- 1Los Angeles Biomedical Research Institute, Harbor-UCLA Medical Center, Torrance, CA, United States
- 2David Geffen School of Medicine at University of California, Los Angeles, CA, United States
- 3NovaDigm Therapeutics, Brookline, MA, United States
A Phase 1b/2a clinical trial of NDV-3A vaccine containing a Candida albicans recombinant Als3 protein formulated with alum protected women <40 years old from recurrent vulvovaginal candidiasis (RVVC). We investigated the potential use of anti-Als3p sera as surrogate marker of NDV-3A efficacy. Pre- and post-vaccination sera from subjects who experienced recurrence of vulvovaginal candidiasis (R) vs. those who were recurrence-free [non-recurrent (NR)] were evaluated. Anti-Als3p antisera obtained were evaluated for (1) titer and subclass profile and (2) their ability to influence C. albicans virulence traits including hyphal elongation, adherence to plastic, invasion of vaginal epithelial cells, biofilm formation on plastic and catheter material, and susceptibility to neutrophil killing in vitro. Serum IgG titers in NR patients were consistently higher than in R patients, particularly for anti-Als3 subclass IgG2. Sera from vaccinated NR patients reduced hyphal elongation, adhesion to plastic, invasion of vaginal epithelial cells, and biofilm formation significantly more than pre-immune sera, or sera from R- or placebo-group subjects. Pre-adsorption of sera with C. albicans germ tubes eliminated these effects, while heat inactivation did not. Finally, sera from NR subjects enhanced neutrophil-mediated killing of C. albicans relative to pre-immune sera or sera from R patients. Our results suggest that higher Als3p antibody titers are associated with protection from RVVC, attenuate C. albicans virulence, and augment immune clearance of the fungus in vitro. Thus, Als3p serum IgG antibodies are likely useful markers of efficacy in RVVC patients vaccinated with NDV-3A.
Introduction
Candida species cause distressing mucocutaneous infections of the integument, oral, and genitourinary tracts. Vulvovaginal candidiasis (VVC) is estimated to occur in 50–75% of women in their childbearing years (1–3) and recurrence of vulvovaginal candidiasis is common (4). Hematogenously disseminated candidiasis is a life-threatening condition of increasing incidence in recent decades (1). Despite the use of antifungal therapy, candidemia is associated with ~40% attributable mortality (5). Compounding these concerns is the alarming rise in emergence of Candida species resistant to antifungal drugs (6).
Candida albicans has multiple putative virulence capabilities including avid adherence to abiotic and host surfaces (7), the capacity to produce tissue-invading filaments (hyphae) (8), and the development of biofilms that promote immune evasion and impede efficacy of antifungal therapy (6). Targeting of these key virulence mechanisms provides opportunities for developing novel therapeutic interventions with minimal effects on the host mycobiome, and reduction in selection pressures that favor drug resistance (9).
NDV-3 is a vaccine containing a His-tagged recombinant version of the C. albicans Als3 protein (Als3p) N-terminus formulated with alum. Expressed on C. albicans hyphae, Als3p promotes adhesion of the fungus to biotic and abiotic substrates, enables invasion of host cell tissues, and facilitates biofilm formation (10, 11). Deletion of the Als3 gene significantly impairs these virulence traits of C. albicans in vitro (10, 11). Consistent with these themes, NDV-3 decreases disease severity caused by Candida species in mice (12–15).
A Phase 1 clinical trial in healthy adults demonstrated safety and immunogenicity of the NDV-3 vaccine as evidenced by robust antibody and T-cell immune responses (13). Furthermore, a single dose of recombinant N-terminus of Als3p without the His-tag formulated with alum (NDV-3A) administered intramuscularly was safe and induced strong antibody and T-cell immune responses in patients with recurrent vulvovaginal candidiasis (RVVC) in a recent exploratory Phase 1b/2a study. This immune response protected patients <40 years of age with a history of RVVC from recurrence over a 12-month study period (16). Specifically, post hoc exploratory analysis revealed a statistically significant increase in the percent of the symptoms-free patients at 12 months post-vaccination (42% vaccinated vs. 22% placebo; p = 0.029) and a doubling time to first symptomatic episode (210 days vaccinated vs. 105 days placebo) for the subset for the patients <40 years of age (n = 137).
The objective of this study is to investigate the role of Als3p antibodies induced by NDV-3A as biomarkers of vaccine efficacy by quantitative and qualitative analysis of antibody titers and by evaluating the effect of these antibodies on C. albicans virulence traits.
Materials and Methods
Serum Samples
All sera used in this study were prepared from blood collected from NDV-3A or placebo recipients in a Phase 1b/2a study in women with RVVC (ClinicalTrials.gov access number, NCT01926028) (16) using previously described methods (13) and were stored at −80°C until analyzed. Sera were obtained from 64 of 66 NDV3-A recipients and 53 of 60 placebo recipients using appropriate collection, processing, and storage practices. In the NDV3-A group, 27 patients had no recurrence of VVC during the 12-month follow-up period and were classified as “non-recurrent” (NR), while 37 patients had one or more recurrences of VVC and were designated “recurrent” (R). For the placebo group, only seven patients were classified as NR, while the rest were classified as R. Because of the low number of NR patients in the placebo arm all comparisons among NR and R patients were confined to NDV-3A vaccinated subjects. For the in vitro studies, matched sera from pre-immune (day 0) and post-vaccination (day 14 or 28) patients or placebo control were compared.
Candida Strain
Candida albicans SC5314 is a well-characterized strain and was the source of the N-terminus of Als3 used to develop the NDV-3A vaccine (17). Routinely, the organism was cultured overnight in yeast peptone dextrose (YPD) broth (Difco) at 30°C with shaking prior to use for in vitro assays. To induce germination, C. albicans blastospores (5 × 106) were grown in RPMI 1640 with l-glutamine (Gibco BRL) for 1 h at 37°C. In all studies, C. albicans cells were washed twice with endotoxin-free Dulbecco’s PBS, suspended in PBS or yeast nitrogen base (YNB, Difco), and counted with a hemocytometer to prepare the final inoculum.
Analysis of Sera Components
Als3p antibody titers in sera were measured using an enzyme-linked immunosorbent assay (ELISA) as previously described (13). To inactivate complement, aliquots of patient sera were independently heated at 55°C for 1 h, added to wells containing C. albicans in YNB medium, and incubated for 24 h at 37°C to permit biofilm development. To adsorb anti-C. albicans antibodies, the sera were incubated with C. albicans germ tubes for 1 h with gentle shaking at room temperature. The mixture was centrifuged at 21,000 g prior to using the cell-free supernatant in the biofilm assay. The presence and/or extent of removal of anti-Als3 antibodies (total IgG, IgG1, and/or IgG2) was measured by ELISA (18).
Peripheral Blood Mononuclear Cell (PBMC) Analysis
Peripheral blood mononuclear cells were collected from vaccinees as previously described (13). PBMCs were evaluated by ELISpot analysis to determine the portion of cells that could be stimulated to produce interferon (IFN)-γ and IL-17A. Results are expressed in spots forming units per 106 cells.
Adhesion and Biofilm Assays
Adhesion and biofilm formation were measured in 96-well polystyrene microtiter plates as previously described (19). Briefly, a 95 µL of C. albicans blastospores (2 × 105 cells/ml in YNB medium) was added to wells containing 5 µL of patient serum (5% serum vol/vol), and incubated at 37°C. Control wells had no serum. After 2 h, wells were washed twice with PBS, and the extent of adhesion was quantified by XTT assay (490 nm) (20). In parallel, cells were grown in the presence of 5% serum for 24 h to promote biofilm formation. Biofilms were washed twice prior to examining by bright field microscopy and quantification by XTT assay (20). Formation of biofilm on the catheter material silicone elastomer (SE) was also assessed (19). Briefly, circular SE pieces were pre-incubated with fetal bovine serum overnight at 25°C, washed twice, and then introduced into the wells. The biofilm assay was conducted as above, in the presence or absence of patient sera.
Invasion Assay
The human Ect1/E6E7 vaginal epithelial cell line was maintained in keratinocyte serum-free medium (Gibco) supplemented with bovine pituitary extract, epidermal growth factor, penicillin/streptomycin, and passaged every 3–4 days as previously described (21). To study the effect of patient sera on C. albicans invasion, fibronectin-coated plastic coverslips were placed in a 24-well plate, and the cells allowed to adhere overnight. After two washes, C. albicans cells were added to wells (fungus:host cell ratio of 5:1) for 12 h in the presence or absence of 5% patient serum. Non-adherent C. albicans was washed away, and the coverslips were stained with concanavalin A (ConA) for 30 min at 37°C. The extent of epithelial cell invasion was visualized by differential staining using a confocal scanning laser microscope (Leica SP2) by overlaying the bright field image with a 594-nm excitation filter (red laser) for ConA. The non-invading yeast was stained, while the invading cells were unstained. The ability of C. albicans to invade the epithelium was expressed as % Invasion defined as: number of C. albicans cells invaded into the epithelium (i.e., unstained hyphae)/total number of C. albicans cells in a single bright field (stained + unstained cells) × 100. At least 20 field per slide were blindly scored and presented as mean % invasion.
Neutrophil Killing Assay
After obtaining institutional review board approved consent (LA Biomed protocol # 11672-07), neutrophils were isolated from blood collected from non-vaccinated human volunteers using endotoxin-free Ficoll-Paque Plus reagent (Amersham Biosciences) (12). Neutrophils were incubated with C. albicans germ tubes containing YNB with 5% serum at 37°C without shaking (neutrophil:fungus ratio, 5:1). Controls contained C. albicans without neutrophils. After 90 min, the mixtures were sonicated to disrupt neutrophils and the surviving fungi quantitatively cultured. The percentage of opsonophagocytic killing (OPK) was calculated by dividing the number of colony forming unit (CFU) in the tubes containing neutrophils by the number of CFU in tubes without neutrophils.
Statistical Analysis
All in vitro studies were performed in triplicate at a minimum, with two biological replicates. Different groups were compared using the non-parametric Wilcoxon rank sum test for pairwise comparisons, and Mann–Whitney test for comparison of unmatched groups. Data were analyzed in GraphPad Prism software (La Jolla, CA, USA), and a p-value <0.05 was considered statistically significant. We estimated how well the in vitro assays of adhesion, biofilm formation, neutrophil killing, and IgG2 titers discriminated between sera of R and NR patients. We performed receiver-operating characteristic (ROC) analysis on GraphPad Prism, which visualizes the sensitivity and specificity characteristics of a particular assay. The y-axis of the ROC graph represents sensitivity, or the true positive rate, i.e., the proportion correctly discriminated or predicted as by the assays. The x-axis represents the component complement of specificity (100% − specificity). Area under the ROC curve [area under the curve (AUC)] is a commonly used measure, where AUC of 1.0 represents a perfect curve fit, while an AUC of 0.5 represents random classification (22). Using ROC, we determined the cut point that maximized the sum of sensitivity and specificity for all the assays.
Results
Antisera From R and NR Subjects Had Distinct Quantitative and Qualitative Antibody Profiles
We analyzed the antibody titers of sera from R or NR patient in an attempt to understand the partial protection elicited by NDV-3A vaccine. We conducted an AUC analysis of the total IgG titers of sera collected from NDV-3A vaccinated subjects over the 12-month period. AUC of total IgG titers from NR patients was significantly higher than those in sera from R patients during the early time points of collection (day 0–90) (Figure 1A). Similarly, the geometric mean of IgG titers of the NR patients at 14 or 28 days post-vaccination was approximately twice as high as that detected in R patients (Figure S1 in Supplementary Material). At later time points (days 90–360), and despite the general drop in Ab titers for both patient populations, the difference in AUC titers of the NR vs. R patients was even greater (Figure 1B). Importantly, while the vast majority of placebo patients had first recurrence within the first 90 days post-vaccination (median recurrence of 53 days), most of the vaccinated subjects had their first recurrence later than this (median recurrence of 94 days) (Figure 1C). Consistent with the Phase 1b/2a clinical trial (16), the enhanced time to recurrence was significant among vaccinees who are <40 years old (p = 0.043). Interestingly, the later recurrence corresponded with the decreased IgG levels beyond 90 days.
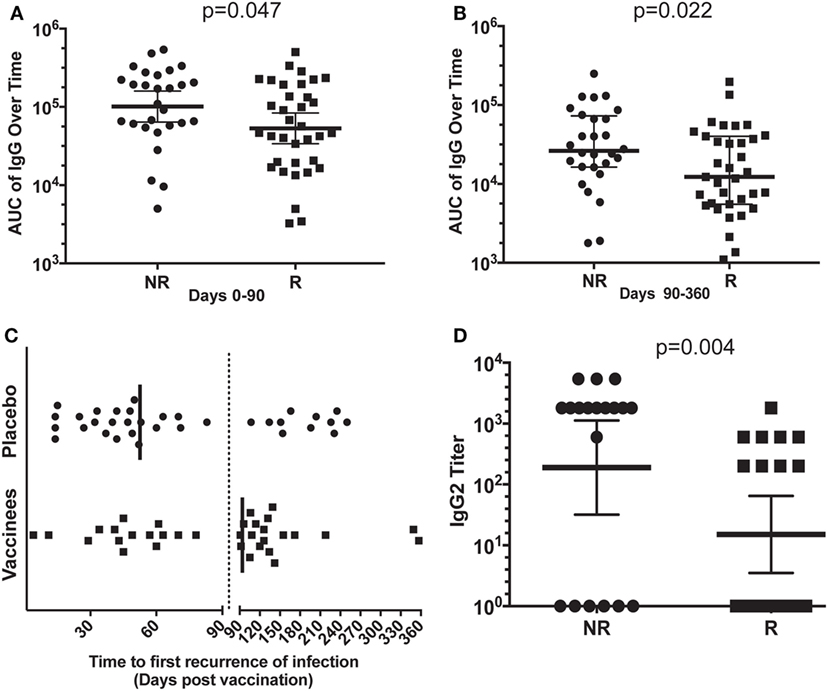
Figure 1. Analysis of the antibody response in vaccinated patients. Mean area under the curve (AUC) of the total IgG titers over time (0–90 and 90–360 days) for each patient, in the non-recurrent (NR) and recurrent (R) NDV-3A-vaccinated subjects was plotted. In the first 3-month post-vaccination, AUC of NR patients was significantly higher (p = 0.046) than that of R patients (A). In months 3–12, this difference in AUC was also significant (p = 0.022) (B). The decrease in AUC of the IgG titers in R patient sera in the later months corresponded with the increase in recurrent episodes of vulvovaginal candidiasis during this period (C). Finally, significantly more number of NR patients displayed IgG2 antibodies in their sera, also mean (geometric) IgG2 antibody titer was higher in NR patients, compared with R patients (p = 0.003) (D). Each dot in (A,B,D) represents antibody titers in each analyzed serum samples from the indicated individual patients. Each dot in (C) represents a first relapse in infection as a function of time post-vaccination. Data in (A,B,D) are presented as geometric mean with 95% confidence interval.
In parallel, the serum antibody profiles were evaluated for anti-Als3 IgG subclasses. The IgG1 subclass comprised the predominant isotype in vaccinated NR and R sera (NR vs. R IgG1 titer, p = 0.9, data not shown). Remarkably, the IgG2 titer in NR sera was much higher than in the R patient sera (Figure 1D), suggesting an isotype-specific enrichment in NR immune responses. Titers of IgG3 and IgG4 were not significantly different in sera of R vs. NR patients (data not shown). Also, we did not find any differences among IFN-γ or IL-17 levels between R vs. NR patients, despite the enhanced level of these two cytokines upon vaccination with NDV-3A (Figure S2 in Supplementary Material). The quantitative and qualitative differences among serum antibodies from R vs. NR patients prompted us to test their effect on C. albicans virulence traits below.
Sera From NR Subjects Reduced C. albicans Adhesion to Plastic
Post-vaccination sera from NR subjects who received NDV-3A significantly reduced adhesion of C. albicans to plastic, compared with pre-vaccination sera from the same patients (Figure 2A). By contrast, post-vaccination sera obtained from R patients who received NDV-3A did not significantly alter C. albicans adhesion relative to pre-vaccination sera (Figure 2B). As expected, sera from patients who received the placebo did not influence C. albicans adhesion to plastic (Figure 2C). Sera from the NR-NDV-3A cohort were the only one that significantly reduced adhesion, compared with the other two groups (Figure 2D).
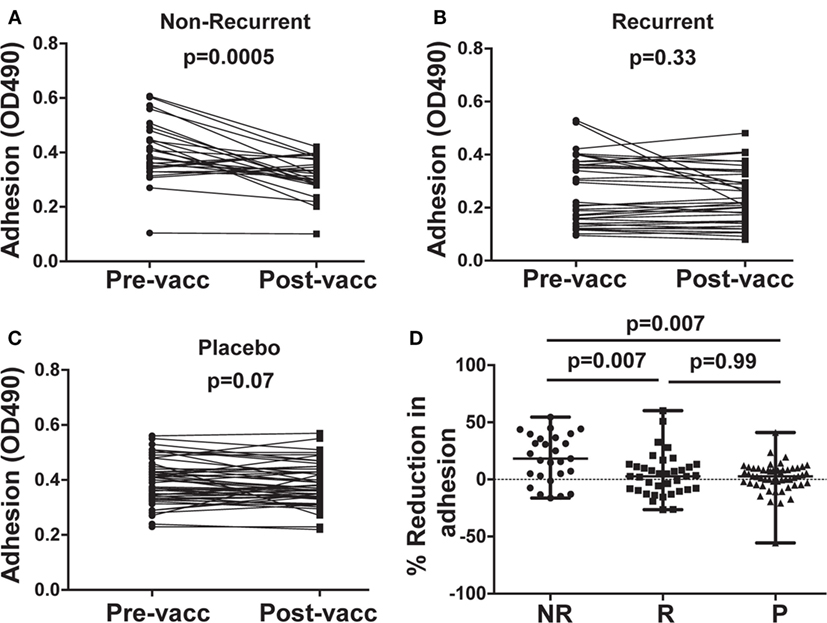
Figure 2. In vitro assessment of Candida albicans adherence to plastic in presence of patient sera. Post-vaccination sera from 27 non-recurrent (NR) patients significantly (p = 0.0005) reduced C. albicans adhesion when compared to their respective pre-vaccination sera (A). There was no difference in the extent of adhesion between pre- and post-vaccination sera from 37 recurrent (R) patients (p = 0.33) (B), or 53 placebo (P) patients (p = 0.067) (C). Percent inhibition of C. albicans adhesion to plastic was significantly higher in post-vaccination sera of NR patients vs. that of R or P patients (D). Data in (D) are presented as median ± interquartile range. Each dot represents alteration in C. albicans adhesion due to an individual patient sample.
Sera From NR Patients Reduced C. albicans Biofilm Development
Post-vaccination sera from NR subjects who received NDV-3A reduced biofilm development when compared with their paired pre-vaccination sera (Figure 3A). This reduction was not observed when cells were incubated with sera from R subjects who received NDV-3A or sera from placebo recipients (Figures 3B,C). As in the adhesion assay, a significantly greater reduction in biofilm formation was observed in sera from NR vs. R patients who received NDV-3A or placebo recipients (Figure 3D).
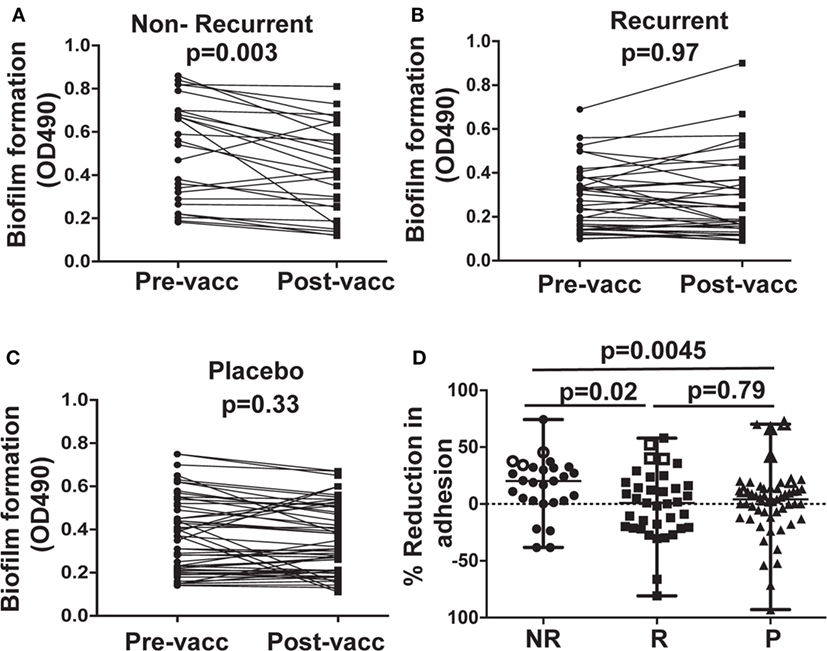
Figure 3. In vitro assessment of Candida albicans biofilm formation in presence of patient sera. Post-vaccination sera from 27 non-recurrent (NR) patients significantly (p = 0.003) reduced C. albicans biofilm formation on 96-well microtiter plates, when compared to their respective pre-vaccination sera (A). There was no difference in the extent of biofilm growth between pre- and post-vaccination sera from 37 recurrent (R) patients (p = 0.97) (B), or 53 placebo (P) patients (p = 0.33) (C). The percent inhibition of C. albicans biofilms to plastic was significantly higher in post-vaccination sera of NR patients vs. that from R or P patients (D). Data in (D) are presented as median ± interquartile range. Each dot represents alteration in C. albicans biofilm formation due to an individual patient sample. The open data points in (D) represent six placebo and six NDV3-A vaccinated patients (three R and three NR) whose sera showed the highest reduction in biofilm formation and were chosen for the vaginal epithelial cell invasion assay presented in (B).
Bare plastic is the gold standard to measure biofilm formation (20). We wanted to confirm that sera samples that prevented biofilm formation on bare-plastic also prevent biofilm formation on SE used in manufacturing catheters. Thus, antisera of NR patients displaying the highest extent of biofilm inhibition were tested for their ability to impede biofilm formation on SE. These sera reduced C. albicans biofilm formation on the SE substrate to an extent similar to that observed in 96-well plates (Figure S3 in Supplementary Material).
Consistent with reduction in biofilm formation, wells containing post-vaccination sera from NR patients also displayed reduced C. albicans adhesion by bright field microscopy, as depicted by reduced density of cells in the bottom of the wells (Figure 4A). This reduction in adhesion to plastic was accompanied by reduced hyphal elongation, but not germ tube induction (Figure S4 in Supplementary Material). By contrast, C. albicans growing in wells containing pre-vaccination sera from the same patient or commercially obtained pooled human serum (controls) displayed robust filamentation and biofilm formation (Figure 4A).
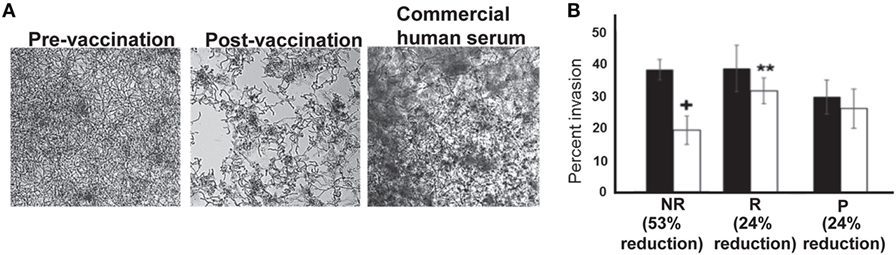
Figure 4. In vitro assessment of Candida albicans filamentation, and invasion of vaginal epithelial cells in presence of patient sera. Representative micrographs (from six different samples in each arm) showing that post-vaccination sera from non-recurrent (NR) patients who abrogated biofilm formation displayed short and wavy hyphae, compared with the normal robust hyphae in the biofilms formed in pre-vaccination serum, or the control commercial pooled human serum (A). Six samples from placebo or NDV-3A-vaccinated patients (three R and three NR) were selected from Figure 2D (open symbols) for analysis in invasion of vaginal epithelial cells. Post-vaccination sera from NR patients inhibited invasion of vaginal epithelial cells twofold more than R patient or P patient sera (B). **p < 0.01 for post- vs. pre-vaccination sera from R patients. + p < 0.05 for post-vaccination sera vs. pre-vaccination sera from NR and vs. post-vaccination sera from R or placebo patients. Data in (B) are presented as mean ± SD.
Sera From NR Patients Reduced C. albicans Invasion of Vaginal Epithelial Cells
Although sera preventing adhesion and biofilm formation were predominantly from the NR group, some R and placebo subject sera also impeded these C. albicans virulence functions. Therefore, sera from the three patients exhibiting the greatest inhibitory effect in each group were compared for pre- and post-vaccination impact on C. albicans capacity to invade vaginal epithelial cells (Figure S5 in Supplementary Material). Antisera from NR patients reduced C. albicans invasion of epithelial cells by ~53%, approximately twofold higher than inhibition displayed by antisera of R patients (24%) (Figure 4B). The sera from placebo patients did not inhibit Candida invasion in this assay (Figure 4B).
The Inhibitory Function of Serum Was Independent of Complement and Likely Associated With Antibodies
To evaluate the possible role of complement, sera from 12 NR patients who received NDV-3A and had the highest decrease in biofilm development were heat-inactivated and then retested in the C. albicans biofilm assay. Inactivated sera retained biofilm inhibition equivalent to that of native sera (Figure 5A), indicating that complement does not play a significant role in the capacity of the sera to inhibit biofilm formation.
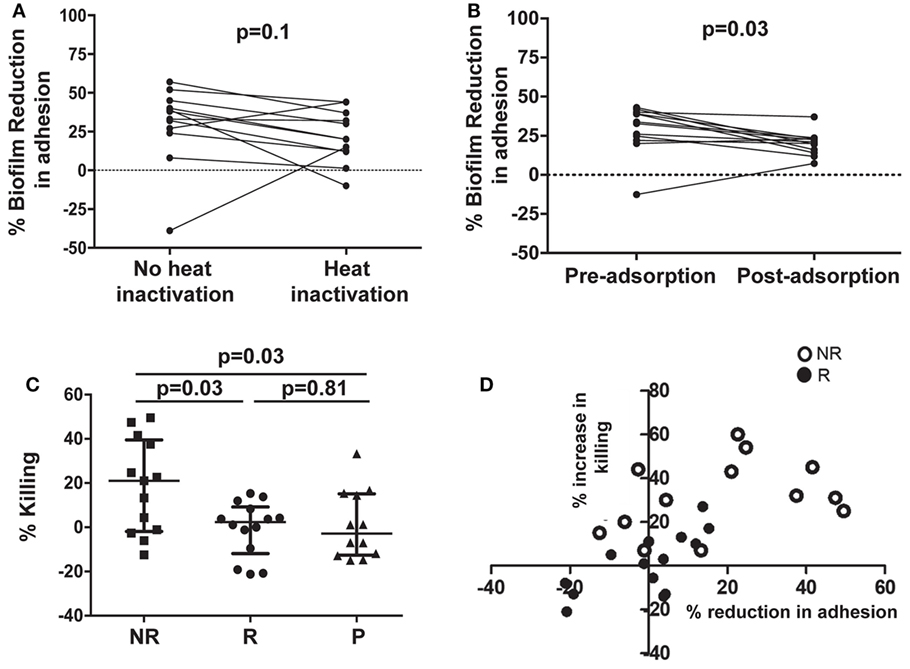
Figure 5. Assessment of the role of antibodies in affecting virulence, and evaluation of opsonophagocytic killing (OPK) of Candida albicans germ tubes in the presence of patient sera. Heat treatment of post-vaccination sera from non-recurrent (NR) patients does not significantly reduce its biofilm-inhibitory activity, compared with paired untreated sera (A). Adsorption of antibodies in post-vaccination sera from NR patients with C. albicans germ tubes significantly (p = 0.03) abolishes the biofilm-inhibitory activity of the sera, when compared with paired adsorbed pre-vaccination sera (B). Only post-vaccination sera from NR patients significantly (p = 0.03) enhance OPK and killing of C. albicans germ tubes by human neutrophils, compared with post-vaccination sera from R or P patients (C). A comparison between percent increase in OPK activity and percent reduction in adhesion, in post-vaccination sera from NR and R patients, resulted in significant correlation within the respective subject sera (D). Each open circle represents individual NR sera, which displayed both an overall greater reduction in adhesion and increase in neutrophil killing. Solid circles denote the individual R patients those who compared with the NR patients, show a smaller % decrease in adhesion as well as neutrophil killing. Negative values on the graph represent % increase in adhesion or % decrease in neutrophil killing.
To determine whether anti-C. albicans antibodies were the active constituent of serum, we incubated the pre- and post-sera with C. albicans germ tubes to adsorb antibodies against Als3p. This process will adsorb antibodies targeting all surface proteins that are expressed on C. albicans germ tubes including those targeting Als3p. Indeed, ELISA plates coated with rAls3p for both day 0 and post-vaccination confirmed that the absorption process significantly reduced the anti-Als3p IgG titers in the samples (Figure S6 in Supplementary Material). Next, these sera were used in C. albicans biofilm assays as detailed above. Adsorption of antibodies from post-vaccination serum reduced their ability to inhibit biofilm formation (Figure 5B).
Sera From NR Patients Enhanced Neutrophil-Mediated Killing of C. albicans
We questioned whether such functionally active sera influenced interactions of the fungus with neutrophils from unvaccinated human volunteers ex vivo. As displayed in Figure 5C, sera from NR patients enhanced neutrophil killing of fungal cells, compared with sera from R or P patients. We also determined whether sera from NR patients who demonstrated the highest reduction in C. albicans adhesion to plastic also exhibited the highest level of neutrophil-mediated killing. We found a strong correlation between the ability of sera from NR patients to reduce adhesion and increased neutrophil-mediated killing (p < 0.05 and R2 of 0.66). Furthermore, overall larger numbers of NR patients (13 NR subjects) induced OPK and prevented adhesion than the R group (13 subjects) (Figure 5D; Figure S7 in Supplementary Material).
ROC Analysis of the In Vitro Assays Predicts Biomarkers of Vaccine Efficacy
To statistically further validate the sensitivity and specificity of each of the in vitro assays, we performed an area under the ROC curve (cvAUROC) analysis for four assays comparing R to NR patient sera for patients who received NDV-3A. Our analysis of IgG2 predict that NR patients have higher IgG2 antibodies than 75% of the R patients (area under curve 0.75, p = 0.008). Furthermore, our data reveal that an IgG2 antibody titer cutoff of above 1,680 (100% sensitivity and >63% specificity) would predict the vaccinated patient to be protected (Figure 6A). Similarly, a high sensitivity/specificity was obtained for the remaining three assays: adhesion (area under curve 0.7, p = 0.007), biofilm reduction (area under curve 0.68, p = 0.012), and neutrophil killing (area under curve 0.75, p = 0.029) (Figures 6B–D). These analyses support such correlations as potential biomarkers of vaccine efficacy.
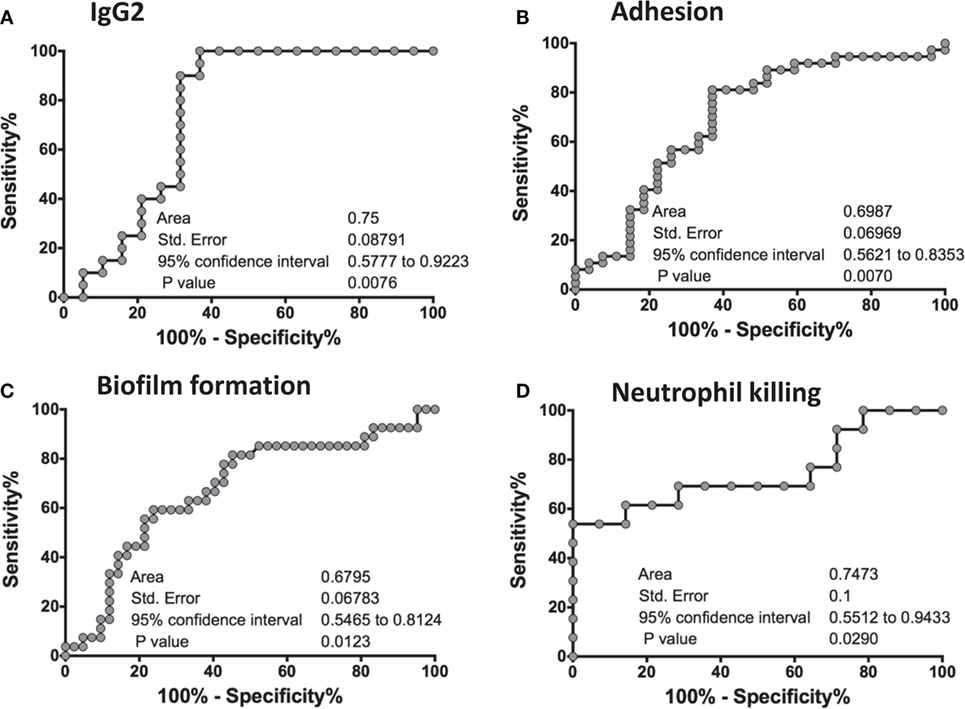
Figure 6. Receiver-operating characteristic (ROC) analysis of the in vitro assays. An ROC analyses for four in vitro studies was performed on GraphPad Prism software, where a graph was generated of 100% − (minus) Specificity % vs. Sensitivity % for each of the assays: IgG2 titers (A), adhesion (B), biofilm formation (C), and neutrophil killing (D). For each graph, an area under the curve (AUC), standard error of the ROC curve, as well as the 95% confidence interval are reported. A p-value of <0.05 in each of the ROC curves concludes that the results are significant, and robust.
Discussion
In this study, we had the unique opportunity to compare humoral immune responses in patients who derived a measurable health benefit from the NDV-3A vaccine vs. those who did not, and vs. placebo patients. In addition, sera from R vs. NR subjects could be compared for their ability to impede key virulence functions of C. albicans in vitro. The study goals were to explore potential surrogate biomarkers of protection that might be useful in future studies of this and more serious Candida infections and to gain insight into the potential mechanism(s) contributing to protective efficacy of the vaccine. Our studies focused on determining the impact of serum antibodies on selected putative virulence factors of C. albicans for three reasons: (1) the NDV-3 vaccine (based on C. albicans Als3 antigen with 6× His tag) induced high antibody titers (13), (2) high antibody titers predict NDV-3 vaccine efficacy in mice (15), and (3) antibodies, including those targeting hyphal regulating protein 1 (23, 24), secreted aspartyl proteinase 2 (25), and Candida cell wall glycopeptides (26, 27), protect against experimental candidiasis. We focused also on Candida adhesion and invasion of vaginal epithelial cells, as well as biofilm formation, since Als3p is a known mediator of these putative virulence functions (10, 11, 28).
Non-recurrent patients maintained a high antibody median titer (AUC) of ≥25,000, while R patients had a median AUC titer of 10,000 after 90 days post-vaccination. This temporal waning of antibody response coincided with the increased frequency to first relapse in R patients. The decrease in antibody titer raises the possibility that R patients may benefit from a booster dose following priming with the vaccine. There was also a significant increase in IgG2 subclass titers in NR patients who received NDV-3A when compared with the R patients who received the vaccine, suggesting that this IgG subclass may be a surrogate marker of protection post-vaccination. Human IgG2 and IgG4, but not IgG1 or IgG3, have been reported to protect mice against Cryptococcus neoformans infection (29), most likely by enhancing the fungicidal activity of macrophages (30). Based on our current data, it is possible that the IgG2 subclass antibody component could impair C. albicans interactions with host tissues, and contribute to neutrophil activation leading to enhanced C. albicans killing by NR antisera. However, an important alternative hypothesis is also of interest that IgG2 and IgG4 antibody are surrogates for non-inflammatory skewing of immune responses, biasing against symptoms of relapse.
Sera from NR patients who received NDV-3A significantly reduced C. albicans adherence to plastic and SE, and impeded invasion of vaginal epithelial cells by C. albicans hyphae more than antisera obtained from R patients who received NDV-3A or those from placebo. Biofilm formation is a function of the ability of C. albicans to adhere to abiotic surfaces. Thus, it was not unexpected that higher levels of anti-Als3p antibodies, as seen in antisera from NR patients but not R patients, would also significantly reduced Candida biofilm formation. As determined from antibody adsorption and complement inactivation studies, such abrogation of these C. albicans virulence functions was due, at least in part, to anti-Als3p antibodies and did not require complement fixation.
Our group previously demonstrated that NDV-3 protects mice from VVC by a mechanism that involves priming of both B cell- and T cell-mediated adaptive immune responses (12). Specifically, anti-Als3p antibodies enhanced the ex vivo killing of C. albicans by neutrophils primed with IFN-γ (12). Although in the current study we could not detect a correlation between IgG titers of NR and R vaccinated subjects and their corresponding IFN-γ levels, antisera from NR patients significantly enhanced the ability of human neutrophils to kill C. albicans ex vivo as compared to antisera from R patients or patients administered placebo. These results are concordant with the finding that RVVC is a disease in which a discordance of exacerbated neutrophil influx often occurs in the face of inefficiency in clearing the infection (31–33). We postulate that in NR women, the vaccine was able to induce an antibody response that: (1) protected against C. albicans adherence to and invasion of mucocutaneous barriers, (2) reduced the capability of the organism to form biofilm from which persistent infection occurs, (3) induced a coordinated phagocyte response that is more efficacious in clearing the infection, and/or (4) modulated profusive inflammatory responses of the host associated with relapse.
The statistical robustness of the in vitro assays of IgG2, adhesion, biofilm formation, and neutrophil killing which were validated in our ROC analyses, revealed that any of these tests could be used as biomarkers of vaccine efficacy. While the latter three assays are likely too cumbersome to support larger clinical trials, an ELISA measuring IgG2 in the sera of vaccinated patients would be a simple yet robust method to predict the protective efficacy of a vaccine in a human clinical study like the current one, or future studies on disseminated candidiasis.
Our forthcoming studies are planned to precisely define the roles of antibody isotypes, the impact of boosting, the combination of multiple antigen vaccines, and the influence of advanced adjuvants to further optimize vaccine and immunotherapeutic strategies targeting Candida species.
Ethics Statements
This study was carried out in accordance with the recommendations of National Institutes of Health guidelines for human subject policies and ethical guidance and regulations with written informed consent from all subjects. All subjects gave written informed consent in accordance with the Declaration of Helsinki. The protocol was approved by the “Los Angeles Biomedical Research Institute IRB.”
Author Contributions
PU designed, performed, supervised the project, and wrote the manuscript. SS performed experiments, designed the ROC, analyzed the data, and revised the manuscript. AA performed the experiments and analyzed the data. CS provided materials and revised the manuscript. JH provided materials and revised the manuscript. MY revised the manuscript. SF contributed to the study design and revised the manuscript. JE contributed to the study design and revised the manuscript. AI designed and supervised the project and wrote the manuscript.
Conflict of Interest Statement
CS and JH are employees and shareholders of NovaDigm Therapeutics. MY, SF, JE, and AI are founders and shareholders of NovaDigm Therapeutics. All other co-authors have no formal association with NovaDigm.
Funding
This work was supported by NIH grant R01 AI063382 to JE, DOD grant W81XWH-11-1-0686 to JH, American Heart Association 16SDG30830012 to PU, and by NovaDigm Therapeutics.
Supplementary Material
The Supplementary Material for this article can be found online at https://www.frontiersin.org/articles/10.3389/fimmu.2018.01349/full#supplementary-material.
Abbreviations
Als3p, agglutinin-like sequence 3 protein; AUC, area under the curve; CFU, colony forming unit; ConA, concanavalin A; ELISA, enzyme-linked immunosorbent assay; Hyr1p, hyphal regulating protein 1; IRB, institutional review board; OPK, opsonophagocytic killing; NR, non-recurrent; NDV-3, recombinant His-tagged N-terminus of Als3p formulated with alum; NDV-3A, recombinant N-terminus of Als3p formulated with alum; RVVC, recurrent vulvovaginal candidiasis; ROC, receiver-operating characteristic; Sap2, secreted aspartyl proteinase 2; SE, silicone elastomer; VVC, vulvovaginal candidiasis; YNB, yeast nitrogen base; YPD, yeast peptone dextrose.
References
1. Brown GD, Denning DW, Gow NA, Levitz SM, Netea MG, White TC. Hidden killers: human fungal infections. Sci Transl Med (2012) 4(165):165rv13. doi:10.1126/scitranslmed.3004404
2. Sobel JD. Vaginal infections in adult women. Med Clin North Am (1990) 74(6):1573–602. doi:10.1016/S0025-7125(16)30496-5
3. Sobel JD, Faro S, Force RW, Foxman B, Ledger WJ, Nyirjesy PR, et al. Vulvovaginal candidiasis: epidemiologic, diagnostic, and therapeutic considerations. Am J Obstet Gynecol (1998) 178(2):203–11. doi:10.1016/S0002-9378(98)80001-X
4. Blostein F, Levin-Sparenberg E, Wagner J, Foxman B. Recurrent vulvovaginal candidiasis. Ann Epidemiol (2017) 27(9):575–82.e3. doi:10.1016/j.annepidem.2017.08.010
5. Wenzel RP. Nosocomial candidemia: risk factors and attributable mortality. Clin Infect Dis (1995) 20(6):1531–4. doi:10.1093/clinids/20.6.1531
6. Ramage G, Rajendran R, Sherry L, Williams C. Fungal biofilm resistance. Int J Microbiol (2012) 2012:528521. doi:10.1155/2012/528521
7. Tsui C, Kong EF, Jabra-Rizk MA. Pathogenesis of Candida albicans biofilm. Pathog Dis (2016) 74(4):ftw018. doi:10.1093/femspd/ftw018
8. Williams DW, Jordan RP, Wei XQ, Alves CT, Wise MP, Wilson MJ, et al. Interactions of Candida albicans with host epithelial surfaces. J Oral Microbiol (2013) 5:22434. doi:10.3402/jom.v5i0.22434
9. Pierce CG, Chaturvedi AK, Lazzell AL, Powell AT, Saville SP, McHardy SF, et al. A novel small molecule inhibitor of Candida albicans biofilm formation, filamentation and virulence with low potential for the development of resistance. NPJ Biofilms Microbiomes (2015) 1:15012. doi:10.1038/npjbiofilms.2015.12
10. Phan QT, Myers CL, Fu Y, Sheppard DC, Yeaman MR, Welch WH, et al. Als3 is a Candida albicans invasin that binds to cadherins and induces endocytosis by host cells. PLoS Biol (2007) 5(3):e64. doi:10.1371/journal.pbio.0050064
11. Zhao X, Daniels KJ, Oh SH, Green CB, Yeater KM, Soll DR, et al. Candida albicans Als3p is required for wild-type biofilm formation on silicone elastomer surfaces. Microbiology (2006) 152(Pt 8):2287–99. doi:10.1099/mic.0.28959-0
12. Ibrahim AS, Luo G, Gebremariam T, Lee H, Schmidt CS, Hennessey JP Jr, et al. NDV-3 protects mice from vulvovaginal candidiasis through T- and B-cell immune response. Vaccine (2013) 31(47):5549–56. doi:10.1016/j.vaccine.2013.09.016
13. Schmidt CS, White CJ, Ibrahim AS, Filler SG, Fu Y, Yeaman MR, et al. NDV-3, a recombinant alum-adjuvanted vaccine for Candida and Staphylococcus aureus, is safe and immunogenic in healthy adults. Vaccine (2012) 30(52):7594–600. doi:10.1016/j.vaccine.2012.10.038
14. Lin L, Ibrahim AS, Avanesian V, Edwards JE Jr, Fu Y, Baquir B, et al. Considerable differences in vaccine immunogenicities and efficacies related to the diluent used for aluminum hydroxide adjuvant. Clin Vaccine Immunol (2008) 15(3):582–4. doi:10.1128/CVI.00427-07
15. Spellberg B, Ibrahim AS, Lin L, Avanesian V, Fu Y, Lipke P, et al. Antibody titer threshold predicts anti-candidal vaccine efficacy even though the mechanism of protection is induction of cell-mediated immunity. J Infect Dis (2008) 197(7):967–71. doi:10.1086/529204
16. Edwards JJE, Schwartz MS, Schmidt CS, Sobel J, Nyirjesy P, Schodel F, et al. A fungal immunotherapeutic vaccine (NDV-3A) for treatment of recurrent vulvovaginal candidiasis—a phase 2 randomized, double-blind, placebo-controlled trial. Clin Infect Dis (2018) 66(12):1928–36. doi:10.1093/cid/ciy185
17. Spellberg BJ, Ibrahim AS, Avanesian V, Fu Y, Myers C, Phan QT, et al. Efficacy of the anti-Candida rAls3p-N or rAls1p-N vaccines against disseminated and mucosal candidiasis. J Infect Dis (2006) 194(2):256–60. doi:10.1086/504691
18. Ibrahim AS, Spellberg BJ, Avanesian V, Fu Y, Edwards JE Jr. The anti-Candida vaccine based on the recombinant N-terminal domain of Als1p is broadly active against disseminated candidiasis. Infect Immun (2006) 74(5):3039–41. doi:10.1128/IAI.74.5.3039-3041.2006
19. Uppuluri P, Chaturvedi AK, Srinivasan A, Banerjee M, Ramasubramaniam AK, Kohler JR, et al. Dispersion as an important step in the Candida albicans biofilm developmental cycle. PLoS Pathog (2010) 6(3):e1000828. doi:10.1371/journal.ppat.1000828
20. Pierce CG, Uppuluri P, Tristan AR, Wormley FL Jr, Mowat E, Ramage G, et al. A simple and reproducible 96-well plate-based method for the formation of fungal biofilms and its application to antifungal susceptibility testing. Nat Protoc (2008) 3(9):1494–500. doi:10.1038/nprot.2008.141
21. Steele C, Fidel PL Jr. Cytokine and chemokine production by human oral and vaginal epithelial cells in response to Candida albicans. Infect Immun (2002) 70(2):577–83. doi:10.1128/IAI.70.2.577-583.2002
22. Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology (1982) 143(1):29–36. doi:10.1148/radiology.143.1.7063747
23. Luo G, Ibrahim AS, French SW, Edwards JE Jr, Fu Y. Active and passive immunization with rHyr1p-N protects mice against hematogenously disseminated candidiasis. PLoS One (2011) 6(10):e25909. doi:10.1371/journal.pone.0025909
24. Luo G, Ibrahim AS, Spellberg B, Nobile CJ, Mitchell AP, Fu Y. Candida albicans Hyr1p confers resistance to neutrophil killing and is a potential vaccine target. J Infect Dis (2010) 201(11):1718–28. doi:10.1086/652407
25. Vilanova M, Teixeira L, Caramalho I, Torrado E, Marques A, Madureira P, et al. Protection against systemic candidiasis in mice immunized with secreted aspartic proteinase 2. Immunology (2004) 111(3):334–42. doi:10.1111/j.1365-2567.2004.01819.x
26. Xin H, Cartmell J, Bailey JJ, Dziadek S, Bundle DR, Cutler JE. Self-adjuvanting glycopeptide conjugate vaccine against disseminated candidiasis. PLoS One (2012) 7(4):e35106. doi:10.1371/journal.pone.0035106
27. Xin H, Cutler JE. Vaccine and monoclonal antibody that enhance mouse resistance to candidiasis. Clin Vaccine Immunol (2011) 18(10):1656–67. doi:10.1128/CVI.05215-11
28. Nobile CJ, Andes DR, Nett JE, Smith FJ, Yue F, Phan QT, et al. Critical role of Bcr1-dependent adhesins in C. albicans biofilm formation in vitro and in vivo. PLoS Pathog (2006) 2(7):e63. doi:10.1371/journal.ppat.0020063
29. Beenhouwer DO, Yoo EM, Lai CW, Rocha MA, Morrison SL. Human immunoglobulin G2 (IgG2) and IgG4, but not IgG1 or IgG3, protect mice against Cryptococcus neoformans infection. Infect Immun (2007) 75(3):1424–35. doi:10.1128/IAI.01161-06
30. Subramaniam KS, Datta K, Marks MS, Pirofski LA. Improved survival of mice deficient in secretory immunoglobulin M following systemic infection with Cryptococcus neoformans. Infect Immun (2010) 78(1):441–52. doi:10.1128/IAI.00506-09
31. Fidel PL Jr, Barousse M, Espinosa T, Ficarra M, Sturtevant J, Martin DH, et al. An intravaginal live Candida challenge in humans leads to new hypotheses for the immunopathogenesis of vulvovaginal candidiasis. Infect Immun (2004) 72(5):2939–46. doi:10.1128/IAI.72.5.2939-2946.2004
32. Peters BM, Yano J, Noverr MC, Fidel PL Jr. Candida vaginitis: when opportunism knocks, the host responds. PLoS Pathog (2014) 10(4):e1003965. doi:10.1371/journal.ppat.1003965
Keywords: Candida albicans, RVVC, Als3p, vaccine, virulence, NDV-3, NDV-3A
Citation: Uppuluri P, Singh S, Alqarihi A, Schmidt CS, Hennessey JP Jr, Yeaman MR, Filler SG, Edwards JE and Ibrahim AS (2018) Human Anti-Als3p Antibodies Are Surrogate Markers of NDV-3A Vaccine Efficacy Against Recurrent Vulvovaginal Candidiasis. Front. Immunol. 9:1349. doi: 10.3389/fimmu.2018.01349
Received: 30 January 2018; Accepted: 31 May 2018;
Published: 15 June 2018
Edited by:
Denise Doolan, James Cook University, AustraliaReviewed by:
Aaron Neumann, University of New Mexico, United StatesFabio Bagnoli, GlaxoSmithKline, Italy
Copyright: © 2018 Uppuluri, Singh, Alqarihi, Schmidt, Hennessey, Yeaman, Filler, Edwards and Ibrahim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ashraf S. Ibrahim, aWJyYWhpbSYjeDAwMDQwO2xhYmlvbWVkLm9yZw==
 Priya Uppuluri
Priya Uppuluri Shakti Singh
Shakti Singh Abdullah Alqarihi
Abdullah Alqarihi Clint S. Schmidt3
Clint S. Schmidt3 John P. Hennessey Jr
John P. Hennessey Jr Michael R. Yeaman
Michael R. Yeaman Ashraf S. Ibrahim
Ashraf S. Ibrahim