- Department of Rheumatology, ASST G. Pini-CTO, Milano, Italy
Systemic sclerosis (SSc) is a connective tissue disease characterized by a complex pathological process where the main scenario is represented by progressive loss of microvascular bed, with the consequent progressive fibrotic changes in involved organ and tissues. Although most aspects of vascular injury in scleroderma are poorly understood, recent data suggest that the scleroderma impairment of neovascularization could be related to both angiogenesis and vasculogenesis failure. Particularly, compensatory angiogenesis does not occur normally in spite of an important increase in many angiogenic factors either in SSc skin or serum. Besides insufficient angiogenesis, the contribution of defective vasculogenesis to SSc vasculopathy has been extensively studied. Over the last decades, our understanding of the processes responsible for the formation of new vessels after tissue ischemia has increased. In the past, adult neovascularization was thought to depend mainly on angiogenesis (a process by which new vessels are formed by the proliferation and migration of mature endothelial cells). More recently, increased evidence suggests that stem cells mobilize from the bone marrow into the peripheral blood (PB), differentiate in circulating endothelial progenitors (EPCs), and home to site of ischemia to contribute to de novo vessel formation. Significant advances have been made in understanding the biology of EPCs, and molecular mechanisms regulating EPC function. Autologous EPCs now are becoming a novel treatment option for therapeutic vascularization and vascular repair, mainly in ischemic diseases. However, different diseases, such as cardiovascular diseases, diabetes, and peripheral artery ischemia are related to EPC dysfunction. Several studies have shown that EPCs can be detected in the PB of patients with SSc and are impaired in their function. Based on an online literature search (PubMed, EMBASE, and Web of Science, last updated December 2017) using keywords related to “endothelial progenitor cells” and “Systemic Sclerosis,” “scleroderma vasculopathy,” “angiogenesis,” “vasculogenesis,” this review gives an overview on the large body of data of current research in this issue, including controversies over the identity and functions of EPCs, their meaning as biomarker of SSc microangiopathy and their clinical potency.
Introduction
Systemic sclerosis (SSc) is a chronic connective tissue disease of unknown etiology characterized by immunologic abnormalities, microangiopathy, and excessive deposition of collagen in the skin and different internal organs (1). Clinical and pathologic findings of vascular damage and endothelial activation strongly support the hypothesis that the vascular involvement could be the most important and the primary process in the pathogenesis of scleroderma (2). Morphological changes in the vessels of patients with SSc range from considerable initial derangement with capillary thrombosis, to often abortive reparative neoangiogenesis with abnormal capillary proliferation, and to almost complete loss of vessels with persistent ischemic injury in target tissues (3, 4). Although tissue hypoxia is a strong inducer of neovascularization, new microvessel formation appears to be defective in SSc patients and no evidence exists of an effective replacement of damaged capillaries (5). Recent data suggest that the scleroderma impairment of neovascularization could be related to both angiogenesis and vasculogenesis failure (6). Angiogenesis is defined as the creation of new vessels sprouting out of pre-existing ones, whereas vasculogenesis refers to the in situ formation of blood vessels from hemangioblasts or vascular stem/progenitor cells (7, 8). In SSc, serum levels of both pro-angiogenic mediators and powerful inhibitors of angiogenesis are largely alterated, especially in the active phases of the disease. In addition, abnormalities in pro-angiogenic signal transduction pathways have been reported, suggesting an intrinsic impaired response of SSc endothelial cells to the mechanisms of vascular angiogenic repair (9). In this scenario, the endothelial cell apoptosis could be recognized as an additional feature of disturbed angiogenesis (10, 11).
In contrast to angiogenesis, during vasculogenesis the formation of new blood vessels can occur in the absence of pre-existing blood vessels through the recruitment and differentiation of endothelial progenitor cells (EPCs). Interest in EPC biology has been growing continuously since their discovery (12) and now EPCs are regarded as biomarkers in cardiovascular diseases and also potential sources of cell for revascularization strategies, which may include direct cellular transplantation and tissue engineering. Significant advances have been made in understanding the biology of EPCs, and preclinical studies using transplanted EPCs provided promising results in the treatment of ischemic diseases (13). Altogether, in the last decade, these data have given rise to several studies regarding the role of EPCs in SSc vasculopathy. The purpose of this review is to evaluate the relevant scientific literature to determine the current state of knowledge on EPCs in the context of the scleroderma vasculopathy. We conducted an online literature search (PubMed, EMBASE, and Web of Science, last updated December 2017) using keywords related to “endothelial progenitor cells” and “Systemic Sclerosis,” “scleroderma vasculopathy,” “angiogenesis,” “vasculogenesis.” Eligible papers were evaluated on four pertinent criteria: (1) SSc study populations and appropriate controls, (2) markers used to define EPC phenotype, (3) methods used for assessing EPC function, and (4) evaluation of the possible correlation between EPC detection and angiogenesis/vasculogenesis processes in SSc.
Herein, we summarize the pertinent findings of these studies and discuss the potential role of EPCs as biomarker of scleroderma microangiopathy, the controversies over the identity and functions of EPCs, and their potential clinical potency.
Characterization and Biology of EPCs
The term “Endothelial Progenitor Cells” (EPCs) should be basically used to refer to populations of cells that are capable of differentiation into mature endothelial cells in vasculogenesis (de novo formation of vascular networks) (14). Many studies have attempted to identify cell surface markers that are unique to EPCs and distinguish them from mature endothelial cells. EPCs were identified for the first time in 1997 by Asahara et al. in human peripheral blood (PB) as a subset of hematopoietic cells with vasculogenic properties in vivo and in vitro (12). They identified these cells as CD34+ (a protein with unknown function expressed in early hematopoietic cells) and KDR+ [kinase-insert domain containing receptor that encodes for vascular endothelial growth factor receptor 2 (VEGFR2)]. Since CD34+/VEGFR+ cells may also identify circulating mature endothelial cells shed from damaged vessel, subsequent works have included CD133 as the stemness marker of EPCs (15). However, the use of CD133 remains controversial. Case et al. showed that mobilized adult PB CD34+/VEGFR2+/CD133+ cells represent an enriched population of CD45+ hematopoietic precursors, which do not differentiate into endothelial cells in vitro (16). Other authors suggested VE-cadherin and E-selectin as additional surface markers to identify progenitor cells in a more advanced stage of endothelial maturation (17). Due to this controversial scenario, it is evident that the family of EPCs is characterized by lineage and functional heterogeneities, with a spectrum of phenotypes not yet fully defined.
Another approach to EPC isolation and characterization is represented by the use of defined culturing assays to culture unselected PB mononuclears cells (MNCs). For EPCs isolated by cell culture assays, there is now agreement that two different populations can be identified. Hur et al. (18) plated MNCs on a fibronectin- and/or gelatin-coated plate with VEGF containing medium to obtain two types of EPCs, early and late EPCs. Early EPCs with spindle-shaped morphology showed peak growth at 2–3 weeks, whereas late EPCs, cobblestone-shaped, showed exponential growth at 4–8 weeks. The definition of early and late EPCs, based on their time of appearance in culture, reflects a very different phenotype, one being hematopoietic and the other endothelial, respectively (19). Recently, Medina et al. recommended identifying in early EPCs myeloid angiogenic cells (MACs) to clarify their lineage and function. MACs are defined as cultured cells derived from PB mononuclear cells which are grown under endothelial cell culture conditions. These cells share multiple surface antigens of monocytes (CD45, CD14, CD31) and are negative for CD133, CD146, and Tie2 (20). Most studies suggest that these short-term cultures of MNCs fail to differentiate themselves into functional endothelial cells, but predominantly promote vessel formation by activating resident endothelial cells through paracrine mechanisms (21–23).
Late EPCs are often called out growth EPCs and have a more mature phenotype. These cells lack hematopoietic and myeloid markers and are usually derived from long-term cultures of at least 2–4 weeks in vitro (24, 25). They are now commonly named as endothelial colony-forming cells. They generate mature endothelial progeny in vitro and it has also been observed that they physically contribute to formation of new capillaries (26, 27).
The exact definition and characterization of EPCs is still an on-going and unresolved issue. EPC populations represent a heterogeneous mix of progenitors, in terms of lineage, proliferative potential, and mechanism of action. Experimental models suggest that there is no evidence for the superiority of one cell type over another, and different signaling pathways co-work in regulating EPC commitment (28). Furthermore, Yoon et al. showed that in vitro, the angiogenic capability of the two cell types (early and late EPCs) was augmented by mutual interaction through cytokines and metalloproteinases. In addition, the injection of a mixture of the two cells resulted in a superior neovascularization than that obtained by using any one of the single-cell-types (29).
The characteristics of the in vivo environment may influence the fate and function of EPCs. In normal homeostatic conditions, there is a low number of circulating EPC populations in the PB. EPCs reside within a stem cell niche in the bone marrow (BM) and complex mechanisms regulate EPC trafficking from the BM to the bloodstream (30). In reality, mechanisms that trigger the regeneration process of EPCs are not well understood and are still under investigation. Tissue ischemia is believed to be the most powerful physiological stimulus for mobilizing EPCs from the BM to the site of new vessel growth (31, 32). EPC recruitment requires a coordinated sequence of multi-step adhesive and signaling events, including chemoattraction, adhesion, and migration. Once at the site of tissue repair, EPCs contribute to new vessel formation in different ways: direct incorporation into the neovessel wall, differentiation into mature endothelial cells, and production of paracrine signals including growth factors, such as VEGF, stromal derived factor, monocyte chemotactic protein 1, and platelet-derived growth factor. Altogether, these pro-angiogenic molecules might further activate resident endothelial cells toward proliferation and vascular repair (28, 33).
EPCs in SSc
In spite of the on-going controversy over EPC identity, the clinical significance and therapeutic potential of EPCs in vascular regenerative applications has been extensively studied in the last few decades. The existence of postnatal vasculogenesis, mediated by a population of endothelial progenitors identified in both the BM and PB, represents an important tool to better understand the biological contribution of EPCs in different vascular diseases, including SSc.
Several studies have demonstrated that the number and function of EPCs (both in circulation and BM) may be impaired in some disorders characterized by prolonged chronic endothelial damage, such as chronic coronary artery disease, hypertension, congestive heart disease, and diabetes mellitus (34, 35). It has, therefore, been postulated that impaired vascular repair may contribute to the pathogenesis of these chronic disorders and that a small number of EPCs may also be a risk factor for atherosclerotic plaque instability (36). By contrast, an increased number of EPCs is often observed in patients experiencing a myocardial infarction (37) or acute vascular trauma (38). Several studies have suggested that EPCs have a role in the pathogenesis of different autoimmune diseases (34, 39). On average in rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE), studies have found decreased peripheral counts of EPCs with altered function (39). The interpretation of these observations is quite difficult at the moment because of the presence of an intrinsic increased cardiovascular risk related to these inflammatory disorders that alone might be responsible for EPC involvement.
Several studies have shown that EPCs can be detected in the PB of patients with SSc and are impaired in their function (40–44). However, the numbers and functions of EPCs in SSc is still a matter of debate, since conflicting reports have been released. Whereas some studies reported a significant depletion in the count of CD34+/CD133+/VEGFR2+ cells in SSc patients using flow-cytometry (40, 45–47), other studies found increased circulating EPC counts, mainly in the early and active stages of the disease (41–43). By contrast, lower EPC counts were associated with long disease duration, higher Medsger’s severity scores, and ischemic digital ulcers (41–44). These different findings may be ascribed to difficulties in correctly assessing patients with different scleroderma subtypes, disease duration, and level of disease activity.
The complexity of this scenario is further increased by the identification of a subpopulation of multipotent circulating monocytes able to differentiate along the endothelial lineage and promote neovascularization in vitro and in vivo (48). Really, the current consensus is that these monocytic cells, termed monocytic pro-angiogenic hematopoietic cells (PHCs) and characterized by the positive expression of CD14, CD45, CD34 and type I collagen, do not give rise to endothelial cells in vivo, but can support vascular repair through their rapid recruitment to the site of endothelial injury, local secretion of pro-angiogenic factors, and differentiation into mural cells (49). Increased circulating PHCs have been found in patients with SSc (50, 51) and some correlation with the fibrotic clinical features of the disease was observed (50).
Recent studies showed that a specific T cell population, called angiogenic T cells (Tang) may contribute to the formation of new vessels enhancing endothelial cell proliferation and function. Hur et al. demonstrated that these T cell subtype, characterized by the surface phenotype CD3+CD31+CXCR4+, constituted the center of EPC colonies during cultures of human PB mononuclear cells. These Tang were required for colony formation and differentiation of early EPCs, actively participating in postnatal vasculogenesis and vascular repair (52). Clinical studies showed an inverse relationship of Tang with cardiovascular risk factors, even in autoimmune diseases, such as RA, SLE, and ANCA-associated vasculitis (53–55). In SSc, Tang cell counts have been reported to be selectively increased in the PB of SSc patients with severe vascular complications like digital ulcers and there was an inverse correlation between circulating Tang and EPCs (defined as CD34+CD133+VEGFR2+). Furthermore, the presence of circulating Tang cells positively correlated with the levels of pro-angiogenic factors, namely VEGF and MMP-9 (56). Such Tang cell expansion has, therefore, been suggested as a possible ineffective attempt to compensate the decrease in EPC number (56).
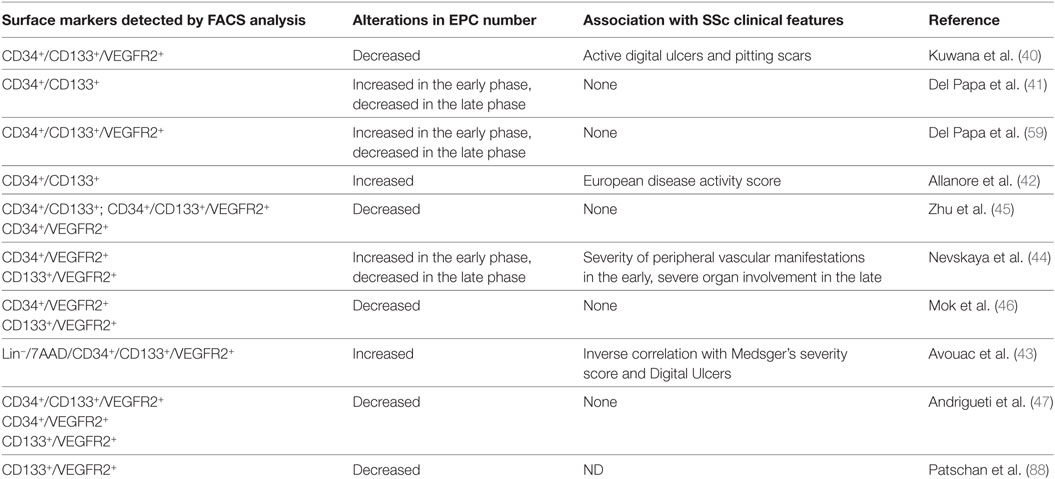
Indeed, a growing body of evidence suggests that there is a large heterogeneity of EPC populations, which include both hematopoietic and non-hematopoietic lineages. Thus, different subtypes of circulating progenitor cells with vasculogenic and/or angiogenic potentialities have been identified in patients with SSc (see Table 1). However, the methods of characterization and quantification of EPCs are not standardized, and the protocols used to count EPCs vary in the different studies (57). Methodological issues are further increased by the difficulties in reliably identifying EPCs. In view of this complexity, the European League against Rheumatism Scleroderma Trials and Research (EUSTAR) recommendations appear to have a limited application (58).
Additional in vitro studies showed that, despite having a normal phenotype, EPCs isolated from SSc patients had an impaired ability to differentiate into mature endothelial cells (40). These functional changes have been further confirmed by the examination of BM aspirated from SSc patients that showed low numbers of EPCs characterized by impaired capacity for endothelial differentiation (59). Interestingly, only the BM EPCs from patients with early disease led to some degree of endothelial differentiation, thus suggesting a probable progressive exhaustion of the pool of the BM resident EPCs during the disease progression. The increased levels of VEGF observed in SSc sera (40, 59–62) and the high expression of VEGF receptor on the surface of BM EPCs generated the intriguing hypothesis of a powerful angiogenic “push” without an appropriate vessel formation in SSc patients. Really, no study has shown a direct evidence of correlation between VEGF levels and circulating EPCs in SSc patients. In addition, recent studies have shown that the “VEGF scenario” is rather more complex in SSc and cannot be simply explained by a general insufficient angiogenic/vasculogenic response to important promoters of new vessel formation. Indeed, the overexpression of the anti-angiogenic splice isoform VEGF165b in the plasma, microvascular endothelial cells dermis, and in the platelets of SSc, adds a new play actor in the set of the mechanisms of scleroderma dysfunctional angiogenesis (63–65).
However, the hypothesis of a direct link between EPCs and pro-angiogenic soluble factors has been further sustained by recent data showing a positive correlation between EPC mobilization and circulating levels of Fractalkine in a context of endothelial activation in SSc patients (66). Fractalkine, the only member of the CX3C chemokine family, has recently been described as an angiogenic chemokine. Previous studies have shown that fractalkine induced endothelial cell migration and proliferation, EPC migration and tube-like structure formation in vitro, and stimulated new blood vessel formation in vivo even in the context of ischemic diseases (67, 68).
An alternative to the hypothesis that prolonged and continuous endothelial cell recruitment may exhaust the BM reservoir of resident EPCs is that disease-related toxic mechanisms can negatively influence the half-life and mobilization of BM EPCs. Since EPCs share the phenotypic and functional properties of mature endothelial cells (15), it is possible that the same immuno-mediated mechanisms capable of inducing peripheral endothelial injury in SSc (i.e., apoptotic phenomena and/or anti-endothelial activity) might also be found in the BM environment. This hypothesis has been confirmed by the detection of significant titers of anti-endothelial cell antibodies (AECA) in the SSc BM plasma (69). Furthermore, their presence correlated with that of activated and apoptotic progenitors (59, 69). These findings were further confirmed by an in vitro assay in which the apoptosis of normal progenitors was induced by the addition of AECA+ purified IgG (69). The role of apoptosis in SSc endothelial impairment, even at progenitor level, has been further elucidated by Zhu et al. (45). They reported an increase rate of apoptosis in EPCs isolated from PB of patients with SSc. Depletion of IgG fraction from SSc sera completely abolished the apoptotic effects, suggesting that EPCs may be destroyed upon their mobilization from the BM by serological factors, may be auto-antibodies, present in the SSc sera. As expected, SSc sera induced apoptosis even in human mature microvascular endothelial cells, although these cells were less susceptible than EPCs to the toxic factors present in the SSc sera (45). Similar to what happens in other cell types, the Akt-FOXO3a–Bim axis is the key pathway implicated in apoptotic process in SSc EPCs (45). In summary, these findings support the hypothesis that AECA may play a pathogenetic role by affecting the BM EPC machinery that should repair the peripheral vascular lesions.
Recently, Shirai et al. proposed Pentraxin 3 (PTX3) might represent another intriguing candidate as a contrasting mediator of EPC-mediated vasculogenesis in SSc (70). The expression of PTX3 is induced by inflammatory cytokines in response to inflammatory stimuli in several mesenchymal and epithelial cell types, particularly endothelial cells and mononuclear phagocytes. Additional properties of PTX3 other than those related to innate immunity and inflammation have been described, such as extracellular matrix deposition (ECM), tissue remodeling, and angiogenesis. PTX3 is involved in a variety of molecular mechanisms leading to vascular damage, and its elevated plasma levels were associated with endothelial dysfunction in different human diseases (71). Elevated plasma level of PTX3 have been found in patients with SSc and correlated with vascular manifestations such as digital ulcers and pulmonary hypertension (PH) (70, 72). Interestingly, EPC counts negatively correlated with circulating PTX3 levels (69). These observations suggest a potential role of PTX3 in regulating vascular homeostasis in SSc. In an experimental model, the exposure to a high concentration of PTX3 inhibited EPC differentiation. Based on these data, PTX3 seems to be an additional contributor to worsening the outcome of vascular EPC-mediated repair in SSc (70).
To further investigate these functional changes in SSc EPCs, the gene expression profiles were investigated by Avouac et al. (73). In this study, a different gene expression profile was observed in EPCs from SSc patients compared to control subjects. Interestingly, many of these genic alterations were associated with a proadhesive, proinflammatory, and activated phenotype. Furthermore, experimental hypoxia conditions modulated the gene expression profile of late-growth EPC-derived endothelial cells (73) showing a further upregulation of genes involved in inflammatory and immune response and a downregulation of HOXA9, a factor necessary for endothelial tube formation during angiogenesis (74) and a key regulator of adult progenitor cell commitment to the endothelial lineage (75).
Further studies on late-outgrowth EPC-derived endothelial cells from SSc patients have revealed that when compared with patient mature dermal microvascular endothelial cells, these EPC-derived cells may already present or not alterations in the expression of key regulators of vascular integrity and angiogenesis, such as epidermal growth factor-like domain 7 (EGFL7) (76),the key VEGF co-receptor neuropilin-1 (NRP-1), and Fli1 transcription factor (77). EGFL7 is an important pro-angiogenic molecule, almost exclusively expressed by and active on endothelial cells and their progenitors. EGFL7 expression is highest when the endothelium is in an active, proliferating state, and is a key actor in controlling vascular patterning and integrity (78). EGFL7 expression in progenitor and mature endothelial cells is deeply downregulated in SSc patients, suggesting its role in the mechanisms of defective vascular repair machinery characteristic of SSc (76). On the contrary, it has been demonstrated that SSc dermal microvascular endothelial cells exhibit impaired expression of NRP-1 due to Fli1 deficiency, while SSc late-outgrowth EPC-derived endothelial cells have a genuine phenotype characterized by normal expression levels of either Fli1 or NRP-1 (77). In another study, gene expression profiling of EPC-derived endothelial cells identified matrix metalloproteinase 10 (MMP10) as a novel candidate gene in SSc-associated PH (79). MMP10 seems to have a predominant role in pathologic conditions related to tissue repair and inflammation (80) and more recently its role in neovascularization has also been proposed (81, 82). Circulating serum proMMP10 concentrations were markedly increased in patients with SSc-associated PH compared to SSc patients without PH and healthy controls. Microarray experiments showed that the MMP10 gene was the top upregulated gene in EPC-derived ECs from patients with SSc-associated PH (79).
Another hypothesis has been made on the mechanisms underlying SSc pathogenesis. Once recruited at sites of vascular damage and after exposition to TGFβ, EPCs might transdifferentiate into myofibroblasts, which are the effector cells ultimately responsible for the severe fibrotic process in SSc. Thus, instead of promoting vasculogenic and angiogenic processes, EPCs and endothelial cells might undergo a phenotypic modification, called EndoMT (83, 84). In this context, recent studies showed the presence of cells in intermediate stages of EndoMT in vessels of lung and dermal tissues of patients with SSc (85–87).Furthermore, the treatment of human normal dermal microvascular endothelial cells with TGFβ and SSc sera induced a myofibroblast morphology and the expression of markers of myofroblasts (a-SMA and type I collagen) with downregulation of endothelial markers (CD31, VE-cadherin) (87). More recently, the occurrence of such a phenotypical change from endothelial cells to myofibroblasts has been further demonstrated in the early circulating EPCs from SSc patients (88).
EPC-Based Therapies for SSc
In view of the fact that EPCs are defective in several chronic diseases, including SSc, different therapeutic strategies could be postulated to stimulate the production of EPCs or directly use these cells for vascular repair in these conditions. From a theoretical point of view, stem/progenitor therapies might be superior to pharmacological therapy not only because of their direct vasculogenic properties but also paracrine action related to the secretion of multiple growth factors known to be effective in promoting angiogenic processes (89).
Pharmacological Approaches to Improve Endothelial Repair Mechanisms
Several pharmacological agents have been shown to impact on the number and function of EPCs in animal models and small clinical studies. Here, we focus on recent data concerning the effects of pharmacological agents in clinical use for the treatment of SSc vasculopathy.
3-Hydroxy-3 methylglutaryl coenzime A reductase inhibitors, or statins, have been developed as lipid lowering drugs, but besides this well-known effect, statins are capable of having anti-inflammatory and immunoregulatory effects (90). In particular, statin therapy improves endothelial function by decreasing platelet aggregation and increasing endothelial-derived nitric oxide production (91). Moreover, statins induce mobilization of EPCs from BM (92, 93), increase their functional activity and, probably, their homing to sites of vascular injury (94). The beneficial effect of statins in the treatment of different vascular diseases led several investigators to propose them as a potential treatment for SSc vasculopathy (95), although only a few studies have evaluated the clinical effect of statins in SSc patients. Two studies measured the impact of statin treatment on EPCs and mature endothelial cells (96, 97). They were both open-label studies in which all the participants received statins, and the EPC counts were made before therapy, upon completion of the trial, and throughout. Kuwana et al. treated 13 SSc patients with atorvastatin 10 mg/day for 12 weeks. The authors observed a significant improvement in peripheral vascular manifestations during the treatment period. Atorvastatin treatment resulted in a 1.7- to 8.0-fold increase in the EPC number from baseline, and the number returned to baseline after treatment with atorvastatin was stopped. Circulating levels of the angiogenic factors VEGF and basic fibroblast growth factor, which are believed to be upregulated in SSc to compensate for the inability EPCs to respond adequately to angiogenic stimuli, were significantly reduced during the atorvastatin treatment. In addition, the circulating levels of soluble vascular cell adhesion molecule-1 and E-selectin, which reflect the status of endothelial activation and injury, decreased (96).
Del Papa et al. compared the effects of simvastatin on EPC mobilization in a hypercholesterolemic group and in 20 normocholesterolemic patients suffering from the limited form of SSc. The therapy significantly increased the number of circulating EPCs in the hypercholesterolemic group, but failed to improve the EPC levels in the SSc patients, mainly in those with long-standing disease. In addition, baseline levels of mature circulating endothelial cells were significantly higher in SSc patients compared with controls and, at the end of the treatment, they were significantly decreased. Regarding other markers of endothelial activation, they found the levels of endothelial activation-related markers decreased in a statistically significant manner in the treated patients (97). The different results observed in the two studies could be partially ascribed to the different statins used and to a different selection of patients enrolled (96, 97). Anyway, the fact that EPC mobilization from BM is always reduced, in comparison with non-scleroderma controls, confirms the hypothesis that impaired vascular repair mediated by EPCs may have a role in the progression of the scleroderma vasculopathy. Different mechanisms may be postulated to explain the failure of EPC recruitment in the BM of SSc patients treated with statins. First, as observed in patients with cardiovascular risk factors, the prolonged request from the peripheral damaged vessels may induce the exhaustion of the BM reservoir of EPCs (36). Second, as other organ and tissues involved in SSc, the involvement of the BM microvascular set could interfere with the processes driving the mobilization of EPCs, including those mediated by statins.
Erythropoietin (EPO) was originally described as a hematopoietic cytokine, regulating proliferation and differentiation of erythroid precursor cells. However, several recent studies have suggested that EPO exerts other important anti-apoptotic and anti-inflammatory effects, beyond the regulation of hematopoiesis. EPO induces mobilization of EPCs from the BM as shown in animal models and humans. Heeschen et al. demonstrated that EPO treatment improved neovascularization in a murine hindlimb ischemia model, and this effect was associated with an increase in the number and proliferation of EPCs (98). These results have been confirmed in humans by the demonstration of a correlation between serum concentration of EPO and number and function of both BM-derived and circulating EPCs in patients with coronary artery disease (99). With regard to SSc vasculopathy, Ferri et al. reported an SSc patient with nonhealing cutaneous ulcers successfully treated with recombinant human erythropoietin (rHuEPO). Before rHuEPO treatment, the BM sample from this patient contained reduced numbers of EPCs, which were functionally impaired. After a 6-month rHuEPO cycle, a marked increase in endothelial progenitor markers was seen, along with a significant reduction in their apoptotic rates (100).
Furuya et al. assessed EPC counts in a small group of patients with SSc and alveolitis. They showed that low-dose i.v. cyclophosphamide (CYC) plus corticosteroid, but not corticosteroid alone, increased the EPC levels. Moreover CYC-induced EPC recruitment was less efficient in SSc patients in comparison with those with other connective tissue disease, thus confirming the well-known impaired differentiation potential of EPC in scleroderma (101).
Other EPC mobilizing cytokines are under investigation in cardiovascular diseases (i.e., G-CSF, VEGF) (94). However, their use at present is hampered by the fact that the intrinsic mechanisms, whereby they alter number and function of EPCs, should be determined in more detail.
Transplantation of EPCs
Direct injection of EPCs into circulation or into the injury site (namely ischemic site) is a therapeutic option that has been shown effective and safe in animal models. However, although promising, little is known about the real benefit of EPC transplantation in several clinical trials. The heterogeneity of the definition and characterization of EPCs results in the use of different cell populations for vascular repair. In this context, interpretation of results from human studies cannot be definitive. In addition, most trials are pilot studies, not controlled and involving small numbers of patients. Furthermore, the rate of homing, incorporation, survival, and long-term follow-up observations are still lacking, with no results regarding the potential cancer risk and/or immunoreactivity. Finally, the consistent evidence of the impairment and reduction of EPCs in diabetes, atherosclerosis, and autoimmune diseases, represents a theoretical obstacle against cell-based therapies with autologous cells. This feature is further evident with regard to SSc, as suggested either by the in vitro evidences (40, 58) and the experimental demonstration of an impaired in vivo neovascularization capacity of EPCs from SSc patients in SCID mice (102). On the other hand, the improvement of EPC isolation and amplification techniques or the use of heterologous cord blood-derived cells might represent a valid future direction of these strategies.
With regard to SSc, similarly to other vascular diseases, the use of autologous EPCs has been largely suggested as a therapeutic option for ulcer healing and other vascular complications related to scleroderma vasculopathy. Local injections of isolated CD34+ cells obtained from PB after mobilization by G-CSF or isolated from BM, probably including EPCs, have been shown to be effective in inducing a rapid and evident beneficial effect on vascular symptoms and ulcer healing (103).
Adipose tissue has been also proposed as a cell source for therapeutic angiogenesis in ischemic diseases. Adipose tissue is mainly composed of two types of cells: mature adipocyte and their precursors, the so-called stromal vascular fraction (SVF) which contains multipotent mesenchymal stem cells, EPCs, pericytes, and macrophages. The ability of SVF to promote angiogenesis and neovascularization has major implications for diseases characterized by poor vascularization, ischemia, and necrosis. Its application has resulted in neovascular formation when applied to acute myocardial infarction, cosmetic procedures, burn wounds, diabetic foot ulcers, and ischemic muscle (104). Prompted by these results, the simple autologous fat grafting (AFG) and other evolved procedures have been used for the treatment of SSc cutaneous complications, such as perioral changes and hand involvement, including skin fibrosis and ulcers (105–107). The present data do not allow us to attribute the reported clinical benefit common to all the studies to a specific subpopulation of cells or a specific mechanism. However, these studies clearly provide evidence that AFG was able to induce neoangiogenesis in the lip and fingers after treatment as suggested by the significant improvement induced by lipofilling in microvascular patterns at labial and digital capillaroscopy (105, 106). Moreover, the AFG in the perioral area induced a neoproliferation of dermal capillaries and reduced the fibrotic changes with partial restoration of the dermal structure, suggesting that the local tissue improvement observed after AFG occurs via a pro-angiogenic process (105). To date, therapeutic mechanisms responsible for angiogenic properties of adipose tissue implantation have not been fully understood. It is likely that both the heterogeneous cellular mixture and growth factors may account for the robust angiogenic and vasculogenic potential confirmed in experimental and human studies (104).
Conclusion
Despite the presence of several stimuli that induce the formation of new vessels such as tissue hypoxia and increased levels of VEGF, appropriate vessel repair does not occur in SSc patients. In SSc, EPCs, usually involved in the mechanisms of vascular repair, have been deeply investigated, with consistent findings showing a significant dysfunction and/or altered cell counts in both PB and BM environment.
In this scenario, a possible therapeutic strategy could be the use of drugs able to induce mobilization, homing, and proliferation of EPCs or alternatively the local or systemic use of purified EPCs or their precursors to treat microvascular damage. Pharmacological interventions with statins or growth factors have shown partially positive results. Few but encouraging studies with different tissue sources of progenitors (including EPCs, mesenchymal stem cells, pericytes) have been carried out in a limited number of patients and so their results are far from conclusive (107–110). Adipose tissue is now under investigation as an alternative source of pro-angiogenic stem cells and pilot studies have demonstrated that these cells may be useful in improving scleroderma-related fibrotic and vascular complications when grafted locally.
Author Contributions
NNDP designed the review organization and wrote it, based on her expertise in the field. FP helped in revising the literature on the subject and writing the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Gabrielli A, Avvedimento E, Krieg T. Scleroderma. N Engl J Med (2009) 360:1989–2003. doi:10.1056/NEJMra0806188
2. Desbois AC, Cacoub P. Systemic sclerosis: an update in 2016. Autoimmun Rev (2016) 15:417–26. doi:10.1016/j.autrev.2016.01.007
3. LeRoy EC, Black C, Fleischmajer R, Jablonska S, Krieg T, Medsger TA Jr, et al. Scleroderma (systemic sclerosis): classification, subset and pathogenesis. J Rheumatol (1988) 15:202–5.
4. Matucci-Cerinic M, Kahaleh B, Wigley FM. Review: evidence that systemic sclerosis is a vascular disease. Arthritis Rheum (2013) 65:1953–62. doi:10.1002/art.37988
5. Asano Y, Sato S. Vasculopathy in scleroderma. Semin Immunopathol (2015) 37:489–500. doi:10.1007/s00281-015-0505-5
6. Rabquer BJ, Koch AE. Angiogenesis and vasculopathy in systemic sclerosis: evolving concepts. Curr Rheumatol Rep (2012) 14(1):56–63. doi:10.1007/s11926-011-0219-1
8. Qian H, Yang Y, Li J, Huang J, Dou K, Yang G. The role of vascular stem cells in atherogenesis and post-angioplasty restenosis. Ageing Res Rev (2007) 6:109–27. doi:10.1016/j.arr.2007.01.001
9. Mostmans Y, Cutolo M, Giddelo C, Decuman S, Melsens K, Declercq H, et al. The role of endothelial cells in the vasculopathy of systemic sclerosis: a systematic review. Autoimmun Rev (2017) 16:774–86. doi:10.1016/j.autrev.2017.05.024
10. Trojanowska M. Cellular and molecular aspects of vascular dysfunction in systemic sclerosis. Nat Rev Rheumatol (2010) 6:453–60. doi:10.1038/nrrheum.2010.102
11. Varga J, Trojanowska M, Kuwana M. Pathogenesis of systemic sclerosis: recent insights of molecular and cellular mechanisms and therapeutic opportunities. J Scleroderma Relat Disord (2017) 2(3):137–52. doi:10.5301/jsrd.5000249
12. Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science (1997) 275:964–7. doi:10.1126/science.275.5302.964
13. Chong MS, Ng WK, Chan JK. Concise review: endothelial progenitor cells in regenerative medicine: applications and challenges. Stem Cells Transl Med (2016) 5:530–8. doi:10.5966/sctm.2015-0227
14. Sukmawati D, Tanaka R. Introduction to next generation of endothelial progenitor cell therapy: a promise in vascular medicine. Am J Transl Res (2015) 7(3):411–21.
15. Peichev M, Naiyer AJ, Pereira D, Zhu Z, Lane WJ, Williams M, et al. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood (2000) 95:952–8.
16. Case J, Mead LE, Besler WK, Prater D, White HA, Saadatzadeh MR, et al. Human CD34+AC133+VEGFR-2+ cells are not endothelial progenitor cells but distinct, primitive hematopoietic progenitors. Exp Hematol (2007) 3:1109–18. doi:10.1016/j.exphem.2007.04.002
17. Tian F, Liang PH, Li LY. Inhibition of endothelial progenitor cell differentiation by VEGI. Blood (2009) 113:5352–60. doi:10.1182/blood-2008-08-173773
18. Hur J, Yoon CH, Kim HS, Choi JH, Kang HJ, Hwang KK, et al. Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arterioscler Thromb Vasc Biol (2004) 24:288–93. doi:10.1161/01.ATV.0000114236.77009.06
19. Medina RJ, O’Neill CL, Sweeney M, Guduric-Fuchs J, Gardiner TA, Simpson DA, et al. Molecular analysis of endothelial progenitor cell (EPC) subtypes reveals two distinct cell populations with different identities. BMC Med Genomics (2010) 3:18–30. doi:10.1186/1755-8794-3-18
20. Medina RJ, Barber CL, Sabatier F, Dignat-George F, Melero-Martin JM, Khosrotehrani K, et al. Endothelial progenitors: a consensus statement on nomenclature. Stem Cells Transl Med (2017) 6:1316–20. doi:10.1002/sctm.16-0360
21. Medina RJ, O’Neill CL, O’Doherty TM, Knott H, Guduric-Fuchs J, Gardiner TA, et al. Myeloid angiogenic cells act as alternative M2 macrophages and modulate angiogenesis through interleukin-8. Mol Med (2011) 17:1045–55. doi:10.2119/molmed.2011.00129
22. Hirschi KK, Ingram DA, Yoder MC. Assessing identity, phenotype, and fate of endothelial progenitor cells. Arterioscler Thromb Vasc Biol (2008) 28:1584–95. doi:10.1161/ATVBAHA.107.155960
23. Kanzler I, Tuchscheerer N, Steffens G, Simsekyilmaz S, Konschalla S, Kroh A, et al. Differential roles of angiogenic chemokines in endothelial progenitor cell-induced angiogenesis. Basic Res Cardiol (2013) 108:310–24. doi:10.1007/s00395-012-0310-4
24. Zhang SJ, Zhang H, Wei YJ, Su WJ, Liao ZK, Hou M, et al. Adult endothelial progenitor cells from human peripheral blood maintain monocyte/macrophage function throughout in vitro culture. Cell Res (2006) 16:577–84. doi:10.1038/sj.cr.7310075
25. Yoder MC, Mead LE, Prater D, Krier TR, Mroueh KN, Li F, et al. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood (2007) 109:1801–9. doi:10.1182/blood-2006-08-043471
26. Gulati R, Jevremovic D, Peterson TE, Chatterjee S, Shah V, Vile RG, et al. Diverse origin and function of cells with endothelial phenotype obtained from adult human blood. Circ Res (2003) 93:1023–5. doi:10.1161/01.RES.0000105569.77539.21
27. Melero-Martin JM, Khan ZA, Picard A, Wu X, Paruchuri S, Bischoff J. In vivo vasculogenic potential of human blood-derived endothelial progenitor cells. Blood (2007) 109:4761–8. doi:10.1182/blood-2006-12-062471
28. Lu W, Li X. Vascular stem/progenitor cells: functions and signaling pathways. Cell Mol Life Sci (2017) 75(5):859–69. doi:10.1007/s00018-017-2662-2
29. Yoon CH, Hur J, Park KW, Kim JH, Lee CS, Oh IY, et al. Synergistic neovascularization by mixed transplantation of early endothelial progenitor cells and late outgrowth endothelial cells: the role of angiogenic cytokines and matrix metalloproteinases. Circulation (2005) 112:1618–27. doi:10.1161/CIRCULATIONAHA.104.503433
30. Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, et al. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res (1999) 85(3):221–8. doi:10.1161/01.RES.85.3.221
31. Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, et al. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med (1999) 5:434–8. doi:10.1038/7434
32. Kopp HG, Ramos CA, Rafii S. Contribution of endothelial progenitors and proangiogenic hematopoietic cells to vascularization of tumor and ischemic tissue. Curr Opin Hematol (2006) 13:175–81. doi:10.1097/01.moh.0000219664.26528.da
33. Aicher A, Zeiher AM, Dimmeler S. Mobilizing endothelial progenitor cell. Hypertension (2005) 45:1–5. doi:10.1161/01.HYP.0000154789.28695.ea
34. Grisar JC, Haddad F, Gomari FA, Wu JC. Endothelial progenitor cells in cardiovascular disease and chronic inflammation: from biomarker to therapeutic agent. Biomark Med (2011) 5:731–44. doi:10.2217/bmm.11.92
35. Menegazzo L, Albiero M, Avogaro A, Fadini GP. Endothelial progenitor cells in diabetes mellitus. Biofactors (2012) 38(3):194–202. doi:10.1002/biof.1016
36. Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, et al. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med (2003) 348(7):593–600. doi:10.1056/NEJMoa022287
37. Shintani S, Murohara T, Ikeda H, Ueno T, Honma T, Katoh A, et al. Mobilization of endothelial progenitor cells in patients with acute myocardial infarction. Circulation (2001) 103(23):2776–9. doi:10.1161/hc2301.092122
38. Gill M, Dias S, Hattori K, Rivera ML, Hicklin D, Witte L, et al. Vascular trauma induces rapid but transient mobilization of VEGFR2(+)/AC133(+) endothelial precursor cells. Circ Res (2001) 88(2):167–74. doi:10.1161/01.RES.88.2.167
39. Distler JH, Beyer C, Schett G, Lüscher TF, Gay S, Distler O. Endothelial progenitor cells: novel players in the pathogenesis of rheumatic diseases. Arthritis Rheum (2009) 60(11):3168–79. doi:10.1002/art.24921
40. Kuwana M, Okazaki Y, Yasuoka H, Kawakami Y, Ikeda Y. Defective vasculogenesis in systemic sclerosis. Lancet (2004) 364(9434):603–10. doi:10.1016/S0140-6736(04)16853-0
41. Del Papa N, Colombo G, Fracchiolla N, Moronetti LM, Ingegnoli F, Maglione W, et al. Circulating endothelial cells as a marker of ongoing vascular disease in systemic sclerosis. Arthritis Rheum (2004) 50(4):1296–304. doi:10.1002/art.20116
42. Allanore Y, Batteux F, Avouac J, Assous N, Weill B, Kahan A. Levels of circulating endothelial progenitor cells in systemic sclerosis. Clin Exp Rheumatol (2007) 1:60–6.
43. Avouac J, Juin F, Wipff J, Couraud PO, Chiocchia G, Kahan A, et al. Circulating endothelial progenitor cells in systemic sclerosis: association with disease severity. Ann Rheum Dis (2008) 67:1455–60. doi:10.1136/ard.2007.082131
44. Nevskaya T, Bykovskaia S, Lyssuk E, Shakhov I, Zaprjagaeva M, Mach E, et al. Circulating endothelial progenitor cells in systemic sclerosis: relation to impaired angiogenesis and cardiovascular manifestations. Clin Exp Rheumatol (2008) 26:421–9.
45. Zhu S, Evans S, Yan B, Povsic TJ, Tapson V, Goldschmidt-Clermont PJ, et al. Transcriptional regulation of Bim by FOXO3a and Akt mediates scleroderma serum-induced apoptosis in endothelial progenitor cells. Circulation (2008) 118:2156–65. doi:10.1161/CIRCULATIONAHA.108.787200
46. Mok MY, Yiu KH, Wong CY, Qiuwaxi J, Lai WH, Wong WS, et al. Low circulating level of CD133+KDR+cells in patients with systemic sclerosis. Clin Exp Rheumatol (2010) 28:S19–25.
47. Andrigueti FV, Arismendi MI, Ebbing PC, Kayser C. Decreased numbers of endothelial progenitor cells in patients in the early stages of systemic sclerosis. Microvasc Res (2015) 98:82–7. doi:10.1016/j.mvr.2015.01.004
48. Kuwana M, Okazaki Y, Kodama H, Satoh T, Kawakami Y, Ikeda Y. Endothelial differentiation potential of human monocyte-derived multipotential cells. Stem Cells (2006) 24(12):2733–43. doi:10.1634/stemcells.2006-0026
49. Yamaguchi Y, Kuwana M. Proangiogenic hematopoietic cells of monocytic origin: roles in vascular regeneration and pathogenic processes of systemic sclerosis. Histol Histopathol (2013) 28(2):175–83. doi:10.14670/HH-28.175
50. Campioni D, Lo Monaco A, Lanza F, Moretti S, Ferrari L, Fotinidi M, et al. CXCR4 pos circulating progenitor cells coexpressing monocytic and endothelial markers correlating with fibrotic clinical features are present in the peripheral blood of patients affected by systemic sclerosis. Haematologica (2008) 93(8):1233–7. doi:10.3324/haematol.12526
51. Yamaguchi Y, Okazaki Y, Seta N, Satoh T, Takahashi K, Ikezawa Z, et al. Enhanced angiogenic potency of monocytic endothelial progenitor cells in patients with systemic sclerosis. Arthritis Res Ther (2010) 12(6):R205. doi:10.1186/ar3180
52. Hur J, Yang HM, Yoon CH, Lee CS, Park KW, Kim JH, et al. Identification of a novel role of T cells in postnatal vasculogenesis: characterization of endothelial progenitor cell colonies. Circulation (2007) 116(15):1671–82. doi:10.1161/CIRCULATIONAHA.107.694778
53. Rodríguez-Carrio J, Alperi-López M, López P, Alonso-Castro S, Ballina-García FJ, Suárez A. Angiogenic T cells are decreased in rheumatoid arthritis patients. Ann Rheum Dis (2015) 74(5):921–7. doi:10.1136/annrheumdis-2013-204250
54. Miao J, Qiu F, Li T, Zhao P, Zhang K, Lv M, et al. Circulating angiogenic T cells and their subpopulations in patients with systemic lupus erythematosus. Mediators Inflamm (2016) 2016:2842143. doi:10.1155/2016/2842143
55. Wilde B, Mertens A, Arends SJ, Rouhl RP, Bijleveld R, Huitema J, et al. Endothelial progenitor cells are differentially impaired in ANCA-associated vasculitis compared to healthy controls. Arthritis Res Ther (2016) 18:147. doi:10.1186/s13075-016-1044-8
56. Manetti M, Pratesi S, Romano E, Bellando-Randone S, Rosa I, Guiducci S, et al. Angiogenic T cell expansion correlates with severity of peripheral vascular damage in systemic sclerosis. PLoS One (2017) 12(8):e0183102. doi:10.1371/journal.pone.0183102
57. Kuwana M, Okazaki Y. Quantification of circulating endothelial progenitor cells in systemic sclerosis: a direct comparison of protocols. Ann Rheum Dis (2012) 71:617–20. doi:10.1136/annrheumdis-2011-200713
58. Distler JH, Allanore Y, Avouac J, Giacomelli R, Guiducci S, Moritz F, et al. EULAR Scleroderma Trials and Research group statement and recommendations on endothelial precursor cells. Ann Rheum Dis (2009) 68:163–8. doi:10.1136/ard.2008.091918
59. Del Papa N, Quirici N, Soligo D, Scavullo C, Cortiana M, Borsotti C, et al. Bone marrow endothelial progenitors are defective in systemic sclerosis. Arthritis Rheum (2006) 54:2605–15. doi:10.1002/art.22035
60. Distler O, Distler JH, Scheid A, Acker T, Hirth A, Rethage J, et al. Uncontrolled expression of vascular endothelial growth factor and its receptor leads to insufficient skin angiogenesis in patients with systemic sclerosis. Circ Res (2004) 95:109–16. doi:10.1161/01.RES.0000134644.89917.96
61. Choi JJ, Min DJ, Cho ML, Min SY, Kim SJ, Lee SS, et al. Elevated vascular endothelial growth factor in systemic sclerosis. J Rheumatol (2003) 30:1529–33.
62. Avouac J, Vallucci M, Smith V, Senet P, Ruiz B, Sulli A, et al. Correlations between angiogenic factors and capillaroscopic patterns in systemic sclerosis. Arthritis Res Ther (2013) 15:R55. doi:10.1186/ar4217
63. Manetti M, Guiducci S, Romano E, Ceccarelli C, Bellando-Randone S, Conforti ML, et al. Overexpression of VEGF165b, an inhibitory splice variant of vascular endothelial growth factor, leads to insufficient angiogenesis in patients with systemic sclerosis. Circ Res (2011) 109(3):e14–26. doi:10.1161/CIRCRESAHA.111.242057
64. Hirigoyen D, Burgos PI, Mezzano V, Duran J, Barrientos M, Saez CG, et al. Inhibition of angiogenesis by platelets in systemic sclerosis patients. Arthritis Res Ther (2015) 17:332. doi:10.1186/s13075-015-0848-2
65. Manetti M, Guiducci S, Matucci-Cerinic M. The crowded crossroad to angiogenesis in systemic sclerosis: where is the key to the problem? Arthritis Res Ther (2016) 18:36. doi:10.1186/s13075-016-0937-x
66. Benyamine A, Magalon J, Cointe S, Lacroix R, Arnaud L, Bardin N, et al. Increased serum levels of fractalkine and mobilisation of CD34+CD45- endothelial progenitor cells in systemic sclerosis. Arthritis Res Ther (2017) 19:60. doi:10.1186/s13075-017-1271-7
67. Qin W, Li Z, Luo S, Wu R, Pei Z, Huang R. Exogenous fractalkine enhances proliferation of endothelial cells, promotes migration of endothelial progenitor cells and improves neurological deficits in a rat model of ischemic stroke. Neurosci Lett (2014) 569:80–4. doi:10.1016/j.neulet.2014.03.052
68. Herlea-Pana O, Yao L, Heuser-Baker J, Wang Q, Wang Q, Georgescu C, et al. Chemokine receptors CXCR2 and CX3CR1 differentially regulate functional responses of bone-marrow endothelial progenitors during atherosclerotic plaque regression. Cardiovasc Res (2015) 106:324–37. doi:10.1093/cvr/cvv111
69. Del Papa N, Quirici N, Scavullo C, Gianelli U, Corti L, Vitali C, et al. Antiendothelial cell antibodies induce apoptosis of bone marrow endothelial progenitors in systemic sclerosis. J Rheumatol (2010) 37:2053–63. doi:10.3899/jrheum.091346
70. Shirai Y, Okazaki Y, Inoue Y, Tamura Y, Yasuoka H, Takeuchi T, et al. Elevated levels of pentraxin 3 in systemic sclerosis: associations with vascular manifestations and defective vasculogenesis. Arthritis Rheumatol (2015) 67(2):498–507. doi:10.1002/art.38953
71. Fornai F, Carrizzo A, Forte M, Ambrosio M, Damato A, Ferrucci M, et al. The inflammatory protein pentraxin 3 in cardiovascular disease. Immun Ageing (2016) 13(1):25. doi:10.1186/s12979-016-0080-1
72. Iwata Y, Yoshizaki A, Ogawa F, Komura K, Hara T, Muroi E, et al. Increased serum pentraxin 3 in patients with systemic sclerosis. J Rheumatol (2009) 36(5):976–83. doi:10.3899/jrheum.080343
73. Avouac J, Cagnard N, Distler JH, Schoindre Y, Ruiz B, Couraud PO, et al. Insights into the pathogenesis of systemic sclerosis based on the gene expression profile of progenitor-derived endothelial cells. Arthritis Rheum (2011) 63:3552–62. doi:10.1002/art.30536
74. Bruhl T, Urbich C, Aicher D, Acker-Palmer A, Zeiher AM, Dimmeler S. Homeobox A9 transcriptionally regulates the EphB4 receptor to modulate endothelial cell migration and tube formation. Circ Res (2004) 94:743–51. doi:10.1161/01.RES.0000120861.27064.09
75. Rössig L, Urbich C, Brühl T, Dernbach E, Heeschen C, Chavakis E, et al. Histone deacetylase activity is essential for the expression of HoxA9 and for endothelial commitment of progenitor cells. J Exp Med (2005) 201:1825–35. doi:10.1084/jem.20042097
76. Manetti M, Guiducci S, Romano E, Avouac J, Rosa I, Ruiz B, et al. Decreased expression of the endothelial cell-derived factor EGFL7 in systemic sclerosis: potential contribution to impaired angiogenesis and vasculogenesis. Arthritis Res Ther (2013) 15(5):R165. doi:10.1186/ar4349
77. Romano E, Chora I, Manetti M, Mazzotta C, Rosa I, Bellando-Randone S, et al. Decreased expression of neuropilin-1 as a novel key factor contributing to peripheral microvasculopathy and defective angiogenesis in systemic sclerosis. Ann Rheum Dis (2016) 75(8):1541–9. doi:10.1136/annrheumdis-2015-207483
78. Nichol D, Stuhlmann H. EGFL7: a unique angiogenic signaling factor in vascular development and disease. Blood (2012) 119(6):1345–52. doi:10.1182/blood-2011-10-322446
79. Avouac J, Guignabert C, Hoffmann-Vold AM, Ruiz B, Dorfmuller P, Pezet S, et al. Role of stromelysin 2 (matrix metalloproteinase 10) as a novel mediator of vascular remodeling underlying pulmonary hypertension associated with systemic sclerosis. Arthritis Rheumatol (2017) 69(11):2209–21. doi:10.1002/art.40229
80. Kassim SY, Gharib SA, Mecham BH, Birkland TP, Parks WC, McGuire JK. Individual matrix metalloproteinases control distinct transcriptional responses in airway epithelial cells infected with Pseudomonas aeruginosa. Infect Immun (2007) 75(12):5640–50. doi:10.1128/IAI.00799-07
81. Burbridge MF, Cogé F, Galizzi JP, Boutin JA, West DC, Tucker GC. The role of the matrix metalloproteinases during in vitro vessel formation. Angiogenesis (2002) 5(3):215–26. doi:10.1023/A:1023889805133
82. Gomez-Rodriguez V, Orbe J, Martinez-Aguilar E, Rodriguez JA, Fernandez-Alonso L, Serneels J, et al. Functional MMP-10 is required for efficient tissue repair after experimental hind limb ischemia. FASEB J (2015) 29(3):960–72. doi:10.1096/fj.14-259689
83. Jimenez SA. Role of endothelial to mesenchymal transition in the pathogenesis of the vascular alterations in systemic sclerosis. ISRN Rheumatol (2013) 2013:835948. doi:10.1155/2013/835948
84. Pardali E, Sanchez-Duffhues G, Gomez-Puerto MC, Ten Dijke P. TGF-β-induced endothelial-mesenchymal transition in fibrotic diseases. Int J Mol Sci (2017) 18:E2157. doi:10.3390/ijms18102157
85. Mendoza FA, Piera-Velazquez S, Farber JL, Feghali-Bostwick C, Jiménez SA. Endothelial cells expressing endothelial and mesenchymal cell gene products in lung tissue from patients with systemic sclerosis-associated interstitial lung disease. Arthritis Rheumatol (2016) 68(1):210–7. doi:10.1002/art.39421
86. Jimenez SA, Piera-Velazquez S. Endothelial to mesenchymal transition (EndoMT) in the pathogenesis of systemic sclerosis-associated pulmonary fibrosis and pulmonary arterial hypertension. Myth or reality? Matrix Biol (2016) 51:26–36. doi:10.1016/j.matbio.2016.01.012
87. Manetti M, Romano E, Rosa I, Guiducci S, Bellando-Randone S, De Paulis A, et al. Endothelial-to-mesenchymal transition contributes to endothelial dysfunction and dermal fibrosis in systemic sclerosis. Ann Rheum Dis (2017) 76(5):924–34. doi:10.1136/annrheumdis-2016-210229
88. Patschan S, Tampe D, Müller C, Seitz C, Herink C, Müller GA, et al. Early Endothelial Progenitor Cells (eEPCs) in systemic sclerosis (SSc) – dynamics of cellular regeneration and mesenchymal transdifferentiation. BMC Musculoskelet Disord (2016) 17:339. doi:10.1186/s12891-016-1197-2
89. Fujita Y, Kawamoto A. Stem cell-based peripheral vascular regeneration. Adv Drug Deliv Rev (2017) 120:25–40. doi:10.1016/j.addr.2017.09.001
90. Khattri S, Zandman-Goddard G. Statins and autoimmunity. Immunol Res (2013) 56:348–57. doi:10.1007/s12026-013-8409-8
91. Pedersen TR. Pleiotropic effects of statins: evidence against benefits beyond LDL-cholesterol lowering. Am J Cardiovasc Drugs (2010) 10:10–7. doi:10.2165/1158822-S0-000000000-00000
92. Llevadot J, Murasawa S, Kureishi Y, Uchida S, Masuda H, Kawamoto A, et al. HMG-CoA reductase inhibitor mobilizes bone marrow-derived endothelial progenitor cells. J Clin Invest (2001) 108:399–405. doi:10.1172/JCI200113131
93. Dimmeler S, Aicher A, Vasa M, Mildner-Rihm C, Adler K, Tiemann M, et al. HMG-CoA reductase inhibitors (statins) increase endothelial progenitor cells via the PI 3-kinase/Akt pathway. J Clin Invest (2001) 108:391–7. doi:10.1172/JCI200113152
94. Besler C, Doerries C, Giannotti G, Lüscher TF, Landmesser U. Pharmacological approaches to improve endothelial repair mechanisms. Expert Rev Cardiovasc Ther (2008) 6:1071–82. doi:10.1586/14779072.6.8.1071
95. Ladak K, Pope JE. A review of the effects of statins in systemic sclerosis. Semin Arthritis Rheum (2016) 45:698–705. doi:10.1016/j.semarthrit.2015.10.013
96. Kuwana M, Kaburaki J, Okazaki Y, Yasuoka H, Kawakami Y, Ikeda Y. Increase in circulating endothelial precursors by atorvastatin in patients with systemic sclerosis. Arthritis Rheum (2006) 54:1946–51. doi:10.1002/art.21899
97. Del Papa N, Cortiana M, Vitali C, Silvestris I, Maglione W, Comina DP, et al. Simvastatin reduces endothelial activation and damage but is partially ineffective in inducing endothelial repair in systemic sclerosis. J Rheumatol (2008) 35:1323–8.
98. Heeschen C, Aicher A, Lehmann R, Fichtlscherer S, Vasa M, Urbich C, et al. Erythropoietin is a potent physiologic stimulus for endothelial progenitor cell mobilization. Blood (2003) 102:1340–6. doi:10.1182/blood-2003-01-0223
99. Lipsic E, Schoemaker RG, van der Meer P, Voors AA, van Veldhuisen DJ, van Gilst WH. Protective effects of erythropoietin in cardiac ischemia: from bench to bedside. J Am Coll Cardiol (2006) 48:2161–7. doi:10.1016/j.jacc.2006.08.031
100. Ferri C, Giuggioli D, Manfredi A, Quirici N, Scavullo C, Colaci M, et al. Recombinant human erythropoietin stimulates vasculogenesis and wound healing in a patient with systemic sclerosis complicated by severe skin ulcers. Clin Exp Dermatol (2010) 35:885–7. doi:10.1111/j.1365-2230.2010.03847.x
101. Furuya Y, Okazaki Y, Kaji K, Sato S, Takehara K, Kuwana M. Mobilization of endothelial progenitor cells by intravenous cyclophosphamide in patients with systemic sclerosis. Rheumatology (Oxford) (2010) 49:2375–80. doi:10.1093/rheumatology/keq259
102. Kuwana M, Okazaki Y. Brief report: impaired in vivo neovascularization capacity of endothelial progenitor cells in patients with systemic sclerosis. Arthritis Rheumatol (2014) 66(5):1300–5. doi:10.1002/art.38326
103. Nevskaya T, Ananieva L, Bykovskaia S, Eremin I, Karandashov E, Khrennikov J, et al. Autologous progenitor cell implantation as a novel therapeutic intervention for ischaemic digits in systemic sclerosis. Rheumatology (Oxford) (2009) 48:61–4. doi:10.1093/rheumatology/ken407
104. Del Papa N, Zaccara E, Andracco R, Maglione W, Vitali C. Adipose-derived cell transplantation in systemic sclerosis: state of the art and future perspectives. J Scleroderma Relat Disord (2017) 2:33–41. doi:10.5301/jsrd.5000222
105. Del Papa N, Caviggioli F, Sambataro D, Zaccara E, Vinci V, Di Luca G, et al. Autologous fat grafting in the treatment of fibrotic perioral changes in patients with systemic sclerosis. Cell Transplant (2015) 24:63–72. doi:10.3727/096368914X674062
106. Del Papa N, Di Luca G, Sambataro D, Zaccara E, Maglione W, Gabrielli A. Regional implantation of autologous adipose tissue-derived cells induces a prompt healing of long-lasting indolent digital ulcers in patients with systemic sclerosis. Cell Transplant (2015) 24:2297–305. doi:10.3727/096368914X685636
107. Granel B, Daumas A, Jouve E, Harlé JR, Nguyen PS, Chabannon C, et al. Safety, tolerability and potential efficacy of injection of autologous adipose-derived stromal vascular fraction in the fingers of patients with systemic sclerosis: an open-label phase I trial. Ann Rheum Dis (2015) 74:2175–82. doi:10.1136/annrheumdis-2014-205681
108. Christopeit M, Schendel M, Föll J, Müller LP, Keysser G, Behre G. Marked improvement of severe progressive systemic sclerosis after transplantation of mesenchymal stem cells from an allogeneic haploidentical-related donor mediated by ligation of CD137L. Leukemia (2008) 22(5):1062–4. doi:10.1038/sj.leu.2404996
109. Guiducci S, Porta F, Saccardi R, Guidi S, Ibba-Manneschi L, Manetti M, et al. Autologous mesenchymal stem cells foster revascularization of ischemic limbs in systemic sclerosis: a case report. Ann Intern Med (2010) 153(10):650–4. doi:10.7326/0003-4819-153-10-201011160-00007
Keywords: systemic sclerosis, endothelial progenitors, stem cells, vasculogenesis, angiogenesis
Citation: Del Papa N and Pignataro F (2018) The Role of Endothelial Progenitors in the Repair of Vascular Damage in Systemic Sclerosis. Front. Immunol. 9:1383. doi: 10.3389/fimmu.2018.01383
Received: 25 December 2017; Accepted: 04 June 2018;
Published: 18 June 2018
Edited by:
Maria Carolina Oliveira, Universidade de São Paulo, BrazilReviewed by:
Mirko Manetti, Università degli Studi di Firenze, ItalyMarco Matucci Cerinic, Università degli Studi di Firenze, Italy
Copyright: © 2018 Del Papa and Pignataro. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nicoletta Del Papa, bmljb2xldHRhLmRlbHBhcGFAYXNzdC1waW5pLWN0by5pdA==
 Nicoletta Del Papa
Nicoletta Del Papa Francesca Pignataro
Francesca Pignataro