tumors. Science (2018) 359:91–7. doi:10.1126/science.aan3706
105. Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, Mcdermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med (2012) 366:2443–54. doi:10.1056/NEJMoa1200690
106. Taube JM, Klein A, Brahmer JR, Xu H, Pan X, Kim JH, et al. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res (2014) 20:5064–74. doi:10.1158/1078-0432.CCR-13-3271
107. Herbst RS, Baas P, Kim DW, Felip E, Perez-Gracia JL, Han JY, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet (2016) 387:1540–50. doi:10.1016/S0140-6736(15)01281-7
108. Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med (2016) 375:1823–33. doi:10.1056/NEJMoa1606774
109. Carbone DP, Reck M, Paz-Ares L, Creelan B, Horn L, Steins M, et al. First-line nivolumab in stage IV or recurrent non-small-cell lung cancer. N Engl J Med (2017) 376:2415–26. doi:10.1056/NEJMoa1613493
110. Borghaei H, Brahmer J, Horn L, Ready N, Steins M, Felip E, et al. P2.35: nivolumab vs docetaxel in advanced NSCLC: CheckMate 017/057 2-Y update and exploratory cytokine profile analysis: track: immunotherapy. J Thorac Oncol (2016) 11:S237–8. doi:10.1016/j.jtho.2016.08.106
111. Sharma P, Retz M, Siefker-Radtke A, Baron A, Necchi A, Bedke J, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol (2017) 18:312–22. doi:10.1016/S1470-2045(17)30065-7
112. Chow LQM, Haddad R, Gupta S, Mahipal A, Mehra R, Tahara M, et al. Antitumor activity of pembrolizumab in biomarker-unselected patients with recurrent and/or metastatic head and neck squamous cell carcinoma: results from the phase Ib KEYNOTE-012 expansion cohort. J Clin Oncol (2016) 34:3838–45. doi:10.1200/JCO.2016.68.1478
113. Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature (2014) 515:563–7. doi:10.1038/nature14011
114. Wolchok JD, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob JJ, Cowey CL, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med (2017) 377:1345–56. doi:10.1056/NEJMoa1709684
115. Hamid O, Schmidt H, Nissan A, Ridolfi L, Aamdal S, Hansson J, et al. A prospective phase II trial exploring the association between tumor microenvironment biomarkers and clinical activity of ipilimumab in advanced melanoma. J Transl Med (2011) 9:204. doi:10.1186/1479-5876-9-204
116. Daud AI, Loo K, Pauli ML, Sanchez-Rodriguez R, Sandoval PM, Taravati K, et al. Tumor immune profiling predicts response to anti-PD-1 therapy in human melanoma. J Clin Invest (2016) 126:3447–52. doi:10.1172/JCI87324
117. Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature (2014) 515:568–71. doi:10.1038/nature13954
118. Johnson DB, Estrada MV, Salgado R, Sanchez V, Doxie DB, Opalenik SR, et al. Melanoma-specific MHC-II expression represents a tumour-autonomous phenotype and predicts response to anti-PD-1/PD-L1 therapy. Nat Commun (2016) 7:10582. doi:10.1038/ncomms10582
119. Johnson DB, Frampton GM, Rioth MJ, Yusko E, Xu Y, Guo X, et al. Targeted next generation sequencing identifies markers of response to PD-1 blockade. Cancer Immunol Res (2016) 4:959–67. doi:10.1158/2326-6066.CIR-16-0143
120. Snyder A, Wolchok JD, Chan TA. Genetic basis for clinical response to CTLA-4 blockade. N Engl J Med (2015) 372:783. doi:10.1056/NEJMc1415938
121. Van Allen EM, Miao D, Schilling B, Shukla SA, Blank C, Zimmer L, et al. Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science (2015) 350:207–11. doi:10.1126/science.aad0095
122. Hugo W, Zaretsky JM, Sun L, Song C, Moreno BH, Hu-Lieskovan S, et al. Genomic and transcriptomic features of response to anti-PD-1 therapy in metastatic melanoma. Cell (2016) 165:35–44. doi:10.1016/j.cell.2016.02.065
123. McGranahan N, Furness AJ, Rosenthal R, Ramskov S, Lyngaa R, Saini SK, et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science (2016) 351:1463–9. doi:10.1126/science.aaf1490
124. Turajlic S, Litchfield K, Xu H, Rosenthal R, Mcgranahan N, Reading JL, et al. Insertion-and-deletion-derived tumour-specific neoantigens and the immunogenic phenotype: a pan-cancer analysis. Lancet Oncol (2017) 18:1009–21. doi:10.1016/S1470-2045(17)30516-8
125. Riaz N, Havel JJ, Makarov V, Desrichard A, Urba WJ, Sims JS, et al. Tumor and microenvironment evolution during immunotherapy with nivolumab. Cell (2017) 171:934–49.e915. doi:10.1016/j.cell.2017.09.028
126. Johnson DB, Lovly CM, Flavin M, Panageas KS, Ayers GD, Zhao Z, et al. Impact of NRAS mutations for patients with advanced melanoma treated with immune therapies. Cancer Immunol Res (2015) 3:288–95. doi:10.1158/2326-6066.CIR-14-0207
127. Eroglu Z, Zaretsky JM, Hu-Lieskovan S, Kim DW, Algazi A, Johnson DB, et al. High response rate to PD-1 blockade in desmoplastic melanomas. Nature (2018) 553:347–50. doi:10.1038/nature25187
128. Ji RR, Chasalow SD, Wang L, Hamid O, Schmidt H, Cogswell J, et al. An immune-active tumor microenvironment favors clinical response to ipilimumab. Cancer Immunol Immunother (2012) 61:1019–31. doi:10.1007/s00262-011-1172-6
129. Karachaliou N, Gonzalez-Cao M, Crespo G, Drozdowskyj A, Aldeguer E, Gimenez-Capitan A, et al. Interferon gamma, an important marker of response to immune checkpoint blockade in non-small cell lung cancer and melanoma patients. Ther Adv Med Oncol (2018) 10:1758834017749748. doi:10.1177/1758834017749748
130. Sachpekidis C, Larribere L, Pan L, Haberkorn U, Dimitrakopoulou-Strauss A, Hassel JC. Predictive value of early 18F-FDG PET/CT studies for treatment response evaluation to ipilimumab in metastatic melanoma: preliminary results of an ongoing study. Eur J Nucl Med Mol Imaging (2015) 42:386–96. doi:10.1007/s00259-014-2944-y
131. Cho SY, Lipson EJ, Im HJ, Rowe SP, Gonzalez EM, Blackford A, et al. Prediction of response to immune checkpoint inhibitor therapy using early-time-point (18)F-FDG PET/CT imaging in patients with advanced melanoma. J Nucl Med (2017) 58:1421–8. doi:10.2967/jnumed.116.188839
132. Anwar H, Sachpekidis C, Winkler J, Kopp-Schneider A, Haberkorn U, Hassel JC, et al. Absolute number of new lesions on (18)F-FDG PET/CT is more predictive of clinical response than SUV changes in metastatic melanoma patients receiving ipilimumab. Eur J Nucl Med Mol Imaging (2018) 45:376–83. doi:10.1007/s00259-017-3870-6
133. Seith F, Forschner A, Schmidt H, Pfannenberg C, Guckel B, Nikolaou K, et al. 18F-FDG-PET detects complete response to PD1-therapy in melanoma patients two weeks after therapy start. Eur J Nucl Med Mol Imaging (2018) 45:95–101. doi:10.1007/s00259-017-3813-2
134. Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med (2003) 348:203–13. doi:10.1056/NEJMoa020177
135. Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science (2006) 313:1960–4. doi:10.1126/science.1129139
136. Thomas NE, Busam KJ, From L, Kricker A, Armstrong BK, Anton-Culver H, et al. Tumor-infiltrating lymphocyte grade in primary melanomas is independently associated with melanoma-specific survival in the population-based genes, environment and melanoma study. J Clin Oncol (2013) 31:4252–9. doi:10.1200/JCO.2013.51.3002
137. Gibney GT, Weiner LM, Atkins MB. Predictive biomarkers for checkpoint inhibitor-based immunotherapy. Lancet Oncol (2016) 17:e542–51. doi:10.1016/S1470-2045(16)30406-5
138. Budczies J, Bockmayr M, Klauschen F, Endris V, Frohling S, Schirmacher P, et al. Mutation patterns in genes encoding interferon signaling and antigen presentation: a pan-cancer survey with implications for the use of immune checkpoint inhibitors. Genes Chromosomes Cancer (2017) 56:651–9. doi:10.1002/gcc.22468
139. Zaretsky JM, Garcia-Diaz A, Shin DS, Escuin-Ordinas H, Hugo W, Hu-Lieskovan S, et al. Mutations associated with acquired resistance to PD-1 blockade in melanoma. N Engl J Med (2016) 375:819–29. doi:10.1056/NEJMoa1604958
140. Shin DS, Zaretsky JM, Escuin-Ordinas H, Garcia-Diaz A, Hu-Lieskovan S, Kalbasi A, et al. Primary resistance to PD-1 blockade mediated by JAK1/2 mutations. Cancer Discov (2017) 7:188–201. doi:10.1158/2159-8290.CD-16-1223
141. Liu W, Ye H, Liu YF, Xu CQ, Zhong YX, Tian T, et al. Transcriptome-derived stromal and immune scores infer clinical outcomes of patients with cancer. Oncol Lett (2018) 15:4351–7. doi:10.3892/ol.2018.7855
142. Lawrence MS, Stojanov P, Mermel CH, Robinson JT, Garraway LA, Golub TR, et al. Discovery and saturation analysis of cancer genes across 21 tumour types. Nature (2014) 505:495–501. doi:10.1038/nature12912
143. Van Allen EM, Wagle N, Sucker A, Treacy DJ, Johannessen CM, Goetz EM, et al. The genetic landscape of clinical resistance to RAF inhibition in metastatic melanoma. Cancer Discov (2014) 4:94–109. doi:10.1158/2159-8290.CD-13-0617
144. Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science (2015) 348:124–8. doi:10.1126/science.aaa1348
145. Balar AV, Galsky MD, Rosenberg JE, Powles T, Petrylak DP, Bellmunt J, et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet (2017) 389:67–76. doi:10.1016/S0140-6736(16)32455-2
146. Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med (2015) 372:2509–20. doi:10.1056/NEJMoa1500596
147. Kim ST, Klempner SJ, Park SH, Park JO, Park YS, Lim HY, et al. Correlating programmed death ligand 1 (PD-L1) expression, mismatch repair deficiency, and outcomes across tumor types: implications for immunotherapy. Oncotarget (2017) 8:77415–23. doi:10.18632/oncotarget.20492
148. Teo MY, Seier K, Ostrovnaya I, Regazzi AM, Kania BE, Moran MM, et al. Alterations in DNA damage response and repair genes as potential marker of clinical benefit from PD-1/PD-L1 blockade in advanced urothelial cancers. J Clin Oncol (2018) 36(17):1685–94. doi:10.1200/JCO.2017.75.7740
149. Colli LM, Machiela MJ, Myers TA, Jessop L, Yu K, Chanock SJ. Burden of nonsynonymous mutations among TCGA cancers and candidate immune checkpoint inhibitor responses. Cancer Res (2016) 76:3767–72. doi:10.1158/0008-5472.CAN-16-0170
150. Beaver JA, Hazarika M, Mulkey F, Mushti S, Chen H, He K, et al. Patients with melanoma treated with an anti-PD-1 antibody beyond RECIST progression: a US Food and Drug Administration pooled analysis. Lancet Oncol (2018) 19:229–39. doi:10.1016/S1470-2045(17)30846-X
151. Balachandran VP, Luksza M, Zhao JN, Makarov V, Moral JA, Remark R, et al. Identification of unique neoantigen qualities in long-term survivors of pancreatic cancer. Nature (2017) 551:512–6. doi:10.1038/nature24462
152. Luksza M, Riaz N, Makarov V, Balachandran VP, Hellmann MD, Solovyov A, et al. A neoantigen fitness model predicts tumour response to checkpoint blockade immunotherapy. Nature (2017) 551:517–20. doi:10.1038/nature24473
153. Nathanson T, Ahuja A, Rubinsteyn A, Aksoy BA, Hellmann MD, Miao D, et al. Somatic mutations and neoepitope homology in melanomas treated with CTLA-4 blockade. Cancer Immunol Res (2017) 5:84–91. doi:10.1158/2326-6066.CIR-16-0019
154. Shahabi V, Whitney G, Hamid O, Schmidt H, Chasalow SD, Alaparthy S, et al. Assessment of association between BRAF-V600E mutation status in melanomas and clinical response to ipilimumab. Cancer Immunol Immunother (2012) 61:733–7. doi:10.1007/s00262-012-1227-3
155. Larkin J, Lao CD, Urba WJ, Mcdermott DF, Horak C, Jiang J, et al. Efficacy and safety of nivolumab in patients with BRAF V600 mutant and BRAF wild-type advanced melanoma: a pooled analysis of 4 clinical trials. JAMA Oncol (2015) 1:433–40. doi:10.1001/jamaoncol.2015.1184
156. Ribas A, Puzanov I, Dummer R, Schadendorf D, Hamid O, Robert C, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol (2015) 16:908–18. doi:10.1016/S1470-2045(15)00083-2
157. Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med (2015) 372:2521–32. doi:10.1056/NEJMoa1503093
158. Jakob JA, Bassett RL Jr, Ng CS, Curry JL, Joseph RW, Alvarado GC, et al. NRAS mutation status is an independent prognostic factor in metastatic melanoma. Cancer (2012) 118:4014–23. doi:10.1002/cncr.26724
159. Lee WC, Diao L, Wang J, Zhang J, Roarty EB, Varghese S, et al. Multiregion gene expression profiling reveals heterogeneity in molecular subtypes and immunotherapy response signatures in lung cancer. Mod Pathol (2018) 31(6):947–55. doi:10.1038/s41379-018-0029-3
160. Spigel D, Betzig-Schrock A, Fabrizio D, Frampton GM, Sun J, He J. Total mutation burden in lung cancer and relationship with response to PD-1/PD-L1 targeted therapies. J Clin Oncol (2016) 34:9017. doi:10.1200/JCO.2016.34.15_suppl.9017
161. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer (2009) 45:228–47. doi:10.1016/j.ejca.2008.10.026
162. Joseph RW, Elassaiss-Schaap J, Wolchok JD, Joshua AM, Ribas A, Hodi FS, et al. Baseline tumor size as an independent prognostic factor for overall survival in patients with metastatic melanoma treated with the anti-PD-1 monoclonal antibody MK-3475. J Clin Oncol (2014) 32:3015. doi:10.1200/jco.2014.32.15_suppl.3015
163. Wolchok JD, Hoos A, O’Day S, Weber JS, Hamid O, Lebbe C, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res (2009) 15:7412–20. doi:10.1158/1078-0432.CCR-09-1624
164. Young H, Baum R, Cremerius U, Herholz K, Hoekstra O, Lammertsma AA, et al. Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: review and 1999 EORTC recommendations. European Organization for Research and Treatment of Cancer (EORTC) PET study group. Eur J Cancer (1999) 35:1773–82. doi:10.1016/S0959-8049(99)00229-4
165. Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med (2009) 50(Suppl 1):122S–50S. doi:10.2967/jnumed.108.057307
166. Gilardi L, Grana CM, Paganelli G. Evaluation of response to immunotherapy: new challenges and opportunities for PET imaging. Eur J Nucl Med Mol Imaging (2014) 41:2090–2. doi:10.1007/s00259-014-2848-x
167. Kaira K, Higuchi T, Naruse I, Arisaka Y, Tokue A, Altan B, et al. Metabolic activity by (18)F-FDG-PET/CT is predictive of early response after nivolumab in previously treated NSCLC. Eur J Nucl Med Mol Imaging (2018) 45:56–66. doi:10.1007/s00259-017-3806-1
168. Herrmann K, Buck AK. Proliferation imaging with (1)(8)F-fluorothymidine PET/computed tomography: physiologic uptake, variants, and pitfalls. PET Clin (2014) 9:331–8. doi:10.1016/j.cpet.2014.03.005
169. Enslow MS, Zollinger LV, Morton KA, Butterfield RI, Kadrmas DJ, Christian PE, et al. Comparison of 18F-fluorodeoxyglucose and 18F-fluorothymidine PET in differentiating radiation necrosis from recurrent glioma. Clin Nucl Med (2012) 37:854–61. doi:10.1097/RLU.0b013e318262c76a
170. Wieder H, Beer AJ, Siveke J, Schuster T, Buck AK, Herrmann K, et al. (18)F-fluorothymidine PET for predicting survival in patients with resectable pancreatic cancer. Oncotarget (2018) 9:10128–34. doi:10.18632/oncotarget.24176
171. Mogensen MB, Loft A, Aznar M, Axelsen T, Vainer B, Osterlind K, et al. FLT-PET for early response evaluation of colorectal cancer patients with liver metastases: a prospective study. EJNMMI Res (2017) 7:56. doi:10.1186/s13550-017-0302-3
172. Nguyen NC, Yee MK, Tuchayi AM, Kirkwood JM, Tawbi H, Mountz JM. Targeted therapy and immunotherapy response assessment with F-18 fluorothymidine positron-emission tomography/magnetic resonance imaging in melanoma brain metastasis: a pilot study. Front Oncol (2018) 8:18. doi:10.3389/fonc.2018.00018
173. Poulsen SH, Urup T, Grunnet K, Christensen IJ, Larsen VA, Jensen ML, et al. The prognostic value of FET PET at radiotherapy planning in newly diagnosed glioblastoma. Eur J Nucl Med Mol Imaging (2017) 44:373–81. doi:10.1007/s00259-016-3494-2
174. Unterrainer M, Galldiks N, Suchorska B, Kowalew LC, Wenter V, Schmid-Tannwald C, et al. (18)F-FET PET uptake characteristics in patients with newly diagnosed and untreated brain metastasis. J Nucl Med (2017) 58:584–9. doi:10.2967/jnumed.116.180075
175. Sanghera B, Wong WL, Sonoda LI, Beynon G, Makris A, Woolf D, et al. FLT PET-CT in evaluation of treatment response. Indian J Nucl Med (2014) 29:65–73. doi:10.4103/0972-3919.130274
176. Kebir S, Rauschenbach L, Galldiks N, Schlaak M, Hattingen E, Landsberg J, et al. Dynamic O-(2-[18F]fluoroethyl)-L-tyrosine PET imaging for the detection of checkpoint inhibitor-related pseudoprogression in melanoma brain metastases. Neuro Oncol (2016) 18:1462–4. doi:10.1093/neuonc/now154
177. Little RA, Barjat H, Hare JI, Jenner M, Watson Y, Cheung S, et al. Evaluation of dynamic contrast-enhanced MRI biomarkers for stratified cancer medicine: how do permeability and perfusion vary between human tumours? Magn Reson Imaging (2018) 46:98–105. doi:10.1016/j.mri.2017.11.008
178. Lee M, Baek SE, Moon J, Roh YH, Lim JS, Park MS, et al. Dynamic contrast-enhanced MRI coupled with a subtraction technique is useful for treatment response evaluation of malignant melanoma hepatic metastasis. Oncotarget (2016) 7:38513–22. doi:10.18632/oncotarget.9567
179. Ahrens ET, Bulte JW. Tracking immune cells in vivo using magnetic resonance imaging. Nat Rev Immunol (2013) 13:755–63. doi:10.1038/nri3531
180. Rashidian M, Ingram JR, Dougan M, Dongre A, Whang KA, Legall C, et al. Predicting the response to CTLA-4 blockade by longitudinal noninvasive monitoring of CD8 T cells. J Exp Med (2017) 214:2243–55. doi:10.1084/jem.20161950
181. Tavare R, Escuin-Ordinas H, Mok S, Mccracken MN, Zettlitz KA, Salazar FB, et al. An effective immuno-PET imaging method to monitor CD8-dependent responses to immunotherapy. Cancer Res (2016) 76:73–82. doi:10.1158/0008-5472.CAN-15-1707
182. Natarajan A, Mayer AT, Reeves RE, Nagamine CM, Gambhir SS. Development of novel ImmunoPET tracers to image human PD-1 checkpoint expression on tumor-infiltrating lymphocytes in a humanized mouse model. Mol Imaging Biol (2017) 19:903–14. doi:10.1007/s11307-017-1060-3
183. Lesniak WG, Chatterjee S, Gabrielson M, Lisok A, Wharram B, Pomper MG, et al. PD-L1 detection in tumors using [(64)Cu]atezolizumab with PET. Bioconjug Chem (2016) 27:2103–10. doi:10.1021/acs.bioconjchem.6b00348
184. Kikuchi M, Clump DA, Srivastava RM, Sun L, Zeng D, Diaz-Perez JA, et al. Preclinical immunoPET/CT imaging using Zr-89-labeled anti-PD-L1 monoclonal antibody for assessing radiation-induced PD-L1 upregulation in head and neck cancer and melanoma. Oncoimmunology (2017) 6:e1329071. doi:10.1080/2162402X.2017.1329071
Keywords: biomarker, checkpoint inhibition, PD-1 antibody, PD-L1 antibody, CTLA-4 antibody, cancer, melanoma
Citation: Buder-Bakhaya K and Hassel JC (2018) Biomarkers for Clinical Benefit of Immune Checkpoint Inhibitor Treatment—A Review From the Melanoma Perspective and Beyond. Front. Immunol. 9:1474. doi: 10.3389/fimmu.2018.01474
Received: 30 March 2018; Accepted: 13 June 2018;
Published: 28 June 2018
Edited by:
Alexandr Bazhin, Klinikum der Universität München, GermanyReviewed by:
Ekaterina Jordanova, Center for Gynaecologic Oncology Amsterdam, NetherlandsRodabe N. Amaria, University of Texas MD Anderson Cancer Center, United States
Copyright: © 2018 Buder-Bakhaya and Hassel. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jessica C. Hassel, amVzc2ljYS5oYXNzZWxAbWVkLnVuaS1oZWlkZWxiZXJnLmRl
 Kristina Buder-Bakhaya
Kristina Buder-Bakhaya Jessica C. Hassel*
Jessica C. Hassel*